Abstract
Introduction
SGLT2 inhibitors are used to reduce the risk of progression of chronic kidney disease (CKD). In patients with type 2 diabetes, they have been found to reduce extracellular volume. Given the high prevalence of extracellular volume expansion and overhydration (OH) in CKD, we investigated whether SGLT2 inhibitors might correct these disturbances in CKD patients.
Methods
CKD patients who started treatment with an SGLT2 inhibitor were investigated in this prospective observational study for 6 months. Body composition and fluid status were measured by bioimpedance spectroscopy. In addition, spot urine samples were analyzed for albuminuria, glucosuria, and urinary aprotinin-sensitive serine protease activity.
Results
Forty-two patients (29% with diabetic/hypertensive CKD, 31% with IgA nephropathy; 88% dapagliflozin 10 mg, 10% dapagliflozin 5 mg, 2% empagliflozin 20 mg; median eGFR 46 mL/min/1.73 m2 and albuminuria 1,911 mg/g creatinine) participated in the study. Median glucosuria increased to 14 (10–19) g/g creatinine. At baseline, patients displayed OH with +0.4 (−0.2 to 2.2) L/1.73 m2, which decreased by 0.5 (0.1–1.2) L/1.73 m2 after 6 months. Decrease of OH correlated with higher OH at BL, decrease of albuminuria, glucosuria, and urinary aprotinin-sensitive protease activity. Adipose tissue mass was not significantly reduced after 6 months.
Conclusion
SGLT2 inhibitors reduce OH in patients with CKD, which is pronounced in the presence of high albuminuria, glucosuria, and urinary aprotinin-sensitive protease activity.
Keywords: SGLT2 inhibitor, Bioimpedance spectroscopy, Chronic kidney disease, Body composition, Overhydration, Proteasuria
Introduction
SGLT2 inhibitors are potent glucose-lowering drugs that also lower body weight. Utilizing bioimpedance spectroscopy, our group has shown that in patients with type 2 diabetes mellitus and normal kidney function, SGLT2 inhibitors reduce body weight by a two-fold effect on extracellular volume and adipose tissue index [1]. Initially, within a few days, SGLT2 inhibition was found to reduce extracellular water (ECW), which was counter-regulated by stimulation of renin activity and normalized after 3 months. Subsequently, body weight loss was driven by reduction of adipose tissue mass of ∼7–12% after 6 months. These effects are in line with the natriuretic and glucosuric effects of SGLT2 inhibitors, leading to a mild volume depletion and calorie loss.
In the meantime, SGLT2 inhibitors emerged as a new therapeutic approach in patients with chronic kidney disease (CKD), independent from the presence of diabetes. They are now used to halt CKD progression and to delay dialysis-dependent CKD on top of blockade of the renin-angiotensin system [2–5]. SGLT2 inhibitors reduce glomerular filtration rate (GFR) up to 15% [6] and proteinuria by one-third [7]. Both effects can be explained by reduction of intraglomerular pressure, which is thought to underly the nephroprotective effects of SGLT2 inhibitors in CKD. However, other mechanisms might also be operative.
CKD patients frequently develop fluid overload and overhydration (OH). In a previous study involving bioimpedance spectroscopy, our group found a gradual increase of OH with advanced stages of CKD and identified proteinuria as the most potent independent predictor of OH [8]. Proteinuria leads to aberrant filtration of large molecular weight serine proteases also termed proteasuria, which are activated in the tubular system and have the potential to also activate the epithelial sodium channel (ENaC) by proteolysis [9–11], thus leading to sodium retention and OH. In a follow-up (FU) study, proteasuria reflected by aprotinin-sensitive urinary serine protease activity and OH was independently associated with a higher risk of CKD progression to end-stage renal disease after 6 years [12].
Currently, it is unknown whether SGLT2 inhibitors affect body composition and fluid status in CKD patients, given that their natriuretic and glucosuric effect is reduced due to a lower GFR. On the other hand, their antiproteinuric effects might facilitate reduction of OH by lowering urinary serine protease activity, all of which might contribute to the nephroprotective effect of SGLT2 inhibitors. Therefore, the present study investigated whether SGLT2 inhibitors had an impact on body composition and fluid status in CKD patients beyond its glucosuric effects.
Methods
Study Design
The study cohort comprised stable CKD patients who consecutively presented to the nephrology outpatient clinic of the University Hospital of Tübingen from February 2021 until July 2022 and were deemed to be treated with an SGLT2 inhibitor by the treating nephrologist. The indication for treatment with an SGLT2 inhibitor was to slow CKD progression after disease-specific treatments such as immunosuppressive treatments and other options were exhausted. Patients were included independently of CKD etiology and the SGLT2 inhibitor prescribed (dapagliflozin or empagliflozin). All patients received maximally tolerated doses of ACE inhibitors/angiotensin receptor blockers. Specified time points for the study visits were baseline (BL, the day of the prescription of the SGLT2 inhibitor) and 1 week, 1 month, 3 months, and 6 months of FU after initiation of the SGLT2 inhibitor. The study was approved by the local Ethics Committee of the University of Tübingen (648/2016BO1). A written informed consent was obtained from all patients. The study was registered at the German Clinical Trials Register (DRKS00028560).
