Abstract
INTRODUCTION:
Proton pump inhibitors (PPIs) modulate the progression of cirrhosis to hepatic encephalopathy (HE) and can affect the bacterial microbiome. However, the impact of PPI on the virome in cirrhosis using viral-like particle (VLP) analysis is unclear.
METHODS:
We determined the VLP in the stool microbiome in patients with cirrhosis cross-sectionally (ascites, HE, and PPI use analyzed) who were followed up for 6-month hospitalizations and through 2 clinical trials of PPI withdrawal and initiation.
RESULTS:
In a cross-sectional study, PPI users had greater ascites prevalence and 6-month hospitalizations, but VLP α diversity was similar. Among phages, PPI users had lower Autographviridae and higher Streptococcus phages and Herelleviridae than nonusers, whereas opposite trends were seen in ascites and HE. Trends of eukaryotic viruses (higher Adenoviridae and lower Virgaviridae/Smacoviridae) were similar for PPI, HE, and ascites. Twenty-one percent were hospitalized, mostly due to HE. α Diversity was similar in the hospitalized/nonhospitalized/not groups. Higher Gokushovirinae and lower crAssphages were related to hospitalizations such as HE-related cross-sectional VLP changes. As part of the clinical trial, PPIs were added and withdrawn in 2 different decompensated groups over 14 days. No changes in α diversity were observed. Withdrawal reduced crAssphages, and initiation reduced Gokushovirinae and Bacteroides phages.
DISCUSSION:
In cirrhosis, PPI use has a gut microbial VLP phage signature that is different from that in HE and ascites, and VLP changes are linked with hospitalizations over 6 months, independent of clinical biomarkers. Eukaryotic viral patterns were consistent across PPI use, HE, and ascites, indicating a relationship with the progression of cirrhosis. PPIs alone showed modest VLP changes with withdrawal or initiation. Distinct phage and eukaryotic viral patterns are associated with the use of PPIs in cirrhosis.
KEYWORDS: cirrhosis, hepatic encephalopathy, clinical trial, viral-like particles, hospitalizations, bacteriophage, decompensated
INTRODUCTION
Cirrhosis is associated with alterations in the gut microbiota, which can affect outcomes and progression toward decompensation (1). Proton pump inhibitors (PPIs) are major modifiers of the gut microbiome and are used extensively in patients with cirrhosis (2). PPI use adversely affects the bacterial structure and function in compensated and decompensated cirrhosis (2,3). Moreover, PPI use is associated with complications of gut microbial origin, such as hepatic encephalopathy (HE) and spontaneous bacterial peritonitis (SBP) (4,5). However, the unique impact of PPI rather than HE or ascites on nonbacterial components of the gut microbiome, such as the virome, is important because they constitute a large fraction of this microbial population (6). Of importance, bacterial pathobionts such as bacteriophages have been associated with liver disease because phages can affect the intestinal bacteriome (7). Prior research on cirrhosis has shown alterations in virome using shotgun metagenomic sequencing in decompensated patients and in infected patients (8,9). While this technique is sensitive in detecting most DNA phages, there could remain a significant proportion of viral dark matter that can only be detected using other direct techniques that isolate viral-like particles (VLPs) (10), which include self-assembled structures that resemble intact viral particles but lack the viral genome or possess a noninfectious genome (11). VLP analysis in the precirrhotic stages of liver diseases has shown changes between healthy controls and patients with liver disease (12), but their analysis in cirrhosis and the impact of PPI needs to be investigated.
Our aim was to determine changes in gut microbial VLPs in patients with cirrhosis with PPI use and complications of cirrhosis cross-sectionally and over 6-month follow-up for hospitalizations and to further evaluate the impact of PPI using trials of PPI withdrawal and initiation.
METHODS
We performed a cross-sectional analysis and 2 trials (4):
Cross-sectional analysis
Patients with cirrhosis were included after informed consent was obtained. Cirrhosis was diagnosed using either liver biopsy, evidence of decompensation, evidence of high stiffness on transient elastography, or a platelet count <150,000 in the setting of liver disease and/or radiological evidence of cirrhosis. We collected data on demographics, cirrhosis details (etiology, model for end-stage liver disease [MELD] score), prior complications (ascites, variceal bleeding, SBP), and medications (SBP prophylaxis, PPIs, lactulose, rifaximin, and recent antibiotics beyond 1 month). Stool was collected to determine gut viral composition. PPI use was defined as the continuous use of PPIs daily for at least 1 month before stool collection; patients using less than this were considered nonusers.
PPI withdrawal trial
PPI withdrawal trial was performed in patients with decompensated cirrhosis defined by prior variceal bleeding or controlled ascites (on diuretics) who were on PPI for an indication other than a Food and Drug Administration (FDA)–approved indication for at least 1 month before enrollment were included (4). The FDA-approved indications for PPI were (i) erosive esophagitis healing and maintenance, (ii) gastroesophageal reflux disease therapy, (iii) nonsteroidal anti-inflammatory drug–related peptic ulcer risk reduction, (iv) Helicobacter pylori eradication with antibiotics, (v) pathological hypersecretory conditions, and (vi) short-term treatment and maintenance of peptic ulcers.
We excluded patients who did not consent to withdraw PPI, patients on therapy for HE (rifaximin, lactulose), those on SBP prophylaxis, and those on recent (6 weeks) antibiotics or probiotics.
Stool was collected when patient was on PPI (baseline) and then patients were asked to withdraw from PPI therapy for 14 days. Phone calls to assess safety, tolerability, and adherence were made on day 7 and on day 14 when the study ended, and a repeat stool collection was performed on day 14. All the subjects were instructed to follow their usual diet. Safety and tolerability, emergence of new cirrhosis complications, MELD score, and gut virus composition were analyzed at baseline and 14 days after PPI withdrawal.
PPI initiation trial
A separate cohort of outpatients with decompensated cirrhosis (as described earlier) who were not on PPI was recruited (4). We excluded those who were allergic to PPI, those on HE treatments, those on SBP prophylaxis, or those on recent (6 weeks) antibiotics/probiotics. We performed this only for 2 weeks for the PPI initiation study because prior studies have shown that use of PPI for >2 weeks can result in symptoms after PPI withdrawal (13). All patients underwent stool collection at baseline before PPIs were started and were administered oral omeprazole (40 mg) for 14 days. A phone call to assess safety, tolerability, and adherence was made on day 7, and repeat stool collection was performed on day 14. The participants were again instructed to follow their usual diet. Safety and tolerability, emergence of new cirrhosis complications, MELD score, and gut virus composition were analyzed before and after PPI therapy.
