Abstract
The recent COVID-19 (Coronavirus Disease 2019) pandemic by SARS-CoV2 infection has caused millions of deaths and hospitalizations across the globe. In the early pandemic phases, the infection had been initially considered a primary pulmonary disease. However, increasing evidence has demonstrated a wide range of possible cardiac involvement. Most of systemic and cardiac damage is likely sustained by a complex interplay between inflammatory, immune-related and thrombotic mechanisms. Biventricular failure and myocardial damage with elevation of cardiac biomarkers have been reported in COVID-19 patients, although histological demonstration of acute myocarditis has been rarely documented. Indeed while cardiac magnetic resonance findings include different patterns of myocardial involvement in terms of late gadolinium enhancement, histological data from necropsy and endomyocardial biopsy showed peculiar inflammatory patterns, mostly composed by macrophages. On the other hand COVID-19 vaccines based on mRN technology have been also associated with increased risk of myocarditis. COVID-19 and mRNA vaccine-related myocarditis present different clinical and imaging presentations and recent data suggest the presence of distinctive immunological mechanisms involved.
Introduction
During the early phases of COVID-19 pandemic, pulmonary involvement was deemed to be the predominant systemic involvement in SARS-CoV-2 infection. Over the course of the pandemic, cardiovascular involvement has emerged as an important negative prognostic factor1,2. Increases in troponin, natriuretic peptide and D-dimer levels have been independently associated with increased mortality in COVID-19 patient1–3. However, these initial studies were limited by the heterogeneity of the cohorts and by the restricted availability of invasive and advanced evaluation (such as coronary angiography and cardiac magnetic resonance, CMR). Most of these studies focused on moderate and severe COVID-19 patients, often presenting elevated cardiovascular risk profiles and ongoing acute respiratory distress (ARDS) syndrome. These elements reduced the specificity of circulating biomarkers in the definition of cardiovascular involvement in COVID-19 infection.
Myocardial inflammation in patients with COVID-19 has been reported by CMR with evidence of oedema on T2-weighted sequences and presence of both ischaemic and non-ischaemic late gadolinium enhancement (LGE)4–6.The advent of COVID-19 vaccines based on messenger ribonucleic acid (mRNA) technology represented a milestone in limiting and mitigating COVID-19 pandemic. However, this class of drugs has been associated with myocarditis onset, especially in young males7–10. Clinical and CMR features of mRNA vaccine-related myocarditis differ from COVID-19 myocarditis and present many similarities with classical acute myocarditis11–14.
In this review we will discuss the clinical, imaging and immunological aspects of COVID-19 and mRNA vaccine related myocarditis.
COVID-19-related cardiac damage: Pathophysiological features
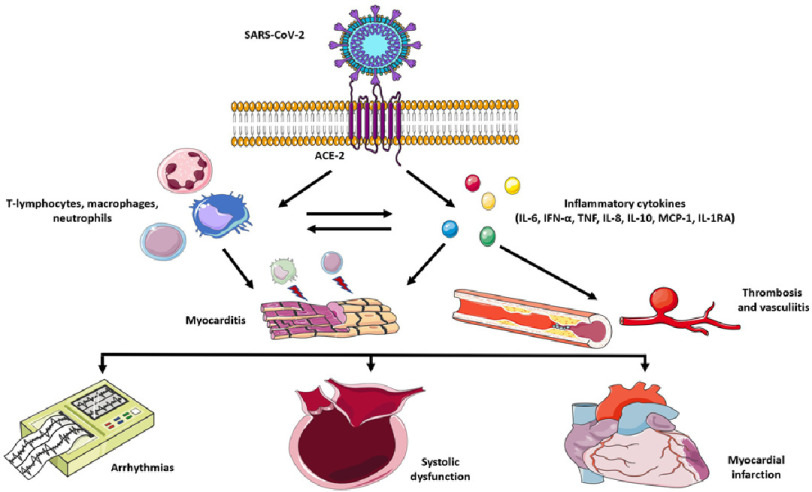
SARS-CoV-2 presents significant vascular tropism due to its binding affinity for angiotensin-converting-enzyme 2 (ACE2) as receptor15 (Figure 1).
Figure 1. Mechanisms of cardiovascular complications of SARS-CoV2 infection.
SARS-CoV-2 enters the cellular membrane due to its affinity to ACE-2 as a receptor. The viral infection determines a marked dysregulated innate immune response with important cellular (T-lymphocytes, macrophages, neutrophils) and cytokine (IL-6, IFN-α, TNF, IL-8, IL-10, MCP-1, IL-1RA) activation. These immune and inflammatory processes can provoke myocarditis, thrombosis, and vasculitis in the myocardium. Arrhythmias, biventricular dysfunction and acute coronary syndromes can occur in these patients. Key: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IL-6, interleukin 6; IFN-α, interferon alpha; TNF, tumor necrosis factor; IL-8, interleukin 8; IL-10 interleukin 10; MCP-1, Monocyte chemoattractant protein-1; IL-1RA, interleukin 1 receptor antagonist.
Lung autopsies performed in the early pandemic phases showed presence of viral localization in the pulmonary vessels with consequential endothelial damage and intussusceptive angiogenesis16. Multiple studies demonstrated marked dysregulation of the innate immunity leading to significant cytokine (such as IFN-α, TNF, IL-6, IL-8, IL-10, MCP-1, IL-1RA) release, complement and neutrophil activation17–20.
Cytokine storm syndrome has emerged as a hallmark feature of moderate and severe COVID-19 infections, leading to multiorgan failure and worse outcome18,21,22. Accordingly, multisystemic inflammatory syndrome in children (MIS-C) represents a serious complication of SARS-CoV-2 infection in the paediatric population23.
Left ventricular dysfunction, haemodynamic instability, troponin release, pericardial effusion and coronary aneurysms have been reported in MIS-C24. Of note these aneurysms may completely regress after treatment with steroids25. Rare reports of coronary aneurysms possibly related to concomitant or recent SARS-CoV2 infection have been described also in adults26.
Taken together, these data suggest that most of COVID-19 related damage could be mediated by direct and indirect effects of marked dysregulated immune response rather than direct viral damage. Evidence of direct myocardial damage by SARS-CoV2 is limited to some cases demonstrating viral localization in interstitial cells and positive genome analysis in endomyocardial biopsies (EMBs)27,28.
Beta-coronaviruses, including SARS-CoV-2, use ACE-2 as cell entry by its receptor-binding domain (RBD)15 and this element could explain its preferential vascular tropism. Bradley et al. described the presence of myocardial fibrosis in 14 patients undergoing autopsies after severe COVID-19 infection with evidence of lymphocytic infiltrates in only one case29. Notably, immunohistochemistry did not detect SARS-CoV-2 in any tissue sample. Other authors reported the presence of extensive inflammatory and thrombotic processes in 12 COVID-19 lung autopsies, but they detected right ventricular (RV) lymphocytic myocarditis in only one case30.
Similarly, Lax et al. described one case of lymphocytic infiltrates in 48 cardiac autopsies with evidence of fibrosis in 10 patients31. Another group reported only one case of lymphocytic myocarditis in 23 COVID cardiac autopsies32 presenting viral particles in interstitial and perivascular cells. Moreover, small vessel vasculitis was found in some tissue samples. The presence of SARS-CoV-2 viral genome was detected by quantitative polymerase chain reaction in 5/104 subjects undergoing EMBs in the early pandemic phase28. Infiltrates of lymphocytes, macrophages and memory T cells were detected, but the Dallas criteria for active myocarditis were reached in only one case. Tanacli et al. reported the presence of fibrosis and macrophage infiltration in 5/10 subjects undergoing EMB5. Lymphocytic infiltrates were found in 5/5 COVID-19 patients undergoing EMB due to the presence of increased cardiac biomarkers by Weckbach et al.33. Notably, CMR was able to detect active myocarditis in only 39% of subjects. Moreover, the same group described the presence of predominant T-lymphocytes and macrophages infiltrates and prominent complement and mitogen activated protein kinase (MAPK) pathways activation in COVID-19 EMBs34 (Table 1).
