Abstract
In human fibroblasts, growth arrest at the end of the normal proliferative life span (induction of senescence) is dependent on the activity of the tumor suppressor protein p53. In contrast, once senescence has been established, it is generally accepted that reinitiation of DNA synthesis requires loss of multiple suppressor pathways, for example, by expression of Simian virus 40 (SV40) large T antigen, and that even this will not induce complete cell cycle traverse. Here we have used microinjection of monoclonal antibodies to the N terminus of p53, PAb1801 and DO-1, to reinvestigate the effect of blocking p53 function in senescent human fibroblasts. Unexpectedly, we found that both antibodies induce senescent cells to reenter S phase almost as efficiently as SV40, accompanied by a reversion to the “young” morphology. Furthermore, this is followed by completion of the cell division cycle, as shown by the appearance of mitoses, and by a four- to fivefold increase in cell number 9 days after injection. Immunofluorescence analysis showed that expression of the p53-inducible cyclin/kinase inhibitor p21sdi1/WAF1 was greatly diminished by targeting p53 with either PAb1801 or DO-1 but remained high and, moreover, still p53 dependent in cells expressing SV40 T antigen. As previously observed for induction, the maintenance of fibroblast senescence therefore appears to be critically dependent on functional p53. We suggest that the previous failure to observe this by using SV40 T-antigen mutants to target p53 was most probably due to incomplete abrogation of p53 function.
Normal human fibroblasts are capable of only a finite number of cell divisions even under optimum culture conditions, after which they enter a state of viable but permanent growth arrest (29). This phenomenon of cellular senescence has been observed in many other normal cell types (15, 23, 54) and represents a natural obstacle to clonal expansion (14, 58) which is thought to be an important restriction on the progression of many (although probably not all) human cancers (19).
One currently popular model for senescence proposes that an intrinsic cell division clock, possibly based on telomere erosion (4), triggers one or more signal pathways which inhibit key components of the cell cycle regulatory machinery. Two candidate inhibitors whose levels increase with proliferative life span are p16 (2, 25) and p21sdi1/WAF1 (46); these proteins inhibit the cyclin-dependent kinases CDK4/6 and CDK2, which are required for passage through and exit from the G1 phase of the cell cycle (51). A related protein, p24 (40), may represent a third inhibitor. One consequence (55) of this inhibition is the failure of senescent cells to phosphorylate a major downstream target of these enzymes—the product of the retinoblastoma (Rb) sensitivity gene, pRb—which in its unphosphorylated form sequesters transcription factors needed for G1-S progression (61). Not surprisingly, therefore, escape from senescence is often associated with deregulation of the Rb pathway, either directly through loss of Rb itself or indirectly by loss of p16, which presumably thereby uncouples Rb from the senescence “clock.” Furthermore, experimental abrogation of Rb function, for example, by expression of the viral oncoprotein human papillomavirus (HPV) E7, results in the extension of life span in many (although not all) cell types; in the human fibroblast this amounts to around 15 to 25 population doublings (p.d.) (50, 63, 65).
Escape from senescence is also strongly associated with loss of another tumor suppressor gene product, p53 (37, 62, 64). A major biological property of p53, its transcriptional transactivation function, is activated as human fibroblasts approach senescence (3, 9), possibly as a direct response to telomere erosion, and activated p53 is a potent inducer of the CDK inhibitor p21 (20), making p53 a potential link between the aging clock and cell cycle inhibition. However, it has been suggested that the induction of p21 in senescence is only partially dependent on p53 (48, 57), and there is evidence that it is not sufficient to account for growth arrest by p53 in senescent cells (8), indicating the presence of other p53-activated growth inhibitors (p16 does not appear to be a candidate, and its upstream inducer is currently unknown). As with Rb, experimental interference with p53 function, e.g., by expression of HPV E6 protein or dominant-negative p53 mutants, can prevent fibroblasts (and some other cell types [50, 60]) from entering normal senescence, again conferring an extension in human fibroblasts of around 15 to 25 p.d. (7, 65).
Evidence from gene transfer experiments using presenescent cells therefore suggests that normal senescence can be prevented by abrogating either Rb or p53. In both cases, however, escape is only temporary, with cells again arresting after an extension of around 15 to 25 p.d. Escape from this backup senescent state requires that both Rb and p53 function be lost, e.g., by expression of Simian virus 40 (SV40) T antigen (T), coexpression of E6 and E7, or mutation of p53 and p16 (50, 63). This shows that p53 and Rb do not form a simple linear pathway and suggests a model in which a p53-dependent and a p16-Rb-dependent pathway act cooperatively to bring about normal senescence, even though either pathway can eventually compensate for loss of the other to bring about a delayed growth arrest.
These conclusions are based mostly on transfection or retroviral approaches which of necessity use dividing cells and hence have addressed the prevention of senescence in presenescent cultures. Interestingly, quite different results have been reported from attempts to reverse senescence in cells which have already undergone growth arrest. By using microinjection of expression vector plasmids, reinitiation of DNA synthesis in senescent cells has consistently been found to require abrogation of both Rb and p53 function, e.g., by SV40 T (15, 26). Use of HPV E7 or of T mutants defective in one or the other function gave a much reduced or undetectable response. This is particularly clear with the T(K1) mutant, which lacks Rb binding, to the extent that this has been used as a screen to uncover potentially novel inhibitors of the Rb pathway (26).
Given its importance for genetic models of senescence, we have readdressed this apparent discrepancy between the requirements for prevention and reversal of senescence by making use of microinjected antibodies, PAb1801 (5) and DO-1 (59), directed against the N-terminal transactivation domain of p53, rather than expression of DNA viral oncogenes, to inhibit p53 function. In contrast to previous reports, our data suggest that loss of p53 function is sufficient by itself to efficiently reinitiate DNA synthesis in senescent fibroblasts, raising the possibility that previous manipulations may have failed to abrogate p53 function completely.
MATERIALS AND METHODS
Cells and culture conditions.
Normal human diploid neonatal foreskin fibroblasts (HCA2 cells, a gift from J. Smith, Houston, Tex.) were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS). (All media and components were obtained from Gibco/BRL, Paisley, United Kingdom). A subclone of HCA2 cells (LacZ21) expressing a p53 reporter construct was generated by stable transfection of plasmid RGCΔFos-LacZ (9). Determination of in vitro life span and passage protocols were as described previously (8).
To be consistent with other studies (26, 34), the following minimum criteria were used for senescence: (i) failure to reach confluence up to 3 weeks after a final 1:2 passage despite regular refeeding; (ii) over 90% of cells showing the characteristic enlarged, flattened shape; and (iii) over 90% of cells failing to incorporate bromodeoxyuridine (BrdU) after a 72-h labelling period, as detected by immunofluorescence (see below). (In most experiments, however, the 72-h BrdU labelling index [LI] was well below 10%, with a 1-h LI of less than 1%.)
Senescence occurred at estimated population doubling levels (PDL) of 64 to 65 for the parent HCA2 cells and 32 to 33 for LacZ21 cells (the latter being counted from the origin of the subclone).
Microinjection.
Microinjections were performed as described previously (9) under a Zeiss (Axiovert 35M) phase-contrast inverted microscope equipped with an Eppendorf microinjection system (Micromanipulator 5171 and Microinjector 5246; Carl Zeiss, Oberkochen, Germany).