Both trials were published for clinical and 16SrRNA analysis data and were registered at www.clinicaltrials.gov (4). This is an analysis of the virome of the previously collected data.
This protocol was approved by the IRBs of Virginia Commonwealth University and Richmond VA Medical Center, and all participants provided written informed consent before study participation.
Stool collection
Stool was collected using previously published home collection kits within 3 hours of spontaneous passage and stored in glycerol at −80 °C until analysis (4,8).
VLP analysis
VLP analysis was performed as published articles (14,15). Specifically, stool VLP was prepared from all samples as previously described (14,16). These were performed under the supervision of Scott Handley.
Only abundant contigs were analyzed, those with at least 100 (transcripts per million) tpm in the dataset, with a threshold of at least 5 tpm mapped to the contig by Kallisto and at least 5 tpm present in 3% of the samples. Taxonomy was assigned to each contig using blastn queries against a viral genome database composed of all viral genomes in NCBI RefSeq. Mean log10 scaled abundances were analyzed for differences between patients with cirrhosis ± PPI use, with and without HE, and with and without ascites. We also followed up the patients for 6 months for nonelective hospitalizations.
Statistical analysis
Initially, we applied 2-sample Wilcoxon tests and Fisher exact tests for the cross-sectional cohort (PPI/not, HE/not, and ascites/not), while for the trials, we applied the pairwise 2-sample Wilcoxon test for clinical characteristics. MAAsLin2 was used for factors (viral and others) associated with hospitalizations, while controlling for demographics, cirrhosis severity, and medications using false discovery rate correction with q value <0.10 (17). TMM normalization and LOG transformation was applied for the linear model. P value threshold was set to 0.05, and false discovery rate correction was adjusted with the Benjamini-Hochberg procedure for multivariate comparison control.
All analyses were performed using R (R Foundation for Statistical Computing, Vienna, Austria) version 4.2, α diversity indexes were obtained with the phyloseq package (18), and significance was assessed using the Kruskal-Wallis test for cross-sectional studies and Wilcoxon paired test for the trails for VLP. Differential abundance test was performed with the DESeq2 package (19), which uses a negative binomial distribution to model the data, hypothesis testing was performed with the Wald test, and multiple inference correction with Benjamini-Hochberg, a P value <0.05, and a log2 fold change >2 were considered statistically significant.
RESULTS
Cross-sectional analysis
Ninety patients with cirrhosis were enrolled in this study (Table 1). The mean age was 62.1 ± 7.0 years, with most of them (N = 72) being men. The predominant etiology was alcohol in 29 patients, followed by hepatitis C plus alcohol (n = 12), hepatitis C alone (n = 15), metabolic dysfunction–associated steatotic liver disease (n = 17), and other etiologies (N = 3). Thirty-eight patients had prior HE, all of whom were on lactulose and 28 on additional rifaximin therapy. Forty-one patients had ascites controlled with spironolactone and furosemide, and 52 patients were on PPI therapy. The PPI used was omeprazole in all patients, with a mean dose of 31 ± 17 mg/d. PPI users had similar MELD scores and rates of prior HE, rifaximin, and lactulose use (25% vs 31%, P = 0.19) but were higher than those of nonusers (Table 1). They were also more likely to be men and were hospitalized at 6 months. Patients on PPI were mainly due to gastroesophageal reflux disease (n = 31), Barrett's esophagus (n = 7), prior peptic ulcer disease (n = 3), and no specific indications (n = 11), and the average duration of PPI use before study enrollment was 17 ± 7 months.
Table 1.
Comparison between PPI users and nonusers in the cross-sectional analysis
| No PPI (n = 38) | PPI (n = 52) | P value | |
| Age, mean ± SD | 62.0 ± 6.2 | 63.4 ± 7.7 | 0.38 |
| Men, n (%) | 35 (9) | 37 (71) | 0.02 |
| Diabetes, n (%) | 12 (32) | 19 (27) | 0.29 |
| Etiology of cirrhosis, n (%) | 0.36 | ||
| Alcohol | 10 (26) | 10 (19) | 0.44 |
| HCV only | 9 (24) | 20 (38) | 0.58 |
| HCV + alcohol | 8 (21) | 4 (8) | 0.11 |
| MASH | 9 (24) | 15 (29) | 0.44 |
| Others | 2 (6) | 3 (6) | 1.0 |
| MELD score, mean ± SD | 10.9 ± 6.3 | 11.7 ± 5.4 | 0.52 |
| Ascites, n (%) | 11 (30) | 30 (58) | 0.006 |
| Prior HE, n (%) | 14 (37) | 24 (46) | 0.38 |
| Prior upper GI bleeding, n (%) | 2 (6) | 5 (10) | 0.69 |
| Lactulose use, n (%) | 14 (37) | 24 (46) | 0.38 |
| Rifaximin use, n (%) | 9 (24) | 19 (27) | 0.07 |
| 6-mo hospitalizations, n (%) | 4 (11) | 15 (29) | 0.04 |
Comparisons made using nonparametric (the χ2 or Fisher exact test) and parametric tests (t test).
GI, gastrointestinal; HCV, hepatitis C virus; HE, hepatic encephalopathy; MASH, metabolic dysfunction–associated steatohepatitis; MELD, model for end-stage liver disease; PPI, proton pump inhibitor.
During the 6-month follow-up period, 19 (21%) patients were hospitalized. Most hospitalizations were due to HE (n = 9), followed by infections (n = 5), ascites and acute kidney injury (n = 3), and liver-related hospitalizations (n = 2). Hospitalized patients were more likely to have ascites, have a higher MELD score, and be on a PPI (Table 3). There was a trend toward higher lactulose and rifaximin use but statistically similar age, gender, diabetes, and cirrhosis etiology between groups that were hospitalized and those that were not.
Table 3.
