Abstract
Fermented foods (FFs) are part of the cultural heritage of several populations, and their production dates back 8000 years. Over the last ~150 years, the microbial consortia of many of the most widespread FFs have been characterised, leading in some instances to the standardisation of their production. Nevertheless, limited knowledge exists about the microbial communities of local and traditional FFs and their possible effects on human health. Recent findings suggest they might be a valuable source of novel probiotic strains, enriched in nutrients and highly sustainable for the environment. Despite the increasing number of observational studies and randomised controlled trials, it still remains unclear whether and how regular FF consumption is linked with health outcomes and enrichment of the gut microbiome in health‐associated species. This review aims to sum up the knowledge about traditional FFs and their associated microbiomes, outlining the role of fermentation with respect to boosting nutritional profiles and attempting to establish a link between FF consumption and health‐beneficial outcomes.
Mechanism for γ‐PGA electrofermentation (left); charge output and γ‐PGA production in electrofermentation (center, top); SEM of γ‐PGA‐containing EPS on carbon fiber in electrofermentation and conventional ferementation (center, bottom); (1H)‐NMR of γ‐PGA‐enriched EPS in electrofermentation (right).
INTRODUCTION
For centuries, food fermentation has been used to preserve foods and ameliorate their sensorial quality, safety and shelf‐life (Marsh et al., 2014). The first evidence of fermentation dates back 8000 years to cheese production (Ross et al., 2002), although recent evidence suggests that fermented foods (FFs) could have been consumed at the dawn of human evolution (Amato et al., 2021).
Since then, the mode of FF production has greatly evolved, owing to developments in technology, with the food industry moving from spontaneous to well‐controlled and even precision fermentations (Chai et al., 2022). Advances in research methods, including improvements in the capacity to isolate of microbial strains and the widespread use of “omics sciences to explore the genetic profiles the microbiomes”, have led to the selection of well‐characterised strains to be used as starter cultures, providing highly standardised FFs on a global scale.
However, in parallel to the industrialisation of practices associated with the production of some specific FFs, it is estimated that a broader range of ~5000 varieties of, primarily artisanally produced, FFs might be consumed nowadays worldwide (Tamang et al., 2020). These traditional FFs are predominantly embedded within local population cultures, harbouring a plethora of microbial species and strains that might be valuable after ad hoc screening and selection of their technologic and probiotic properties. Further research efforts are needed to better describe the microbial diversity within traditional FFs and depict the metabolic activities exerted by product‐specific microbiome.
Therefore, the purpose of this review is to summarise the knowledge about FFs and their associated microbiome, highlighting the relevance of food fermentation for humans, focusing on its technological and nutritional outcomes, as well as on the beneficial effects of FFs. Furthermore, we describe the microbial communities residing in traditional FFs worldwide, focusing on the role of each microbial group in the production and stability of the final product.
FERMENTATION AS A SUSTAINABLE TOOL TO ENSURE QUALITY AND SAFETY OF PERISHABLE PRODUCTS
According to the International Scientific Association for Probiotics and Prebiotics (ISAPP), FFs are “food made through desired microbial growth and enzymatic conversions of food components.” The fermentation process is among the most effective ways for extending raw material shelf‐life, especially for world areas with limited access to electricity (Marco et al., 2021). The utility of fermentation as a means food preservation is due to the biosynthesis of organic acids, alcohols, bacteriocins and other antimicrobials as a result of metabolism by the FF microbiome (Ross et al., 2002). Thus, during fermentation, multiple biochemical and physical modifications of food components occur, resulting in the improvement of food safety and shelf‐life. For example, during lactic acid fermentation, bacteria use six‐carbon mono‐ or oligosaccharides as carbon sources and mainly produce lactic acid (Amit et al., 2017), which leads to a drop in the pH (Zapaśnik et al., 2022), thus inhibiting several spoilage and pathogenic species that might be present in the raw materials. Besides this, alcoholic fermentation consists of the anaerobic conversion of sugars into ethanol and carbon dioxide (Buglass, 2011). High concentrations of ethanol might lead to cell death through disruption of membrane integrity, as well as altering the biosynthesis of essential components such as fatty acids, lipids and outer membranes (Liu & Qureshi, 2009). In some settings, alcoholic is followed by acetic fermentation, which involves the aerobic oxidation of ethanol to acetic acid (Gomes et al., 2018). This fermentation is the pivotal step in the production of vinegar and kombucha, albeit being undesirable in the production of wine and beer. Even though lactic and alcoholic are the most studied and employed form of food fermentation, a number of other fermentation types exist, for example, the malolactic and the alkaline fermentations. The former is relevant in wine ageing and consists of the conversion of malic acid (present in grapes) into lactic acid and carbon dioxide, with considerable consequences on sensory profile and long‐term stability of wines (Paramithiotis et al., 2022), whereas the latter involves protein hydrolysis with the release of ammonia, typically occurring in traditional high‐pH FFs from Africa and Asia such as nattō (Owusu‐Kwarteng et al., 2022). A plethora of microorganisms can be involved in food fermentation. Generally, Lactic Acid Bacteria (LAB) perform lactic fermentation, whereas yeasts and a few bacteria produce ethanol from sugar metabolism (Malakar et al., 2020). Furthermore, Acetic Acid Bacteria (AAB), a group comprising 19 genera from the Acetobacteriaceae family, can perform the acetic fermentation (Gomes et al., 2018; Table 1). During fermentation, the accumulation of metabolites, such as ethanol and acids, is not the sole mechanism protecting foods from harmful microbes. On the contrary, other beneficial compounds produced by fermenting microbes, or even their sole presence, might preserve food matrices. For example, several LAB species are known to excrete bacteriocins, short peptides with antimicrobial activity (De Filippis et al., 2020; Mokoena, 2017). Several studies have shown that bacteriocins are being active against foodborne pathogens, both in vitro and in situ. For example, Ye et al. (2021) recently characterised a novel bacteriocin produced by a Lacticaseibacillus paracasei (formerly Lactobacillus paracasei; Zheng et al., 2020) strain which is active against the foodborne pathogens Listeria monocytogenes and Salmonella typhimurium, while Martinez et al. (2016) assessed the inhibitory effect of nisin on the growth of Listeria monocytogenes and germination of Bacillus cereus spores in milk. Interestingly, several FFs are sources of strains belonging to species that have been shown to be bacteriocin‐producing. Indeed, the FFs' milk kefir (Petrova et al., 2021) and table olives (Hurtado et al., 2012) are frequently reported to be a source of bacteriocin‐producing LAB. This is also true of cheeses, with soft cheese harbouring greater amounts compared to hard and semihard cheeses (Trejo‐González et al., 2022). Besides bacteriocin production, food stability is further improved during fermentation due to the fact that technologically active bacteria frequently outcompete potential pathogenic and spoilage taxa (Rul & Monnet, 2015). Indeed, LAB can inhibit the growth of pathogens in FFs by competing for nutrients (Ibrahim et al., 2021; Vieco‐Saiz et al., 2019). For example, Martín et al. (2022) showed that the addition of strains of Lacticaseibacillus casei and Lactococcus garvieae during the production of a Spanish cheese effectively inhibited the growth of Listeria monocytogenes, while Siedler et al. (2020) showed that LAB species may counteract the growth of pathogens and spoilage microbes by depriving them of manganese.
TABLE 1.
Overview of the most common food fermentations.
| Fermentation | Main taxa involved | Main products | Main FFs produced |
|---|---|---|---|
| Lactic | Lactobacillaceae, Leuconostocaceae, Streptococcaceae | Lactic acid (homolactic), CO2, ethanol (heterolactic) | Dairy (yogurt, cheeses, kefir), sauerkraut, kimchi, pickles, tempeh, fermented meats |
| Alcoholic | Saccharomyces spp., Kloeckera spp. | Ethanol, CO2 | Wine, beer, kefir |
| Acetic | Acetobacter spp., Gluconacetobacter, Gluconobacter | Acetate, EPS | Chocolate, coffee, vinegar, specialty beers, water kefir |
| Propionic | Propionibacterium spp. | Propionate, acetate, CO2, succinate (Wood–Werkman pathway) | Swiss‐type cheeses |
In some FFs, microbial fermentation of the raw materials is necessary to make them edible. For example, multiple fermentations by a mix of yeasts, AAB and LAB are necessary to digest the pulp covering the cocoa beans and develop chocolate flavour precursors and antioxidants that would otherwise be absent (Goya et al., 2022; Rahardjo et al., 2022).
INNOVATIONS IN FOOD FERMENTATION TO IMPROVE GLOBAL HEALTH
Besides being a tool for improving the shelf‐life of foods, making them edible or safer for human consumption, fermentation can be considered one of the solutions to reduce the dramatically high environmental impact of the food industry. Indeed, fermentation is widely recognised as a sustainable process, with very low gas emission and a high production yield (Rastogi et al., 2022) and is a possible route to produce nutrient foods from alternative sources (e.g., legumes and vegetables), potentially offering alternatives to less sustainable foods. For instance, fermentation of protein‐rich foods such as legumes with LAB strains increases the concentration of bioactive compounds (Kårlund et al., 2020) and might ameliorate the amino acid profiles (Emkani et al., 2022), thus providing a sustainable and nutritionally valid alternative protein source compared to meats, as well as helping in fighting hunger and malnutrition in low‐income countries.
FFs have been a consistent part of human diets across various cultures for thousands of years, and different FFs and beverages are widespread across the world (Tamang et al., 2020). However, the production and consumption of FFs traditionally consumed only in specific countries are changing with globalisation (Rastogi et al., 2022). For instance, products obtained from fermented legumes (e.g., tempeh) are traditionally consumed in several Asiatic countries and are a consistent part of their cultural heritage, but have attracted greater interest from Western populations quite recently (Vinderola et al., 2023). While some of these FFs have continued to be produced using traditional methods of production, that is, depending on local resources and environmental conditions, most have undergone significant technological advancements in order to meet global demand (Panda & Shetty, 2018). Despite the limited scientific understanding of fermentation and microorganisms in the past, microbial communities in small‐scale fermentations using traditional methods have been identified (Holzapfel, 2002; Panda & Shetty, 2018). However, the challenge for scientists lies in managing large scale production without compromising the distinctive characteristics of the traditional products. Therefore, the characterisation of traditional fermentations followed by their optimisation and application on a large‐scale might represent a promising starting point towards a more sustainable food system, providing nutrient‐rich products (see Section: “Fermentation boosts the nutritional value of foods”) with limited waste produced. The need for novel sustainable food production strategies is pushing the food industry to explore paths for innovation in food fermentation. Whole‐genome sequencing and metabolic network reconstruction can help to identify safe and productive microbial strains prior to any in vitro test, overcoming the “trial and error” approach and leading us towards precision fermentation. These technologies open large‐scale frontiers, such as the production of nutrients and the development of appealing novel foods from previous waste streams (Jahn et al., 2023). Indeed, several companies are testing the production of food products with complete amino acid profiles and acceptable sensorial characteristics from microbial biomass, to use them for the production of surrogate meat (Humpenöder et al., 2022; Wackett, 2020) and dairy products (Linder, 2019). Therefore, revalorisation of agricultural wastes through cost‐effective fermentations at industrial scale might soon represent the turning point in fighting deficiencies in macro and micronutrients and in limiting the food industry environmental impact through production of stable and easy‐to‐delivery foods.
Overall, fermentation is a sustainable, efficient and low‐cost way to provide highly valuable, stable, safe and nutritional sources to vulnerable populations in low‐income countries, thus preventing malnutrition. This approach can further help to fulfil the United Nations Sustainable Development Goals (SDG) 2 and 3, which aim to end hunger and improve global health, respectively.
FERMENTED FOODS FROM THE WORLD, A TAXONOMIC CHARACTERISATION
Each area of the world has its own indigenous FFs. The wide differentiation in raw materials and production technologies employed is linked with the availability of local food sources and with the cultural and religious heritage of the populations. Therefore, fermented meats are far less widespread in Far East regions (e.g., Japan and Korea), which most frequently ferment rice, sprouts and soy, whereas fermentation of milk and cheese production mostly developed in areas where the pastoral practices were more common in ancient times, such as India and European countries (Tamang et al., 2020). For the same reason, fermented fish are traditionally consumed by populations living in coastal regions who generally rely on fishing, such as Scandinavian countries (Tamang et al., 2016), while sorghum‐, maize‐ and wheat‐based FFs are typical from Africa (Tamang et al., 2020). All of these foods host diverse microbial communities, for which differences and similarities remain undescribed.
In order to depict a map of worldwide FF microbiota, we screened the available literature. Only studies using 16S rRNA gene sequencing were included. The studies were further filtered considering the availability of raw fastq sequences and metadata for each sample. Furthermore, we only included samples that were not spoiled. According to these criteria, 72 studies were selected, with a total of 2013 samples (Table S1). Data were downloaded and analysed through the QIIME 2 pipeline (Bolyen et al., 2019; q2cli version 2020.11.1). More specifically, raw reads from each BioProject were denoised independently using dada2 (options “‐‐p‐chimera‐method pooled,” “‐‐p‐pooling‐method pseudo,” “‐‐p‐min‐fold‐parent‐over‐abundance 10” and “‐‐p‐max‐ee 2”), producing Amplicon Sequence Variants (ASVs). Therefore, the ASVs were mapped against the Greengenes 13_8 database (McDonald et al., 2012) using the command “qiime feature‐classifier classify‐consensus‐vsearch,” and taxonomic information was used to independently collapse the ASVs tables at species level. Finally, all the collapsed abundance tables were merged to perform statistical comparisons.
Overall, the most studied FFs are cheeses from European countries (mainly Italy, France and United Kingdom), with a total of 33 studies and 1158 samples. However, European fermented meats (sausages and salami, n = 7 studies and 101 samples) were also frequently studied (Figure 1).
FIGURE 1.
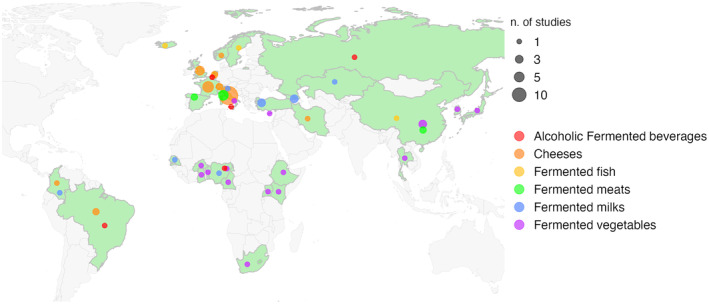
World map showing the number of studies included in the metataxonomic analysis focusing on traditional fermented foods from different countries (highlighted in green). Bubbles are colour‐coded according to the fermented food type. Bubble size is proportional to the number of different studies found in literature for each FF type.
Interestingly, the microbiota of Asian and African fermented vegetables and cereals were also frequently studied. The high humidity and temperature of tropical and subtropical countries in Asia and Africa can cause perishable products to spoil quickly. To overcome this, ancient populations fermented vegetables and cereals to extend their quality and ensure safety. These traditional methods have been passed down, leading to a variety of FFs still consumed in Asia and Africa (Swain et al., 2014). Moreover, 5 studies focused on fermented milk from Caucasian area (i.e., kefir and yogurt), although the microbial composition of local fermented milk from Nigeria, Senegal, Italy and Colombia was also characterised by single studies (Figure 1). Fermented fish and alcoholic fermented beverages were generally less characterised, with only 3 and 5 studies, respectively.
For each type of FF, Figure 2 shows the average composition of its microbiota (occurring in >25% of the samples of the group with a relative abundance >0.5%). Interestingly, Lactobacillus spp. (that includes also the genera arising from its recent reclassification, Zheng et al., 2020) is highly prevalent in all the groups except fermented fish, with an intragroup average abundance ranging from 4.68% (in fermented meats) to 32.7% (in fermented milk). The result is not surprising: Lactobacillus spp. have a pivotal role in food fermentations, given the massive phenotypic and genotypic diversity, which led to the selection of multiple species, well adapted to different food niches (Widyastuti et al., 2021).
FIGURE 2.
