Abstract
IMPORTANCE
Estimating the US burden of methicillin-resistant Staphylococcus aureus (MRSA) infections is important for planning and tracking success of prevention strategies.
OBJECTIVE
To describe updated national estimates and characteristics of health care– and community-associated invasive methicillin-resistant Staphylococcus aureus (MRSA) infections in 2011.
DESIGN, SETTING, AND PARTICIPANTS
Active laboratory-based case finding identified MRSA cultures in 9 US metropolitan areas from 2005 through 2011. Invasive infections (MRSA cultured from normally sterile body sites) were classified as health care–associated community-onset (HACO) infections (cultured ≤3 days after admission and/or prior year dialysis, hospitalization, surgery, long-term care residence, or central vascular catheter presence ≤2 days before culture); hospital-onset infections (cultured >3 days after admission); or community-associated infections if no other criteria were met. National estimates were adjusted using US census and US Renal Data System data.
MAIN OUTCOMES AND MEASURES
National estimates of invasive HACO, hospital-onset, and community-associated MRSA infections using US census and US Renal Data System data as the denominator.
RESULTS
An estimated 80 461 (95% CI, 69 515–93 914) invasive MRSA infections occurred nationally in 2011. Of these, 48 353 (95% CI, 40 195–58 642) were HACO infections; 14 156 (95% CI, 10 096–20 440) were hospital-onset infections; and 16 560 (95% CI, 12 806–21 811) were community-associated infections. Since 2005, adjusted national estimated incidence rates decreased among HACO infections by 27.7% and hospital-onset infections decreased by 54.2%; community-associated infections decreased by only 5.0%. Among recently hospitalized community-onset (nondialysis) infections, 64% occurred 3 months or less after discharge, and 32% of these were admitted from long-term care facilities.
CONCLUSIONS AND RELEVANCE
An estimated 30 800 fewer invasive MRSA infections occurred in the United States in 2011 compared with 2005; in 2011 fewer infections occurred among patients during hospitalization than among persons in the community without recent health care exposures. Effective strategies for preventing infections outside acute care settings will have the greatest impact on further reducing invasive MRSA infections nationally.
Methicillin-resistant Staphylococcus aureus (MRSA) continues to be one of the most common antimicrobial-resistant pathogens causing invasive infections, both in health care settings and in the community.1–4 In the United States, MRSA is the predominant pathogen causing skin and soft tissue infections,5 and reports using administrative data documented increases in the number of estimated hospitalizations in which International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes indicating MRSA infection were present.6,7
However, recent reports of surveillance programs in geographically distinct areas suggest there have been recent declines in the incidence of the most serious types of MRSA infections (ie, bloodstream infections), including those among critical care patients in US hospital settings2,8 and both community- and hospital-onset MRSA bacteremia among military families, active duty personnel, and military retirees starting in 2005.9 The Emerging Infections Program–Active Bacterial Core surveillance system (EIP-ABCs) at the Centers for Disease Control and Prevention (CDC) has been tracking invasive MRSA infections in 9 diverse US metropolitan areas since 2005. Reports using this system documented an 11% annual decline in the incidence of hospital-onset MRSA bloodstream infections between 2005 and 2008 in these metropolitan areas.2 The cause of these declines is uncertain but may be due to improvements in hospital-based infection prevention practices, improved early management of noninvasive infections, or changes in the virulence of the circulating strains.
A prior CDC study estimated the national burden of invasive MRSA in 20053; however, this report did not adjust for dialysis or include patients with recurrent infections.10 Therefore, to better understand the current national burden of these most serious MRSA infections, spanning both community and health care settings, we used EIP-ABCs’ data and updated methodology to describe the types and outcomes of invasive MRSA infections in 2011 and to estimate the infection burden in the United States in 2011 and 2005. These estimates are necessary to improve the assessment of invasive MRSA infection nationally, to inform prevention policies in health care settings, and to measure progress toward public health goals.11
Methods
Human Subjects Considerations
This surveillance activity was reviewed by human subject personnel at the CDC and was determined to be nonresearch. Local institutional review boards also reviewed when indicated and approved this activity.
Invasive MRSA Surveillance
The EIP-ABCs is a population-based surveillance system used by the CDC and collaborating agencies and organizations to estimate the incidence of invasive MRSA infections in selected counties in 9 US states (California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New York, Oregon, and Tennessee). The methods of surveillance have been described previously,3 with modifications to include recurrent infections (ie, multiple infections in a single patient during the calendar year) in incidence calculations. In brief, surveillance personnel at participating sites investigated all laboratory reports where MRSA was isolated. An invasive MRSA case was defined as a positive MRSA culture from a normally sterile site in a surveillance area resident at least 30 days apart of an initial invasive MRSA culture. For each invasive MRSA case, information on hospitalization and health care risk factors (ie, culture obtained after hospital day 3; presence of a central vascular catheter [CVC] at the time of infection; and history of MRSA infection or colonization, surgery, hospitalization, dialysis, or residence in a long-term care facility in the 12 months preceding the culture) were collected. Underlying conditions were obtained and used to calculate the Charlson index.12
Study Population
We used MRSA surveillance data from January through December of 2005 and 2011. The surveillance areas represented 16 489 254 and 19 393 677 persons in 2005 and 2011, respectively.13,14
Definitions
Cases were classified as (1) “hospital onset,” if culture was obtained after hospital day 3 (with admission being day 1), (2) “health care–associated community onset” (HACO) if culture was obtained as an outpatient on or before hospital day 3 in a patient with a documented health care risk factor, or (3) “community associated” if culture was obtained as an outpatient or before hospital day 3 in a patient without documentation of a health care risk factor. Of note, the definition of hospital-onset infections differ from previous reports where MRSA culture was obtained after hospital day 2 in prior methods; this refinement is consistent with classifying MRSA bloodstream infections as hospital-onset in the National Healthcare Safety Network’s (NHSN) Laboratory Identified Event Reporting and is applied to all analysis in this article.
