Abstract
Tumor-associated macrophages (TAMs) are the major component of the tumor microenvironment (TME), where they sustain tumor progression and or-tumor immunity. Due to their plasticity, macrophages can exhibit anti- or pro-tumor functions through the expression of different gene sets leading to distinct macrophage phenotypes: M1-like or pro-inflammatory and M2-like or anti-inflammatory. NF-κB transcription factors are central regulators of TAMs in cancers, where they often drive macrophage polarization toward an M2-like phenotype. Therefore, the NF-κB pathway is an attractive therapeutic target for cancer immunotherapy in a wide range of human tumors. Hence, targeting NF-κB pathway in the myeloid compartment is a potential clinical strategy to overcome microenvironment-induced immunosuppression and increase anti-tumor immunity. In this review, we discuss the role of NF-κB as a key driver of macrophage functions in tumors as well as the principal strategies to overcome tumor immunosuppression by targeting the NF-κB pathway.
Keywords: NF-κB, tumor-associated macrophages (TAMs), tumor microenvironment (TME)
1. Introduction
Macrophages represent one of the major lines of host defense in the innate immune system, able to kill pathogens and induce inflammatory response [1]. They control tissue repair and homeostasis via the extracellular matrix remodeling and scavenging of cellular debris and apoptotic cells [2]. Macrophages are highly versatile and can exert several different functions by changing their transcriptional profile based on the anatomic location and physiologic or pathophysiologic conditions [1,3].
In the tumor microenvironment (TME), the complex interactions between tumor cells, immune cells, endothelial cells, fibroblasts, and the extracellular matrix shapes tumor clinical behavior through the release and uptake of several angiogenic, mitogenic, immunosuppressive, or pro-migratory factors that may either inhibit or stimulate tumor progression [4]. Chemoattractants, produced by malignant cells and the stromal tumor compartment, such as C-C Motif Chemokine Ligand 2 (CCL2), vascular endothelial growth factor (VEGF), CXCL12 (SDF1) and colony-stimulating factor-1 (CSF-1), recruit monocytes from the bloodstream that migrate into tumor site [5,6]. Different studies showed that the tissue-resident macrophages (MTR) (e.g., microglia, Kupffer cells, alveolar macrophages) are responsible for regulating tissues homeostasis and inflammation, and during tumorigenesis, they developed progressively into pro-tumoral phenotype within the TME in presence of various molecules such as interleukin-10 (IL-10) [7,8,9]. Macrophages recruited in the tumor site, called tumor-associated macrophages (TAMs), are the major population of leukocytes in the TME, and display a high phenotype plasticity [10]. TAMs acquire a distinct phenotype and activation status and can exert anti- or pro-tumor activities through the expression of different functional programs [11].
There is a common agreement that macrophages can polarize into two different subtypes, the so-called M1-like and M2-like macrophages according to different stimuli. The M1 macrophages are “classically” activated by microbial products or soluble cytokines (e.g., lipopolysaccharide (LPS), tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interferon-γ (IFN-γ) produced by activated CD4+ T helper (Th) 1 cells, CD8+ T cytotoxic cells, and natural killer (NK) cells, and show specific surface markers such as toll-like receptor(TLR)-2 (TLR-2), TLR-4, CD80, CD86, inducible nitric oxide synthase (iNOS), and major histocompatibility complex-II (MHC-II) [12]. M1 macrophages secrete nitric oxide (NO), reactive oxygen species (ROS) and various cytokines and chemokines (e.g., TNF-α, interleukin(IL)-1α (IL-1α), IL-1β, IL-6, IL-12, C-X-C motif chemokine ligand (CXCL) 9 (CXCL9), and CXCL10) which trigger the activity of NK and cytotoxic T cells [13]. In addition, these secreted factors activate unpolarized macrophages promoting the M1 state, in a positive feedback loop [14].
The M2 macrophages are “alternatively” activated by anti-inflammatory molecules such as glucocorticoid hormones and Th2 cytokines (interleukin (IL)-4 (IL–4), IL-10, and IL-13), as well as apoptotic cells and immune complexes [15]. As for M1, specific surface markers such as CD206, CD163, CD209, mannitol receptor, Ym1/2 and FIZZ1 characterize M2 macrophages. They also express anti-inflammatory cytokines such as IL-10, transforming growth factor-β (TGF-β), and chemokines (e.g., chemokine (C-C motif) Ligand(CCL) 1 (CCL1), CCL17, CCL22, CCL24) that contribute to dampen the inflammatory response and maintain macrophages into M2 phenotype by acting autocrinally [16,17].
Given the high plasticity of macrophages, the M2 phenotype has been subdivided into M2a, M2b, M2c and M2d subtypes, that differ both for stimuli and exerted functions. M2a macrophages, activated by IL-4 and IL-13, express higher levels of IL-10, TGF-β, CCL17 and CCL22 and promote cell growth, tissue repair and endocytic activity. Immune complexes, TLR ligands and interleukin-1 receptor (IL-1R) agonists activate M2b macrophages that regulate the intensity of inflammatory response and immune reaction via releasing TNF-α, IL-1β, IL-6 and IL-10. M2c macrophages, induced by IL-10, suppress immune responses and are responsible for tissue remodeling. Finally, M2d macrophages release IL-10 and VEGF and thus promote tumor progression and angiogenesis [10,17,18,19,20].
Recently the transcriptomic and proteomic analyzes of TAMs have identified different macrophage subpopulations in the TME, that go beyond the simple dichotomy “M1-M2” system, highlighting the presence of a more complex population of macrophages, highly plastic and heterogeneous [13,14]. Hence, several pieces of evidence indicated that microRNAs (miRNAs) [21], non-coding RNAs [22], extracellular vesicles (EVs) [23,24], and epigenetic modification [25] contribute to shape TAM phenotype in the TME, suggesting that the M1/M2 model has numerous limitations [13]. Although the current classification of macrophages is challenging as several heterogeneous subsets have been identified within the TME, here we use the common M1 and M2 classification to explain the role of NF-κB in TAM polarization.
TAMs respond to the local signals provided by TME depending on tumor type and stage [26,27,28]. Although in the TME M2 macrophages are the most abundant population, M1 macrophages can be present in the TME during pathological conditions [29,30].
The balance between M1/M2 phenotypes and the switch between these two extreme borders of macrophage polarization is finely regulated by an intricate network of receptors and signaling pathways (e.g., JAK/STATs, MAPK, PI3K/AKT, NOTCH and NF-κB) [31,32].
Among these crucial signaling pathways, NF-κB is a key regulator of macrophage function in cancers, tipping the balance between the immunosuppressive, pro-tumoral activity and the pro-inflammatory, protective functions of TAMs [15]. Given the central role during tumorigenesis, TAMs represent a potential target for cancer treatment. NF-κB family of transcription factors plays a critical role in most physiological and pathological processes such as cell proliferation, survival, apoptosis [33,34], inflammation [35], immune response [36,37] tumor progression, invasion, metastasis, and angiogenesis [38]. NF-κB is also responsible for the activation and differentiation of innate immune cells and T cells [39] and the regulation of macrophage gene expression patterns [15].
The NF-κB family is composed of five structurally related DNA-binding subunits, consisting of the homo- and heterodimers of p50, p52, c-Rel, RelA (p65), RelB and c-Rel, which control transcription of target genes by binding specific DNA elements, called κB enhancers [40,41,42]. In resting cells, the NF-κB complexes are retained into the cytoplasm, bound to the inhibitory proteins of IκB family [43]. A wide range of stimuli, including microbial and viral infection products, stress, pro-inflammatory cytokines, and antigen receptors can trigger NF-κB activation [44] leading to the phosphorylation of IκBs by the IκB kinase (IKK) complex. In turn, IκBs trigger the polyubiquitination and proteolysis of the IκB inhibitors, leading to the translocation of the NF-κB complexes in the nucleus, where they drive the transcription of several target genes [45]. Based on stimuli, NF-κB activation involves different signaling pathways including the canonical, the non-canonical and the atypical pathways [39].
The canonical pathway is activated by microbial products, IL-1β, damage-associated molecular patterns (DAMPs), pattern-recognition receptors (PRRs), T-cell receptor (TCR) and B-cell receptor (BCR) [46,47] and leads to the phosphorylation of the IκB-family members (IκBα, IκBβ and IκBɛ) by the IκB kinase (IKK), complex, which is composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ (also named NF-κB essential modulator, or NEMO). The IκB kinase IKKβ phosphorylates the IκB inhibitor molecules via an IKKγ/NEMO mechanism leading to their proteasomal degradation, via a ubiquitin-proteasome system. The active NF-κB dimers, predominantly the heterodimer p50/p65, translocate to the nucleus and induce the expression of several target genes [40,48].
The non-canonical pathway responds to different stimuli, such as LTβR, BAFFR, CD40 (belonging to TNFR superfamily members) and RANK, and activates a different response signal. The non-canonical pathway relies on NF-κB-inducing kinase (NIK) for the phosphorylation of IKKα and the subsequent processing of the NF-κB2 precursor protein (p100) that leads to the release of mature NF-κB2/p52-RelB heterodimers. The NF-κB2/p52-RelB complex translocates to the nucleus and regulates the transcription of non-canonical NF-κB target genes [49].
The atypical NF-κB activation pathway is triggered by factors involved in the ageing process, such as endoplasmic stress response, oxidative stress, mitochondrial dysfunction, and DNA damage. In the atypical pathway, NF-κB induces the transcription of pro-survival genes and activates genes responsible of ROS scavenging and inhibition of calcium release from the ER, to protect organelles from stress [50].
In this review, we will discuss the role of NF-κB in TAMs and how targeting the NF-κB pathway could be a promising approach to overcome tumor immunosuppression.
