Abstract
Signals propagated via the gp130 subunit of the interleukin-6 (IL-6)-type cytokine receptors mediate, among various cellular responses, proliferation of hematopoietic cells and induction of acute-phase plasma protein (APP) genes in hepatic cells. Hematopoietic growth control by gp130 is critically dependent on activation of both STAT3 and protein tyrosine phosphatase 2 (SHP-2). To investigate whether induction of APP genes has a similar requirement for SHP-2, we constructed two chimeric receptors, G-gp130 and G-gp130(Y2F), consisting of the transmembrane and cytoplasmic domains of gp130 harboring either a wild-type or a mutated SHP-2 binding site, respectively, fused to the extracellular domain of the granulocyte colony-stimulating factor (G-CSF) receptor. Rat hepatoma H-35 cells stably expressing the chimeric receptors were generated by retroviral transduction. Both chimeric receptors transmitted a G-CSF-induced signal characteristic of that triggered by IL-6 through the endogenous gp130 receptor; i.e., both activated the appropriate JAK, induced DNA binding activity by STAT1 and STAT3, and up-regulated expression of the target APP genes, those for α-fibrinogen and haptoglobin. Notwithstanding these similarities in the patterns of signaling responses elicited, mutation of the SHP-2 interaction site in G-gp130(Y2F) abrogated ligand-activated receptor recruitment of SHP-2 as expected. Moreover, the tyrosine phosphorylation state of the chimeric receptor, the associated JAK activity, and the induced DNA binding activity of STAT1 and STAT3 were maintained at elevated levels and for an extended period of time in G-gp130(Y2F)-expressing cells following G-CSF treatment compared to that in cells displaying the G-gp130 receptor. H-35 cells ectopically expressing G-gp130(Y2F) were also found to display an enhanced sensitivity to G-CSF and a higher level of induction of APP genes. Overexpression of the enzymatically inactive SHP-2 enhanced the signaling by the wild-type but not by the Y2F mutant G-gp130 receptor. These results indicate that gp130 signaling for APP gene induction in hepatic cells differs qualitatively from that controlling the proliferative response in hematopoietic cells in not being strictly dependent on SHP-2. The data further suggest that SHP-2 functions normally to attenuate gp130-mediated signaling in hepatic (and, perhaps, other) cells by moderating JAK action.
The engagement of the JAK/STAT pathway by gp130, the common signal-transducing subunit of interleukin-6 (IL-6)-type cytokine receptors, has been well established (30, 42, 43). The ligand-mediated oligomerization of receptor subunits leads to the activation of gp130-associated Janus protein tyrosine kinases (JAKs). This process is in part determined by the Box B1 and Box B2 motifs in the cytoplasmic gp130 domain. The activated JAKs mediate cross-phosphorylation as well as phosphorylation of gp130, in particular, at the four Box B3 motifs, which then serve as docking elements for STAT1 and STAT3 (42). The receptor-recruited STAT proteins are in turn subject to tyrosine phosphorylation, causing their dimerization, acquisition of DNA binding activity, and nuclear translocation for action as transcriptional inducers of IL-6-responsive genes. Phosphorylation of additional receptor or JAK tyrosine residues provides binding sites for other SH-2 domain-containing signaling molecules, such as protein tyrosine phosphatase 2 (SHP-2), SHC, and Src-related kinases (16, 25, 31, 32, 42, 47, 49).
Deletions and residue substitutions have defined the functional relevance of the Box B1, B2, and B3 motifs for controlling cellular functions. Some of these analyses have utilized the experimental model of chimeric gp130 subunits, granulocyte colony-stimulating factor receptor (G-CSFR)–gp130 (5) or IL-5 receptor–gp130 (6), which permitted characterization of the signaling response independent of endogenous gp130. The data suggest that gp130 acts through at least two separate pathways, one that depends on the Box B3 element and involves STAT3 as a mediator and another that is independent of STAT3, appears to engage STAT5, and induces a restricted set of genes (27).
Analyses of gp130 domains involved in controlling proliferation and differentiation of hematopoietic cells have provided support for a model of multiple signaling pathways. The membrane-proximal region, including Box B1 and B2, was noted to be sufficient for cell survival, whereas proliferation as well as differentiation requires minimally one Box B3 motif and the activation of STAT3 (14, 36). Additional mutations in gp130 have identified tyrosine 759 (the second tyrosine located further downstream from Box B1 and B2, termed Y2) as part of the binding sequence for SHP-2 (14). SHP-2 is a ubiquitous enzyme of 72 kDa that contains two SH-2 domains and has been found to interact with a broad spectrum of signal-transducing molecules (1, 9, 11, 12, 19, 20, 24, 28, 48). gp130-recruited SHP-2 has also proved to be involved in the transmission of a proliferative signal in various hematopoietic cell model systems (8, 14, 36). These findings suggested that STAT3 and SHP-2 are critical mediators of gp130 signaling. A role of SHP-2 in mitogenic signaling was also demonstrated for other hematopoietin receptors (48) and growth factor receptors (7, 51).
A major function of IL-6-type cytokine receptors in hepatic cells is the induction of acute-phase plasma protein (APP) genes (3, 15). The requirement of Box B3 of gp130 in this process has been shown previously (26), but the relevance of the Y2-dependent signaling function for the APP response has not yet been demonstrated. Therefore, by retroviral transduction, we established rat hepatoma cells stably expressing chimeric G-CSFR–gp130 proteins with or without an intact SHP-2 binding site. Here, we report that the induction of APP genes by gp130 signaling differs from gp130-mediated growth control in that it is not abrogated by the Y2 mutation; instead, prevention of SHP-2 recruitment enhances the signaling reaction and responsiveness of the cells to the cytokine, in part by prolonging the activity of JAKs.
MATERIALS AND METHODS
Cytokines and antibodies.
Human recombinant IL-6 and G-CSF were gifts from Genetics Institute and Immunex (Seattle, Wash.), respectively. Insulin was obtained from Sigma. Antisera against JAK1, JAK2, and TYK2 were purchased from Upstate Biotechnology, Inc. (Lake Placid, N.Y.); anti-FLAG antibody (M2) was from Eastman Kodak (Rochester, N.Y.); anti-SHP-1 antibody (C-19) and anti-SHP-2 antibody (C-18) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.); antiphosphotyrosine (PY20) and anti-STAT3 antibodies were from Transduction (Lexington, Ky.); and anti-phospho-STAT3 antibody was from New England Biolabs (Beverly, Mass.).
Plasmid construction and cell lines.
To change tyrosine 759 to phenylalanine (Y2F) in the cytoplasmic domain of gp130, a substitution mutation was introduced by overlap extension PCR with an oligonucleotide containing the desired amino acid codon sequence. A C-terminal epitope (DYKDDDDK) in gp130 was created by introducing the FLAG sequence and termination codon at the end of gp130. All mutations were confirmed by sequence analysis. The chimeric receptors were constructed by inserting the EcoRI-NotI fragment encoding the FLAG-tagged gp130 (amino acid residues 561 to 874) into the EcoRI-NotI site of pSVsport1–G-CSFR, encoding the extracellular domain of G-CSFR (amino acid residues 1 to 623), as previously described (5). The construct G-CSFR–gp130(wild type)–FLAG was termed G-gp130, and G-CSFR–gp130(Y2F)–FLAG was termed G-gp130(Y2F). To generate cell lines stably expressing the chimeric receptors, G-gp130 and G-gp130(Y2F) were cloned into the retroviral vector MINV (17). The receptor cDNA is expressed from the murine stem cell virus (MSCV) long terminal repeat on a bicistronic transcript which contains a downstream neomycin phosphotransferase (neo) gene linked via an internal ribosome entry site from encephalomyocarditis virus. Recombinant MINV–G-gp130 retroviruses were produced in PA317 packaging cells (33) (CRL 9078; American Type Culture Collection). Rat hepatoma H-35 cells (clone T-7-18) (4) were transduced and selected by culturing in Dulbecco’s modified essential medium containing 10% fetal calf serum and G418 (2 mg/ml) (50). Proliferating cells were cloned by limiting dilution. The initial pools of transduced H-35 cells and individual clonal lines were screened for chimeric receptor expression by immunoblotting with anti-FLAG antibodies and for G-CSF-stimulated APP expression. All cytokine treatments were carried out in serum-free minimal essential medium.
