Abstract
Natural products with health benefits, nutraceuticals, have shown considerable promise in many studies; however, this potential has yet to translate into widespread clinical use for any condition. Notably, many drugs currently on the market, including the first analgesic aspirin, are derived from plant extracts, emphasizing the historical significance of natural products in drug development. Curcumin and resveratrol, well-studied nutraceuticals, have excellent safety profiles with relatively mild side effects. Their long history of safe use and the natural origins of numerous drugs contrast with the unfavorable reputation associated with nutraceuticals. This review aims to explore the nutraceutical potential for treating pseudoachondroplasia, a rare dwarfing condition, by relating the mechanisms of action of curcumin and resveratrol to molecular pathology. Specifically, we will examine the curcumin and resveratrol mechanisms of action related to endoplasmic reticulum stress, inflammation, oxidative stress, cartilage health, and pain. Additionally, the barriers to the effective use of nutraceuticals will be discussed. These challenges include poor bioavailability, variations in content and purity that lead to inconsistent results in clinical trials, as well as prevailing perceptions among both the public and medical professionals. Addressing these hurdles is crucial to realizing the full therapeutic potential of nutraceuticals in the context of pseudoachondroplasia and other health conditions that might benefit.
Keywords: nutraceuticals, resveratrol, curcumin, turmeric, dwarfism, growth plate chondrocyte, articular cartilage, joint degeneration, joint pain
1. Introduction
The mechanisms of action of curcumin and resveratrol target many of the pathologic stress mechanisms involved in pseudoachondroplasia (PSACH). Specifically, resveratrol and curcumin, a compound in turmeric, reduce inflammation [1,2,3,4] and oxidative stress [2,5,6,7,8,9,10,11,12,13] and increase autophagy [6,7,14,15]. Testing of a coconut oil dispersion of curcumin, CurQ+ [16], in young MT-COMP mice, a model of PSACH, completely restored limb growth [17]. Resveratrol treatment resolved pain and prevented joint degeneration in adult MT-COMP mice [18]. These findings suggest that resveratrol and curcumin may provide a therapeutic benefit for PSACH. Curcumin/turmeric and resveratrol have a long, safe history of consumption in humans and have been shown to reduce joint degeneration in osteoarthritis (OA) [7,19,20,21,22,23,24,25]. Despite these positive outcomes, there are significant challenges to nutraceutical therapy. In this review, the PSACH pathology will be discussed along with the relevant stresses that curcumin and resveratrol impact [1,10,12,13,14,17,18,26,27,28]. Finally, the hurdles to using natural compounds as therapeutics will be discussed, including one obstacle that is inherent to the compounds: bioavailability. Another barrier is the lack of Food and Drug Administration (FDA) regulation, which leaves the consumer in the dark about the product they are purchasing and creates variation in studies such that they cannot be compared equally. These issues have limited physicians’ interest in using supplements/nutraceuticals and have led to skepticism in the public. Curcumin and resveratrol come from the diet and, therefore, have fewer and less significant side effects than pharmaceuticals and typically cost less than prescription drugs. Overcoming these obstacles could lead to the first treatment for PSACH and perhaps prevention of OA joint damage if caught early enough, safer pain management for joint degeneration, more healthy and active years in late adulthood, and the alleviation of a great deal of the health care burden on society.
2. PSACH Pathology
Cartilage oligomeric matrix protein (COMP) is a large, pentameric, matricellular protein that binds to many extracellular (ECM) proteins. COMP contributes to cartilage homeostasis [29,30,31,32] by sponsoring multiple interactions among ECM components, including collagens and proteoglycans [29,30,31]. Mutations in COMP cause PSACH, a severe dwarfing condition characterized by disproportionate short stature (average height of 3′ 9″ (females) and 3′ 11″ (males)) with short limbs [30,33,34,35,36,37,38,39,40,41,42,43]. Joint pain, extreme laxity, and very early-onset joint degeneration are the most significant clinical outcomes [30,33,34,35,36,37,38,39,40,41,42,43].
2.1. Chondrocyte Pathology and Mechanisms
In order to study PSACH pathologic mechanisms and test therapeutics in vivo, we generated the MT-COMP mouse that expresses mutant human D469del-COMP (deletion of one of five consecutive aspartic acid residues) in tissues expressing type II collagen with doxycycline (DOX) administration. The expression of the most common PSACH mutation (D469del-COMP) in addition to the endogenous wild-type mouse COMP recapitulates the clinical phenotype and PSACH chondrocyte pathology in a mouse designated the MT-COMP [44]. The MT-COMP mouse allowed for us to demonstrate that accumulation of mutant-COMP in the rER (rough endoplasmic reticulum) due to misfolding is cytotoxic to chondrocytes in the growth plate and articular cartilage [38,45,46]. Accumulation of mutant-COMP in chondrocytes induces ER stress, driving both oxidative stress and inflammation, creating a self-perpetuating pathologic loop that leads to an autophagy block [11] (Figure 1). The autophagy blockade is particularly harmful given that autophagy is the primary means to clear the ER of misfolded protein. Autophagy is blocked by high levels of mTORC1 signaling stimulated via TNFα and CHOP [11]. Increased mTORC1 signaling supports general protein synthesis at the detriment of autophagy, directly inhibiting autophagic clearance of the ER in chondrocytes [11,12]. Protein synthesis, in the context of accumulated misfolded protein in the ER, likely exacerbates ER stress [11,12]. In the growth plate, the multitude of stresses driven by mutant-COMP accumulation leads to a loss of chondrocytes needed to generate the matrix necessary to make the cartilage model of long bones, thereby decreasing long bone growth. In articular chondrocytes, the collection of stresses induces degenerative changes in the cartilage related to inflammation, oxidative stress, and autophagy blockade. Moreover, the cooperative action of these stresses drives a senescent phenotype in MT-COMP mice [27]. Senescence is known to propagate degenerative changes to nearby cells and tissues of the joint, hastening joint damage.
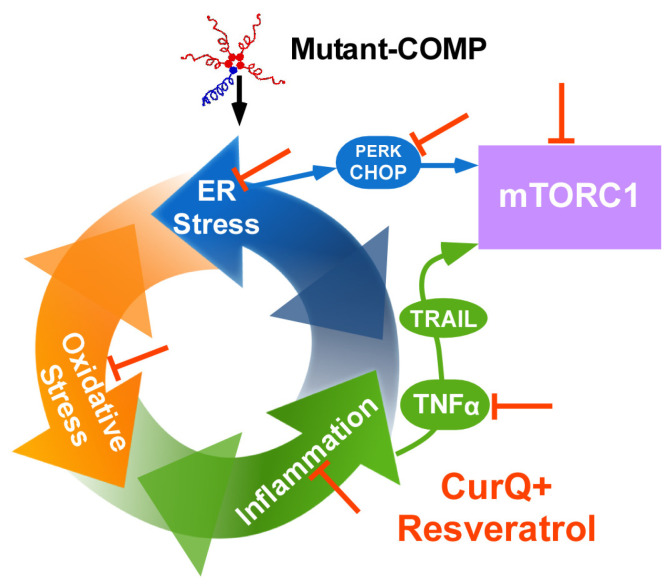
Figure 1.
Stress processes involved in mutant-COMP pathology. This schematic briefly summarizes the pathologic processes in PSACH chondrocytes. Mutant-COMP is a pentamer; mutant subunits are shown in red, and wild type is shown in blue. Quality control systems recognize mutant-COMP as improperly folded, and it is held in the ER [47]. This accumulation generates ER stress that leads to oxidative stress and inflammation, and each makes the other stress worse [10]. Prolonged ER stress results in TNFα/TRAIL and CHOP activation that, in turn, elevate mTORC1 signaling, blocking autophagy—thereby preventing the clearance of the ER [11,27]. Orange bar block (⊥) represents inhibition associated with resveratrol and CurQ+, including inflammation, TNFα, oxidative stress, ER stress, PERK, and CHOP [10,12,13,17,18,48].