2. The NF-κB Pathway in TAMs
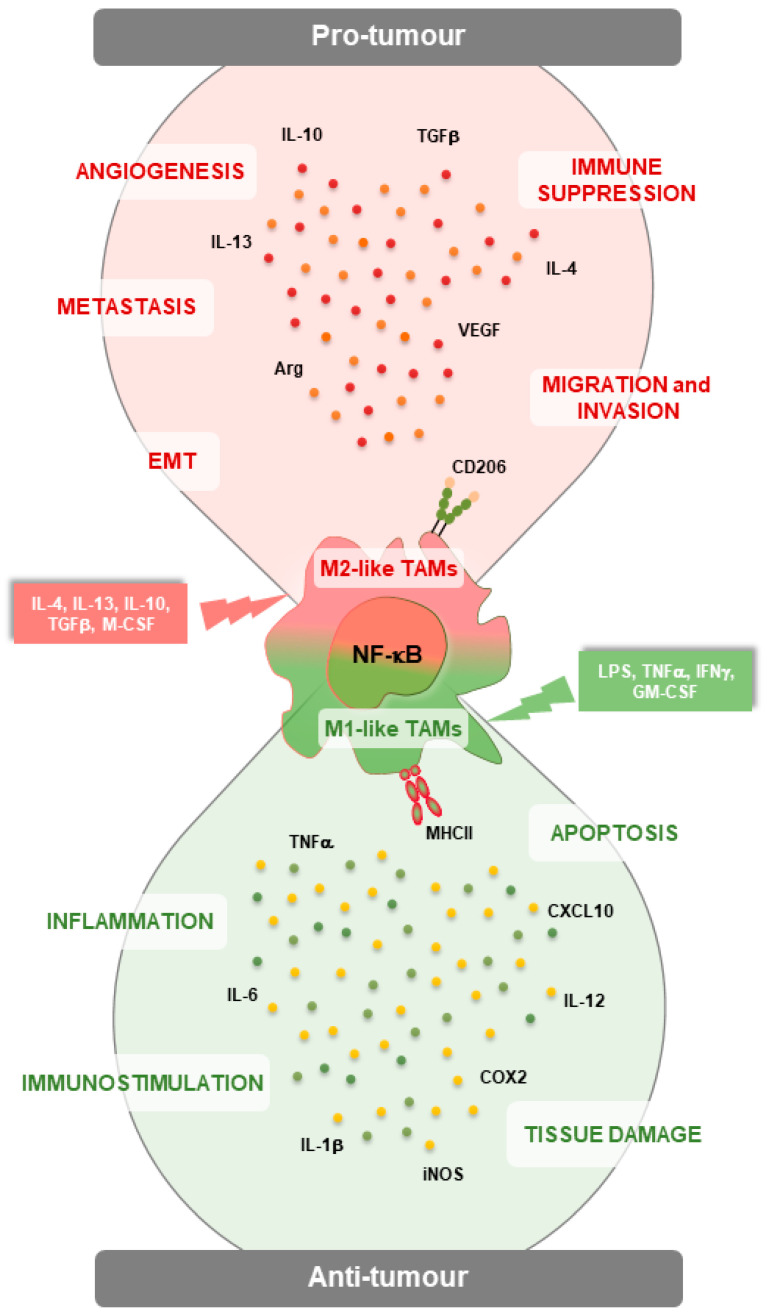
NF-κB plays a key role in TAM polarization during tumorigenesis (Figure 1) [15,38]. Accordingly, NF-κB, in response to activating stimuli such as TLR ligands, IL-1β, and TNF-α, can directly regulate the transition of macrophages toward M1 phenotype, usually exerting a tumor suppressor function, while, in different contexts, NF-κB activation can induce the transcription of many genes responsible for M2 polarization, thus promoting tumor growth (Table 1) [14,51,52,53].
Figure 1.
NF-κB signaling in M1- and M2-like tumor-associated macrophages (TAMs) in the tumor microenvironment (TME). NF-κB activation can polarize myeloid cells towards M1-like macrophages, which counteract tumorigenesis by promoting inflammation, immunostimulation, tissue damage, and apoptosis of cancer cells by inducing several molecules such as TNF-α, IL-12, iNOS, COX2 and IL-6. By contrast, NF-κB activation can shift macrophages towards M2-like anti-inflammatory TAMs, which promote tumor growth, angiogenesis, metastasis, and EMT, as well as the establishment of an immunosuppressive TME.
Weigert and collaborators demonstrated that sphingosine-1-phosphate (S1P) produced by apoptotic tumor cells suppresses TNF-α production and increases interleukin-8 (IL-8) and IL-10 levels, thus promoting macrophage polarization toward an alternative activated (M2) phenotype in vitro. They showed that in response to LPS, S1P and apoptotic cancer cells inhibit the activation of NF-κB in macrophages. Accordingly, the reduced levels of S1P after genetic inhibition of sphingosine kinase 2 (Sphk2), restored M1 macrophages in vitro [54,55,56]. Recently, Shan and colleagues demonstrated that mechanical stretch (MS) (e.g., Flexcell Tension system) promotes M1 macrophage phenotype in an NF-κB-dependent manner, thus increasing tumoricidal effects in vitro and reducing tumor growth in vivo. The authors demonstrated that macrophages stretched with the Flexcell Tension system increase the levels of M1-related genes such as iNOS, TNF-α, IL-1β, and IL-6, as well as the release of M1 cytokines. The MS induces the up-regulation of focal adhesion kinase (FAK) that, in turn, activates NF-κB signaling, thus promoting the transcription of genes responsible of the M1 phenotype activity. Accordingly, NF-κB inhibition reduces the expression of M1 target genes in vitro [57]. In vivo study demonstrated that an intratumoral injection of macrophages treated with MS enhances M1 macrophage polarization within the TME and increases apoptosis of cancer cells, thus reducing melanoma growth [58].
In accordance with the role of MS to promote M1 polarization, Gao and collaborators demonstrated that tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) plays an important role in re-educating macrophages towards an antitumor phenotype by inducing the activation of NF-κB as well as the expression of pro-inflammatory cytokines such as IL-1β, IL-6 and TNF-α that in turn promote a cytotoxic effect in the tumor cells [59]. The authors showed that TRAIL also enhanced the expression of miR-146a via NF-κB and its overexpression blocked the production of pro-inflammatory cytokines suggesting that miR146a negatively controls the immunosuppressive phenotype. The modulation of this immune response regulated by the TRAIL/NF-κB/miR-146a axis identified TRAIL as a potential target to re-educate macrophages in tumor tissues.
Studies conducted by Lee and collaborators pointed out how NF-κB activation plays an important role in controlling the communication between tumor cells and TAMs through TLR4, reporting how NF-κB activation, on one hand, sustains cancer cell proliferation and invasion, and on the other hand, induces TAMs to release inflammatory cytokines and angiogenic factors in the TME, that in turn, support tumor proliferation, thus creating a vicious circle. They demonstrated that TLR4 signaling is the mediator of the NF-κB activation in TAMs. Consistently, TLR4 deficient TAMs showed a decreased NF-κB activity, and a reduced production of inflammatory and angiogenic factors, thus limiting tumor growth in vivo. Furthermore, TLR4 KO TAMs were not able to induce the activation of NF-κB in tumor cells. In contrast, macrophages TLR4 wild-type adoptive transferred in TLR4-deficient mice bearing tumor, showed a significantly higher NF-κB activity, enhanced release of inflammatory factors such as TNF-α and VEGF, thus prompting an increased NF-κB activity in tumor cells and tumor growth in vivo [60]. Therefore, targeting TLR4 in TAMs could be an attractive therapeutic strategy to counteract tumor growth in cancer patients.
It is recognized that NF-κB can exert both anti-tumor and pro-tumor functions within the TME (Figure 1). In fact, the NF-κB activation can either promote the polarization of macrophages toward a pro-inflammatory anti-tumor phenotype or, at the same time, sustain the immunosuppressive activity of other cells such as Treg, macrophages, and dendritic cells, leading to tumor growth [61]. Yang and collaborators demonstrated that M-CSF stimulation increased the expression of c-Jun, a member of the AP-1 family, which in turn induces the macrophage polarization toward the M2 phenotype. Additionally, they showed that NF-κB synergizes with c-Jun to promote macrophage transformation from M1 to M2. Immunoprecipitation experiments confirmed the interaction between c-Jun and p50 after M-CSF stimulation, interaction that weakened in absence of macrophage colony-stimulating factor (M-CSF) or after treatment with a p50 inhibitor, andrographolide [62].
Although the activation of NF-κB is important for inducing the M2 phenotype, isolated TAMs from several well-established tumors have reduced NF-κB [15,63,64]. Saccani and collaborators demonstrated that high expression of the p50 NF-κB inhibitory homodimer inhibits M1 activation of TAMs and fosters tumor progression. Accordingly, TAMs isolated from mice knockout for p50 showed normal M1 activation with secretion of inflammatory cytokines and reduced tumor growth [65].
Kühnemuth and Michl demonstrated that the Cut-like homeobox 1 (CUX1), a homeodomain transcription factor expressed in different tumor types, acts as an antagonist of NF-κB signaling in TAMs. CUX1, which is the transcriptional target of the immunosuppressive cytokine TGFβ, exerts its action by displacing RelA from the promoters of several genes (e.g., CXCL10, CCL5) related to the M1-phenotype and by mediating the deacetylation of RelA through the recruitment of histone deacetylase 1 (HDAC1) to the promoters of NF-κB target genes. Furthermore, CUX1 inhibits the secretion of pro-inflammatory factors and supports tumorigenesis [66]. The inactivation of NF-κB by CUX1 inhibits the transactivation of inflammatory cytokines regulated by this transcription factor in established tumors [66]. Another mechanism that inhibits TAM anti-tumor activities is the degradation of NF-κB via selective autophagy. In vitro studies demonstrated that TLR2 signaling induces the accumulation of ubiquitinated NF-κB p65, that in turn forms aggresome-like structures (ALS) in the cytoplasm of M2 but not in M1 polarized macrophages. These structures are then recognized by the ubiquitin-binding proteins p62/SQSTM1 (sequestosome 1) and degraded via lysosomes. In addition, the authors showed that autophagy-dependent NF-κB p65 degradation is supported by sustained ERK1/2 phosphorylation that is triggered by TLR signaling [67].
