Abstract
Aims:
We examined associations between lipoprotein subfractions and prevalent and incident T2D in two race/ethnically diverse cohort studies.
Methods:
Adults self-identifying as White, Black, Chinese, Hispanic and South Asian-American without cardiovascular disease, with fasting serum, demographic, and clinical data at enrollment and after 5 years of follow-up were included. Lipoprotein subfractions were measured at enrollment using NMR spectrometry. LASSO regularized logistic regression models adjusted for age, sex, race/ethnicity, lipid-lowering agent use, and waist circumference assessed odds of incident T2D in pooled analyses.
Results:
There were 4474 participants with lipoprotein subfraction data at enrollment and 3839 participants without prevalent diabetes, mean age 62 years, 51% women, with 234 incident T2D cases at 5 years. Triglycerides in small, dense LDL-5 [OR 1.26 (95% CI 1.11,1.43)], VLDL triglycerides 1.30** [1.16,1.46] and phospholipids in VLDL-1 [OR 1.31 (1.17,1.47)] were associated with higher odds of incident T2D, while free cholesterol in large HDL-1 [OR 0.75 (95% CI 0.63,0.89)] was inversely associated. The results were similar for prevalent diabetes and did not vary by race/ethnic group.
Conclusions:
Composition of lipoprotein subfractions is differentially associated with prevalent and incident T2D without difference by race/ethnic group. Assessment of lipoprotein composition may enhance targeted risk reduction for T2D.
Keywords: South Asian, disparities, lipidomics, type 2 diabetes
INTRODUCTION
Atherogenic dyslipidemia, defined as low HDL cholesterol, high triglycerides, and higher numbers of small, dense low-density lipoprotein (LDL) particles are common in individuals at risk for diabetes and cardiovascular disease. In particular, South Asians have a unique lipid profile which consists of elevated triglycerides and low HDL in spite of lower or normal LDL cholesterol (LDL-C) concentrations (1). It is unclear whether ethnic differences in lipoprotein subfraction influence type 2 diabetes (T2D) prevalence and incidence.
Recent use of NMR-directed technologies has allowed for lipoprotein size and subclass measurement and highlighted the relationship between T2D and both large VLDL and small, dense LDL particles (2, 3). Lipoprotein subclass analysis has identified associations between LDL particle number (LDL-P) and HDL-P and content of lipoprotein particles with cardiovascular disease (4, 5) that are robust and sometimes discordant with associations with typical clinical measurements of LDL-C and HDL-C. Other investigations have noted associations in lipoprotein subclasses with peripheral arterial disease (6), insulin resistance, and both type 1 and T2D and its associated complications (7). As changes in lipoprotein composition may precede insulin resistance and T2D, investigations centering on these profiles before the onset of T2D may yield insights into prediabetes risk.
This investigation aimed to determine the composition of lipoprotein subfractions in participants without cardiovascular disease in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) study compared with White, Black, Chinese American and Hispanic American participants of the Multi-Ethnic Study of Atherosclerosis (MESA) cohort study. The objective was to characterize and compare associations between the composition of lipid subfractions and prevalent and incident type 2 diabetes in MASALA and MESA participants of diverse ethnic groups.
METHODS
Participants
We included 3809 men and women from the Multi-Ethnic Study of Atherosclerosis (MESA) and 665 men and women from the Mediators of Atherosclerosis in South Asians Living in America (MASALA) longitudinal cohort studies with metabolomic profile data and no known cardiovascular disease at enrollment. Participants were excluded if they did not have NMR lipoprotein subfraction data available, (3005 participants from MESA and 241 participants from MASALA). Participants with prevalent diabetes (495 participants in MESA and 136 participants in MASALA) were excluded from analysis of incident diabetes. Three further participants were excluded from MASALA as they did not have data on prevalent diabetes at enrollment. (Figure 1)
