Abstract
Skin photoaging, resulting from prolonged exposure to ultraviolet radiation, is a form of exogenous aging that not only impacts the aesthetic aspect of the skin but also exhibits a strong correlation with the onset of skin cancer. Nonetheless, the safety profile of non-natural anti-photoaging medications and the underlying physiological alterations during the process of photoaging remain inadequately elucidated. Consequently, there exists a pressing necessity to devise more secure interventions involving anti-photoaging drugs. Multiple studies have demonstrated the noteworthy significance of marine biomolecules in addressing safety concerns related to anti-photoaging and safeguarding the skin. Notably, bioactive peptides have gained considerable attention in anti-photoaging research due to their capacity to mitigate the physiological alterations associated with photoaging, including oxidative stress; inflammatory response; the abnormal expression of matrix metalloproteinase, hyaluronidase, and elastase; and excessive melanin synthesis. This review provides a systematic description of the research progress on the anti-photoaging and skin protection mechanism of marine bioactive peptides. The focus is on the utilization of marine bioactive peptides as anti-photoaging agents, aiming to offer theoretical references for the development of novel anti-photoaging drugs and methodologies. Additionally, the future prospects of anti-aging drugs are discussed, providing an initial reference for further research in this field.
Keywords: skin photoaging, anti-photoaging, peptides, marine bioactive peptides
1. Introduction
The examination of marine bioactive peptides has garnered significant interest over the past two decades owing to their extensive occurrence in various marine sources and their notable biological effectiveness [1]. These peptides are primarily derived from mollusks, crustaceans, fish, algae, and certain marine by-products (such as shellfish, fish skin, offal, and muscle) [2,3]. The investigation of marine bioactive peptides has received substantial attention in recent years, primarily due to their widespread presence in diverse marine sources and their potent biological activity [4]. Currently, Peptides derived from marine sources are employed for their advantageous biological properties, including but not limited to anti-aging [5], anti-oxidant [6], anti-inflammatory [7], anti-microbial [8], anti-hypertensive [9], and anti-tumor activities [10]. Moreover, peptide compounds are extensively utilized in the advancement of various novel food, cosmetic, and pharmaceutical products owing to their minimal toxicity, functional versatility, specificity, and wide-ranging efficacy [3,11]. Notably, marine bioactive peptides have been documented to mitigate the likelihood of photoaging induced by UV radiation, thereby exerting a regulatory influence on skin aging [3,12]. Hence, marine bioactive peptides possess promising potential in the realm of skin protection.
Skin aging is a multifaceted phenomenon encompassing both endogenous and exogenous processes [13]. Among the external factors, ultraviolet (UV) radiation stands out as the primary contributor to skin photoaging [14]. Currently, the predominant approaches to counteract photoaging involve pharmaceutical interventions, physical and chemical therapies, and surgical interventions [15,16]. One such pharmaceutical intervention is retinoic acid, which has received approval from the U.S. Food and Drug Administration (FDA) for the treatment of skin photoaging [17]. However, its application is constrained by the occurrence of adverse reactions, such as burning, flaking, and dermatitis. Hence, the exploration of innovative pharmaceutical candidates pertaining to anti-photoaging mechanisms has emerged as a prominent subject of scientific inquiry. Marine bioactive peptides, originating from the metabolites of marine organisms, constitute a significant component of the human dietary intake [18,19]. Nevertheless, research investigating the anti-photoaging properties of peptides remains limited, thereby impeding their potential application within industries focused on enhancing skin quality. Nonetheless, numerous conjectures and perspectives exist regarding the underlying mechanisms and pathways associated with these peptides.
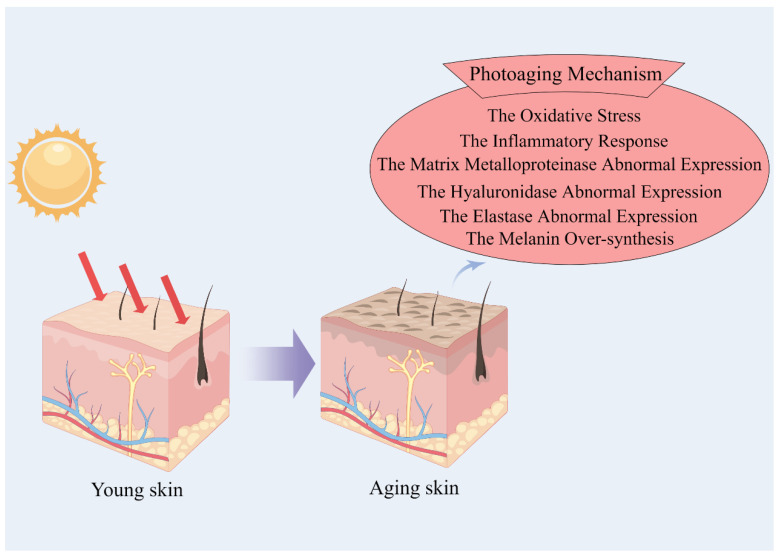
Research has demonstrated that marine bioactive peptides play a significant role in numerous anti-photoaging hypotheses [20], encompassing the oxidative stress theory [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37], the inflammatory response theory [38,39,40], the matrix metalloproteinase abnormal expression theory [41,42,43,44,45], the hyaluronidase abnormal expression theory [46,47,48,49,50], the elastase abnormal expression theory [47,51,52], and the melanin over-synthesis theory [53,54,55,56,57] (Figure 1). Based on this premise, the present study critically examines the anti-photo-aging properties of marine bioactive peptides, encompassing their molecular characterization and underlying mechanisms associated with photoaging. The findings elucidate the protective attributes of marine bioactive peptides in mitigating photoaging and promoting skin well-being. The objective of this study is to explore the potential of marine bioderived products in promoting industrialization and the development of natural anti-photoaging agents for the food, pharmaceutical, and cosmetic industries. Additionally, this research aims to establish a theoretical foundation for the study and application of marine functionalized products. Ultimately, this investigation seeks to contribute to the understanding of marine bioactive peptides’ efficacy in combating skin photoaging, thereby providing valuable insights for future research in this field.
Figure 1.
The factors in photoaging.
2. Anti-Photoaging Mechanism of Marine Bioactive Peptides
2.1. Peptide Anti-Skin-Photoaging by Inhibiting Oxidative Stress Damage
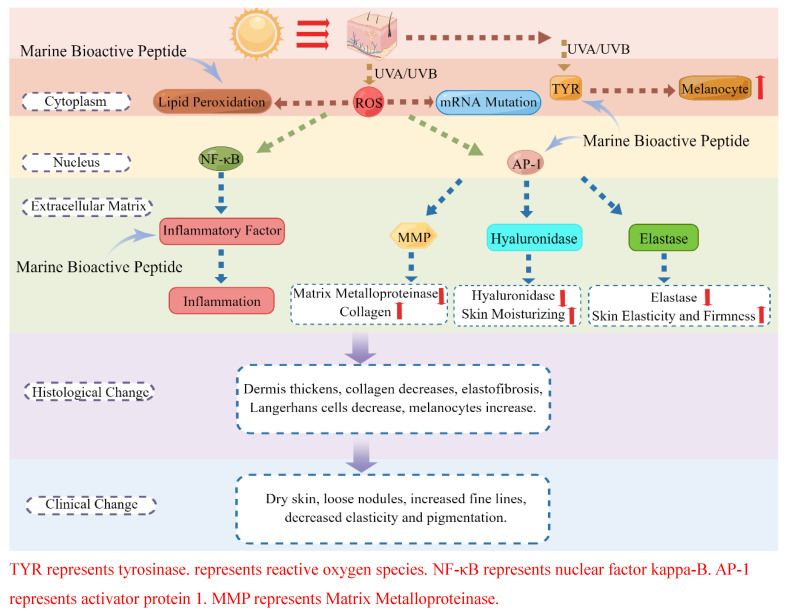
Oxidative stress, characterized by an imbalance between oxidation and antioxidant mechanisms within the body, constitutes a significant contributor to skin photoaging [58,59] (Figure 2). In the context of normal physiological conditions, a limited quantity of reactive oxygen species (ROS) exerts an immune defense effect and confers benefits to the body [60] (Table 1). However, excessive ROS production induced by ultraviolet radiation disrupts the cellular REDOX capacity, thereby impairing the oxidative stress defense system [61]. Consequently, the regulation of ROS levels assumes paramount importance in preserving the equilibrium of skin homeostasis [62].
Figure 2.
Schematic diagram of anti-photoaging mechanisms.
Table 1.
Potential bioactive antioxidant peptides from marine resources.
| Source | Enzyme Used | Peptides (Amino Acid Sequence) | Mechanism of Action | In Vivo or In Vitro | Reference |
|---|---|---|---|---|---|
| Tuna eggs | - | Ile-Cys-Arg-Asp and Leu-Cys-Gly-Glu-Cys | Inhibition of DPPH radicals and activation of SOD and GSH-Px | in vivo | [21] |
| Boiled abalone by-products | - | Ala-Thr-Pro-Gly-Asp-Glu-Gly | Inhibition of ROS radicals | in vitro | [22] |
| Jellyfish collagen | Pepsin | - | Activation of total antioxidant activity | in vitro | [23] |
| Rhopilema esculentum | Pepsin | - | Activation of SOD, CAT, and GSH-Px | in vivo | [24] |
| Salmon skin | - | - | Activation of SOD, CAT, and GSH-Px | in vivo | [25] |
| Katsuwonus pelamis | - | TCP3, TCP6, and TCP9 | Activation of SOD, CAT, and GSH-Px | in vitro | [26] |
| Tilapia gelatin | - | Leu-Ser-Gly-Tyr-Gly-Pro | Scavenging free radicals | in vitro | [27] |
| Katsuwonus pelamis | - | - | Scavenging free radicals | in vitro | [28] |
| Monkfish | Trypsin | Glu-Trp-Pro-Ala-Gln, Phe-Leu-His-Arg-Pro, and Leu-Met-Gly-Gln-Trp | Inhibition of DPPH radicals and hydroxyl radicals; activation of SOD, CAT, and GSH-Px |
in vitro | [29] |
| Macroalga P. palmata | Corolase PP | Ser-Asp-Ile-Thr-Arg-Pro-Gly-Gly-Asn-Met | Activation of oxygen radical absorption capacity (ORAC) and iron reduction antioxidant capacity (FRAP) | in vitro | [30] |
| Thunnus obesus | Alcalase, α-chymotrypsin, neutrase, papain, pepsin, and trypsin | H-Leu-Asn-Leu-Pro-Thr-Ala-Val-Tyr-Met-Val-Thr-OH | Inhibition of DPPH, hydroxyl, superoxide, and alkyl radicals | in vitro | [31] |
| Magalaspis cordyla | Pepsin/trypsin, and α-chymotrypsin | Asn-His-Arg-Tyr-Asp-Arg | Inhibition of DPPH and hydroxyl radicals | in vitro | [32] |
| Otolithes ruber | pepsin/trypsin and α-chymotrypsin | Gly-Asn-Arg-Gly-Phe-Ala-Cys-Arg-His-Ala | Inhibition of DPPH and hydroxyl radicals | in vitro | [32] |
| Hypoptychus dybowskii | Alcalase, neutrase, α-chymotrypsin, papain, pepsin, and trypsin | Ile–Val–Gly–Gly–Phe–Pro–His–Tyr–Leu | Inhibition of DPPH radicals | in vitro | [33] |
| Oreochromis niloticus | Alcalase, pronase E, pepsin, and trypsin | Asp-Pro-Ala-Leu-Ala-Thr-Glu-Pro-Asp-Pro-Met-Pro-Phe | Inhibition of DPPH, hydroxyl, and superoxide radicals | in vitro | [34] |
| Decapterus maruadsi | Alcalase, neutral protease, papain, pepsin, and trypsin | His-Asp-His-Pro-Val-Cys and His-Glu-Lys-Val-Cys | Inhibition of DPPH and hydroxyl radicals | in vitro | [35] |
| Johnius belengerii | Trypsin, R-chymotrypsin, and pepsin | His-Gly-Pro-Leu-Gly-Pro-Leu | Inhibition of DPPH radicals | in vitro | [36] |
| Paralichthys olivaceus | Papain, pepsin, trypsin, neutrase, alcalase, kojizyme, protamex, and α-chymotrypsin | Val-Cys-Ser-Val and Cys-Ala-Ala-Pro | Inhibition of DPPH radicals | in vitro | [37] |
Marine-derived peptides have garnered considerable interest among researchers due to their potent antioxidant characteristics [63]. Specifically, the peptides ICRD and LCGEC, derived from tuna eggs, exhibit robust in vitro DPPH free-radical scavenging activity and effectively protect HaCaT cells from ultraviolet B (UVB) radiation by upregulating the expression of SOD and GSH-Px [21]. Additionally, the abalone peptide ATPGEG demonstrates the capacity to mitigate UVB-induced ROS levels in HaCaT cells and inhibit cellular DNA damage resulting from UVB exposure [22]. According to reports, jellyfish collagen exhibits notable antioxidant activity and holds significant promise for the development of nutritional health products [23]. In the context of UV-induced skin photoaging in mice, the application of jellyfish collagen hydrolysate (JCH) has been found to augment the protective effect by elevating the levels of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) [24]. Similarly, the gelatin hydrolysate AMW derived from salmon skin has been observed to diminish malondialdehyde (MDA) content and enhance antioxidant enzyme and glutathione (GSH) levels while mitigating the oxidative damage inflicted by ultraviolet radiation on the skin [25]. Three antioxidant peptides, namely TCP3 (PKK), TCP6 (YEGGD), and TCP9 (GPGLM), derived from the bonipjack heart artery balls of Skipjack tuna, have been found to enhance the activity of SOD, CAT, and GSH-P, effectively eliminating reactive oxygen species (ROS) and reducing intracellular malondialdehyde (MDA) levels [28]. Consequently, the protective capacity of HaCaT cells against UVB irradiation has been significantly enhanced [26]. Moreover, the impact of tilapia gelatin peptides on UV-induced skin damage in mice has also been investigated [27]. The findings demonstrate that the tilapia gelatin peptide LSGTGP can effectively neutralize hydroxyl radicals, thereby preventing UV-induced damage [27].
