Keywords: ACE2, bile acid, farnesoid X receptor, intestinal epithelium, SARS-CoV-2
Abstract
Intestinal inflammation and diarrhea are often associated with SARS-CoV-2 infection. The angiotensin converting enzyme 2 (ACE2) receptor plays a key role in SARS-CoV-2 pathogenesis, facilitating entry of the virus into epithelial cells, while also regulating mucosal inflammatory responses. Here, we investigated roles for the nuclear bile acid receptor farnesoid X receptor (FXR) in regulating ACE2 expression and virally mediated inflammatory responses in intestinal epithelia. Human colonic or ileal enteroids and cultured T84 and Caco-2 monolayers were treated with the FXR agonists, obeticholic acid (OCA) or GW4064, or infected with live SARS-CoV-2 (2019-nCoV/USA_WA1/2020). Changes in mRNA, protein, or secreted cytokines were measured by qPCR, Western blotting, and ELISA. Treatment of undifferentiated colonic or ileal enteroids with OCA increased ACE2 mRNA by 2.1 ± 0.4-fold (n = 3; P = 0.08) and 2.3 ± 0.2-fold (n = 3; P < 0.05), respectively. In contrast, ACE2 expression in differentiated enteroids was not significantly altered. FXR activation in cultured epithelial monolayers also upregulated ACE2 mRNA, accompanied by increases in ACE2 expression and secretion. Further experiments revealed FXR activation to inhibit IL-6 release from both Caco-2 cells infected with SARS-CoV-2 and T84 cells treated with the viral mimic, polyinosinic:polycytidylic acid, by 46 ± 12% (n = 3, P < 0.05) and 35 ± 6% (n = 8; P < 0.01), respectively. By virtue of its ability to modulate epithelial ACE2 expression and inhibit virus-mediated proinflammatory cytokine release, FXR represents a promising target for the development of new approaches to prevent intestinal manifestations of SARS-CoV-2.
NEW & NOTEWORTHY Activation of the nuclear bile acid receptor, farnesoid X receptor (FXR), specifically upregulates ACE2 expression in undifferentiated colonic epithelial cells and inhibits virus-induced proinflammatory cytokine release. By virtue of these actions FXR represents a promising target for the development of new approaches to prevent intestinal manifestations of SARS-CoV-2 infection.
INTRODUCTION
Although most often associated with respiratory infections and symptoms, much has been learned about the effects of SARS-CoV-2 on the gastrointestinal tract since the outbreak of the pandemic in January 2020. Originally reported to be relatively rare manifestations of the disease (1, 2), gastrointestinal symptoms are now recognized to occur in 40%–50% of infected patients (3, 4), with diarrhea being the most common. Although the pathophysiological mechanisms underlying SARS-CoV-2-associated diarrhea are likely to be multifactorial (3), numerous studies suggest that mucosal inflammation is a key feature (5–8).
Viral entry into cells occurs via the ACE2 receptor, which is abundantly expressed along the intestinal tract, particularly in the epithelial cells of the ileum and colon where it mediates nutrient absorption and exerts anti-inflammatory actions by converting proinflammatory angiotensin II to anti-inflammatory angiotensin 1–7 (9, 10). SARS-CoV-2 infection is associated with downregulation of ACE2 expression, an effect that has been strongly linked to the progression of mucosal inflammation (11, 12). Recent studies by Brevini et al. (13) have shown that ACE2 expression is regulated by the nuclear bile acid receptor farnesoid X receptor (FXR), which, like ACE2, is expressed at high levels in epithelial cells of the ileum and colon. FXR agonists were shown to promote ACE2 expression in undifferentiated ileal enteroids, whereas the FXR antagonists, ursodeoxycholic acid (UDCA) and guggulsterone, reduced its expression and prevented SARS-CoV-2 entry. Although these studies suggest FXR to have potential for development as a target to protect against SARS-CoV-2 infection of the intestine, it is important to more fully understand how the receptor regulates ACE2 expression along the crypt/villus axis.
In addition to regulating ACE2 expression, FXR is also well established to exert barrier-promoting and anti-inflammatory actions in the intestine (14). However, whether it can prevent virus-induced inflammatory responses in intestinal epithelial cells is still unknown. With this in mind, the aims of the current study were to 1) elucidate FXR effects on ACE2 expression in intestinal epithelial cells and 2) determine whether FXR activation has the capacity to inhibit virally induced inflammatory responses.