Cases were classified as a bloodstream infection if they had a positive blood culture for MRSA. Bloodstream infection cases were further classified as either (1) an access infection (physician-documented arteriovenous fistula, dialysis access site, or CVC exit site infection), an infection of uncertain focus either (2) with a CVC present or (3) without a CVC present, or (4) as having other infection foci if there were either positive MRSA cultures from sterile sites other than blood or additional infectious clinical diagnosis in the admission or discharge summary. An MRSA clinical diagnosis of bacteremia, pneumonia, osteomyelitis, or other infection was assigned to each case based on documentation of such diagnosis in the admission or discharge summary or by the sterile site from which MRSA was isolated. Each case was assigned additional MRSA clinical diagnosis if multiple diagnoses (eg, bacteremia and osteomyelitis) were documented within 30 days of the initial MRSA culture. Health care–associated infection criteria defined by the NHSN were not applied to cases because NHSN definitions only apply to infections not present on admission.
Cases with a clinical diagnosis of pneumonia were further classified as having confirmed lower respiratory tract infections if radiographic evidence was present (ie, bronchopneumonia, pneumonia, air space density or opacity, or new or changed infiltrates documented in a radiologist report) and the patient had MRSA recovered from a respiratory tract specimen within 3 days of the sterile site MRSA culture.
Statistical Analysis
Differences in baseline characteristics among cases in each epidemiologic category (hospital onset, HACO, and community associated) were analyzed using the χ2 test or Fisher exact test (where appropriate) for categorical variables and the Wilcoxon rank sum test for continuous variables.
Overall, 9% of cases were reported with unknown race in medical charts. To facilitate extrapolation to national estimates, cases for which the race was unknown were assigned a race based on the known population distributions of race by sex, age, and receipt of chronic dialysis in each surveillance site. National estimates were determined by calculating EIP-ABC–specific incidence rates stratified by age group, race, sex, and receipt of dialysis, and extrapolated nationally by multiplying each stratum-specific incidence rate by the national population estimate for each stratum based on US census and US Renal Data System data.15–18 In previous reports and publications, national estimates were adjusted for only age, race, and sex.3 Confidence intervals for nationally estimated incidence rates of disease and mortality were calculated based on the gamma distribution.19 Analyses were performed using SAS statistical software, version 9.3 (SAS Institute Inc), and Stata version 11.1 (StataCorp). We defined statistical significance as P ≤ .05 for a 2-tailed test.
Results
Case Characteristics
The EIP-ABCs sites reported 4872 cases of invasive MRSA infections among 4445 persons (8% had >1 infection) in 2011. Among these cases, 4746 cases (97%) were classified into epidemiological classes: 2912 (60%) were HACO, 868 (18%) were hospital onset, and 966 (20%) were community associated (Table 1).
Table 1.
Demographics of Health Care–Associated Community-Onset (HACO), Hospital-Onset (HO), and Community-Associated (CA) MRSA Infectiona Reported to the Emerging Infections Program–Active Bacterial Core Surveillance, United States, 2011
| Demographic | Infections, No. (%) | P Valuec | |||
|---|---|---|---|---|---|
| HACO | HO | CA | Totalb | ||
| Total | 2912 (100) | 868 (100) | 966 (100) | 4872 (100) | |
| Sex | |||||
| Male | 1724 (59) | 527 (61) | 619 (64) | 2934 (60) | .03 |
| Female | 1185 (41) | 341 (39) | 347 (36) | 1935 (40) | |
| Not specified | 3(0) | 0 | 0 | 3 | |
| Age, y | …d,e,f | ||||
| <1 | 9(0) | 12 (1) | 22 (2) | 50 (1) | |
| 1–17 | 18(1) | 17 (2) | 51 (5) | 87 (2) | |
| 18–34 | 156(5) | 64 (7) | 122 (13) | 349 (7) | |
| 35–49 | 401 (14) | 135 (16) | 216 (22) | 769 (16) | |
| 50–64 | 920 (32) | 288 (33) | 290 (30) | 1536 (32) | |
| ≥65 | 1408 (48) | 345 (40) | 265 (27) | 2081 (43) | |
