Abstract
Aedes aegypti is the primary vector of dengue fever virus (DENV) worldwide. Infusions made from organic materials have been shown to act as oviposition attractants for Ae. aegypti; however, studies on locally suitable infusion materials are lacking. The current study assessed the suitability of 4 locally available materials as oviposition infusions for use in surveillance and control of Ae. aegypti in Kwale County, Kenya. Oviposition infusion preferences were assessed in laboratory, semifield, and field conditions, using 4 infusions made from banana, grass, neem, and coconut. In addition, ovitrapping in wall, grass, bush, and banana microhabitats was done in 10 houses each in urban and rural coastal households to determine suitable oviposition microhabitats. Overall, the highest oviposition responses were observed for banana infusion, followed by neem and grass infusions, which were comparable. Coconut infusion resulted in the lowest oviposition response. Although female Ae. aegypti did not show preference for any microhabitat, the oviposition activity across all the microhabitats was highly enhanced by use of the organic infusions. Banana, neem, and grass infusions could be used to attract gravid mosquitoes to oviposition sites laced with insecticide to kill eggs. Additionally, banana plantings could be important targets for integrated vector control programs.
Keywords: Aedes aegypti, dengue virus, infusion, microhabitats, oviposition
INTRODUCTION
Aedes aegypti (L.) is the primary vector of dengue fever virus (DENV) worldwide (WHO 2020). This mosquito is also known to transmit other arboviruses, including chikungunya, Zika, and yellow fever viruses (Bancroft 1906, Paupy et al. 2010, Chepkorir et al. 2014). Dengue is the most prevalent mosquito-borne viral illness in the world, and its impact continues to increase, as the virus and its vector expand into more tropical and subtropical regions (Bhatt et al. 2013, WHO 2020). Kenya has reported increased outbreaks of dengue fever along its coast in the last decade (Akhwale 2013, Ellis et al. 2015, WHO 2016, Vu et al. 2017, WHO 2018), corresponding to an increase in abundance of Ae. aegypti in the region (Lutomiah et al. 2016; Agha et al. 2017; Ngugi et al. 2017, 2020). Aedes aegypti are aggressive container breeders that spend their life in defined areas near human blood meal sources owing to their low flight range (Harrington et al. 2005, Guerra et al. 2014). Limiting contact between the vector and the human host is the primary and core strategy exploited for dengue control (WHO 2009).
Although the ecology of Ae. aegypti was actively studied in the coastal area of Kenya in the 1970s–1990s (McDonald 1977, Moore 1979, Trpis and Hausermann 1986, Trpis et al. 1995), there have been few studies during the last 2 decades (Midega et al. 2006, Lutomiah et al. 2016, Ndenga et al. 2017, Ngugi et al. 2017). Particularly, local studies on Ae. aegypti oviposition preferences are lacking. Studies in other regions have postulated that gravid Ae. aegypti actively choose oviposition sites guided by certain factors, including container characteristics such as container size, container fill method, and sun exposure (Harrington et al. 2008, Wong et al. 2011). Other factors which influence Ae. aegypti oviposition site selection include conspecific eggs, larvae, and pupae (Chadee 1993, Wong et al. 2011) and chemical and biological components of the water (Reiter and Colon 1991, Ritchie 2001, Afify et al. 2014, Afify and Galizia 2015). Decomposing organic matter has been shown to produce pheromones that attract or stimulate oviposition activity for a wide range of mosquito species (Santana et al. 2006). The commonly used organic materials for infusions are grass and plant leaves (Reiter and Colon 1991, Chadee 1993, Santana et al. 2006). The organic material generates microbial activity, which in turn is known to mediate oviposition by producing olfactory cues (Afify and Galizia 2015). Age, concentration, and the organic material used influence the efficiency of the organic infusions in attracting gravid female mosquitoes (Chadee 1993, Santana et al. 2006, Santos et al. 2010, Day 2016).
Organic infusions made from different species of grass have been shown to act as oviposition attractants for Ae. aegypti (Reiter and Colon 1991, Chadee 1993). Similarly, organic infusions made from different species of leaves acted as oviposition attractants for Ae. aegypti (Allan and Kline 1995, Ponnusamy et al. 2010). The oviposition attractant and stimulant properties found in organic infusions have been used widely in vector surveillance (Reiter and Colon 1991, Ritchie 2001, Russell and Ritchie 2004, Estallo et al. 2011) and during trapping with organic infusion baited traps (Russell and Ritchie 2004, Maciel-de-Freitas et al. 2008). The Centers for Disease Control and Prevention (CDC) autocidal gravid ovitrap, which exploits grass infusion to attract gravid mosquitoes, has demonstrated effectiveness in suppressing Ae. aegypti population (Barrera et al. 2014a). In other studies, potential for population-wide mosquito density reductions was shown by attracting ovipositing female Ae. aegypti using organic infusions to ovitraps whose substrates were laced with Bacillus thuringiensis israelensis (de Barjac 1978, Santos et al. 2003, Regis et al. 2008).
Gravid mosquitoes consider many factors during selection of oviposition sites, including the nature of the surrounding environment. Studies have demonstrated that there is higher survival of Ae. aegypti eggs and larvae in shaded and/or vegetated surroundings (Vezzani et al. 2005, Vezzani and Albicócco 2009). Whether the gravid mosquitoes preferentially oviposit in in shaded and/or vegetated microhabitats compared to short grass or other nonvegetated microhabitats (bare soil and impervious surfaces) remains unknown.
The current study assessed the suitability of 4 locally available materials as oviposition infusions for use in control of Ae. aegypti. Assessment of local accessible and cheap organic infusions that could be used as bait in available or new traps is required in surveillance and control of Ae. aegypti. A secondary objective of the study was to identify Ae. aegypti preferred oviposition microhabitats. Identification of potential oviposition microhabitats will enhance targeted interventions.
MATERIALS AND METHODS
Study area and population
This study was conducted for 6 months in 2 sites within Kwale County, South Coast, Kenya, each covering 25 km2. The urban site is Ukunda (4°17′59.9994″, 39°31′59.8794″), and the rural site is Msambweni (4°28′0.0114″, 39°28′0.12″), located at approximately 30 and 60 km south of Mombasa, respectively. The coastal climate is tropical: hot and humid throughout the year with annual mean temperatures of 23–34°C and average relative humidity (RH) of 60–80%. Ukunda is a rapidly growing urban center with total population of about 82,651 people (Kwale County Government 2018).
The area is characterized by a proliferation of unplanned residential houses, with unreliable water, sewer, and waste management systems (Kwale County Government 2018, Kenya National Bureau of Statistics 2019a). Most residents engage in small-scale trade, fishing, and casual labor in the tourist industry along the Indian Ocean coast. Msambweni is a rural area with a total population of about 22,524 people, where most of the residents are fisherfolk or subsistence farmers (Kenya National Bureau of Statistics 2019a, 2019b). Residents rely mainly on wells and rainfall for domestic use water, since the piped water system is inadequate and unreliable. The study sites are characterized by 4 seasons: long dry (January–March), long rainy (April–June), short dry season (July–September), and short rainy (October–December) (Mutuku et al. 2011).
