Abstract
Technologies in the 21st century provide increasingly detailed and accurate maps of brain structure and function. So why don’t psychiatrists order brain imaging on all our patients? Here we briefly review major neuroimaging methods and some of their findings in psychiatry. As clinicians and neuroimaging researchers, we are eager to bring brain imaging into daily clinical practice. However, to be clinically useful, any test in medicine must demonstrate adequate test statistics, and show proven benefits that outweigh its risks and costs. In 2024, beyond certain limited circumstances, we have no imaging tests that can meet those standards to provide diagnosis or guide treatment. This cold fact explains why for most psychiatric patients, neuroimaging is not currently recommended by professional organizations or the National Institute of Mental Health.
Introduction
Contrary to a popular myth,1 psychiatrists routinely look at the brain for some aspects of patient care. For instance, PET is increasingly used for dementia assessment,2 and MRI is recommended for some patients with new-onset psychosis.3 However, we still do not recommend brain imaging for most of our patients. This leads to some consternation, with patients asking questions like:
‘If this is a brain disorder, can’t you tell what’s wrong by looking at my brain?’
‘Are you sure this is the right diagnosis? Isn’t there some kind of brain scan you can do?’
‘How do you know this is the right medication for me?’
As psychiatrists who are also NIH-funded researchers using neuroimaging to understand psychopathology, these questions are understandable but the answer is as frustrating for us as it is our patients: “We don’t have any brain imaging techniques that can reliably diagnose you or guide your treatment.” Medicine in general, and psychiatry specifically, has made amazing strides in understanding the neurobiological basis of psychiatric symptoms and disorders over the last 50 years. We have learned that essentially all psychiatric diagnoses are caused in part by genes the patient carried,4 sometimes with higher genetic contributions than many non-psychiatric conditions.5,6 We know that brain lesions can produce psychiatric symptoms.7,8 We have even identified neurobiological mechanisms by which social and psychological stress affects the brain.9 Yet, there are still no standard lab or imaging tests to diagnose psychiatric disorders, sometimes leading to frustration and confusion as well as opening the door to potentially exaggerated claims. Here, we review what is needed to make any medical test clinically useful, then overview various neuroimaging methods and how they have contributed to our understanding of the neurobiology of psychiatric illness and how these techniques may fit into the framework of medical testing for individual patients in the future.
Demonstrating the Clinical Utility of Any Test in Medicine
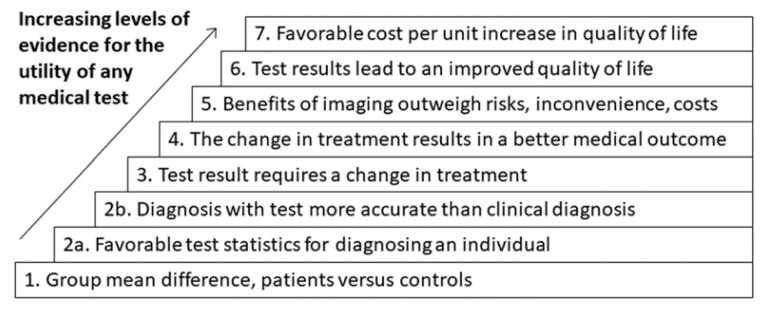
As with any diagnostic test in medicine, differentiating patients and unaffected persons is important, but is only the first hurdle the test must pass to be clinically useful. One may view the needed steps as a hierarchy, each step building on the previous step, as shown in Figure 1. After first defining whether any group mean difference exists, it is then important to know if the statistical differences are actually meaningful in diagnoses. The test must be consistent and replicable enough in a single individual to allow for a single measurement to reflect that person’s disease “state”.
Figure 1.
Hierarchy of clinical utility of medical tests.
For example, in a patient with non-insulin dependent diabetes when a single blood glucose level (BGL) is checked, it may or may not be within the normal range at that given time. Thus, the single BGL is not a reliable diagnostic test and measures that are integrated over time are necessary. Moreover, there must a reliable separation between the test results in someone with and without the diagnosis.
For example, the presence of antinuclear antibodies, particularly with mild abnormalities, shows overlap between the range seen in those with and without autoimmune diseases and is better seen as only one part of a diagnostic assessment.10 Moreover, even if a test can reliably distinguish between those with and without a certain diagnosis, it must add value to the clinical assessment or treatment algorithm. It should be more accurate than, or at least add diagnostic accuracy to a clinical diagnosis and/or result in a change in treatment.