NF-κB is also involved in the sophisticated mechanisms that regulate TAMs’ action in the processes of cancer cell invasion and metastasis [68]. A characteristic feature of the TME is the crosstalk between pericytes (PCs), cancer-associated fibroblasts (CAFs) and TAMs, that together coordinate the molecular mechanisms responsible of metastasis [68]. TAMs play important roles in promoting cancer cell dissemination [69]. Accordingly, interleukin-33 (IL-33) produced by CAFs drives the release of Th2-associated cytokines that polarize macrophages toward the M2 phenotype. IL-33-stimulated TAMs show an increase in NF-κB-mediated Matrix metalloproteinase-9 (MMP9) expression, that in turn degrades the extracellular matrix protein laminin and allows the extravasation and dissemination of tumor cells, suggesting that the IL-33-NF-κB-MMP9-laminin axis moderates the CAF-TAM crosstalk to foster cancer metastasis [68].
It Is well known that TME is characterized by low levels of oxygen (hypoxia) which promotes tumor progression and resistance to therapy. In addition, hypoxia enhances macrophage recruitment, thus conferring aggressiveness [70,71]. In this scenario, tumor cells and macrophages activate pro-angiogenic programs mediated by NF-κB-regulated hypoxia inducible factor 1 (HIF-1) that promote tumor cell adaptation and proliferation as well as TAM recruitment and oncogenic activities [72,73].
Studies showed that TAMs, together with other immune cells (e.g., myeloid-derived suppressor cells (MDSCs), T-regulatory cells (Treg)), infiltrate the hypoxic regions within the tumor and inhibit their anti-tumor function [71,74]. A study conducted by Delprat and colleagues indicated that cycling hypoxia (cyH), also called intermittent hypoxia, promotes the M1-like phenotype of macrophages via activation of JNK/p65 signaling pathway [75]. In this study the authors demonstrated that cyH promotes and amplifies a pro-inflammatory phenotype in non-activated (M0) and M1 macrophages by increasing the expression of M1 markers such as TNF-α, IL-8, CXCL10, Macrophage inflammatory protein 2 (MIP-2). This pro-inflammatory phenotype in human M0 and M1 macrophages was due to an increased activation of c-jun/NF-κB signaling. Accordingly, p65 and JNK ablation inhibits the pro-inflammatory phenotype induced by cyH, suggesting that c-jun-p65 axis regulates the cyH-mediated M1 macrophages [75].
TAMs support tumor resistance by regulating drug metabolism and/or secreting cytokines such as IL-6 in several cancer types. Additionally, M2-TAMs promote angiogenesis and tumor relapse [76]. A recent work showed that NF-κB is involved in the development of chemo and radiotherapy resistance, as well as in tumor response to therapy. In particular, several chemotherapeutic agents, such as taxol, cyclophosphamide, and cisplatin induce the up-regulation of proinflammatory cytokines such as TNF-α, IL-12, INOS, cyclooxygenase-2 (COX2), and the downregulation of anti-inflammatory factors like IL-10 and TGFβ via NF-κB activation. In addition, cisplatin and carboplatin treatments enhance the activation of the NF-κB pathway through the chemotherapy-induced DNA damage response (DDR), thus sustaining the polarization of monocytes toward M2-like macrophages in the TME [77]. Although radiotherapy affects TAM recruitment and phenotype in cancer, its role in reprogramming TAMs towards an anti-tumor phenotype remains unclear [78]. It is known that NF-κB plays an opposite role in TAM response during radiotherapy depending on the dose irradiation. Indeed, low radiation doses polarize macrophages towards a pro-tumoral phenotype by reducing the expression of IL-1β through the increase in the nuclear translocation of p50-p50 homodimer and the inhibition of p65 translocation [79]. On the contrary, moderate doses of radiations reprogram macrophages into M1 phenotype by inducing higher p65-p50 transcriptional activity, which in turn results in increased TNF-α, IL-6 and IL-8 production [80]. The immunosuppressive phenotype is maintained at high irradiation doses, where sustained activation of p50 keeps TAMs in an M2 polarization state (Table 1) [81].
Table 1.
The NF-κB pathway in TAMs.
| Tumor/Cell Type | Stimuli | NF-κB Pathway Component |
TAMs Phenotype |
Effect | Ref. |
|---|---|---|---|---|---|
| Breast carcinoma | SP1 | p65/p50 | M2 | Pro-tumor | [54,55,56] |
| Melanoma | Mechanical stretch |
p65 | M1 | Anti-tumor | [57,58] |
| Lung cancer | TRAIL | p65 | M1 | Anti-tumor | [59] |
| Melanoma, Bladder cancer | HSPs/TLR4 | NF-κB | M2 | Pro-tumor | [60] |
| Breast cancer | M-CSF | p50 | M2 | Pro-tumor | [62] |
| Pancreatic cancer | CUX1 | p65 (RelA) | M2 | Pro-tumor | [66] |
| Hepatoma | TLR2 | p65 | M2 | Pro-tumor (Autophagy) |
[67] |
| Pancreatic cancer | IL-33 | IκBα | M2 | Pro-tumor (Dissemination/ Metastasis) |
[68,69] |
| Hepatocellular carcinoma |
Hypoxia | IKKβ | M2 | Pro-tumor (Angiogenesis, EMT) |
[71] |
| hTHP1/mBMDM (Human monocytic cell line/ murine bone marrow-derived macrophage) |
Cycling hypoxia | p65 | M1 | Anti-tumor (M0-M1 transition) |
[75] |
| Ovarian cancer | Chemotherapeutic agents | IKK | M2 | Pro-tumor | [77] |
| THP1 (monocytic cell line) |
Low radiation doses |
p50/p50; p65 | M2 | Pro-tumor (Reduced M1 cytokines) |
[79] |
| THP1 (monocytic cell line) |
Moderate radiation doses |
p65/p50 | M1 | Anti-tumor | [80] |
| Mammary Carcinoma, Pancreatic adenocarcinoma |
High radiation doses |
p50 | M2 | Pro-tumor | [81] |
3. NF-κB Signaling in TAMs: The Lesson from Different Human Cancer Types
3.1. Hepatocellular Carcinoma
According to the Global Cancer Statistics 2020, hepatocellular carcinoma (HCC) is the third leading cause of cancer death and the sixth most common cancer worldwide [82]. Many patients cannot benefit from the most common effective treatments, such as surgical resection, local tumor ablation, and liver transplant due to tumor size or the invasion and spreading of cancerous cells in other tissues and sites [83]. TME plays an important role in HCC progression and TAMs can mediate the acquisition and maintenance of tumor cell stemness conferring chemo and radiotherapy tolerance and resistance [84]. It is known that high density of TAMs in HCC has been associated to unfavorable prognosis [85]. This relationship is due to the mutual influence that tumor cells and TAMs exert on each other. In fact, the release of several factors such as M-CSF, CCL2, VEGF, and TGF-β and the expression of surface markers (e.g., glypican-3) on cancer cells are responsible of TAM recruitment and polarization. On the other hand, recruited and activated TAMs in HCC release many cytokines, chemokines and growth factors that are responsible for tumor cell proliferation, angiogenesis, extravasation, invasion and metastasis, as well as the suppression of anti-tumor immune response [72]. The NF-κB pathway plays a fundamental role in the establishment of HCC. It has been demonstrated that it exerts opposite functions based on the stage and development of the disease as well as the cell type where NF-κB is activated. NF-κB activation showed a tumor suppressor function in cancerous epithelial and parenchymal cells, while explaining a tumor promoting function if activated in Kupffer cells, the resident macrophages of the liver. In this scenario, the inflammatory cytokines released by activated Kupffer cells provide a pro-survival and proliferative stimulus to malignant hepatocytes mediated by myeloid-differentiation-factor-88 (MyD-88)/NF-κB pathway, thus sustaining tumorigenesis. The inhibition of NF-κB signaling in macrophages but not in hepatocytes reduces the production of pro-inflammatory cytokines and tumor growth [86,87,88,89].
A comparative analysis of RNA sequencing and whole-genome expression profiling of TAMs and M0 macrophages in HCC identified the upregulation in TAMs of the S100 calcium-binding protein A9 (S100A9) gene, which has a potential impact on HCC prognosis. In fact, the expression of S100A9 is correlated with poor clinical outcomes in patients with HCC [90]. It has been found that the S100A9 stimulates the NF-κB-dependent secretion of CCL2, the major chemoattractant for TAMs, thus promoting the recruitment, infiltration, and activation of TAMs. Furthermore, S100A9 secreted by TAMs induces a strong NF-κB activation and an increased expression of stemness-associated genes in HCC cells in a dose-dependent manner, resulting in an enhanced sphere formation ability and self-renewal in vitro. The knockdown of advanced glycosylation end product-specific receptor (AGER), the receptor of S100A9, eradicates the NF-κB-mediated pro-tumorigenic effects of S100A9, suggesting that S100A9 controls the crosstalk between tumor cells and TAMs and could be a potential target for treating HCC patients [90,91].
