Abstract
Different factors, including antimicrobial resistance, may diminish the effectiveness of antibiotic therapy, challenging the management of post-transplant urinary tract infection (UTI). The association of acidic urine pH with microbiological and clinical outcomes was evaluated after fosfomycin or ciprofloxacin therapy in 184 kidney transplant recipients (KTRs) with UTI episodes by Escherichia coli (N = 115) and Klebsiella pneumoniae (N = 69). Initial urine pH, antimicrobial therapy, and clinical and microbiological outcomes, and one- and six-month follow-up were assessed. Fosfomycin was prescribed in 88 (76.5%) E. coli and 46 (66.7%) K. pneumoniae UTI episodes in the total cohort. When the urine pH ≤ 6, fosfomycin was prescribed in 60 (52.2%) E. coli and 29 (42.0%) K. pneumoniae. Initial urine pH ≤ 6 in E. coli UTI was associated with symptomatic episodes (8/60 vs. 0/55, p = 0.04) at one-month follow-up, with a similar trend in those patients receiving fosfomycin (7/47 vs. 0/41, p = 0.09). Acidic urine pH was not associated with microbiological or clinical cure in K. pneumoniae UTI. At pH 5, the ciprofloxacin MIC90 increased from 8 to >8 mg/L in E. coli and from 4 to >8 mg/L in K. pneumoniae. At pH 5, the fosfomycin MIC90 decreased from 8 to 4 mg/L in E. coli and from 512 to 128 mg/L in K. pneumoniae. Acidic urine is not associated with the microbiological efficacy of fosfomycin and ciprofloxacin in KTRs with UTI, but it is associated with symptomatic UTI episodes at one-month follow-up in E. coli episodes.
Keywords: urinary tract infections, urine pH, ciprofloxacin, fosfomycin, kidney transplant recipients, Escherichia coli, Klebsiella pneumoniae
1. Introduction
Urinary tract infections (UTIs) remain a major issue in kidney transplant recipients (KTRs), with an incidence of 7.3% and increased mortality in the first year [1,2]. The incidence of UTIs is even higher in the six months (36.5–47%) after transplantation, being the most frequent etiologies Escherichia coli, Klebsiella spp., and Pseudomonas aeruginosa [1,3,4]. In addition to its clinical impact, the treatment of UTI in KTRs poses a serious problem owing to the antimicrobial resistance of the most frequent Gram-negative bacilli [1,3]. In a multicenter European study, the resistance rates among 775 E. coli isolates were 15.1% and 1.3% for ciprofloxacin and fosfomycin, respectively, with multidrug resistance (MDR) in 13.9% [5]. In two multicenter studies from China and South Korea, the fosfomycin resistance rates among E. coli urine isolates were 3.9% and 5% [6,7]. For K. pneumoniae, fosfomycin resistance rates in urine isolates in two Spanish cohorts of KTRs were 22%–25% [8].
A retrospective, multicenter, cohort study evaluated the efficacy of oral fosfomycin in KTRs with cystitis, caused mainly by E. coli and K. pneumoniae [9]; the clinical and microbiological cure was achieved in 83.9% and 70.2% of cases, respectively. In three clinical trials performed in KTRs, the screening and treatment with different antimicrobials for asymptomatic bacteriuria (AB) caused mostly by E. coli and Klebsiella spp. did not reduce the occurrence of later symptomatic UTI [10] or acute pyelonephritis [11,12] compared with no treatment and seemed to promote the emergence of resistant organisms [10,12]. In this context, the 2023 EAU Guidelines consider fosfomycin trometamol among the first-line treatments for uncomplicated cystitis and recommend against the screening and treatment of asymptomatic bacteriuria, including in KTRs [13]. Moreover, the EMA recommends avoiding the use of quinolones for mild/moderate bacterial infections and considering patients with organ transplantation at a higher risk [14].
Low-level resistance to ciprofloxacin (LLQR) has been described in E. coli, with a frequency of 17% to 39% [15,16]. In LLQR E. coli strains, either isogenic derivatives of ATCC 25922, carrying combinations of the most prevalent chromosomal mutations, or uropathogenic LLQR E. coli clinical isolates, the acidic pH of the medium increases the ciprofloxacin minimum inhibitory concentration (MIC) against E. coli between 32-fold and 256-fold at pH 5 [17]. The same effect has been reported in E. oli ATCC 25922 derivatives harboring the qnrA1, qnrB1, qnrC, qnrD1, and qnrS1 genes and in nine E. coli clinical strains harboring well-characterized Qnr determinants, with an increase in MIC from 16- to 128-fold when urine pH decreased from 7 to 5 [18]. Other studies using E. coli 25922 and Klebsiella oxytoca showed that the acidification of urine led to a major impairment of the antimicrobial activity of all tested fluoroquinolones, with near-total neutralization of activity in time-kill experiments [19].
In contrast to ciprofloxacin, the acidic pH of the culture medium seems to increase the activity of fosfomycin [20,21]. Using E. coli BW25113 and 10 isogenic strains carrying fosfomycin chromosomal mutations and five fosfomycin-resistant E. coli urine isolates, the MIC decreased steadily from pH 7 to pH 5 in most cases; at pH 8, a 2-fold to 16-fold increase in the MIC was observed [20]. Another study showed that the fosfomycin MIC90 against 158 E. coli urine isolates was lower at pH 6 compared with pH 7, although the pH conditions did not affect the activity against Klebsiella spp. [21].
Nevertheless, the impact of physiological urine pH changes on the clinical and microbiological efficacy of the therapy in E. coli and K. pneumoniae lower UTIs has not been analyzed in patients, including KTRs. Thus, in this study, we aimed to evaluate the microbiological and clinical outcomes, during a six-month follow-up period, of the treatment of E. coli and K. pneumoniae UTIs with fosfomycin and ciprofloxacin in KTRs and determine associations with the urine pH, and also to assess the changes produced at different pH in the MIC distribution of fosfomycin and ciprofloxacin in the urine isolates of E. coli and K. pneumoniae and in their bactericidal activity.
2. Results
2.1. Characteristics of KTRs with E. coli and K. pneumoniae UTI Episodes
We included E. coli and K. pneumoniae UTI episodes in 115 and 69 KTRs, respectively. Among the E. coli episodes, 19 (16.5%) and 96 (83.5%) were cystitis and AB, respectively, and among the K. pneumoniae episodes, 33 (47.8%) and 36 (52.2%) were cystitis and AB. The most common immunosuppressive drug combination in the two etiologies was mycophenolate (MMF), prednisone, and tacrolimus. Demographics, Charlson comorbidity index, characteristics of the transplantation, data on underlying end-stage renal disease, previous allograft rejection, plasma creatinine and urine pH at inclusion, antimicrobial susceptibilities of E. coli and K. pneumoniae isolates from the episodes, and antimicrobial therapy are detailed in Table 1. No differences regarding cystitis or AB episodes were found for any variables (Supplementary Tables S1–S6).
Table 1.