Moving up in the hierarchy, an increasingly useful test not only provides a reliable diagnosis or dictates a change in treatment but does so in a way that results in a better medical outcome or, even better, and improved quality of life. Moreover, it should do so in a cost-effective way, in that both the personal costs of inconvenience and potential harm, as well as the financial costs are outweighed by the benefit. Let us now review neuroimaging techniques and how they might fit into this framework.
What is Neuroimaging and How Has it Been Used in Psychiatry?
Broadly speaking, neuroimaging methods can be defined into those that assess structure, or what the brain looks like (often in considerable detail), and those that assess function, which measure what parts of the brain are most active at a given time, or locate and measure specific neurotransmitters or their receptors. For each of these, neuroimagers can use radiation (i.e., computed tomography (CT) scans) and/or radiolabeled markers (i.e., positron emission tomography (PET) and single photon emission computed tomography (SPECT)), magnetic field-based imaging (i.e., magnetic resonance imaging (MRI), functional MRI and magnetic spectroscopy), or measurements of changes in the electrical field (i.e., electroencephalography (EEG)). First, we briefly review each of these techniques and how they have contributed to our understanding of psychiatric disorders.
Structural Neuroimaging: CT, MRI, and DTI
Structural neuroimaging, looking at the structure of the gray matter and white matter of the cortex, subcortical areas, the cerebellum, ventricles, and blood vessels has become increasingly high resolution. Currently, CT scans can accurately render single millimeter slices and MRI can give sub-millimeter details.11 CT scans utilize x-ray based technology, meaning there is a small but measurable dose of radiation for each CT scan done, and images are reconstructed to a 3D model. MRI does not utilize any ionizing radiation, but rather capitalizes on differential changes in the magnetic field produced by the water content of diverse tissues. While there are no radiation risks, as the magnet strength increases above 3 tesla, patients can experience some discomfort during scans. Special techniques, such as diffusion tensor imaging (DTI), utilize MR-based imaging to assess the integrity of the white matter tracts.11
Together, structural imaging techniques allow us to see many types of brain injury, including both ischemic and hemorrhagic strokes, congenital malformations, malignancies of all types, and even some traumatic brain injuries, when these are large enough to be accompanied by white matter damage. Some key findings in primary psychiatric disorders are shown in Table 1.
Table 1.
Selected structural brain imaging findings in primary psychiatric disorders
|
Functional Neuroimaging: EEG, PET, fMRI, SPECT
In contrast to structural neuroimaging, functional neuroimaging attempts to discern the neural activity in the brain at a given time. A variety of techniques for measuring function exist, capitalizing on the changes that occur in the brain when neurons are firing or are in activated and/or deactivated states. We will review many of these techniques and some major contributions they have provided in our understanding of psychiatric disorders in Table 2.
Table 2.
Selected functional brain imaging findings in primary psychiatric disorders
|
Electroencephalography (EEG) is one of the oldest and also the most direct non-invasive measure of function. EEG measures electrical field changes from neural activity—both the neurons firing as well as more general changes in local field potentials which allow neurons to be in a state where they are more or less likely to fire.11,12 Unfortunately, as EEG uses electrodes that are on the scalp, it has limited ability to determine the specific part of the brain causing any particular signal as each electrode is receiving information from many parts of the brain. Placing the electrode directly on the dura, as is done for neurosurgical assessments, improves spatial resolution, but is clearly not a good solution for use as a diagnostic test.
To improve spatial resolution in the 1980s, researchers, including those at Washington University, developed positron emission tomography (PET) imaging for use in studying neural activity. In the most common application of PET, radiolabeled glucose is injected into patients, which can then be measured to see which parts of the brain are most metabolically active at that time, or when performing a specific task, though other radiotracers can be used as well.13 PET can also measure neurochemical receptors on neurons. PET imaging provided the foundation for assessing anterior cingulate cortex dysfunction in MDD.14,15 Further psychiatric research has utilized PET to understand serotonin and serotonin receptors in mood disorders.16 However, use of PET in psychiatric research is limited by exposure to ionizing radiation, expense and limited spatial resolution.11
Single-photon emission computer tomography (SPECT) is a highly-related technique, also utilizing a radiomarker.12 Often SPECT in neuroimaging utilizes technetium-based isotopes to assess blood flow, though as with PET, other compounds can be used as well. This technique does carry some radiation risk and by measuring the emission of single photons, spatial resolution in limited to around 1cm; temporal resolution is also limited.11
In the 1990s it was recognized that MRI could provide similar information on regional brain activity without the radiation exposure inherent to prior imaging modalities. Magnetic resonance based functional neuroimaging most often uses changes in the level of oxygenated and deoxygenated hemoglobin in blood to determine which areas are most metabolically active. The MRI detects the differential magnetic field properties of oxygenated vs deoxygenated blood at a spatial resolution of a about a millimeter, much higher than any prior neuroimaging technique.11,12 This method is called BOLD (blood oxygen level–dependent) fMRI.
