Abstract
Context:
The Centers for Disease Control and Prevention awarded $85 million to health care–associated infection and antibiotic resistance (HAI/AR) programs in March 2015 as part of Infection Control Assessment and Response (ICAR) activities in the Epidemiology and Laboratory Capacity cooperative agreement Domestic Ebola Supplement.
Program:
One goal of this funding was to assess and improve program capacity to respond to potential health care outbreaks (eg, HAI clusters). All 55 funded programs (in 49 state and 6 local health departments) participated.
Implementation:
The Centers for Disease Control and Prevention developed guidance and tools for HAI/AR programs to document relevant response capacities, assess policies, and measure progress. HAI/AR programs completed an interim assessment in 2016 and a final progress report in 2017.
Evaluation:
During the project period, 78% (n = 43) of the programs developed new investigation tools, 85% (n = 47) trained staff on outbreak response, and 96% (n = 53) of the programs reported hiring staff to assist with outbreak response activities. Staffing and expertise to support HAI outbreak response increased substantially among awardees reporting staffing limitations on the interim assessment, including in domains such as on-site infection control assessment (n = 20; 83%), laboratory capacity (n = 20; 91%), and data management/analytics (n = 14; 67%). By 2017, reporting requirements in 100% of the programs addressed possible HAI/AR outbreaks; 93% (n = 51) also addressed sentinel events such as identification of novel AR threats. More than 90% (n = 50) of programs enhanced capacities for tracking response activities; in 2016, these systems captured 6665 events (range, 3–1379; median = 46). Health departments also reported wide-ranging efforts to engage regulatory, public health, and health care partners to improve HAI/AR outbreak reporting and investigation.
Discussion:
Broad capacity for responding to HAI/AR outbreaks was enhanced using Ebola ICAR supplemental funding. As response activities expand, health department programs will be challenged to continue building expertise, reporting infrastructure, investigation resources, and effective relations with health care partners.
Keywords: antimicrobial resistance, HAI program, outbreak reporting, outbreak response
In 2014, 2 nurses in Texas were infected with Ebola virus while caring for a patient who had traveled from Liberia during the height of the epidemic in West Africa. These infections catalyzed action to improve health care infection control practices and better coordinate efforts by health care providers and public health agencies to respond to emerging disease threats and potential outbreaks in health care settings.1
The Centers for Disease Control and Prevention (CDC) awarded approximately $85 million for health care Infection Control Assessment and Response (ICAR) as part of the Domestic Ebola Supplement2 to the Epidemiology and Laboratory Capacity for Infectious Diseases (ELC) cooperative agreement.3 Awards were distributed to health care–associated infection and antibiotic resistance (HAI/AR) programs in 49 state and 6 local health departments in March 2015. The purpose of this funding was to help address infection control gaps in US health care facilities as well as enhance state and local health department infection prevention and control activities led by the HAI/AR programs to better respond to emerging infectious diseases in health care. HAI/AR programs not only perform the traditional core public health function of responding to outbreaks and clusters but also implement containment strategies for antimicrobial-resistant (AR) pathogens4 and respond to other threats including serious infection control breaches.5,6 The wide range of health care–associated response activities conducted by these HAI programs7–12 requires epidemiology and laboratory expertise, knowledge of the public health regulatory framework, and strong communication channels.
There were several discrete components within the ICAR project to address preparedness for Ebola and other disease threats; this article presents the results and discusses the implications for activity A.4, which had a project period of May 2015-July 2017. The goal of this activity was to improve health department response capacities in relation to potential outbreaks and emerging threats associated with health care delivery. This capacity includes detection of threats (eg, receiving reports of sentinel events or HAI clusters) and efforts to investigate and mitigate those threats.
Methods
The term “HAI” is used to refer to an infection acquired in a health care setting or while accessing medical care. “Response” refers to efforts on the part of public health authorities to assist with investigation of specific HAI risks, which may take the form of clusters of infection, sentinel events including AR organisms, and serious breaches in infection control practice (eg, gross error in aseptic practice such as reusing syringes or failing to clean and sterilize a surgical instrument).
As part of ELC technical monitoring, CDC developed 2 self-assessment tools for HAI programs to evaluate their outbreak response capacities and document progress. HAI programs submitted an interim assessment in year 1 (July 2016) and a final progress report in year 2 (July 2017) to CDC. The assessments documented health care–associated response capacities and activities in 4 areas: Detection and Reporting; Investigation; Tracking; and Coordination, Communication, and Outreach. CDC received completed assessments from 55 health departments, representing 49 states (all but Oklahoma) and 6 ELC-eligible local health departments (Chicago, Houston, Los Angeles, New York City, Philadelphia, and the District of Columbia).
The “Detection and Reporting” section assessed the comprehensiveness of requirements (eg, in code, statute, or regulation) for health department notification when potential outbreaks are identified. The use of various communication channels to distribute these requirements and the clarity of instructions for health care providers and laboratory staff to report these events were also evaluated.