Clinical Assessment of Body Composition and Fluid Status
Body composition and fluid status was measured at every study visit using bioimpedance spectroscopy using the body composition monitor (BCM, Fresenius Medical Care). The device was developed for the detection of fluid overload and the determination of dry weight in patients undergoing dialysis [13] and can be used to detect and quantify fluid overload in CKD patients as described before [8, 12]. Due to measurement of bioimpedance at a spectrum of 50 frequencies between 5 and 1,000 kHz, whereby the low frequencies cannot pass cell membranes, the device differentiates between intra- and extracellular water [14]. Parameters of body composition and body fluid status are calculated by the BCM device using physiological calculation models: the body volume model for intracellular, ECW, and total body water and the body composition model for parameters of lean and adipose tissue. Excess fluid is mainly located in the extracellular compartment and calculated by the BCM device as so-called OH out of normally hydrated lean and adipose tissue masses. Reference values for OH in healthy individuals lie between −1 and +1 L [15]. Values obtained for OH, ECW, intracellular water, and total body water were normalized to a body surface area of 1.73 m2.
Laboratory Analysis and Measurement of Urinary Serine Protease Activity against the Polybasic Tract of Gamma ENaC
Blood and spot urine samples were obtained from every patient at every study visit. Routine laboratory parameters were measured at the Institute for Clinical Chemistry and Pathobiochemistry (Central Laboratory) of the University Hospital Tübingen under accredited conditions and obtained from the LAURIS Order Communication System (Nexus AG/Swisslab). Serum aldosterone concentration and plasma renin activity were measured manually using radioimmunoassays (Immunotech, Prague, Czech Republic; Zentech, Angleur, Belgium) at the nephrology laboratory of the University Hospital of Tübingen.
For the measurement of urinary serine protease activity, an own synthesized substrate with the amino acid sequence FTGRKR was used representing the polybasic tract of the gamma subunit of the ENaC as cleavage site by serine proteases [16]. The peptide substrate was conjugated with the fluorophore 7-amino-4-methylcoumarin, which gives a fluorogenic signal if cleaved from the amino acid sequence. Measurements were done in spot urine samples from BL and 6 months FU. To further improve specificity of the signal, protease activity was measured as aprotinin-sensitive activity with and without coincubation with the serine protease inhibitor aprotinin. Overall, 20 µL spot urine, 10 µL substrate (0.2 mg/mL), and 5 μL aprotinin (0.1 mg/mL) were incubated in a total volume of 100 µL of phosphate-buffered saline for 4 h at 37°C in a black 96-well plate. The fluorescence signal was read at a fluorescence microplate reader (380/460 nm) and normalized for urinary creatinine concentration.
Statistical Analysis
Parameters are reported and illustrated as delta values from BL (or factor of BL in case of plasma renin activity and serum aldosterone concentration due to lognormal distribution), respectively. For description of BL values and delta from BL, median and interquartile range or number and percent of patients are reported. To test for significant changes during the course of complete FU, Friedman test was performed. To test for differences between two respective FU time points or between groups, Wilcoxon-Mann-Whitney test (Wilcoxon signed-rank test/shortly referred to as Wilcoxon test) for paired/dependent or independent samples was performed, respectively. Bonferroni correction was used to correct for multiple testing. For proteasuria, binning of data was performed to respect zero inflation of the data.
For univariate correlations, the Pearson product-moment correlation coefficient was calculated and linear regression model was fitted. For a multivariate linear regression model, variables were selected from all variables with significant correlation in univariate analysis in a stepwise model with forward and backward selection mode.
Statistical significance was defined as a significance threshold of p < 0.05. Data analysis was performed using the statistical software packages R studio version 4.1.2 and Microsoft Office Professional Plus 2019 Excel version 1808.
Results
Characterization of the Study Cohort and Course of General Parameters
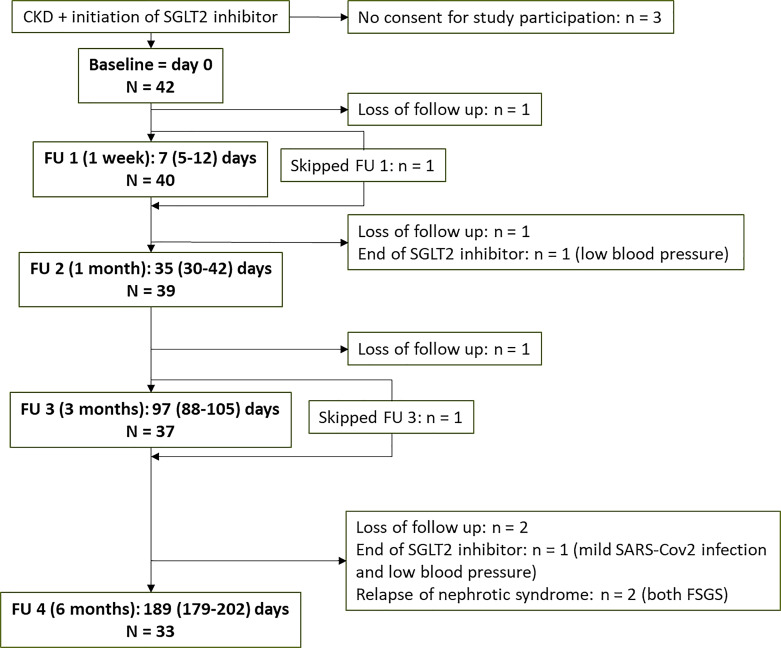
The timeline of the study is shown in Figure 1. N = 42 CKD patients who received SGLT2 inhibitor therapy participated in the study. N = 33 patients completed the full FU at 6 months; in 2 patients, SGLT2 inhibitor was discontinued due to low blood pressure and the patient’s preferences; 2 patients suffered a relapse of nephrotic syndrome and were therefore excluded from further follow-up (Fig. 1).
Fig. 1.