Comparison of clinical and VLP characteristics in those who were hospitalized over 6 months vs those who did not
| Not hospitalized (n = 71) | Hospitalized (N = 19) | P value | |
| Age, mean ± SD | 62.7 ± 7.5 | 60.7 ± 7.4 | 0.30 |
| Men, n (%) | 68 (96) | 19 (100) | 1.0 |
| Diabetes, n (%) | 27 (38) | 8 (42) | 0.75 |
| Etiology of cirrhosis, n (%) | 0.50 | ||
| Alcohol | 22 (31) | 8 (42) | 0.89 |
| HCV only | 16 (23) | 3 (16) | 0.75 |
| HCV + alcohol | 9 (13) | 3 (16) | 0.72 |
| MASH | 19 (27) | 5 (26) | 0.96 |
| Others | 5 (7) | 0 (0) | 0.58 |
| MELD score, mean ± SD | 10.0 ± 3.5 | 16.4 ± 9.2 | 0.008 |
| Ascites, n (%) | 26 (37) | 15 (79) | 0.001 |
| Prior HE, n (%) | 27 (38) | 11 (58) | 0.12 |
| PPI use, n (%) | 37 (48) | 15 (79) | 0.04 |
| Lactulose use, n (%) | 24 (34) | 11 (58) | 0.06 |
| Rifaximin use, n (%) | 22 (31) | 10 (52) | 0.09 |
| VLP characteristics | |||
| Observed species, mean ± SD | 4.04 ± 1.70 | 4.53 ± 2.20 | 0.38 |
| Chao1, mean ± SD | 7.01 ± 7.24 | 10.63 ± 12.02 | 0.23 |
| Shannon, mean ± SD | 1.13 ± 0.51 | 1.27 ± 0.60 | 0.36 |
| Viruses higher on DESeq2a | CrAssphage sp. cat21691 | crAss-like viruses sp. cat29880, 28533, 23050 Human mastadenovirus A sp. cat27160 Gokushovirus WZ-2015a sp. cat4053 |
<0.05 |
Kruskal-Wallis tests were applied for statistical analysis, and no significance was found among alpha diversity indexes.
HCV, hepatitis C virus; HE, hepatic encephalopathy; MASH, metabolic dysfunction–associated steatohepatitis; MELD, model for end-stage liver disease; PPI, proton pump inhibitor; VLP, viral-like particle.
DESeq2 was used for differential abundance analysis, and all results shown have a Benjamini and Hochberg false discovery rate–adjusted P value <0.05 and a log2 fold change >2.0.
VLP enrichment analysis
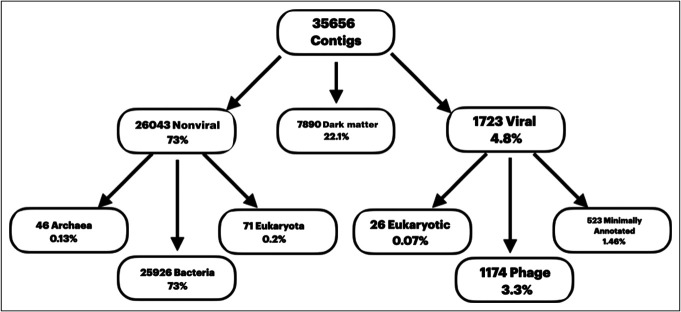
As shown in Figure 1, most contigs were nonviral, followed by dark matter, and 4.8% were viral. Of these, most viruses are phages, followed by eukaryotic viruses.
Figure 1.
Results of the cross-sectional analysis with viral and nonviral taxa and dark matter distribution.
Cross-sectional comparisons
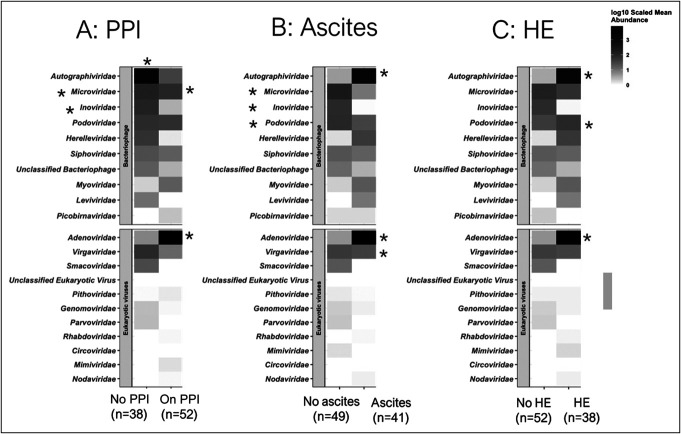
When we created heatmaps based on viral family abundance between HE/no HE, ascites/no ascites, PPI/no PPI, and hospitalization/no hospitalization groups, we found similarities and key differences between the groups (Figure 2, Table 3) using DESeq2.
Figure 2.
Family-level heatmap of differences using Kruskal-Wallis tests. Data are presented as log-scaled abundance, and asterisks indicate significant changes. (a) Comparison between PPI and no PPI, (b) comparison between ascites and no ascites, (c) comparison between HE and no HE. HE, hepatic encephalopathy; PPI, proton pump inhibitor.
Alpha diversity
Cross-sectional comparison between HE, ascites, and hospitalization showed no significant changes in Shannon, observed species, or richness using Kruskal-Wallis tests.
Family-level analysis
Phage families
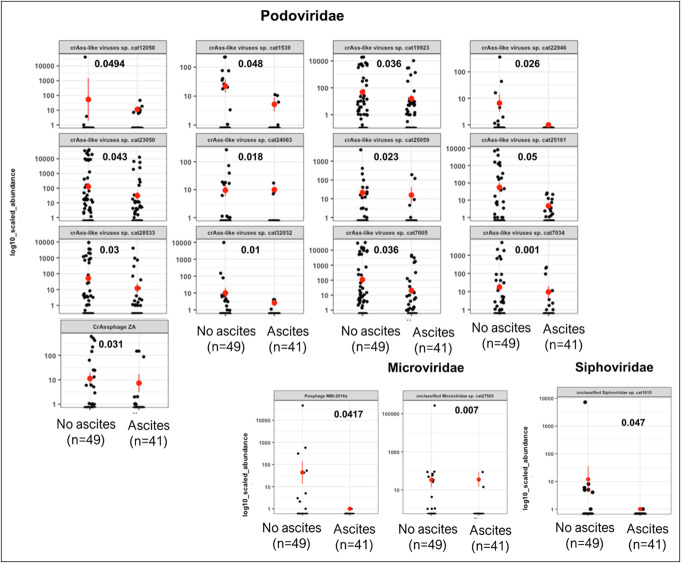
Podoviridae were largely similar across all comparisons (Figures 3–5, Table 2 and Supplementary Figures S1–3, Supplementary Digital Content 2, http://links.lww.com/CTG/B41). Similar patterns for Myoviridae were observed, that is, they were higher in ascites, HE, and PPI users than in their counterparts. By contrast, Microviridae were only lower in patients with ascites but were relatively similar between PPI users/not and HE/no HE patients.