To test the influence of overexpressed SHP-2 on gp130 signaling, the two chimeric constructs, G-gp130 and G-gp130(Y2F), were inserted into the expression vector pDC containing the human cytomegalovirus immediate-early gene promoter (35). The pcDNA3 vector (Invitrogen) containing the full-length murine phosphatase-inactive SHP-2 (SHP-2*), was generously provided by Gen-Sheng Feng (Walther Oncology Center, Indiana University School of Medicine, Indianapolis) and has been described previously (52).
CAT assay.
HepG2 cells were transfected by the calcium phosphate method (38). The DNA mixture consisted of the IL-6-responsive reporter gene constructs of pHPX(5×IL-6RE)-CAT containing five tandem copies of the STAT3-sensitive regulatory elements from the rat hemopexin gene (18) (15 μg/ml) and the expression vectors for G-gp130 or G-gp130(Y2F) (1 μg/ml) and for SHP-2* (4 μg/ml). The plasmid pIE-MUP (1 μg/ml) was included in all transfections as an internal marker (39). The transfected subcultures were treated for 24 h either with serum-free medium alone (control) or with medium containing 100 ng of G-CSF or IL-6 per ml. The chloramphenicol acetyltransferase (CAT) activities were determined in serially diluted cell extracts to ensure measurements in the linear range of the enzyme assay. The activities were normalized to the amounts of immunodetectable major urinary protein derived from the transfection marker in each culture (5, 34). The normalized values for CAT activities were then expressed relative to the values in control cultures in each experimental series (fold stimulation).
125I-G-CSF binding assay.
G-CSF was labeled with 125I by the Iodobead method according to the instructions of the manufacturer (Pierce) and purified on a Sephadex G-25 column. The labeled G-CSF had a specific activity of 81,000 cpm/ng and was fully functional as judged from the ability to induce APP expression in G-gp130-expressing H-35 cells. Binding was determined on confluent cell monolayers in six-well cluster plates by using 50 pM 125I-G-CSF in phosphate-buffered saline with 1% bovine serum albumin alone or in the presence of 100 nM unlabeled G-CSF. After incubation for 4 h at 4°C, the cultures were washed four times with 2 ml of binding buffer and two times with 2 ml of phosphate-buffered saline. The cells were solubilized in 0.1 N NaOH–0.1% sodium dodecyl sulfate (SDS), and the radioactivity was measured in a gamma counter (Beckman). The radioactivity specifically competed for by cold G-CSF was taken as a measure of specific G-CSF binding activity and was calculated as 125I-G-CSF molecules bound per cell. In each experimental series, the average number of cells per well was counted in extra wells not used for the radioactive-ligand binding assay but which had been subjected to the same procedures as the assay cells. The number of H-35 cells per confluent monolayer in one well was 2.1 × 106 ± 0.2 × 106 (n = 36) and was highly consistent from experiment to experiment.
Electrophoretic mobility shift assay. (EMSA).
Whole-cell extracts were prepared as described previously (40). The double-stranded DNA probe SIEm67 was used for detecting binding activity of STAT1 and STAT3 (34, 40).
Immunoprecipitation and Western blotting.
Before cytokine treatment, cells were incubated for 6 h in serum-free minimal essential medium. Then, cells were treated with medium alone (control) or medium containing G-CSF (50 ng/ml) or IL-6 (10 ng/ml) for various lengths of time and lysed in modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl [pH 7.4], 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mM NaF, 1 mM sodium orthovanadate, 1 μg of leupeptin and 1 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride, 10% glycerol). The soluble fraction after centrifugation at 15,000 × g for 10 min was used for immunoprecipitation with the appropriate antibodies. The immune complexes were recovered with protein A-Sepharose or protein G-Sepharose (Pharmacia) by incubating for 16 h and then were washed five times with modified RIPA buffer. Immunoprecipitated proteins were separated on an SDS–7.5% polyacrylamide gel and transferred to polyvinylidine difluoride membranes (Bio-Rad). Proteins on membranes were then reacted with antibodies, and immune complexes were visualized with horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin G (Cappel) and enhanced chemiluminescence reagent (Amersham). Sequential immunoblotting reactions were performed following stripping of the membranes in 0.1 N glycine (pH 2.7) for 4 h. All data on coimmunoprecipitated proteins shown in the figures in this paper were obtained by using the modified RIPA buffer containing Nonidet P-40 as the detergent. We have, however, performed complementary immunoprecipitations by using RIPA buffer that contains 1% of the milder detergent Brij 96. The immunoprecipitates recovered with Brij-containing buffers were comparable to those described below, but the electrophoretic gel analyses showed more-complex patterns, probably due to less-stringent washing conditions achieved with Brij 96 (data not presented).
Northern hybridization.
Total cellular RNA (25 or 5 μg) was separated on a 1.5% agarose–formaldehyde gel and blotted onto a positively charged nylon filter (Schleicher and Schuell). cDNAs encoding the extracellular domain of G-CSFR, rat haptoglobin, or rat α-fibrinogen (3) were labeled with 32P by using an oligolabeling kit (Pharmacia). Hybridizations were performed for 16 h at 65°C, and filters were washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.2% SDS at 65°C four times for 30 min each.
Quantitation of secreted plasma proteins.
Aliquots (25 μl) of medium from H-35 cells treated with medium alone, G-CSF, or IL-6 for 24 h were analyzed by immunoelectrophoresis for rat fibrinogen (2). The precipitation peaks were measured by scanning and integrated by using the National Institutes of Health Image program, version 1.6. The data are expressed in arbitrary immunoelectrophoretic units. The detection limit of the method is <1 immunoelectrophoretic unit.
RESULTS
Stable expression of G-gp130 in hepatoma cells.
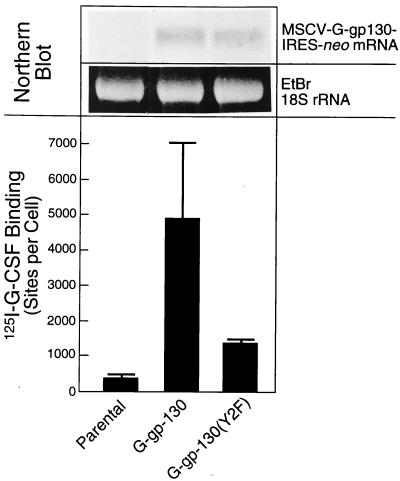
H-35 cells transduced with retroviral vectors for G-gp130 and G-gp130(Y2F) yielded significant expression of the chimeric receptors. From several hundred individual clones tested, we selected for functional characterization those clones that showed an induction of APP production by G-CSF treatment that was equal to or greater than that by IL-6. By comparing receptor expression detectable by anti-FLAG antibodies and induction of APP genes in the initial pools of transduced and G-418-selected cells and in individual clones, we noted that the magnitudes of APP gene induction were generally correlated with receptor levels (for example, see Fig. 7). Similarly, clonal cell lines with different amounts of expressed receptors showed a DNA binding activity of STAT proteins following G-CSF treatment that correlated in most instances with the relative receptor level and APP induction (data not shown). The few clonal lines (<5% of all clones) that did not respond to G-CSF treatment by a measurable induction of APPs had also undetectable amounts of transduced receptors. Interestingly, the cell lines with G-gp130(Y2F) showed consistently less receptor expression than those with G-gp130. This difference is demonstrated in Fig. 1 for two representative lines chosen for this study and termed H-35 G-gp130 and H-35 G-gp130(Y2F). The lines had been initially selected because of their comparable levels of maximally G-CSF-induced APP expression. Parental H-35 cells showed a low level of 125I-G-CSF binding, although no G-CSFR mRNA could be detected by Northern blot analysis (data not shown). The receptor-transduced cells displayed a significantly increased ligand binding activity, with cells expressing G-gp130 binding on average four times more G-CSF than those expressing G-gp130(Y2F). Northern blot analysis confirmed the differences in expression determined by the ligand binding assay. However, there was only a twofold difference in the mRNA hybridization signals (Fig. 1, upper panel), suggesting some effect of the Y2F mutation on posttranslational receptor processing (see also the severalfold difference in immunodetectable receptor proteins in Fig. 2B).