Demographics and characteristics of the kidney transplant recipients with urinary tract infection by Escherichia coli and Klebsiella pneumoniae.
| Variables |
E. coli 115 Episodes N (%) |
K. pneumoniae 69 Episodes N (%) |
|---|---|---|
| Age (years; median [IQR]) | 58 (50–67) | 61 (50–69) |
| Female patients | 71 (61.7) | 42 (60.9) |
| Charlson Comorbidity Index (median [IQR]) | 3 (3–5) | 5 (3–5) |
| Months from transplantation (median [IQR]) | 14 (4–77) | 6 (1–77) |
| <2 months from transplantation | 21 (18.3) | 41 (59.4) |
| Previous kidney transplantation | 10 (8.7) | 8 (11.6) |
| Living donor | 11 (9.5) | 6 (8.7) |
Induction therapy within 3 previous months:
|
73 (63.5) 26 (22.6) 41 (35.7) 6 (5.2) |
28 (40.6) 12 (17.4) 14 (20.3) 2 (2.9) |
Current immunosuppression:
|
107 (93.0) 107 (93.0) 90 (78.3) 8 (6.9) 4 (3.4) |
58 (84.1) 64 (92.8) 54 (78.3) 4 (5.8) 3 (4.3) |
| Acute rejection within the previous 6 months | 11 (9.6) | 0 (0.0) |
Rejection treatment in the previous 6 months:
|
9 (7.9) 1 (0.9) 1 (0.9) |
- - - |
| Creatinine (mg/dL; median [IQR]) | 1.57 (1.21–1.95) | 1.56 (1.25–1.99) |
| Bacteriuria within the previous 6 months | 57 (49.6) | 49 (71.0) |
Antibiotic use within the previous 3 months
|
48 (41.7) 11 (9.6) 6 (5.2) 14 (12.2) 15 (13.0) 2 (1.7) |
30 (43.5) 3 (4.3) 8 (11.6) 10 (14.5) 7 (10.1) 2 (2.9) |
| Cystitis | 19 (16.5) | 33 (47.8) |
| Asymptomatic bacteriuria | 96 (83.5) | 36 (52.2) |
| Urinary pH (median [IQR]) | 6 (6–6.5) | 6.5 (6–6.5) |
Baseline antibiotic resistance:
|
66 (57.4) 30 (26.1) 24 (20.9) 10 (8.7) 2 (1.7) 2 (1.7) |
42 (60.9) 20 (29.0) 15 (21.7) 8 (11.6) 21 (30.4) 20 (29.0) |
Antibiotic therapy of the UTI episodes
|
88 (76.5) 27 (23.5) |
46 (66.7) 23 (33.3) |
IQR: Interquartile range; MMF: Mycophenolate mofetil; mTOR inhibitors: Sirolimus, everolimus; ESBL: Extended spectrum beta-lactamases; * Quinolones: ciprofloxacin or levofloxacin; ** Cephalosporins: cefixime or cefuroxime; *** Others: Ertapenem, cloxacillin and rifaximine; **** Cephalosporins: cefuroxime, cefotaxime, ceftazidime, cefixime or cefepime.
2.2. Association of Urine pH with Microbiological and Clinical Outcomes of E. coli and K. pneumoniae UTI Episodes
The 115 patients with E. coli UTI episodes were treated with fosfomycin and ciprofloxacin in 88 (76.5%) and 27 (23.5%) cases, respectively. At inclusion, urine pH was acidic (≤6) in 60 (52.1%) episodes. Acidic urine, compared to neutral or alkaline urine, was not associated with microbiological cure one month after therapy (61.7% vs. 69.1%) in all episodes, nor in episodes treated with fosfomycin or ciprofloxacin (Table 2). Regarding clinical outcome, acidic urine at inclusion was associated with more symptomatic events at one-month follow-up (13.3% vs. 0%, p = 0.045) in all episodes and in those treated with fosfomycin (14.9% vs. 0%, p = 0.013). By the six-month follow-up, renal function had worsened in fourteen (12.3%) patients, one (0.8%) patient lost the graft, and one (0.8%) patient died in the fourth month with acute pyelonephritis by E. coli and renal failure.
Table 2.
Microbiological and clinical outcomes, in patients with acidic vs. non-acidic urine, after fosfomycin or ciprofloxacin therapy of urinary tract infection by Escherichia coli and Klebsiella pneumoniae.
| Variable | Urinary pH ≤ 6 | Urinary pH > 6 | p | |
|---|---|---|---|---|
| N (%) | N (%) | |||
| Escherichia coli UTI Episodes (N = 115) | ||||
| Microbiological cure during one-month follow-up |
Total | 37/60 (61.7) | 38/55 (69.1) | 0.41 |
| Episodes treated with fosfomycin | 29/47 (61.7) | 27/41 (65.9) | 0.69 | |
| Episodes treated with ciprofloxacin | 8/13 (61.5) | 11/14 (78.6) | 0.42 | |
| Symptomatic UTI during one-month follow-up |
Total | 8/60 (13.3) | 0/55 (0.0) | 0.006 |
| Episodes treated with fosfomycin | 7/47 (14.9) | 0/41 (0.0) | 0.013 | |
| Episodes treated with ciprofloxacin | 1/13 (7.7) | 0/14 (0.0) | 0.48 | |
| Symptomatic UTI during six-month follow-up |
Total | 11/60 (18.3) | 9/55 (16.4) | 0.78 |
| Episodes treated with fosfomycin | 10/47 (21.3) | 9/41 (22.0) | 0.94 | |
| Episodes treated with ciprofloxacin | 1/13 (7.7) | 0/14 (0.0) | 0.48 | |
| Klebsiella pneumoniae UTI episodes (N = 69) | ||||
| Microbiological cure during one-month follow-up |
Total | 10/29 (34.5) | 15/40 (37.5) | 0.69 |
| Episodes treated with fosfomycin | 4/16 (25.0) | 8/30 (26.7) | 1.00 | |
| Episodes treated with ciprofloxacin | 6/13 (46.2) | 7/10 (70.0) | 0.16 | |
| Symptomatic UTI during one-month follow-up |
Total | 4/29 (13.8) | 4/40 (10.0) | 0.71 |
| Episodes treated with fosfomycin | 3/16 (18.8) | 4/30 (13.3) | 0.69 | |
| Episodes treated with ciprofloxacin | 1/13 (7.7) | 0/10 (0.0) | 1.00 | |
| Symptomatic UTI during six-month follow-up |
Total | 3/29 (10.3) | 6/40 (15.0) | 0.75 |
| Episodes treated with fosfomycin | 1/16 (6.3) | 4/30 (13.3) | 1.00 | |
| Episodes treated with ciprofloxacin | 2/13 (15.4) | 2/10 (20.0) | 1.00 | |
p: Chi-square or Fisher exact tests.
Among the 69 patients with K. pneumoniae UTI episodes, 46 (66.7%) and 23 (33.3%) were treated with fosfomycin and ciprofloxacin, respectively. At inclusion, urine pH was acidic (≤6) in 29 (42.0%) episodes. Acidic urine, compared to neutral or alkaline urine, was not associated with microbiological cure at one month in all episodes, nor in episodes treated with fosfomycin or ciprofloxacin, nor were there differences in symptomatic UTI episodes at one- and six-month follow-up (Table 2). By the six-month follow-up, renal function worsened in seven (10.1%) patients, one (1.4%) patient lost the graft, and four (5.8%) patients died in the second month of follow-up, in the context of bloodstream infections by K. pneumoniae in two patients.
