Abstract
Fluorocarbons are fluorinated organic molecules widely used in industry and commerce. Nomenclature has changed over the years, with PFAS becoming the accepted umbrella term. The environment is heavily polluted with these toxins. Worldwide research shows that they contribute to almost every chronic disease. The primary source of human contamination is food packaging. There are significant concerns that the available research has not adequately addressed ultrashort-chain PFAS, which are breakdown products of longer-chain versions and accumulate in the environment at almost 100 times higher concentrations than the longer-chain versions.
Introduction
Much of the research for the basic science aspects of this editorial came from the Toxicology Profile for Perfluoroalkyls published by the US Agency for Toxic Substances and Disease Registry in 2021. (Toxicological Profile for Perfluoroalkyls (cdc.gov)) Those interested in more deeply studying PFAS will find it an excellent resource.
Definition and Terminology
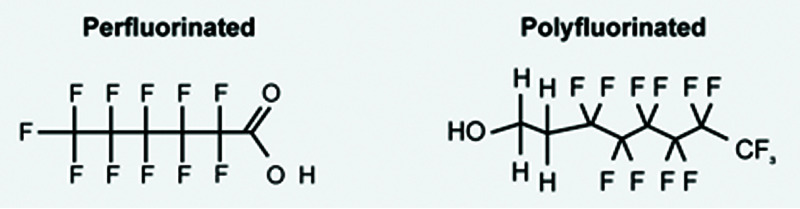
Perfluorocarbons are organofluorine compounds with the formula CxFy.
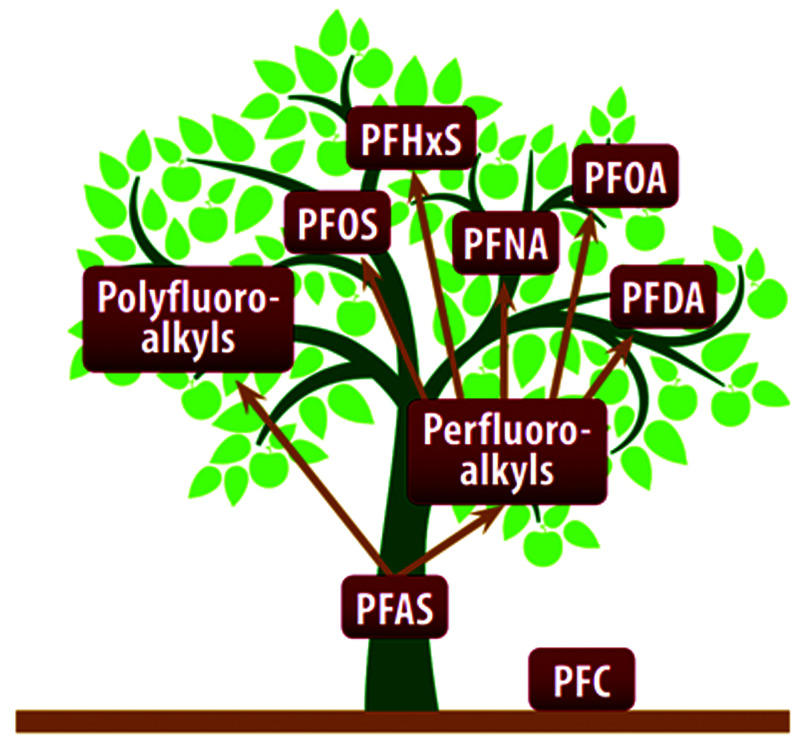
Reading the research reveals that this class of chemicals has seen diverse names used interchangeably over the years. While perfluorocarbon (PFC) was used most in the past, the research community has settled on PFAS. This editorial uses PFAS as the generic term for all per- and poly-fluorinated compounds. Figure 1 lays out the PFAS tree.
Figure 1.
The PFAS Family Tree1
PFAS are fluorinated molecules that are widely used in industry and in diverse products and processes. Examples include water-repelling textiles, grease-resistant paper, nonstick packaging and cooking appliances, medical and laboratory tubing, aqueous film-forming foams, and industrial detergents.