FIG. 7.
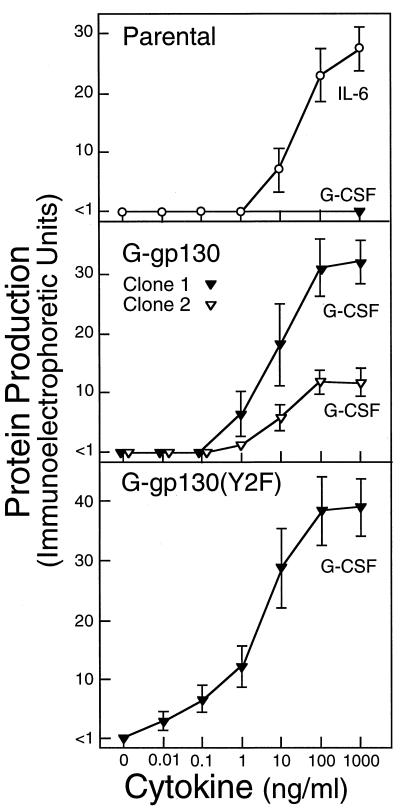
Cytokine-regulated production of fibrinogen. Parental H-35 cells and H-35 cell lines transduced with G-gp130(wild type) (clone 1 represents the line used in all previous experiments; clone 2 represents a separate line with four-times-lower G-gp130 expression) and G-gp130(Y2F) were grown to confluency in 24-well cluster plates. The cells were treated for 24 h with serum-free medium containing increasing concentrations of G-CSF or IL-6. Aliquots from the culture media were analyzed for the concentration of fibrinogen by immunoelectrophoresis (expressed in arbitrary immunoelectrophoretic units). The means ± standard deviations for three separate treatment series are shown.
FIG. 1.
Expression of G-gp130 forms in H-35 cells. (Upper panel) Total cellular RNAs (5 μg) from parental H-35 cells and from the transduced clonal lines G-gp130 and G-gp130(Y2F) were analyzed by Northern blot hybridization. The hybridization of the G-CSFR probe to the fusion transcripts MSCV-G-gp130/IRES-neo at 7 kb (3-day exposure) and ethidium bromide (EtBr)-stained 18S rRNA of the separated RNA are shown. (Lower panel) Binding of 125I-G-CSF to the same cell cultures was determined by the single-point binding assay. The specific 125I-G-CSF binding was calculated as sites per cell (means ± standard deviations of three separate measurements).
FIG. 2.
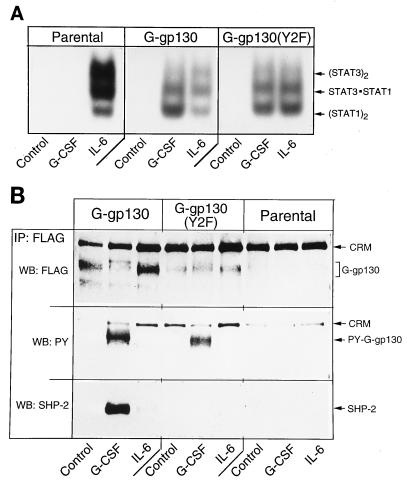
Identification of gp130 proteins and functions. (A) Parental and transduced H-35 cells were treated for 15 min as indicated. Whole-cell extracts were subjected to EMSA. Binding complexes with the SIE probe and positions of the specific STAT combinations are shown. (B) Lysates from the same cell cultures used in panel A were immunoprecipitated (IP) with anti-FLAG antibodies, and the recovered proteins were separated by SDS-polyacrylamide gel electrophoresis and sequentially reacted in Western blotting (WB) with antibodies against FLAG, phosphotyrosine, and SHP-2 (FLAG, PY, and SHP-2, respectively). H-35 cells contain a protein of 190 kDa (CRM) that cross-reacts with anti-FLAG antibodies.
The expression of functional receptor proteins was assessed by the G-CSF-induced activation of STAT proteins (Fig. 2A) and phosphorylation of receptor and binding of SHP-2 to G-gp130 but not G-gp130(Y2F) (Fig. 2B). The specificity of the cell response and of the biochemical analytical techniques was demonstrated by comparing the transduced cells with parental H-35 cells and by comparing the effects of G-CSF and IL-6. Although parental H-35 cells exhibited a low level of G-CSF binding, treatment with G-CSF did not result in any detectable activation of DNA binding activity of STAT1 and STAT3 (Fig. 2A). In both transduced cell lines, a strong STAT activation that was comparable to that elicited by IL-6 was observed. Immunoprecipitation of cell lysates with anti-FLAG antibody yielded receptor proteins with the expected size of 130 kDa. The antibody also recognized an endogenous cross-reacting protein (Fig. 2B) with an apparent molecular size of 190 kDa. This protein conveniently served as internal marker for protein loading (see Fig. 4). The immunoblot detection of G-gp130 proteins by anti-FLAG antibody verified a severalfold-lower level of G-gp130(Y2F) compared to G-gp130.
FIG. 4.
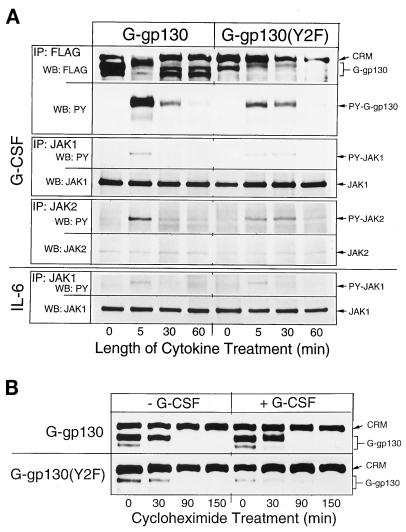
Time course of signaling by G-gp130 and G-gp130(Y2F). (A) Monolayers of the two transduced H-35 cell lines were treated for the indicated lengths of time with either G-CSF or IL-6. Separate cell lysates were immunoprecipitated (IP) with antibodies against FLAG, JAK1, or JAK2. The proteins were immunoblotted (WB) as indicated. PY, phosphotyrosine. (B) To determine turnover of G-gp130 proteins, monolayers of the two transduced cell lines were preincubated with cycloheximide (30 μg/ml) for 10 min, and then the cells were treated for the indicated lengths of time in the presence of cycloheximide with or without G-CSF. Cell lysates from each time point were immunoprecipitated with anti-FLAG antibodies, followed by immunoblot analysis of the proteins with anti-FLAG antibodies. The gel areas of G-gp130 proteins and cross-reacting material (CRM) are shown.
G-CSF treatment led to tyrosine phosphorylation of the chimeric receptor that was detected by phosphotyrosine immunoblotting and recognized by the slower electrophoretic mobility of the anti-FLAG-detected G-gp130 proteins (Fig. 2B). A similar phosphorylation-induced change in electrophoretic mobility was reported for the native gp130, which showed an increase in the apparent molecular mass from ∼ 155 to ∼170 kDa (30). As expected, G-CSF induced association of SHP-2 with G-gp130 but not G-gp130(Y2F). Moreover, the data also demonstrated that there was no constitutive binding of SHP-2 to G-gp130 and no IL-6-mediated modification of the G-gp130 forms. H-35 cells express immunodetectable amounts of SHP-1; however, we could not detect the phosphorylation of SHP-1 by G-CSF or IL-6 treatment, nor was the association of SHP-1 with anti-FLAG-immunoprecipitated proteins from either G-gp130 or G-gp130(Y2F) cells observed (data not shown).
