Photobiomodulation targets mitochondrial signaling systems to improve cellular bioenergetic processes. Age-related macular degeneration shows mitochondrial dysfunction contributing to disease development and progression. Multiwavelength PBM improved clinical and anatomical outcomes in early-intermediate nonexudative (dry) age-related macular degeneration. Photobiomodulation offers a novel treatment with a unique mechanism and mode of delivery for dry AMD.
Key words: photobiomodulation, multiwavelength, age related macular degeneration, mitochondria, ocular disease, vision, retina, nonexudative macular degeneration, light therapy
Abstract
Purpose:
The LIGHTSITE III study evaluated multiwavelength photobiomodulation (PBM) therapy in nonexudative (dry) age-related macular degeneration (AMD) using the LumiThera Valeda Light Delivery System.
Methods:
LIGHTSITE III is a randomized, controlled trial to assess the safety and effectiveness of PBM in dry AMD. Subjects were given multiwavelength PBM (590, 660, and 850 nm) or Sham treatment delivered in a series of nine sessions over 3 to 5 weeks every four months over 24 months. Subjects were assessed for efficacy and safety outcomes. Data from the 13-month analysis are presented in this report.
Results:
A total of 100 subjects (148 eyes) with dry AMD were randomized. LIGHTSITE III met the primary efficacy best-corrected visual acuity endpoint with a significant difference between PBM (n = 91 eyes) and Sham (n = 54 eyes) groups (Between group difference: 2.4 letters (SE 1.15), CI: −4.7 to −0.1, P = 0.02) (PBM alone: 5.4 letters (SE 0.96), CI: 3.5 to 7.3, P < 0.0001; Sham alone: 3.0 letters (SE 1.13), CI: 0.7–5.2, P < 0.0001). The PBM group showed a significant decrease in new onset geographic atrophy (P = 0.024, Fisher exact test, odds ratio 9.4). A favorable safety profile was observed.
Conclusion:
LIGHTSITE III provides a prospective, randomized, controlled trial showing improved clinical and anatomical outcomes in intermediate dry AMD following PBM therapy.
Age-related macular degeneration (AMD) is a retinal disease that causes irreversible, severe loss of vision and blindness. Age-related macular degeneration is classified into two categories: exudative (wet) and nonexudative (dry) AMD. Dry AMD affects 90% of AMD patients and is characterized by the accumulation of extracellular material under the retinal pigment epithelium (RPE). Geographic atrophy (GA) results from atrophy of the retinal pigment epithelium cell layer, which leads to vision loss secondary to death of macular photoreceptors.1 Global prevalence of AMD is expected to reach 288 million by 2040. In the United States, it is estimated that 18.34 million individuals (11.64%) are living with early-stage AMD and 1.49 million (0.94%) are living with late-stage AMD (choroidal neovascularization (CNV)/neovascular AMD (nAMD) and/or GA).2,3 There are currently no approved treatments for dry AMD in early/intermediate stages beyond antioxidant supplementation, which only delay progression in 20%–25% of eyes.1,4
Photobiomodulation (PBM) is an established biotechnology that involves light from the visible spectrum to near infrared (NIR) (500-1,000 nm) applied to selected tissue to produce beneficial cellular effect.5–8 The mechanism of PBM is ascribed to stimulation of mitochondrial respiratory chain components resulting in stabilization of metabolic function and initiation of signaling cascades promoting cellular proliferation and cytoprotection. Cytochrome C oxidase is recognized as a key photoacceptor of light in the far red to NIR spectral range.9–12 Cytochrome C oxidase activation enhances electron transport pathway function and promotes ATP production, the cell's major source of energy.10,13–16
Collectively, studies across multiple indications show improvements in clinical and anatomical outcomes following PBM treatment. Recent ophthalmologic clinical trials, including LIGHTSITE I and II, evaluated the effect of multiwavelength PBM using the Valeda light delivery system and found improvement in clinical vision outcomes and anatomical correlates of the disease.17–19 LIGHTSITE III further investigates the effects of PBM treatment in early/intermediate dry AMD.
Methods
Study Participants
Subjects were eligible for enrollment (NCT04065490) if they were at least 50 years and had a diagnosis of dry AMD defined by the presence of drusen and/or nonfoveal center GA with best-corrected visual acuity (BCVA) scores determined by Early Treatment Diabetic Retinopathy Study (ETDRS) between 50 and 75 (snellen equivalent: 20/32 to 20/100) (see Table, Supplemental Digital Content 1, http://links.lww.com/IAE/C119). Subjects were excluded with a history of CNV, presence of center involving GA, or other significant retinal disease. Each eye was individually assessed for drusen, GA, and CNV by a central reading center (Duke Reading Center, Durham, NC).19
Subjects were enrolled across 10 centers throughout the United States. This study was conducted in compliance with the protocol, Good Clinical Practice guidelines, the guidelines of the Declaration of Helsinki and all other applicable regulatory requirements.
Study Design
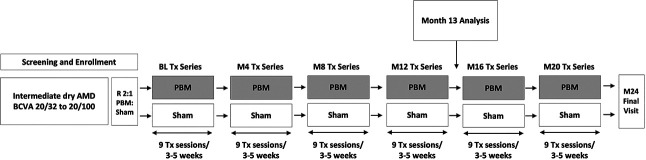
LIGHTSITE III was a double-masked, randomized, sham-controlled, parallel-group, multicenter, prospective study. Subjects who met the inclusion criteria, had none of the exclusion criteria, and provided their informed consent underwent PBM or Sham treatment randomized in a 2:1 ratio. Subjects were treated with the Valeda Light Delivery System (LumiThera, Inc., Poulsbo, WA) and received treatment in a series of nine sessions over a period of 3 to 5 weeks. The 24-month study included six series of treatment delivered every 4 months. A prespecified primary analysis was conducted at Month 13 after four series of treatments. Data were collected during 61 visits over the 24-month study with a 13-month analysis (Figure 1). The study has been completed, and 24-month data are under analysis.
Fig. 1.