2.2. Peptide Anti-Skin-Photoaging via Anti-Inflammation
When cells are exposed to UV radiation and other environmental stimuli, they have the ability to release a group of small molecular peptides or glycoproteins known as cytokines, which play a crucial role in regulating the inflammatory response [64,65] (Figure 2). In normal physiological conditions, the production of cytokines remains at low levels, thereby avoiding any harm to the cells [66]. However, following exposure to ultraviolet irradiation, both epidermal and dermal cells can activate NF-κB, leading to the synthesis and secretion of inflammatory factors such as IL-1, IL-6, cyclooxygenase-2 (COX-2), and TNF-α, consequently inducing an inflammatory response [67]. Simultaneously, the presence of these cytokines induces mitochondrial impairment, leading to heightened levels of reactive oxygen species (ROS) and subsequent augmentation in the release of inflammatory mediators [68].
The potent anti-inflammatory properties of marine peptides have been extensively documented in the scientific literature [69]. Specifically, gelatin hydrolysate derived from the skin of Pacific cod has been found to effectively mitigate inflammation caused by UV radiation [38]. This is achieved through the suppression of pro-inflammatory cytokines IL-1α and TNF-α, thereby preventing UV-radiation-induced skin damage. The polypeptide (WNLNP) extracted from oyster protein can significantly down-regulate the inflammatory pathway of MAPK/NF-κB and reduce the overexpression of bax, which has a good protective effect against skin injury [70]. The hydrolysate (PWG) extracted from Pacific cod skin reduced the cytokines TNF-α, IL-6, and IL-1β associated with inflammation and inhibited inflammation by inhibiting the nuclear factor-κB (NF-κB) pathway, suggesting that PWG may be an effective anti-photoaging material [71]. Additionally, the impact of hydrolyzing six collagens extracted from sturgeon skin on photodamage induced by UVB radiation has been investigated [39]. The study revealed that the peptides DPFRHY and PEG, derived from M. maritima, effectively suppressed the abnormal expression of pro-inflammatory cytokines IL-1β, IL-6, TNF-α, and Cox-2. Specifically, DPFRHY demonstrated notable anti-inflammatory and cell repair properties [39]. Furthermore, PEG exhibited the inhibition of IL-1β, IL-6, PGE, TNF-α, and COX-2 production while also providing enhanced protection against UV-induced photoaging in mice [40]. The polypeptide (SEP) extracted from by-products of bonito fish can significantly reduce the expression levels of IL-6, IL-10, and TNF-α and has a good anti-inflammatory effect [72]. In addition, it has also been reported that SEP-3 significantly inhibits the inflammatory pathway of NF-κB [72], which has a strong potential to prevent photoaging and inflammatory diseases. Meanwhile, peptides isolated from marine actinomycetes have attracted attention for their unique biological activities [73]. The cyclic peptide isolated from Streptomyces maritimus CNB-091 can be used as an anti-inflammatory agent [74], while the peptide isolated from Streptomyces maritimus CNB-982 also has good anti-inflammatory activity and is expected to be used as an anti-photoaging agent [75]. However, it is important to note that limited research has been conducted in this particular field, thus necessitating further investigation.
2.3. Peptide Anti-Skin-Photoaging via Inhibition of Matrix Metalloproteinases
Under typical physiological circumstances, matrix metalloproteinases (MMPs) exhibit minimal expression within the human body [76] (Figure 2). However, their expression escalates swiftly upon exposure to various stimuli such as ultraviolet (UV) radiation, inflammation, and cancer [77]. Specifically, UVB radiation has been observed to induce the secretion of MMPs in a dosage-dependent manner [78]. This augmented expression of MMPs facilitates the degradation of the dermal extracellular matrix (ECM), particularly type I and type III procollagen, while concurrently impeding collagen synthesis [79,80,81]. As a result, these processes contribute to the desiccation and diminished elasticity of the skin [79,80,81]. Consequently, the inhibition of MMP expression and the promotion of collagen synthesis are crucial strategies in combating photoaging [82].
Inhibiting the aberrant expression of MMPs represents a significant approach in investigating the effects of skin anti-photoaging [83]. Notably, the peptides derived from the skin gelatin hydrolysate of Pacific cod (GEIGPSGGRGKPGKDGDAGPK and GFSGLDGAKGD) exhibited inhibitory properties against MMP-1 expression in mouse skin fibroblasts subjected to UV radiation [41]. By suppressing MMP-1 activity, the process of skin photoaging can be ameliorated. Additionally, peptides obtained from Pinctada martensii meat have been found to mitigate UVB-radiation-induced damage in HaCaT cells by inhibiting the expression of MMPs [42]. The levels of interstitial collagenase (MMP-1) and stromal lyase (MMP-3) were found to be reduced, leading to an improvement in UVB-induced cell damage in HaCaT cells. Peptides derived from Pyropia yezoensis (specifically peptide PYP1-5) were observed to inhibit the expression of the MMP-1 protein, thereby mitigating skin aging and demonstrating potential in combating photoaging [43]. Additionally, the enzymatic hydrolysate (OAH) obtained from oyster exhibited the ability to suppress the expression of MMP-1 and alleviate UV-induced cytotoxicity, thus exhibiting anti-photoaging properties [44]. The application of tlapia collagen hydrolysate YGDE resulted in a reduction in the enzymatic activity of MMP-1 and MMP-9, mitigated cellular damage induced by ultraviolet rays (UVB), and ameliorated the effects of skin photoaging [45].
2.4. Peptide Anti-Skin-Photoaging via Inhibition of Hyaluronidase
Hyaluronic acid, a biopolymer constituent of the dermal extracellular matrix, is naturally present in various tissues of the body, such as the synovial fluid, eyes, gums, bone tissue, and heart valves [84,85]. Its capacity to bind water enables it to contribute to the preservation of skin moisture and serves a significant function in skin rejuvenation by enhancing viscosity and reducing extracellular fluid permeability [86]. In the context of normal skin, hyaluronic acid synthesis is responsible for maintaining skin moisture [87]. Nevertheless, the reduction in hyaluronic acid levels is attributed to the excessive production of hyaluronidase [88] (Figure 2). Consequently, the inhibition of hyaluronic acid degradation is imperative for safeguarding the integrity of the skin.
The impact of hyaluronidase on skin photoaging has garnered significant scholarly interest [89]. Research has indicated that peptides derived from a variety of microalgae species (including Sukka’s algae, Dunaliella, and Nanophyllum) possess the ability to diminish hyaluronidase activity [46]. Specifically, peptides extracted from three microalgae species (Dunaliella tertiolecta, Tetraselmis suecica, and Nannochloropsis sp.) exhibit inhibitory effects on hyaluronidase [47]. Simultaneously, previous studies have provided evidence that the peptide levels derived from the maximum biomass of spirochete, namely pepsin (PHP), Subtilin A (PHA), and both PHS enzymes, possess anti-hyaluronidase activities [48]. Furthermore, the collagen-derived peptides from squid (Todarodes pacificus) generated through alkaline enzymes exhibit dose-dependent characteristics and display promising efficacy as anti-photoaging agents [49]. The administration of low-molecular-weight collagen peptides derived from fish scales has been demonstrated to stimulate the synthesis of hyaluronic acid in HaCaT cells. This process counteracts photoaging damage by upregulating the expression of the hyaluronate synthase 2 (HAS2) gene and downregulating the expression of the hyaluronase 1 (HYAL1) gene, thereby promoting improvement [50].
2.5. Peptide Anti-Skin-Photoaging via Inhibition of Elastase
Elastin, an extracellular matrix protein, imparts elasticity and resilience to various connective tissues including the aorta, lungs, cartilage, elastic ligaments, and skin [90]. In comparison to collagen, elastin exhibits a significantly higher degree of flexibility, approximately 1000 times greater [91]. Consequently, the principal role of elastin lies in conferring tissue elasticity [92]. The synthesis and secretion of elastin occur through the activity of vascular smooth muscle cells and fibroblasts [93]. This physiological phenomenon typically ceases shortly after the onset of puberty, coinciding with the maturation of the body. Alongside collagen, the production of elastin is initiated to uphold the suppleness and tautness of the skin [91]. Nevertheless, an excessive production of elastase leads to a decline in elastin fibers, thereby compromising the mechanical characteristics of the tissue (Figure 2). Consequently, the inhibition of elastase becomes imperative in safeguarding the integumentary system [94].
Marine bioactive peptides have demonstrated their potential in effectively inhibiting elastase activity within skin anti-photoaging pathways [42,89]. Previous studies have reported the beneficial impact of squid skin collagen hydrolysate in inhibiting elastin and serving as an effective anti-photoaging agent [51]. Notably, two peptides derived from bonito’s elastin hydrolysate, namely TGVLTVM and NHIINGW, have exhibited protective effects against UVA-irradiation-induced skin damage by effectively inhibiting elastase [52]. Furthermore, additional peptides with elastase inhibition properties have been identified from Duneria, Susica, and Nannochloropsis sp. [47]. This implies that the utilization of these peptides may potentially enhance skin health by preventing the deterioration of the protein matrix within the skin, although the precise mechanism has yet to be fully understood.
2.6. Peptide Anti-Skin-Photoaging via Inhibition of Melanin Over-Synthesis
Melanin serves as the primary protective mechanism against ultraviolet radiation in the skin [95]. Following exposure to UVB, melanocytes situated in the basal layer of the skin generate an excessive amount of melanin, leading to the manifestation of skin pigmentation [96] (Figure 2). The formation of this pigment is facilitated by a series of oxidation reactions mediated by the enzyme tyrosinase (TYR), with the synthesis, transportation, and catalytic activity of TYR playing pivotal roles in melanin synthesis [97]. Hence, directing attention towards the active constituents of marine bioactive peptides towards melanocytes, impeding the overproduction of melanin, and exploring novel tyrosinase inhibitors have emerged as a viable approach to mitigate the manifestations associated with skin photoaging.
The TYR inhibitory peptide derived from marine organisms exhibits the ability to hinder melanin production and enhance skin lightening, thereby demonstrating promising prospects in combating skin photoaging [98]. Notably, research has demonstrated that the protein hydrolysate obtained from the shrimp by-product Kirin polysavone exhibits significant TYR inhibitory activity [56]. This inhibitory effect is concentration-dependent, with a complete TYR inhibition observed at a concentration of 400 μg/mL [54]. Additionally, tilapia scale polypeptides possess the capacity to chelate copper ions, thereby affecting TYR activity [55]. In vitro investigations have demonstrated the potent inhibition of TYR activity and effective reduction of melanin synthesis in mouse melanoma cells through the utilization of polypeptide hydrolysates derived from tilapia by-products [55]. Furthermore, there have been numerous in vivo and in vitro evaluations of the use of active collagen peptides derived from fish by-products. Notably, the marine bioactive peptide DLGFLARGF has exhibited the ability to impede tyrosinase activity, consequently hindering melanin production [57]. The squamosal fish’s collagen peptide demonstrates a remarkable moisture absorption capacity of 20%, effectively inhibits tyrosinase activity and melanin synthesis, and exhibits promising anti-photoaging properties [53].