Activation of signaling reaction.
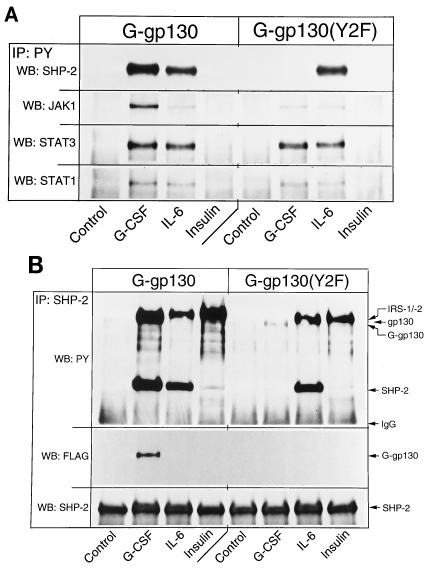
G-gp130-initiated signaling was compared to those of the endogenous receptors for IL-6 and insulin. The insulin response was selected because, while ineffective at inducing the JAK/STAT pathway, insulin prominently activates the mitogen-activated protein kinase (MAPK) pathway (45) and involves SHP-2 (24). H-35 cells expressing G-gp130 and G-gp130(Y2F) responded to G-CSF and IL-6 by engagement of JAK1, STAT1, and STAT3 as detected by immunoprecipitation with antiphosphotyrosine antibodies (Fig. 3A). The somewhat stronger G-CSF response of the G-gp130 cells was attributed to the higher level of chimeric receptors in these cells. Phosphorylation of SHP-2 was observed in G-gp130 but not G-gp130(Y2F) cells following G-CSF treatment; this was higher than seen after IL-6 treatment, in correlation with the stronger signaling response. By comparison, insulin treatment did not yield significant tyrosine phosphorylation of the analyzed proteins or a significant association of these proteins. However, SHP-2, probably through association with insulin receptor substrate 1/2 (24), could be recovered from antiphosphotyrosine immunoprecipitates of insulin-treated H-35 cells when these precipitates had been prepared in Brij 96-containing buffers and subjected to less-stringent washing conditions (data not shown; see also Fig. 3B). SHP-2 receptor recruitment by each treatment was investigated directly by immunoprecipitation with anti-SHP-2 antibody (Fig. 3B). The association of SHP-2 with G-gp130, but not with G-gp130(Y2F), was further demonstrated by the coprecipitation of only G-gp130 with SHP-2 (Fig. 3B). The same analysis also illustrated that the cross-reactive material observed in the anti-FLAG immunoprecipitates (Fig. 2B) was not associated with the proteins recovered with the anti-SHP-2 antibody.
FIG. 3.
Comparison of the signaling reactions initiated by G-CSF, IL-6, and insulin. Monolayers of G-gp130 and G-gp130(Y2F) cells were treated for 10 min as indicated. (A) One half of the cell lysate was immunoprecipitated (IP) with antiphosphotyrosine antibody (PY). The immunoprecipitated proteins were analyzed sequentially by immunoblotting (WB) with antibodies against SHP-2, JAK1, STAT3, and STAT1. (B) The other half of the cell lysate was reacted with anti-SHP-2 antibodies, and the immunoprecipitated proteins were analyzed by sequential immunoblotting with antibodies against phosphotyrosine, FLAG, and SHP-2. In the upper panel, the positions of the several proteins are indicated only for reference. It is not proven whether the antiphosphotyrosine-immunoreactive bands shown represent the comigrating proteins. IgG, immunoglobulin G; IRS, insulin receptor substrate.
Loss of SHP-2 recruitment correlates with prolonged JAK action.
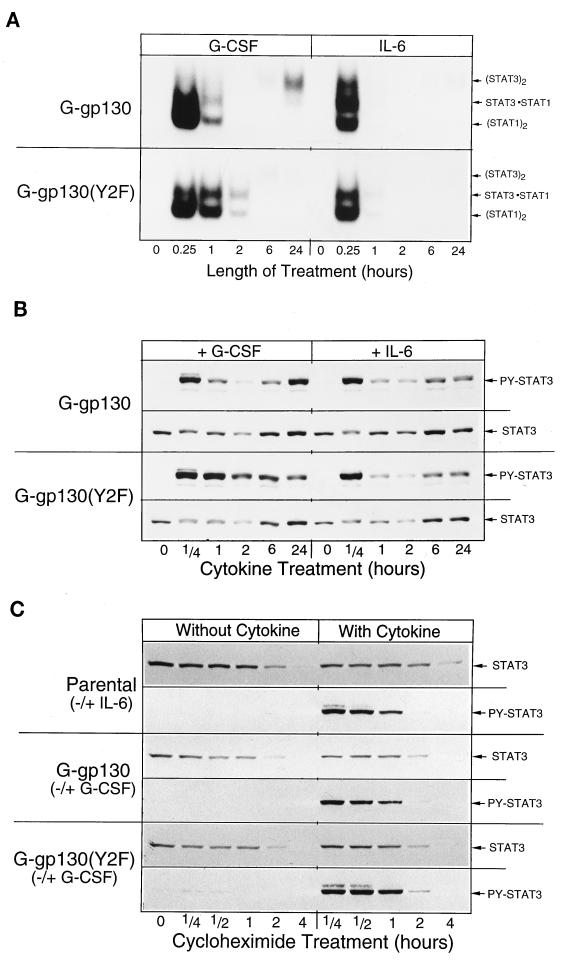
To assess the potential effect of the Y2F mutation on signaling, we compared time-dependent changes of JAK phosphorylation in G-gp130 and G-gp130(Y2F) cells (Fig. 4A). In G-gp130 cells, maximal tyrosine phosphorylation of the receptors, JAK1, JAK2, and TYK2 was attained after a 5-min treatment with G-CSF, with rapid loss of signal by 30 min. A similar time course was obtained for JAK1 activation by IL-6 (Fig. 4A, bottom, and data not shown). In contrast, in G-CSF-treated G-gp130(Y2F) cells, elevated levels of tyrosine-phosphorylated receptors and JAKs persisted for 30 min before declining to basal levels.
Since the distinct patterns of temporal changes in the phosphorylation of receptors and JAKs (Fig. 4A) could conceivably be caused by differences in ligand-induced receptor turnover, we measured immunodetectable G-gp130 proteins during the course of cycloheximide treatment (Fig. 4B). In so far as this technique allowed an estimate of receptor amounts, the cellular concentrations of both G-gp130 and G-gp130(Y2F) appeared to decline with roughly the same kinetics, regardless of whether the cells had been treated with G-CSF. Most of the receptor proteins were lost by 90 min. Thus, relatively fast turnover of the chimeric receptors, rather than the specific action of a tyrosine phosphatase, may account for the loss of tyrosine-phosphorylated proteins noted after 1 h of cytokine treatment (Fig. 4A).
Prolonged JAK activity enhances STAT activation.
Observing that the inability of G-gp130(Y2F) to recruit SHP-2 resulted in prolonged JAK activity, we expected a corresponding prolongation or even an enhancement of STAT activation. Time course analysis of G-CSF treatment indeed indicated that the DNA binding activity of STAT1 and STAT3 (Fig. 5A) and the relative amounts of tyrosine-phosphorylated STAT3 (Fig. 5B) persisted longer in G-gp130(Y2F) cells than in G-gp130 cells. IL-6 treatment elicited the same relative temporal changes in STAT activity in both cell types, which were essentially identical to those produced by G-CSF in G-gp130 cells. The higher STAT3 activity following a 24-h G-CSF treatment in G-gp130 cells was tentatively attributed to the relatively high level of the chimeric receptor in these cells.
FIG. 5.