| Median (range) | 64 (0–103) | 60 (0–96) | 54 (0–103) | 61 (0–103) | |
| Race | |||||
| White | 1610 (55) | 480 (55) | 576 (60) | 2743 (56) | .046 |
| Black | 996 (34) | 295 (34) | 230 (24) | 1542 (32) | <.001 |
| Other | 80 (3) | 18 (2) | 24(2) | 126 (3) | .54 |
| Unknown | 226 (8) | 75 (9) | 136 (14) | 461 (9) | <.001 |
| Dialysis within 1 y | |||||
| Yes | 891 (31) | 122 (14) | 0 | 1013 (21) | …g |
| No | 2021 (69) | 746 (86) | 966 (100) | 3859 (79) | |
| Underlying conditions: MRSA risk factors | |||||
| Abscess/boil | 89 (3) | 35 (4) | 86 (9) | 216 (4) | <.001 |
| Current smoker | 456 (16) | 162 (19) | 294 (30) | 933 (19) | <.001 |
| Decubitus/pressure ulcer | 394 (14) | 81 (9) | 52 (5) | 536 (11) | <.001 |
| Diabetes | 1355 (47) | 331 (38) | 276 (29) | 1999 (41) | <.001 |
| Intravenous drug use | 99 (3) | 30 (3) | 133 (14) | 272 (6) | <.001 |
| Metastatic solid tumor | 126 (4) | 34(4) | 14(1) | 176 (4) | <.001 |
| Obesity | 372 (13) | 117 (13) | 77 (8) | 569 (12) | <.001 |
| Solid organ malignancy | 256 (9) | 99 (11) | 43 (4) | 402 (8) | <.001 |
| Other underlying conditions | |||||
| Charlson Index, median (range) | 3 (0–12) | 2 (0–13) | 0 (0–12) | 2 (0–13) | …d,f |
| Chronic liver disease | 123 (4) | 58 (7) | 41 (4) | 223 (5) | .008 |
| Chronic pulmonary disease | 719 (25) | 211 (24) | 182 (19) | 1145 (24) | .001 |
| Chronic renal insufficiency | 1260 (43) | 247 (28) | 83 (9) | 1604(33) | <.001 |
| Chronic skin breakdown | 359 (12) | 108 (12) | 95 (10) | 572 (12) | .09 |
| Congestive heart failure | 689 (24) | 202 (23) | 69 (7) | 985 (20) | <.001 |
| Stroke (not TIA) | 430 (15) | 93 (11) | 47 (5) | 577 (12) | <.001 |
| Dementia | 388 (13) | 77 (9) | 44 (5) | 525 (11) | <.001 |
| HIV/AIDS | 134(5) | 32 (4) | 60 (6) | 227 (5) | .03 |
| Myocardial infarction | 236 (8) | 69 (8) | 30 (3) | 336 (7) | <.001 |
| Unknown | 20(1) | 2 (0) | 3 (0) | 47 (1) | .21 |
| Other | 857 (29) | 254 (29) | 152 (16) | 1282 (26) | <.001 |
| Health care | |||||
| Hospitalization in the past 1 yearh | 2291 (79) | 501 (58) | 0 | 2792 (57) | …g |
| Surgery within 1 year prior to culture | 1164 (40) | 333 (38) | 0 | 1497 (31) | …i |
| Long-term care facility within 1 year | 1057 (36) | 182 (21) | 0 | 1239 (25) | …g |
| Central venous catheter within 2 days | 790 (27) | 303 (35) | 0 | 1093 (22) | …g |
Abbreviations: HIV/AIDS, human immunodeficiency virus/acquired immunodeficiency syndrome; MRSA, methicillin-resistant Staphylococcus aureus; TIA, transient ischemic attack.
Defined as MRSA isolated from a normally sterile site.
Twenty-six additional cases with unknown epidemiological category included in total.
χ2 Test (or Fisher exact test if values ≤5) comparing all epidemiologic categories.
HACO significantly different from non-HACO (P < .001).
HO significantly different from non-HO (P < .01).
CA significantly different from non-CA (P < .001).
HACO and HO significantly different (P < .001).
For infants younger than 12 months, hospitalization for a normal delivery was not counted as a hospitalization.
HACO and HO not significantly different (P = .40).
The median age of cases was 61 years (range, 0–103 years) (Table 1). The majority of cases were male (60%) and of either white (56%) or black (32%) race. Diabetes was a diagnosis in 41% of cases. There was receipt of hemodialysis or peritoneal dialysis within the year before invasive MRSA infection in 21% of cases (61% of cases receiving dialysis also had diabetes).
Most invasive MRSA infections had a positive blood culture (3907 [80%]) and were classified as a bloodstream infection (Table 2). Overall, 55% of infections had a nonvascular focus, and 45% had a vascular or nonspecific focus (Table 2). Among the 760 invasive MRSA infections with clinician-defined pneumonia, based on radiographic and respiratory specimen findings, 293 (31%) had confirmed MRSA lower respiratory tract infections (eTable in the Supplement). Other common MRSA syndromes included skin infections (1081 [22%]) and osteomyelitis (629 [13%]) (Table 2). There were 650 in-hospital deaths (13%), and the majority of these were within 7 days of initial MRSA culture (393 [60%]).
Table 2.