EXPERIMENTAL PROCEDURES
Ex vivo human ileal and colonic enteroids, T84 colonic epithelial cells, Caco-2 intestinal epithelial cells, and human embryonic kidney 293 cells were used for these studies. Gene expression in enteroids was measured by bulk RNA-Seq and in cultured cells lines gene expression was measured by qRT-PCR, whereas protein expression was measured by Western blotting. Protein secretion into the cell culture medium was measured by ELISA. NF-κB transcriptional activity was measured using the Dual-Luciferase Reporter Assay System from Promega. For detailed protocols see Supplemental Methods: https://doi.org/10.6084/m9.figshare.24053331.
RESULTS
FXR Activation Upregulates ACE2 Expression in Undifferentiated Colonic and Ileal Enteroids
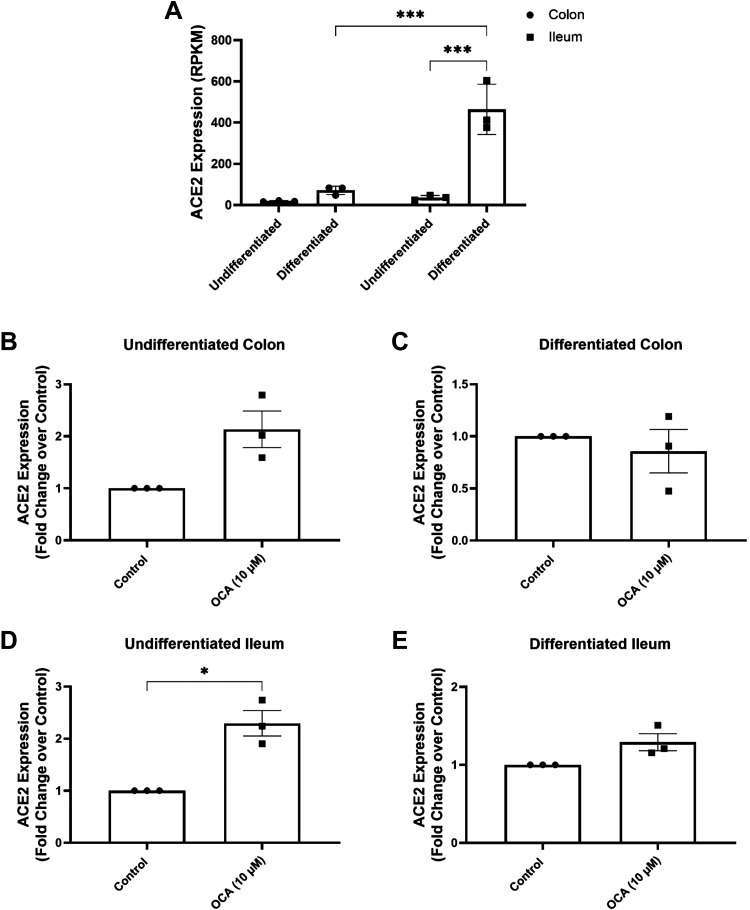
We first investigated the effects of FXR activation on ACE2 expression in undifferentiated (UD) and differentiated (DF) human colonic and ileal enteroids. Cells were differentiated by removal of Wnt, R-spondin, and noggin from the culture media for 5 days, and effective differentiation was evidenced by decreased expression of LGR5 and increased expression of the differentiated cell markers, SI, ALPI, and SLC28A3 (Supplemental Fig. S1). Analysis of basal ACE2 expression in the enteroids revealed that it is more highly expressed in differentiated tissues compared with undifferentiated tissues and that its expression is more abundant in the ileum than the colon (Fig. 1A). Treatment of undifferentiated colonic and ileal enteroids with OCA (10 µM; 24 h) caused 2.1 ± 0.4-fold (n = 3; P = 0.08) and 2.3 ± 0.2-fold (n = 3; P < 0.001) increases in ACE2 expression, respectively, compared with that of untreated controls (Fig. 1, B and D). In contrast, OCA was without effect in differentiated enteroids from either ileum or colon (Fig. 1, C and E). Expression levels of the ACE2 homolog, ACE, were unaltered in both the colonic and ileal enteroids (data not shown), indicating the effects of FXR to be specific for ACE2.
Figure 1.
FXR activation upregulates ACE2 expression in undifferentiated colonic and ileal enteroids. Enteroids prepared from crypts isolated from human colonic and ileal tissue specimens were cultured as monolayers on semipermeable supports. Enteroids were studied in the undifferentiated state by exposure to medium containing Wnt, R-spondin, and noggin, or in the differentiated state by withdrawal of these growth factors for 5 days, and treated with OCA (10 µM) for 24 h. A: basal ACE2 expression in undifferentiated and differentiated colonic and ileal enteroids (n = 3; ***P < 0.001). Changes in ACE2 expression in response to OCA treatment in undifferentiated (B) and differentiated (C) colonic enteroids and undifferentiated (D) and differentiated (E) ileal enteroids were analyzed by bulk RNA-Seq, with results normalized to changes in the untreated conditions (n = 3; *P < 0.05). Results are expressed as means ± SE. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test or paired t test. FXR, farnesoid X receptor; OCA, obeticholic acid.