Time course of STAT protein activation and degradation. (A and B) Monolayers of the two transduced H-35 cell lines were treated for the indicated lengths of time with either G-CSF or IL-6. Whole-cell extracts were prepared and subjected to EMSA (A) or Western blot analysis for proteins reacting to anti-phosphotyrosine (PY)-STAT3 and STAT3 antibody (B). (C) Parental H-35 cells and H-35 cells expressing G-gp130 or G-gp130(Y2F) were preincubated for 10 min with cycloheximide (30 μg/ml) (0-h time point), and then treatment was started and continued for the indicated lengths of time in the presence of cycloheximide with or without cytokines. The cells were solubilized directly in SDS buffer. Equal aliquots of extracts were analyzed by immunoblotting by reaction first with anti-STAT3 antibody and, after stripping of the membrane, then with anti-phospho-STAT3 antibody.
The prolonged high STAT activity in G-gp130(Y2F) cells could be explained by (i) a prolonged tyrosine phosphorylation through the receptor-associated kinases that was not suppressed by SHP-2, (ii) a reduced dephosphorylation of receptor-recruited STATs by SHP-2, or (iii) a change in STAT protein turnover. Using the same experimental procedure for G-gp130 (Fig. 4B) except that cycloheximide treatment was for 4 h, we determined the effect of SHP-2 recruitment on the turnover of STAT3 and phospho-STAT3 proteins (Fig. 5C). The kinetic analysis indicated a half-life of immunodetectable STAT3 of approximately 100 min in H-35 cells treated with or without cytokines. Although the measured STAT3 half-life was likely influenced by the prolonged treatment of cells with cycloheximide and showed no statistically significant differences, a minor IL-6- or G-CSF-induced enhanced turnover of STAT3 seems to take place within the first hours of treatment in cells not exposed to cycloheximide, as evident from the reduced immunoblot signal for STAT3 in the extract from cells after a 2-h treatment (Fig. 5B). Essentially the same time course of STAT3 reduction was detected in G-gp130 and G-gp130(Y2F) cells after either G-CSF or IL-6 treatment. The analysis also revealed that the cellular level of STAT3 increased again following extended hours of treatment and even exceeded the level detected in cells at 0 h. This increase was previously shown to be the result of cytokine-induced STAT3 expression (10). Taking these data together, we interpret the elevated DNA binding activity of STATs (Fig. 5A) and the phosphotyrosine STAT3 level (Fig. 5B) to be the result of prolonged kinase activity and not of a slower STAT3 turnover.
Gene induction is enhanced in G-gp130(Y2F) cells.
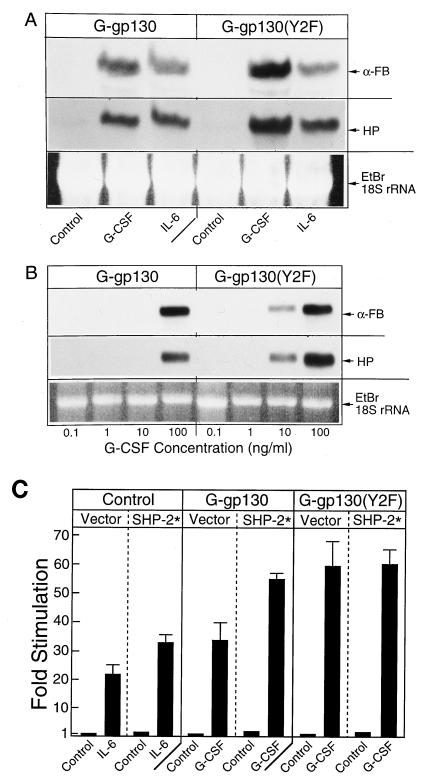
Regardless of the mechanism by which the level of activated STAT3 was enhanced in G-gp130(Y2F) cells, a corresponding effect with regard to the regulation of APP genes was expected. Since the relative difference in activated STAT3 protein between G-gp130 and G-gp130(Y2F) cells was most prominent after the initial phase of cytokine treatment, we determined the mRNA levels of the marker APPs, α-fibrinogen and haptoglobin, after a 2-h treatment that allowed for accumulation of sufficient mRNA to be detected by Northern blot hybridization (Fig. 6A). Whereas IL-6 induced α-fibrinogen and haptoglobin mRNAs in both cell lines to the same levels, G-CSF was approximately twice as effective in G-gp130(Y2F) cells than in G-gp130 cells, in agreement with the noted difference in STAT3 activation in these cells. The greater induction of APP mRNA in G-gp130(Y2F) cells was also manifested in an enhanced sensitivity of the cells to G-CSF (Fig. 6B). For instance, 10 ng of G-CSF per ml induced a strong APP mRNA signal in G-gp130(Y2F) cells but not in G-gp130 cells. The comparison also indicated that the APP genes were not identically regulated, in that α-fibrinogen attained the same maximal mRNA level in both cell types after G-CSF treatment, whereas the haptoglobin mRNA level in G-gp130(Y2F) cells was approximately twice that in G-CSF-treated G-gp130 cells.
FIG. 6.
Induction of APP mRNA and CAT gene expression. (A) The two transduced H-35 cell lines were treated for 2 h with serum-free medium alone (control) or medium containing G-CSF or IL-6. Total cellular RNA (20 μg per lane) was analyzed by sequential Northern blot hybridization for the mRNAs for α-fibrinogen (α-FB) and haptoglobin (HP). The autoradiograms are after a 24-h (HP) or 6-h (α-Fb) exposure. Equal RNA loading is indicated by the ethidium bromide (EtBr)-stained 18S rRNA bands. (B) Receptor-transduced H-35 cells were treated for 24 h with increasing doses of G-CSF. Total cellular RNAs (5 μg per lane) were analyzed as for panel A. In order to demonstrate the quantitative differences in hybridization signals, the autoradiographic image after a 6-h exposure is shown. (C) HepG2 cells were transfected with pHPX(5×IL-6RE)-CAT and the expression vectors for the chimeric receptors and SHP-2* as indicated. Subcultures were treated with the appropriate cytokines, and the fold inductions of the CAT activity were determined (means ± standard deviations of three determinations).
The data thus far suggested that the APP gene-inducing action of wild-type gp130 was moderated by the recruitment of SHP-2. Recently, Symes et al. (46) demonstrated that the phosphatase activity of SHP-2 was necessary to achieve this moderating effect on gene induction in neuroblastoma cells. To assess whether the catalytic activity of SHP-2 was also critical for G-gp130-mediated induction of genes via the STAT3-sensitive IL-6-responsive APP gene elements, we overexpressed the phosphatase-inactive SHP-2* together with the chimeric receptors and the pHPX(5×IL-6RE)-CAT reporter gene in transfected HepG2 cells (Fig. 6C). HepG2 cells were chosen for this assay because in this cell line, in contrast to in H-35 cells, a significantly larger overexpression of transfected vectors can be achieved (22, 26, 27, 34). In agreement with the prediction that enzymatically inactive SHP-2 acts as a dominant-negative protein (46), overexpression of SHP-2* enhanced the gene-regulatory effects by endogenous IL-6 receptor and G-gp130. Also, as expected, SHP-2* was ineffective on the signaling by the G-gp130(Y2F). The rather modest effects of the overexpressed SHP-2* may in part be explained by the relatively high levels of endogenous SHP-2 proteins, which, based on comparative Western blot data, were similar in HepG2 and H-35 cells (data not shown). The endogenous SHP-2 protein likely prevented an effective competition by the transfected SHP-2* and thus yielded a result that was less prominent than that seen in neuroblastoma cells (46).
