Abstract
Simple Summary
In the context of increasing global meat consumption, the meat production industry is facing significant challenges as it strives to enhance attributes related to livestock and meat quality. This is of paramount importance. Vertebral traits are among the factors influencing the overall body size and carcass weight of animals, with profound implications for both meat quality and quantity. This issue is particularly prominent in the livestock breeding sector, especially in China, where the augmentation of body size traits has become a central objective due to its consequential impact on carcass quality. Vertebral traits in livestock are complex, polygenic characteristics, and numerous genetic markers and pathways associated with vertebral development, size, and length have already been identified. This review article emphasizes the significant genes linked to vertebral traits in animals such as pigs, sheep, and donkeys based on the existing literature. It further underscores the importance of exploring deeper into molecular mechanisms to gain a more comprehensive understanding of the relationship between vertebral traits and genes, facilitating their effective utilization in successful breeding programs.
Abstract
In livestock breeding, the number of vertebrae has gained significant attention due to its impact on carcass quality and quantity. Variations in vertebral traits have been observed across different animal species and breeds, with a strong correlation to growth and meat production. Furthermore, vertebral traits are classified as quantitative characteristics. Molecular marker techniques, such as marker-assisted selection (MAS), have emerged as efficient tools to identify genetic markers associated with vertebral traits. In the current review, we highlight some key potential genes and their polymorphisms that play pivotal roles in controlling vertebral traits (development, length, and number) in various livestock species, including pigs, donkeys, and sheep. Specific genetic variants within these genes have been linked to vertebral development, number, and length, offering valuable insights into the genetic mechanisms governing vertebral traits. This knowledge has significant implications for selective breeding strategies to enhance structural characteristics and meat quantity and quality in livestock, ultimately improving the efficiency and quality of the animal husbandry industry.
Keywords: livestock, vertebral traits, meat production, genetic markers, genomic selection
1. Introduction
The meat production industry encounters challenges in augmenting carcass and meat quality attributes, significantly influencing consumer preferences and demand amid the global upsurge in meat consumption [1,2]. Key attributes encompassing body morphology, skeletal architecture, vertebral characteristics, muscular development, tenderness, and adipose deposition in livestock species hold paramount significance in governing both meat quality and quantity [3,4,5,6,7,8,9].
In the domain of livestock breeding, the pursuit of augmenting traits related to body size has emerged as a central objective, particularly in China, due to its substantial influence on meat production and carcass traits [10,11,12]. Recent research underscores the economic significance of vertebral count in determining carcass length, weight, and body size traits [10,11,12,13]. Variations in body size have emerged within and between domestic animal species or breeds during livestock evolution [14]. Notably, the number of thoracolumbar vertebrae plays a crucial role in carcass characteristics, particularly carcass length [15]. Across different pig breeds [16], sheep breeds [17], donkeys [10,18], and yaks [19], variations in the number of thoracolumbar vertebrae have been observed. This trait has been considered a selection criterion in commercial animal breeding due to its strong correlations with growth and meat production, exhibiting high heritability (0.60–0.62) and a positive correlation with body length [15].
China has made significant advancements in livestock genetic improvement, particularly in pig breeding [20]. However, enhancing breeding efficiency and accuracy remains a challenge. Molecular marker techniques, such as marker-assisted selection (MAS), have emerged as a rapid and effective approach [21,22,23]. MAS relies on the linkage between phenotypic traits and molecular markers within the genome, enabling the swift and accurate identification of individuals with desired traits, thus improving breeding efficiency. Identifying candidate genes and their polymorphisms associated with vertebral traits holds scientific and economic significance, providing markers for genetic enhancement in livestock.
The influence of genetic factors and signaling pathways on multi-vertebrae traits in livestock is a complex and multifaceted area of research. Several studies have shed light on the genetic underpinnings of these traits, with a focus on genes such as PLAG1, VRTN, PRKG2, MMP4, NR6A1, LTBP2, DCAF7, NCAPG-LCORL, ActRIIB, and TGFβ3, as well as their associations with specific vertebral characteristics in various livestock species, especially pigs, donkeys, and sheep. Consistently, Yan et al. [10] and Liu Z et al. [18] emphasized the quantitative nature of these traits, indicating that they are influenced by multiple genes and intricate biological signaling pathways. This highlights the polygenic nature of multi-vertebrae traits, where numerous genetic factors interplay to determine the final outcome. The genes PLAG1 and NCAPG-LCORL, originally known for their roles in human height, carcass weight, and body length, have emerged as key players in livestock vertebral traits, underscoring their pleiotropic effects [24]. Furthermore, specific mutations in genes like ActRIIB and TGFβ3 have been linked to variations in vertebral number in sheep and pigs, respectively. Liu J et al. [25] identified a point mutation in intron 4 of the ActRIIB gene in Small-Tailed Han sheep associated with vertebral number variation. Similarly, Yue J et al. [26] reported that the mutation g.105179474 G > A in the TGFβ3 gene was associated with rib and thoracolumbar vertebrae numbers in pigs, highlighting the genetic diversity that can underlie these traits. In Beijing Black pigs, Niu N et al. [27] identified a significant link between variant-g.19034 A > C of the VRTN gene and multiple thoracic vertebrae numbers, further exemplifying the genetic complexity of vertebral traits in sheep and pigs.
Donkeys are a comparatively less explored area in this context and have shown promising insights. Shi et al. [28] reported significant associations between specific genetic loci, HOXC8 g.15179224C > T and g.15179674G > A, with lumbar vertebrae length and the number of lumbar vertebrae. This highlights the potential for genetic selection and breeding strategies to influence vertebral traits in donkeys, similar to other livestock species. Nevertheless, the existing body of literature to date has exclusively focused on the investigation of genetic markers associated with vertebral traits in donkeys, sheep, and pigs. However, it is noteworthy that comprehensive investigations into the association of genetic markers with vertebral traits in cattle and horses have been absent from the existing body of research. Drawing upon the aforementioned evidence, it becomes evident that variations in vertebral traits among livestock species (donkeys, sheep, and pigs) can significantly influence changes in body size, carcass length, and weight. Furthermore, it is essential to recognize that vertebral traits are inherently quantitative in nature, controlled by a complex interplay of multiple genes. Thus, the exploration and screening of genes associated with these vertebral traits hold substantial potential for enhancing meat production within the livestock sector, thereby making a notable contribution to the meat industry. Therefore, the primary objective of this review article is to elucidate the recent advancements in the study of genetic markers linked to vertebral traits in pigs, donkeys, and sheep. The purpose is to provide comprehensive insights that can serve as foundational references for future research endeavors within this domain.
2. Methodology and Criteria for Literature Search and Selection
In the course of this current review, our methodology for the selection of relevant literature was established with careful consideration. We focused our attention primarily on articles that had been published from the year 2015 to the present day. To broaden the scope of our discussion, we also took into account pertinent data from articles published after the year 2000. Our search strategy entailed the utilization of specific keywords to identify relevant articles. The keywords employed encompassed subjects pertaining to livestock, specifically pigs, sheep, and donkeys. Additionally, we sought articles encompassing genetic markers, vertebral traits, growth traits, and both the quality and quantity of meat. It is worth noting that we exercised selectivity in our inclusion criteria. Articles published in journals not indexed in the Science Citation Index (SCI), books, or book chapters, as well as those published in languages other than English, were excluded from our review.
3. Genetic Markers Associated with Number of Vertebrae in Pigs, Donkeys, and Sheep
3.1. Genetic Markers Associated with Number of Vertebrae in Pigs
Pig vertebral classification comprises five distinct segments: cervical, thoracic, lumbar, sacral, and caudal vertebrae. The precise count of cervical, sacral, and caudal vertebrae in pigs is consistent at seven, four, and five, respectively [15]. The key constituents of the vertebral column are the thoracic and lumbar vertebrae, exhibiting considerable variability in their numbers. In Western modern breeds, the thoracic vertebral number spans from 13 to 17, while the lumbar vertebral number ranges from five to seven [13,15]. Wild boars possess 19 thoracic-lumbar vertebrae, whereas Chinese indigenous breeds exhibit a total thoracic and lumbar vertebral count ranging from 19 to 20 [13,15]. Notably, Western commercial breeds such as Large White, Duroc, and Landrace feature a higher number of thoracic-lumbar vertebrae (n = 21 to 23) due to rigorous selective breeding [13,15]. In pigs, some causal or tightly linked genes affecting crucial vertebral traits and used in practical production have been reported in previous studies [29,30,31,32]. In practical pig production, the integration of genetic knowledge regarding vertebral traits allows producers to make informed breeding decisions. Selecting breeding stock based on favorable genetic markers can lead to more robust, efficient, and economically viable pig populations. In addition, vertebral malformations can lead to structural deformities, reduced growth rates, and increased susceptibility to injuries. This, in turn, contributes to the sustainability and competitiveness of the swine industry. The summary of genes associated with vertebral traits is provided in Table 1.
Table 1.