LIGHTSITE III study Design. Subjects were randomized in a 2:1 fashion (PBM:Sham treatment) and followed for 24 months. Data analyses were planned for Month 13 and Month 24. The PBM mode delivered 590, 660, and 850 nm multiwavelength treatment. The Sham mode delivered a 50x reduction of the 590 nm and a 100x reduction of the 660 nm wavelengths; the 850 nm was omitted. BL, baseline; M, month; R, randomized; Tx, treatment.
Evaluated Parameters
Clinical classification of disease stage followed Beckman categorization.20 The prespecified primary endpoint was the 13-month difference in BCVA (change from baseline) between the PBM and Sham groups. A second primary endpoint was the 21-month data if the study did not achieve statistical significance at 13 months. A statistically significant difference was defined with a P value threshold of P = 0.025 for both the primary endpoints (accounting for both possible endpoints). The ETDRS BCVA examination was employed before and after each treatment series. The BCVA evaluation included a thorough protocol refraction and visual acuity examination with certified equipment and examination rooms. Subjects were also assessed for low-luminance BCVA (LLBCVA), Mars letter contrast sensitivity, Radner reading speed, Farnsworth–Munsell D-15 dichotomous color vision testing, and completed the visual function questionnaire-25 (VFQ-25) at selected time points. Certifications for clinical outcomes were performed by specialists (Global Eye Trials, London, United Kingdom). Subjects were assessed with 20 × 20 mm high-speed spectral-domain optical coherence tomography (OCT) volume scans, fundus autofluorescence (FAF), and fundus photography (Spectralis optical coherence tomography, Heidelberg Engineering, Heidelberg, Germany), as described previously.17,19 An independent, masked, imaging center reviewed and graded all images. Safety information was collected at all time points through the Month 13 visit.
Photobiomodulation Treatment with Valeda Light Delivery System
Subjects were treated with Valeda using three distinct wavelengths in the yellow (590 nm; 4 mW/cm2; 2× 35 seconds), red (660 nm; 65 mW/cm2; 2× 90 seconds), and NIR (850 nm; 0.6 mW/cm2; 2× 35 seconds) range. A complete masked control is not possible (i.e., a true sham would deliver zero light fluence, which would be observable to patients and study staff). Therefore, the Sham treatment consisted of an active control, which delivered a lower fluence of selected wavelengths. The Sham mode delivered a 50x and 100x reduction in treatment fluence compared with the PBM mode of the 590 and 660 nm wavelengths, respectively; the 850 nm wavelength was omitted.
Statistical Analysis
Statistical analyses were performed using SAS or R Version 3.4.4 (SAS Institute, Cary, NC).21 Based on previous studies, a within-group SD of 5.0 in BCVA change from baseline was assumed. A total of 119 eyes (40 sham and 79 PBM) completing the study provided power of 0.84 to detect a difference of 3.18 between the groups in BCVA with a two-sided alpha of 0.025. Allowing for a 10.0% dropout, assuming an average of 1.5 eligible eyes per subject, and potentially smaller effect size and larger SD, a sample size of at least 96 subjects, giving 144 eyes, was planned.
All analyses are based on individual eyes rather than subjects unless otherwise indicated. All subjects enrolled (n = 144 eyes) and modified intent-to-treat (mITT) (n = 142 eyes) analyses were conducted across outcomes and study time points. Nonstudy eye analyses included companion eyes that were not enrolled in the study and did not receive treatment. Analyses of change from baseline following treatment and the treatment effect on the change from baseline used linear mixed-effects models that account for correlation between eyes within subject. Efficacy analyses were implemented using 1) the measured value of each outcome and 2) the rank value of each outcome. For each efficacy analysis, the model residuals from the measured values were examined using the Anderson–Darling test for normality. If the residuals were not normally distributed (P < 0.05), the analysis using the rank values was considered the principal analysis, with the analysis of measured values considered to be a sensitivity analysis.
Results
Participants
A total of 178 subjects were screened for the study with 100 subjects (56.2%) eligible for randomization. At Month 13, a total of 17 subjects discontinued the study (nine PBM; eight Sham): 10 withdrew consent, three were unable to return to the facility, and four discontinued because of adverse events (AEs) not considered related to the treatment (Figure 2). Baseline characteristics are provided in Table 1. Subjects were enrolled with baseline BCVA scores between 50 and 75 letters. At baseline, 45 of 148 eyes (30.0%) had baseline BCVA <70 letters (20/100–20/40 Snellen) and 103 of 148 eyes (70.0%) have a baseline BCVA between 70 and 75 letters (20/40–20/32 Snellen). AREDS category and clinical classification of subjects in the MITT group showed that a majority were enrolled in an AREDS category 3 (86.9%) and classification of intermediate AMD (72.0%). A total of 13.1% (n = 19) of subjects were AREDS category 2 and 86.9% (n = 126) were AREDS category 3. A total of 20.0% (n = 29) of subjects were categorized as early-stage AMD, 72.0% (n = 105) were intermediate-stage AMD, and 8.0% (n = 11) were late-stage AMD (GA, no CNV).
Fig. 2.
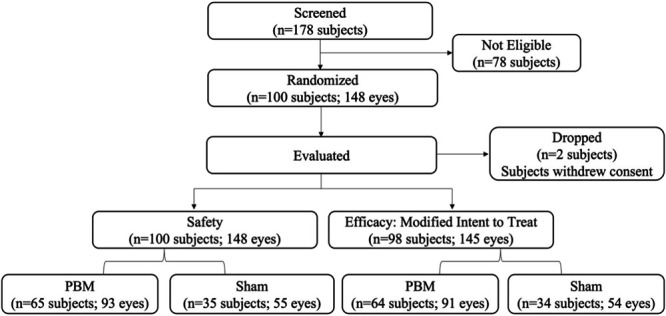
Diagram of LIGHTSITE III subject enrollment. A total of 100 subjects and 148 eyes were enrolled into the study.
Table 1.