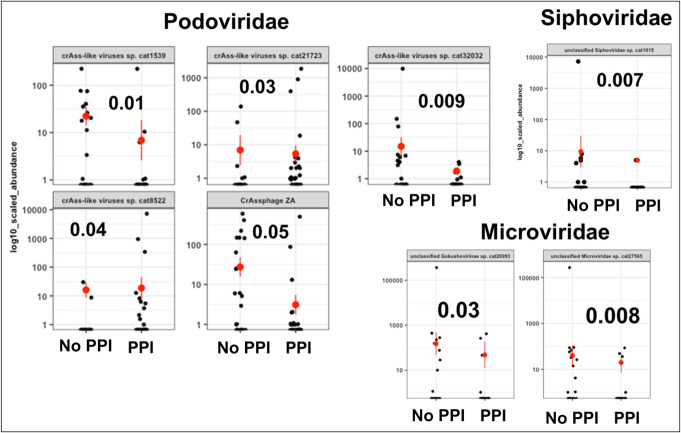
Figure 3.
Representative changes in viral families in patients with and without PPI using Kruskal-Wallis tests. PPI, proton pump inhibitor.
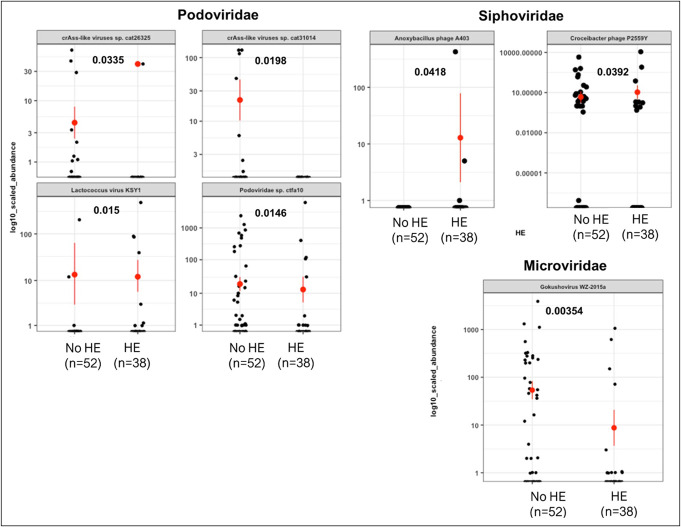
Figure 5.
Representative changes in viral families in patients with and without HE using Kruskal-Wallis tests. HE, hepatic encephalopathy.
Table 2.
Viral-like particle analysis on the cross-sectional analysis
| Mean ± SD for α diversity values | PPIs | HE | Ascites | |||
| On PPI (n = 38) | Not on PPI (n = 52) | No HE (N = 52) | HE (N = 38) | No ascites (n = 49) | Ascites (N = 41) | |
| Observed | 13.72 ± 7.12 | 12.89 ± 7.34 | 3.98 ± 1.68 | 4.14 ± 2.08 | 3.92 ± 1.77 | 4.20 ± 1.92 |
| Chao1 | 21.22 ± 14.64 | 19.76 ± 15.27 | 6.69 ± 6.60 | 8.27 ± 9.74 | 6.71 ± 6.90 | 8.03 ± 9.13 |
| Shannon | 1.67 ± 0.71 | 1.56 ± 0.81 | 1.11 ± 0.51 | 1.15 ± 0.60 | 1.08 ± 0.54 | 1.81 ± 0.57 |
| Viruses higher on DESeq2a | Microviridae sp.cat27563 crAss-like viruses (sp. Cat 25161, 28533, 7034, 7055) Tobamavirus cat 26963 Streptococcus virus C1 cat 27547 Bacteroides phage crass 001 cat 27495 Bacteriophage cat 28848, 28849 |
Siphoviridae sp. cat16818 crAss-like viruses sp. cat8522, 21723, 13435 Human mastadenovirus A sp. cat27160, 28854 Bacteroides phage crAss001 sp. Cat14543, 27059 |
Drulisvirus sp. cat28994, 27004 Unclassified Siphoviridae sp. cat16818 Bacteroides phage crAss001 sp. cat27059 |
Uncultured crAssphage sp. cat33595 Unclassified Microviridae sp. cat27563 Gokushovirus WZ-2015a sp. cat 4053 Unclassified Gokushovirinae sp cat26993 crAss-like viruses sp. cat8522 Bacteroides phage crAss001 sp. Cat14543 |
Unclassified Microviridae sp. cat27563 CrAssphage cat21691 unclassified Siphoviridae cat16818 Drulisvirus cat27004 crAss-like viruses cat 21723 |
CrAss-like viruses (sp. cat 25161, 21132, 8522, 25059) Bacteriophage sp. cat28848 |
Kruskal-Wallis tests were applied for statistical analysis, and no significance was found among alpha diversity indexes.
HE, hepatic encephalopathy; PPI, proton pump inhibitor.
DESeq2 was used for differential abundance analysis, and all results shown have a Benjamini and Hochberg false discovery rate-adjusted P value <0.05 and a log2 fold change >2.0.
Figure 4.
Representative changes in viral families in patients with and without ascites using Kruskal-Wallis tests.
Patients on PPI showed unique changes that were opposite to those observed in ascites and HE. PPI users had lower Autographviridae, and higher Levidviridae and Herelleviridae compared with nonusers, while the opposite (higher Autographviridae and lower Leviviridae and Herelleviridae) patterns were seen in ascites vs no ascites and HE vs no HE.
Eukaryotic families
PPI users and those with HE and ascites had similar patterns of change compared with nonusers and those without HE or ascites, respectively. These were higher in Adenoviridae and lower in Virgaviridae and Smacoviridae.
DESeq2 cross-sectional analysis for PPI
On DESeq2, PPI use was associated with higher Microviridae, crAss-like viruses, Bacteroides phage crAss001, and Streptococcus virus C1. Patients without PPI had greater unclassified Siphoviridae, along with human mastadenovirus, crAss-like viruses, and a Bacteroides phage crAss001 cat 27495 (Table 2).