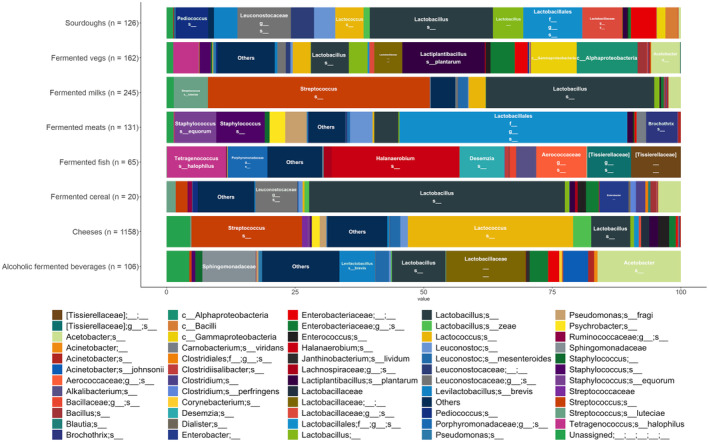
Bar plot showing, for each fermented food category, the average relative abundance of the taxa occurring in >25% of the group samples with an abundance >0.5%. All taxa with abundance <0.5% were included in “Others.”
However, apart from Lactobacillus spp., several group‐specific prevalent taxa can be identified. Indeed, Tetragenococcus halophilus, Halanaerobium sp. and members of the Tissierellaceae and Aerococcaceae families are exclusively found in fermented fish. The occurrence of the halophilic T. halophilus and Halanaerobium sp. (Kim et al., 2022; Lipus et al., 2017) can be explained by the addition of salt to fermented fish (e.g., a Chinese fish sauce and the Swedish Surströmming). Interestingly, a T. halophilus strain isolated from a Korean fermented soybean has been identified as a potential probiotic starter culture for salty fermented foods (Kim et al., 2022). Similarly, Tissierellaceae have been reported as dominant in hákarl, traditional Icelandic fermented shark also included in this review (Osimani et al., 2019, Table S1).
Lactiplantibacillus plantarum (formerly Lactobacillus plantarum) showed high prevalence and average relative abundance in fermented vegetables (that includes pickles, olives and fermented soybeans; Figure 2). Research reports this species as being dominant in spontaneously fermented vegetables (Filannino et al., 2016; Owade et al., 2021; Xiong et al., 2012; Yang et al., 2014), and a recent review summarised the protechnological role of L. plantarum during fermentation of vegetables, which included enhancement of flavour, competition against pathogens in silage and boost of nutritional properties (Yilmaz et al., 2022). Hence, some L. plantarum strains have been characterised in depth to produce fine‐tuned starter cultures widely used nowadays (Li et al., 2022; Zhang et al., 2022).
Fermented vegetables included in this review were also dominated by Acetobacter sp., being present in high abundance in cocoa beans, with an average relative abundance >25% (Figure 2). Acetobacter spp. perform acetic fermentation (see par. 1), described as a key process in chocolate production as it triggers a cascade of reactions resulting in the release of chocolate aroma precursors (Soumahoro et al., 2020). In addition, Acetobacter was also found in alcoholic fermented beverages, where it may cause spoilage. Indeed, when alcoholic concentration is <10%, Acetobacter spp. can oxidise ethanol into acetic acid, developing off‐flavours and ropiness in wines, beers and cider (Bisson & Walker, 2015; Kubizniaková et al., 2021). However, in some specific brewery technologies, a well‐controlled acetic acid fermentation by Acetobacter spp. might lead to the production of sour beers (Bouchez & De Vuyst, 2022).
Overall, fermented dairy products and meats harbour the lowest biodiversity (Figure 3).
FIGURE 3.
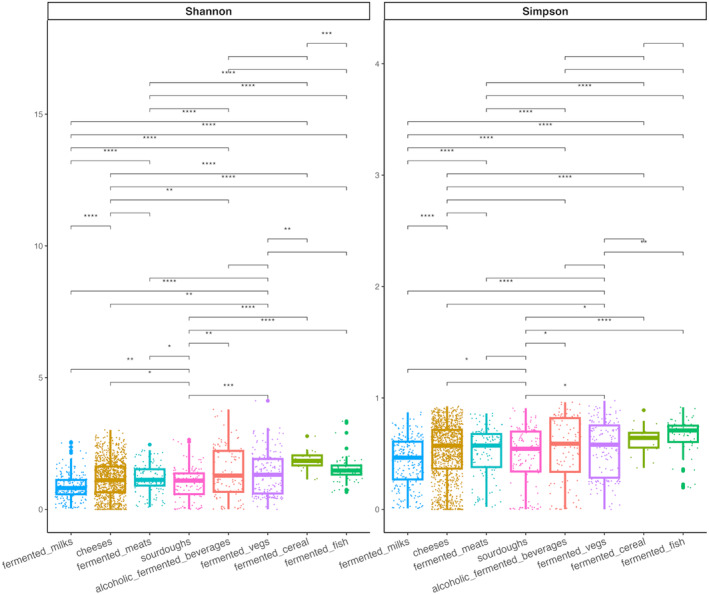
Box plot showing the Shannon's and Simpson's alpha diversity indices in each fermented food group. The means of the indices are compared across the groups using paired Wilcoxon's rank‐sum test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
This suggests that these products host a selected microbiota and is consistent with observations that these foods are mainly composed of Lactobacillus, Lactococcus and Streptococcus in dairy products and Lactobacillaceae, Staphylococcaceae and Brochotrix in fermented meats. While the main genera occurring in dairy products are involved in fermentation and are recognised as “Generally Recognized As Safe” (GRAS) and/or the “Qualified Presumption of Safety” (QPS) status in the United States and UE, respectively, some of the microorganisms present in fermented meats might cause spoilage (e.g., Brochothrix, linked with off‐odours and discoloration; Stanborough et al., 2017) or raise safety concerns. Generally, fermented meats are populated by coagulase‐negative staphylococci (CNS), differing from the coagulase‐positive staphylococci (e.g., Staphylococcus aureus) that include human pathogens (Lee et al., 2018). The CNS group includes S. carnosum, S. xylosus and S. equorum, a bacterium firstly isolated from a healthy horse which enhances the colour of fermented meats through reduction of nitrates to nitrites. In addition, reports suggest that this species might influence the sensorial profile of sausages and cheeses through the production of butan‐3‐diol, acetoin and diacetyl (Irlinger et al., 2012; Lee et al., 2018; Stavropoulou et al., 2018), generating pleasant or off‐flavours depending on the ratio between these molecules and other volatile organic compounds (VOCs). However, it was also proved that S. equorum strains isolated from FFs may become resistant to multiple antibiotics through acquisition of mobile genetic elements (Heo et al., 2022).
As the samples were not reported as spoiled in the original publications, the high abundance of Brochothrix sp. and Pseudomonas fragi, coming from the raw meat used and considered as the main meat spoilers (Sequino et al., 2022), might be counteracted by Lactobacillaceae (Barcenilla et al., 2022; Favaro & Todorov, 2017; Zhang et al., 2018).
Despite the limited taxonomic resolution of 16S rRNA sequencing‐based approaches (Gupta et al., 2019), we provided valuable information about the average taxonomic composition and microbial diversity of several FF categories. Although not exhaustive, these results show that LAB are widespread in different FFs from all over the world, highlighting their key role in defining the sensory characteristics and protecting FFs from spoilage and pathogenic microorganisms. Besides LAB, each FF group showed its own microbial community that resulted from the composition of the raw materials (Leech et al., 2020), from the production environment (De Filippis, Valentino, et al., 2021) and from the selection occurring during the specific fermentation, as a result of the technological parameters applied.
FERMENTATION BOOSTS THE NUTRITIONAL VALUE OF FOODS
Fermentation can also boost nutritional properties of the food matrices by either enhancing mineral bioavailability or reducing the concentration of toxic molecules such as mycotoxins (Adebo et al., 2019). Yogurt is one of the most studied FF, and research data suggest that fermentation of milk into yogurt increases the concentration and the bioavailability of several essential minerals, such as calcium and potassium (Hadjimbei et al., 2022). In addition, LAB may synthetise group B vitamins—mainly B2 and B12—which are essential vitamins for humans (Fabian et al., 2008).
Similarly, Lactobacillus kefiranofaciens produces kefiran, a peculiar exopolysaccharide (EPS) of kefir lacking in milk, which shows both prebiotic and protechnological properties (Moradi & Kalanpour, 2019; Vieira et al., 2021).
The enrichment in nutrients as a consequence of fermentation has been also observed in different food matrices. Indeed, Verni et al. (2019) showed that fermentation of faba bean flour with a Lactiplantibacillus plantarum strain improved the release of essential free amino acids and their bioactive peptide derivatives such as γ‐aminobutyric acid (GABA; Verni et al., 2019), a neurotransmitter often linked with stress reduction and benefiting brain health (see Section: “Fermented foods and their effects on human health through the gut microbiome: evidence from clinical trials”). Moreover, Jiang et al. (2021) observed similar results after fermentation of corn gluten meal and wheat bran with Lb. delbrueckii subsp. bulgaricus and Lb. acidophilus. Generally, improvements in the amino acid composition of FFs can be linked to LAB, which show a wide range of exo‐ and endopeptidases needed to release the free amino acids necessary for their growth (Kieliszek et al., 2021).
In addition, a decrease in antinutritional compounds, allergens or other molecules that can cause gastrointestinal discomfort may occur after food fermentation. Indeed, several LAB harbour the β‐galactosidase (lactase) enzyme, which hydrolyses lactose into glucose and galactose. Consequently, fermented milk products like yogurt and long‐ripened cheeses contain little or no lactose, which makes them suitable for lactose‐intolerant people (Melini et al., 2019). Moreover, the action of LAB proteases and peptidases on milk proteins such as alpha‐lacto‐albumin and beta‐lacto‐globulin enhances the release of short bioactive peptides with radical scavenging, metal chelating and peroxidation‐preventing activities, protecting human cells from oxidative stress (Pessione & Cirrincione, 2016). Several Lb. helveticus strains produce lactotripeptides (e.g., Val‐Pro‐Pro and Ile‐Pro‐Pro) with antihypertensive activities (Raveschot et al., 2018), and it is also known that Lactobacillus spp. proteolytic systems lead to the release of peptides enhancing the activity of anti‐inflammatory cytokines involved in allergies and macrophage phagocytosis. Importantly, the biosynthesis of most of the bioactive peptides can be linked to Lactobacillus spp. (and all genera arising from its reclassification, Zheng et al., 2020), which generally encode more peptidases, proteases and transport systems than other LAB, such as Lactococcus spp. (Pessione & Cirrincione, 2016). Also, these proteolytic systems provide LAB with the ability to release free amino acids (Nielsen et al., 2022). Indeed, all these reports highlighted the role of fermentation in improving the nutritional value of foods, possibly contributing to prevent malnutrition and contribute to well‐being, as auspicated in the SDG3.
Fermentation may also reduce antinutritional compounds naturally occurring in the raw materials. As an example, cereals and legumes naturally have high concentration of phytates, derivatives of phytic acids that are negatively charged and considered antinutritional compounds as they are able to bind positively charged minerals (e.g., zinc, calcium and iron), thus chelating them and reducing intestinal absorption upon consumption. It has been shown that fermentation results in phytate reduction, and several phytase‐producing strains from both bacterial and fungal species have been identified (Greppi et al., 2015; Mohammadi‐Kouchesfahani et al., 2019). Similarly, food fermentation of vegetable raw materials facilitated by Lactobacillaceae can denature cyanogenic glycosides, a class of toxic compounds present in >2000 plant species which causes acute intoxication (Bolarinwa et al., 2016).
Furthermore, several studies highlighted the ability of Lactobacillus and Bifidobacterium spp. probiotic strains to denature the indigested gliadin‐derived peptides, responsible of the immune response in celiac disease, thus suggesting an exciting possibility of exploiting fermentation to produce gluten‐free products (Norouzbeigi et al., 2020).
FERMENTED FOODS: AN EXPLOITABLE SOURCE OF POTENTIALLY PROBIOTIC STRAINS
According to the ISAPP, probiotics are “live microorganisms which confer a health benefit on the host when administered in adequate amounts” (Hill et al., 2014). As such, microbial strains have to satisfy several criteria to be considered probiotic. First of all, a probiotic candidate must be safe for its intended use to be administered to humans (Sanders et al., 2010), and the safety of an isolate has to be proven at strain‐level (European Food Safety Authority, 2007). However, some taxonomic groups are not of concern from a pathogenicity perspective as research and a long history of use has established their safety. These microbial groups have gained the aforementioned GRAS and/or QPS status, which provides a simplified pathway for the application of isolates from widely recognised safe species. Secondly, beneficial activities of putative probiotics must be proven by multiple randomised double‐blind placebo‐controlled trials, which represent the gold standard in intervention studies (Misra, 2012). Moreover, high‐quality systematic reviews can provide evidence of a causal effect between probiotic administration and health effects (Hill et al., 2014). Finally, beneficial outcomes on human health are dose‐dependent, therefore probiotics should reach the body site where the positive action is exerted (e.g., the gut) in adequate amounts.
Given these assumptions, FFs represent an optimal source of potentially probiotic strains, although fermentation does not necessarily lead to the selection of probiotics, therefore the terms “fermented food” and “probiotic” cannot be used interchangeably (Marco et al., 2021; Vinderola et al., 2023). Firstly, most of the confirmed probiotic strains belong to genera often involved in food fermentations such as the former Lactobacillus and Lactococcus (Lavefve et al., 2019). Indeed, several species from these genera have been assigned QPS and GRAS status, given the long history of safe consumption and commensalism with humans. Streptococci represent an exception: Although S. thermophilus is frequently involved in food fermentations and probiotics have been characterised from this species, the genus also includes some human pathogens (Plummer et al., 2021). However, phylogenetic studies suggest that S. thermophilus followed a separate evolutionary path from other Streptococcus species, leading to the loss of virulence genes (De Filippis et al., 2020).
Microbial strains and whole communities might reach loads up to 108 CFU/g in some traditional FFs (Leeuwendaal et al., 2022; Rezac et al., 2018). This might ease the technological process of isolating autochthonous potentially probiotic strains with an enhanced growth rate. Once isolated and characterised, these strains might be inoculated into food matrices with chemical and physical properties that mimic those of the isolation source. In such a way, the FF might become a carrier of a new probiotic (Papadopoulou et al., 2023), which might in turn reach the human gut in adequate amounts to have a beneficial impact. This strategy, also known as “probiotication,” is widely used for the production of dairy products (Mojikon et al., 2022), but recent advances aim to extend it to the production of plant‐based FFs (Di Cagno et al., 2013; Kumar et al., 2015; Mustafa et al., 2019).
After ingestion, one desirable trait of probiotic strains is to tolerate adverse conditions encountered through the gastrointestinal tract that might pose a limit to the survival of an adequate number of cells. For example, microorganisms targeting the human gut should tolerate acid environments and high concentrations of bile salts and digestive enzymes (e.g., lipases, amylases and nucleases) while transiting through the stomach and duodenum, respectively. Interestingly, Wang et al. (2018) isolated >30 LAB strains resistant to low pH and high bile concentrations from spontaneously fermented vegetables and meats, similarly to Meena et al. (2022), who found 6 candidate probiotic strains of Lb. delbrueckii subsp. bulgaricus and Lacticaseibacillus rhamnosus in fermented cereals from India. To explain this, Di Cagno et al. (2013) suggested that autochthonous and potentially probiotic strains from fermented and non‐fermented vegetables might naturally show resistance to stressful environmental conditions thanks to some traits shared between their isolation source and the gut, such as the high acidity and concentration of antinutritional components. Interestingly, some structures produced by LAB such as exopolysaccharides (EPS) can be both an important component for the texture of certain fermented foods (Han et al., 2016) and a tool to protect cells from environmental stress (De Filippis et al., 2020).
Another desirable trait of probiotic strains is adherence to the host gut epithelium, which promotes its persistence in the gut after the consumption. Several cell structures can equip probiotics with this ability; membrane receptors like lipoteichoic acids (LTA) and mucin‐binding proteins spread in some LAB genera enhance the bonds between the microorganism and the glycosylated part of the gut mucin (Monteagudo‐Mera et al., 2019). Overall, these traits are highly prevalent within the species commonly found in FFs (De Filippis et al., 2020; Garcia‐Gonzalez et al., 2018), although defining the ability of probiotics to establish in the gut is still challenging (Roselli et al., 2021). However, recent evidence suggests that either a permanent or transitory establishment of microorganisms introduced with FFs in the gut might occur. For example, a large‐scale analysis of LAB genomes reconstructed from gut and FF metagenomes highlighted that for several LAB species (e.g., S. thermophilus and Lc. lactis), genomes from the two environments have a high genomic similarity, suggesting that the intake of FFs might be the primary source of these species in the human gut (Pasolli et al., 2020). This hypothesis was further supported by focusing on the LAB prevalence in different cohorts, which showed that several Leuconostocaceae and Weissellaceae taxa, commonly found in traditional Asiatic fermented vegetables and cereals, were more frequently detected in the gut metagenomes of non‐Westernised cohorts (e.g., Far East populations).