Hospitalization Characteristics of Health Care–Associated Community-Onset (HACO), Hospital-Onset (HO), and Community-Associated (CA) MRSA Infectiona Reported to the Emerging Infections Program–Active Bacterial Core Surveillance, United States, 2011
| Characteristic | Infections, No. (%) | P Valuec | |||
|---|---|---|---|---|---|
| HACO | HO | CA | Totalb | ||
| Total | 2912 (100) | 868 (100) | 966 (100) | 4872 (100) | |
| Hospitalized | 2590 (89) | 868 (100) | 876 (91) | 4416 (91) | <.001 |
| Location prior to date of initial culture | |||||
| Incarcerated | 16(1) | 5(1) | 12 (1) | 33 (1) | .07 |
| Homeless | 32(1) | 17 (2) | 25 (3) | 79 (2) | .003 |
| Long-term acute care hospital | 50(3) | 7(1) | 0 | 64 (0) | .053 |
| Long-term care facility | 876 (30) | 139 (16) | 0 | 1046 (21) | <.001 |
| Private residence | 1812(62) | 646 (74) | 910 (94) | 3428 (70) | <.001 |
| Transferred from hospital/acute care facility | 52(2) | 29 (3) | 0 | 83 (2) | <.001 |
| Unknown | 59(2) | 14(2) | 18 (2) | 104 (2) | .73 |
| Other | 12 (0) | 11 (1) | 0 | 31 (1) | <.001 |
| Location of culture collection | |||||
| Emergency department | 1692 (58) | 35 (4) | 584 (60) | 2368 (49) | <.001 |
| Inpatient unit, nonintensive care | 389 (13) | 427 (49) | 133 (14) | 962 (20) | <.001 |
| Intensive care unit | 118 (4) | 224 (26) | 59 (6) | 412 (8) | <.001 |
| Long-term acute care hospital | 57(2) | 3 (0) | 0 | 64(1) | <.001 |
| Long-term care facility | 52(2) | 2 (0) | 0 | 57 (1) | <.001 |
| Outpatient | 299 (10) | 7 (1) | 54(6) | 380 (8) | <.001 |
| Surgery/operating room | 206 (7) | 127 (15) | 101 (10) | 439 (9) | <.001 |
| Unknown | 34(1) | 19 (2) | 25 (3) | 89 (2) | .004 |
| Other | 65 (2) | 24(3) | 10 (1) | 101 (2) | .02 |
| MRSA infections diagnosis type (multiple categories possible for each case)d | |||||
| Any BSI | 2452 (84) | 619 (71) | 738 (76) | 3907 (80) | <.001 |
| With CVC exit site or AV fistula infection | 160 (7) | 21 (3) | 0 | 182 (5) | …e |
| BSI with uncertain focus | 996 (41) | 312 (50) | 226 (31) | 1572 (40) | <.001 |
| Presence of CVC in prior 2 days | 377 (15) | 137 (22) | 0 | 514(13) | …e |
| No CVC documented | 619 (25) | 175 (28) | 226 (31) | 1058 (27) | .21 |
| BSI with other infection focus | 1296 (53) | 286 (46) | 512 (69) | 2153 (55) | <.001 |
| Skin infection (cellulitis, abscess, ulceration) | 656 (27) | 162 (26) | 241 (33) | 1082 (22) | .005 |
| Pneumonia | 441 (15) | 146 (17) | 154 (16) | 760 (16) | .47 |
| Confirmed lower respiratory tract infection | 127 (29) | 58 (40) | 51 (33) | 239 (31) | .02 |
| Osteomyelitis | 346 (12) | 123 (14) | 139 (14) | 629 (13) | .054 |
| Arthritis, joint infection, or bursitis | 227 (8) | 48 (6) | 157 (16) | 440 (9) | <.001 |
| Abscess (not skin) | 180 (6) | 52 (6) | 133 (14) | 370 (8) | <.001 |
| Endocarditis | 150(5) | 48 (6) | 73 (8) | 277 (6) | .02 |
| Urinary tract infection | 191 (7) | 33 (4) | 56 (6) | 285 (6) | .009 |
| Surgical site infection | 119 (4) | 15 (2) | 11 (1) | 146 (3) | <.001 |
| Other | 328 (11) | 109 (13) | 165 (17) | 610 (13) | <.001 |
| Patient outcomes | |||||
| Death (all causes) | 350 (12) | 182 (21) | 94 (10) | 650 (13) | <.001 |
| Death within 7 days of culture (all causes) | 213 (7) | 103 (12) | 59 (6) | 393 (8) | .53 |
Abbreviations: BSI, bloodstream infection; CVC, central vascular catheter; MRSA, methicillin-resistant Staphylococcus aureus.
Defined as MRSA isolated from a normally sterile site.
One hundred twenty-six additional cases with unknown epidemiological category included in total.
χ2 Test (or Fisher exact test if values ≤5) comparing all epidemiologic categories.
Based on physician documentation in admission or discharge summary and/or sterile sites where MRSA was isolated. A single case could have multiple infection types within a 30-day period.
HACO and HO significantly different (P < .05).