Timeline of the study. CKD, chronic kidney disease; FU, follow-up; FSGS, focal segmental glomerulosclerosis.
BL characteristics of the patients are shown in Table 1. Median age was 55 (interquartile range 38; 70) years, 60% of patients were male, and 40% were female (Table 1). Underlying disease of CKD was diabetic/hypertensive nephropathy in 29%, IgA nephropathy in 31%, ANCA-associated vasculitis in 7%, membranous nephropathy in 7%, lupus nephritis in 7%, and focal segmental glomerulosclerosis in 5% (Table 1). SGLT2 inhibitor dose was dapagliflozin 10 mg in 88%, dapagliflozin 5 mg in 10%, and empagliflozin 20 mg (2 × 10 mg tablet according to the patient’s preferences; Table 1).
Table 1.
Characteristics of study cohort
| BL value (n = 42) | 1 month FU (n = 39) | 6 months FU (n = 33) | ||
|---|---|---|---|---|
| delta to BL | delta to BL | p value | ||
| Dose of SGLT2 inhibitor | Empagliflozin 20 mg (2 × 10 mg tablet), n = 1 (2%), dapagliflozin 5 mg, n = 4 (10%), dapagliflozin 10 mg, n = 37 (88%) | |||
| Cause of CKD | Diabetic/hypertensive nephropathy, n = 12 (29%), IgAN, n = 13 (31%), AAV, n = 3 (7%), MN, n = 3 (7%), SLE, n = 3 (7%), FSGS, n = 2 (5%), other, n = 6 (14%) | |||
| Patients with diabetes mellitus | n = 13 (31%) | |||
| Age, years | 55 (38; 70) | |||
| Sex | Male 25 (60%); female 17 (40%) | |||
| Use of diuretics | ||||
| No diuretic therapy | n = 27 (64%) | n = 29 | n = 26 | |
| Torasemide | n = 7 (21%) | n = 3 (×4 stopped/×1 loss of FU/×1 new) | n = 3 (×1 stopped/×1 loss of FU/×1 restarted) | |
| HCT | 2 (4%) | n = 2 | n = 2 | |
| Chlortalidone | 1 (2%) | n = 0 (stopped) | n = 0 (stopped) | |
| Indapamide | 1 (2%) | n = 1 | n = 1 | |
| Eplerenone | n = 1 (2%) | n = 1 | n = 1 | |
| Torasemide/HCT | n = 1 (2%) | n = 1 | n = 0 (HCT stopped) | |
| Amiloride/HCT | n = 1 (2%) | n = 1 | n = 1 | |
| Torasemide/amiloride/HCT | n = 1 (2%) | n = 1 | n = 1 | |
| BMI, kg/m2 | 27.6 (24.4; 33.6) | −0.2 (−0.6; +0.1) | −0.2 (−0.9; +0.4) | ns |
| GFR (CKD-EPIcrea), mL/min/1.73 m2 | 46 (34; 68) | −1 (−5; 0) | −5 (−9; −1) | 0.0006 |
| Albuminuria, mg/g creatinine | 1,881 (479; 3,788) | −248 (−945; 143) | −680 (−1,225; 32) | 0.0003 |
| OH, L/1.73 m2 | +0.4 (−0.2; +2.2) | −0.4 (−0.9; 0.1) | −0.3 (−0.7; 0) | 0.0065 |
| ECW, L/1.73 m2 | 16.4 (14.8; 18.4) | −0.3 (−1.0; +0.3) | −0.6 (−1.2; +0.2) | 0.0083 |
| FTI, kg/m2 | 12.4 (7.9; 17.8) | 0.1 (−1.2; +1.1) | 0.4 (−1.2; +1.5) | ns |
| Plasma renin act., ng Ang L/mL/h | 6.2 (2.5; 11.7) | 2.1 (−1.2; 5.1) | 0.8 (−0.6; 4.8) | ns |
| Serum aldosterone, pg/mL | 111 (63; 191) | 17 (−19; 80) | 31 (−35; 71) | ns |
| NT-pro-BNP, pg/mL | 211 (94; 589) | −54 (−213; −1) | −16 (−222; 28) | ns |
| Hb, g/dL | 13.3 (12.4; 13.9) | 0.2 (−0.4; 0.9) | 0.5 (0.1; 1.0) | 0.0053 |
| CRP, mg/dL | 0.09 (0.02; 0.42) | 0.00 (−0.03; 0.06) | 0.02 (−0.03; 0.14) | ns |
Values are median (interquartile range) or number (percent). p values are from Wilcoxon tests, with Bonferroni correction for multiple testing.
BL, baseline; FU, follow-up; HCT, hydrochlorothiazide; BMI, body mass index; eGFR, glomerular filtration rate estimated by CKD-EPIcrea formula; OH, overhydration (measured by bioimpedance spectroscopy); ECW, extracellular water (measured by bioimpedance spectroscopy); FTI, fat tissue index (measured by bioimpedance spectroscopy); act., activity; CKD, chronic kidney disease; IgAN, IgA nephropathy; AAV, ANCA-positive vasculitis; MN, membranous glomerulonephritis; SLE, systemic lupus erythematosus, FSGS, focal segmental glomerulosclerosis; ns, not significant.
