Abstract
Obesity is a significant health problem with a continuously increasing prevalence among children and adolescents that has become a modern pandemic during the last decades. Nowadays, the genetic contribution to obesity is well-established. For this narrative review article, we searched PubMed and Scopus databases for peer-reviewed research, review articles, and meta-analyses regarding the genetics of obesity and current pharmacological treatment, published in the English language with no time restrictions. We also screened the references of the selected articles for possible additional articles in order to include most of the key recent evidence. Our research was conducted between December 2022 and December 2023. We used the terms “obesity”, “genetics”, “monogenic”, “syndromic”, “drugs”, “autosomal dominant”, “autosomal recessive”, “leptin-melanocortin pathway”, and “children” in different combinations. Recognizing the genetic background in obesity can enhance the effectiveness of treatment. During the last years, intense research in the field of obesity treatment has increased the number of available drugs. This review analyzes the main categories of syndromic and monogenic obesity discussing current data on genetic-based pharmacological treatment of genetic obesity and highlighting the necessity that cases of genetic obesity should follow specific, pharmacological treatment based on their genetic background.
Keywords: genetic obesity, monogenic obesity, syndromic obesity, Prader–Willi syndrome, Bardet–Biedl syndrome, melanocortin 4 receptor, congenital leptin deficiency, setmelanotide, GLP-1 R agonists, semaglutide
1. Introduction
Obesity is a metabolic disorder in which energy balance is dysregulated, leading to weight gain. It is a major medical problem beginning from childhood and seems to have an epidemic size as the World Health Organization has estimated the number of children < 5 years old who are obese or overweight to be ~39 million [1]. Children are considered overweight when their BMI is between the 85th and 95th percentile for their age and gender, obese with a BMI ≥ 95th percentile, and extremely obese with a BMI ≥ 120% of the 95th percentile [2]. According to the World Obesity Atlas 2023 report, 38% of the global population is currently either overweight or obese. In a recent WHO report based on data collected from 411,000 children aged 6–9 years from 33 countries of the WHO European Region, 29% of children aged 7–9 years were found to live as overweight or obese, thus almost 1 in 3 children were overweight or obese. According to the WHO organization, Greece ranks first in the European Union in childhood obesity, with 20.6% of children aged 4–6 years being obese or overweight with this percentage increasing to 38.5% and 41.2% in children aged 6–10 and 10–12 years, respectively [1]. Moreover, 37.9% of Greek adults are overweight and 24.9% are obese. Importantly, a large percentage of obese children become obese adults with many comorbidities such as type 2 diabetes (T2D), hypertension, and cardiovascular diseases (CVD) and an increased risk of early death [2]. Thus, early detection and the prompt use of strategies against obesity are of crucial importance.
Previous genetic studies have shown that there is a genetic factor in the development of obesity [3]. According to the genetic involvement, obesity is classified into (i) “common polygenic obesity,” which results from the interplay of genetic and environmental factors. Hundreds of polymorphisms that each have a small effect contribute to common obesity. With the advent of genome-wide association studies, multiple genes and loci such as FTO, TMEM18, CADM1, CADM2, and NEGR1 have been associated with increased susceptibility to common, polygenic obesity; (ii) “syndromic obesity”, which except for obesity is characterized by neurodevelopmental delay or dysmorphic features such as Prader–Willi and Bardet–Biedl syndrome and “monogenic obesity”, which is typically rare, early-onset, severe, inherited in a Mendelian pattern, and results from single gene mutations with large effects. This classification has been reconsidered, as cases with monogenic obesity may be accompanied by neurodevelopmental characteristics or psychiatric conditions that are usually seen in syndromic cases. Thus, obesity syndromes can be evaluated separately from polygenic obesity [4]. In this review “syndromic” and “monogenic” forms of obesity are discussed focusing on the most well-known representatives of each category.
2. Syndromic Obesity
Syndromic obesity refers to obesity that is associated with intellectual disability, dysmorphic features, or abnormalities affecting different organs and systems, with low frequency, high variability, and a Mendelian pattern of inheritance [5].
2.1. Prader–Willi Syndrome (PWS)
PWS is an imprinting disorder with an incidence of ~1/15,000–20,000 [6]. Babies with this syndrome are born “floppy”, with hypotonia and up-slanted palpebral fissures, and feed poorly. However, over the first two years, the infant slowly develops hyperphagia and shows developmental and cognitive delays and behavioral problems. PWS obesity is age-dependent, being ~40% and ~85% in children/adolescents and in adulthood, respectively [7]. Multiple endocrine abnormalities such as growth hormone deficiency, hypothyroidism, hypogonadism, and leptin resistance are also seen in PW patients, due to hypothalamus–pituitary–gonadal axis dysregulation. Moreover, PW patients are characterized by an increased incidence of metabolic complications, such as CVD, T2D, and hypertension [6]. The basic genetic types of PW are due to paternal 15q11.2–q13 deletion present in ~70% of PWS cases, maternal uniparental disomy 15 present in ~30% of PWS cases, and imprinting defects in about ~3%. [6].
2.2. Bardet–Biedl Syndrome (BBS)
BBS is a clinically and genetically heterogeneous, autosomal recessive syndrome with a prevalence of about 1/125,000 [8,9], affecting multiple organs in the body. The core clinical characteristics of BBS are severe visual impairment, deformities in extremities, central obesity, mental retardation, renal dysfunction, and male hypogonadism. Obesity in BBS patients involves the dysregulation of the hypothalamic leptin–melanocortin signaling pathway. Commonly associated secondary features of BBS include liver fibrosis, T2D, short stature, speech problems, and neurodevelopmental delay [10].
BBS is a disorder of genetic heterogeneity. Currently, 19 BBS genes have been described. BBS1 and BBS10 are the most highly mutated genes, reaching percentages of 70–80% in certain Caucasian and ~40–50% in patients from Northern Europe [11]. Most commonly, BBS follows an autosomal recessive mode of inheritance but also ”triallelic inheritance” has been observed [12]. Genetic modifiers may also determine clinical variability in BBS patients.
2.3. Pseudohypoparathyroidism (PHP) Type 1a
Pseudohypoparathyroidism (PHP) is a rare disorder characterized by low levels of calcium, high levels of phosphorus, elevated PTH levels in the blood, and parathyroid hormone (PTH) resistance [13]. It is divided into three categories, PHP-1, PHP-2, and pseudopseudohypoparathyroidism (PPHP). PHP-1 has three subtypes: 1a, 1b, and 1c. PHP type 1a and 1c display Albright’s hereditary osteodystrophy (AHO) features, including obesity. Mutations in the GNAS on chromosome 20q13.2–13.3 have been recognized due to spontaneous mutation or inherited in an autosomal dominant manner. PHP 1b has no AHO characteristics and is restricted only to the kidney. Recently, GNAS and STX16 deletions were associated with PHP 1b [14]. PHP-2 patients have PTH resistance but with no AHO features. Regarding, PPHP patients, they inherit the mutation from the father and GSa is maternally expressed. In PHP 1a, obesity is associated with alterations in the MC4R pathway [15].
2.4. Alström Syndrome (ALMS)
ALMS is a rare autosomal recessive syndrome caused by ALMS mutations located on chromosome 2. Current incidence is unknown with estimates ranging from 1 in 500,000 to 1 in 1,000,000 [16,17]. ALMS affects multiple organs and is characterized by clinical heterogeneity. ALMS patients usually suffer from vision and hearing loss, central obesity, T2D, dilated cardiomyopathy or congestive heart failure, infertility, acanthosis nigricans, hypothyroidism, and short stature. Fibrosis in the kidneys, liver, and lungs can lead to its dysfunction. Delayed learning skills are also common [18].