Figure 1:
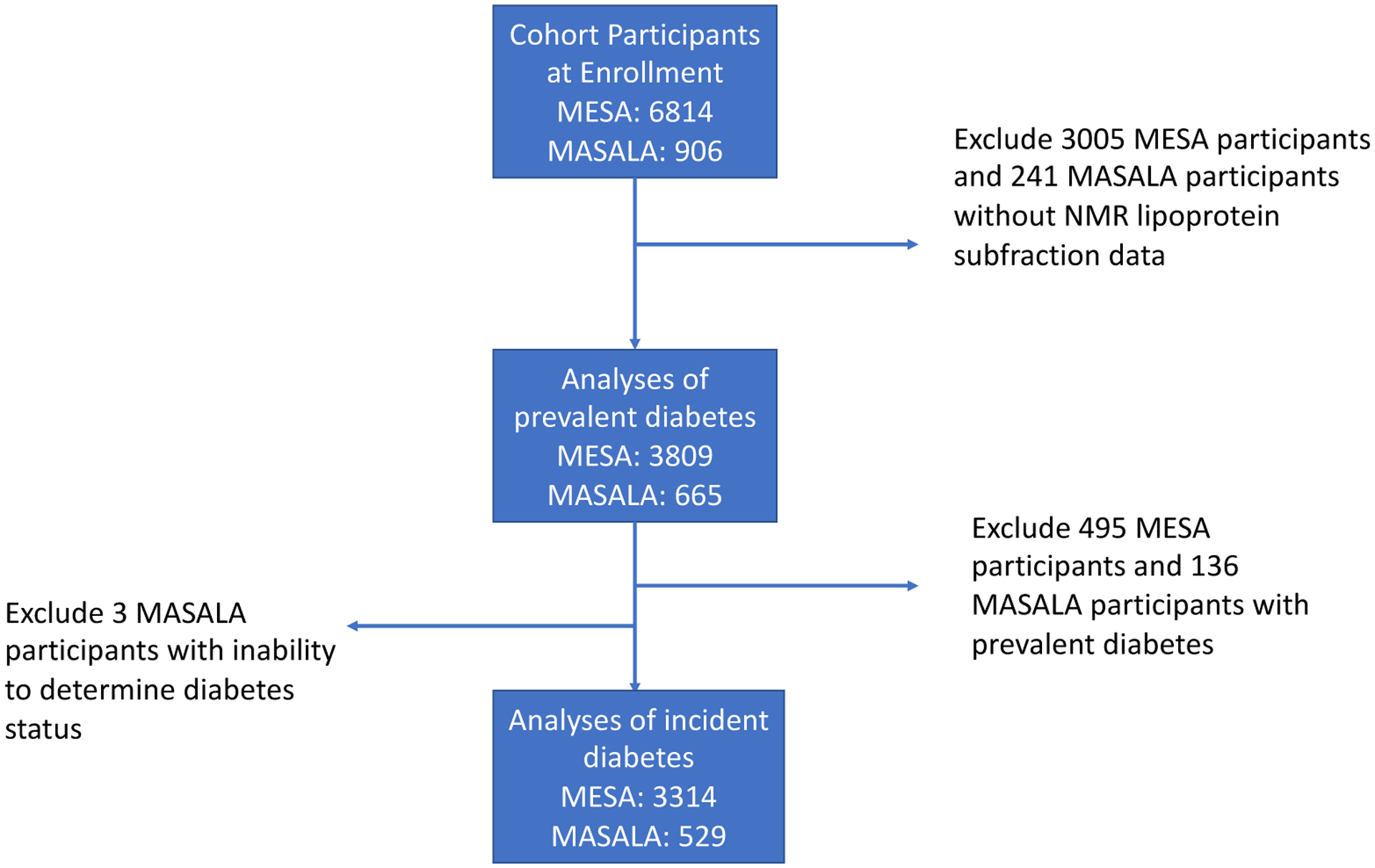
Flow Diagram for Study Inclusion
MESA is a U.S.-based prospective cohort study of 6814 participants between the ages of 45 to 84 years recruited at six sites (Baltimore City and County, Maryland; Chicago, Illinois; Forsyth County, North Carolina; New York, New York; Los Angeles County, California; and St. Paul, Minnesota), designed to investigate the development and progression of subclinical atherosclerotic disease. Participants were enrolled between 2000–2002 (8), did not have cardiovascular disease at enrollment and were purposively recruited from four race/ethnicity categories (Black, White, Chinese American and Hispanic). Institutional review board approval was obtained at all participating centers, and all participants gave informed consent.
MASALA is a prospective cohort study of community-dwelling individuals living in the San Francisco Bay Area and the greater Chicago areas from 2010–2013. Participants self-identified as having South Asian ancestry were aged 40–84 years and had no known cardiovascular disease, similar criteria to the MESA study (9, 10). The University of California, San Francisco and Northwestern University Institutional Review Board approved the study protocol and all study participants provided written informed consent.
In each cohort, participants underwent in-person interviews to determine age, gender, medical history, physical activity, smoking status and alcohol intake. Weight was determined using a digital scale, height with a stadiometer, and waist circumference using a measuring tape halfway between the lower ribs and the anterior superior iliac spine, at the site of greatest circumference.
A random sample of participants from MESA were retrospectively chosen for lipoprotein subfraction data generation using serum samples from the enrollment exam (2000–2002) as part of the COMBInatorial BIOmarkers for subclinical atherosclerosis (COMBI-Bio) consortium metabolomics analyses.(11, 12) In MASALA, only participants who had follow-up exam 2 data were chosen retrospectively for lipoprotein subfraction data from samples gathered at enrollment (2010–2013). The most recent follow-up data in MASALA is available for Exam 2 (2015–2018; mean of 4.8 years follow-up), therefore we used the same follow-up time in the MESA study which occurred at Exam 4 (2005–2007; mean of 4.8 years follow-up).
Cardiometabolic factors measured at enrollment
MESA:
Serum glucose was measured from fasting samples by the glucose oxidase method (Ortho Clinical Diagnostics, Johnson & Johnson). Serum insulin was measured from enrollment samples with the Beckman Access assay. To harmonize this insulin assay with newer-generation assays with the Roche Elecsys assay that were used in future MESA exams (as well as the MASALA study), a calibration study was performed to calculate a formula for serum insulin values that correlated with the Roche method. The calibration formula is as follows: calibrated insulin = 1.656 + [0.208 3 (Beckman Access assay result 3 6)].
MASALA:
Fasting plasma glucose was measured using the hexokinase method (Quest diagnostics, San Jose, CA).
Diabetes was defined as a fasting glucose ≥126 mg/dl or use of a glucose-lowering medication in both cohort studies. Prevalent diabetes indicates those who meet this definition at enrollment examination.
Incident Diabetes
Data on incident diabetes, using the same definition as described for assessment of type 2 diabetes at enrollment, in each cohort was obtained at a mean follow-up time of 4.8 years.