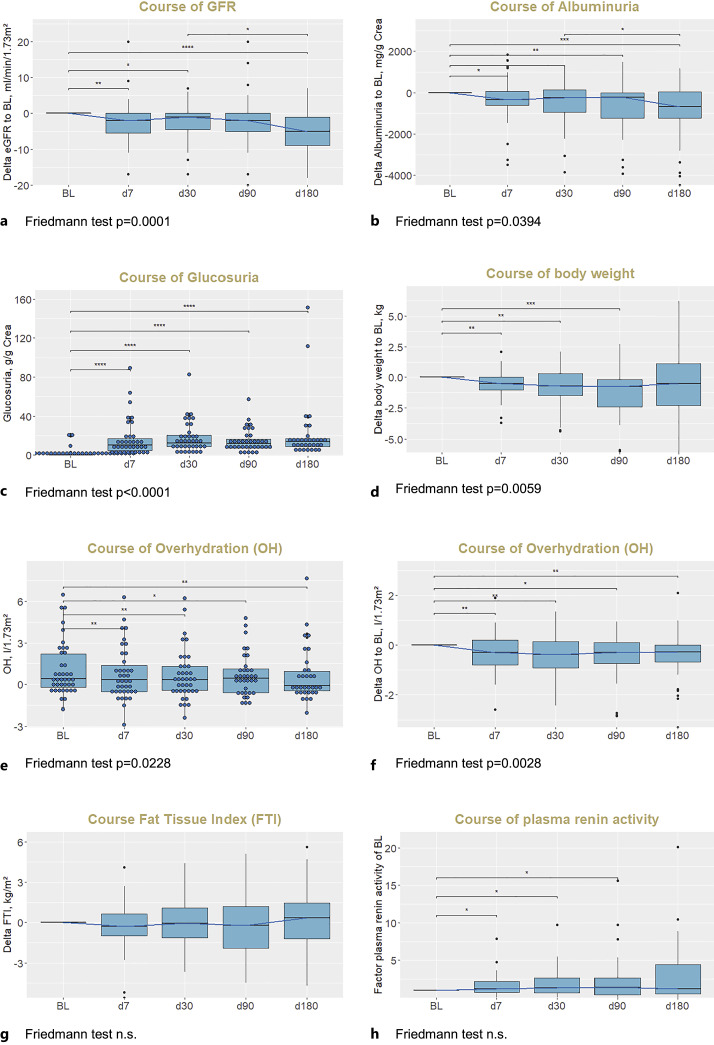
BL estimated GFR by CKD-EPI formula (median and interquartile range) was 46 (34; 68) mL/min/1.73 m2 and decreased significantly by −2 (−6; 0) mL/min/1.73 m2 at FU 1 after 1 week and by −5 (−9; −1) mL/min/1.73 m2 after 6 months (Table 1; Fig. 2a). BL albuminuria was 1,881 (479; 3,788) mg/g creatinine and decreased significantly from the first FU on by −680 (−1,225; 32) mg/g creatinine after 6 months (Table 1; Fig. 2b).
Fig. 2.
Course of GFR (a), albuminuria (b), glucosuria (absolute values, c), body weight (d), OH (absolute values, e, and delta values, f), FTI (g), and plasma renin activity (h). p values are from Wilcoxon tests, with Bonferroni correction for multiple testing: *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, ****p ≤ 0.0001; ns, not significant. Note that the distances of x axis are not representative of FU time intervals. To correct for differences in available values at different FU time points, delta values normalized to the respective BL value are plotted. (e)GFR, (estimated) glomerular filtration rate calculated from plasma creatinine with CKD-EPI formula; BL, baseline, OH, overhydration; FTI, fat tissue index.
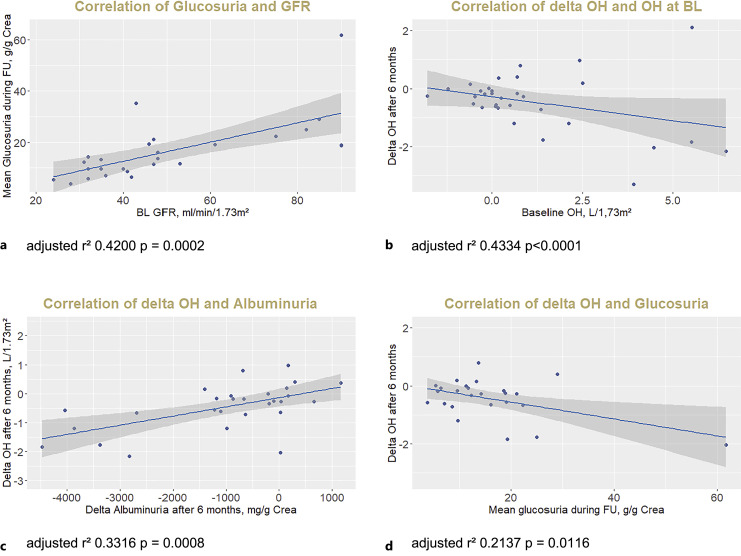
Glucosuria was present in all patients at FU 1 and was stable during FU, with mean value of 14 (10; 19) g/g creatinine (Fig. 2c). Glucosuria was slightly pronounced in patients with diabetes (16 [13; 25] g/g creatinine). Extent of glucosuria significantly correlated with estimated GFR; that is, glucosuria was lower at lower GFR values (Fig. 3a). Natriuresis and kaliuresis measured in spot urine samples did not change significantly during FU (online suppl. Fig. 1a, b; for all online suppl. material, see https://doi.org/10.1159/000535643). Extent of glucosuria did not correlate with delta natriuresis or delta albuminuria (online suppl. Fig. 1c, d).
Fig. 3.
Correlation of glucosuria with GFR (a) and correlation of delta OH with BL OH (b), delta albuminuria (c), and glucosuria (d). GFR, glomerular filtration rate calculated from plasma creatinine with CKD-EPI formula; OH, overhydration (measured by bioimpedance spectroscopy); BL, baseline.
