Abstract
Structurally unique among ion channels, ATP-sensitive K+ (KATP) channels are essential in coupling cellular metabolism with membrane excitability, and their activity can be reconstituted by coexpression of an inwardly rectifying K+ channel, Kir6.2, with an ATP-binding cassette protein, SUR1. To determine if constitutive channel subunits form a physical complex, we developed antibodies to specifically label and immunoprecipitate Kir6.2. From a mixture of Kir6.2 and SUR1 in vitro-translated proteins, and from COS cells transfected with both channel subunits, the Kir6.2-specific antibody coimmunoprecipitated 38- and 140-kDa proteins corresponding to Kir6.2 and SUR1, respectively. Since previous reports suggest that the carboxy-truncated Kir6.2 can form a channel independent of SUR, we deleted 114 nucleotides from the carboxy terminus of the Kir6.2 open reading frame (Kir6.2ΔC37). Kir6.2ΔC37 still coimmunoprecipitated with SUR1, suggesting that the distal carboxy terminus of Kir6.2 is unnecessary for subunit association. Confocal microscopic images of COS cells transfected with Kir6.2 or Kir6.2ΔC37 and labeled with fluorescent antibodies revealed unique honeycomb patterns unlike the diffuse immunostaining observed when cells were cotransfected with Kir6.2-SUR1 or Kir6.2ΔC37-SUR1. Membrane patches excised from COS cells cotransfected with Kir6.2-SUR1 or Kir6.2ΔC37-SUR1 exhibited single-channel activity characteristic of pancreatic KATP channels. Kir6.2ΔC37 alone formed functional channels with single-channel conductance and intraburst kinetic properties similar to those of Kir6.2-SUR1 or Kir6.2ΔC37-SUR1 but with reduced burst duration. This study provides direct evidence that an inwardly rectifying K+ channel and an ATP-binding cassette protein physically associate, which affects the cellular distribution and kinetic behavior of a KATP channel.
Potassium channels are the most diverse group of ion channels, with molecular cloning revealing a number of structurally distinct families, including the subfamily of inwardly rectifying K+ (Kir) channels (11, 27, 35). Channel diversity is increased by the ability of constitutive subunits to form not only homomeric but also heteromultimeric complexes with distinct functional and regulatory properties (8, 9, 15, 21, 27, 30, 39, 53). Present in most excitable tissues, ATP-sensitive K+ (KATP) channels belong to the Kir family and are involved in signaling networks that transduce cellular metabolic events into membrane potential changes (1, 9, 40). These channels are regulated by intracellular nucleotides and have been implicated in hormone secretion, cardioprotection, and neurotransmitter release, with their function best understood in the pancreatic β cell, where KATP channels are essential in glucose-mediated membrane depolarization and insulin secretion (7, 9, 14, 31, 34, 42, 44, 52). Structurally unique among K+ channels, KATP channel activity can be reconstituted by coexpressing two unrelated proteins: the Kir channel Kir6.2 and the ATP-binding cassette (ABC) protein SUR, specifically the SUR1 isoform for the pancreatic channel phenotype (2, 22, 38).
Expression of Kir6.2 alone does not result in functional ion channels, suggesting an intimate and required interaction between Kir6.2 with SUR1 (1, 7, 40, 41). Actually, expression of Kir6.2-SUR1 fusion constructs indicates that a subunit stoichiometry of 1:1 is necessary for assembly of active KATP channels (10, 24). Furthermore, Kir6.2 and SUR1 genes are clustered on chromosome 11 (p15.1), separated by a short intergenic sequence of 4.3 kb, suggesting that these genes could be cotranscribed and cotranslated to form a functional heteromultimeric channel (1, 9, 22, 40). To date, evidence for physical association between Kir6.2 and SUR1 is based on photoaffinity labeling of both channel subunits by radioactive sulfonylurea (10). Labeling of Kir6.2 was dependent on coexpression of SUR1, suggesting close association between the two subunits (10). However, photoaffinity labeling is based primarily on proximity rather than physical interaction between proteins (18).
Recent evidence indicates that K+ channels are tetramers of single subunits comprising the K+-selective pore (27). The measurement of KATP channel activity in cells expressing mutant carboxy-truncated Kir6.2 has been interpreted to mean that the presence of the carboxy terminus in Kir6.2 prevents functional expression of the channel in the absence of SUR (51). However, it is not known whether the distal carboxy terminus of Kir6.2 merely serves as a suppressor of channel activity or is also important in regulating physical interaction between Kir6.2 and SUR1.
To determine whether Kir6.2 and SUR1 can physically associate with each other, and to investigate the role of the carboxy terminus of Kir6.2 in complex formation, we used a Kir6.2-specific antibody to coimmunoprecipitate and to immunostain channel subunits. We truncated the carboxy terminus of Kir6.2 polypeptide to yield functional channels in the absence of SUR1 (49, 51) and then used such mutants to measure single-channel properties when expressed alone or with SUR1. We demonstrate that Kir6.2 and SUR1 physically associate in functional complexes and that the carboxy terminus of Kir6.2 is not required for subunit association. Furthermore, we provide evidence that the intraburst behavior of KATP channels is defined by Kir6.2 alone, whereas burst channel behavior is modulated by association with SUR1.
MATERIALS AND METHODS
Plasmid construction and in vitro translation.
For in vitro translation, the coding region of Kir6.2 (kind gift from S. Seino, Chiba University) was cloned as an BamHI-EcoRI fragment into the BamHI-EcoRI sites of vector pcDNA3.1+ (Invitrogen). Similarly, the open reading frame of SUR1 (kind gift from L. Aguilar-Bryan and J. Bryan, Baylor College of Medicine) was cloned as an EcoRI fragment into the EcoRI site of pcDNA1Amp vector (Invitrogen). For construction of the carboxy-terminus deletion mutant of Kir6.2, the naturally occurring PshA1 restriction site at position 1057 of the Kir6.2 open reading frame was used. Kir6.2 in pcDNA3.1+ was cut with PshA1 and XbaI, blunt ended, and ligated. Correct wild-type (Kir6.2) and mutant (Kir6.2ΔC37) protein expression was verified by in vitro translation, using a T7 TNT reticulocyte lysate coupled transcription-translation kit (Promega).
Antibody preparation and immunoprecipitation.