The mRNA analysis, as well as the CAT regulation (Fig. 6), indicated a more prominent signaling action by G-gp130(Y2F) than by G-gp130. The difference in the G-CSF dose response between the two G-gp130 cell lines was more clearly seen in the amounts of APP secreted into the culture medium over the 24-h treatment period (Fig. 7 shows fibrinogen as an example). The IL-6 responses of the parental and the transduced cell lines proved to be essentially indistinguishable (Fig. 7 shows only the parental H-35 cells). While the G-CSF responses in the two transduced cell lines were similar, in that approximately 5 ng of G-CSF per ml was required for half-maximal stimulation, the production of fibrinogen was already detectable in G-gp130(Y2F) cells at the lowest concentration tested (0.01 ng/ml), and the maximal level of expression was somewhat higher. The data strongly suggest that the Y2F mutation of gp130 had not appreciably altered the affinity of the chimeric receptor for G-CSF interaction (i.e., the same concentration of G-CSF induced the half-maximal response) but enhanced the signaling activity of the receptor such that many fewer receptors were needed to attain the level of APP induction mediated by the wild-type receptor. This higher signaling action was highlighted by the comparison of the APP response of the G-gp130(Y2F) cells with that of a separate clonal line of G-gp130 cells (clone 2 in Fig. 7) that expressed the same amount of G-gp130 proteins as the G-gp130(Y2F) cells. While showing essentially the same G-CSF dose response as clone 1 or the G-gp130(Y2F) cells, the magnitude of induction of fibrinogen was threefold lower in this cell line.
DISCUSSION
In this study, we attempted to identify the specific contribution of SHP-2 to the signaling by gp130 that controls induction of IL-6-responsive APP genes in hepatic cells. The experimental approach was to examine the effect of mutating the suggested SHP-2 binding site in the chimeric G-gp130 constructs that could be tested in hepatoma cells independently of the endogenous gp130. The results showed that SHP-2 is recruited to gp130 upon activation by ligand binding. Prevention of this binding by the Y2F mutation led to an enhanced signaling response that was associated with prolonged phosphorylation of the receptors, JAK, and STATs, extended DNA binding of the STAT proteins, and elevated transcription of APP genes. From this information, we conclude that in the wild-type gp130 the recruited SHP-2 attenuates the signaling intensity but that SHP-2 is not essential for APP gene expression. A similar conclusion has been reached about the gp130-recruited SHP-2 in neuroblastoma cells to induce ciliary neurotrophic factor-sensitive gene constructs (46).
SHP-1, expressed exclusively in hematopoietic and epithelial cells, is thought to play a negative role in signal transduction of growth factors or cytokines, whereas SHP-2 has been suggested as a positive regulator, in particular of proliferation (8, 9, 14, 23, 28, 29, 48). Surprisingly, our data show that SHP-2 recruited into gp130 can act as a negative regulator of JAK in hepatoma cells. The specific effects of SHP-2 were determined by eliminating its docking site on the receptor. The loss of SHP-2 from immunoprecipitated G-gp130(Y2F) attested to the relevance of the tyrosine residue (Y2) as being critical for binding of SHP-2 through its SH-2 domain (14). It is less certain whether the G-gp130(Y2F) signaling in transduced H-35 cells is indeed independent of SHP-2. An indirect recruitment of SHP-2 by tyrosine-phosphorylated downstream substrates of gp130 action is conceivable, such as that suggested in the case of insulin receptor action (12, 24). In fact, we noted a low-level recovery of a G-CSF-induced association of SHP-2 with tyrosine-phosphorylated protein in G-gp130(Y2F) cells (Fig. 3B) that may arise from such an indirect pathway.
It has been suggested that SHP-2 serves as a link between the hematopoietin or growth factor receptors and the control of cell proliferation. This connection is invariably associated with the activation of the Raf-MAPK (ERK1 and -2) pathway. Abrogation of SHP-2 recruitment by gp130 has been seen to negatively affect proliferation of hematopoietic cells, which was in part correlated with lower MAPK activity (8, 14). However, truncated gp130 forms that lack the SHP-2 binding site still deliver a detectable MAPK activation (36, 41) that is interpreted to account for minimal growth stimulation (36). Similarly, analysis of transduced G-gp130(Y2F) cells showed detectable ERK1 and ERK2 tyrosine phosphorylation following G-CSF treatment, although the magnitude of activation was much lower than that observed for G-gp130 cells (22a). Hence, while SHP-2 may play a role in engaging the MAPK pathway as part of the gp130 signaling, a critical contribution of the gp130-regulated MAPK pathway to the induction of APP genes appears unlikely.
What is a conceivable mechanism of SHP-2 action in our more restricted experimental model of APP gene induction? Upon receptor engagement, the gp130 cytoplasmic domain, including the Y2 site, is phosphorylated in a ligand-dependent manner. The gp130-associated JAKs are probably the principal kinases in this process. However, it has also been observed that members of other cellular protein tyrosine kinase families, including Fyn, Fes, and Tec, are associated with ligand-activated gp130 and may contribute to the gp130 phosphorylation process (30, 31, 48). After SHP-2 binds to the phosphorylated Y2 site, it may exert a phosphatase action on phosphorylated sites on gp130 and its associated factors, including JAKs and STATs. However, at present, there is no convincing experimental evidence that the negative regulation is achieved by the phosphatase action of SHP-2 on JAKs. Although the direct association of SHP-2 with JAK1 and JAK2 has been demonstrated in COS-1 cells overexpressing these proteins, a reduced tyrosine phosphorylation of the JAK proteins was not evident in those analyses (52). Substrate specificity of SHP-2 appears to be determined by its association with the receptor rather than by direct binding to a JAK or STAT, since we did not observe in our receptor-transduced hepatoma cell line an association of the endogenous SHP-2 with STAT3 or JAKs (data not shown). Moreover, it is likely that SHP-2 is also a target for phosphorylation by gp130-associated kinases. Since the SHP-2 that coprecipitated with gp130 barely reacted with antiphosphotyrosine antibody (22a), which is in contrast to the relatively strong signal seen for tyrosine-phosphorylated SHP-2 recovered from the fraction that was not associated with gp130, we assume that SHP-2, once phosphorylated at the receptor site, disassociates from gp130 and may associate with various cellular and membrane proteins (9, 19, 21, 28, 29).
Based on the observation that phosphorylation of JAKs is maintained longer in G-gp130(Y2F) cells (Fig. 4A), we intuitively assume that SHP-2 acts soon after signal initiation and explains the effects on the downstream targets of JAKs, e.g., STAT recruitment and activation by phosphorylation. In fact, the consequence of the proposed SHP-2 action is equivalent to the negative-feedback action demonstrated for the JAK-associated protein inducible by IL-6 (13, 37, 44). The difference is that the moderating action of SHP-2 is practically immediate, whereas the JAK-associated protein first requires protein synthesis.
SHP-2 is not crucial for APP gene induction or for eventual down-regulation of the gp130 signal. Since IL-6 induction of APP genes can be maintained for days (i.e., long after the dramatic changes in phosphorylation of gp130, JAKs, and STATs are observed at signal initiation) (Fig. 4A and 5A), the influence of SHP-2 on gp130 action is expected to continue, albeit at a much lower, barely detectable level. The fact that this process does take place is highlighted by the striking effect of the Y2F mutation on the APP level (Fig. 7). Even slightly enhanced gp130 signaling that is maintained for 24 h leads to a significantly elevated accumulation of stable APP mRNA and, consequently, to a corresponding accumulation of plasma protein in the medium. In conclusion, this study has defined a component of the gp130 signal pathway that distinguishes the regulation of “differentiated” genes from that of those that control cell proliferation.
ACKNOWLEDGMENTS
We are greatly indebted to Immunex Corporation and Genetics Institute for their generous supply of cytokines, to D. Gearing for providing the original chimeric G-CSFR–gp130 construct, to G.-S. Feng for the SHP-2* expression vectors, to Olivier Robledo for 125I-G-CSF, and to Marcia Held for secretarial assistance.
This work was supported by NIH grant CA26122 to H.B. and by NCI grant 8398 to R.G.H.