Hospitalizations
As summarized in Table 3, there were no changes in α diversity in those who were hospitalized or not. In those who were hospitalized, DESeq2 showed higher phages (Gokushovirus, crAss-like) and eukaryotic viruses (human mastadenovirus, Supplementary Figure S4, Supplementary Digital Content 2, http://links.lww.com/CTG/B41, Supplementary Table S1, Supplementary Digital Content 1, http://links.lww.com/CTG/B40)
Species-level analysis
Because phages showed major differences, we used Wilcoxon and Fisher exact tests to compare individual genera and species (Figures 3–5). Among Podoviridae, most crAss-like phages were lower in PPI users, patients with ascites, and patients with HE compared with their respective counterparts. In Microviridae, there was a lower Gokushovirinae abundance in both patients with HE and those with PPI use, but this change was not observed in patients with or without ascites. In patients with ascites, there was a lower Poophage (Microviridae), which was not observed in other comparisons. In addition, in patients with HE, there was a higher abundance of Anoxybacillus phage A403 and Croceibacter phage P2559Y and a lower Lactococcus phage than in patients without HE.
PPI initiation and withdrawal trials
PPI withdrawal trial
Eight men with cirrhosis and controlled ascites (on diuretics) who were on PPI for an unapproved indication (all were on omeprazole at a mean dose of 36 ± 47 mg/d for an unclear indication) for 15 ± 6 months underwent successful withdrawal of PPI over 13 ± 3 days. No clinical symptoms related to PPI withdrawal or cirrhosis-related complications were observed. The MELD score remained unchanged from baseline to end (12.2 ± 3.6 vs 12.5 ± 2.9).
PPI initiation trial
Eight men with cirrhosis and ascites controlled with diuretics were included. All subjects were started on omeprazole (40 mg/d), which was continued for 14 ± 4 days. No interval issues were noted, and the MELD score was statistically similar at both time points (12.7 ± 5.6 vs 12.1 ± 3.8).
VLP changes with PPI initiation and withdrawal trials
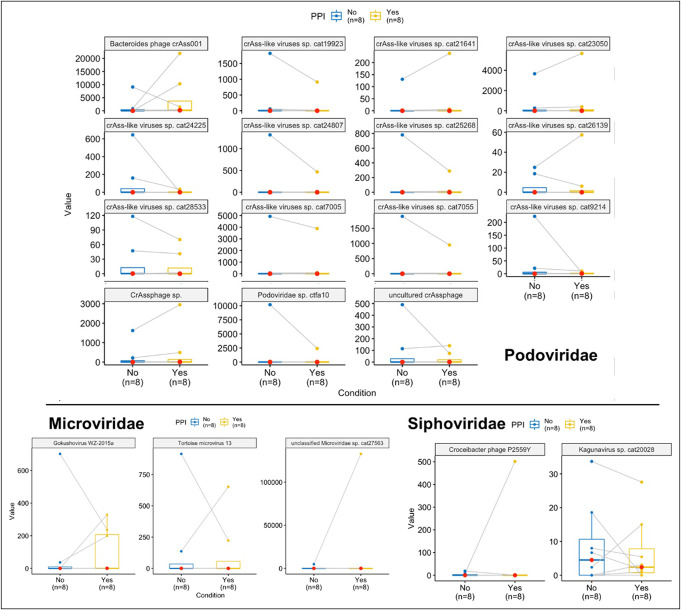
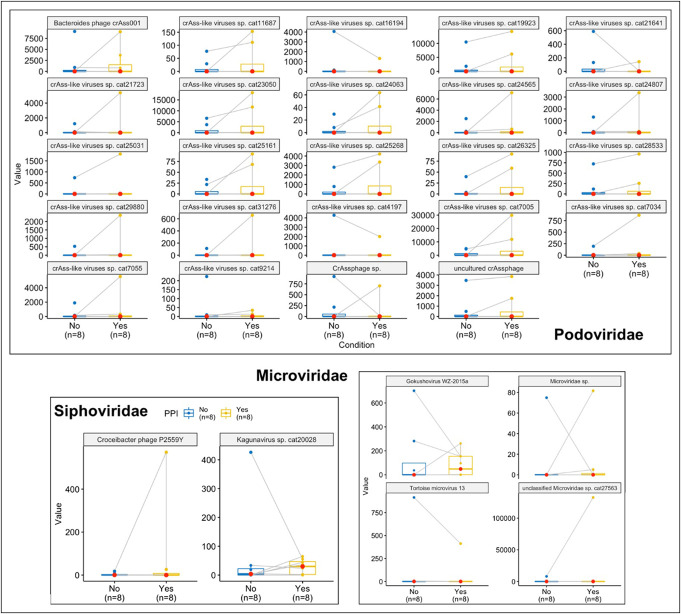
As summarized in Table 4, there were no changes in the alpha and beta diversity of VLP either in the initiation or withdrawal trials. Using DESEq2, addition of PPI reduced Bacteriodes phage, crAss-like phage, and Gokushoviruses, while withdrawal reduced Tomato mottle mosaic virus and crAss-like phages. As shown in Figures 6 and 7, there were no major differences using Wilcoxon paired tests, even when the outlier(s) were removed.
Table 4.
Changes in VLP in PPI addition and withdrawal trials
| Clinical details | PPI initiation (n = 8) | PPI withdrawal (n = 8) | ||
| Pre-PPI | Post-PPI | On PPI | Off PPI | |
| Age, mean ± SD | 61.5 ± 7.1 | 62.4 ± 5.1 | ||
| Male gender, n (%) | 8 (100) | 8 (100) | ||
| Etiology of cirrhosis, n (%) | ||||
| Alcohol | 4 (50) | 5 (63) | ||
| HCV | 3 (38) | 2 (25) | ||
| HCV + alcohol | 0 (0) | 0 (0) | ||
| MASH | 1 (12) | 1 (12) | ||
| Others | 0 (0) | 0 (0) | ||
| Ascites | 8 (100) | 8 (100) | ||
| HE, n (%) | 0 (0) | 0 (0) | ||
| MELD score, mean ± SD | 12.2 ± 3.6 | 12.5 ± 2.9 | 12.7 ± 5.6 | 12.1 ± 3.8 |
| α diversity, mean ± SD | ||||
| Observed | 1.44 ± 0.88 | 1.66 ± 0.87 | 1.19 ± 0.20 | 1.12 ± 0.91 |
| Chao1 | 10.43 ± 6.80 | 14.13 ± 7.36 | 10.50 ± 10.00 | 8.29 ± 6.65 |
| Shannon | 14.21 ± 9.77 | 20.00 ± 11.17 | 14.13 ± 15.35 | 12.04 ± 11.64 |
| Viruses higher on DESeq2a | cat28842 Bacteroides phage crAss001 sp. cat27161 Gokushovirus WZ-2015a sp. Cat4053 |
ns | Tomato mottle mosaic virus sp.cat 25494 crAss-like viruses sp. Cat24225 |
ns |
Kruskal-Wallis tests were used for statistical analysis, and no significance was found among alpha diversity indexes.