2.2. PSACH Pain
Pain in the MT-COMP mice has been established through multiple assays, including alterations of gait [27], voluntary running, and grooming [18]. The parents of PSACH young children describe their children as resting more than their peers, tiring easily, and frequently complaining of leg pain that limits stamina (personal communication). This pain in childhood is likely attributable to inflammation-related processes that occur in growth plate chondrocytes due to the accumulated mutant-COMP [34,38,46,49]. PSACH joint pain in adulthood is a chronic problem that affects ambulation, mood, and quality of life [50]. Eighty-one percent of PSACH adults report chronic pain [50]. PSACH pain is described as tiring, exhausting, nagging, aching, throbbing, sharp, and miserable [50]. Joint pain and degenerative changes in the joints along with micro-injuries from extremely lax joints necessitate joint replacements in the late 20′s [36,44]. Typically, hips are replaced first, followed by knees, and some individuals have shoulders and elbows replaced. MT-COMP mice joint degeneration studies demonstrate multiple sources of nociceptive pain present, including subchondral bone remodeling, meniscus damage, synovitis, and inflammation [27]. A non-surgical approach is desperately needed since all joints are affected, but not all joints are replaceable, and numerous years of uncontrolled pain are endured before joint replacement [38,44,50,51].
3. Modulation of ER Stress
ER stress arises when the capacity of the ER to synthesize correctly folded proteins is exceeded, and secretory cells, such as chondrocytes, are particularly susceptible [52]. In PSACH, the residues deleted or mutated in COMP impact calcium binding, which is crucial for correct folding [53,54,55], leading to a protein that cannot be folded correctly. Three sensors, PERK, ATF6, and IRE1, detect ER stress. BiP selectively binds to misfolded proteins, releasing the sensors and activating downstream targets. PERK suppresses global translation while inducing the transcription of genes involved in the unfolded protein response (UPR), including CHOP, a mediator of apoptosis (Figure 2). Activation of ATF6 and IRE1 triggers the expression of UPR-related targets, such as chaperones and ER-assisted degradation (ERAD). These systems collaborate to restore ER homeostasis. However, if homeostasis is not promptly reestablished, ERAD and autophagy are activated. Cell death or apoptosis is triggered if these mechanisms fail to clear the ER of misfolded proteins.
Mutant-COMP-induced ER stress results in the intracellular retention of mutant-COMP, leading to elevated expression of CHOP and GADD34, consequently reactivating protein translation and exacerbating the intracellular retention of MT-COMP [56] (Figure 2). Reactive oxygen species (ROS) are generated by an increase in endoplasmic reticulum receptor stress-inducible 1 beta (Ero1β) [56]. This oxidative stress triggers DNA damage and upregulates the expression of growth arrest and DNA damage (GADD) genes [56]. The absence of activated caspases coupled with the presence of cleaved apoptosis-inducing factor suggest that mutant-COMP-induced premature chondrocyte death occurs through necroptosis [56] (Figure 2).
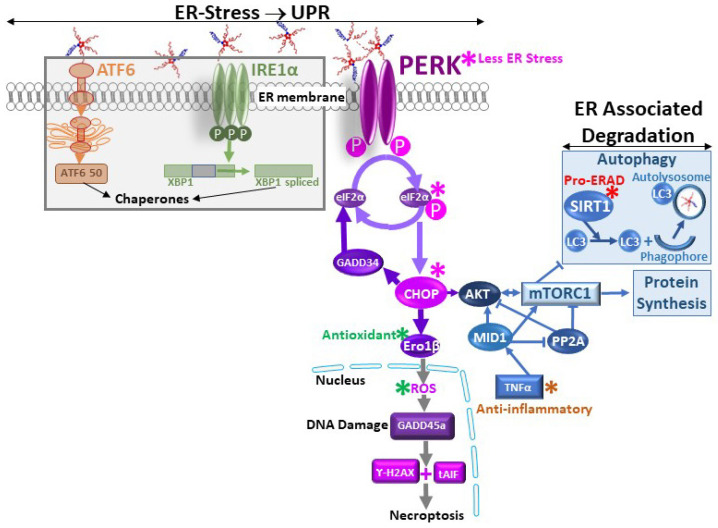
Figure 2.
Mechanisms of mitigating ER stress with CurQ+ and resveratrol. ER stress stimulates the unfolded protein response (UPR) that uses three sensors, PERK, ATF6, and IRE1, and only the PERK branch proceeds beyond initial activation with the mutant-COMP response [57] (pink asterisks*). The PERK branch leads to CHOP activation, generating ROS from Ero1β, which is dampened by CurQ+ and resveratrol (green asterisks*) [57]. Of particular importance, the mutant-COMP stimulated increase in PERK, P-eIF2α, and CHOP is reversed by CurQ+ and resveratrol. Less oxidative stress prevents additional ER stress/inflammation driven by ROS. Anti-inflammatory activity of resveratrol and CurQ+ diminishes TNFα [5,12,17,48,58,59,60], lessening mTORC1 activation and exacerbation of oxidative and ER stress (brown asterisks*). SIRT1 upregulation by curcumin and resveratrol [61] promotes ERAD/autophagy, allowing for accumulated protein to be cleared (red asterisks*).
Curcumin and resveratrol mitigate ER stress through various mechanisms, including the repression of ER sensors and CHOP expression, the elevation of silent information regulator factor 2-related enzyme 1 (SIRT1), chaperones, and ERAD (Figure 2). Specifically, curcumin and resveratrol decrease the PERK pathway by upregulating SIRT1 expression. This leads to the suppression of activated forms of PERK and eIF2α (Figure 2). Decreasing CHOP indirectly induces autophagy by upregulating SIRT1 by directly stimulating translation of SIRT1 and indirectly increasing SIRT1 levels through phosphorylated AMPKα [61]. This increase in autophagy promotes clearance of mutant-COMP. The activation of SIRT1 by curcumin and resveratrol also inhibits inflammation and supports cellular survival. Furthermore, curcumin and resveratrol enhance heat shock protein levels, aiding in protein refolding [62,63,64]; however, since mutant-COMP cannot be folded properly, this likely does not play a role in this case. ER stress is associated with ROS production and oxidative stress-induced inflammation, and therefore, the antioxidant and anti-inflammatory properties of curcumin and resveratrol indirectly dampen ER stress.
4. Anti-Inflammatory Effects
Inflammatory markers are increased in chondrocytes expressing mutant-COMP as early as 2–3 weeks of age [10,27]. Importantly, IL1β, IL6, and TNFα are elevated in MT-COMP mice [10,27], and all of these pro-inflammatory molecules are involved in OA joint degeneration [65,66]. Curcumin exerts its anti-inflammatory effects by reducing various cytokines, including those involved in interleukin (IL) activation, tumor necrosis factor-alpha (TNF-α), and the nuclear factor-kappa B pathway [5,58,67] (Figure 2). On the other hand, resveratrol targets IL-1β, TNF-α, and cyclooxygenase-2 to achieve its anti-inflammatory activity [18,27,48,59,60,68,69,70]. The upregulation of SIRT1 by curcumin and resveratrol plays a pivotal role in driving their anti-inflammatory activities [28,63,71,72,73,74,75] (Figure 1 and Figure 2). In the context of cartilage, TNF-α and IL-1β hold particular significance as pro-inflammatory molecules as they induce the expression of enzymes that contribute to the degradation of extracellular matrix proteins, including matrix metalloproteinases (MMPs) [2,19,20,24,27,48,76,77,78]. The ability of curcumin and resveratrol to modulate these key inflammatory mediators underscores their potential to address inflammation-related issues in cartilage.
5. Antioxidant Effects
Oxidative stress has been observed in chondrocytes expressing mutant-COMP [57]. Prolonged ER stress drives the expression of CHOP, which induces endoplasmic reticulum oxidoreductase 1 beta (ERO1β), that works together with protein disulfide isomerase (PDI) to eliminate free radicals [79] (Figure 1 and Figure 2). In vitro oxidative stress in chondrocytes with accumulation of mutant-COMP was demonstrated by increased expression of ERO1β and NADPH oxidase 4 (NOX4) and reduction in mitochondrial membrane potential [57]. Each of these changes indicates that, in the presence of mutant-COMP, there are excessive ROS that drive oxidative stress.
Curcumin and resveratrol play a role in neutralizing free radicals, mitigating the oxidative stress involved in joint damage [10,12,19,28,69,78,80,81,82,83,84,85,86]. Oxidative stress is a key factor in cartilage damage, affecting the tissue at various levels that include damage to cartilage proteins, contributions to chondrocyte dysfunction, and premature cell death. Moreover, oxidative stress induces inflammation that drives painful synovitis, associated with joint degeneration [65,87,88]. Furthermore, inflammation is directly correlated with pain as it stimulates the expression of molecules known to induce pain [89,90]. By addressing oxidative stress and its downstream effects, curcumin and resveratrol may provide a multi-faceted approach to mitigating joint damage, inflammation, and associated pain in conditions like osteoarthritis.