Table 1. Main cardiac histopathological studies in COVID-19 patients.
| Study | Population | Design | Pathological findings | Notes |
|---|---|---|---|---|
| Tavazzi et al., Eur J Heart Fail33 | 1 COVID-19 patients with ARDS and cardiogenic shock undergoing circulatory mechanical support | The patient underwent EMB. Histological and ultrastructural analysis were performed. | Evidence of interstitial and endocardial inflammatory infiltrates (CD68+ macrophages). Presence of viral particles in interstitial cells with signs of cytopathic damage. | Presence of mild interstitial and perivascular fibrosis. |
| Bradley et al., Lancet14 | 14 patients deceased due to severe COVID-19 | All patients underwent lung, cardiac and kidney autopsies. Histological, Immunohistochemistry and ultrastructural evaluations were performed. |
Presence of cardiac fibrosis in 100% of subjects and myocyte hypertrophy in 93% of them. Evidence of lymphocytic myocarditis in 1 patient with myocyte necrosis. | Immunohistochemical analysis for SARS-CoV-2 in cardiac samples turned out to be negative. |
| Wichmann et al., Ann Intern Med15 | 12 consecutive subjects deceased due to severe COVID-19 | Autoptic cardiac and pulmonary evaluation. | Evidence of RV lymphocytic myocarditis in 1 patient. | Massive pulmonary thrombosis in 4 cases, presence of DVT in 3 subjects. |
| Lax et al., Ann Intern Med16 | 48 patients deceased due to severe COVID-19 | All subjects underwent lung, heart, liver and kidney autopsies. | Presence of enlarged myocytes with nuclear polymorphism. 10/48 subjects presented patchy fibrosis. Presence of lymphocytic infiltrates in 1 case. | Lung autopsies demonstrated presence of extensive parenchymal and intravascular inflammatory infiltrates with frequent signs of thrombosis. |
| Buja et al., Cardiovasc Pathol17 | 23 deceased patients due to severe COVID-19 | Cardiac, pulmonary, and splenic autopsies of the included subjects. | Viral localization in the cardiac interstitial and perivascular cells. Evidence of small vessel vasculitis. Lymphocytic myocarditis in 1 case. | Presence of ARDS and mononuclear infiltrates in lung autopsies. Evidence of large and small pulmonary vessel thrombosis. |
| Escher et al., ESC Heart Fail34 | 104 patients undergoing EMB due to suspected myocarditis or unexplained heart failure during early pandemic phases. | All patients underwent EMB with immunohistological evaluation and qRT-PCR for SARS-CoV-2 genome. | Positive qRT-PCR ofr SARS-CoV-2 in 5/104 EMBs. Presence of active myocarditis following Dallas criteria in 1/5 patients. Presence of elevated levels of T-cless, macrophages, lymphocytes and memory T-memory cells in 4 subjects. | All COVID-19 patients presented increased levels of cell adhesion molecules. Presence of vasculitis in one patient. |
| Weckbach et al., Circ Cardiovasc Imaging48 | 18 subjects with SARS-CoV-2 infection and increased cardiac damage biomarkers. | All patients underwent CMR. EMB performed in 5/18 patients. | 5/5 patients presented lymphocytic myocarditis at histology with no evidence of SARS-CoV-2 genome on qRT-PCR. | CMR demonstrated active myocarditis in only 38.9% of subjects following revised Lake-Louise criteria. |
| Tanacli et al., Front Cardiovasc Med13 | 32 patients with persisting cardiac symptoms after SARS-CoV-2 infection. | All subjects underwent CMR. 10/32 patients underwent EMB. | Presence of fibrosis and mild macrophage infiltration in 5/10 subjects. | Presence of active myocarditis in only 9% of COVID-19 patients following revised Lake-Louise criteria at CMR. |
| Weckbach et al., JAMA Cardiol35 | 5 patients with SARS-CoV-2 infection. | All patients underwent EMB, immunohistochemical, immunofluorescence proteomic and RNA analysis. | Predominant macrophage infiltration in COVID-19 EMBs and significant activation of serine/threonine kinase, MAPK and complement pathways. | Increased macrophage co-expression of the CD-163 scavenger receptor (also expressed by pulmonary macrophages in COVID-19 pneumonia). |
Notes.
- COVID-19
- coronavirus disease 2019
- ARDS
- acute respiratory distress syndrome
- EMB
- endomyocardial biopsy
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- RV
- right ventricle
- DVT
- deep venous thrombosis
- q-RT-PCR
- quantitative real time polymerase chain reaction
- CMR
- cardiac magnetic resonance
- RNA
- ribonucleic acid
- MAPK
- mitogen activated protein kinases
COVID-19 CARDIAC DAMAGE: CLINICAL, IMAGING AND PATHOLOGICAL FINDINGS
Clinical presentation and imaging findings
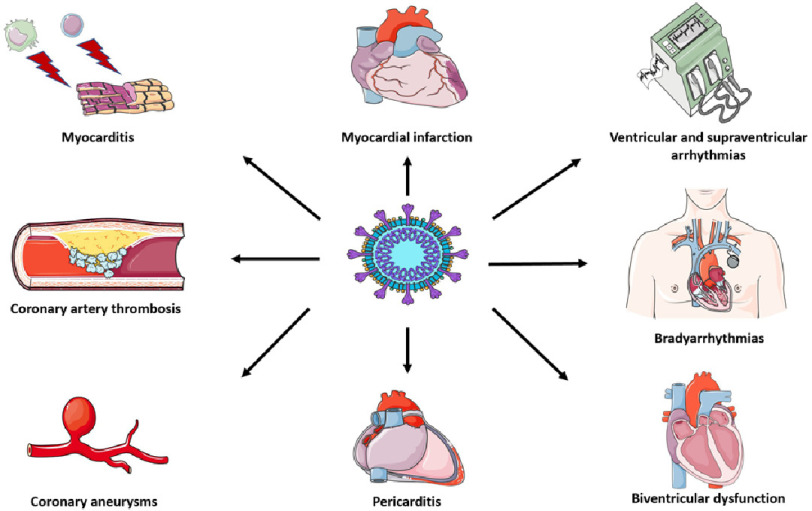
Left and right ventricular dysfunction are relatively common in severe COVID-19 and have been associated with worse outcomes in literature35–38. Ammirati et al. reported high rates (39%) of fulminant presentation in a cohort of COVID-19 myocarditis39. Presence of concomitant pneumonia has been associated with worse prognosis in patients with COVID-19 myocarditis39. Ventricular arrhythmia, high-grade atrioventricular block, pericardial effusion and cardiac tamponade can complicate the clinical course26. Notably, Barhoum et al. reported lower left ventricular ejection fraction, increased use of mechanical circulatory support and higher intensive care unit complications in COVID-19 myocarditis without multisystem inflammatory syndrome (MIS-) compared to MIS+ subjects (Figure 2)25,39.
Figure 2. Cardiovascular manifestations of SARS-CoV2 infection.
SARS-CoV-2 mainly causes macrophage and lymphocytic myocarditis, while its direct pathogenetic role is still debated. Due to its peculiar vascular tropism, many cases of arterial and venous thrombosis and coronary vasculitis have been described in literature. These pathogenetic mechanisms can lead to biventricular dysfunction, brady and tachyarrhythmias and myocardial infarction (even with non-obstructive coronary arteries). Pericarditis is another possible complication of COVID-19 infection. Key: COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Electrocardiographic signs of RV strain, presence of left bundle branch block, QRS fragmentation, repolarization abnormalities and supraventricular arrhythmias have been associated with a worse prognosis in COVID-19 patients40–43. Advanced atrioventricular block and ventricular arrhythmias are relatively uncommon and limited to most severe cases26.
Echocardiography can demonstrate the presence of right and left ventricular dysfunction, reduced biventricular strain and pericardial effusion26,35–39. CMR studies performed in patients hospitalized for COVID-19 demonstrated the presence of ischaemic LGE patterns in 6–22% of patients, even in patients without significant lesions at coronary angiography4,44. Coronary vasculitis and biventricular dysfunction have been described in paediatric patients with COVID-19 related MIS-c23,24. Presence of multisystemic inflammatory syndrome has been reported also in the adult population (MIS-A)26.
