Abstract
The Rho family GTP-binding proteins play a critical role in a variety of cytoskeleton-dependent cell functions. In this study, we examined the role of Rho family G proteins in muscle differentiation. Dominant negative forms of Rho family proteins and RhoGDI, a GDP dissociation inhibitor, suppressed transcription of muscle-specific genes, while mutationally activated forms of Rho family proteins strongly activated their transcription. C2C12 cells overexpressing RhoGDI (C2C12RhoGDI cells) did not differentiate into myotubes, and expression levels of myogenin, MRF4, and contractile protein genes but not MyoD and myf5 genes were markedly reduced in C2C12RhoGDI cells. The promoter activity of the myogenin gene was suppressed by dominant negative mutants of Rho family proteins and was reduced in C2C12RhoGDI cells. Expression of myocyte enhancer binding factor 2 (MEF2), which has been reported to be required for the expression of the myogenin gene, was reduced at the mRNA and protein levels in C2C12RhoGDI cells. These results suggest that the Rho family proteins play a critical role in muscle differentiation, possibly by regulating the expression of the myogenin and MEF2 genes.
Myogenic basic helix-loop-helix (bHLH) proteins are master regulatory proteins that activate the transcription of many muscle-specific genes during myogenesis (reviewed in references 60 and 89). Each of the four myogenic bHLH proteins, MyoD (17), myogenin (18, 90), myf5 (7), and MRF4 (8, 47, 67), can activate the skeletal myogenic program when introduced into a variety of cells derived from all three germ layers of the embryo. The bHLH motif mediates dimerization of myogenic factors with ubiquitous bHLH proteins such as E12/E47, and these heterodimeric complexes bind to a conserved DNA sequence known as the E box, which is present in the promoters and enhancers of most muscle-specific genes (54). Myocyte enhancer binding factor 2 (MEF2), which is a member of the MADS box family, also plays an important role in muscle differentiation (reviewed in reference 61). MEF2 activates transcription by binding to the consensus sequence, called the MEF2-binding site, which is also found in the control regions of numerous muscle-specific genes (23, 64). In embryos with loss-of-function mutations of the single mef2 gene in Drosophila (D-mef2), somatic, cardiac, and visceral muscle cells did not differentiate (6, 38, 66). These results indicated that MEF2 is necessary for the differentiation of all types of muscle cells. MEF2 and myogenic bHLH proteins have been suggested to activate mutual expression in an autoregulatory network and maintain the expression of muscle-specific genes (5, 16, 31, 43, 56). Moreover, a recent study demonstrated that MEF2 and myogenic bHLH proteins synergistically activate expressions of muscle-specific genes via protein-protein interactions between DNA-binding domains of these heterologous classes of transcription factors (51). In addition, it has been reported that a variety of factors, such as fibroblast growth factor, transforming growth factor β, and Ras, modulate muscle differentiation by regulating expression of myogenic bHLH proteins (9, 34, 36, 37).
The Rho family GTP-binding proteins (G proteins) consist of three subfamilies, Rho, Rac, and Cdc42 (25, 58, 77, 78). All members of the Rho family exhibit both GDP/GTP binding and GTPase activities; they are inactive when bound to GDP and active when bound to GTP (58). The GDP/GTP exchange reaction is regulated by guanine nucleotide exchange factors and GTPase-activating proteins. Among guanine nucleotide exchange factors, GDP dissociation stimulators catalyze the dissociation of GDP and convert Rho proteins into an active GTP-bound form, while RhoGDI, a GDP dissociation inhibitor, inhibits the dissociation of GDP (reviewed in references 58 and 78). The intrinsic GTPase activity of the GTP-bound form is stimulated by GTPase-activating proteins. The Rho family proteins have been shown to regulate a variety of cytoskeleton-dependent cell functions, such as cell morphology (11, 63, 71), formation of focal adhesions and stress fibers (63, 68, 75), cell motility (79, 80), platelet and lymphocyte aggregation (53, 85), growth factor- and phorbol ester-induced membrane rufflings (57, 69), contractile ring formation and cytokinesis (33, 39), smooth muscle contraction (29), cell cycle progression (91), neurite retraction (30), and bud formation in the yeast Saccharomyces cerevisiae (92). There is a regulatory cascade among the three subfamily members. Bradykinin stimulates the formation of filopodia by activating Cdc42 first, which is followed by the formation of lamelipodia induced by Rac activation, leading finally to the formation of stress fibers and focal contacts induced by Rho activation (35, 59). Recently, the Rho family of G proteins has also been shown to function in protein kinase cascades. Phosphatidylinositol 3-kinase (PI3K) has been reported to function upstream (27, 58, 69) and downstream (84, 94) of the Rho family proteins. It has been reported that Cdc42 and Rac proteins directly bind to and activate a family of highly related serine/threonine kinases referred to as p21-activated kinases (PAKs) (41, 42). They also regulate members of the mitogen-activated protein kinase (MAPK) family, such as c-Jun N-terminal kinase (JNK) and p38MAPK (4, 15, 46) and 70-kDa S6 kinase (13).
Recently, the Rho family of G proteins has been reported to be required for transcriptional activation of the c-fos serum response element (SRE) induced by extracellular signals and to activate its transcription via the MADS box-containing transcription factor serum response factor (SRF) (28). SRE in the promoter of the c-fos gene contains a core sequence known as the CArG box (48). The CArG box sequence is also found in the promoters of many muscle-specific genes and is essential for the expression of these genes (49, 50, 65). Although the precise mechanism by which the CArG box acts in the expression of these muscle-specific genes remains unknown, SRF has been shown to bind to the CArG box (81) and to be required for muscle differentiation (87). It has also been reported that SRF is necessary for the expression of some muscle-specific genes in concert with other myogenic factors (72) and that SRF physically interacts with myogenic bHLH proteins (24). In the present study, we examined the role of Rho family proteins in muscle differentiation. We show here that inhibition of Rho family functions suppresses transcription of muscle-specific genes irrespective of the existence of CArG box in their promoters. C2C12 cells overexpressing RhoGDI did not differentiate into myotubes, and expression levels of the myogenin, MRF4, and contractile protein genes but not the MyoD and myf5 genes were markedly reduced in the cell lines. The promoter activity of the myogenin gene was suppressed by dominant negative mutants of the Rho family and was reduced in RhoGDI-expressing cells. Expression of MEF2, which has been shown to be required for expression of the myogenin gene, was reduced at the mRNA and protein levels in those cells. These results suggest that the Rho family plays a critical role in muscle differentiation, possibly by regulating the expression of myogenin and MEF2.
MATERIALS AND METHODS
Cell culture and transfections.