Metabolic Profiling by NMR
Lipid subfractions were measured by Bruker IVDr Lipoprotein Subclass Analysis (B.I. LISA) NMR mass spectrometry from baseline samples in both cohorts. Full details of NMR metabolomic analysis protocols have been previously published (11, 13). Quantification of 105 lipoprotein subclasses for targeted NMR analyses was performed by the in vitro diagnostics platform (IVDr) from Bruker Biospin (www.bruker.com).(14) This analysis included the following lipoprotein subfractions: intermediate-density lipoprotein (density 1.006–1.019 kg/L), very low-density lipoproteins (VLDL, 0.950–1.006kg/L), LDL (density 1.09–1.63 kg/L), and HDL (density 1.063–1.210 kg/L). The LDL subfraction was fractionated into six density classes (LDL1 1.019–1.031 kg/L, LDL2 1.031–1.034 kg/L, LDL3 1.034–1.037 kg/L, LDL4 1.037–1.040 kg/L, LDL5 1.040–1.044 kg/L, and LDL6 1.044–1.063 kg/L), VLDL and the HDL subfraction in four density classes (HDL1 1.063–1.100 kg/L, HDL2 1.100–1.125 kg/L, HDL3 1.125–1.175 kg/L, and HDL4 1.175–1.210 kg/L (11, 15). Naming of lipoprotein subfractions, (e.g. LDL1 to LDL6), is such that size and density decrease as the subfraction number increases. Thus, higher subfraction numbers represent smaller, more dense lipoproteins.
Statistical methods
Before modeling, relative abundance of lipoprotein subfractions were log-transformed to reduce the potential for outliers to influence the model. Lipoprotein subfraction profile comparisons were assessed at enrollment using t tests, ANOVA, Spearmen rank correlation or Kruskal Wallis tests by each of the 5 race/ethnic groups. To adjust for unreliable parameter estimates that may occur when using multiple regression models in the setting of multicollinearity, we performed a least absolute shrinkage and selection operator (Lasso) regression model to evaluate metabolites that were significant in independent analyses. The Lasso model allowed for a penalized logistic regression on all biomarkers simultaneously to identify the most predictive metabolites for incident diabetes.
Multiple logistic regression analyses using standardized regression estimates were used to determine associations of relative abundance of each log-transformed lipoprotein subfraction chosen by the Lasso model with prevalent diabetes and incident diabetes at 5 years, adjusted for age, sex, cholesterol-lowering medication use and waist circumference. We assessed the association of lipoprotein subfractions with incident diabetes in a subset of participants who were not taking cholesterol-lowering medications at enrollment in a subgroup analysis. BMI was excluded due to collinearity with waist circumference. In pooled analyses of the MESA and MASALA cohorts, after excluding those with prevalent diabetes at enrollment, multivariable logistic regression modeling was used to assess lipoprotein subfractions at enrollment with 5-year incident diabetes. We performed a GLM model including an interaction term to determine if there were different associations by race/ethnicity and the conservative Bonferroni method to adjust for multiple comparisons, with a p<0.002 deemed significant.
The analysis was completed using STATA (version 16.1, 2022, College Station, TX, USA).
RESULTS
A total of 4474 MASALA and MESA study participants with lipoprotein subfraction data at enrollment were analyzed. (Table 1 and Figure 1) South Asian study participants from MASALA were approximately five years younger, with a higher prevalence of any lipid-lowering medication use overall (43%) than participants of the MESA study, who were enrolled 10 years earlier. At enrollment, South Asians had the lowest total cholesterol [Mean 187 (SD 36)] mg/dL and LDL-C 110 (SD 32) mg/dL compared to the other groups. Chinese American and South Asian participants had a lower BMI and lower waist circumference, and Chinese Americans had the highest fasting triglycerides [132 mg/dL (SD 74 mg/dL; p<0.0001) compared with other race/ethnic groups.
Table 1:
Characteristics of MESA (n=3809) and MASALA (n=665) study participants at enrollment, mean (SD) unless otherwise specified
| White (N=1479) | Chinese (N=516) | Black (N=920) | Hispanic (N=894) | South Asian (N=665) | pvalue | |
|---|---|---|---|---|---|---|
| Age, years | 63.0 (10.1) | 63.5 (10.4) | 62.6 (10.2) | 62.6 (10.5) | 57.0 (8.5) | <0.001 |
| Men, n (%) | 742 (50.2) | 258 (50.0) | 453 (49.2) | 435 (48.7) | 296 (44.7) | 0.20 |
| BMI (kg/m2) | 27.7 (4.9) | 23.9 (3.3) | 29.8 (5.8) | 29.4 (5.2%) | 25.9 (4.0) | <0.001 |
| Waist circumference (cm) | 97.9 (14.1) | 87.6 (9.4) | 100.8 (14.5) | 101.0 (13.1) | 92.9 (10.2) | <0.001 |
| Hemoglobin A1c (%) | 6.8 (1.1) | 7.3 (1.5) | 7.6 (1.8) | 7.8 (1.9) | 7.3 (1.2) | <0.001 |
| Type 2 diabetes, n (%) | 89 (6%) | 74 (14.4%) | 157 (17.1%) | 175 (19.6%) | 136 (20.5%) | <0.001 |
| Lipid lowering medication use, n (%) | 261 (17.6%) | 84 (16.3%) | 155 (16.8%) | 130 (14.5%) | 287 (43.2%) | <0.001 |
| Statin use, n (%) | 247 (16.7%) | 71 (13.8%) | 139 (15.2%) | 117 (13.1%) | 185 (27.8%) | <0.001 |
| Total cholesterol (mg/dL) | 196 (35) | 194 (32) | 189 (38) | 198 (38) | 187 (37) | <0.001 |
| LDL Cholesterol (mg/dL) | 117 (29) | 116 (29) | 116 (34) | 120 (33) | 110.7 (32) | <0.001 |
| HDL Cholesterol (mg/dL) | 52 (16) | 50 (12) | 52 (15) | 47 (13) | 50.2 (13) | <0.001 |
| Triglycerides (mg/dL) | 134 (101) | 145 (91) | 105 (64) | 161 (101) | 132 (74) | <0.001 |
Lipoprotein subfractions were examined among participants who were not taking lipid-lowering medications, and the results were comparable to the larger population. (Supplemental Table 1) Among these participants, South Asians had lower concentrations of LDL 118 (26) mg/dL than White participants with 140 (34) mg/dL, 136±35mg/dL in Chinese Americans, 136 (40) mg/dL in Black and 138 (38) mg/dL in Hispanic participants). South Asians also had lower triglycerides in LDL 16 (4) than other groups. Participants identifying as Hispanic had the highest total plasma triglycerides at 175 (95) and triglycerides in VLDL particles 112 (68) compared with other ethnic groups (p<0.001). South Asian participants had lower free cholesterol in HDL particles 12 (3) than other groups. Anti-atherogenic particles in South Asians, HDL-1 to HDL-4, also carried lower concentrations of triglycerides, cholesterol, and free cholesterol (p<0.0001).