Aedes aegypti: Aedes aegypti eggs were collected using ovitraps from the 2 sites (Msambweni and Ukunda) and then submerged in seasoned water in the laboratory. Emerging larvae were reared to adults with fishmeal used as food source (TetraMinbaby® fish food, Melle, Germany) at an average temperature of 28°C and RH of 81% (Ngugi et al. 2017). The emerging adults were identified to species level using standard taxonomic keys (Edwards 1941, Mattingly 1958, Huang 2004). These mosquitoes were fed on 6% glucose solution soaked in cotton wool. The reared mosquitoes were used to perform oviposition experiments I–III. The mosquitoes were fed on fresh cow blood by membrane feeding using a Hemotekt® machine (Hemotek Limited, Unit 5 Union Court, Great Harwood Business Zone, Blackburn, UK) 2 days before each of the experiments. Cow blood was obtained from a slaughterhouse at a nearby market. Twenty milliliters of cow blood were added into the feeder and placed on top of each of the mosquito cages for a maximum of 50 min.
Preparation of infusions
Sun dried plant leaves from Cocos nucifera (L.) (coconut tree), Musa sp. (banana plantain), Azadirachta indica (A. Juss) (neem tree), and mixed grass were used. All the leaves used in this study area are widely distributed in coastal Kenya and thus readily available for vector control, if deemed appropriate. Each type of leaf and grass measuring 33.6 g (Trexler et al. 1998) was chopped into small pieces using a knife and placed in separate 5 liter plastic jerrycans. Four liters of rainwater were added to each jerrycan; then jerrycans were covered and allowed to ferment for 7 days (Trexler et al. 1998). Each infusion was filtered using a plastic sieve, and the filtrate was collected in separate containers that were labeled appropriately. To achieve the desired infusion concentration (100%, 75%, 50%, and 25%), the initial fermented froth was diluted in the ratio (infusion:water) of 1:0, 3:1, 1:1, and 1:3, respectively.
Experiment I: Suitable infusion concentration for oviposition
To determine the ideal infusion concentration for each of the prepared organic infusions, 80 ml of 100%, 75%, 50%, and 25% of each of the infusions were added to separate plastic cups (100 ml) lined with paper towels (10 cm × 5 cm) for oviposition. Each infusion concentration was placed in such a way that they were 30 cm apart in 1 cage by infusion type and labeled appropriately. Twenty gravid Ae. aegypti were aspirated into each cage. Cotton wool soaked in 6% glucose solution was placed on top of each cage for mosquito feeding (Fig. 1A). The cages were separated by 2 feet (60 cm) and were rotated by 90 degrees every 6 h during daytime to minimize shade and light confounding effects (Fig. 1A). The set-up was maintained for 4 days, after which the ovicups were removed from each cage and the oviposited eggs counted under a dissecting microscope (40×) (Nikon®, SMZ Japan) using a manual counter. Ten replicates were used for this experiment.
Fig. 1.
Set-up for suitable infusion concentration for (A) oviposition and (B) preferred infusion in laboratory setting experiments.
Experiment II: Preferred infusion in a laboratory setting
Ovipositional attractiveness of neem, grass, banana, and coconut leaf infusions was evaluated in a laboratory setting using F1 4–5 days old Ae. aegypti collected from the 2 study sites. A measure of 80 ml of the 4 different infusion types at 50% concentration was put in each of the100 ml ovicups lined with paper towels (Fig. 1B). All 4 ovicups were placed 30 cm apart in the same cage. An additional ovicup with 80 ml of tap water was added in the cage as a control (Fig. 1B). Twenty blood fed Ae. aegypti were aspirated into the cage and allowed to lay eggs within 4 days. Cotton wool soaked in 6% glucose solution was placed at the top of the cage for mosquito feeding. Ten replicates of this experiment were performed with the cages placed 2 feet apart. After 4 days, the ovicups were removed from each cage and the oviposited eggs counted using a counter under a dissecting microscope.
Experiment III: Preferred infusion in a semifield setting
A measure of 300 ml of each of 50% concentration of neem, banana, coconut, and grass infusions, and untreated tap water (as control) were added to respective 350 ml black plastic ovicups. A brown paper towel (20 cm × 8 cm) was lined on the inside of the cup and partially submerged to act as an oviposition substratum. The infusion concentrations were placed 80 cm apart in same cage (1 m×1 m×1 m) placed in a sheltered place in the field to prevent further dilution from rain. Twenty gravid Ae. aegypti were aspirated into the cage. Cotton wool soaked in 6% glucose solution was placed at the top of the cage for mosquito feeding. The cages were placed 10 m apart, and the set-up allowed to stay for 4 days, after which ovicups were removed from each cage and the oviposited eggs counted using a counter under the dissecting microscope. This experiment was replicated 10 times.
Experiment IV: Preferred oviposition microhabitats in a field setting
Bushes (comprising of mainly shrubs), bananas, and grass were observed as common types of vegetation in both rural (Msambweni) and urban (Ukunda) study areas. We sought to find out if Ae. aegypti preferred to oviposit in containers located in any of these microhabitats. A microhabitat was identified when 75% of flora within a 5 m radius comprised of either bananas, grass, or bushes. A wall was included as the 4th microhabitat to act as a control. Ten houses each in Msambweni and Ukunda with all 4 microhabitats were identified in the peridomestic area for this experiment. One modified ovitrap was placed at ground level for every microhabitat weekly for 6 months (July to December 2017). Except for the wall, all the other microhabitats were located 5–20 m from the house. For the wall microhabitat, the ovitrap was placed right beside the wall at locations where there is likely to be minimum disturbance. The ovitraps consisted of half liter black plastic cups filled with 350 ml of water. The water used in the ovitraps was sourced from the respective households. The common sources of water for the study area households included taps, borehole, and rain. The oviposition substratum was a brown paper towel (20 cm×8 cm) lining the inside of the cup and partially submerged. The ovitraps were exposed for oviposition for 5 days, after which the paper towels were removed, wrapped in white tissue paper (to absorb excess water), and placed in plastic ziplock bags which were labeled with date, house number, and microhabitat. They were then placed in a cool box and transported to the laboratory. In the laboratory, each paper towel was removed from its protective bag and examined under a dissecting microscope (40×) for presence of eggs, which were counted and recorded. To confirm the species, the eggs were submerged in seasoned tap water in rearing trays to hatch. The resulting larvae were reared at an average temperature of 28°C and RH of 81%, and the emerging adults identified using taxonomic keys (Edwards 1941, Mattingly 1958, Huang 2004).
Experiment V: Preferred infusion in different microhabitats in a field setting
Microhabitats of banana, bush, grass, and wall were randomly selected in Msambweni rural households. For banana, bush, and grass microhabitats, a specific microhabitat was selected if 75% of flora within 5 m radius comprised the respective plants. Only houses where all 4 microhabitats could be identified in the peridomestic environment were included in this experiment. Five ovicups each containing 50% of neem, grass, coconut, banana, and tap water (control) infusions were placed in each of the 4 microhabitats. The ovicups were separated at a distance of 50 cm apart on the ground. The oviposition substratum was a brown paper towel (20 cm×8 cm) lining the inside of the cup and partially submerged. The ovitraps were exposed for oviposition for 5 days, after which the paper towels were removed, wrapped in white tissue paper (to absorb excess water) and placed in plastic ziplock bags, which were labeled appropriately. They were then placed in a cool box and transported to the laboratory. In the laboratory, each paper towel was removed from its protective bag and examined under a dissecting microscope (40×) for presence of eggs, which were counted and recorded. To confirm the species, the eggs were submerged in seasoned tap water in rearing trays to hatch. The resulting larvae were reared at an average temperature of 28°C and RH of 81%, and the emerging adults identified using taxonomic keys (Edwards 1941, Mattingly 1958, Huang 2004). The experiment was performed in different households weekly for 12 consecutive weeks (12 replicates).
