Abstract
Neutrophil released peptidyl arginine deiminase 4 (PAD4) converts arginine residues on plasma proteins into citrulline. Here, we developed an assay to quantify citrullinated fibrinogen. We employed a biotin‐conjugated phenylglyoxal (biotin‐phenylglyoxal (PG)) compound that selectively labels citrulline. Patient samples were derived from a multicenter prospective cohort study that aimed to identify cancer patients at high risk for venous thromboembolism (VTE). Our data show that cancer patients have higher (median 2‐fold increased) citrullinated fibrinogen levels when compared to normal human plasma and a cohort of healthy donors. Our results show that citrullination of fibrinogen is a common posttranslational modification in patients with cancer.
Keywords: cancer, citrullination, coagulation, fibrinogen, thrombosis
Citrullination is a post‐translational modification of arginine. Recent research has shown that citrullination can affect the activity of coagulation factors like fibrinogen. Quantitative analysis of citrullinated fibrinogen showed that cancer patients who are at increased risk of developing venous thromboembolism have significantly increased levels of circulating citrullinated fibrinogen compared to healthy controls. This finding is indicative of widespread plasma protein citrullination in cancer patients.
1. INTRODUCTION
Citrullination is the posttranslational modification that constitutes the conversion of a positively charged arginine residue into neutral citrulline. Citrullination impacts the structure and function of proteins and produces neoepitopes that can be targeted by anti‐citrullinated protein antibodies, which are an early diagnostic biomarker of rheumatoid arthritis [1]. Synovial fluid and serum from patients with rheumatoid arthritis are known to contain multiple citrullinated proteins, including fibrinogen [2]. Rheumatoid arthritis patients suffer an increased risk of both arterial and venous thrombosis, and recent research has shown that clots derived from rheumatoid arthritis patients in acute phase are rich in citrullinated fibrin [3]. Citrullinated fibrinogen produces clots with higher density and lower permeability, which are resistant to lysis [4, 5]. Citrullination is directly related to inflammation due to release of citrullinating peptidyl‐arginine‐deiminase enzymes (PADs) from neutrophils [6]. PAD isoform 4 specifically is capable of citrullinating many proteins including antithrombin [7], antiplasmin [7], and TFPI [8]. Cancer is characterized by chronic inflammation, and citrullination is involved in the progression of cancer [9]. Venous thromboembolism (VTE) occurs frequently in patients with cancer [10]. It is currently not known whether plasma protein citrullination contributes to cancer‐associated thrombosis. Phenyl‐glyoxal derived probes have been developed for detection of citrullination in complex samples [11]. Here, we report a novel quantification method for citrullinated fibrinogen using biotinylated phenyl‐glyoxal (biotin‐PG) [12]. We quantified citrullinated fibrinogen in plasma samples collected in a multicenter prospective cohort study of patients with cancer at risk of developing VTE (multinational cohort study to identify cancer patients at high risk of venous thromboembolism; MICA) [13]. We measured significantly increased levels of citrullinated fibrinogen in plasma samples of patients with cancer compared to healthy controls. We propose that citrullination of fibrinogen and (regulators of) other blood coagulation factors may modulate coagulation in cancer patients.