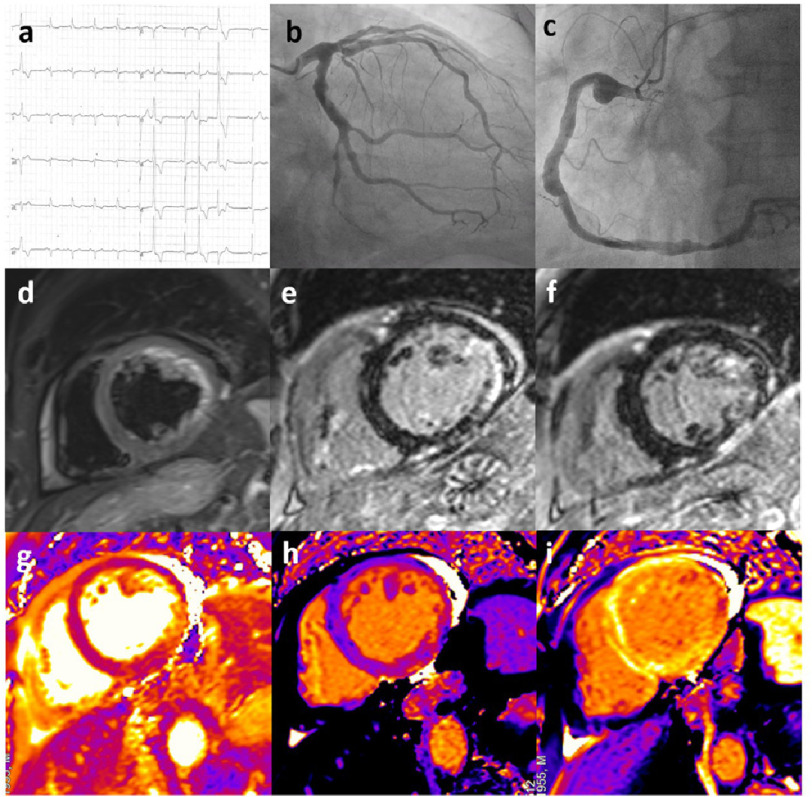
Presence of oedema and non-ischaemic LGE patterns compatible with myocarditis have been reported in a significant proportion of patients after SARS-CoV-2 infection4,5,44,45. Although the authors could not exclude the presence of concomitant inherited or acquired cardiac diseases in all cases, the relatively high prevalence of these alterations and the frequent association with CMR signs of oedema (in terms of positive T2-weighted or T2 mapping sequences) suggest a pathogenetic role of SARS-Cov-2 infection. Ammirati etal.reported high rates of biventricular dysfunction in patients with COVID-19 related myocarditis with frequent need of vasoactive drugs and/or mechanical support39. A case of severe COVID-19 patient with complex cardiovascular involvement is described in Figure 3.
Figure 3. A case of COVID-19-related cardiovascular damage.
A 65-year-old male presenting with severe left ventricular dysfunction and non-sustained ventricular tachycardia shortly after hospitalization due to COVID-19 pneumonia. (a) ECG at presentation showed the presence of sinus rhythm with normal atrioventricular conduction, left anterior hemiblock, QRS fragmentation in V1–V2 leads and negative lateral T waves; polymorphic ventricular beats were present. (b–c) Coronary angiography showed diffuse aneurysmatic lesions in the absence of critical stenosis in the circumflex (b) and right (c) coronary artery. (d) CMR demonstrated the presence of myocardial oedema in the LV inferolateral walls on T2-weighted images (short-tau inversion recovery, STIR) with mild pericardial effusion. (e–f) Late sequences after contrast administration showed the presence of extensive ischaemic LGE in the mid LV lateral wall and midwall LGE in the apical anterolateral wall. LGE was detected also in the anterior RV wall. (g) T2 mapping confirmed the presence of inflammation in the lateral LV wall. h and i: Image analysis of Native and post-contrast T1 mapping (ShMOLLI at 1.5 T) showed increased T1 and ECV values matching LGE sequences in the inferior and lateral wall. Key: COVID-19, coronavirus disease 2019; CMR, cardiac magnetic resonance; LV, left ventricle; STIR, short-tau inversion recovery; LGE, late gadolinium enhancement; RV, right ventricle; ECV, extracellular volume.
Cardiac screening performed on athletes recovering from SARS-CoV-2 infection revealed the presence of cardiac abnormalities in 0.7−2.3% of subjects by use of CMR in terms of pathological T2-derived sequences, presence of increased native T1 mapping and non-ischaemic LGE46,47. Patients with cardiopulmonary symptoms and abnormal ECG, stress testing or echocardiographic findings more likely presented pathological findings at CMR.
Histology and proposed mechanisms
Cardiac autopsies in COVID-19 patients demonstrated the presence of fibrosis and macrophage infiltration in tissue samples with low prevalence of active lymphocytic myocarditis following Dallas criteria29–32,48. Small vessel vasculitis has been detected in a cardiac autoptic COVID-19 cohort32.
EMBs demonstrated the presence of SARS-CoV-2 viral genome in only a minority of cases27,28,39. Macrophages, neutrophils and T lymphocytes were the main cellular lines found in tissue samples and many cases did not reach the classical Dallas criteria for the histological diagnosis of acute myocarditis33,34,39. The low prevalence of pathological infiltrates could be potentially explained by sample biases related to EMB technique, especially when performed only in the RV49. However, these findings were concordant across multiple studies, suggesting the presence of peculiar pathological features in COVID-19 related myocarditis. These elements, together with the evidence of MAPK and complement pathways activation, further support the hypothesis of a predominant immune response activation in patients with COVID-19 cardiac involvement.
Management and outcome
Treatment of hospitalized patients with SARS-CoV-2 infection is based on corticosteroids, heparin and remdesivir50–52. The important role of cytokines in the pathogenesis of COVID-19 related systemic damage led many groups to use specific immunosuppressive drugs in the management of these patients. Use of interleukin-6 (IL-6) inhibitors has been associated with mixed results in literature53 although a recent meta-analysis reported improved outcomes in COVID-19 patients receiving IL-6 inhibitors in combination with steroid therapy54. Interestingly, the use of baricitinib (a janus kinase inhibitor) in combination with remdesivir has been associated with improved outcomes compared to remdesivir alone in a randomized controlled trial55. Steroid therapy is recommended in case of COVID-19 related myocarditis with heart failure, MIS-A or haemodynamic instability56. Ammirati etal. reported the use of remdesivir, IL-6 inhibitors and intravenous immunoglobulins in a large cohort of patients with COVID-19 related myocarditis39. Anecdotal use of immunoadsorption in severe cardiac and pulmonary COVID-19 infection has been also described57.
COVID-19 VACCINES AND MYOCARDITIS
COVID-19 vaccines based on messenger RNA technology have played a crucial role in limiting and mitigating the pandemic due to their efficacy and safety profile58–61. The registration trials did not demonstrate serious adverse effects, but many cases of myocarditis have emerged after widespread vaccination10–13. COVID-19 vaccines-related myocarditis occurred in young males, usually a few days after second dose administration10–13,62. Estimated prevalence ranges from 0.2 to 38.9 cases per million doses, depending on age, sex and geographical distribution12,13,63.
Clinical presentation and imaging findings
Chest pain is the most typical symptom, sometimes associated with fever and flu-like symptoms62. Heart failure and cardiogenic shock are uncommon (less than 10% of cases)12. Ammirati etal.reported the presence of ST segment elevation in 60% of cases and non-sustained ventricular tachycardia in 11% of subjects62. Most of the patients presented preserved biventricular function with pericardial effusion in 16% of them. CMR demonstrated the presence of oedema on T2-weighted images and T2 mapping sequences14,62,64. Signs of oedema, late gadolinium enhancement and increased T1 mapping values were mostly observed in inferolateral and anterolateral segments14,62,64.
Histology and proposed mechanisms
EMBs performed in patients with clinically suspected vaccine-related myocarditis demonstrated the presence of B and T lymphocytes, plasma cells, macrophages and degranulated eosinophils65–67. Notably, EMBs revealed the presence of chronic healed myocarditis and sarcoidosis in patients with clinically suspected COVID-19 vaccine related myocarditis66.