C2C12 mouse myoblasts were maintained in Dulbecco’s minimum essential medium (DMEM) supplemented with 15% fetal bovine serum (FBS). Differentiation of C2C12 cells was induced by changing the culture medium from growth medium (GM) (DMEM with 15% FBS) to differentiation medium (DM) (DMEM with 0.1% FBS). To isolate stable transfectants, a 1.8-kb human RhoGDI cDNA (22) subcloned into the pMAM2-BSD vector (Kaken Pharmaceutical Co. Ltd., Tokyo, Japan) (32), which harbors a blasticidin S resistance gene and a dexamethasone (Dexa)-inducible mouse mammary tumor virus long terminal repeat-Rous sarcoma virus promoter, was transfected into C2C12 myoblasts by the calcium phosphate method. Permanently transfected cells were selected with 20 μg of blasticidin S per ml, and two independent cell lines designated C2C12RhoGDI-1 and -2 were isolated. Transient transfections were performed by the calcium phosphate method. At 36 h after transfection, culture media were changed from GM to DM and cells were incubated for an additional 36 h so that they fully differentiated into myocytes. Then cells were harvested and subjected to luciferase assays. In preliminary experiments, we cotransfected simian virus 40 (SV40)-driven β-galactosidase as an internal control. The relative promoter activity was not changed with or without the correction by the β-galactosidase activity. Thus, the luciferase activity in the same amount of cell extracts was measured and presented without the correction by the β-galactosidase activity. Western blot analysis revealed that various transfected proteins were expressed at almost the same levels from 2 days after transfection through at least 1 week.
Plasmids.
Expression vectors encoding RhoGDI and various mutants of RhoA, Rac1, and Cdc42 were provided by Y. Takai (22) and J. S. Gutkind (15), respectively. pMHC25, pTnT15, and pSRMyoDneo were provided by T. Endo (20). EMSV-Myo8 and MEF2C were gifts from E. Olson (18). pMSVmyf5 and pEMSVmyf6 were gifts from H. Arnold (7, 8). The myogenin promoter was from J. Schmidt (40). The reporter constructs of the skeletal α-actin promoter with various mutations were provided by M. D. Schneider (62). All plasmid DNAs were prepared by using Qiagen (Chatsworth, Calif.) plasmid DNA preparation kits.
Immunofluorescence.
To assess the myogenic conversion of C2C12 myoblasts to myotubes, cells were immunostained with a monoclonal antibody (MF20) against sarcomeric myosin heavy chain (MHC) as described previously (3). Fluorescein isothiocyanate-conjugated goat anti-mouse immunoglobulin G was used as the secondary antibody.
Northern blot analysis.
Stable transfectants were maintained in GM, and 1 μM Dexa was added to the culture medium 24 h before confluence. When cells were grown to 100% confluence, the culture medium was changed to DM with Dexa (1 μM), and cells were maintained under these conditions for 3 days. Total RNA was extracted by the acid guanidine method, and 10 μg of total RNA was loaded in each lane for Northern blot analysis. The following cDNA fragments were used as probes: the PstI fragment of pMHC25 containing the rat skeletal muscle MHC cDNA, the PstI fragment of pTnT15 containing the rat skeletal muscle troponin T (TnT) cDNA, the EcoRI fragment of pSRMyoDneo containing the MyoD cDNA (17), the EcoRI fragment of EMSV-Myo8 containing the murine myogenin cDNA (18), the EcoRI fragment of pMSVmyf5 containing human myf5 cDNA (7), and the EcoRI fragment of pEMSVmyf6 containing human MRF4 cDNA (8).
Gel mobility shift assay.
Nuclear extracts were prepared from C2C12 cells or C2C12RhoGDI cells as described previously (23). Ten micrograms of nuclear extracts was incubated with the 32P-labeled oligonucleotide probe corresponding to the MEF2 site of the myogenin promoter. The electrophoretic mobility shift assay was performed as described previously (23).
RESULTS
Rho family proteins are required for the transcription of muscle-specific genes.
To test whether Rho family G proteins are involved in transcription of muscle-specific genes, we first examined the role of Rho family proteins in the transcription of the skeletal α-actin gene, which depends on the CArG box (14). A luciferase reporter plasmid containing the skeletal α-actin promoter (bp −394 to +24) was transiently transfected into C2C12 myoblasts together with dominant interfering mutants of Rac1 (Rac1N17), RhoA (RhoAN17), and Cdc42 (Cdc42N17) (15) or the GDP dissociation inhibitor RhoGDI (22). At 36 h after induction of C2C12 cell differentiation by changing the culture medium from GM to DM, luciferase activities were measured. The transcriptional activity of the skeletal α-actin gene was much higher in differentiated myotubes than in undifferentiated myoblasts (Fig. 1A, bars 1 and 2) and was reduced by cotransfection of either dominant interfering plasmid and RhoGDI (Fig. 1A, bars 2 to 6). RhoGDI, which inhibits the functions of all Rho family members, most strongly inhibited the transcriptional activity of the skeletal α-actin gene during muscle differentiation (Fig. 1A, bar 6), and the inhibitory activity was strongest in Rac1N17 among the three dominant interfering mutants (Fig. 1A, bar 3). Transfection of these interfering mutants of the Rho family proteins and RhoGDI did not show such a strong inhibitory effect on transcription of nonmuscle gene promoters such as the SV40-derived promoter (Fig. 1A, bars 11 and 12). We next examined the effects of wild-type and mutationally activated forms of Rho family G proteins (wild-type Rac1, Rac1V12, RhoAV12, or Cdc42V12) (35, 68, 69) on the activity of the skeletal α-actin promoter (Fig. 1A, bars 7 to 10). Although overexpression of wild-type Rac1 had no significant effects on the activity of the skeletal α-actin promoter (Fig. 1A, bar 7), all constitutively active mutants of Rho family proteins activated the promoter to various degrees (Fig. 1A, bars 8 to 10). Rac1V12 most strongly activated the transcription, by more than 10-fold (Fig. 1A, bar 8).
FIG. 1.