Health Care–Associated Infections
Health Care–Associated Community-Onset Infections
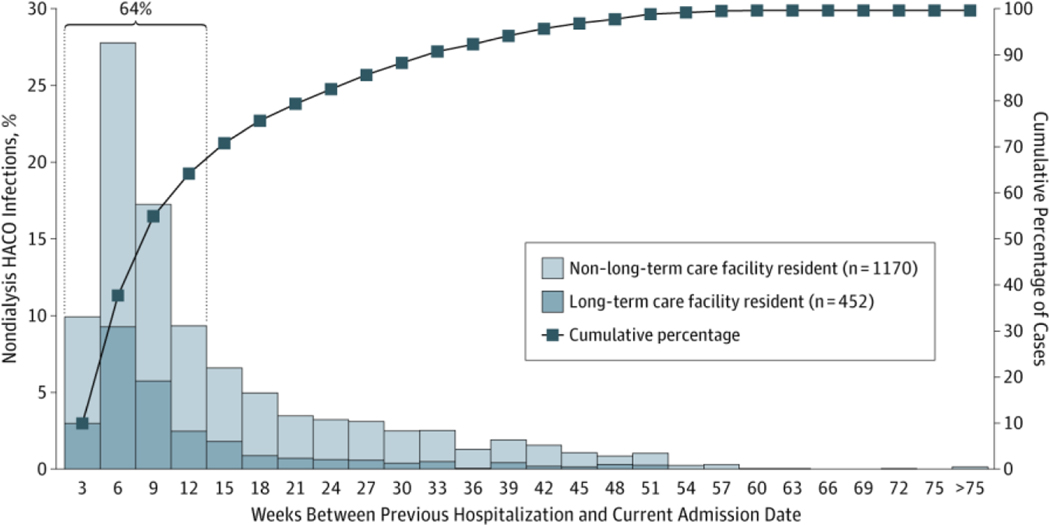
Of the 3780 health care–associated cases, 2912 (77%) had onset in the community and were classified as HACO; median age was 64 years, 59% were male, and 34% were black (Table 1). The median Charlson index score was 3 (range, 0–12), with specific underlying conditions including diabetes (1355 [47%]), decubitus and/or pressure ulcers (394 [14%]), chronic renal insufficiency (1260 [43%]), prior stroke (430 [15%]), and dementia (388 [13%]) all more prevalent compared with cases in other epidemiologic categories. Receipt of dialysis was recorded in 891 cases (31%) in the prior year; a significantly higher proportion compared with hospital-onset cases. Most had resided at a private residence (1812 [62%]) (Table 2), or long-term care facility (876 [30%]) at the time of culture. Few had resided in long-term acute care hospitals, or were homeless, incarcerated, or transferred from another hospital or acute care facility (1%−3% each). Hospitalization in the prior year was a common source of health care exposure (2291 [79%]) (Table 1]), especially among those not receiving dialysis (1622 [80%]). Most cases in this latter group had been hospitalized in the 12 weeks prior to their MRSA infection (1042 [64%]) (Figure 1), and nearly a third of those cases (331 [32%]) were residents of a long-term care facility.
Figure 1. Distribution of Weeks Between Previous Hospitalization and Current Admission Date, Stratified by Long-term Care Facility Residence.
Data are given for nondialysis cases of health care–associated community-onset (HACO) invasive methicillin-resistant Staphylococcus aureus (MRSA) infectiona with hospitalization in the prior year in 2011 (n = 1622b).
aDefined as MRSA isolated from a normally sterile source.
bA total of 399 cases of HACO infections in nondialysis patients who had no prior hospitalization date in the prior year are not represented in this figure.
Hospital-Onset Infections
Underlying conditions among hospital-onset cases were similar to health care–associated community-onset cases except as previously noted. Among hospital-onset cases, 26% were in the intensive care unit, whereas 49% were in other inpatient wards (Table 2). The median length of hospitalization prior to MRSA infection was 7 days (range, 3–366 days). Bloodstream infection was the most common type of hospital-onset infection (619 [71%]), and had either a physician-diagnosed arteriovenous fistula or CVC exit site infection (21 [3%]), an uncertain focus with a CVC (137 [22%]) or without a CVC present (175 [28%]), or other infection foci (286 [46%]). Seven day all-cause mortality was more common among cases with hospital-onset infections (103 [12%]) compared with other epidemiologic categories, as was in-hospital all-cause mortality (182 [21%]).
Community-Associated Infections
Community-associated cases were more commonly male (619 [64%]) and younger than were cases in other epidemiologic categories (54 years) (P < .001) Table 1). Cases had significantly fewer comorbidities, with a median Charlson index score of 0 (range, 0–12). However, this group had a higher proportion of smoking (294 [30%]) (Table 1), intravenous drug use (133 [14%]), and human immunodeficiency virus (HIV)/AIDS (60 [6%]). Most cases lived in private residences prior to positive culture (910 [94%]) (Table 2). This group also had higher proportions of several infection types, including invasive infections associated with skin infections (241 [33%]), arthritis, joint infection, or bursitis (157 [16%]), or nonskin or internal abscess (133 [14%]), compared with cases in other epidemiologic categories. Community-associated cases had a lower in-hospital all-cause mortality rate (10%) compared with other epidemiologic categories.
National Estimated Incidence and All-Cause Mortality
An estimated 80 461 (95% CI, 69 516–93 914) invasive MRSA infections occurred nationally in 2011 (compared with an estimated 111 261 in 2005) (Table 3); of these, 48 353 (95% CI, 40 195–58 642) were HACO, 14 156 (95% CI, 10 096–20 440) were hospital onset, and 16 560 (95% CI, 12 806–21 811) were community associated.
Table 3.