Plasmid SVori−, containing an SV40 genome lacking a functional viral origin of replication due to a 6-bp deletion (22, 53), or a control plasmid (vector only) was injected at a concentration of 500 μg/ml in phosphate-buffered saline (PBS), pH 7.2.
Affinity-purified mouse monoclonal anti-p53 antibody PAb1801 (5, 59) (Oncogene Science Ab-2), DO-1 (59) (Oncogene Science Ab-6), or PAb421 (Oncogene Science Ab-1), or control mouse immunoglobulin (IgG) (Sigma), was injected at 2 mg/ml. Affinity-purified rat IgG (Sigma) was coinjected (10 mg/ml) in each case to facilitate subsequent immunodetection of the injected cells. All antibodies were prepared similarly by resuspension of lyophilized preparations in PBS (pH 7.2). Plasmids and antibodies were injected into the nuclei of target cells.
At 72 h before microinjection, HCA2 cells were trypsinized and plated into 60-mm-diameter culture dishes in DMEM–10% FCS. Just before microinjection, the medium was replaced with Leibovitz’s L-15 medium containing 10% FCS to provide control of pH in air. Following microinjection, cells were returned to DMEM–10% FCS.
UV irradiation.
LacZ21 cells were washed twice in serum-free medium. The medium was then removed, and dishes, without lids, were placed under a UVG-11 Mineralight lamp (U.V. Products, San Gabriel, Calif.) and exposed to 10 J of UVC per m2 over a period of 25 s. Fresh complete medium was then added, and the cells were analyzed 24 h later.
Detection of β-gal activity.
β-Galactosidase (β-gal) expression from the RGCΔfosLacZ reporter in LacZ21 cells was assessed histochemically as previously described (9). Briefly, cells were fixed in 0.5% glutaraldehyde, permeabilized in 0.02% Nonidet P-40–0.01% sodium deoxycholate, and stained with 1 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactoside (X-Gal substrate) per ml in 5 mM potassium ferricyanide–5 mM potassium ferrocyanide–2 mM magnesium chloride for 16 h at 37°C, using an alkaline pH (7.8) to prevent interference by endogenous senescence-associated mammalian β-galactosidase (SAβ-gal) (18). Microinjected cells were identified by immunoperoxidase staining with a mixture of horseradish peroxidase-conjugated anti-rat and anti-mouse antibodies (Southern Biotechnology) as described previously (6).
Endogenous SAβ-gal was assessed in HCA2 cells by carrying out the above-described histochemical reaction at an acidic pH (6.0) as described by Dimri et al. (18).
Immunofluorescence.
Monolayers were fixed in 3.7% formaldehyde, washed in PBS, and permeabilized in 0.2% Triton X-100 in PBS. Samples were then quenched in 100 mM glycine, and nonspecific binding was blocked with 10% FCS in PBS. The samples were then treated with the following primary antibodies as appropriate for 1 h at a 1:500 dilution in PBS-bovine serum albumin: (i) rabbit polyclonal anti-p53 antibody CM1 (kindly provided by D. Lane) (43), (ii) rabbit polyclonal anti-p21 antibody (Santa-Cruz Biotechnology); and (iii) mouse monoclonal antibody PAb419 recognizing SV40 T (27).
After being washed, cells were incubated as appropriate in secondary antibody (rhodamine-conjugated goat anti-rabbit IgG or anti-mouse IgG [Southern Biotechnology]) at 1:100 in PBS-bovine serum albumin for 1 h. Fluorescein isothiocyanate (FITC)-conjugated goat anti-rat IgG (Southern Biotechnology) was also added simultaneously to detect microinjected rat IgG and hence permit independent identification of microinjected cells. Dishes were mounted in Fluoromount G (Southern Biotechnology) and viewed with an Olympus IMT-2 fluorescence microscope.
BrdU incorporation.
Cells were incubated with BrdU (10 μM) for 1 or 72 h, following which they were washed, fixed, and permeabilized for immunofluorescence as described above. BrdU incorporation into DNA was detected by incubating the fixed cells with a mouse monoclonal anti-BrdU antibody (Boehringer Mannheim) at a 1:100 dilution in the presence of 10 U of DNase I per ml for 1 h at 37°C, followed by FITC-conjugated goat anti-mouse IgG. Microinjected cells were identified as described above by immunostaining the injected rat IgG, in this case with rhodamine-conjugated anti-rat IgG. The BrdU LI was expressed as the percentage of microinjected cells labelled. Similar data (not shown) were also obtained with a rat anti-BrdU antibody (SeraLabs).
Determination of cell number after microinjection.
Zones of 200 to 300 cells in replicate sets of dishes were microinjected with rat carrier IgG (10 mg/ml) together with either PAb1801, DO-1, or control mouse IgG (2 mg/ml).
To determine the efficiency of microinjection, three parallel dishes injected with rat IgG were fixed 8 h later, and the number of cells containing IgG was determined by immunofluorescence. This number, divided by the number of cells actually injected, provided a correction factor for efficiency of microinjection, which was applied to all other dishes in the same experiment in order to estimate, for each dish, the number of cells which had been successfully injected.
Dishes from each treatment group were subsequently immunostained after 3, 7, and 9 days, and the number of cells positive for rat IgG was determined by immunofluorescence. This number was then expressed as a percentage of the number of cells estimated (as described above) to have been successfully injected at day 0. (The use of a high concentration of carrier IgG allowed immunodetection even after 9 days of subsequent cell division.)
Statistics.
At least 100 to 200 cells were injected in each microinjection experiment. Results are expressed as the means from at least three independent experiments, together with standard errors (SE) and total numbers of cells injected.
RESULTS
Microinjection of antibody PAb1801 or DO-1 blocks activation of the transcriptional function of p53.
To validate directly the ability of PAb1801 and DO-1 to effectively block the transcriptional transactivation activity of p53 in our experimental system, we microinjected these antibodies into our previously described subclone of human fibroblasts, LacZ21 (9), which stably expresses a p53-dependent β-gal reporter. We made use of two well-characterized stimuli to activate p53 in these cells, UV irradiation and microinjection of antibody PAb421 (9, 33); the latter is thought to act by relieving inhibition from a C-terminal regulatory domain of p53 (32).
As expected (9), irradiation of early-passage LacZ21 cells with the optimum dose of UV (10 J · m−2) induced expression of the β-gal reporter in the majority of control (IgG-injected) cells (Fig. 1A), with approximately 75% being positive when analyzed 24 h after irradiation (compared to <3% under untreated basal conditions). Nuclear microinjection of PAb1801 or DO-1, 24 h prior to irradiation, almost completely blocked this response, with the percentage of β-gal-positive cells decreasing to 5 and 7%, respectively (Fig. 1B and C).