As a sensitivity analysis, in the 184 E. coli and K. pneumoniae UTI episodes, the urine pH was acidic (≤6) at inclusion in 89 (48.4%) cases, which was associated with more symptomatic events by the one-month follow-up (13.5% vs. 4.2%, p = 0.052) (Supplementary Tables S7–S10).
2.3. Antimicrobial and Bactericidal Activities of Ciprofloxacin and Fosfomycin against E. coli and K. pneumoniae Clinical Isolates at Neutral, Acidic, and Alkaline pH
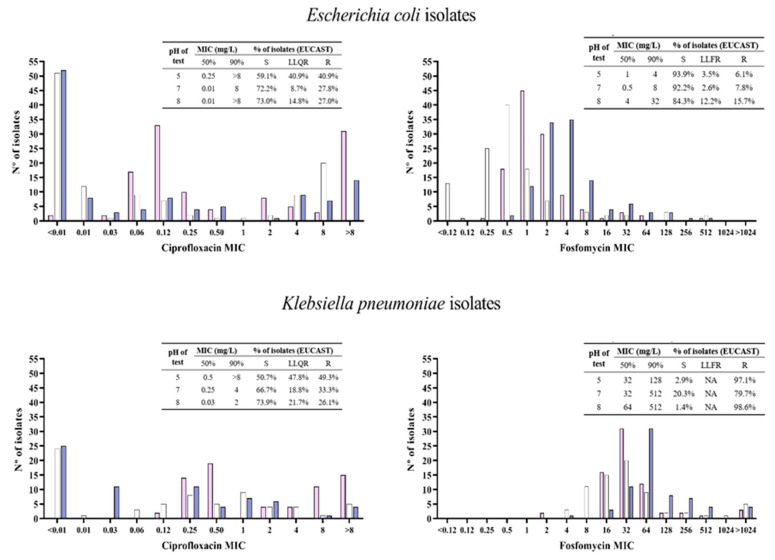
Among the 115 E. coli initial isolates, 32 (27.8%) were resistant to ciprofloxacin, and 10 (8.7%) were LLQR. When the MIC of ciprofloxacin was determined at pH 5, the resistance and LLQR rates increased to 40.9%; minimal changes in the MIC occurred at pH 8. Nine (7.8%) isolates were resistant to fosfomycin and three (2.6%) were LLFR. In the MIC determination of fosfomycin at pH 5, there were no appreciable changes; at pH 8, the resistance increased to 15.7%, and 12.2% had LLQR. Regarding the 69 initial K. pneumoniae isolates, 23 (33.3%) were resistant to ciprofloxacin, and 13 (18.8%) were LLQR. The MIC determination of ciprofloxacin at pH 5 increased resistance to 49.3%, and 47.8% exhibited LLQR; at pH 8, the MIC50 and MIC90 values decreased from 0.25 and 4 to 0.03 and 2 mg/L, respectively. Fifty-five (79.7%) isolates were resistant to fosfomycin. When the MIC of fosfomycin was determined at pH 5 and pH 8, the resistance increased to 97.1% and 98.6%, respectively. Details on the MIC distributions of ciprofloxacin and fosfomycin at different pH are in Figure 1 and Supplementary Tables S11–S15.
Figure 1.
Ciprofloxacin and fosfomycin MIC values distributions determined at pH 5 (pink bars), pH 7 (white bars), and pH 8 (blue bars) against Escherichia coli (N = 115) and Klebsiella pneumoniae (N = 69) urine clinical isolates. S: susceptible; LLQR: Low-level quinolone resistance; LLFR: Low-level fosfomycin resistance; R: resistant; EUCAST: European Committee on Antimicrobial Susceptibility Testing interpretative criteria 2023 (ciprofloxacin resistant: MIC > 0.5 g/L; fosfomycin resistant: MIC > 8 g/L).
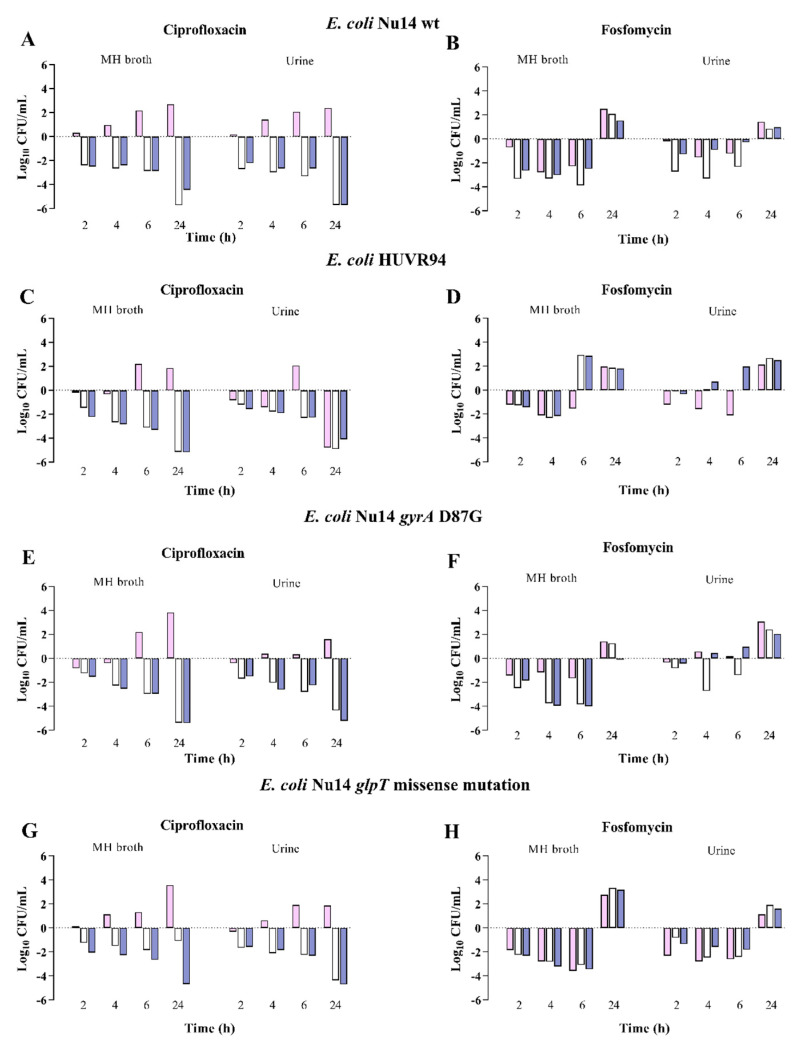
The growth of the E. coli and K. pneumoniae strains in Müller-Hinton Broth (MHB) or urine was not different depending on the pH, but it was approximately 1 log10 CFU/mL lower in urine. Against the E. coli strains at neutral pH, ciprofloxacin was bactericidal at 24 h against three and four strains in MHB and urine, respectively; fosfomycin was bactericidal at six hours, with regrowth at 24 h, against three strains in MHB. At acidic pH, ciprofloxacin at 24 h and fosfomycin at 6 h, with regrowth at 24 h, were bactericidal only against one strain, respectively. Finally, at alkaline pH, ciprofloxacin was bactericidal at 24 h against the four strains in MHB and urine, and fosfomycin at six hours, with regrowth at 24 h, against two strains in MHB (Figure 2, Supplementary Tables S16 and S17). In the experiments with regrowth at 24 h, E. coli Nu14, at neutral pH, developed resistant mutants to fosfomycin in 60% and 20% of strains, respectively, in MHB and urine, and at alkaline pH, it also showed a 20% mutant resistance in MHB.