National Estimated Incidence and Mortality of Invasive MRSA Infections,a United States, 2005 and 2011
| Incidence |
Mortalityb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Epidemiologic Category | Year | Surveillance Population | Case Incidence | Crude Ratec | Adjusted National Ratec (95% CI) | Estimated National Incidence (95% CI) | Case Deaths | Crude Ratec | Adjusted National Ratec (95% CI) | Estimated National Deaths (95% CI) |
| HACO | 2005 | 16 489 254 | 3463 | 21.00 | 21.46 (18.06–25.72) | 63 598 (5531–76 237) | 559 | 3.39 | 3.69 (2.58–5.35) | 10 934 (7647–15 858) |
|
| ||||||||||
| 2011 | 19 393 677 | 2912 | 15.02 | 15.52 (12.90–18.82) | 48 353 (40 195–58 642) | 350 | 1.80 | 1.95 (1.26–3.12) | 6071 (3926–9722) | |
|
| ||||||||||
| HO | 2005 | 16 489 254 | 1601 | 9.71 | 9.91 (7.75–12.91) | 29 373 (22 972–38 267) | 418 | 2.53 | 2.71 (1.77–4.36) | 8042 (5246–12 924) |
|
| ||||||||||
| 2011 | 19 393 677 | 868 | 4.48 | 4.54 (3.24–6.56) | 14 156 (10 096–20 440) | 182 | 0.94 | 1.00 (0.51–2.06) | 3126 (1589–6419) | |
|
| ||||||||||
| CA | 2005 | 16 489 254 | 966 | 5.86 | 5.59 (4.31–7.33) | 16 566 (12 775–21 726) | 105 | 0.64 | 0.64 (0.31–1.25) | 1905 (919–3705) |
|
| ||||||||||
| 2011 | 19 393 677 | 1010 | 5.21 | 5.31 (4.11–7.00) | 16 560 (12 806–21 811) | 100 | 0.52 | 0.57 (0.30–1.04) | 1764 (935–3241) | |
|
| ||||||||||
| Overall | 2005 | 16 489 254 | 6134 | 37.20 | 37.54 (32.93–43.06) | 111 261 (97 608–127 634) | 1098 | 6.66 | 7.13 (5.47–9.52) | 21 138 (16 21428 218) |
|
| ||||||||||
| 2011 | 19 393 677 | 4872 | 25.12 | 25.82 (22.31–30.14) | 80 461 (69 516–93 914) | 650 | 3.35 | 3.62 (2.58–5.31) | 11 285 (8039–16 545) | |
Abbreviations: CA, community-associated; HACO, health care–associated community-onset; HO, hospital-onset; MRSA, methicillin-resistant Staphylococcus aureus.
Defined as MRSA isolated from a normally sterile site.
Mortality due to all causes during hospitalization where invasive MRSA infection was diagnosed.
Rate per 100 000 persons.
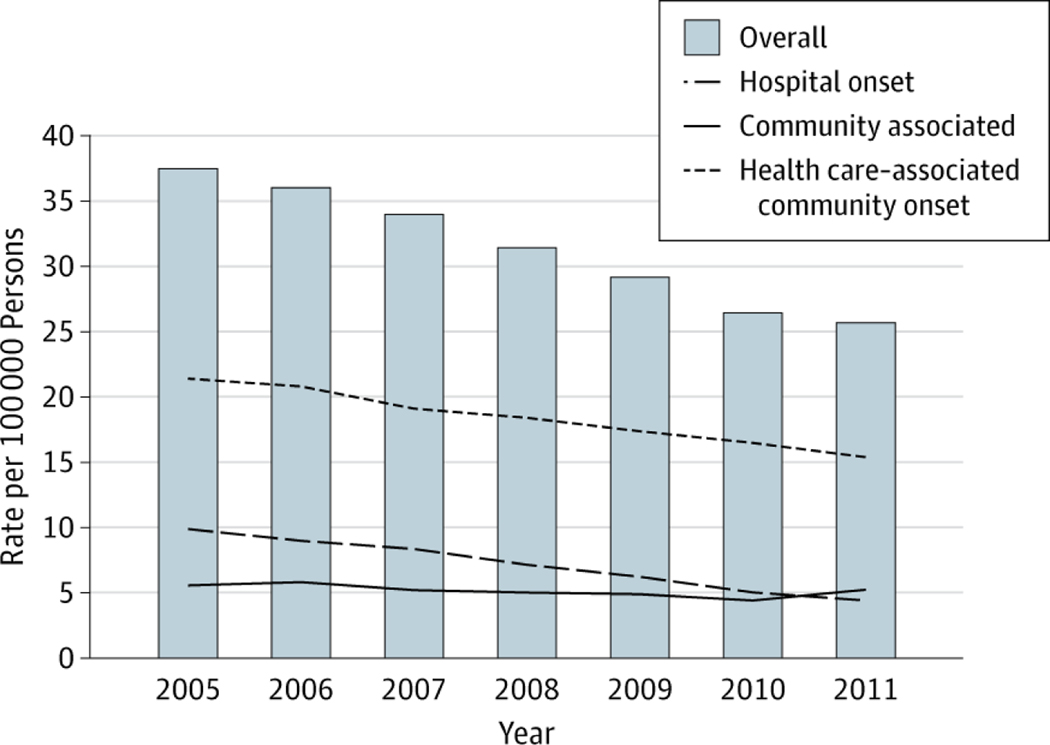
National estimated incidence rates from 2005 through 2011 are illustrated in Figure 2. Compared with 6 years earlier (2005), the estimated national rate of invasive MRSA has decreased by 31.2%. Although this rate decrease was most precipitous among hospital-onset infections, at 54.2%, rate decreases were evident among other categories as well: health care– associated community-onset by 27.7% and community-associated infections by 5.0%.
Figure 2. National Estimated Incidence Rates of Invasive MRSA Infections, Stratified by Epidemiologic Categorya.
Data are given for methicillin-resistant Staphylococcus aureus (MRSA) infections reported to the Emerging Infections Program–Active Bacterial Core surveillance (United States, 2005–2011).
aDefined as MSRA isolated from a normally sterile source.
An estimated 11 285 (95% CI, 8039–16 545) persons with invasive MRSA infections died of all causes during their hospitalizations in 2011 (Table 3); of these estimated deaths, 6071 (95% CI, 3926–9722) had health care–associated community-onset, 3126 (95% CI, 1589–6419) had hospital-onset, and 1764 (95% CI, 935–3241) had community-associated infections.