Demographics and Baseline Characteristics
| Variable | PBM (N = 65) n (%) |
Sham (N = 35) n (%) |
Total (N = 100) n (%) |
| Age (years) | |||
| Mean (SD) | 74.4 (7.3) | 77.1 (6.2) | 75.4 (7.1) |
| Minimum-maximum | 53–91 | 66–88 | 53–91 |
| Gender | |||
| Female | 46 (70.8) | 22 (62.9) | 68 (68.0) |
| Male | 19 (29.2) | 13 (37.1) | 32 (32.0) |
| Ethnicity | |||
| Hispanic or Latino | 3 (4.6) | 3 (8.6) | 6 (6.0) |
| Not Hispanic or Latino | 62 (95.4) | 32 (91.4) | 94 (94.0) |
| Race | |||
| Black or African American | 0 (0.0) | 1 (2.9) | 1 (1.0) |
| White | 65 (100) | 34 (97.1) | 99 (99.0) |
| AREDS supplementation | |||
| Yes | 57 (87.6) | 29 (82.8) | 86 (86.0) |
| No | 8 (12.3) | 6 (17.2) | 14 (14.0) |
| Eye color | |||
| Blue | 24 (36.9) | 9 (25.7) | 33 (33.0) |
| Green | 8 (12.3) | 3 (8.6) | 11 (11.0) |
| Brown | 18 (27.7) | 15 (42.9) | 33 (33.0) |
| Hazel | 14 (21.5) | 7 (20.0) | 21 (21.0) |
| Other | 1 (1.5) | 1 (2.9) | 2 (2.0) |
| Diabetes | |||
| Yes | 2 (5.7) | 7 (10.8) | 9 (9.0) |
| Type I | 0 (0.0) | 1 (1.5) | 1 (1.0) |
| Type II | 2 (5.7) | 6 (9.2) | 8 (8.0) |
| Unknown | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| No | 33 (94.3) | 58 (89.2) | 91 (91.0) |
| Hypertension | |||
| Yes | 23 (65.7) | 32 (49.2) | 55 (55.0) |
| No | 12 (34.3) | 33 (50.8) | 45 (45.0) |
Presented data is from subjects and not eyes.
Efficacy Assessments
At Month 13 (4 series of treatment), the average change from baseline in BCVA was an increase of 5.4 letters (SE 0.96; SD 9.15) in PBM and 3.0 letters (SE 1.13; SD 8.30) in Sham-treated eyes. The BCVA change from baseline to Month 13 was not normally distributed (Anderson–Darling P-value = 0.04); therefore, the rank analysis was considered the principal analysis, giving the rank P value of 0.0204 for the primary analysis comparing PBM and Sham groups at Month 13 (Table 2; Figure 3). Approximately 55.0% of PBM-treated eyes showed ≥ 5 letter gain compared with 40.8% of Sham, 26.4% of PBM-treated eyes showed ≥ 10 letter gain compared with 14.9% of Sham, and 5.5% of PBM-treated eyes showed ≥ 15 letter gain compared with 1.9% of Sham. Sham-treated eyes showed a two-fold decrease in BCVA letter count and an increased number of eyes with a letter loss ≥ 10 at each visit compared with PBM-treated eyes. A significant difference in BCVA between PBM and Sham groups was also observed at Months 5, 9, and 12. A significant difference in the PBM group was observed at all time points assessed (P < 0.05; Table 2; Figure 4).
Table 2.
Clinical Outcomes
| Clinical Parameter | PBM (N = 91)* | Sham (N = 54)* |
| BCVA | ||
| Mean baseline BCVA score, letters (SE) [SD] | 70.7 (0.55) [5.23] | 70.1 (0.58) [4.29] |
| Primary BCVA endpoint† | ||
| Change in BCVA from baseline at Month 13 | ||
| ETDRS letters, LS mean (SE) [SD] | 5.4 (0.96) [9.16] | 3.0 (1.13) [8.30] |
| 95% CI | 3.5–7.3 | 0.7–5.2 |
| Within-group comparison | P < 0.0001 | P = 0.0094 |
| Between-group comparison | P = 0.0204 | |
| Secondary and exploratory BCVA endpoints | ||
| No. of subjects BCVA ≥5 letter improvement, n (%) | 50 (54.9) | 22 (40.7) |
| No. of subjects BCVA ≥10 letter improvement, n (%) | 24 (25.8) | 8 (15.1) |
| No. of subjects BCVA ≥15 letter improvement, n (%) | 4 (4.4) | 1 (1.9) |
| BCVA ≥5 mean letter improvement, mean (SE) [SD] | 9.7 (0.5) [3.7] | 8.7 (0.7) [3.1] |
| BCVA ≥10 mean letter improvement, mean (SE) [SD] | 12.8 (0.5) [2.7] | 11.9 (0.6) [1.8] |
| Secondary and exploratory endpoints | ||
| Change from baseline, mean (SE) [SD] | ||
| Month 1 | 3.0 (0.68) [6.49] | 2.0 (0.80) [5.88] |
| PBM within-group comparison | P < 0.0001 | |
| Between-group comparison | P = 0.22 | |
| Month 4 | 4.1 (0.85) [8.1] | 3.2 (0.98) [7.20] |
| PBM within-group comparison | P < 0.0001 | |
| Between-group comparison | P = 0.37 | |
| Month 5 | 4.8 (0.75) [7.15] | 2.7 (0.89) [6.54] |
| PBM within-group comparison | P < 0.0001 | |
| Between-group comparison | P = 0.027 | |
| Month 8 | 4.8 (0.9) [8.59] | 3.0 (1.06) [7.79] |
| PBM within-group comparison | P < 0.0001 | |
| Between-group comparison | P = 0.027 | |
| Month 9 | 5.5 (0.88) [8.39] | 3.3 (1.04) [7.64] |
| PBM within-group comparison | P < 0.0001 | |
| Between-group comparison | P = 0.045 | |
| Month 12 | 6.0 (1.01) [9.63] | 3.4 (1.20) [8.82] |
| PBM within-group comparison | P < 0.0001 | |
| Between-group comparison | P = 0.04 | |
| Secondary and exploratory endpoints | ||
| Macular drusen volume | ||
| Baseline, mean (SE) [SD] | 0.947 (0.03) [0.29] | 0.973 (0.04) [0.27] |
| Month 13 | 0.947 (0.03) [0.29] | 1.02 (0.04) [0.29] |
| Within-group comparison | P = 0.57 | P = 0.36 |
| PBM versus sham difference in means | P = 0.36 | |
| New-onset geographic atrophy, No. of events (%)‡ | ||
| No. of subjects at baseline | 6 (6.5) | 5 (9.1) |
| No. of new onset subjects at Month 13 | 1 (1.1) | 5 (10.0) |
| Between-group comparison | P = 0.024 |
n = Number of eyes with data available. LS mean = least squares estimation of mean based on a liner mixed effect model with eye nested within subject and use of AREDS supplements as a covariate.