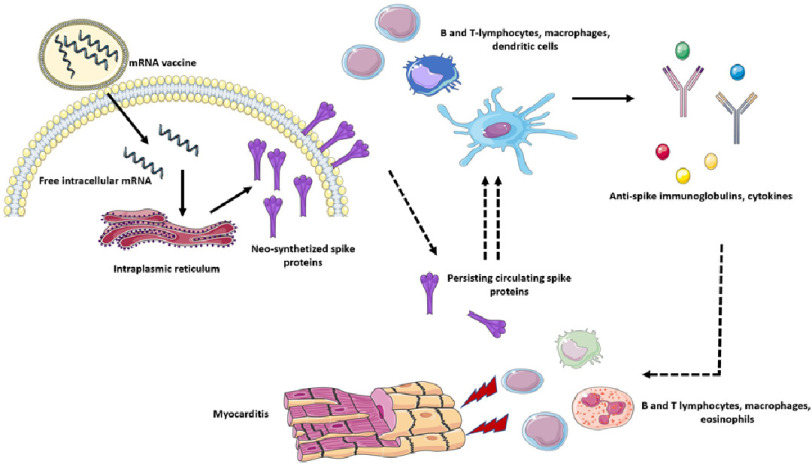
The mechanisms leading to myocarditis following mRNA vaccine administration remain largely unclarified. An exaggerated immune response to vaccine has been claimed but not conclusively demonstrated65. Interestingly, a recent work by Yonker and colleagues68 demonstrated that patients with myocarditis following mRNA vaccine administration present high levels of unbound circulating spike protein compared to vaccinated controls without myopcarditis. Extensive immunophenotype characterization also demonstrated the presence of increased effector memory T cells and PD-1–expressing bulk CD4+ T cells in patients with myocarditis, suggesting a potential exhaustion in these cellular lines. Moreover, the author reported increased levels of IL-8, IL-6, tumor necrosis factor-α, IL-10, interferon-γ, and IL-1 β and lower IL-4 levels in subjects with myocarditis compared to controls (Figure 4).
Figure 4. Proposed mechanisms of COVID-19 mRNA vaccine-related myocarditis.
COVID-19 vaccines based on mRNA technology enter cells by endocytosis and release their mRNA (coding for COVID-19 spike protein) content in the cytoplasm. The mRNA is therefore transcripted in the endoplasmic reticulum into numerous spike proteins. These molecules are then exposed in the outer cellular membrane and presented to B and T-lymphocytes, dendritic cells, dedicated macrophages and other antigen presenting cells. The antigen exposure and recognition lead to marked immune cells activation and expansion with consequent immunoglobulin and cytokine production. It has been proposed that persistent free circulating spike proteins could determine an excessive and dysregulated adaptive immune response. These immune pathways could therefore play a role in determining myocarditis with extensive lymphocytes, macrophages and eosinophilic infiltrates. Key: COVID-19, coronavirus disease 2019; mRNA, messenger ribonucleic acid.
Compared to COVID-19 myocarditis, vaccine-related myocarditis present a milder clinical course with frequent infarct-like presentation (Table 2). CMR usually demonstrates preserved biventricular function, while COVID-19 myocarditis can be frequently associated with RV or LV impairment. Oedema and non-ischaemic LGE has been usually detected in the inferolateral segments, similarly to classical viral myocarditis. CMR signs of ischaemic scars have not been described in these patients, differently from COVID-19 myocarditis. Tissue samples obtained from EMB demonstrated florid lymphocytic, macrophage and eosinophilic infiltrates, while COVID-19 EMBs usually present scarce macrophage infiltration and rare cases of lymphocytic myocarditis (Table 2).
Table 2. Comparison between COVID-19 related and COVID-19 mRNA vaccine-related myocarditis: clinical, imaging and pathological features.
| COVID-19 related myocarditis | COVID-19 mRNA vaccine-related myocarditis |
|---|---|
| Pathophysiology | |
| • Innate immunity dysregulation and cytokine pathways activation • Direct viral damage (limited evidence) |
• Persistent elevated circulatory levels of spike protein (limited data) • Increased expression of inflammatory cytokines and activated T lymphocytes (limited data) |
| Clinical presentation | |
| • Acute myocarditis (infarct-like, arrhythmic, heart failure or fulminant presentation) • Chronic inflammatory cardiomyopathies and long-term biventricular dysfunction |
• Infarct-like presentation (most common) • Heart failure/fulminant myocarditis (uncommon) • Serious brady-tachyarrhythmia (uncommon) |
| ECG | |
| • Signs of RV strain • Bundle branch blocks • Fragmented QRS • Repolarization abnormalities • Atrial fibrillation/supraventricular arrhythmia • Ventricular arrhythmia • Bradyarrhythmia |
• ST segment alterations • NSVTs • Sustained ventricular arrhythmia (uncommon) |
| Imaging | |
| • Echocardiography: RV and LV dysfunction, reduced biventricular strain, coronary aneurysms in MIS-A/MIS-C • CMR: biventricular dysfunction, signs of oedema on T2-weighted sequences, ischaemic and non-ischaemic LGE |
• Echocardiography: LV/RV wall motion abnormalities, LV/RV dysfunction (uncommon) • CMR: LV/RV dysfunction (uncommon), signs of oedema on T2-weighted sequences, non-ischaemic LGE |
| Histology | |
| • SARS-Cov-2 localization in the cardiac interstitial space (limited data) • Limited amount of oedema in tissue samples from autopsies or EMBs • Limited T-lymphocyte and macrophages infiltrates • MAPK and complement pathways activation |
• Infiltrates of B and T lymphocytes, plasma cells, macrophages and degranulating eosinophils |
| Treatment | |
| • Inotropes and mechanical support in case of haemodynamic instability • Corticosteroids • Antivirals (remdesivir) • Heparin (in case of moderate-to-severe systemic COVID-19) • IL-6 inhibitors (contrasting evidence) • Janus kinase inhibitors (limited evidence) • Immunoglobulins (limited data) • Immunoadsorption (anecdotal) |
• Inotropes and mechanical support in case of haemodynamic instability (uncommon) • Corticosteroids and immunosuppressive therapies (limited data) |
Notes.
- COVID-19
- coronavirus disease 2019
- mRNA
- messenger ribonucleic acid
- RV
- right ventricle
- NSVT
- non sustained ventricular tachycardia
- MIS-A
- multisystem inflammatory syndrome in adults
- MIS-C
- multisystem inflammatory syndrome in children
- CMR
- cardiac magnetic resonance
- LGE
- late gadolinium enhancement
- LV
- left ventricle
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus 2
- EMB
- endomyocardial biopsy
- MAPK
- mitogen activated protein kinase
- IL-6
- interleukin-6
Management and outcome
From a clinical point of view, most of these patients present a benign course with low rates of fulminant myocarditis or sustained ventricular arrhythmias10–13,62. Ammirati et al. reported persistence of oedema in only 20% of subjects and significant LGE reduction at 3-months CMR follow-up, in line with imaging findings of classical myocarditis62. A case of acute myocarditis following mRNA vaccination is illustrated in Figure 5.
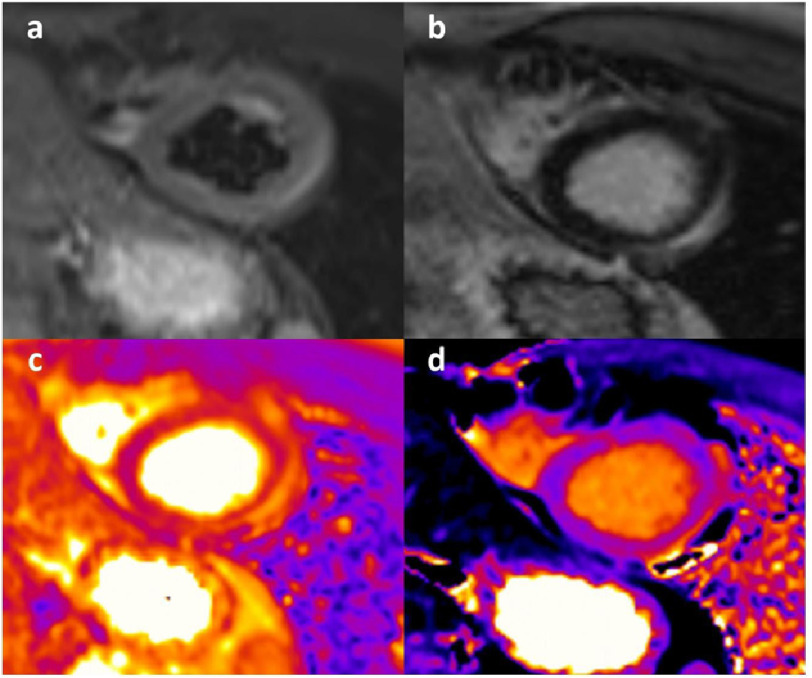
Figure 5. A case of CIVID-19 mRNA vaccine-related acute myocarditis.