A rabbit polyclonal antibody was raised against the synthetic peptide comprised of residues 19 to 39 in the Kir6.2 protein (EDPAEPRYRARQRRARFVSKK), conjugated to a carrier protein, keyhole limpet hemocyanin, and used for immunoprecipitation (30). cDNAs encoding Kir6.2 or Kir6.2ΔC37 and SUR1 were in vitro translated by using a T7 TNT transcription-translation kit to generate recombinant proteins. In vitro-translated products for each protein (5 μl) were solubilized in 100 μl of immunoprecipitation buffer (53) and incubated at 30°C for 30 min to allow for formation of the complex. Following preincubation, 20 μl of prewashed protein A-Sepharose Fast Flow beads was added together with 1 μl of the epitope-specific Kir6.2 antibody, and incubation continued at 4°C with rotation for 90 min. Precipitates were sedimented by centrifugation at 4°C and washed three times in immunoprecipitation buffer and once in phosphate-buffered saline (PBS; pH 7.4). Prior to loading on a 10% polyacrylamide–sodium dodecyl sulfate (SDS) gel, samples were solubilized in twofold-concentrated alkaline sample buffer without boiling. Following electrophoresis, gels were stained and destained, signals were enhanced by a 20-min incubation in Amplify (Amersham), and dry gels were exposed overnight at −70°C. For single-protein immunoprecipitation, the preincubation step was omitted. To test for antibody specificity, 10 μl of the antigenic peptide (1 mg/ml in PBS) was incubated for 1 h with 1 μg of the Kir6.2 antibody at room temperature prior to immunoprecipitation.
Heterologous expression.
African green monkey kidney COS cells do not natively express Kir6.2 or SUR1 that produces KATP channel activity or proteins that can be recognized by antibodies (see also reference 22). COS-7 cells were cultured (at 5% CO2) in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum and 2 mM glutamine. COS-7 cells (2 × 106) plated on a 100-mm2-diameter culture dish were transfected according to the manufacturer’s instructions at 60% confluence, using 8 μl of Lipofectamine (Gibco) and 2 μg of total plasmid DNA in low-serum OPTI-MEM medium (Gibco/BRL). For cotransfection, plasmid cDNAs encoding the two subunits were added in equal amounts.
Immunoprecipitation from solubilized membrane proteins.
Membranes were isolated from COS cells coexpressing Kir6.2 and SUR1, labeled with the radioactive sulfonyurea 125I-azidoglyburide as described previously (10) (kindly provided by J. Bryan, Baylor College of Medicine). Membranes were solubilized in buffer containing 20 mM HEPES, 150 mM NaCl, and 1% digitonin (pH 7.5), and 25 μg of solubilized protein was immunoprecipitated with 3 μg of the anti-Kir6.2 antibody. In control experiments, the antibody was preblocked with 50 μM antigenic peptide. Immunoprecipitates were washed with the same buffer and solubilized in SDS loading buffer.
Immunostaining and immunofluorescence microscopy.
Transfected COS-7 cells, grown on sterile 25-mm-diameter coverslips, were imaged by laser confocal microscopy. Twenty-four hours posttransfection, cells were washed three times in Dulbecco’s phosphate-buffered saline (DPBS) and fixed for 15 min in 4% formaldehyde. After two washes in DPBS, cells were permeabilized and immersed in blocking solution (DPBS, 0.2% Triton X-100, 3% bovine serum albumin) for 45 min. The anti-Kir6.2 specific antibody (at 2 to 3 μg/ml) served as the primary antibody and was added to the blocking solution for 1 h (22°C). Cells were then washed three times for 10 min each in DPBS containing 0.2% Triton X-100. Donkey anti-rabbit immunoglobulin G fluorescein-conjugated antibody (Chemicon), used as a secondary antibody, was added at a 1/200 dilution to the blocking solution (1 h, 22°C). Following two washes for 10 min with DPBS and 0.2% Triton X-100, cells were mounted on slides by using Poly-Aquamount (Polysciences). Immunostained cells were viewed via a Zeiss LSM 410 confocal microscope coupled to an argon-krypton laser illumination beam (488 nm). Untransfected COS-7 cells and cells that did not incorporate foreign DNA during the transfection procedure served as negative controls.
Patch-clamp recording.
To aid in visualizing transfected cells, green fluorescent protein was added as a reporter gene to the DNA–Opti-MEM mixture at 0.2 μg for each 2 μg of total DNA used. A day after transfection, cells were lifted off the culture dish, using 0.05% trypsin-EDTA to prevent tight attachment to coverslips, prior to electrophysiological measurements, performed about 4 h later. Transfected COS-7 cells, selected by green fluorescence under the microscope, were superfused with 140 mM KCl–1 mM MgCl2–5 mM EGTA–5 mM HEPES-KOH (pH 7.3), and recordings were made at room temperature (20 to 22°C). Fire-polished pipettes, coated with Sylgard (resistance, 8 to 10 MΩ), were filled with 140 mM KCl–1 mM CaCl2–1 mM MgCl2–5 mM HEPES-KOH (pH 7.3) (45). Channel activity was monitored on-line and stored on tape, using a pulse code modulation converter system (VR-10; Instrutech) (43). Data were reproduced, low pass filtered at 4 kHz (−3 dB) by a Bessel filter (Frequency Devices 902), sampled at a rate of 80 μs, and analyzed off-line with BioQuest software (5, 13). Only patches displaying single-channel activity were used for kinetic analysis. It should be pointed, however, that due to overexpression of recombinant channel subunits such patches were rare to obtain compared to patches with multiple-channel activity. For analysis of intraburst channel behavior, closed times that exceeded 2.5 ms were omitted, as previously established for reconstituted Kir6.2-SUR1 channel activity (6). By using this criterion, intraburst closed-time distribution was well fitted by a single exponential. Fitting of closed- and open-time distributions by exponentials was carried out by using minimization of the χ2 criterion with the Nelder-Meed method of deformed polyhedrons (4). Results are expressed as mean ± standard error of the mean, with n referring to the number of experiments used in each analysis.
RESULTS
Kir6.2-specific antibody coimmunoprecipitates Kir6.2 and SUR1.
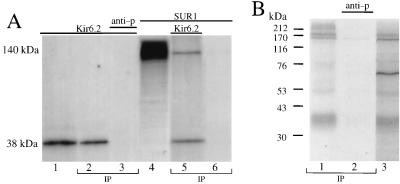
The product of in vitro-translated Kir6.2 migrates on gel electrophoresis consistent with a 38-kDa protein (predicted molecular mass, 43 kDa) (Fig. 1A, lane 1). An amino-terminus-directed anti-Kir6.2 antibody immunoprecipitated Kir6.2 (lane 2); the epitope specificity of this antibody was verified by competition with the corresponding antigenic peptide (lane 3). SUR1 in vitro translated in the absence of microsomes yielded a 140-kDa protein (lane 4). Following coincubation, in vitro-translated Kir6.2 and SUR1 were coimmunoprecipitated by the anti-Kir6.2 antibody (lane 5). No 140-kDa protein was detected in the absence of coincubation with Kir6.2 (lane 6), indicating absence of cross-reactivity. In the presence of microsomes, a 170-kDa isoform of SUR1 was also coimmunoprecipitated with Kir6.2 by the Kir6.2-specific antibody (not shown). Thus, the Kir6.2-specific antibody recognized SUR1 only when SUR1 formed a complex with Kir6.2.