MITT population analysis.
The Anderson–Darling test for normality indicated that the model residuals from measured values were not normally distributed (P = 0.04), which lead to rank assessment.
Subject eyes included at screening/baseline removed.
Fig. 3.
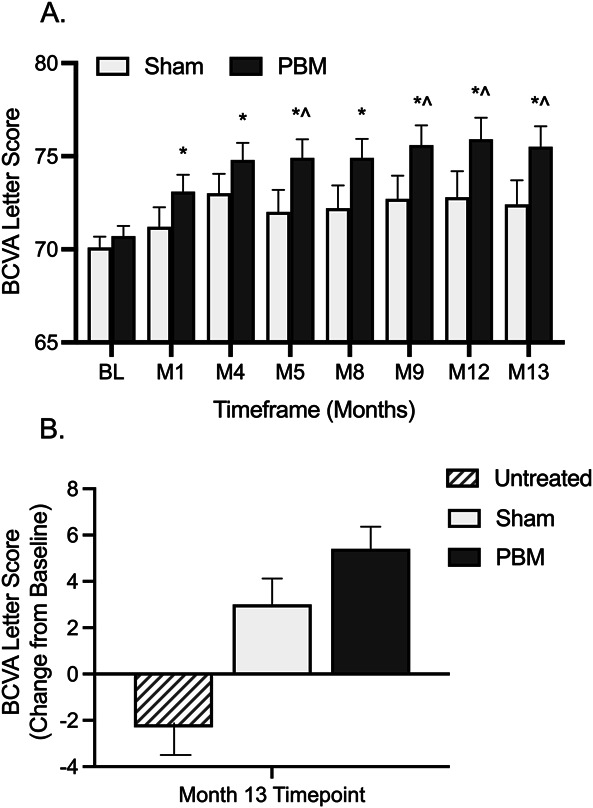
Photobiomodulation improves BCVA in early/intermediate stage dry AMD. A. Subjects received PBM or Sham treatment at baseline, Month 4, Month 8 and Month 12 time points. Significant improvements in BCVA were observed through Month 13 following PBM treatment. B. At Month 13, nonstudy eyes showed a mean letter loss of 2.3 letters, Sham-treated eyes showed a mean letter gain of 3.0 letters, and PBM-treated eyes showed a mean letter gain of 5.4 letters. *Within-group (PBM) comparison, P < 0.005, ^PBM versus sham between-group comparison, P < 0.05. BL, baseline; M, month.
Fig. 4.
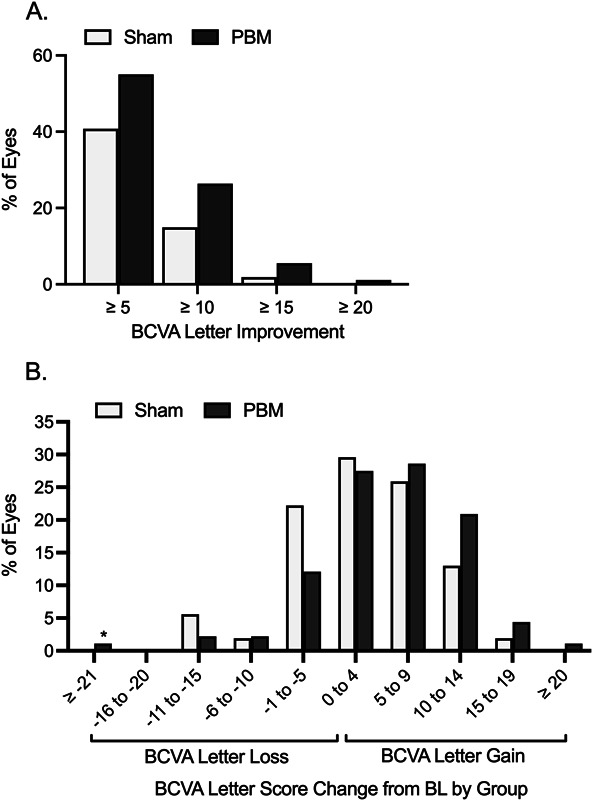
Distribution of BCVA letter gain and loss by treatment group at Month 13. A. Approximately 55.0% of PBM-treated eyes showed ≥5 letter gain (mean of 9.7 letters, SD 3.7) compared with 40.8% of Sham, 26.4% of PBM-treated eyes showed ≥ 10 letter gain (mean of 12.8 letters, SD 2.7) compared with 14.9% of Sham, and 5.5% of PBM-treated eyes showed ≥ 15 letter gain compared with 1.9% of Sham. B. A higher number of Sham-treated and nonstudy eyes showed BCVA letter losses compared with PBM as noted in the −11 to −15, −6 to −10, and −1 to −5 letter loss groups. A higher number of PBM-treated eyes showed BCVA letter gains in the 5 to 9, 10 to 14, 15 to 19, and ≥ 20 letter gain groups. * Patient had vision loss because of worsening of posterior capsule opacity. BL, baseline.
Secondary and exploratory evaluation of LLBCVA, contrast sensitivity, Radner reading charts, and the VFQ-25 showed normal or near normal visual outcomes at baseline, which were stable through the 13-month time point in both groups (see Table, Supplemental Digital Content 1, http://links.lww.com/IAE/C119).