Discussion
In 2011, we estimated the overall number of invasive MRSA infections was 80 461; 31% lower than when estimates were first available in 2005. Also, for the first time since the CDC began tracking invasive MRSA infections nationally, the estimated incidence of invasive hospital-onset infections is lower than that among persons residing in the community without significant health care contact. However, the largest burden of disease remains among patients with infection onset outside of acute care hospitals but with recent or ongoing exposure to health care services, such as recent discharge from acute care hospitals. In these patients, infection rates only decreased moderately. Overall, these estimates indicate the United States is on track to meet the Department of Health and Human Services 2013 target of reducing health care–associated MRSA invasive infections by 50%.11 However, little progress has been made in reducing invasive community-associated MRSA infections during this time.
Our findings strongly support those from other studies that have documented decreasing incidence of invasive MRSA infections among health care–related infections since 2005.2,9 While prior studies focused on invasive MRSA incidence for a specific population or region,9,20 our study used data from the largest US population surveillance system for invasive MRSA to provide robust national burden estimates of this disease. The methodology in this study was improved from our prior methods3 (and applied to historical 2005 data) by (1) including all cases, both recurrent infections in the same patient (at least 30 days apart) and new infections, (2) adjusting national estimates for the receipt of dialysis, a critical risk factor for invasive MRSA,10 and (3) including confidence intervals of national estimates.
The large decrease (54%) in hospital-onset invasive MRSA infections between 2005 and 2011 is highly encouraging and may be attributable to increased awareness and implementation of local and nationwide infection prevention measures in many health care settings, including those targeting intravascular catheter-related infections21,22 and health care transmission of multidrug-resistant organisms.23 National survey data suggest that the number of hospitalizations and the average length of stay were stable between 2007 and 2010,24,25 making it unlikely that the reductions in hospital-onset MRSA we observed could be attributable to reductions in length of hospital stays.
It is notable that the incidence of community-associated invasive MRSA infections, although relatively stable, has not increased over this time, despite increases in hospitalizations related to MRSA skin and soft-tissue infections (ie, mostly noninvasive infections) documented in discharge data.7 Progress in reducing infections among this population is likely to be most challenging due to a lack of clearly effective strategies to control endemic MRSA transmission in the community setting. While guidance exists for the prevention of transmission in some institutions such as schools, athletic facilities, and correctional facilities,26 prevention of community-associated MRSA transmission outside of these settings is not well described. Changes in this setting may related to transmission in households, prevention of invasive disease from improved early treatment of noninvasive infections,27 or the natural evolution of this pathogen.
Approximately 78% of all invasive MRSA infections in 2011 had onset in the outpatient or community setting (health care–associated community-onset and community-associated infections), an increase from 72% in 2005. Of these infections, 77% were classified as HACO, which comprised mostly recently discharged patients, long-term care residents, and dialysis patients. The moderate decrease of these infections could be attributable to decreased transmission in hospital settings or prevention efforts outside of the acute care hospital. Among nondialysis cases with prior hospitalization, nearly two-thirds developed their infections within 3 months of hospital discharge. This suggests a higher risk of invasive MRSA in the weeks following hospitalization. Invasive devices that remain placed during the postdischarge period, progression from colonization to clinical infection, and breakdowns in host defense and skin integrity during hospitalization may account for this increased risk. Further research is needed to understand (1) risk factors for progression of colonization and noninvasive infection to invasive infections and (2) transmission dynamics within health care settings after discharge from acute care hospitals. Significant progress in preventing invasive MRSA infections in the dialysis and postdischarge settings is needed to substantially reduce the overall burden of invasive MRSA infections.
There are several limitations to this study. First, although this program operates in several metropolitan areas with diverse populations, these specific areas were not randomly selected to be representative; however, when calculating national estimates, we adjusted for regional differences in sex, age, race, and use of dialysis to produce estimates that more accurately represent the entire US population. In addition, this surveillance system does not track MRSA colonization or noninvasive MRSA infections, such as skin and soft-tissue infections. Therefore, we cannot draw any conclusions about the relatedness between MRSA colonization or prior noninvasive infection and invasive infection. However, this system was designed to create national estimates capturing infections in both inpatient and outpatient settings and therefore delivers a more comprehensive assessment of invasive MRSA than systems focused predominantly on acute care facility–specific performance measures, including NHSN.
In conclusion, a substantial decrease in the national burden of invasive MRSA infections has been observed in the United States between 2005 and 2011, with the largest decreases among hospital-onset infections and the smallest among community-associated infections. Despite these decreases, invasive MRSA infections with onset in the community or outpatient setting remain problematic and represent the majority of invasive MRSA infections. Future research is needed to understand the progression of colonization and noninvasive MRSA infection to invasive infection in outpatient settings. Future prevention efforts should target both community and health care transmission, especially among patients with recent hospitalization.
Supplementary Material
Funding/Support:
This work was supported by the Emerging Infections Program at the Centers for Disease Control and Prevention (CDC) and the National Center for Emerging and Zoonotic Infectious Diseases.
Role of the Sponsors:
The Emerging Infections Program at the CDC provided financial and material support for conduction of the study, data collection, and management. The National Center for Emerging and Zoonotic Infectious Diseases at the CDC provided financial and material support for conduction of the study, study design, analysis, interpretation of data, preparation, review, and approval of the manuscript.