Ethical Considerations
The study was part of a larger study that had been ethically approved by the scientific and ethics review unit (SERU) of Kenya Medical Research Institute (KEMRI; SSC No. 2611) and Stanford University IRB (protocol number 31488). Study objectives and procedures were explained to the community leaders and residents of the study area. Written, informed consent was obtained from household owners/heads to permit mosquito surveys.
Data analysis
Descriptive analyses were used to analyze the data. The preferred Ae. aegypti organic infusion concentration (experiment I) was determined by comparing ovipositional responses among the different concentrations using 1-factor analysis of variance (ANOVA) test, with Tukey’s HSD post hoc test. Oviposition activity in the laboratory and semifields setting (experiments II and III) was also determined by comparing ovipositional responses in the different organic infusions using 1-factor analysis of variance (ANOVA) test, with Tukey’s HSD post hoc test. Distribution of egg count data from preferred microhabitats and preferred infusion in different microhabitat experiments in the field setting (experiments IV and V respectively) fitted a negative binomial frequency distribution. A negative binomial regression was therefore used to compare oviposition activity in different microhabitats and in different infusions per microhabitat. Egg counts in the different microhabitats were compared by negative binomial regression using the generalized estimating equations (GEE) procedure (PROC GENMOD, SAS version 9.3; SAS Institute 2011), with microhabitats and site (rural/urban) as the factors. Because eggs were collected from the same house every 5 days for 6 months, the model included an exchangeable correlation structure to adjust for correlations due to repeated eggs collections at the same house. Oviposition activity of the different organic infusions in the different microhabitats was assessed by negative binomial regression using a generalized estimating equations (GEE) procedure (PROC GENMOD, SAS version 9.3; SAS Institute 2011), with microhabitats and organic infusions as the factors. A new set of microhabitats was selected for every replicate, and thus the repeated measurement option was not included in the model.
The oviposition attractant/repellent property of each organic infusion was scored by oviposition activity index (OAI) (Kramer and Mulla 1979) and calculated as follows.
where NT denotes the mean number of eggs laid in the infusion and NS denotes the mean number of eggs laid in the control water. All the index values fall within the range of +1 to −1. The positive value indicates that more eggs were laid by the gravid mosquito females in the infusion than in the control (water), which provides an indication that the infusion was an oviposition attractant/stimulant. Conversely, more eggs laid in the control than infusion would result in a negative OAI value, signifying the treated chemical to be an oviposition repellent/deterrent.
In this paper, the phrase “oviposition attractant” refers to the positive ovipositional responses which may include oviposition attractance or stimulant property or a combination of both. Similarly, an “oviposition repellent” refers to the negative ovipositional responses, which may act on the ovipositing gravid females by repellent or deterrent mode or a combination of both properties. Accordingly, infusions with OAI of +0.30 and above are considered as oviposition attractants/stimulants, while those with −0.30 and below are considered as repellents/deterrents. The OAI value was multiplied by 100 to get the corresponding percentage equivalents of oviposition response for each observation.
RESULTS
Experiment I: Suitable infusion concentration
There was no significant difference in the number of eggs oviposited per infusion concentration (F = 1.52; df = 3; P = 0.2119). However, the highest number of eggs were retrieved from the 50% infusion concentration (Table 1) and the least from 25% infusion concentration. Therefore, 50% infusion concentration was used in all the subsequent experiments.
Table 1.
Ovipositional responses of Aedes aegypti eggs to 4 concentrations of 4 different organic infusions.
| Infusion concentration | Banana mean ± SE |
Coconut mean ± SE |
Grass mean ± SE |
Neem mean ± SE |
Total mean ± SE |
|---|---|---|---|---|---|
| 25% | 78.7 ± 11.6 | 58.5 ± 9.2 | 65 ± 27.0 | 63.4 ± 19.4 | 66.4 ± 8.8 |
| 50% | 118.5 ± 36.3 | 61.4 ± 15.1 | 80.4 ± 20.6 | 106.2 ± 31.0 | 91.6 ± 13.5 |
| 75% | 65 ± 16.4 | 62.3 ± 9.9 | 61.8 ± 13.4 | 71.7 ± 16.9 | 65.2 ± 7.0 |
| 100% | 132.8 ± 8 | 58.8 ± 18.2 | 90.1 ± 28.5 | 51.4 ± 19.5 | 83.3 ± 11.6 |
| Mean ± SE | 98.8 ± 11.8 | 60.3 ± 6.5 | 74.3 ± 11.3 | 74.2 ± 11.2 |
Experiment II: Preferred infusion in laboratory setting
In the laboratory, Ae. aegypti females strongly preferred to lay eggs in the banana infusion (F = 12.25; df = 4; P < 0.0001) compared to all the other infusions. Even though greater numbers of eggs were laid in the grass infusion compared to the coconut infusion, the difference was not significant (Fig. 2). The number of eggs laid in the neem infusion was comparable to that of the control (water) and was significantly lower compared to grass and coconut infusions. Compared to the control, all the infusions except neem were good oviposition stimulants in the laboratory setting. Oviposition activity index (OAI) indicated all the infusions were oviposition stimulants, with banana and grass infusions showing strongest attractance and neem infusion the weakest (Table 2).
Fig. 2.
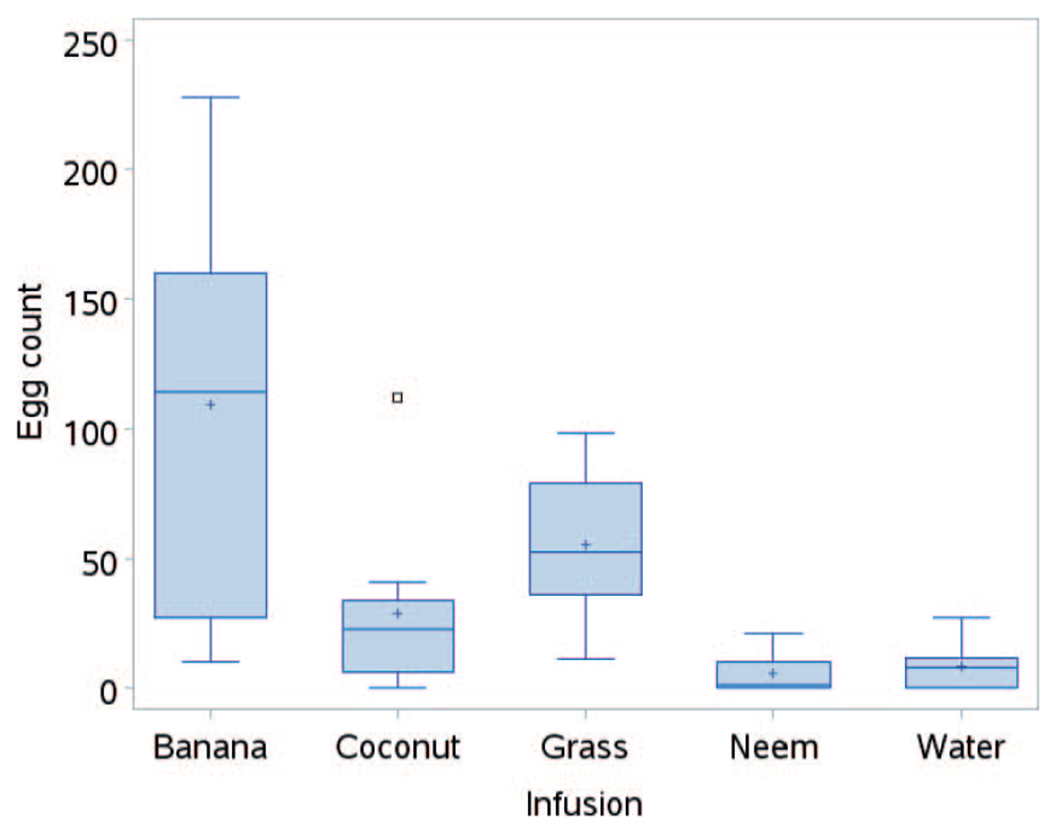
Boxplots of number of eggs laid by Aedes aegypti females in different infusions in the laboratory.