6. Cartilage Health
MT-COMP mice develop joint degeneration as early as 20 weeks compared to 9–12 months in the background strain, which is similar to the premature joint degeneration observed in PSACH [27]. Numerous studies have demonstrated the chondroprotective effects of curcumin and resveratrol, effectively slowing or inhibiting joint degeneration [1,4,5,6,7,10,12,13,18,19,20,24,27,28,48,77,91,92,93,94,95,96]. Both resveratrol and curcumin enhance SIRT1 and autophagy, mechanisms that safeguard chondrocyte viability—essential for the optimal function of the growth plate and articular cartilage [63,97,98,99]. The deceleration of degradation is likely attributed to reductions in the matrix metalloproteinases (MMP)-1, MMP-3, MMP-13, and ADAMTS5, which are responsible for breaking down collagen and aggrecan, the primary components of cartilage [2,19,20,24,27,48,76,77,78]. Notably, resveratrol has been shown to increase the expression of type II collagen and aggrecan [94,100]. Additionally, both curcumin and resveratrol exhibit the ability to alleviate chondrocyte senescence, mitigate stiffening, and counteract cartilage glycation—factors that contribute to degeneration progression [14,27,48,94]. These multifaceted effects underscore the potential of curcumin and resveratrol as therapeutic agents for mutant-COMP growth plate pathology and the prevention of joint degeneration processes [66] (Figure 1 and Figure 3).
Figure 3.
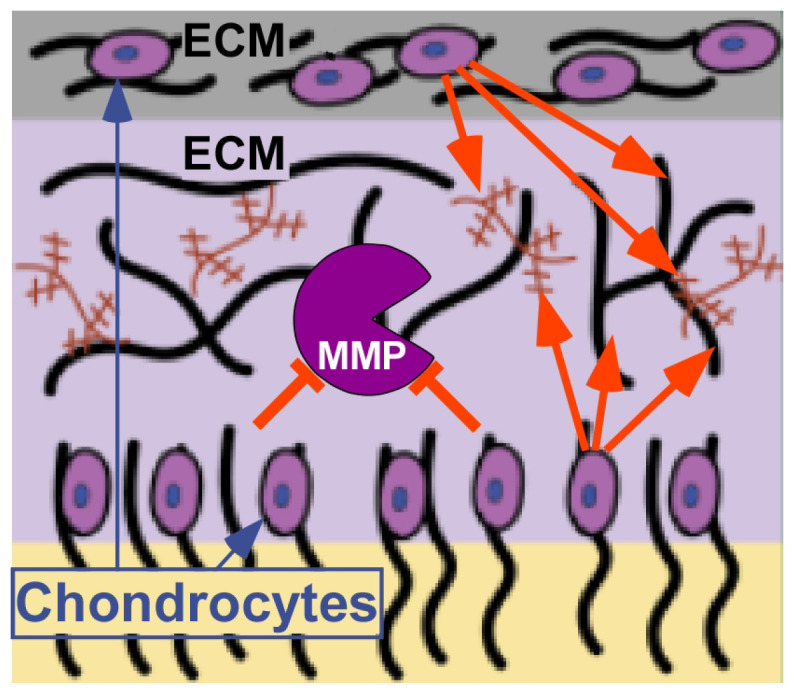
CurQ+ and resveratrol impacts in cartilage. This schematic shows cartilage built of ECM generated by chondrocytes. Orange red block bar (⊥) represents inhibition of MMPs by CurQ+ and resveratrol. Orange arrows depict resveratrol’s enhancement of ECM synthesis. Image shown is modified from https://sitn.hms.harvard.edu/flash/2021/treating-osteoarthritis-the-smart-way/ (accessed on 20 Jan 2004).
7. Pain
MT-COMP mice show signs of pain in multiple assays that are proxies for pain in rodents, including gait changes [27,48], voluntary running, and grooming [18]. Pain modulation can occur at various levels, encompassing the origin of pain, sensory perception, and the transmission of signals. The anti-inflammatory properties of both curcumin and resveratrol play a role in dampening sources of pain [1,3,4,28,87,101,102,103]. Resveratrol specifically inhibits nociceptive (pain-sensing) pathways [104,105,106]. At the same time, curcumin intervenes by inhibiting the activity of key pain mediators, including bradykinin, substance P, and TRPV1 (transient receptor potential vanilloid 1), as well as by modulating the neuronal excitability of sodium channels [20,107,108,109,110,111,112,113]. Moreover, both curcumin and resveratrol alter neurotransmitter levels, influencing the perception and transmission of pain signals to the central nervous system [114,115,116,117,118,119,120,121]. Curcumin modifies serotonin and dopamine levels, whereas resveratrol impacts glutamate and serotonin [116,117,118,119,120,121]. By targeting these multiple facets of pain processing, curcumin and resveratrol present a comprehensive approach to pain modification, potentially offering relief across various dimensions of the pain experience. Importantly, resveratrol treatment of MT-COMP mice abrogates pain (discussed in Section 9) [18].
8. CurQ+ Normalizes Limb Growth in MT-COMP Mice
Curcumin, an active ingredient in turmeric, has been used in traditional medicine and cooking for decades. CurQ+, a unique coconut oil-based dispersion technology, is better absorbed, up to 35-fold more, than 95% dry curcumin in capsules [16]. CurQ+ treatment of MT-COMP mice from 1 to 4 weeks postnatally restored IL10 levels, which control MMP13 cartilage degradation and decrease the pro-inflammatory molecules IL6, IL1β, and TNFα [17]. As expected, IL6, IL1β, TNFα, and MMP13, which drive high levels of chondrocyte stress, are all dampened in growth plate chondrocytes with CurQ+ treatment of MT-COMP mice [17]. CurQ+ treatment of MT-COMP mice from 1 to 4 weeks effectively eliminated mutant-COMP accumulation in the ER and ER stress (CHOP), and preserved growth plate chondrocyte viability (TUNEL), autophagy (MID1, pS6), and proliferation (PCNA) [17]. The most important outcome of this study is the normalization of long bone growth in MT-COMP mice with CurQ+ treatment [17]. Restoring long bone growth is the gold standard for treating a dwarfing condition. This dramatic result occurred after three weeks of CurQ+ treatment and is significant given that autophagy blockage jeopardizes chondrocyte viability and proliferation, which are both crucial to growth plate function and long bone growth [17]. Significantly, the dosages of curcumin used in these studies did not negatively impact weight or compromise pup health and are within the range safely consumed by humans.
9. Resveratrol Preserves Joint Health and Abrogates Pain in MT-COMP Mice
Previously, we showed that resveratrol treatment of MT-COMP mice from birth to 4 weeks recovers approximately 50% of lost limb growth [10,12]. This partial rescue of limb growth led us to study the effect of resveratrol on premature joint degeneration and pain in MT-COMP mice [18]. Resveratrol was administered to MT-COMP mice beginning at birth, 4, 6, and 8 weeks to 20 weeks. Resveratrol dampens articular chondrocyte stress, as demonstrated by the reduction in ER stress, inflammation, autophagy block, and degenerative enzymes (MMP13) [18]. The inflammatory proteins reduced by resveratrol include TNFα, IL1β, IL6, and IL18 [12,48,60,122]. IL6 and TNFα play a pro-inflammatory role in joint degeneration (OA) [123,124,125], and both IL-6 and TNFα stimulate MMP13 expression [48,76,126], an enzyme that degrades articular cartilage. Clearance of mutant-COMP from the ER of chondrocytes restored homeostasis, normalized function, and alleviated the multiple associated stresses. Early resveratrol treatment (beginning at birth or four weeks) preserved joint health, as joint degeneration scores were equivalent to controls [18]. Specifically, resveratrol treatment decreases synovitis and bone/cartilage damage and diminishes the loss of proteoglycans in the articular cartilage [18]. Importantly, resveratrol treatment ameliorated pain in MT-COMP mice whether administration began at birth or at 4 or 6 weeks [18]. Grooming, a natural behavior, is reduced or less efficient when the mouse is in pain [127]. This assay was validated by the administration of a pain reliever (ibuprofen) or by withholding the induction agent, which normalized grooming scores in the MT-COMP mice [18]. Direct joint pain is associated with synovitis, meniscal damage, and subchondral bone remodeling, while inflammation primarily drives indirect joint pain; all are observed in the MT-COMP mice [58,59]. Given that the MT-COMP mice treated with resveratrol from 6 to 20 and 8 to 20 weeks have joint degeneration in the absence of pain suggests that resveratrol suppresses mutant-COMP-induced pain primarily by dampening inflammation [18]. This study reveals that resveratrol not only improves chondrocyte health but also addresses clinically relevant issues of structural joint degeneration and pain.