Several factors might contribute to defining the extent of establishment in the gut. Firstly, strain‐specific metabolic adaptive traits can be involved in engraftment in the human gut. Indeed, it was shown that Lactiplantibacillus plantarum 299v is able to adapt its metabolic potential towards carbohydrate hydrolysation and production of compounds involved in adherence (Derrien & van Hylckama Vlieg, 2015). Furthermore, the personalised gut microbiome response can influence the establishment of food‐introduced microbes. Maldonado‐Gómez et al. (2016) observed that the engraftment of a Bifidobacterium bifidum strain was strongly dependent on the gut microbiome composition of the individuals and favoured by the presence of phylogenetically related taxa in the gut. However, the mechanisms underlying this interaction are mostly not yet explained. Lastly, the persistence of a strain in the gut is also strongly linked with the intake. Indeed, it was estimated that the consumption of 1010 cells might alter the gut microbiota composition temporarily, potentially modulating its immune and neuroendocrine activities in the short term (Derrien & van Hylckama Vlieg, 2015).
Notably, even though the human gut still remains the main source of commercially available probiotic strains (i.e., those strains with beneficial effects supported by clinical trials; Hill et al., 2014), some probiotics have been isolated from FFs. Indeed, Lb. helveticus Rosell‐52, a strain that can contribute to ameliorate immune functions and modulate the gut–brain axis (Murina et al., 2021), was firstly isolated from dairy‐fermented products (McFarland, 2015), while Lacticaseibacillus rhamnosus HN001 originates from cheese (Ceapa et al., 2015). Besides bacteria, some yeast strains commonly found in FFs have been screened for probiotic activities. For example, Saccharomyces cerevisiae var. boulardii was first isolated from lychee and mangosteen, and is frequently detected in kombucha and kefir (Ansari et al., 2023). Collectively, these recent outcomes suggest that FFs might represent an underexplored source of highly competitive, stress‐tolerant and easy‐to‐propagate strains with potential beneficial outcomes on human health, therefore further efforts should focus on the screening of FF microbial communities for novel probiotics.
FERMENTED FOODS AND THEIR EFFECTS ON HUMAN HEALTH THROUGH THE GUT MICROBIOME: EVIDENCE FROM CLINICAL TRIALS
The gut microbiome, a diverse and dynamic community of microorganisms that inhabit the human gastrointestinal tract, has a crucial function in host physiology and state of health (De Filippis et al., 2018). For instance, a disequilibrium in the gut microbiome composition may lead to abnormalities in the immune system, resulting in diseases such as obesity, diabetes, allergies and inflammatory bowel disease (Lin & Zhang, 2017). Among the variety of factors, food is thought to be one of the most important variables in modulating the gut microbiome throughout life (Dominianni et al., 2015; Wang et al., 2020). Indeed, FFs that are rich in living and/or inactivated microorganisms and nutritional components released from fermentation, may modulate the gut microbiome (Zhang et al., 2016). In order to investigate how consuming FFs may have a crucial role on human health, we performed a search using the MeSH (Medical Subject Headings) terms in the PubMed database (https://pubmed.ncbi.nlm.nih.gov/) with the query reported in Appendix S1. This search resulted in about 59,000 results when filtered by year (from 1980 to 2023) in March 2023. Abstracts were screened to include observational and intervention studies that used qPCR, DGGE, 16S rRNA and shotgun sequencing to analyse the gut microbiome and evaluated health outcomes; although it is clear that gut microbiome analyses and health outcome investigations are not always conducted within the same study. Interventions consisting of only probiotics were also not considered in this review. As reported above, the fermentation process might improve nutritional value of the FFs beyond mere nourishment. This hypothesis is supported by human clinical investigations on FFs, as reported in Tables 2 and 3, summarising observational and intervention studies. In particular, Table 2 summarises observational studies on the effects of diets containing different FFs on gut microbiome and associated health benefits. In this case, the studies vary in the whole diet composition of the participants, as well as the study design, the control group used for comparison, the participant characteristics and health‐related parameters measured. Smith‐Brown et al. (2016) investigated the effect of a diet rich in dairy products, including cheese and yogurt, on the gut microbiome of a cohort of 37 normal‐weight children, comparing them with a plant‐based diet group. The study reports changes in the relative abundance of several bacterial taxa, including Streptococcus thermophilus, Erysipelatoclostridium ramosum and Faecalibacterium prausnitzii; however, no health effect was monitored. Taylor et al. (2020) evaluated the effect of the habitual consumption of a diet rich in various plant‐based FFs on the gut microbiome, compared with a group of non‐consumers. The study reports differences in the relative abundance of several bacterial taxa in the gut microbiome, including several taxa with a potential positive effect on human health, such as Bacteroides, Dorea, Prevotella and Faecalibacterium prausnitzii, which were higher in the consumer group. These taxa have been suggested as potential next‐generation probiotics (NGPs), that are “microbial taxa that conform to the traditional definition of probiotics, but do not have an history of use for health promotion.” (De Filippis et al., 2022). Anyway, the metabolome of FFs consumers was also analysed in the same study (Taylor et al., 2020), finding an enrichment in conjugated linoleic acid, a molecule supposed to be health‐promoting (Marangoni et al., 2020). Another observational study by González et al. (2019) demonstrated an increase of taxa suggested as NGPs upon FFs consumption. In particular, Akkermansia muciniphila, a promising NGP (Zhang et al., 2019), increased in consumers of yogurt (González et al., 2019). Moreover, in the same study, yogurt consumption was positively correlated with healthier metabolic profile in terms of lower inflammation and serum lipid peroxidation. In Table 3, several studies on the effects of dietary intervention with FFs on the gut microbiome and health outcomes are summarised. The studies vary in the FF matrices analysed and in the daily quantity consumed, as well as in the study design, participant characteristics and health outcome targeted. These studies suggest that FFs, such as kefir, kombucha, yogurt and fermented milk, may have a positive effect on gut microbiome composition, reducing the abundance of detrimental bacteria and increasing the abundance of beneficial taxa. A visualisation of microbial genera that increase after intervention, based on the results from studies reported in Table 3, is shown in Figure 4.
TABLE 2.
Observational studies investigating the effects of fermented food consumption on human health.
| Diet and fermented food matrix | Brief diet description | Study design | Control group | Participants | Participant characteristics | Gut microbiome analysis method | Variation in gut microbiome detected | Health outcome targeted | Health outcome achieved | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Lacto‐fermented vegetables | Balanced diet including a 2‐year‐long daily consumption of lacto‐fermented vegetables | Cohort, comparison of consumer groups versus non‐consumers | Non‐consumers | 47 | Male (29.79%), adults (20–50 years), normal‐weight | 16S rRNA and ITS2 |
Bacteria: ↑ Leuconostoc mesenteroides, Parabacteroides distasonis, Lachnospira, Ruminococcaceae, Coproccocus, Blautia, Clostridiales, Coprobacillus, Alphaproteobacteria Fungi: ↑ Rhodotorula mucilaginosa, Penicillium, Starmerella, Cryptococcus laurentii and Vishniacozyma carnescens |
None | ‐ | Guse et al. (2023) |
| Dairy products | Balanced diet including cheese, yogurt and dairy milk among fermented foods high‐intake | Cohort, comparison of groups with high versus low intake of FFs | Plant‐based diet | 37 | Male (56.76%), children (2–3 years), normal‐weight | 16S rRNA |
↑ Streptococcus salivarius subsp. thermophilus, Erysipelatoclostridium ramosum, Lachnoclostridium ↓ Faecalibacterium prausnitzii, Fusicatenibacter, Alistipes, Bacteroides, Parabacteroides |
None | ‐ | Smith‐Brown et al. (2016) |
| Dairy products | Balanced diets including yogurt, natural yogurt, sweetened yogurt, cheese, matured/semi‐matured cheese, fresh cheese, fermented milk | Cross‐sectional, comparison of consumer groups versus non‐consumers | Non‐consumers | 130 | Male (29.23%), adults (58.18 ± 17.10 years), normal‐weight and overweight | qPCR |
↑ Akkermansia muciniphila ↓ Bacteroides |
Cardiometabolic Disease and metabolic syndrome |
Yes | González et al. (2019) |
| Fermented milk | Balanced with a fermented milk high‐intake | Case–control, comparison of groups affected by ASD versus not affected | Children not affected by ASD | 46 | Male (100%), children (4–9 years), normal‐weight and obese | 16S rRNA | ↑ Clostridia, Lactobacillus, Blautia, Anaerostipes and Fusicatenibacter | Autism spectrum disorder (ASD) | Yes | Tomova et al. (2020) |
| Fermented products | Diet including yogurt, wine, sauerkraut, pickled vegetables, kombucha, kimchi and beer, aside from fermented plants | Cohort, comparison of consumer groups versus non‐consumers | Non‐consumers | 115 | Male (52.60%), adults (19–70 years), normal‐weight | 16S rRNA | ↑ Bacteroides, Pseudomonas, Dorea, Lachnospiraceae, Prevotella, Alistipes putredinis, Oscillospira, Enterobacteriaceae, Fusobacterium, Actinomyces, Achromobacter, Clostridium clostridioforme, Faecalibacterium prausnitzii, Bacteroides uniformis, Clostridiales and Delftia | None | ‐ | Taylor et al. (2020) |
| Yogurt and Probiotic Fermented Milk (PFM) | Not available | Cohort, comparison of consumer groups versus non‐consumers | Non‐consumers | 272 | Male (49.26%), adults (25–50 years), normal‐weight and overweight | 16S rRNA | ↑ Bifidobacterium | None | ‐ | Redondo‐Useros et al. (2019) |
| Asian and African diet | Lacto‐vegetarian diet | Cross‐sectional, comparison between Asian versus African diet | Control‐based Asian/African | 100 | Male (0%), adults (16–35 years), normal‐weight | 16S rRNA |
↑ Bifidobacterium, Succinivibrio and Escherichia‐Shigella in Asian population ↑ Ruminococcus, Lachnospiraceae, Succinivibrio and Faecalibacterium in African population |
None | ‐ | Tang et al. (2019) |
| Kazakh nomads diet | High‐fat, high‐meat and low‐vegetable diet | Cross‐sectional, comparison among interindividual variations | Control‐based interindividual variations | 29 | Male (37.93%), adults (6–84 years), normal‐weight and overweight | 16S rRNA | ↑ Bifidobacterium and Collinsella aerofaciens | None | ‐ | Li et al. (2019) |
| Rural Saudi Arabian diet | Diet characterised by six plant‐based fermented foods, called Lohoh in the local language | Cross‐sectional, comparison between rural versus urban Arabian diet | Urban Saudi Arabian diet | 28 | Male (92.86%), adults (>18 years), weight not reported | 16S rRNA | ↑ Verrucomicrobaeota, Acetobacter, Mycoplasma, Treponema berlinense and Treponema succinifaciens | None | ‐ | Angelakis et al. (2016) |
| Sakura diet | Not available | Cohort, comparison among interindividual and intraindividual variations | Control‐based interindividual and intraindividual variations | 10 | Male (50.00%), adults (men, 37.2 years; women, 38.2 years) normal‐weight | 16S rRNA | ↑ Bifidobacterium | None | ‐ | Hisada et al. (2015) |
TABLE 3.
Intervention studies investigating the effects of fermented food consumption on human health.