Disclaimer:
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. Some of the data reported herein have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of the US government.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Hidron AI, Edwards JR, Patel J, et al. ; National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare–associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29(11):996–1011. [DOI] [PubMed] [Google Scholar]
- 2.Kallen AJ, Mu Y, Bulens S, et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators of the Emerging Infections Program. Health care–associated invasive MRSA infections, 2005–2008. JAMA. 2010;304(6):641–648. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, et al. ; Active Bacterial Core surveillance (ABCs) MRSA Investigators. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298(15):1763–1771. [DOI] [PubMed] [Google Scholar]
- 4.Weber JT. Community-associated methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2005;41(suppl 4):S269–S272. [DOI] [PubMed] [Google Scholar]
- 5.Talan DA, Krishnadasan A, Gorwitz RJ, et al. ; EMERGEncy ID Net Study Group. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin Infect Dis. 2011;53(2):144–149. [DOI] [PubMed] [Google Scholar]
- 6.Klein E, Smith DL, Laxminarayan R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg Infect Dis. 2007;13(12):1840–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.David MZ, Medvedev S, Hohmann SF, Ewigman B, Daum RS. Increasing burden of methicillin-resistant Staphylococcus aureus hospitalizations at US academic medical centers, 2003–2008. Infect Control Hosp Epidemiol. 2012;33(8):782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301(7):727–736. [DOI] [PubMed] [Google Scholar]
- 9.Landrum ML, Neumann C, Cook C, et al. Epidemiology of Staphylococcus aureus blood and skin and soft tissue infections in the US military health system, 2005–2010. JAMA. 2012;308(1):50–59. [DOI] [PubMed] [Google Scholar]
- 10.Patel PR, Kallen AJ, Arduino MJ. Epidemiology, surveillance, and prevention of bloodstream infections in hemodialysis patients. Am J Kidney Dis. 2010;56(3):566–577. [DOI] [PubMed] [Google Scholar]
- 11.US Department of Health & Human Services. National Targets and Metrics: Monitoring Progress Toward Action Plan Goals. Washington, DC: US Department of Health & Human Services; 2011. [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. Vintage 2005 Bridged-race postcensal population estimates for July 1, 2000–July 1, 2005, by year, county, single-year of age (0 to 85+ years), bridged-race, Hispanic origin, and sex. 2006. www.cdc.gov/nchs/nvss/bridged_race.htm. Accessed July 3, 2012.
- 14.National Center for Health Statistics. Vintage 2011 Bridged-race postcensal population estimates for July 1, 2010–July 1, 2011, by year, county, single-year of age (0 to 85+ years), bridged-race, Hispanic origin, and sex. 2012. www.cdc.gov/nchs/nvss/bridged_race.htm. Accessed March 3, 2013.
- 15.US Census Bureau. Annual Estimates of the Population for the United States and States, and for Puerto Rico: April 1, 2000 to July 1, 2005. www.census.gov/popest/data/historical/2000s/vintage_2005/index.html. Accessed July 10, 2012.
- 16.US Census Bureau. Annual Estimates of the Resident Population for the United States, Regions, States, and Puerto Rico: April 1, 2010 to July 1, 2011. www.census.gov/popest/data/historical/2010s/vintage_2011/index.html. Accessed March 28, 2013.
- 17.US Renal Data System. USRDS 2005 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Vol 2005. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 18.US Renal Data System. USRDS 2011 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. Vol 2012. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2011. [Google Scholar]
- 19.Fay MP, Feuer EJ. Confidence intervals for directly standardized rates: a method based on the gamma distribution. Stat Med. 1997;16(7):791–801. [DOI] [PubMed] [Google Scholar]
- 20.Hadler JL, Petit S, Mandour M, Cartter ML. Trends in invasive infection with methicillin-resistant Staphylococcus aureus, Connecticut, USA, 2001–2010. Emerg Infect Dis. 2012;18(6):917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health Research & Education Trust of the American Hospital Association. On the CUSP: Stop BSI, funded by the Agency for Healthcare Research and Quality. 2009. www.onthecuspstophai.org/on-the-cuspstop-bsi/. Accessed August 17, 2012.
- 22.O’Grady NP, Alexander M, Burns LA, et al. Guidelines for the Prevention of Intravascular Catheter-Related Infections. Atlanta, GA: Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 23.Siegel JD, Rhinehard E, Jackson M, Chiarello L. Management of Multidrug-Resistant Organisms in Healthcare Settings, 2006. Atlanta, GA: Centers for Disease Control and Prevention; 2006. [Google Scholar]
- 24.National Center for Health Statistics. National Hospital Discharge Survey: number, percent distribution, rate, days of care with average length of stay, and standard error of discharges from short-stay hospitals, by sex and age: United States, 2007. www.cdc.gov/nchs/data/nhds/2average/2007ave2_ratesexage.pdf. Accessed May 21, 2013.
- 25.National Center for Health Statistics. National Hospital Discharge Survey: number, percent distribution, rate, days of care with average length of stay, and standard error of discharges from short-stay hospitals, by sex and age: United States, 2010. www.cdc.gov/nchs/data/nhds/2average/2010ave2_ratesexage.pdf. Accessed May 25, 2013.
- 26.Centers for Disease Control and Prevention. MRSA infections: prevention of MRSA Infections. 2010; www.cdc.gov/mrsa/prevent/index.html. Accessed July 11, 2012.
- 27.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.