PFAS are highly persistent toxins with half-lives ranging from 2 to 7 years. There are hundreds of unique PFAS, and the rate of detoxification is, in general, inversely proportional to the number of fluorine substitutes.
As might be expected with their wide range of use and long persistence, PFAS heavily contaminate the environment.
Classification and Naming
PFAS are a class of over 12 000 compounds, each with a carbon chain of varying length that is either fully or partially saturated with fluorine atoms and has a polar end group.
Key identification criteria for PFAS are
- Per- or poly-
- End molecule hydroxyl or sulfate
Length (the third letter in the abbreviation usually indicates the carbon-chain length)
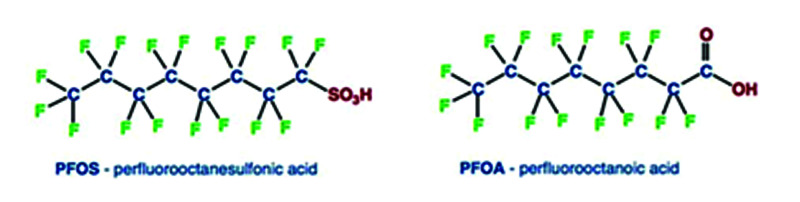
Example names are in Table 1.
Table 1.
Common PFAS Compound Names2
| X | Y | Acronym | Name | Formula |
|---|---|---|---|---|
| B = buta (4 carbon) | A = Carboxulate or carboxlic acid | PFBA | Perfluorobutanoate | C3F7CO2- |
| Perfluorobutanoate acid | C3F7COOH | |||
| S = sulfonate or sulfonic acid | PFBS | Perfluorobutane sulfonate | C4F9SO3- | |
| Perfluorobutane sulfonic acid | C4F7SO3H | |||
| O = octa (8 carbon) | A = Carboxylate or carboxylic acid | PFOA | Perfluorooctanoate | C7F15CO2- |
| Perfluorooctanoic acid | C7F15COOH | |||
| S = sulfonate or sulfonic acid | PFOS | Perfluoroocatane sulfonate | C8F17SO3-- | |
| Perfluoroctane sulfonic acid | C8F17SO3H |
Chain Length
PFAS are classified according to chain length:
Long-chain: 7 or more perfluorocarbons for perfluorocarboxylates (PFCAs), and 6 or more perfluorocarbons for perfluorosulfonates (PFSAs)
Short-chain: 3 to 7 perfluorocarbons for PFCAs, and 4 to 5 perfluorocarbons for PFSAs
Ultrashort-chain: 2 or fewer perfluorocarbons for PFCAs, and 3 or fewer perfluorocarbons for PFSAs and other PFAS
Challenges
This has been a challenging editorial to write. Normally, when I study an environmental metal or chemical toxicant, the patterns of exposure and disease causations and associations quickly become clear. For PFAS, a number of problems showed up immediately:
The mechanisms of damage are poorly understood.
Most of the safety research is with animals. However, rodents detoxify PFAS 10 to 1000 times faster than humans do.
The PFAS that have been measured and studied may not be the worst fluorocarbons. Specifically, virtually all the research is on short-, medium-, and long-length perfluorinated compounds, but emerging research suggests that the ultrashort perfluorinated compounds are the worst for human health AND that their concentrations in water are typically 100 times higher.
The actual exposure has shifted a lot in the past decade as we transitioned from the now banned perfluorooctanoic acid (PFOA) to supposedly safer perfluorooctane sulfonic acid (PFOS) and other perfluorinated compounds. Unfortunately, like so many other substitutions of toxic chemicals we often don’t have much long term safety data.
Exposure is so widespread and persistent that there is virtually no human control population.
Further complicating our understanding is that several PFAS have nonlinear health damaging effects.
There are many disease associations, but they are of marginal statistical significance—probably because of the issues in this list.