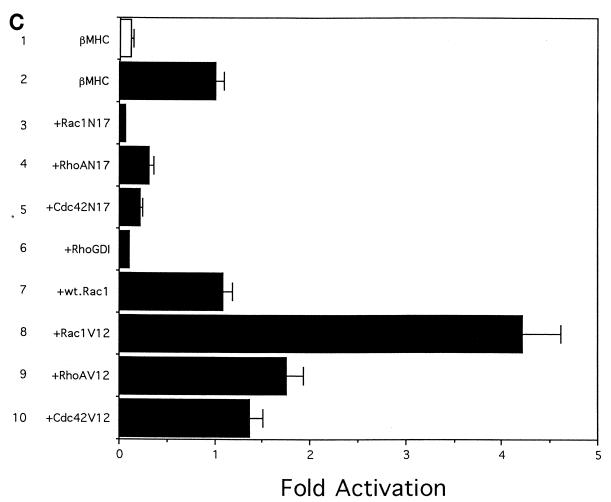
Rho family proteins are involved in transcriptional regulation of muscle-specific genes. Luciferase reporter plasmids containing the wild-type (wt) skeletal α-actin promoter (A), mutants of the skeletal α-actin promoter (B), or the βMHC promoter (C) were transiently transfected into C2C12 myoblasts by the calcium phosphate method with effector plasmids encoding dominant interfering mutants (Rac1N17, RhoAN17, or Cdc42N17) or constitutively active mutants (Rac1V12, RhoAV12, or Cdc42V12) of Rac1, RhoA, or Cdc42, RhoGDI, or wild-type Rac1. Cell differentiation was induced by changing the culture medium from GM to DM at 36 h after transfection. Cells were cultured for an additional 36 h, and luciferase activities were measured by using 10 μg of cell extracts. (A) Luciferase reporter plasmids containing the skeletal α-actin promoter (bars 1 to 10) and the SV40 promoter (bars 11 and 12) were transfected with various mutants of Rho proteins. (B) Luciferase reporter plasmids containing the wild-type skeletal α-actin promoter (bars 1 and 2) or the skeletal α-actin promoter with mutations in the CArG box (bars 3 and 4), the TEF-1 site (bars 5 and 6), the SP1 site (bars 7 and 8), or the TATA box (bars 9 and 10) were transfected with Rac1V12. (C) Luciferase reporter plasmids containing the βMHC promoter were transfected with various mutants of Rho proteins. Bars 1 in panels A and C represent the luciferase activity in undifferentiated myoblasts. Results are expressed relative to the levels of luciferase activity in cells transfected with vector alone (bars 2 and 11 in panel A, bar 1 in panel B, and bar lane 2 in panel C). The results are shown as means ± standard errors for four independent experiments.
To examine whether the regulation of the skeletal α-actin gene by Rho proteins requires the CArG box, luciferase reporter plasmids containing various mutations in the skeletal α-actin promoter were transiently cotransfected with Rac1V12, which activated the skeletal α-actin promoter most strongly among Rho family proteins (Fig. 1B). Mutations in the CArG box (62) more strongly reduced responsiveness to Rac1V12 as compared with mutations in the TEF-1 binding site or SP1 binding site (62) (Fig. 1B, bars 1 to 8). These results suggest that the CArG box plays an important role in Rho protein-induced activation of skeletal α-actin transcription. However, since there remains some responsiveness to Rac1V12 even in the CArG box mutant, there may be CArG box-dependent and -independent pathways in Rho protein-induced transactivation of the skeletal α-actin gene.
We next examined the role of Rho proteins in transcriptional regulation of the βMHC gene, whose transcription does not depend on the CArG box (83). When dominant interfering mutants of Rac1, RhoA, and Cdc42 or RhoGDI were overexpressed, luciferase activity of the rat βMHC promoter (bp −354 to +34) was also suppressed (Fig. 1C, bars 1 to 6). Among the interfering mutants, Rac1N17 showed the strongest inhibitory activity on the βMHC promoter (Fig. 1C, bar 3). Although wild-type Rac1 had no effects on the promoter activity of the βMHC gene (Fig. 1C, bar 7), constitutively active mutants of Rho proteins activated transcription of the βMHC gene to a lesser extent than that of the skeletal α-actin gene (Fig. 1C, bars 8 to 10). Rac1V12 most strongly (by ∼4-fold) among the three constitutively active mutants activated the expression of the βMHC gene. These results suggest that Rho proteins may regulate the expression of muscle-specific genes during muscle differentiation, regardless of the presence of the CArG box in their promoters.
Inhibition of Rho protein functions suppresses myogenesis.
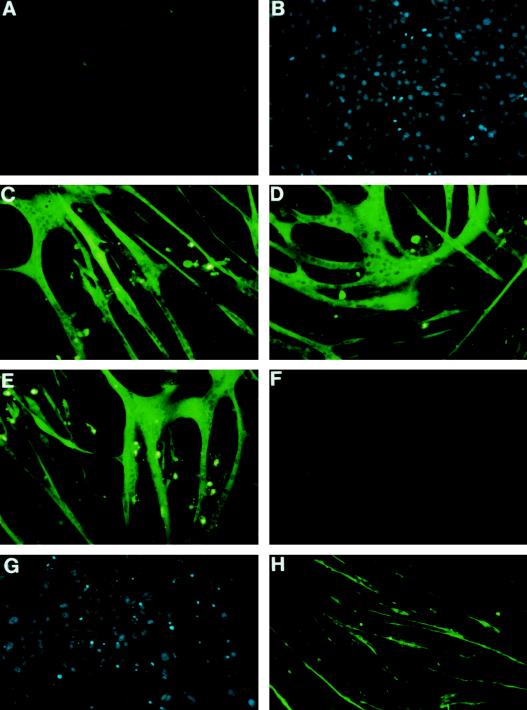
To determine whether Rho family G proteins are necessary for myogenesis, we isolated two independent C2C12 cell lines which were permanently transfected by human RhoGDI cDNA in a Dexa-inducible promoter vector and designated them C2C12RhoGDI-1 and -2 cells. When the culture medium was changed from GM to DM, C2C12 myoblasts differentiated into myocytes, and many anti-MHC antibody (MF20)-positive myotubes were formed (Fig. 2A to C). The addition of 1 μM Dexa to the culture medium had no effect on muscle differentiation in parental C2C12 cells (Fig. 2D). When cultured in DM, C2C12RhoGDI-1 cells also differentiated into myotubes as did parental C2C12 cells in the absence of Dexa (Fig. 2E). When expression of RhoGDI was induced by the addition of 1 μM Dexa, however, myogenesis was completely suppressed (Fig. 2F and G). There were no MF20-positive myotubes in C2C12RhoGDI-1 cells in the presence of Dexa. When C2C12 myoblasts were pretreated for 24 h before differentiation with the ADP-ribosylation exoenzyme C3 from Clostridium botulinum (C3 exoenzyme), which selectively ribosylates and inhibits functions of Rho protein but not of Rac or Cdc42 protein, C2C12 cells formed very thin myotubes that were stained faintly with MF20 (Fig. 2H). Similar results were obtained with C2C12RhoGDI-2 cells. These results suggest that Rho family proteins play a critical role in terminal muscle differentiation.
FIG. 2.
Inhibition of muscle differentiation by overexpression of RhoGDI. C2C12 cells (A, B, C, D, and H) or C2C12RhoGDI-1 cells (E, F, and G), which bear the Dexa-inducible RhoGDI, were cultured in GM (A and B) or in DM (C, D, E, F, G, and H). For differentiation, the culture medium was changed from GM to DM after confluency, and the cells were cultured for additional 3 days. A separate set of cultures was treated identically but was exposed to 1 μM Dexa (D and F) or to 10 μg of C. botulinum ADP-ribosyltransferase C3 (H) from 24 h before the culture medium was changed. The cells were stained with anti-sarcomeric MHC antibody (MF20) (A, C, D, E, F, and H) or Hoechst dye (B and G).