HCV, hepatitis C virus; HE, hepatic encephalopathy; MASH, metabolic dysfunction–associated steatohepatitis; MELD, model for end-stage liver disease; PPI, proton pump inhibitor; VLP, viral-like particle.
DESeq2 was used for differential abundance analysis, and all results shown have a Benjamini and Hochberg false discovery rate–adjusted P value <0.05 and a log2 fold change >2.0.
Figure 6.
Representative changes in viral families before and after PPI withdrawal using Wilcoxon paired tests. PPI, proton pump inhibitor.
Figure 7.
Representative changes in viral families before and after PPI addition using Wilcoxon paired tests. PPI, proton pump inhibitor.
DISCUSSION
This study showed changes in the virome in patients with cirrhosis that span eukaryotic and phage factions using VLP analysis, which have unique features depending on PPI use, ascites, or HE, which are associated with hospitalization over 6 months. PPI use is associated with specific changes in the virome across eukaryotic viruses and phages. Trials of PPI withdrawal and initiation also showed changes in the virome in patients with decompensated cirrhosis.
PPIs have been associated with several negative outcomes in patients with cirrhosis and are often related to gut microbial changes (20). These involve oralization of the lower intestinal microbiome using cross-sectional, addition, and withdrawal trials in compensated and decompensated cirrhosis (2–4). The impact of PPI on other kingdoms within the gastrointestinal (GI) tract is unclear. Our previous study using ITS sequencing showed that the effect on fungi was minimal (21). In another metagenomic analysis that studied PPI cross-sectionally, the impact of phages was mostly related to advanced liver disease, which is usually associated with greater use of PPIs (22). We extended prior studies by using VLP rather than metagenomics, which provides a more holistic view of the virome, including RNA viruses and eukaryotic viruses.
Our results show that there are unique VLP signatures at the family and species levels between patients with cirrhosis on PPI, patients with HE, and patients with ascites compared with their counterparts. The lower Autographviridae and higher Herelleviridae and Leviviridae or Fiersviridae were unique to PPI, with the opposite patterns observed in ascites and HE. Autographviridae family comprises lytic phages with typical hosts in the Enterobacteriaceae family. The relative decrease in PPI users and increase in the HE and ascites groups is likely related to the higher Enterobacteriaceae as patients develop ascites and HE, but greater Gram-positive taxa are observed with PPI use.
On the contrary, Herelleviridae is a newly described family in Caudovirales, whose primary hosts are Firmicutes family members (23–25) Higher abundance of this species in PPI users could be due to the increase in oral-origin Gram-positive pathobionts with PPI. Fiersviridae or Leviviridae is an RNA coliphage with specificity for enterobacteria, including Pseudomonas and Escherichia, whose log abundance change was relatively lower than the other 2 families mentioned earlier (26). While it is unclear why this pattern is opposite to that seen with Autographviridae, RNA viruses such as Fiersviridae are relatively sparse compared with DNA viruses such as Autographviridae. Unlike the phage families mentioned earlier, crAssphages were significantly lower in patients with HE, patients with ascites, and PPI users. CrAssphages are usually stable colonizers and likely an integral part that may have coevolved with the human lineage. These phages are often observed in greater abundance in healthy subjects and are reduced in the presence of disease processes (27–29). Therefore, it is not surprising that PPI users, patients with HE and ascites, and those who were hospitalized for more than 6 months had a lower crAssphage abundance than others. Apart from the overall VLP trends that were similar across ascites, HE, or PPI, there were individual phages whose relative abundance changed. Streptococcus spp. is a major biomarker of PPI therapy, and the bacterial analysis of PPI withdrawal and addition showed a higher relative abundance of these taxa with PPIs. This could explain the higher relative abundance of Streptococcus virus C1, which predominantly infects Streptococcus spp., and could be a biomarker of PPI presence that is secondary to changes in bacterial abundance. Tobamovirus is a member of Virgaviridae, which is higher in PPI, and is an environmental or food-related virus that was likely ingested by patients. With PPI use, it likely survives to be isolated in the stool. Similarly, lower Mastadenovirus is a species-level change that was observed at the family level Adenoviridae, which was lower in PPI users.
Gokushovirus, which was lower in HE and PPI and in those who were hospitalized over 6 months in a univariate and multivariable analysis, was initially believed to only be a lytic phage affecting intracellular bacteria, but recent studies have shown the prophage potential in several other bacterial classes, including a wide range of Gram-negative and Gram-positive taxa (30). Lactococcus phage reduction found only in patients with HE may be related to lactulose therapy, which modulates the growth of the target bacterium. Anoxybacillus and Croceibacter phages, which were higher in HE only, were more likely to be related to the environment (31). Similarly, Poophages as a whole need better characterization, and their reduction in ascites needs further study (32).
The association with phages could be due to the primary impact on bacteria by PPI use and other complications of cirrhosis, but changes in eukaryotic taxa in the GI tract in cirrhosis are novel. Usually, eukaryotic viruses are relatively rare compared with phages in the GI tract. We found that eukaryotic patterns were similar regardless of HE, ascites, or PPI with higher adenovirus and lower Virgaviridae and Smacoviridae. Members of Adenoviridae could lead to human diseases, and it is hypothesized that gastric acid suppression due to PPI could be associated with a lack of defense against external eukaryotic viruses (33,34). The other 2 families, Virgaviridae and Smacoviridae, have not been shown to have major disease potential and could be environment related or food related. While the reasons for a similar pattern of eukaryotes across PPI, HE, and ascites are unclear, this could also be associated with the impairment of the immune response in advancing cirrhosis. Because most hospitalizations were linked with HE, it was not surprising that similar changes in crAssphages were also seen in those who were ultimately hospitalized (1).