Summary of genes associated with vertebral traits in pigs.
| Genes | Associated Traits | Breeds | Country | Reference |
|---|---|---|---|---|
| RSAD2-CMPK2, COL3A1 |
|
Meishan pigs | China | [30] |
| HMGA1, VRTN, BMP2 |
|
Duroc × Landrace × Yorkshire crossbred pigs | [31,32] | |
| TIMP2, EML1, SMN1 |
|
Pigs | [33] | |
| NR6A1, LTBP2 |
|
Xiang pigs | [34] | |
| GREB1L, ABCD4, VRTN, MIB1 |
|
Beijing Black pigs | [27] | |
| ABCD4 Hox family genes (HOXB 1–7, 9, and 13), NTRK2 |
|
Beijing Black pigs | [35] | |
|
NR6A1, VRTN PLAG1, BMP2 MC4R |
|
Shanxia Black pigs | [36] | |
| HOXA10 |
|
Pigs | [37] | |
| BMP2 |
|
Duroc × (Landrace × Yorkshire) hybrid pigs | [38] | |
| VRTN, LTBP2, BMPR1A, FOS |
|
White × Minzhu crossbred pigs | [39] | |
| VRTN |
|
Sujiang, Meishan, Bama, Erhualian, and Tibetan pigs | [40] | |
| VRTN |
|
Suhuai pigs | [13] | |
| VRTN |
|
Pigs | [41] | |
| VRTN |
|
Duroc, Landrace, and Large White pigs | Norway | [42] |
| MMP9, VEGF |
|
Pigs | USA | [43] |
| NR6A1 |
|
Large White × Minzhu pigs | China | [44] |
|
VRTN, FOS, PROX2, TGFB3 |
|
3.2. Genetic Markers Associated with Number of Vertebrae in Donkeys
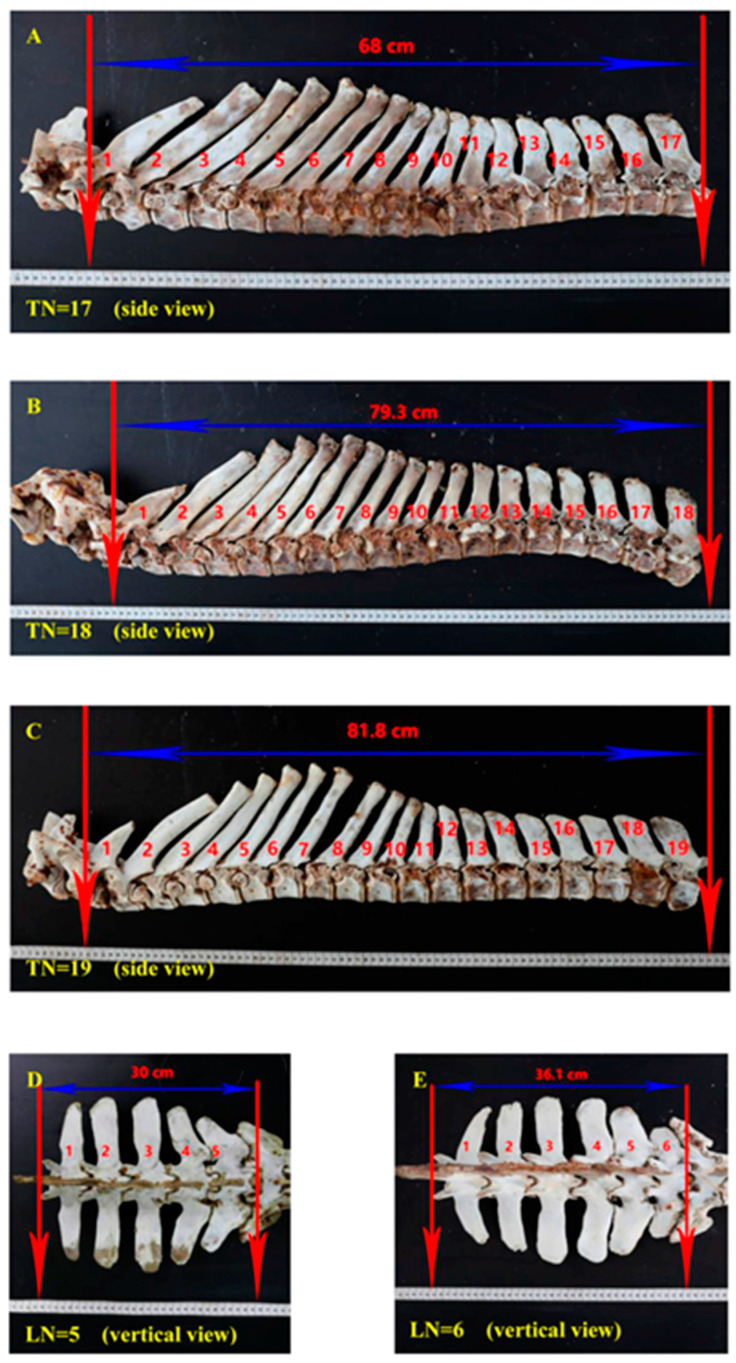
The study by Liu Z et al. [18], which involved a comprehensive survey of 455 donkeys, highlighted the presence of diverse configurations of thoracic and lumbar vertebrae in the Dezhou donkey population [18]. These configurations were identified as T18L5, T18L6, T17L6, T17L5, and T19L5. Notably, T18L5 was the most prevalent, accounting for 75.8% of the population. This finding suggests a certain level of variability in vertebral numbers among Dezhou donkeys. Moreover, research conducted by Liu Z et al. also established a correlation between the body size and weight of donkeys and the number of vertebrae (Figure 1) [18,45]. This correlation could potentially open up avenues for selective breeding and manipulation of vertebral numbers to enhance the structural characteristics of donkeys within the industry. However, it is important to note that the genetic mechanisms governing these traits require further investigation.
Figure 1.
Association of number of vertebrae with body size traits [46]. (A) Side overview of 17 thoracic vertebrae; (B) Eighteen thoracic vertebrae side overview of seventeen; (C) Side overview of 19 thoracic vertebrae; (D) Five lumber vertebrae vertical overview; (E) Six lumber vertebrae vertical overview.
In the context of understanding the genetic basis of vertebral number determination in donkeys, recent studies have made significant contributions [12,18,45,46]. Specifically, the PRKG2 gene has emerged as a key player associated with both the number and length of thoracic and lumbar vertebrae in donkeys [12]. Specific genetic variants within PRKG2 were found to be significantly correlated with thoracic and lumbar vertebrae numbers as well as their length. This genetic insight provides valuable information for further research into the molecular mechanisms behind vertebral development.
It is worth noting that PRKG2, located on chromosome 3, consists of 18 exons and 17 introns in donkeys, as outlined by Wang C et al. [22]. Interestingly, PRKG2 has also been implicated in dwarfism in various species, including American Angus cattle [47], humans [48,49], and dogs [50], underscoring its importance in skeletal development across different organisms. Additionally, its involvement in adipocyte and osteoblast differentiation in the human body, as reported by Yi et al., highlights the multifaceted nature of PRKG2’s functions [51]. In conclusion, the research on vertebral variations in Dezhou donkeys, coupled with the identification of the PRKG2 gene and its associated genetic variants, provides a foundation for further exploration into the genetic mechanisms governing vertebral development. The summary of genes associated with vertebral traits in donkeys is provided in Table 2.
Table 2.
Summary of genes associated with vertebral traits in donkeys.
| Genetic Markers | Biological Effect | Breed | Country | Reference |
|---|---|---|---|---|
| DCAF7 |
|
Dezhou donkeys | China | [11] |
| PRKG2 |
|
[12] | ||
| NR6A1 |
|
[18] | ||
| LTBP2 |
|
[46] | ||
| HOXC8 |
|
[28] | ||
|
NLGN1, DCC, FBXO4 SLC26A7, TOX, LRP5 WNT7A, LOC123286078, LOC123280142, GABBR2, LOC123277146, LOC123277359, BMP7, B3GAT1, EML2 |
|
[10] |
3.3. Genetic Markers Associated with Number of Vertebrae in Sheep
In the context of sheep anatomy, sheep typically possess a vertebral arrangement comprising seven cervical vertebrae (C), 13 thoracic vertebrae (T), six lumbar vertebrae (L), and four sacral vertebrae (S), totaling 30 vertebrae. Notably, mutations in the thoracolumbar region, such as T14L6 or T13L7, have been identified as the most prevalent [52]. These mutations are associated with multi-vertebrae sheep, which exhibit enhanced adaptability and meat production performance [52]. The cultivation of such multi-spine sheep carries substantial benefits for the economy, society, and ecology, making it a pivotal endeavor for improving the quality and efficiency of the animal husbandry industry.
In the case of Kazakh sheep, an indigenous breed in Western Xinjiang, China, there exists variability in the number of lumbar vertebrae. While most sheep conform to the standard configuration of 13 thoracic vertebrae and six lumbar vertebrae, denoted as T13L6, Kazakh sheep exhibit variations, specifically in T13L7 and T14L6. These variations correspondingly lead to an increase in carcass length by 2.22 cm and 2.93 cm compared to the typical T13L6 Kazakh sheep. Moreover, there is a corresponding elevation in carcass weight by 1.68 kg and 1.90 kg, respectively [53,54,55]. Significant progress has been achieved in China regarding the screening of genetic markers associated with vertebral traits in sheep [56,57,58,59,60,61,62]. For the sake of clarity, we compiled a concise summary of genes related to vertebral count and bone development, presented in Table 3.