2.5. 16p11.2 Deletion Syndrome
16p11.2 deletion syndrome is a microdeletion syndrome with an estimated prevalence of 1–5/10,000. It is usually caused by a small deletion of chromosome 16 at position p11.2. De novo deletions are frequent [19]. The main clinical characteristics of this syndrome are neurodevelopmental delay, intellectual disability, and autism [20]. A strong association between 16p11.2 microdeletion and obesity has been reported [21]. Among the genes involved in this microdeletion syndrome is SH2B1, which participates in the leptin–melanocortin pathway. Some other physical abnormalities such as finger clinodactyly and syndactyly, craniofacial or dermatological abnormalities, anxiety disorders, or reduced fertility may also be present. The size of the lost chromosome region actually determines the severity of the syndrome [19].
2.6. WAGR Syndrome
WAGR is a syndrome with a prevalence of 1/500,000, which is caused by a deletion of chromosome 11p13 that encompasses the WT1 and PAX6 genes [22]. It is an autosomal dominant syndrome that has a susceptibility to Wilms tumor, absence of the iris, genital and urinary abnormalities, and developmental delay. A specific phenotype of WAGR, WAGRO includes obesity, too [23]. Brain-derived neurotrophic factor (BDNF) gene haploinsufficiency has been implicated in childhood-onset obesity, intellectual disability, and autism [24]. In a study of 33 patients with WAGR, it was observed that patients with BDNF haploinsufficiency had significantly higher BMIs and this was directly associated with childhood-onset obesity [25].
2.7. Smith–Magenis Syndrome (SMS)
SMS mainly occurs by a small deletion of chromosome 17p11.2 [26]. Mutations in RAI1 leading to its haploinsufficiency are more rarely found [27]. SMS is a developmental disorder accompanied by intellectual disability, dysmorphic characteristics, and sleep and behavioral problems. Early in their lives, the majority of patients with SMS become overweight/obese [26].
2.8. Cohen Syndrome (CS)
CS is caused by mutations in the vacuolar protein sorting 13 homolog B (VPS13B) gene located on chromosome 8q22.2. The so-called “brain-obesity-eye-bone” syndrome is an autosomal recessive disease, with developmental delay, intellectual disability, hypotonia, and specific facial characteristics [28]. Currently, >200 VPS13B mutations have been detected in ~200 patients with CS of various ethnicities [29,30]. VPS13B is a transmembrane protein with functional and structural role in the Golgi apparatus [31].
2.9. MYT1L-Variants Syndrome
Mutations in the myelin transcription factor 1-like (MYT1L) gene have been associated with obesity following an autosomal dominant mode of inheritance, which is mainly accompanied by developmental and behavioral problems, intellectual disability, and epilepsy. MYT1L belongs to the myelin transcription factor 1 (MYT1) family and in humans is only expressed in the brain. Mutations in the MYT1L affect the expression of genes that have a crucial role in the neurodevelopment and function of the hypothalamus [32].
2.10. Börjeson–Forssman–Lehmann Syndrome (BFLS)
BFLS is an X-linked recessive genetic disease caused by mutations in PHF6 [33]. It is accompanied by intellectual disability, growth defects, hypogonadism, seizures, and specific facial characteristics. Obesity emerging in late childhood is found in ~75% of BFLS patients who also can have microcephaly (6%) or macrocephaly (15%) [34].
2.11. Carpenter Syndrome (CRPT1)
CRPT1 is an autosomal recessive disease caused by homozygous or compound heterozygous loss-of-function mutations in RAB23. It is characterized by craniosynostosis, polysyndactyly, obesity mental retardation, hypogonadism, and lateralization defects [35]. RAB23 encodes a small GTPase of the Ras superfamily and participates in membrane trafficking and vesicular transport, exerting a crucial role in the Sonic hedgehog signaling pathway [36].
2.12. Down Syndrome (DS)
DS also known as trisomy 21 occurs in about 1:600–700 newborns. The main causes of the extra copy of chromosome 21 are chromosomal non-disjunction, Robertsonian translocations, and mosaicism. Patients with DS are more often obese probably due to increased levels of leptin, decreased activity, bad eating habits, and abnormal metabolism. Interestingly, in a previous study, the possibility of patients with DS being overweight or obese compared to individuals without developmental disabilities was significantly increased [37,38].
2.13. Kallmann Syndrome (KS)
KS is characterized by hypogonadotropic hypogonadism and anosmia. Multiple genes have been implicated in the pathogenesis of KS including KAL1, FGFR1, FGF8, PROKR2, and PROK2. KS is mainly an X-linked recessive disorder but cases with autosomal recessive or dominant patterns of inheritance have been described [39]. Unilateral renal agenesis, cleft palate, abnormal eye movements, hearing loss, as well as obesity are also seen in these patients.
3. Monogenic Obesity
Monogenic obesity follows a Mendelian pattern of inheritance and patients are usually characterized by severe obesity due to mutations in a specific gene. Mutations in genes implicated in the leptin–melanocortin pathway have been mostly associated with monogenic obesity. In this pathway, leptin—an adipose tissue hormone—binds to the hypothalamic leptin receptor, stimulating the production of proopiomelanocortin (POMC). Proprotein convertase subtilisin/kexin type 1 (PCSK1) cleaves POMC into melanocortin ligands, such as α- and β-melanocyte–stimulating hormone, expediting binding and activation of the melanocortin-4 receptor (MC4R), thus reducing food intake and increasing energy consumption. BDNF has also a regulatory role in this pathway [5].
3.1. Congenital Leptin Deficiency
Leptin is mainly produced by adipose tissue and has a crucial role in energy metabolism. More specifically, leptin acts in the hypothalamus activating anorexigenic POMC, as well as cocaine- and amphetamine-related transcript preprotein (CARTPT) derivatives that control food consumption. Leptin has also an inhibitory action on the orexigenic peptides neuropeptide Y (NPY) and Agouti-related protein (AGRP), which increase food consumption. When leptin binds to its receptor, a cascade of signals is triggered that can possibly lead to leptin resistance. Serum leptin levels are usually undetectable and clinically heterozygous patients are characterized by early-onset obesity, whereas in a homozygous state, hypogonadotropic hypogonadism and hypothyroidism can also be seen [40,41].
3.2. Congenital Leptin Receptor Deficiency
Leptin receptors are present mainly in the hypothalamus but also in other organs, thus, leptin can exert its role in multiple different ways. The LEPR gene encodes for six different isoforms (LEPR a–f), among which the LEPR-b receptor has been mostly associated with severe forms of monogenic obesity [42]. Both homozygous or compound heterozygous LEPR mutations can lead to leptin receptor deficiency inherited in an autosomal recessive manner. Serum leptin levels are usually increased and severe obesity is prominent in these patients [43].
3.3. POMC Deficiency
POMC-derived peptides, such as α-MSH, are produced in the arcuatus nucleus and act via binding to the MC4R, suppressing appetite and food intake, thus having a fundamental role in weight regulation. Patients with POMC deficiency are characterized by severe early-onset obesity and follow an autosomal recessive mode of inheritance. Among clinical symptoms are polyphagia, pigmentary abnormalities, and adrenal insufficiency [44].
3.4. PCSK1 Deficiency
PCSK1 can activate by cleaving many precursor molecules such as POMC, proinsulin, proglucagon, and proghrelin, which are important for energy metabolism [45]. Individuals with PCSK1 mutations show severe obesity, polyphagia, diarrhea, hypoglycemia, and other symptoms of hormonal imbalance [46,47]). Biallelic PCSK1 mutations are associated with impaired prohormone processing [46,47].
3.5. MC4R Deficiency
MC4R, also expressed in the hypothalamus, has a crucial effect on energy homeostasis and food consumption. Clinically, mutation carriers are characterized by severe obesity, severe hyperinsulinemia, increased lean body mass, and linear growth, whereas MC4R homozygous patients—although rarely identified—are more severely affected [45].