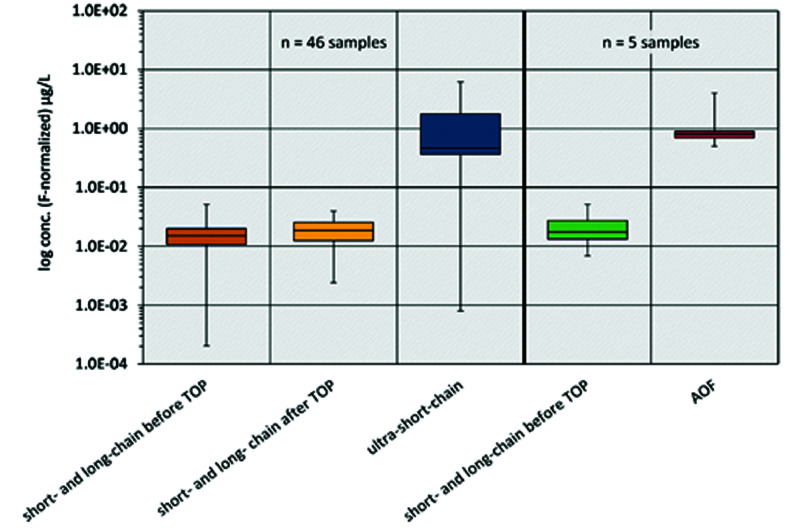
The Problem of Under-Recognized Ultrashort-Chain PFAS
The ultrashort-chain PFAS are degradation products of longer-chain PFAS. Commercial laboratories do not currently measure most ultrashort-chain PFAS. Ultrashort-chain PFAS commonly contaminate drinking water and are found in high concentrations in the body. Worse, these molecules are water soluble, and there are no current environmental regulations.3
Figure 2, from a recent study of drinking water in Germany, illustrates the problem well. Note that the vertical axis is a log scale.
Figure 2.
PFAS in Drinking Water in Germany
Routes of Exposure
The routes of exposure to PFAS are diverse and common. In general, they come from food, water, clothing, dust, industrial processes—basically they come from everywhere. Table 2 provides an example of their use in industry.
Table 2.
Industrial Uses of PFAS4
| Point Source Category | Description | Usaes or Sources of PFASa |
|---|---|---|
| Organic Chemicals, Plastics, and Synthetic Fibers (OCPSF) | Industrial facilities that manufacture organic chemicals, plastics, synthetic fibers or resin products, including those that manufacture PFAS or process PFAS in production of such products. Subject to ELGs in 40 CFR Part 414. |
|
| Metal Finishing | Industrial facilities that change the surface of an object to improve its appearance or durability. Includes six primary operations: electroplating, electroless plating, anodizing, coating, printed circuit board manufacturing, and chemical etching and milling. Subject to ELGs in 40 CFR Part 433. | - PFAS-containing chemicals used as wetting agents, mist and fume suppressants to prevent air emissions of toxic metal fumes, agents to reduce mechanical wear, and surface coatings to impart certain characteristics (e.g., reduced corrosion, enhanced appearance). |
| Pulp, Paper, and Paperboard | Mills that convert wood into pulp, paper, paperboard, and other cellulose-based products. Subject to ELGs in 40 CFR Part 430. |
|
| Textile Mills | Mills that receive and prepare fibers; transform materials into yarn, thread, or webbing; convert yarn and webbing into fabric or related products; or finish these materials to produce consumer products (e.g., thread, yarn, bolt fabric, hosiery, towels, sheets, carpet). Subject to ELGs in 40 CFR Part 410. | - PFAS-containing chemicals used to impart outdoor gear, clothing, household, and other textile products with water, oil, soil, and heat resistance. |
| Commercial Airports | Commercial facilities associated with commercial air transport or aircraft flight operations. Excludes facilities operated by the United States Department of Defense (DOD). Subject to ELGs in 40 CFR Part 449. | - PFAS are a component of aqueous film-forming foam (AFFF), used for exterminating hydrocarbon fuel fires and firefighting training. |
a - In general, PFAS may be used as coatings of surfactants for mechanical components (e.g., semiconductors, wiring, tubing, piping, seals, gaskets, etc.) used at many types of industrial facilities.