Prevalent Diabetes
In a pooled analysis of MESA and MASALA participants, triglycerides in multiple lipoprotein subfractions were associated with prevalent diabetes, with results depicted in Figure 2 and Supplemental Table 3. Total cholesterol (OR 1.29 [95% CI 1.16,1.43]), triglycerides (OR 1.58 [95% CI 1.44, 1.73]) and phospholipids in VLDL-5 1.31** [1.19,1.46] were associated with incident T2D. Triglycerides in HDL-4 (OR 1.15 [95% CI 1.08, 1.22]), HDL-3 (OR 1.20 [95% CI 1.11, 1.29]) and HDL-2 (OR 1.22 [95% CI 1.12, 1.32]) were positively associated with prevalent type 2 diabetes. (Table 2) Conversely, free cholesterol in HDL-3 (OR 0.78 [95% CI 0.71, 0.85]), HDL-4 (OR 0.79 [95% CI 0.73, 0.85]) and LDL-4 (OR 0.88 [95% CI 0.85, 0.91]) were associated with lower odds of prevalent diabetes. There were no interactions between race/ethnicity and lipoprotein subfraction for prevalent diabetes.
Figure 2:
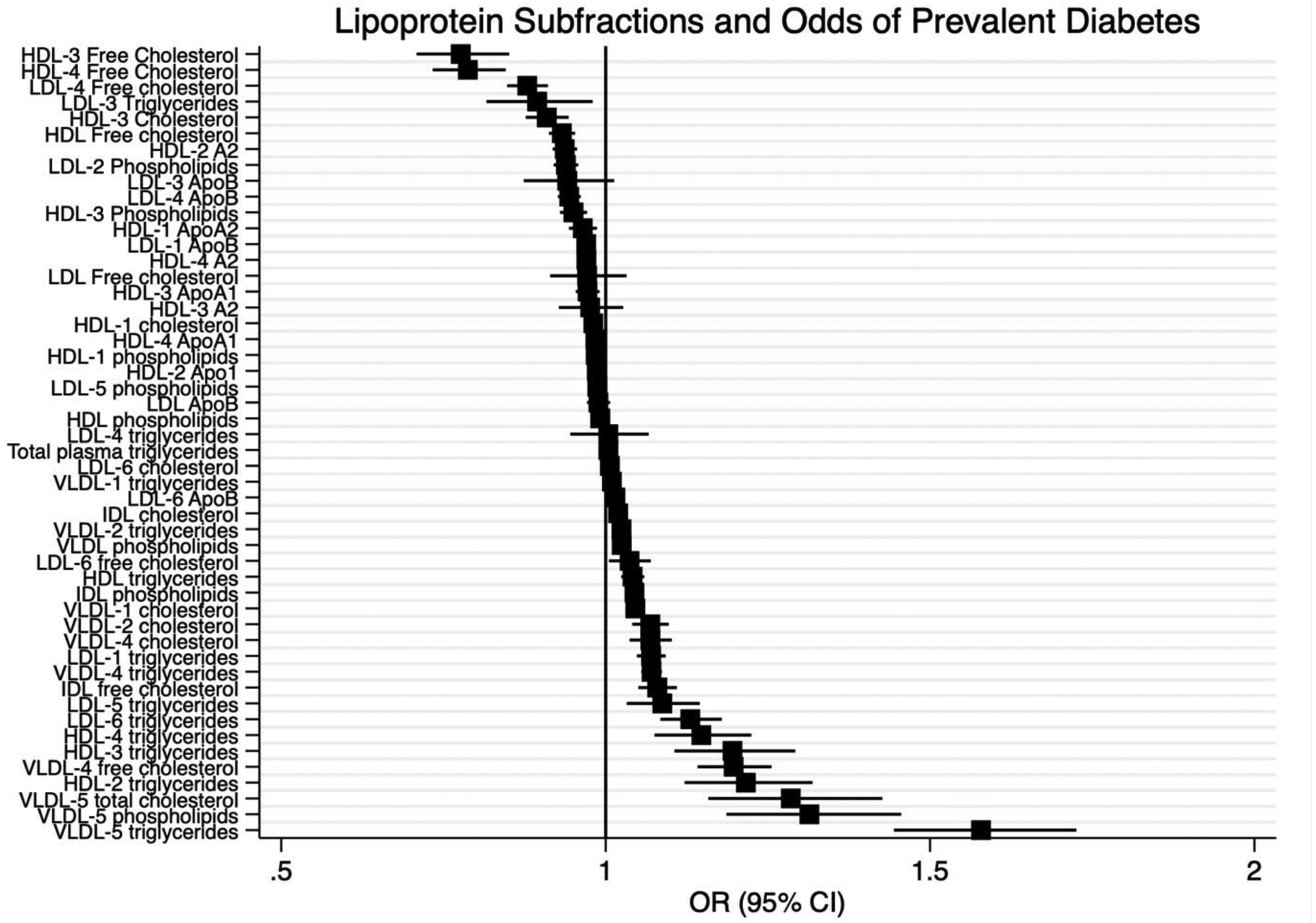
Lipoprotein Subfractions and Odds of Prevalent Diabetes; We used logistic regression to determine an odds ratio for each lipoprotein subfraction and odds of prevalent diabetes in the pooled MESA and MASALA cohort studies. Odds Ratio (95% CI) standardized regression coefficients (n=4477).