REFERENCES
- 1.Adachi M, Ishino M, Torigoe T, Minami Y, Matozaki T, Miyazaki T, Taniguchi T, Hinoda Y, Imai K. Interleukin-2 induces tyrosine phosphorylation of SHP-2 through IL-2 receptor beta chain. Oncogene. 1997;14:1629–1633. doi: 10.1038/sj.onc.1200981. [DOI] [PubMed] [Google Scholar]
- 2.Baumann H, Hill R E, Sauder D M, Jahreis G P. Regulation of major acute-phase plasma proteins by hepatocyte-stimulating factors of human squamous carcinoma cells. J Cell Biol. 1986;102:370–383. doi: 10.1083/jcb.102.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumann H, Richards C, Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1 and glucocorticoid for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987;139:1422–1428. [PubMed] [Google Scholar]
- 4.Baumann H, Prowse K R, Marinkovic S, Won K-A, Jahreis G P. Stimulation of hepatic acute phase response by cytokines and glucocorticoids. Ann NY Acad Sci. 1989;557:280–295. doi: 10.1111/j.1749-6632.1989.tb24021.x. [DOI] [PubMed] [Google Scholar]
- 5.Baumann H, Symes A J, Comeau M R, Morella K K, Wang Y, Friend D, Ziegler S F, Fink J S, Gearing D P. Multiple regions within the cytoplasmic domains of the leukemia inhibitory factor receptor and gp130 cooperate in signal transduction in hepatic and neuronal cells. Mol Cell Biol. 1994;14:138–146. doi: 10.1128/mcb.14.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrmann I, Janzen C, Gerhartz C, Schmitz-Van de L H, Hermanns H, Heesel B, Graeve L, Horn F, Tavernier J, Heinrich P C. A single STAT recruitment module in a chimeric cytokine receptor complex is sufficient for STAT activation. J Biol Chem. 1997;272:5269–5274. doi: 10.1074/jbc.272.8.5269. [DOI] [PubMed] [Google Scholar]
- 7.Bennett A M, Hausdorff S F, O’Reilly A M, Freeman R M, Jr, Nell B G. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger L C, Hawley R G. Interferon-β interrupts interleukin-6 dependent signaling events in myeloma cells. Blood. 1997;89:261–271. [PubMed] [Google Scholar]
- 9.Bone H, Dechert U, Jirik F, Schrader J W, Welham M J. SHP1 and SHP2 protein-tyrosine phosphatases associate with betac after interleukin-3-induced receptor tyrosine phosphorylation. J Biol Chem. 1997;272:14470–14476. doi: 10.1074/jbc.272.22.14470. [DOI] [PubMed] [Google Scholar]
- 10.Campos S P, Wang Y, Baumann H. Insulin modulates STAT3 protein activation and gene in hepatic cells. J Biol Chem. 1996;271:24418–24424. doi: 10.1074/jbc.271.40.24418. [DOI] [PubMed] [Google Scholar]
- 11.Carlberg K, Rohrschneider L R. Characterization of a novel tyrosine phosphorylated 100-kDa protein that binds to SHP-2 and phosphatidylinositol 3′-kinase in myeloid cells. J Biol Chem. 1997;272:15943–15950. doi: 10.1074/jbc.272.25.15943. [DOI] [PubMed] [Google Scholar]
- 12.Case R D, Piccione E, Wolf G, Benett A M, Lechleider R J, Neel B G, Shoelson S E. SH-PTP2/Syp SH2 domain binding specificity is defined by direct interactions with platelet-derived growth factor beta-receptor, epidermal growth factor receptor, and insulin receptor substrate-1-derived phosphopeptides. J Biol Chem. 1994;269:10467–10474. [PubMed] [Google Scholar]
- 13.Endo T A, Masuhara M, Yokouchi M, Suzuki R, Sakamoto H, Mitsui K, Matsumoto A, Tanimura S, Ohtsubo M, Misawa H, Miyazaki T, Leonor N, Taniguchi T, Fujita T, Kanakura Y, Komiya S, Yoshimura A. A new protein containing an SH2 domain that inhibits JAK kinases. Nature. 1997;387:921–924. doi: 10.1038/43213. [DOI] [PubMed] [Google Scholar]
- 14.Fukada T, Hibi M, Yamanaka Y, Takahashi-Tezuka M, Fujitani Y, Yamaguchi T, Nakajima K, Hirano T. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in antiapoptosis. Immunity. 1996;5:449–460. doi: 10.1016/s1074-7613(00)80501-4. [DOI] [PubMed] [Google Scholar]
- 15.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon-β2/BSF-2 shares identity with monocytoe derived hepatocyte stimulating factor (HSF) and regualtes the major acute phase protein response in liver cells. Proc Natl Acad Sci USA. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giordano V, De Falco G, Chiari R, Quinto I, Pelicci P G, Bartholomew L, Delmastro P, Gadina M, Scala G. Shc mediates IL-6 signaling by interacting with gp130 and Jak2 kinase. J Immunol. 1997;158:4097–4103. [PubMed] [Google Scholar]
- 17.Hawley R G, Lieu F H, Fong A Z, Goldman S J, Leonard J P, Hawley T S. Retroviral vectors for production of interleukin-12 in the bone marrow to induce a graft-versus-leukemia effect. Ann NY Acad Sci. 1996;795:341–345. doi: 10.1111/j.1749-6632.1996.tb52687.x. [DOI] [PubMed] [Google Scholar]
- 18.Immenschuh S, Nagae Y, Satoh H, Baumann H, Muller-Eberhard U. The rat and human hemopexin genes contain an identical interleukin-6 response element that is not a target of CAT enhancer-binding protein isoforms. J Biol Chem. 1994;269:12654–12661. [PubMed] [Google Scholar]
- 19.Jackson D E, Ward C M, Wang R, Newman P J. The protein-tyrosine phosphatase SHP-2 binds platelet/endothelial cell adhesion molecule-1 (PECAM-1) and forms a distinct signaling complex during platelet aggregation. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 20.Kazlauskas A, Feng G-S, Pawson T, Valius M. The 64-kDa protein that associates with the platelet-derived growth factor receptor β subunit via Tyr-1009 is the SH-2 containing phosphotyrosine phosphatase Syp. Proc Natl Acad Sci USA. 1993;90:6939–6942. doi: 10.1073/pnas.90.15.6939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. A family of proteins that inhibit signalling through tyrosine kinase receptors. Nature. 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Baumann H. The carboxyl-terminal region of STAT3 controls gene induction by the mouse haptoglobin promoter. J Biol Chem. 1997;272:14571–14579. doi: 10.1074/jbc.272.23.14571. [DOI] [PubMed] [Google Scholar]
- 22a.Kim, H., and H. Baumann. Unpublished data.