Figure 2.
Bactericidal activity of ciprofloxacin and fosfomycin at MIC concentrations in MHB and urine, determined at pH 5 (pink bars), pH 7 (white bars), and pH 8 (blue bars) (dark grey bars) against Escherichia coli strains. Panels (A,B) E. coli NU14 wild-type strain, susceptible to ciprofloxacin (MIC 0.03 mg/L) and fosfomycin (MIC 2 mg/L). Panels (C,D) E. coli HUVR94 clinical strain, susceptible to ciprofloxacin (MIC 0.03 mg/L) and fosfomycin (MIC 0.5 mg/L). Panels (E,F) E. coli Nu79 gyrA (D87G) strain with low-level quinolone resistance (MIC 0.12 mg/L) and susceptible to fosfomycin (MIC 0.5 mg/L). Panels (G,H) E. coli Nu14 glpT missense mutation strains with low-level fosfomycin resistance (MIC 32 mg/L) and susceptible to ciprofloxacin (MIC 0.01 mg/L). Results are represented as differences (log10 CFU/mL) relative to the initial time-point (0 h).
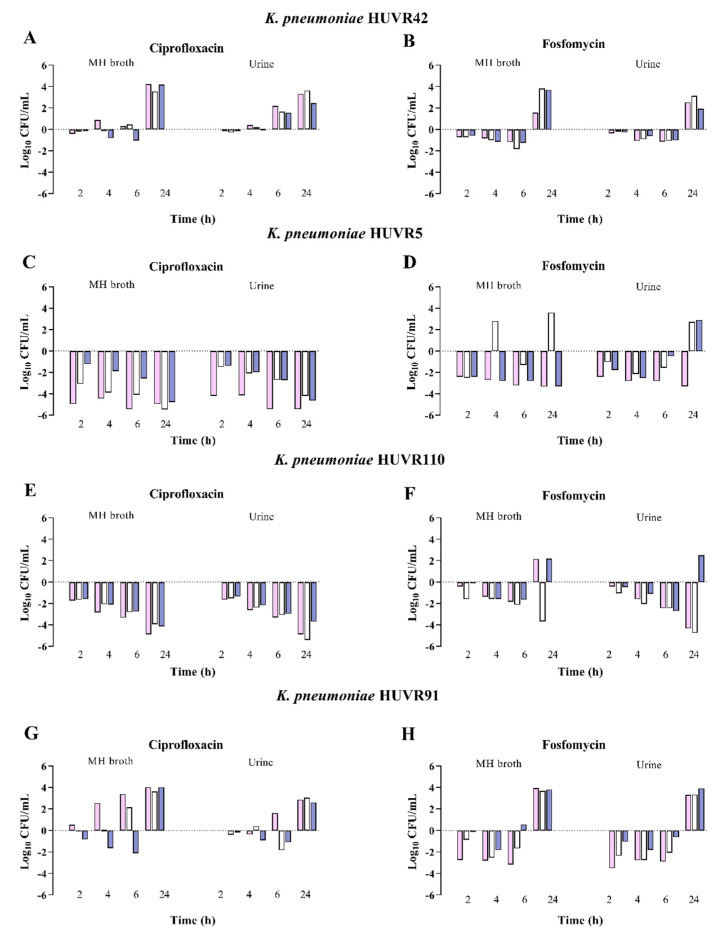
Regarding K. pneumoniae strains, ciprofloxacin was bactericidal at 24 h against two strains, at neutral, acidic, and alkaline pH, in both MHB and urine. Fosfomycin, at neutral pH, was bactericidal at 24 h against one strain in both MHB and urine. At acidic pH, fosfomycin was bactericidal at 24 h against one and two strains in MHB and urine, respectively, and at 2 h against one strain in urine, with regrowth at 24 h, without developing resistance to fosfomycin. At alkaline pH, fosfomycin was bactericidal at 24 h against one strain in MHB (Figure 3, Supplementary Tables S18 and S19).
Figure 3.
Bactericidal activity of ciprofloxacin and fosfomycin at MIC concentrations in MHB and urine, determined at pH 5 (pink bars), pH 7 (white bars), and pH 8 (blue bars) against Klebsiella pneumoniae strains. Panels (A,B) K. pneumoniae HUVR42 susceptible to ciprofloxacin (MIC 0.007 mg/L) and fosfomycin (4 mg/L). Panels (C,D) K. pneumoniae HUVR5 resistant to ciprofloxacin (MIC 8 mg/L) and fosfomycin (128 mg/L. Panels (E,F) K. pneumoniae HUVR110 resistant to ciprofloxacin (MIC 8 mg/L) and susceptible to fosfomycin (MIC 4 mg/L). Panels (G,H) K. pneumoniae HUVR91 resistant fosfomycin (MIC 64 mg/L) and susceptible to ciprofloxacin (MIC 0.06 mg/L). Results are represented as differences (log10 CFU/mL) relative to the initial time-point (0 h).
3. Discussion
The present study shows that in KTRs with UTI caused by E. coli, cystitis, and asymptomatic bacteriuria, the acidic urine pH (≤6) at diagnosis, although physiological, is associated with a higher frequency of symptomatic UTI at one month of follow-up, particularly in those treated with oral fosfomycin. The acidic urine was not associated with the microbiological and clinical cure at one and six months after therapy, respectively, in patients with E. coli UTI, nor with the microbiological and clinical outcomes in KTRs with UTI caused by K. pneumoniae. At pH 5, the ciprofloxacin MIC90 increased in E. coli and K. pneumoniae. Fosfomycin MIC90 increased at pH 8 in E. coli and decreased at pH 5 in K. pneumoniae. The bactericidal in vitro activity of ciprofloxacin and fosfomycin against the E. coli strains decreased at acidic pH. At acidic pH, there was a slight increase of the bactericidal activity in fosfomycin against K. pneumoniae without changes with ciprofloxacin.
The clinical outcome of fosfomycin in the present study is inconsistent with its reported pharmacodynamics, including urine acidification. Using a Monte Carlo simulation of the urinary fosfomycin area under the concentration–time curve, after a single oral dose of 3 g, fosfomycin was effective against E. coli (MIC90 ≤ 16 mg/L) but not against Klebsiella spp. Acidification increased the susceptibility of 71% of the bacterial isolates, and the cumulative fractions of the bacterial responses were 99% and 55% against E. coli and Klebsiella spp., respectively, based on simulated drug exposure in urine with an acidic pH of 6 [21]. However, a retrospective study, including 48 cases of asymptomatic bacteriuria (AB) and cystitis, found fosfomycin resistance after treatment in six (12.5%) episodes caused by Enterobacterales [22], as we have observed against one E. coli strain after exposition to fosfomycin in the time-kill assays. In addition, a multicenter study showed 9.1% heteroresistance to fosfomycin among 66 E. coli urine isolates, with overexpression of metabolic genes increasing their survival rate [23], which may play a role in the failure of antibiotic treatments.