Expression of muscle-specific genes in C2C12RhoGDI cells.
To elucidate the mechanism of how Rho family proteins are involved in muscle differentiation, expression of muscle-specific genes was examined by Northern blot analysis. When C2C12 myoblasts were induced to differentiate into myotubes by serum deprivation, expression of myogenic bHLH protein genes such as the myogenin and MRF4 genes and of muscle-specific contractile protein genes such as the MHC and TnT genes was induced (Fig. 3, lanes 1 and 2). Transcripts of myf5 and MyoD were detected in both myoblasts and myotubes, as reported previously (7, 17). The addition of Dexa to parental C2C12 cells did not affect the expression levels of any of these genes (Fig. 3, lanes 2 and 3). When induced to differentiate, C2C12RhoGDI-1 cells also expressed these muscle-specific genes as abundantly as parental C2C12 cells in the absence of Dexa (Fig. 3, lanes 2 and 5). When expression of RhoGDI was induced by Dexa, although the mRNA levels of MyoD and myf5 were not changed, induction of the myogenin and MRF4 genes was markedly suppressed (Fig. 3, lanes 5 and 6). All contractile protein genes were also expressed at very low levels in C2C12RhoGDI-1 cells in the presence of Dexa. Similar expression patterns were obtained with C2C12RhoGDI-2 cells. Since myogenin and MRF4 have been reported to play important roles in terminal muscle differentiation, we speculate that Rho family proteins are involved in muscle differentiation, possibly by regulating expression of these myogenic genes.
FIG. 3.
RhoGDI inhibits induction of myogenin and muscle-specific genes. RNA was prepared from C2C12 cells and C2C12RhoGDI cells cultured in GM or in DM for 3 days. Ten micrograms of RNA from each sample was subjected to Northern blot analysis. Ethidium bromide staining of rRNA is presented at the bottom to show that same amount of intact RNA was loaded in each lane.
Rho family proteins are required for the transcription of the myogenin gene during myogenesis.
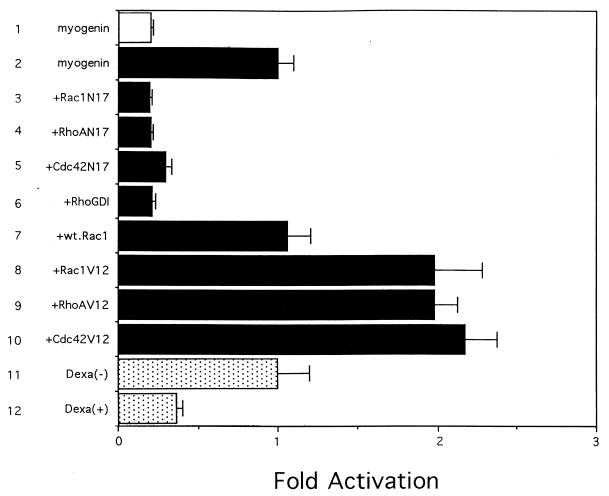
Among myogenic bHLH genes, induction of the myogenin and MRF4 genes was inhibited during muscle differentiation by overexpressing RhoGDI. During mouse development, each myogenic bHLH factor is expressed in a precise temporal and spatial pattern to give rise to muscle. myf5 is the first of the bHLH factors to be expressed during myogenesis; this is followed shortly thereafter by the expression of MyoD and myogenin and finally by that of MRF4 (73, 89). Myogenin has been shown to activate the MRF4 promoter during myogenesis (5, 56), and knockout experiments have clearly demonstrated that myogenin is required for differentiation from myoblasts to myocytes (26, 55). These previous observations suggest that reduced myogenin gene expression could result in low MRF4 and contractile protein mRNA levels and failure in myogenesis. We therefore examined the transcriptional activity of the myogenin gene in C2C12RhoGDI cells. A reporter gene containing the myogenin promoter (bp −222 to +40), which can confer its muscle-specific expression (40), was transfected together with the expression plasmid encoding either RhoGDI or dominant interfering or constitutively active mutants of the Rho family proteins. The activity of the myogenin promoter was upregulated during muscle differentiation (Fig. 4, bars 1 and 2) and was strongly suppressed by RhoGDI or dominant interfering mutants of Rho family proteins (Fig. 4, bars 3 to 6). The promoter activity was, conversely, upregulated by the activated mutants of Rho family proteins (Fig. 4, bars 8 to 10). In C2C12RhoGDI cells, the luciferase activity of the reporter gene increased during muscle differentiation in the absence of Dexa but was markedly suppressed when expression of RhoGDI was induced by Dexa (Fig. 4, bars 11 and 12). These results suggest that Rho family G proteins are critically involved in the transcription of the myogenin gene.
FIG. 4.
Rho family proteins regulate the transcription of the myogenin gene. The luciferase reporter gene containing the myogenin promoter was transiently transfected into C2C12 cells together with effector plasmids encoding dominant interfering mutants, RhoGDI, wild-type (wt) Rac1, or constitutively active mutants of Rac1, RhoA, and Cdc42 (bars 1 to 10). The reporter plasmids were also transiently transfected into C2C12RhoGDI cells in the absence (bar 11) or presence (bar 12) of 1 μM Dexa. The culture medium was changed from GM to DM at 36 h after transfection, and luciferase activity was determined 36 h later by using cell extracts prepared from myoblasts (bar 1) or myocytes (bars 2 to 12). The results are expressed relative to the luciferase activity in cells cotransfected with the vehicle vector (bar 2) or C2C12RhoGDI cells in the absence of Dexa (bar 11). The results are shown as means ± standard errors for four independent experiments.
Rho family proteins are involved in transcription of the myogenin gene through MEF2.
We next examined how the Rho family is involved in the transcription of the myogenin gene. It has been demonstrated that an E box and a MEF2-binding site in the myogenin promoter are required for the transcription of the myogenin gene (10, 12, 19, 40, 93). Because the E box-binding proteins myf5 and MyoD, which are thought to be involved in the regulation of myogenin gene expression during terminal muscle differentiation, were expressed in cells overexpressing RhoGDI as abundantly as in parental cells (Fig. 3), we focused on MEF2 proteins. Electrophoretic mobility shift assay revealed that the level of proteins bound to the MEF2 site of the myogenin promoter was much less in cells overexpressing RhoGDI than in C2C12 cells and C2C12RhoGDI cells without Dexa (Fig. 5A). Northern blot analysis showed that the expression of MEF2C was induced in both parental and C2C12RhoGDI cells without Dexa by serum deprivation (Fig. 5B, lanes 2, 3, and 5). When Dexa was added to the culture medium, expression levels of MEF2C were markedly reduced in C2C12RhoGDI cells (Fig. 5B, lane 6). These results suggest that low expression levels of the myogenin gene may be due to reduced expression of the MEF2 gene in C2C12RhoGDI cells.
FIG. 5.