Table 3.
Summary of genes associated with vertebral traits and bone development in sheep.
| Genes | Associated Traits | Breeds | Country | Reference |
|---|---|---|---|---|
| NR6A1 |
|
Xinjiang Kazakh sheep | China | [56] |
| SFRP4 |
|
Duolang sheep | [57] | |
| SYNDIG1L, UNC13C |
|
Han sheep and Sunite sheep | [52] | |
| TBXT |
|
Sheep | [58] | |
|
MGAT4A, KCNH1 CPOX, CPQ |
|
Hu sheep | [59] | |
| LTBP2, SYNDIG1L |
|
Large fat-tailed sheep, Altay sheep, Tibetan sheep |
[60] | |
| VRTN, HoxA |
|
Xinjiang Kazakh sheep | [61] | |
| NDRG2 |
|
Kazakh sheep | [62] | |
| VRTN |
|
China Kazakh sheep | [55] | |
| NID2, ACAN |
|
Afghani sheep | Iran | [63] |
|
ALX4, HOXB13, BMP4 EYA2, SULF2 |
|
Ethiopian indigenous sheep | Ethiopia | [64] |
4. Comparative Analysis of Overlapping Genes Linked to Vertebral Traits in Pigs, Donkeys, and Sheep
For detail discussion, we selected some overlapping genes, including NR6A1, VRTN, LTBP2, BMPs, and Hox, in donkeys, sheep, and pigs for their association with vertebral traits.
4.1. Nuclear Receptor Subfamily 6, Group A, Member 1 (NR6A1)
NR6A1, a member of the nuclear receptor family, exerts a pivotal role in regulating various biological processes such as growth, metabolism, and embryonic stem cell differentiation [65,66]. Furthermore, it is essential for orchestrating Hox signatures and determining the fate between neural and mesodermal cells within axial progenitors, which is crucial for vertebral column development in mice [65]. The involvement of NR6A1 in vertebral development extends beyond mice, as it has been extensively investigated in livestock species.
Studies in sheep [56,67], pigs [16,68,69], and donkeys [18] have consistently demonstrated the association between NR6A1 and the number of vertebrae. Specific polymorphisms in the NR6A1 gene, including IVS8-281G > A in intron 8 and rs414302710: A > C in exon 8, have been identified as contributors to the variation in lumbar spine number and the number of lumbar vertebrae, particularly in Xinjiang Kazakh sheep [56,67]. These variations in vertebrae have significant implications for the carcass length and weight of Kazakh sheep [54]. Furthermore, in Dezhou donkeys, specific single-nucleotide polymorphisms (SNPs) within NR6A1 have been linked to thoracic and lumbar vertebrae, highlighting the role of NR6A1 in controlling the vertebral structure in this species [18]. Additionally, Fang et al. [70] reported a correlation between higher NR6A1 gene expression and body size as well as carcass weight in Dezhou donkeys.
Pigs have also been a subject of investigation regarding the genetic basis of vertebral number, with NR6A1 identified alongside PLAG1 and LCORL as genes associated with this trait [69]. These findings have been further corroborated in European commercial pig breeds [71]. Moreover, a specific mutation, c.575T > C-NR6A1, has been linked to increased vertebral number in Licha Black and Laiwu pigs [72]. In Duroc × Landrace/Large White cross pigs, polymorphisms 748 C > T-NR6A1 and insertion g.20311_20312ins291-VRTN have been found to influence vertebral number [16], with similar associations verified in Chinese pigs [73]. Additionally, Zhang et al. [44] documented a positive correlation between NR6A1 and lumbar vertebrae in Large White × Minzhu pigs. These cumulative findings underscore the significance of NR6A1 in the regulation of vertebral development and its potential impact on livestock characteristics.
4.2. Vertnin (VRTN)
The significant association of the VRTN gene with the number of vertebrae and ribs has been extensively investigated in pigs. Consistently, Jiang et al. [13] systematically investigated the relationship between the g.20311_20312ins291 polymorphism within the VRTN gene and the augmentation of rib count. Their study revealed a consistent association between this polymorphism and an increase in the number of ribs. Additionally, Jiang et al. reported a positive correlation between rib count and carcass length (CL), body size, and cannon bone circumference. It is worth noting that cannon bone circumference is well-established for its role in enhancing body size traits, including the ability to support excessive body weight, engage in strenuous physical activity, and exhibit resistance to injuries. Multiple studies have reported this association [31,74,75,76], with consistent findings. Similarly, Xie L et al. [31,32] identified key candidate genes, including HMGA1, VRTN, and BMP2, linked to body length and size in pigs. They observed a positive correlation of HMGA1 with leg bone size, VRTN with the number of vertebrae, and BMP2 with the length of vertebrae. In line with these findings, other studies have also confirmed the association of the VRTN gene with skeletal development in pigs. Danish pigs were found to exhibit this association [75], as well as Duroc pigs [77], with an increase in the number of thoracic vertebrae and ribs. Consequently, Nakano et al. [78] reported that VRTN is not only associated with the number of thoracic vertebrae and length but also with carcass weight and body length in Duroc pigs. Furthermore, the pleiotropic effect of the VRTN gene has been documented, as it is associated with increased teat numbers and vertebral numbers in Chinese indigenous pigs [79,80,81]. Variations such as g.20311_20312ins291-VRTN have been linked to the number of ribs in Chinese Suhuai pigs [13] and the number of vertebrae in Duroc × Landrace/Large White crossbreeds [16]. Similar associations were found in Chinese Erhualian and White Duroc pigs [82] and in Duroc, Landrace, and Large White pigs [42]. Additionally, polymorphisms in VRTN-g.19034 A > C, LTBP2 c.4481A > C, and BMPR1A genes have been linked to the number of thoracic vertebrae in Large White × Minzhu pig crossbreeds [40]. Zheng Y et al. [40] also found an association between SINE retrotransposon insertion polymorphism (sRTIP) in VRTN and the number of vertebrae [40].
In sheep, VRTN gene studies have also explored its association with the number of thoracic vertebrae [54,61]. These variations in vertebrae have been suggested to contribute to carcass and body length in sheep [54]. Notably, the polymorphism rs426367238-VRTN has been statistically correlated with thoracic vertebral numbers in Kazakh sheep [55]. In summary, the extensive body of research discussed here establishes a robust and consistent link between the VRTN gene and vertebral traits in both pigs and sheep. These findings contribute to our understanding of the genetic factors influencing skeletal development and provide valuable insights for breeding and selection programs in these livestock species.
4.3. Latent TGFβ Binding Protein-2 (LTBP2)
LTBP2, a pivotal component in the microfibril structure, exerts its influence on bone formation by virtue of its binding affinity with fibrillin 1 [83,84]. This interaction assumes critical significance as it actively regulates bone development through the intricate modulation of endogenous signaling pathways, particularly those governed by transforming growth factor-β (TGF-β) and bone morphogenetic protein (BMP) [85,86]. A seminal investigation by Park et al. [87] underscored the pivotal role played by LTBP2 in bone metabolism and its intriguing association with the number of thoracic vertebrae observed in porcine species [87].
Recent genetic inquiries have unraveled intriguing variations within the NR6A1 and LTBP2 genes that appear to account for the observed disparities in vertebral numbers between Xiang pigs and European pig breeds [34]. Furthermore, the involvement of LTBP2 in shaping rib development has surfaced in multiple species, including sheep and pigs [60,76]. In a striking alignment with these findings, previous studies have posited LTBP2 as an indirect regulator of growth differentiation factor (Gdf11), a factor known to influence rib count in knockout mice [88]. Expanding the scope of LTBP2’s impact, a genome-wide association analysis in donkeys illuminated its potential involvement in determining vertebral numbers, with particular reference to Dezhou donkeys [22].
Further unraveling the genetic intricacies, Liu Z et al. [18] identified significant associations between specific LTBP2 polymorphisms (c.5547 + 860 C > T, c.5251 + 281 A > C, c.3769 + 40 C > T, c.2782 + 3975 A > G) and thoracic vertebrae number. Additionally, variants at the c.1381 + 768 T > G and c.1381 + 763 G > T loci within LTBP2 have been correlated with lumbar vertebrae numbers [46]. These insights collectively contribute to our understanding of LTBP2’s multifaceted role in vertebral development and underscore its significance in shaping skeletal phenotypes across various species.