The clinical outcomes in the present study did not confirm the in vitro studies showing that the acidic pH of the medium increased the ciprofloxacin MIC against E. coli in LLQR [17], strains harboring well-characterized Qnr determinants [18], or E. coli 25922 [19]. Although our in vitro studies showed an increase in the MIC90 and a decrease in the susceptibility rate to ciprofloxacin in E. coli at diagnosis, the clinical and microbiological outcomes in patients treated with ciprofloxacin did not differ depending on the pH (acidic vs. neutral or alkaline).
The time-kill results were in accordance with those previously published [17,18,19], showing that the growth of E. coli and K. pneumoniae strains was similar, independent of pH conditions, and that E. coli growth was lower in urine than in MHB [18,19,20]. As reported [17,18,19], the present study shows less bactericidal in vitro activity of ciprofloxacin against E. coli in acidic pH conditions, independent of the medium. Likewise, at acidic pH conditions, fosfomycin activity against E. coli and K. pneumoniae was found to be marginal. Burian et al. [24] also found that pH acidification decreases the activity of different antibiotics, including fosfomycin. Martín-Gutiérrez et al. [20] found reduced fosfomycin activity in MHB at alkaline pH with an increase in the fosfomycin MIC against susceptible E. coli strains and those with LLFR. Nevertheless, in our in vitro studies, fosfomycin was bactericidal against three and two out of four isolates at neutral and alkaline pH, respectively, and at acidic pH only against one isolate.
Our study had several limitations. It was not a controlled study, although it reflected the results obtained in daily clinical practice. The number of patients treated with ciprofloxacin was limited following the EMA recommendation [14]. Moreover, the small number of patients with symptomatic UTIs in the follow-up precluded the exploration of possible confounding variables. The strengths were the inclusion of 184 UTI episodes by E. coli and K. pneumoniae and treatment only with fosfomycin or ciprofloxacin, considering that clinical trials to evaluate the therapeutic efficacy in AB included 112 and 205 KTRs with UTI of any etiology and receiving nonhomogeneous therapies [10,11,12]. Moreover, physiologically acidic urine was a common event, occurring in 48.4% of the 184 KTRs in the present study.
The study had several implications for clinical practice. First, it pointed out the need to consider an initial acidic urine pH as a factor for the strict follow-up of KTRs with E. coli UTI, especially in those treated with fosfomycin. Regarding the future, controlled and randomized clinical trials may answer the question of what the better therapy for cystitis is in KTRs with the most frequent gram-negative bacilli etiologies.
4. Materials and Methods
4.1. Study Design and Setting
We carried out an observational cohort of adult KTRs with E. coli and K. pneumoniae UTI episodes (cystitis and asymptomatic bacteriuria ([AB]), who attended as outpatients at the Virgen del Rocío University Hospital, Seville, Spain, from January 2017 to December 2019 and in a second period from March 2021 to June 2022 to collect additional K. pneumoniae episodes.
Physicians in charge of patients asked them to participate in the study, and they were prospectively included if (i) they provided informed consent; (ii) the requested urine cultures identified E. coli or K. pneumoniae; (iii) the attendant physicians prescribed fosfomycin or ciprofloxacin therapy in accordance with their clinical criteria; (iv) urine cultures were performed between 14 and 30 days after beginning the treatment; and (iv) a six-month follow-up was available, with an optional urine culture if new UTI symptoms occurred. Patients who did not fulfill these criteria were excluded. Fosfomycin trometamol was administered as two oral doses of 3 g administered 48 h apart [25], and ciprofloxacin was administered as 250 mg orally every 12 h for 5 days [26]. The outcomes investigated were microbiological cure at one month and clinical cure at one- and six-month follow-ups.
The following data were recorded from the digital charts at inclusion: demographics, chronic underlying diseases, time since kidney transplantation, immunosuppressive regimens, clinical data, plasma creatinine, urinary pH (pH ≤ 6 was defined as acidic), leukocyturia, urine nitrites in samples processed 4–8 h after collection, and antimicrobial therapy. The GESITRA/REIPI UTI guidelines were followed for clinical definitions [3]. Bacteriuria was defined as urine specimens isolated with quantitative counts of ≥105 CFU/mL. Asymptomatic bacteriuria was defined as the presence of bacteriuria in the absence of any UTI symptoms. Cystitis was considered for bacteriuria and clinical manifestations such as dysuria, frequency and urgency of urination, suprapubic pain, and/or hematuria in the absence of pyelonephritis symptoms. Acute pyelonephritis (APN) was considered the simultaneous presence of bacteriuria and/or bacteremia and fever, with one or more of the following: lumbar pain (if native kidney involved), renal allograft tenderness (if the kidney was transplanted), chills, or cystitis symptoms. Microbiological cure was achieved when urine culture was negative at 14–30 days. Clinical cure was considered the resolution of symptoms in the case of cystitis. Mortality was considered as death occurring within six months of follow-up. Impairment of renal function was defined as a ≥0.5 mg/dL increase in plasma creatinine.
4.2. Antimicrobial Susceptibility of E. coli and K. pneumoniae Clinical Isolates at Different pH Conditions
Clinical isolates were collected at inclusion and during follow-up and processed within 4–8 h after collection, and urine pH was measured. The hospital microbiology service identified the bacterial isolates and performed susceptibility testing with standard tests. Causative organisms were identified using a MicroScan WalkAway® Plus system (Beckman Coulter, Nyon, Switzerland). In cases where identification was uncertain, verification was obtained using a Bruker Biotyper MALDI-TOF MS system (Bruker Daltonik GmbH, Leipzig, Germany). The antimicrobial susceptibility testing and interpretation were in accordance with the yearly European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria [27].
Moreover, ciprofloxacin and fosfomycin MICs were determined in duplicate, in solutions with pH 8, 7, and 5 and interpreted following the 2023 EUCAST criteria (www.eucast.org/clinical_breakpoints, accessed on 22 December 2023): in the absence of defined breakpoints for oral fosfomycin in Enterobacterales other than E. coli, the same criteria were applied for K. pneumoniae. Antimicrobials were purchased as standard powders (Sigma-Aldrich, Madrid, Spain). The in vitro ciprofloxacin susceptibility assay was determined by microdilution method adjusting MHB (Thermo Scientific, Oxford, UK) to obtain acidic (pH = 5) or alkaline (pH = 8) pH values by adding 0.012% (v/v) of 12 N HCl or 0.072% (v/v) of 2 N NaOH, respectively (Sigma-Aldrich, Spain). Increasing concentrations of ciprofloxacin (from 0.01 to 8 mg/L) were tested with a starting inoculum of 5 × 105 CFU/mL. Fosfomycin susceptibility assays were carried out by agar diffusion. Aliquots of LB-agar (Sigma-Aldrich, Spain) were supplemented with glucose-6-phosphate (25 mg/L), increasing concentrations of fosfomycin (from 0.12 to 1024 mg/L) and, finally, 0.05% (v/v) of 12 N HCl or 0.07% (v/v) of 10 N NaOH to adjust acidic (pH = 5) or alkaline (pH = 8) pH values, respectively. Aliquots were plated and gel, then 2 µL of inoculum (final concentration of 5 × 105 CFU/mL) was plated and incubated overnight at 37 °C. For E. coli, ciprofloxacin, and fosfomycin with MIC values of >0.06 to 0.5 mg/L and >4 to 8 mg/L were considered the LLQR and LLFR, respectively. For K. pneumoniae, ciprofloxacin MIC values of >0.12 to 0.5 mg/L were considered the LLQR; no isolates were classified as LLFR because of the 128 mg/L ECOFF (https://mic.eucast.org/; accessed on 21 July 2023).