Table 2.
Aedes aegypti infusion preference in laboratory, semifield, and field settings
| Infusion | Laboratory (N = 50) |
Semifield (N = 50) |
Field (N = 240) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Mean ± SE | OAI | Total | Mean ± SE | OAI | Total | Mean ± SE | OAI | |
| Banana | 1,093 | 109.3 ± 23.2 | 0.86 | 1,355 | 135.5 ± 26.6 | 0.82 | 5,017 | 104.52 ± 12.19 | 0.52 |
| Coconut | 286 | 28.6 ± 10.3 | 0.55 | 733 | 73.3 ± 31.4 | 0.69 | 3,153 | 65.69 ± 6.94 | 0.33 |
| Grass | 551 | 55.1 ± 9.4 | 0.74 | 775 | 77.5 ± 19.8 | 0.71 | 4,369 | 91.02 ± 13.39 | 0.46 |
| Neem | 56 | 5.6 ± 2.5 | −0.20 | 415 | 41.5 ± 11.0 | 0.51 | 5,455 | 113.65 ± 15.69 | 0.55 |
| Water | 84 | 8.4 ± 2.6 | — | 133 | 13.3 ± 3.3 | — | 1,596 | 33.25 ± 7.48 | — |
| Total | 2,070 | 41.4 ± 7.6 | 3,411 | 68.2 ± 10.8 | 19,590 | 81.62 ± 5.50 | |||
Experiment III: Preferred infusion in semifield setting
In the semifield setting, banana infusion remained the overall preferred infusion (F = 4.72; df = 4; P < 0.0029). The mean numbers of eggs laid in grass (77.5) and coconut (73.3) infusions were not significantly different from those in the banana (135.5) infusion (Fig. 3). Significantly fewer eggs were laid in neem and water (control) infusions compared to all the other infusions (Table 2; Fig. 3). The numbers of eggs laid in neem and water infusions were comparable. Ovipositional responses ranged from 51% to 82%, indicating that all the organic infusions were oviposition stimulants (Table 2).
Fig. 3.
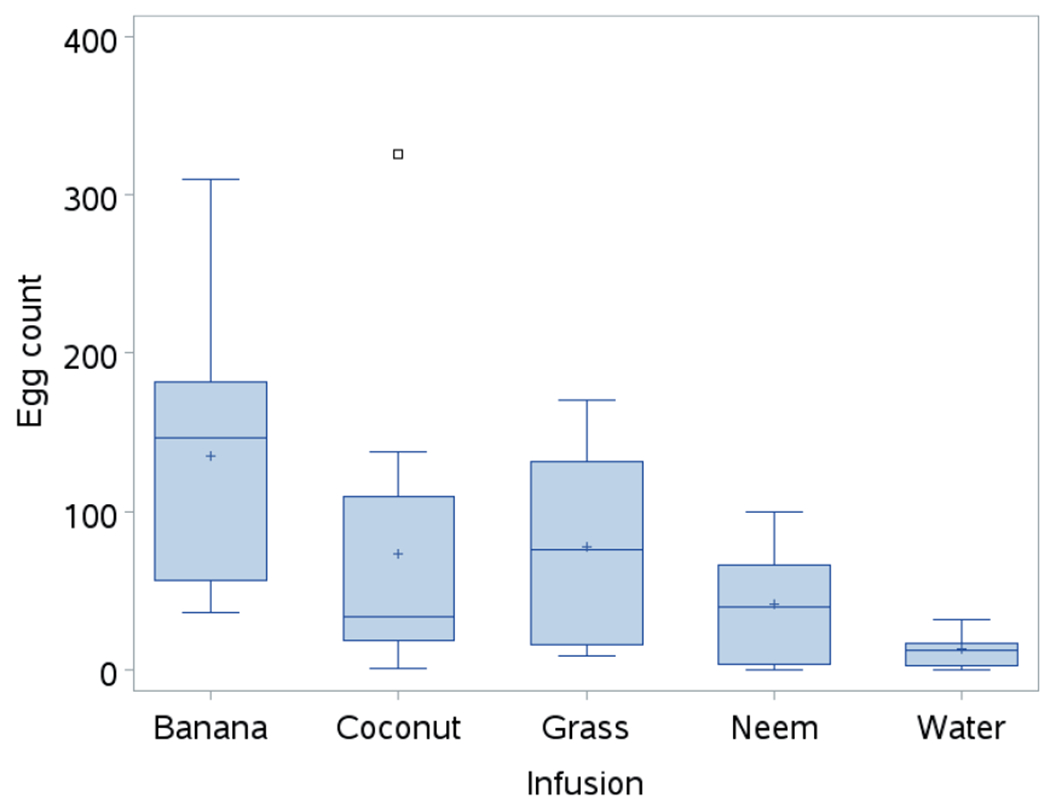
Mean number of eggs laid by Aedes aegypti females in different infusions in the semifield conditions.
Experiment IV: Preferred oviposition microhabitats in field setting
In total, 73,876 eggs were collected from 2080 ovitraps during the 6 months of trapping (July to December 2017) in Msambweni (rural) and Ukunda (urban). The overall ovitrap positivity index (OPI; the percentage of positive ovitraps) was 36%. OPI was significantly higher in the urban site compared to the rural site (χ2 = 4.22, P = 0.0398). However, OPI was not different among the microhabitats (χ2 = 5.73, P = 0.1254), with wall having the largest positive ovitraps and banana having the least (Table 3). Slightly more eggs were trapped in the urban site compared to the rural site, but the difference was not significant (Table 3). In the field setting, Ae. aegypti females laid significantly more eggs in banana and bush microhabitats (vegetated microhabitats) compared to the wall (Table 3). The mean numbers of eggs laid in grass and wall microhabitats were comparable (Table 3).
Table 3.