Rho family proteins regulate the expression of the MEF2 protein. (A) The MEF2 DNA binding activity was determined by electrophoretic mobility shift assay as described in Materials and Methods. Nuclear extracts were prepared from C2C12 cells (lanes 1 and 2) or C2C12RhoGDI cells (lanes 3 and 4) after inducing differentiation into myotubes in the absence (lanes 1 and 3) or presence (lanes 2 and 4) of 1 μM Dexa. Equal amounts (10 μg) of nuclear extracts were incubated with radiolabeled myogenin MEF2-binding site and electrophoresed in Tris-acetate-buffered gels. (B) RNA was prepared from C2C12 cells (lanes 1 to 3) and C2C12RhoGDI cells (lanes 4 to 6) cultured in GM (lanes 1 and 4) or in DM (lanes 2, 3, 5, and 6) for 3 days. Ten micrograms of RNA from each sample was subjected to Northern blot analysis. Human MEF2C cDNA was used as a probe.
DISCUSSION
The Rho family of GTP-binding proteins consists of the Rho, Rac, and Cdc42 subfamilies and has been demonstrated to regulate numerous aspects of cytoskeleton function (25, 58, 77, 78). Recently it has been reported that Rho family proteins also play a critical role in transcriptional regulation of the c-fos gene by modulating the transcription factor SRF (28). Since SRF also binds to the CArG box, which is a critical cis element in the promoters of many muscle-specific genes (49, 50, 65, 81, 87), we examined whether the Rho family plays an important role in the expression of muscle-specific genes. Since the skeletal α-actin promoter with mutations in the CArG box showed less transactivation by constitutively activated mutants of the Rho family proteins than did the wild-type promoter (Fig. 1B) and since effects of Rho proteins were more prominent in the CArG box-containing skeletal α-actin promoter than the CArG box-less βMHC promoter (Fig. 1A and C), the CArG box may play an important role in Rho-induced gene expression. However, since Rho proteins have some effects on the skeletal α-actin promoter with mutations in the CArG box (Fig. 1B) and on βMHC genes which have no CArG box in their promoters (Fig. 1C), Rho family proteins are involved in muscle gene expression through CArG box-dependent and -independent mechanisms. Since neither RhoGDI nor dominant negative mutants of the Rho family showed such a strong inhibitory activity on the promoter of SV40 (Fig. 1A) or thymidine kinase (data not shown), the inhibitory effects of these molecules were considered specific to muscle genes, unlike dominant negative Ras mutants (1). We isolated C2C12 cell lines which were permanently transfected with RhoGDI in an inducible promoter vector. Two independent cell lines, C2C12RhoGDI-1 and -2 cells, differentiated into myotubes like wild-type parental C2C12 cells in the absence of Dexa. When RhoGDI was induced by Dexa, however, C2C12RhoGDI cells did not differentiate into myotubes. Since Dexa itself did not have any effects on differentiation of parental cells, the inhibition of myogenesis in C2C12RhoGDI cells in the presence of Dexa should be due to overexpression of RhoGDI. These results strongly suggest that Rho family G proteins play a critical role in muscle differentiation. There is a study suggesting that Rho family proteins are required for epidermal cell differentiation (76).
Although inhibition of muscle differentiation was not complete as in the case of cells overexpressing RhoGDI, C3 exoenzyme also inhibited muscle differentiation (Fig. 2H). The different effects of C3 exoenzyme and RhoGDI may come from the specificities of the molecules. C3 exoenzyme selectively inhibits the functions of Rho protein but not those of Rac or Cdc42 protein, while RhoGDI suppressed the functions of all three Rho family proteins. Although there were differences in inhibitory activity among dominant interfering mutants of the three Rho family members (Rac1N17, RhoAN17, and Cdc42N17), all of the mutants strongly suppressed the transcription of muscle-specific genes. These results suggest that each member of the Rho family proteins may be involved in the transcription of muscle-specific genes or that there may be a hierarchy among the three subfamilies of Rho proteins and muscle gene transcription may be activated by the cascade of these Rho subfamilies (35, 59). It remains to be determined how each of these Rho family proteins is involved in the regulation of muscle differentiation.
The myogenic bHLH proteins MyoD, myf5, myogenin, and MRF4 are master regulatory proteins and regulate the transcription of all muscle-specific genes during muscle differentiation (60, 89). Each bHLH protein shows auto- and cross-activation of each protein (7, 18, 47, 67, 82, 89). Although all four bHLH proteins have many common features, the temporal and spatial expression patterns of individual genes are different (73). It has been reported that both the MyoD and myf5 genes are expressed in myoblasts as well as in myotubes, while myogenin and MRF4 are not expressed in undifferentiated myoblasts and their expression was induced during terminal differentiation (7, 17, 18, 47, 67, 73, 82, 90). Evidence provided from mouse mutants carrying an inactivated myogenin gene suggests that the expression of myogenin leads to overt terminal muscle differentiation (12, 55). In cells overexpressing RhoGDI, MyoD and myf5 were expressed as abundantly as in parental C2C12 cells but expression levels of myogenin and MRF4 were very low, suggesting that inhibition of Rho family functions did not change the state of committed myoblasts but inhibited terminal differentiation. Since MRF4 functions downstream of the other myogenic bHLH factors (5, 8, 47, 67) and since myogenin and MEF2 synergistically activated the MRF4 promoter during myogenesis (5, 56), we speculated that a lack of an increase in expression of the myogenin gene, but not the MRF4 gene, in C2C12RhoGDI cells may be a primary cause for failure of myogenesis. The transcriptional activity of the myogenin gene was low in cells overexpressing RhoGDI and was suppressed by cotransfection with dominant interfering mutants of Rho family proteins. It has been reported that transcription of the myogenin gene is regulated by bHLH proteins and preexisting MEF2 (10, 12, 19, 40, 93). Since a recent study demonstrated that MEF2 and myogenic bHLH proteins synergistically activate muscle gene expression via protein-protein interactions between the DNA-binding domains of these heterologous classes of transcription factors, it is difficult to dissect the roles of two different transcription factors in the activation of the myogenin gene. However, because MyoD and myf5, which might regulate myogenin gene expression during terminal muscle differentiation, were expressed abundantly in C2C12RhoGDI cells, we postulated that loss of the upregulation of myogenin gene expression after serum deprivation may be due to low expression of MEF2 proteins but not of bHLH proteins.