The oxidored-nitro domain-containing protein1 (NOR1) is overexpressed in human HCC tissues and correlates with advanced clinical stage and poor prognosis [92]. In vivo study demonstrated that in diethylnitrosamine (DEN)-induced HCC, NOR1 is overexpressed in F4/80 positive macrophages and decreases M1-like markers (e.g., iNOS) and increases M2-like markers, such as arginase-1 (Arg1) and IL-10, thus promoting M2 polarization. Consistently, loss of NOR1 ablates NF-κB p65 expression, impairs the production of inflammatory cytokines such as IL-6 and TNF-α in mice and reduces DEN-induced HCC, suggesting that NOR1/NF-κB plays a role in TAMs polarization and HCC development [92].
Another protein involved in promoting tumorigenesis and M2-like polarization is growth arrest and DNA damage β (GADD45β), a NF-κB-regulated anti-apoptotic protein, able to suppress the JNK-mediated pro-apoptotic signaling by targeting MKK7 [93,94,95,96,97]. Recently GADD45β has been identified as an essential modulator of TAMs reprogramming and of the CD8+ T-cell trafficking in HCC tumors [98]. The authors demonstrated that Gadd45b−/− mice displayed a reduced number of HCCs compared to WT mice. Indeed, Gadd45b−/− HCC tumors, but not Gadd45b+/+ tumors, showed an enhanced F4/80+ and IBA1+ TAMs and T-cells infiltration as well as a higher number of tertiary lymphoid structures (TLSs), which are correlated to a favorable clinical outcome in most human cancers. Strikingly, M1-like macrophages were observed in Gadd45b-deficient HCC tumors, characterized by high expression of inflammatory markers, such as iNOS, COX-2 and MHC-II than Gadd45b+/+ HCCs, suggesting that Gadd45β loss increases the M1-like polarization state via upregulation of p38 signaling. These findings show that Gadd45β supports anti-inflammatory TAM activation and blocks TLS formation and lymphocyte infiltration within tumors, thus sustaining tumor growth [98]. However, in other tumors, the authors also demonstrated that macrophage-specific Gadd45β loss blocks oncogenesis, indicating that Gadd45β governs the immunosuppressive activity of the TME across multiple cancer types [98,99]. Therefore, targeting Gadd45β, in combination with conventional immunotherapies, could be a potential new therapeutic approach to counterstain Gadd45β-mediated tumorigenesis in human cancers.
Receptor-interacting protein 140 (RIP140) is a protein widely expressed in macrophages, responsible for the regulation of TAMs energy metabolism and inflammatory response that has been associated with NF-κB pathway [100]. In HCC, NF-κB/IL-6 axis induces the alternative polarization of TAMs. In contrast, the overexpression of RIP140 in TAMs can reduce the expression of M2-like polarized markers (Arg-1, Peroxisome proliferator-activated receptors (PPAR), CD206) and enhance the expression of TNF-α, an M1-like marker. In vivo studies demonstrated that the inhibition of M2 polarization reduces tumor burden and invasiveness (proliferating cell nuclear antigen (PCNA) and VEGF ratio) and increases HCC cell apoptosis. In addition, they observed a decrease of p-p65, p-c-Jun and tumor necrosis factor receptor-associated factor 3 (TRAF3) expression levels, as well as of IL-6. These findings suggest that the overexpression of RIP140 inhibits NF-κB activation and influences TAMs polarization in HCC [100].
Contrastingly, Sharen et al. demonstrated that the infiltration of M1-TAM in HCCs induces a reduction of the efficacy of postoperative transcatheter arterial chemoembolization (TACE) in patients with liver cancer. In particular, the infiltration of M1 TAMs promotes the hyperactivation of NF-κB pathway, the increase in anti-apoptotic activity, the upregulation of the expression of cyclin-dependent kinase (CDK)1, CDK2 and cyclin D1, and the reduction of p21 expression in HCC cells, sustaining tumor proliferation. The pro-tumoral effect of M1-like TAMs was reverted by the administration of the p65 inhibitor, JSH-23, underlying the controversial role of NF-κB in M1 TAMs in HCC progression [101].
The use of immunotherapy for HCC treatment is still far from achieving its intended effect in the clinical setting, even if increasing number of patients have greatly benefited from this therapeutic approach [102,103,104,105,106,107].
The inhibitors of the programmed cell death protein-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathway are one of the most used immune checkpoint inhibitors able to re-modulate T-cells and TAMs immune response [108]. Recently, Xu and collaborators demonstrated that the combination therapy using a Listeria monocytogenes-based tumor vaccine, Lmdd-MPFG, and anti-PD-1 reduced HCC tumor volume and increased survival rates, by inducing CD8 T-cells activation and IFN-γ signals. No effects are shown following monotherapy with anti-PD-1. In addition, they observed a shift of TAMs from M2 to M1 phenotype, an increased autophagy, driven by enhanced activation of the NF-κB pathway. This evidence highlighted that the combination therapy synergizes to affect TAM polarization and TME composition in a NF-κB dependent manner [109].
The liver is the preferred metastatic site for several tumors, such as colorectal cancer (CRC), lung cancer, melanoma, and gastric carcinoma. TAMs promote tumor progression by increasing tumor metastasis and stabilizing a pre-metastatic microenvironment [110,111]. Studies conducted by Li and collaborators demonstrated that N-myc downstream-regulated gene 2 (NDRG2), a protein that binds PTEN via protein phosphatase 2A (PP2A) recruitment, controls TME remodeling, and TAMs polarization during metastatic processes through activation of the NF-κB pathway. Interestingly, in a liver metastasis model, they found that Ndrg2−/− macrophages have a tumor-suppressor phenotype compared to WT macrophages, and the M1–like polarization is driven by enhanced activation of the NF-κB pathway. Accordingly, the inhibition of IκBα phosphorylation abolishes the tumor-suppressor function of Ndrg2−/− macrophages, thus inhibiting cancer liver metastasis [112].
3.2. Breast Cancer
The role of M2 TAMs in promoting proliferation, invasion, migration and angiogenesis in human breast cancer seems to be related to the NF-κB-mediated expression and secretion of galectin-3, a member of the β-galactoside binding proteins. Galectin-3 results particularly expressed in hypoxic tumor regions, where it promotes the migration and invasiveness of breast cancer cells and enhances angiogenesis and vascular mimicry. The expression of galectin-3 in TAMs is dramatically reduced in the presence of the NF-κB inhibitor, pyrrolidine dithiocarbamate (PDTC), both in normoxia and hypoxia conditions. In contrast, the presence of ROS induces the nuclear accumulation of NF-κB and the consequent upregulation of galectin-3 [113].
The chemokine profiling of murine and human breast cancer models indicated that CXCL1 is one of the most abundant chemokines secreted by TAMs. The levels of CXCL1 are significantly higher in metastatic lung cancer specimens compared to primary breast cancer tumor, suggesting the active role of CXCL1 in the promotion of breast cancer cell migration and invasiveness. Indeed, the presence of CXCL1 significantly increased breast cancer 4T1 cell migration and invasiveness ability, matrix metalloproteinase-2 (MMP-2) and MMP-9 secretion, as well as the expression of EMT-related proteins in vitro. TAMs-secreted CXCL1 mediates the EMT in breast cancer cells and induces the NF-κB-mediated expression of several metastatic genes, such as SRY-box transcription factor 4 (SOX4). ChIP assay showed that NF-κB actively binds the predicted binding region on the SOX4 promoter after CXCL1 treatment. Accordingly, the inhibition of NF-κB blocks CXCL1-induced SOX4 overexpression, increases the E-cadherin and reduces both vimentin and β-catenin expression, underlying the importance of CXCL1/SOX4/NF-κB axis in sustaining breast cancer [114].
Annexin 1 (ANXA1) is a protein member of the annexin family with multifunctional roles in cancer development and progression. ANXA1 is highly expressed in metastatic and triple negative (estrogen, progesterone and HER2 receptor) breast cancer (TNBC). Studies conducted on ANXA1+/+ and ANXA1−/− mice indicate that ANXA1 is necessary for a macrophage phenotypic switch from M1 to M2. Interestingly, ANXA1 directly induces ERK and NF-κB activation via the formyl peptide receptors 2 (FPR2), leading to a macrophage polarization and tumor cell proliferation [115].
The physical structure of the tumoral extracellular matrix (tECM) in breast cancer has an important role in the protection of the tumor niche and in the penetrance of the immune system. TAMs redefine the composition of the tECM by enhancing the deposition of the heparan sulfate proteoglycan 2 (HSPG2), known as perlecan, that confers mechanical rigidity to the ECM and generates a favorable gradient for cancer cell development. NF-κB p65/p52 complex is responsible of the transcriptional regulation of HSPG2 in M2 TAMs and is associated with the altered expression of perlecan. Accordingly, the treatment with QNZ, the NF-κB inhibitor, on TAMs significantly downregulates the expression of HSPG2, indicating that NF-κB pathway is involved in the remodeling of the tECM and in the tumor escaping from the immune system [116].
The acquisition of properties associated with mammary stem cells (MaSCs) and cancer stem cells (CSCs) is one of the key passages that enables breast cancer cells to perform the epithelial mesenchymal transition (EMT) and develop malignancy, invasiveness and therapy resistance [117,118,119]. Interestingly, human monocytes or TAMs fail to promote tumor-initiation or progression in breast cancer cells expressing an NF-κB super-repressor, indicating that NF-κB activation and cytokines production have a critical role in the CSCs and in the tumorigenic effects of the TAMs [119].