3.6. SH2B1 Deficiency
Src homology 2B1 (SH2B1) protein binds to a number of tyrosine kinases, such as Janus kinase 2 (JAK2) and the insulin receptor. SH2B1 is abundantly expressed in the brain and in various tissues including the adipose tissue. SH2B1 participates in the leptin-mediated signal pathway regulating body weight, insulin sensitivity, and glucose metabolism. Individuals with SH2B1 mutations show obesity, polyphagia, severe insulin resistance, as well as maladaptive behaviors, and follow an autosomal recessive mode of inheritance [48].
3.7. CPE Deficiency
Carboxypeptidase E (CPE) catalyzes the activation of hormone precursors to active molecules [49]. CPE is highly expressed in the central nervous system as well as in other tissues including adipose tissue. In 2015, a truncating pathogenic CPE mutation was detected in a female patient suffering from severe obesity, hyperglycemia, intellectual disability, and hypogonadotropic hypogonadism [50] and, recently, a new syndrome Blakemore–Durmaz–Vasileiou was described with homozygous CPE mutations and similar characteristics plus central hypothyroidism [51].
3.8. SRC1 Deficiency
Steroid receptor coactivator-1 (SRC1) affects the expression of many target genes [52]. In a previous large exome-sequencing study of patients with severe obesity, rare heterozygous variants in SRC1 were detected and were associated with the dysregulation of POMC expression [53,54]. Importantly, SRC-1 deletion also caused obesity in mice [55]. In a recent study, it was shown that in the hypothalamus, SRC-1 interacts with phosphorylated signal transducer and activator of transcription-3 (STAT3), affecting POMC expression [56]. Patients with SRC1 mutations and severe obesity have been enrolled in phase 2 clinical trials of setmelanotide, an MC4R agonist, approved for obese patients with POMC or LEPR mutations [54].
4. Genetic-Based Pharmacological Treatment of Obesity
Obesity has become a major health problem worldwide due to its increasing prevalence and comorbidities. About 20% of the children and adolescents in the United States suffer from obesity, nearly 6% of them have severe obesity, and ~7% of severe pediatric obesity has a genetic background [57,58] with almost 3% having LEPR mutations [59] and 3–6% MC4R mutations [60]. It is well-known that children suffering from obesity are at increased risk for several medical health problems, thus, early diagnosis and intervention are of fundamental importance; however, the available pharmacological armamentarium is currently restricted [61]. Moreover, identifying a child with polyphagia based on a genetic background can be of crucial importance for treatment. Lately, intense research has emerged focusing especially on genetic-based pharmacological treatment of obesity with quite enthusiastic results, which have been accompanied by FDA approval of new agents, eventually increasing the available armamentarium for the pharmacological treatment of genetic obesity (Table 1).
Table 1.
New pharmacological agents approved for genetic obesity.
| Drug Class | Dose | Route | Approved for | Most Common Side Effects | |
|---|---|---|---|---|---|
| Metreleptin | Recombinant analog of leptin | 0.03 mg/kg | subcutaneous | LEP deficiency | production of anti-leptin antibodies, increased risk of lymphomas |
| Setmelanotide | MC4R agonist | Max 3 mg | subcutaneous | POMC deficiency PCSK1 deficiency LEPR deficiency BBS |
hyperpigmentation, nausea, vomiting, and injection site reactions |
| Semaglutide | GLP-1 receptor agonist | 2.4 mg | subcutaneous | chronic weight management BMI ≥ 27 kg/m2, at least one weight-related ailment or BMI of ≥30 kg/m2. Age limit 12 years. | Nausea, vomiting, diarrhea, constipation |
| Liraglutide | GLP-1 receptor agonist | 3 mg | subcutaneous | chronic weight management among pediatric patients aged ≥ 12 who are obese | nausea, vomiting, diarrhea, dizziness fever |
| Tirzepatide | GIP receptor and GLP-1 receptor agonist | 2.5 mg | subcutaneous | chronic weight management BMI ≥ 27 kg/m2, at least one weight-related ailment or BMI of ≥30 kg/m2. It is only approved for adults. | nausea, diarrhea, vomiting, constipation, abdominal discomfort and pain, injection site reactions |
In 1999, leptin deficiency—diagnosed in homozygous LEP gene mutation carriers with severe obesity—was successfully treated with leptin administration [62]. Indeed, subcutaneous injection of human recombinant leptin (metreleptin) is notably beneficial for patients with leptin deficiency, leading to improved food control and weight reduction, as well as improvement of metabolic and endocrine dysregulations [63]. Reported side effects include the production of antibodies against leptin and an increased susceptibility to lymphomas [62].
In 2016, a breakthrough regarding the drug therapy of genetic obesity was made introducing setmelanotide, a new MC4R agonist [64,65]. Initially, in a phase II trial, in two POMC homozygous patients, setmelanotide resulted in better food control and considerable weight improvement [64]. In 2020, in a phase III trial, 80% of POMC-deficient patients and 45% of LEPR-deficient patients had ~10% weight loss at ~1 year. The adverse events of setmelanotide were hyperpigmentation, nausea, vomiting, and injection site reactions [65]. In 2020, setmelanotide was approved by the US Food and Drug Administration (FDA) for chronic weight management in obese adult and pediatric patients ≥ 6 years old with POMC, PCSK1, or LEPR receptor deficiency [66]. In 2022, a long-term evaluation of the two initial POMC-deficient obese patients who were administered setmelanotide for 7 years verified the sustained decrease in BMI and hunger and the absence of serious side effects, apart from hyperpigmentation [67]. Collet et al. also evaluated setmelanotide in patients with MC4R deficiency. In this study, the effectiveness of setmelanotide was closely related to the type of MC4R mutation [68]. Semelanotide has also been examined in obese BBS patients and obese patients with Alström syndrome. In a phase II study, 10 BBS patients ≥ 12 years old were administered setmelanotide once/day for 1 year, with significant weight loss (16.3%) and reduction in polyphagia [69]. In a phase III trial, setmelanotide was tested in 38 BBS patients and 34% of patients had a weight loss > 10% after 52 weeks of drug administration, without serious side effects [70]. Moreover, in a recent Phase 3 trial after 1 year of use of setmelanotide, adults and children with BBS syndrome reported a better quality of life [71]. In 2022, setmelanotide was also approved by the FDA for the treatment of obesity and binge-eating disorders in > 6 years of age BBS patients. The results of ongoing clinical trials for the use of setmelanotide in patients having mutations in multiple genes connected with the MC4R pathway and in patients with deletions on chromosome 16p11.2 are also awaited with a lot of interest [72].
GLP-1R agonists may also be a novel alternative drug for patients with genetic obesity [73]. GLP-1R is expressed in the central nervous system and peripherally, such as in the β-cells of the pancreas [73]. The use of semaglutide, a long-acting GLP-1R analog, once weekly in patients with obesity has been associated with ≥ 10% weight loss after 52 weeks of drug administration [74,75]. The use of 3 mg liraglutide/day, another long-acting GLP-1R analog, in patients with MC4R variants or PWS for 16 weeks has also been tested, leading to ~6% weight loss in both MC4R-variant carriers and non-carriers, indicating that liraglutide has an appetite-reducing effect and acts independently of the MC4R pathway [76]. In another study, 3 mg liraglutide/day was administered for 16 weeks in a woman with homozygous pathogenic MC4R mutation, morbid obesity, and T2D, resulting in a decrease in body weight of ~10 kg, similar to weight loss in heterozygous MC4R mutation carriers and common obesity; thus, it can act independently of the MC4R mutation status [76,77]. Currently, liraglutide and semaglutide have been approved for obesity treatment. Moreover, tirzepatide, a novel agonist that acts at both the gastric inhibitory polypeptide receptor (GIPR) and GLP-1R, has been documented to lower food consumption as well as affect energy metabolism [78]. GIPRs are found in the central nervous system and in adipose tissue [79]. Interestingly, in a phase III clinical trial, tirzepatide was administered in obese individuals and led to significant weight loss [80,81]. Recently, tirzepatide also received FDA approval for obesity treatment. GLP-1/GIP/glucagon receptor triple agonists are also examined in obesity [82] and many other novel molecules and combinations of agonists are still in development and tested, with promising results.