FIG. 1.
Complex formation between Kir6.2 and SUR1. (A) Coimmunoprecipitation of in vitro-translated SUR1 with Kir6.2 by an anti-Kir6.2 antibody. Lane 1, SDS electrophoresis of in vitro-translated Kir6.2, which migrated to 38 kDa. Kir6.2 was immunoprecipitated (IP) by the epitope-specific anti-Kir6.2 antibody in the absence (lane 2) but not in the presence (lane 3) of the corresponding antigenic peptide (anti-p). In vitro-translated SUR1 migrated to 140 kDa (lane 4). When coincubated (30 min), in vitro-translated Kir6.2 and SUR1 were coimmunoprecipitated by the Kir6.2-specific antibody (lane 5). In the absence of Kir6.2, the anti-Kir6.2 antibody failed to immunoprecipitate SUR1 (lane 6). Immunoprecipitation of respective in vitro-translated products was confirmed in three to five additional experiments. (B) Coimmunoprecipitation of SUR1 with Kir6.2 from solubilized membrane proteins of COS cells expressing SUR1 and Kir6.2 and labeled with the radioactive sulfonylurea 125I-azidoglyburide. Lane 1 demonstrates that the epitope-specific anti-Kir6.2 antibody precipitates Kir6.2 (38-kDa band) and coimmunoprecipitates SUR1 (140- to 180-kDa bands). Lane 2 shows that the antigenic peptide (anti-p) prevented immunoprecipitation (IP) of both proteins. Lane 3 shows the pattern of 125I-azidoglyburide-labeled membrane proteins. Molecular masses are indicated to the left of the gels.
In membranes from mammalian COS cells heterologously expressing Kir6.2 and SUR1, the radioactive sulfonylurea 125I-azidoglyburide labeled not only SUR1 but also a number of other proteins, including a protein with a molecular mass of approximately 38 kDa (Fig. 1B, lane 1; see also reference 10). From 125I-azidoglyburide-labeled membranes, the anti-Kir6.2 antibody immunoprecipitated the 38-kDa protein band (1B, lane 3). This immunoprecipitation was blocked by the antigenic peptide, demonstrating that the 38-kDa band was Kir6.2 (lane 2). The only protein that coimmunoprecipitated with Kir6.2 was SUR1 (140- and 170-kDa bands). Since SUR1 does not cross-react with the anti-Kir6.2 antibody (Fig. 1A, lane 6), this finding demonstrates that when coexpressed in mammalian cells, SUR1 and Kir6.2 are strongly physically associated.
Coimmunoprecipitation of the carboxy-terminus-truncated Kir6.2 with SUR1.
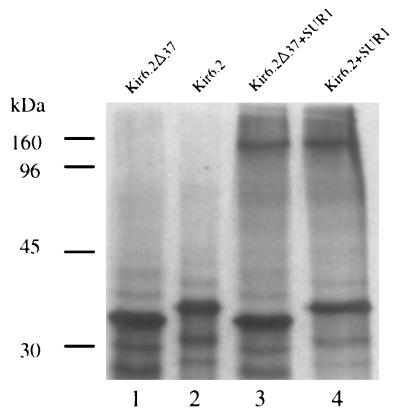
Deletion of the carboxy-terminal 37 amino acids (114 nucleotides) of Kir6.2 produced a truncated Kir6.2 (Kir6.2ΔC37) in vitro-translated product which was recognized by the amino-terminus-directed Kir6.2-specific antibody (Fig. 2, lane 1). Due to its smaller size, Kir6.2ΔC37 migrated faster than full-length Kir6.2 (lane 2). Following coincubation of Kir6.2ΔC37 or Kir6.2 with SUR1 translated product, the Kir6.2 antibody coimmunoprecipitated Kir6.2ΔC37 and SUR1 (lane 3), as well as Kir6.2 with SUR1 (lane 4). We conclude that the carboxy-terminus deletion mutant, Kir6.2ΔC37, can physically associate with SUR1.
FIG. 2.
Complex formation between carboxy-terminus-truncated Kir6.2 and SUR1. In the absence of SUR1, an amino-terminus Kir6.2-specific antibody immunoprecipitated in vitro-translated Kir6.2Δ37 (lane 1) and Kir6.2 (lane 2). When coincubated with equal amounts of either mutant Kir6.2Δ37 or wild-type Kir6.2, SUR1 (lanes 3 and 4) was coimmunoprecipitated by the anti-Kir6.2 antibody. Immunoprecipitation of respective in vitro-translated products was confirmed in three additional experiments. Molecular masses are indicated to the left of the SDS gel.
Coexpression with SUR1 alters the pattern of distribution of Kir6.2.
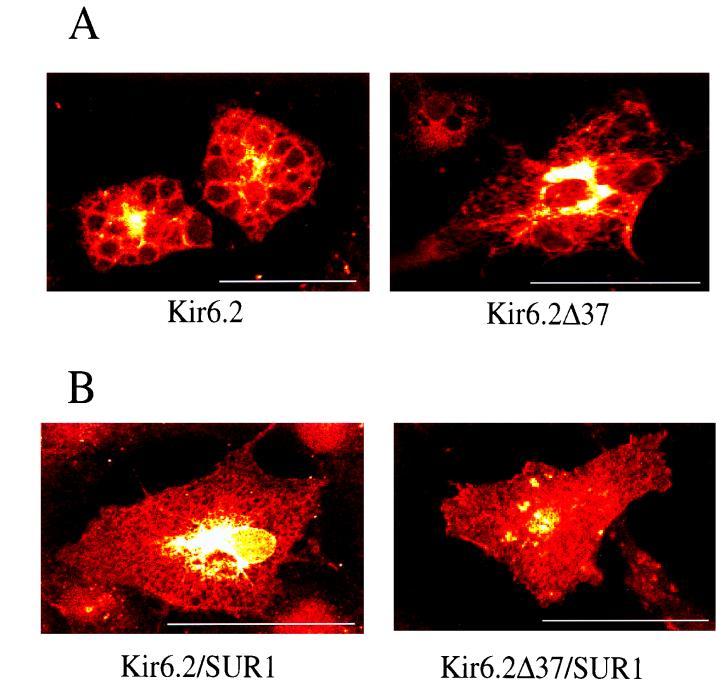
On immunostaining with the Kir6.2-specific antibody (Fig. 3), COS cells transfected with Kir6.2 or Kir6.2ΔC37 displayed a honeycomb pattern. Staining could be blocked by the corresponding antigenic peptide (not shown). After cotransfection of Kir6.2 or Kir6.2ΔC37 with SUR1 into COS cells, immunostaining revealed an evenly distributed pattern of fluorescence (Fig. 3). We detected no fluorescence when SUR1-transfected COS cells were stained with the same antibody (not shown). Thus, the cellular distribution of Kir6.2 appears to depend on coexpression of SUR1 but not on the presence of the carboxy terminus of Kir6.2. A similar honeycomb pattern of protein distribution was seen when COS cells cotransfected with Kir6.2 and SUR1 were incubated (for 16 h) in the presence of brefeldin A (5 μg/ml), a known disrupter of intracellular protein transport between the endoplasmic reticulum and the Golgi apparatus (33). Although we do not yet understand the significance of the honeycomb pattern, this finding suggests that SUR1 is required for the intracellular transport and distribution of Kir6.2.