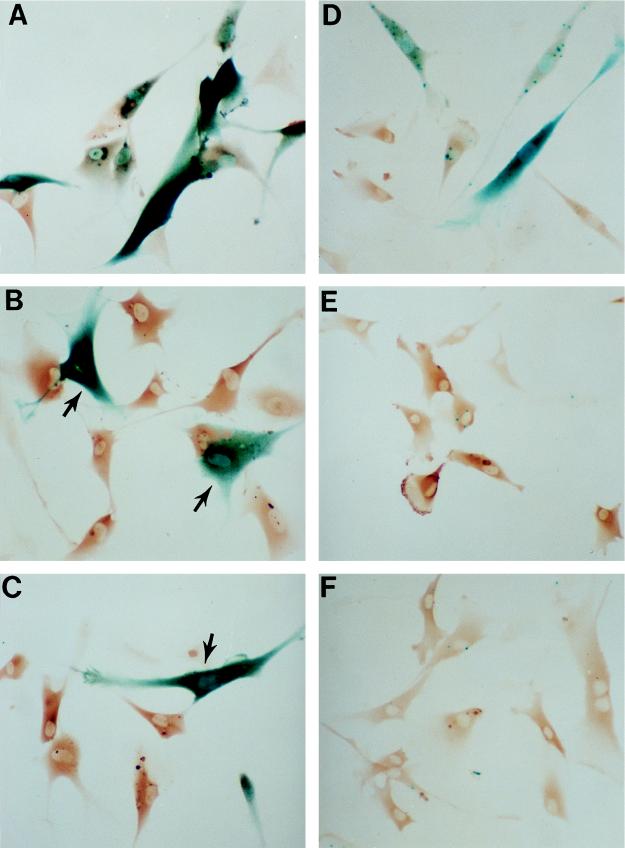
FIG. 1.
Inhibition of p53-dependent transactivation by anti-p53 antibodies. p53 transactivation activity was stimulated in early-passage LacZ21 fibroblasts either by irradiation with 10 of UV J · m−2 (A to C) or by microinjection of the activating antibody PAb421 (D to F). Expression of the p53-dependent reporter was assessed 24 h later by β-gal histochemistry (punctate and/or diffuse blue staining). Microinjected cells were identified by immunoperoxidase detection of injected IgG (brown staining). Injection of either DO-1 (B) or PAb1801 (C) 24 h prior to UV treatment greatly reduced reporter expression compared to that with mouse IgG controls (A). Similarly, the response to PAb421 (D) was inhibited by coinjection of DO-1 (E) or PAb1801 (F). (Note that the β-gal-positive [blue] cells in panels B and C [arrows] were not microinjected.) Magnification, ×140.
Microinjection of PAb421 at 2 mg/ml also induced β-gal expression but less dramatically, with 30 to 40% of cells being positive 24 h after injection (Fig. 1D). Coinjection of equal amounts of PAb1801 or DO-1 with PAb421 completely abolished this response, reducing the percentage of positive cells to 1.5% in both cases (Fig. 1E and F).
Microinjection of antibody PAb1801 or DO-1 reinitiates DNA synthesis in senescent fibroblasts and induces a reversion to the “young” morphology.
At an estimated PDL of 65, HCA2 cultures demonstrated the characteristic morphology of senescence in over 95% of the population and gave an overall 1-h BrdU LI of 0.5 to 1.0%. Cultures were first used at this stage, but as a further assurance against partial senescence, experiments were repeated (with similar results) with cultures which had been maintained (with regular refeeding) for up to a further 19 days (Table 1). Also, in all cases, microinjection was targeted specifically at morphologically senescent cells.
TABLE 1.
Stimulation of DNA synthesis (BrdU nuclear labelling index) by antibody PAb1801 or plasmid pSVori− in senescent human fibroblasts
| Injection | LIa at the following culture ageb
|
||
|---|---|---|---|
| PDL65 | PDL65 + 5 days | PLD65 + 19 days | |
| IgG (control) | 0.9 ± 0.2 (645) | 1.0 ± 0.02 (727) | 0.8 ± 0.2 (686) |
| PAb1801 | 17.4 ± 0.4 (531) | 15.0 ± 1.6 (774) | 14.0 ± 0.5 (458) |
| Vector (control) | 1.2 ± 0.2 (607) | 0.8 ± 0.1 (304) | 0.6 ± 0.3 (610) |
| pSVori− | 25.9 ± 1.9 (680) | 20.8 ± 4.2 (395) | 28.4 ± 2.2 (747) |
One-hour LI; mean of at least three experiments ± SE. The total number of cells injected is given in parentheses.
See text for explanation.
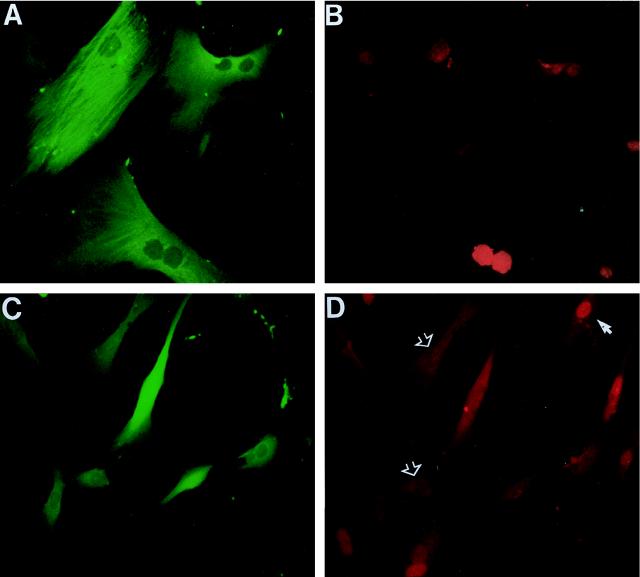
At 72 h following injection with control IgG, less than 1% of injected cells (identified by immunocytochemical staining of coinjected rat IgG) incorporated BrdU after a 1-h labelling period, and nearly all retained a senescent morphology (Table 1; Fig. 2B). In sharp contrast, microinjection with anti-p53 antibody PAb1801 (or DO-1 [data not shown]) resulted (within 48 h) in a reversion to a much more compact, fusiform morphology resembling that of young cultures (compare Fig. 2A with C), which was sustained for at least 7 days. This was accompanied by a marked reduction in the expression of SAβ-gal activity, with the proportion of cells with undetectable activity increasing from 1 to 40%.
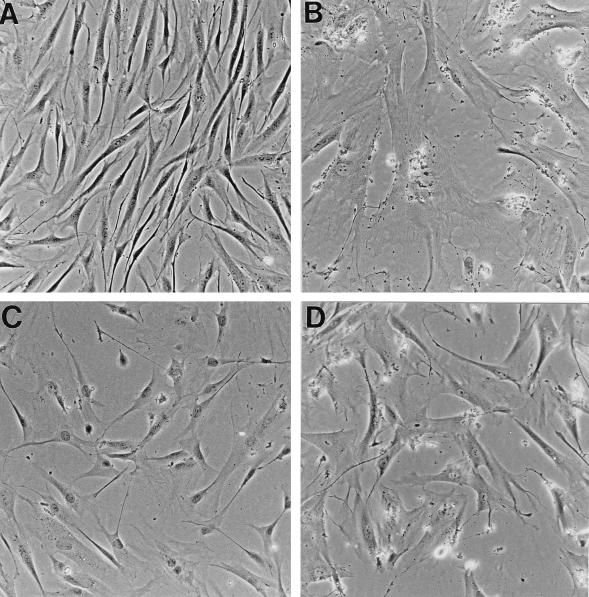
FIG. 2.
Morphological reversion and reinitiation of DNA synthesis in senescent human fibroblasts induced by anti-p53 antibody PAb1801. Senescent HCA2 cells (corresponding to PDL65 plus 19 days in Table 1) were microinjected with control mouse IgG (B, E, and F), PAb1801 (C, G, and H), or plasmid SVori− (D) and analyzed 72 h later (all cells in the fields shown were injected). (B to D) Effects on morphology determined by phase-contrast microscopy compared to untreated young cultures (A). (E to H) Effects on DNA synthesis as revealed by BrdU labelling. Injected cells are identified by immunofluorescence of coinjected rat IgG with a rhodamine label (red) (E and G); nuclear BrdU incorporation (examples are indicated by arrows) is shown by immunofluorescence with FITC (green) (F and H). (Note that the weak cytoplasmic FITC fluorescence visible in panels F and H is due to detection of the injected mouse IgGs by the rabbit anti-mouse–FITC used to detect the BrdU.) Magnification, ×160.