FXR Activation Upregulates ACE2 Expression and Secretion by T84 Colonic Epithelial Cells
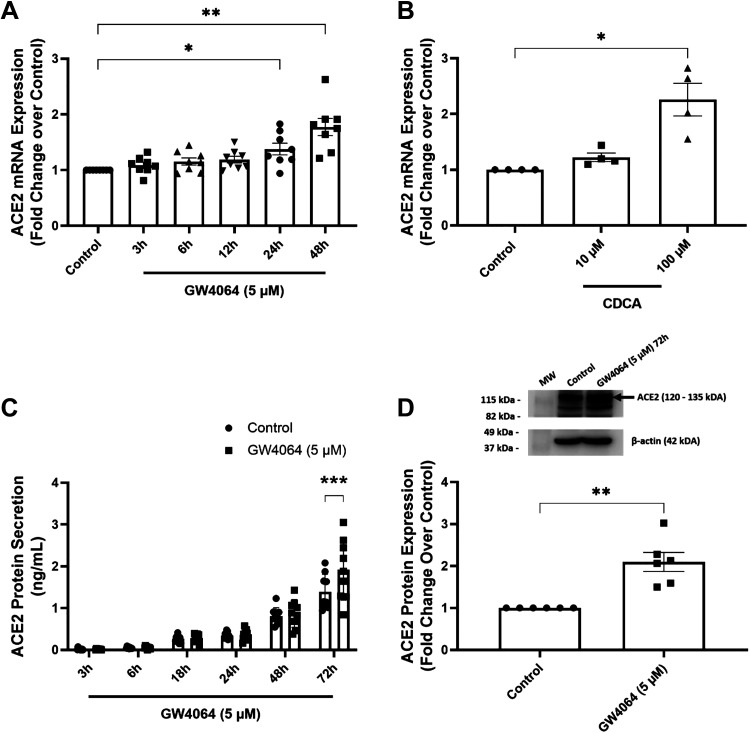
T84 colonic epithelial cells were used to further study the effects of FXR activation on ACE2 expression. Similar to enteroids, but in this case with the synthetic FXR agonist, GW4064, FXR activation upregulated ACE2 mRNA expression by 1.4 ± 0.1-fold (P < 0.05) and 1.8 ± 0.2-fold (P < 0.01) of that in control cells after 24 and 48 h of treatment, respectively (n = 8) (Fig. 2A). Treatment with the endogenous bile acid, chenodeoxycholic acid (CDCA) (100 µM but not 10 µM) also increased ACE2 mRNA expression by 2.3 ± 0.3-fold relative to controls (n = 4; P < 0.05) (Fig. 2B). FXR-induced increases in ACE2 mRNA were translated into increased levels in cellular expression of the protein (2.3 ± 0.3-fold) and enhanced secretion of soluble ACE2 into the apical medium from 1.4 ± 0.1 ng/mL to 1.9 ± 0.2 ng/mL (n = 9; P < 0.001) (Fig. 2, C and D).
Figure 2.
FXR activation upregulates ACE2 expression and secretion in T84 colonic epithelial cells. Serum-starved monolayers of T84 colonic epithelial cells bilaterally treated with GW4064 (A) (5 µM) for 3–48 h (n = 8; *P < 0.05, **P < 0.01) or CDCA (B) (10 and 100 µM) for 48 h (n = 4; *P < 0.05) were analyzed for ACE2 mRNA expression by qRT-PCR. T84 cells were treated with GW4064 (5 µM) at various time points between 3 and 72 h and aliquots of medium collected from the apical compartment of matched control and GW4064-treated cells were analyzed for levels of soluble ACE2 by ELISA (C) (n = 9; ***P < 0.001). D: ACE2 expression in the protein lysates was measured by Western blotting (n = 6; **P < 0.01). Results are expressed as means ± SE. Statistical analysis was performed using one-way ANOVA with Dunnett’s multiple comparisons test, two-way ANOVA with Šidák’s multiple comparisons test, or paired t test. FXR, farnesoid X receptor.