The “Investigation and Control” section assessed the expertise and capacity of health department staff to conduct health care–associated response activities, including evaluations of internal staffing, expertise, and training, as well as the health departments’ legal authorities to conduct investigations and implement control measures.
The “Tracking” section assessed the types of software and electronic systems (eg, spreadsheets or databases) used to document details of health care- associated outbreak reports and response activities; the capacities of these systems were also evaluated. Health departments reported the total numbers of health care–associated response activities recorded for the calendar year 2016; these figures were further stratified by health care setting type and health department level of involvement (eg, on-site visit).
The “Coordination, Communication, and Outreach” section assessed efforts made by the HAI program to strengthen communication among governmental partners and with external stakeholders, including health care associations and professional societies.
The interim (year 1) assessment summarized “baseline” capacities and was designed to stimulate thinking within health departments about gaps, including identifying areas that would benefit most from efforts to increase capacity during the project period. The final progress report (year 2) gave health departments the opportunity to report how they utilized the funding to strengthen their health care–associated response capacities. Both the interim assessment and the final progress report were fillable Microsoft Word documents that included a combination of true or false, select-the-best-response, and free-text fields. The health departments answered questions from the perspective of the HAI/AR program or the department that houses the HAI/AR program. CDC received responses from both assessments and extracted and aggregated responses into Microsoft Excel files; frequencies and proportions were analyzed for each jurisdiction and at the national level.
Results
Detection and reporting
Requirements for HAI/AR outbreak event reporting (Table 1) were strengthened in several key respects. By the close of the project period, all 55 health departments indicated that their reporting requirements addressed reporting clusters of infections prior to confirmation of a true outbreak; this type of requirement covers all pathogens and is not limited to those pathogens already required to be reported as individual cases (eg, acute hepatitis B). Likewise, 93% (n = 51) indicated their requirements address sentinel events such as identification of a novel multidrug-resistant organism (MDRO) or emerging pathogen. The specific addition of reporting requirements for isolates of carbapenem-resistant Enterobacteriaceae (CRE) was noted by 8 health departments (data not shown). Examples of other newly introduced reporting requirements included Candida auris isolates, extrapulmonary nontuberculous mycobacteria infections, Ebola virus infections, Enterobacteriaceae isolates with plasmid-mediated colistin resistance, and episodes of drug diversion9 (eg, tampering). The mechanisms (n = 50; 91%) and time frames (n = 48; 87%) for reporting were specified in most of the requirements, with many health departments indicating these aspects were expanded or introduced during the project period.
TABLE 1.
Health Department Requirements and Activities Promoting HAI/AR Outbreak Reporting, 2015–2017
| Present at Baseline, Maintained | Present at Baseline, Strengthened/Expanded | Newly Added During Project Period | Absent | ||
|---|---|---|---|---|---|
| a. Reporting requirement characteristics on interim (baseline) and final progress report, n (%) | |||||
| Addresses clusters of infections including pathogens not included under general reportable conditions as single cases (N = 55) | 43 (78%) | 9 (16%) | 3 (5%) | 0 | |
| Addresses sentinel cases such as novel MDROs or emerging pathogens (N = 55) | 30 (55%) | 18 (33%) | 3 (5%) | 4 (7%) | |
| Specifies mechanism(s) for reporting (eg, phone number, e-mail address) (N = 53) | 25 (47%) | 18 (34%) | 5 (9%) | 5 (9%) | |
| Specifies time frame (eg, within 24 h) for reporting (N = 54) | 28 (52%) | 14 (26%) | 5 (9%) | 7 (13%) | |
| Acute Care | Long-term Care | Dialysis | Outpatient | Laboratory | |
| b. Distribution channels for educating partners about reporting requirements on final progress report, by setting (n = 55), n (%) | |||||
| Mass e-mails | 47 (85%) | 43 (78%) | 31 (56%) | 32 (58%) | 38 (69%) |
| Mass mailings | 9 (16%) | 9 (16%) | 5 (9%) | 9 (16%) | 11 (20%) |
| Training/presentations | 48 (87%) | 51 (93%) | 24 (44%) | 30 (55%) | 33 (60%) |
| Social media | 7 (13%) | 7 (13%) | 5 (9%) | 6 (11%) | 4 (7%) |
| Web site posting | 42 (76%) | 41 (75%) | 35 (64%) | 37 (67%) | 36 (65%) |
| Press releases | 12 (22%) | 9 (16%) | 8 (15%) | 9 (16%) | 8 (15%) |
Abbreviations: AR, antimicrobial-resistant organism; HAI, health care–associated infection; MDRO, multidrug-resistant organism.
Health departments used a variety of methods to inform and educate partners about their reporting requirements. E-mail communications, trainings/presentations, and Web site postings were the most common distribution channels. Hospitals and long-term care settings were the most frequent targets. For example, during the project period, 51 (93%) health departments utilized training sessions and presentations to inform long-term care facilities about reporting requirements. Health departments from 35 (74%) jurisdictions indicated plans to further improve the distribution of reporting requirements (data not shown).