A 23-year-old male presenting with chest pain and elevation of markers of cardiac damage 3 days after the administration of a second dose of mRNA vaccine. (a) STIR sequences showed presence of subepicardial oedema in the mid-apical wall. (b) late sequences after contrast demonstrated corresponding LGE in the same segments. (c) T2 mapping sequences confirmed the presence of inflammation in the lateral wall. (d) presence of increased T1 mapping values in the corresponding segments on native T1 mapping sequences. Key: mRNA, messenger ribonucleic acid; STIR, short-tau inversion recovery; LGE, late gadolinium enhancement.
Rare cases of fulminant myocarditis with haemodynamic instability should be treated with inotropes and mechanical support24,66. The use of corticosteroids, colchicine and nonsteroidal anti-inflammatory drugs has been described in literature62,69, but comparative efficacy data are still lacking.
It has been debated whether patients with a previous non-COVID-19-related myocarditis could present a higher risk of myocarditis recurrence following COVID-19 vaccination. Pieroni et al. reported no cases of COVID-19 vaccine related myocarditis in a cohort of patients with a history of a previous acute myocarditis undergoing mRNA COVID-19 vaccination70. These preliminary data suggest that a clinical history of previous acute myocarditis does not portend per se an increased risk for myocarditis following mRNA-based vaccination.
CONCLUSIONS
Myocardial damage in COVID-19 can be extremely heterogeneous and only in a subset of cases can be properly classified as myocarditis. While CMR can frequently show signs of acute and chronic myocarditis in patients with COVID-19, in these subjects histologic findings usually differ from those obderved in classical myocarditis, while a direct SARS-CoV-2 myocaridal damage is still debated.
COVID-19 vaccines based on mRNA technology have been associated with an increased risk of myocarditis, especially in young males. In these patients CMR findings are comparable to those observed in classical myocarditis, while histology demonstrated the presence of lymphocytic and eosinophilic infiltrates. Most patients present mild forms with a favourable outcome also in terms of residual scar at follow-up CMR, with a possible beneficial effect of steroids and immunomodulatory drugs being reported.
References
- 1.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Yan X, Fan Q, Liu H, Liu X, Liu Z, Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotecha T, Knight DS, Razvi Y, Kumar K, Vimalesvaran K, Thornton G, Patel R, Chacko L, Brown JT, Coyle C, Leith D, Shetye A, Ariff B, Bell R, Captur G, Coleman M, Goldring J, Gopalan D, Heightman M, Hillman T, Howard L, Jacobs M, Jeetley PS, Kanagaratnam P, Kon OM, Lamb LE, Manisty CH, Mathurdas P, Mayet J, Negus R, Patel N, Pierce I, Russell G, Wolff A, Xue H, Kellman P, Moon JC, Treibel TA, Cole GD, Fontana M. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur Heart J. 2021;42(19):1866–1878. doi: 10.1093/eurheartj/ehab075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanacli R, Doeblin P, Götze C, Zieschang V, Faragli A, Stehning C, Korosoglou G, Erley J, Weiss J, Berger A, Pröpper F, Steinbeis F, Kühne T, Seidel F, Geisel D, Cannon Walter-Rittel T, Stawowy P, Witzenrath M, Klingel K, Van Linthout S, Pieske B, Tschöpe C, Kelle S. COVID-19 vs. classical myocarditis associated myocardial injury evaluated by cardiac magnetic resonance and endomyocardial biopsy. Front Cardiovasc Med. 2021;8:737257. doi: 10.3389/fcvm.2021.737257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mascia G, Pescetelli F, Baldari A, Gatto P, Seitun S, Sartori P, Pieroni M, Calò L, Della Bona R, Porto I. Interpretation of elevated high-sensitivity cardiac troponin I in elite soccer players previously infected by severe acute respiratory syndrome coronavirus 2. Int J Cardiol. 2021;326:248–251. doi: 10.1016/j.ijcard.2020.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, Reilly N, Ottaviani G, Elghetany MT, Trujillo DO, Aisenberg GM, Madjid M, Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, Damluji AA, de Lemos JA, Desai SS, Emaminia A, Flanagan MC, Khera A, Maghsoudi A, Mekonnen G, Muthukumar A, Saeed IM, Sherwood MW, Sinha SS, O’Connor CM, de Filippi CR. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144(6):502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US Military. JAMA Cardiol. 2021;6(10):1202–1206. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Witberg G, Barda N, Hoss S, Richter I, Wiessman M, Aviv Y, Grinberg T, Auster O, Dagan N, Balicer RD, Kornowski R. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021;385(23):2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mevorach D, Anis E, Cedar N, Bromberg M, Haas EJ, Nadir E, Olsha-Castell S, Arad D, Hasin T, Levi N, Asleh R, Amir O, Meir K, Cohen D, Dichtiar R, Novick D, Hershkovitz Y, Dagan R, Leitersdorf I, Ben-Ami R, Miskin I, Saliba W, Muhsen K, Levi Y, Green MS, Keinan-Boker L, Alroy-Preis S. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N Engl J Med. 2021;385(23):2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel YR, Shah NR, Lombardi K, Agarwal S, Salber G, Patel R, Poppas A, Atalay MK. Follow-up cardiovascular magnetic resonance findings in patients with COVID-19 vaccination-associated acute myocarditis. JACC Cardiovasc Imaging. 2022;15(11):2007–2010. doi: 10.1016/j.jcmg.2022.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5(4):562–69. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383(2):120–28. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW, Dentali F, Montecucco F, Massberg S, Levi M, Abbate A. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol. 2021;21(5):319–329. doi: 10.1038/s41577-021-00536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanderbeke L, Van Mol P, Van Herck Y, De Smet F, Humblet-Baron S, Martinod K, Antoranz A, Arijs I, Boeckx B, Bosisio FM, Casaer M, Dauwe D, De Wever W, Dooms C, Dreesen E, Emmaneel A, Filtjens J, Gouwy M, Gunst J, Hermans G, Jansen S, Lagrou K, Liston A, Lorent N, Meersseman P, Mercier T, Neyts J, Odent J, Panovska D, Penttila PA, Pollet E, Proost P, Qian J, Quintelier K, Raes J, Rex S, Saeys Y, Sprooten J, Tejpar S, Testelmans D, Thevissen K, Van Buyten T, Vandenhaute J, Van Gassen S, Velásquez Pereira LC, Vos R, Weynand B, Wilmer A, Yserbyt J, Garg AD, Matthys P, Wouters C, Lambrechts D, Wauters E, Wauters J. Monocyte-driven atypical cytokine storm and aberrant neutrophil activation as key mediators of COVID-19 disease severity. Nat Commun. 2021;12(1):4117. doi: 10.1038/s41467-021-24360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaath H, Vishnubalaji R, Elkord E, Alajez NM. Single-cell transcriptome analysis highlights a role for neutrophils and inflammatory macrophages in the pathogenesis of severe COVID-19. Cells. 