While the unique impact of PPI beyond that of ascites and HE was found in the phage VLP analysis, addition and withdrawal studies were able to further determine whether these changed over time with the modulation of PPI therapy. In our previous study, we showed that oralization of stool bacteria was reduced after withdrawal and increased with the addition of PPI in decompensated patients. To reduce the impact of HE-related therapies, we only included those patients who had decompensated without HE in these small trials. While the nonparametric tests were not significantly different, DESeq2 showed relatively minor changes with higher Gokushovirus, crAssphages, and Bacteroides phages observed before PPI initiation, while other crAssphages and a food-derived phage (tomatomosaic virus) reduced after withdrawal. Ultimately, these trials did not show a major impact of PPI on the virome using VLP analysis. Unlike prior studies, we used patients who were PPI users with similar clinical and cirrhosis characteristics, including demographics, MELD score, prior HE, ascites, and use of concomitant medications. We found similar alpha diversity measures, likely because of the similarity in major cirrhosis complications between PPI users and nonusers. This extends a previous VLP-based study of patients with precirrhotic fatty liver, where alpha diversity was not affected by PPI use, although the rate of PPI use in that study was relatively low (12). Similar weak effects of PPI on alpha diversity were also found in an unselected group of healthy individuals, which we extended into cirrhosis realm (35).
This study was limited by the relatively small number of participants who underwent VLP analysis; however, our sample size was robust in determining cross-sectional changes and similarities and differences between PPI-associated, HE-associated, and ascites-associated VLP signatures in cirrhosis. The withdrawal and initiation trials were also limited by the small sample size and the short duration of the study; a longer follow-up could have given information regarding longer-term changes. This small number may be also associated with the lack of overlap of VLP taxa between the trials and the cross-sectional analysis. However, the predesign/postdesign was a strength. We did not perform a simultaneous metagenomic exploration of the bacteria because the focus was on the virome, especially VLP, RNA, and eukaryotic viral changes, which are not easily determined by metagenomics. We did not coculture phages with bacteria; therefore, direct linkage with bacterial hosts was not studied. We included only omeprazole as a PPI in this study; it is possible that other PPIs could have different effects on the virome. Given the major variations in individual viral taxa between groups, these data can be seen as potential indications of the impact but may not be ready for use as biomarkers (36).
We conclude that PPI use, HE, and ascites have unique VLP signatures in the stool of patients with cirrhosis that span DNA phages, RNA phages, and eukaryotic viruses and can be associated with hospitalizations over 6 months. PPI use alone did not significantly change alpha diversity cross-sectionally or after withdrawal or addition of PPIs. Patterns of eukaryotic viruses are consistent across PPI use, HE, and ascites, indicating that cirrhosis progression, rather than individual medications or events, is likely associated with these changes in eukaryotic gut microbial composition. PPI use uniquely showed lower Autographviridae, which infects Enterobacteria, and higher Herelleviridae and Streptococcus phages, which infect bacteria, usually increased with PPI use. The patterns observed in HE or ascites were opposite to those observed with PPI use. Further studies are needed to evaluate the impact of VLP analysis on outcomes in patients with cirrhosis.
CONFLICTS OF INTEREST
Guarantor of the article: Jasmohan S. Bajaj, MD, MS, FACG.
Specific author contributions: J.S.B. conceptualized the manuscript and participated in all aspects. P.M.G., M.S., and M.P. were associated with analysis and interpretation. A.F. participated in enrollment of patients and study conduct.
Financial support: VA Merit review 2I0CX1001076 and 101CX1002472, NIAAA RO1AA029398, NCATS R21TR003095, and Richmond Institute for Veterans Research to J.S.B.
Potential competing interests: None to report.
Presentations: Portions of this manuscript were presented in poster form at the EASL congress in Vienna in June 2023.
Data availability statement: The participants of this study did not provide written consent for their data to be shared publicly; therefore, supporting data were not available.
IRB approval: Approved by VCU and Richmond VA IRBs.
Trial registration: Both trials were published with respect to clinical and 16SrRNA analysis data and were registered at www.clinicaltrials.gov (NCT01458990).
Study Highlights.
WHAT IS KNOWN
✓ Proton pump inhibitors (PPIs) are used widely and often without reason in patients with cirrhosis
✓ PPI use is associated with infections, and hepatic encephalopathy (HE), and results in alteration in gut bacteria in compensated and decompensated cirrhosis.
✓ The effect of PPI on viral-like particles (VLPs), which can influence humans directly or through alteration of gut bacteria, is unclear.
WHAT IS NEW HERE
✓ Using a cross-sectional study and 2 small prospective trials of omeprazole initiation and withdrawal, we found significant changes in eukaryotic viruses and bacteriophages associated with PPI use
✓ PPI use has a gut microbial VLP phage signature that is different from that in HE and ascites, and VLP changes were linked with hospitalizations over 6 months, independent of clinical biomarkers.
✓ Eukaryotic viral patterns were consistent across PPI use, HE, and ascites, indicating a relationship with the progression of cirrhosis.
✓ PPIs alone showed modest VLP changes with withdrawal or initiation.
✓ Distinct phage and eukaryotic viral patterns are associated with the use of PPIs in cirrhosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Scott Handley and Leran Wang for the VLP virome processing at the Washington University School of Medicine in St. Louis (they consented to being listed here).
Footnotes
SUPPLEMENTARY MATERIAL accompanies this paper at http://links.lww.com/CTG/B40; http://links.lww.com/CTG/B41
Contributor Information
Marcela Peña Rodríguez, Email: marcee24.p.r@gmail.com.
Andrew Fagan, Email: andrew.fagan@va.gov.
Masoumeh Sikaroodi, Email: msikaroo@gmu.edu.
Patrick M. Gillevet, Email: pgilleve@gmu.edu.