5. Other Current and Promising Therapeutic Approaches
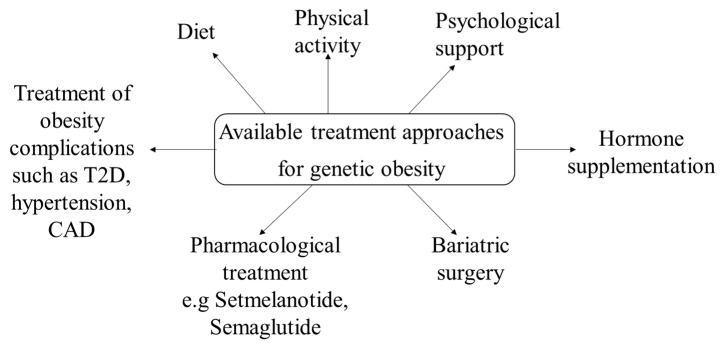
Except for the genetic-based pharmacological treatment of obesity—which is mainly discussed in this review article and currently applies mostly in monogenic obesity and in BBS syndrome—as well, there are also other current therapeutic approaches that should be considered in genetic obesity (Figure 1).
Figure 1.
Available treatment approaches for genetic obesity.
In patients with genetic obesity restriction of food consumption, psychological and behavioral support, increased physical activity, and reduced carbohydrate consumption are generally recommended. Possible obesity complications and comorbidities also require appropriate treatment. Importantly, these measures should be initiated as soon as possible during childhood [3]. For instance, in BBS and AS patients and especially in PWS patients, restriction of food intake and a routine regarding the timing of meals is greatly beneficial [83,84]. Decreased carbohydrate intake and increased dietary fiber intake are also recommended in PWS patients [85]. The possible benefits of probiotics in these patients are under investigation. In addition, GH therapy has been approved for the growth development of PWS children and is used after the establishment of the PWS diagnosis [86]. The results of the use of GLP-1 receptor agonists like exenatide, liraglutide, and semaglutide in PWS are currently inconsistent, and additional studies are needed in order for more definite conclusions to be drawn [87,88,89]. Oxytocin and carbetocin, a synthetic analog of oxytocin, have also been administered in PWS patients. In an RCT with 119 PWS patients, those who were given 3.2 mg of carbetocin displayed substantial improvements regarding hyperphagia [90]. Diazoxide choline-controlled release, an extended-release form of diazoxide choline, was also examined in a phase III PWS RCT study and was associated with significantly reduced fat mass [91]. Moreover, a methionine aminopeptidase-2 inhibitor, belanorib, was examined in a phase III PWS trial, with improvement in hyperphagia and weight loss; however, the study was discontinued due to the high incidence of thrombotic events [92]. A combination of tesofensine and metoprolol has also been used in a study including 21 adults with hypothalamic obesity, with a mean weight change of −6.3% in the patient group [93]. A few studies have also examined phentermine/topiramate and bupropion/naltrexone in PWS patients with positive results, however, after treatment discontinuation, weight regain was observed [93,94]. Regarding bariatric surgery, in cases with syndromic obesity, it should be generally considered with caution, as severe behavioral problems, compulsive food behavior, and developmental delays can lead to worse results [3]. Interestingly, in a recent systematic review of metabolic and bariatric surgery in PWS a significant decrease in BMI was observed with promising long-term outcomes [95]. Regarding monogenic obesity, the long-term outcomes of bariatric surgery are questionable, and considering the increasing availability and effectiveness of pharmacological agents, bariatric surgery should be avoided as long as possible [3].
Other alternative promising strategies to counteract obesity include the use of human-induced pluripotent stem cells (hiPSCs). hiPSCs can be differentiated into multiple cell types in vitro; thus, hiPSCs are a promising therapeutic approach for obese patients as they can provide an unlimited amount of brown adipose progenitor cells [96], which in contrast to white adipocyte cells, can dissipate stored energy. With the iPSC methodology, brown adipose progenitor cells can be used for both cell transplantation as well as for anti-obesity drug discovery. In vitro representations of human genetic conditions can also improve the examination of the molecular and cellular impact of certain genetic mutations in energy homeostasis pathways [97,98,99,100,101,102].
Targeted genome editing mediated by clustered, regularly interspaced, short palindromic repeat (CRISPR)/CRISPR-associated nuclease 9 (Cas9) technology has become a robust alternative to examine gene functions and treat genetic disorders, including genetic obesity. Interestingly, in a recent study, the mutated leptin gene in ob/ob mice was edited using CRISPR/Cas9. The ob/ob mice displayed a correction of 1.67% of leptin alleles, which was sufficient to restore the production and function of leptin [103]. Moreover, regulating the expression of obesity-causing genes, like MC4R, by using the CRISPRi technique gene expression has also been suggested to be useful in genetic obesity therapy [104].
The exploitation of small extracellular vesicles (sEVs) In obesity is also promising. sEVs are small cellular vesicles that can deliver specific molecules including lipids, proteins, mRNAs, and noncoding RNAs extracellularly to target cells, affecting their function. Interestingly, recently regulation of hypothalamic AMPK using sEV methodology in obese mouse models resulted in significant weight loss [105].
6. Conclusions
Obesity is a modern pandemic, with the WHO recently reporting that almost 1 in 3 children are overweight or obese and, among the European Union, Greece is first in childhood obesity. Importantly, a large percentage of obese children become obese adults with many comorbidities such as type 2 diabetes (T2D), hypertension, cardiovascular diseases (CVD), and an increased risk of early death [2]. This review highlights the importance of genetics in obesity, discussing cases of syndromic obesity such as Prader–Willi and Bardet–Biedl and of common monogenic obesity that are mainly characterized by mutations in the leptin–melanocortin pathway. This review also emphasizes the fact that, in patients with genetic obesity, general measures such as restriction of food consumption, psychological and behavioral support, increased physical activity, and reduced carbohydrate consumption should be initiated as soon as possible during childhood but also focuses on current progress that has been recently made in terms of pharmacological treatment. The MC4R agonist, setmelanotide, should be administered in patients with POMC, PCSK1, or LEPR deficiency and BBS, and the GLP-1 receptor agonists, semaglutide and liraglutide are also novel pharmacological agents that have been FDA approved for the treatment of childhood obesity. The importance of this review is that it contributes to the awareness of healthcare professionals that identifying a child with polyphagia based on a genetic background can be of crucial importance for its treatment and enhance knowledge on the available armamentarium for the pharmacological treatment of genetic obesity. Alternative strategies against obesity such as hiPSCs, CRISPR technology, and the use of sEVs are also promising future methodologies. In the meanwhile, future long-term studies in an increased number of patients are recommended and awaited to increase the list of available drugs that target certain genetic types of obesity. Combinations of anti-obesity drugs also warrant further study in patients with genetic obesity, aiming to facilitate genetic-based individualized treatment.