The results of Northern blot analysis and electrophoretic mobility shift assay showed that the expression of MEF2 was reduced in C2C12RhoGDI cells. Although the regulatory mechanism of MEF2 gene transcription has not been clarified as yet, overexpression of bHLH proteins was reported to induce the expression of MEF2 in fibroblasts (16, 43). Thus, there seems to be a cross-activating loop between bHLH proteins and MEF2. The molecular mechanism involved in the reduced expression of MEF2 in C2C12RhoGDI cells is unknown at present. Recently, many target proteins of the Rho family have been identified, and Rho family proteins have been shown to participate in the protein kinase cascade. Rac1 and Cdc42 activate serine/threonine kinase PAKp65 (41, 42), and the p85 subunit of PI3K is also directly associated with activated Rac and Cdc42 (84, 94). Rho binds to and activates protein kinase N (2, 88) and a novel serine/threonine kinase, Rho kinase (44). Rac1 and Cdc42 have also been reported to activate JNK and the 70-kDa S6 kinase (13, 15, 46). It has been reported that phosphorylation of the serine residue located between the MADS and MEF2 domains of the MEF2 protein enhances its DNA binding activity and transcriptional activity (52). These observations suggest that Rho family proteins may be involved in the increase of the binding activity of MEF2 to the myogenin promoter by phosphorylation. Upregulation of myogenin gene expression may in turn increase MEF2 expression. There have also been many reports suggesting that occupation of the extracellular matrix receptor, integrin, is required for terminal muscle differentiation (21, 45, 70, 74). A recent report has suggested that Rho family proteins work as mediators of integrin signaling (86). Taking these observations and our results together, it is tempting for us to speculate that integrin regulates muscle differentiation via the Rho family proteins. How Rho family proteins regulate the expression of MEF2 and myogenin and how the members of this family interact with each other during muscle differentiation remain to be clarified.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
We thank Y. Takai, J. S. Gutkind, T. Endo, J. Schmidt, E. N. Olson, M. D. Schneider, and H. H. Arnold for providing plasmids and S. Narumiya for providing C3 exoenzyme.
This study was supported by a grant-in-aid for scientific research and developmental science research from the Ministry of Education, Science and Culture; a grant from Pfeizer Pharmaceutical Co. Ltd.; and a grant from Tanabe Medical Frontier (all to I.K.).
REFERENCES
- 1.Abdellatif M, MacLellan W R, Schneider M D. p21 Ras as a governor of global gene expression. J Biol Chem. 1994;269:15423–15426. [PubMed] [Google Scholar]
- 2.Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for Rho as the serine-threonine kinase protein kinase N. Science. 1996;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- 3.Bader D, Masaki T, Fischman D A. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagrodia S, Derijard B, Davis R J, Cerione R A. Cdc42 and PAK-mediated signaling leads to Jun kinase and p38 mitogen-activated protein kinase activation. J Biol Chem. 1995;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- 5.Black B L, Martin J F, Olson E N. The mouse MRF4 promoter is trans-activated directly and indirectly by muscle-specific transcription factors. J Biol Chem. 1995;270:2889–2892. doi: 10.1074/jbc.270.7.2889. [DOI] [PubMed] [Google Scholar]
- 6.Bour B A, O’Brien M A, Lockwood W L, Goldstein E S, Bodmer R, Taghert P H, Abmayr S M, Nguyen H T. Drosophila MEF2, a transcription factor that is essential for myogenesis. Genes Dev. 1995;9:730–741. doi: 10.1101/gad.9.6.730. [DOI] [PubMed] [Google Scholar]
- 7.Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold H H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989;8:701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun T, Bober E, Winter N, Rosenthal R, Arnold H H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome. EMBO J. 1990;9:821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brennan T J, Edmondson D G, Li L, Olson E N. Transforming growth factor β represses the actions of myogenin through a mechanism independent of DNA binding. Proc Natl Acad Sci USA. 1991;88:3822–3826. doi: 10.1073/pnas.88.9.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchberger A, Ragge K, Arnold H H. The myogenin gene is activated during myocyte differentiation by pre-existing, not newly synthesized transcription factor MEF-2. J Biol Chem. 1994;269:17289–17296. [PubMed] [Google Scholar]
- 11.Chardin P, Boquet P, Maduale P, Popoff M R, Rubin E J, Gill D M. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng T-C, Wallace M C, Merlie J P, Olson E N. Separable regulatory elements governing myogenin transcription in mouse embryogenesis. Science. 1993;261:215–218. doi: 10.1126/science.8392225. [DOI] [PubMed] [Google Scholar]
- 13.Chou M M, Blenis J. The 70 kDa S6 kinase complexes with and is activated by the Rho family G proteins Cdc42 and Rac1. Cell. 1996;85:573–583. doi: 10.1016/s0092-8674(00)81257-x. [DOI] [PubMed] [Google Scholar]
- 14.Chow K L, Schwartz R J. A combination of closely associated positive and negative cis-acting promoter elements regulates transcription of the skeletal alpha-actin gene. Mol Cell Biol. 1990;10:528–538. doi: 10.1128/mcb.10.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coso O A, Chiariello M, Yu J-C, Teramoto H, Crespo P, Xu N, Miki T, Gutkind J S. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- 16.Cserjesi P, Olson E N. Myogenin induces muscle-specific enhancer binding factor MEF-2 independently of other muscle-specific gene products. Mol Cell Biol. 1991;11:4854–4862. doi: 10.1128/mcb.11.10.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis R L, Weintraub H, Lassar A B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- 18.Edmondson D G, Olson E N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- 19.Edmondson D G, Cheng T-C, Cserjesi P, Chakraborty T, Olson E N. Analysis of the myogenin promoter reveals an indirect pathway for positive autoregulation mediated by the muscle-specific enhancer factor MEF-2. Mol Cell Biol. 1992;12:3665–3677. doi: 10.1128/mcb.12.9.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Endo T. SV40 large T inhibits myogenic differentiation partially through inducing c-jun. J Biochem. 1992;112:321–329. doi: 10.1093/oxfordjournals.jbchem.a123899. [DOI] [PubMed] [Google Scholar]
- 21.Enomoto M I, Boettiger D, Menko A S. Alpha 5 integrin is a critical component of adhesion plaques in myogenesis. Dev Biol. 1993;155:180–197. doi: 10.1006/dbio.1993.1017. [DOI] [PubMed] [Google Scholar]
- 22.Fukumoto Y, Kaibuchi K, Hori Y, Fujioka H, Araki S, Ueda T, Kikuchi A, Takai Y. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for the rho proteins, ras p21-like small GTP-binding proteins. Oncogene. 1990;5:1321–1328. [PubMed] [Google Scholar]