Anatomical Outcomes
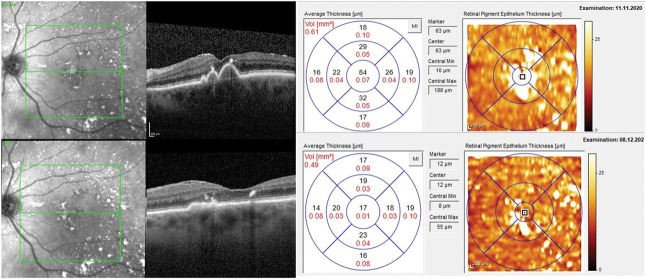
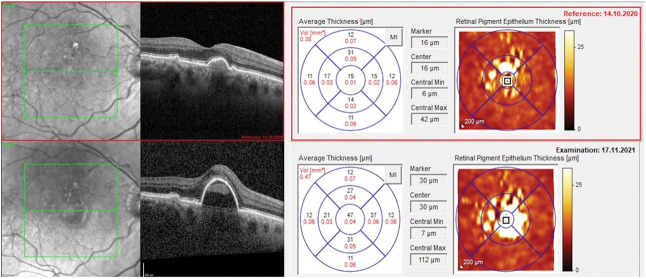
A total of 138 eyes were enrolled with drusen at baseline (PBM, n = 86; Sham, n = 52). No change in subRPE macular drusen volume was seen in PBM-treated eyes (0.006 mm3); however, an increase was seen in the Sham group (0.049 mm3; Figure 5). At Month 13, a statistically significant correlation was observed for measured BCVA scores and measured macular drusen volume in PBM-treated eyes. Subjects with higher BCVA scores showed lower values for macular drusen volume in the PBM group (P = 0.004). Representative images are presented in Figures 6 and 7.
Fig. 5.
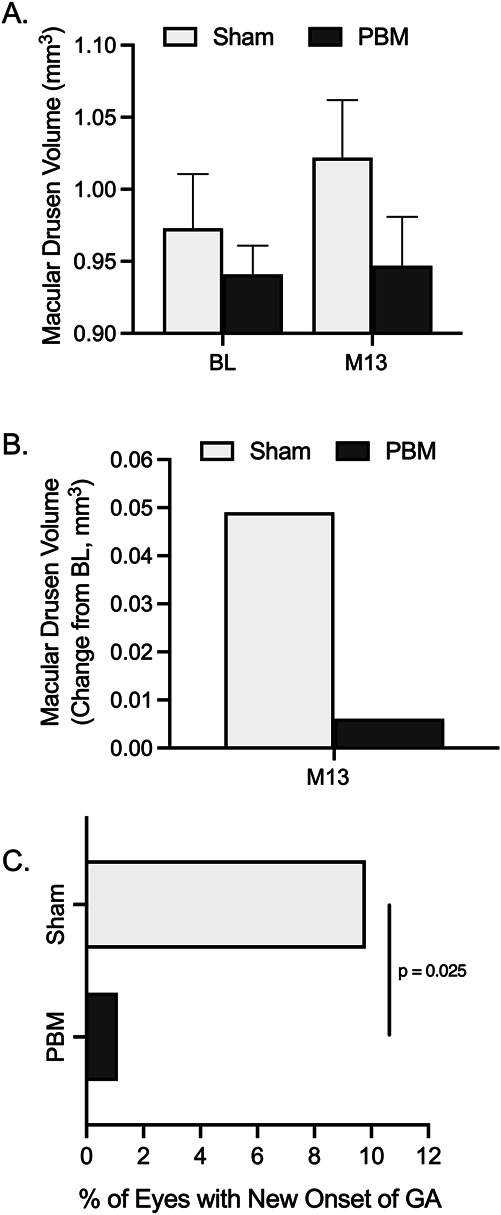
Impact of photobiomodulation on anatomical outcomes. A. A numerical increase in macular drusen volume was observed in Sham-treated eyes, ns, P > 0.05. B. Macular drusen volume increased 0.049 mm3 in Sham-treated eyes and 0.006 mm3 in PBM-treated eyes. The occurrence of new GA was observed in 5 of 51 (9.8%) Sham-treated eyes and one of 88 (1.1%) PBM-treated eyes. C. The occurrence of new GA was significantly higher in the Sham group than in the PBM group (P = 0.025, Fisher exact test, odds ratio 9.3). BL, baseline; M, month.
Fig. 6.
Representative imaging of macular drusen reduction following photobiomodulation treatment. A significant reduction in macular drusen volume was observed following four series of PBM treatment without the loss of photoreceptor or retinal pigment epithelium visible. A 4-letter increase in BCVA was observed from 75 letters to 79 letters at Month 13.
Fig. 7.
Representative imaging of macular drusen increase following Sham treatment. A significant increase in macular drusen volume was observed following four series of sham treatment with confluent drusen that further developed into large RPE detachments. A 3-letter decrease in BCVA was observed from 72 letters to 69 letters at Month 13. The subject subsequently converted to nAMD.
Development of Advanced Age-Related Macular Degeneration
A very small number of eyes presented with noncenter involving GA at baseline (PBM, n = 6; Sham, n = 5). Using FAF analysis, a numerical trend showed an increase in GA lesion area in Sham (1.48, SE 0.94; SD 1.62) compared with PBM-treated eyes (1.16, SE 0.66; SD 1.32, P = 0.75) at Month 13 (change from baseline; Table 2; Figure 5). The occurrence of new GA over the course of 13 months was observed in six additional eyes. A total of 10.0% (n = 5/50) of new-onset GA events were observed in Sham eyes compared to in Sham, and 1.1% (n = 1/87) observed in PBM-treated eyes. The occurrence of new GA was significantly higher in the Sham versus PBM group (P = 0.024, Fisher exact test, odds ratio 9.4) (Table 2; Figure 5).
A total of one Sham eye (1.8%), five PBM-treated eyes (5.4%), and three nonstudy eyes (8.3%) converted to nAMD by Month 13. A total of 16 subjects were randomized with one nonstudy eye that had nAMD. Of the 16 eyes with the nonstudy companion eye with nAMD, 12 (75.0%) were in the PBM group, providing a higher risk proportion for conversion to nAMD. The single sham eye that converted was at high risk, and 4 of 5 (80.0%) of the PBM-treated eyes were high-risk eyes.