Incident Diabetes
In a pooled analysis of MESA and MASALA participants without diabetes at enrollment, we included 3839 participants with an average age of 62 years of which 51% were women, with a mean BMI 28 kg/m2 and observed 234 incident T2D cases after approximately 5 years of follow-up. (Supplemental Table 2) The results are shown in Figure 3 and Supplemental Table 4. Free cholesterol in HDL-1 (OR 0.75 [95% CI 0.63, 0.89]) was inversely associated with incident diabetes. Triglycerides in overall VLDL (OR 1.30 [95% CI 1.16, 1.46]), IDL (OR 1.21 95% [CI 1.08, 1.36]), LDL-6 (OR 1.25 [95% CI 1.10, 1.47]), LDL-5 (OR 1.26 [95% CI 1.11, 1.43]) and in VLDL-2 (OR 1.25 [95% CI [1.10, 1.41]) were associated with incident diabetes (all p<0.002). Phospholipids in VLDL-1 (OR 1.31 [95% CI 1.17, 1.47]) were also associated with incident diabetes. There were no interactions between race/ethnicity and lipoprotein subfraction for incident diabetes. In a subset of 3143 participants without diabetes who were not taking lipid-lowering medications at enrollment, (Supplemental Table 5) only VLDL-phospholipids (OR 1.05 [95% CI 1.03, 1.07]) and LDL-6 Triglycerides (OR 1.11 [95% CI 1.04, 1.19]) were associated with odds of incident T2D. Overall, triglycerides in VLDL and small, dense LDL were associated with incident T2D.
Figure 3:
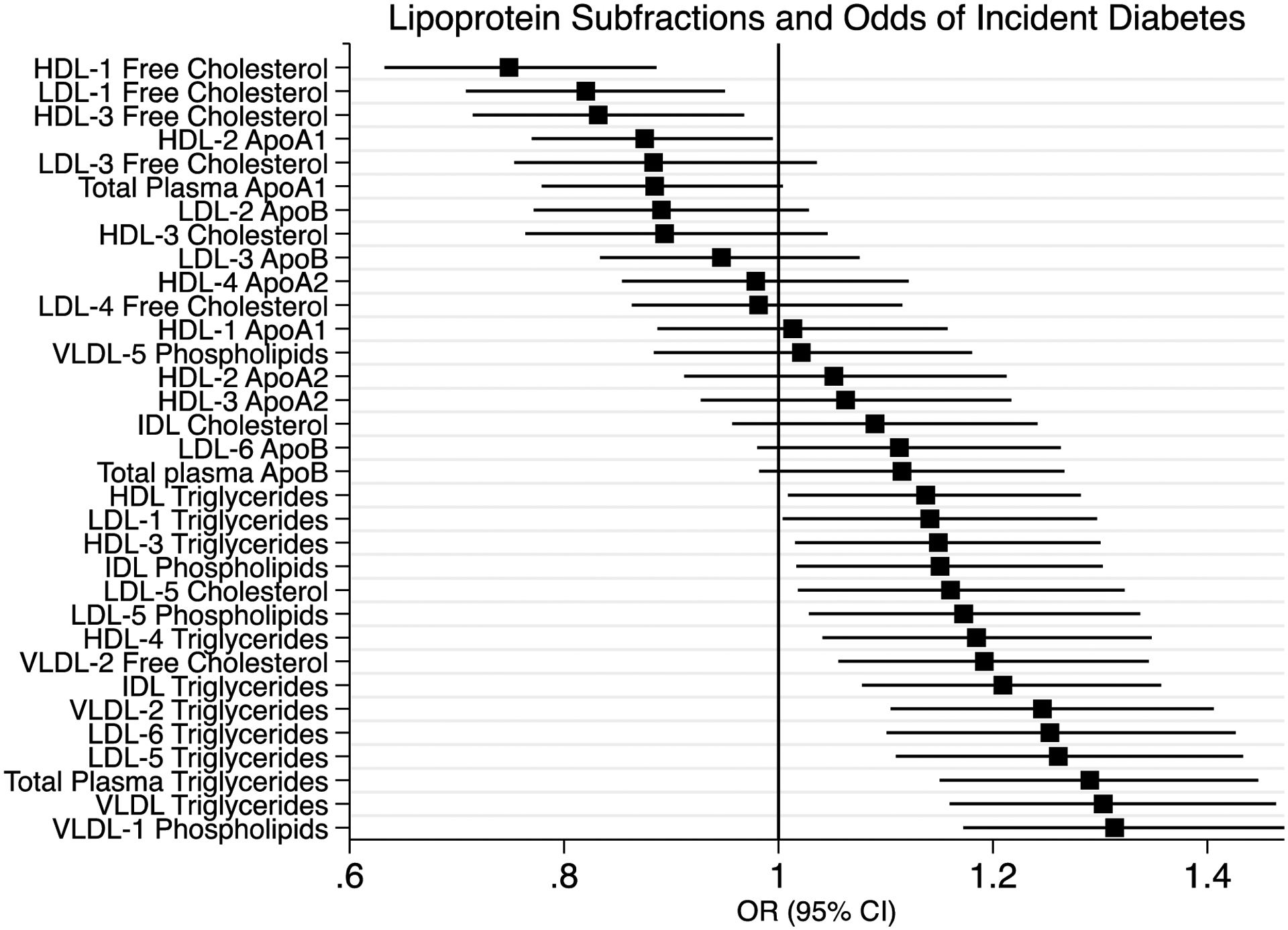
Lipoprotein Subfractions and Odds of Incident Diabetes; We used logistic regression to determine an odds ratio for each lipoprotein subfraction and odds of incident diabetes at 5 years in the pooled MESA and MASALA cohort studies. We excluded participants with diagnosed type 2 diabetes at enrollment and determine Odds Ratio (95% CI) standardized regression coefficients (n=3839).