MRI can also measure local blood flow. Perfusion MRI has lower spatial and temporal resolution than BOLD, but is quantitatively much more stable over time, making it potentially more useful to examine slow changes in the brain such as between wakefulness and sleep. The temporal stability of perfusion MRI has also been exploited to quantify drug activity and receptor sensitivity in the brain, since pharmacological effects often require measurements separated by hours or days in time.17
Magnetic spectroscopy, like MRI, capitalizes on the magnetic properties of excited protons, for example in the amino acid neurotransmitter glutamate. However, to do so reliably, the overwhelming water signal must be suppressed in data analyses. Practically, this means that to get good signal to noise, MRS must focus on very small areas of the brain, on the order of several square centimeters and/or utilize long scan times to maximize the number of measurements taken.11 The potential to measure neurotransmitters such as GABA and glutamate have made it an appealing methodology for psychiatric research.
Around 20 years ago, researchers recognized that not only could fMRI be used to assess which brain regions were most active when doing specific activities, but that regions intrinsically varied (at rest) in how metabolically active they were in interesting and consistent ways. For instance, the left and right motor cortex tend to be active more often than not at the same time. Assessing the relationships between which regions were consistently co-activated is the basis of resting state functional connectivity, one of the most commonly used neuroimaging techniques in modern psychiatric research.18
How Has Neuroimaging Fallen Short?
While neuroimaging has given us a multitude of information about how the brain is generally affected in psychiatric diagnoses, the neuroimaging techniques described above are not yet meeting the criteria for a useful or high quality medical test as defined above. First, all of the neuroimaging techniques described above generally rely on group-based analyses. That is to say that in order to get a reliable enough signal to see differences related to depression, for example, researchers measure which areas differ on average between those with depression and those without. Second, the distribution of values for those who have a diagnosis and those who do not often overlap.
First, while large deviations from typical neuroimaging values or patterns may be more indicative of pathology, there are people who will have psychopathology but no abnormalities in brain imaging and those with differences in neuroimaging that have no specific psychiatric diagnosis. In this way, neuroimaging techniques struggle to meet criterion 2a in the above referenced hierarchy of clinical test utility – they often cannot reliably distinguish between those with and without a diagnosis. Moreover, there is considerable overlap in neuroimaging findings across psychiatric diagnoses. To draw another analogy to antinuclear antibody testing, ANA can be elevated in multiple autoimmune conditions and cannot alone be used to give a particular diagnosis but only in conjunction with other tests and the clinical picture. Neuroimaging abnormalities such as decreased anterior cingulate cortex activity is seen across psychiatric conditions from depression and anxiety to bipolar disorder and obsessive-compulsive disorder19 and cannot be used to give a specific diagnosis. Abnormalities in reward processing occur in both depressive disorder and attention deficit hyperactivity disorder.20 For both, it is the context in which these abnormalities occur (meaning other symptoms and time course) that provide the diagnosis.
Second, in functional neuroimaging, the signal itself is not reliable enough to get a single (or even several) time measurement in an individual person and determine where that sits on the spectrum of “normal” or even “depression.” As referenced above, by failing to reliably differentiate between a person with and without a disorder at any given time, these techniques fail step 2a in the hierarchy of clinical utility in medical testing. While resting state neuroimaging techniques are less affected by the individual measure or “state” as they either are thought to reflect a variation overall, “average” patterns of neural activations across the brain, there remains considerable variability in these measurements as these patterns can systematically change throughout the day and with training or experience.21,22 Even state-of-the-art machine learning algorithms, which have excellent performance differentiating between groups of people with and without symptoms (i.e., psychosis), struggle to reliably label an individual as one experiencing psychosis.23 Moreover, current imaging “diagnoses” are still being compared to clinical diagnoses, indicating that at the current time these tests also fail 2b in the hierarchy as they are using clinical diagnoses as the “gold standard,” not performing better than or even adding to these diagnoses in most studies.