Investigation and control
During the project period, expanded staffing and expertise helped address gaps that were noted during the interim assessment, including in domains such as on-site infection control assessment (n = 20; 83%), laboratory (n = 20; 91%), and data management/analytics (n = 14; 67%) (Table 2). Nearly all health departments (n = 53; 96%) hired new staff to support HAI/AR outbreak response. Most health departments also indicated that they provided training (n = 47; 85%) or developed investigation tools (n = 43; 78%) to improve outbreak response. Other staffing limitations specified by health departments on the interim assessment included insufficient senior staff to lead investigations when multiple response activities occur concurrently, reliance on clinical experience from health department staff outside the HAI/AR program, and needing to subcontract infection prevention experts from outside the health department (data not shown).
TABLE 2.
Health Department Final Progress Report on HAI/AR Outbreak Investigation, 2015–2017
| n (%) | ||
|---|---|---|
| a. Staffing/expertise (N = 55) | ||
| Hired new staff to participate in HAI/AR response activities | 53 (96%) | |
| Provided training for existing staff | 47 (85%) | |
| Developed new or expanded investigation tools/resources | 43 (78%) | |
| Staffing Limitation Present at Baseline, n/N (%) | Expertise Added During Project Period, n/N (%) | |
| b. Staffing/expertise development among those who reported staffing limitations on interim (baseline) assessment | ||
| Infection control assessment | 24/55 (44%) | 20/24 (83%) |
| Laboratory | 22/55 (40%) | 20/22 (91%) |
| Data management/analytics | 21/55 (38%) | 14/21 (67%) |
| Communications | 15/55 (27%) | 7/15 (47%) |
| General epidemiology | 11/55 (20%) | 10/11 (91%) |
| n (%) | ||
| c. Scope of investigation authority (N = 55) | ||
| Pathogens not on reportable diseases list | 53 (96%) | |
| Unlicensed health care facilities | 51 (93%) | |
| Infection control breaches absent confirmed case of disease | 43 (78%) | |
| Monitoring implementation/effectiveness of control measures | 46 (84%) | |
| n (%) | ||
| d. Documentation available for health care providers (N = 55) | ||
| Describing authority to investigate, conduct chart reviews, and observe practices | 50 (91%) | |
| Describing application of HIPAA protections to public health investigations | 52 (95%) | |
Abbreviations: AR, antimicrobial-resistant organism; HAI, health care–associated infection; HIPAA, Health Insurance Portability and Accountability Act; ICAR, Infection Control Assessment and Response.
In nearly all jurisdictions, health department investigation authorities covered pathogens that are not on their reportable diseases list (n = 53; 96%) as well as facilities such as doctors’ offices and clinics that may operate without the requirement for a state-issued facility license (n = 51; 93%). Most (n = 43; 78%) health departments reported having authority to investigate reports of infection control breaches without a confirmed case of disease. Likewise, 84% reported having authority to monitor implementation and effectiveness of control measures recommended in the context of an investigation. Health departments from 36 (65%) jurisdictions reported having authority for all 4 of the assessed elements (Table 2). Finally, more than 85% (n = 47) reported having forms of documentation that can be used to explain (a) their authority to investigate and (b) the application of Health Insurance Portability and Accountability Act (HIPAA) protections.13
Tracking
Health department capacities for tracking their HAI/AR outbreak response activities matured substantially over the project period. More than 90% (n = 50) expanded or added to their capacity for documenting information related to reports and investigations involving clusters and sentinel events (Table 3). On the Final Progress Report, all responding jurisdictions reported being able to enter HAI outbreaks and investigation of sentinel events into a tracking system. Laboratory information could be entered into the response activity tracking systems for 96% of jurisdictions. However, 18% (n = 9) reported no capacity to track reports and assessments of serious infection control breaches.
TABLE 3.
Health Department Final Progress Report on HAI/AR Outbreak Activity Tracking, 2015–2017
| Present at Baseline, Maintained | Present at Baseline, Strengthened/Expanded | Newly Added During Project Period | Absent | |
|---|---|---|---|---|
| a. Capacity to enter HAI/AR responses into an electronic database, n (%) | ||||
| HAI outbreaks including clusters of infections (N = 52) | 8 (15%) | 34 (65%) | 10 (19%) | 0 |
| Investigation of sentinel cases of infection, novel AR/emerging pathogen (N = 52) | 8 (15%) | 32 (62%) | 12 (23%) | 0 |
| Laboratory information (N = 53) | 16 (30%) | 26 (49%) | 9 (17%) | 2 (4%) |
| Serious infection control breaches (N = 51) | 8 (16%) | 26 (51%) | 8 (16%) | 9 (18%) |
| n (%) | ||||
| b. Characteristics of HAI/AR responses in 2016 (N = 6665) | ||||
| Active participation from staff in the health department | 5604 (84%) | |||
| Active participation from staff in the HAI program | 2838 (43%) | |||
| Public health laboratory support | 1859 (28%) | |||
| On-site visit by health department | 417 (6%) | |||
| Investigations of sentinel cases of infection, novel AR/emerging pathogen | 274 (4%) | |||
| Investigations of serious infection control breaches | 136 (2%) | |||
| n (%) | ||||
| c. Use of software platforms for response activity tracking (N = 55, multiple selections allowed) | ||||
| Microsoft Excel | 38 (69%) | |||
| Custom system (eg, incorporated into disease surveillance system, .NET application) | 26 (47%) | |||
| CDC National Outbreak Reporting System (NORS) | 20 (36%) | |||
| Microsoft Access | 19 (35%) | |||
| REDCap | 6 (11%) | |||
| Maven | 7 (13%) | |||
| Microsoft SharePoint | 3 (5%) | |||
| Other | 10 (18%) | |||
Abbreviations: AR, antimicrobial-resistant organism; CDC, Centers for Disease Control and Prevention; HAI, health care–associated infection.