REFERENCES
- 1.Trebicka J, Macnaughtan J, Schnabl B, et al. The microbiota in cirrhosis and its role in hepatic decompensation. J Hepatol 2021;75(Suppl 1):S67–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvath A, Rainer F, Bashir M, et al. Biomarkers for oralization during long-term proton pump inhibitor therapy predict survival in cirrhosis. Sci Rep 2019;9(1):12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bajaj JS, Cox IJ, Betrapally NS, et al. Systems biology analysis of omeprazole therapy in cirrhosis demonstrates significant shifts in gut microbiota composition and function. Am J Physiol Gastrointest Liver Physiol 2014;307(10):G951–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajaj JS, Acharya C, Fagan A, et al. Proton pump inhibitor initiation and withdrawal affects gut microbiota and readmission risk in cirrhosis. Am J Gastroenterol 2018;113(8):1177–86. [DOI] [PubMed] [Google Scholar]
- 5.Dam G, Vilstrup H, Watson H, et al. Proton pump inhibitors as a risk factor for hepatic encephalopathy and spontaneous bacterial peritonitis in patients with cirrhosis with ascites. Hepatology 2016;64(4):1265–72. [DOI] [PubMed] [Google Scholar]
- 6.Cao Z, Sugimura N, Burgermeister E, et al. The gut virome: A new microbiome component in health and disease. EBioMedicine 2022;81:104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu CL, Duan Y, Fouts DE, et al. Intestinal virome and therapeutic potential of bacteriophages in liver disease. J Hepatol 2021;75(6):1465–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajaj JS, Sikaroodi M, Shamsaddini A, et al. Interaction of bacterial metagenome and virome in patients with cirrhosis and hepatic encephalopathy. Gut 2021;70(6):1162–73. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj JS, Rodriguez MP, Fagan A, et al. Impact of bacterial infections and spontaneous bacterial peritonitis prophylaxis on phage-bacterial dynamics in cirrhosis. Hepatology 2022;76(6):1723–34. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Wang Y, Tang M, et al. The microbial dark matter and “wanted list” in worldwide wastewater treatment plants. Microbiome 2023;11(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nooraei S, Bahrulolum H, Hoseini ZS, et al. Virus-like particles: Preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnology 2021;19(1):59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lang S, Demir M, Martin A, et al. Intestinal virome signature associated with severity of nonalcoholic fatty liver disease. Gastroenterology 2020;159(5):1839–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niklasson A, Lindström L, Simrén M, et al. Dyspeptic symptom development after discontinuation of a proton pump inhibitor: A double-blind placebo-controlled trial. Am J Gastroenterol 2010;105:1531–7. [DOI] [PubMed] [Google Scholar]
- 14.Kim AH, Armah G, Dennis F, et al. Enteric virome negatively affects seroconversion following oral rotavirus vaccination in a longitudinally sampled cohort of Ghanaian infants. Cell Host Microbe 2022;30(1):110–23.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adiliaghdam F, Amatullah H, Digumarthi S, et al. Human enteric viruses autonomously shape inflammatory bowel disease phenotype through divergent innate immunomodulation. Sci Immunol 2022;7(70):eabn6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkbeiner SR, Holtz LR, Jiang Y, et al. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol J 2009;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mallick H, Rahnavard A, McIver LJ, et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput Biol 2021;17(11):e1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McMurdie PJ, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS One 2013;8(4):e61217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65(5):740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajaj JS, Liu EJ, Kheradman R, et al. Fungal dysbiosis in cirrhosis. Gut 2018;67(6):1146–54. [DOI] [PubMed] [Google Scholar]
- 22.Shamsaddini A, Gillevet PM, Acharya C, et al. Impact of antibiotic resistance genes in gut microbiome of patients with cirrhosis. Gastroenterology 2021;161(2):508–21.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barylski J, Kropinski AM, Alikhan NF, et al. ; ICTV Report C. ICTV virus taxonomy profile: Herelleviridae. J Gen Virol 2020;101(4):362–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barylski J, Enault F, Dutilh BE, et al. Analysis of spounaviruses as a case study for the overdue reclassification of tailed phages. Syst Biol 2020;69(1):110–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Andrea MM, Frezza D, Romano E, et al. The lytic bacteriophage vB_EfaH_EF1TV, a new member of the Herelleviridae family, disrupts biofilm produced by Enterococcus faecalis clinical strains. J Glob Antimicrob Resist 2020;21:68–75. [DOI] [PubMed] [Google Scholar]
- 26.Rumnieks J, Tars K. Diversity of pili-specific bacteriophages: Genome sequence of IncM plasmid-dependent RNA phage M. BMC Microbiol 2012;12:277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulyaeva A, Garmaeva S, Ruigrok R, et al. Discovery, diversity, and functional associations of crAss-like phages in human gut metagenomes from four Dutch cohorts. Cell Rep 2022;38(2):110204. [DOI] [PubMed] [Google Scholar]
- 28.Edwards RA, Vega AA, Norman HM, et al. Global phylogeography and ancient evolution of the widespread human gut virus crAssphage. Nat Microbiol 2019;4(10):1727–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koonin EV, Yutin N. The crAss-like phage group: How metagenomics reshaped the human virome. Trends Microbiol 2020;28(5):349–59. [DOI] [PubMed] [Google Scholar]
- 30.Kirchberger PC, Ochman H. Resurrection of a global, metagenomically defined gokushovirus. Elife 2020;9:e51599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh KM, Gan HM, Chan KG, et al. Analysis of anoxybacillus genomes from the aspects of lifestyle adaptations, prophage diversity, and carbohydrate metabolism. PLoS One 2014;9(6):e90549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santiago-Rodriguez TM, Garoutte A, Adams E, et al. Metagenomic information recovery from human stool samples is influenced by sequencing depth and profiling method. Genes (Basel) 2020;11:1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vilcu AM, Sabatte L, Blanchon T, et al. Association between acute gastroenteritis and continuous use of proton pump inhibitors during winter periods of highest circulation of enteric viruses. JAMA Netw Open 2019;2(11):e1916205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charpiat B, Bleyzac N, Tod M. Proton pump inhibitors are risk factors for viral infections: Even for COVID-19? Clin Drug Investig 2020;40(10):897–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishijima S, Nagata N, Kiguchi Y, et al. Extensive gut virome variation and its associations with host and environmental factors in a population-level cohort. Nat Commun 2022;13(1):5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Espen L, Bak EG, Beller L, et al. A previously undescribed highly prevalent phage identified in a Danish enteric virome catalog. mSystems 2021;6(5):e0038221. [DOI] [PMC free article] [PubMed] [Google Scholar]