DISCUSSION
In a pooled analysis of five race and ethnic groups in the United States (non-Hispanic White, Black, Hispanic, Chinese, and South Asian Americans), triglycerides in small, dense LDL and large VLDL were positively associated with incidence of type 2 diabetes while free cholesterol in large HDL were inversely associated after 5 years of follow-up. We did not find significant race/ethnic differences in association of lipoprotein subfractions with odds of incident diabetes. Our findings suggest that lipoprotein subfraction analysis assessing the composition of lipoproteins may be a tool in risk analysis for personalized medicine to determine risk for T2D.
Atherogenic dyslipidemia is represented by a triad of lipid changes: high plasma triglycerides, low HDL levels and higher concentrations of small, dense LDL (7). This triad has been associated with glycemic impairment and often precedes the onset of type 2 diabetes (1, 16). Our findings are in line with this prior work; in this analysis, triglycerides in small, dense LDL were associated with greater odds of incident T2D. Prior investigation has focused more frequently on lipoprotein particle size, with various epidemiologic studies showing associations between small LDL and HDL particles and prevalent diabetes(3, 17), including in the MESA cohort.(2) Another analysis created a “Diabetes Risk Index” including lipoprotein subfractions based on size and determined to be associated with insulin resistance as well as branched-chain amino acids.(18) This risk index was associated with a 12-fold higher hazard of developing T2D over eight years. Prior work has also shown that lipoprotein particle numbers are modifiable; participation in the intensive lifestyle group of the Diabetes Prevention Program study led to decreases in small and dense LDL-P (19). Our analysis focused on the composition of lipoprotein subfractions, and found a small increase in odds of incident diabetes with a higher concentration of triglycerides in large VLDL particles, concordant with prior investigations. This builds upon work in the MESA study showing that larger VLDL size and the presence of overall triglycerides are associated with incident T2D.(2)
There is a strong evidence base for the association between high triglyceride levels and future T2D.(20–22) The mechanisms of lipid-induced glucose intolerance are under investigation, and are hypothesized to be the consequence of a dual metabolic actions which drive hyperinsulinemia in the context of a glucose challenge. First, it is postulated that the presence of excess lipids, specifically triglycerides, lowers whole-body insulin sensitivity, and therefore a degradation in the response to an oral glucose challenge. Second, elevated triglycerides decrease insulin clearance, and may have direct effects on insulin secretion in the context of a glucose challenge, compounding a state of hyperinsulinemia.(23)
In the current analysis, triglycerides in small LDL and large VLDL were associated with incident T2D. Triglyceride-rich LDL particles are formed when the concentrations of triglyceride-rich remnant proteins overwhelm the activity of lipoprotein lipase, leading to aberrant VLDL metabolism (24). This enzyme is then unable to convert the remnant proteins into LDL particles depleted of triglycerides, therefore concentrations of triglyceride-rich LDL particles increase and there is decreased clearance of large VLDL remnant particles (24). In a prior analysis of triglyceride-rich lipoprotein subfractions, large and very large triglyceride-rich lipoprotein particles were associated with higher risk for T2D, suggesting triglyceride-rich large VLDL accumulation (25). In that study, investigators postulated that increasing ectopic adiposity leads to VLDL and LDL accumulation in pancreatic tissue, causing direct toxicity to pancreatic beta cells and ultimately tipping glucose homeostasis to T2D (25, 26). Individuals who go on to develop T2D have early excess hepatic production of VLDL, resulting in higher large VLDL particle number and increased VLDL size.(7, 17) Separately, an investigation in 630 adolescents without diabetes showed an association between triglyceride-rich VLDL and insulin secretion from beta cells, both basal and when stimulated by a glucose challenge.(27) Taken together, the presence of LDL particles rich in triglycerides may signal an overall increase in triglyceride remnant particles and direct effects on insulin secretion leading to hyperinsulinemia associated with preclinical metabolic abnormalities and high risk for T2D. This investigation bolsters and extends earlier work on the presence of triglycerides in LDL and VLDL and their associations with incident T2D through investigating size and composition of lipoprotein subfractions.
Higher concentrations of free cholesterol in large HDL were associated with lower risk for incident diabetes in this study. It has been suggested that free, or unesterified, cholesterol is a marker of glycemic control and plays a role in atherogenesis. In the Diabetes Prevention Program (DPP) study, the intensive lifestyle intervention was associated with increases in large HDL-P concentrations and an overall increase in HDL size (19). In the PREDIMED study, participants in the highest tertile of nut consumption over 1 year had higher increases in large HDL particles, together suggesting that healthy lifestyle interventions can beneficially influence a profile of atherogenic dyslipidemia (28). Separately, HDL particle size and subclass has previously been linked with incident coronary heart disease in individuals with diabetes; large and medium HDL subclasses were inversely associated with incident CHD while small and very small HDL were positively associated (29).