3. Skin Protective Effects of Marine Bioactive Peptides
3.1. Peptides Improve Skin via Photoprotective Mechanisms
Skin aging encompasses both intrinsic aging and photoaging, with research indicating that photoaging contributes to over 80% of facial aging [99]. External factors that contribute to skin photoaging primarily consist of ultraviolet (UV) radiation, infrared radiation, chemical smoke, dust, and haze, with UV radiation being the most influential [100]. Numerous studies have demonstrated the efficacy of marine biopeptides in combating photoaging, making them a promising ingredient for the development of cosmeceuticals aimed at reducing skin aging.
The present study investigated the anti-photoaging effects of the peptide LSGYGP, isolated from the skin of tilapia [27]. It was observed that this peptide exerted its beneficial effects on the skin by utilizing its antioxidant activity to ameliorate UV-induced photoaging in mice [27]. In addition, it was found that cod skin gelatin hydrolysate (CGH) can inhibit MMP-1 and contribute to its anti-photoaging expression [41]. Protein hydrolysates derived from various sources such as fish bones, scales, and digestive organs were also identified as potential agents for improving skin aging [101,102,103]. Research findings indicate that starfish collagen peptides possess the ability to diminish the expression of MMP-1, which is induced by UV radiation photoaging, thereby exhibiting anti-photoaging properties [44]. Polypeptides isolated from scallops can inhibit UVA-induced ROS production and protect HaCaT cells from UVA-induced apoptosis [70]. Polypeptide (JCH) extracted from jellyfish (Rhopilema esculentum) mitigated abnormal UV-induced changes in antioxidant defense systems such as superoxide dismutase and glutathione peroxidase, effectively protecting skin from UV radiation [104]. The hydrolysate (PWG) extracted from Pacific cod skin decreased the cytokines TNF-α, IL-6, and IL-1β associated with inflammation and increased the contents of antioxidant enzymes, HO-1, SOD, GPx, CAT, and GSH. These multi-target mechanisms suggest that PWG may be an effective anti-photoaging material [71]. Additionally, the UVB irradiation of human immortalized keratinocytes (Hacats) and mouse aging models have been studied. The hexapeptide (AAH) extracted from spirulina can significantly increase the expression of SOD and GSH-P and reduce the expression of MMP-1 and MMP-3, which has potential applications in preventing skin photoaging [105]. The derived peptide (CDP) extracted from Chlorella can inhibit UVB-induced MMP-1 expression in skin fibroblasts and achieve photoprotection [106].
3.2. Peptides Improve Skin via Anti-Microbial Mechanisms
The skin is frequently exposed to environmental factors and can be harmed by microbial agents and ultraviolet radiation [107]. The aging process of the skin diminishes the production of protective bacteria, resulting in skin damage [108]. Consequently, it is imperative to investigate bioactive peptides that exhibit resistance against various bacteria such as Staphylococcus aureus, propionibacterium acnes, Pseudomonas aeruginosa, Enterococcus faecium, Acinetobacter baumannii, Klebsiella pneumoniae, propionibacterium acnes, and Escherichia coli. Numerous studies have demonstrated the efficacious antibacterial activity of marine-derived biopeptides, thereby highlighting their considerable potential for application in the realm of skin protection.
Marine antimicrobial peptides exhibit a diverse array of antibacterial and bactericidal properties, rendering them suitable for employment as fungicides [109]. Moreover, these peptides possess substantial potential in the realm of skin protection [110]. They are extensively present in various marine fish species [111], such as Capitella teleta, Porphyra yezoensis, Octopus minor, Olivancillaria hiatula, Mytilus coruscus, Green tiger shrimp (Peaneaus semisulcatus), Hypoptychus dybowskii, and Cyanobacteria, demonstrating notable efficacy against bacterial pathogens (Table 2) [112,113,114,115,116,117,118]. The proteolytic peptides derived from S. longicruris, a brown seaweed, exhibited notable antibacterial efficacy and demonstrated significant activity against Gram-positive Staphylococcus aureus [119]. Specifically, HAHp2-3-I, consisting of five cationic peptides (MLTTPPHAKYVLQW, SHAATKAPPKNGNY, PTAGVANALQHA, QLGTHSAQPVPF, and VNVDERWRKL) and obtained from the hydrolysate of semi-engraan pepsin, displayed robust resistance against Escherichia coli [8]. Additionally, a separate investigation revealed that the half anchoa pepsin hydrolysate (HAHp) exhibited a potent inhibitory effect against Escherichia coli, suggesting its potential as a protective agent for the skin [120]. The potential of protamex hydrolysate, derived from Atlantic mackerel, to provide skin protection through the inhibition of both Gram-positive (intrinsic listeria) and Gram-negative bacteria (Escherichia coli) has been observed in various studies [121]. Furthermore, these studies have consistently shown that the inhibitory activity against both Gram-positive (intrinsic listeria) and Gram-negative bacteria (Escherichia coli) is more pronounced in Atlantic mackerel [122].
Table 2.
Potential bioactive antimicrobial peptides from marine resources.
| Source | Enzyme Used | Peptides (Amino Acid Sequence) | Microorganisms | Reference |
|---|---|---|---|---|
| Capitella teleta | - | - | E. coli BL21 | [110] |
| Porphyra yezoensis | Pepsin | Thr-Pro-Asp-Ser-Glu-Ala-Leu | Staphylococcus aureus | [112] |
| Octopus minor | - | Gly-Trp-Leu-Ile-Arg-Gly-Ala-Ile-His-Ala-Gly-Lys-Ala-Ile-His-Gly-Leu-Ile-His-Arg-Arg-Arg-His | Candida albicans | [113] |
| Olivancillaria hiatula | - | - | Pseudomonas aeruginosa | [114] |
| Mytilus coruscus | - | - | Gram-positive bacteria —Bacillus, Bacillus subtilis, Clostridium perfringens, Staphylococcus aureus, Streptococcus, Streptococcus mutans; Gram-negative bacteria— Escherichia coli, Pseudomonas aeruginosa, Vibrio alginolyticus | [115] |
| Green tiger shrimp (Peaneaus semisulcatus) | - | - | Staphylococcus aureus | [116] |
| Hypoptychus dybowskii | - | Ser-Arg-Ser-Ser-Arg-Ala-Gly-Leu-Gln-Phe-Pro-Val-Gly-Arg-Ile-His-Arg-Leu-Leu-Arg-Lys | Staphylococcus aureus and Escherichia coli | [117] |
| Cyanobacteria | - | - | Candida albicans | [118] |
3.3. Peptides Improve Skin via Skin Repair
Skin is an important immune organ of the human body, but it easily experiences health problems under the influence of physiological factors and external environmental factors. At the same time, as the efficacy of collagen peptides in improving the skin has become apparent, the research in this area has been increasing in recent years (Table 3). Studies have shown that the ingestion of collagen peptides inhibits UVB-induced reduced skin hydration, epidermal hyperplasia, and decreased soluble type I collagen. These results suggest that collagen peptides as a dietary supplement may be beneficial in inhibiting UVB-induced skin damage and photoaging [123]. The oral administration of marine collagen peptides from salmon skin promoted skin wound healing and angiogenesis in rats in [124]. Meanwhile, the oral administration of marine collagen peptides from salmon skin also promoted skin wound healing in rats in [125]. Cod skin collagen polypeptides have good moisture absorption and moisture retention properties and can reduce the damage from ultraviolet light on the skin [126]. At the same time, some studies have shown that cod skin gelatin peptides can inhibit the production of melanin [127]. Paralichthys olivaceus (PO) and Alaska pollock Gadus chalcogrammus (AP) proteolytic substance increased the viability of UVB-irradiated HaCaT cells and decreased the intracellular and extracellular melanin content of stimulated B16F10 cells. These results indicate that PO and AP have potential applications in the cosmetics industry [128]. In a UVB-irradiated HDF cell model, Pacific cod protein hydrolysate (PWG) had a protective effect against photoaging by down-regulating MMP1 [71]. In addition, there are clinical trials showing that marine collagen peptide (MCP) can improve skin properties without the risk of oxidative damage [125].
Table 3.
Potential skin-protective bioactive peptides from marine resources.
| Source | Functional Product | Processing Method | Cosmeceutical Function | Reference |
|---|---|---|---|---|
| Salmon skin | Collagen peptides | Water, protease | Wound healing | [124] |
| Fish scales | Collagen peptides | Hot water, enzymatic | Improving skin elasticity | [125] |
| Codfish skin | Collagen polypeptides | Water, pepsin, and alkaline protease | Moisturizer, antioxidant | [126] |
| Pacific whiting skin | Hydrolysate gelatin | Hot water | Anti-photoaging, delayed skin wrinkling | [71] |
| Pacific cod skin | Gelatin and polypeptides | Hot water extraction, pepsin, and alkaline protease hydrolysis | Melanogenesis inhibition | [127] |
| Olive flounder and Alaska pollock skins | Fish skin hydrolysates | Enzymatic hydrolysis (pepsin, alcalase, protemax) | Minimizing ROS levels, enhancing the viability of UVB-irradiated HaCat cells and human dermal fibroblasts | [128] |
| Scales of Tilapia zillii | Polypeptides | Pepsin | Increasing skin hydration and decreasing epidermal hyperplasia | [123] |
The reparative properties of marine biological collagen and its hydrolysate for skin damage caused by UV exposure have been observed [129]. In vitro cell experiments have demonstrated that the hydrolysate derived from sponge collagen possesses wound healing capabilities and tissue repair functions for UV-irradiated fibroblasts and keratinocytes [130]. Additionally, scholars have conducted mouse experiments and discovered that gelatin hydrolysate from trilefish exhibits a reparative effect on UV-induced skin damage [25]. The reparative properties of jellyfish collagen peptide have been observed in its ability to restore endogenous collagen and elastin fibers in compromised skin [104]. A study conducted on mice with cortical injury demonstrated that the administration of nude sidereal collagen peptide resulted in successful wound healing [131]. Furthermore, the application of marine collagen peptides (MCPs) derived from salmon skin exhibited a substantial enhancement in the tensile strength of skin wounds in rats [132]. The specific peptides employed in cosmetic formulations exhibit diverse effects. Notably, collagen peptides derived from deep-sea fish possess a range of functionalities [133], such as skin whitening, freckle removal, moisturization, nutritional repair, and anti-aging properties. Consequently, these peptides hold significant promise in safeguarding the skin.
4. Bioavailability of Marine Bioactive Peptides
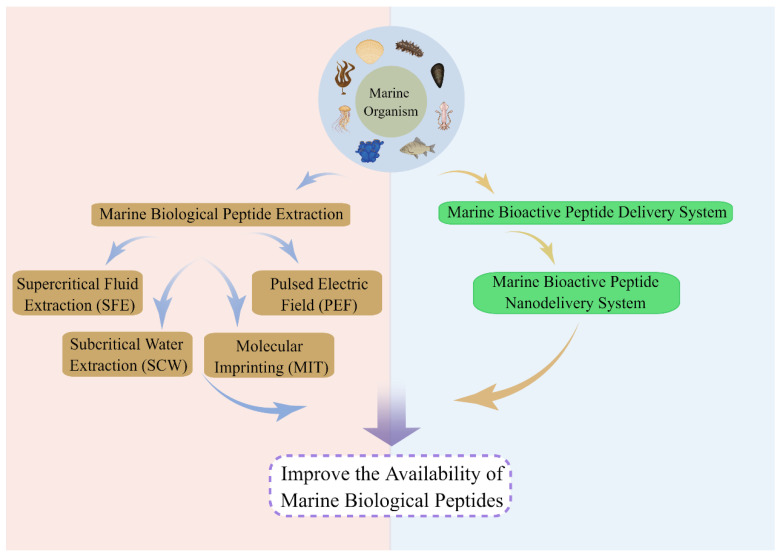
The photoaging of the skin is a notable characteristic associated with the aging process, particularly concerning women [134]. Statistical data reveal that a mere 2% of Chinese women undertake anti-aging measures, while the anti-aging market in China reached a substantial value of RMB 6.4 billion in 1990 [135]. In addition, by 2027, the global market for skin whitening and anti-aging products is expected to reach USD 1.23 billion [136] and USD 83.2 billion [137], respectfully. Marine bioactive peptide substances exhibit potent anti-photoaging and skin protection properties with minimal toxicity; however, the investigation into their underlying mechanisms remains at a nascent stage. Therefore, it is imperative to undertake the isolation and purification of marine bioactive peptides, as well as to conduct extensive investigations into their safety and bioavailability. Additionally, there is a need to further explore the relevant indicators and pathways associated with photoaging. It is crucial to note that the development of marine bioactive peptide drugs is still in its nascent stage and lacks clinical application. Consequently, enhancing the bioavailability of marine bioactive peptides through increased modifications and more favorable methods of separation and purification has become highly significant. Therefore, efforts should be made to improve the separation and purification techniques for marine bioactive peptides in order to enhance their bioavailability (Figure 3).