Human mammary epithelial cells responsible for EMT were characterized as CD44+ and CD24− [120]. Interestingly, mammary epithelial cells cocultured with M2 TAMs exhibit increased percentage of CD44+/CD24− and elevated levels of HIF-1α, α-catenin, NF-κB, and twist family bHLH transcription factor 1 (Twist 1). In fact, the silencing of NF-κB in the M2-like MDA-MB-231 activated macrophages decreases the migration and invasion abilities of breast cancer cells. Mechanistically, NF-κB directly downregulates the anti-metastatic miR-488 and enhances special AT-rich sequence-binding protein-1 (SATB1) and Twist1 expression, the major initiators of the EMT, suggesting that the NF-κB/miR-488 axis is responsible for cancer cells migration and metastasis in presence of M2-TAMs [121].
It is known that the intercellular communication between TAMs, TME and several cancer cells, including breast cancer cells, is also mediated by EVs [122]. A recent study uncovered that TAM-derived EVs enriched with miR-660 inhibit Kelch-like protein 21 (KLHL21) in breast cancer cells leading to the activation of NF-κB p65 signaling pathway. In turn, the activation of NF-κB controls the cancer cells invasiveness, migration, and metastasis by modulating the expression of E-cadherin, N-cadherin, MMP-9, MMP-3 and β-actin [123].
3.3. Colon Cancer
Several pieces of evidence showed that TAMs sustain the initiation and progression of CRC by regulating several processes including tumor proliferation, metabolism, immunosuppression, angiogenesis and metastasis [124]. In CRC, intratumoral macrophages exert tumor-promoting functions, while TAMs found in the tumor invasive front correlate with better prognosis [122,123]. However, mixed macrophage populations are observed in human colorectal cancer tumors [125].
Studies in colitis-associated cancer (CAC) and in genetically driven ApcMin mouse models showed that NF-κB pathway regulates the shift from M1 to M2 macrophages and the transition from colitis to colon cancer. While the accumulation of p50 induces the activation of the M2-like transcriptional program, p50 deficiency reduces inflammation and number and size of neoplastic lesions in chemically induced CAC. Similar results were found in genetically driven CRC, where tumor incidence and growth are reduced in ApcMinp50−/− mice compared to ApcMin mice. Accordingly, tumor inhibition was observed in the immunogenic MC38 transplantable CRC model in p50−/− mice compared to WT mice, as well as an increased expression of M1 genes and cytokines. Moreover, p50 ablation reduces TAMs accumulation indicating the fundamental role of NF-κB in modulating macrophage infiltration [126]. Consistently, the inhibition of NF-κB (p50) in M2-TAMs by siRNAs induces M2 macrophages to switch towards M1-like phenotype, thus promoting the production of pro-inflammatory and tumoricidal factors, as well as growth factors and NO. These findings suggest that NF-κB plays a central role in the assessment and maintenance of TAMs phenotype in colon cancer [127].
Prolyl hydroxylases domain proteins (PHDs) are dioxygenases activated in response to oxygen availability. The overexpression of PHD2 isoform is associated with the suppression of colon cancer growth and invasiveness [128]. Interestingly, the reduced p65 phosphorylation and the downregulation of NF-κB-related downstream genes involved in cell cycle, EMT, and inflammation (e.g., CyclinD1, E-cadherin, and TNF-α) as well as in the recruitment of M2-like TAMs within the TME, has been found in colon cancer cells expressing PHD2 and in PHD2-overexpressing colon cancer xenografts, suggesting that NF-κB mediates the anti-inflammatory and anti-cancer effects of PHD2 in colon cancers [129].
Recent studies demonstrated that prolonged and enhanced NF-κB signaling activation sustains TAMs F4/80-positive recruitment and angiogenesis via P2X purinoceptor 7 (P2X7R) in CRC cells [130]. It is known that NF-κB sustains cancer cells survival and progression by regulating metabolic switch and adaptation to low nutrient conditions [33,131]. In vivo studies demonstrated that a ketogenic diet (KD) induces a strong p65 inhibition in both TAMs and CRC cells, that in turn, promotes TAMs M1 polarization and reduces tumor growth and metastasis, suggesting that NF-κB plays a role in the modulation of tumor metabolic adaptation and inflammatory response [132]. It has been demonstrated that metabolism is also associated to the TAMs ability to migrate with cancer cells to metastatic sites [133,134].
In CRC, macrophages have distinct metabolic profiles based on their migratory abilities. In fact, migration-active macrophages express lower abhydrolase domain containing 5 (ABHD5), a catalysator of triglyceride hydrolysis, compared to the nonmigratory TAMs. Interestingly, ABHD5 deficiency and, consequently, triglyceride accumulation, stimulate NLRP3 inflammasome activation, in a ROS-dependent manner, and induce IL-1β secretion, that in turn, stimulates p65 signaling. Conversely, gene microarray showed that ABHD5 suppresses NF-κB-dependent MMPs expression and activity, fundamental for cancer cell migration. In addition, the authors demonstrated that ABHD5-mediated knockdown (ABHD5-KD) macrophages have an enrichment of JAK–STAT and TLR pathways, that stimulate the NF-κB and JNK signaling compared to controls. In conclusion, TAMs expressing ABHD5 exert an inhibitory effect on colon cancer cell migration, while ABHD5low macrophages promote NF-κB-dependent colon cancer cells invasiveness [135].
3.4. Glioblastoma
It is known that up to 30% of the total glioblastoma (GBM) composition is represented by peripheral macrophages and microglia [136,137]. In a GBM syngeneic immune competent mouse model, it has been shown that the LysM-Cre-mediated conditional deletion of p65 in myeloid lineage induces the TAMs polarization from the M2 to M1 phenotype. Indeed, p65 deficiency enhances CD8+T cells proliferation and increases levels of IFN-γ, TNF-α and IL-1β, which lead to a decreased GBM tumor growth. In contrast, where p65 KO and control donor mouse bone marrow was transplanted in immune-deficient mice, no reduction in tumor growth was observed in p65 KO chimaera mice compared to control mice. These findings suggest that the anti-tumor immunity in GBM is inextricably linked to T cells activity and is negatively regulated by the NF-κB pathway [138]. Interestingly, in a GL261-implanted mouse model, the treatment with curcumin (CC) induced an overall TAM repolarization from the M2 to the M1 phenotype via STAT1 and NF-κB signaling activation. Indeed, the CC treatment suppresses p50 homodimers in the microglia and promotes the activation of the p50/p65 NF-κB pathway, which leads to the M1 tumoricidal phenotype activation. Moreover, the concerted activation of STAT1 and NF-κB pathways induces apoptosis of GBM cancer cells and increases iNOS and Iba1+ expression in the microglia, indicating the presence of activated M1 macrophages which eliminate the remaining GBM cells and apoptotic debris, and a tumor remission in 50–60% of treated mice [139].
GBM growth is significantly accelerated by IL-1β, a cytokine highly expressed in TAMs and neutrophils localized in the perinecrotic tumor areas. IL-1β expression in TAMs is synergistically activated by CXCL8 and IL-6, which in turn activate NF-κB and STAT3 pathways. TAMs-derived IL-1β stimulates GBM tumor cells and induces the expression of IL-1β itself and CCL2, which actively recruits TAMs in the tumor site. Furthermore, damage-associated molecular patterns (DAMPs) released from necrotic cells stimulate IL-1β expression in TAMs with an autocrine/paracrine feedback loop that accelerates TAMs migration and tumor growth [140]. Consistently, higher IL-1β expression is correlated with a worse clinical course in GBM [33,131] suggesting that NF-κB /IL-1β axis in TAMs has a key role in GBM growth and progression.
3.5. Gynecologic Cancer
As for HCC, NF-κB/Gadd45β axis governs the immunosuppressive activity of the TME and the M2 macrophage polarization in ovarian cancer (OC) [98]. Therefore, the Gadd45b ablation in macrophages re-establishes proinflammatory TAM activation and CD8+ T-cell infiltration into the tumor, thus inhibiting ovarian adenocarcinoma growth [98].
The crosstalk between endothelial ovarian cancer cells (EOC) and TAMs promotes the progression of OC [141], and exosomes have been identified as principal mediators of this interplay [133]. In fact, exosomes isolated from TAMs in the ascites of EOC patients and carrying high levels of miR-146b-5p are then internalized in the cytoplasm of EOCs as endosome-like vesicles. Once internalized in EOCs, miR-146b-5p directly targets and inhibits the TRAF6/NF-κB/MMP2 axis, suppressing endothelial cell migration. In contrast, exosomes derived from EOCs, that transport 2 lncRNAs, restore the endothelial cell migration by activating NF-κB pathway in the recipient cells [142].
Mitochondrial transcription factor B2 (TFB2M), responsible for the mitochondrial DNA (mtDNA) transcription and compacting, is expressed in ovarian cancer patients, and is associated with poor prognosis, immunosuppressive TME and macrophage polarization. In fact, IHC analysis on primary OC specimens indicates a striking correlation between TFB2M expression and M2 TAM infiltration. Interestingly, the overexpression of TFB2M increases cytosolic mtDNA and IL-6 expression in OC cells via TLR9/NF-κB signaling pathway activation, contributing to M2 macrophage polarization and infiltration in the TME [134]. Accordingly, the inhibition of cytosolic mtDNA, TLR9 or NF-κB pathway blocks the TFB2M-induced IL-6 expression and abrogates the M2 macrophage polarization and the immunosuppressive TME in OC.
In other types of gynecologic cancers, TLR9 pathway activates lymphocyte T cell shift towards Th1 profile and decreases the number of MDSCs, TAMs and Tregs in the TME, displaying anti-tumor properties [133]. However, the TLR9 pathway stimulated by radiotherapy, cell death, and CpG release, can also induce tumor progression, angiogenesis and invasiveness in cervical cancers through the activation of the NF-κB and STAT3 transcription factors [143].