This investigation did not find differences in the relationship of lipoprotein subfractions with prevalent or incident diabetes by race or ethnicity. In analyses at enrollment, there were notable differences in the lipoprotein subfraction profiles of South Asian participants, including lower concentrations of triglycerides and cholesterol in LDL particles and higher ApoB100 in large LDL particles. Prior work has shown differences in lipid profiles in South Asians, including smaller, more dense LDL (30), as well as lower HDL-2 (31) that limits cholesterol-clearing capacity. These findings suggest that the composition of lipoprotein subfractions do not have differential relationships by race/ethnicity with future T2D. However, the relationships between free cholesterol in HDL and incident T2D in this analysis suggest that metabolic dyslipidemia and the presence of triglyceride remnant particles and lower HDL subfractions may be a culprit in increased risk for T2D across all populations. It is also possible that evaluation of lipoprotein subfractions in a sample of participants of younger age may show more prominent lipoprotein profile differences preceding the onset of T2D and cardiovascular disease, and thus support the role for NMR-based lipoprotein subfraction analysis to target primary prevention.
The goal of this analysis was to identify whether sub-fractionation of lipoproteins and more detailed information about lipid composition helps to explains differential diabetes risk. We acknowledge limitations in this analysis, including measurement of NMR spectroscopy at only one time point, which does not allow evaluation of changes in lipoprotein subfractions over time. The definitions of prevalent and incident diabetes used in this analysis are based on a single time point fasting glucose and use of glucose-lowering medications and do not match current ADA/EASD criteria as they were captured in the context of data collection at enrollment in each study. There has been limited follow-up in the MASALA study, therefore assessment of incident diabetes in both cohorts was limited to 5 years, and longer follow-up may yield more robust insights. Additionally, the limited number of participants in each race/ethnic group, especially after excluding those on any lipid lowering medications, may not provide adequate power to see differences between these groups. Given the study design, were unable to compare trajectories to the onset of diabetes, therefore could not determine if this information would improve the timing of prediction for diabetes. Despite these limitations, the strength of this investigation remains the comprehensive demographic, clinical, longitudinal data on two prospective cohorts of individuals without known cardiovascular disease and with NMR spectroscopy measured on the same platform.
In conclusion, in both the MESA and MASALA studies, triglycerides in small, dense LDL and large VLDL were associated with higher odds of incident T2D, while the presence of free cholesterol in large HDL was linked with lower odds of incident T2D. Future work is needed to determine whether using NMR-based measurement of lipoprotein subfractions may lead to earlier diagnosis and lifestyle management and the potential for targeted pharmacologic therapies in all race/ethnic groups.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the other investigators, the staff, and the participants of the MESA and MASALA studies for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
FUNDING SOURCES
MDG is supported by K23DK119404 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The MASALA study was supported by Grant Number R01HL093009 from the National Heart, Lung, And Blood Institute and the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through UCSF-CTSI Grant Number UL1RR024131. The metabolomic measurements were supported by an anonymous gift to Dr. Kanaya. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health.
COMBI-BIO Research supported by EU COMBI-BIO project (FP7, 305422), and NIH/NHLBI (R01HL133932).
MESA Study research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS).
DISCLOSURE
Dr. Gadgil is supported by funding from NIH-NIDDK and the MASALA and MESA studies are supported by funding from NIH-NHLBI. The authors declare no financial conflicts of interest.
REFERENCES CITED
- 1.Misra A, Shrivastava U. Obesity and dyslipidemia in South Asians. Nutrients. 2013;5(7):2708–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mackey RH, Mora S, Bertoni AG, Wassel CL, Carnethon MR, Sibley CT, et al. Lipoprotein particles and incident type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 2015;38(4):628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao X, Zhang HW, Zhang Y, Li S, Xu RX, Sun J, et al. Analysis of Lipoprotein Subfractions in 920 Patients With and Without Type 2 Diabetes. Heart Lung Circ. 2017;26(3):211–8. [DOI] [PubMed] [Google Scholar]
- 4.Holmes MV, Millwood IY, Kartsonaki C, Hill MR, Bennett DA, Boxall R, et al. Lipids, Lipoproteins, and Metabolites and Risk of Myocardial Infarction and Stroke. J Am Coll Cardiol. 2018;71(6):620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansen MO, Vedel-Krogh S, Nielsen SF, Afzal S, Davey Smith G, Nordestgaard BG. Per-Particle Triglyceride-Rich Lipoproteins Imply Higher Myocardial Infarction Risk Than Low-Density Lipoproteins: Copenhagen General Population Study. Arterioscler Thromb Vasc Biol. 2021;41(6):2063–75. [DOI] [PubMed] [Google Scholar]
- 6.Tikkanen E, Jagerroos V, Holmes MV, Sattar N, Ala-Korpela M, Jousilahti P, et al. Metabolic Biomarker Discovery for Risk of Peripheral Artery Disease Compared With Coronary Artery Disease: Lipoprotein and Metabolite Profiling of 31 657 Individuals From 5 Prospective Cohorts. J Am Heart Assoc. 2021;10(23):e021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225–36. [DOI] [PubMed] [Google Scholar]