Figure 3.
Marine bioactive peptide bioavailability enhancement pathway.
4.1. Improvement of Bioavailability of Marine Bioactive Peptides via Isolation and Purification
The majority of polypeptide bioactive compounds derived from marine organisms exist as intricate mixtures, and the presence of these complex constituents can impede the extraction procedure of polypeptide compounds. Consequently, prior to conducting an in-depth investigation of polypeptide compounds, it is imperative to effectively extract and purify these compounds from marine organisms to facilitate a more comprehensive and meticulous analysis. A fundamental requirement for identifying polypeptide compounds in marine organisms is the establishment of effective extraction technology. Consequently, the exploration of novel approaches for extracting polypeptide compounds with high purity has emerged as a new area of research. The conventional method of extracting and purifying polypeptides relies on the utilization of organic solvents, which is frequently associated with inefficiency, time consumption, and labor intensiveness [138]. Furthermore, the utilization of organic solvents is restricted in the food and pharmaceutical industries due to certain limitations. Moreover, the viability of their application is further compromised by the potential generation of degradation products during the extraction process. As scientific and technological advancements continue to unfold, numerous intricate extraction and purification methodologies have been developed and implemented, such as supercritical fluid extraction (SFE) [139], subcritical water extraction (SCW) [140], pulsed electric fields (PEFs) [140], and molecular imprinting technology (MIT) [141]. These technologies provide enhanced functionalities by mitigating the constraints of conventional extraction methods.
Supercritical fluid extraction (SFE) is a technique wherein a supercritical fluid is employed as a solvent to facilitate the separation of a mixture, owing to the notable permeability and solubility of the fluid in this state. In contrast to conventional toxic, flammable, and volatile organic solvents, SFE predominantly employs carbon dioxide as an extractant [142]. SFE technology holds significance in the extraction of marine polypeptides. Moreover, it possesses the ability to effectively isolate and extract various other marine biological active substances, including marine biotoxins, essential oils, marine natural pigments, and select rare amino acids [143].
Subcritical water extraction (SCW) refers to the treatment of liquid water under high pressure at temperatures above its boiling point (100–374 °C). This process utilizes subcritical water as a solvent for both polar and non-polar compounds due to its lower dielectric constant in the subcritical state, which enhances its affinity for less polar compounds and facilitates excellent protein solubility [144]. Furthermore, the application of a high temperature and pressure during SCW also triggers protein hydrolysis, leading to the generation of peptides and amino acids. The SCW process resulted in a peptide yield of 87.4% from sardine by-products [144]. Additionally, the hydrolysis rate of squid viscera treated with SCW reached 95% [145]. By employing SCW in the extraction process, the need for enzyme and acid–base ion removal is eliminated, thereby establishing SCW as an environmentally friendly technology for enhancing the yield of marine bioactive peptides [146].
The utilization of pulsed electric field (PEF) technology has gained prominence in the fields of the low-temperature sterilization and preservation of agricultural products. In recent times, there has been a growing trend towards employing PEF for the extraction of bioactive substances from food-source substrates. PEF facilitates the enhancement of membrane permeability through mechanisms such as membrane electroporation or electroosmosis, thereby enabling the release of intracellular proteins and exogenous enzymes. Consequently, the integration of PEF with enzymatic or solvent extraction methods presents a promising approach to augment the production yield of marine bioactive peptides [147]. For instance, the utilization of a pulsed electric field (PEF) in conjunction with enzymes, such as flavor enzymes and trypsin, during the treatment of abalone (Haliotis discus hannai Ino) viscera resulted in a significantly higher yield of hydrolysate compared to extraction using a single enzyme [148]. Furthermore, the application of PEF to senedesmus almeriensis demonstrated enhanced enzymatic (specifically alkaline enzyme) hydrolysis, leading to an increase in the yield of bioactive peptides from 40.8% to 50.6% [149]. The utilization of a pulsed electric field (PEF) in marine bioprocessing remains constrained primarily by equipment costs. Nevertheless, the inherent attributes of PEFs, such as their low energy consumption, rapid processing time, and comparatively gentle extraction conditions, hold significant promise for augmenting the production capacity of bioproducts derived from marine organisms.
Molecular imprinting (MIT) is a novel interdisciplinary technique that integrates receptor–antibody mechanisms and expertise in biochemistry, structural chemistry, and materials chemistry [150]. Due to its remarkable selectivity, robust stability, and broad applicability, MIT has garnered significant attention and extensive investigation in recent years [149]. The advancement of molecular imprinting technology holds the potential to facilitate the efficient and expeditious separation of biomolecules, including polypeptides and proteins, from intricate mixtures [149]. However, during the preparation procedure of molecularly imprinted polymers, it is typically imperative to incorporate biomolecules as imprint templates, some of which possess conformational flexibility, volatility, and inactivation.
4.2. Improvement of Bioavailability of Marine Bioactive Peptides via Nanodelivery Systems
The potential disparity between the activity of peptides in vitro and in vivo is influenced by the presence of enzymes and stomach acids during gastrointestinal digestion, which can impact peptide bioavailability. Consequently, it is imperative to carry out both in vitro and in vivo experiments to ascertain the validity of these findings. In particular, the outcomes of in vivo studies need to be verified in order to confirm the observed results. In the event that biological activity is compromised in vitro, it is essential to re-evaluate peptide concentrations or matrix properties prior to conducting in vivo testing. In order to ascertain the gastrointestinal digestibility and solubility, absorption, distribution, and utilization of each peptide, as well as to determine the required dosage for achieving its efficacy, it is imperative to conduct in vivo studies encompassing both animal and human subjects [151]. Various models, including invertebrate C. elegans and fruit flies, vertebrate rats and mice, and human subjects, have been employed for in vivo investigations of peptide functionality, particularly in the context of functional foods. In vivo experiments serve as a reliable means of observing the potential outcomes associated with the consumption of a substrate containing peptides. However, these experiments are intricate and costly, necessitating the involvement of either animals or humans and often requiring an extended duration. To date, there has been a scarcity of research conducted on the bioavailability of marine compounds.
Despite the demonstrated diverse biological activities of marine bioactive peptides, their practical application is hindered by challenges such as hydrophobicity, chemical instability, and limited bioavailability. Consequently, the preservation of peptide activity post-digestion necessitates the exploration of protective measures. Among these measures, nanodelivery has emerged as the most extensively investigated and documented method.
Nanotechnology has emerged as a promising strategy for addressing the challenges associated with incorporating bioactive peptides into food [151]. Recent studies have demonstrated the efficacy of utilizing nanosized carriers, such as nanoemulsions, nanoliposomes, microemulsions, micelles, nanostructured lipid carriers, solid lipid nanoparticles, and polymer nanoparticles, to encapsulate hydrophobic bioactive peptides. These encapsulated peptides have exhibited enhanced biological effects, highlighting the potential of nanotechnology in developing effective delivery systems for bioactive peptides in food [152]. Furthermore, nanometer-based delivery systems possess the ability to modify the uptake pathway of bioactive substances by manipulating their metabolism and bioactivity within an organism [153]. This enhanced biological performance can be attributed to the diminutive size, augmented surface area, and refined surface chemistry of these systems. For instance, the encapsulation of peptide grades derived from fish skin gelatin within a nanoliposome system resulted in a prolonged release rate, decreasing from 41% to 24% over a span of 30 h, accompanied by a comparatively elevated antioxidant activity ranging from 15.7% to 74.7% when compared to unencapsulated peptide grades [154]. In two additional autonomous investigations, the nanoencapsulation of fish protein peptides was achieved through ion-to-gel techniques, yielding improved gastrointestinal stability and bioavailability outcomes [155]. Nevertheless, the safety considerations associated with nanoencapsulated marine peptides should not be disregarded. Ultimately, comprehending the destiny and conduct of nanoparticles in food and the human organism is imperative for safety evaluation, with their capacity to maintain structural integrity within the gastrointestinal tract serving as the principal determining factor. In the presence of digestive enzymes, strong acids, and bile salts, nanoparticles have a tendency to aggregate or experience alterations in size, consequently impacting their capacity to be absorbed and traverse biological barriers within the body [156]. Moreover, nanoparticles derived from digestible organic substances, such as proteins, lipids, or starches, present a lesser risk compared to nanoparticles obtained from indigestible inorganic materials, such as metal or metal oxide nanoparticles.
5. Conclusions and Prospects
The investigation of marine active peptides constitutes a crucial domain within marine research and development in the 21st century, currently experiencing rapid advancements and yielding significant outcomes. Nevertheless, the present research endeavors in this area are insufficient within our nation, necessitating an augmentation in research funding for marine peptides. This would enable the further examination and elucidation of the mechanism of action of marine peptides, the exploration of the inherent characteristics of active peptides, and the fortification of the application research pertaining to marine active peptides across diverse domains.
Marine bioactive peptides have emerged as a significant asset in combating skin photoaging. Extensively investigated for their diverse biological attributes, including theories related to oxidative stress, the abnormal expression of matrix metalloproteinase, inflammatory response, the abnormal expression of hyaluronidase, the abnormal expression of elastase, and the excessive synthesis of melanin, these peptides have garnered considerable attention for their potential in enhancing skin health. Novel extraction methods, such as supercritical fluid extraction (SFE), subcritical water extraction (SCW), pulsed electric fields (PEFs), and molecular imprinting technology (MIT), have successfully yielded several bioactive peptides from marine fish. These methods are regarded as safer alternatives for the development of cosmeceutical products. Simultaneously, the utilization of marine bioactive peptides aids in mitigating environmental pollution resulting from waste generated by the fish processing industry. Furthermore, the potential topical applications or oral utilization of marine bioactive peptides for safeguarding the skin highlight their significant biological activities (Table 4). However, it is crucial to acknowledge that despite the immense potential of marine fish-derived proteins and peptides in the field of cosmeceuticals, the majority of these compounds remain in the experimental phase. Consequently, additional investigations pertaining to their formulations and long-term safety are imperative for their successful commercialization. Additionally, it is imperative to explore the development of supplementary products that can enhance the bioavailability and efficacy of proteins and peptides derived from marine sources, thereby augmenting their potential in the field of cosmeceuticals, particularly in terms of tissue regeneration.
Table 4.
Marine bioactive peptides as cosmetic ingredients.
| Company | Country | By-Product Resource | Bioactive Compounds |
Cosmeceutical Function |
Reference |
|---|---|---|---|---|---|
| Rousselot | France | Fisk skin and bone | Collagen peptides | Skin moisturization, enhanced skin collagen density | [157] |
| Celergen Inc | Switzerland | Fish skin | Collagen hydrolysate | Enhanced skill elasticity | [125] |
| Abyss | France | Fish skin | Collagen hydrolysate | Reduced appearance of wrinkles | [158] |
| Finn Canada | Canada | Salmon skin | Collagen | Improved skin condition; treatment of various skin problems, such as wrinkles, spots, dryness, dullness, and acne | [159] |
| Kenney and Ross Limited | Canada | Fish skin | Collagen | Stimulates healthy skin, nails, and hair | [160] |
| Nuwen | France | Fish skin | Collagen hydrolysate | Skin moisturization | [161] |
| One Ocean | United States | Fish skin | Collagen | Skin moisturization, anti-wrinkle | [162] |
| Osteralia | France | Mother-of-pearl | Oyster shell | Anti-aging, skin nourishment | [163] |
In summary, researchers can obtain a large number of bioactive peptides with strong light protection and light repair properties from marine organisms, which have a series of important functions such as blocking light penetration, anti-oxidant activity, anti-inflammatory behavior, damage repair, delaying degradation, promoting synthesis, and stabilizing the skin barrier and can be applied to various scenarios such as external use, oral use, and product addition. This is of great value for future research in light damage and primary skin disease prevention, product development, and other fields. In addition, marine bioactive peptides, which are generally more acceptable due to their “natural and healthy” characteristics, can promote a positive response from consumers, provide social impetus, and help to further tap the great potential of ocean treasures, providing valuable insights for future research in this field and thus laying a solid foundation for the global anti-aging molecule market.
Acknowledgments
The figures in the manuscript were all drawn with Figdraw.