10. Obstacles to Nutraceutical Treatments
10.1. Bioavailability
The low bioavailability of curcumin is primarily linked to its inadequate water solubility, poor absorption, and rapid elimination from the body [128]. CurQ+ has been developed to address these challenges, demonstrating enhanced absorption and elevated serum plasma levels [16,129]. Similar to curcumin, dry resveratrol powder has limited water solubility, a short half-life, and low oral bioavailability [130,131,132]. While liquid resveratrol formulations circumvent the solubility issues associated with desiccation, they introduce complexities by incorporating additional compounds. These co-purifying compounds may be beneficial (or the source of the benefit), but they obscure the specific impact of resveratrol.
10.2. Lack of Regulation
Both resveratrol and curcumin are classified as supplements; the United States Federal FDA does not regulate these products. While this may be advantageous to some manufacturers as it eliminates the FDA regulatory burden, the lack of stringent regulations for supplements leads to considerable variations in formulations and concentrations of specific compounds in the product [133]. Our analysis using mass spectroscopy on five different commercially available resveratrol preparations revealed substantial disparities between the reported concentrations and the concentrations measured with mass spectroscopy. The concentrations ranged from 16% to 80% of the labeled concentration. Nature’s Answer liquid resveratrol demonstrated the concentration closest to the reported labeled amount and was selected for use in our resveratrol study [18]. The inconsistency in supplement formulations poses a challenge for consumers in accurately determining the dosage they are actually taking. This lack of uniformity also hinders clinical evaluations of efficacy, as variations in concentrations make it challenging to establish standardized and reliable outcomes.
10.3. Perspectives
Prudent skepticism toward any new treatment is justified [134,135]. Communication regarding prescription medications is typically guided by reputable manufacturers and confined to approved uses. The classification of curcumin and resveratrol as supplements constrains manufacturers from making therapeutic or treatment claims. In contrast, naturopathic groups and fringe elements within the medical community have exaggerated claims for supplements, and regulations have not eliminated this problem. Unfortunately, the designation of resveratrol and curcumin as supplements unintentionally fosters a perception among the public that supplements lack the efficacy and potency necessary for legitimate consideration as treatments [136,137]. It also implies that potential dangers do not necessitate stringent regulation, and in the case of resveratrol and curcumin, the risks are low, but other supplements can pose significant risks. Additionally, the low cost of supplements produced by multiple manufacturers and the limited profit potential from a patent introduce financial constraints. The limited profit margins associated with these products hinder the ability to support comprehensive large-scale clinical trials and fund rigorous FDA approval processes.
11. Conclusions
Curcumin normalizes growth in young MT-COMP mice [17], while resveratrol prevents early joint degeneration and the associated pain in adult MT-COMP mice [18]. This suggests that these natural products warrant investigation for their efficacy in PSACH. However, conducting clinical trials for a rare condition like PSACH poses significant challenges due to the limited availability of participants. Many individuals with PSACH manage pain using NSAIDs that thin the blood. Complicating matters more, both curcumin and resveratrol possess weak blood-thinning properties, and it is unsafe to combine blood-thinning agents. Consequently, the already restricted pool of participants for a PSACH clinical trial is further diminished.
The multifaceted impacts of curcumin and resveratrol, encompassing anti-inflammatory, antioxidant, pro-autophagy, and chondroprotective effects, pose a challenge in isolating the essential therapeutic attribute for addressing mutant-COMP pathology. Determining whether the improvements in MT-COMP mice are solely linked to a specific therapeutic property or the result from the synergistic cross-talk of these properties presents a complex issue that is impossible to tease out. Our work with the anti-inflammatory aspirin has shown that the stress cross-talk in the mutant-COMP pathology allows for the dampening of one stress to reduce others [10]. While this intricacy of synergistic cross-talk might be perceived as a drawback, especially in the context of FDA applications, it can be argued that the complex pathology of PSACH benefits from a multi-target approach offered by curcumin and resveratrol.
Collectively, these formidable challenges, compounded by the inherent complexities of nutraceutical development, create substantial hurdles for curcumin and resveratrol as potential treatments for PSACH. Despite these impediments, the life-altering impact of intractable pain in PSACH that significantly diminishes the quality of life for affected individuals motivates ongoing research efforts to address this pressing issue.
Acknowledgments
I thank Jacqueline T. Hecht for her expertise and guidance, crucial to our work and played a significant role in the research reviewed here.
Data Availability Statement
Data are available upon request by writing to karen.posey@uth.tmc.edu. K.L.P. is the first and senior author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This review work was not directly supported but summarized data coming from original published studies funded in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (NIAMS) Award 5R01AR057117-10 (J.T.H. and K.L.P.) and Stratum Nutrition (K.L.P.). A patent application related to this work (USE OF BIOAVAILABLE CURCUMIN CONTAINING COMPOSITIONS FOR THE TREATMENT OF PSEUDOACHONDROPLASIA) has been filed by McGovern Medical School UTHealth. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Stratum Nutrition.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chin K.Y. The spice for joint inflammation: Anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des. Dev. Ther. 2016;10:3029–3042. doi: 10.2147/DDDT.S117432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han G., Zhang Y., Li H. The Combination Treatment of Curcumin and Probucol Protects Chondrocytes from TNF-alpha Induced Inflammation by Enhancing Autophagy and Reducing Apoptosis via the PI3K-Akt-mTOR Pathway. Oxidative Med. Cell. Longev. 2021;2021:5558066. doi: 10.1155/2021/5558066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollazadeh H., Cicero A.F.G., Blesso C.N., Pirro M., Majeed M., Sahebkar A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019;59:89–101. doi: 10.1080/10408398.2017.1358139. [DOI] [PubMed] [Google Scholar]
- 4.Oliviero F., Scanu A., Zamudio-Cuevas Y., Punzi L., Spinella P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018;98:1653–1659. doi: 10.1002/jsfa.8664. [DOI] [PubMed] [Google Scholar]
- 5.Chen T., Zhou R., Chen Y., Fu W., Wei X., Ma G., Hu W., Lu C. Curcumin ameliorates IL-1beta-induced apoptosis by activating autophagy and inhibiting the NF-kappaB signaling pathway in rat primary articular chondrocytes. Cell Biol. Int. 2021;45:976–988. doi: 10.1002/cbin.11541. [DOI] [PubMed] [Google Scholar]