2020;9(11):2374. doi: 10.3390/cells9112374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Georg P, Astaburuaga-García R, Bonaguro L, Brumhard S, Michalick L, Lippert LJ, Kostevc T, Gäbel C, Schneider M, Streitz M, Demichev V, Gemünd I, Barone M, Tober-Lau P, Helbig ET, Hillus D, Petrov L, Stein J, Dey HP, Paclik D, Iwert C, Mülleder M, Aulakh SK, Djudjaj S, Bülow RD, Mei HE, Schulz AR, Thiel A, Hippenstiel S, Saliba AE, Eils R, Lehmann I, Mall MA, Stricker S, Röhmel J, Corman VM, Beule D, Wyler E, Landthaler M, Obermayer B, von Stillfried S, Boor P, Demir M, Wesselmann H, Suttorp N, Uhrig A, Müller-Redetzky H, Nattermann J, Kuebler WM, Meisel C, Ralser M, Schultze JL, Aschenbrenner AC, Thibeault C, Kurth F, Sander LE, Blüthgen N, Sawitzki B. PA-COVID-19 Study Group. Complement activation induces excessive T cell cytotoxicity in severe COVID-19. Cell. 2022;185(3):493–512.e25. doi: 10.1016/j.cell.2021.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Muñoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S, Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu Y, Sofra V, Cano F, Greco M, Theodoridis E, Freedman JD, Gee S, Chan JNE, Ryan S, Bugallo-Blanco E, Peterson P, Kisand K, Haljasmägi L, Chadli L, Moingeon P, Martinez L, Merrick B, Bisnauthsing K, Brooks K, Ibrahim MAA, Mason J, Lopez Gomez F, Babalola K, Abdul-Jawad S, Cason J, Mant C, Seow J, Graham C, Doores KJ, Di Rosa F, Edgeworth J, Shankar-Hari M, Hayday AC. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26(10):1623–1635. doi: 10.1038/s41591-020-1038-6. [DOI] [PubMed] [Google Scholar]
- 22.Lucas C, Wong P, Klein J, Castro TBR, Silva J, Sundaram M, Ellingson MK, Mao T, Oh JE, Israelow B, Takahashi T, Tokuyama M, Lu P, Venkataraman A, Park A, Mohanty S, Wang H, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Muenker MC, Fournier JB, Campbell M, Odio CD, Casanovas-Massana A, Yale IMPACT Team, Herbst R, Shaw AC, Medzhitov R, Schulz WL, Grubaugh ND, Dela Cruz C, Farhadian S, Ko AI, Omer SB, Iwasaki A. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584(7821):463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whittaker E, Bamford A, Kenny J, et al. PIMS-TS study group and EUCLIDS and PERFORM Consortia. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324(3):259–69. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alsaied T, Tremoulet AH, Burns JC, et al. Review of cardiac involvement in multisystem inflammatory syndrome in children. Circulation. 2021;143(1):78–88. doi: 10.1161/CIRCULATIONAHA.120.049836. [DOI] [PubMed] [Google Scholar]
- 25.Diakite S, Bousdira N, Tachon G, Ackermann F, Groh M, Rohmer J. Regression of coronary aneurysms with intravenous immunoglobulins and steroids for COVID-19 adult multisystem inflammatory syndrome. JACC Case Rep. 2021;3(4):581–585. doi: 10.1016/j.jaccas.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barhoum P, Pineton de Chambrun M, Dorgham K, Kerneis M, Burrel S, Quentric P, Parizot C, Chommeloux J, Bréchot N, Moyon Q, Lebreton G, Boussouar S, Schmidt M, Yssel H, Lefevre L, Miyara M, Charuel JL, Marot S, Marcelin AG, Luyt CE, Leprince P, Amoura Z, Montalescot G, Redheuil A, Combes A, Gorochov G, Hékimian G. Phenotypic heterogeneity of fulminant COVID-19–related myocarditis in adults. J Am Coll Cardiol. 2022;80(4):299–312. doi: 10.1016/j.jacc.2022.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22(5):911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Escher F, Pietsch H, Aleshcheva G, Bock T, Baumeier C, Elsaesser A, Wenzel P, Hamm C, Westenfeld R, Schultheiss M, Gross U, Morawietz L, Schultheiss HP. Detection of viral SARS-CoV-2 genomes and histopathological changes in endomyocardial biopsies. ESC Heart Fail. 2020;7(5):2440–2447. doi: 10.1002/ehf2.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H, Najafian B, Deutsch G, Lacy JM, Williams T, Yarid N, Marshall DA. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396(10247):320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy findings and venous thromboembolism in patients with COVID-19: A prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, Reilly N, Ottaviani G, Elghetany MT, Trujillo DO, Aisenberg GM, Madjid M, Kar B. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weckbach LT, Curta A, Bieber S, Kraechan A, Brado J, Hellmuth JC, Muenchhoff M, Scherer C, Schroeder I, Irlbeck M, Maurus S, Ricke J, Klingel K, Kääb S, Orban M, Massberg S, Hausleiter J, Grabmaier U. Myocardial inflammation and dysfunction in COVID-19-associated myocardial injury. Circ Cardiovasc Imaging. 2021;14(1):e012220. doi: 10.1161/CIRCIMAGING.120.011713. [DOI] [PubMed] [Google Scholar]
- 34.Weckbach LT, Schweizer L, Kraechan A, Bieber S, Ishikawa-Ankerhold H, Hausleiter J, Massberg S, Straub T, Klingel K, Grabmaier U, Zwiebel M, Mann M, Schulz C, Group EMBStudy. Association of complement and MAPK activation with SARS-CoV-2-associated myocardial inflammation. JAMA Cardiol. 2022;7(3):286–297. doi: 10.1001/jamacardio.2021.5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karagodin I, Carvalho Singulane C, Woodward GM, Xie M, Tucay ES, Tude Rodrigues AC, Vasquez-Ortiz ZY, Alizadehasl A, Monaghan MJ, Ordonez Salazar BA, Soulat-Dufour L, Mostafavi A, Moreo A, Citro R, Narang A, Wu C, Descamps T, Addetia K, Lang RM, Asch FM, WASE-COVID Investigators Echocardiographic correlates of in-hospital death in patients with acute COVID-19 infection: The World Alliance Societies of Echocardiography (WASE-COVID) study. J Am Soc Echocardiogr. 2021;34(8):819–830. doi: 10.1016/j.echo.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, Sun W, Zhang Y, Li M, Cui L, Cai Y, Wang J, Yang Y, Lv Q, Zhang L, Xie M. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2287–2299. doi: 10.1016/j.jcmg.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rothschild E, Baruch G, Szekely Y, Lichter Y, Kaplan A, Taieb P, Laufer-Perl M, Beer G, Kapusta L, Topilsky Y. The predictive role of left and right ventricular speckle-tracking echocardiography in COVID-19. JACC Cardiovasc Imaging. 2020;13(11):2471–2474. doi: 10.1016/j.jcmg.2020.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Creel-Bulos C, Hockstein M, Amin N, Melhem S, Truong A, Sharifpour M. Acute cor pulmonale in critically ill patients with Covid-19. N Engl J Med. 2020;382(21):e70. doi: 10.1056/NEJMc2010459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ammirati E, Lupi L, Palazzini M, Hendren NS, Grodin JL, Cannistraci CV, Schmidt M, Hekimian G, Peretto G, Bochaton T, Hayek A, Piriou N, Leonardi S, Guida S, Turco A, Sala S, Uribarri A, Van de Heyning CM, Mapelli M, Campodonico J, Pedrotti P, Barrionuevo Sánchez MI, Ariza Sole A, Marini M, Matassini MV, Vourc’h M, Cannatà A, Bromage DI, Briguglia D, Salamanca J, Diez-Villanueva P, Lehtonen J, Huang F, Russel S, Soriano F, Turrini F, Cipriani M, Bramerio M, Di Pasquale M, Grosu A, Senni M, Farina D, Agostoni P, Rizzo S, De Gaspari M, Marzo F, Duran JM, Adler ED, Giannattasio C, Basso C, McDonagh T, Kerneis M, Combes A, Camici PG, de Lemos JA, Metra M. Prevalence, characteristics, and outcomes of COVID-19-associated acute myocarditis. Circulation. 