4.3. Bactericidal Activity of Ciprofloxacin and Fosfomycin against E. coli and K. pneumoniae Strains at Different pH Conditions
To evaluate the impact of different pH conditions on the bactericidal activities of ciprofloxacin and fosfomycin against uropathogenic E. coli and K. pneumoniae strains, with different susceptibility patterns, we used eight strains: (i) E. coli Nu14 [28], ciprofloxacin- and fosfomycin-susceptible (MIC 0.03 and 2 mg/L, respectively); (ii) E. coli HUVR94, ciprofloxacin- and fosfomycin-susceptible (MIC 0.03 and 0.5 mg/L, respectively); (iii) E. coli Nu79 gyrA D87G with LLQR (MIC 0.12 mg/L) and fosfomycin-susceptible (MIC 0.5 mg/L) [29]; (iv) E. coli Nu14 with a glpT missense mutation, ciprofloxacin-susceptible (MIC 0.01 mg/L) and previously defined as LLFR (MIC 32 mg/L) [30]; (v) K. pneumoniae HUVR42, ciprofloxacin- and fosfomycin-susceptible (MIC 0.007 and 4 mg/L, respectively); (vi) K. pneumoniae HUVR5, ciprofloxacin- and fosfomycin-resistant (MIC 8 and 128 mg/L, respectively); (vii) K. pneumoniae HUVR110, ciprofloxacin-resistant (MIC 8 mg/L) and fosfomycin-susceptible (MIC 4 mg/L); and (viii) K. pneumoniae HUVR91, ciprofloxacin-susceptible (MIC 0.06 mg/L) and fosfomycin-resistant (MIC 64 mg/L).
The bactericidal activity of both antimicrobials against the eight strains was determined, in triplicate, by time-kill assays at concentrations equivalent to their MIC on MHB (Thermo-Scientific, UK) and urine from three healthy volunteers who had not undergone antibiotic treatment in the previous three months. Urine samples were pooled and sterilized by filtration (polyether-sulphone membrane filters, 0.22 mm, VWR; Leicestershire, UK) and stored at 4 °C until analysis. MHB and/or urine were adjusted to obtain acidic (pH = 5) or alkaline (pH = 8) pH values by adding 0.012% (v/v) of 12 N HCl or 0.072% (v/v) of 2 N NaOH, respectively (Sigma-Aldrich, Spain). The initial concentration of inoculum was 5 × 105 CFU/mL, and samples were taken at 0, 2, 4, 6, and 24 h. Bactericidal activity was defined as a decrease of ≥3 log10 CFU/mL based on the initial concentration of inoculum [27].
In these assays, mutant-resistant development was determined if the strains were ciprofloxacin- or fosfomycin-susceptible, and there was regrowth at 24 h after a bactericidal effect at previous time points. In these cases, five colonies were randomly picked up to perform susceptibility assays, as previously described. The mutant resistant rate was calculated as (number of resistant colonies/total number of colonies) × 100.
4.4. Statistical Analysis
Continuous variables are expressed as median and interquartile range (IQR), and qualitative variables as proportions. The microbiological cure at one month and clinical cure at one- and six-months follow-up were analyzed separately in patients with E. coli or K. pneumoniae episodes and treated with ciprofloxacin or fosfomycin, comparing the results in patients with urine pH at diagnosis of ≤6 and >6, through chi-square and Fisher exact tests. The association of urine pH with the microbiological and clinical outcomes was adjusted by age (≤60 vs. >60 years) and cystitis or AB. Several sensitivity analyses were performed to compare patients with urine pH ≤ 6 and > 6 in all patients (N = 184), patients with cystitis (N = 52), and patients with AB (N = 132), independently of the etiology. The bactericidal activities of ciprofloxacin and fosfomycin in time-kill studies are presented as differences in the log10 CFU/mL with respect to the initial bacterial concentrations. A p value of <0.05 was considered significant. The statistical package SPSS v24.0 (SPSS Inc., Chicago, IL, USA) was used.
5. Conclusions
The results of the present study suggest that low urine pH is associated with a greater frequency of symptomatic UTI in the first month after the antimicrobial treatment when the etiology of the initial episode is E. coli. However, the data in the follow-up show that acidic urine does not affect the microbiological efficacy of ciprofloxacin and fosfomycin in KTRs with cystitis and asymptomatic bacteriuria.
Acknowledgments
S.H.-E. is supported by the program PFIS (Contratos Predoctorales de Formación en Investigación en Salud), Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Ciencia e Innovación under grant [FI21/00280]. M.E.P.-I. is a “Nicolás Monardes” researcher (C1-0038-2019), Servicio Andaluz de Salud, Junta de Andalucía, Spain. M.E.P.-I. and E.C. also received support from the CIBER de Enfermedades Infecciosas (CIBERINFEC, CB21/13/00006), Instituto de Salud Carlos III, Ministerio de Ciencia e Innovación, co-financed by the European Development Regional Fund.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics13020116/s1, Table S1: Demographics and characteristics of the 115 kidney transplant recipients (KTR) with urinary tract infection (UTI) by Escherichia coli, and according the asymptomatic bacteriuria (AB) and cystitis episodes; Table S2: Demographics and characteristics of the 115 kidney transplant recipients with urinary tract infection by Escherichia coli according the initial urine pH; Table S3: Demographics and characteristics of the 115 kidney transplant recipients with urinary tract infection by Escherichia coli according symptomatic UTI during one-month follow-up; Table S4: Demographics and characteristics of the 69 kidney transplant recipients (KTR) with urinary tract infection (UTI) by Klebsiella pneumoniae, and according the asymptomatic bacteriuria (AB) and cystitis episodes; Table S5: Demographics and characteristics of the 69 kidney transplant recipients with urinary tract infection by Klebsiella pneumoniae according the initial urine pH; Table S6: Demographics and characteristics of the 69 kidney transplant recipients with urinary tract infection by Klebsiella pneumoniae according symptomatic UTI during one-month follow-up; Table S7: Symptomatic urinary tract infection (UTI) during one-month follow-up according the acidic urine pH, after fosfomycin or ciprofloxacin therapy of UTI by Escherichia coli or Klebsiella pneumoniae (N = 184); Table S8: Symptomatic urinary tract infection (UTI) during one-month follow-up according the acidic urine pH, after fosfomycin or ciprofloxacin therapy of UTI by Escherichia coli; Table S9: Symptomatic urinary tract infection (UTI) during one-month follow-up according the acidic urine pH, after fosfomycin or ciprofloxacin therapy of UTI by Klebsiella pneumoniae (N = 69); Table S10: Microbiological and clinical outcomes, in patients with acidic (pH ≤ 6) vs. non-acidic (pH > 6) urine, after fosfomycin or ciprofloxacin therapy of urinary tract infection (UTI) regardless of aetiology; Table S11: Initial clinical, urine pH, and microbiological characteristics of patients with symptomatic Escherichia coli and Klebsiella pneumoniae UTI episodes during one-month follow-up after fosfomycin or ciprofloxacin therapy; Table S12: Ciprofloxacin and fosfomycin MIC distribution and frequencies of low-level quinolone and fosfomycin resistance rates in the 115 isolates of Escherichia coli and according the AB and cystitis episodes; Table S13: Ciprofloxacin and fosfomycin MIC distribution and frequencies of low-level quinolone and fosfomycin resistance rates in the 69 isolates of Klebsiella pneumoniae and according the AB and cystitis episodes; Table S14: In vitro MIC distribution of ciprofloxacin at pH 5.0 (acidic) and 8.0 (alkaline) of E. coli and K. pneumoniae clinical isolates with low-level ciprofloxacin resistance at pH 7.0 (neutral); Table S15: In vitro MIC distribution of fosfomycin at pH 5.0 (acidic) and 8.0 (alkaline) of E. coli clinical isolates with low-level fosfomycin resistance at pH 7.0 (neutral); Table S16: pH effect on the bactericidal activity of ciprofloxacin MIC, in MHB and urine, against uropathogenic Escherichia coli strains; Table S17: pH effect on the bactericidal activity of fosfomycin MIC, in MHB and urine, against uropathogenic Escherichia coli strains; Table S18: pH effect on the bactericidal activity of ciprofloxacin MIC, in MHB and urine, against uropathogenic Klebsiella pneumoniae strains; Table S19: pH effect on the bactericidal activity of fosfomycin MIC; in MHB and urine, against uropathogenic Klebsiella pneumoniae strains.