Safety and Compliance Outcomes
A total of 33 study eyes (22.3%) presented with at least one ocular-specific AE. Four ocular-specific AEs were considered related to the treatment (none led to study discontinuation and were mild or moderate in intensity). Only one ocular-specific SAE was reported and was not considered related to the treatment. No ocular-specific AE was reported at a frequency of more than 5%. A total of three device-related AEs were reported. All device-related AEs were dry eye and considered possibly or probably related to the device. A total of three subjects had nonocular SAEs that resulted in death. All SAEs were considered not related to the treatment (Table 3). No significant changes were observed in perimetry or color vision assessment. The majority of subjects were compliant with all treatment visits. At Month 13, 88.2% of eyes receiving PBM and 74.5% of eyes receiving Sham treatment were fully compliant with the treatment protocol.
Table 3.
Ocular AEs by System Organ Class and Preferred Term in Study Eyes
| Preferred Term | Study Eyes | Nonstudy Eyes | ||
| PBM (N = 93) n (%) | Sham (N = 55) n (%) | Total (N = 148) n (%) | Total (N = 52) n (%) | |
| Eye disorders | 21 (22.6) | 12 (21.8) | 33 (22.3) | 18 (34.6) |
| Neovascular age-related macular degeneration | 5 (5.4) | 1 (1.8) | 6 (4.1) | 3 (8.3)* |
| Vitreous floaters | 1 (1.1) | 4 (7.3) | 5 (3.4) | 0 (0.0) |
| Dry eye | 1 (1.1) | 2 (3.6) | 3 (2.0) | 2 (3.8) |
| Punctate keratitis | 1 (1.1) | 2 (3.6) | 3 (2.0) | 0 (0.0) |
| Vitreous detachment | 2 (2.2) | 1 (1.8) | 3 (2.0) | 0 (0.0) |
| Blepharitis | 2 (2.2) | 0 (0.0) | 2 (1.4) | 1 (1.9) |
| Conjunctival hemorrhage | 2 (2.2) | 0 (0.0) | 2 (1.4) | 0 (0.0) |
| Conjunctivitis allergic | 2 (2.2) | 0 (0.0) | 2 (1.4) | 0 (0.0) |
| Eye pain | 2 (2.2) | 0 (0.0) | 2 (1.4) | 0 (0.0) |
| Foreign body sensation in eyes | 2 (2.2) | 0 (0.0) | 2 (1.4) | 0 (0.0) |
| Lacrimation increased | 2 (2.2) | 0 (0.0) | 2 (1.4) | 0 (0.0) |
| Photopsia | 2 (2.2) | 0 (0.0) | 2 (1.4) | 1 (1.9) |
| Posterior capsule opacification | 1 (1.1) | 1 (1.8) | 2 (1.4) | 0 (0.0) |
| Abnormal sensation in eye | 0 (0.0) | 1 (1.8) | 1 (0.7) | 0 (0.0) |
| Amaurosis fugax | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Angle closure glaucoma | 0 (0.0) | 1 (1.8) | 1 (0.7) | 1 (1.9) |
| Cataract | 0 (0.0) | 1 (1.8) | 1 (0.7) | 3 (5.8) |
| Cataract subcapsular | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.8) |
| Cystoid macular oedema | 0 (0.0) | 1 (1.8) | 1 (0.7) | 0 (0.0) |
| Diplopia | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Eye discharge | 0 (0.0) | 1 (1.8) | 1 (0.7) | 0 (0.0) |
| Eye irritation | 1 (1.1) | 0 (0.0) | 1 (0.7) | 2 (3.8) |
| Eye pruritus | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Macular hole | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Open angle glaucoma | 0 (0.0) | 1 (1.8) | 1 (0.7) | 1 (1.9) |
| Photophobia | 1 (1.1) | 0 (0.0) | 1 (0.7) | 1 (1.9) |
| Retinal vein occlusion | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Retinopathy hypertensive | 0 (0.0) | 1 (1.8) | 1 (0.7) | 0 (0.0) |
| Vitreous degeneration | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Vitreous hemorrhage | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.8) |
| Diplopia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (3.8) |
| Amaurosis fugax | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) |
| Chalazion | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (1.9) |
| General disorders and administration site conditions | 3 (3.2) | 0 (0.0) | 3 (2.0) | 0 (0.0) |
| Pain | 2 (2.2) | 0 (0.0) | 2 (1.4) | 0 (0.0) |
| Application site warmth | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Infections and infestations | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
| Hordeolum | 1 (1.1) | 0 (0.0) | 1 (0.7) | 0 (0.0) |
A total of 33 ocular-specific AEs categorized as eye disorders were observed in study eyes. In total, no ocular-specific AE was reported at a frequency of more than 5% in study eyes. N = number of eyes treated in the group; n = number of eyes reported such event. Percentages are based on the number of eyes treated in the group.
Sixteen nonstudy eyes presented with nAMD at study enrollment. These eyes were removed from the total number of eyes for the development of new nAMD.