4.4. Bone Morphogenetic Proteins (BMPs)
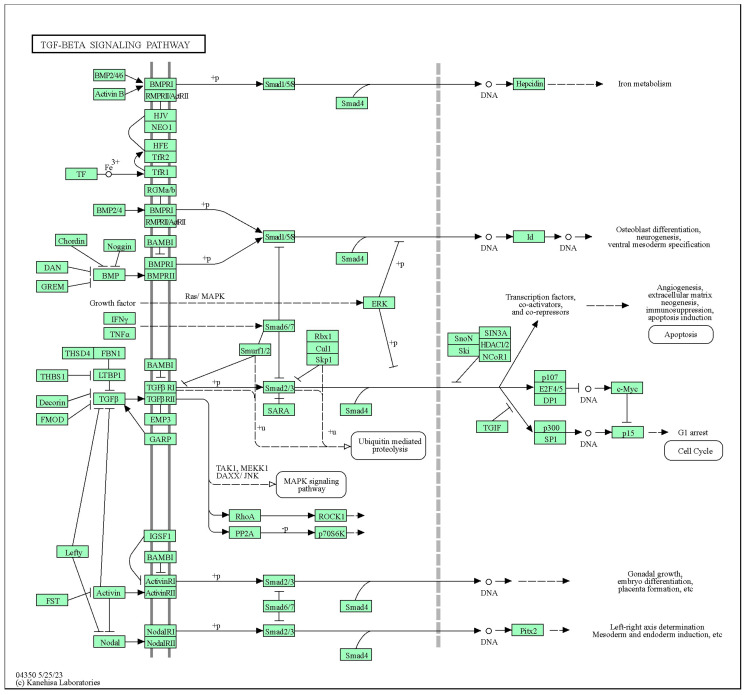
BMPs belong to the transforming growth factor (TGF)-β superfamily of signaling molecules, known for their pivotal involvement in a wide spectrum of biological processes spanning from early embryonic tissue development to the maintenance of postnatal tissue equilibrium [89,90]. The principal mechanism through which BMPs elicit cellular responses is via the canonical signaling pathway, wherein intracellular Smads assume a central role in relaying extracellular signals to the cellular nucleus [91]. Notably, while BMPs engage the same Smads across diverse cell types, the functional diversity of BMPs is, in part, attributed to distinct transcription factors [92]. These transcription factors are recruited by Smads to orchestrate the regulation of specific subsets of target genes, a process contingent upon the cellular context, as shown in Figure 2 [93]. Within this group of transcriptional regulators, Hox proteins emerge as noteworthy constituents. Empirical investigations involving gain-of-function and loss-of-function experiments, coupled with the scrutiny of naturally occurring Hox gene mutations, have provided compelling evidence underscoring their indispensable contributions to embryonic skeletal patterning [94,95,96,97].
Figure 2.
Regulatory mechanism of TGF-beta signaling pathway (hsa04350) in bone development.
Numerous studies have consistently identified the significant association of a single nucleotide polymorphism (SNP), specifically rs320706814, within the BMP2 gene, with its involvement in the regulation of growth and bone development [38]. Additionally, MBP4 has been correlated with an increase in carcass length of up to 4 cm in Duroc × (Landrace × Yorkshire) pig breeds [37], while BMP15 was found to be associated with litter size in sheep [98]. This effect has also been observed in Duroc × (Landrace × Yorkshire) crossbreeds. Furthermore, a separate investigation reported that this SNP influences the length of vertebral structures and the size and development of hind leg bones in pigs [31,32].
The BMP receptors constitute a family of transmembrane serine/threonine kinases, which encompass the type I receptors BMPR1A and BMPR1B. These receptors serve as docking points for ligands belonging to the transforming growth factor-beta (TGF-beta) superfamily. Consistent with these findings, a GWAS study revealed that mutations in BMPR1A are associated with the number of thoracic vertebrae in Large White × Minzhu pig crossbreeds [39]. Recent GWAS studies have also shed light on the regulatory roles of BMP7 and BMP4 in bone development. These studies have elucidated their involvement in key signaling pathways, including in hippos (accession number: ssc04390), TGF-beta signaling pathways (accession number: hsa04350) and Wnt signaling pathways (accession number: hsa04310) in donkeys [10], and in mice [99,100,101,102]. Additionally, the association of BMP4 with limb and skeleton development, as well as tail formation, has been identified in Ethiopian fat-tailed sheep through the utilization of GWAS studies [64].
4.5. Homeobox (Hox) Genes
Hox genes have been recognized for their pivotal role in the determination of body segment identity throughout the course of embryonic development [103,104,105]. Furthermore, it is well established that HOX genes actively participate in shaping the anterior–posterior axis and contribute significantly to the intricate process of forming various anatomical structures, including limbs and the vertebral column [106,107]. Furthermore, studies involving gene mutation have demonstrated that variations in the sequence of the mouse HOXC8 gene are linked to the addition of a thoracic vertebrae and an extra pair of ribs [108]. Similarly, differential expression of the HOXC8 gene has been found to cause vertebral changes in livestock, resulting in the appearance of multi-vertebral variants in pigs [109] and Mongolian sheep [110]. Another investigation highlighted the significant role of HOXB13 in the embryonic development of tendons, limbs, the skeleton, and tail formation in Ethiopian indigenous sheep [64]. Additionally, research has indicated a positive association between HoxA and the development of thoracic and lumbar vertebrae in Kazak sheep [61]. Recent studies have reported significant associations between polymorphisms in HOX8 genes and body size, lumbar vertebrae length, and numbers in Dezhou donkeys [28]. Similarly, in pigs, the HOX family genes, including HOXA10 and HOXB7, have been found to be associated with variations in thoraco-lumbar vertebrae [35,37]. Collectively, these findings highlight the critical role of HOX genes in vertebral development across various species and provide valuable insights into the molecular mechanisms governing this process.
5. Future Directions and Limitations
Based on the available published studies, our review article presented significant strides in exploring key genes associated with vertebral traits in pigs, donkeys, and sheep. The exploration of these genetic factors has opened up exciting avenues for further research and applications in animal breeding and genetics. Based on the facts and findings, one of the promising directions for future research in this domain is conducting more extensive genome-wide association studies (GWASs). GWASs have proven to be a valuable tool in identifying genetic variants linked to vertebral characteristics. These studies can help uncover additional variants contributing to the complexity of vertebral traits in various livestock species. The knowledge gained from such studies would provide a deeper understanding of the genetic intricacies governing vertebral development. Furthermore, it is crucial to advance our understanding of the functional characterization of the identified genes. This entails gene expression studies, knockout experiments, and pathway analysis to elucidate how these genes precisely influence vertebral development. Investigating the molecular mechanisms behind these genetic influences is pivotal for a comprehensive understanding of vertebral traits. Consequently, the genetic markers unearthed through these studies can be seamlessly integrated into selective breeding programs for livestock improvement. Breeders can employ this genetic information to make well-informed decisions about mating pairs with the aim of enhancing desirable vertebral traits in their herds or flocks.
Another intriguing avenue of research lies in comparative genomics across different livestock species. This approach can provide valuable insights into the evolutionary history of vertebral traits. It offers the opportunity to investigate why certain genes are more prominent in specific species and how these genetic factors have evolved to shape vertebral characteristics.
Finally, validating the effects of specific genetic variants on vertebral traits is essential. Modern genetic editing techniques, such as CRISPR/Cas9, offer a means to manipulate genes of interest and observe resulting phenotypic changes in vertebral development.
While these recent advancements are commendable, it is important to acknowledge the limitations and areas for further exploration. Presently, research primarily focuses on pigs, donkeys, and sheep. However, the diversity of livestock species with varying vertebral characteristics necessitates expanding the research to include species like horses and cattle. Furthermore, the genetic markers identified may not universally apply to all livestock breeds, and further research is imperative to encompass a broader spectrum of breeds and species. It is important to note that while genetics play a significant role, vertebral traits are polygenic and influenced by intricate environmental factors. Future research should consider the interplay between genes and the environment in shaping these traits. Donkeys remain relatively less explored in this context, emphasizing the need for comprehensive genetic investigations to fully comprehend vertebral traits in this species.
6. Conclusions
The research on genetic markers associated with vertebral traits in pigs, donkeys, and sheep has provided critical insights into the genetic basis of the number and length of vertebrae. Genes such as PRKG2, NR6A1, LTBP2, VRTN, BMP, and the HOX family genes have been identified as key players in controlling vertebral number and length, with specific genetic variants associated with these traits in donkeys, sheep, and pigs. This information offers a foundation for selective breeding strategies in these livestock species with the potential to enhance carcass quality and quantity. Moreover, the study of these genes highlights their pleiotropic effects and their significance in skeletal development across different organisms. Further research into the molecular pathways and interactions involved in vertebral traits is warranted to fully harness the potential of these genetic markers in improving livestock breeding programs.