2. METHODS
2.1. Biotin‐PG mediated enrichment of citrullinated plasma proteins
Our method is based on ADAMTS13 citrullination analysis as published by Sorvillo et al. [14] The protocol used is described in detail in the Supplementary Information. In brief, 300‐μg plasma proteins were labeled using biotin‐PG at low pH. Subsequently citrullinated proteins were bound to streptavidin‐agarose beads and washed extensively. Citrullinated proteins were eluted from the beads and run on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS‐PAGE) gel and subsequently transferred to a PDVF membrane by Western‐blotting. Membranes were blocked using 5% milk powder (Protifar, Nutricia) in phosphate‐buffered saline (PBS)‐0.1%Tween20. Polyclonal rabbit antihuman fibrinogen antibody (DAKO A0080) which detects all chains of human fibrinogen was used as primary antibody at 1:500 dilution. Goat‐anti‐rabbit horseradish peroxidase (HRP) labeled secondary antibody was applied at 1:5000 dilution. Blots were developed using chemiluminescent substrate and imaged by Amersham ImageQuant 800. Quantification of lanes was performed using ImageJ densitometry. Normalized citrullination index (NCI) for all samples were calculated by dividing the citrullinated fibrinogen α‐chain band intensity for the patient samples over the intensity of normal human plasma (NHP) citrullinated fibrinogen. For one of the patient samples (sample 2098), we originally observed an exceptionally low NCI of 0.13. This patient did not show a significant α‐chain band of fibrinogen on Western blot, although the β‐chain was present. Based on the aberrant pattern on Western blot we performed an independent repeat for sample 2098 which gave a value of 0.95 for this sample. We used this value for data analysis.
Citrate plasma samples from the MICA study were obtained from the Amsterdam University Medical Center. D‐Dimer levels were measured using the INNOVANCE assay (Siemens Healthcare, Marburg, Germany). Fibrinogen antigen was determined by ELISA. A 96‐wells plate was coated with anti‐human fibrinogen antibody (DAKO, A0080, lot. 097/602, rabbit polyclonal). HRP‐conjugated anti‐human fibrinogen antibody was used for detection (DAKO, P0445, lot. no. 114/501, rabbit polyclonal). Values were calculated using a calibration curve of pooled human plasma. Statistical analyses were performed in GraphPad Prism, version 9.1.1.
3. RESULTS
Citrullinated fibrinogen is detected following citrulline‐specific labeling by biotin‐PG at low pH (Figure 1A) [12]. The biotin moiety is subsequently used to capture labeled proteins with streptavidin‐agarose beads (Figure 1B). Following washing and elution, enriched proteins are separated by SDS‐PAGE and bound fibrinogen, is detected by conventional Western blotting We validated our protocol using in vitro citrullinated fibrinogen (Figure S1). Subsequently, we analyzed a series of samples of patients with cancer (Figure 1C). Each blot is organized in a similar manner: lanes 1 and 2 contain NHP, whereas lanes 3 and 4 contain patient sample 2071 which is included to evaluate assay reproducibility. Remaining lanes 5–8 contain individual cancer patient plasma samples. For samples in lanes 1 and 3, no biotin‐PG was added; small amounts of fibrinogen detected in lanes 1 and 3 reflect a specific binding of fibrinogen to streptavidin‐agarose. A relatively high intensity was observed for biotin‐PG treated plasma of patient 2071 indicated that elevated levels of citrullinated fibrinogen were present in this sample (Figure 1C). Interestingly, low levels of citrullinated fibrinogen were also observed as evidenced by the signal obtained for biotin‐PG treated NHP (Figure 1C; lane 2), suggesting that citrullinated fibrinogen is present in healthy individuals. Importantly, the intensity of lane 4 shows patient 2071 having increased levels of fibrinogen citrullination when compared to NHP. Bands were quantified based on the intensity of the signal obtained for the fibrinogen α‐chain as determined by densitometric scanning using Image J. The results were used to calculate a citrullination index, which is defined as the ratio between the intensity of the band obtained for the patient samples and the signal‐intensity obtained for NHP. Based on 8 separate citrullination indices obtained for sample 2071, an average index of 2.8 was calculated with an SD of 0.27, corresponding to an assay coefficient of variation (CV) of 9.6%. This value indicates that the citrullinated fibrinogen level in sample 2071 is 2.8‐fold higher when compared to normal human plasma (Figure 2A). Based on the CV we conclude that this assay provides consistent results over multiple experimental runs. Having validated our method we analyzed a small cohort of cancer patients and 10 healthy volunteers. We included plasma of patients who developed VTE during cancer treatment (VTE+) and matched these samples to cancer patients who did not develop VTE (VTE‐) based on tumor type, age and sex [15]. An overview of the cohort is given in Table S1. We measured the citrullination index for the cohort (Figure 2B). The median citrullination index of 2.21 was significantly higher than the value of 0.85 obtained for plasma samples of healthy controls (p < 0.001, Mann–Whitney U‐test). We measured fibrinogen levels in patient samples and controls to correct for possible variations in fibrinogen levels (Figure S2A). As fibrinogen concentrations may vary for the different samples analyzed, we normalized the citrullination index values for fibrinogen concentration as determined by ELISA. The results of this analysis are shown in Figure 2C. Correction for fibrinogen levels results in lower citrullination index values, however still the median normalized citrullination index of 1.53 is significantly higher when compared to healthy controls (p < 0.001, Mann–Whitney U‐test).