Results of regression analysis using generalized estimating equations of eggs trapping in 4 microhabitats in Ukunda and Msambweni sites in Kwale County.
| Parameter | OPI | Total eggs | Mean ± SE | Parameter estimate1 | 95% CI | Z score | P |
|---|---|---|---|---|---|---|---|
| Microhabitat | |||||||
| Banana | 33.85 | 22,155 | 42.61 ± 2.05 | 0.32 | 0.18, 0.46 | 4.51 | <0.0001 |
| Bush | 34.23 | 18,653 | 35.87 ± 1.71 | 0.15 | 0.01, 0.28 | 2.06 | 0.0390 |
| Grass | 35.77 | 16,924 | 32.55 ± 1.66 | 0.05 | −0.09, 0.19 | 0.6 | 0.4965 |
| Wall (reference) | 40.19 | 16,144 | 31.05 ± 1.66 | — | — | — | — |
| Site (rural/urban) | |||||||
| Msambweni | 33.85 | 36,584 | 35.14 ± 1.21 | −0.07 | −0.17, 0.11 | −0.40 | 0.6922 |
| Ukunda (reference) | 38.17 | 37,328 | 35.89 ± 1.32 | — | — | — | — |
Wall and Ukunda were used as reference categories.
Experiment V: Preferred infusion in different microhabitats in a field setting
In total, 19,590 eggs were collected from 240 infusion-enriched ovitraps in the 4 microhabitats: banana, bush, grass, and wall at Msambweni site. Although female Ae. aegypti did not show preferences for any microhabitat, the oviposition activity across all the microhabitats were highly enhanced by use of the organic infusions (Table 4). The mean numbers of eggs laid in banana and bush microhabitats were higher compared to grass and wall microhabitats. All the organic infusions performed significantly better compared to the control (Table 4). The overall OPI for the microhabitats and organic infusions was each 93%. This was significantly higher in the organic infusions compared to the control (χ2 = 18.74, P=0.0009). However, OPI was not different between microhabitats (χ2 = 2.22, P=0.5288) with wall having the largest positive ovitraps and banana having the least (Tables 4 and 5). Ovipositional responses ranged from 33% to 55%, indicating that all the organic infusions were oviposition stimulants (Table 4).
Table 4.
Results of regression analysis using generalized estimating equations of eggs trapping in 4 microhabitats using 4 organic infusions in Msambweni, Kwale County, Coastal Kenya.
| Parameter | OPI | Total eggs | Mean ± SE | Parameter estimate1 | 95% CI | Z score+2 |
|---|---|---|---|---|---|---|
| Microhabitat | ||||||
| Banana | 90.00 | 5,919 | 98.65 ± 12.93 | 0.09 | −0.29, 0.46 | 0.16ns |
| Bush | 91.67 | 4,998 | 83.30 ± 12.68 | 0.02 | −0.35, 0.39 | 0.01ns |
| Grass | 93.33 | 4,479 | 74.65 ± 9.85 | −0.12 | −0.49, 0.25 | 0.34ns |
| Wall | 96.67 | 4,194 | 69.90 ± 7.52 | — | — | — |
| Total | 19,590 | |||||
| Organic infusion | ||||||
| Banana | 100.00 | 5,017 | 104.52 ± 12.19 | 1.12 | 0.65, 1.59 | 21.50*** |
| Coconut | 93.75 | 3,153 | 65.69 ± 6.94 | 0.67 | 0.21, 1.13 | 8.21** |
| Grass | 95.83 | 4,369 | 91.02 ± 13.39 | 1.01 | 0.54, 1.48 | 17.96*** |
| Neem | 95.83 | 5,455 | 113.65 ± 15.69 | 1.22 | 0.75,1.70 | 26.13*** |
| Water (control) | 79.17 | 1,596 | 33.25 ± 7.48 | — | — | — |
| Total | 19,590 | |||||
Wall and water were used as reference categories.
ns, not significant;
P < 0.01;
P < 0.001.
Table 5.
Oviposition activity index (OAI) for different organic infusions within different microhabitats.
| Microhabitat | Infusion | Mean No. of eggs (±SE) | OAI |
|---|---|---|---|
| Banana | Banana | 187.6 ± 24.7 | 0.80 |
| Coconut | 53.3 ± 15.8 | 0.44 | |
| Grass | 73.1 ± 10.6 | 0.56 | |
| Neem | 158.5 ± 40.4.7 | 0.77 | |
| Water (control) | 20.8 ± 5.5 | ||
| Bush | Banana | 90.2 ± 27 | 0.50 |
| Coconut | 70.4 ± 12 | 0.40 | |
| Grass | 129.3 ± 43.4 | 0.62 | |
| Neem | 96.5 ± 31.2 | 0.52 | |
| Water (control) | 30.1 ± 10.0 | ||
| Grass | Banana | 73.6 ± 16.5 | 0.47 |
| Coconut | 47.4 ± 10.7 | 0.28 | |
| Grass | 97 ± 27.3 | 0.57 | |
| Neem | 128.4 ± 28.8 | 0.65 | |
| Water (control) | 26.8 ± 7.6 | ||
| Wall | Banana | 66.8 ± 8.1 | 0.09 |
| Coconut | 91.7 ± 14.4 | 0.25 | |
| Grass | 64.7 ± 9.7 | 0.08 | |
| Neem | 71.2 ± 19.4 | 0.13 | |
| Water (control) | 55.3 ± 26.6 |
Organic infusions performance in laboratory, semifield, and field settings
Oviposition responses improved in the different experimental settings, with lowest responses in laboratory and highest in the field (Table 2). All the infusions performed significantly better in the semifield and field settings compared to laboratory (F = 11.49; df = 2; P = <0.0001). Overall, the highest oviposition responses were observed for banana infusion, followed by neem and grass infusions. Oviposition responses in neem and grass infusion were comparable. Coconut infusion significantly recorded the least oviposition response (Tables 2 and 5).
Discussion
The attractive properties for oviposition of organic infusions have been used widely for monitoring mosquito vector densities, for targeted interventions, and to achieve area-wide mosquito population control by leveraging the use of lethal traps (Barrera et al. 2014a, 2014b; Johnson et al. 2017). In this exploratory study where highly locally available organic materials were assessed as oviposition attractants for Ae. aegypti, infusions from banana leaves, mixed grass, neem tree leaves, and coconut leaves were better oviposition attractants compared to water (control) in laboratory, semifield, and field settings. Of the 4 organic materials assessed, banana leaves and mixed grass showed consistently strong oviposition responses in laboratory, semifield, and field settings and are therefore recommended for use in vector surveillance and suppression strategies. Neem tree leaf infusions are good sources of infusion material when preferentially used outdoors where there is enough light. Additionally, our results suggest that unlike in unbaited traps, gravid Ae. aegypti do not discriminate between microhabitats where ovipositional sites are located when traps are infused.
Strong oviposition activity was reported around banana plantings in a study in Argentina (Estallo et al. 2013), but we did not find any report that assessed banana leaf infusion as oviposition attractant. Banana leaf infusions exhibited the strongest oviposition responses across the different settings evaluated in this study. Aedes mosquitoes are historically associated with banana plantations in 2 ways: 1) they are known to breed in banana leaf axils, and 2) banana plants are assumed to be good resting places. These associations and the strong oviposition response demonstrated in this study make banana leaf infusions stand out as the best candidate for use in mosquito population suppression traps, such as autocidal gravid ovitraps. Banana plantings are common near most households in the study area; thus, banana infusion baited ovitraps could be important Ae. aegypti surveillance tool (Barrera et al. 2014a, 2014b, 2018).
Grass infusion was consistently found to be a good oviposition attractant across the laboratory, semifield, and field settings. Grass infusions have been shown to be attractants for oviposition activity against a variety of mosquitoes, including Culex spp. (Allan et al. 2005), Ae. aegypti (Allan and Kline 1995), and Ae. albopictus (Holck et al. 1988, Allan and Kline 1995). In a study done in Brazil, oviposition responses did not differ between different species of grass evaluated (Santana et al. 2006). Grass species-specific oviposition responses remain unknown in the study area because mixed species of grass were used in this study. Grass is highly ubiquitous, and thus can easily be used for Aedes spp. vector surveillance and suppression programs in the study area.