Author Contributions
Conceptualization: M.Z.K. and C.W., Data curation; M.Z.K., W.C. (Wenting Chen), X.L., Y.L., B.H., X.W., W.C. (Wenqiong Chai) and C.W., Formal analysis; M.Z.K. and C.W., Funding acquisition; C.W., Investigation; Methodology; M.Z.K. and C.W., Project administration; M.Z.K. and C.W., Resources; C.W., Software; M.Z.K., W.C. (Wenting Chen), X.L., Y.L., B.H., X.W., W.C. (Wenqiong Chai) and C.W., Supervision; M.Z.K. and C.W., Validation; M.Z.K., W.C. (Wenting Chen), X.L., Y.L., B.H., X.W., W.C. (Wenqiong Chai) and C.W., Visualization; M.Z.K. and C.W., Roles/Writing—original draft: M.Z.K. and C.W., and Writing—review & editing: M.Z.K., W.C. (Wenting Chen), X.L., Y.L., B.H., X.W., W.C. (Wenqiong Chai) and C.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are available in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Key R&D Program of China (grant number 2022YFD1600103), The Shandong Province Modern Agricultural Technology System Donkey Industrial Innovation Team (grant no. SDAIT-27), Livestock and Poultry Breeding Industry Project of the Ministry of Agriculture and Rural Affairs (grant number 19211162), The National Natural Science Foundation of China (grant no. 31671287), The Open Project of Liaocheng University Animal Husbandry Discipline (grant no. 319312101-14), The Open Project of Shandong Collaborative Innovation Center for Donkey Industry Technology (grant no. 3193308), Research on Donkey Pregnancy Improvement (grant no. K20LC0901), and Liaocheng University scientific research fund (grant no. 318052025).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Whitton C., Bogueva D., Marinova D., Phillips C.J.C. Are We Approaching Peak Meat Consumption? Analysis of Meat Consumption from 2000 to 2019 in 35 Countries and Its Relationship to Gross Domestic Product. Animals. 2021;11:3466. doi: 10.3390/ani11123466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hötzel M.J., Vandresen B. Brazilians’ attitudes toward meat consumption and production: Present and future challenges to the sustainability of the meat industry. Meat Sci. 2022;192:108893. doi: 10.1016/j.meatsci.2022.108893. [DOI] [PubMed] [Google Scholar]
- 3.Chai W., Qu H., Ma Q., Zhu M., Li M., Zhan Y., Liu Z., Xu J., Yao H., Li Z., et al. RNA-seq analysis identifies differentially expressed genes in different types of donkey skeletal muscles. Anim. Biotechnol. 2023;34:1786–1795. doi: 10.1080/10495398.2022.2050920. [DOI] [PubMed] [Google Scholar]
- 4.Ma Q., Kou X., Yang Y., Yue Y., Xing W., Feng X., Liu G., Wang C., Li Y. Comparison of Lipids and Volatile Compounds in Dezhou Donkey Meat with High and Low Intramuscular Fat Content. Foods. 2023;12:3269. doi: 10.3390/foods12173269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai W., Xu J., Qu H., Ma Q., Zhu M., Li M., Zhan Y., Wang T., Gao J., Yao H., et al. Differential proteomic analysis to identify potential biomarkers associated with quality traits of Dezhou donkey meat using a data-independent acquisition (DIA) strategy. LWT. 2022;166:113792. doi: 10.1016/j.lwt.2022.113792. [DOI] [Google Scholar]
- 6.Dagevos H., Verbeke W. Meat consumption and flexitarianism in the Low Countries. Meat Sci. 2022;192:108894. doi: 10.1016/j.meatsci.2022.108894. [DOI] [PubMed] [Google Scholar]
- 7.Realini C.E., Ares G., Antúnez L., Brito G., Luzardo S., Del Campo M., Saunders C., Farouk M.M., Montossi F.M. Meat insights: Uruguayan consumers’ mental associations and motives underlying consumption changes. Meat Sci. 2022;192:108901. doi: 10.1016/j.meatsci.2022.108901. [DOI] [PubMed] [Google Scholar]
- 8.Wang T., Shi X., Liu Z., Ren W., Wang X., Huang B., Kou X., Liang H., Wang C., Chai W. A novel A>G polymorphism in the intron 1 of LCORL gene is significantly associated with hide weight and body size in Dezhou donkey. Animals. 2022;12:2581. doi: 10.3390/ani12192581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao G., Gao N., Li S., Kuang W., Zhu L., Jiang W., Yu W., Guo J., Li Z., Yang C., et al. Genome-wide association study of meat quality traits in a three-way crossbred commercial pig population. Front. Genet. 2021;12:614087. doi: 10.3389/fgene.2021.614087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan S.U., Li Y.H., Zhao C.H., Jun T.E., Wang Y.H., Wang T.Q., Shi X.Y., Liu Z.W., Li H.J., Wang J.J., et al. Genome-wide association study for numbers of vertebrae in Dezhou donkey population reveals new candidate genes. J. Integr. Agric. 2023;22:3159–3169. [Google Scholar]
- 11.Wang T., Wang X., Liu Z., Shi X., Ren W., Huang B., Liang H., Wang C., Chai W. Genotypes and haplotype combination of DCAF7 gene sequence variants are associated with the number of thoracolumbar vertebrae and carcass traits in Dezhou donkey. J. Appl. Anim. Res. 2023;51:31–39. doi: 10.1080/09712119.2022.2149538. [DOI] [Google Scholar]
- 12.Wang T., Liu Z., Wang X., Li Y., Akhtar F., Li M., Zhang Z., Zhan Y., Shi X., Ren W., et al. Polymorphism Detection of PRKG2 Gene and Its Association with the Number of Thoracolumbar Vertebrae and Carcass Traits in Dezhou Donkey. BMC Genom. Data. 2023;24:2. doi: 10.1186/s12863-022-01101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang N., Liu C., Lan T., Zhang Q., Cao Y., Pu G., Niu P., Zhang Z., Li Q., Zhou J., et al. Polymorphism of VRTN Gene g. 20311_20312ins291 Was Associated with the Number of Ribs, Carcass Diagonal Length and Cannon Bone Circumference in Suhuai Pigs. Animals. 2020;10:484. doi: 10.3390/ani10030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fogel J.L., Lakeland D.L., Mah I.K., Mariani F.V. A minimally sufficient model for rib proximal-distal patterning based on genetic analysis and agent-based simulations. eLife. 2017;6:e29144. doi: 10.7554/eLife.29144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borchers N., Reinsch N., Kalm E. The number of ribs and vertebrae in a Piétrain cross: Variation, heritability and effects on performance traits. J. Anim. Breed. Genet. 2004;121:392–403. doi: 10.1111/j.1439-0388.2004.00482.x. [DOI] [Google Scholar]
- 16.Burgos C., Latorre P., Altarriba J., Carrodeguas J., Varona L., López Buesa P. Allelic frequencies of NR6A1 and VRTN, two genes that affect vertebrae number in diverse pig breeds: A study of the effects of the VRTN insertion on phenotypic traits of a Duroc×Landrace–Large White cross. Meat Sci. 2015;100:150–155. doi: 10.1016/j.meatsci.2014.09.143. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson C.L., Lambe N.R., Maltin C.A., Knott S., Bunger L. Between-and within-breed variations of spine characteristics in sheep. J. Anim. Sci. 2013;91:995–1004. doi: 10.2527/jas.2012-5456. [DOI] [PubMed] [Google Scholar]
- 18.Liu Z., Gao Q., Wang T., Chai W., Zhan Y., Akhtar F., Zhang Z., Li Y., Shi X., Wang C. Multi-Thoracolumbar Variations and NR6A1 Gene Polymorphisms Potentially Associated with Body Size and Carcass Traits of Dezhou Donkey. Animals. 2022;12:1349. doi: 10.3390/ani12111349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J., Wen Y., Feng Z., Ke M., Gao X., An D. Correlation analysis for beef performance and multi-vertebra properties of Jinchuan yak. J. Domest. Anim. Ecol. 2015;36:26–30. [Google Scholar]
- 20.Palombo V., D’Andrea M., Licastro D., Dal Monego S., Sgorlon S., Sandri M., Stefanon B. Single-step genome-wide association study identifies QTL signals for untrimmed and trimmed thigh weight in Italian crossbred pigs for dry-cured ham production. Animals. 2021;11:1612. doi: 10.3390/ani11061612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang W., Liu Z., Zhao Q., Du H., Yu J., Wang H., Liu X., Liu H., Jing X., Yang H., et al. Population Genetic Structure and Selection Signature Analysis of Beijing Black Pig. Front. Genet. 2022;13:860669. doi: 10.3389/fgene.2022.860669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang C., Li H., Guo Y., Huang J., Sun Y., Min J., Wang J., Fang X., Zhao Z., Wang S., et al. Donkey genomes provide new insights into domestication and selection for coat color. Nat. Commun. 2020;1:6014. doi: 10.1038/s41467-020-19813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W., Fang G.F., Wang S.D., Wang H., Zeng Y.-Q. Longissimus lumborum muscle transcriptome analysis of Laiwu and Yorkshire pigs differing in intramuscular fat content. Genes Genom. 2017;39:759–766. doi: 10.1007/s13258-017-0540-9. [DOI] [Google Scholar]