- 6.Li X., Feng K., Li J., Yu D., Fan Q., Tang T., Yao X., Wang X. Curcumin Inhibits Apoptosis of Chondrocytes through Activation ERK1/2 Signaling Pathways Induced Autophagy. Nutrients. 2017;9:414. doi: 10.3390/nu9040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao J., Liu X., Sun Y., Dong X., Liu L., Gu H. Curcumin-Alleviated Osteoarthritic Progression in Rats Fed a High-Fat Diet by Inhibiting Apoptosis and Activating Autophagy via Modulation of MicroRNA-34a. J. Inflamm. Res. 2021;14:2317–2331. doi: 10.2147/JIR.S312139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito M., Yurube T., Kakutani K., Maeno K., Takada T., Terashima Y., Kakiuchi Y., Takeoka Y., Miyazaki S., Kuroda R., et al. Selective interference of mTORC1/RAPTOR protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism with Akt and autophagy induction. Osteoarthr. Cartil. 2017;25:2134–2146. doi: 10.1016/j.joca.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Posey K.L., Coustry F., Veerisetty A.C., Liu P., Alcorn J.L., Hecht J.T. Chondrocyte-specific pathology during skeletal growth and therapeutics in a murine model of pseudoachondroplasia. J. Bone Miner. Res. 2014;29:1258–1268. doi: 10.1002/jbmr.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posey K.L., Coustry F., Veerisetty A.C., Hossain M., Alcorn J.L., Hecht J.T. Antioxidant and anti-inflammatory agents mitigate pathology in a mouse model of pseudoachondroplasia. Hum. Mol. Genet. 2015;24:3918–3928. doi: 10.1093/hmg/ddv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Posey K.L., Coustry F., Veerisetty A.C., Hossain M.G., Gambello M.J., Hecht J.T. Novel mTORC1 Mechanism Suggests Therapeutic Targets for COMPopathies. Am. J. Pathol. 2019;189:132–146. doi: 10.1016/j.ajpath.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecht J.T., Coustry F., Veerisetty A.C., Hossain M.G., Posey K.L. Resveratrol Reduces COMPopathy in Mice through Activation of Autophagy. JBMR Plus. 2021;5:e10456. doi: 10.1002/jbm4.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posey K.L., Hecht J.T. Novel therapeutic interventions for pseudoachondroplasia. Bone. 2017;102:60–68. doi: 10.1016/j.bone.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kakiuchi Y., Yurube T., Kakutani K., Takada T., Ito M., Takeoka Y., Kanda Y., Miyazaki S., Kuroda R., Nishida K. Pharmacological inhibition of mTORC1 but not mTORC2 protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction. Osteoarthr. Cartil. 2019;27:965–976. doi: 10.1016/j.joca.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L., Ding B., Gao J., Yang B., Wang J., Xu H. Curcumin prevents tension-induced endplate cartilage degeneration by enhancing autophagy. Life Sci. 2020;258:118213. doi: 10.1016/j.lfs.2020.118213. [DOI] [PubMed] [Google Scholar]
- 16.Stohs S.J., Ji J., Bucci L.R., Preuss H.G. A Comparative Pharmacokinetic Assessment of a Novel Highly Bioavailable Curcumin Formulation with 95% Curcumin: A Randomized, Double-Blind, Crossover Study. J. Am. Coll. Nutr. 2018;37:51–59. doi: 10.1080/07315724.2017.1358118. [DOI] [PubMed] [Google Scholar]
- 17.Hecht J.T., Veerisetty A.C., Hossain M.G., Chiu F., Posey K.L. CurQ+, a Next-Generation Formulation of Curcumin, Ameliorates Growth Plate Chondrocyte Stress and Increases Limb Growth in a Mouse Model of Pseudoachondroplasia. Int. J. Mol. Sci. 2023;24:3845. doi: 10.3390/ijms24043845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hecht J.T., Veerisetty A.C., Patra D., Hossain M.G., Chiu F., Mobed C., Gannon F.H., Posey K.L. Early Resveratrol Treatment Mitigates Joint Degeneration and Dampens Pain in a Mouse Model of Pseudoachondroplasia (PSACH) Biomolecules. 2023;13:1553. doi: 10.3390/biom13101553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yabas M., Orhan C., Er B., Tuzcu M., Durmus A.S., Ozercan I.H., Sahin N., Bhanuse P., Morde A.A., Padigaru M., et al. A Next Generation Formulation of Curcumin Ameliorates Experimentally Induced Osteoarthritis in Rats via Regulation of Inflammatory Mediators. Front. Immunol. 2021;12:609629. doi: 10.3389/fimmu.2021.609629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z., Leong D.J., Xu L., He Z., Wang A., Navati M., Kim S.J., Hirsh D.M., Hardin J.A., Cobelli N.J., et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016;18:128. doi: 10.1186/s13075-016-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 21.Almeida L., Vaz-da-Silva M., Falcao A., Soares E., Costa R., Loureiro A.I., Fernandes-Lopes C., Rocha J.F., Nunes T., Wright L., et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009;53:S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 22.Hussain S.A., Marouf B.H., Ali Z.S., Ahmmad R.S. Efficacy and safety of co-administration of resveratrol with meloxicam in patients with knee osteoarthritis: A pilot interventional study. Clin. Interv. Aging. 2018;13:1621–1630. doi: 10.2147/CIA.S172758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawda C., Moussa C., Turner R.S. Resveratrol for Alzheimer′s disease. Ann. N. Y. Acad. Sci. 2017;1403:142–149. doi: 10.1111/nyas.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shakibaei M., Csaki C., Nebrich S., Mobasheri A. Resveratrol suppresses interleukin-1beta-induced inflammatory signaling and apoptosis in human articular chondrocytes: Potential for use as a novel nutraceutical for the treatment of osteoarthritis. Biochem. Pharmacol. 2008;76:1426–1439. doi: 10.1016/j.bcp.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 25.Singh N., Agrawal M., Dore S. Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. Neurosci. 2013;4:1151–1162. doi: 10.1021/cn400094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calamini B., Ratia K., Malkowski M.G., Cuendet M., Pezzuto J.M., Santarsiero B.D., Mesecar A.D. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem. J. 2010;429:273–282. doi: 10.1042/BJ20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hecht J.T., Veerisetty A.C., Hossain M.G., Patra D., Chiu F., Coustry F., Posey K.L. Joint Degeneration in a Mouse Model of Pseudoachondroplasia: ER Stress, Inflammation, and Block of Autophagy. Int. J. Mol. Sci. 2021;22:9239. doi: 10.3390/ijms22179239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng K., Ge Y., Chen Z., Li X., Liu Z., Li X., Li H., Tang T., Yang F., Wang X. Curcumin Inhibits the PERK-eIF2alpha-CHOP Pathway through Promoting SIRT1 Expression in Oxidative Stress-induced Rat Chondrocytes and Ameliorates Osteoarthritis Progression in a Rat Model. Oxid. Med. Cell Longev. 2019;2019:8574386. doi: 10.1155/2019/8574386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holden P., Meadows R.S., Chapman K.L., Grant M.E., Kadler K.E., Briggs M.D. Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J. Biol. Chem. 2001;276:6046–6055. doi: 10.1074/jbc.M009507200. [DOI] [PubMed] [Google Scholar]
- 30.Thur J., Rosenberg K., Nitsche D.P., Pihlajamaa T., Ala-Kokko L., Heinegard D., Paulsson M., Maurer P. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J. Biol. Chem. 2001;276:6083–6092. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- 31.Mann H.H., Ozbek S., Engel J., Paulsson M., Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J. Biol. Chem. 2004;279:25294–25298. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]
- 32.Di Cesare P.E., Chen F.S., Moergelin M., Carlson C.S., Leslie M.P., Perris R., Fang C. Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002;21:461–470. doi: 10.1016/S0945-053X(02)00015-X. [DOI] [PubMed] [Google Scholar]
- 33.Briggs M.D., Chapman K.L. Pseudoachondroplasia and multiple epiphyseal dysplasia: Mutation review, molecular interactions, and genotype to phenotype correlations. Hum. Mutat. 2002;19:465–478. doi: 10.1002/humu.10066. [DOI] [PubMed] [Google Scholar]
- 34.Briggs M.D., Hoffman S.M.G., King L.M., Olsen A.S., Mohrenweiser H., Leroy J.G., Mortier G.R., Rimoin D.L., Lachman R.S., Gaines E.S., et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat. Genet. 1995;10:330–336. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 35.Briggs M.D., Brock J., Ramsden S.C., Bell P.A. Genotype to phenotype correlations in cartilage oligomeric matrix protein associated chondrodysplasias. Eur. J. Hum. Genet. 2014;22:1278–1282. doi: 10.1038/ejhg.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kung L.H.W., Mullan L., Soul J., Wang P., Mori K., Bateman J.F., Briggs M.D., Boot-Handford R.P. Cartilage endoplasmic reticulum stress may influence the onset but not the progression of experimental osteoarthritis. Arthritis Res. Ther. 2019;21:206. doi: 10.1186/s13075-019-1988-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiCesare P.E., Morgelin M., Carlson C.S., Pasumarti S., Paulsson M. Cartilage oligomeric matrix protein: Isolation and characterization from human articular cartilage. J. Orthop. Res. 1995;13:422–428. doi: 10.1002/jor.1100130316. [DOI] [PubMed] [Google Scholar]