Health departments utilized a wide variety of software platforms for response activity tracking. Health departments from 43 (78%) jurisdictions reported using multiple tracking systems concurrently on the final progress report. The most common software used for tracking was Microsoft Excel (n = 38, 69%), although only 4 (7%) health departments reported using Microsoft Excel exclusively (data not shown). Custom-made tracking systems were used by 26 (47%) health departments. A minority of health departments (n = 22; 42%) reported tracking health care–associated response activities separately from other response activities (eg, foodborne outbreaks). However, all health departments that were utilizing a shared tracking system reported being able to query health care–associated responses separately (data not shown).
A total of 6665 (range, 3–1379; median 46) response activities were reported by health departments in calendar year 2016, with the majority affecting long-term care facilities (n = 5191; 78%) (Figure). Other health care settings included acute care facilities (n = 1014; 15%), outpatient (n = 159; 2%), and hemodialysis (n = 74; 1%). These events involved varying levels of support including active health department participation (84%), HAI program involvement (43%), public health laboratory support (28%), and on-site visits (6%) (Table 3).
FIGURE.
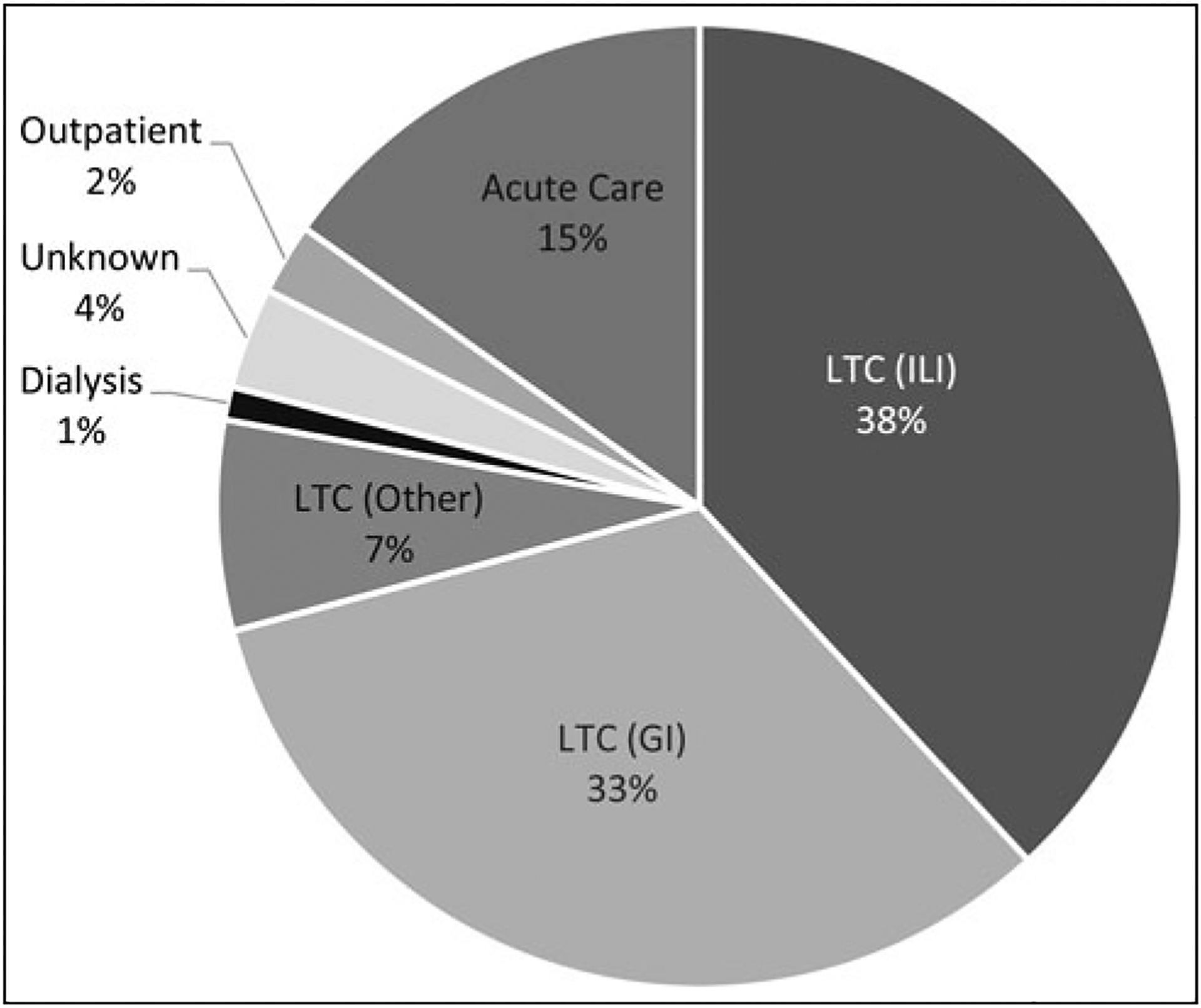
HAI/AR Outbreak Response Activities in 2016 Reported by Health Departments, Stratified by Health Care Setting (N = 6665 Events). Abbreviations: AR, antimicrobial-resistant organism; GI, gastrointestinal illness; HAI, health care–associated infection; ILI, influenza-like illness; LTC, long-term care facility.