Author Contributions
Conceptualization, J.P., M.E.P.-I. and E.C.; methodology, J.P., M.E.P.-I. and E.C.; software, S.H.-E., C.I. and S.F.; validation, J.P., M.E.P.-I., S.H.-E., C.I. and S.F.; formal analysis, J.P., M.E.P.-I., E.C. and S.H.-E.; investigation, M.E.P.-I., S.H.-E., M.C.-L., G.M.-G. and J.A.P.-C.; resources, M.E.P.-I., E.C., G.M.-G., J.A.P.-C., A.S.-B., M.S.-P., C.G.-C. and G.B.; data curation, M.E.P.-I., S.H.-E., M.S.-P., C.G.-C. and G.B.; writing—original draft preparation, S.H.-E.; writing—review and editing, J.P., M.E.P.-I. and E.C.; visualization, S.F., C.I., M.C.-L., G.M.-G., J.A.P.-C., A.S.-B., M.S.-P., C.G.-C. and G.B.; supervision, J.P., M.E.P.-I. and E.C.; project administration, M.E.P.-I.; funding acquisition, M.E.P.-I. and E.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Virgen Macarena and Virgen del Rocío University Hospitals (FIS-CIP-2016-01 on 7 July 2016, FIS-FOS-2020-01 on 6 November 2020), Seville, Spain.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
This study has been funded by Instituto de Salud Carlos III, through the projects PI17/01405 and PI20/01255, by the Subdirección General de Evaluación y Fomento de la Investigación, Ministerio de Economía, Industria y Competitividad, US-1381501 US/JUNTA/FEDER, UE, by the Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, and the Spanish Network for Research in Infectious Diseases (REIPI, RD16/0016/0009) and co-funded by the European Union.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bodro M., Sanclemente G., Lipperheide I., Allali M., Marco F., Bosch J., Cofan F., Ricart M.J., Esforzado N., Oppenheimer F., et al. Impact of urinary tract infections on short-term kidney graft outcome. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2015;21:1104.e1–1104.e8. doi: 10.1016/j.cmi.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 2.Naik A.S., Dharnidharka V.R., Schnitzler M.A., Brennan D.C., Segev D.L., Axelrod D., Xiao H., Kucirka L., Chen J., Lentine K.L. Clinical and economic consequences of first-year urinary tract infections, sepsis, and pneumonia in contemporary kidney transplantation practice. Transpl. Int. 2016;29:241–252. doi: 10.1111/tri.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontserè S., Infante-Domínguez C., Suárez-Benjumea A., Suñer-Poblet M., González-Corvillo C., Martín-Gutiérrez G., Bernal G., Pachón J., Pachón-Ibáñez M.E., Cordero E. Impact of Treating Asymptomatic Bacteriuria in Kidney Transplant Recipients: A Prospective Cohort Study. Antibiotics. 2021;10:218. doi: 10.3390/antibiotics10020218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosseinpour M., Pezeshgi A., Mahdiabadi M.Z., Sabzghabaei F., Hajishah H., Mahdavynia S. Prevalence and risk factors of urinary tract infection in kidney recipients: A meta-analysis study. BMC Nephrol. 2023;24:284. doi: 10.1186/s12882-023-03338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ny S., Edquist P., Dumpis U., Gröndahl-Yli-Hannuksela K., Hermes J., Kling A.M., Klingeberg A., Kozlov R., Källman O., Lis D.O., et al. Antimicrobial resistance of Escherichia coli isolates from outpatient urinary tract infections in women in six European countries including Russia. J. Glob. Antimicrob. Resist. 2019;17:25–34. doi: 10.1016/j.jgar.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Li Y., Zheng B., Li Y., Zhu S., Xue F., Liu J. Antimicrobial Susceptibility and Molecular Mechanisms of Fosfomycin Resistance in Clinical Escherichia coli Isolates in Mainland China. PLoS ONE. 2015;10:e0135269. doi: 10.1371/journal.pone.0135269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seok H., Choi J.Y., Wi Y.M., Park D.W., Peck K.R., Ko K.S. Fosfomycin Resistance in Escherichia coli Isolates from South Korea and in vitro Activity of Fosfomycin Alone and in Combination with Other Antibiotics. Antibiotics. 2020;9:112. doi: 10.3390/antibiotics9030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Origüen J., Fernández-Ruiz M., López-Medrano F., Ruiz-Merlo T., González E., Morales J.M., Fiorante S., San-Juan R., Villa J., Orellana M., et al. Progressive increase of resistance in Enterobacteriaceae urinary isolates from kidney transplant recipients over the past decade: Narrowing of the therapeutic options. Transpl. Infect. Dis. Off. J. Transplant. Soc. 2016;18:575–584. doi: 10.1111/tid.12547. [DOI] [PubMed] [Google Scholar]
- 9.López-Medrano F., Silva J.T., Fernández-Ruiz M., Vidal E., Origüen J., Calvo-Cano A., Luna-Huerta E., Merino E., Hernández D., Jironda-Gallegos C., et al. Oral fosfomycin for the treatment of lower urinary tract infections among kidney transplant recipients-Results of a Spanish multicenter cohort. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020;20:451–462. doi: 10.1111/ajt.15614. [DOI] [PubMed] [Google Scholar]
- 10.Coussement J., Kamar N., Matignon M., Weekers L., Scemla A., Giral M., Racapé J., Alamartine É., Mesnard L., Kianda M., et al. Antibiotics versus no therapy in kidney transplant recipients with asymptomatic bacteriuria (BiRT): A pragmatic, multicentre, randomized, controlled trial. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021;27:398–405. doi: 10.1016/j.cmi.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Origüen J., López-Medrano F., Fernández-Ruiz M., Polanco N., Gutiérrez E., González E., Mérida E., Ruiz-Merlo T., Morales-Cartagena A., Pérez-Jacoiste Asín M.A., et al. Should Asymptomatic Bacteriuria Be Systematically Treated in Kidney Transplant Recipients? Results From a Randomized Controlled Trial. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2016;16:2943–2953. doi: 10.1111/ajt.13829. [DOI] [PubMed] [Google Scholar]
- 12.Sabé N., Oriol I., Melilli E., Manonelles A., Bestard O., Polo C., Los Arcos I., Perelló M., Garcia D., Riera L., et al. Antibiotic Treatment Versus No Treatment for Asymptomatic Bacteriuria in Kidney Transplant Recipients: A Multicenter Randomized Trial. Open Forum. Infect. Dis. 2019;6:ofz243. doi: 10.1093/ofid/ofz243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonkat G., Bartoletti R., Bruyère F., Cai T., Geerlings S.E., Köves B., Schubert S., Pilatz A., Veeratterapillay R., Wagenlehnerand F. EAU Guidelines on Urological Infections. EAU Guidelines Office; Arnhem, The Netherlands: 2023. [Google Scholar]
- 14.EMA (European Medicines Agency) Disabling and Potentially Permanent Side Effects Lead to Suspension or Restrictions of Quinolone and Fluoroquinolone Antibiotics. [(accessed on 8 November 2023)]; Available online: https://www.ema.europa.eu/en/documents/referral/quinolone-fluoroquinolone-article-31-referral-disabling-potentially-permanent-side-effects-lead_en.pdf.