Author Contributions
Conceptualization, writing—original draft preparation, K.K.; literature search and analysis, V.G. and V.R.T.; writing—review and editing E.S.; visualization, supervision, L.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization Obesity and Overweight. 2021. [(accessed on 9 June 2021)]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Hampl S.E., Hassink S.G., Skinner A.C., Armstrong S.C., Barlow S.E., Bolling C.F., Avila Edwards K.C., Eneli I., Hamre R., Joseph M.M., et al. Clinical Practice Guideline for the Evaluation and Treatment of Children and Adolescents with Obesity. Pediatrics. 2023;151:e2022060640. doi: 10.1542/peds.2022-060640. [DOI] [PubMed] [Google Scholar]
- 3.Faccioli N., Poitou C., Clément K., Dubern B. Current Treatments for Patients with Genetic Obesity. J. Clin. Res. Pediatr. Endocrinol. 2023;15:108–119. doi: 10.4274/jcrpe.galenos.2023.2023-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sohn Y.B. Genetic obesity: An update with emerging therapeutic approaches. Ann. Pediatr. Endocrinol. Metab. 2022;27:169–175. doi: 10.6065/apem.2244188.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koves I.H., Roth C. Genetic and Syndromic Causes of Obesity and Its Management. Indian J. Pediatr. 2018;85:478–485. doi: 10.1007/s12098-017-2502-2. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy S.B., Schwartz S., Miller J.L., Driscoll D.J. Prader-Willi syndrome. Genet. Med. 2012;14:10–26. doi: 10.1038/gim.0b013e31822bead0. [DOI] [PubMed] [Google Scholar]
- 7.Haqq A.M., Muehlbauer M.J., Newgard C.B., Grambow S., Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: Heightened insulin sensitivity relative to body mass index. J. Clin. Endocrinol. Metab. 2011;96:E225–E232. doi: 10.1210/jc.2010-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bardet G. On congenital obesity syndrome with polydactyly and retinitis pigmentosa (a contribution to the study of clinical forms of hypophyseal obesity) Obes. Res. 1995;3:387–399. doi: 10.1002/j.1550-8528.1995.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 9.Biedl A. A pair of siblings with adiposo-genital dystrophy. Obes. Res. 1995;3:404. doi: 10.1002/j.1550-8528.1995.tb00167.x. [DOI] [PubMed] [Google Scholar]
- 10.Schachat A.P., Maumenee I.H. Bardet Biedl syndrome and related disorders. Arch. Ophthalmol. 1982;100:285–288. doi: 10.1001/archopht.1982.01030030287011. [DOI] [PubMed] [Google Scholar]
- 11.M’hamdi O., Ouertani I., Chaabouni-Bouhamed H. Update on the genetics of bardet-biedl syndrome. Mol. Syndromol. 2014;5:51–56. doi: 10.1159/000357054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beales P.L., Badano J.L., Ross A.J., Ansley S.J., Hoskins B.E., Kirsten B., Mein C.A., Froguel P., Scambler P., Lewis R.A., et al. Genetic interaction of BBS1 mutations with alleles at other BBS loci can result in non-Mendelian Bardet-Biedl syndrome. Am. J. Hum. Genet. 2003;72:1187–1199. doi: 10.1086/375178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albright F., Burnett C.H., Smith P.H., Parson W. Pseudohypoparathyroidism—An example of “Seabright–Bantam Syndrome”. Endocrinology. 1942;30:922–932. [Google Scholar]
- 14.Jüppner H. Molecular Definition of Pseudohypoparathyroidism Variants. J. Clin. Endocrinol. Metab. 2021;106:1541–1552. doi: 10.1210/clinem/dgab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levine M.A. An update on the clinical and molecular characteristics of pseudohypoparathyroidism. Curr. Opin. Endocrinol. Diabetes Obes. 2012;19:443–451. doi: 10.1097/MED.0b013e32835a255c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alstrom C.H., Hallgren B., Nilsson L.B., Asander H. Retinal degeneration combined with obesity, diabetes mellitus and neurogenous deafness: A specific syndrome (not hitherto described) distinct from the Laurence-Moon-Bardet-Biedl syndrome: A clinical, endocrinological and genetic examination based on a large pedigree. Acta Psychiatr. Neurol. Scand. Suppl. 1959;129:1–35. [PubMed] [Google Scholar]
- 17.Marshall J.D., Maffei P., Beck S., Barrett T.G., Paisey R., Naggert J.K. Clinical utility gene card for: Alstrom Syndrome—Update 2013. Eur. J. Hum. Genet. 2013;21:3–4. doi: 10.1038/ejhg.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Álvarez-Satta M., Castro-Sánchez S., Valverde D. Alström syndrome: Current perspectives. Appl. Clin. Genet. 2015;8:171–179. doi: 10.2147/TACG.S56612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szelest M., Stefaniak M., Ręka G., Jaszczuk I., Lejman M. Three case reports of patients indicating the diversity of molecular and clinical features of 16p11.2 microdeletion anomaly. BMC Med. Genom. 2021;14:76. doi: 10.1186/s12920-021-00929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finelli P., Natacci F., Bonati M.T., Gottardi G., Engelen J.J., de Die-Smulders C.E., Sala M., Giardino D., Larizza L. FISH characterisation of an identical (16)(p11.2p12.2) tandem duplication in two unrelated patients with autistic behaviour. J. Med. Genet. 2004;41:e90. doi: 10.1136/jmg.2003.016311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zufferey F., Sherr E.H., Beckmann N.D., Hanson E., Maillard A.M., Hippolyte L., Mace A., Ferrari C., Kutalik J., Andrieux J., et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J. Med. Genet. 2012;49:660. doi: 10.1136/jmedgenet-2012-101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marakhonov A.V., Vasilyeva T.A., Voskresenskaya A.A., Sukhanova N.V., Kadyshev V.V., Kutsev S.I., Zinchenko R.A. LMO2 gene deletions significantly worsen the prognosis of Wilms’ tumor development in patients with WAGR syndrome. Hum. Mol. Genet. 2019;28:3323–3326. doi: 10.1093/hmg/ddz168. [DOI] [PubMed] [Google Scholar]
- 23.Turleau C., de Grouchy J., Dufier J.L., Phuc L.H., Schmelck P.H., Rappaport R., Nihoul-Fekete C., Diebold N. Aniridia, male pseudohermaphroditism, gonadoblastoma, mental retardation, and del 11p13. Hum. Genet. 1981;57:300–306. doi: 10.1007/BF00278949. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Lopez R., Perez J.M., Balsera A.M., Rodriguez G.G., Moreno T.H., Garcia de Caceres M., Serrano M.G., Freijo F.C., Ruiz J.R., Angueira F.B., et al. The modifier effect of the BDNF gene in the phenotype of the WAGRO syndrome. Gene. 2013;516:285–290. doi: 10.1016/j.gene.2012.11.073. [DOI] [PubMed] [Google Scholar]
- 25.Han J.C., Liu Q.R., Jones M., Levinn R.L., Menzie C.M., Jefferson-George K.S., Adler-Wailes D.C., Sanford E.L., Lacbawan F.L., Uhl G.R., et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N. Engl. J. Med. 2008;359:918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith A.C.M., Magenis R.E., Elsea S.H. Overview of Smith-Magenis Syndrome. J. Assoc. Genet. Technol. 2005;31:163–167. [PubMed] [Google Scholar]
- 27.Slager R.E., Newton T.L., Vlangos C.N., Finucane B., Elsea S.H. Mutations in RAI1 Associated with Smith-Magenis Syndrome. Nat. Genet. 2003;33:466–468. doi: 10.1038/ng1126. [DOI] [PubMed] [Google Scholar]
- 28.Cohen M.M., Hall B.D., Smith D.W., Graham C.B., Lampert K.J. A new syndrome with hypotonia, obesity, mental deficiency, and facial, oral, ocular, and limb anomalies. J. Pediatr. 1973;83:280–284. doi: 10.1016/S0022-3476(73)80493-7. [DOI] [PubMed] [Google Scholar]