FIGURE 1.
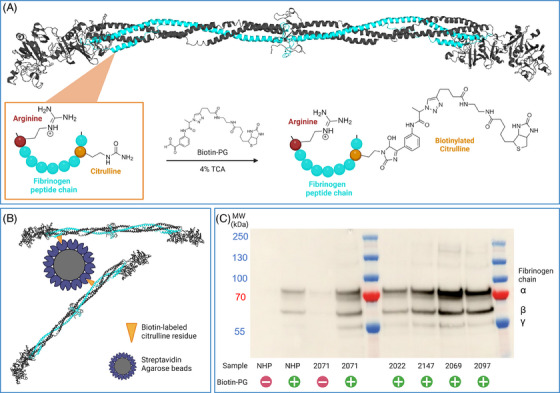
Biotin‐PG mediated capture of citrullinated fibrinogen. (A) Citrulline residues of fibrinogen are selectively labeled with a biotin‐containing linker at low pH. (B) Biotinylated residues are exploited to capture citrullinated fibrinogen with streptavidin‐agarose beads. (C) A representative western blot of fibrinogen after citrulline‐specific protein capture, elution and sodium dodecyl sulfate (SDS) gel electrophoresis. The α‐, β‐, and γ‐chain of fibrinogen are detected. Control samples without biotin‐PG (‐) give low background bands compared to samples where biotin‐PG was added (+).
FIGURE 2.
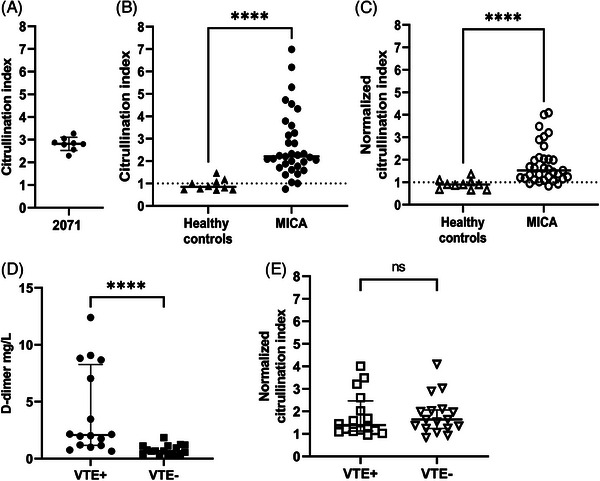
MICA patient plasma samples contain elevated levels of citrullinated fibrinogen. (A) Interassay control samples 2071 gives a mean citrullination index of 2.8 with a CV of 9,6%. (B) Fibrinogen citrullination index was determined for 10 healthy controls and the MICA patient cohort. For the MICA cohort a median index of 2.21 was determined, which is significantly higher than the median of 0.85 obtained for healthy controls (p < 0.001, Mann–Whitney U‐test). (C) Citrullination index values were normalized based on fibrinogen concentration. The normalized fibrinogen index (NCI) mean was determined at 1.53, which is significantly higher than the median of 0.9 obtained for normalized healthy controls (p < 0.001, Mann–Whitney U‐test). (D) D‐dimer levels are significantly higher for cancer patients who develop venous thromboembolism (VTE) (VTE+) compared to patients who do not develop VTE (VTE‐) (Mann–Whitney U test). (E) MICA patient citrullinated fibrinogen index does not differ between VTE+ patients and VTE‐patients (Mann–Whitney U‐test). (F) The normalized citrullinated fibrinogen index does not differ between VTE+ patients and VTE‐ patients (Mann–Whitney U‐test).