Furthermore, the BrdU LI 72 h after injection increased markedly, to between 14 and 17% depending on the age of the culture (Table 1). Averaging of results from all ages analyzed in Table 1 shows that PAb1801 stimulated a 21-fold increase in the BrdU LI (15.9%, compared to 0.76% in controls) (Fig. 2E to H). Closely similar results were obtained with DO-1, which induced a 24-fold increase in the BrdU LI (Table 1).
As a further control against any artifactual, nonspecific stimulatory effect of antibody microinjection, we also microinjected PAb421, which, as shown above, activates (rather than inhibits) p53 transcriptional activity. This antibody failed to induce either a morphological or proliferative response in senescent HCA2 cells.
As a positive control, and to permit comparison with earlier work, senescent HCA2 cells were also injected with plasmid SVori− (encoding origin-defective SV40). At 72 h after microinjection, injected cells stained positively for SV40 T and showed elevated levels of nuclear p53 by immunofluorescence (data not shown). As expected, this was accompanied by a stimulation of BrdU incorporation, with the 1-h LI increasing from 0.9% ± 0.1% (average ± SE for all three sets of experiments in Table 1) in vector-only controls to 25.0% ± 1.8% in cells injected with SVori−. Interestingly, though, the morphological change induced by SVori− was much less marked than that with PAb1801 or DO-1 (compare Fig. 2D with C).
PAb1801 and DO-1, as well as SV40, induce full cell cycle traverse in senescent human diploid fibroblasts.
Contrary to expectations (15, 24), mitotic figures were clearly evident in senescent cells (including those of the oldest cultures) at 6 days following injection with PAb1801 or DO-1 but not control IgG (data not shown). Mitotic activity was also induced by plasmid SVori− but not by the vector control.
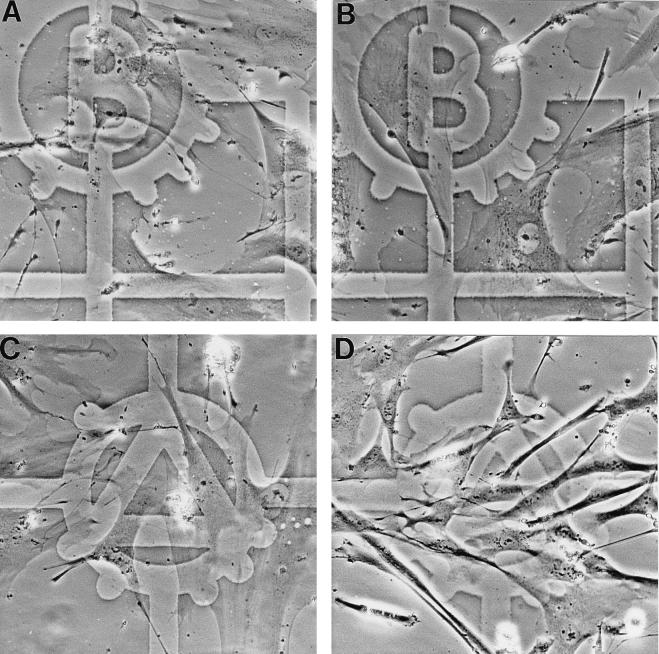
This evidence for full cell cycle traverse by PAb1801 was supported by demonstration of cell proliferation. This was visualized directly by photographing predefined zones before (Fig. 3A and C) and after (Fig. 3B and D) microinjection. This clearly revealed both the switch from the senescent to young morphology and a striking increase in cell density following injection of PAb1801 (Fig. 3A and C).
FIG. 3.
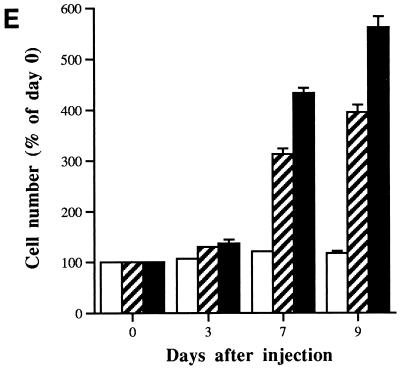
Stimulation of cell proliferation in senescent fibroblasts by microinjection of antibody PAb1801 or DO-1. (A to D) Photomicrographs of senescent fibroblasts in zones predefined by reference to an underlying grid, before (A and C) and 7 days after (B and D) microinjection with either control IgG (A and B) or PAb1801 (C and D). (Note the increase in cell density in panel D and reversion of most cells to a young morphology.) Magnification, ×200. (E) Histogram showing increasing cell number with time after microinjection of DO-1 (solid bars) or PAb1801 (hatched bars) compared to control IgG (open bars). Injected cells and their progeny were identified by immunostaining of coinjected rat IgG, and their numbers are expressed as a percentage of the day 0 value. Values are means from at least three independent experiments ± SE.
To exclude any contribution from migration of noninjected cells, serial counts were also performed following coinjection of sufficient rat IgG to permit immunofluorescence detection at least 9 days later, thereby providing an objective marker of injected cells and their progeny. PAb1801 and DO-1 (but not control IgG) stimulated a progressive increase in the number of immunopositive cells, reaching ∼4- and ∼5.6-fold, respectively, by day 9 (Fig. 3E).
PAb1801 blocks senescence-induced transactivation by p53.
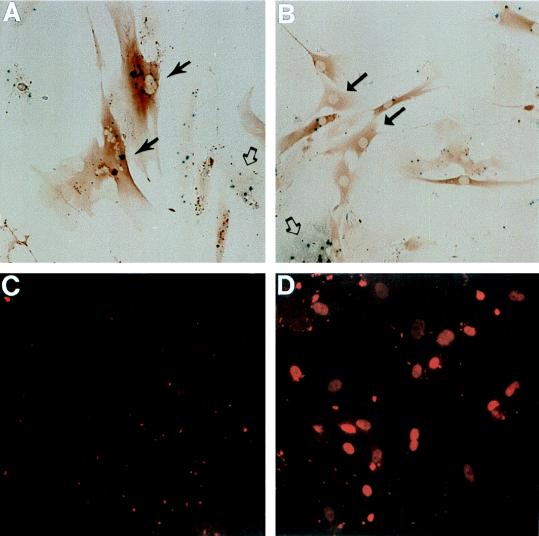
Using LacZ21 fibroblasts, we previously showed that growth arrest in senescent fibroblasts is closely associated with induction of p53 transactivation activity (9). To investigate the effect of PAb1801 on p53 function in senescent cells, LacZ21 cells were grown to senescence prior to microinjection. At 72 h after injection, expression of β-gal from the reporter construct was assessed by staining with the chromogenic substrate X-Gal.
Approximately 82% ± 6% (mean for three experiments ± SE) of cells injected with control IgG (Fig. 4A) expressed β-gal, consistent with previous findings for uninjected cells (9). This was dramatically reduced, to 11% ± 2%, by injection of PAb1801 (Fig. 4B).