- 15.Komp Lindgren P., Karlsson A., Hughes D. Mutation rate and evolution of fluoroquinolone resistance in Escherichia coli isolates from patients with urinary tract infections. Antimicrob. Agents Chemother. 2003;47:3222–3232. doi: 10.1128/AAC.47.10.3222-3232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi A., Muratani T., Yasuda M., Takahashi S., Monden K., Ishikawa K., Kiyota H., Arakawa S., Matsumoto T., Shima H., et al. Genetic profiles of fluoroquinolone-resistant Escherichia coli isolates obtained from patients with cystitis: Phylogeny, virulence factors, PAIusp subtypes, and mutation patterns. J. Clin. Microbiol. 2009;47:791–795. doi: 10.1128/JCM.01740-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martín-Gutiérrez G., Rodríguez-Beltrán J., Rodríguez-Martínez J.M., Costas C., Aznar J., Pascual Á., Blázquez J. Urinary Tract Physiological Conditions Promote Ciprofloxacin Resistance in Low-Level-Quinolone-Resistant Escherichia coli. Antimicrob. Agents Chemother. 2016;60:4252–4258. doi: 10.1128/AAC.00602-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martín-Gutiérrez G., Rodríguez-Martínez J.M., Pascual Á., Rodríguez-Beltrán J., Blázquez J. Plasmidic qnr Genes Confer Clinical Resistance to Ciprofloxacin under Urinary Tract Physiological Conditions. Antimicrob. Agents Chemother. 2017;61:e02615-16. doi: 10.1128/AAC.02615-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erdogan-Yildirim Z., Burian A., Manafi M., Zeitlinger M. Impact of pH on bacterial growth and activity of recent fluoroquinolones in pooled urine. Res. Microbiol. 2011;162:249–252. doi: 10.1016/j.resmic.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 20.Martín-Gutiérrez G., Docobo-Pérez F., Rodriguez-Beltrán J., Rodríguez-Martínez J.M., Aznar J., Pascual A., Blázquez J. Urinary Tract Conditions Affect Fosfomycin Activity against Escherichia coli Strains Harboring Chromosomal Mutations Involved in Fosfomycin Uptake. Antimicrob. Agents Chemother. 2018;62:e01899-17. doi: 10.1128/AAC.01899-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fedrigo N.H., Mazucheli J., Albiero J., Shinohara D.R., Lodi F.G., Machado A., Sy S.K.B., Tognim M.C.B. Pharmacodynamic Evaluation of Fosfomycin against Escherichia coli and Klebsiella spp. from Urinary Tract Infections and the Influence of pH on Fosfomycin Activities. Antimicrob. Agents Chemother. 2017;61:e02498-16. doi: 10.1128/AAC.02498-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ten Doesschate T., van Werkhoven H., Meijvis S., Stalenhoef J., van Zuilen A., de Vries A., Bonten M. Fosfomycin-trometamol for Urinary Tract Infections in Kidney Transplant Recipients. Transplantation. 2019;103:1272–1276. doi: 10.1097/TP.0000000000002427. [DOI] [PubMed] [Google Scholar]
- 23.Campos A., Andrade N.L., Couto N., Mutters N.T., de Vos M., Rosa A.C.P., Damasco P.V., Lo Ten Foe J.R., Friedrich A.W., Chlebowicz-Flissikowska M.A., et al. Characterization of fosfomycin heteroresistance among multidrug-resistant Escherichia coli isolates from hospitalized patients in Rio de Janeiro, Brazil. J. Glob. Antimicrob. Resist. 2020;22:584–593. doi: 10.1016/j.jgar.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 24.Burian A., Erdogan Z., Jandrisits C., Zeitlinger M. Impact of pH on activity of trimethoprim, fosfomycin, amikacin, colistin and ertapenem in human urine. Pharmacology. 2012;90:281–287. doi: 10.1159/000342423. [DOI] [PubMed] [Google Scholar]
- 25.AEMPS (Agencia Española de Medicamentos y Productos Sanitarios) Fosfomicina Kern Pharma. AEMPS; Madrid, Spain: 2021. [Google Scholar]
- 26.AEMPS (Agencia Española de Medicamentos y Productos Sanitarios) Ciprofloxacino Normon. AEMPS; Madrid, Spain: 2020. [Google Scholar]
- 27.EUCAST (The European Committee on Antimicrobial Susceptibility Testing) Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 11.0. ESCMID; Base, Switzerland: 2021. [Google Scholar]
- 28.Mehershahi K.S., Chen S.L. Complete Genome Sequence of the Uropathogenic Escherichia coli Strain NU14. Genome Announc. 2017;5:e00306-17. doi: 10.1128/genomeA.00306-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Komp Lindgren P., Marcusson L.L., Sandvang D., Frimodt-Møller N., Hughes D. Biological cost of single and multiple norfloxacin resistance mutations in Escherichia coli implicated in urinary tract infections. Antimicrob. Agents Chemother. 2005;49:2343–2351. doi: 10.1128/AAC.49.6.2343-2351.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsson A.I., Berg O.G., Aspevall O., Kahlmeter G., Andersson D.I. Biological costs and mechanisms of fosfomycin resistance in Escherichia coli. Antimicrob. Agents Chemother. 2003;47:2850–2858. doi: 10.1128/AAC.47.9.2850-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.