We subsequently investigated the citrullination index as a marker for cancer‐related thrombosis. Due to the limited size of this cohort we were unable to evaluate different tumor types (Figure S2C). As expected VTE+ patients had significantly higher D‐dimer levels than VTE‐ patients (p value < 0.0001, Mann–Whitney U‐test; Figure 2D). However, values of the normalized citrullination index obtained did not correlate with D‐dimer levels (Figure S2B). Figure 2E shows the normalized citrullination index of fibrinogen for samples derived from patients with and without VTE. Based on this, no significant difference in levels of citrullinated fibrinogen was detected for patients with or without VTE (p value = 0.65 respectively, Mann–Whitney U‐test). Therefore, although citrullinated fibrinogen has been proposed as a thrombotic risk marker [3], we did not find an association between citrullinated fibrinogen levels and cancer‐related VTE. The effect of increased citrullinated fibrinogen levels in cancer on clot morphology, structure and fibrinolysis needs to be assessed in a separate study. In summary, we have developed a novel assay that allows for quantitative analysis of citrullinated fibrinogen in patient plasma and can be easily modified for quantification of other proteins of interest. We are the first to show that plasma fibrinogen citrullination is significantly increased in cancer compared to healthy controls, which may be indicative of global plasma protein citrullination during cancer which may potentially promote cancer‐related thrombosis.
AUTHOR CONTRIBUTIONS
T.A. performed experiments and wrote the paper. V.Z. and N.S. designed experiments. N.v.E. and F.T.M.B. coordinated clinical samples. G.A.F.N. and J.V. provided supervision of the study. All authors participated in writing of the manuscript and approved the final version.
CONFLICT OF INTEREST STATEMENT
None of the authors have conflict of interest to report.
CLINICAL TRIAL REGISTRATION
The MICA study was registered at clinicaltrials.gov (identifier: 02095925) after the enrollment of the first patient.
PATIENT CONSENT STATEMENT
Patients included in the MICA study provided written informed consent in accordance with the Declaration of Helsinki. The MICA study was registered at clinicaltrials.gov (identifier: 02095925) after the enrollment of the first patient.
Supporting information
MICA citrullinated fibrinogen supplementary file
ACKNOWLEDGMENTS
The authors thank W. Kopatz, Department of Experimental Vascular Medicine, Amsterdam University Medical Center, for performing the fibrinogen antigen ELISA. T. A. is supported by a grant from the Dutch Thrombosis Society, (grant number: TSN2019.3).