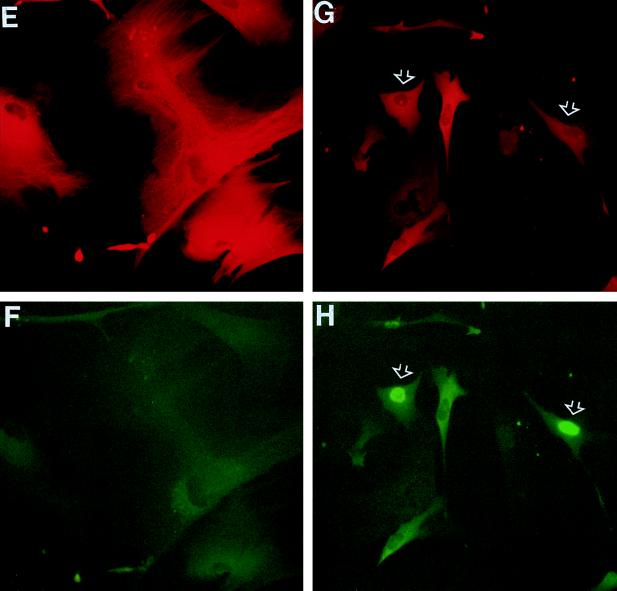
FIG. 4.
Inhibition of p53 transactivating activity and increased nuclear content of p53 protein induced by PAb1801 in senescent fibroblasts. Senescent LacZ21 cells were microinjected with control IgG (A and C) or PAb1801 (B and D) and analyzed 72 h later. (A and B) β-gal expression assessed by X-Gal histochemistry (blue product); injected cells are identifiable by immunoperoxidase detection of coinjected rat IgG (brown). In panel A injected (solid arrows) and uninjected (open arrow) cells have similar β-gal activities. In panel B most injected cells have reverted to a young morphology and have undetectable β-gal activity (examples are shown by solid arrows). An uninjected β-gal-positive cell is also shown (open arrow). Magnification, ×130. (C and D) Nuclear p53 analyzed by immunofluorescence with antibody CM1 and a rhodamine label (red). Nearly all of the cells in these fields were injected. Note the heterogeneous nuclear p53 content in cells injected with PAb1801 (D), in contrast to the absence of detectable p53 in the control cells (C). Magnification, ×160.
In contrast to the increase in p53 activity at senescence, but in agreement with our own and most other previous reports (3, 9), amounts of nuclear p53 protein remained close to or below the detection limit of our immunocytochemical analysis in senescent HCA2 cells, whether untreated or injected with control IgG (Fig. 4C). Interestingly, PAb1801 (or DO-1) led to a marked increase in immunodetectable endogenous p53, with more than 90% of nuclei immunostained to various degrees by polyclonal antibody CM1, 72 h after injection (Fig. 4D). This heterogeneous pattern was maintained for at least 6 days and probably represents stabilization of the protein, although direct assessment of the half-life was of course not possible with the cell numbers available.
Recent work (28, 35) suggests that the rapid turnover of p53 is dependent on binding of its N terminus to mdm-2 protein. Interruption of this interaction by the N-terminally directed antibodies used here, resulting in stabilization of p53, is therefore a plausible explanation for the observed increase in nuclear content of the protein after microinjection.
PAb1801 or DO-1, but not SV40, reduces expression of p21WAF1 in senescent cells.
We next investigated whether the abrogation of p53 transactivation revealed by our reporter construct would be similarly reflected in a reversal of the senescence-associated increase in expression of the p53-inducible CDK inhibitor p21sdi1/WAF1.
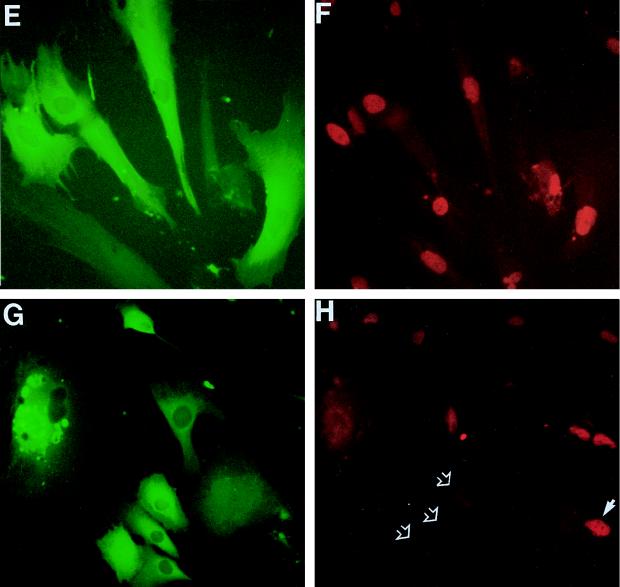
Consistent with previous results for senescent HCA2 cells (8), 82% ± 5% of senescent cells injected with control IgG or noninjected cells displayed readily detectable nuclear p21 protein by immunofluorescence analysis, with marked heterogeneity in intensity among cells (Fig. 5A and B). At 72 h after microinjection with PAb1801, this percentage had fallen to 7.0% ± 2.5% (mean ± SE, n = 3) (Fig. 5C and D), and the overall level of immunofluorescence was comparable to that observed in actively growing young cells (data not shown). Similar results were obtained following microinjection of DO-1 (but not PAb421).
FIG. 5.
Inhibition of p21sdi1/WAF1 expression in senescent fibroblasts by PAb1801 but not SV40. Senescent HCA2 cells (as for Fig. 1) were microinjected with control IgG (A and B), PAb1801 (C and D), plasmid SVori− plus control IgG (E and F), or SVori− plus PAb1801 (G and H) and analyzed 72 h later by double immunofluorescence. Injected cells are shown by immunofluorescence detection of coinjected rat IgG with an FITC label (green) (A, C, E, and G). p21 content is shown by immunofluorescence with a rhodamine label (red) (B, D, F, and H). Examples of nuclei lacking detectable p21 after injection with PAb1801 are indicated with open arrows in panel D; a p21-positive nucleus in a neighboring uninjected cell is also visible (solid arrow). Note that PAb1801 (D) but not SVori− (F) causes loss of detectable nuclear p21 and that the persistent elevation in SVori−-injected cells is also abolished by coinjection of PAb1801 (H) (compare injected cells [open arrows] with uninjected cell [closed arrow]). Magnification, ×160.
In contrast, injection of SVori− produced no significant reduction in the proportion of nuclei containing detectable p21 (72% ± 4.5%, compared to 82% ± 5% in controls) or any obvious reduction in intensity (Fig. 5E and F). However, coinjection of PAb1801 with SVori− led to a striking loss of detectable p21, similar to that observed with PAb1801 alone (Fig. 5G and H).
It should be noted that these results were obtained with HCA2 cells at PDL65 plus 19 days (Table 1), in which parallel dishes showed a marked stimulation of DNA synthesis by SVori−, with a 1-h BrdU LI of nearly 30%.
DISCUSSION
Our data show that microinjection into senescent fibroblasts of monoclonal antibodies directed at the N-terminal transactivation region of p53 results in a 20-fold increase in the proportion of cells synthesizing DNA at 3 days after injection. Since, as in most other studies, no attempts were made here to first remove residual cycling cells, we cannot formally distinguish true reentry of arrested cells into the cycle from the alternative of a contraction of G1 phase duration in a very slowly cycling compartment (39). However, the vast majority of cells which synthesized DNA as a result of PAb1801 and DO-1 (or SVori−) injection were derived from cells which, prior to injection, displayed the conventional criteria of terminal senescence observed in cultures from which cycling cells have been selectively depleted (12, 24, 52), i.e., large, flattened shape and absence of labelling by BrdU. Indeed the switch from this phenotype to one resembling that of young fibroblasts was readily observable by phase-contrast microscopy of predefined zones before and after microinjection and was accompanied by loss of the senescence marker, SAβ-gal (18).