Plans to further enhance response activity tracking were reported by more than 80% of health departments (data not shown); specific examples noted by several health departments were enhancements to CRE/MDRO investigation data elements and plans to cease the use of multiple concurrent systems in favor of a single system with enhanced capacity.
Coordination, communication, and outreach
Health departments made wide-ranging efforts to engage partners to improve their HAI/AR response activities and included regulatory, public health, and health care partners (Table 4). During the project period, 91% (n = 50) of health departments engaged their state health care facility licensing and survey agency. Of note, 27% (n = 15) of jurisdictions reported no active relationship with this agency at baseline (data not shown). Efforts to improve engagement included increased communication through regular meetings, formalized data sharing agreements, invitations to participate on the HAI/AR program’s advisory committee, and efforts to align infection prevention training with new Centers for Medicare & Medicaid Services (CMS) requirements for long-term care facilities.14 More than two-thirds (n = 38; 69%) reported efforts to strengthen outbreak response through enhanced partnerships between state and local health departments. With regard to health care partners, jurisdictions targeted their state hospital associations (n = 32; 58%) and CMS-funded Quality Innovation Network-Quality Improvement Organizations (n = 24; 47%); other targets included groups representing the long-term care and dialysis provider communities.
TABLE 4.
Health Department HAI/AR Outbreak Response Coordination, Communication and Outreach, 2015–2017 (N = 55)
| n (%) | ||||
|---|---|---|---|---|
| a. Efforts to strengthen partnerships to improve HAI/AR outbreak response on final progress report | ||||
| State health care facility licensing and survey agencies | 50 (91%) | |||
| Between state and local health departments | 38 (69%) | |||
| Hospital associations | 32 (58%) | |||
| Quality Innovation Network-Quality Improvement Organizations (QIN-QIO) | 24 (47%) | |||
| Nursing home/long-term care associations | 13 (24%) | |||
| Association for Professionals in Infection Control (eg, APIC chapters) | 13 (24%) | |||
| End-stage renal disease networks | 8 (15%) | |||
| Present at Baseline, Maintained | Present at Baseline, Strengthened | Newly Added During Project Period | Absent | |
| b. Health department communication with state licensing boards to facilitate better coordination of HAI/AR response activities on interim (baseline) and final progress report, n (%) | ||||
| Medical board | 12 (22%) | 9 (16%) | 11 (20%) | 23 (42%) |
| Nursing board | 7 (13%) | 6 (11%) | 10 (18%) | 32 (58%) |
| Dental board | 7 (13%) | 8 (15%) | 10 (18%) | 30 (55%) |
| Pharmacy board | 4 (7%) | 10 (18%) | 10 (18%) | 31 (56%) |
Abbreviations: AR, antimicrobial-resistant organism; HAI, health care–associated infection.
Engagement with professional licensing boards was also encouraged as part of efforts to improve coordination of HAI/AR response. At baseline, only 24% to 38% of health departments reported communication with medical, nursing, dental, or pharmacy boards. Overall, these engagement levels increased over the course of the project period (and were highest for medical boards at 58%) but left substantial room for improvement.
Discussion
CDC-funded health department HAI/AR programs have expanded and developed broad capacities for responding to HAI outbreaks and AR threats. Timely and appropriate investigation of potential outbreaks involving medical care is needed to prevent the spread of HAIs and AR pathogens and minimize patient harm. Public health departments are uniquely positioned to play a central role in such investigations.15 Health care facilities sometimes lack internal capacities for effective investigation and control activities. Infections and resistant organisms can move across facilities and within the communities these facilities serve. As the value added by an active public health engagement in HAI/AR response activities becomes increasingly evident, challenges will include strengthening relations with the health care community, regulatory, and professional bodies and maintaining capacities to meet the demand for these services.