| Diet and fermented food matrix | Daily quantity of fermented food | Study design | Control group | Participants | Participant characteristics | Length of intervention | Wash out | Gut microbiome analysis method | Variation in gut microbiome detected | Health outcome targeted | Health outcome achieved | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| High‐fermented foods diet (cottage cheese, kefir, kombucha, vegetable brine drinks, vegetables, yogurt, other foods and drinks) | 6 servings | Randomised, prospective | High‐fibre diet (fruits, grains, legumes, nuts/seeds, vegetables, meat, dairy and other) | 36 | Male (30.56%), adults (52 ± 11 years), normal‐weight | 17 weeks | No | 16S rRNA and Shotgun |
↑ Ruminococcaceae and Streptococcaceae ↓ Lachnospira |
Inflammation | Yes | Wastyk et al. (2021) |
| Fermented milk | 80 g | Cross‐over trial | No intervention | 35 | Male (60.00%), children (7–10 years), normal‐weight and overweight | 4 weeks | Yes | 16S rRNA | ↑ Bacteroides ovatus, Lachnospira and Ruminococcus | Obesity | No | Joseph et al. (2020) |
| Fermented milk | 100 g | Before/after trial | Control‐based before/after | 21 | Male (33.33%), adults (18–25 years), normal‐weight | 2 weeks | Yes | 16S rRNA | None | None | ‐ | Khine et al. (2019) |
| Fermented milk | 100/300 g | Randomised, double‐blind, placebo‐controlled trial | Acidified milk | 96 | Male (45.83%), adults (18–55 years), normal‐weight | 4 weeks | Yes | 16S rRNA and Shotgun | None | None | ‐ | Alvarez et al. (2020) |
| Fermented milk | 110 g | Randomised, double‐blind, placebo‐controlled trial | Pasteurised acidified milk | 25 | Male (36.00%), adults (treated, 36.9 ± 6.9 years; control, 36.5 ± 6.1 years), normal‐weight | 10 weeks | No | 16S rRNA |
↑ Collinsella, Lactobacillus, Blautia and Ruminococcus ↓ Bacteroides, Parabacteroides, Prevotella and Oscillospira |
Cedar pollinosis | Yes | Harata et al. (2017) |
| Fermented milk | 250 g | Randomised, double‐blind, parallel and controlled trial | Non‐fermented milk product | 106 | Male (35.80%), adults (18–65 years), normal‐weight | 2 weeks | Yes | 16S rRNA | None | Irritable bowel syndrome | No | Le Nevé et al. (2019) |
| Fermented milk | 250 g | Randomised, double‐blind, parallel and placebo‐controlled trial | Acidified milk | 28 | Male (0%), adults (20–69 years), weight not reported | 4 weeks | Yes | Shotgun |
↑ Clostridiales ↓ Bilophila wadsworthia |
Irritable bowel syndrome | Yes | Veiga et al., 2014 |
| Fermented milk | 180 g | Double‐blind, randomise, placebo‐controlled, multicentric trial | Heat‐treated fermented milk | 30 | Gender not reported, adults (18–65 years), weight not reported | 4 weeks | Yes | 16S rRNA and qPCR | ↑ Streptococcus thermophilus (short term, both treatment and control) | Irritable bowel syndrome | No | Bogovič Matijašić et al. (2016) |
| Fermented milk | 140 g | Before/after trial | Control‐based before/after | 6 | Male (0%), adults (20–24 years), normal‐weight | 3 weeks | Yes | 16S rRNA |
↑ Bacteroidaceae and Prevotellaceae ↓ Ruminococcaceae and Lachnospiraceae |
None | ‐ | Unno et al. (2015) |
| Fermented milk | 600 mL | Randomised, double‐blind and placebo‐controlled trial | Placebo | 18 | Male (100%), adults (21.6 ± 0.8 years), normal‐weight | 2 days | No | Not detected | ‐ | Muscle soreness | Yes | Iwasa et al. (2013) |
| Fermented milk | 125 g | Randomised, controlled, parallel trial | Non‐fermented milk and no intervention | 36 | Male (0%), adults (18–55 years), normal‐weight | 4 weeks | No | 16S rRNA | None | Brain intrinsic activity or emotional attention | Yes | Tillisch et al. (2013) |
| Fermented milk | 80 mL | Randomised, placebo‐controlled, double‐blind trial | Placebo | 72 | Male (26.39%), (85 years), normal‐weight | 6 months | No | qPCR |
↑ Bifidobacterium, Lactobacillus ↓ Clostridium difficile, C. perfringens, Enterobacteriaceae, Staphylococcus and Pseudomonas |
Infection control, bowel movement normalcy | Yes | Nagata et al. (2016) |
| Yogurt | 125 g | Cross‐over trial, double blind | Pasteurised yogurt | 79 | Male (40.51%), adults (mean 23.6 years), normal‐weight | 2 weeks | Yes | DGGE and qPCR |
LAB and C. perfringens ↓ Bacteroides |
None | ‐ | García‐Albiach et al. (2008) |
| Yogurt | 500 g | Before/after trial | Control‐based before/after | 20 | Male (20.00%), adults (30 ± 5 years), normal‐weight | 2 weeks | Yes | DGGE and qPCR |
↑ LAB and Clostridium perfringens ↓ Bacteroides, Prevotella, Porphyromonas |
None | ‐ | Vázquez et al. (2013) |
| Yogurt | 220 g | Randomised, controlled trial | Milk | 92 | Male (0%), adults (milk, 51.2 ± 10.2 years; yogurt, 48.9 ± 10.5 years), obese | 24 weeks | Yes | 16S rRNA |
↑ Phascolarctobacterium ↓ Bacillaeota, Clostridiales, Blautia, Eubacterium ventriosum, Erysipelotrichaceae, Ruminococcus, Pseudobutyrivibrio, Dialister |
Metabolic syndrome | Yes | Chen et al. (2019) |
| Yogurt | 400 g | Randomised, double‐blind, cross‐over trial | Acidified milk | 14 | Male (100%), adults (18–40 years), normal‐weight | 2 weeks | Yes | 16S rRNA | None | Postprandial inflammation | No | Burton et al. (2017) |
| Yogurt | 250 g | Before/after trial | Control‐based before/after | 135 | Male (31.11%), adults (18–40 years), normal‐weight | 30 days | No | 16S rRNA | ↑ Bifidobacterium, Streptococcus, Actinobacteraeota and Erysipelotrichaceae | None | ‐ | Volokh et al. (2019) |
| Yogurt | 250 g | Open label trial, longitudinal over pregnancy and after delivery | No intervention | 56 | Male (0%), adults (18–40 years), pregnant | 88 ± 31 days | No | 16S rRNA |
Mother's microbiota: no effects Newborns: ↑ Bifidobacterium ↓ Enterobacteriaceae |
Undernourishment |
Mother's microbiota: no Newborn faeces: yes |
Bisanz et al. (2015) |
| Kefir | 180 mL | Randomised, parallel and controlled trial | Milk | 22 | Male (27.27%), adults (18–65 years), normal‐weight and overweight | 12 weeks | No | 16S rRNA | ↑ Actinobacteria | Metabolic syndrome | Yes | Bellikci‐Koyu et al. (2019) |
| Kefir | 500 mL | Non‐randomised, uncontrolled intervention study | None | 20 | Male (40%), adults (27–78 years), normal‐weight | 4 weeks | No | Not detected | ‐ | Functional constipation | Yes | Turan et al. (2015) |
| Kefir | 500 mL | Randomised controlled trial, double‐blind | Milk | 82 | Male (56.10%), children and adults (12–46 years), normal‐weight | 2 weeks | No | Not detected | ‐ | Dyspepsia and H. pylori infection | Yes | Bekar et al. (2011) |
| Kefir | 508 mL (plain kefir); 519 g (raspberry‐flavoured kefir) | Randomised control trial, cross‐over | Low‐fat cow milk; plain yogurt; flavoured yogurt | 15 | Male (53.33%), adults (20–34 years), normal‐weight | 5 days | No | Not detected | ‐ | Lactose malabsorption | Yes | Hertzler and Clancy (2003) |
| Kefir | 75–150 mL | Randomised control trial, double‐blind | Heat‐treated kefir | 125 | Male (51.20%), children (1–5 years), normal‐weight | 2 weeks | No | Not detected | ‐ | Antibiotic‐associated diarrhoea | No | Merenstein et al. (2009) |
| Kefir | 400 mL | Randomised control trial | No intervention | 45 | Male (51.11%), adults (19–68 years), normal‐weight | 4 weeks | No | qPCR | None | Irritable bowel disease | Yes | Yilmaz et al. (2020) |
| Kefir | 1600 mg (supplemented with 1500 mg of CaCO3) | Randomised, parallel, controlled, double‐blind trial | Unfermented raw milk | 40 | Male (43.80%), adults (control group, 67.94 ± 8.37 years; kefir group 64.08 ± 14.51 years), normal‐weight | 6 months | No | Not detected | ‐ | Osteoporosis | Yes | Tu et al. (2015) |
| Parmesan | 45 g | Before/after trial | Parmesan with milk | 20 | ‐ | 2 weeks | Yes | 16S rRNA and Shotgun | None | None | ‐ | Milani et al. (2019) |
| Cured meats and cheeses | Ad libitum | Cross‐over trial | Plant‐based diet | 20 | Male (60%), adults (21–33 years), normal‐weight | 15 days | Yes | 16S rRNA and ITS |
↑ Alistipes, Bilophila and Bacteroides ↓ Roseburia, Eubacterium rectale and Ruminococcus bromii |
None | ‐ | David et al. (2014) |
| Chungkookjang (Korean fermented soy product) | 35 g | Randomised, placebo‐controlled, cross‐over trial | Placebo pills | 120 | Male (60%), adults (19–29 years), normal‐weight | 12 weeks | Yes | Not detected | ‐ | Obesity | Yes | Byun et al. (2016) |
| Fermented soy product supplemented with isoflavones | 200 mL | Randomised, placebo‐controlled, double‐blind trial | Unfermented soy product | 49 | Male (100%), adults (37–57 years), normal‐weight | 42 days | No | Not detected | ‐ | Cardiometabolic disease | Yes | Cardoso Umbelino Cavallini et al. (2016) |
| Kochujang (Korean fermented sauce) | 34.5 g | Randomised, placebo‐controlled, double‐blind trial | Placebo pills | 30 | Male (43.33%), adults (19–55 years), normal‐weight | 12 weeks | No | Not detected | ‐ | Hyperlipidemia | Yes | Lim et al. (2015) |
| Kimchi | 180 g | Randomised, parallel and controlled trial | Unfermented kimchi | 23 | Male (0%), adults (30–60 years), obese | 8 weeks | No | 16S rRNA |
↑ Prevotella and Bacteroides ↓ Blautia |
Metabolic syndrome | Yes | Han et al. (2015) |
| Kimchi | 300 g | Randomised, controlled, cross‐over trial | Unfermented kimchi | 21 | Male (33.3%), adults (31–65 years), normal‐weight and overweight | 8 weeks | Yes | Not detected | ‐ | Prediabetes | Yes | An et al. (2013) |
| Kimchi | 210 g | Randomised, parallel and controlled trial | Low kimchi consumption (15 g/day) | 100 | Male (50%), adults (low‐intake group, 22.5 ± 3.1 years; high‐intake group 23.0 ± 2.8 years), normal‐weight | 1 week | No | Not detected | ‐ | Cardiometabolic disease | Yes | Choi et al. (2013) |
| Kimchi | 100 g | Randomised, open‐labelled, prospective, and controlled trial | No intervention | 43 | Gender not reported, adults (over 20 years), normal‐weight | 4 weeks | Yes | Not detected | ‐ | Immune system | No | Lee et al. (2014) |
| Kimchi | 210 g | Randomised, parallel and controlled trial | Functional kimchi (210 g/day) | 28 | Male (64.29%), adults (standard kimchi, 22.6 ± 2.2 years functional kimchi, 24.1 ± 4.8 years), normal‐weight | 4 weeks | No | 16S rRNA |
Kimchi: ↑ Bacteroidaeota and Actinobacteraeota ↓ Bacillaeota, Alphaproteobacteraeota and Tenericutes. Bifidobacterium adolescentis Functional kimchi: ↑ Bacteroidaeota and Alphaproteobacteraeota. Bifidobacterium adolescentis ↓ Bacillaeota and Actinobacteraeota |
Metabolic syndrome and irritable bowel syndrome | Yes | Kim and Park (2018) |
| Psychobiotic diet (high in prebiotic fibres, legumes and FFs) | 6–8 servings/day (prebiotic fibres); 5–8 servings/day (grains); 3–4 servings/week (legumes), 2–3 servings/day (FFs) | Single‐blind, randomised, controlled trial | No intervention | 45 | Male (37.78%), adults (18–59 years), normal‐weight | 4 weeks | No | Shotgun | None | Perceived stress | Yes | Berding et al. (2023) |
| Sauerkraut (unpasteurised and containing LAB) | 75 g | Randomised, double‐blind controlled trial | Pasteurised sauerkraut | 58 | Male (62.50%), adults (21–59 years), normal‐weight | 6 weeks | No | 16S rRNA | ↑ Clostridiales, Lachnospiraceae, Ruminococcaceae Faecalibacterium, Eubacterium and Bacteroides | Irritable bowel syndrome | Yes | Nielsen et al. (2018) |
| Sourdough | 145 g | Randomised cross‐over trial | White wheat bread | 20 | Male (45%), adults (18–70 years), normal‐weight | 1 week | Yes | 16S rRNA and Shotgun | White wheat bread: ↑ Eubacterium ventriosum and Anaerostipes spp. | Cardiometabolic disease | ‐ | Korem et al. (2017) |
| Sourdough | 2 sourdough croissants | Randomised, double‐blind, cross‐over, controlled trial | Traditional yeast croissants | 17 | Male (48%), adults (18–40 years), normal‐weight | Single study day | Yes | Not detected | ‐ | Gastric emptying and gastrointestinal fermentation symptoms | Yes | Polese et al. (2018) |
| Sourdough | 6–10 slices | Randomised cross‐over trial | Wheat bread enriched with fermented rye bran | 7 | Male (57.14%), adults (38–61 years), normal‐weight | 4 weeks | No | Not detected | ‐ | Gastrointestinal symptoms | Yes | Raninen et al. (2017) |
| Sourdough | 7–8 slices | Randomised, double‐blinded, cross‐over trial | Traditional sourdough rye bread | 87 | Male (8.7%), adults (21–64 years), normal‐weight | 4 weeks | Yes | Not detected | ‐ | Irritable bowel syndrome | Yes | Laatikainen et al. (2016) |
| Sourdough | 6 slices | Randomised controlled trial, double‐blinded | Yeast‐fermented wheat bread | 26 | Male (4%), adults (21–64 years), normal‐weight | 7 days | No | Not detected | ‐ | Irritable bowel syndrome | No | Laatikainen et al. (2017) |
| Sourdough | 200 g | Non‐randomise, uncontrolled trial | None | 8 | Gender not reported, children (8–17 years), normal‐weight | 60 days | No | Not detected | ‐ | Coeliac disease | Yes | Di Cagno et al. (2010) |
| Sourdough | 200 g | Randomised controlled trial | Traditional wheat bread | 20 | Male (30%), children and adults (12–24 years), normal‐weight | 3 days | No | Not detected | ‐ | Coeliac disease | Yes | Mandile et al. (2017) |
FIGURE 4.
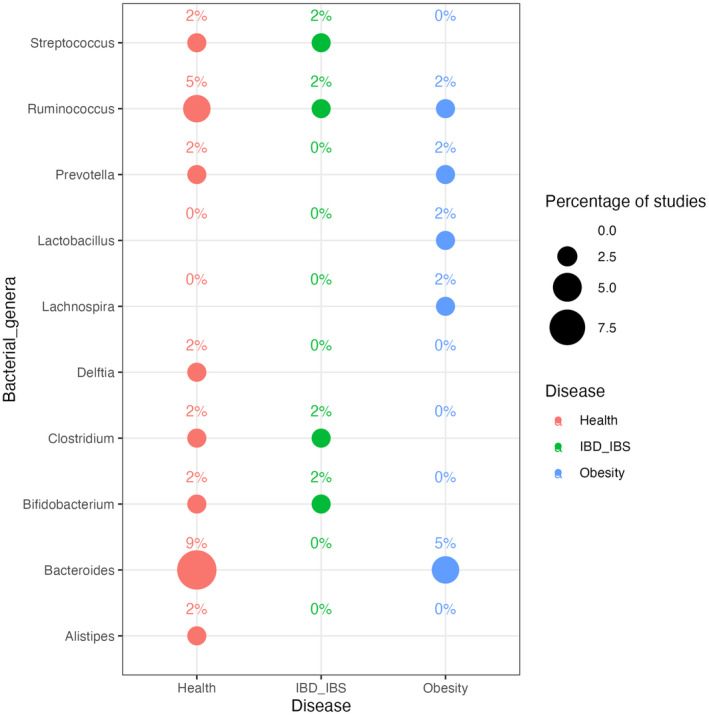
Bubble plot showing the percentage of studies (from those summarised in Table 3) in which microbial genera increase after an intervention with different FFs. Bubble size is proportional to the number of different studies reporting the same association. The group labelled as “Health” corresponds to those intervention studies where the population was described as “Healthy.” The total number of intervention studies reported in this analysis is 44.