FXR Activation Inhibits Virally Induced IL-6 Expression and Secretion NF-κB Signaling
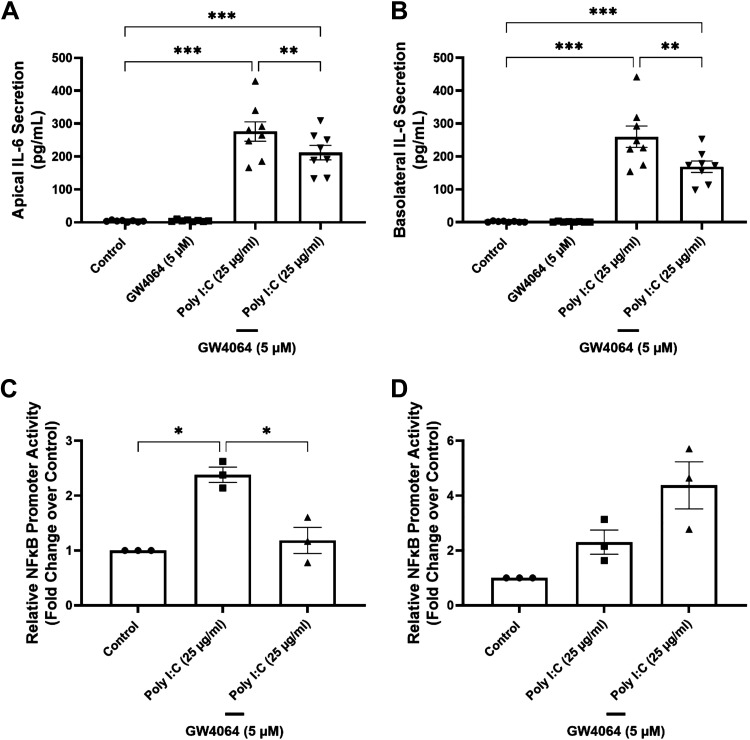
We investigated a potential role for FXR in modulating inflammatory responses to SARS-CoV-2 by analyzing its effects on the expression and secretion of IL-6, a proinflammatory cytokine known to be important in initiating SARS-CoV-2-induced inflammatory responses and a biomarker of severe disease. First, we investigated the effects of FXR activation on IL-6 secretion in response to the viral mimic/TLR3 agonist, polyinosinic:polycytidylic acid (poly I:C), in T84 cells. Treatment of the cells with GW4064 significantly reduced poly I:C-induced IL-6 secretion into the apical medium from 275.9 ± 29.6 pg/mL to 211.8 ± 22 pg/mL (n = 8; P < 0.01) (Fig. 3A) and in the basolateral medium from 259.8 ± 32.4 pg/mL to 168.7 ± 17.3 pg/mL (n = 8; P < 0.01) (Fig. 3B). Since TLR-3-mediated IL-6 secretion has been previously shown to be mediated by NF-κB signaling (15), we went on to investigate whether FXR might exert its actions through inhibiting this pathway. In these experiments, HEK293 cells transfected with constructs containing an NF-κB-luc reporter gene, FXR, RXR, or empty vector were used. In cells cotransfected with FXR and RXR, treatment with GW4064 reduced poly I:C-induced luciferase activity from 2.4 ± 0.1-fold to 1.2 ± 0.2-fold of that in untreated controls (Fig. 3C) (n = 3; P < 0.05). However, this effect was not apparent in cells that were not transfected with nuclear receptors (Fig. 3D).
Figure 3.
FXR activation inhibits Poly I:C-induced IL-6 secretion and NF-κB signaling. T84 cell monolayers were serum starved for 24 h and treated apically and basolaterally with either GW4064 (5 µM) or poly I:C (25 µg/mL) alone, or GW4064 in combination with poly I:C for 24 h. IL-6 secretion into the apical (A) and basolateral (B) compartments was measured by ELISA (n = 8; *P < 0.001, **P < 0.01, ***P < 0.001). HEK293 cells were transfected with constructs containing NF-κB response elements linked to the firefly luciferase reporter gene, FXR, RXR, or empty vector. A pRL-SV40 vector, containing Renilla luciferase, was used to control for transfection efficiency. Cells transfected with FXR/RXR (C) or with empty vector (D) were treated with either poly I:C (25 µg/mL) or poly I:C in combination with GW4064 for 24 h, after which they were lysed and NF-κB luciferase activity measured (n = 3; *P < 0.05). Results are expressed as means ± SE. Statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparisons test. FXR, farnesoid X receptor.