- 38.Cooper R.R., Ponseti I.V., Maynard J.A. Pseudoachondroplasia dwarfism. A rough-surfaced endoplasmic reticulum disorder. J. Bone Jt. Surg. Am. 1973;55A:475–484. doi: 10.2106/00004623-197355030-00003. [DOI] [PubMed] [Google Scholar]
- 39.Dinser R., Zaucke F., Kreppel F., Hultenby K., Kochanek S., Paulsson M., Maurer P. Pseudoachondroplasia is caused through both intra- and extracellular pathogenic pathways. J. Clin. Investig. 2002;110:505–513. doi: 10.1172/JCI0214386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ikegawa S., Ohashi H., Nishimura G., Kim K.C., Sannohe A., Kimizuka M., Fukushima Y., Nagai T., Nakamura Y. Novel and recurrent COMP (cartilage oligomeric matrix protein) mutations in pseudoachondroplasia and multiple epiphyseal dysplasia. Hum. Genet. 1998;103:633–638. doi: 10.1007/s004390050883. [DOI] [PubMed] [Google Scholar]
- 41.Briggs M.D., Mortier G.R., Cole W.G., King L.M., Golik S.S., Bonaventure J., Nuytinck L., De Paepe A., Leroy J.G., Biesecker L., et al. Diverse mutations in the gene for cartilage oligomeric matrix protein in the pseudoachondroplasia-multiple epiphyseal dysplasia disease spectrum. Am. J. Hum. Genet. 1998;62:311–319. doi: 10.1086/301713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hecht J.T., Nelson L.D., Crowder E., Wang Y., Elder F.F., Harrison W.R., Francomano C.A., Prange C.K., Lennon G.G., Deere M., et al. Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat. Genet. 1995;10:325–329. doi: 10.1038/ng0795-325. [DOI] [PubMed] [Google Scholar]
- 43.Bonafe L., Cormier-Daire V., Hall C., Lachman R., Mortier G., Mundlos S., Nishimura G., Sangiorgi L., Savarirayan R., Sillence D., et al. Nosology and classification of genetic skeletal disorders: 2015 revision. Am. J. Med. Genet. A. 2015;167A:2869–2892. doi: 10.1002/ajmg.a.37365. [DOI] [PubMed] [Google Scholar]
- 44.Hecht J.T., Chiu F., Veerisetty A., Hossain M., Posey K.L. Health consequences of mutant cartilage oligomeric matrix protein and its relationship to abnormal growth and joint degeneration. Matrix Biol. 2023;119:101–111. doi: 10.1016/j.matbio.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Kung L.H., Rajpar M.H., Preziosi R., Briggs M.D., Boot-Handford R.P. Increased classical endoplasmic reticulum stress is sufficient to reduce chondrocyte proliferation rate in the growth plate and decrease bone growth. PLoS ONE. 2015;10:e0117016. doi: 10.1371/journal.pone.0117016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Posey K.L., Veerisetty A.C., Liu P., Wang H.R., Poindexter B.J., Bick R., Alcorn J.L., Hecht J.T. An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am. J. Pathol. 2009;175:1555–1563. doi: 10.2353/ajpath.2009.090184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKeand J., Rotta J., Hecht J.T. Natural history study of pseudoachondroplasia. Am. J. Med. Genet. 1996;63:406–410. doi: 10.1002/(SICI)1096-8628(19960517)63:2<406::AID-AJMG16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 48.Hecht J.T., Veerisetty A.C., Wu J., Coustry F., Hossain M.G., Chiu F., Gannon F.H., Posey K.L. Primary Osteoarthritis Early Joint Degeneration Induced by Endoplasmic Reticulum Stress Is Mitigated by Resveratrol. Am. J. Pathol. 2021;191:1624–1637. doi: 10.1016/j.ajpath.2021.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maynard J.A., Cooper R.R., Ponseti I.V. A unique rough surfaced endoplasmic reticulum inclusion in pseudoachondroplasia. Lab. Investig. 1972;26:40–44. [PubMed] [Google Scholar]
- 50.Gamble C., Nguyen J., Hashmi S.S., Hecht J.T. Pseudoachondroplasia and painful sequelae. Am. J. Med. Genet. A. 2015;167:2618–2622. doi: 10.1002/ajmg.a.37253. [DOI] [PubMed] [Google Scholar]
- 51.Hall J.G. Pseudoachondroplasia. Birth Defects Orig. Artic. Ser. 1975;11:187–202. [PubMed] [Google Scholar]
- 52.Moore K.A., Hollien J. The unfolded protein response in secretory cell function. Annu. Rev. Genet. 2012;46:165–183. doi: 10.1146/annurev-genet-110711-155644. [DOI] [PubMed] [Google Scholar]
- 53.Posey K.L., Hayes E., Haynes R., Hecht J.T. Role of TSP-5/COMP in pseudoachondroplasia. Int. J. Biochem. Cell Biol. 2004;36:1005–1012. doi: 10.1016/j.biocel.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Maddox B.K., Mokashi A., Keene D.R., Bachinger H.P. A cartilage oligomeric matrix protein mutation associated with pseudoachondroplasia changes the structural and functional properties of the type 3 domain. J. Biol. Chem. 2000;275:11412–11417. doi: 10.1074/jbc.275.15.11412. [DOI] [PubMed] [Google Scholar]
- 55.Kvansakul M., Adams J.C., Hohenester E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. EMBO J. 2004;23:1223–1233. doi: 10.1038/sj.emboj.7600166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coustry F., Posey K.L., Liu P., Alcorn J.L., Hecht J.T. D469del-COMP retention in chondrocytes stimulates caspase-independent necroptosis. Am. J. Pathol. 2012;180:738–748. doi: 10.1016/j.ajpath.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Posey K.L., Coustry F., Veerisetty A.C., Liu P., Alcorn J.L., Hecht J.T. Chop (Ddit3) is essential for D469del-COMP retention and cell death in chondrocytes in an inducible transgenic mouse model of pseudoachondroplasia. Am. J. Pathol. 2012;180:727–737. doi: 10.1016/j.ajpath.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sethi V., Garg M., Herve M., Mobasheri A. Potential complementary and/or synergistic effects of curcumin and boswellic acids for management of osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2022;14:1759720X221124545. doi: 10.1177/1759720X221124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marouf B.H., Hussain S.A., Ali Z.S., Ahmmad R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food. 2018;21:1253–1259. doi: 10.1089/jmf.2017.4176. [DOI] [PubMed] [Google Scholar]
- 60.Wei Y., Jia J., Jin X., Tong W., Tian H. Resveratrol ameliorates inflammatory damage and protects against osteoarthritis in a rat model of osteoarthritis. Mol. Med. Rep. 2018;17:1493–1498. doi: 10.3892/mmr.2017.8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang C., Xu X., Dong X., Yang B., Dong W., Luo Y., Liu X., Wu Y., Wang J. DDIT3/CHOP promotes autophagy in chondrocytes via SIRT1-AKT pathway. Biochim. Biophys. Acta Mol. Cell Res. 2021;1868:119074. doi: 10.1016/j.bbamcr.2021.119074. [DOI] [PubMed] [Google Scholar]
- 62.Dvir-Ginzberg M., Mobasheri A., Kumar A. The Role of Sirtuins in Cartilage Homeostasis and Osteoarthritis. Curr. Rheumatol. Rep. 2016;18:43. doi: 10.1007/s11926-016-0591-y. [DOI] [PubMed] [Google Scholar]
- 63.Lee J., Hong S.W., Kwon H., Park S.E., Rhee E.J., Park C.Y., Oh K.W., Park S.W., Lee W.Y. Resveratrol, an activator of SIRT1, improves ER stress by increasing clusterin expression in HepG2 cells. Cell Stress Chaperones. 2019;24:825–833. doi: 10.1007/s12192-019-01012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Maiti P., Manna J., Veleri S., Frautschy S. Molecular chaperone dysfunction in neurodegenerative diseases and effects of curcumin. Biomed. Res. Int. 2014;2014:495091. doi: 10.1155/2014/495091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Eitner A., Hofmann G.O., Schaible H.G. Mechanisms of Osteoarthritic Pain. Studies in Humans and Experimental Models. Front. Mol. Neurosci. 2017;10:349. doi: 10.3389/fnmol.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wojdasiewicz P., Poniatowski L.A., Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014;2014:561459. doi: 10.1155/2014/561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rahimnia A.R., Panahi Y., Alishiri G., Sharafi M., Sahebkar A. Impact of Supplementation with Curcuminoids on Systemic Inflammation in Patients with Knee Osteoarthritis: Findings from a Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2014;65:521–525. doi: 10.1055/s-0034-1384536. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H., Zhang J., Ungvari Z., Zhang C. Resveratrol improves endothelial function: Role of TNFα and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Z.M., Chen Y.C., Wang D.P. Resveratrol, a natural antioxidant, protects monosodium iodoacetate-induced osteoarthritic pain in rats. Biomed. Pharmacother. 2016;83:763–770. doi: 10.1016/j.biopha.2016.06.050. [DOI] [PubMed] [Google Scholar]