- 29.Balikova I., Lehesjoki A.E., de Ravel T.J., Thienpont B., Chandler K.E., Clayton-Smith J., Traskelin A.L., Fryns J.P., Vermeesch J.R. Deletions in the VPS13B (COH1) gene as a cause of Cohen syndrome. Hum. Mutat. 2009;30:E845–E854. doi: 10.1002/humu.21065. [DOI] [PubMed] [Google Scholar]
- 30.Parri V., Katzaki E., Uliana V., Scionti F., Tita R., Artuso R., Longo I., Boschloo R., Vijzelaar R., Selicorni A., et al. High frequency of COH1 intragenic deletions and duplications detected by MLPA in patients with Cohen syndrome. Eur. J. Hum. Genet. 2010;18:1133–1140. doi: 10.1038/ejhg.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duplomb L., Duvet S., Picot D., Jego G., El Chehadeh-Djebbar S., Marle N., Gigot N., Aral B., Carmignac V., Thevenon J., et al. Cohen syndrome is associated with major glycosylation defects. Hum. Mol. Genet. 2014;23:2391–2399. doi: 10.1093/hmg/ddt630. [DOI] [PubMed] [Google Scholar]
- 32.Blanchet P., Bebin M., Bruet S., Cooper G.M., Thompson M.L., Duban-Bedu B., Gerard B., Piton A., Suckno S., Deshpando C., et al. MYT1L mutations cause intellectual disability and variable obesity by dysregulating gene expression and development of the neuroendocrine hypothalamus. PLoS Genet. 2017;13:e1006957. doi: 10.1371/journal.pgen.1006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Börjeson M., Forssman H., Lehmann O. An X-linked, Recessively Inherited Syndrome Characterized by Grave Mental Deficiency, Epilepsy, and Endocrine Disorder. Acta Medica Scand. 1962;171:13–22. doi: 10.1111/j.0954-6820.1962.tb04162.x. [DOI] [PubMed] [Google Scholar]
- 34.Jahani-Asl A., Cheng C., Zhang C., Bonni A. Pathogenesis of Börjeson-Forssman-Lehmann syndrome: Insights from PHF6 function. Neurobiol. Dis. 2016;96:227–235. doi: 10.1016/j.nbd.2016.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carpenter G. Acrocephaly, with other congenital malformations-autopsy. Proc. R. Soc. Med. 1909;2:199–201. doi: 10.1177/003591570900201481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins D., Seelow D., Jehee F.S., Perlyn C.A., Alonso L.G., Bueno D.F., Donnai D., Josifova D., Mathijssen I.M., Morton J.E., et al. RAB23 mutations in Carpenter syndrome imply an unexpected role for hedgehog signaling in cranial-suture development and obesity. Am. J. Hum. Genet. 2007;80:1162–1170. doi: 10.1086/518047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havercamp S.M., Tasse M.J., Navas P., Benson B.A., Allain D., Manickam K. Exploring the weight and health status of adults with Down syndrome. J. Educ. Train. Stud. 2017;5:97–108. doi: 10.11114/jets.v5i6.2343. [DOI] [Google Scholar]
- 38.Artioli T. Understanding obesity in Down’s syndrome children. J. Obes. Metab. 2017;1:1–3. [Google Scholar]
- 39.Stamou M.I., Georgopoulos N.A. Kallmann syndrome: Phenotype and genotype of hypogonadotropic hypogonadism. Metabolism. 2018;86:124–134. doi: 10.1016/j.metabol.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubern B., Clement K. Leptin and leptin receptor-related monogenic obesity. Biochimie. 2012;94:2111–2115. doi: 10.1016/j.biochi.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Funcke J.B., von Schnurbein J., Lennerz B., Lahr G., Debatin K.M., Fischer-Posovszky P., Wabitsch M. Monogenic forms of childhood obesity due to mutations in the leptin gene. Mol. Cell. Pediatr. 2014;1:3. doi: 10.1186/s40348-014-0003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cottrell E.C., Mercer J.G. Handbook of Experimental Pharmacology. Springer; Berlin/Heidelberg, Germany: 2012. Leptin receptors; pp. 3–21. [DOI] [PubMed] [Google Scholar]
- 43.Farooqi I.S., Wangensteen T., Collins S., Kimber W., Matarese G., Keogh J.M., Lank E., Bottomley B., Lopez-Fernandez J., Ferraz-Amaro I., et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cetinkaya S., Guran T., Kurnaz E., Keskin M., Sagsak E., Savas E.S., Suntharalingham J.P., Buonocore F., Achermann J.C., Aycan Z. A patient with proopiomelanocortin deficiency: An increasingly important diagnosis to make. J. Clin. Res. Pediatr. Endocrinol. 2018;10:68–73. doi: 10.4274/jcrpe.4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cone R.D. Studies on the physiological functions of the melanocortin system. Endocr. Rev. 2006;27:736–749. doi: 10.1210/er.2006-0034. [DOI] [PubMed] [Google Scholar]
- 46.Farooqi I.S., Volders K., Stanhope R., Heuschkel R., White A., Lank E., Keogh J., O’Rahilly S., Creemers J.W.M. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J. Clin. Endocrinol. Metab. 2007;92:3369–3373. doi: 10.1210/jc.2007-0687. [DOI] [PubMed] [Google Scholar]
- 47.Jackson R.S., Creemers J.W., Ohagi S., Raffin-Sanson M.L., Sanders L., Montague C.T., Hutton J.C., O’Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat. Genet. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 48.Doche M.E., Bochukova E.G., Su H.W., Pearce L.R., Keogh J.M., Henning E., Cline J.M., Saeed S., Dale A., Cheetman T., et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Investig. 2012;122:4732–4736. doi: 10.1172/JCI62696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ji L., Wu H.T., Qin X.Y., Lan R. Dissecting carboxypeptidase E: Properties, functions and pathophysiological roles in disease. Endocr. Connect. 2017;6:R18–R38. doi: 10.1530/EC-17-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alsters S.I., Goldstone A.P., Buxton J.L., Zekavati A., Sosinsky A., Yiorkas S., Holder S., Klaber R.E., Bridges N., van Haelst M.M., et al. Truncating homozygous mutation of carboxypeptidase E (CPE) in a morbidly obese female with type 2 diabetes mellitus, intellectual disability and hypogonadotrophic hypogonadism. PLoS ONE. 2015;10:e0131417. doi: 10.1371/journal.pone.0131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosch E., Hebebrand M., Popp B., Penger T., Behring B., Cox H., Towner S., Kraus C., Wilson W.G., Khan S., et al. BDV syndrome: An emerging syndrome with profound obesity and neurodevelopmental delay resembling Prader-Willi syndrome. J. Clin. Endocrinol. Metab. 2021;106:3413–3427. doi: 10.1210/clinem/dgab592. [DOI] [PubMed] [Google Scholar]
- 52.York B., O’Malley B.W. Steroid receptor coactivator (SRC) family: Masters of systems biology. J. Biol. Chem. 2010;285:38743–38750. doi: 10.1074/jbc.R110.193367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hendricks A.E., Bochukova E.G., Marenne G., Koegh J.M., Atanassova N., Bounds R., Wheeler E., Mistry V., Henning E., Korner A., et al. Rare variant analysis of human and rodent obesity genes in individuals with severe childhood obesity. Sci. Rep. 2017;7:4394. doi: 10.1038/s41598-017-03054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cacciottolo T.M., Henning E., Keogh J.M., Bel Lassen P., Lawler K., Bounds R., Ahmed R., Perdikari A., Mendes de Oliveira E., Smith M., et al. Obesity Due to Steroid Re-ceptor Coactivator-1 Deficiency Is Associated with Endocrine and Metabolic Abnormalities. J. Clin. Endocrinol. Metab. 2022;107:e2532–e2544. doi: 10.1210/clinem/dgac067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Picard F., Gehin M., Annicotte J., Rocchi S., Champy M.F., O’Malley B.W., Chambon P., Auwerx J. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell. 2002;111:931–941. doi: 10.1016/S0092-8674(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 56.Yang Y., van der Klaauw A.A., Zhu L., Cacciottolo T.S., He Y., Stadler L.K.J., Wang C., Xu P., Saito K., Hinton A., et al. Steroid receptor coactivator-1 modulates the function of Pomc neurons and energy homeostasis. Nat. Commun. 2019;10:1718. doi: 10.1038/s41467-019-08737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogden C.L., Carroll M.D., Lawman H.G. Trends in obesity prevalence among children and adolescents in the United States, 1988–1994 through 2013–2014. JAMA. 2016;315:2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thaker V.V. Genetic and epigenetic causes of obesity. Adolesc. Med. State Art Rev. 2017;28:379–405. [PMC free article] [PubMed] [Google Scholar]