Author Contributions
X.Z.: Investigation, Writing—original draft. H.Z.: Methodology, Writing—review & editing. S.W.: Data curation. C.M.: Project administration. Y.D.: Conceptualization. H.Y.: Supervision. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Program of Science and Technology Development Plan of Jilin Province (20230508018RC).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Vodouhe M., Marois J., Guay V., Leblanc N., Weisnagel S.J., Bilodeau J.-F., Jacques H. Marginal Impact of Brown Seaweed Ascophyllum nodosum and Fucus vesiculosus Extract on Metabolic and Inflammatory Response in Overweight and Obese Prediabetic Subjects. Mar. Drugs. 2022;20:174. doi: 10.3390/md20030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malve H. Exploring the ocean for new drug developments: Marine pharmacology. J. Pharm. Bioallied Sci. 2016;8:83–91. doi: 10.4103/0975-7406.171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Venkatesan J., Anil S., Kim S.-K., Shim M.S. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs. 2017;15:143. doi: 10.3390/md15050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smichi N., Parsiegla G., Achouri N., Zarai Z., Abousalham A., Fendri A. Intestinal phospholipase A2 from Sparidae species: Functional properties and cytotoxic potential evaluation. Int. J. Biol. Macromol. 2020;143:881–890. doi: 10.1016/j.ijbiomac.2019.09.149. [DOI] [PubMed] [Google Scholar]
- 5.Yang H., Zhang Q., Zhang B., Zhao Y., Wang N. Potential Active Marine Peptides as Anti-Aging Drugs or Drug Candidates. Mar. Drugs. 2023;21:144. doi: 10.3390/md21030144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N., Lv S., Ma Y., Liu N., Zhou S., Zhou D. In vitro antioxidant and anti-aging properties of swim bladder peptides from Atlantic cod (Gadus morhua) Int. J. Food Prop. 2020;23:1416–1429. doi: 10.1080/10942912.2020.1807565. [DOI] [Google Scholar]
- 7.Zhang Z., Hu X., Lin L., Ding G., Yu F. Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-kappa B Pathway. Mar. Drugs. 2019;17:404. doi: 10.3390/md17070404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song R., Wei R.-B., Luo H.-Y., Wang D.-F. Isolation and Characterization of an Antibacterial Peptide Fraction from the Pepsin Hydrolysate of Half-Fin Anchovy (Setipinna taty) Molecules. 2012;17:2980–2991. doi: 10.3390/molecules17032980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jensen I.-J., Maehre H.K. Preclinical and Clinical Studies on Antioxidative, Antihypertensive and Cardioprotective Effect of Marine Proteins and Peptides A Review. Mar. Drugs. 2016;14:211. doi: 10.3390/md14110211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frau J., Flores-Holguin N., Glossman-Mitnik D. Chemical Reactivity Theory and Empirical Bioactivity Scores as Computational Peptidology Alternative Tools for the Study of Two Anticancer Peptides of Marine Origin. Molecules. 2019;24:1115. doi: 10.3390/molecules24061115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khazaei Monfared Y., Mahmoudian M., Cecone C., Caldera F., Zakeri-Milani P., Matencio A., Trotta F. Stabilization and Anticancer Enhancing Activity of the Peptide Nisin by Cyclodextrin-Based Nanosponges against Colon and Breast Cancer Cells. Polymers. 2022;14:594. doi: 10.3390/polym14030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sridhar K., Inbaraj B.S., Chen B.-H. Recent developments on production, purification and biological activity of marine peptides. Food Res. Int. 2021;147:468. doi: 10.1016/j.foodres.2021.110468. [DOI] [PubMed] [Google Scholar]
- 13.Elsheikh M.A., Gaafar P.M.E., Khattab M.A., Helwah M.K.A., Noureldin M.H., Abbas H. Dual-effects of caffeinated hyalurosomes as a nano-cosmeceutical gel counteracting UV-induced skin ageing. Int. J. Pharm.-X. 2023;5:100170. doi: 10.1016/j.ijpx.2023.100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh S., Zheng S., Fang M., Kim M., Bellere A.D., Jeong J., Yi T.-H. Anti-Photoaging Effect of Phaseolus angularis L. Extract on UVB-Exposed HaCaT Keratinocytes and Possibilities as Cosmetic Materials. Molecules. 2023;28:1407. doi: 10.3390/molecules28031407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukherjee S., Date A., Patravale V., Korting H.C., Roeder A., Weindl G. Retinoids in the treatment of skin aging: An overview of clinical efficacy and safety. Clin. Interv. Aging. 2006;1:327–348. doi: 10.2147/ciia.2006.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng R., Kang S.-G., Huang K., Tong T. Boosting the Photoaged Skin: The Potential Role of Dietary Components. Nutrients. 2021;13:1691. doi: 10.3390/nu13051691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honeybrook A., Bernstein E. Oral isotretinoin and photoaging: A review. J. Cosmet. Dermatol. 2020;19:1548–1554. doi: 10.1111/jocd.13467. [DOI] [PubMed] [Google Scholar]
- 18.Guan B., Wang F., Jiang H., Zhou M., Lin H. Preparation of Mesoporous Silica Nanosphere-Doped Color-Sensitive Materials and Application in Monitoring the TVB-N of Oysters. Foods. 2022;11:817. doi: 10.3390/foods11060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Glukhov E., He Y., Liu Y., Zhou L., Ma X., Hu X., Hong P., Gerwick W.H., Zhang Y. Secondary Metabolite Variation and Bioactivities of Two Marine Aspergillus Strains in Static Co-Culture Investigated by Molecular Network Analysis and Multiple Database Mining Based on LC-PDA-MS/MS. Antibiotics. 2022;11:513. doi: 10.3390/antibiotics11040513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallela R., Na-Young Y., Kim S.-K. Anti-photoaging and Photoprotective Compounds Derived from Marine Organisms. Mar. Drugs. 2010;8:1189–1202. doi: 10.3390/md8041189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han J., Huang Z., Tang S., Lu C., Wan H., Zhou J., Li Y., Ming T., Wang Z.J., Su X. The novel peptides ICRD and LCGEC screened from tuna roe show antioxidative activity via Keap1/Nrf2-ARE pathway regulation and gut microbiota modulation. Food Chem. 2020;327:94. doi: 10.1016/j.foodchem.2020.127094. [DOI] [PubMed] [Google Scholar]
- 22.Chen J., Liang P., Xiao Z., Chen M.-F., Gong F., Li C., Zhou C., Hong P., Jung W.-K., Qian Z.-J. Antiphotoaging effect of boiled abalone residual peptide ATPGDEG on UVB-induced keratinocyte HaCaT cells. Food Nutr. Res. 2019;63:3508. doi: 10.29219/fnr.v63.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leone A., Lecci R.M., Durante M., Meli F., Piraino S. The Bright Side of Gelatinous Blooms: Nutraceutical Value and Antioxidant Properties of Three Mediterranean Jellyfish (Scyphozoa) Mar. Drugs. 2015;13:4654–4681. doi: 10.3390/md13084654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhuang Y., Hou H., Zhao X., Zhang Z., Li B. Effects of Collagen and Collagen Hydrolysate from Jellyfish (Rhopilema esculentum) on Mice Skin Photoaging Induced by UV Irradiation. J. Food Sci. 2009;74:H183–H188. doi: 10.1111/j.1750-3841.2009.01236.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen T., Hou H., Lu J., Zhang K., Li B. Protective effect of gelatin and gelatin hydrolysate from salmon skin on UV irradiation-induced photoaging of mice skin. J. Ocean Univ. China. 2016;15:711–718. doi: 10.1007/s11802-016-2953-5. [DOI] [Google Scholar]
- 26.Kong J., Hu X.-M., Cai W.-W., Wang Y.-M., Chi C.-F., Wang B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs (II): Protective Function on UVB-Irradiated HaCaT Cells through Antioxidant and Anti-Apoptotic Mechanisms. Mar. Drugs. 2023;21:105. doi: 10.3390/md21020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun L., Zhang Y., Zhuang Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods. 2013;5:154–162. doi: 10.1016/j.jff.2012.09.006. [DOI] [Google Scholar]
- 28.Cai W.-W., Hu X.-M., Wang Y.-M., Chi C.-F., Wang B. Bioactive Peptides from Skipjack Tuna Cardiac Arterial Bulbs: Preparation, Identification, Antioxidant Activity, and Stability against Thermal, pH, and Simulated Gastrointestinal Digestion Treatments. Mar. Drugs. 2022;20:626. doi: 10.3390/md20100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu X.-M., Wang Y.-M., Zhao Y.-Q., Chi C.-F., Wang B. Antioxidant Peptides from the Protein Hydrolysate of Monkfish (Lophius litulon) Muscle: Purification, Identification, and Cytoprotective Function on HepG2 Cells Damage by H2O2. Mar. Drugs. 2020;18:153. doi: 10.3390/md18030153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harnedy P.A., O’Keeffe M.B., FitzGerald R.J. Fractionation and identification of antioxidant peptides from an enzymatically hydrolysed Palmaria palmata protein isolate. Food Res. Int. 2017;100:416–422. doi: 10.1016/j.foodres.2017.07.037. [DOI] [PubMed] [Google Scholar]
- 31.Je J.-Y., Qian Z.-J., Lee S.-H., Byun H.-G., Kim S.-K. Purification and Antioxidant Properties of Bigeye Tuna (Thunnus obesus) Dark Muscle Peptide on Free Radical-Mediated Oxidative Systems. J. Med. Food. 2008;11:629–637. doi: 10.1089/jmf.2007.0114. [DOI] [PubMed] [Google Scholar]
- 32.Kumar N.S.S., Nazeer R.A., Jaiganesh R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber) Amino Acids. 2012;42:1641–1649. doi: 10.1007/s00726-011-0858-6. [DOI] [PubMed] [Google Scholar]
- 33.Lee W.-S., Jeon J.-K., Byun H.-G. Characterization of a novel antioxidative peptide from the sand eel Hypoptychus dybowskii. Process Biochem. 2011;46:1207–1211. doi: 10.1016/j.procbio.2011.02.001. [DOI] [Google Scholar]
- 34.Ngo D.-H., Qian Z.-J., Ryu B., Park J.W., Kim S.-K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods. 2010;2:107–117. doi: 10.1016/j.jff.2010.02.001. [DOI] [Google Scholar]
- 35.Jiang H., Tong T., Sun J., Xu Y., Zhao Z., Liao D. Purification and characterization of antioxidative peptides from round scad (Decapterus maruadsi) muscle protein hydrolysate. Food Chem. 2014;154:158–163. doi: 10.1016/j.foodchem.2013.12.074. [DOI] [PubMed] [Google Scholar]
- 36.Mendis E., Rajapakse N., Kim S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005;53:581–587. doi: 10.1021/jf048877v. [DOI] [PubMed] [Google Scholar]
- 37.Ko J.-Y., Lee J.-H., Samarakoon K., Kim J.-S., Jeon Y.-J. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food Chem. Toxicol. 2013;52:113–120. doi: 10.1016/j.fct.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 38.Chen T., Hou H. Protective effect of gelatin polypeptides from Pacific cod (Gadus macrocephalus) against UV irradiation-induced damages by inhibiting inflammation and improving transforming growth factor-beta/Smad signaling pathway. J. Photochem. Photobiol. B-Biol. 2016;162:633–640. doi: 10.1016/j.jphotobiol.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 39.Chen B., Yu L., Wu J., Qiao K., Cui L., Qu H., Su Y., Cai S., Liu Z., Wang Q. Effects of Collagen Hydrolysate From Large Hybrid Sturgeon on Mitigating Ultraviolet B-Induced Photodamage. Front. Bioeng. Biotechnol. 2022;10:908033. doi: 10.3389/fbioe.2022.908033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Xu Y., Lai R., Deng H., Zhou F., Wang P., Pang X., Huang G., Chen X., Lin H., et al. Protective Effect of the Pearl Extract from Pinctada fucata martensii Dunker on UV-Induced Photoaging in Mice. Chem. Biodivers. 2022;19:876. doi: 10.1002/cbdv.202100876. [DOI] [PubMed] [Google Scholar]
- 41.Lu J., Hou H., Fan Y., Yang T., Li B. Identification of MMP-1 inhibitory peptides from cod skin gelatin hydrolysates and the inhibition mechanism by MAPK signaling pathway. J. Funct. Foods. 2017;33:251–260. doi: 10.1016/j.jff.2017.03.049. [DOI] [Google Scholar]