Course of Body Composition and OH and Correlations of Delta OH
Body weight decreased during initial follow-up; however, decrease of body weight was not sustained and did not significantly differ from BL after 6 months (Fig. 2d). OH decreased persistently after initiation of SGLT2 inhibitor with significant difference of OH to BL at all FU time points (Fig. 2e, f). In n = 4 of n = 7 patients with loop diuretic therapy at BL, loop diuretic was stopped during FU (Table 1). In contrast, fat tissue index did not change significantly during FU (Fig. 2g). Parallel to decrease of OH, plasma renin activity increased during FU (Fig. 2h; Table 1) while serum aldosterone concentration did not change significantly (online suppl. Fig. 1e; Table 1); plasma NT-pro-BNP decreased (online suppl. Fig. 1f; Table 1) during FU; and plasma Hb was significantly increased after 6 months compared to BL (Table 1).
Factors associated with the decrease of OH (delta OH) are shown in Table 2. There was a strong univariate correlation of delta OH after 6 months with OH at BL: patients with higher OH at BL showed greater reduction of OH, whereas OH of patients without elevated OH at BL did not decrease during FU (Fig. 2e, Fig. 3b). Delta OH also correlated with delta albuminuria after 6 months (Fig. 3c), mean glucosuria during FU (Fig. 3d), BL P-NT-pro-BNP and delta P-NT-pro-BNP (Table 2). In a multivariate model, BL OH, delta albuminuria, and mean glucosuria during follow-up were independent predictors of delta OH (Table 2). Delta OH did not correlate with BL GFR, delta GFR, BL albuminuria, BL renin or aldosterone, delta Hb, or blood pressure (Table 2).
Table 2.
Factors associated with change of OH after 6 months and univariate and multivariate correlations of delta OH after 6 months
| Univariate correlation | Multivariate model stepwise with forward + backward selection, adjusted r2 = 0.5278, p = 0.0006 | |
|---|---|---|
| Age, years | ns | – |
| Sex | ns | – |
| BL GFR (CKD-EPI), mL/min/1.73 m2 | ns | – |
| Delta GFR after 6 months (CKD-EPI), mL/min/1.73 m2 | ns | – |
| BL albuminuria, mg/g creatinine | ns | – |
| Delta albuminuria after 6 months, mg/g creatinine | Estimate = 0.00032, adjusted r2 = 0.3316, p = 0. 0.0008 | Estimate = 0.00021, p = 0.0192 |
| BL OH, L/1.73 m2 | Estimate = −0.16556, adjusted r2 = 0.08077, p = 0.06314 | Estimate = −0.12999, p = 0.1203 |
| Glucosuria (mean during FU), g/g creatinine | Estimate = −0.02908, adjusted r2 = 0.2137, p = 0.01159 | Estimate = −0.022912, p = 0.0353 |
| BL plasma renin activity, ng Ang L/mL/h | ns | – |
| BL serum aldosterone, pg/mL | ns | – |
| BL plasma NT-pro-BNP, pg/mL | Estimate = −0.0003842, adjusted r2 = 0.0945, p = 0.05454 | Not selected in stepwise model |
| Delta plasma NT-pro-BNP after 6 months, pg/mL | Estimate = 0.61915, adjusted r2 = 0.3587, p = 0.00008 | Not selected in stepwise model |
| Delta Hb after 6 months, g/dL | ns | – |
| BL systolic blood pressure, mm Hg | ns | – |
Delta is delta values at 6 months of FU to BL.
BL, baseline; GFR, glomerular filtration rate (estimated by CKD-EPI formula); OH, overhydration (measured by bioimpedance spectroscopy); ns, not significant.
Course of Urinary Serine Protease Activity against the Polybasic Tract of Gamma ENaC
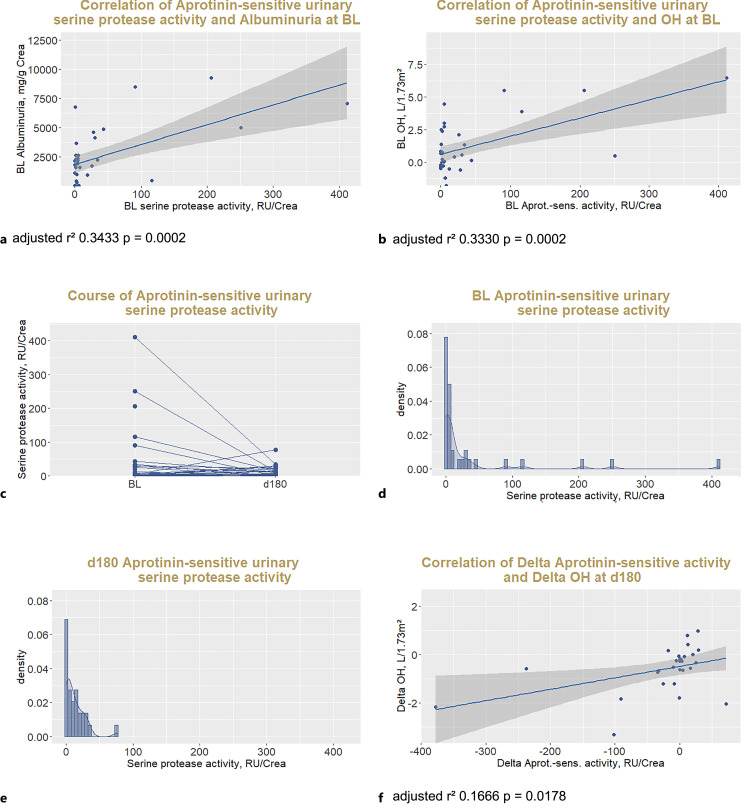
At BL, aprotinin-sensitive urinary serine protease activity against the polybasic tract of gamma ENaC represented by the fluorogenic FTGRKR-amino-4-methylcoumarin substrate was detected in all but n = 9 (22%) of n = 41 patients with available spot urine samples. Aprotinin-sensitive urinary serine protease activity was positively correlated to albuminuria at BL (Fig. 4a).