- 23.Klingmuller U, Lorenz U, Cantley L C, Neel B G, Lodish H F. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- 24.Kuhne M R, Pawson T, Lienhard G E, Feng G-S. The insulin receptor substrate 1 associates with the SH-2 containing phophotyrosine phosphatase Syp. J Biol Chem. 1993;268:11479–11481. [PubMed] [Google Scholar]
- 25.Kumar G, Gupta S, Wang S, Nel E. Involvement of Janus kinases, p52shc, Raf-1, and MEK-1 in the IL-6 induced mitogen-activated protein kinase cascade of growth responsive B cell line. J Immunol. 1994;152:4436–4447. [PubMed] [Google Scholar]
- 26.Lai C-F, Ripperger J, Morella K K, Wang Y, Gearing D P, Fey G H, Baumann H. Separate signaling mechanisms are involved in the control of STAT protein activation and gene regulation via the interleukin 6 response element by the box 3 motif of gp130. J Biol Chem. 1995;270:14847–14850. doi: 10.1074/jbc.270.25.14847. [DOI] [PubMed] [Google Scholar]
- 27.Lai C-F, Ripperger J, Morella K K, Wang Y, Gearing D P, Horseman N D, Campos S P, Fey G H, Baumann H. STAT3 and STAT5B are targets of two different signal pathways activated by hematopoietin receptors and control transcription via separate cytokine response elements. J Biol Chem. 1995;270:23254–23257. doi: 10.1074/jbc.270.40.23254. [DOI] [PubMed] [Google Scholar]
- 28.Li W, Nishimura R, Kashishian A, Batzer A G, Kim W J, Cooper J A, Schlessinger J. A new function for a phosphotyrosine phosphatase: linking GRB2-Sos to a receptor tyrosine kinase. Mol Cell Biol. 1994;14:509–517. doi: 10.1128/mcb.14.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Damen J E, Ware M D, Krystal G. Interleukin-3 induces the association of the inositol 5-phosphatase SHIP with SHP2. J Biol Chem. 1997;272:10998–11001. doi: 10.1074/jbc.272.17.10998. [DOI] [PubMed] [Google Scholar]
- 30.Lutticken C, Wegenka U M, Yuan J, Buschmann J, Schindle C, Ziemiecki A, Harpur A G, Wilks A F, Yasukawa K, Taga T, Kishimoto T. Association of transcription factor APRF and protein kinase Jak1 with the interleukin-6 signal transducer gp130. Science. 1994;263:89–92. doi: 10.1126/science.8272872. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda T, Fukada T, Takahashi-Tezuka M, Okuyama Y, Fujitani Y, Hanazono Y, Hirai H, Hirano T. Activation of Fes tyrosine kinase by gp130, an interleukin-6 family cytokine signal transducer, and their association. J Biol Chem. 1995;270:11037–11039. doi: 10.1074/jbc.270.19.11037. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda T, Takahashi-Tezuka M, Fukada T, Okuyama Y, Fujitani Y, Tsukada S, Mano H, Hirai H, Witte O N, Hirano T. Association and activation of Btk and Tec tyrosine kinases by gp130, a signal transducer of the interleukin-6 family of cytokines. Blood. 1995;85:627–633. [PubMed] [Google Scholar]
- 33.Miller A D, Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986;6:2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morella K K, Lai C-F, Kumaki S, Kumaki N, Wang Y, Bluman E M, Witthuhn B A, Ihle J N, Giri J, Gearing D P, Cosman D, Ziegler S F, Tweardy D J, Campos S P, Baumann H. The action of interleukin-2 receptor subunits defines a new type of signaling mechanism for hematopoietic receptors in hepatic cells and fibroblasts. J Biol Chem. 1995;270:8298–8310. doi: 10.1074/jbc.270.14.8298. [DOI] [PubMed] [Google Scholar]
- 35.Mosley B, Beckmann M P, March C J, Idzerda R L, Gimpel S D, Vanden Bos T, Friend D, Anderson D, Jackson J, Wignall J M, Smith C, Gallis B, Sims J E, Urdal D, Widmer M B, Cosman D. The murine interleukin-4 receptor: molecular cloning and characterization of secreted and membrane bound forms. Cell. 1989;59:335–348. doi: 10.1016/0092-8674(89)90295-x. [DOI] [PubMed] [Google Scholar]
- 36.Murakami M, Narazaki M, Hibi M, Yawata H, Yasukawa K, Hamaguchi M, Taga T, Kishimoto T. Critical cytoplasmic region of the interleukin 6 signal transducer gp130 is conserved in the cytokine receptor family. Proc Natl Acad Sci USA. 1991;88:11349–11353. doi: 10.1073/pnas.88.24.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naka T, Narazaki M, Hirata M, Matsumoto T, Minamoto S, Aono A, Nishimoto N, Kajita T, Taga T, Yoshizaki K, Akira S, Kishimoto T. Structure and function of a new STAT-induced STAT inhibitor. Nature. 1997;387:924–929. doi: 10.1038/43219. [DOI] [PubMed] [Google Scholar]
- 38.O’Mahoney J V, Adams T E. Optimization of experimental variables influencing reporter gene expression in hepatoma cells following calcium phosphate transfection. DNA Cell Biol. 1994;13:1227–1232. doi: 10.1089/dna.1994.13.1227. [DOI] [PubMed] [Google Scholar]
- 39.Prowse K R, Baumann H. Hepatocyte-stimulating factor, beta-2 interferon, and interleukin-1 enhance expression of the rat alpha 1-acid glycoprotein gene via a distal upstream regulatory element. Mol Cell Biol. 1988;8:42–51. doi: 10.1128/mcb.8.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadowski H B, Shuai K, Darnell J E, Jr, Gilman M Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993;261:1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- 41.Schiemann W P, Bartoe J L, Nathanson N M. Box 3-independent signaling mechanisms are involved in leukemia inhibitory factor receptor alpha- and gp130-mediated stimulation of mitogen-activated protein kinase. J Biol Chem. 1997;272:16631–16636. doi: 10.1074/jbc.272.26.16631. [DOI] [PubMed] [Google Scholar]
- 42.Stahl N, Farruggella T J, Boulton T G, Zhong Z, Darnell J E, Jr, Yancopoulos G D. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353. doi: 10.1126/science.7871433. [DOI] [PubMed] [Google Scholar]
- 43.Stahl N, Boulton T G, Farruggella T, Ip N Y, Davis S, Witthuhn B A, Quelle F W, Silvennoinen O, Barbieri G, Pellegrini S, et al. Association and activation of Jak-Tyk kinases by CNTF-LIF-OSM-IL-6 beta receptor components. Science. 1994;263:92–95. doi: 10.1126/science.8272873. [DOI] [PubMed] [Google Scholar]
- 44.Starr R, Willson T A, Viney E M, Murray L J, Rayner J R, Jenkins B J, Gonda T J, Alexander W S, Metcalf D, Nicola N A, Hilton D J. A family of cytokine-inducible inhibitors of signalling. Nature. 1997;387:917–921. doi: 10.1038/43206. [DOI] [PubMed] [Google Scholar]
- 45.Sturgill T W, Ray L B, Erikson E, Maller J L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- 46.Symes A, Stahl N, Reeves S A, Farruggella T, Servidei T, Gearan T, Yancopoulos G, Fink J S. The protein tyrosine phosphatase SHP-2 negatively regulates ciliary neurotrophic factor induction of gene expression. Curr Biol. 1997;7:697–700. doi: 10.1016/s0960-9822(06)00298-3. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi-Tezuka M, Hibi M, Fujitani Y, Fukada T, Yamaguchi T, Hirano T. Tec tyrosine kinase links the cytokine receptors to PI-3 kinase probably through JAK. Oncogene. 1997;14:2273–2282. doi: 10.1038/sj.onc.1201071. [DOI] [PubMed] [Google Scholar]
- 48.Tauchi T, Damen J E, Toyama K, Feng G S, Broxmeyer H E, Krystal G. Tyrosine 425 within the activated erythropoietin receptor binds Syp, reduces the erythropoietin required for Syp tyrosine phosphorylation, and promotes mitogenesis. Blood. 1996;87:4495–4501. [PubMed] [Google Scholar]
- 49.Wang X Y, Fuhrer D K, Marshall M S, Yang Y C. Interleukin-11 induces complex formation of Grb2, Fyn, and Jak2 in 3T3L1 cells. J Biol Chem. 1995;270:27999–28002. doi: 10.1074/jbc.270.47.27999. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Kuropatwinski K K, White D W, Hawley T S, Hawley R G, Tartaglia L A, Baumann H. Leptin receptor action in hepatic cells. J Biol Chem. 1997;272:16216–16223. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- 51.Xia S, Rose D W, Sasuoka T, Maegawa H, Burke T R, Roller P P, Shoelson S E, Olefsky J M. SYP(SH-PTP2) is a positive mediator of growth factor-stimulated signal transduction. J Biol Chem. 1994;269:21244–21248. [PubMed] [Google Scholar]
- 52.Yin T, Shen R, Feng G-S, Yang Y-C. Molecular characterization of specific interactions between SHP-2 phosphatase and JAK tyrosine kinases. J Biol Chem. 1997;272:1032–1037. doi: 10.1074/jbc.272.2.1032. [DOI] [PubMed] [Google Scholar]