Response to an emerging threat begins with detecting or receiving a signal. In public health practice, these signals traditionally come from one of 2 sources. First, monitoring for clusters is part of routine surveillance for conditions that are reportable as single cases. Second, most jurisdictions have made outbreaks reportable, which provides a helpful catchall to supplement the list of reportable conditions. This second element is especially relevant for HAI/AR response as it provides coverage for the immense variety of pathogens, infection types, and resistance mechanisms that may be spread by health care delivery. As described earlier, health departments have strengthened reporting requirements by making them more specific and working to educate partners so that reporting becomes more consistent. There remains potential to improve the language within reporting requirements that often utilizes terms such as “baseline,” “expected,” or “background” that may be difficult for health care partners to interpret and apply. Efforts are underway to provide more granular guidance on thresholds for investigation and reporting of HAI clusters and sentinel events. For example, the Council for Outbreak Response: Healthcare-Associated Infections and Antimicrobial-Resistant Pathogens (CORHA) has been assembling guidance for specific pathogens on the numbers of infection reports per unit of time that should trigger reporting.16
Going forward, there are also opportunities to expand use of nontraditional sources of information to identify signals that warrant a public health response. For example, while communicable disease reporting is the backbone of public health surveillance, health care systems often utilize electronic information and surveillance systems for both internal use and external reporting. Recognizing this, CDC has funded research to help develop algorithmic detection of infection clusters17 and also encouraged active monitoring of event reports captured in CDC’s National Healthcare Safety Network (NHSN).18 In addition, owing in large part to the launch of the Antibiotic Resistance Laboratory Network (AR Lab Network) in 2016, health care facilities and health departments have gained unique capabilities to accelerate detection of emerging AR threats and support coordinated local responses to prevent their spread.19 Another nontraditional source of information being used to prompt public health response comes from health care facility licensing and accreditation surveys. CMS introduced a policy6 in 2014 that indicates that surveyors who identify serious infection control deficiencies should relay these concerns to public health for evaluation, including considerations for patient notification.
To be successful, health departments that investigate HAI/AR outbreaks require certain technical capabilities, resources, and authorities. During 2015–2017, HAI/AR programs made substantial gains in adequately trained and qualified staff. On-site assessment of infection control practices represents a core competency and critical element; meaningful public health HAI/AR response often hinges on this capability. Investigators who lack this capability risk delays or may fail to implement appropriate interventions. This is particularly true in supporting facilities such as nursing homes or outpatient clinics that lack infection control expertise. Even acute care hospitals often benefit from additional expertise and recognize health department contributions in this area. In fact, recognition of public health HAI/AR program value in addressing emerging infectious disease threats grew largely from the 2014 domestic Ebola hospital preparedness activities, which were founded on structured on-site infection control assessments.1 These activities spawned the ELC Ebola Supplement-supported ICAR work, with 55 health departments conducting several thousand on-site assessments across a spectrum of health care facility types.20 Infection control assessment capacity is now integrated in most HAI programs and helps underpin the CDC AR threat containment strategy.6 Laboratory capacity is the other key area where staffing and technical capabilities were developed beginning with ELC Ebola Supplement funds and then expanded and sustained in conjunction with the AR Lab Network investments.19,21 Areas for improvement include establishment or expansion of investigation-related policies and authorities.22 Some jurisdictions reported not having sufficient authority to investigate infection control breach reports or monitor implementation of recommended control measures. Finally, there exists a need for more consistent approaches to outbreak investigation and control. The CDC AR containment strategy is a prime example of how investigation guidance and technical support can foster a more uniform and effective response.4 With active support from CDC and other partner organizations, CORHA is engaged in complementary efforts to package tools and best practices for supporting various types of HAI/AR response.16
HAI/AR outbreak reporting and investigation tracking remains a work in progress. Work begun during the project period by CDC and CORHA marked a significant step toward a more standard national approach to tracking outbreaks.23 The data presented here provide a glimpse of the breadth and scope of the public health contributions, with more than 6000 events captured in these nascent systems in 2016 alone. More systematic tracking of HAI outbreaks and response activities will provide information that is helpful locally to evaluate the effectiveness of reporting requirements and investigation activities. Tracking also informs prevention efforts by identifying facilities, settings, or issues that might benefit from technical assistance or other forms of outreach. Nationally, systematic HAI/AR outbreak response tracking has the potential to inform development of prevention guidance and investigation tools. CDC has come to recognize the need for tracking systems to be flexible to accommodate a variety of event types, such as investigations of sentinel events, clusters and confirmed outbreaks, including AR threat containment efforts, as well as investigations stemming from recognition of hazardous practices (eg, serious infection control breaches or drug tampering) even when associated infections have not been identified. Tracking systems should also accommodate relatively common and simple response events (eg, cluster of gastrointestinal illness in a nursing home investigated at the local health department level) as well as more complex and protracted investigations. Interoperability with other systems, such as those that record patient-level laboratory, clinical, or epidemiologic data, is a desirable feature. This also speaks to the need to pursue linkage with health systems to collaborate more efficiently during responses by granting health care facility and public staff shared access to select electronic information systems. It is worth noting that the investigation tracking capabilities described here for public health response are equally important for hospitals and other health systems to adopt or expand.24