Fig. 4.
Effect of SGLT2 inhibitor treatment on proteasuria. Correlation of aprotinin-sensitive urinary serine protease activity with albuminuria (a) and OH (b) at BL, course of aprotinin-sensitive activity against the polybasic tract of g-ENaC represented by the substrate FTGRKR-AMC (c), histograms showing the distribution of urinary proteas activity at BL (d) and at 6 months (e), and correlation of delta aprotinin-sensitive urinary serine protease activity with delta OH after 6 months (f). BL, baseline; RU, relative units.
Aprotinin-sensitive urinary serine protease activity correlated positively with OH at BL (Fig. 4b). Aprotinin-sensitive protease activity did not change significantly after initiation of SGLT2 inhibitor as measured after 6 months in the total cohort (p = 0.52, Fig. 4c), however, decreased particularly in patients with high BL aprotinin-sensitive protease activity as seen in the histogram analysis of its skewed distribution (Fig. 4d, e). Decrease of aprotinin-sensitive protease activity correlated with decrease of OH after 6 months (Fig. 4f).
Discussion
This study demonstrates that treatment of CKD patients with an SGLT2 inhibitor leads to a reduction of OH that relates to BL OH, the glucosuric response, and the antialbuminuric effect. At the same time, treatment did not reduce adipose mass in contrast to previous findings in obese patients with diabetes mellitus and normal kidney function in which after an initial reduction of OH adipose tissue continuously decreased [1, 17]. This might be due to the fact that glucosuria and hence loss of calories are blunted in CKD patients with reduced GFR who also are not hyperglycemic. In our study, median glucosuria was 13 g/g creatinine which assuming a daily creatinine excretion of 1.0–1.5 g is far below values reported for patients with diabetes mellitus (up to 67 g/day) [18].
Another important difference to patients with obesity and diabetes mellitus is the finding that OH was highly prevalent in CKD patients reaching values of >6 L/1.73 m2 at BL (Fig. 2e). Our data show that these patients particularly had a benefit from treatment with an SGLT2 inhibitor. Conversely, CKD patients without significant OH did not experience fluid loss and volume depletion, underscoring the good safety profile of SGLT2 inhibitors in CKD patients.
SGLT2 inhibitors have shown blood pressure-lowering effects [19]. We did not find significant changes of blood pressure, which was measured as office blood pressure and not the primary focus of the study. Reduction of fluid overload in addition to natriuretic effects might still be an underlying mechanism of blood pressure control by SGLT2 inhibitors.
Since OH in CKD is the consequence of renal sodium and water retention, reduction of OH in CKD by SGLT2 inhibitors must relate to a natriuretic effect followed by loss of excess water. The failure to note any significant change in natriuresis does not abrogate this notion. First, spot urine samples do not allow to infer sodium excretion over a time period. Second, it also does not take into reflect sodium intake, which still can exceed renal sodium excretion. Also, it must be remembered that natriuresis follows an infradian rhythm with a period up to 1 week [20]. In addition, natriuresis may be altered by activation of renin-angiotensin-aldosterone system [21]. Despite use of renin-angiotensin-aldosterone system inhibitors including mineralocorticoid receptor antagonists in participants, there was evidence of increased renin activity without a significant change of serum aldosterone concentration (Fig. 2g), which might be explained by the fact that all patients received maximally tolerated doses of ACE inhibitors/angiotensin receptor blockers. Of note, renoprotective effects of SGLT2 inhibitors were found to be still present in patients with mineralocorticoid receptor antagonists [22]. In our experience, OH measured by bioimpedance spectroscopy is an excellent surrogate of sodium and water retention, reflecting their balance over the previous days and weeks. Increase of Hb after initiation of SGLT2i was described previously [23] and might also reflect the changes of fluid status with Hb values less diluted after decrease of OH.