Last, a number of the neuroimaging techniques discussed above do carry small but significant risk. PET and SPECT all include exposure to radiolabeled markers and exposure to ionizing radiation (albeit in relatively small quantities) as well as intravenous access. Research indicates that more than 20 minutes of high quality resting state scans are needed to be able to reliably asses an individual’s functional connectivity patterns.24 The long amounts of time needed to obtain MR imaging in a small, contained space where patients must lie flat results in significant inconvenience. Furthermore, relative contraindications to MRI exist, from pacemakers to tattoos to claustrophobia. The use of these expensive imaging techniques, which require trained technicians and intensive computational analysis, also makes these techniques currently much more expensive than clinical diagnoses, indicating an additional failure in step 5 of the testing hierarchy.
To put this all together, consider the example of perfusion SPECT, which some have advocated to diagnose and guide treatment in children with ADHD and related symptoms. Some patients and clinicians are already convinced that the scan was useful in that setting. However, for these judgments, the quality of the evidence must be high, because a happy outcome after a test may be chance. As the old saying puts it, even a stopped clock is right twice a day. Furthermore, since patients and doctors both are hoping for the test to mean something, they are likely to interpret their experience with favorable bias. Therefore we can trust only research that keeps them both from knowing whether the test results are real or simulated until the outcome of interest is recorded without bias. Judged in this light, what does the evidence say?
Average brain activity probably differs in ADHD compared with people without ADHD (step 1 in the box). However, there is no reliable published information on step 2a, e.g., what is the positive predictive value of a specific finding on the SPECT scan for diagnosis? And given that the current standard for diagnosis of ADHD is driven by clinical assessment, it is not surprising that step 2b is hard to achieve. Even if the scan could provide clinically important superior diagnostic information, that information is largely irrelevant to the patient unless it makes a difference to treatment (step 3) and ultimately to the effect on the patient of changing treatment (step 4). Any such benefits must of course be weighed against the downsides of imaging, such as financial cost, time and inconvenience, and in this example involving irradiating children, risks to health (step 5). Furthermore, ideally the downstream effects of improving a diagnosis by imaging would change not only a medically measurable outcome but raise the quality of the patient’s life (step 6). Finally, from a public health perspective, test results should improve quality of life at least as much as other interventions of similar cost (step 7). Collectively, these points remind us that any medical diagnostic test needs to pass quite a high bar for us to conclude that they are worth it to the patient (step 6) or to society (step 7). Unfortunately, at this time, imaging tests for common psychiatric illnesses are almost all stuck at step 1.
Where is Neuroimaging Going?
Ultimately, this is not an indictment of the use of neuroimaging in psychiatry—it’s an acknowledgment of the complexity of the human brain and psychiatric conditions, which are themselves part of the human experience. There are 100 billion neurons in the human brain and the activity patterns that correlate with “normal” or “typical” human experience is not fully understood. Those activity patterns we do see differ across people and types of tasks themselves can differ depending on the context—in what part of the circadian cycle the data is collected, how sleepy the participant feels, whether another person is in the room, or whether they have just experienced intense emotions. This is consistent with the human experience, but makes measurement extremely difficult. Additionally, our current psychiatric diagnoses are (well validated) conjunctions of symptoms. While this does capture important information about the diagnoses, there are also more than 100 combinations of symptoms that can result in a diagnosis of major depressive disorder.25 Between the complexity of the human brain and the variety of symptoms, it is not surprising that neuroimaging findings related to a specific diagnosis have produced variable results. As a result, the field, guided by the NIMH, has increasingly moved to investigating neuroimaging markers of individual psychiatric symptoms rather than diagnoses.26 While this approach holds promise, the difficulty in identifying reliable, specific markers continues.
As reviewed in this journal by Siegel et al., in July 2023, researchers continue to work to identify patterns of brain responses that may indicate a particular diagnosis or possible treatment response to improve our ability to “personalize” psychiatric medicine. Much of this research is focused on using complex computer algorithms and machine learning to identify complex spatial or temporal patterns of resting state functional connectivity associated with disease states or treatment response, or on combinations of imaging modalities.25 However, even when initial studies have found patterns consistent with subtypes of depression,27 follow up studies using different populations have often found the initial results are not generalizable.28
Further work in these areas promises to improve on these limitations,29 but currently the results reported in neuroimaging studies can tell us about a group, but cannot reliably tell us about an individual within that group.