Discussion
The current report provides details of the 13-month analysis of the LIGHTSITE III 24-month study. The study met the predetermined primary efficacy endpoint with a statistically significant difference in BCVA between the PBM versus Sham treatment groups. A mean letter gain ≥ 5 letters was observed following PBM. Furthermore, 55.0% of PBM-treated eyes showed ≥ 5 letter gain, and 26.4% showed ≥ 10 letter gain. A total of 92.0% of eyes were categorized as early/intermediate dry AMD and showed limited visual impairment at study enrollment. Best-corrected visual acuity letter scores of 60 to 70 letters (Snellen 20/40–20/64) are considered mild vision loss/near-normal vision and 40 to 60 letters (Snellen 20/64–20/160) are considered moderate visual impairment or moderate low vision. The majority of eyes enrolled in this study had an initial BCVA letter score consistent with very mild or near-normal vision.20,22 Earlier stage dry AMD patients with better vision are not capable of large magnitude gains as seen in later stage patients with worse BCVA. Subjects who were enrolled had good vision with 70.0% of eyes showing a baseline BCVA of 70 to 75 letters (20/40–20/32 Snellen), which made these improvements in BCVA more noteworthy. Stabilization of BCVA, that is, a reduction in further decline, is also of critical consideration in degenerative disease. Treatment with PBM showed a reduction in the number of eyes that lost BCVA letters. The nonstudy eye subgroup with good vision (>75 letters) further documents the loss of BCVA over time with a 2.3 letter loss. This BCVA letter loss per year is consistent with natural history studies of earlier/intermediate dry AMD.23
A loss of 2 to 3 letters per year (as assessed via ETDRS BCVA) in intermediate dry AMD has been shown to increase to >5 letters per year, followed by the development of GA and irreversible loss of viable retinal tissue.23,24 This decline in vision impacts on patient independence and quality of life. Clinical outcomes, such as contrast sensitivity, color vision, VFQ-25 and reading speed, are also of interest to provide a well-rounded assessment of visual function. Secondary outcomes included these measures and showed normal to near normal vision scores at baseline. These values did not allow sufficient room to determine beneficial effect; however, no decreases in outcome scores were observed supporting safety of PBM and potential to prevent progressive decline in visual function. However, central fovea–mediated improvements in BCVA were statistically significant with a mean increase of nearly two lines in 55.0% of PBM-treated eyes, demonstrating high impact of PBM effect on BCVA in early/intermediate dry AMD subjects with better vision.
Drusen, a hallmark pathologic feature for AMD, provides a risk factor for the development of inflammation, ischemia, and further complications of AMD. Previous studies show progression rates to advanced AMD (CNV and GA for more than 5 years) of 1.3% with many small or few medium drusen, 18% if many medium or any large drusen and 43% if unilateral advanced AMD is present.25,26 Higher frequency and larger drusen deposits are indicative of disease progression. Pegcetacoplan is the only approved treatment for GA having recently received FDA approval indicated for GA secondary to AMD. The pegcetacoplan studies show slowing of GA lesion progression with no impact on other clinical outcomes such as BCVA.27,28 Anatomical markers such as GA and drusen represent appealing treatment targets in AMD. After GA onset, central GA is observed at 2.5 years accompanied by a BCVA loss of 3.7 letters; a 22-letter loss is expected at 5 years.29 This study showed the occurrence of new GA in 9.8% of Sham-treated eyes and 1.1% of PBM-treated eyes, demonstrating a statistically significant reduction in new-onset GA in the PBM group. A numerical trend showed an increase in GA lesion area in Sham compared with PBM-treated eyes. No macular drusen volume increase was observed in PBM-treated eyes, whereas the volume showed trends for increase over time in Sham-treated eyes. These effects support the potential disease-modifying effects of PBM on dry AMD development and progression. Slowing of drusen and/or GA lesion growth or progression should be recognized as important to delay disease progression, and any improvement in vision or visual stabilization should be considered clinically important. A recent (2022) retrospective observational case series published by Le et al assessed the impact of multiwavelength PBM using Valeda in subjects with reticular pseudodrusen (RPD). Treatment with PBM showed stabilization of RPD and reductions in Stage 2 and Stage 3 RPD following PBM. No progression of RPD into greater stages was observed.18
Photobiomodulation was well tolerated, with a favorable safety and compliance profile. Compliance rates for both PBM and Sham groups were high throughout each treatment series with a higher rate noted in the PBM group. Similar to previous clinical PBM studies, subjects showed a positive benefit–risk profile with high subject compliance rates and a low rate of AEs.17,19,30
Study limitations include masking of the study. A Sham arm was included to ensure masking and emitted a reduced light dose compared with the PBM mode. A 50 to 100× reduction in light fluency parameters was assumed to provide a significant reduction in the biological effect being studied. Although reduced, these wavelengths still produce a treatment that is visible to the eye and activates photoreceptors, thus could be anticipated to activate cytochrome C oxidase and other cellular targets that may produce a small biologic effect. Therefore, the Sham arm in this study could be considered an active control arm, which also showed moderate improvements in BCVA that were inferior to the full PBM active dose. In support for this limitation, nonstudy eyes with no other ocular variable and good vision (>75 letters at baseline) lost 2.3 letters at Month 13. This loss is consistent with the published literature in intermediate dry AMD studies.23 Change from baseline within groups provides a secondary measure of improvements in BCVA letter score following PBM or Sham treatments over time and confirmed the BCVA improvements. These sham effects are consistent with prior reports from the LIGHTSITE I and II studies, which used the same fluency doses.17,19 The study required extensive visits from subjects (i.e., 40 visits over 13 months). Regardless of this burden, subject compliance was 100% in the majority of subjects (PBM: 88.2%; Sham: 74.5%). Although study visits were extensive (and took place during the COVID-19 pandemic), treatment visits were < 5 minutes per eye, and subjects were motivated to attend.
The LIGHTSITE III 13-month analysis evaluating multiwavelength PBM in subjects with early/intermediate stage dry AMD showed statistically significant improvements in BCVA across time points collected during the first four treatment series. Improvements in clinical and anatomical endpoints following PBM treatment suggest disease-modifying effects. Safety data show a strong profile with AEs consistent with the patient population and no signs of phototoxicity. Multiwavelength PBM therapy may offer a new treatment strategy with a unique mechanism and modality for subjects with dry AMD. Additional data will be reported on the 24-month outcomes in a secondary report.
Supplementary Material
Acknowledgments
The authors thank Jing Shi, MD, PhD, for statistical analysis support.
Footnotes
The LIGHTSITE III study was supported in part by LumiThera, Inc. and the National Eye Institute (Grant #1R43EY025508-03).
S. E. Tedford, C. L. Croissant, M. Walker, R. Rückert and C. Tedford are all employees/contractors of LumiThera.
This study involved human subjects, was approved by the institutional review board, and adhered to the tenets of the Declaration of Helsinki.
Written informed consent was obtained from all subjects in this study.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
Contributor Information
David Boyer, Email: vitdoc@aol.com.
Allen Hu, Email: AllenH@retinacare.net.
David Warrow, Email: dwarrow22@gmail.com.
Samantha Xavier, Email: Samantha.Xavier@floridaeyeclinic.com.
Victor Gonzalez, Email: maculadoc@aol.com.