In contrast to the accepted consensus (15, 24), we also observed mitotic activity induced not only by SVori− but also by PAb1801 and DO-1, supported by direct evidence of increasing cell number. It is widely believed that even the most potent viral oncogenes are not capable of inducing cell cycle progression beyond G2 phase in senescent fibroblasts (15, 24). In the earliest studies this may have related to the use of origin-containing SV40 sequences (even whole virus [24]). This would enable a viral DNA replication cycle to occur in semipermissive human cells, thereby potentially activating a G2/M cell cycle checkpoint, which may not be overcome even by the effects of T. Such a potential artifact would not occur with our SVori− plasmid or, of course, with the anti-p53 antibody. However, this argument does not account for later failures to observe mitoses by using plasmids expressing SV40 early-region genes from a heterologous promoter which also lack a replication origin, and we currently have no satisfactory explanation for this discrepancy.
Several lines of evidence support the conclusion that the effects of PAb1801 and DO-1 observed here are due to specific abrogation of p53 function.
The DO-1 antibody recognizes an epitope (amino acids 20 to 25) within the N terminus of p53 (11, 56) which includes key residues required for transactivation (amino acids 22 and 23) (38). Although the PAb1801 epitope (amino acids 46 to 55) is located approximately 25 residues downstream (36), it too has been shown to block transactivation (1, 44), presumably through steric hindrance. Furthermore, we confirmed directly that both antibodies were indeed inhibitory in our experimental model, by showing efficient blockade of a well-characterized p53-dependent reporter response. Finally, the possibility that a cross-reacting protein, rather than p53 itself, might be the relevant target for the effects observed is made extremely unlikely by the finding of identical results with two anti-p53 antibodies directed at nonoverlapping epitopes.
In this study, therefore, abrogation of p53 function, without any inhibition of the Rb pathway, appears to be sufficient to efficiently stimulate DNA synthesis in senescent fibroblasts, with the response to PAb1801 or DO-1 averaging nearly 70% of that achieved by expression of SV40 T. This contrasts sharply with a series of similar studies (15, 26) using T mutants defective in either p53 or Rb binding, which concluded that both functions needed to be abolished. Indeed a reanalysis of these data shows that the T(K1) mutant, which abrogates p53 but not Rb, produced only 9% of the response obtained with wild-type T. A similar conclusion was reached by using a plasmid electroporation approach (49).
It is unlikely that these contrasts can be explained merely by methodological differences relating to cell kinetics. Both our own and the T(K1) studies (15, 26) used the same strain of human fibroblasts (HCA2) and the same criteria for senescence. Indeed, we extended the period following arrest of culture growth to 19 days to minimize the possibility of using incompletely senescent cells (16). The proliferative response was assessed differently, using a 1-h pulse 72 h after injection instead of the continuous 48-h labelling used previously (15). However, the lower nuclear labelling indices observed with SVori− in our hands (25 versus 80%) are consistent with this shorter labelling time and suggest that there was no significant difference in the biological response to SV40 T in our system.
Clearly the most likely explanation, therefore, lies with our use of inhibitory anti-p53 antibodies, as opposed to a plasmid expressing an Rb-binding-defective mutant of T. There is evidence from our own (21) and other (31) studies that some preparations of plasmid can provoke a nonspecific inhibition of DNA synthesis in primary cells. If this were dependent on both Rb and p53 pathways, it could explain the lack of response to injected mutant T plasmids, despite retention of the response to wild-type T. However, this is made less likely by the ability of the T(K1) mutant to stimulate quiescent cells almost as effectively as wild-type T (26). The key difference therefore almost certainly lies in the biological properties of the antibodies as opposed to the T(K1) protein.
Although we cannot exclude the role of qualitative differences (the T mutant being expected to have activities in addition to p53 abrogation), the simpler quantitative hypothesis would be that T(K1) produces a less complete inhibition of p53 activity than PAb1801 or DO-1, leaving enough free active p53 to sustain G1 arrest in senescence (although not in quiescence) provided that the cooperating Rb pathway is intact. This would be consistent with the long-standing paradox that T binds only a subfraction of wild-type p53 molecules in many cell types (17) (whereas PAb1801 or DO-1 at the concentration used may well achieve near saturation of p53), with early reports that expression of exogenous mutant p53 could enhance transformation by T (42), and finally with a recent report of residual DNA damage-inducible p53 activity in SV40-transformed murine cell lines (30).
Direct evidence for this notion comes from immunofluorescence analysis of the behavior in our model of a key downstream target of p53, p21sdi1/WAF1. Although we have not addressed the effect of T(K1), we have shown that wild-type T produces only a minimal reduction in immunodetectable nuclear p21 levels, in contrast to its near-complete elimination by PAb1801 or DO-1. Furthermore, coinjection of PAb1801 with SVori− showed that this persistent elevation of p21 expression in the presence of SV40 T is still p53 driven.
Incomplete abrogation of p53 function would provide a novel explanation for the frequent finding, in stable transfection models, that senescence-related induction of p21 is reduced minimally (if at all) by SV40 T (48, 57) and at least one mutant p53 (8), which has previously been interpreted as indicating the existence of p53-independent inducers of p21. Our data suggest simply that T (or mutant p53) expression reduces wild-type p53 function enough to turn off an additional effector pathway(s) necessary for entry into senescence but not enough to affect p21 expression, for which a more complete loss of p53 activity (as is produced by PAb1801 or DO-1) is required. Independent evidence that the induction of p21 in senescence is indeed p53 dependent is also provided by its absence in aging p53-deficient fibroblasts derived spontaneously from Li-Fraumeni syndrome (41) or by stable expression of HPV E6 (10). p21 induction is, of course, not always p53 dependent, and it is worth noting here that in similar microinjection experiments we have observed that the early induction of p21 by refeeding of serum-starved young human fibroblasts was not inhibited by PAb1801 (data not shown).
Whereas the sustained induction of p21 in fibroblasts whose life span has been extended by expression of mutant p53 (or T) clearly indicates that a loss of p21 is not necessary for cells to evade senescence (8, 48), the ability of PAb1801 or DO-1, but not p53-binding mutants of T, to reinitiate DNA synthesis in cells which are already senescent, coupled with its ability to abolish p21 expression, suggests intriguingly that loss of p21 may be necessary for reversal of senescence in the presence of a functional Rb pathway.
Our data do not address whether loss of p21 is sufficient for abrogation of normal senescence. An antisense p21 construct which was able to overcome cell cycle arrest in quiescent fibroblasts (45) was reported to be incapable of restimulating senescent cells (47), suggesting that loss of p21 is not sufficient for reversal of senescence. However, a more recent gene targeting approach (13) suggests that loss of p21 may in fact be sufficient, at least for evasion of senescence.