What Do I Tell My Patients?
When patients ask us: “Isn’t there a brain scan you can do?” using the knowledge reviewed here, we tell them: “I wish we could—it’s something we’re working on in our research studies. Unfortunately, right now all types of brain imaging cannot reliably give you a diagnosis or tell us which treatment will be best for you.”
Walking through the information above can also help patients understand why such tests, when offered, are not recommended by most psychiatrists or the National Institute of Mental Health, are not FDA approved, and are not covered by insurance. Fortunately, a long history of research using expert review of symptom patterns does allow us to reliably make a diagnosis, which points us to an algorithm for starting treatments.30 In short, as psychiatrists, we love to look at the brain—but we’re not going to put you through the time, cost, and risks of brain imaging unless there’s evidence that it is likely to benefit you.
Footnotes
Alecia C. Vogel, MD, PhD, (pictured), is Assistant Professor of Psychiatry (Child) and Kevin J. Black, MD, is Professor of Psychiatry, Neurology, Radiology, and Neuroscience; both are at Washington University School of Medicine in St. Louis, Missouri.
Disclosure: As of 2020, KJB received honorarium from: SK Life Science, Inc./scientific advisory board; Medscape/CME program on PD psychosis; Mededicus/CME program on PD psychosis; Informa Pharma Intelligence; and Putnam Associates as a consultant. Artificial intelligence was not used in the study, research, preparation, or writing of this manuscript.
References
- 1.Amen D. Why don’t psychiatrists look at the brain? The case for greater use of SPECT imaging in neuropsychiatry. NeuroPsychiatry Reviews. 2001;2:1–6. [Google Scholar]
- 2.Dubois B, et al. Clinical diagnosis of Alzheimer’s disease: recommendations of the International Working Group. The Lancet Neurology. 2021;20:484–496. doi: 10.1016/S1474-4422(21)00066-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. American Psychiatric Association Publishing; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspi A, Moffitt TE. Gene–environment interactions in psychiatry: joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- 5.Faraone SV, Larsson H. Genetics of attention deficit hyperactivity disorder. Mol Psychiatry. 2019;24:562–575. doi: 10.1038/s41380-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins N, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53:817–829. doi: 10.1038/s41588-021-00857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padmanabhan JL, et al. A Human Depression Circuit Derived From Focal Brain Lesions. Biological Psychiatry. 2019;86:749–758. doi: 10.1016/j.biopsych.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotovio G, et al. Mapping mania symptoms based on focal brain damage. Journal of Clinical Investigation. 2020;130:5209–5222. doi: 10.1172/JCI136096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barch DM, Luby JL. Understanding Social Determinants of Brain Health During Development. AJP. 2023;180:108–110. doi: 10.1176/appi.ajp.20220991. [DOI] [PubMed] [Google Scholar]
- 10.Ali Y. Rheumatologic Tests: A Primer for Family Physicians. American Family Physician. 2018;98:164–170. [PubMed] [Google Scholar]
- 11.Roy AK, Ferrara E, Keesey R, Davis K. Clinical Psychology. Elsevier; 2022. fMRI and Other Neuroimaging Methods. in Comprehensive; pp. 62–82. [DOI] [Google Scholar]
- 12.Martinelli C, Shergill SS. Everything you wanted to know about neuroimaging and psychiatry, but were afraid to ask. BJPsych advances. 2015;21:251–260. [Google Scholar]
- 13.Cervenka S, Frick A, Bodén R, Lubberink M. Application of positron emission tomography in psychiatry—methodological developments and future directions. Transl Psychiatry. 2022;12:248. doi: 10.1038/s41398-022-01990-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drevets WC, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 15.Öngür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savitz JB, Drevets WC. Neuroreceptor imaging in depression. Neurobiology of Disease. 2013;52:49–65. doi: 10.1016/j.nbd.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Zelaya FO, Fernandez-Seara MA, Black KJ, Williams SCR, Mehta MA. Perfusion in pharmacologic imaging. In: Bammer R, editor. MR and CT Perfusion and Pharmacokinetic Imaging: Clinical Applications and Theory. Part II: Clinical Applications. Lippincott Williams & Wilkins; 2016. pp. 1083–1091. [Google Scholar]