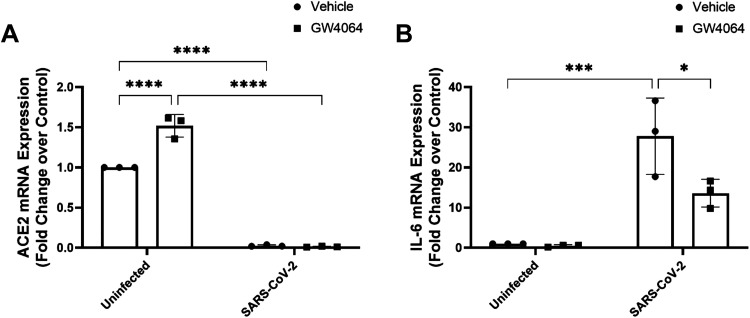
Finally, we examined the effects of FXR activation on ACE2 expression and IL-6 secretion by Caco-2 cells infected with the original Wuhan strain of the SARS-CoV-2 virus. Similar to its effects in enteroids and T84 cells, treatment with GW4064 resulted in a significant 1.5 ± 0.1-fold (n = 3; P < 0.001) increase in ACE2 mRNA expression relative to that of untreated controls (Fig. 4A). Infection of Caco-2 cells with SARS-CoV-2 virus reduced relative ACE2 mRNA expression from 1.0 in uninfected cells to 0.03 ± 0.06 (n = 3; P < 0.001) (Fig. 4A), while increasing IL-6 expression by 25.8 ± 1.9-fold (n = 3; P < 0.001) (Fig. 4B). Treatment with GW4064 did not increase ACE2 expression in SARS-CoV-2-infected cells, but reduced induction of IL-6 expression in response to the virus by ∼50% (n = 3; P < 0.001) (Fig. 4B).
Figure 4.
FXR activation upregulates ACE2 expression and inhibits SARS-CoV-2-induced IL-6 secretion from Caco-2 intestinal epithelial cells. Polarized monolayers of Caco-2 cells apically infected with SARS-CoV-2 were treated bilaterally with GW4064 (5 µM) for 120 h. Levels of ACE2 (A) and IL-6 (B) mRNA expression were measured by qPCR (n = 3; *P < 0.05, ***P < 0.001, ****P < 0.0001). Results are expressed as means ± SE. Statistical analysis was performed using two-way ANOVA with Tukey’s multiple comparisons test.
DISCUSSION
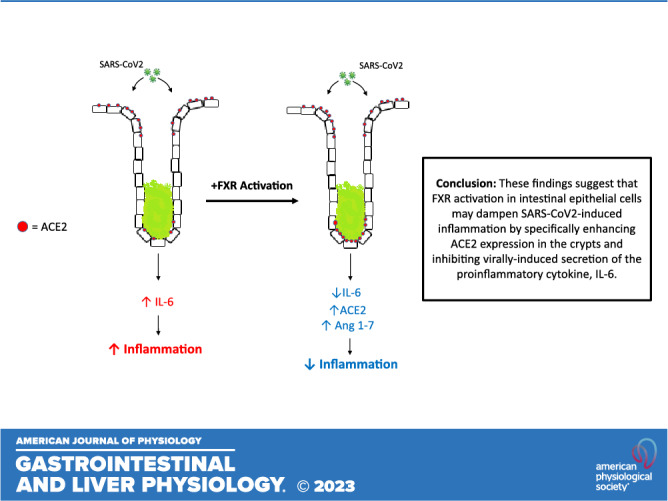
ACE2 plays a complex role in the pathogenesis of SARS-CoV-2; on the one hand, it serves as the primary entry pathway for the virus in epithelial cells, whereas on the other hand, it is an important regulator of mucosal inflammation. Once infected by SARS-CoV-2, ACE2 expression is downregulated, slowing further infection, but also promoting inflammation by preventing local conversion of the proinflammatory ANG II to the anti-inflammatory angiotensin 1–7 (Ang 1–7). Given that there are such opposing facets to its role in determining the severity of SARS-CoV-2 infection, it is important to more fully understand how ACE2 expression and virally induced inflammatory responses are regulated at the molecular level. The emergence of the nuclear bile acid receptor FXR as an important regulator of both mucosal inflammation and intestinal epithelial ACE2 expression represents a significant step forward in developing our understanding of how SARS-CoV-2 infection likely affects the gut.