In summary, we suggest a model in which (i) activation of wild-type p53 makes an essential contribution to both induction (7) and maintenance of growth arrest in normal fibroblast senescence, i.e., in contrast to the conclusion of some studies (26) there is no redundancy at this level; (ii) p53 exerts this action through multiple signal pathways, including p21; (iii) more than one of these signals is required to ensure entry into senescence after the normal life span duration, with at least one being a p21-independent pathway; and (iv) in contrast, the role of p53 in maintenance of senescence requires only a subset of its downstream signals, with p21 being perhaps sufficient alone. Clearly, an important goal now will be to identify the p21-independent pathways involved in p53-mediated growth arrest.
ACKNOWLEDGMENTS
We are grateful to the Cancer Research Campaign for grant support.
We thank James Smith for provision of HCA2 cells, Fiona Wyllie and Jane Bond for LacZ21 cells, and Theresa King for manuscript preparation.
REFERENCES
- 1.Abarzua P, LoSardo J E, Gubler M L, Neri A. Microinjection of monoclonal antibody PAb421 into human SW480 colorectal carcinoma cells restores the transcription activation function to mutant p53. Cancer Res. 1995;55:3490–3494. [PubMed] [Google Scholar]
- 2.Alcorta D A, Xiong Y, Phelps D, Hannon G, Beach D, Barrett J C. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence of normal human fibroblasts. Proc Natl Acad Sci USA. 1996;93:13742–13747. doi: 10.1073/pnas.93.24.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atadja P, Wong H, Garkaavtsev I, Geillette C, Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacchetti S. Telomere dynamics and telomerase activity in cell senescence and cancer. Cell Dev Biol. 1996;7:31–39. [Google Scholar]
- 5.Banks L, Matlashewski G, Crawford L. Isolation of human p53 specific monoclonal antibodies and their use in the study of human p53 expression. Eur J Biochem. 1986;159:529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- 6.Blaydes J P, Gire V, Rowson M, Wynford-Thomas D. Tolerance of high levels of wild-type p53 in transformed epithelial cells dependent on auto-regulation by mdm-2. Oncogene. 1997;14:1859–1868. doi: 10.1038/sj.onc.1201018. [DOI] [PubMed] [Google Scholar]
- 7.Bond J A, Wyllie F S, Wynford-Thomas D. Escape from senescence in human diploid fibroblasts induced directly by mutant p53. Oncogene. 1994;9:1885–1889. [PubMed] [Google Scholar]
- 8.Bond J A, Blaydes J P, Rowson J, Haughton M F, Smith J R, Wynford-Thomas D, Wyllie F S. Mutant p53 rescues human diploid cells from senescence without inhibiting the induction of SDI1/WAF1. Cancer Res. 1995;55:2404–2409. [PubMed] [Google Scholar]
- 9.Bond J A, Haughton M, Blaydes J, Wynford-Thomas D, Wyllie F S. Evidence that transcriptional activation by p53 plays a direct role in the induction of cellular senescence. Oncogene. 1996;13:2097–2104. [PubMed] [Google Scholar]
- 10.Bond J A, Ivan M, Wyllie F, Wynford-Thomas D. Abstracts of the Cancer Research Campaign Beatson International Cancer Conference, Glasgow, Scotland. 1997. Control of proliferative lifespan by tumour suppressor genes (TSGs): cell-type diversity in senescence checkpoints, abstr. 79. [Google Scholar]
- 11.Böttger V, Böttger A, Howard S F, Picksley S M, Chène P, Garcia-Echeverria C, Hochkeppel H-K, Lane D P. Identification of novel mdm2 binding peptides by phage display. Oncogene. 1996;13:2141–2147. [PubMed] [Google Scholar]
- 12.Bowman P D, Meek R L, Daniel C W. Aging of human fibroblasts in vitro. Exp Cell Res. 1975;93:184–190. doi: 10.1016/0014-4827(75)90438-3. [DOI] [PubMed] [Google Scholar]
- 13.Brown J P, Wei W, Sedivy J M. Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts. Science. 1997;177:831–834. doi: 10.1126/science.277.5327.831. [DOI] [PubMed] [Google Scholar]
- 14.Bryan T M, Reddel R R. SV40-induced immortalization of human cells. Crit Rev Oncogenesis. 1994;5:331–357. doi: 10.1615/critrevoncog.v5.i4.10. [DOI] [PubMed] [Google Scholar]
- 15.Campisi J. Replicative senescence: an old lives’ tale? Cell. 1996;84:497–500. doi: 10.1016/s0092-8674(00)81023-5. [DOI] [PubMed] [Google Scholar]
- 16.Cristofalo V J, Pignolo R J. Replicative senescence of human fibroblast-like cells in culture. Phys Rev. 1993;73:617–638. doi: 10.1152/physrev.1993.73.3.617. [DOI] [PubMed] [Google Scholar]
- 17.Deppert W, Haug M, Steinmayer T. Modulation of p53 protein expression during cellular transformation with simian virus 40. Mol Cell Biol. 1987;7:4453–4463. doi: 10.1128/mcb.7.12.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimri G P, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano E E, Linskens M, Rubelj I, Pereira-Smith O, Peacocke M, Campisi J. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA. 1995;92:9363–9367. doi: 10.1073/pnas.92.20.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edington K G, Loughran O P, Berry I J, Parkinson E K. Cellular immortality: a late event in the progression of human squamous cell carcinoma of the head and neck associated with p53 alteration and a high frequency of allele loss. Mol Carcinogen. 1995;13:254–265. doi: 10.1002/mc.2940130408. [DOI] [PubMed] [Google Scholar]
- 20.El-Deiry W S, Tokino T, Velculescu V E, Trent J M, Lin D, Mercer W E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumour suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 21.Gire V, Hall-Jackson C, Paterson H, Marshall C J, Wynford-Thomas D. Induction of DNA synthesis in human primary thyroid epithelial cells by microinjection of mutant p21 ras oncoprotein. J Endocrinol Invest. 1996;19:5. [Google Scholar]
- 22.Gluzman Y, Sambrook J F, Frisque R J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci USA. 1980;77:3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 24.Gorman S D, Cristofalo V J. Reinitiation of cellular DNA synthesis in BrdU-selected nondividing senescent WI-38 cells by simian virus 40 infection. J Cell Phys. 1985;125:122–126. doi: 10.1002/jcp.1041250116. [DOI] [PubMed] [Google Scholar]
- 25.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hara E, Uzman J A, Dimri G P, Nehlin J O, Testori A, Campisi J. The helix-loop-helix protein Id-1 and a retinoblastoma protein binding mutant of SV40 T antigen synergize to reactivate DNA synthesis in senescent human fibroblasts. Dev Genet. 1996;18:161–172. doi: 10.1002/(SICI)1520-6408(1996)18:2<161::AID-DVG9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Harlow E, Crawford L V, Pim D C, Williamsen N W. Monoclonal antibodies specific for simian virus 40 tumour antigens. J Virol. 1981;39:861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 29.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 30.Hess R D, Brandner G. DNA-damage-inducible p53 activity in SV40-transformed cells. Oncogene. 1997;15:2501–2504. doi: 10.1038/sj.onc.1201404. [DOI] [PubMed] [Google Scholar]