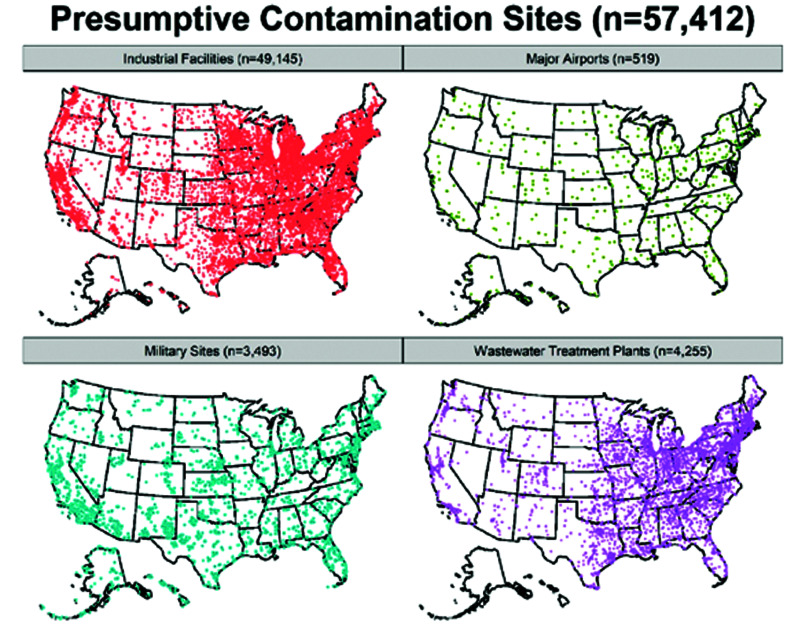
This widespread use has resulted in extensive contamination of the environment, as can be seen in Figure 3.
Figure 3.
Industrial Contamination of the Environment with PFAS5
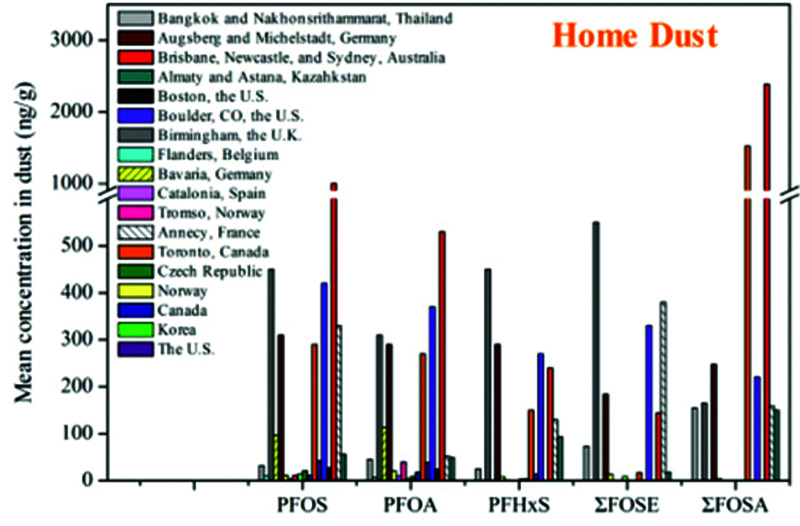
This widespread contamination of the environment shows up in household dust all over the world, as shown in Figure 4. Clearly, a clean home is important for health.
Figure 4.
PFAS in House Dust6
Prepared and packaged foods are the major source of PFAS. The amount leached into food is increased by higher fat, lower pH, increased salt, alcohol, time, heat, and surface area of the food in contact with the packaging.7 While fast food is a significant source of PFAS, prepared popcorn is much worse—by a factor of 5.8 Even straws are a significant source.9
Several professions have unusually high levels of PFAS exposure. Those professions most exposed are firefighters, construction workers, furniture makers, and ski technicians (professional ski waxers have up to 25 times as many PFAS in their blood as the general population).10 Basically everyone who uses nonstick or stain-guard products is exposed. We even have to consider newly installed carpet. Those interested will find Dr Steven Genuis’ case report illuminating.11
Worldwide research to quantify the primary sources of PFAS exposure in the general population found that more than 90% of the average body load comes from diet.12 However, these studies did not include the ultrashort-chain PFAS. If these had been included, it is quite possible that water is also a primary source of exposure.