- 23.Gossett L A, Kelvin D J, Sternberg E A, Olson E N. A new myocyte-specific enhancer-binding factor that recognizes a conserved element associated with multiple muscle-specific genes. Mol Cell Biol. 1989;9:5022–5033. doi: 10.1128/mcb.9.11.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Groisman R, Masutani H, Leibovitch M P, Robin P, Soudant I, Trouche D, Harel-Bellan A. Physical interaction between the mitogen-responsive serum response factor and myogenic basic-helix-loop-helix proteins. J Biol Chem. 1996;271:5258–5264. doi: 10.1074/jbc.271.9.5258. [DOI] [PubMed] [Google Scholar]
- 25.Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- 26.Hasty P, Bradley A, Morris J H, Edmondson D G, Venuti J M, Olson E N, Klein W H. Muscle deficiency and neonatal death in mice with a targetted mutation in the myogenin gene. Nature. 1993;364:501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins P T, Eguinoa A, Qiu R G, Stokoe D, Cooke F T, Walters R, Wennstorm S, Claesson W L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in Rac-GTP via the activation of phophoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- 28.Hill C S, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 29.Hirata K, Kikuchi A, Sasaki T, Kuroda S, Kaibuchi K, Matsuura Y, Seki H, Saida K, Takai Y. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem. 1992;267:8719–8722. [PubMed] [Google Scholar]
- 30.Jalink K, van Corven E J, Hengeveld T, Morii N, Narumiya S, Moolenaar W H. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP-ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaushal S, Schneider J W, Nadal-Ginard B, Mahdavi V. Activation of the myogenic lineage by MEF2A, a factor that induces and cooperates with MyoD. Science. 1994;266:1236–1240. doi: 10.1126/science.7973707. [DOI] [PubMed] [Google Scholar]
- 32.Kimura M, Takatsuki A, Yamaguchi I. Blasticidin S deaminase gene from Aspergillus terreus (BSD): a new drug resistance gene for transfection of mammalian cells. Biochim Biophys Acta. 1994;1219:653–659. doi: 10.1016/0167-4781(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 33.Kishi K, Sasaki T, Kuroda S, Itoh T, Takai Y. Regulation of cytoplasmic division of Xenopus embryo by rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) J Cell Biol. 1993;120:1187–1195. doi: 10.1083/jcb.120.5.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong Y, Johnson S E, Taparowsky E J, Konieczny S F. Ras p21Val inhibits myogenesis without altering the DNA binding or transcriptional activities of the myogenic basic helix-loop-helix factors. Mol Cell Biol. 1995;15:5205–5213. doi: 10.1128/mcb.15.10.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lassar A B, Thayer M J, Overell R W, Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell. 1989;58:659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Zhou J, James G, Heller-Harrison R, Czech M P, Olson E N. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992;71:1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- 38.Lilly B, Zhao B, Ranganayakulu G, Paterson B M, Schulz R A, Olson E N. Requirement of MADS domain transcription factor D-MEF2 for muscle formation in Drosophila. Science. 1995;267:688–693. doi: 10.1126/science.7839146. [DOI] [PubMed] [Google Scholar]
- 39.Mabuchi I, Hamaguchi Y, Fujimoto H, Morii N, Mishima M, Narumiya S. A rho-like protein is involved in the organisation of the contractile ring in dividing sand dollar eggs. Zygote. 1993;1:325–331. doi: 10.1017/s0967199400001659. [DOI] [PubMed] [Google Scholar]
- 40.Malik S, Huang C-F, Schmidt J. The role of the CANNTG promoter element (E box) and the myocyte-enhancer-binding-factor-2 (MEF-2) site in the transcriptional regulation of the chick myogenin gene. Eur J Biochem. 1995;230:88–96. doi: 10.1111/j.1432-1033.1995.tb20537.x. [DOI] [PubMed] [Google Scholar]
- 41.Manser E, Leung T, Salihuddin H, Zhao Z S, Lim L. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature. 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- 42.Martin G A, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin J F, Schwarz J J, Olson E N. Myocyte enhancer factor (MEF) 2C: a tissue-restricted member of the MEF-2 family of transcription factors. Proc Natl Acad Sci USA. 1993;90:5282–5286. doi: 10.1073/pnas.90.11.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP binding protein Rho. EMBO J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 45.Menko A S, Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- 46.Minden A, Lin A, Claret F-X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 47.Miner J H, Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci USA. 1990;87:1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Minty A, Kedes L. Upstream regions of the human cardiac actin gene that modulate its transcription in muscle cells: presence of an evolutionarily conserved repeated motif. Mol Cell Biol. 1986;6:2125–2136. doi: 10.1128/mcb.6.6.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miwa T, Kedes L. Duplicated CArG box domains have positive and mutually dependent regulatory roles in expression of the human α-cardiac actin gene. Mol Cell Biol. 1987;7:2803–2813. doi: 10.1128/mcb.7.8.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mohun T J, Taylor M V, Garrett N, Gurdon J B. The CArG promoter sequence is necessary for muscle-specific transcription of the cardiac actin gene in Xenopus embryos. EMBO J. 1989;8:1153–1161. doi: 10.1002/j.1460-2075.1989.tb03486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molkentin J D, Black B L, Martin J F, Olson E N. Cooperative activation of muscle gene expression by MEF2 and myogenic bHLH proteins. Cell. 1995;83:1125–1136. doi: 10.1016/0092-8674(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 52.Molkentin J D, Li L, Olson E N. Phosphorylation of the MADS-box transcription factor MEF2C enhances its DNA binding activity. J Biol Chem. 1996;271:17199–17204. doi: 10.1074/jbc.271.29.17199. [DOI] [PubMed] [Google Scholar]
- 53.Morii N, Teru-uchi T, Tominaga T, Kumagai N, Kozaki S, Ushikubi F, Narumiya S. A rho gene product in human blood platelets. II. Effects of the ADP-ribosylation by botulinum C3 ADP-ribosyltransferase on platelet aggregation. J Biol Chem. 1992;267:20921–20926. [PubMed] [Google Scholar]
- 54.Murre C, McCaw P S, Vaessin H, Caudy M, Jan L Y, Yan J N, Cabrera C V, Buskin J N, Hauschka S D, Lassar A B, Weintraub H, Baltimore D. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989;58:537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- 55.Nabeshima Y, Hanaoka K, Hayasaka M, Esumi E, Li S, Nonaka I, Nabeshima Y-I. Myogenin gene disruption results in perinatal lethality because of severe muscle defect. Nature. 1993;364:532–535. doi: 10.1038/364532a0. [DOI] [PubMed] [Google Scholar]