- 8.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. American Journal of Epidemiology. 2002;156(9):871–81. [DOI] [PubMed] [Google Scholar]
- 9.Kanaya AM, Kandula N, Herrington D, Budoff MJ, Hulley S, Vittinghoff E, et al. Mediators of Atherosclerosis in South Asians Living in America (MASALA) Study: Objectives, Methods, and Cohort Description. Clinical cardiology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanaya AM, Herrington D, Vittinghoff E, Ewing SK, Liu K, Blaha MJ, et al. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: the MASALA and MESA studies. Diabetes care. 2014;37(6):1621–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karaman I, Ferreira DL, Boulange CL, Kaluarachchi MR, Herrington D, Dona AC, et al. Workflow for Integrated Processing of Multicohort Untargeted 1H NMR Metabolomics Data in Large-Scale Metabolic Epidemiology. Journal of proteome research. 2016;15(12):4188–94. [DOI] [PubMed] [Google Scholar]
- 12.Tzoulaki I, Castagne R, Boulange CL, Karaman I, Chekmeneva E, Evangelou E, et al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur Heart J. 2019;40(34):2883–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.al. DACJBSHe. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Analytical Chemistry. 2014;86(19):9887–94. [DOI] [PubMed] [Google Scholar]
- 14.Jimenez B, Holmes E, Heude C, Tolson RF, Harvey N, Lodge SL, et al. Quantitative Lipoprotein Subclass and Low Molecular Weight Metabolite Analysis in Human Serum and Plasma by (1)H NMR Spectroscopy in a Multilaboratory Trial. Anal Chem. 2018;90(20):11962–71. [DOI] [PubMed] [Google Scholar]
- 15.Petersen M, Dyrby M, Toubro S, Engelsen SB, Norgaard L, Pedersen HT, et al. Quantification of lipoprotein subclasses by proton nuclear magnetic resonance-based partial least-squares regression models. Clin Chem. 2005;51(8):1457–61. [DOI] [PubMed] [Google Scholar]
- 16.Flowers E, Molina C, Mathur A, Reaven GM. Use of plasma triglyceride/high-density lipoprotein cholesterol ratio to identify increased cardio-metabolic risk in young, healthy South Asians. The Indian journal of medical research. 2015;141(1):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Festa A, Williams K, Hanley AJ, Otvos JD, Goff DC, Wagenknecht LE, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111(25):3465–72. [DOI] [PubMed] [Google Scholar]
- 18.Flores-Guerrero JL, Gruppen EG, Connelly MA, Shalaurova I, Otvos JD, Garcia E, et al. A Newly Developed Diabetes Risk Index, Based on Lipoprotein Subfractions and Branched Chain Amino Acids, is Associated with Incident Type 2 Diabetes Mellitus in the PREVEND Cohort. Journal of Clinical Medicine. 2020;9(9):2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldberg R, Temprosa M, Otvos J, Brunzell J, Marcovina S, Mather K, et al. Lifestyle and metformin treatment favorably influence lipoprotein subfraction distribution in the Diabetes Prevention Program. J Clin Endocrinol Metab. 2013;98(10):3989–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tirosh A, Shai I, Bitzur R, Kochba I, Tekes-Manova D, Israeli E, et al. Changes in triglyceride levels over time and risk of type 2 diabetes in young men. Diabetes Care. 2008;31(10):2032–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer EJ, McNamara JR, Shah PK, Nakajima K, Cupples LA, Ordovas JM, et al. Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care. 2002;25(6):989–94. [DOI] [PubMed] [Google Scholar]
- 22.Perry IJ, Wannamethee SG, Walker MK, Thomson AG, Whincup PH, Shaper AG. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. Bmj. 1995;310(6979):560–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tricò D, Mengozzi A, Baldi S, Bizzotto R, Olaniru O, Toczyska K, et al. Lipid-induced glucose intolerance is driven by impaired glucose kinetics and insulin metabolism in healthy individuals. Metabolism. 2022;134:155247. [DOI] [PubMed] [Google Scholar]
- 24.Packard CJ, Boren J, Taskinen MR. Causes and Consequences of Hypertriglyceridemia. Front Endocrinol (Lausanne). 2020;11:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sokooti S, Flores-Guerrero JL, Heerspink HJL, Connelly MA, Bakker SJL, Dullaart RPF. Triglyceride-rich lipoprotein and LDL particle subfractions and their association with incident type 2 diabetes: the PREVEND study. Cardiovasc Diabetol. 2021;20(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007;56(9):2328–38. [DOI] [PubMed] [Google Scholar]
- 27.Tricò D, Natali A, Mari A, Ferrannini E, Santoro N, Caprio S. Triglyceride-rich very low-density lipoproteins (VLDL) are independently associated with insulin secretion in a multiethnic cohort of adolescents. Diabetes Obes Metab. 2018;20(12):2905–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Damasceno NR, Sala-Vila A, Cofan M, Perez-Heras AM, Fito M, Ruiz-Gutierrez V, et al. Mediterranean diet supplemented with nuts reduces waist circumference and shifts lipoprotein subfractions to a less atherogenic pattern in subjects at high cardiovascular risk. Atherosclerosis. 2013;230(2):347–53. [DOI] [PubMed] [Google Scholar]
- 29.Akinkuolie AO, Paynter NP, Padmanabhan L, Mora S. High-density lipoprotein particle subclass heterogeneity and incident coronary heart disease. Circ Cardiovasc Qual Outcomes. 2014;7(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kulkarni KR MJ, Nanda NC, Segrest JP. Increased prevalence of smaller and denser LDL particles in Asian Indians. Arteriosclerosis, Thrombosis, and Vascular Biology. 1999(11):2749–55. [DOI] [PubMed] [Google Scholar]
- 31.Bhalodkar NC, Blum S, Rana T, Bhalodkar A, Kitchappa R, Kim KS, et al. Comparison of levels of large and small high-density lipoprotein cholesterol in Asian Indian men compared with Caucasian men in the Framingham Offspring Study. Am J Cardiol. 2004;94(12):1561–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