As extensively reported, hypoxia plays a fundamental role in cancer progression, and hypoxic regions are often infiltrated by immunosuppressive cells, such as TAMs, MDSCs and Tregs [144,145] and are strongly associated with poor patient outcomes [146]. It has been demonstrated that hypoxia induces the binding of HIF-1α to the proximal promoter of zinc finger E-box binding homeobox 1 (ZEB1), which is highly expressed in cervical cancer hypoxic cells islets and is associated with a stronger pro-tumor phenotype. ZEB1-driven cancer cells produce CCL8, attract and promote macrophages activity through NF-κB activation. Interestingly, TCGA data showed that both ZEB1 and CCL8 overexpression correlate with poor prognosis in human cervical cancer. These findings suggest that the NF-κB pathway is responsible for hypoxia-induced ZEB1-mediated TAM infiltration, and targeting ZEB1-CCL8-NF-κB axis could be a promising strategy for the cervical cancer treatment [147].
3.6. Other Solid Tumors
Other solid tumors in which TAMs have a crucial role in mediating tumor progression and immune escape are neuroblastoma (NB), bladder cancer, renal carcinoma, gastric cancer (GC), basal cell carcinoma (BCC), oral squamous cell carcinoma (OSCC), prostate cancer (PCa), nasopharyngeal carcinoma (NPC), multiple myeloma (MM), sarcoma, pancreatic cancer (PDA) and lung cancer [148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163]. In addition, it is known that in these tumors TAMs correlate with worse prognosis and drug resistance [164].
NB is the most frequent pediatric cancer, with heterogeneous clinical outcomes based on tumor stage [165]. Recently, it has been demonstrated that higher levels of lipid metabolism-related genes are associated with unfavorable histology and an advanced stage of NB compared to the early stage. Fatty acid binding protein 4 (FABP4), a lipid chaperone that facilitates lipid distribution and response in cells, is highly expressed in NB macrophages compared to other cell types of the TME, leading to tumor progression [137]. In NB orthotopic and metastatic mouse models with FABP4 KO macrophages the tumor growth is repressed. Accordingly, the authors observed an increased tumor growth when FABP4 is overexpressed in macrophages. Furthermore, FABP4 inhibits NF-κB activity and IL-1α secretion in TAMs and downregulates ATP production via the ubiquitination of ATPB, maintaining TAMs in an anti-inflammatory state and sustaining the pro-tumorigenic effects on NB cells [137].
Bladder cancer is the fourth most common cancer in men and the overall survival is still low [149]. In vitro studies with HTB-1 and T24 bladder cancer cell lines cocultured with M2-like TAMs, demonstrated that the coculture upregulates the expression of EMT-related genes, such as VEGF, twist, vimentin and NF-κB in bladder cancer cells, leading to increased migratory and tumor sphere generation abilities. Interestingly, the treatment with BAY11-7082, a potent NF-κB signaling inhibitor, reduces the expression of the CD206, a M2-macrophage marker, and increases the expression of TNF-α, a M1 marker. In addition, the treatment reverts the EMT-induced phenotype of bladder cancer cells and reduces the expression of CD133+, a marker produced by M2-like TAMs. The authors also showed that BAY11-7082 induces the expression of miR-30a both in bladder cancer cells and in TAMs, reprogramming M2-like macrophages towards M1 phenotype. These findings underline the role of NF-κB in the regulation of TME and cancer cells metastatic potential in bladder cancer [166].
In human renal cell carcinoma (RCC), the most common type of kidney cancer in humans [167], blood derived monocytes display a distinct transcriptional profile characterized by the upregulation of pro-inflammatory cytokines and chemokines, pro-tumor genes, (e.g., COX2, IL-8, VEGFA, MMP19, MMP10, CXCR4, and HIF1A) and polarization-related genes [168]. Interestingly, RCC-conditioned monocytes display enhanced IκBα phosphorylation and p65 NF-κB nuclear translocation, via IL-1/IL-1R/MyD88 signaling activation. In RCC mouse models, the blockade of IL-1/IL-1R pathway with recombinant IL-1RA or Il1r1 knockdown, inhibits tumor growth and TAMs pro-tumor phenotype. Meta-analysis of tumor gene-expression data from RCC patients highlights that the higher expression of IL-1B correlates with pro-tumor genes induced by TAMs and advanced tumor stages, indicating that IL-1/IL-1R signaling is crucial in shaping the TME and the RCC tumor development through MyD88-dependent NF-κB pathway activation [169].
GC is the fifth leading cause of death worldwide [158]. Immunostaining of primary GC tissue samples suggests that TAMs are preferentially localized in the invasive tumor front and correlate with invasion, lymph node metastasis, TNM stage, and poor prognosis [170]. In vitro co-culture of GC cells and macrophages indicates that TAMs actively produce angiogenic and lymphangiogenic growth factors, VEGF, and VEGF-C, through the activation of NF-κB signaling pathway. Interestingly, NF-κB inhibition in TAMs decreases the expression of VEGF and VEGF-C in both macrophages and cancer cells, but the molecular mechanism is still unclear. However, this study indicates that NF-κB has a role in the interactions between tumor cells and TAMs in GC [170].
BCC is the most common skin cancer with a complicated pathogenesis [160]. In aggressive (micronodular) BCC, increased tumor invasion and angiogenesis correlates with the number of TAMs [171]. Using an in vitro noncontact co-culture system, Tjiu and colleagues demonstrated that both M2-polarized THP-1 and monocyte-derived M2 macrophages promote extracellular matrix degradation, invasiveness and angiogenesis of BCC cells by inducing COX2-dependent MMP-9, VEGF-A and basic fibroblast growth factor (bFGF) expression, via the p38 MAPK/NF-κB cascade [171].
Another skin cancer with a considerable tumor mutational burden is melanoma. Melanoma is the most fatal skin cancer and TAMs play an important role in regulating tumor development [161]. In situ immunofluorescence analysis of human primary melanoma samples indicates that there is a strong correlation between the abundance of TAMs expressing CCL20/TNF/VEGF-A and worse prognosis. The study illustrates that this pro-tumoral M2 phenotype of TAMs is driven by a consistent p53 and NF-κB activation [172].
TAMs promote tumor progression also in OSCC, a common head and neck tumor with a low overall survival [162]. It is known that the TAMs phenotype is related to Axl signaling, an oncogenic pathway activated by the autocrine and paracrine release of growth arrest—specific 6 (Gas6), an antiapoptotic and proliferative protein [173,174]. In vitro studies showed that OSCC cells expressing Axl polarize THP-1 towards M2 phenotype and induce the secretion of pro-tumorigenic factors like MMP2, MMP9 and VEGF, by activating the PI3/Akt/NF-κB pathway. Accordingly, the inhibition of Axl, PI3/Akt and NF-κB on OSCC cells with specific single inhibitors, reduces M2 polarization [175]. Furthermore, co-culture of OSCC cells with THP-1 cells enhances the expression of Axl and its ligand, Gas6, and NF-κB transcription activity in cancer cells that, in turn, acquire tumor invasion/migration abilities and express EMT related genes [176]. These findings suggest the presence of a bidirectional interaction between TAMs and OSCC that promotes malignancy and tumor growth via Gas6/Axl/NF-κB signaling [175,176].
The expression of nephroblastoma overexpressed (NOV)/ cellular communication network factor 3 (CCN3) has been associated with M2 macrophage infiltration in PCa. PCa is the second most common tumor in men. NF-κB plays an important role during prostate cancer progression and its overexpression is correlated with worse prognosis [163,177]. CCN3 secreted by PCa cells recruits macrophages and educates TAMs toward an M2 phenotype, while PCa cells pre-treated with CCN3-neutralizing antibody attenuate CCN3-induced macrophage migration and polarization. Interestingly, in RAW264.7 cells, CCN3 induces the phosphorylation of focal adhesion kinase (FAK), Akt, and NF-κB, in a time-dependent manner, and enhances p65 binding to the NF-κB element on the VEGF promoter, leading to increased angiogenesis. Correspondingly, pre-treatment with FAK, Akt, and NF-κB inhibitors, or transfection with a dominant-negative (DN) mutant of FAK, Akt, IKKα, or IKKβ, abolishes the VEGF expression. Consistently, knockdown of CCN3 in PCa cells inhibits RAW264.7-promoted angiogenesis and tumor growth in mouse models, suggesting that CCN3 secreted by PCa cells regulates TAMs infiltration and function by modulating the FAK/Akt/NF-κB signaling pathway in macrophages [178].
MM cells can modify the bone marrow (BM) niche and induce M2 phenotype polarization by releasing several soluble factors [179]. A recent study showed that circulating miR-16 in MM patients is associated with a better survival, and MM patients with chromosome 13 deletion (Del13) express lower levels of miR-16 compared to non-Del13 patients. Interestingly, primary basal state spleen MΦ (M0-MΦ) isolated from miR-16 KO mouse showed a more pronounced M2 phenotype compared to WT mice. The treatment of MΦ isolated from MM patients and mice, MΦ-like malignant cell line and human stromal cell line with ds-miR-16 significantly downmodulates the expression of both IKKα and IKKβ. Consistently, miR-16 directly binds IKKβ 3′UTR and subsequently affects protein translation. Indeed, miR-16 has a significant additive effect in inhibiting NF-κB activity in MM cells when combined with bortezomib or BAY11-7082 and sensitizes MM-PCs and resident MΦ of the BM-ME to bortezomib treatment. These findings indicate that miR-16 mediated-NF-κB pathway activation has a critical role in the selection and polarization of specific monocytes clones during MM progression [180].