Arfman T, Zollet V, van Es N, et al. Elevated levels of citrullinated fibrinogen in patients with cancer. eJHaem. 2024;5:136–140. 10.1002/jha2.825
DATA AVAILABILITY STATEMENT
Data, protocols, and materials reported in this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Won P, Kim Y, Jung H, Rim YA, Sohn DH, Robinson WH, et al. Pathogenic role of circulating citrullinated antigens and anti‐cyclic monoclonal citrullinated peptide antibodies in rheumatoid arthritis. Front Immunol. 2021;12:2389. 10.3389/FIMMU.2021.692242/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Spengler J, Lugonja B, Ytterberg AJ, Zubarev RA, Creese AJ, Pearson MJ, et al. Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis Rheumatol. 2015;67(12):3135–3145. 10.1002/ART.39313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bezuidenhout JA, Venter C, Roberts TJ, Tarr G, Kell DB, Pretorius E. Detection of citrullinated fibrin in plasma clots of rheumatoid arthritis patients and its relation to altered structural clot properties, disease‐related inflammation and prothrombotic tendency. Front Immunol. 2020;11:3182. 10.3389/FIMMU.2020.577523/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Damiana T, Damgaard D, Sidelmann JJ, Nielsen CH, De Maat MPM, Münster A‐MB, et al. Citrullination of fibrinogen by peptidylarginine deiminase 2 impairs fibrin clot structure. Clin Chim Acta. 2020;501:6–11. 10.1016/J.CCA.2019.10.033 [DOI] [PubMed] [Google Scholar]
- 5. Varjú I, Sorvillo N, Cherpokova D, Farkas ÁZ, Farkas VJ, Komorowicz E, et al. Citrullinated fibrinogen renders clots mechanically less stable, but lysis‐resistant. Circ Res. 2021;129(2):342–344. 10.1161/CIRCRESAHA.121.319061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Makrygiannakis D, Af Klint E, Lundberg IE, Lofberg R, Ulfgren A‐K, Klareskog L, et al. Citrullination is an inflammation‐dependent process. Ann Rheum Dis. 2006;65(9):1219. 10.1136/ARD.2005.049403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tilvawala R, Nemmara VV, Reyes AC, Sorvillo N, Salinger AJ, Cherpokova D, et al. The role of SERPIN citrullination in thrombosis. Cell Chem Biol. 2021;28:1728–1739.e5. 10.1016/j.chembiol.2021.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomassen M, Bouwens BRC, Wichapong K, Suylen DP, Bouwman FG, Hackeng TM, et al. Protein arginine deiminase 4 inactivates tissue factor pathway inhibitor‐alpha by enzymatic modification of functional arginine residues. J Thromb Haemost. 2023;21(5):1214–1226. 10.1016/J.JTHA.2023.01.017 [DOI] [PubMed] [Google Scholar]
- 9. Yuzhalin AE. Citrullination in cancer. Cancer Res. 2019;79(7):1274–1284. 10.1158/0008-5472.CAN-18-2797/661345/P/CITRULLINATION-IN-CANCERCITRULLINATION-IN-CANCER [DOI] [PubMed] [Google Scholar]
- 10. Mulder FI, Horváth‐Puhó E, van Es N, Van Laarhoven HWM, Pedersen L, Moik F, et al. Venous thromboembolism in cancer patients: a population‐based cohort study. Blood. 2021;137(14):1959–1969. 10.1182/BLOOD.2020007338 [DOI] [PubMed] [Google Scholar]
- 11. Clancy KW, Weerapana E, Thompson PR. Detection and identification of protein citrullination in complex biological systems. Curr Opin Chem Biol. 2016;30:1. 10.1016/J.CBPA.2015.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lewallen DM, Bicker KL, Subramanian V, Clancy KW, Slade DJ, Martell J, et al. Chemical proteomic platform to identify citrullinated proteins. ACS Chem Biol. 2015;10(11):2520–2528. 10.1021/ACSCHEMBIO.5B00438/SUPPL_FILE/CB5B00438_SI_002.XLSX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Es N, Di Nisio M, Cesarman G, Kleinjan A, Otten H‐M, Mahé I, et al. Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica. 2017;102(9):1494–1501. 10.3324/HAEMATOL.2017.169060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sorvillo N, De Moraes Mizurini D, Coxon C, Martinod K, Tilvawala R, Cherpokova D, et al. Plasma peptidylarginine deiminase IV promotes VWF‐platelet string formation and accelerates thrombosis after vessel injury HHS public access. Circ Res. 2019;125(5):507–519. 10.1161/CIRCRESAHA.118.314571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pabinger I, van Es N, Heinze G, Posch F, Riedl J, Reitter E‐M, et al. A clinical prediction model for cancer‐associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5(7):e289–e298. 10.1016/S2352-3026(18)30063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MICA citrullinated fibrinogen supplementary file
Data Availability Statement
Data, protocols, and materials reported in this study are available from the corresponding author upon reasonable request.