- 70.Rao C.V. Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol. 2007;595:213–226. doi: 10.1007/978-0-387-46401-5_9. [DOI] [PubMed] [Google Scholar]
- 71.Alcain F.J., Villalba J.M. Sirtuin activators. Expert Opin. Ther. Pat. 2009;19:403–414. doi: 10.1517/13543770902762893. [DOI] [PubMed] [Google Scholar]
- 72.Chung S., Yao H., Caito S., Hwang J.W., Arunachalam G., Rahman I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010;501:79–90. doi: 10.1016/j.abb.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo H., Chen Y., Liao L., Wu W. Resveratrol protects HUVECs from oxidized-LDL induced oxidative damage by autophagy upregulation via the AMPK/SIRT1 pathway. Cardiovasc. Drugs Ther. 2013;27:189–198. doi: 10.1007/s10557-013-6442-4. [DOI] [PubMed] [Google Scholar]
- 74.Kao C.L., Chen L.K., Chang Y.L., Yung M.C., Hsu C.C., Chen Y.C., Lo W.L., Chen S.J., Ku H.H., Hwang S.J. Resveratrol protects human endothelium from H(2)O(2)-induced oxidative stress and senescence via SirT1 activation. J. Atheroscler. Thromb. 2010;17:970–979. doi: 10.5551/jat.4333. [DOI] [PubMed] [Google Scholar]
- 75.Li W., Cai L., Zhang Y., Cui L., Shen G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2alpha. J. Orthop. Res. 2015;33:1061–1070. doi: 10.1002/jor.22859. [DOI] [PubMed] [Google Scholar]
- 76.Liacini A., Sylvester J., Li W.Q., Huang W., Dehnade F., Ahmad M., Zafarullah M. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp. Cell Res. 2003;288:208–217. doi: 10.1016/S0014-4827(03)00180-0. [DOI] [PubMed] [Google Scholar]
- 77.Liu J., He X., Zhen P., Zhou S., Li X. Inflammatory cytokines and oxidative stress markers in the inhibition of osteoarthritis by curcumin. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2016;45:461–468. doi: 10.3785/j.issn.1008-9292.2016.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dave M., Attur M., Palmer G., Al-Mussawir H.E., Kennish L., Patel J., Abramson S.B. The antioxidant resveratrol protects against chondrocyte apoptosis via effects on mitochondrial polarization and ATP production. Arthritis Rheum. 2008;58:2786–2797. doi: 10.1002/art.23799. [DOI] [PubMed] [Google Scholar]
- 79.Marciniak S.J., Yun C.Y., Oyadomari S., Novoa I., Zhang Y., Jungreis R., Nagata K., Harding H.P., Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Collodel G., Federico M.G., Geminiani M., Martini S., Bonechi C., Rossi C., Figura N., Moretti E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011;31:239–246. doi: 10.1016/j.reprotox.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 81.Das S.K., Mukherjee S., Gupta G., Rao D.N., Vasudevan D.M. Protective effect of resveratrol and vitamin E against ethanol-induced oxidative damage in mice: Biochemical and immunological basis. Indian J. Biochem. Biophys. 2010;47:32–37. [PubMed] [Google Scholar]
- 82.Olas B., Nowak P., Kolodziejczyk J., Ponczek M., Wachowicz B. Protective effects of resveratrol against oxidative/nitrative modifications of plasma proteins and lipids exposed to peroxynitrite. J. Nutr. Biochem. 2006;17:96–102. doi: 10.1016/j.jnutbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Ungvari Z., Orosz Z., Rivera A., Labinskyy N., Xiangmin Z., Olson S., Podlutsky A., Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 84.Abrahams S., Haylett W.L., Johnson G., Carr J.A., Bardien S. Antioxidant effects of curcumin in models of neurodegeneration, aging, oxidative and nitrosative stress: A review. Neuroscience. 2019;406:1–21. doi: 10.1016/j.neuroscience.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 85.Panahi Y., Alishiri G.H., Parvin S., Sahebkar A. Mitigation of Systemic Oxidative Stress by Curcuminoids in Osteoarthritis: Results of a Randomized Controlled Trial. J. Diet. Suppl. 2016;13:209–220. doi: 10.3109/19390211.2015.1008611. [DOI] [PubMed] [Google Scholar]
- 86.Suryanarayana P., Satyanarayana A., Balakrishna N., Kumar P.U., Reddy G.B. Effect of turmeric and curcumin on oxidative stress and antioxidant enzymes in streptozotocin-induced diabetic rat. Med. Sci. Monit. 2007;13:BR286–BR292. [PubMed] [Google Scholar]
- 87.Wang G., Hu Z., Song X., Cui Q., Fu Q., Jia R., Zou Y., Li L., Yin Z. Analgesic and Anti-Inflammatory Activities of Resveratrol through Classic Models in Mice and Rats. Evid. Based Complement. Altern. Med. 2017;2017:5197567. doi: 10.1155/2017/5197567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benito M.J., Veale D.J., FitzGerald O., van den Berg W.B., Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005;64:1263–1267. doi: 10.1136/ard.2004.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mackey S. Mechanisms of inflammatory pain: Therapeutic implications. J. Clin. Rheumatol. 2004;10:S5–S11. doi: 10.1097/01.rhu.0000130684.35729.55. [DOI] [PubMed] [Google Scholar]
- 90.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthr. Cartil. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 91.Zhang G., Cao J., Yang E., Liang B., Ding J., Liang J., Xu J. Curcumin improves age-related and surgically induced osteoarthritis by promoting autophagy in mice. Biosci. Rep. 2018;38:BSR20171691. doi: 10.1042/BSR20171691. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Mobasheri A., Henrotin Y., Biesalski H.K., Shakibaei M. Scientific evidence and rationale for the development of curcumin and resveratrol as nutraceutricals for joint health. Int. J. Mol. Sci. 2012;13:4202–4232. doi: 10.3390/ijms13044202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008;75:677–687. doi: 10.1016/j.bcp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 94.Liu F.C., Hung L.F., Wu W.L., Chang D.M., Huang C.Y., Lai J.H., Ho L.J. Chondroprotective effects and mechanisms of resveratrol in advanced glycation end products-stimulated chondrocytes. Arthritis Res. Ther. 2010;12:R167. doi: 10.1186/ar3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shakibaei M., Mobasheri A., Buhrmann C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011;6:171–179. doi: 10.1007/s12263-010-0179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nguyen C., Savouret J.F., Widerak M., Corvol M.T., Rannou F. Resveratrol, Potential Therapeutic Interest in Joint Disorders: A Critical Narrative Review. Nutrients. 2017;9:45. doi: 10.3390/nu9010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Forouzanfar F., Read M.I., Barreto G.E., Sahebkar A. Neuroprotective effects of curcumin through autophagy modulation. IUBMB Life. 2020;72:652–664. doi: 10.1002/iub.2209. [DOI] [PubMed] [Google Scholar]
- 98.Jang J.H., Surh Y.J. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free Radic. Biol. Med. 2003;34:1100–1110. doi: 10.1016/S0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 99.Bournival J., Quessy P., Martinoli M.G. Protective effects of resveratrol and quercetin against MPP+ -induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell. Mol. Neurobiol. 2009;29:1169–1180. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Qin N., Wei L., Li W., Yang W., Cai L., Qian Z., Wu S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017;134:166–174. doi: 10.1016/j.jphs.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 101.de Sa Coutinho D., Pacheco M.T., Frozza R.L., Bernardi A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int. J. Mol. Sci. 2018;19:1812. doi: 10.3390/ijms19061812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Deng Z., Li Y., Liu H., Xiao S., Li L., Tian J., Cheng C., Zhang G., Zhang F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019;39:BSR20190189. doi: 10.1042/BSR20190189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ebrahim H.A., Alzamil N.M., Al-Ani B., Haidara M.A., Kamar S.S., Dawood A.F. Suppression of knee joint osteoarthritis induced secondary to type 2 diabetes mellitus in rats by resveratrol: Role of glycated haemoglobin and hyperlipidaemia and biomarkers of inflammation and oxidative stress. Arch. Physiol. Biochem. 2020;128:1375–1382. doi: 10.1080/13813455.2020.1771378. [DOI] [PubMed] [Google Scholar]
- 104.Granados-Soto V., Arguelles C.F., Ortiz M.I. The peripheral antinociceptive effect of resveratrol is associated with activation of potassium channels. Neuropharmacology. 2002;43:917–923. doi: 10.1016/S0028-3908(02)00130-2. [DOI] [PubMed] [Google Scholar]