- 18.Canario E, Chen D, Biswal B. A review of resting-state fMRI and its use to examine psychiatric disorders. Psychoradiology. 2021;1:42–53. doi: 10.1093/psyrad/kkab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holroyd CB, Umemoto A. The research domain criteria framework: The case for anterior cingulate cortex. Neuroscience & Biobehavioral Reviews. 2016;71:418–443. doi: 10.1016/j.neubiorev.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 20.Nielson DM, et al. Great Expectations: A Critical Review of and Suggestions for the Study of Reward Processing as a Cause and Predictor of Depression. Biological Psychiatry. 2021;89:134–143. doi: 10.1016/j.biopsych.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaisvilaite L, Andersson M, Salami A, Specht K. Time of day dependent longitudinal changes in resting-state fMRI. Front Neurol. 2023;14:1166200. doi: 10.3389/fneur.2023.1166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steel A, Thomas C, Trefler A, Chen G, Baker CI. Finding the baby in the bath water – evidence for task-specific changes in resting state functional connectivity evoked by training. NeuroImage. 2019;188:524–538. doi: 10.1016/j.neuroimage.2018.12.038. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigue AL, et al. Searching for Imaging Biomarkers of Psychotic Dysconnectivity. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2021;6:1135–1144. doi: 10.1016/j.bpsc.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marek S, et al. Identifying reproducible individual differences in childhood functional brain networks: An ABCD study. Developmental Cognitive Neuroscience. 2019;40:100706. doi: 10.1016/j.dcn.2019.100706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lynch CJ, Gunning FM, Liston C. Causes and Consequences of Diagnostic Heterogeneity in Depression: Paths to Discovering Novel Biological Depression Subtypes. Biological Psychiatry. 2020;88:83–94. doi: 10.1016/j.biopsych.2020.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drysdale AT, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinga R, et al. Evaluating the evidence for biotypes of depression: Methodological replication and extension of Drysdale et al. (2017) NeuroImage: Clinical. 2019;22:101796. doi: 10.1016/j.nicl.2019.101796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel JS, Pearson C, Lenze EJ. Better Biomarkers, Faster Drugs, Stronger Models: Progress Towards Precision Psychiatry. Mo Med. 2023;120:292–298. [PMC free article] [PubMed] [Google Scholar]
- 30.Black KJ. In: Diagnosis in Rubin EH, Zorumski CF: Adult Psychiatry (Blackwell ’s Neurology and Psychiatry Access series, series ed. David RB, editor. Blackwell; 2005. pp. 15–23. [Google Scholar]
- 31.Svancer P, Spaniel F. Brain ventricular volume changes in schizophrenia. A narrative review. Neuroscience Letters. 2021;759:136065. doi: 10.1016/j.neulet.2021.136065. [DOI] [PubMed] [Google Scholar]
- 32.Agosta F, Canu E, Sarro L, Comi G, Filippi M. Neuroimaging findings in frontotemporal lobar degeneration spectrum of disorders. Cortex. 2012;48:389–413. doi: 10.1016/j.cortex.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Fonov VS, Dadar M, Manera AL, Ducharme S, Collins L. Clinical subtypes of frontotemporal dementia show different patterns of cortical atrophy. Alzheimer’s & Dementia. 2021;17:e054494. [Google Scholar]
- 34.Draper A, Jackson GM, Morgan PS, Jackson SR. Premonitory urges are associated with decreased grey matter thickness within the insula and sensorimotor cortex in young people with T ourette syndrome. Journal of Neuropsychology. 2016;10:143–153. doi: 10.1111/jnp.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaiser RH, et al. Frontostriatal and Dopamine Markers of Individual Differences in Reinforcement Learning: A Multi-modal Investigation. Cereb Cortex. 2018;28:4281–4290. doi: 10.1093/cercor/bhx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gur RE, Gur RC. Functional magnetic resonance imaging in schizophrenia. Dialogues in Clinical Neuroscience. 2010;12:333–343. doi: 10.31887/DCNS.2010.12.3/rgur. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavanne AV, Robinson OJ. The Overlapping Neurobiology of Induced and Pathological Anxiety: A Meta-Analysis of Functional Neural Activation. AJP. 2021;178:156–164. doi: 10.1176/appi.ajp.2020.19111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry. 2015;72:603. doi: 10.1001/jamapsychiatry.2015.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]