- 59.Farooqi S. Insights from the genetics of severe childhood obesity. Horm. Res. 2007;68:5–7. doi: 10.1159/000110462. [DOI] [PubMed] [Google Scholar]
- 60.Vollbach H., Brandt S., Lahr G. Prevalence and phenotypic characterization of MC4R variants in a large pediatric cohort. Int. J. Obes. 2017;41:13–22. doi: 10.1038/ijo.2016.161. [DOI] [PubMed] [Google Scholar]
- 61.Cuda S., Censani M. Progress in pediatric obesity: New and advanced therapies. Curr. Opin. Pediatr. 2022;34:407–413. doi: 10.1097/MOP.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 62.Farooqi I.S., Jebb S.A., Langmack G.E., Lawrence E.C., Cheetham C.H., Prentice A.M., Hughes I.A., McCamish M.A., O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 63.Wabitsch M., Funcke J.B., Lennerz B., Kuhnle-Krahl U., Lahr G., Debatin K.M., Vatter P., Gierschic P., Moepps B., Fischer-Posovszky O. Biologically inactive leptin and early- onset extreme obesity. N. Engl. J. Med. 2015;372:48–54. doi: 10.1056/NEJMoa1406653. [DOI] [PubMed] [Google Scholar]
- 64.Kόhnen P., Clement K., Wiegand S., Blankenstein O., Gottesdiener K., Martini L.L., Mai K., Blume-Petavi U., Gruters A., Krude H. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 2016;375:240–246. doi: 10.1056/NEJMoa1512693. [DOI] [PubMed] [Google Scholar]
- 65.Clement K., van den Akker E., Argente J., Bahm A., Chung W.K., Connors H., De Waele K., Farooqi A., Gonneau-Lejeune J., Gordon G., et al. Setmelanotide POMC and LEPR Phase 3 Trial investigators. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: Single- arm, open- label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8:960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 66.Markham A. Setmelanotide: First approval. Drugs. 2021;81:397–403. doi: 10.1007/s40265-021-01470-9. [DOI] [PubMed] [Google Scholar]
- 67.Kühnen P., Clement K. Long-term MC4R agonist treatment in POMC-deficient patients. N. Engl. J. Med. 2022;387:852–854. doi: 10.1056/NEJMc2207442. [DOI] [PubMed] [Google Scholar]
- 68.Collet T.H., Dubern B., Mokrosinski J., Connors J., Keogh J.M., de Oliveira E.M., Henning E., Poitou-Bernet C., Oppert J.M., Tounian P., et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol. Metab. 2017;6:1321–1329. doi: 10.1016/j.molmet.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haws R., Brady S., Davis E., Fletty K., Yuan G., Gordon G., Stewart M., Yanovski J. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet–Biedl syndrome. Diabetes Obes. Metab. 2020;22:2133–2140. doi: 10.1111/dom.14133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haqq A.M., Chung W.K., Dollfus H., Haws R.M., Martos-Moreno G.A., Poitou C., Yanovski J.A., Mittleman R.S., Yang G., Forsythe E., et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alstrom syndrome: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 2022;10:859–868. doi: 10.1016/S2213-8587(22)00277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Forsythe E., Haws R.M., Argente J., Beales P., Martos-Moreno G.A., Dollfus A., Chirila C., Gnanasakthy A., Buckley B., Mallya U., et al. Quality of life improvements following one year of setmelanotide in children and adult patients with Bardet-Biedl syndrome: Phase 3 trial results. Orphanet J. Rare Dis. 2023;18:12. doi: 10.1186/s13023-022-02602-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dubern B., Faccioli N., Poitou C., Clément K. Novel therapeutics in rare genetic obesities: A narrative review. Pharmacol. Res. 2023;191:106763. doi: 10.1016/j.phrs.2023.106763. [DOI] [PubMed] [Google Scholar]
- 73.Wang J.Y., Wang Q.W., Yang X.Y., Yang W., Li D.R., Jin J.Y., Zhang H.C., Zhang X.F. GLP-1 receptor agonists for the treatment of obesity: Role as a promising approach. Front. Endocrinol. 2023;14:1085799. doi: 10.3389/fendo.2023.1085799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., McGowan B.M., Rosenstock J., Tran M.T.D., Wadden T.A., et al. Once-weekly semaglutide in adults with overweight or obesity. N. Engl. J. Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 75.Weghuber D., Barrett T., Barrientos-Perez M., Gies I., Hesse D., Jeppesen O.K., Kelly A.S., Mastrandrea L.D., Sorrig R., Arslanian S., et al. Once-weekly semaglutide in adolescents with obesity. N. Engl. J. Med. 2022;387:2245–2257. doi: 10.1056/NEJMoa2208601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iepsen E.W., Zhang J., Thomsen H.S., Hansen E.L., Hollensted M., Madsbad S., Hansen T., Holst J.J., Holm J.C., Torekov S.S. Patients with obesity caused by melanocortin-4 receptor mutations can be treated with a glucagon-like peptide-1 receptor agonist. Cell Metab. 2018;28:23–32.e3. doi: 10.1016/j.cmet.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Iepsen E.W., Have C.T., Veedfald S., Madsbad S., Holst J.J., Grarup N., Pedersen O., Brandslund I., Holm J.C., Hansen T., et al. GLP-1 receptor agonist treatment in morbid obesity and type 2 diabetes due to pathogenic homozygous melanocortin-4 receptor mutation: A case report. Cell Rep. Med. 2020;1:100006. doi: 10.1016/j.xcrm.2020.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Samms R.J., Zhang G., He W., Ilkayeva O., Droz B.A., Bauer S.M., Stutsman C., Pirro V., Collins K.W., Furber E.C., et al. Tirzepatide induces a thermogenic-like amino acid signature in brown adipose tissue. Mol. Metab. 2022;64:101550. doi: 10.1016/j.molmet.2022.101550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Seino Y., Fukushima M., Yabe D. GIP and GLP-1, the two incretin hormones: Similarities and differences. J. Diabetes Investig. 2010;1:8–23. doi: 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boer G.A., Hay D.L., Tups A. Obesity pharmacotherapy: Incretin action in the central nervous system. Trends Pharm. Sci. 2023;44:50–63. doi: 10.1016/j.tips.2022.11.001. [DOI] [PubMed] [Google Scholar]