- 24.Takasuga A. PLAG1 and NCAPG-LCORL in livestock. Anim. Sci. J. 2016;87:159–167. doi: 10.1111/asj.12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J., Sun S., Han L., Li X., Sun Z. Association between single nucleotide polymorphism of ActRIIB gene and vertebra number variation in small tail Han sheep. Acta Vet. Et. Zootech. Sin. 2010;41:951–954. [Google Scholar]
- 26.Yue J., Guo H., Zhou W., Liu X., Wang L., Gao H., Hou X., Zhang Y., Yan H., Wei X. Polymorphism Sites of TGFβ3 Gene and Its Association Analysis with Vertebral Number of Porcine. Chin. Anim. Husb. Vet. Med. 2018;45:738–744. [Google Scholar]
- 27.Niu N., Liu Q., Hou X., Liu X., Wang L., Zhao F., Gao H., Shi L., Wang L., Zhang L. Genome-wide association study revealed ABCD4 on SSC7 and GREB1L and MIB1 on SSC6 as crucial candidate genes for rib number in Beijing Black pigs. Anim. Genet. 2022;53:690–695. doi: 10.1111/age.13237. [DOI] [PubMed] [Google Scholar]
- 28.Shi X., Li Y., Wang T., Ren W., Huang B., Wang X., Liu Z., Liang H., Kou X., Chen Y., et al. Association of HOXC8 Genetic Polymorphisms with Multi-Vertebral Number and Carcass Weight in Dezhou Donkey. Genes. 2022;13:2175. doi: 10.3390/genes13112175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J., Jiang A., Zhang C., Zheng Y., Zhang T., Zhou L. Potential of eight mutations for marker-assisted breeding in Chinese Lulai black pigs. Can. J. Anim. Sci. 2022;102:431–439. doi: 10.1139/cjas-2021-0108. [DOI] [Google Scholar]
- 30.Liu C., Hou L., Zhao Q., Zhou W., Liu K., Liu Q., Zhou T., Xu B., Li P., Huang R. The selected genes NR6A1, RSAD2-CMPK2, and COL3A1 contribute to body size variation in Meishan pigs through different patterns. J. Anim. Sci. 2023;101:skad304. doi: 10.1093/jas/skad304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie L., Qin J., Yao T., Tang X., Cui D., Chen L., Rao L., Xiao S., Zhang Z., Huang L. Genetic dissection of 26 meat cut, meat quality and carcass traits in four pig populations. Genet. Sel. Evol. 2023;55:43. doi: 10.1186/s12711-023-00817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie L., Qin J., Rao L., Cui D., Tang X., Chen L., Xiao S., Zhang Z., Huang L. Genetic dissection and genomic prediction for pork cuts and carcass morphology traits in pig. J. Anim. Sci. Biotechnol. 2023;14:116. doi: 10.1186/s40104-023-00914-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou F., Quan J., Ruan D., Qiu Y., Ding R., Xu C., Ye Y., Cai G., Liu L., Zhang Z., et al. Identification of Candidate Genes for Economically Important Carcass Cutting in Commercial Pigs through GWAS. Animals. 2023;13:3243. doi: 10.3390/ani13203243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Ran X., Niu X., Huang S., Li S., Wang J. Whole-genome sequence analysis reveals selection signatures for important economic traits in Xiang pigs. Sci. Rep. 2022;12:11823. doi: 10.1038/s41598-022-14686-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niu N., Wang H., Shi G., Liu X., Liu H., Liu Q., Yang M., Wang L., Zhang L. Genome scanning reveals novel candidate genes for vertebral and teat number in the Beijing Black Pig. Anim. Genet. 2021;52:734–738. doi: 10.1111/age.13111. [DOI] [PubMed] [Google Scholar]
- 36.Li L.Y., Xiao S.J., Tu J.M., Zhang Z.K., Zheng H., Huang L.B., Huang Z.Y., Yan M., Liu X.D., Guo Y.M. A further survey of the quantitative trait loci affecting swine body size and carcass traits in five related pig populations. Anim. Genet. 2021;52:621–632. doi: 10.1111/age.13112. [DOI] [PubMed] [Google Scholar]
- 37.Li J., Wang L., Yu D., Hao J., Zhang L., Adeola A.C., Mao B., Gao Y., Wu S., Zhu C., et al. Single-cell RNA sequencing reveals thoracolumbar vertebra heterogeneity and rib-genesis in pigs. Genom. Proteom. Bioinform. 2021;19:423–436. doi: 10.1016/j.gpb.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H., Zhuang Z., Yang M., Ding R., Quan J., Zhou S., Gu T., Xu Z., Zheng E., Cai G., et al. Genome-wide detection of genetic loci and candidate genes for body conformation traits in Duroc× Landrace× Yorkshire crossbred pigs. Front. Genet. 2021;12:664343. doi: 10.3389/fgene.2021.664343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q., Yue J., Niu N., Liu X., Yan H., Zhao F., Hou X., Gao H., Shi L., Wang L., et al. Genome-wide association analysis identified BMPR1A as a novel candidate gene affecting the number of thoracic vertebrae in a Large White× Minzhu intercross pig population. Animals. 2020;10:2186. doi: 10.3390/ani10112186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng Y., Cai C.H., Wei C.H., Wang X.Y., Wei W.A., Bo G.A., Wimmers K., Mao J.D., Song C.Y. Two new SINE insertion polymorphisms in pig Vertnin (VRTN) gene revealed by comparative genomic alignment. J. Integr. Agric. 2020;19:2514–2522. doi: 10.1016/S2095-3119(20)63255-5. [DOI] [Google Scholar]
- 41.Duan Y., Zhang H., Zhang Z., Gao J., Yang J., Wu Z., Fan Y., Xing Y., Li L., Xiao S., et al. VRTN is required for the development of thoracic vertebrae in mammals. Int. J. Biol. Sci. 2018;14:667. doi: 10.7150/ijbs.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Son M., Lopes M.S., Martell H.J., Derks M.F., Gangsei L.E., Kongsro J., Wass M.N., Grindflek E.H., Harlizius B. A QTL for the number of teats shows breed-specific effects on the number of vertebrae in pigs: Bridging the gap between molecular and quantitative genetics. Front. Genet. 2019;10:272. doi: 10.3389/fgene.2019.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amundson L.A., Hernandez L.L., Crenshaw T.D. Gene expression of matrix metalloproteinase 9 (MMP9), matrix metalloproteinase 13 (MMP13), vascular endothelial growth factor (VEGF), and fibroblast growth factor 23 (FGF23) in femur and vertebra tissues of the hypovitaminosis D kyphotic pig model. Br. J. Nutr. 2018;120:404–414. doi: 10.1017/S0007114518001605. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L.C., Xin L.I., Liang J., Hua Y.A., Zhao K.B., Na L.I., Lei P.U., Shi H.B., Zhang Y.B., Wang L.G., et al. Quantitative trait loci for the number of vertebrae on Sus scrofa chromosomes 1 and 7 independently influence the numbers of thoracic and lumbar vertebrae in pigs. J. Integr. Agric. 2015;14:2027–2033. doi: 10.1016/S2095-3119(15)61084-X. [DOI] [Google Scholar]
- 45.Liu Z., Wang T., Shi X., Wang X., Ren W., Huang B., Wang C. Identification of LTBP2 gene polymorphisms and their association with thoracolumbar vertebrae number, body size, and carcass traits in Dezhou donkeys. Front. Genet. 2022;13:969959. doi: 10.3389/fgene.2022.969959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang B., Khan M.Z., Chai W., Ullah Q., Wang C. Exploring Genetic Markers: Mitochondrial DNA and Genomic Screening for Biodiversity and Production Traits in Donkeys. Animals. 2023;13:2725. doi: 10.3390/ani13172725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koltes J.E., Mishra B.P., Kumar D., Kataria R.S., Totir L.R., Fernando R.L., Cobbold R., Steffen D., Coppieters W., Georges M., et al. A nonsense mutation in cGMP-dependent type II protein kinase (PRKG2) causes dwarfism in American Angus cattle. Proc. Natl. Acad. Sci. USA. 2009;106:19250–19255. doi: 10.1073/pnas.0904513106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soranzo N., Rivadeneira F., Chinappen-Horsley U., Malkina I., Richards J.B., Hammond N., Stolk L., Nica A., Inouye M., Hofman A., et al. Meta-analysis of genome-wide scans for human adult stature identifies novel Loci and associations with measures of skeletal frame size. PLoS Genet. 2009;5:e1000445. doi: 10.1371/journal.pgen.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsuchida A., Yokoi N., Namae M., Fuse M., Masuyama T., Sasaki M., Kawazu S., Komeda K. Phenotypic Characterization of the Komeda Miniature Rat Ishikawa, an Animal Model of Dwarfism Caused by a Mutation in Prkg2. Comp. Med. 2008;58:560–567. [PMC free article] [PubMed] [Google Scholar]