FIG. 3.
SUR1 alters the immunostaining pattern of Kir6.2- or Kir6.2Δ37-transfected cells. Laser confocal images show COS-7 cells immunostained with the anti-Kir6.2 amino-terminal antibody. (A) Saccular (honeycomb) pattern in cells (n ≥ 8) transfected with Kir6.2 (left) or Kir6.2Δ37 (right) alone. (B) Diffuse pattern in cells (n ≥ 8) cotransfected with Kir6.2-SUR1 (left) or Kir6.2Δ37/SUR1 (right). Bars = 20 μm.
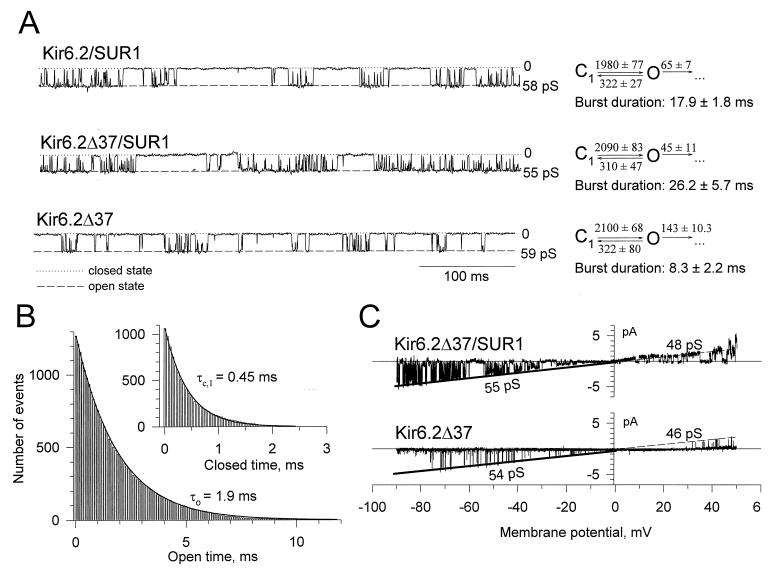
Coexpression of wild-type or carboxy-truncated Kir6.2 with SUR1 produce similar single-channel properties.
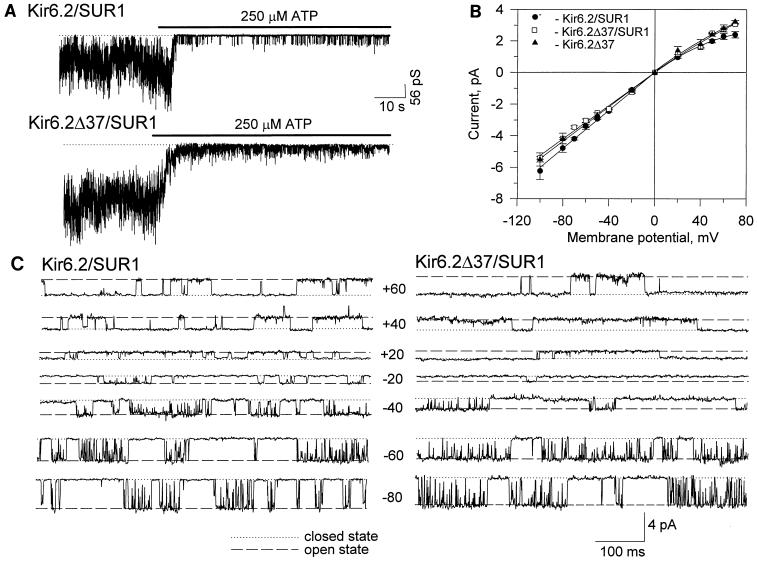
Channel activity, obtained in membrane patches excised from COS cells cotransfected with Kir6.2-SUR1 or Kir6.2ΔC37-SUR1, was inhibited by micromolar concentrations of ATP applied to the intracellular side of the patch (Fig. 4A; n = 10). At a concentration of 250 μM, ATP reduced channel activity by 96% ± 1% (Kir6.2-SUR1; n = 5) and 91% ± 5% (Kir6.2ΔC37-SUR1; n = 5). With symmetrical 140 mM [K+], ionic current produced by Kir6.2-SUR1 and Kir6.2ΔC37-SUR1 reversed at 0 mV. Currents produced by Kir6.2ΔC37 rectified somewhat more weakly than those produced by Kir6.2 (Fig. 4B and C). Fit of the current-voltage relation at negative potentials yielded single-channel conductances of 58.4 ± 2.7 pS (n = 8) for Kir6.2-SUR1 and 54.2 ± 2.8 pS (n = 7) for Kir6.2ΔC37-SUR1 (Fig. 4B). The voltage dependence of the distribution of the open and closed times within a burst was previously used to characterize recombinant and native KATP channel activity (6). As the membrane potential was progressively clamped from −80 to −20 mV, the apparent open-time duration of channel activity increased from 2.1 ± 0.2 to 4.5 ± 0.8 ms (n = 5) for Kir6.2-SUR1 and from 2.2 ± 0.3 to 5.8 ± 0.8 ms (n = 3) for Kir6.2ΔC37-SUR1 (Fig. 4C). In the same voltage range, the mean closed time decreased from 0.56 ± 0.05 to 0.37 ± 0.03 ms (n = 5) for Kir6.2-SUR1 and from 0.46 ± 0.01 to 0.37 ± 0.01 ms (n = 3) for Kir6.2ΔC37-SUR1 (Fig. 4C). As the membrane potential was clamped from +20 to +60 mV, the apparent mean open time decreased from 12.1 ± 2.4 to 7.4 ± 0.2 ms (n = 5) for Kir6.2-SUR1 and from 10.9 ± 3.1 to 6.4 ± 1.7 ms (n = 3) for Kir6.2ΔC37-SUR1 (Fig. 4C). In this voltage range, the mean closed time increased from 0.4 ± 0.1 to 1.7 ± 0.2 ms (n = 5) for Kir6.2-SUR1 and from 0.4 ± 0.1 to 0.6 ± 0.2 ms (n = 3) for Kir6.2ΔC37-SUR1 (Fig. 4C). Thus, heterologous expression of Kir6.2-SUR1 and Kir6.2ΔC37-SUR1 produced similar single-channel conductances, with similar voltage dependence of the intraburst open and closed times defining channel activity.