- 56.Naidu P S, Ludolph D C, To R Q, Hinterberger T J, Konieczny S F. Myogenin and MEF2 function synergistically to activate the MRF4 promoter during myogenesis. Mol Cell Biol. 1995;15:2707–2718. doi: 10.1128/mcb.15.5.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishiyama T, Sasaki T, Takaishi K, Kato M, Yaku H, Araki K, Matsuura Y, Takai Y. rac p21 is involved in insulin-induced membrane ruffling and rho p21 is involved in hepatocyte growth factor- and 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced membrane ruffling in KB cells. Mol Cell Biol. 1994;14:2447–2456. doi: 10.1128/mcb.14.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 59.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 60.Olson E N. The MyoD family, a paradigm for development? Genes Dev. 1990;4:1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- 61.Olson E N, Perry M, Schulz R A. Regulation of muscle differentiation by the MEF2 family of MADS box transcription factors. Dev Biol. 1995;172:2–14. doi: 10.1006/dbio.1995.0002. [DOI] [PubMed] [Google Scholar]
- 62.Paradis P, MacLellan W R, Belaguli N S, Schwartz R J, Schneider M D. Serum response factor mediates AP-1-dependent induction of the skeletal α-actin promoter in ventricular myocytes. J Biol Chem. 1996;271:10827–10833. doi: 10.1074/jbc.271.18.10827. [DOI] [PubMed] [Google Scholar]
- 63.Paterson H F, Self A J, Garrett M D, Just I, Aktories K, Hall A. Microinjection of recombinant p21rho induces rapid changes in cell morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pollock R, Treisman R. Human SRF-related proteins: DNA-binding properties and potential regulatory targets. Genes Dev. 1991;5:2327–2341. doi: 10.1101/gad.5.12a.2327. [DOI] [PubMed] [Google Scholar]
- 65.Quitschke W W, DePonti-Zilli L, Lin Z Y, Paterson B M. Identification of two nuclear factor-binding domains on the chicken cardiac actin promoter: implications for regulation of the gene. Mol Cell Biol. 1989;9:3218–3230. doi: 10.1128/mcb.9.8.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranganayakula G, Zhao B, Dokidis A, Molkentin J D, Olson E N. A series of mutations in the D-MEF2 transcription factor reveal multiple functions in larval and adult myogenesis in Drosophila. Dev Biol. 1995;171:169–181. doi: 10.1006/dbio.1995.1269. [DOI] [PubMed] [Google Scholar]
- 67.Rhodes S J, Konieczny S F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- 68.Ridley A J, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- 69.Ridley A J, Paterson H F, Johnston C L, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 70.Rosen G D, Sanes J R, LaChance R, Cunningham J M, Roman J, Dean D C. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69:1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- 71.Rubin E J, Gill D M, Boquet P, Popoff M R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988;8:418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sartorelli V, Webster K A, Kedes L. Muscle-specific expression of the cardiac α-actin gene requires MyoD1, CArG-box binding factor, and Sp1. Genes Dev. 1990;4:1811–1822. doi: 10.1101/gad.4.10.1811. [DOI] [PubMed] [Google Scholar]
- 73.Sassoon D A. Myogenic regulatory factors: dissecting their role and regulation during vertebrate embryogenesis. Dev Biol. 1993;156:11–23. doi: 10.1006/dbio.1993.1055. [DOI] [PubMed] [Google Scholar]
- 74.Sastry S K, Lakonishok M, Thomas D A, Muschler J, Horwitz A F. Integrin alpha subunit ratios, cytoplasmic domains, and growth factor synergy regulate muscle proliferation and differentiation. J Cell Biol. 1996;133:169–184. doi: 10.1083/jcb.133.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Self A J, Paterson H F, Hall A. Different structural organization of Ras and Rho effector domains. Oncogene. 1993;8:655–661. [PubMed] [Google Scholar]
- 76.Sugai M, Hashimoto K, Kikuchi A, Inoue S, Okumura H, Matsumoto K, Goto Y, Ohgai H, Moriishi K, Syuto B, Yoshikawa K, Suginaka H, Takai Y. Epidermal cell differentiation inhibitor ADP-ribosylates small GTP-binding proteins and induces hyperplasia of epidermis. J Biol Chem. 1992;267:2600–2604. [PubMed] [Google Scholar]
- 77.Takai Y, Kaibuchi K, Kikuchi A, Kawata M. Small GTP-binding proteins. Int Rev Cytol. 1992;133:187–230. doi: 10.1016/s0074-7696(08)61861-6. [DOI] [PubMed] [Google Scholar]
- 78.Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- 79.Takaishi K, Kikuchi A, Kuroda K, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13:72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9:273–279. [PubMed] [Google Scholar]
- 81.Taylor M, Treisman R, Garrett N, Mohun T. Muscle-specific (CArG) and serum responsive (SRE) promoter elements are functionally interchangeable in Xenopus embryos and mouse fibroblasts. Development. 1989;106:67–78. doi: 10.1242/dev.106.1.67. [DOI] [PubMed] [Google Scholar]
- 82.Thayer M J, Tapscott S J, Davis R L, Wright W E, Lassar A B, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58:241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- 83.Thompson W R, Nadal-Ginard B, Mahdavi V. A MyoD1-independent muscle specific enhancer controls the expression of the β-myosin heavy chain gene in skeletal and cardiac muscle cells. J Biol Chem. 1991;266:22678–22688. [PubMed] [Google Scholar]
- 84.Tolias K F, Cantley L C, Carpenter C L. Rho family GTPases bind to phosphoinositide kinases. J Biol Chem. 1995;270:17656–17659. doi: 10.1074/jbc.270.30.17656. [DOI] [PubMed] [Google Scholar]
- 85.Tominaga T, Sugie K, Hirata M, Morii N, Fukata J, Uchida A, Imura H, Narumiya S. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP-ribosylation of the small molecular weight GTP-binding protein, rho. J Cell Biol. 1993;120:1529–1537. doi: 10.1083/jcb.120.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Udagawa T, McIntyre B W. ADP-ribosylation of the G protein Rho inhibits integrin regulation of tumor cell growth. J Biol Chem. 1996;271:12542–12548. doi: 10.1074/jbc.271.21.12542. [DOI] [PubMed] [Google Scholar]
- 87.Vandromme M, Gauthier-Rouviere C, Carnac G, Lamb N, Fernandez A. Serum response factor p67SRF is expressed and required during myogenic differentiation of both mouse C2 and rat L6 muscle cell lines. J Cell Biol. 1992;118:1489–1500. doi: 10.1083/jcb.118.6.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein Rhophilin as targets of small GTPase Rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- 89.Weintraub H. The MyoD family and myogenesis: redundancy, networks, and thresholds. Cell. 1993;75:1241–1244. doi: 10.1016/0092-8674(93)90610-3. [DOI] [PubMed] [Google Scholar]
- 90.Wright W E, Sassoon D A, Lin V K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- 91.Yamamoto M, Marui N, Sakai T, Morii N, Kozaki S, Ikai K, Imamura S, Narumiya S. ADP-ribosylation of the rhoA gene product by botulinum C3 exoenzyme causes Swiss 3T3 cells to accumulate in the G1 phase of cell cycle. Oncogene. 1993;8:1449–1455. [PubMed] [Google Scholar]
- 92.Yamochi W, Tanaka K, Nonaka H, Maeda A, Musha T, Takai Y. Growth site localization of rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J Cell Biol. 1994;125:1077–1093. doi: 10.1083/jcb.125.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yee S P, Rigby P W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993;7:1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]
- 94.Zheng Y, Bagrodia S, Cerione R A. Activation of phosphoinositide-3 kinase activity by Cdc42Hs binding to p85. J Biol Chem. 1994;269:18727–18730. [PubMed] [Google Scholar]