- 31.Huang L-C, Clarkin K C, Wahl G M. Sensitivity and selectivity of the DNA damage sensor responsible for activating p53-dependent G1 arrest. Proc Natl Acad Sci USA. 1996;93:4827–4832. doi: 10.1073/pnas.93.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hupp T R, Lane D P. Allosteric activation of latent p53 tetramers. Curr Biol. 1994;4:865–875. doi: 10.1016/s0960-9822(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 33.Hupp T R, Sparks A, Lane D P. Small peptides activate the latent sequence-specific DNA binding function of p53. Cell. 1995;83:237–245. doi: 10.1016/0092-8674(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 34.Ide T, Tsuji Y, Ishibashi S, Mitsuf Y. Reinitiation of host DNA synthesis in senescent human diploid cells by infection with simian virus 40. Exp Cell Res. 1983;143:343–349. doi: 10.1016/0014-4827(83)90060-5. [DOI] [PubMed] [Google Scholar]
- 35.Kubbutat M H G, Jones S N, Vousden K H. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 36.Legros Y, Lafon C, Soussi T. Linear antigenic sites defined by the B-cell response to human p53 are localized predominantly in the amino and carboxy-termini of the protein. Oncogene. 1994;9:2071–2076. [PubMed] [Google Scholar]
- 37.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Teresky A K, Levine A J. Two critical hydrophobic amino acids in the N-terminal domain of the p53 protein are required for the gain of function phenotypes of human p53 mutants. Oncogene. 1995;10:2387–2390. [PubMed] [Google Scholar]
- 39.Macieira-Coelho A, Taboury F. A re-evaluation of the changes in proliferation in human fibroblasts during ageing in vitro. Cell Tissue Kinet. 1982;15:213–224. doi: 10.1111/j.1365-2184.1982.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 40.Mazars G R, Jat P S. Expression of p24, a novel p21WAF1/Cip1/Sdi1-related protein, correlates with measurement of the finite proliferative potential of rodent embryo fibroblasts. Proc Natl Acad Sci USA. 1997;94:151–156. doi: 10.1073/pnas.94.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Medcalf A S C, Klein-Szanto A J P, Cristofalo V J. Expression of p21 is not required for senescence of human fibroblasts. Cancer Res. 1996;56:4582–4585. [PubMed] [Google Scholar]
- 42.Michael-Michalovitz D, Eliyahu D, Oren M. Overproduction of protein p53 contributes to simian virus 40-mediated transformation. Mol Cell Biol. 1986;6:3531–3536. doi: 10.1128/mcb.6.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Midgley C A, Fisher C J, Bartek J, Vojtesek B, Lane D P, Barnes D M. Analysis of p53 expression in human tumours: an antibody raised against human p53 expressed in E. Coli. J Cell Sci. 1992;101:183. doi: 10.1242/jcs.101.1.183. [DOI] [PubMed] [Google Scholar]
- 44.Mundt M, Hupp T, Frische M, Merkle C, Hansen S, Lane D, Groner B. Protein interactions at the carboxyl terminus of p53 result in the induction of its in vitro transactivation potential. Oncogene. 1997;15:237–244. doi: 10.1038/sj.onc.1201174. [DOI] [PubMed] [Google Scholar]
- 45.Nakanishi M, Adami G R, Robetorye R S, Noda A, Venable S F, Dimitrov D, Pereira-Smith O M, Smith J R. Exit from G0 and entry into the cell cycle of cells expressing p21 Sdi1 antisense RNA. Proc Natl Acad Sci USA. 1995;92:4352–4356. doi: 10.1073/pnas.92.10.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 47.Robetorye R S, Nakanishi M, Venable S F, Pereira-Smith O M, Smith J R. Regulation of p21Sdi/Cip1/Waf1/mda-6 and expression of other cyclin-dependent kinase inhibitors in senescent human cells. Mol Cell Differ. 1996;4:113–126. [Google Scholar]
- 48.Rubelj I, Pereira-Smith O M. SV40-transformed human cells in crisis exhibit changes that occur in normal cellular senescence. Exp Cell Res. 1994;211:82–89. doi: 10.1006/excr.1994.1062. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto K, Howard T, Ogryzko V, Xu N-Z, Corsico C C, Jones D H, Howard B. Relative mitogenic activities of wild-type and retinoblastoma binding-defective SV40 T antigens in serum-deprived and senescent human diploid fibroblasts. Oncogene. 1993;8:1887–1893. [PubMed] [Google Scholar]
- 50.Shay J W, Wright W E, Brasiskyte D, Van der Hagen B A. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalisation in human mammary epithelial cells but not human fibroblasts. Oncogene. 1993;8:1407–1413. [PubMed] [Google Scholar]
- 51.Sherr C J, Roberts J M. Inhibitors of mammalian G (1) cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 52.Sherwood S W, Rush D, Ellsworth J L, Schimke R T. Defining cellular senescence in IMR-90 cells: a flow cytometric analysis. Proc Natl Acad Sci USA. 1988;85:9086–9090. doi: 10.1073/pnas.85.23.9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Small M B, Gluzman Y, Ozer H L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982;296:671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- 54.Smith J R, Pereira-Smith O M. Replicative senescence: implications for in vivo aging and tumour suppression. Science. 1996;273:63–67. doi: 10.1126/science.273.5271.63. [DOI] [PubMed] [Google Scholar]
- 55.Stein G H, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249:666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- 56.Stephen C W, Helminen P, Lane D P. Characterisation of epitopes on human p53 using phage-displayed peptide libraries: insights into antibody-peptide interactions. J Mol Biol. 1995;248:58–78. doi: 10.1006/jmbi.1995.0202. [DOI] [PubMed] [Google Scholar]
- 57.Tahara H, Sato E, Noda A, Ide T. Increase in expression level of p21sdi1/cip1/waf1 with increasing division age in both normal and SV40-transformed human fibroblasts. Oncogene. 1995;10:835–840. [PubMed] [Google Scholar]
- 58.Vojta P J, Barrett J C. Genetic analysis of cellular senescence. Biochim Biophys Acta. 1995;1242:29–41. doi: 10.1016/0304-419x(95)00002-w. [DOI] [PubMed] [Google Scholar]
- 59.Vojtesek B, Bartek J, Midgley C A, Lane D P. An immunochemical analysis of the human nuclear phosphoprotein p53. J Immunol Methods. 1992;151:237–244. doi: 10.1016/0022-1759(92)90122-a. [DOI] [PubMed] [Google Scholar]
- 60.Wazer D E, Liu X-L, Chu Q, Gao Q, Band V. Immortalization of distinct human mammary epithelial cell types by human papillomavirus 16 E6 or E7. Proc Natl Acad Sci USA. 1995;92:3687–3691. doi: 10.1073/pnas.92.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 62.Wynford-Thomas D. p53: guardian of cellular senescence. J Pathol. 1996;180:118–121. doi: 10.1002/(SICI)1096-9896(199610)180:2<118::AID-PATH673>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 63.Wynford-Thomas D. Proliferative lifespan checkpoints: cell-type specificity and influence on tumour biology. Eur J Cancer. 1997;33:716–725. doi: 10.1016/S0959-8049(97)00064-6. [DOI] [PubMed] [Google Scholar]
- 64.Wynford-Thomas D, Jones C J, Wyllie F S. The tumour suppressor gene p53 as a regulator of proliferative life-span and tumour progression. Biol Signals. 1996;5:139–153. doi: 10.1159/000109184. [DOI] [PubMed] [Google Scholar]
- 65.Yan Y, Ouellette M M, Shay J W, Wright W E. Age-dependent alterations of c-fos and growth regulation in human fibroblasts expressing the HPV16 E6 protein. Mol Biol Cell. 1996;7:975–983. doi: 10.1091/mbc.7.6.975. [DOI] [PMC free article] [PubMed] [Google Scholar]