FIG. 4.
Kir6.2-SUR1 and Kir6.2ΔC37-SUR1 channel activity. Channel activity was recorded from inside-out membrane patches excised from COS-7 cells transfected with Kir6.2-SUR1 or Kir6.2ΔC37-SUR1. (A) Both Kir6.2-SUR1 (upper trace) and Kir6.2ΔC37-SUR1 (lower trace) channel activities were inhibited by ATP (250 μM). Dotted lines correspond to zero-current level. The holding membrane potential was −60 mV. (B) Current-voltage relationships for Kir6.2-SUR1 and Kir6.2ΔC37-SUR1. For comparison, the current-voltage relationship for Kir6.2ΔC37 expressed alone is superimposed. (C) Portions of representative single channel records of Kir6.2-SUR1 (left) and Kir6.2DC37-SUR1 (right) obtained at different membrane potentials (indicated in millivolts between traces).
Kir6.2 responsible for intraburst kinetic properties in the absence of SUR1.
In contrast to wild-type Kir6.2, expression of Kir6.2ΔC37 alone produced channel activity in the absence of SUR1 (see also reference 51). To define which channel subunit is responsible for specific single-channel and kinetic properties of recombinant KATP channels, the behavior of Kir6.2-SUR1 and Kir6.2ΔC37-SUR1 was compared to that of Kir6.2ΔC37. The current-voltage relationship for Kir6.2ΔC37 was not distinguishable from that obtained for Kir6.2-SUR1 and Kir6.2ΔC37-SUR1 (Fig. 4B). The slope of the voltage-current relationship for Kir6.2ΔC37, calculated at negative holding potentials by linear regression, was 57.6 ± 2.4 pS (n = 5) (Fig. 4B) and was not significantly different from values obtained for Kir6.2-SUR1 and Kir6.2ΔC37-SUR1 (Fig. 4B). Kir6.2ΔC37 produced a slightly weaker inward rectification compared to Kir6.2-SUR1 (Fig. 4B). At −60 mV, single-channel records of Kir6.2ΔC37 displayed kinetics similar to that of Kir6.2-SUR1 and Kir6.2ΔC37-SUR1 (Fig. 5A). Analysis of transitions between open and closed times within a burst of channel activity revealed that all three channel activities had similar intraburst kinetic properties (Fig. 5A and B). Specifically, the distribution of open and closed times within a burst for Kir6.2ΔC37 (Fig. 5B) was not significantly different from that obtained for Kir6.2-SUR1 (6) or Kir6.2ΔC37-SUR1 (not illustrated). Using times which define distributions of open and closed intervals, we calculated the rates of transitions between closed and open states within a burst for Kir6.2-SUR1, Kir6.2ΔC37-SUR1, and Kir6.2ΔC37 channel activity (Fig. 5A) based on the equations
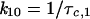 |
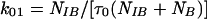 |
where k10 is the rate of transition from the closed to the open state, k01 is the rate of backward transition, τc,1 is the characteristic time for the closed-time distribution, τ0 is the characteristic time for the open-time distribution, NIB is the number of events within a burst, and NB is the number of bursts. Transition rates calculated for Kir6.2-SUR1, Kir6.2ΔC37-SUR1, and Kir6.2ΔC37 were not significantly different from each other (Fig. 5A), indicating that intraburst channel properties are a property of Kir6.2. However, mean burst duration for Kir6.2ΔC37 was 8.3 ± 2.2 ms (n = 5), significantly shorter than that for either Kir6.2-SUR1 (17.9 ± 1.8 ms; n = 5) or Kir6.2ΔC37-SUR1 (26.2 ± 5.7 ms; n = 5). The rate leading away from intraburst transitions was calculated (16, 37) as
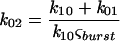 |
where ςburst is the mean burst duration obtained from single-channel tracings and k02 is the rate of transition associated with termination of a burst. This rate was found to be significantly different for Kir6.2ΔC37 (143 ± 10 s−1; n = 5) than for Kir6.2-SUR1 (65 ± 7 s−1; n = 5) or Kir6.2ΔC37-SUR1 (45 ± 11 s−1; n = 5) (Fig. 5A). Such differences in burst duration appeared to exist throughout the range of holding membrane potentials (from −80 to +50 mV) (Fig. 5C). Thus, in contrast to intraburst channel behavior defined by Kir6.2 alone, burst duration was affected by assembly with SUR1.
FIG. 5.
(A) Single-current records obtained in inside-out patches excised from COS-7 cells transfected with Kir6.2-SUR1 (upper trace), Kir6.2ΔC37-SUR1 (middle trace), or Kir6.2ΔC37 alone (lower trace). The holding membrane potential was −60 mV. Corresponding kinetic schemes with calculated rates of transitions (per second) are provided for each record. C1 represents the closed state within a burst, and O represents the open state, whereas transitions between states are defined by forward and backward rates. Termination of a burst of channel activity is defined by transitions leading away from the O state toward an additional closed state(s). (B) Intraburst open-time (and closed-time [inset]) distribution obtained for Kir6.2ΔC37 channel activity based on burst analysis and fitted by single exponentials. Results of fitting are represented by solid lines with corresponding characteristic open (τ0) and closed (τc,1) times. The holding membrane potential was −60 mV. (C) Current-voltage relationships for Kir6.2ΔC37-SUR1 (upper trace) and Kir6.2ΔC37 (lower trace) were obtained from a voltage-ramp protocol from −90 to +50 mV (10-s duration). Lines correspond to single-channel conductance.
DISCUSSION
We have shown that (i) full-length or carboxy-terminus-truncated Kir6.2 physically associates with SUR1, (ii) the cellular distribution of Kir6.2 is dependent on coexpression with SUR1, and (iii) burst duration of channel activity is dependent on association between Kir6.2 and SUR1. These findings provide direct evidence for physical association between subunits of the KATP channel and suggest that the carboxy terminus of Kir6.2 is not required for complex formation with SUR1. Furthermore, these results support the role of Kir6.2 as a pore-forming subunit responsible for gating within a burst, while assembly with SUR1 affects overall burst duration of KATP channel activity.