Unlike all the other organic materials assessed in this study that showed consistent results across laboratory, semifield, and field settings, neem tree leaf infusions did not show any ovipositional responses in the laboratory setting. However, they showed strong attractant properties in semifield and field settings. Our study was able to distinguish the opposite effects of neem in oviposition in laboratory and field settings and demonstrates the importance of field studies. The finding that A. indica leaf infusion was a good oviposition attractant for Ae. aegypti was unexpected, given the wide use of materials from this tree in insect control, including mosquitoes (Su and Mulla 1998, Okumu et al. 2007, Dua et al. 2009). The most important insecticidal compound in neem tree materials is azadirachtin (Schmutterer 2002), which is responsible for deterrent, anti-ovipositional, anti-feedant, growth-disrupting (growth-regulating), fecundity- and fitness-reducing properties on insects (Su and Mulla 1998, Schmutterer 2002, Okumu et al. 2007, Dua et al. 2009, Seenivasagan et al. 2019). However, the azadirachtin in leaves, which breaks down rapidly when exposed to high temperatures and light, can dissipate as quickly as 17 h (Ujváry 2010). Substantial break down of azadirachtin seems not to have occurred in the neem tree infusion in the laboratory, but complete azadirachtin breakdown likely occurred when the infusion was exposed to higher temperature and sunlight in semifield and field experiments. Climatic conditions along the coastal strip of Kenya are characterized by plenty of sunlight and high temperatures rendering utility of neem tree infusion in Ae. aegypti control highly feasible. Although coconut leaf infusions performed better than plain water in attracting gravid Ae. aegypti to oviposit, they were the least attractive among the infusions assessed, especially in the field setting.
Our study demonstrates the importance of microhabitats in Ae. aegypti oviposition site selection. In fact, the banana infusion, the preferred infusion in most experiments, was not preferred in all microhabitats, demonstrating that microhabitat preference likely overrides infusion preference. The ovitrap positivity index was higher among unshaded microhabitats (wall and grass) compared to shaded microhabitats (banana and bush). The interpretation of this finding is that there were more oviposition occurrences among walls and grass microhabitats compared to bush and banana microhabitats. However, egg densities in unshaded microhabitats did not correspond with these higher oviposition occurrences. The lack of correspondence between ovitrap positivity index and egg density index in shaded and unshaded microhabitats was sustained in both baited and unbaited ovitraps. The finding that gravid Ae. aegypti mosquitoes were similarly attracted to oviposition sites in the 4 microhabitats when the traps were baited shows the strong influence that infusions have on oviposition site selection. However, the negative relationship between ovitrap positivity index and the egg density index in the 4 microhabitats is a unique finding. The reasons why Ae. aegypti preferred to oviposit in unshaded microhabitats but higher egg densities are reported in the shaded microhabitats remain unknown. Previous studies have demonstrated better survival of Ae. aegypti larvae and pupae in shaded microhabitats (Vezzani et al. 2005, Barrera et al. 2006, Vezzani and Albicócco 2009, Wong et al. 2011, Kroth et al. 2019). Vegetation, especially trees and shrubs, provide shade and larval nutrients to the larval habitats; explaining the better survival of larvae and pupae in vegetated microhabitats (Vezzani et al. 2005, Barrera et al. 2006, Vezzani and Albicócco 2009). A previous study in Tanzania reported better egg survival in shaded compared to unshaded microhabitats (Trpis 1972). Egg desiccation due to direct sunlight exposure (Juliano et al. 2002, Wong et al. 2011) may not be the reason for the low egg densities in the unshaded microhabitats because potentially desiccated eggs were included in the analysis. It has also been shown that adult mosquitoes have optimal temperatures for biologic processes, and Ae. aegypti has been shown to have an optimal temperature of 29°C. In coastal Kenya, temperatures in unshaded areas far surpass 29°C, which may also explain the preference for shaded sites (Mordecai et al. 2017, 2020).
This study has several limitations, primarily due to our small sample size of 10 houses in each of the 2 study sites. We were not able to survey additional oviposition sites due to limited resources. However, we were able to demonstrate the importance of both microhabitat and infusion preference in real-world field conditions in coastal Kenya. In addition, further research to isolate attractant chemicals in banana leaves and grass is needed, if our findings are to be applied in actual control programs. Our choice of 50% as the preferred concentration for all the infusions tested may not necessarily apply in real life situations, especially since no control was added in the experiment.
In conclusion, a strong oviposition attractant has potential uses in mosquito vector surveillance and suppression programs. Banana leaves, mixed grass, and neem tree leaves are suitable materials for oviposition infusions and outperform water in all microhabitats. Locally made organic infusions should replace water in ovitrap studies to improve egg collections. Using these infusions, gravid mosquito could be attracted to oviposition sites that are laced with an insecticide with ovicidal properties. Additionally, the small pockets of banana plantings could be important targets for vector control in an integrated vector control version.
REFERENCES CITED
- Afify A, Galizia CG. 2015. Chemosensory cues for mosquito oviposition site selection. J Med Entomol 52:120–130. [DOI] [PubMed] [Google Scholar]
- Afify A, Horlacher B, Roller J, Galizia CG. 2014. Different repellents for Aedes aegypti against blood-feeding and oviposition. PLoS One 9:e103765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha SB, Tchouassi DP, Bastos ADS, Sang R. 2017. Dengue and yellow fever virus vectors: seasonal abundance, diversity and resting preferences in three Kenyan cities. Parasit Vectors 10:628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhwale W. 2013. Dengue fever outbreak response. In: Ministry of Health, ed. The East Africa Public Health Lab Network Newsletter, Quarterly Bulletin, Kenya chapter. Nairobi: Kenya Ministry of Health. p 1–2. [Google Scholar]
- Allan SA, Bernier UR, Kline DL. 2005. Evaluation of oviposition substrates and organic infusions on collection of Culex in Florida. J Am Mosq Control Assoc 21:268–273. [DOI] [PubMed] [Google Scholar]
- Allan SA, Kline DL. 1995. Evaluation of organic infusions and synthetic compounds mediating oviposition in Aedes albopictus and Aedes aegypti (Diptera: Culicidae). J Chem Ecol 21:1847–1860. [DOI] [PubMed] [Google Scholar]
- Bancroft TL. 1906. On the aetiology of dengue fever. Austral Med Gaz 25:17–18. [Google Scholar]