The variation in gut microbiome detected was often specific to the type and quantity of FFs consumed. For instance, a recent trial compared the effects of different microbiota‐targeted dietary interventions (i.e., high‐FF and high‐fibre diets) on gut microbiota and immune response (Wastyk et al., 2021). The goal of this study was to increase the number of FFs servings per day from <1 to at least 6 in the high‐FF group. FFs included yogurt, kefir, fermented cottage cheese, kombucha, vegetable brine drinks and fermented vegetables. A high‐fibre diet, on the other hand, was characterised by an average fibre intake of 45.1 ± 10.7 g per day. The authors showed that several taxa were shared between FFs and gut microbiota upon intervention, with the greatest overlap occurring in the early phase of the intervention. Moreover, the investigation revealed compelling evidence of different effects between the two dietary interventions on the diversity of the gut microbiota. Specifically, a high‐FF diet boosted a significant and noteworthy increase in microbial diversity. Conversely, a high‐fibre diet did not result in substantial alterations in the overall abundance of species within the gut microbial community. Notably, an increase in Bacillota was observed, distributed across the Lachnospiraceae, Ruminococcaceae and Streptococcaceae families in the high‐FF group. However, results from clinical trials present in the literature are sometimes discordant, and not all the studies showed a significant improvement in health outcomes, such as inflammation, obesity and irritable bowel syndrome (IBS), suggesting that the relationship among FFs, gut microbiome and health is complex and may depend on various factors. In fact, Joseph et al. (2020) reported an increase in Bacteroides ovatus, Lachnospira and Ruminococcus in the gut microbiome of participants following an intervention of 4 weeks with a fermented milk. The study targeted obesity as the health outcome but did not find a significant effect. The relative abundance of Lachnospira spp., a bacterial genus well‐known for producing short‐chain fatty acids (SCFAs) and associated with a variety of health outcomes, increases significantly in FFs trials targeting obese participants. Several studies reported an increase in Prevotella upon FFs consumption. Prevotella spp. has been mooted as a diet and lifestyle biomarker, owing to its extensive presence in varied worldwide populations (Tett et al., 2021). Indeed, Prevotella species have a unique metabolite profile linked with the breakdown and digestion of complex dietary polysaccharides, indicating that dietary fibre fermentation is occurring (Precup & Vodnar, 2019). Dietary patterns associated with Prevotella spp. are largely plant‐based, with high‐fibre content, and this taxon is often associated with rural and non‐Westernised dietary patterns (Medawar et al., 2019). Furthermore, vegetarian, vegan and Mediterranean diets are linked to increased Prevotella levels (De Filippis et al., 2016), although species and strain‐level differences may subsist (De Filippis et al., 2019; Tett et al., 2021). We also identified several studies consistently reporting an increase in Ruminococcus spp. in different target populations during trials with FFs. Ruminococcus spp. was often reported as correlated with both IBS and inflammatory bowel disease (IBD) (Bolte et al., 2021). In particular, Ruminococcus gnavus showed enrichment in people with IBD (Zhai et al., 2023) and specific R. gnavus strains are linked with inflammation associated with food allergies (De Filippis, Paparo, et al., 2021; De Filippis, Valentino, et al., 2021). However, other Ruminococcus species (e.g., R. bromii and R. albus) have been associated with a positive influence on human health (Scott et al., 2015; Ze et al., 2012). Furthermore, according to Chen et al. (2019), traditionally fermented yogurt was more efficient than milk in decreasing insulin resistance in obese women with metabolic syndrome, potentially through a modulation of lipid metabolism, inflammation, oxidative stress and shifting the composition of the gut microbiota. In another intervention study focused on the consumption of fresh cabbages or fermented kimchi (Han et al., 2015) in obese women, fermented kimchi intake yielded considerable and positive changes in the gut microbiota, including increases in Bifidobacterium and Lactobacillus, while reducing the harmful Clostridium perfringens. Berding et al. (2023) highlighted that the consumption of FFs is also related to mental health. An intervention with a diet rich in FFs and fibre (defined psychobiotic diet, PD) led to a decrease in perceived stress. Moreover, although no significant change in gut microbiome composition was detected, the intervention with the PD boosted the changes in a total of 53 distinct faecal, plasmatic and urinary metabolites compared with the control diet. Thus, a diet rich in FFs may be beneficial for the neuronal networks, suggesting that consuming FFs can affect also mood and brain activity (Kok & Hutkins, 2018; Selhub et al., 2014). Some commensal and mutualistic bacteria may have a key role in treating stress‐related disorders such as anxiety and depression that are closely linked to functional bowel disorders (Bravo et al., 2011). Alterations in the receptor expression of the main central nervous system inhibitory neurotransmitter, γ‐aminobutyric acid (GABA), contribute to the pathogenesis of anxiety and depression (Pehrson & Sanchez, 2015). FFs may alleviate symptoms of depression and anxiety (Aslam et al., 2020; Selhub et al., 2014). A combination between advances in shotgun metagenomic sequencing and new clinical trials should make it possible to characterise the microbial and molecular relationships between FFs and human health in greater depth (Walsh et al., 2023). If consuming FFs can alter and enrich the human gut microbiome in the long term, FFs may help to prevent the significant loss of gut microbial diversity that has been linked with several chronic diseases typical of Western lifestyle (Segata, 2015). However, some of the studies found in the literature highlight inconsistent results (Table 3 and Figure 5). This may be explained by multiple variables, such as different target populations, quantity of FFs examined, and the diversity of microbial species and/or strains contained in FFs (Raoult & Henrissat, 2014), as well as gut microbiome interindividual differences, that may influence the response to dietary interventions (De Filippis et al., 2018). In fact, it was previously shown that gut microbiome interindividual variability, local dietary habits and genetic background are all components of an intricate interplay between dietary intakes and health outcomes (Chen et al., 2022). Indeed, these factors may contribute to the observed discrepancies within the examined literature. As a result, it is critical to evaluate these factors in order to get more accurate conclusions on the possible health effects of FFs. In several cases, intervention studies do not specifically target alterations in the gut microbiome following the consumption of FFs (Figure 5). Furthermore, it is essential to underscore that a significant portion of the analysed studies showed in Figure 5, and aiming to characterise the gut microbiome, employed the 16S rRNA sequencing method or even more outdated molecular analysis (i.e., DGGE and qPCR), that do not target the entire genomic content of a sample and do not allow to reach the strain‐level characterisation. As a consequence, a more in‐depth characterisation of the gut microbiome would be necessary in order to explore FFs‐gut microbiome relationships, since a strain‐level diversity in the gut exists (De Filippis et al., 2019, 2020; Tett et al., 2019) and this may influence the impact of dietary or drug treatments (Vinderola et al., 2023). In addition, most of the available interventions are conducted on yogurt and other dairy products. Thus, expansion of randomised controlled trials to other FFs is needed, in order to address these knowledge gaps. In addition, in‐depth focus on the molecular mechanisms behind the beneficial effects of FFs on human health is also necessary. Overall, we provide a useful summary of different studies on the effects of various FFs on the gut microbiome composition and associated health outcomes.
FIGURE 5.
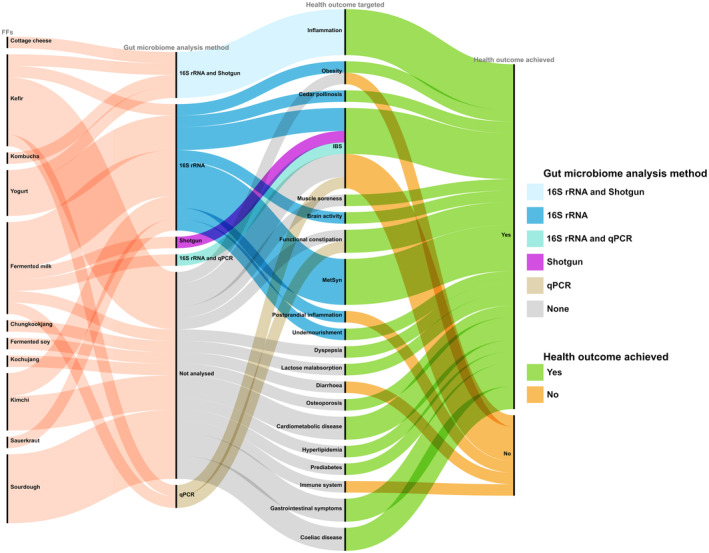
Alluvial diagram highlighting the correlation among FFs, microbiome analysis methods, health outcomes targeted and achieved in trials reported in Table 3.
Specifically, although it has been speculated that consuming FFs may introduce new microorganisms in the human gut, there is still a lack of evidence in tracking microbial strains that pass from FFs to human gut microbiome (Marco et al., 2017). In such a way, the application of shotgun metagenomic sequencing for strain tracking provides an opportunity for future studies that want to further investigate the delivery of microbes and the associations with human health (Quince et al., 2017). All this knowledge may support the development of personalised and tailored interventions targeting the gut microbiome to promote human health (Virgin & Todd, 2011). Anyway, while numerous association and correlation studies have linked the ingestion of live microbes through FFs to various health benefits, a comprehensive understanding of the cause‐and‐effect relationship between their consumption and specific health outcomes remains a field that warrants further investigation and clinical trials. Indeed, Marco et al. (2022) classified different foods according to the quantity of live microbes per gram in high (mainly FFs and raw vegetables), medium and low live microbe content. Using this classification, screened the American National Health and Nutrition Examination Survey (NHANES) database was screened to identify associations between the live microbe intake and several diseases (Hill et al., 2023). They highlighted that the quantity of live microbes in the diet is negatively correlated with systolic blood pressure, inflammation, body mass index, insulin and triglycerides levels. Therefore, they suggested that defining a minimal daily intake dose of live microorganisms is crucial to minimise the risk of chronic diseases and improve population health. Clearly further research and properly designed clinical trials are needed to better understand the mechanisms explaining the link of FF live microbes with specific health outcomes, focusing on determining the optimal type and quantity of FFs that can positively impact human health and if this effect may be modulated by the gut microbiome. Additionally, long‐term interventions with larger populations are needed to confirm the findings present in literature. However, nowadays, ultra‐processed foods pervade Westernised diets, which lead to a decreased diversity of the gut microbiome and can have significant health implications, such as chronic inflammation (Sonnenburg & Sonnenburg, 2019). The human gut microbiome interacts not only with microorganisms, but also with their leftover compounds. Indeed, metabolites like acetic, butyric and lactic acids, EPS, and proteins may improve the immunity associated with the mucosal membrane of the gastrointestinal tract, lowering inflammation (Mathur et al., 2020). Through this mechanism, FFs may protect against gastrointestinal tract disorders, food allergies, bacterial or viral infections, Crohn's disease and more (Parvez et al., 2006). Even though there have been relatively few human dietary intervention studies involving FFs, most of the evidence points to the positive effects of eating FFs on human health, particularly in the treatment of metabolic diseases, the management of weight, mood and mental health, and the reduction of overall mortality (Hill et al., 2023; Marco et al., 2017). Indeed, FF consumption may represent a valuable and low‐cost alternative to promote well‐being and prevent non‐communicable diseases, in line with the objectives of the SDG3.
CONCLUSIONS AND FUTURE PERSPECTIVES
Overall, FFs are widespread and the multiple combinations of matrices and fermentation technologies concur to define ecological niches harbouring microbial communities different in composition and functions, which are still partially unexplored. In this Review, we focused on the complexity of FF microbiota, highlighting that traditional products harbour highly selected microbial communities. Furthermore, we provided an up‐to‐date review of the literature focusing on the effects of FF consumption on the human health, including the most recent observational/intervention studies, which supports the idea that human health might take advantage from FF consumption. Indeed, they may actively modulate both the composition and the functionality of the human gut microbiome, leading to the selection of health‐related species or functions. In addition, since microorganisms are exposed to similar environmental pressures in some FFs and in the human gut, we suggested that traditional FFs might be an exciting and unexplored source of novel probiotic strains, able to survive to the gastrointestinal passage. Unfortunately, the direct transfer of microorganisms from FFs to the human gut remains not proved yet and the possible underlying mechanisms explaining the link between FFs and human health are still to be fully uncovered. However, the field of FFs microbiome research is on an exciting wave with promising advancements, and Next‐Generation Sequencing technologies coupled with machine learning approaches will help researchers to unravel the molecular networks existing between microbial communities in FFs and human health outcomes, as well as to identify new probiotic strains potentially boosting human health.
As we explore the molecular interactions within FFs, refined dietary recommendations are expected, considering both health benefits and ecological impact. The convergence of cutting‐edge research, technological innovations and dietary guidelines place FFs not just as culinary delights but as sustainable contributors to human well‐being.
AUTHOR CONTRIBUTIONS
Vincenzo Valentino: Formal analysis (equal); writing – original draft (equal). Raffaele Magliulo: Formal analysis (equal); writing – original draft (equal). Dominic Farsi: Writing – review and editing (equal). Paul D. Cotter: Funding acquisition (equal); writing – review and editing (equal). Orla O'Sullivan: Funding acquisition (equal); writing – review and editing (equal). Danilo Ercolini: Conceptualization (equal); funding acquisition (equal); writing – review and editing (equal). Francesca De Filippis: Conceptualization (equal); funding acquisition (equal); writing – original draft (equal).
FUNDING INFORMATION
This work was partially supported by the Italian Ministry of Foreign Affairs and International Cooperation, with a grant to the project FOODMICROHERITAGE—“Quality and authenticity protection of artisanal fermented foods through the characterization and conservation of their microbial and genetic heritage” (VN21GR09) and by the project DOMINO—“Harnessing the microbial potential of fermented foods for healthy and sustainable food systems.” This project has received funding from the European Union's Horizon Europe research and innovation programme under grant agreement No 101060218. The manuscript reflects only the authors' views, and the European Commission is not responsible for any use that may be made of the information it contains. The work was also supported by the National Recovery and Resilience Plan (NRRP), Mission 4, Component 2, Investment 1.4 “Strengthening of research structures and creation of R&D ‘national champions’ on some Key Enabling Technologies” ‐ Call for tender No. 3138 of 16 December 2021, rectified by Decree n. 3175 of 18 December 2021 of Italian Ministry of University and Research funded by the European Union—NextGenerationEU; Project code CN_00000033, “National Biodiversity Future Center—NBFC.”
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Supporting information
Table S1
Appendix S1
Valentino, V. , Magliulo, R. , Farsi, D. , Cotter, P.D. , O’Sullivan, O. , Ercolini, D. et al. (2024) Fermented foods, their microbiome and its potential in boosting human health. Microbial Biotechnology, 17, e14428. Available from: 10.1111/1751-7915.14428
Vincenzo Valentino and Raffaele Magliulo contributed equally to this study.
REFERENCES
- Adebo, O. , Kayitesi, E. & Njobeh, P. (2019) Reduction of mycotoxins during fermentation of whole grain sorghum to whole grain ting (a southern African food). Toxins, 11(3), 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez, A.‐S. , Tap, J. , Chambaud, I. , Cools‐Portier, S. , Quinquis, L. , Bourlioux, P. et al. (2020) Safety and functional enrichment of gut microbiome in healthy subjects consuming a multi‐strain fermented milk product: a randomised controlled trial. Scientific Reports, 10(1), 15974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato, K.R. , Chaves, Ó.M. , Mallott, E.K. , Eppley, T.M. , Abreu, F. , Baden, A.L. et al. (2021) Fermented food consumption in wild nonhuman primates and its ecological drivers. American Journal of Physical Anthropology, 175(3), 513–530. [DOI] [PubMed] [Google Scholar]