2022;145(15):1123–1139. doi: 10.1161/CIRCULATIONAHA.121.056817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chevrot G, Hauguel-Moreau M, Pépin M, Vieillard-Baron A, Lot AS, Ouadahi M, Hergault H, Aïdan V, Greffe S, Costantini A, Dubourg O, Beaune S, Mansencal N. Electrocardiogram abnormalities and prognosis in COVID-19. Front Cardiovasc Med. 2022;9:993479. doi: 10.3389/fcvm.2022.993479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chevrot G, Hauguel-Moreau M, Pépin M, Vieillard-Baron A, Lot AS, Ouadahi M, Hergault H, Aïdan V, Greffe S, Costantini A, Dubourg O, Beaune S, Mansencal N. Electrocardiogram abnormalities and prognosis in COVID-19. Front Cardiovasc Med. 2022;9:993479. doi: 10.3389/fcvm.2022.993479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia-Zamora S, Lee S, Haseeb S, Bazoukis G, Tse G, Alvarez-Garcia J, Gul EE, Çinier G, Alexander B, Martins Pinto-Filho M, Liu T, Baranchuk A. Arrhythmias and electrocardiographic findings in Coronavirus disease 2019: A systematic review and meta-analysis. Pacing Clin Electrophysiol. 2021;44(6):1062–1074. doi: 10.1111/pace.14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yildirim A, Karaca IO, Yilmaz FK, Gunes HM, Cakal B. Fragmented QRS on surface electrocardiography as a predictor of cardiac mortality in patients with SARS-CoV-2 infection. J Electrocardiol. 2021;66:108–112. doi: 10.1016/j.jelectrocard.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidula MK, Rajewska-Tabor J, Cao JJ, Kang Y, Craft J, Mei W, Chandrasekaran PS, Clark DE, Poenar AM, Gorecka M, Malahfji M, Cowan E, Kwan JM, Reinhardt SW, Al-Tabatabaee S, Doeblin P, Villa ADM, Karagodin I, Alvi N, Christia P, Spetko N, Cassar MP, Park C, Nambiar L, Turgut A, Azad MR, Lambers M, Wong TC, Salerno M, Kim J, Elliott M, Raman B, Neubauer S, Tsao CW, LaRocca G, Patel AR, Chiribiri A, Kelle S, Baldassarre LA, Shah DJ, Hughes SG, Tong MS, Pyda M, Simonetti OP, Plein S, Han Y. Myocardial injury on CMR in patients with COVID-19 and suspected cardiac involvement. JACC Cardiovasc Imaging. 2022 doi: 10.1016/j.jcmg.2022.10.021. S1936-878X(22)00659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID- 19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moulson N, Petek BJ, Drezner JA, Harmon KG, Kliethermes SA, Patel MR, Baggish AL, Outcomes Registry for Cardiac Conditions in Athletes Investigators SARS-CoV-2 cardiac involvement in young competitive athletes. Circulation. 2021;144(4):256–266. doi: 10.1161/CIRCULATIONAHA.121.054824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daniels CJ, Rajpal S, Greenshields JT, Rosenthal GL, Chung EH, Terrin M, Jeudy J, Mattson SE, Law IH, Borchers J, Kovacs R, Kovan J, Rifat SF, Albrecht J, Bento AI, Albers L, Bernhardt D, Day C, Hecht S, Hipskind A, Mjaanes J, Olson D, Rooks YL, Somers EC, Tong MS, Wisinski J, Womack J, Esopenko C, Kratochvil CJ, Rink LD, Big Ten COVID-19 Cardiac Registry Investigators Prevalence of clinical and subclinical myocarditis in competitive athletes with recent SARS-CoV-2 infection: Results from the big ten COVID-19 cardiac registry. JAMA Cardiol. 2021;6(9):1078–1087. doi: 10.1001/jamacardio.2021.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawakami R, Sakamoto A, Kawai K, Gianatti A, Pellegrini D, Nasr A, Kutys B, Guo L, Cornelissen A, Mori M, Sato Y, Pescetelli I, Brivio M, Romero M, Guagliumi G, Virmani R, Finn AV. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J Am Coll Cardiol. 2021;77(3):314–325. doi: 10.1016/j.jacc.2020.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ammirati E, Buono A, Moroni F, Gigli L, Power JR, Ciabatti M, Garascia A, Adler ED, Pieroni M. State-of-the-art of endomyocardial biopsy on acute myocarditis and chronic inflammatory cardiomyopathy. Curr Cardiol Rep. 2022;24(5):597–609. doi: 10.1007/s11886-022-01680-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.RECOVERY Collaborative Group. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.ATTACC Investigators. ACTIV-4a Investigators. REMAP-CAP Investigators. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, Gong MN, Carrier M, Rosenson RS, Reynolds HR, Turgeon AF, Escobedo J, Huang DT, Bradbury CA, Houston BL, Kornblith LZ, Kumar A, Kahn SR, Cushman M, McQuilten Z, Slutsky AS, Kim KS, Gordon AC, Kirwan BA, Brooks MM, Higgins AM, Lewis RJ, Lorenzi E, Berry SM, Berry LR, Aday AW, Al-Beidh F, Annane D, Arabi YM, Aryal D, Baumann Kreuziger L, Beane A, Bhimani Z, Bihari S, Billett HH, Bond L, Bonten M, Brunkhorst F, Buxton M, Buzgau A, Castellucci LA, Chekuri S, Chen JT, Cheng AC, Chkhikvadze T, Coiffard B, Costantini TW, de Brouwer S, Derde LPG, Detry MA, Duggal A, Džavík V, Effron MB, Estcourt LJ, Everett BM, Fergusson DA, Fitzgerald M, Fowler RA, Galanaud JP, Galen BT, Gandotra S, García-Madrona S, Girard TD, Godoy LC, Goodman AL, Goossens H, Green C, Greenstein YY, Gross PL, Hamburg NM, Haniffa R, Hanna G, Hanna N, Hegde SM, Hendrickson CM, Hite RD, Hindenburg AA, Hope AA, Horowitz JM, Horvat CM, Hudock K, Hunt BJ, Husain M, Hyzy RC, Iyer VN, Jacobson JR, Jayakumar D, Keller NM, Khan A, Kim Y, Kindzelski AL, King AJ, Knudson MM, Kornblith AE, Krishnan V, Kutcher ME, Laffan MA, Lamontagne F, Le Gal G, Leeper CM, Leifer ES, Lim G, Lima FG, Linstrum K, Litton E, Lopez-Sendon J, Lopez-Sendon Moreno JL, Lother SA, Malhotra S, Marcos M, Saud Marinez A, Marshall JC, Marten N, Matthay MA, McAuley DF, McDonald EG, McGlothlin A, McGuinness SP, Middeldorp S, Montgomery SK, Moore SC, Morillo Guerrero R, Mouncey PR, Murthy S, Nair GB, Nair R, Nichol AD, Nunez-Garcia B, Pandey A, Park PK, Parke RL, Parker JC, Parnia S, Paul JD, Pérez González YS, Pompilio M, Prekker ME, Quigley JG, Rost NS, Rowan K, Santos FO, Santos M, Olombrada Santos M, Satterwhite L, Saunders CT, Schutgens REG, Seymour CW, Siegal DM, Silva DG Jr, Shankar-Hari M, Sheehan JP, Singhal AB, Solvason D, Stanworth SJ, Tritschler T, Turner AM, van Bentum Puijk W, van de Veerdonk FL, van Diepen S, Vazquez-Grande G, Wahid L, Wareham V, Wells BJ, Widmer RJ, Wilson JG, Yuriditsky E, Zampieri FG, Angus DC, McArthur CJ, Webb SA, Farkouh ME, Hochman JS, Zarychanski R. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med. 2021;385(9):790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gottlieb RL, Vaca CE, Paredes R, Mera J, Webb BJ, Perez G, Oguchi G, Ryan P, Nielsen BU, Brown M, Hidalgo A, Sachdeva Y, Mittal S, Osiyemi O, Skarbinski J, Juneja K, Hyland RH, Osinusi A, Chen S, Camus G, Abdelghany M, Davies S, Behenna-Renton N, Duff F, Marty FM, Katz MJ, Ginde AA, Brown SM, Schiffer JT, Hill JA, GS-US-540-9012 (PINETREE) Investigators Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386(4):305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosn L, Chaimani A, Evrenoglou T, Davidson M, Graña C, Schmucker C, Bollig C, Henschke N, Sguassero Y, Nejstgaard CH, Menon S, Nguyen TV, Ferrand G, Kapp P, Riveros C, Ávila C, Devane D, Meerpohl JJ, Rada G, Hróbjartsson A, Grasselli G, Tovey D, Ravaud P, Boutron I. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;3(3):CD013881. doi: 10.1002/14651858.CD013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, Spiga F, Savovic J, Tierney J, Baron G, Benbenishty JS, Berry LR, Broman N, Cavalcanti AB, Colman R, De Buyser SL, Derde LPG, Domingo P, Omar SF, Fernandez-Cruz A, Feuth T, Garcia F, Garcia-Vicuna R, Gonzalez-Alvaro I, Gordon AC, Haynes R, Hermine O, Horby PW, Horick NK, Kumar K, Lambrecht BN, Landray MJ, Leal L, Lederer DJ, Lorenzi E, Mariette X, Merchante N, Misnan NA, Mohan SV, Nivens MC, Oksi J, Perez-Molina JA, Pizov R, Porcher R, Postma S, Rajasuriar R, Ramanan AV, Ravaud P, Reid PD, Rutgers A, Sancho-Lopez A, Seto TB, Sivapalasingam S, Soin AS, Staplin N, Stone JH, Strohbehn GW, Sunden-Cullberg J, Torre-Cisneros J, Tsai LW, van Hoogstraten H, van Meerten T, Veiga VC, Westerweel PE, Murthy S, Diaz JV, Marshall JC, Sterne JAC. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: A meta-analysis. JAMA. 2021;326(6):499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH, ACTT-2 Study Group Members Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384(9):795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Writing Committee. Gluckman TJ, Bhave NM, Allen LA, Chung EH, Spatz ES, Ammirati E, Baggish AL, Bozkurt B, Cornwell WK 3rd, Harmon KG, Kim JH, Lala A, Levine BD, Martinez MW, Onuma O, Phelan D, Puntmann VO, Rajpal S, Taub PR, Verma AK. 2022 ACC expert consensus decision pathway on cardiovascular sequelae of COVID-19 in adults: Myocarditis and other myocardial involvement, post-acute sequelae of SARS-CoV-2 infection, and return to play: A report of the American college of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;79(17):1717–1756. doi: 10.1016/j.jacc.2022.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ciabatti M, Martinese L, Quacquarelli A, Pieroni M, Feri M, Bolognese L. Cytosorb treatment in severe COVID-19 cardiac and pulmonary disease. Eur Heart J Case Rep. 2021;5(4):ytab123. doi: 10.1093/ehjcr/ytab123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammit LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T, COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, Groom HC, Dunnigan K, Odean MJ, Hegmann K, Stefanski E, Edwards LJ, Schaefer-Solle N, Grant L, Ellingson K, Kuntz JL, Zunie T, Thiese MS, Ivacic L, Wesley MG, Mayo Lamberte J, Sun X, Smith ME, Phillips AL, Groover KD, Yoo YM, Gerald J, Brown RT, Herring MK, Joseph G, Beitel S, Morrill TC, Mak J, Rivers P, Poe BP, Lynch B, Zhou Y, Zhang J, Kelleher A, Li Y, Dickerson M, Hanson E, Guenther K, Tong S, Bateman A, Reisdorf E, Barnes J, Azziz-Baumgartner E, Hunt DR, Arvay ML, Kutty P, Fry AM, Gaglani M. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, Gower C, Kall M, Groves N, O’Connell AM, Simons D, Blomquist PB, Zaidi A, Nash S, Iwani Binti Abdul Aziz N, Thelwall S, Dabrera G, Myers R, Amirthalingam G, Gharbia S, Barrett JC, Elson R, Ladhani SN, Ferguson N, Zambon M, Campbell CNJ, Brown K, Hopkins S, Chand M, Ramsay M, Lopez Bernal J. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ammirati E, Lupi L, Palazzini M, Ciabatti M, Rossi VA, Gentile P, Uribarri A, Vecchio CR, Nassiacos D, Cereda A, Conca C, Tumminello G, Piriou N, Lelarge C, Pedrotti P, Stucchi M, Peretto G, Galasso M, Huang F, Ianni U, Procopio A, Saponara G, Cimaglia P, Tomasoni D, Moroni F, Turco A, Sala S, Di Tano G, Bollano E, Moro C, Abbate A, Della Bona R, Porto I, Carugo S, Campodonico J, Pontone G, Grosu A, Bolognese L, Salamanca J, Diez-Villanueva P, Ozieranski K, Tyminska A, Sardo Infirri L, Bromage D, Cannatà A, Hong KN, Adamo M, Quattrocchi G, Foà A, Potena L, Garascia A, Giannattasio C, Adler ED, Sinagra G, Ruschitzka F, Camici PG, Metra M, Pieroni M. Outcome and morphofunctional changes on cardiac magnetic resonance in patients with acute myocarditis following mRNA COVID-19 vaccination. Circ Heart Fail. 2023:e010315. doi: 10.1161/CIRCHEARTFAILURE.122.010315. [DOI] [PubMed] [Google Scholar]
- 63.Heidecker B, Dagan N, Balicer R, Eriksson U, Rosano G, Coats A, Tschöpe C, Kelle S, Poland GA, Frustaci A, Klingel K, Martin P, Hare JM, Cooper LT, Pantazis A, Imazio M, Prasad S, Lüscher TF. Myocarditis following COVID-19 vaccine: incidence, presentation, diagnosis, pathophysiology, therapy, and outcomes put into perspective. A clinical consensus document supported by the Heart Failure Association of the European Society of Cardiology (ESC) and the ESC Working Group on Myocardial and Pericardial Diseases. Eur J Heart Fail. 2022;24(11):2000–2018. doi: 10.1002/ejhf.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pareek M, Steele J, Asnes J, Baldassarre LA, Casale LR, Desai NR, Elder RW, Faherty E, Ferguson I, Fishman RF, Ghazizadeh Z, Glick LR, Hall EK, Khera R, Kokkinidis DG, Kwan JM, O’Marr J, Schussheim A, Tuohy E, Wang Y, Spatz ES, Jacoby D, Miller EJ. Short-term outcomes after myopericarditis related to COVID-19 vaccination. JACC Cardiovasc Imaging. 2022;15(11):2002–2005. doi: 10.1016/j.jcmg.2022.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med. 2021;385(14):1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kiblboeck D, Klingel K, Genger M, Traxler S, Braunsteiner N, Steinwender C, Kellermair J. Myocarditis following mRNA COVID-19 vaccination: call for endomyocardial biopsy. ESC Heart Fail. 2022;9(3):1996–2002. doi: 10.1002/ehf2.13791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frustaci A, Verardo R, Galea N, Lavalle C, Bagnato G, Scialla R, Chimenti C. Hypersensitivity myocarditis after COVID-19 mRNA vaccination. J Clin Med. 2022;11(6):1660. doi: 10.3390/jcm11061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yonker LM, Swank Z, Bartsch YC, Burns MD, Kane A, Boribong BP, Davis JP, Loiselle M, Novak T, Senussi Y, Cheng CA, Burgess E, Edlow AG, Chou J, Dionne A, Balaguru D, Lahoud-Rahme M, Arditi M, Julg B, Randolph AG, Alter G, Fasano A, Walt DR. Circulating spike protein detected in post-COVID-19 mRNA vaccine myocarditis. Circulation. 2023;147(11):867–876. doi: 10.1161/CIRCULATIONAHA.122.061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Truong DT, Dionne A, Muniz JC, McHugh KE, Portman MA, Lambert LM, Thacker D, Elias MD, Li JS, Toro-Salazar OH, Anderson BR, Atz AM, Bohun CM, Campbell MJ, Chrisant M, D’Addese L, Dummer KB, Forsha D, Frank LH, Frosch OH, Gelehrter SK, Giglia TM, Hebson C, Jain SS, Johnston P, Krishnan A, Lombardi KC, McCrindle BW, Mitchell EC, Miyata K, Mizzi T, Parker RM, Patel JK, Ronai C, Sabati AA, Schauer J, Sexson Tejtel SK, Shea JR, Shekerdemian LS, Srivastava S, Votava-Smith JK, White S, Newburger JW. Clinically suspected myocarditis Temporally related to COVID-19 vaccination in adolescents and young adults: Suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145(5):345–356. doi: 10.1161/CIRCULATIONAHA.121.056583. [DOI] [PubMed] [Google Scholar]
- 70.Pieroni M, Ciabatti M, Saletti E, D’Aniello E, Bolognese L, On-Behalf-Of-The-Covid-Vaccine-In-Myocarditis-Study-Group COVID-19 mRNA vaccination in patients with previous myocarditis. Eur J Intern Med. 2022;104:116–117. doi: 10.1016/j.ejim.2022.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]