Eleonora Lad, Email: nora.lad@duke.edu.
Richard B. Rosen, Email: richardrosen2@me.com.
Diana Do, Email: dianado@stanford.edu.
Todd Schneiderman, Email: todd@retinacenternw.com.
Allen Ho, Email: acho@midatlanticretina.com.
Marion R. Munk, Email: marion_munk@hotmail.com.
Glenn Jaffe, Email: glenn.jaffe@duke.edu.
Cindy L. Croissant, Email: ccroissant@lumithera.com.
Michael Walker, Email: mwalker@stanfordalumni.org.
Rene Rückert, Email: rene.ruckert@eyegnos.com, rruckert@lumithera.com.
Clark E. Tedford, Email: ctedford@lumithera.com.
References
- 1.Fernandes AR, Zielińska A, Sanchez-Lopez E, et al. Exudative versus nonexudative age-related macular degeneration: physiopathology and treatment options. Int J Mol Sci 2022;23:2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–e116. [DOI] [PubMed] [Google Scholar]
- 3.Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond) 2016;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans J. Antioxidant supplements to prevent or slow down the progression of AMD: a systematic review and meta-analysis. Eye (Lond) 2008;22:751–760. [DOI] [PubMed] [Google Scholar]
- 5.Chung H, Dai T, Sharma SK, et al. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 2012;40:516–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashmi JT, Huang YY, Osmani BZ, et al. Role of low-level laser therapy in neurorehabilitation. PM R 2010;2:S292–S305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tata DB, Waynant RW. Laser Therapy: a review of its mechanism of action and potential medical applications. Laser Photon Rev 2010;5:1–12. [Google Scholar]
- 8.Gonzalez-Lima F, Gonzalaz-Lima F. Low level light therapy of the eye and brain. Eye and Brain 2011;2011:49–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman N, Schneid N, Reuveni H, et al. 780 nm low power diode laser irradiation stimulates proliferation of keratinocyte cultures: involvement of reactive oxygen species. Lasers Surg Med 1998;22:212–218. [DOI] [PubMed] [Google Scholar]
- 10.Karu T, Pyatibrat L, Kalendo G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 1995;27:219–223. [DOI] [PubMed] [Google Scholar]
- 11.Karu TI, Kolyakov SF. Exact action spectra for cellular responses relevant to phototherapy. Photomed Laser Surg 2005;23:355–361. [DOI] [PubMed] [Google Scholar]
- 12.Wong-Riley MT, Liang HL, Eells JT, et al. Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 2005;280:4761–4771. [DOI] [PubMed] [Google Scholar]
- 13.Oron U, Ilic S, De Taboada L, Streeter J. Ga-As (808 nm) laser irradiation enhances ATP production in human neuronal cells in culture. Photomed Laser Surg 2007;25:180–182. [DOI] [PubMed] [Google Scholar]
- 14.Silveira PC, Silva LA, Fraga DB, et al. Evaluation of mitochondrial respiratory chain activity in muscle healing by low-level laser therapy. J Photochem Photobiol B 2009;95:89–92. [DOI] [PubMed] [Google Scholar]
- 15.Passarella S, Casamassima E, Molinari S, et al. Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser. FEBS Lett 1984;175:95–99. [DOI] [PubMed] [Google Scholar]
- 16.Mochizuki-Oda N, Kataoka Y, Cui Y, et al. Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett 2002;323:207–210. [DOI] [PubMed] [Google Scholar]
- 17.Burton B, Parodi MB, Jürgens I, et al. LIGHTSITE II randomized multicenter trial: evaluation of multiwavelength photobiomodulation in non-exudative age-related macular degeneration. Ophthalmol Ther 2023;12:953–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le HM, Mehanna CJ, De Rosa I, et al. Effects of photobiomodulation in patients presenting with reticular pseudodrusen: a retrospective observational case series study. Medicina (Kaunas) 2022;58:1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markowitz SN, Devenyi RG, Munk MR, et al. A double-masked, randomized, sham-controlled, single-center study with photobiomodulation for the treatment of dry age-related macular degeneration. Retina 2020;40:1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferris FL, 3rd, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology 2013;120:844–851. [DOI] [PubMed] [Google Scholar]
- 21.2.33 RCTR. A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 22.Low Vision and Legal Blindness Terms and Descriptions. American Foundation for the Blind; 2023. [Google Scholar]
- 23.Thompson AC, Luhmann UFO, Stinnett SS, et al. Association of low luminance Questionnaire with objective functional measures in early and intermediate age-related macular degeneration. Invest Ophthalmol Vis Sci 2018;59:289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holekamp N, Wykoff CC, Schmitz-Valckenberg S, et al. Natural history of geographic atrophy secondary to age-related macular degeneration: results from the prospective proxima A and B clinical trials. Ophthalmology 2020;127:769–783. [DOI] [PubMed] [Google Scholar]
- 25.Chew EY, Clemons TE, Agrón E, et al. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol 2014;132:272–277. [DOI] [PubMed] [Google Scholar]
- 26.Joachim N, Mitchell P, Burlutsky G, et al. The incidence and progression of age-related macular degeneration over 15 Years: the blue mountains eye study. Ophthalmology 2015;122:2482–2489. [DOI] [PubMed] [Google Scholar]
- 27.Apellis Announces Top-Line Results from Phase 3 DERBY and OAKS Studies in Geographic Atrophy (GA) and Plans to Submit NDA to FDA in the First Half of 2022.
- 28.Holz FG, Sadda SR, Busbee B, et al. Efficacy and safety of lampalizumab for geographic atrophy due to age-related macular degeneration: chroma and spectri phase 3 randomized clinical trials. JAMA Ophthalmol 2018;136:666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindblad AS, Lloyd PC, Clemons TE, et al. Change in area of geographic atrophy in the Age-Related Eye Disease Study: AREDS report number 26. Arch Ophthalmol 2009;127:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JE, Glassman AR, Josic K, et al. A randomized trial of photobiomodulation therapy for center-involved diabetic macular edema with good visual acuity (Protocol AE). Ophthalmol Retina; 2022;6:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]