- 105.Rojas-Aguilar F.A., Briones-Aranda A., Jaramillo-Morales O.A., Romero-Nava R., Esquinca-Aviles H.A., Espinosa-Juarez J.V. The Additive Antinociceptive Effect of Resveratrol and Ketorolac in the Formalin Test in Mice. Pharmaceuticals. 2023;16:1078. doi: 10.3390/ph16081078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Singh A.K., Vinayak M. Anti-Nociceptive Effect of Resveratrol During Inflammatory Hyperalgesia via Differential Regulation of pro-Inflammatory Mediators. Phytother. Res. 2016;30:1164–1171. doi: 10.1002/ptr.5624. [DOI] [PubMed] [Google Scholar]
- 107.Blacona G., Raso R., Castellani S., Pierandrei S., Del Porto P., Ferraguti G., Ascenzioni F., Conese M., Lucarelli M. Downregulation of epithelial sodium channel (ENaC) activity in cystic fibrosis cells by epigenetic targeting. Cell. Mol. Life Sci. 2022;79:257. doi: 10.1007/s00018-022-04190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang C.D., Tliba O., Panettieri R.A., Jr., Amrani Y. Bradykinin induces interleukin-6 production in human airway smooth muscle cells: Modulation by Th2 cytokines and dexamethasone. Am. J. Respir. Cell Mol. Biol. 2003;28:330–338. doi: 10.1165/rcmb.2002-0040OC. [DOI] [PubMed] [Google Scholar]
- 109.Naik G.G., Uniyal A., Chouhan D., Tiwari V., Sahu A.N. Natural Products and some Semi-synthetic Analogues as Potential TRPV1 Ligands for Attenuating Neuropathic Pain. Curr. Pharm. Biotechnol. 2022;23:766–786. doi: 10.2174/1389201022666210719155931. [DOI] [PubMed] [Google Scholar]
- 110.Nalli M., Ortar G., Schiano Moriello A., Di Marzo V., De Petrocellis L. Effects of curcumin and curcumin analogues on TRP channels. Fitoterapia. 2017;122:126–131. doi: 10.1016/j.fitote.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 111.Zhi L., Dong L., Kong D., Sun B., Sun Q., Grundy D., Zhang G., Rong W. Curcumin acts via transient receptor potential vanilloid-1 receptors to inhibit gut nociception and reverses visceral hyperalgesia. Neurogastroenterol. Motil. 2013;25:e429–e440. doi: 10.1111/nmo.12145. [DOI] [PubMed] [Google Scholar]
- 112.Koroljevic Z.D., Jordan K., Ivkovic J., Bender D.V., Peric P. Curcuma as an anti-inflammatory component in treating osteoarthritis. Rheumatol. Int. 2023;43:589–616. doi: 10.1007/s00296-022-05244-8. [DOI] [PubMed] [Google Scholar]
- 113.Tajik H., Tamaddonfard E., Hamzeh-Gooshchi N. The effect of curcumin (active substance of turmeric) on the acetic acid-induced visceral nociception in rats. Pak. J. Biol. Sci. 2008;11:312–314. doi: 10.3923/pjbs.2008.312.314. [DOI] [PubMed] [Google Scholar]
- 114.Kulkarni S.K., Bhutani M.K., Bishnoi M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology. 2008;201:435–442. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 115.Xu Y., Ku B.S., Yao H.Y., Lin Y.H., Ma X., Zhang Y.H., Li X.J. The effects of curcumin on depressive-like behaviors in mice. Eur. J. Pharmacol. 2005;518:40–46. doi: 10.1016/j.ejphar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 116.Quincozes-Santos A., Bobermin L.D., Latini A., Wajner M., Souza D.O., Goncalves C.A., Gottfried C. Resveratrol Protects C6 Astrocyte Cell Line against Hydrogen Peroxide-Induced Oxidative Stress through Heme Oxygenase 1. PLoS ONE. 2013;8:e64372. doi: 10.1371/journal.pone.0064372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sanchez-Melgar A., Albasanz J.L., Pallas M., Martin M. Resveratrol Differently Modulates Group I Metabotropic Glutamate Receptors Depending on Age in SAMP8 Mice. ACS Chem. Neurosci. 2020;11:1770–1780. doi: 10.1021/acschemneuro.0c00067. [DOI] [PubMed] [Google Scholar]
- 118.Anderson G., Maes M., Berk M. Inflammation-related disorders in the tryptophan catabolite pathway in depression and somatization. Adv. Protein Chem. Struct. Biol. 2012;88:27–48. doi: 10.1016/B978-0-12-398314-5.00002-7. [DOI] [PubMed] [Google Scholar]
- 119.Zhao X., Yu C., Wang C., Zhang J.F., Zhou W.H., Cui W.G., Ye F., Xu Y. Chronic resveratrol treatment exerts antihyperalgesic effect and corrects co-morbid depressive like behaviors in mice with mononeuropathy: Involvement of serotonergic system. Neuropharmacology. 2014;85:131–141. doi: 10.1016/j.neuropharm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 120.Abdelrahman K.M., Hackshaw K.V. Nutritional Supplements for the Treatment of Neuropathic Pain. Biomedicines. 2021;9:674. doi: 10.3390/biomedicines9060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao X., Xu Y., Zhao Q., Chen C.R., Liu A.M., Huang Z.L. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: Descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2012;62:843–854. doi: 10.1016/j.neuropharm.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 122.Yuce P., Hosgor H., Rencber S.F., Yazir Y. Effects of Intra-Articular Resveratrol Injections on Cartilage Destruction and Synovial Inflammation in Experimental Temporomandibular Joint Osteoarthritis. J. Oral Maxillofac. Surg. 2020;79:344.e1–344.e12. doi: 10.1016/j.joms.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Goldring M.B., Otero M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Goldring M.B. Articular cartilage degradation in osteoarthritis. HSS J. 2012;8:7–9. doi: 10.1007/s11420-011-9250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burrage P.S., Mix K.S., Brinckerhoff C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 126.Tang C.H., Chen C.F., Chen W.M., Fong Y.C. IL-6 increases MMP-13 expression and motility in human chondrosarcoma cells. J. Biol. Chem. 2011;286:11056–11066. doi: 10.1074/jbc.M110.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deuis J.R., Dvorakova L.S., Vetter I. Methods Used to Evaluate Pain Behaviors in Rodents. Front. Mol. Neurosci. 2017;10:284. doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anand P., Kunnumakkara A.B., Newman R.A., Aggarwal B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 129.Stohs S.J., Chen C.Y.O., Preuss H.G., Ray S.D., Bucci L.R., Ji J., Ruff K.J. The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: A comparative study. BMC Complement. Altern. Med. 2019;19:293. doi: 10.1186/s12906-019-2699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Smoliga J.M., Blanchard O. Enhancing the delivery of resveratrol in humans: If low bioavailability is the problem, what is the solution? Molecules. 2014;19:17154–17172. doi: 10.3390/molecules191117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pangeni R., Sahni J.K., Ali J., Sharma S., Baboota S. Resveratrol: Review on therapeutic potential and recent advances in drug delivery. Expert Opin. Drug Deliv. 2014;11:1285–1298. doi: 10.1517/17425247.2014.919253. [DOI] [PubMed] [Google Scholar]
- 132.Smoliga J.M., Vang O., Baur J.A. Challenges of translating basic research into therapeutics: Resveratrol as an example. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012;67:158–167. doi: 10.1093/gerona/glr062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manasa P.S.L., Kamble A.D., Chilakamarthi U. Various Extraction Techniques of Curcumin-A Comprehensive Review. ACS Omega. 2023;8:34868–34878. doi: 10.1021/acsomega.3c04205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Arvanitoyannis I.S., Van Houwelingen-Koukaliaroglou M. Functional foods: A survey of health claims, pros and cons, and current legislation. Crit. Rev. Food Sci. Nutr. 2005;45:385–404. doi: 10.1080/10408390590967667. [DOI] [PubMed] [Google Scholar]
- 135.Ameye L.G., Chee W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther. 2006;8:R127. doi: 10.1186/ar2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rakofsky J.J., Dunlop B.W. Review of nutritional supplements for the treatment of bipolar depression. Depress. Anxiety. 2014;31:379–390. doi: 10.1002/da.22220. [DOI] [PubMed] [Google Scholar]
- 137.Santos H.O., Cerqueira H.S., Tinsley G.M. The Effects of Dietary Supplements, Nutraceutical Agents, and Physical Exercise on Myostatin Levels: Hope or Hype? Metabolites. 2022;12:1146. doi: 10.3390/metabo12111146. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon request by writing to karen.posey@uth.tmc.edu. K.L.P. is the first and senior author.