- 42.Wei M., Qiu H., Zhou J., Yang C., Chen Y., You L. The Anti-Photoaging Activity of Peptides from Pinctada martensii Meat. Mar. Drugs. 2022;20:770. doi: 10.3390/md20120770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim C.-R., Kim Y.-M., Lee M.-K., Kim I.-H., Choi Y.-H., Nam T.-J. Pyropia yezoensis peptide promotes collagen synthesis by activating the TGF-beta/Smad signaling pathway in the human dermal fibroblast cell line Hs27. Int. J. Mol. Med. 2017;39:31–38. doi: 10.3892/ijmm.2016.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C., Lv J., Qin X., Peng Z., Lin H. Novel Antioxidant Peptides from Crassostrea Hongkongensis Improve Photo-Oxidation in UV-Induced HaCaT Cells. Mar. Drugs. 2022;20:100. doi: 10.3390/md20020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao Z., Liang P., Chen J., Chen M.-F., Gong F., Li C., Zhou C., Hong P., Yang P., Qian Z.-J. A Peptide YGDEY from Tilapia Gelatin Hydrolysates Inhibits UVB-mediated Skin Photoaging by Regulating MMP-1 and MMP-9 Expression in HaCaT Cells. Photochem. Photobiol. 2019;95:1424–1432. doi: 10.1111/php.13135. [DOI] [PubMed] [Google Scholar]
- 46.Juncan A.M., Moisa D.G., Santini A., Morgovan C., Rus L.-L., Vonica-Tincu A.L., Loghin F. Advantages of Hyaluronic Acid and Its Combination with Other Bioactive Ingredients in Cosmeceuticals. Molecules. 2021;26:4429. doi: 10.3390/molecules26154429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Norzagaray-Valenzuela C.D., Valdez-Ortiz A., Shelton L.M., Jimenez-Edeza M., Rivera-Lopez J., Valdez-Flores M.A., German-Baez L.J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J. Appl. Phycol. 2017;29:189–198. doi: 10.1007/s10811-016-0938-9. [DOI] [Google Scholar]
- 48.Barriga Montalvo G.E., Thomaz-Soccol V., Vandenberghe L.P.S., Carvalho J.C., Faulds C.B., Bertrand E., Prado M.R.M., Bonatto S.J.R., Soccol C.R. Arthrospira maxima OF15 biomass cultivation at laboratory and pilot scale from sugarcane vinasse for potential biological new peptides production. Bioresour. Technol. 2019;273:103–113. doi: 10.1016/j.biortech.2018.10.081. [DOI] [PubMed] [Google Scholar]
- 49.Nakchum L., Kim S.M. Preparation of squid skin collagen hydrolysate as an antihyaluronidase, antityrosinase, and antioxidant agent. Prep. Biochem. Biotechnol. 2016;46:123–130. doi: 10.1080/10826068.2014.995808. [DOI] [PubMed] [Google Scholar]
- 50.Hoon K., Jeon B., Lee H.-J., Kyun C.D. Evaluation of the Skin Moisturizing Efficacy of a Collagen Peptide Isolated from Fish Scales, Using HaCaT Keratinocytes. J. Korean Soc. Food Sci. Nutr. 2020;49:454–461. doi: 10.3746/jkfn.2020.49.5.454. [DOI] [Google Scholar]
- 51.Nam K.A., You S.G., Kim S.M. Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates. J. Food Sci. 2008;73:C249–C255. doi: 10.1111/j.1750-3841.2008.00722.x. [DOI] [PubMed] [Google Scholar]
- 52.Amakye W.K., Yang L., Yao M., Yuan E., Ren R., Ren J. Skipjack (Katsuwonus pelamis) elastin hydrolysate-derived peptides attenuate UVA irradiation-induced cell damage in human HaCaT keratinocytes. Food Front. 2021;2:184–194. doi: 10.1002/fft2.74. [DOI] [Google Scholar]
- 53.Chen Y.-P., Liang C.-H., Wu H.-T., Pang H.-Y., Chen C., Wang G.-H., Chan L.-P. Antioxidant and anti-inflammatory capacities of collagen peptides from milkfish (Chanos chanos) scales. J. Food Sci. Technol. Mysore. 2018;55:2310–2317. doi: 10.1007/s13197-018-3148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mechri S., Sellem I., Bouacem K., Jabeur F., Laribi-Habchi H., Mellouli L., Hacene H., Bouanane-Darenfed A., Jaouadi B. A biological clean processing approach for the valorization of speckled shrimp Metapenaeus monoceros by-product as a source of bioactive compounds. Environ. Sci. Pollut. Res. 2020;27:15842–15855. doi: 10.1007/s11356-020-08076-w. [DOI] [PubMed] [Google Scholar]
- 55.Ju X., Cheng S., Li H., Xu X., Wang Z., Du M. Tyrosinase inhibitory effects of the peptides from fish scale with the metal copper ions chelating ability. Food Chem. 2022;390:133146. doi: 10.1016/j.foodchem.2022.133146. [DOI] [PubMed] [Google Scholar]
- 56.Mechri S., Sellem I., Bouacem K., Jabeur F., Chamkha M., Hacene H., Bouanane-Darenfed A., Jaouadi B. Antioxidant and Enzyme Inhibitory Activities of Metapenaeusmonoceros By-Product Hydrolysates Elaborated by Purified Alkaline Proteases. Waste Biomass Valorization. 2020;11:6741–6755. doi: 10.1007/s12649-020-00942-5. [DOI] [Google Scholar]
- 57.Hu Z.-Z., Sha X.-M., Zhang L., Zha M.-J., Tu Z.-C. From Fish Scale Gelatin to Tyrosinase Inhibitor: A Novel Peptides Screening Approach Application. Front. Nutr. 2022;9:853442. doi: 10.3389/fnut.2022.853442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang J., Xie X., Deng Y., Yang H., Du X., Liu P., Du Y. SOX9 in Keratinocytes Regulates Claudin 2 Transcription during Skin Aging. Contrast Media Mol. Imaging. 2022;2022:6884308. doi: 10.1155/2022/6884308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhao X.-Y., Zhang X.-L. DNA Methyltransferase Inhibitor 5-AZA-DC Regulates TGF beta 1-Mediated Alteration of Neuroglial Cell Functions after Oxidative Stress. Oxid. Med. Cell. Longev. 2022;2022:9259465. doi: 10.1155/2022/9259465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Chu N., Yao G., Liu Y., Cheng M., Ikejima T. Newly synthesized bis-benzimidazole compound 8 induces apoptosis, autophagy and reactive oxygen species generation in HeLa cells. Bioorg. Med. Chem. Lett. 2016;26:227–231. doi: 10.1016/j.bmcl.2016.01.085. [DOI] [PubMed] [Google Scholar]
- 61.Jesus A., Mota S., Torres A., Cruz M.T., Sousa E., Almeida I.F., Cidade H. Antioxidants in Sunscreens: Which and What For? Antioxidants. 2023;12:138. doi: 10.3390/antiox12010138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Khan M.A., Siddiqui S., Ahmad I., Singh R., Mishra D.P., Srivastava A.N., Ahmad R. Phytochemicals from Ajwa dates pulp extract induce apoptosis in human triple-negative breast cancer by inhibiting AKT/mTOR pathway and modulating Bcl-2 family proteins. Sci. Rep. 2021;11:10322. doi: 10.1038/s41598-021-89420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhao R., Jiang X.-X., Zhao Q.-L., Ye H.-W., Lin Y., Huang J., Tang Y.-P. Immunoenhancing Effects of Cyclina sinensis Pentadecapeptide through Modulation of Signaling Pathways in Mice with Cyclophosphamide-Induced Immunosuppression. Mar. Drugs. 2022;20:560. doi: 10.3390/md20090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lembo S., Balato A., Di Caprio R., Cirillo T., Giannini V., Gasparri F., Monfrecola G. The Modulatory Effect of Ellagic Acid and Rosmarinic Acid on Ultraviolet-B-Induced Cytokine/Chemokine Gene Expression in Skin Keratinocyte (HaCaT) Cells. Biomed Res. Int. 2014;2014:346793. doi: 10.1155/2014/346793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan H., Zhang X., Zhang Q., Wang Y., Wang S., Li Y., Zhang Y., Jing J., Qiu J., Wang Z., et al. Comparative transcriptome profiles of Lindian chicken eyelids identify melanin genes controlling eyelid pigmentation. Br. Poult. Sci. 2019;60:15–22. doi: 10.1080/00071668.2018.1544414. [DOI] [PubMed] [Google Scholar]
- 66.Wang J., Zhang W., Wang S., Liu H., Zhang D., Wang Y., Ji H. Swine-Derived Probiotic Lactobacillus plantarum Modulates Porcine Intestinal Endogenous Host Defense Peptide Synthesis Through TLR2/MAPK/AP-1 Signaling Pathway. Front. Immunol. 2019;10:02691. doi: 10.3389/fimmu.2019.02691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiu L.-Y., Wu N.-L., Hung C.-F., Bai P., Dai Y.-S., Lin W.-W. PARP-1 involves in UVB-induced inflammatory response in keratinocytes and skin injury via regulation of ROS-dependent EGFR transactivation and p38 signaling. FASEB J. 2021;35:e21393. doi: 10.1096/fj.202002285RR. [DOI] [PubMed] [Google Scholar]
- 68.Samivel R., Nagarajan R.P., Subramanian U., Khan A.A., Masmali A., Almubrad T., Akhtar S. Inhibitory Effect of Ursolic Acid on Ultraviolet B Radiation-Induced Oxidative Stress and Proinflammatory Response-Mediated Senescence in Human Skin Dermal Fibroblasts. Oxid. Med. Cell. Longev. 2020;2020:1246510. doi: 10.1155/2020/1246510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Q., Wang Y., Jiang X., Ma L., Li Z., Chang Y., Wang Y., Xue C. Amino Acid Profiling with Chemometric Analysis as a Feasible Tool for the Discrimination of Marine-Derived Peptide Powders. Foods. 2021;10:1294. doi: 10.3390/foods10061294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng Z., Gao J., Su W., Cao W., Zhu G., Qin X., Zhang C., Qi Y. Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells. Mar. Drugs. 2022;20:749. doi: 10.3390/md20120749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han S.H., Ballinger E., Choung S.-Y., Kwon J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs. 2022;20:308. doi: 10.3390/md20050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z.-G., Ying X.-G., Gao P., Wang C.-L., Wang Y.-F., Yu X.-W., Chen J., Wang B., Luo H.-Y. Anti-Inflammatory Activity of a Peptide from Skipjack (Katsuwonus pelamis) Mar. Drugs. 2019;17:582. doi: 10.3390/md17100582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zotchev S.B. Marine actinomycetes as an emerging resource for the drug development pipelines. J. Biotechnol. 2012;158:168–175. doi: 10.1016/j.jbiotec.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Moore B.S., Trischman J.A., Seng D., Kho D., Jensen P.R., Fenical W. Salinamides, antiinflammatory depsipeptides from a marine streptomycete. J. Org. Chem. 1999;64:1145–1150. doi: 10.1021/jo9814391. [DOI] [Google Scholar]
- 75.Schultz A.W., Oh D.-C., Carney J.R., Williamson R.T., Udwary D.W., Jensen P.R., Gould S.J., Fenical W., Moore B.S. Biosynthesis and structures of cyclomarins and cyclomarazines, prenylated cyclic peptides of marine actinobacterial origin. J. Am. Chem. Soc. 2008;130:4507–4516. doi: 10.1021/ja711188x. [DOI] [PubMed] [Google Scholar]
- 76.Eisner L., Vambutas A., Pathak S. The Balance of Tissue Inhibitor of Metalloproteinase-1 and Matrix Metalloproteinase-9 in the Autoimmune Inner Ear Disease Patients. J. Interferon Cytokine Res. 2017;37:354–361. doi: 10.1089/jir.2017.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi S.-H., Choi S.-I., Jung T.-D., Cho B.-Y., Lee J.-H., Kim S.-H., Yoon S.-A., Ham Y.-M., Yoon W.-J., Cho J.-H., et al. Anti-Photoaging Effect of Jeju Putgyul (Unripe Citrus) Extracts on Human Dermal Fibroblasts and Ultraviolet B-induced Hairless Mouse Skin. Int. J. Mol. Sci. 2017;18:2052. doi: 10.3390/ijms18102052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vicentini F.T.M.C., He T., Shao Y., Fonseca M.J.V., Verri W.A., Jr., Fisher G.J., Xu Y. Quercetin inhibits UV irradiation-induced inflammatory cytokine production in primary human keratinocytes by suppressing NF-kappa B pathway. J. Dermatol. Sci. 2011;61:162–168. doi: 10.1016/j.jdermsci.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 79.Wang M., Charareh P., Lei X., Zhong J.L. Autophagy: Multiple Mechanisms to Protect Skin from Ultraviolet Radiation-Driven Photoaging. Oxid. Med. Cell. Longev. 2019;2019:8135985. doi: 10.1155/2019/8135985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiao Z., Yang S., Chen J., Li C., Zhou C., Hong P., Sun S., Qian Z.-J. Trehalose against UVB-induced skin photoaging by suppressing MMP expression and enhancing procollagen I synthesis in HaCaT cells. J. Funct. Foods. 2020;74:104198. doi: 10.1016/j.jff.2020.104198. [DOI] [Google Scholar]