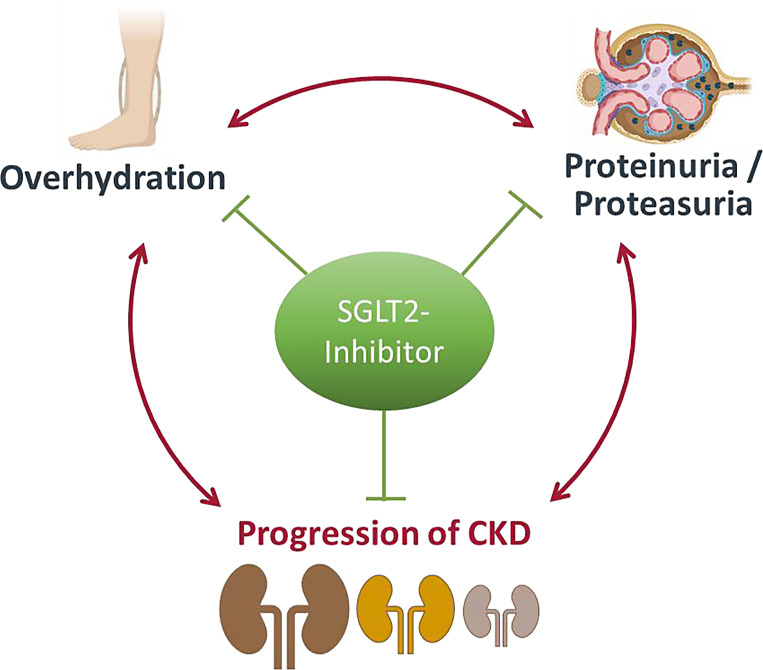
OH is strongly associated with proteinuria and albuminuria in CKD [8]. Confirming earlier findings from phase 3 studies such as the DAPA-CKD or EMPA-KIDNEY, we also noted the reduction of albuminuria by SGLT2 inhibitors in our cohort. We also found that SGLT2 inhibitors reduced proteasuria in patients with high BL urinary serine protease activity. Moreover, reduction of proteasuria was positively correlated with correction of OH at 6 months. Therefore, reduction of proteasuria and attenuation of proteolytic ENaC activation by SGLT2 inhibition might constitute another mechanism by which SGLT2 inhibitors affect OH in proteinuric CKD patients. Both OH and proteasuria are associated with progression of CKD [12] and it is conceivable that both factors might promote structural damage to the nephron. Hence, the correction of OH and reduction of proteasuria by SGLT2 inhibitors might represent another explanation for their nephroprotective effect. Given the interrelation of OH, proteasuria, and progression of CKD, patients with (latent) OH and high proteinuria/proteasuria might benefit particularly from SGLT2 inhibition (Fig. 5). In this context, SGLT2 inhibitors seem to have volume-controlling but not a volume-depleting effect and can therefore be used safely in these patients.
Fig. 5.
Interrelation of OH, proteinuria, and progression of CKD.
The study is limited by its small study size and its observational character and a comparatively short FU period. On the other hand, our analysis is among the first to explore body composition and fluid status in the context of CKD without type 2 diabetes. The use of bioimpedance spectroscopy provides an objective method and results with parameters directly transferable into daily clinical application. Our study focused on the changes of fluid status in particular, which are expected to occur directly after initiation of therapy, and we therefore carried out the first FU measurement after 1 week already. Longer FU might be necessary to fully evaluate changes of body composition, notably changes of lean and fat tissue. We present clinically important findings with implications for the use of SGLT2 inhibitors in CKD patients. We additionally present reduction of proteasuria and implications on the interrelation of OH and proteinuria/proteasuria as new aspects of underlying mechanisms of the nephroprotective effects of SGLT2 inhibitors. Moreover, we have very few losses to FU.
Conclusion
In conclusion, SGLT2 inhibitors inhibit the pathophysiological interaction of OH, proteinuria/proteasuria, and progression of CKD, which presents a potential mechanism for the nephroprotective effects of SGLT2 inhibitors.
Acknowledgments
We thank Dr. Hubert Kalbacher for providing the synthesized peptide substrate. We thank Martina Buttschardt for the support of the measurement of urinary serine protease activity.
Statement of Ethics
The study was approved by the local Ethics Committee of the University of Tübingen (648/2016BO1). A written informed consent was obtained from all patients. The study was registered at the German Clinical Trials Register (DRKS00028560).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
A.S. was supported by the TÜFF program of the Medical Faculty of Tübingen University (2729-0-0). This study was supported by a grant from the German Research Foundation to F.A. (AR 1092/3-1). The funders had no role in the design, data collection, data analysis, and reporting of this study.
Author Contributions
Detailed author contributions are as follows: A.S. obtained the ethics vote, carried out the clinical measurements and collected the follow-up data together with M.-L.E., analyzed and interpreted the data, and drafted the manuscript. M.W. performed the laboratory analyses in the Nephrology Laboratory. B.N.B., D.H., F.E., and E.V. helped with the clinical measurements. N.H. and A.L.B. helped analyzing and interpreting the data and revised the manuscript. F.A. planned the study and helped analyzing and interpreting the data and revised the manuscript. All authors approved the final version of the manuscript submitted.
Funding Statement
A.S. was supported by the TÜFF program of the Medical Faculty of Tübingen University (2729-0-0). This study was supported by a grant from the German Research Foundation to F.A. (AR 1092/3-1). The funders had no role in the design, data collection, data analysis, and reporting of this study.
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request. A preprint version of this article is available on Research Square [https://doi.org/10.21203/rs.3.rs-3343672/v1] [24].