We found that treatment of either ileal or colonic enteroids with the semisynthetic FXR agonist, OCA, upregulated expression of ACE2, an effect that was recapitulated at both the mRNA and protein level in response to the naturally occurring FXR agonist, CDCA, or the synthetic agonist, GW4064, in cultured colonic epithelial cell lines. Thus, our studies confirm the recent findings of Brevini et al. (13) that FXR activation promotes ileal epithelial ACE2 expression and extend those findings to show the effects are also evident in the colon. Furthermore, our studies provide new insights with respect to differential effects of FXR activation along the crypt villus axis. In agreement with previous studies, we found that there is more abundant expression of ACE2 mRNA in ileal epithelial cells than that of colonic epithelial cells (16) and in differentiated cells compared with that of undifferentiated cells (17, 18). This is probably not surprising given the well-established role of ACE2 in dietary amino acid absorption (9). Interestingly, we found that FXR activation stimulated expression of ACE2 only in undifferentiated (i.e., crypt-like) ileal and colonic enteroids, whereas it was without effect in differentiated enteroids. This observation is important, given that viral infection in the intestinal tract occurs primarily through differentiated enterocytes of the ileal villus or colonic surface cells (19), whereas viral entry via the crypts is prevented by the mucus barrier secreted by goblet cells (20). Thus, although FXR promotes ACE2 expression in intestinal epithelia, our findings that these effects may be restricted to undifferentiated cells of the crypts combined with the already much higher basal level of ACE2 expression in the differentiated epithelial cells, the FXR-induction of ACE2 may not necessarily be associated with increased intestinal viral infection in vivo. Thus, although FXR promotes ACE2 expression in intestinal epithelia, by virtue of its effects being restricted to undifferentiated cells of the crypts, this may not necessarily be associated with increased viral infection in vivo. Indeed, given its classical role in converting the proinflammatory angiotensin II to anti-inflammatory angiotensin 1–7, one might expect FXR-induced ACE2 expression in the crypts to dampen virally induced inflammatory responses. Further studies are clearly warranted to more fully understand the physiological and pathophysiological consequences of FXR-induced ACE2 expression in the gut.
In addition to its effects on ACE2 expression, our studies show that FXR is also likely to dampen inflammatory responses to SARS-CoV-2 infection via other pathways. Indeed, FXR has well-established anti-inflammatory and barrier-promoting properties in the intestine, with such effects being attributed, at least partly, to inhibition of mucosal cytokine accumulation (14, 21–24). Similar to its effects in the airways, SARS-CoV-2 infection of the gut leads to the generation of a “cytokine storm” with consequent mucosal inflammation, effects that likely contribute to diarrheal symptoms associated with the disease (5–8). However, whether FXR activation has the capacity to dampen virally induced inflammatory responses has not yet been established. Here, we found that treatment with the FXR agonist, GW4064, significantly inhibited expression and secretion of IL-6 in response to either infection with live SARS-CoV-2 in Caco-2 cells or TLR3 activation with Poly I:C in T84 cells. TLR3 has been previously shown to, at least partly, mediate SARS-CoV-2-induced IL-6 secretion from intestinal epithelial cells (25, 26). Furthermore, studies using a luciferase-reporter construct in HEK293 cells suggest that FXR activation likely has this effect by inhibiting NF-κB signaling, a critical step in the pathway linking TLR3 activation to IL-6 secretion (15). Given the central role of IL-6 in initiating and propagating inflammatory responses to SARS-CoV-2 infection (27), our data suggest that FXR activation would likely serve to limit such responses.
In summary, our data confirm recent findings that activation of FXR upregulates expression of epithelial ACE2, the primary entry pathway for SARS-CoV-2 in intestinal epithelial cells (13). Although these data indicate that FXR activation may be detrimental in terms of enhancing viral infection and increasing disease severity, they must be interpreted in light of our current findings showing differential regulation of ACE2 along the crypt villus axis and a dampening of virus-induced proinflammatory signals in response to FXR activation. Thus, FXR upregulation of ACE2 may have minimal impact on viral entry but be more associated with anti-inflammatory actions mediated by angiotensin 1–7 and inhibition of IL-6 release. Interestingly, this hypothesis is supported by recent studies demonstrating FXR activation does not alter early SARS-CoV-2 infection in mouse ileum but does enhance survival of infected animals (28). Our current studies highlight the need for further research to better understand how FXR modulates CoV infection-associated actions in the gut and ultimately, how to treat gastrointestinal symptoms in patients with COVID-19.
DATA AVAILABILITY
Data will be made available upon reasonable request.
SUPPLEMENTAL DATA
Supplemental Methods and Supplemental Fig.S1: https://doi.org/10.6084/m9.figshare.24053331.