Evidence that a member of the Kir6.0 subfamily of inwardly rectifying K+ channels (11, 27, 35) could associate with a member of the SUR subfamily of ABC proteins (19) was first obtained through functional studies (22). Coexpression of recombinant Kir6.2 and SUR1, in heterologous expression systems, reconstituted pancreatic KATP channel activity (10, 17, 22, 38, 48). Loss of functional KATP channels was found in pancreatic β cells from patients with familial persistent hyperinsulinemic hypoglycemia, which usually carry mutations in the SUR1 gene (9, 12, 28, 36, 46). Coexpression of other Kir6.0 and SUR isoforms reconstituted cardiac and vascular KATP channel-related phenotypes (23, 26, 54). Chromosomal clustering of Kir6.2 and SUR1 genes also supports the notion of their related functions (9, 22, 40). More recent evidence for association between Kir6.2 and SUR1 was obtained by photoaffinity colabeling of Kir6.2 with a radioactive sulfonylurea, indicating close proximity between SUR1 with Kir6.2 in pancreatic cellular membranes (10). Thus, in addition to previously established functional coupling and chromosomal and membrane colocalization, our demonstration of complex formation between Kir6.2 and SUR1 provides direct proof for physical association between channel subunits which constitute the pancreatic KATP channel. In this regard, SUR proteins are unique, since no other member of the ABC superfamily has been reported to functionally assemble with a K+ channel, although similar associations might occur between the ABC family cystic fibrosis transmembrane regulator and other channels (3, 19).
In this study, complex formation was determined by coimmunoprecipitation of either in vivo- or in vitro-translated Kir6.2 and SUR1. Thus, Kir6.2 and SUR1 when heterologously expressed physically associated within cellular membranes, although formation of a complex also occurred in the absence of plasmalemma or microsomes. Recently, it has been reported that the highly glycosylated form of SUR1 (170 kDa) is part of the KATP channel complex and that coexpression of Kir6.2 facilitates this glycosylation state (10). In the case of other inward rectifying K+ channels, glycosylation of channel subunits may promote heteromultimer formation (30, 53). We show here that glycosylation is not a prerequisite for interaction with Kir6.2 and that the unglycosylated form of SUR1 can form a stable complex with Kir6.2. This, however, does not exclude the possibility that higher glycosylated forms of SUR1 possess higher affinity for Kir6.2 or are otherwise functionally important. Our study indicates that sequence motifs required for protein-protein interaction are inherent to Kir6.2 and SUR1 sequences and suggests that no cofactor is required for channel subunit assembly.
Previously, coimmunoprecipitation was established as a reliable approach to determine heteromultimeric formation among structurally related K+-selective inwardly rectifying channel proteins, such as Kir3.1 and Kir3.4 (30). Coimmunoprecipitation has also revealed complex formation between a K+ channel and nonhomologous proteins within, or even outside, the K+ channel family (8, 20, 21, 25, 39). In this regard, association between Kir6.2 and SUR1 indicates that a K+ inwardly rectifying channel protein can create a complex with a structurally unrelated protein of the ABC family. The present findings also indicate a role for SUR1 in regulating the cellular distribution of Kir6.2, in accord with the regulation of intracellular protein transport by proteins structurally related to the ABC family (33), as well as with the requirement for chaperonin-like proteins for proper membrane localization of K+ channels (29).
Physical interaction among subunits forming K+ channels is complex (15, 30, 32, 50), with an intact carboxy terminus required for certain inwardly rectifying K+ channels (47, 53). In our case, truncation of 37 amino acids from the carboxy terminus of Kir6.2 did not prevent complex formation with SUR1, suggesting that other domains within Kir6.2 may serve as molecular determinants for subunit assembly. The absence of the carboxy terminus of Kir6.2 also did not alter the pattern of distribution of channel subunits within a cell. Although expendable for association, deletion of the carboxy terminus led to Kir6.2 channel activity in the absence of SUR1, as previously obtained for Kir6.2 mutants lacking 26 or 36 amino acids from the carboxy terminus (51). That study concluded that the carboxy terminus of Kir6.2 inhibits independent channel activity, which could be restored following coexpression with SUR1 (51). In principle, SUR1 could rescue Kir6.2 channel activity by binding directly to the carboxy terminus of Kir6.2, thereby masking the inhibitory property of this domain. This appears not to be the case since carboxy-terminus-truncated Kir6.2 retained the ability to complex with SUR1. Moreover, carboxy-terminus-truncated Kir6.2 complexed with SUR1 retained the ATP sensitivity and voltage dependence, similar to native KATP channels (6, 7, 55).
The present study provides further evidence for the individual contribution of Kir6.2 and SUR1 to the behavior of the channel. The single-channel conductance of Kir6.2Δ37 was similar to that of Kir6.2-SUR1 or Kir6.2Δ37-SUR1, supporting the notion that Kir6.2 serves as the pore-forming channel subunit (40, 51). However, while Kir6.2Δ37 defined the intraburst kinetic behavior (6, 23), SUR1 conferred the overall characteristic burst duration (23). In particular, prolongation of burst duration obtained by coexpression of Kir6.2 with SUR1 explains larger whole-cell currents previously recorded in cells expressing both subunits compared to currents produced by the carboxy-terminus-truncated Kir6.2 alone (51).
In summary, this study demonstrates physical association between subunits of the KATP channel and establishes an assay system with which to further study the structural determinants of subunit interaction between an ABC protein and an inwardly rectifying K+ channel. With respect to the role of constitutive subunits in defining KATP channel behavior, single-channel analysis suggests that Kir6.2 serves as the pore-forming region of the channel, while assembly with SUR1 modulates the overall channel behavior.
ACKNOWLEDGMENTS
This work was supported by the Bruce and Ruth Rappaport Program in Vascular Biology and Gene Delivery, the Miami Heart Research Institute, the American Heart Association, the George M. Eisenberg Cardiovascular Research Fund, the John Tainsh Heart Research Fund, and the Harrington Professorship Fund (all to A.T.) and by a grant from the NIH (D.E.C.). A.J.C. was supported by NIH grant R25GM55252.
We thank S. Seino (Chiba University) for the gift of the Kir6.2 clone; we thank J. Bryan and L. Aguilar-Bryan (Baylor College of Medicine) for the gift of the SUR1 clone and for generously providing membranes labeled with radioactive sulfonylurea. We also acknowledge the Rappaport Program Vector Core (Mayo Foundation) for plasmid purification.