- Amit, S.K. , Uddin, M.M. , Rahman, R. , Islam, S.M.R. & Khan, M.S. (2017) A review on mechanisms and commercial aspects of food preservation and processing. Agriculture & Food Security, 6(1), 51. [Google Scholar]
- An, S.‐Y. , Lee, M.S. , Jeon, J.Y. , Ha, E.S. , Kim, T.H. , Yoon, J.Y. et al. (2013) Beneficial effects of fresh and fermented kimchi in prediabetic individuals. Annals of Nutrition & Metabolism, 63(1–2), 111–119. [DOI] [PubMed] [Google Scholar]
- Angelakis, E. , Yasir, M. , Bachar, D. , Azhar, E.I. , Lagier, J.‐C. , Bibi, F. et al. (2016) Gut microbiome and dietary patterns in different Saudi populations and monkeys. Scientific Reports, 6(1), 32191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari, F. , Alian Samakkhah, S. , Bahadori, A. , Jafari, S.M. , Ziaee, M. , Khodayari, M.T. et al. (2023) Health‐promoting properties of Saccharomyces cerevisiae var. boulardii as a probiotic; characteristics, isolation, and applications in dairy products. Critical Reviews in Food Science and Nutrition, 63(4), 457–485. [DOI] [PubMed] [Google Scholar]
- Aslam, H. , Green, J. , Jacka, F.N. , Collier, F. , Berk, M. , Pasco, J. et al. (2020) Fermented foods, the gut and mental health: a mechanistic overview with implications for depression and anxiety. Nutritional Neuroscience, 23(9), 659–671. [DOI] [PubMed] [Google Scholar]
- Barcenilla, C. , Ducic, M. , López, M. , Prieto, M. & Álvarez‐Ordóñez, A. (2022) Application of lactic acid bacteria for the biopreservation of meat products: a systematic review. Meat Science, 183, 108661. [DOI] [PubMed] [Google Scholar]
- Bekar, O. , Yilmaz, Y. & Gulten, M. (2011) Kefir improves the efficacy and tolerability of triple therapy in eradicating Helicobacter pylori . Journal of Medicinal Food, 14(4), 344–347. [DOI] [PubMed] [Google Scholar]
- Bellikci‐Koyu, E. , Sarer‐Yurekli, B.P. , Akyon, Y. , Aydin‐Kose, F. , Karagozlu, C. , Ozgen, A.G. et al. (2019) Effects of regular kefir consumption on gut microbiota in patients with metabolic syndrome: a parallel‐group, randomized, controlled study. Nutrients, 11(9), 2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berding, K. , Bastiaanssen, T.F.S. , Moloney, G.M. , Boscaini, S. , Strain, C.R. , Anesi, A. et al. (2023) Feed your microbes to deal with stress: a psychobiotic diet impacts microbial stability and perceived stress in a healthy adult population. Molecular Psychiatry, 28(2), 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisanz, J.E. , Enos, M.K. , PrayGod, G. , Seney, S. , Macklaim, J.M. , Chilton, S. et al. (2015) Microbiota at multiple body sites during pregnancy in a rural Tanzanian population and effects of Moringa‐supplemented probiotic yogurt. Applied and Environmental Microbiology, 81(15), 4965–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson, L.F. & Walker, G.A. (2015) The microbial dynamics of wine fermentation. In: Holzapfel, W. (Ed.) Advances in fermented foods and beverages. Amsterdam: Elsevier, pp. 435–476. [Google Scholar]
- Bogovič Matijašić, B. , Obermajer, T. , Lipoglavšek, L. , Sernel, T. , Locatelli, I. , Kos, M. et al. (2016) Effects of synbiotic fermented milk containing Lactobacillus acidophilus La‐5 and Bifidobacterium animalis ssp. lactis BB‐12 on the fecal microbiota of adults with irritable bowel syndrome: a randomized double‐blind, placebo‐controlled trial. Journal of Dairy Science, 99(7), 5008–5021. [DOI] [PubMed] [Google Scholar]
- Bolarinwa, I.F. , Oke, M.O. , Olaniyan, S.A. & Ajala, A.S. (2016) A review of cyanogenic glycosides in edible plants. In: Soloneski, S. & Larramendy, M.L. (Eds.) Toxicology ‐ new aspects to this scientific conundrum. London: IntechOpen. [Google Scholar]
- Bolte, L.A. , Vich Vila, A. , Imhann, F. , Collij, V. , Gacesa, R. , Peters, V. et al. (2021) Long‐term dietary patterns are associated with pro‐inflammatory and anti‐inflammatory features of the gut microbiome. Gut, 70(7), 1287–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolyen, E. , Rideout, J.R. , Dillon, M.R. , Bokulich, N.A. , Abnet, C.C. , Al‐Ghalith, G.A. et al. (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nature Biotechnology, 37(8), 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchez, A. & De Vuyst, L. (2022) Acetic acid bacteria in sour beer production: friend or foe? Frontiers in Microbiology, 13, 957167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo, J.A. , Forsythe, P. , Chew, M.V. , Escaravage, E. , Savignac, H.M. , Dinan, T.G. et al. (2011) Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proceedings of the National Academy of Sciences of the United States of America, 108(38), 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buglass, A.J. (2011) Handbook of alcoholic beverages, 2 volume set: technical, analytical and nutritional aspects. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Burton, K.J. , Rosikiewicz, M. , Pimentel, G. , Bütikofer, U. , von Ah, U. , Voirol, M.‐J. et al. (2017) Probiotic yogurt and acidified milk similarly reduce postprandial inflammation and both alter the gut microbiota of healthy, young men. The British Journal of Nutrition, 117(9), 1312–1322. [DOI] [PubMed] [Google Scholar]
- Byun, M.‐S. , Yu, O.‐K. , Cha, Y.‐S. & Park, T.‐S. (2016) Korean traditional Chungkookjang improves body composition, lipid profiles and atherogenic indices in overweight/obese subjects: a double‐blind, randomized, crossover, placebo‐controlled clinical trial. European Journal of Clinical Nutrition, 70(10), 1116–1122. [DOI] [PubMed] [Google Scholar]
- Cardoso Umbelino Cavallini, D. , Jovenasso Manzoni, M. , Bedani, R. , Roselino, M. , Celiberto, L. , Vendramini, R. et al. (2016) Probiotic soy product supplemented with isoflavones improves the lipid profile of moderately hypercholesterolemic men: a randomized controlled trial. Nutrients, 8(1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceapa, C. , Lambert, J. , van Limpt, K. , Wels, M. , Smokvina, T. , Knol, J. et al. (2015) Correlation of Lactobacillus rhamnosus genotypes and carbohydrate utilization signatures determined by phenotype profiling. Applied and Environmental Microbiology, 81(16), 5458–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai, K.F. , Ng, K.R. , Samarasiri, M. & Chen, W.N. (2022) Precision fermentation to advance fungal food fermentations. Current Opinion in Food Science, 47, 100881. [Google Scholar]
- Chen, L. , Zhernakova, D.V. , Kurilshikov, A. , Andreu‐Sánchez, S. , Wang, D. , Augustijn, H.E. et al. (2022) Influence of the microbiome, diet and genetics on inter‐individual variation in the human plasma metabolome. Nature Medicine, 28, 2333–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Feng, R. , Yang, X. , Dai, J. , Huang, M. , Ji, X. et al. (2019) Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. The American Journal of Clinical Nutrition, 109(6), 1611–1619. [DOI] [PubMed] [Google Scholar]
- Choi, I.H. , Noh, J.S. , Han, J.‐S. , Kim, H.J. , Han, E.‐S. & Song, Y.O. (2013) Kimchi, a fermented vegetable, improves serum lipid profiles in healthy young adults: randomized clinical trial. Journal of Medicinal Food, 16(3), 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, L.A. , Maurice, C.F. , Carmody, R.N. , Gootenberg, D.B. , Button, J.E. , Wolfe, B.E. et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis, F. , Esposito, A. & Ercolini, D. (2022) Outlook on next‐generation probiotics from the human gut. Cellular and Molecular Life Sciences, 79(2), 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis, F. , Paparo, L. , Nocerino, R. , Della Gatta, G. , Carucci, L. , Russo, R. et al. (2021) Specific gut microbiome signatures and the associated pro‐inflamatory functions are linked to pediatric allergy and acquisition of immune tolerance. Nature Communications, 12(1), 5958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis, F. , Pasolli, E. & Ercolini, D. (2020) The food‐gut axis: lactic acid bacteria and their link to food, the gut microbiome and human health. FEMS Microbiology Reviews, 44(4), 454–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippis, F. , Pasolli, E. , Tett, A. , Tarallo, S. , Naccarati, A. , De Angelis, M. et al. (2019) Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host & Microbe, 25(3), 444–453. [DOI] [PubMed] [Google Scholar]
- De Filippis, F. , Pellegrini, N. , Vannini, L. , Jeffery, I.B. , La Storia, A. , Laghi, L. et al. (2016) High‐level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut, 65(11), 1812–1821. [DOI] [PubMed] [Google Scholar]
- De Filippis, F. , Valentino, V. , Alvarez‐Ordóñez, A. , Cotter, P.D. & Ercolini, D. (2021) Environmental microbiome mapping as a strategy to improve quality and safety in the food industry. Current Opinion in Food Science, 38, 168–176. [Google Scholar]
- De Filippis, F. , Vitaglione, P. , Cuomo, R. , Berni Canani, R. & Ercolini, D. (2018) Dietary interventions to modulate the gut microbiome—how far away are we from precision medicine. Inflammatory Bowel Diseases, 24(10), 2142–2154. [DOI] [PubMed] [Google Scholar]
- Derrien, M. & van Hylckama Vlieg, J.E.T. (2015) Fate, activity, and impact of ingested bacteria within the human gut microbiota. Trends in Microbiology, 23(6), 354–366. [DOI] [PubMed] [Google Scholar]
- Di Cagno, R. , Barbato, M. , Di Camillo, C. , Rizzello, C.G. , De Angelis, M. , Giuliani, G. et al. (2010) Gluten‐free sourdough wheat baked goods appear safe for young celiac patients: a pilot study. Journal of Pediatric Gastroenterology and Nutrition, 51(6), 777–783. [DOI] [PubMed] [Google Scholar]
- Di Cagno, R. , Coda, R. , De Angelis, M. & Gobbetti, M. (2013) Exploitation of vegetables and fruits through lactic acid fermentation. Food Microbiology, 33(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Dominianni, C. , Sinha, R. , Goedert, J.J. , Pei, Z. , Yang, L. , Hayes, R.B. et al. (2015) Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS One, 10(4), e0124599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emkani, M. , Oliete, B. & Saurel, R. (2022) Effect of lactic acid fermentation on legume protein properties, a review. Fermentation, 8(6), 244. [Google Scholar]
- European Food Safety Authority (EFSA) . (2007) Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA ‐ opinion of the Scientific Committee. EFSA Journal, 5(12), 587. [Google Scholar]
- Fabian, E. , Majchrzak, D. , Dieminger, B. , Meyer, E. & Elmadfa, I. (2008) Influence of probiotic and conventional yoghurt on the status of vitamins B1, B2 and B6 in young healthy women. Annals of Nutrition & Metabolism, 52(1), 29–36. [DOI] [PubMed] [Google Scholar]
- Favaro, L. & Todorov, S.D. (2017) Bacteriocinogenic LAB strains for fermented meat preservation: perspectives, challenges, and limitations. Probiotics and Antimicrobial Proteins, 9(4), 444–458. [DOI] [PubMed] [Google Scholar]
- Filannino, P. , Di Cagno, R. , Crecchio, C. , De Virgilio, C. , De Angelis, M. & Gobbetti, M. (2016) Transcriptional reprogramming and phenotypic switching associated with the adaptation of Lactobacillus plantarum C2 to plant niches. Scientific Reports, 6(1), 27392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Albiach, R. , José, M. , de Felipe, P. , Angulo, S. , Morosini, M.‐I. , Bravo, D. et al. (2008) Molecular analysis of yogurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in human intestinal microbiota. The American Journal of Clinical Nutrition, 87(1), 91–96. [DOI] [PubMed] [Google Scholar]
- Garcia‐Gonzalez, N. , Prete, R. , Battista, N. & Corsetti, A. (2018) Adhesion properties of food‐associated Lactobacillus plantarum strains on human intestinal epithelial cells and modulation of IL‐8 release. Frontiers in Microbiology, 9, 2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, R.J. , Borges, M.F. , Rosa, M.F. , Castro‐Gómez, R.J.H. & Spinosa, W.A. (2018) Acetic acid bacteria in the food industry: systematics, characteristics and applications. Food Technology and Biotechnology, 56(2), 139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González, S. , Fernández‐Navarro, T. , Arboleya, S. , de Los Reyes‐Gavilán, C.G. , Salazar, N. & Gueimonde, M. (2019) Fermented dairy foods: impact on intestinal microbiota and health‐linked biomarkers. Frontiers in Microbiology, 24(10), 1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goya, L. , Kongor, J.E. & de Pascual‐Teresa, S. (2022) From cocoa to chocolate: effect of processing on flavanols and methylxanthines and their mechanisms of action. International Journal of Molecular Sciences, 23(22), 14365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greppi, A. , Krych, Ł. , Costantini, A. , Rantsiou, K. , Hounhouigan, D.J. , Arneborg, N. et al. (2015) Phytase‐producing capacity of yeasts isolated from traditional African fermented food products and PHYPk gene expression of Pichia kudriavzevii strains. International Journal of Food Microbiology, 205, 81–89. [DOI] [PubMed] [Google Scholar]
- Gupta, S. , Mortensen, M.S. , Schjørring, S. , Trivedi, U. , Vestergaard, G. , Stokholm, J. et al. (2019) Amplicon sequencing provides more accurate microbiome information in healthy children compared to culturing. Communications Biology, 2(1), 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guse, K. , Sharma, A. , Weyenberg, E. , Davison, S. , Ma, Y. , Choi, Y. et al. (2023) Regular consumption of lacto‐fermented vegetables has greater effects on the gut metabolome compared with the microbiome. Gut Microbes, 4, e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimbei, E. , Botsaris, G. & Chrysostomou, S. (2022) Beneficial effects of yoghurts and probiotic fermented milks and their functional food potential. Food, 11(17), 2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, K. , Bose, S. , Wang, J.‐H. , Kim, B.‐S. , Kim, M.J. , Kim, E.‐J. et al. (2015) Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. Molecular Nutrition & Food Research, 59(5), 1004–1008. [DOI] [PubMed] [Google Scholar]
- Han, X. , Yang, Z. , Jing, X. , Yu, P. , Zhang, Y. , Yi, H. et al. (2016) Improvement of the texture of yogurt by use of exopolysaccharide producing lactic acid bacteria. BioMed Research International, 2016, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harata, G. , Kumar, H. , He, F. , Miyazawa, K. , Yoda, K. , Kawase, M. et al. (2017) Probiotics modulate gut microbiota and health status in Japanese cedar pollinosis patients during the pollen season. European Journal of Nutrition, 56(7), 2245–2253. [DOI] [PubMed] [Google Scholar]
- Heo, S. , Kim, T. , Na, H.‐E. , Lee, G. , Lee, J.‐H. & Jeong, D.‐W. (2022) Transcriptomic analysis of Staphylococcus equorum KM1031 from the high‐salt fermented seafood jeotgal under chloramphenicol, erythromycin and lincomycin stresses. Scientific Reports, 12(1), 15541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzler, S.R. & Clancy, S.M. (2003) Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. Journal of the American Dietetic Association, 103(5), 582–587. [DOI] [PubMed] [Google Scholar]
- Hill, C. , Guarner, F. , Reid, G. , Gibson, G.R. , Merenstein, D.J. , Pot, B. et al. (2014) The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11(8), 506–514. [DOI] [PubMed] [Google Scholar]
- Hill, C. , Tancredi, D.J. , Cifelli, C.J. , Slavin, J.L. , Gahche, J. , Marco, M.L. et al. (2023) Positive health outcomes associated with live microbe intake from foods, including fermented foods, assessed using the NHANES database. The Journal of Nutrition, 153(4), 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisada, T. , Endoh, K. & Kuriki, K. (2015) Inter‐ and intra‐individual variations in seasonal and daily stabilities of the human gut microbiota in Japanese. Archives of Microbiology, 197(7), 919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel, W.H. (2002) Appropriate starter culture technologies for small‐scale fermentation in developing countries. International Journal of Food Microbiology, 75(3), 197–212. [DOI] [PubMed] [Google Scholar]
- Humpenöder, F. , Bodirsky, B.L. , Weindl, I. , Lotze‐Campen, H. , Linder, T. & Popp, A. (2022) Projected environmental benefits of replacing beef with microbial protein. Nature, 605, 90–96. [DOI] [PubMed] [Google Scholar]
- Hurtado, A. , Reguant, C. , Bordons, A. & Rozès, N. (2012) Lactic acid bacteria from fermented table olives. Food Microbiology, 31(1), 1–8. [DOI] [PubMed] [Google Scholar]
- Ibrahim, S.A. , Ayivi, R.D. , Zimmerman, T. , Siddiqui, S.A. , Altemimi, A.B. , Fidan, H. et al. (2021) Lactic acid bacteria as antimicrobial agents: food safety and microbial food spoilage prevention. Food, 10(12), 3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irlinger, F. , Loux, V. , Bento, P. , Gibrat, J.‐F. , Straub, C. , Bonnarme, P. et al. (2012) Genome sequence of Staphylococcus equorum subsp. equorum Mu2, isolated from a French smear‐ripened cheese. Journal of Bacteriology, 194(18), 5141–5142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa, M. , Aoi, W. , Mune, K. , Yamauchi, H. , Furuta, K. , Sasaki, S. et al. (2013) Fermented milk improves glucose metabolism in exercise‐induced muscle damage in young healthy men. Nutrition Journal, 12(1), 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn, L.J. , Rekdal, V.M. & Sommer, M.O.A. (2023) Microbial foods for improving human and planetary health. Cell, 186(3), 469–478. [DOI] [PubMed] [Google Scholar]