Body Load
All these exposures add up to a significant body burden13:
Industrial workers: 1000 ng/mL
Highly exposed residents (without occupational exposure): 423 ng/mL
US population: 4.9 ng/mL
There is good news and bad. The good news is that the body load of PFOA has decreased 90% since these compounds were banned 10 years ago.14 The bad news is that they have been replaced with other PFAS.
Excretion and Detoxification
PFAS are difficult for humans to detoxify and have half-lives measured in years. They are excreted through urine, menstrual blood, breast milk, and stool but not through sweat. Most of the detoxification is through the liver, though mechanisms are unclear.
In general, the longer the chain and the more saturation with fluorine the slower the detoxification.
Table 3 shows the rates of detoxification of the most common PFAS. Note especially the dramatic differences in detoxification rates between the rodents typically used for toxicology research and humans and nonhuman primates. Also of interest is that female rats are much more effective at this detoxification than male rats.
Table 3.
Half-Lives of Common PFAS15
| Humans | Nonhuman primates | Rats | Mice | |
|---|---|---|---|---|
| PFOA | 2.1–10.1 years | 20.1–32.6 days | Males: 44–322 hours Females: 1.9–16.2 hours |
|
| PFOS | 3.3–27 years | 110–170 days | 179–1.968 hours | 731–1.027 hours |
| PFHxS | 4.7–35 years | 87–141 days | Males: 382–688 hours Females: 1.03–41.28 hours |
597–643 hours |
| PFNA | 2.5–4.3 years | Males: 710-1.128 hours Females: 33.6–58.6 hours |
619.2–1,653 hours | |
| PFBS | 665 hours | 8.0–95.2 hours | 2.1–7.42 hours | |
| PFBA | 72–81 hours | 40.3–41.0 hours | 1.03–9.22 hours | 2.79–13.34 hours |
Disease Associations
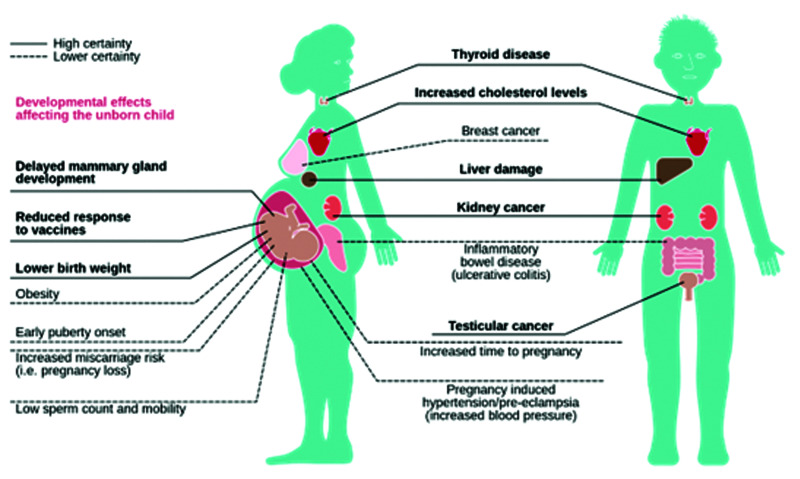
This is where the research is so frustrating. While PFAS body load correlates with many diseases, the actual increase is apparently relatively modest. Figure 5 provides a clear graphical presentation of how pervasively PFAS damage human tissues.
Figure 5.
PFAS Damage Many Tissues16
Table 4 lists the diseases that have research-documented correlations with the body load of PFAS.
Table 4.
Diseases Correlated with Body Load of PFAS16-22
|
Assessment
Assessment of body load of PFAS is currently very difficult. Some commercial laboratories are starting to implement the technology needed to measure these molecules in human fluids. However, the scientists have many challenges, including the use of equipment that contains Teflon tubing or other PFAS.