- 81.Bang J.S., Choung S.-Y. Inhibitory effect of oyster hydrolysate on wrinkle formation against UVB irradiation in human dermal fibroblast via MAPK/AP-1 and TGF beta/Smad pathway. J. Photochem. Photobiol. B-Biol. 2020;209:111946. doi: 10.1016/j.jphotobiol.2020.111946. [DOI] [PubMed] [Google Scholar]
- 82.Oh J.H., Kim J., Karadeniz F., Kim H.R., Park S.Y., Seo Y., Kong C.-S. Santamarine Shows Anti-Photoaging Properties via Inhibition of MAPK/AP-1 and Stimulation of TGF-beta/Smad Signaling in UVA-Irradiated HDFs. Molecules. 2021;26:3585. doi: 10.3390/molecules26123585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huang K.-F., Ma K.-H., Chang Y.-J., Lo L.-C., Jhap T.-Y., Su Y.-H., Liu P.-S., Chueh S.-H. Baicalein inhibits matrix metalloproteinase 1 expression via activation of TRPV1-Ca-ERK pathway in ultraviolet B-irradiated human dermal fibroblasts. Exp. Dermatol. 2019;28:568–575. doi: 10.1111/exd.13912. [DOI] [PubMed] [Google Scholar]
- 84.Kulaberoglu Y., Bhushan B., Hadi F., Chakrabarti S., Khaled W.T., Rankin K.S., Smith E.S.J., Frankel D. The material properties of naked mole-rat hyaluronan. Sci. Rep. 2019;9:6632. doi: 10.1038/s41598-019-43194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swathi N., Srikanth K., Venipriya S. Does the addition of hyaluronidase improve the quality of peribulbar anesthesia in cataract surgery?—A randomized double blinded study. Saudi J. Ophthalmol. 2018;32:204–210. doi: 10.1016/j.sjopt.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Robinson D.M., Vega J., Palm M.D., Bell M., Widgerow A.D., Giannini A. Multicenter evaluation of a topical hyaluronic acid serum. J. Cosmet. Dermatol. 2022;21:3848–3858. doi: 10.1111/jocd.15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee H.P., Kim D.S., Park S.H., Shin C.Y., Woo J.J., Kim J.W., An R.-B., Lee C., Cho J.Y. Antioxidant Capacity of Potentilla paradoxa Nutt. and Its Beneficial Effects Related to Anti-Aging in HaCaT and B16F10 Cells. Plants. 2022;11:873. doi: 10.3390/plants11070873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morgan D.J., Casulli J., Chew C., Connolly E., Lui S., Brand O.J., Rahman R., Jagger C., Hussell T. Innate Immune Cell Suppression and the Link with Secondary Lung Bacterial Pneumonia. Front. Immunol. 2018;9:02943. doi: 10.3389/fimmu.2018.02943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee J.-O., Kim E., Kim J.H., Hong Y.H., Kim H.G., Jeong D., Kim J., Kim S.H., Park C., Seo D.B., et al. Antimelanogenesis and skin-protective activities of Panax ginseng calyx ethanol extract. J. Ginseng Res. 2018;42:389–399. doi: 10.1016/j.jgr.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shukla S., Park J., Park J.H., Lee J.S., Kim M. Development of novel Meju starter culture using plant extracts with reduced Bacillus cereus counts and enhanced functional properties. Sci. Rep. 2017;7:11409. doi: 10.1038/s41598-017-09551-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han N., Kim J., Bae J.H., Kim M., Lee J.Y., Lee Y.-Y., Kang M.S., Han D., Park S., Kim H.-J. Effect of Atmospheric-Pressure Plasma on Functional Compounds and Physiological Activities in Peanut Shells. Antioxidants. 2022;11:2214. doi: 10.3390/antiox11112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith M.J., White K.L., Jr., Smith D.C., Bowlin G.L. In vitro evaluations of innate and acquired immune responses to electrospun polydioxanone-elastin blends. Biomaterials. 2009;30:149–159. doi: 10.1016/j.biomaterials.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 93.Rossetti D., Kielmanowicz M.G., Vigodman S., Hu Y.P., Chen N., Nkengne A., Oddos T., Fischer D., Seiberg M., Lin C.B. A novel anti-ageing mechanism for retinol: Induction of dermal elastin synthesis and elastin fibre formation. Int. J. Cosmet. Sci. 2011;33:62–69. doi: 10.1111/j.1468-2494.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- 94.Choi D., Min S.-G., Jo Y.-J. Functionality of porcine skin hydrolysates produced by hydrothermal processing for liposomal delivery system. J. Food Biochem. 2018;42:12464. doi: 10.1111/jfbc.12464. [DOI] [Google Scholar]
- 95.Malaspina P., Catellani E., Burlando B., Brignole D., Cornara L., Bazzicalupo M., Candiani S., Obino V., De Feo V., Caputo L., et al. Depigmenting potential of lichen extracts evaluated by in vitro and in vivo tests. PeerJ. 2020;8:9150. doi: 10.7717/peerj.9150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roh E., Kim J.-E., Kwon J.Y., Park J.S., Bode A.M., Dong Z., Lee K.W. Molecular mechanisms of green tea polyphenols with protective effects against skin photoaging. Crit. Rev. Food Sci. Nutr. 2017;57:1631–1637. doi: 10.1080/10408398.2014.1003365. [DOI] [PubMed] [Google Scholar]
- 97.Yu S., Wang G., Liao J., Tang M. Five alternative splicing variants of the TYR gene and their different roles in melanogenesis in the Muchuan black-boned chicken. Br. Poult. Sci. 2019;60:8–14. doi: 10.1080/00071668.2018.1533633. [DOI] [PubMed] [Google Scholar]
- 98.Li L., Tang Y., Li X., Zhou T., Song Q., Li A. Mechanism of skin whitening through San-Bai decoction-induced tyrosinase inhibition and discovery of natural products targeting tyrosinase. Medicine. 2023;102:e33420. doi: 10.1097/MD.0000000000033420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cui B., Wang Y., Jin J., Yang Z., Guo R., Li X., Yang L., Li Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell. Longev. 2022;2022:6037303. doi: 10.1155/2022/6037303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gao X., Luo F., Zhao H. Cloves Regulate Na+-K+-ATPase to Exert Antioxidant Effect and Inhibit UVB Light-Induced Skin Damage in Mice. Oxid. Med. Cell. Longev. 2021;2021:5197919. doi: 10.1155/2021/5197919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cunha S.A., Pintado M.E. Bioactive peptides derived from marine sources: Biological and functional properties. Trends Food Sci. Technol. 2022;119:348–370. doi: 10.1016/j.tifs.2021.08.017. [DOI] [Google Scholar]
- 102.Oh S.-J., Park J.-Y., Won B., Oh Y.-T., Yang S.-C., Shin O.S. Asterias pectinifera-Derived Collagen Peptides Mixed with Halocynthia roretzi Extracts Exhibit Anti-Photoaging Activities during Exposure to UV Irradiation, and Antibacterial Properties. J. Microbiol. Biotechnol. 2022;32:1382–1389. doi: 10.4014/jmb.2207.07018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Han S.-B., Won B., Yang S.-c., Kim D.-H. Asterias pectinifera derived collagen peptide-encapsulating elastic nanoliposomes for the cosmetic application. J. Ind. Eng. Chem. 2021;98:289–297. doi: 10.1016/j.jiec.2021.03.039. [DOI] [Google Scholar]
- 104.Fan J., Zhuang Y., Li B. Effects of Collagen and Collagen Hydrolysate from Jellyfish Umbrella on Histological and Immunity Changes of Mice Photoaging. Nutrients. 2013;5:223–233. doi: 10.3390/nu5010223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeng Q., Jiang J., Wang J., Zhou Q., Zhang X. N-Terminal Acetylation and C-Terminal Amidation of Spirulina platensis-Derived Hexapeptide: Anti-Photoaging Activity and Proteomic Analysis. Mar. Drugs. 2019;17:520. doi: 10.3390/md17090520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen C.-L., Liou S.-F., Chen S.-J., Shih M.-F. Protective effects of Chlorella-derived peptide on UVB-induced production of MMP-1 and degradation of procollagen genes in human skin fibroblasts. Regul. Toxicol. Pharmacol. 2011;60:112–119. doi: 10.1016/j.yrtph.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 107.Yazdi A.S., Roecken M., Ghoreschi K. Cutaneous immunology: Basics and new concepts. Semin. Immunopathol. 2016;38:3–10. doi: 10.1007/s00281-015-0545-x. [DOI] [PubMed] [Google Scholar]
- 108.Wang L.-F., Hsu C.-J., Miaw S.-C., Chiu H.-C., Liu C.-Y., Yu H.-S. Cross-priming with an epicutaneously introduced soluble protein antigen generates Tc1 cells. Eur. J. Immunol. 2006;36:2904–2911. doi: 10.1002/eji.200535770. [DOI] [PubMed] [Google Scholar]
- 109.Semreen M.H., El-Gamal M.I., Abdin S., Alkhazraji H., Kamal L., Hammad S., El-Awady F., Waleed D., Kourbaj L. Recent updates of marine antimicrobial peptides. Saudi Pharm. J. 2018;26:396–409. doi: 10.1016/j.jsps.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Safronova V.N., Panteleev P.V., Sukhanov S.V., Toropygin I.Y., Bolosov I.A., Ovchinnikova T.V. Mechanism of Action and Therapeutic Potential of the β-Hairpin Antimicrobial Peptide Capitellacin from the Marine Polychaeta Capitella teleta. Mar. Drugs. 2022;20:167. doi: 10.3390/md20030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ford P.W., Gustafson K.R., McKee T.C., Shigematsu N., Maurizi L.K., Pannell L.K., Williams D.E., de Silva E.D., Lassota P., Allen T.M., et al. Papuamides A-D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 1999;121:5899–5909. doi: 10.1021/ja990582o. [DOI] [Google Scholar]
- 112.Jiao K., Gao J., Zhou T., Yu J., Song H., Wei Y., Gao X. Isolation and purification of a novel antimicrobial peptide from Porphyra yezoensis. J. Food Biochem. 2019;43:12864. doi: 10.1111/jfbc.12864. [DOI] [PubMed] [Google Scholar]
- 113.Nikapitiya C., Dananjaya S.H.S., Chandrarathna H.P.S.U., De Zoysa M., Whang I. Octominin: A Novel Synthetic Anticandidal Peptide Derived from Defense Protein of Octopus minor. Mar. Drugs. 2020;18:56. doi: 10.3390/md18010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gasu E.N., Ahor H.S., Borquaye L.S. Peptide Mix from Olivancillaria hiatula Interferes with Cell-to-Cell Communication in Pseudomonas aeruginosa. Biomed Res. Int. 2019;2019:5313918. doi: 10.1155/2019/5313918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Oh R., Lee M.J., Kim Y.-O., Nam B.-H., Kong H.J., Kim J.-W., Park J.-Y., Seo J.-K., Kim D.-G. Myticusin-beta, antimicrobial peptide from the marine bivalve, Mytilus coruscus. Fish Shellfish Immunol. 2020;99:342–352. doi: 10.1016/j.fsi.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 116.Sivakamavalli J., James R.A., Park K., Kwak I.-S., Vaseeharan B. Purification of WAP domain-containing antimicrobial peptides from green tiger shrimp Peaneaus semisulcatus. Microb. Pathog. 2020;140:103920. doi: 10.1016/j.micpath.2019.103920. [DOI] [PubMed] [Google Scholar]
- 117.Sruthy K.S., Nair A., Antony S.P., Puthumana J., Singh I.S.B., Philip R. A histone H2A derived antimicrobial peptide, Fi-Histin from the Indian White shrimp, Fenneropenaeus indicus: Molecular and functional characterization. Fish Shellfish Immunol. 2019;92:667–679. doi: 10.1016/j.fsi.2019.06.044. [DOI] [PubMed] [Google Scholar]
- 118.Humisto A., Jokela J., Teigen K., Wahlsten M., Permi P., Sivonen K., Herfindal L. Characterization of the interaction of the antifungal and cytotoxic cyclic glycolipopeptide hassallidin with sterol-containing lipid membranes. Biochim. Biophys. Acta-Biomembr. 2019;1861:1510–1521. doi: 10.1016/j.bbamem.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 119.Beaulieu L., Bondu S., Doiron K., Rioux L.-E., Turgeon S.L. Characterization of antibacterial activity from protein hydrolysates of the macroalga Saccharina longicruris and identification of peptides implied in bioactivity. J. Funct. Foods. 2015;17:685–697. doi: 10.1016/j.jff.2015.06.026. [DOI] [Google Scholar]