In responding to potential outbreaks and emerging threats, health departments serve the broader community, acting as a bridge between health care facilities, regulatory bodies, and patients. It follows that a foundation based on strong partnerships and effective communication pathways is critical to support all aspects of HAI/AR response activities. Our findings were encouraging in this regard and suggest that health department HAI/AR programs should continue to build relationships with licensing boards, state survey agencies, and accreditation organizations to encourage exchange of information and collaboration, balancing technical assistance with the appropriate enforcement of regulatory authority.22 Likewise, HAI/AR programs should continue to expand their partnerships with professional societies, health care industry associations, and other organizations charged with patient safety and quality improvement to facilitate uptake of recommended outbreak reporting policies and control strategies. An area for future growth involves engagement and communication directly with patients and the public. Transparency during and after responses is critical and can be used as an opportunity to build trust with the public.25
The data presented here are subject to limitations. Each health department was responsible for completing the 2 self-assessments as part of their ELC award performance measures. Health departments are evaluated by CDC on their achievements during the project period based on the successful completion of each performance measure, and this evaluation may influence future CDC award determinations. One of the limitations with the self-reported nature of these assessments is the desire for health department staff to present their program in a positive light. Second, there was potential for questions to be interpreted differently by staff across health departments. To help address this, CDC provided supplementary coaching and instruction during the project period through regular conference calls and individual assistance. Third, the numbers of outbreak response events that each jurisdiction provided likely underestimated the true levels of HAI/AR outbreaks and related investigations. Eligible events could have been missed (not detected or reported), and events that were investigated by local health departments might not have been communicated or captured by the HAI/AR program tracking systems. Fourth, this article did not evaluate the ability of health care staff to identify potential outbreaks or describe the likelihood of reporting to public health. The completeness of health department tracking requires further evaluation.
In summary, broad capacity for responding to HAI/AR outbreaks was enhanced using Ebola ICAR supplemental funding. There is a wide variety of HAI/AR outbreak response activities being conducted by US health departments, each of which has varying expertise and resources to support these investigations. Nonetheless, a more comprehensive and systematic public health approach to health care outbreak response activity is taking shape. We expect this will help highlight the growing contributions of public health in this arena and identify additional unmet needs related to HAI/AR surveillance, outbreak investigation, and prevention. As response activities expand, HAI programs will be challenged to continue building expertise, reporting infrastructure, investigation resources, and effective relations with health care partners.
Implications for Policy & Practice.
Health department HAI/AR programs should periodically review public health reporting requirements, looking for opportunities to enhance specificity and performance in relation to HAI outbreaks; stay informed about changes proposed by other jurisdictions that address emerging AR threats.
Confirm investigation authority in various health care settings with legal counsel and have informational handouts available to present to health care partners. Consider ways to establish health department authority to investigate situations where unsafe practices have been reported, if needed.
Health department tracking systems should be able to accommodate all types of health care–associated response activities. Health department staff should be able to retrieve historical data to inform new responses and regularly review aggregate data to inform prevention priorities.
Build partnerships with licensing boards, state survey agencies, and accreditation organizations to encourage exchange of information and collaboration, balancing technical assistance with the appropriate enforcement of regulatory authority.
Foster partnerships with professional societies, hospital and long-term care associations, and other organizations that are charged with patient safety and quality improvement to facilitate systems-level uptake of recommended outbreak reporting, response, and control strategies.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Van Beneden CA, Pietz H, Kirkcaldy RD, et al. Early identification and prevention of the spread of Ebola—United States. In: CDC’s response to the 2014–2016 Ebola epidemic—West Africa and United States. MMWR Suppl. 2016;65(S3):75–84. https://www.cdc.gov/mmwr/volumes/65/su/su6503a11.htm. Accessed January 21, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Domestic Ebola Supplement to Epidemiology and Laboratory Capacity for Infectious Diseases (ELC)—building and strengthening epidemiology, laboratory and health information systems capacity in state and local health departments. https://www.grants.gov/web/grants/view-opportunity.html?oppId=271714. Accessed January 21, 2020.
- 3.Centers for Disease Control and Prevention. Epidemiology and Laboratory Capacity for Infectious Diseases (ELC) cooperative agreement. https://www.cdc.gov/ncezid/dpei/epidemiology-laboratory-capacity.html. Accessed January 21, 2020.