At this time, indirect measures to detect significant PFAS exposure may be clinically more useful, and certainly much less expensive. Alanine transaminase (ALT), bilirubin, γ-glutamyltransferase, (GGTP) and uric acid all increase within the “normal range” after PFAS exposure.24-26 Once again, the normal range includes a lot of sickness and is NOT the healthy range.
My recommendation is to directly measure PFAS to establish a baseline. Then use the inexpensive indirect measures to monitor intervention efficacy.
Treatment
As is usual with toxins, avoidance is the key. Since the main source of PFAS, by far, is food (but may also be water, if the ultrashort-chain PFAS are shown to be significant), addressing the food-packaging problem will be hugely successful. The best way to accomplish this is to not eat out, NEVER eat microwave popcorn, and replace all kitchen storage and cookware with those made from glass, stainless steel, or ceramics. Carbon block filters are effective at removing PFAS from water.
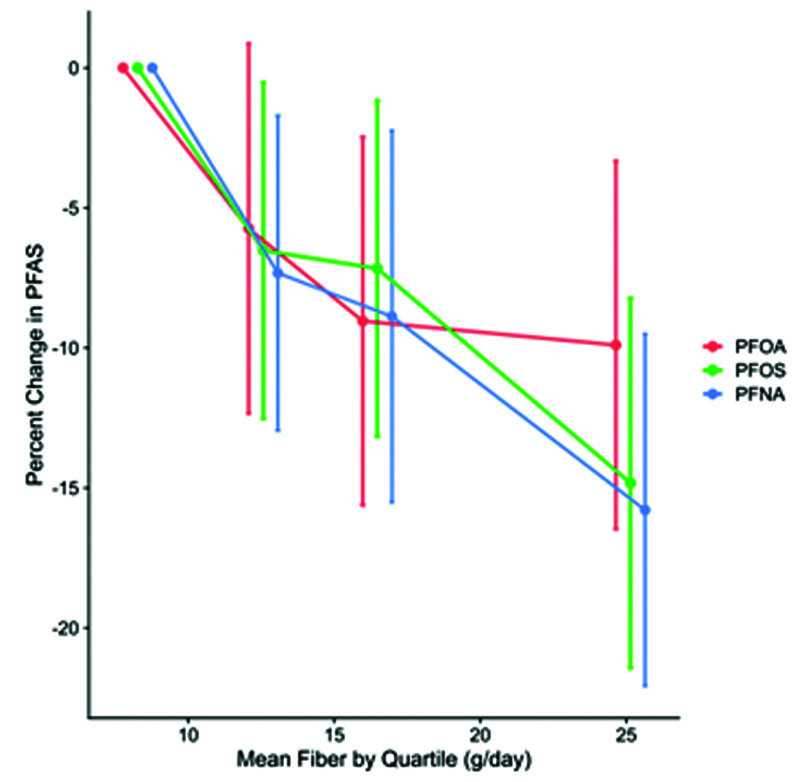
Of great importance is recognizing that enterohepatic recirculation is a significant factor impairing PFAS excretion from the body. As would be expected, increasing dietary fiber definitely increases the rate of excretion in the stool. Figure 6 presents this very clearly. However, note that the top dosage was only 25 grams per day—far less than the amount in human evolutionary diet. The rate of excretion can also be increased through the use of bile sequestrants, such as cholestyramine.27
Figure 6.
Dietary Fiber Increases PFAS Excretion28
Finally, several herbs and nutrients can help prevent the damage caused by PFAS. Curcumin has been shown in cell cultures to decrease DNA damage.29 Vitamin C blunts insulin resistance in humans.30 Blueberries were shown in an animal study to decrease PFAS neurotoxicity.31
Conclusion
Exposure to PFAS is ubiquitous but is primarily from food. The entire population has a body load of PFAS that increases their risk of the most common chronic diseases. The very long half-lives of PFAS mean that, while avoidance is important, intervention is required to decrease body load.
I do not think the research is clear enough yet to determine exactly how much disease is caused by PFAS. I suspect we will find it significant as the ultrashort-chain PFAS are researched.