- Jiang, X. , Liu, X. , Xu, H. , Sun, Y. , Zhang, Y. & Wang, Y. (2021) Improvement of the nutritional, antioxidant and bioavailability properties of corn gluten‐wheat bran mixture fermented with lactic acid bacteria and acid protease. Lebensmittel‐Wissenschaft und Technologie, 144, 111161. [Google Scholar]
- Joseph, N. , Clayton, J.B. , Hoops, S.L. , Linhardt, C.A. , Mohd Hashim, A. , Mohd Yusof, B.N. et al. (2020) Alteration of the gut microbiome in normal and overweight school children from Selangor with Lactobacillus fermented milk administration. Evolutionary Bioinformatics Online, 16, 117693432096594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kårlund, A. , Gómez‐Gallego, C. , Korhonen, J. , Palo‐oja, O.‐M. , El‐Nezami, H. & Kolehmainen, M. (2020) Harnessing microbes for sustainable development: food fermentation as a tool for improving the nutritional quality of alternative protein sources. Nutrients, 12(4), 1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khine, W.W.T. , Ang, X.J. , Chan, Y.S. , Lee, W.Q. , Quek, S.Y. , Tan, S.H. et al. (2019) Recovery of Lactobacillus casei strain Shirota (LcS) from faeces of healthy Singapore adults after intake of fermented milk. Beneficial Microbes, 10(7), 721–728. [DOI] [PubMed] [Google Scholar]
- Kieliszek, M. , Pobiega, K. , Piwowarek, K. & Kot, A.M. (2021) Characteristics of the proteolytic enzymes produced by lactic acid bacteria. Molecules, 26(7), 1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.H. , Kim, S.‐A. , Jo, Y.M. , Seo, H. , Kim, G.Y. , Cheon, S.W. et al. (2022) Probiotic potential of Tetragenococcus halophilus EFEL7002 isolated from Korean soy Meju. BMC Microbiology, 22(1), 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.‐Y. & Park, K.‐Y. (2018) Clinical trials of kimchi intakes on the regulation of metabolic parameters and colon health in healthy Korean young adults. Journal of Functional Foods, 47, 325–333. [Google Scholar]
- Kok, C.R. & Hutkins, R. (2018) Yogurt and other fermented foods as sources of health‐promoting bacteria. Nutrition Reviews, 76(Supplement_1), 4–15. [DOI] [PubMed] [Google Scholar]
- Korem, T. , Zeevi, D. , Zmora, N. , Weissbrod, O. , Bar, N. , Lotan‐Pompan, M. et al. (2017) Bread affects clinical parameters and induces gut microbiome‐associated personal glycemic responses. Cell Metabolism, 25(6), 1243–1253. [DOI] [PubMed] [Google Scholar]
- Kubizniaková, P. , Kyselová, L. , Brožová, M. , Hanzalíková, K. & Matoulková, D. (2021) The role of acetic acid bacteria in brewing and their detection in operation. Kvasny Prumysl, 67(5), 511–522. [Google Scholar]
- Kumar, B.V. , Sreedharamurthy, M. & Reddy, O.V.S. (2015) Probiotication of mango and sapota juices using Lactobacillus plantarum NCDC LP 20. Nutrafoods, 14(2), 97–106. [Google Scholar]
- Laatikainen, R. , Koskenpato, J. , Hongisto, S.‐M. , Loponen, J. , Poussa, T. , Hillilä, M. et al. (2016) Randomised clinical trial: low‐FODMAP rye bread vs. regular rye bread to relieve the symptoms of irritable bowel syndrome. Alimentary Pharmacology & Therapeutics, 44(5), 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laatikainen, R. , Koskenpato, J. , Hongisto, S.‐M. , Loponen, J. , Poussa, T. , Huang, X . et al. (2017) Pilot Study: Comparison of Sourdough Wheat Bread and Yeast‐Fermented Wheat Bread in Individuals with Wheat Sensitivity and Irritable Bowel Syndrome. Nutrients 9(11): 1215. 10.3390/nu9111215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavefve, L. , Marasini, D. & Carbonero, F. (2019) Microbial ecology of fermented vegetables and non‐alcoholic drinks and current knowledge on their impact on human health. In: Toldra, F. (Ed.) Advances in food and nutrition research. Amsterdam: Elsevier, pp. 147–185. [DOI] [PubMed] [Google Scholar]
- Le Nevé, B. , Derrien, M. , Tap, J. , Brazeilles, R. , Cools Portier, S. , Guyonnet, D. et al. (2019) Fasting breath H2 and gut microbiota metabolic potential are associated with the response to a fermented milk product in irritable bowel syndrome. PLoS One, 14(4), e0214273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, H. , Kim, D.Y. , Lee, M.A. , Jang, J.‐Y. & Choue, R. (2014) Immunomodulatory effects ofKimchiin Chinese healthy college students: a randomized controlled trial. Clinical Nutrition Research, 3(2), 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.‐H. , Heo, S. & Jeong, D.‐W. (2018) Genomic insights into Staphylococcus equorum KS1039 as a potential starter culture for the fermentation of high‐salt foods. BMC Genomics, 19(1), 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech, J. , Cabrera‐Rubio, R. , Walsh, A.M. , Macori, G. , Walsh, C.J. , Barton, W. et al. (2020) Fermented‐food metagenomics reveals substrate‐associated differences in taxonomy and health‐associated and antibiotic resistance determinants. mSystems, 5(6), e00522‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwendaal, N.K. , Stanton, C. , O'Toole, P.W. & Beresford, T.P. (2022) Fermented foods, health and the gut microbiome. Nutrients, 14(7), 1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Zheng, X. , Yang, J. , Shen, X. , Jiao, L. , Yan, Z. et al. (2019) Relation between gut microbiota composition and traditional spontaneous fermented dairy foods among Kazakh nomads in Xinjiang, China. Journal of Food Science, 84(12), 3804–3814. [DOI] [PubMed] [Google Scholar]
- Li, Y. , Ten, M.M.Z. , Zwe, Y.H. & Li, D. (2022) Lactiplantibacillus plantarum 299v as starter culture suppresses Enterobacteriaceae more efficiently than spontaneous fermentation of carrots. Food Microbiology, 103, 103952. [DOI] [PubMed] [Google Scholar]
- Lim, J.‐H. , Jung, E.‐S. , Choi, E.‐K. , Jeong, D.‐Y. , Jo, S.‐W. , Jin, J.‐H. et al. (2015) Supplementation with Aspergillus oryzae‐fermented kochujang lowers serum cholesterol in subjects with hyperlipidemia. Clinical Nutrition, 34(3), 383–387. [DOI] [PubMed] [Google Scholar]
- Lin, L. & Zhang, J. (2017) Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunology, 18(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, T. (2019) Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Security, 11(2), 265–278. [Google Scholar]
- Lipus, D. , Vikram, A. , Ross, D. , Bain, D. , Gulliver, D. , Hammack, R. et al. (2017) Predominance and metabolic potential of Halanaerobium spp. in produced water from hydraulically fractured Marcellus shale wells. Applied and Environmental Microbiology, 83(8), e02659‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S. & Qureshi, N. (2009) How microbes tolerate ethanol and butanol. New Biotechnology, 26(3–4), 117–121. [DOI] [PubMed] [Google Scholar]
- Malakar, S. , Paul, S.K. & Jolvis Pou, K.R. (2020) Biotechnological interventions in beverage production. In: Grumezescu, A. & Holban, A.M. (Eds.) Biotechnological progress and beverage consumption. Amsterdam: Elsevier, pp. 1–37. [Google Scholar]
- Maldonado‐Gómez, M.X. , Martínez, I. , Bottacini, F. , O'Callaghan, A. , Ventura, M. , van Sinderen, D. et al. (2016) Stable engraftment of Bifidobacterium longum AH1206 in the human gut depends on individualized features of the resident microbiome. Cell Host & Microbe, 20(4), 515–526. [DOI] [PubMed] [Google Scholar]
- Mandile, R. , Picascia, S. , Parrella, C. , Camarca, A. , Gobbetti, M. , Greco, L. et al. (2017) Lack of immunogenicity of hydrolysed wheat flour in patients with coeliac disease after a short‐term oral challenge. Alimentary Pharmacology & Therapeutics, 46(4), 440–446. [DOI] [PubMed] [Google Scholar]
- Marangoni, F. , Agostoni, C. , Borghi, C. , Catapano, A.L. , Cena, H. , Ghiselli, A. et al. (2020) Dietary linoleic acid and human health: focus on cardiovascular and cardiometabolic effects. Atherosclerosis, 292, 90–98. [DOI] [PubMed] [Google Scholar]
- Marco, M.L. , Heeney, D. , Binda, S. , Cifelli, C.J. , Cotter, P.D. , Foligné, B. et al. (2017) Health benefits of fermented foods: microbiota and beyond. Current Opinion in Biotechnology, 44, 94–102. [DOI] [PubMed] [Google Scholar]
- Marco, M.L. , Hutkins, R. , Hill, C. , Fulgoni, V.L. , Cifelli, C.J. , Gahche, J. et al. (2022) A classification system for defining and estimating dietary intake of live microbes in US adults and children. The Journal of Nutrition, 152(7), 1729–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco, M.L. , Sanders, M.E. , Gänzle, M. , Arrieta, M.C. , Cotter, P.D. , De Vuyst, L. et al. (2021) The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nature Reviews Gastroenterology & Hepatology, 18(3), 196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh, A.J. , Hill, C. , Ross, R.P. & Cotter, P.D. (2014) Fermented beverages with health‐promoting potential: past and future perspectives. Trends in Food Science and Technology, 38(2), 113–124. [Google Scholar]
- Martín, I. , Rodríguez, A. & Córdoba, J.J. (2022) Application of selected lactic‐acid bacteria to control Listeria monocytogenes in soft‐ripened “Torta del Casar” cheese. Lebensmittel‐Wissenschaft und Technologie, 168, 113873. [Google Scholar]
- Martinez, R.C.R. , Alvarenga, V.O. , Thomazini, M. , Fávaro‐Trindade, C.S. & Sant'Ana, A.S. (2016) Assessment of the inhibitory effect of free and encapsulated commercial nisin (Nisaplin®), tested alone and in combination, on Listeria monocytogenes and Bacillus cereus in refrigerated milk. Lebensmittel‐Wissenschaft und Technologie, 68, 67–75. [Google Scholar]
- Mathur, H. , Beresford, T.P. & Cotter, P.D. (2020) Health benefits of lactic acid bacteria (LAB) fermentates. Nutrients, 12(6), 1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald, D. , Price, M.N. , Goodrich, J. , Nawrocki, E.P. , DeSantis, T.Z. , Probst, A. et al. (2012) An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME Journal, 6(3), 610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland, L.V. (2015) From yaks to yogurt: the history, development, and current use of probiotics. Clinical Infectious Diseases, 60(suppl 2), S85–S90. [DOI] [PubMed] [Google Scholar]
- Medawar, E. , Huhn, S. , Villringer, A. & Veronica Witte, A. (2019) The effects of plant‐based diets on the body and the brain: a systematic review. Translational Psychiatry, 9(1), 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena, K.K. , Taneja, N.K. , Jain, D. , Ojha, A. , Kumawat, D. & Mishra, V. (2022) In vitro assessment of probiotic and technological properties of lactic acid bacteria isolated from indigenously fermented cereal‐based food products. Fermentation, 8(10), 529. [Google Scholar]
- Melini, F. , Melini, V. , Luziatelli, F. , Ficca, A.G. & Ruzzi, M. (2019) Health‐promoting components in fermented foods: an up‐to‐date systematic review. Nutrients, 11(5), 1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merenstein, D.J. , Foster, J. & D'Amico, F. (2009) A randomized clinical trial measuring the influence of kefir on antibiotic‐associated diarrhea. Archives of Pediatrics & Adolescent Medicine, 163(8), 750–754. [DOI] [PubMed] [Google Scholar]
- Milani, C. , Duranti, S. , Napoli, S. , Alessandri, G. , Mancabelli, L. , Anzalone, R. et al. (2019) Colonization of the human gut by bovine bacteria present in Parmesan cheese. Nature Communications, 10(1), 1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra, S. (2012) Randomized double blind placebo control studies, the “gold standard” in intervention based studies. Indian Journal of Sexually Transmitted Diseases and AIDS, 33(2), 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi‐Kouchesfahani, M. , Hamidi‐Esfahani, Z. & Azizi, M.H. (2019) Isolation and identification of lactic acid bacteria with phytase activity from sourdough. Food Science & Nutrition, 7(11), 3700–3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojikon, F.D. , Kasimin, M.E. , Molujin, A.M. , Gansau, J.A. & Jawan, R. (2022) Probiotication of nutritious fruit and vegetable juices: an alternative to dairy‐based probiotic functional products. Nutrients, 14(17), 3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokoena, M.P. (2017) Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini‐review. Molecules, 22(8), 1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteagudo‐Mera, A. , Rastall, R.A. , Gibson, G.R. , Charalampopoulos, D. & Chatzifragkou, A. (2019) Adhesion mechanisms mediated by probiotics and prebiotics and their potential impact on human health. Applied Microbiology and Biotechnology, 103(16), 6463–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi, Z. & Kalanpour, N. (2019) Kefiran, a branched polysaccharide: preparation, properties and applications: a review. Carbohydrate Polymers, 223, 115100. [DOI] [PubMed] [Google Scholar]
- Murina, F. , Vicariotto, F. & Lubrano, C. (2021) Efficacy of an orally administered combination of Lactobacillus paracasei LC11, cranberry and D‐mannose for the prevention of uncomplicated, recurrent urinary tract infections in women. Urologia, 88(1), 64–68. [DOI] [PubMed] [Google Scholar]
- Mustafa, S.M. , Chua, L.S. , El‐Enshasy, H.A. , Abd Majid, F.A. , Hanapi, S.Z. & Abdul Malik, R. (2019) Effect of temperature and pH on the probiotication ofPunica granatumjuice using Lactobacillus species. Journal of Food Biochemistry, 43(4), e12805. [DOI] [PubMed] [Google Scholar]
- Nagata, S. , Asahara, T. , Wang, C. , Suyama, Y. , Chonan, O. , Takano, K. et al. (2016) The effectiveness of Lactobacillus beverages in controlling infections among the residents of an aged care facility: a randomized placebo‐controlled double‐blind trial. Annals of Nutrition & Metabolism, 68(1), 51–59. [DOI] [PubMed] [Google Scholar]
- Nielsen, E.S. , Garnås, E. , Jensen, K.J. , Hansen, L.H. , Olsen, P.S. , Ritz, C. et al. (2018) Lacto‐fermented sauerkraut improves symptoms in IBS patients independent of product pasteurisation – a pilot study. Food & Function, 9(10), 5323–5335. [DOI] [PubMed] [Google Scholar]
- Nielsen, S.D. , Jakobsen, L.M.A. , Geiker, N.R.W. & Bertram, H.C. (2022) Chemically acidified, live and heat‐inactivated fermented dairy yoghurt show distinct bioactive peptides, free amino acids and small compounds profiles. Food Chemistry, 376, 131919. [DOI] [PubMed] [Google Scholar]
- Norouzbeigi, S. , Vahid‐Dastjerdi, L. , Yekta, R. , Sohrabvandi, S. , Zendeboodi, F. & Mortazavian, A.M. (2020) Celiac therapy by administration of probiotics in food products: a review. Current Opinion in Food Science, 32, 58–66. [Google Scholar]
- Osimani, A. , Ferrocino, I. , Agnolucci, M. , Cocolin, L. , Giovannetti, M. , Cristani, C. , et al. (2019) Unveiling hákarl: A study of the microbiota of the traditional Icelandic fermented fish. Food Microbiology 82: 560–572. 10.1016/j.fm.2019.03.027 [DOI] [PubMed] [Google Scholar]
- Owade, J.O. , Abong', G.O. , Okoth, M.W. , Mwang'ombe, A.W. & Jobor, J.O. (2021) Comparative profiling of lactic acid bacteria isolates in optimized and spontaneous fermentation of cowpea leaves. Food Science & Nutrition, 9(3), 1651–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu‐Kwarteng, J. , Agyei, D. , Akabanda, F. , Atuna, R.A. & Amagloh, F.K. (2022) Plant‐based alkaline fermented foods as sustainable sources of nutrients and health‐promoting bioactive compounds. Frontiers in Sustainable Food Systems, 6, 885328. [Google Scholar]
- Panda, S.K. & Shetty, P.H. (2018) Innovations in technologies for fermented food and beverage industries. Berlin: Springer. [Google Scholar]
- Papadopoulou, O.S. , Doulgeraki, A. , Panagou, E. & Argyri, A.A. (2023) Editorial: recent advances and future perspective in probiotics isolated from fermented foods: from quality assessment to novel products. Frontiers in Microbiology, 14, 1150175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paramithiotis, S. , Stasinou, V. , Tzamourani, A. , Kotseridis, Y. & Dimopoulou, M. (2022) Malolactic fermentation—theoretical advances and practical considerations. Fermentation, 8(10), 521. [Google Scholar]
- Parvez, S. , Malik, K.A. , Ah Kang, S. & Kim, H.‐Y. (2006) Probiotics and their fermented food products are beneficial for health. Journal of Applied Microbiology, 100(6), 1171–1185. [DOI] [PubMed] [Google Scholar]