GRANTS
This study was supported by National Institutes of Health Grant DK047987 (to P.A.D., A.R., and J.K.T.), NIH/NIDDK P30DK089502, NIH RO1DK116352, and NIH RO16523 (to M.D.) and by Science Foundation Ireland (SFI) Grants 16-IA-4445 (to S.J.K.) and 17TIDA5050 (to S.J.K).
DISCLOSURES
Luciano Adorini is a consultant of Intercept Pharmaceuticals. None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
AUTHOR CONTRIBUTIONS
J.S.S., J.K.T., M.D., P.A.D., and S.J.K. conceived and designed research; J.S.S., J.K.T., A.R., R.L., and J.F.-A. performed experiments; J.S.S., J.K.T., A.R., R.L., J.F.-A., and S.J.K. analyzed data; J.S.S., J.K.T., A.R., J.F.-A., L.A., M.D., P.A.D., and S.J.K. interpreted results of experiments; J.S.S. and J.K.T. prepared figures; J.S.S., P.A.D., and S.J.K. drafted manuscript; J.S.S., J.K.T., A.R., L.A., M.D., P.A.D., and S.J.K. edited and revised manuscript; J.S.S., J.K.T., A.R., J.F.-A., L.A., M.D., P.A.D., and S.J.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Drs. Leda Bassett and Raymond Schinazi for their support using the BL3 facility in the Laboratory of Biochemical Pharmacology at Emory University.
REFERENCES
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395: 507–513, 2020. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jin B, Singh R, Ha SE, Zogg H, Park PJ, Ro S. Pathophysiological mechanisms underlying gastrointestinal symptoms in patients with COVID-19. World J Gastroenterol 27: 2341–2352, 2021. doi: 10.3748/wjg.v27.i19.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aguila EJT, Cua IHY, Fontanilla JAC, Yabut VLM, Causing MFP. Gastrointestinal manifestations of COVID-19: impact on nutrition practices. Nutr Clin Pract 35: 800–805, 2020. doi: 10.1002/ncp.10554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, Hilbe R, Seiwald S, Scholl-Buergi S, Fritsche G, Bellmann-Weiler R, Weiss G, Müller T, Adolph TE, Tilg H. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut 69: 1543–1544, 2020. doi: 10.1136/gutjnl-2020-321388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang X, Wei J, Zhu R, Chen L, Ding F, Zhou R, Ge L, Xiao J, Zhao Q. Contribution of CD4+ T cell-mediated inflammation to diarrhea in patients with COVID-19. Int J Infect Dis 120: 1–11, 2022. doi: 10.1016/j.ijid.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zerbato V, Di Bella S, Giuffrè M, Jaracz AW, Gobbo Y, Luppino D, Macor P, Segat L, Koncan R, D'Agaro P, Valentini M, Crocé LS, Ruscio M, Luzzati R. High fecal calprotectin levels are associated with SARS-CoV-2 intestinal shedding in COVID-19 patients: a proof-of-concept study. World J Gastroenterol 27: 3130–3137, 2021. doi: 10.3748/wjg.v27.i22.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ellakany WI, AbdelHady AM, Nassar MW, Elwafa RAHA. Faecal calprotectin in COVID-19 patients with intestinal symptoms. Prz Gastroenterol 17: 332–337, 2022. doi: 10.5114/pg.2022.114685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camargo SMR, Vuille-Dit-Bille RN, Meier CF, Verrey F. ACE2 and gut amino acid transport. Clin Sci (Lond) 134: 2823–2833, 2020. doi: 10.1042/CS20200477. [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487: 477–481, 2012. doi: 10.1038/nature11228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maranduca MA, Tanase DM, Cozma CT, Dima N, Clim A, Pinzariu AC, Serban DN, Serban IL. The impact of angiotensin-converting enzyme-2/angiotensin 1-7 axis in establishing severe COVID-19 consequences. Pharmaceutics 14: 1906, 2022. doi: 10.3390/pharmaceutics14091906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alabsi S, Dhole A, Hozayen S, Chapman SA. Angiotensin-converting enzyme 2 expression and severity of SARS-CoV-2 infection. Microorganisms 11: 612, 2023. doi: 10.3390/microorganisms11030612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brevini T, Maes M, Webb GJ, John BV, Fuchs CD, Buescher G, et al. FXR inhibition may protect from SARS-CoV-2 infection by reducing ACE2. Nature 615: 134–142, 2023. doi: 10.1038/s41586-022-05594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S, Penna G, Laverny G, Adorini L, Moschetta A, van Mil SW. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 60: 463–472, 2011. doi: 10.1136/gut.2010.212159. [DOI] [PubMed] [Google Scholar]