Biography

Joseph Pizzorno, ND, Editor in Chief, IMCJ; co-author, Textbook of Natural Medicine; Founding President, Bastyr University; Chair, Board of Directors, Institute for Functional Medicine.
References
- 1.ATSDR. NCEH Fact Sheet. Agency for Toxic Substances and Disease Registry. Accessed December 2023. https://www.atsdr.cdc.gov/pfas/docs/pfas_fact_sheet.pdf
- 2. Naming Conventions of Per- and Polyfluoroalkyl Substances (PFAS) ( itrcweb.org) (accessed January 2024)f. [Google Scholar]
- 3.Neuwald IJ, Hübner D, Wiegand HL, et al. Ultra-short-chain PFASs in the sources of German drinking water: prevalent, overlooked, difficult to remove, and unregulated. Environ Sci Technol. 2022;56(10):6380-6390. doi:10.1021/acs.est.1c07949 [DOI] [PubMed] [Google Scholar]
- 4.United States Environmental Protection Agency. Multi-industry per- and polyfluoroalkyl substances (PFAS) study—2021 preliminary report. Accessed September 2023. https://www.epa.gov/system/files/documents/2021-09/multi-industry-pfas-study_preliminary-2021-report_508_2021.09.08.pdf
- 5.Salvatore D, Mok K, Garrett KK, et al. Presumptive contamination: a new approach to PFAS contamination based on likely sources. Environ Sci Technol Lett. 2022;9(11):983-990. doi:10.1021/acs.estlett.2c00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jian JM, Guo Y, Zeng L, et al. Global distribution of perfluorochemicals (PFCs) in potential human exposure source-A review. Environ Int. 2017;108:51-62. doi:10.1016/j.envint.2017.07.024 [DOI] [PubMed] [Google Scholar]
- 7.Ramírez Carnero A, Lestido-Cardama A, Vazquez Loureiro P, Barbosa-Pereira L, Rodríguez Bernaldo de Quirós A, Sendón R. Presence of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in food contact materials (FCM) and its migration to food. Foods. 2021;10(7):1443. doi:10.3390/foods10071443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Susmann HP, Schaider LA, Rodgers KM, Rudel RA. Dietary habits related to food packaging and population exposure to PFASs. Environ Health Perspect. 2019;127(10):107003. doi:10.1289/EHP4092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boisacq P, De Keuster M, Prinsen E, et al. Assessment of poly- and perfluoroalkyl substances (PFAS) in commercially available drinking straws using targeted and suspect screening approaches. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2023;40(9):1230-1241. doi:10.1080/19440049.2023.2240908 [DOI] [PubMed] [Google Scholar]
- 10.van Deelen G. Workers exposed to PFAS in a variety of industries. Environmental Health News. May 16, 2022. Accessed September 2023. https://www.ehn.org/pfas-in-workers-2657293887.html
- 11.Beesoon S, Genuis SJ, Benskin JP, Martin JW. Exceptionally high serum concentrations of perfluorohexanesulfonate in a Canadian family are linked to home carpet treatment applications. Environ Sci Technol. 2012;46(23):12960-12967. doi:10.1021/es3034654 [DOI] [PubMed] [Google Scholar]
- 12.Fromme H, Tittlemier SA, Völkel W, Wilhelm M, Twardella D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int J Hyg Environ Health. 2009;212(3):239-270. doi:10.1016/j.ijheh.2008.04.007 [DOI] [PubMed] [Google Scholar]
- 13.Agency for Toxic Substances and Disease Registry. Toxicological profile for perfluoroalkyls. Accessed September 2023. https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf
- 14.Agency for Toxic Substances and Disease Registry. PFAS in the US population. Accessed September 2023. https://www.atsdr.cdc.gov/pfas/docs/PFAS-and-the-US-Population-FS-H.pdf
- 15.Agency for Toxic Substances and Disease Registry. Toxicological profile for perfluoroalkyls. Accessed September 2023. https://www.atsdr.cdc.gov/toxprofiles/tp200.pdf
- 16.Per-and polyfluoroalkyl substances. Wikipedia. Accessed September 2023. https://en.wikipedia.org/wiki/Per-_and_polyfluoroalkyl_substances