- 50.Garces G.R., Turba M.E., Muracchini M., Diana A., Jagannathan V., Gentilini F., Leeb T. PRKG2 Splice Site Variant in Dogo Argentino Dogs with Disproportionate Dwarfism. Genes. 2021;12:1489. doi: 10.3390/genes12101489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yi X., Wu P., Liu J., He S., Gong Y., Xiong J., Xu X., Li W. Candidate kinases for adipogenesis and osteoblastogenesis from human bone marrow mesenchymal stem cells. Mol. Omics. 2021;17:790–795. doi: 10.1039/D1MO00160D. [DOI] [PubMed] [Google Scholar]
- 52.Zhong Y.J., Yang Y., Wang X.Y., Di R., Chu M.X., Liu Q.Y. Expression analysis and single-nucleotide polymorphisms of SYNDIG1L and UNC13C genes associated with thoracic vertebral numbers in sheep (Ovis aries) Arch. Anim. Breed. 2021;64:131–138. doi: 10.5194/aab-64-131-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S., Luo R., Lai D., Ma M., Hao F., Qi X., Liu X. Whole-genome resequencing of Ujumqin sheep to investigate the determinants of the multi-vertebral trait. Genome. 2018;61:653–661. doi: 10.1139/gen-2017-0267. [DOI] [PubMed] [Google Scholar]
- 54.Li C., Zhang X., Cao Y., Wei J., You S., Jiang Y., Chen C. Multivertebrae variation potentially contributes to carcass length and weight of Kazakh sheep. Small Rumin. Res. 2017;150:8–10. doi: 10.1016/j.smallrumres.2017.02.021. [DOI] [Google Scholar]
- 55.Zhang Z., Sun Y., Du W., He S., Liu M., Tian C. Effects of vertebral number variations on carcass traits and genotyping of Vertnin candidate gene in Kazakh sheep. Asian-Australas. J. Anim. Sci. 2017;30:123. doi: 10.5713/ajas.16.0959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mi T., Liu K., Guo T., Li L., Wang Y., Li C., Cui Y., Dai J., Zhang Y., Hu S. Analysis of the eighth intron polymorphism of NR6A1 gene in sheep and its correlation with lumbar spine number. Anim. Biotechnol. 2023;34:218–224. doi: 10.1080/10495398.2021.1954529. [DOI] [PubMed] [Google Scholar]
- 57.Li C., Liu K., Dai J., Li X., Liu X., Ni W., Li H., Wang D., Qiao J., Wang Y., et al. Whole-genome resequencing to investigate the determinants of the multi-lumbar vertebrae trait in sheep. Gene. 2022;809:146020. doi: 10.1016/j.gene.2021.146020. [DOI] [PubMed] [Google Scholar]
- 58.Kalds P., Luo Q., Sun K., Zhou S., Chen Y., Wang X. Trends towards revealing the genetic architecture of sheep tail patterning: Promising genes and investigatory pathways. Anim. Genet. 2021;52:799–812. doi: 10.1111/age.13133. [DOI] [PubMed] [Google Scholar]
- 59.Zhang D., Zhang X., Li F., Liu T., Hu Z., Gao N., Yuan L., Li X., Zhao Y., Zhao L., et al. Whole-genome resequencing identified candidate genes associated with the number of ribs in Hu sheep. Genomics. 2021;113:2077–2084. doi: 10.1016/j.ygeno.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 60.Zhao F., Deng T., Shi L., Wang W., Zhang Q., Du L., Wang L. Genomic scan for selection signature reveals fat deposition in Chinese indigenous sheep with extreme tail types. Animals. 2020;10:773. doi: 10.3390/ani10050773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li C., Li M., Li X., Ni W., Xu Y., Yao R., Wei B., Zhang M., Li H., Zhao Y., et al. Whole-genome resequencing reveals loci associated with thoracic vertebrae number in sheep. Front. Genet. 2019;10:674. doi: 10.3389/fgene.2019.00674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang X., Li C., Li X., Liu Z., Ni W., Hazi W., Cao Y., Yao Y., Wang D., Hou X., et al. Expression profiles of MicroRNAs from multiple lumbar spine in sheep. Gene. 2018;678:105–114. doi: 10.1016/j.gene.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Moradi M.H., Mahmodi R., Farahani A.H., Karimi M.O. Genome-Wide Evaluation of Copy Gain and Loss Variations in Three Afghan Sheep Breeds. Sci. Rep. 2022;12:14286. doi: 10.1038/s41598-022-18571-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahbara A., Bahbahani H., Almathen F., Al Abri M., Agoub M.O., Abeba A., Kebede A., Musa H.H., Mastrangelo S., Pilla F., et al. Genome-Wide Variation, Candidate Regions, and Genes Associated With Fat Deposition and Tail Morphology in Ethiopian Indigenous Sheep. Front. Genet. 2019;9:699. doi: 10.3389/fgene.2018.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chang Y.C., Manent J., Schroeder J., Wong S.F., Hauswirth G.M., Shylo N.A., Moore E.L., Achilleos A., Garside V., Polo J.M., et al. Nr6a1 controls Hox expression dynamics and is a master regulator of vertebrate trunk development. Nat. Commun. 2022;13:7766. doi: 10.1038/s41467-022-35303-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y., Wan X., Hao Y., Zhao Y., Du L., Huang Y., Liu Z., Wang Y., Wang N., Zhang P. NR6A1 regulates lipid metabolism through mammalian target of rapamycin complex 1 in HepG2 cells. Cell Commun. Signal. 2019;17:77. doi: 10.1186/s12964-019-0389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang X., Li C., Li X., Liu Z., Ni W., Cao Y., Yao Y., Islamov E., Wei J., Hou X., et al. Association analysis of polymorphism in the NR6A1 gene with the lumbar vertebrae number traits in sheep. Genes Genom. 2019;41:1165–1171. doi: 10.1007/s13258-019-00843-5. [DOI] [PubMed] [Google Scholar]
- 68.Yan G., Qiao R., Zhang F., Xin W., Xiao S., Huang T., Zhang Z., Huang L. Imputation-based whole-genome sequence association study rediscovered the missing QTL for lumbar number in Sutai pigs. Sci. Rep. 2017;7:615. doi: 10.1038/s41598-017-00729-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rubin C.J., Megens H.J., Martinez B.A., Maqbool K., Andersson L. Strong signatures of selection in the domestic pig genome. Proc. Natl. Acad. Sci. USA. 2012;109:19529–19536. doi: 10.1073/pnas.1217149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang X., Lai Z., Liu J., Zhang C., Li S., Wu F., Zhou Z., Lei C., Dang R. A Novel 13 bp Deletion within the NR6A1 Gene Is Significantly Associated with Growth Traits in Donkeys. Animals. 2019;9:681. doi: 10.3390/ani9090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mikawa S., Morozumi T., Shimanuki S.I., Hayashi T., Uenishi H., Domukai M., Okumura N., Awata T. Fine mapping of a swine quantitative trait locus for number of vertebrae and analysis of an orphan nuclear receptor, germ cell nuclear factor (NR6A1) Genome Res. 2007;17:586–593. doi: 10.1101/gr.6085507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang G., Ren J., Zhang Z., Huang L. Genetic evidence for the introgression of Western NR6A1 haplotype into Chinese Licha breed associated with increased vertebral number. Anim. Genet. 2009;40:247–250. doi: 10.1111/j.1365-2052.2008.01820.x. [DOI] [PubMed] [Google Scholar]
- 73.Huang J., Zhang M., Ye R., Ma Y., Lei C. Effects of increased vertebral number on carcass weight in PIC pigs. Anim. Sci. J. 2017;88:2057–2062. doi: 10.1111/asj.12881. [DOI] [PubMed] [Google Scholar]
- 74.Green H.E., Oliveira H.R., Alvarenga A.B., Scramlin-Zuelly S., Grossi D., Schinckel A.P., Brito L.F. Genomic background of biotypes related to growth, carcass and meat quality traits in Duroc pigs based on principal component analysis. J. Anim. Breed. Genet. 2023:1–16. doi: 10.1111/jbg.12831. [DOI] [PubMed] [Google Scholar]
- 75.Le T.H., Christensen O.F., Nielsen B., Sahana G. Genome-wide association study for conformation traits in three Danish pig breeds. Genet. Sel. Evol. 2017;49:12. doi: 10.1186/s12711-017-0289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang L.C., Yue J.W., Pu L., Wang L.G., Liu X., Liang J., Yan H., Zhao K.B., Li N., Shi H.B., et al. Genome-wide study refines the quantitative trait locus for number of ribs in a Large White× Minzhu intercross pig population and reveals a new candidate gene. Mol. Genet. Genom. 2016;291:1885–1890. doi: 10.1007/s00438-016-1220-1. [DOI] [PubMed] [Google Scholar]
- 77.Mikawa S., Sato S., Nii M., Morozumi T., Yoshioka G., Imaeda N., Yamaguchi T., Hayashi T., Awata T. Identification of a second gene associated with variation in vertebral number in domestic pigs. BMC Genet. 2011;12:5. doi: 10.1186/1471-2156-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakano H., Sato S., Uemoto Y., Kikuchi T., Shibata T., Kadowaki H., Kobayashi E., Suzuki K. Effect of VRTN gene polymorphisms on D uroc pig production and carcass traits, and their genetic relationships. Anim. Sci. J. 2015;86:125–131. doi: 10.1111/asj.12260. [DOI] [PubMed] [Google Scholar]