REFERENCES
- 1.Aguilar-Bryan L, Bryan J. ATP-sensitive potassium channels, sulfonylurea receptors and persistent hyperinsulinemic hypoglycemia of infancy. Diabetes Rev. 1996;4:336–346. [Google Scholar]
- 2.Aguilar-Bryan L, Nichols C G, Wechsler S W, Clement J P, Boyd A E, Gonzalez G, Herrera-Sosa H, Nguy K, Bryan J, Nelson D A. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 3.al-Awqati Q. Regulation of ion channels by ABC transporters that secrete ATP. Science. 1995;269:805–806. doi: 10.1126/science.7543697. [DOI] [PubMed] [Google Scholar]
- 4.Alekseev A E, Markevich N I, Korystova A F, Terzic A, Kokoz Y M. Comparative analysis of the kinetic characteristics of L-type calcium channels in cardiac cells of hibernators. Biophys J. 1996;70:786–797. doi: 10.1016/S0006-3495(96)79618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alekseev A E, Gomez L A, Aleksandrova L A, Brady P A, Terzic A. Opening of cardiac sarcolemmal KATP channels by dinitrophenol separate from metabolic inhibition. J Membr Biol. 1997;157:203–214. doi: 10.1007/s002329900229. [DOI] [PubMed] [Google Scholar]
- 6.Alekseev A E, Kennedy M E, Navarro B, Terzic A. Burst kinetics of co-expressed Kir6.2/SUR1 clones: comparison of recombinant with native ATP-sensitive K+ channel behavior. J Membr Biol. 1997;159:161–168. doi: 10.1007/s002329900279. [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft S J, Ashcroft F M. Properties and functions of ATP-sensitive K-channels. Cell Signalling. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- 8.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and IsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 9.Bryan J, Aguilar-Bryan L. The ABCs of ATP-sensitive potassium channels—more pieces of the puzzle. Curr Opin Cell Biol. 1997;9:553–559. doi: 10.1016/s0955-0674(97)80033-6. [DOI] [PubMed] [Google Scholar]
- 10.Clement J P, Kunjilwar K, Gonzalez G, Schwanstecher M, Panten U, Aguilar-Bryan L, Bryan J. Association and stoichiometry of K-ATP channel subunits. Neuron. 1997;18:827–838. doi: 10.1016/s0896-6273(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 11.Doupnik C A, Davidson N, Lester H A. The inward rectifier potassium channel family. Curr Opin Neurobiol. 1995;5:268–277. doi: 10.1016/0959-4388(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 12.Dunne M J, Kane C, Shepherd R M, Sanchez J A, James R F, Johnson P R, Aynsley-Green A, Lu S, Clement J P, Lindley K J, Aguilar-Bryan L. Familial persistent hyperinsulinemic hypoglycemia of infancy and mutations in the sulfonylurea receptor. N Engl J Med. 1997;336:703–706. doi: 10.1056/NEJM199703063361005. [DOI] [PubMed] [Google Scholar]
- 13.Elvir-Mairena J R, Jovanovic A, Gomez L A, Alekseev A E, Terzic A. Reversal of the ATP-liganded state of ATP-sensitive K+ channels by adenylate kinase activity. J Biol Chem. 1996;271:31903–31908. doi: 10.1074/jbc.271.50.31903. [DOI] [PubMed] [Google Scholar]
- 14.Findlay I. Interactive regulation of the ATP-sensitive potassium channel of cardiac muscle. J Cardiovasc Pharmacol. 1994;24:S6–S11. [PubMed] [Google Scholar]
- 15.Fink M, Duprat F, Heurteaux C, Lesage F, Romey G, Barhanin J, Lazdunski M. Dominant negative chimeras provide evidence for homo and heteromultimeric assembly of inward rectifier K+ channel proteins via their N-terminal end. FEBS Lett. 1996;378:64–68. doi: 10.1016/0014-5793(95)01388-1. [DOI] [PubMed] [Google Scholar]
- 16.Gillis K D, Gee W M, Hammoud A, McDaniel M L, Falke L C, Misler S. Effects of sulfonamides on a metabolite-regulated ATPi-sensitive K+ channel in rat pancreatic B-cells. Am J Physiol. 1989;257:C1119–C1127. doi: 10.1152/ajpcell.1989.257.6.C1119. [DOI] [PubMed] [Google Scholar]
- 17.Gribble F M, Tucker S J, Ashcroft F M. The essential role of the walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillory R J. Design, implementation and pitfalls of photoaffinity labeling experiments in in vitro preparations. General principles. Pharmacol Ther. 1989;41:1–25. doi: 10.1016/0163-7258(89)90100-9. [DOI] [PubMed] [Google Scholar]
- 19.Higgins C F. The ABC of channel regulation. Cell. 1995;82:693–696. doi: 10.1016/0092-8674(95)90465-4. [DOI] [PubMed] [Google Scholar]
- 20.Holmes T C, Fadool D A, Ren R, Levitan I B. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science. 1996;274:2089–2091. doi: 10.1126/science.274.5295.2089. [DOI] [PubMed] [Google Scholar]
- 21.Horio Y, Hibino H, Inanobe A, Yamada M, Ishii M, Tada Y, Satoh E, Hata Y, Takai Y, Kurachi Y. Clustering and enhanced activity of an inwardly rectifying potassium channel, Kir4.1, by an anchoring protein, PSD-95/SAP90. J Biol Chem. 1997;272:12885–12888. doi: 10.1074/jbc.272.20.12885. [DOI] [PubMed] [Google Scholar]
- 22.Inagaki N, Gonoi T, Clement J P, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 23.Inagaki N, Gonoi T, Clement J P, Wang C Z, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 24.Inagaki N, Gonoi T, Seino S. Subunit stoichiometry of the pancreatic beta-cell ATP-sensitive K+ channel. FEBS Lett. 1997;409:232–236. doi: 10.1016/s0014-5793(97)00488-2. [DOI] [PubMed] [Google Scholar]
- 25.Inanobe A, Ito H, Ito M, Hosoya Y, Kurachi Y. Immunological and physical characterization of the brain G protein-gated muscarinic potassium channel. Biochem Biophys Res Commun. 1995;217:1238–1244. doi: 10.1006/bbrc.1995.2901. [DOI] [PubMed] [Google Scholar]
- 26.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 27.Jan L Y, Jan Y N. Cloned potassium channels from eukaryotes and prokaryotes. Annu Rev Neurosci. 1997;20:91–123. doi: 10.1146/annurev.neuro.20.1.91. [DOI] [PubMed] [Google Scholar]
- 28.Kane C, Shepherd R M, Squires P E, Johnson P R, James R F, Milla P J, Aynsley-Green A, Lindley K J, Dunne M J. Loss of functional KATP channels in pancreatic beta-cells causes persistent hyperinsulinemic hypoglycemia of infancy. Nat Med. 1996;2:1344–1347. doi: 10.1038/nm1296-1344. [DOI] [PubMed] [Google Scholar]