The development and progression of nasopharyngeal carcinoma (NPC) is associated with Epstein–Barr virus (EBV) infection and sustained inflammation, due to the inhibition of forkhead box P1 (FOXP1) tumor-suppressive activity driven by one of the 44 EBV miRNAs, EBV-miR-BART11. In fact, EBV-miR-BART11 has been found to be significantly higher in NPC biopsies compared to non-tumor nasopharyngeal epithelial tissues. Inhibition of FOXP1 induces enhanced NPC cells proliferation and the expression of inflammatory factors (e.g., IL-1β, IL-6, and IL-8) leading to a sustained local inflammation. Moreover, macrophages transfected with EBV-miR-BART11 are hyperresponsive to LPS and promote epithelial cell proliferation. Indeed, FOXP1 overexpression decreases NF-κB p65 protein expression, while EBV-miR-BART11 overexpression or FOXP1 knockdown increases NF-κB p65 expression, indicating the role of NF-κB in the crosstalk between NPC cell and TAMs in response to inflammation and tumor development [181].
In Ewing sarcoma (ES), the regulation of macrophages infiltration is driven by let-7a expression. The let-7a has a tumor suppressive activity and inhibits TAMs recruitment on tumor site. The overexpression of let-7a represses STAT3 signaling pathway, which is constitutively activated in ES cells. Accordingly, STAT3 upregulation suppresses let-7a activity by inducing NF-κB pathway, that inhibits the expression of mature let-7a through lin-28B, leading to enhanced TAMs infiltration and activation [182].
It has been demonstrated that acidic polysaccharide IAPS-2 exhibits an anti-tumor effect on sarcoma by re-educating TAMs towards an anti-tumor phenotype. The IAPS-2 treatment induces NF-κB and STAT1 pathways and inhibits STAT3 signaling, that in turn, modulates TAM gene expression and cytokine profile. In S180 tumor-bearing mice, IAPS-2 promotes the secretion of several M1 markers and reduces the concentration of MMP-9 and VEGF in the tumor, restoring the immunosurveillance with an anti-angiogenetic effect [183].
In pancreatic (PDA) cancer, the transcription factor Cut like homeobox 1 (CUX1) is an important modulator of TAM phenotype plasticity and functions and it is highly expressed not only in PDA tumor cells but also in PDA TAMs. It is recognized that CUX1 displaces NF-κB p65 binding at the promoter level of CXCL10 and recruits HDAC1, which decreases the acetylation and transcriptional activity of NF-κB, leading to a reduced expression of M1 phenotype-related cytokines. As a result, CUX1 reduces the recruitment and the activation of M1 TAMs, promoting tumor progression and angiogenesis in PDA. These finding suggest that CUX1 regulates TAMs phenotype by counteracting NF-κB activity [184]. It is established that ApoE mediates cholesterol metabolism, is highly expressed by TAMs and CAFs both in mouse and human PDA and correlates with patient survival. Recently, Kemp and colleagues showed that apolipoprotein E (ApoE) sustains immunosuppressive TME in PDA via NF-κB-mediated CXCL1/5. Specifically, ApoE expressed in tumor macrophages binds LDLR and induces CXCL1/5 expression through NF-κB signaling [185]. Accumulating evidence showed that lung cancer displays alveolar macrophages (AM), the tissue-resident macrophages, which exhibit different functions within the TME. As for other cancers, TAMs sustain proliferation, immunosuppression and invasion in lung cancer via secretion of several cytokines, chemokines and growth factors [155]. Analysis of non-small cell lung cancer (NSCLC) tumors from 60 patients indicate that plasminogen activator inhibitor-1 (PAI-1) expression correlates with TGF-β expression and the percentage of TAMs. In vitro study has demonstrated that PAI-1 on cancer cells binds TLR4 and promotes TGF-β secretion in macrophages by increasing IL-6 production via NF-κB activation. At the same time, TGF-β promotes PAI-1 expression in NSCLC cells in a R1/SMAD3-dependent manner. However, while a high concentration of TGF-β can inhibit NF-κB activation, optimal concentration of TGF-β activates the NF-κB pathway and IL-6-induced TGF-β production. These findings indicate the presence of an auto-regulatory or auto-inhibitory loop regulated by NF-κB activation responsible for the immunosuppressive TME in NSCLC [186].
4. Targeting NF-κB Pathway in TAMs
It is recognized that TAMs are involved in orchestrating the immune response, tumor growth and progression as well as metastasis and TME remodeling. In particular, the polarization of TAMs to the M1 phenotype exerts important tumor suppressing effects on the TME, while the M2-like macrophages display pro-tumoral and angiogenetic activity [11]. NF-κB plays a key role in the regulation of TAMs as it swings the scale between tumor suppression and tumor progression [15]. Thus, there is a rationale to target NF-κB pathway to counteract TAMs activity within the TME. In this contest, the principal strategies explored are (a) re-education of TAMs towards the antitumoral (M1) phenotype; (b) TAMs depletion; (c) termination/blocking of macrophage recruitment and accumulation into the TME (Table 2). Although all these strategies showed efficacy in early-stage diseases, recent evidence suggests that the TAM reprogramming approach could be safer and more effective.
Table 2.
Molecules/drugs targeting NF-κB pathways to reshape tumor microenvironment via termination of macrophage recruitment, TAMs depletion, and TAMs repolarization.
| TAM REPROGRAMMING | |||
|---|---|---|---|
| Molecules/Drug | Target | Cancers | Ref. |
| Chrysin thiazole derivative (ChR-TD) |
TLR4/NF-κB | Breast cancer cell line (4T1) | [187] |
| Anemoside A3 (A3) | TLR4/NF-κB | Breast cancer | [188] |
| β-D-(1→6) glucan (AAMP-A70) |
TLR2/Akt/NF-κB | Colon cancer cells | [189] |
| Aqueous Extract of Cimicifuga dahurica (CRAE) | TLR4/MyD88/TAK1/NF-κB | MM | [190] |
| Homogeneous Polyporus Polysaccharide (HPP) | TLR2/NF-κB/NLRP3 | Bladder cancer | [191] |
| Baicalein | PI3K/NF-κB | Breast cancer, melanoma | [57] |
| IKK2 | NF-κB | OC | [192] |
| BAY11-7082 | NF-κB/miR30a | Bladder cancer cells | [166] |
| IncRNA DCST1-AS1 | NF-κB | OSCC | [193] |
| Glycocalyx-mimicking nanoparticles (GNPs) | STAT6 and NF-κB | LLC | [194] |
| Mannose modified lipid nanoparticles (M-IMD-LNP) with IMD-0354 |
NF-κB | Melanoma cells (B16) | [195] |
| Hyaluronic acid (HA) nanoparticles, loaded with micro-RNA miR-125 |
NF-κB | NSCLC | [196] |
| Porous hollow iron oxide nanoparticles (PHNPs) loaded with 3-methyladenine (3-MA) | PI3Kγ/Akt/NF-κB | Breast cancer cell line (MDA-MB-231) | [197] |
| PLGA-ION-R837@M | TLR7/IRF5/NF-κB | Breast cancer cell line (4T1) | [198] |
| Gd@C82 nanoparticles modified with b-alanines (GF-Ala) | NF-κB/IRF5 | Breast cancer cell line (4T1) | [199] |
| Copper sulfide nanoparticles (CuS-NP) | NF-κB | Melanoma | [200] |
| Cetuximab | NF-κB and STAT3 | CRC | [201] |
| Cabazitaxel | TLR/NF-κB | Breast cancer cells | [202] |
| Proton irradiation | NF-κB | THP1 cells | [80] |
| TAM DEPLETION AND TERMINATION OF RECRUITMENT | |||
| CCR2 antagonist or knocking out of host CCR2 | CCL2/CCR2/NF-κB | HCC | [203] |
| Total glucosides of paeony (TGP) | NF-κB/CCL2 | Breast cancer | [204] |
| IκBα si-RNA encapsulated into mannosylated siRNA-delivering NPs | NF-κB | OC, breast cancer | [205] |
| Trans-retinoic acid (ATRA) | NF-κB | PCa | [206] |
4.1. Re-Educating TAMs via NF-κB Modulation
A promising and highly investigated approach engages toll like receptors (TLRs) to induce an immunostimulatory response and reshape TAMs into M1 phenotype. Several studies using bioactive compounds such as chrysin thiazole derivative (ChR-TD), anemoside A3 (A3), β-D-(1→6) glucan (AAMP-A70), aqueous extract of Cimicifuga dahurica (CRAE) as well as antineoplastic agent (e.g., paclitaxel) demonstrated that these drugs activate TLR2 or TLR4 that in turn directly trigger the NF-κB pathway leading to TAMs reprogramming toward M1-like antitumor phenotype. In vitro studies demonstrated that these compounds induce a significant increase of TNF-α, IL-12, IL-6 and IL-1β mRNA expression and downregulate M2-like genes like IL-10, Ym-1, CD206 and Arg-1 in several models of cancer (e.g., breast cancer, colon cancer, MM) [187,188,189,190,207]. Accordingly, homogeneous polyporus polysaccharide (HPP) induces M1 polarization via TLR2/NF-κB/NLRP3 signaling activation and reduces the progression of bladder cancer [191].
The PI3K/NF-κB axis is another molecular target investigated for its capability to promote M1 polarization. He and colleagues demonstrated that the inhibition of PI3Kγ with the natural compound baicalein potentiates the M1 macrophage polarization and inhibits tumor growth via NF-κB/TNF-α inflammatory signaling in both breast cancer and melanoma mouse [57].
Hoover and collaborators showed that increased IKK2 activity, an enzyme involved in the NF-κB signaling pathway, in macrophages contributes to M1 TAMs polarization and abrogates ovarian cancer growth in vivo [192].
It has been shown that the use of BAY11-7082, a selected inhibitor of the NF-κB pathway, inhibits anti-inflammatory macrophage phenotype and reduces the invasiveness of bladder cancer cells by increasing the expression of miR30a [166]. Additionally, targeting IncRNA DCST1-AS1/NF-κB axis in oral epithelial squamous cell carcinoma (OSCC) blocks the M2 polarization and tumor progression [193].