- Barrera R, Amador M, Acevedo V, Caban B, Felix G, Mackay AJ. 2014a. Use of the CDC autocidal gravid ovitrap to control and prevent outbreaks of Aedes aegypti (Diptera: Culicidae). J Med Entomol 51:145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Amador M, Acevedo V, Hemme RR, Félix G. 2014b. Sustained, area-wide control of Aedes aegypti using CDC autocidal gravid ovitraps. Am J Trop Med Hyg 91:1269–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera R, Amador M, Clark GG. 2006. Ecological factors influencing Aedes aegypti (Diptera: Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol 43:484–492. [DOI] [PubMed] [Google Scholar]
- Barrera R, Amador M, Munoz J, Acevedo V. 2018. Integrated vector control of Aedes aegypti mosquitoes around target houses. Parasit Vectors 11:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt S, Gething P, Brady O, Messina J, Farlow, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers F, George DB, Jaenisch TT, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadee DD. 1993. Oviposition response of Aedes aegypti (L.) to the presence of conspecific eggs in the field in Trinidad, W.I. J Fla Mosq Control Assoc 64:63–66. [Google Scholar]
- Chepkorir E, Lutomiah J, Mutisya J, Mulwa F, Limbaso K, Orindi B, Ng’ang’a Z, Sang RC. 2014. Vector competence of Aedes aegypti populations from Kilifi and Nairobi for dengue 2 virus and the influence of temperature. Parasit Vectors 7:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JF. 2016. Mosquito oviposition behavior and vector control. Insects 7:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Barjac H. 1978. Toxicity of Bacillus thuringienses var. israelensis for larvae of Aedes aegypti and Anopheles stephensi. Comptes rendus hebdomadaires des seances de l’Academie des sciences. Serie D: Sciences naturelles 286:1175–1178. [PubMed] [Google Scholar]
- Dua VK, Pandey AC, Raghavendra K, Gupta A, Sharma T, Dash AP. 2009. Larvicidal activity of neem oil (Azadirachta indica) formulation against mosquitoes. Malar J 8:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F. 1941. Mosquitoes of the Ethiopian region III: Culicine adults and pupae. London:The Trustees of the British Museum. [Google Scholar]
- Ellis E, Neatherlin J, Delorey M, Ochieng M, Mohamed A, Mogeni D, Hunsperger E, Patta S, Gikunju S, Waiboic L, Fields B, Ofula V, Konongoi S, Torres-Velasquez B, Marano N, Sang R, Margolis H, Montgomery J, Tomashek K. 2015. A household serosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Negl Trop Dis 9:e0003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estallo EL, Ludueña-Almeida FF, Visintin AM, Scavuzzo CM, Introini MV, Zaidenberg M, Almirón WR. 2011. Prevention of dengue outbreaks through Aedes aegypti oviposition activity forecasting method. Vector Borne Zoonotic Dis 11:543–549. [DOI] [PubMed] [Google Scholar]
- Estallo EL, Más G, Vergara-Cid C, Lanfri MA, Ludueña-Almeida F, Scavuzzo CM, Introini MV, Zaidenberg M, Almirón WR. 2013. Spatial patterns of high Aedes aegypti oviposition activity in northwestern Argentina. PloS One 8:e54167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra C, Reiner R, Perkins T, Lindsay S, Midega J, Brady O, Barker C, Reisen W, Harrington L, Takken W, Kitron U, Lloyd A, Hay S, Scott T, Smith D. 2014. A global assembly of adult female mosquito mark-release-recapture data to inform the control of mosquito-borne pathogens. Parasit Vectors 7:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LC, Ponlawat A, Edman JD, Scott TW, Vermeylen F. 2008. Influence of container size, location, and time of day on oviposition patterns of the dengue vector, Aedes aegypti, in Thailand. Vector Borne Zoonotic Dis 8:415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington L, Scott T, Lerdthusnee K, Coleman R, Costero A, Clark G, Jones J, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. 2005. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg 72:209–220. [PubMed] [Google Scholar]
- Holck A, Meek C, Holck J. 1988. Attractant enhanced ovitraps for the surveillance of container breeding mosquitoes. J Am Mosq Control Assoc 4:97–98. [PubMed] [Google Scholar]
- Huang Y. 2004. The subgenus Stegomyia of Aedes in the Afrotropical region with keys to the species (Diptera: Culicidae). Zootaxa 700:1–120. [Google Scholar]
- Johnson BJ, Ritchie SA, Fonseca DM. 2017. The state of the art of lethal oviposition trap-based mass interventions for arboviral control. Insects 8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano SA, O’Meara GF, Morrill JR, Cutwa MM. 2002. Desiccation and thermal tolerance of eggs and the coexistence of competing mosquitoes. Oecologia 130:458–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenya National Bureau of Statistics. 2019a. The 2019 Kenya Population and Housing Census “Counting Our People for Sustainable development and devolution of services” Volume II Distribution of population by administrative units. Nairobi: Kenya National Bureau of Statistics. [Google Scholar]
- Kenya National Bureau of Statistics. 2019b. The 2019 Kenya Population and Housing Census “Counting Our People for Sustainable development and devolution of services” Volume IV Distribution of population by Socio-economic characteristics. Nairobi: Kenya National Bureau of Statistics. [Google Scholar]
- Kramer WL, Mulla MS. 1979. Oviposition attractants and repellents of mosquitoes: oviposition responses of Culex mosquitoes to organic infusions. Environ Entomol 8:1111–1117. [Google Scholar]
- Kroth N, Cozzer GD, de Carvalho G, Cassol AS, Breaux J, Lutinski JA, Busato MA, Roman Junior WA, Dos Santos JJ, Albeny-Simões D. 2019. Oviposition preferences of the mosquito Aedes aegypti Linnaeus, 1762 (Culicidae): an urban environment bioassay. Bull Entomol Res 109:762–770. [DOI] [PubMed] [Google Scholar]
- Kwale County Government. 2018. Kwale County Integrated Development Plan (2018–2022). Kwale: Kwale County Government. [Google Scholar]
- Lutomiah J, Barrera R, Makio A, Mutisya J, Koka H, Owaka S, Koskei E, Nyunja A, Eyase F, Coldren R, Sang R. 2016. Dengue outbreak in Mombasa city, Kenya, 2013–2014: entomologic investigations. PLoS Negl Trop Dis 10:e0004981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R, Peres RC, Alves F, Brandolini MB. 2008. Mosquito traps designed to capture Aedes aegypti (Diptera: Culicidae) females: preliminary comparison of Adultrap, MosquiTRAP and backpack aspirator efficiency in a dengue-endemic area of Brazil. Memórias do Instituto Oswaldo Cruz 103:602–605. [DOI] [PubMed] [Google Scholar]
- Mattingly P. 1958. Genetical aspects of the Aedes aegypti problem. I: taxonomy and bionomics. Ann Trop Med Parasitol 51:392–408. [PubMed] [Google Scholar]
- McDonald P. 1977. Population characteristics of domestic Aedes Aegypti (Diptera: Gulicidae) in villages on the Kenya Coast II. Dispersal within and between villages. J Med Entomol 14:49–53. [DOI] [PubMed] [Google Scholar]
- Midega JT, Nzovu J, Kahindi S, Sang RC, Mbogo C. 2006. Application of the pupal/demographic-survey methodology to identify the key container habitats of Aedes aegypti (L.) in Malindi district, Kenya. Ann Trop Med Parasitol. 100:S61–S72. [DOI] [PubMed] [Google Scholar]
- Moore DF. 1979. Hybridization and mating behavior in Aedes aegypti (Diptera: Culicidae). J Med Entomol 16:223–226. [DOI] [PubMed] [Google Scholar]