Supplementary Material
References
- 1. Schork A, Saynisch J, Vosseler A, Jaghutriz BA, Heyne N, Peter A, et al. Effect of SGLT2 inhibitors on body composition, fluid status and renin-angiotensin-aldosterone system in type 2 diabetes: a prospective study using bioimpedance spectroscopy. Cardiovasc Diabetol. 2019;18(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436–46. [DOI] [PubMed] [Google Scholar]
- 3. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cherney DZI, Dekkers CCJ, Barbour SJ, Cattran D, Abdul Gafor AH, Greasley PJ, et al. Effects of the SGLT2 inhibitor dapagliflozin on proteinuria in non-diabetic patients with chronic kidney disease (DIAMOND): a randomised, double-blind, crossover trial. Lancet Diabetes Endocrinol. 2020;8(7):582–93. [DOI] [PubMed] [Google Scholar]
- 5. The EMPA-KIDNEY Collaborative Group; Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023;388(2):117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jongs N, Chertow GM, Greene T, McMurray JJV, Langkilde AM, Correa-Rotter R, et al. Correlates and consequences of an acute change in eGFR in response to the SGLT2 inhibitor dapagliflozin in patients with CKD. J Am Soc Nephrol. 2022;33(11):2094–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jongs N, Greene T, Chertow GM, McMurray JJV, Langkilde AM, Correa-Rotter R, et al. Effect of dapagliflozin on urinary albumin excretion in patients with chronic kidney disease with and without type 2 diabetes: a prespecified analysis from the DAPA-CKD trial. Lancet Diabetes Endocrinol. 2021;9(11):755–66. [DOI] [PubMed] [Google Scholar]
- 8. Schork A, Woern M, Kalbacher H, Voelter W, Nacken R, Bertog M, et al. Association of plasminuria with overhydration in patients with CKD. Clin J Am Soc Nephrol. 2016;11(5):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Artunc F, Worn M, Schork A, Bohnert BN. Proteasuria-The impact of active urinary proteases on sodium retention in nephrotic syndrome. Acta Physiol. 2019;225(4):e13249. [DOI] [PubMed] [Google Scholar]
- 10. Svenningsen P, Friis UG, Versland JB, Buhl KB, Møller Frederiksen B, Andersen H, et al. Mechanisms of renal NaCl retention in proteinuric disease. Acta Physiol. 2013;207(3):536–45. [DOI] [PubMed] [Google Scholar]
- 11. Wörn M, Bohnert BN, Alenazi F, Boldt K, Klose F, Junger K, et al. Proteasuria in nephrotic syndrome-quantification and proteomic profiling. J Proteomics. 2021;230:103981. [DOI] [PubMed] [Google Scholar]
- 12. Schork A, Bohnert BN, Heyne N, Birkenfeld AL, Artunc F. Overhydration measured by bioimpedance spectroscopy and urinary serine protease activity are risk factors for progression of chronic kidney disease. Kidney Blood Press Res. 2020;45(6):955–68. [DOI] [PubMed] [Google Scholar]
- 13. Moissl U, Arias-Guillen M, Wabel P, Fontsere N, Carrera M, Campistol JM, et al. Bioimpedance-guided fluid management in hemodialysis patients. Clin J Am Soc Nephrol. 2013;8(9):1575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moissl UM, Wabel P, Chamney PW, Bosaeus I, Levin NW, Bosy-Westphal A, et al. Body fluid volume determination via body composition spectroscopy in health and disease. Physiol Meas. 2006;27(9):921–33. [DOI] [PubMed] [Google Scholar]
- 15. Chamney PW, Wabel P, Moissl UM, Muller MJ, Bosy-Westphal A, Korth O, et al. A whole-body model to distinguish excess fluid from the hydration of major body tissues. Am J Clin Nutr. 2007;85(1):80–9. [DOI] [PubMed] [Google Scholar]
- 16. Wörn M, Kalbacher H, Artunc F. Proteolytic activity against the distal polybasic tract of the gamma subunit of the epithelial sodium channel ENaC in nephrotic urine. Curr Med Chem. 2022;29(42):6433–45. [DOI] [PubMed] [Google Scholar]
- 17. Matsuba I, Takihata M, Takai M, Maeda H, Kubota A, Iemitsu K, et al. Effects of 1-year treatment with canagliflozin on body composition and total body water in patients with type 2 diabetes. Diabetes Obes Metab. 2021;23(12):2614–22. [DOI] [PubMed] [Google Scholar]
- 18. Hu S, Lin C, Cai X, Zhu X, Lv F, Nie L, et al. The urinary glucose excretion by sodium-glucose cotransporter 2 inhibitor in patients with different levels of renal function: a systematic review and meta-analysis. Front Endocrinol. 2021;12:814074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Q, Zhou S, Liu L. Efficacy and safety evaluation of SGLT2i on blood pressure control in patients with type 2 diabetes and hypertension: a new meta-analysis. Diabetol Metab Syndr. 2023;15(1):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Titze J, Dahlmann A, Lerchl K, Kopp C, Rakova N, Schröder A, et al. Spooky sodium balance. Kidney Int. 2014;85(4):759–67. [DOI] [PubMed] [Google Scholar]
- 21. Eickhoff MK, Dekkers CCJ, Kramers BJ, Laverman GD, Frimodt-Møller M, Jørgensen NR, et al. Effects of dapagliflozin on volume status when added to renin-angiotensin system inhibitors. J Clin Med. 2019;8(6):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Provenzano M, Jongs N, Vart P, Stefánsson BV, Chertow GM, Langkilde AM, et al. The kidney protective effects of the sodium-glucose cotransporter-2 inhibitor, dapagliflozin, are present in patients with CKD treated with mineralocorticoid receptor antagonists. Kidney Int Rep. 2022;7(3):436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanbay M, Tapoi L, Ureche C, Tanriover C, Cevik E, Demiray A, et al. Effect of sodium-glucose cotransporter 2 inhibitors on hemoglobin and hematocrit levels in type 2 diabetes: a systematic review and meta-analysis. Int Urol Nephrol. 2022;54(4):827–41. [DOI] [PubMed] [Google Scholar]
- 24. Schork A, Eberbach M-L, Bohnert BN, Woern M, Heister DJ, Eisinger F, et al. SGLT2 inhibitors decrease overhydration and proteasuria in patients with chronic kidney disease: a longitudinal observational study. Kidney Blood Press Res. 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to privacy reasons but are available from the corresponding author upon reasonable request. A preprint version of this article is available on Research Square [https://doi.org/10.21203/rs.3.rs-3343672/v1] [24].