- Pasolli, E. , De Filippis, F. , Mauriello, I.E. , Cumbo, F. , Walsh, A.M. , Leech, J. et al. (2020) Large‐scale genome‐wide analysis links lactic acid bacteria from food with the gut microbiome. Nature Communications, 11(1), 2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehrson, A. & Sanchez, C. (2015) Altered γ‐aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Design, Development and Therapy, 9, 603–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessione, E. & Cirrincione, S. (2016) Bioactive molecules released in food by lactic acid bacteria: encrypted peptides and biogenic amines. Frontiers in Microbiology, 7, 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova, P. , Ivanov, I. , Tsigoriyna, L. , Valcheva, N. , Vasileva, E. , Parvanova‐Mancheva, T. et al. (2021) Traditional Bulgarian dairy products: ethnic foods with health benefits. Microorganisms, 9(3), 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer, E.L. , Danielewski, J.A. , Garland, S.M. , Su, J. , Jacobs, S.E. & Murray, G.L. (2021) The effect of probiotic supplementation on the gut microbiota of preterm infants. Journal of Medical Microbiology, 70(8), 001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polese, B. , Nicolai, E. , Genovese, D. , Verlezza, V. , La Sala, C.N. , Aiello, M. et al. (2018) Postprandial gastrointestinal function differs after acute administration of sourdough compared with brewer's yeast bakery products in healthy adults. The Journal of Nutrition, 148(2), 202–208. [DOI] [PubMed] [Google Scholar]
- Precup, G. & Vodnar, D.‐C. (2019) Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: a comprehensive literature review. The British Journal of Nutrition, 122(2), 131–140. [DOI] [PubMed] [Google Scholar]
- Quince, C. , Walker, A.W. , Simpson, J.T. , Loman, N.J. & Segata, N. (2017) Shotgun metagenomics, from sampling to analysis. Nature Biotechnology, 35(9), 833–844. [DOI] [PubMed] [Google Scholar]
- Rahardjo, Y.P. , Rahardja, S. , Samsudin, Saidah, Dalapati, A. , Amalia, A.F. et al. (2022) A literature review on cocoa fermentation techniques to shorten fermentation time. IOP Conference Series: Earth and Environmental Science, 974(1), 012111. [Google Scholar]
- Raninen, K. , Lappi, J. , Kolehmainen, M. , Kolehmainen, M. , Mykkänen, H. , Poutanen, K. et al. (2017) Diet‐derived changes by sourdough‐fermented rye bread in exhaled breath aspiration ion mobility spectrometry profiles in individuals with mild gastrointestinal symptoms. International Journal of Food Sciences and Nutrition, 68(8), 987–996. [DOI] [PubMed] [Google Scholar]
- Raoult, D. & Henrissat, B. (2014) Are stool samples suitable for studying the link between gut microbiota and obesity? European Journal of Epidemiology, 29(5), 307–309. [DOI] [PubMed] [Google Scholar]
- Rastogi, Y.R. , Thakur, R. , Thakur, P. , Mittal, A. , Chakrabarti, S. , Siwal, S.S. et al. (2022) Food fermentation – significance to public health and sustainability challenges of modern diet and food systems. International Journal of Food Microbiology, 371, 109666. [DOI] [PubMed] [Google Scholar]
- Raveschot, C. , Cudennec, B. , Coutte, F. , Flahaut, C. , Fremont, M. , Drider, D. et al. (2018) Production of bioactive peptides by Lactobacillus species: from gene to application. Frontiers in Microbiology, 9, 2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo‐Useros, N. , Gheorghe, A. , Díaz‐Prieto, L. , Villavisencio, B. , Marcos, A. & Nova, E. (2019) Associations of probiotic fermented milk (PFM) and yogurt consumption with Bifidobacterium and Lactobacillus components of the gut microbiota in healthy adults. Nutrients, 11(3), 651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezac, S. , Kok, C.R. , Heermann, M. & Hutkins, R. (2018) Fermented foods as a dietary source of live organisms. Frontiers in Microbiology, 9, 1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli, M. , Natella, F. , Zinno, P. , Guantario, B. , Canali, R. , Schifano, E. et al. (2021) Colonization ability and impact on human gut microbiota of foodborne microbes from traditional or probiotic‐added fermented foods: a systematic review. Frontiers in Nutrition, 8, 689084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, P.R. , Morgan, S. & Hill, C. (2002) Preservation and fermentation: past, present and future. International Journal of Food Microbiology, 79(1–2), 3–16. [DOI] [PubMed] [Google Scholar]
- Rul, F. & Monnet, V. (2015) How microbes communicate in food: a review of signaling molecules and their impact on food quality. Current Opinion in Food Science, 2, 100–105. [Google Scholar]
- Sanders, M.E. , Akkermans, L.M.A. , Haller, D. , Hammerman, C. , Heimbach, J.T. , Hörmannsperger, G. et al. (2010) Safety assessment of probiotics for human use. Gut Microbes, 1(3), 164–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, K.P. , Antoine, J.‐M. , Midtvedt, T. & van Hemert, S. (2015) Manipulating the gut microbiota to maintain health and treat disease. Microbial Ecology in Health and Disease, 26, 25877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata, N. (2015) Gut microbiome: westernization and the disappearance of intestinal diversity. Current Biology, 25(14), R611–R613. [DOI] [PubMed] [Google Scholar]
- Selhub, E.M. , Logan, A.C. & Bested, A.C. (2014) Fermented foods, microbiota, and mental health: ancient practice meets nutritional psychiatry. Journal of Physiological Anthropology, 33(1), 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequino, G. , Valentino, V. , Villani, F. & De Filippis, F. (2022) Omics‐based monitoring of microbial dynamics across the food chain for the improvement of food safety and quality. Food Research International, 157, 111242. [DOI] [PubMed] [Google Scholar]
- Siedler, S. , Rau, M.H. , Bidstrup, S. , Vento, J.M. , Aunsbjerg, S.D. , Bosma, E.F. et al. (2020) Competitive exclusion is a major bioprotective mechanism of lactobacilli against fungal spoilage in fermented milk products. Applied and Environmental Microbiology, 86(7), e02312‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith‐Brown, P. , Morrison, M. , Krause, L. & Davies, P.S.W. (2016) Dairy and plant based food intakes are associated with altered faecal microbiota in 2 to 3 year old Australian children. Scientific Reports, 6(1), 32385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenburg, E.D. & Sonnenburg, J.L. (2019) The ancestral and industrialized gut microbiota and implications for human health. Nature Reviews Microbiology, 17(6), 383–390. [DOI] [PubMed] [Google Scholar]
- Soumahoro, S. , Ouattara, H.G. , Droux, M. , Nasser, W. , Niamke, S.L. & Reverchon, S. (2020) Acetic acid bacteria (AAB) involved in cocoa fermentation from Ivory Coast: species diversity and performance in acetic acid production. Journal of Food Science and Technology, 57(5), 1904–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanborough, T. , Fegan, N. , Powell, S.M. , Tamplin, M. & Chandry, P.S. (2017) Insight into the genome of Brochothrix thermosphacta, a problematic meat spoilage bacterium. Applied and Environmental Microbiology, 83(5), e02786‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavropoulou, D.A. , De Maere, H. , Berardo, A. , Janssens, B. , Filippou, P. , De Vuyst, L. et al. (2018) Species pervasiveness within the group of coagulase‐negative staphylococci associated with meat fermentation is modulated by pH. Frontiers in Microbiology, 9, 2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain, M.R. , Anandharaj, M. , Ray, R.C. & Parveen Rani, R. (2014) Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnology Research International, 2014, 250424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang, J.P. , Cotter, P.D. , Endo, A. , Han, N.S. , Kort, R. , Liu, S.Q. et al. (2020) Fermented foods in a global age: East meets West. Comprehensive Reviews in Food Science and Food Safety, 19(1), 184–217. [DOI] [PubMed] [Google Scholar]
- Tamang, J.P. , Watanabe, K. & Holzapfel, W.H. (2016) Review: diversity of microorganisms in global fermented foods and beverages. Frontiers in Microbiology, 7, 377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, M. , Frank, D.N. , Tshefu, A. , Lokangaka, A. , Goudar, S.S. , Dhaded, S.M. et al. (2019) Different gut microbial profiles in sub‐Saharan African and South Asian women of childbearing age are primarily associated with dietary intakes. Frontiers in Microbiology, 10, 1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor, B.C. , Lejzerowicz, F. , Poirel, M. , Shaffer, J.P. , Jiang, L. , Aksenov, A. et al. (2020) Consumption of fermented foods is associated with systematic differences in the gut microbiome and metabolome. mSystems, 5(2), e00901‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett, A. , Huang, K. D. , Asnicar, F. , Fehlner‐Peach, H. , Pasolli, E. , Karcher, N . et al. (2019) The Prevotella copri Complex Comprises Four Distinct Clades Underrepresented in Westernized Populations. Cell Host & Microbe 26(5): 666–679.e7. 10.1016/j.chom.2019.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tett, A. , Pasolli, E. , Masetti, G. , Ercolini, D. & Segata, N. (2021) Prevotella diversity, niches and interactions with the human host. Nature Reviews Microbiology, 19(9), 585–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch, K. , Labus, J. , Kilpatrick, L. , Jiang, Z. , Stains, J. , Ebrat, B. et al. (2013) Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology, 144(7), 1394–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova, A. , Soltys, K. , Kemenyova, P. , Karhanek, M. & Babinska, K. (2020) The influence of food intake specificity in children with autism on gut microbiota. International Journal of Molecular Sciences, 21(8), 2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo‐González, L. , Gutiérrez‐Carrillo, A.‐E. , Rodríguez‐Hernández, A.‐I. , del Rocío López‐Cuellar, M. & Chavarría‐Hernández, N. (2022) Bacteriocins produced by LAB isolated from cheeses within the period 2009–2021: a review. Probiotics and Antimicrobial Proteins, 14(2), 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu, M.‐Y. , Chen, H.‐L. , Tung, Y.‐T. , Kao, C.‐C. , Hu, F.‐C. & Chen, C.‐M. (2015) Short‐term effects of kefir‐fermented milk consumption on bone mineral density and bone metabolism in a randomized clinical trial of osteoporotic patients. PLoS One, 10(12), e0144231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan, I. , Dedeli, O. , Bor, S. & Ilter, T. (2015) Effects of a kefir supplement on symptoms, colonic transit, and bowel satisfaction score in patients with chronic constipation: a pilot study. The Turkish Journal of Gastroenterology, 25(6), 650–656. [DOI] [PubMed] [Google Scholar]
- Unno, T. , Choi, J.‐H. , Hur, H.‐G. , Sadowsky, M.J. , Ahn, Y.‐T. , Huh, C.‐S. et al. (2015) Changes in human gut microbiota influenced by probiotic fermented milk ingestion. Journal of Dairy Science, 98(6), 3568–3576. [DOI] [PubMed] [Google Scholar]
- Vázquez, C. , Botella‐Carretero, J.I. , García‐Albiach, R. , Pozuelo, M.J. , Rodríguez‐Baños, M. , Baquero, F. et al. (2013) Screening in a Lactobacillus delbrueckii subsp. bulgaricus collection to select a strain able to survive to the human intestinal tract. Nutrición Hospitalaria, 28(4), 1227–1235. [DOI] [PubMed] [Google Scholar]
- Veiga, P. , Pons, N. , Agrawal, A. , Oozeer, R. , Guyonnet, D. , Brazeilles, R. et al. (2014) Changes of the human gut microbiome induced by a fermented milk product. Scientific Reports, 4(1), 6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verni, M. , De Mastro, G. , De Cillis, F. , Gobbetti, M. & Rizzello, C.G. (2019) Lactic acid bacteria fermentation to exploit the nutritional potential of Mediterranean faba bean local biotypes. Food Research International, 125, 108571. [DOI] [PubMed] [Google Scholar]
- Vieco‐Saiz, N. , Belguesmia, Y. , Raspoet, R. , Auclair, E. , Gancel, F. , Kempf, I. et al. (2019) Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food‐animal production. Frontiers in Microbiology, 10, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, C.P. , Rosario, A.I.L.S. , Lelis, C.A. , Rekowsky, B.S.S. , Carvalho, A.P.A. , Rosário, D.K.A. et al. (2021) Bioactive compounds from kefir and their potential benefits on health: a systematic review and meta‐analysis. Oxidative Medicine and Cellular Longevity, 2021, 9081738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinderola, G. , Cotter, P.D. , Freitas, M. , Gueimonde, M. , Holscher, H.D. , Ruas‐Madiedo, P. et al. (2023) Fermented foods: a perspective on their role in delivering biotics. Frontiers in Microbiology, 14, 1196239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgin, H.W. & Todd, J.A. (2011) Metagenomics and personalized medicine. Cell, 147(1), 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokh, O. , Klimenko, N. , Berezhnaya, Y. , Tyakht, A. , Nesterova, P. , Popenko, A. et al. (2019) Human gut microbiome response induced by fermented dairy product intake in healthy volunteers. Nutrients, 11(3), 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett, L.P. (2020) Microbial meat substitutes. Microbial Biotechnology, 13(4), 1284–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh, A.M. , Leech, J. , Huttenhower, C. , Delhomme‐Nguyen, H. , Crispie, F. , Chervaux, C. et al. (2023) Integrated molecular approaches for fermented food microbiome research. FEMS Microbiology Reviews, 47(2), fuad001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wang, J. , Yang, K. , Liu, M. , Zhang, J. , Wei, X. et al. (2018) Screening for potential probiotic from spontaneously fermented non‐dairy foods based on in vitro probiotic and safety properties. Annales de Microbiologie, 68(12), 803–813. [Google Scholar]
- Wang, Z. , Neupane, A. , Vo, R. , White, J. , Wang, X. & Marzano, S.‐Y.L. (2020) Comparing gut microbiome in mothers' own breast milk‐ and formula‐fed moderate‐late preterm infants. Frontiers in Microbiology, 11, 891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wastyk, H.C. , Fragiadakis, G.K. , Perelman, D. , Dahan, D. , Merrill, B.D. , Yu, F.B. et al. (2021) Gut‐microbiota‐targeted diets modulate human immune status. Cell, 184(16), 4137–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widyastuti, Y. , Febrisiantosa, A. & Tidona, F. (2021) Health‐promoting properties of lactobacilli in fermented dairy products. Frontiers in Microbiology, 12, 673890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, T. , Guan, Q. , Song, S. , Hao, M. & Xie, M. (2012) Dynamic changes of lactic acid bacteria flora during Chinese sauerkraut fermentation. Food Control, 26(1), 178–181. [Google Scholar]
- Yang, H. , Zou, H. , Qu, C. , Zhang, L. , Liu, T. , Wu, H. et al. (2014) Dominant microorganisms during the spontaneous fermentation of Suan Cai, a Chinese fermented vegetable. Food Science and Technology Research, 20(5), 915–926. [Google Scholar]
- Ye, P. , Wang, J. , Liu, M. , Li, P. & Gu, Q. (2021) Purification and characterization of a novel bacteriocin from Lactobacillus paracasei ZFM54. Lebensmittel‐Wissenschaft und Technologie, 143, 111125. [Google Scholar]
- Yilmaz, B. , Bangar, S.P. , Echegaray, N. , Suri, S. , Tomasevic, I. , Manuel Lorenzo, J. et al. (2022) The impacts of Lactiplantibacillus plantarum on the functional properties of fermented foods: a review of current knowledge. Microorganisms, 10(4), 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz, I. , Dolar, M.E. & Ozpinar, H. (2020) Effect of administering kefir on the changes in fecal microbiota and symptoms of inflammatory bowel disease: a randomized controlled trial. The Turkish Journal of Gastroenterology, 30(3), 242–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapaśnik, A. , Sokołowska, B. & Bryła, M. (2022) Role of lactic acid bacteria in food preservation and safety. Food, 11(9), 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ze, X. , Duncan, S.H. , Louis, P. & Flint, H.J. (2012) Ruminococcus bromii is a keystone species for the degradation of resistant starch in the human colon. The ISME Journal, 6(8), 1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, L. , Huang, C. , Ning, Z. , Zhang, Y. , Zhuang, M. , Yang, W. et al. (2023) Ruminococcus gnavus plays a pathogenic role in diarrhea‐predominant irritable bowel syndrome by increasing serotonin biosynthesis. Cell Host & Microbe, 31(1), 33–44. [DOI] [PubMed] [Google Scholar]
- Zhang, C. , Derrien, M. , Levenez, F. , Brazeilles, R. , Ballal, S.A. , Kim, J. et al. (2016) Ecological robustness of the gut microbiota in response to ingestion of transient food‐borne microbes. The ISME Journal, 10(9), 2235–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Li, Q. , Cheng, L. , Buch, H. & Zhang, F. (2019) Akkermansia muciniphila is a promising probiotic. Microbial Biotechnology, 12(6), 1109–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Han, J. , Zheng, X. , Yan, J. , Chen, X. , Zhou, Q. et al. (2022) Use of Lactiplantibacillus plantarum ZJ316 as a starter culture for nitrite degradation, foodborne pathogens inhibition and microbial community modulation in pickled mustard fermentation. Food Chemistry: X, 14, 100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Zhu, L. , Dong, P. , Liang, R. , Mao, Y. , Qiu, S. et al. (2018) Bio‐protective potential of lactic acid bacteria: effect of Lactobacillus sakei and Lactobacillus curvatus on changes of the microbial community in vacuum‐packaged chilled beef. Asian‐Australasian Journal of Animal Sciences, 31(4), 585–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, J. , Wittouck, S. , Salvetti, E. , Franz, C.M.A.P. , Harris, H.M.B. , Mattarelli, P. et al. (2020) A taxonomic note on the genus Lactobacillus: description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae . International Journal of Systematic and Evolutionary Microbiology, 70(4), 2782–2858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Appendix S1