- 81.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., Kiyosue A., Zhang S., Liu B., Bunck M.C., et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 82.Coskun T., Urva S., Roell W.C., Qu H., Loghin C., Moyers J.S., O’Farrell L.S., Briere D.A., Sloop K.W., Thomas M.K., et al. LY3437943, a novel triple glucagon, GIP, and GLP-1 receptor agonist for glycemic control and weight loss: From discovery to clinical proof of concept. Cell Metab. 2022;34:1234–1247.e9. doi: 10.1016/j.cmet.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 83.Tahani N., Maffei P., Dollfus H., Paisey R., Valverde D., Milan G., Han J.C., Favaretto F., Madathil S.C., Dawson C., et al. Consensus clinical management guidelines for Alstrom syndrome. Orphanet J. Rare Dis. 2020;15:253. doi: 10.1186/s13023-020-01468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feuillan P.P., Ng D., Han J.C., Sapp J.C., Wetsch K., Spaulding E., Zheng Y.C., Caruso R.C., Brooks B.P., Johnston J.J., et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. J. Clin. Endocrinol. Metab. 2011;96:E528–E535. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pedemonti B., Ceccomancini R., D’Acunti A., Stegmann J. Effectiveness of a transdisciplinary approach on hyperphagia management among patients with Prader Willi syndrome. Endocrinol. Diabetes Nutr. 2023;70:347–351. doi: 10.1016/j.endinu.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 86.Irizarry K.A., Miller M., Freemark M., Haqq A.M. Prader Willi Syndrome: Genetics, Metabolomics, Hormonal Function, and New Approaches to Therapy. Adv. Pediatr. 2016;63:47–77. doi: 10.1016/j.yapd.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ng N.B.H., Low Y.W., Rajgor D.D., Low J.M., Lim Y.Y., Loke K.Y., Lee Y.S. The effects of glucagon-like peptide (GLP)-1 receptor agonists on weight and glycaemic control in Prader-Willi syndrome: A systematic review. Clin. Endocrinol. 2022;96:144–154. doi: 10.1111/cen.14583. [DOI] [PubMed] [Google Scholar]
- 88.Diene G., Angulo M., Hale P.M., Jepsen C.H., Hofman P.L., Hokken-Koelega A., Ramesh C., Turan S., Tauber M. Liraglutide for Weight Management in Children and Adolescents with Prader-Willi Syndrome and Obesity. J. Clin. Endocrinol. Metab. 2022;108:4–12. doi: 10.1210/clinem/dgac549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Han J.C., Rasmussen M.C., Forte A.R., Schrage S.B., Zafar S.K., Haqq A.M. Management of Monogenic and Syndromic Obesity. Gastroenterol. Clin. N. Am. 2023;52:733–750. doi: 10.1016/j.gtc.2023.08.005. [DOI] [PubMed] [Google Scholar]
- 90.Roof E., Deal C.L., McCandless S.E., Cowan R.L., Miller J.L., Hamilton J.K., Roeder E.R., McCormack S.E., Roshan Lal T.R., Abdul-Latif H.D., et al. Intranasal Carbetocin Reduces Hyperphagia, Anxiousness and Distress in Prader-Willi Syndrome: CARE-PWS Phase 3 Trial. J. Clin. Endocrinol. Metab. 2023;108:1696–1708. doi: 10.1210/clinem/dgad015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miller J.L., Gevers E., Bridges N., Yanovski J.A., Salehi P., Obrynba K.S., Felner E.I., Bird L.M., Shoemaker A.H., Angulo M., et al. Diazoxide Choline Extended-Release Tablet in People with Prader-Willi Syndrome: A Double-Blind, Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2023;108:1676–1685. doi: 10.1210/clinem/dgad014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCandless S.E., Yanovski J.A., Miller J., Fu C., Bird L.M., Salehi P., Chan C.L., Stafford D., Abuzzahab M.J., Viskochil D., et al. Effects of MetAP2 inhibition on hyperphagia and body weight in Prader-Willi syndrome: A randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 2017;19:1751–1761. doi: 10.1111/dom.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huynh K., Klose M., Krogsgaard K., Drejer J., Byberg S., Madsbad S., Magkos F., Aharaz A., Edsberg B., Tfelt-Hansen J., et al. Randomized controlled trial of Tesomet for weight loss in hypothalamic obesity. Eur. J. Endocrinol. 2022;186:687–700. doi: 10.1530/EJE-21-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nolan B.J., Proietto J., Sumithran P. Intensive management of obesity in people with Prader-Willi syndrome. Endocrine. 2022;77:57–62. doi: 10.1007/s12020-022-03064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wolfe G., Salehi V., Browne A., Riddle R., Hall E., Fam J., Tichansky D., Myers S. Metabolic and bariatric surgery for obesity in Prader Willi syndrome: Systematic review and meta-analysis. Surg. Obes. Relat. Dis. 2023;19:907–915. doi: 10.1016/j.soard.2023.01.017. [DOI] [PubMed] [Google Scholar]
- 96.Wang L., Liu Y., Stratigopoulos G., Panigrahi S., Sui L., Zhang Y., Leduc C.A., Glover H.J., De Rosa M.C., Burnett L.C., et al. Bardet-Biedl syndrome proteins regulate intracellular signaling and neuronal function in patient-specific iPSC-derived neurons. J. Clin. Investig. 2021;131:e146287. doi: 10.1172/JCI146287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shi Y., Kim H., Hamann C.A., Rhea E.M., Brunger J.M., Lippmann E.S. Nuclear receptor ligand screening in an iPSC-derived in vitro blood-brain barrier model identifies new contributors to leptin transport. Fluids Barriers CNS. 2022;19:77. doi: 10.1186/s12987-022-00375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yao X., Dani V., Dani C. Human Pluripotent Stem Cells: A Relevant Model to Identify Pathways Governing Thermogenic Adipocyte Generation. Front. Endocrinol. 2020;10:932. doi: 10.3389/fendo.2019.00932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yao L., Liu Y., Qiu Z., Kumar S., Curran J.E., Blangero J., Chen Y., Lehman D.M. Molecular Profiling of Human Induced Pluripotent Stem Cell-Derived Hypothalamic Neurones Provides Developmental Insights into Genetic Loci for Body Weight Regulation. J. Neuroendocrinol. 2017:29. doi: 10.1111/jne.12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stelzer Y., Sagi I., Yanuka O., Eiges R., Benvenisty N. The noncoding RNA IPW regulates the imprinted DLK1-DIO3 locus in an induced pluripotent stem cell model of Prader-Willi syndrome. Nat. Genet. 2014;46:551–557. doi: 10.1038/ng.2968. [DOI] [PubMed] [Google Scholar]
- 101.Soeda S., Saito R., Fujii A., Tojo S., Tokumura Y., Taniura H. Abnormal DNA methylation in pluripotent stem cells from a patient with Prader-Willi syndrome results in neuronal differentiation defects. Stem Cell Res. 2021;53:102351. doi: 10.1016/j.scr.2021.102351. [DOI] [PubMed] [Google Scholar]
- 102.Sledziowska M., Winczura K., Jones M., Almaghrabi R., Mischo H., Hebenstreit D., Garcia P., Grzechnik P. Non-coding RNAs associated with Prader-Willi syndrome regulate transcription of neurodevelopmental genes in human induced pluripotent stem cells. Hum. Mol. Genet. 2023;32:608–620. doi: 10.1093/hmg/ddac228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu L., Yang X., Li J., Jia X., Bai X., Zhao Y., Cheng W., Shu M., Zhu Y., Jin S. Leptin gene-targeted editing in ob/ob mouse adipose tissue based on the CRISPR/Cas9 system. J. Genet. Genom. 2021;48:134–146. doi: 10.1016/j.jgg.2021.01.008. [DOI] [PubMed] [Google Scholar]
- 104.Wang Z., Yang L., Qu S., Zhang C. CRISPR-mediated gene editing to rescue haploinsufficient obesity syndrome. Protein Cell. 2019;10:705–708. doi: 10.1007/s13238-019-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Milbank E., Dragano N., Vidal-Gómez X., Rivas-Limeres V., Garrido-Gil P., Wertheimer M., Recoquillon S., Pata M.P., Labandeira-Garcia J.L., Diéguez C., et al. Small extracellular vesicle targeting of hypothalamic AMPKα1 promotes weight loss in leptin receptor deficient mice. Metabolism. 2023;139:155350. doi: 10.1016/j.metabol.2022.155350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.