- 15. Kanmani P, Kim H. Immunobiotic strains modulate toll-like receptor 3 agonist induced innate antiviral immune response in human intestinal epithelial cells by modulating IFN regulatory factor 3 and NF-κB signaling. Front Immunol 10: 1536, 2019. doi: 10.3389/fimmu.2019.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McAllister MJ, Kirkwood K, Chuah SC, Thompson EJ, Cartwright JA, Russell CD, Dorward DA, Lucas CD, Ho GT. Intestinal protein characterisation of SARS-CoV-2 entry molecules ACE2 and TMPRSS2 in inflammatory bowel disease (IBD) and fatal COVID-19 infection. Inflammation 45: 567–572, 2022. doi: 10.1007/s10753-021-01567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang H, Kang Z, Gong H, Xu D, Wang J, Li Z, Li Z, Cui X, Xiao J, Zhan J, Meng T, Zhou W, Liu J, Xu H. Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 69: 1010–1018, 2020. doi: 10.1136/gutjnl-2020-320953. [DOI] [Google Scholar]
- 18. Pearce SC, Suntornsaratoon P, Kishida K, Al-Jawadi A, Guardia J, Nadler I, Flores J, Shiarella R, Auvinen M, Yu S, Gao N, Ferraris RP. Expression of SARS-CoV-2 entry factors, electrolyte, and mineral transporters in different mouse intestinal epithelial cell types. Physiol Rep 9: e15061, 2021. doi: 10.14814/phy2.15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Salim AF, Phillips AD, Farthing MJ. Pathogenesis of gut virus infection. Baillieres Clin Gastroenterol 4: 593–607, 1990. doi: 10.1016/0950-3528(90)90051-h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schwegmann C, Zimmer G, Yoshino T, Enss M, Herrler G. Comparison of the sialic acid binding activity of transmissible gastroenteritis coronavirus and E. coli K99. Virus Res 75: 69–73, 2001. doi: 10.1016/s0168-1702(01)00228-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Massafra V, Ijssennagger N, Plantinga M, Milona A, Ramos Pittol JM, Boes M, van Mil SW. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. Biochim Biophys Acta 1862: 166–173, 2016. doi: 10.1016/j.bbadis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 22. Liu HM, Liao JF, Lee TY. Farnesoid X receptor agonist GW4064 ameliorates lipopolysaccharide-induced ileocolitis through TLR4/MyD88 pathway related mitochondrial dysfunction in mice. Biochem Biophys Res Commun 490: 841–848, 2017. doi: 10.1016/j.bbrc.2017.06.129. [DOI] [PubMed] [Google Scholar]
- 23. Zhao D, Cai C, Chen Q, Jin S, Yang B, Li N. High-Fat Diet Promotes DSS-Induced Ulcerative Colitis by Downregulated FXR Expression through the TGFB Pathway. Biomed Res Int 2020: 3516128, 2020. doi: 10.1155/2020/3516128. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Tang K, Kong D, Peng Y, Guo J, Zhong Y, Yu H, Mai Z, Chen Y, Chen Y, Cui T, Duan S, Li T, Liu N, Zhang D, Ding Y, Huang J. Ginsenoside Rc attenuates DSS-induced ulcerative colitis, intestinal inflammatory, and barrier function by activating the farnesoid X receptor. Front Pharmacol 13: 1000444, 2022. doi: 10.3389/fphar.2022.1000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bortolotti D, Gentili V, Rizzo S, Schiuma G, Beltrami S, Strazzabosco G, Fernandez M, Caccuri F, Caruso A, Rizzo R. TLR3 and TLR7 RNA Sensor Activation during SARS-CoV-2 Infection. Microorganisms 9: 1820, 2021. doi: 10.3390/microorganisms9091820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manik M, Singh RK. Role of toll-like receptors in modulation of cytokine storm signaling in SARS-CoV-2-induced COVID-19. J Med Virol 94: 869–877, 2022. doi: 10.1002/jmv.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol 146: 518–534.e1, 2020. doi: 10.1016/j.jaci.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nagai M, Moriyama M, Ishii C, Mori H, Watanabe H, Nakahara T, Yamada T, Ishikawa D, Ishikawa T, Hirayama A, Kimura I, Nagahara A, Naito T, Fukuda S, Ichinohe T. High body temperature increases gut microbiota-dependent host resistance to influenza A virus and SARS-CoV-2 infection. Nat Commun 14: 3863, 2023. doi: 10.1038/s41467-023-39569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Methods and Supplemental Fig.S1: https://doi.org/10.6084/m9.figshare.24053331.
Data Availability Statement
Data will be made available upon reasonable request.