- 120.Song R., Wei R., Zhang B., Wang D. Optimization of the Antibacterial Activity of Half-Fin Anchovy (Setipinna taty) Hydrolysates. Food Bioprocess Technol. 2012;5:1979–1989. doi: 10.1007/s11947-010-0505-3. [DOI] [Google Scholar]
- 121.Ennaas N., Hammami R., Beaulieu L., Fliss I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015;462:195–200. doi: 10.1016/j.bbrc.2015.04.091. [DOI] [PubMed] [Google Scholar]
- 122.Ennaas N., Hammami R., Beaulieu L., Fliss I. Production of antibacterial fraction from Atlantic mackerel (Scomber scombrus) and its processing by-products using commercial enzymes. Food Bioprod. Process. 2015;96:145–153. doi: 10.1016/j.fbp.2015.07.014. [DOI] [Google Scholar]
- 123.Tanaka M., Koyama Y.-i., Nomura Y. Effects of Collagen Peptide Ingestion on UV-B-Induced Skin Damage. Biosci. Biotechnol. Biochem. 2009;73:930–932. doi: 10.1271/bbb.80649. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Z., Wang J., Ding Y., Dai X., Li Y. Oral administration of marine collagen peptides from Chum Salmon skin enhances cutaneous wound healing and angiogenesis in rats. J. Sci. Food Agric. 2011;91:2173–2179. doi: 10.1002/jsfa.4435. [DOI] [PubMed] [Google Scholar]
- 125.De Luca C., Mikhal’chik E.V., Suprun M.V., Papacharalambous M., Truhanov A.I., Korkina L.G. Skin Antiageing and Systemic Redox Effects of Supplementation with Marine Collagen Peptides and Plant-Derived Antioxidants: A Single-Blind Case-Control Clinical Study. Oxid. Med. Cell. Longev. 2016;2016:4389410. doi: 10.1155/2016/4389410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hou H., Li B., Zhang Z., Xue C., Yu G., Wang J., Bao Y., Bu L., Sun J., Peng Z., et al. Moisture absorption and retention properties, and activity in alleviating skin photodamage of collagen polypeptide from marine fish skin. Food Chem. 2012;135:1432–1439. doi: 10.1016/j.foodchem.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 127.Hou H., Zhao X., Li B., Zhang Z., Zhuang Y. Inhibition of melanogenic activity by gelatin and polypeptides from pacific cod skin in b16 melanoma cells. J. Food Biochem. 2011;35:1099–1116. doi: 10.1111/j.1745-4514.2010.00437.x. [DOI] [Google Scholar]
- 128.Oh J.-Y., Lee H.G., Je J.-G., Wang L., Kim H.-S., Jeon Y.-J. Evaluation of Cosmeceutical Properties of Fish Skin By-product Hydrolysates Collected during Surimi Manufacturing Process. Korean J. Fish. Aquat. Sci. 2020;53:297–307. doi: 10.5657/kfas.2020.0297. [DOI] [Google Scholar]
- 129.Al-Atif H. Collagen Supplements for Aging and Wrinkles: A Paradigm Shift in the Fields of Dermatology and Cosmetics. Dermatol. Pract. Concept. 2022;12:e2022018. doi: 10.5826/dpc.1201a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pozzolini M., Millo E., Oliveri C., Mirata S., Salis A., Damonte G., Arkel M., Scarfi S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia reniformis. Mar. Drugs. 2018;16:465. doi: 10.3390/md16120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lin H., Zheng Z., Yuan J., Zhang C., Cao W., Qin X. Collagen Peptides Derived from Sipunculus nudus Accelerate Wound Healing. Molecules. 2021;26:1385. doi: 10.3390/molecules26051385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J., Xu M., Liang R., Zhao M., Zhang Z., Li Y. Oral administration of marine collagen peptides prepared from chum salmon (Oncorhynchus keta) improves wound healing following cesarean section in rats. Food Nutr. Res. 2015;59:26411. doi: 10.3402/fnr.v59.26411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leon-Lopez A., Morales-Penaloza A., Manuel Martinez-Juarez V., Vargas-Torres A., Zeugolis D.I., Aguirre-Alvarez G. Hydrolyzed Collagen-Sources and Applications. Molecules. 2019;24:4031. doi: 10.3390/molecules24224031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsitsipatis D., Martindale J.L., Ubaida-Mohien C., Lyashkov A., Yanai H., Kashyap A., Shin C.H., Herman A.B., Ji E., Yang J.-H., et al. Proteomes of primary skin fibroblasts from healthy individuals reveal altered cell responses across the life span. Aging Cell. 2022;21:13609. doi: 10.1111/acel.13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shin J.-W., Kwon S.-H., Choi J.-Y., Na J.-I., Huh C.-H., Choi H.-R., Park K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019;20:2126. doi: 10.3390/ijms20092126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Burki T. Skin-whitening creams: Worth the risk? Lancet Diabetes Endocrinol. 2021;9:10. doi: 10.1016/S2213-8587(20)30400-9. [DOI] [PubMed] [Google Scholar]
- 137.Boyajian J.L., Ghebretatios M., Schaly S., Islam P., Prakash S. Microbiome and Human Aging: Probiotic and Prebiotic Potentials in Longevity, Skin Health and Cellular Senescence. Nutrients. 2021;13:4550. doi: 10.3390/nu13124550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mansinhos I., Goncalves S., Rodriguez-Solana R., Ordonez-Diaz J.L., Moreno-Rojas J.M., Romano A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants. 2021;10:582. doi: 10.3390/antiox10040582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Costa C., Anselmo H., Ferro R., Matos A.S., Casimiro T., Aguiar-Ricardo A. Dry Dosage Forms of Add-Value Bioactive Phenolic Compounds by Supercritical CO2-Assisted Spray-Drying. Molecules. 2022;27:2001. doi: 10.3390/molecules27062001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Xing L., Wang Z., Hao Y., Zhang W. Marine Products As a Promising Resource of Bioactive Peptides: Update of Extraction Strategies and Their Physiological Regulatory Effects. J. Agric. Food Chem. 2022;70:3081–3095. doi: 10.1021/acs.jafc.1c07868. [DOI] [PubMed] [Google Scholar]
- 141.Zhang Y., Zhao G., Han K., Sun D., Zhou N., Song Z., Liu H., Li J., Li G. Applications of Molecular Imprinting Technology in the Study of Traditional Chinese Medicine. Molecules. 2023;28:301. doi: 10.3390/molecules28010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barzegar F., Kamankesh M., Mohammadi A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019;280:240–254. doi: 10.1016/j.foodchem.2018.12.058. [DOI] [PubMed] [Google Scholar]
- 143.Wu H., Li J., Jia Y., Xiao Z., Li P., Xie Y., Zhang A., Liu R., Ren Z., Zhao M., et al. Essential Oil Extracted from Cymbopogon citronella Leaves by Supercritical Carbon Dioxide: Antioxidant and Antimicrobial Activities. J. Anal. Methods Chem. 2019;2019:8192439. doi: 10.1155/2019/8192439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Marcet I., Alvarez C., Paredes B., Diaz M. The use of sub-critical water hydrolysis for the recovery of peptides and free amino acids from food processing wastes. Review of sources and main parameters. Waste Manag. 2016;49:364–371. doi: 10.1016/j.wasman.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 145.Melgosa R., Trigueros E., Teresa Sanz M., Cardeira M., Rodrigues L., Fernandez N., Matias A.A., Bronze M.R., Marques M., Paiva A., et al. Supercritical CO2 and subcritical water technologies for the production of bioactive extracts from sardine (Sardina pilchardus) waste. J. Supercrit. Fluids. 2020;164:104943. doi: 10.1016/j.supflu.2020.104943. [DOI] [Google Scholar]
- 146.Rivas-Vela C.I., Amaya-Llano S.L., Castano-Tostado E., Castillo-Herrera G.A. Protein Hydrolysis by Subcritical Water: A New Perspective on Obtaining Bioactive Peptides. Molecules. 2021;26:6655. doi: 10.3390/molecules26216655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gomez B., Munekata P.E.S., Gavahian M., Barba F.J., Marti-Quijal F.J., Bolumar T., Bastianello Campagnol P.C., Tomasevic I., Lorenzo J.M. Application of pulsed electric fields in meat and fish processing industries: An overview. Food Res. Int. 2019;123:95–105. doi: 10.1016/j.foodres.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 148.Li M., Lin J., Chen J., Fang T. pulsed electric field-assisted enzymatic extraction of protein from abalone (haliotis discus hannai ino) viscera. J. Food Process Eng. 2016;39:702–710. doi: 10.1111/jfpe.12262. [DOI] [Google Scholar]
- 149.Akaberi S., Gusbeth C., Silve A., Senthilnathan D.S., Navarro-Lopez E., Molina-Grima E., Muller G., Frey W. Effect of pulsed electric field treatment on enzymatic hydrolysis of proteins of Scenedesmus almeriensis. Algal Res.-Biomass Biofuels Bioprod. 2019;43:101656. doi: 10.1016/j.algal.2019.101656. [DOI] [Google Scholar]
- 150.Di D.-L., Zheng Y.-Y., Chen X.-F., Huang X.-Y., Feng S.-L. Advance of Application of High Speed Counter-current Chromatography in Separation and Purification of Flavonoids. Chin. J. Anal. Chem. 2011;39:269–275. doi: 10.1016/S1872-2040(10)60419-7. [DOI] [Google Scholar]
- 151.Ramezanzade L., Hosseini S.F., Nikkhah M. Biopolymer-coated nanoliposomes as carriers of rainbow trout skin-derived antioxidant peptides. Food Chem. 2017;234:220–229. doi: 10.1016/j.foodchem.2017.04.177. [DOI] [PubMed] [Google Scholar]
- 152.McClements D.J. Nanoscale Nutrient Delivery Systems for Food Applications: Improving Bioactive Dispersibility, Stability, and Bioavailability. J. Food Sci. 2015;80:N1602–N1611. doi: 10.1111/1750-3841.12919. [DOI] [PubMed] [Google Scholar]
- 153.Yao M., Xiao H., McClements D.J. Delivery of Lipophilic Bioactives: Assembly, Disassembly, and Reassembly of Lipid Nanoparticles. Annu. Rev. Food Sci. Technol. 2014;5:53–81. doi: 10.1146/annurev-food-072913-100350. [DOI] [PubMed] [Google Scholar]
- 154.Choonpicharn S., Tateing S., Jaturasitha S., Rakariyatham N., Suree N., Niamsup H. Identification of bioactive peptide from Oreochromis niloticus skin gelatin. J. Food Sci. Technol.-Mysore. 2016;53:1222–1229. doi: 10.1007/s13197-015-2091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Desai K.G.H. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carrier Syst. 2016;33:107–158. doi: 10.1615/CritRevTherDrugCarrierSyst.2016014850. [DOI] [PubMed] [Google Scholar]
- 156.Hosseini S.F., Ramezanzade L., McClements D.J. Recent advances in nanoencapsulation of hydrophobic marine bioactives: Bioavailability, safety, and sensory attributes of nano-fortified functional foods. Trends Food Sci. Technol. 2021;109:322–339. doi: 10.1016/j.tifs.2021.01.045. [DOI] [Google Scholar]
- 157.Asserin J., Lati E., Shioya T., Prawitt J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015;14:291–301. doi: 10.1111/jocd.12174. [DOI] [PubMed] [Google Scholar]
- 158.Maia Campos P.M.B.G., Franco R.S.B., Kakuda L., Cadioli G.F., Costa G.M.D.A., Bouvret E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Molecules. 2021;26:4880. doi: 10.3390/molecules26164880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Finn Canada. [(accessed on 29 September 2022)]. Available online: https://www.finncanada.com/
- 160.Kenney & Ross Limited. Kenney and Ross. [(accessed on 11 November 2022)]. Available online: https://www.kenneyandross.com/index.php?option=com_content&view=featured&Itemid=101.
- 161.Nuwen. Marine Active. [(accessed on 18 November 2022)]. Available online: https://www.nuwen.com/en/gamme/ingredients-en/marine-actives-ingredients-en/
- 162.OneOcean. Collagen Boosting Powerhouses. [(accessed on 15 November 2022)]. Available online: https://oneoceanbeauty.com/collections/collagen.
- 163.Ostrealia. [(accessed on 11 November 2022)]. Available online: https://www.ostrealia.fr/en/categorie-produit/cosmetics/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.