- 17.Ji J, Song L, Wang J, et al. Association between urinary per- and poly-fluoroalkyl substances and COVID-19 susceptibility. Environ Int. 2021;153:106524. doi:10.1016/j.envint.2021.106524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji J, Song L, Wang J, et al. Association between urinary per- and poly-fluoroalkyl substances and COVID-19 susceptibility. Environ Int. 2021;153:106524. doi:10.1016/j.envint.2021.106524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valvi D, Højlund K, Coull BA, Nielsen F, Weihe P, Grandjean P. Life-course exposure to perfluoroalkyl substances in relation to markers of glucose homeostasis in early adulthood. J Clin Endocrinol Metab. 2021;106(8):2495-2504. doi:10.1210/clinem/dgab267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steenland K, Kugathasan S, Barr DB. PFOA and ulcerative colitis. Environ Res. 2018;165:317-321. doi:10.1016/j.envres.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod. 2009;24(5):1200-1205. doi:10.1093/humrep/den490 [DOI] [PubMed] [Google Scholar]
- 22.Cathey AL, Nguyen VK, Colacino JA, Woodruff TJ, Reynolds P, Aung MT. Exploratory profiles of phenols, parabens, and per- and poly-fluoroalkyl substances among NHANES study participants in association with previous cancer diagnoses. J Expo Sci Environ Epidemiol. 2023;33(5):687-698. doi:10.1038/s41370-023-00601-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scinicariello F, Buser MC, Balluz L, et al. Perfluoroalkyl acids, hyperuricemia and gout in adults: analyses of NHANES 2009-2014. Chemosphere. 2020;259:127446. doi:10.1016/j.chemosphere.2020.127446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gleason JA, Post GB, Fagliano JA. Associations of perfluorinated chemical serum concentrations and biomarkers of liver function and uric acid in the US population (NHANES), 2007-2010. Environ Res. 2015;136:8-14. doi:10.1016/j.envres.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 25.Borghese MM, Liang CL, Owen J, Fisher M. Individual and mixture associations of perfluoroalkyl substances on liver function biomarkers in the Canadian Health Measures Survey. Environ Health. 2022;21(1):85. doi:10.1186/s12940-022-00892-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin CY, Chen PC, Lin YC, Lin LY. Association among serum perfluoroalkyl chemicals, glucose homeostasis, and metabolic syndrome in adolescents and adults. Diabetes Care. 2009;32(4):702-707. doi:10.2337/dc08-1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genuis SJ, Birkholz D, Ralitsch M, Thibault N. Human detoxification of perfluorinated compounds. Public Health. 2010;124(7):367-375. doi:10.1016/j.puhe.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 28.Dzierlenga MW, Keast DR, Longnecker MP. The concentration of several perfluoroalkyl acids in serum appears to be reduced by dietary fiber. Environ Int. 2021;146:106292. doi:10.1016/j.envint.2020.106292 [DOI] [PubMed] [Google Scholar]
- 29.Çelik A, Eke D, Ekinci SY, Yıldırım S. The protective role of curcumin on perfluorooctane sulfonate-induced genotoxicity: single cell gel electrophoresis and micronucleus test. Food Chem Toxicol. 2013;53:249-255. doi:10.1016/j.fct.2012.11.054 [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Park HY, Jeon JD, et al. The modifying effect of vitamin C on the association between perfluorinated compounds and insulin resistance in the Korean elderly: a double-blind, randomized, placebo-controlled crossover trial. Eur J Nutr. 2016;55(3):1011-1020. doi:10.1007/s00394-015-0915-0 [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Shao X, Zhao B, et al. Neurotoxicity of perfluorooctanoic acid and post-exposure recovery due to blueberry anthocyanins in the planarians Dugesia japonica. Environ Pollut. 2020;263(pt B):114471. doi:10.1016/j.envpol.2020.114471 [DOI] [PubMed] [Google Scholar]