- 4.Centers for Disease Control and Prevention. Containment strategy responding to emerging AR threats. https://www.cdc.gov/hai/containment/index.html. Accessed January 21, 2020. [Google Scholar]
- 5.Patel PR, Srinivasan A, Perz J. Developing a broader approach to management of infection control breaches in health care settings. Am J Infect Control. 2008;36(10):685–690. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Medicare & Medicaid Services. Infection control breaches which warrant referral to public health authorities. https://www.cms.gov/Medicare/Provider-Enrollment-and-Certification/SurveyCertificationGenInfo/Downloads/Survey-and-Cert-Letter-14-36.pdf. Accessed January 21, 2020.
- 7.Vannice K, Benoliel E, Kauber K. Notes from the Field: clinical Klebsiella pneumoniae isolate with three carbapenem resistance genes associated with urology procedures—King County, Washington, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(30):667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross KM, Mehr JS, Carothers BL, et al. Bacterial septic arthritis infections associated with intra-articular injection practices for osteoarthritis knee pain—New Jersey, 2017. Infect Control Hosp Epidemiol. 2019;40(9):1013–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Njuguna HN, Stinson D, Montgomery P, et al. Hepatitis C virus potentially transmitted by opioid drug diversion from a nurse— Washington, August 2017-March 2018. MMWR Morb Mortal Wkly Rep. 2019;68(16):374–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perkins KM, Reddy SC, Fagan R, Arduino MJ, Perz JF. Investigation of healthcare infection risks from water-related organisms: summary of CDC consultations, 2014–2017. Infect Control Hosp Epidemiol. 2019;40(6):621–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nanduri SA, Metcalf BJ, Arwady MA, et al. Prolonged and large outbreak of invasive group A Streptococcus disease within a nursing home: repeated intrafacility transmission of a single strain. Clin Microbiol Infect. 2019;25(2):248.e1–248.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peña SA, Davis SS, Lu X, et al. Severe respiratory illness associated with human metapneumovirus in nursing home, New Mexico, USA. Emerg Infect Dis. 2019;25(2):383–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. HIPAA privacy rule and public health: guidance from CDC and the U.S. Department of Health and Human Services. MMWR Morb Mortal Wkly Rep. 2003;52;1–12.12549898 [Google Scholar]
- 14.Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; reform of requirements for long-term care facilities. Federal Register Doc. 2016–23503. https://www.federalregister.gov/documents/2016/10/04/2016-23503/medicare-and-medicaid-programs-reform-of-requirements-for-long-term-care-facilities. Accessed January 21, 2020. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Making health care safer: stop spread of antibiotic resistance. CDC Vital Signs. https://www.cdc.gov/vitalsigns/pdf/2015-08-vitalsigns.pdf. Published August 2015. Accessed January 21, 2020. [Google Scholar]
- 16.Council for Outbreak Response: Healthcare-Associated Infections and Antimicrobial-Resistant Pathogens. Home page. https://corha.org. Accessed January 21, 2020.
- 17.Harvard Pilgrim Health Care Institute. CLUSTER (Cluster Linkage Using Statistics to Trigger and Evaluate Response) trial. https://www.populationmedicine.org/research/therapeutics-research-infectious-disease/research/CLUSTER. Accessed January 21, 2020.
- 18.Centers for Disease Control and Prevention. National Healthcare Safety Network. https://www.cdc.gov/nhsn/. Accessed January 21, 2020.
- 19.Centers for Disease Control and Prevention. Lab capacity: Antibiotic Resistance Laboratory Network (AR Lab Network). https://www.cdc.gov/drugresistance/solutions-initiative/ar-lab-network.html. Accessed January 21, 2020.
- 20.Centers for Disease Control and Prevention. Antibiotic Resistance Investment Map. https://wwwn.cdc.gov/arinvestments/. Accessed January 21, 2020.
- 21.Taylor D, Rebmann R, Ashley M, et al. Evolution of ELC Healthcare-Associated Infections/Antimicrobial Resistance (HAI/AR) programs, 2009–2018. 2018 CSTE Conference Abstract 133. https://cste.confex.com/cste/2018/meetingapp.cgi/Paper/9828. Accessed January 21, 2020. [Google Scholar]
- 22.Centers for Disease Control and Prevention. Outpatient settings policy options for improving infection prevention. https://www.cdc.gov/hai/pdfs/prevent/Outpatient-Settings-Policy-Options.pdf. Accessed January 21, 2020.
- 23.Council for Outbreak Response: Healthcare-Associated Infections and Antimicrobial-Resistant Pathogens. HAI-AR Outbreak Investigation and Response Tracking System. https://corha.org/resources/corha-hai-ar-outbreak-and-response-tracking-system. Accessed January 21, 2020.
- 24.Kanamori H, Weber DJ, Gergen MF, et al. Epidemiologic characteristics of health care-associated outbreaks and lessons learned from multiple outbreak investigations with a focus on the usefulness of routine molecular analysis. Am J Infect Control. 2018;46(8):893–898. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher TH, Waterman AD. Patients’ and physicians’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289(8):1001–1007. [DOI] [PubMed] [Google Scholar]