- 79.Yang J., Huang L., Yang M., Fan Y., Li L., Fang S., Deng W., Cui L., Zhang Z., Ai H., et al. Possible introgression of the VRTN mutation increasing vertebral number, carcass length and teat number from Chinese pigs into European pigs. Sci. Rep. 2016;6:19240. doi: 10.1038/srep19240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rohrer G.A., Nonneman D.J., Wiedmann R.T., Schneider J.F. A study of vertebra number in pigs confirms the association of vertnin and reveals additional QTL. BMC Genet. 2015;16:129. doi: 10.1186/s12863-015-0286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hirose K., Mikawa S., Okumura N., Noguchi G., Fukawa K., Kanaya N., Mikawa A., Arakawa A., Ito T., Hayashi Y., et al. Association of swine vertnin (VRTN) gene with production traits in D uroc pigs improved using a closed nucleus breeding system. Anim. Sci. J. 2013;84:213–221. doi: 10.1111/j.1740-0929.2012.01066.x. [DOI] [PubMed] [Google Scholar]
- 82.Ren D.R., Ren J., Ruan G.F., Guo Y.M., Wu L.H., Zhou L.H., Li L., Zhang Z.Y., Huang L.S. Mapping and fine mapping of quantitative trait loci for the number of vertebrae in a W hite D uroc× C hinese E rhualian intercross resource population. Anim. Genet. 2012;43:545–551. doi: 10.1111/j.1365-2052.2011.02313.x. [DOI] [PubMed] [Google Scholar]
- 83.Bodmer N.K., Knutsen R.H., Roth R.A., Castile R.M., Brodt M.D., Gierasch C.M., Broekelmann T.J., Gibson M.A., Haspel J.A., Lake S.P., et al. Multi-organ phenotypes in mice lacking latent TGFβ binding protein 2 (LTBP2) Dev. Dyn. 2023;253:233–254. doi: 10.1002/dvdy.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dabovic B., Chen Y., Colarossi C., Zambuto L., Obata H. BEYOND CARRIER PROTEINS Bone defects in latent TGF-β binding protein (Ltbp)-3 null mice; a role for Ltbp in TGF-β presentation. J. Endocrinol. 2002;175:129–141. doi: 10.1677/joe.0.1750129. [DOI] [PubMed] [Google Scholar]
- 85.Du X., Cai L., Xie J., Zhou X. The role of TGF-beta3 in cartilage development and osteoarthritis. Bone Res. 2023;11:2. doi: 10.1038/s41413-022-00239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nistala H., Lee-Arteaga S., Smaldone S., Siciliano G., Carta L., Ono R.N., Sengle G., Arteaga-Solis E., Levasseur R., Ducy P., et al. Fibrillin-1 and-2 differentially modulate endogenous TGF-β and BMP bioavailability during bone formation. J. Cell Biol. 2010;190:1107–1121. doi: 10.1083/jcb.201003089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Park H.B., Han S.H., Lee J.B., Cho I.C. Rapid Communication: High-resolution quantitative trait loci analysis identifies LTBP2 encoding latent transforming growth factor beta binding protein 2 associated with thoracic vertebrae number in a large F2 intercross between Landrace and Korean native pigs. J. Anim. Sci. 2017;95:1957–1962. doi: 10.2527/jas.2017.1390. [DOI] [PubMed] [Google Scholar]
- 88.Sun X., Essalmani R., Susan-Resiga D., Prat A., Seidah N.G. Latent transforming growth factor β-binding proteins-2 and-3 inhibit the proprotein convertase 5/6A. J. Biol. Chem. 2011;286:29063–29073. doi: 10.1074/jbc.M111.242479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma Q., Yang Y., Mao F., Zhou Q., Wang L., Chen G. Genome-wide identification, phylogeny and expression analysis of the bmp gene family associated with development and skeleton deformity in cobia (Rachycentron canadum) Aquac. Rep. 2023;31:101644. doi: 10.1016/j.aqrep.2023.101644. [DOI] [Google Scholar]
- 90.Li X., Cao X. BMP signaling and skeletogenesis. Ann. N. Y. Acad. Sci. 2006;1068:26–40. doi: 10.1196/annals.1346.006. [DOI] [PubMed] [Google Scholar]
- 91.Cao X., Chen D. The BMP signaling and in vivo bone formation. Gene. 2005;357:1–8. doi: 10.1016/j.gene.2005.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pregizer S., Mortlock D.P. Control of BMP gene expression by long-range regulatory elements. Cytokine Growth Factor. Rev. 2009;20:509–515. doi: 10.1016/j.cytogfr.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peskin B., Norman J., Bagwell J., Lin A., Adhyapok P., Di Talia S., Bagnat M. Dynamic BMP signaling mediates notochord segmentation in zebrafish. Curr. Biol. 2023;33:P2574–P2581.E3. doi: 10.1016/j.cub.2023.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pogoda H.M., Riedl-Quinkertz I., Hammerschmidt M. Direct BMP signaling to chordoblasts is required for the initiation of segmented notochord sheath mineralization in zebrafish vertebral column development. Front. Endocrinol. 2023;14:1107339. doi: 10.3389/fendo.2023.1107339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Costantini A., Guasto A., Cormier-Daire V. TGF-β and BMP Signaling Pathways in Skeletal Dysplasia with Short and Tall Stature. Annu. Rev. Genom. Hum. Genet. 2023;24:225–253. doi: 10.1146/annurev-genom-120922-094107. [DOI] [PubMed] [Google Scholar]
- 96.Bandyopadhyay A., Tsuji K., Cox K., Harfe B.D., Rosen V., Tabin C.J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wan M., Cao X. BMP signaling in skeletal development. Biochem. Biophys. Res. Commun. 2005;328:651–657. doi: 10.1016/j.bbrc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y., Shan X., Jiang H., Sun L., Guo Z. Regulation of litter size in sheep (Ovis aries) by the GDF9 and BMP15 genes. Ann. Agric. Sci. 2023;68:148–158. doi: 10.1016/j.aoas.2023.12.004. [DOI] [Google Scholar]
- 99.Lu X., Li L., Wu N., Chen W., Hong S., Xu M., Ding Y., Gao Y. BMP9 functions as a negative regulator in the myogenic differentiation of primary mouse myoblasts. Biosci. Biotechnol. Biochem. 2023;87:1255–1264. doi: 10.1093/bbb/zbad104. [DOI] [PubMed] [Google Scholar]
- 100.Li Z., Liu G., Yang L., Sun M., Zhang Z., Xu Z., Gao Y., Jiang X., Su Z., Li X., et al. BMP7 expression in mammalian cortical radial glial cells increases the length of the neurogenic period. Protein Cell. 2023;15:21–35. doi: 10.1093/procel/pwad036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bottasso-Arias N., Burra K., Sinner D., Riede T. Disruption of BMP4 signaling is associated with laryngeal birth defects in a mouse model. Dev. Biol. 2023;500:10–21. doi: 10.1016/j.ydbio.2023.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bottasso-Arias N., Leesman L., Burra K., Snowball J., Shah R., Mohanakrishnan M., Xu Y., Sinner D. BMP4 and Wnt signaling interact to promote mouse tracheal mesenchyme morphogenesis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2022;322:L224–L242. doi: 10.1152/ajplung.00255.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mallo M. Reassessing the role of Hox genes during vertebrate development and evolution. Trends Genet. 2018;34:209–217. doi: 10.1016/j.tig.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 104.Iimura T., Denans N., Pourquié O. Establishment of Hox vertebral identities in the embryonic spine precursors. Curr. Top. Dev. Biol. 2009;88:201–234. doi: 10.1016/S0070-2153(09)88007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lappin T.R., Grier D.G., Thompson A., Halliday H.L. HOX genes: Seductive science, mysterious mechanisms. Ulst. Med. J. 2006;75:23. [PMC free article] [PubMed] [Google Scholar]
- 106.Carpenter E.M. Hox genes and spinal cord development. Dev. Neurosci. 2002;24:24–34. doi: 10.1159/000064943. [DOI] [PubMed] [Google Scholar]
- 107.Hauswirth G.M., Garside V.C., Wong L.S., Bildsoe H., Manent J., Chang Y.C., Nefzger C.M., Firas J., Chen J., Rossello F.J., et al. Breaking constraint of mammalian axial formulae. Nat. Commun. 2022;13:243. doi: 10.1038/s41467-021-27335-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Van Den Akker E., Fromental-Ramain C., de Graaff W., Le Mouellic H., Brûlet P., Chambon P., Deschamps J. Axial skeletal patterning in mice lacking all paralogous group 8 Hox genes. Development. 2001;128:1911–1921. doi: 10.1242/dev.128.10.1911. [DOI] [PubMed] [Google Scholar]
- 109.Tang J., Liu Y., Xie S., Ma S., Jiang M. Advances in Candidate Genes on Vertebral Number Trait of Pig. Chin. J. Anim. Sci. 2022;58:92–98. [Google Scholar]
- 110.Zhao J., Zhang L., Qi C., Batu Relationship between methylation of Hoxc8 gene and the numbers of thoracic vertebrae in Mongolia sheep. Heilongjiang Anim. Sci. Vet. 2011;5:5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are available in the manuscript.