- 29.Kennedy M E, Nemec J, Clapham D E. Localization and interaction of epitope-tagged GIRK1 and CIR inward rectifier K+ channel subunits. Neuropharmacology. 1996;35:831–839. doi: 10.1016/0028-3908(96)00132-3. [DOI] [PubMed] [Google Scholar]
- 30.Krapivinsky G, Gordon E A, Wickman K, Velimirovic B, Krapivinsky L, Clapham D E. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K+-channel proteins. Nature. 1995;374:135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 31.Lazdunski M. ATP-sensitive potassium channels: an overview. J Cardiovasc Pharmacol. 1994;24:S1–S5. [PubMed] [Google Scholar]
- 32.McDonald T V, Yu Z, Ming Z, Palma E, Meyers M B, Wang K W, Goldstein S A N, Fishman G I. A minK-HERG complex regulates the cardiac potassium current Ikr. Nature. 1997;388:289–292. doi: 10.1038/40882. [DOI] [PubMed] [Google Scholar]
- 33.Nagao K, Taguchi Y, Arioka M, Kadokura H, Takatsuki A, Yoda K, Yamasaki M. bfr1+, a novel gene of Schizosaccharomyces pombe which confers brefeldin A resistance, is structurally related to the ATP-binding cassette superfamily. J Bacteriol. 1995;177:1536–1543. doi: 10.1128/jb.177.6.1536-1543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols C G, Lederer W J. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–H1686. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 35.Nichols C G, Lopatin A N. Inward rectifier potassium channels. Annu Rev Physiol. 1997;59:171–191. doi: 10.1146/annurev.physiol.59.1.171. [DOI] [PubMed] [Google Scholar]
- 36.Nichols C G, Shyng S L, Nestorowicz A, Glaser B, Clement J P, Gonzalez G, Aguilar-Bryan L, Permutt M A, Bryan J. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–1787. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- 37.Sakmann B, Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakura H, Ammala C, Smith P A, Gribble F M, Ashcroft F M. Cloning and functional expression of the cDNA encoding a novel ATP-sensitive potassium channel subunit expressed in pancreatic beta-cells, brain, heart and skeletal muscle. FEBS Lett. 1995;377:338–344. doi: 10.1016/0014-5793(95)01369-5. [DOI] [PubMed] [Google Scholar]
- 39.Sanguinetti M C, Curran M E, Zou A, Shen J, Spector P S, Atkinson D L, Keating M T. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 40.Seino S, Inagaki N, Namba N, Gonoi T. Molecular biology of the β-cell ATP-sensitive K+ channel. Diabetes Rev. 1996;4:177–190. [Google Scholar]
- 41.Shyng S L, Ferrigni T, Nichols C G. Control of rectification and gating of cloned K-ATP channels by the Kir6.2 subunit. J Gen Physiol. 1997;110:141–153. doi: 10.1085/jgp.110.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suzuki M, Fujikura K, Inagaki N, Seino S, Takata K. Localization of the ATP-sensitive K+ channel subunit Kir6.2 in mouse pancreas. Diabetes. 1997;46:1440–1444. doi: 10.2337/diab.46.9.1440. [DOI] [PubMed] [Google Scholar]
- 43.Terzic A, Findlay I, Hosoya Y, Kurachi Y. Dualistic behavior of ATP-sensitive K+ channels toward intracellular nucleoside diphosphates. Neuron. 1994;12:1049–1058. doi: 10.1016/0896-6273(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 44.Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269:C525–C545. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- 45.Terzic A, Kurachi Y. Actin microfilament disrupters enhance KATP channel opening in patches from guinea-pig cardiomyocytes. J Physiol. 1996;492:395–404. doi: 10.1113/jphysiol.1996.sp021316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas P M, Cote G J, Wohllk N, Haddad B, Mathew P M, Rabl W, Aguilar-Bryan L, Gagel R F, Bryan J. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- 47.Tinker A, Jan Y N, Jan L Y. Regions responsible for the assembly of inwardly rectifying potassium channels. Cell. 1996;87:857–868. doi: 10.1016/s0092-8674(00)81993-5. [DOI] [PubMed] [Google Scholar]
- 48.Tokuyama Y, Fan Z, Furuta H, Makielski J C, Polonsky K S, Bell G I, Yano H. Rat inwardly rectifying potassium channel Kir6.2: cloning electrophysiological characterization, and decreased expression in pancreatic islets of male Zucker diabetic fatty rats. Biochem Biophys Res Commun. 1996;220:532–538. doi: 10.1006/bbrc.1996.0439. [DOI] [PubMed] [Google Scholar]
- 49.Trapp S, Tucker S J, Ashcroft F M. Activation and inhibition of K-ATP currents by guanine nucleotides is mediated by different channel subunits. Proc Natl Acad Sci USA. 1997;94:8872–8877. doi: 10.1073/pnas.94.16.8872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucker S J, Bond C T, Herson P, Pessia M, Adelman J P. Inhibitory interactions between two inward rectifier K+ channel subunits mediated by the transmembrane domains. J Biol Chem. 1996;271:5866–5870. doi: 10.1074/jbc.271.10.5866. [DOI] [PubMed] [Google Scholar]
- 51.Tucker S J, Gribble F M, Zhao C, Trapp S, Ashcroft F M. Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature. 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 52.Ueda K, Inagaki N, Seino S. MgADP antagonism to Mg2+-independent ATP binding of the sulfonylurea receptor SUR1. J Biol Chem. 1997;272:22983–22986. doi: 10.1074/jbc.272.37.22983. [DOI] [PubMed] [Google Scholar]
- 53.Woodward R, Steven E B, Murrell-Lagnado R D. Molecular determinants for assembly of G-protein-activated inwardly rectifying K+ channels. J Biol Chem. 1997;272:10823–10830. doi: 10.1074/jbc.272.16.10823. [DOI] [PubMed] [Google Scholar]
- 54.Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499:715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zilberter Y, Burnashev N, Papin A, Portnov V, Khodorov B. Gating kinetics of ATP-sensitive single potassium channels in myocardial cells depends on electromotive force. Pflugers Arch. 1988;411:584–589. doi: 10.1007/BF00582382. [DOI] [PubMed] [Google Scholar]