- Mordecai EA, Cohen JM, Evans MV, Gudapati P, Johnson LR, Lippi CA, Miazgowicz K, Murdock CC, Rohr JR, Ryan SJ, Savage V, Shocket MS, Stewart Ibarra A, Thomas MB, Weikel DP. 2017. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis 11:e0005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordecai E, Sadie R, Caldwell J, Shah M, LaBeaud ALPH. 2020. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet Healt 4:e416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutuku F, King C, Mungai P, Charles Mbogo C, Mwangangi J, Muchiri E, Walker E, Kitron U. 2011. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J 10:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndenga B, Mutuku F, Ngugi H, Mbakaya J, Aswani P, Musunzaji P, Vulule J, Mukoko D, Kitron U, LaBeaud A. 2017. Characteristics of Aedes aegypti adult mosquitoes in rural and urban areas of western and coastal Kenya. PLoS One 2:e0189971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngugi H, Mutuku F, Ndenga B, Musunzaji P, Mbakaya J, Aswani P, Irungu L, Mukoko D, Vulule J, Kitron U, LaBeaud A. 2017. Characterization and productivity profiles of Aedes aegypti (L.) breeding habitats across rural and urban landscapes in western and coastal Kenya. Parasit Vectors 10:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngugi H, Nyathi S, Krystosik A, Ndenga B, Mbakaya J, Aswani P, Musunzaji P, Irungu L, Bisanzio D, Kitron U, LaBeaud AD, Mutuku FM. 2020. Risk factors for Aedes aegypti household pupal persistence in longitudinal entomological household surveys in urban and rural Kenya. Parasit Vectors 13:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumu FO, Knols BGJ, Fillinger U. 2007. Larvicidal effects of a neem (Azadirachta indica) oil formulation on the malaria vector Anopheles gambiae. Malar J 6:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paupy C, Ollomo B, Kamgang B, Moutailler S, Rousset D, Demanou M, Hervé J-P, Leroy E, Simard F. 2010. Comparative role of Aedes albopictus and Aedes aegypti in the emergence of Dengue and Chikungunya in central Africa. Vector Borne Zoonotic Dis 10:259–266. [DOI] [PubMed] [Google Scholar]
- Ponnusamy L, Xu N, Böröczky K, Wesson DM, Abu Ayyash L, Schal C, Apperson CS. 2010. Oviposition responses of the mosquitoes Aedes aegypti and Aedes albopictus to experimental plant infusions in laboratory bioassays. J Chem Ecol 36:709–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regis L, Monteiro AM, Melo-Santos MA, Silveira JC Jr, Furtado AF, Acioli RV, Santos GM, Nakazawa M, Carvalho MS, Ribeiro PJ Jr, Souza WV. 2008. Developing new approaches for detecting and preventing Aedes aegypti population outbreaks: basis for surveillance, alert and control system. Mem Inst Oswaldo Cruz 103:50–59. [DOI] [PubMed] [Google Scholar]
- Reiter P, Colon M. 1991. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc 7:52–55. [PubMed] [Google Scholar]
- Ritchie SA. 2001. Effect of some animal feeds and oviposition substrates on Aedes oviposition in ovitraps in Cairns, Australia. J Am Mosq Control Assoc 17:206–208. [PubMed] [Google Scholar]
- Russell RC, Ritchie SA. 2004. Surveillance and behavioral investigations of Aedes aegypti and Aedes polynesiensis in Moorea, French Polynesia, using a sticky ovitrap. J Am Mosq Control Assoc 20:370–375. [PubMed] [Google Scholar]
- Santana AL, Roque RA, Eiras AE. 2006. Characteristics of grass infusions as oviposition attractants to Aedes (Stegomyia) (Diptera: Culicidae). J Med Entomol 43:214–220. [DOI] [PubMed] [Google Scholar]
- Santos E, Correia J, Muniz L, Meiado M, Albuquerque C. 2010. Oviposition activity of Aedes aegypti L. (Diptera: Culicidae) in response to different organic infusions. Neotrop Entomol 39:299–302. [DOI] [PubMed] [Google Scholar]
- Santos SRA, Melo-Santos MAV, Regis L, Albuquerque CMR. 2003. Field evaluation of ovitraps consociated with grass infusion and Bacillus thuringiensis var. israelensis to determine oviposition rates of Aedes aegypti. New Delhi: WHO Regional Office for South-East Asia. [Google Scholar]
- Schmutterer H. 2002. The neem tree (Azadirachta indica) and other Meliceous plants. In Source of Unique Natural Products for Integrated Pest Management, Medicine, Industry and other purposes. Mumbai: Neem Foundation. [Google Scholar]
- Seenivasagan T, Sharma A, Yadav R, Tyagi V, Singh R, Sukumaran D. 2019. Plant infusions mediate oviposition of malaria, dengue and filariasis vectors: push-pull approach for vector surveillance and control. J Biopest 12:95–103. [Google Scholar]
- Su T, Mulla M. 1998. Antifeedancy of neem products containing Azadirachtin against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J Vector Ecol 23:114–122. [PubMed] [Google Scholar]
- Trexler JD, Apperson CS, Schal C. 1998. Laboratory and field evaluations of oviposition responses of Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) to oak leaf infusions. J Med Entomol 35:967–976. [DOI] [PubMed] [Google Scholar]
- Trpis M. 1972. Dry season survival of Aedes aegypti eggs in various breeding sites in the Dar es Salaam area, Tanzania. Bull World Health Organ 47:433–437. [PMC free article] [PubMed] [Google Scholar]
- Trpis M, Hausermann W. 1986. Dispersal and other population parameters of Aedes aegypti in African village and their possible significance in epidemiology of vector-borne diseases. Am J Trop Med Hyg 35:1263–1279. [DOI] [PubMed] [Google Scholar]
- Trpis M, Häusermann W, Craig GB Jr. 1995. Estimates of population size, dispersal, and longevity of domestic Aedes aegypti aegypti (Diptera: Culicidae) by mark–release–recapture in the village of Shauri Moyo in eastern Kenya. J Med Entomol 32:27–33. [DOI] [PubMed] [Google Scholar]
- Ujváry I. 2010. Pest control agents from natural products. In: Krieger R, ed. Hayes’ handbook of pesticide toxicology. New York: Academic Press. p 119–229. [Google Scholar]
- Vezzani D, Albicócco AP. 2009. The effect of shade on the container index and pupal productivity of the mosquitoes Aedes aegypti and Culex pipiens breeding in artificial containers. Med Vet Entomol 23:78–84. [DOI] [PubMed] [Google Scholar]
- Vezzani D, Rubio A, Velázquez SM, Schweigmann N, Wiegand T. 2005. Detailed assessment of microhabitat suitability for Aedes aegypti (Diptera: Culicidae) in Buenos Aires, Argentina. Acta Trop 95:123–131. [DOI] [PubMed] [Google Scholar]
- Vu D, Banda T, Teng C, Heimbaugh C, Muchiri E, Mungai P, Mutuku F, Brichard J, Gildengorin G, Borland E, Powers A, Kitron U, King C, LaBeaud A. 2017. Dengue and West Nile Virus transmission in children and adults in coastal Kenya. Am J Trop Med Hyg. 96:141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. [World Health Organization]. 2009. Dengue; guidelines for diagnosis, treatment, prevention and control. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
- WHO. [World Health Organization]. 2016. Emergencies preparedness and response. Disease outbreak news: Chikungunya—Kenya. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO. [World Health Organization]. 2018. Chikungunya—Mombasa Kenya. Geneva, Switzerland: World Health Organization. [Google Scholar]
- WHO. [World Health Organization]. 2020. Dengue and severe dengue. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Wong J, Stoddard ST, Astete H, Morrison AC, Scott TW. 2011. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl Trop Dis 5:e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]