Recent and innovative approaches have exploited nanoparticle technology to restrain TAM activity. An interesting attempt has been made with glycocalyx-mimicking nanoparticles (GNPs), both in vitro and in vivo. These nanoparticles are specifically internalized by TAMs and can neutralize and reprogram M2 TAMs through the reciprocal STAT6 suppression and NF-κB phosphorylation. In addition, the authors demonstrated that GNPs improve the therapeutic efficacy of anti-PDL1 and reduce Lewis lung carcinoma (LLC) growth [194]. Congruently, mannose modified lipid nanoparticles (M-IMD-LNP), containing the NF-κB inhibitor IMD-0354, as well as hyaluronic acid (HA) nanoparticles, loaded with the micro-RNA miR-125, showed that M2 TAMs are able to repolarize toward the M1 phenotype, in an NF-κB dependent manner, both in melanoma cells and in an in vivo model of non-small cell lung cancer (NSCLC) [195,196]. Furthermore, Li and collaborators showed that a modified porous hollow iron oxide nanoparticles (PHNPs) loaded with a P13Kγ small molecule inhibitor (3-methyladenine, 3-MA), (PHNPs@DPA-S-S BSA-MA@3-MA), selectively inhibit the PI3Kγ/Akt signaling in TAMs by inducing a prolonged activation of NF-κB, through the reduction of the P13Kγ protein in macrophages and cancer cells, that in turn, promote the switch of TAMs toward pro-inflammatory M1 phenotype and the activation of immune response leading to reduced tumor growth and immunosuppressive TME [197].
The use of cell membrane-coated nanocarrier system such as PLGA-ION-R837@M, is another strategy to repolarize macrophages toward M1 and mitigate immunosuppressive TME by activating interferon regulatory factor 5 (IRF5) and NF-κB pathway using the synergy of Fe3O4 NPs and R837, a TLR7 antagonist [198]. As for PLGA-ION-R837@M, Gd@C82 nanoparticles modified with b-alanines (GF-Ala) showed an anticancer effect in vivo by activating the anti-tumor immune response and relieving the immunosuppressive TME via NF-κB/IRF5 activation [199].
In addition, the reprogramming of M2 macrophage in M1 via NF-κB pathway activation and the consequently tumor inhibition, was also observed in melanoma-bearing mice using copper sulfide nanoparticles (CuS-NP) [200].
Recently, Zhao and collaborators demonstrated that cetuximab exerts its antitumoral effect in colon cancer attenuating the M2 TAMs pro-tumorigenic activity and triggering the repolarization of TAMs from the M2 to the M1 phenotype. Cetuximab inhibits EGFR signaling and suppresses IL-6 expression in M2 TAMs by directly inhibiting the NF-κB and STAT3 pathways. Cetuximab-induced TAM repolarization strongly affects the immunosuppressive TME by reducing the tumor burden and inflammation in both the xenograft and azoxymethane/dextran sodium sulfate (AOM/DSS)-induced mouse colon cancer model [201]. Another drug able to re-programming TAMs toward a M1 phenotype is cabazitaxel. The RNA profiling of bone marrow–derived macrophage (BMDM) treated with cabazitaxel displays a significant TLR/NF-κB signaling activation and consequently the upregulation of NF-κB-mediated cytokines and chemokines expression compared to control. Indeed, rather than exerting a direct cytotoxic effect on breast cancer cells, cabazitaxel appears to induce an NF-κB-mediated macrophage’s polarization toward a M1 state stimulating the programmed cell removal (PrCR) macrophage activity [202].
According to the TAM re-polarization strategies, the treatment with proton irradiation has shown to be effective in the reprogramming of TAMs via modulation of NF-κB signaling. Proton therapy applied on THP1-derived M0, M1 and M2 phenotype macrophages promotes an enhanced nuclear translocation of NF-κB p65 in all three groups after 2h of irradiation. Both gene expression and cytokines analysis showed that moderate doses of proton irradiation promote the reprogramming of M0 and M2 macrophages towards an M1 phenotype. However, the combined treatment of irradiation with the NF-κB inhibitor Bay 11-7082 (IKK inhibitor) produces a clear induction of the macrophages towards the M2 phenotype. In particular, the M0 macrophages shifted to the M2 phenotype rather than M1 polarization, while a reinforcement of the M2 phenotype was observed in M2 macrophages [80].
4.2. TAMs Depletion and Termination of Recruitment via NF-κB Modulation
Recently, it has been demonstrated that CCL2, a pro-inflammatory and NF-κB-mediated chemokine, promotes TAM infiltration. In addition, NF-κB/CCL2 signaling pathway plays a key role in tumor progression, invasion and metastasis in a wide range of human cancers [203]. Therapeutic inhibition of CCL2/CCR2 axis through NF-κB ablation, abrogates inflammatory monocyte recruitment and TAM infiltration in different mice models of several cancer types such as primary and metastatic breast cancer, HCC and lung cancer [204,208]. In this contest, the natural compound total glucosides of paeony (TGP) downregulates the expression of genes involved in the formation and function of TAMs via NF-κB/CCL2 ablation as well as the secretion of CCL2 in primary and metastatic breast cancer. Furthermore, it decreases tumor growth and mitigates the immunosuppressive TME by decreasing CD45+CD11b+F4/80+ TAMs population and promoting CD4+ and CD8+ T cell infiltration in vivo [204].
The knocking down of IκBα protein obtained through the transfection of the IκBα si-RNA encapsulated into mannosylated siRNA-delivering NPs, promotes the recruitment of T-cells within the TME and inhibits macrophage infiltration both in ovarian and in breast cancer [205,209,210].
All trans-retinoic acid (ATRA) is a clinically available molecule able to immune-modulate myeloid cells. In a study on prostate cancer, it was shown that the treatment of primary macrophages with medium conditioned with PC3 prostate cancer cells induces a clear activation of the NF-κB and ERK pathways, and M2 macrophages polarization. Exposure to ATRA suppresses the production of the M2-like released factors (e.g., IL-10, IL-1β, indoleamine-pyrrole 2,3-dioxygenase (IDO) and VEGF) and downregulates MHC class I and II and Fas ligand (FasL) molecules expression, through a specific inhibition of NF-κB p50 without effects on ERK phosphorylation [206].
4.3. Targeting TAMs in Clinical Setting
It is known that NF-κB transcription factors are major drivers of most human cancers and yet there are no clinically useful NF-κB inhibitors to treat most of them, given the on-target toxicities of IKK/NF-κB targeting drugs. Currently, numerous cancer-selective agents targeting upstream activators or downstream effectors of the NF-κB pathway that are able to circumvent the adverse effects associated with the systemic targeting of NF-κB, are being tested in clinical studies [40,41,211,212,213,214,215]. However, none of these agents showed the capability to specifically target NF-κB in TAMs. Unlike NF-κB inhibitors, several other agents in clinical trials were proven effective in polarizing TAMs toward an anti-tumor phenotype when used alone or in combination therapy [216,217]. The use of TLR agonists, either as monotherapy or more commonly in combination with targeted therapy or immunotherapy, promotes TAMs-specific antitumor activity in advanced and refractory solid tumors [218,219]. Additionally, several agents targeting CSF1(R), CCR5, CD40, CD47 as well as CCL2-CCR2 have shown efficacy both as a single agent or in combination in patients with solid tumors [216,220,221,222,223,224,225,226,227]. Yet, recent evidence arising from ongoing clinical trials indicates that the functional reprogramming of TAMs could be a promising therapeutic approach to ameliorate the outcomes of cancer patients.
5. Conclusions
TAMs are the principal component of the TME, and infiltrating macrophages are associated with worse clinical outcome and drug resistance. Although numerous progresses have been made for cancer treatment, targeting macrophages represent a new approach in cancer immunotherapy to improve tumor immune microenvironment. NF-κB is a master regulator of innate immune responses and plays a key role in TAM reprogramming within the TME. However, while a great bulk of evidence has been collected, there are still controversies about the functions of NF-κB in myeloid cells within the TME. Hence, NF-κB activation in TAMs has been associated to the acquisition of both immunosuppressive and immunopermissive TAM phenotype, with opposite effects in terms of tumor progression. Therefore, further studies are needed to better understand in which tumoral contexts the inhibition of NF-κB signaling to reprogram TAMs could be of benefit.
Acknowledgments
D.V. (Daniela Verzella) has been supported by National Operational Programme (PON)-Attraction and International Mobility (AIM) Research and Innovation 2014–2020, Project AIM1855453; D.V. (Davide Vecchiotti) has been supported by National Operational Programme (PON)-Attraction and International Mobility (AIM) Research and Innovation 2014–2020, Project AIM1887574.
Author Contributions
Writing—original draft preparation, J.C.; writing—review and editing, D.V. (Daniela Verzella), D.C., F.Z. and G.F.; figure and table preparation, D.V. (Davide Vecchiotti) and P.A. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no competing financial interests.
Funding Statement
This research was funded by Cancer Research UK, grant number A15115; NIHR Imperial Biomedical Research Centre (BRC), grant number P81135; Medical Research Council (MRC), grant number MR/V027581/1; Imperial College London Joint Translational Fund, Medical Research Council (MRC) and Yuhan Corporation, Imperial Confidence in Concept (ICiC) 2021, grant number RSRO_PA2579, RSRO_P87961 to G.F.; Progetto di Ateneo per la Ricerca di Base 2022, University of L’Aquila, grant number 07_Progetto_Ricerca_Ateneo_Verzella to D.V. (Daniela Verzella); Intramural DISCAB GRANT 2023, Department of Biotechnological and Applied Clinical Sciences, University of L’Aquila, grant number 07_DG_2023_22 to D.V. (Daniela Verzella).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hirayama D., Iida T., Nakase H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017;19:92. doi: 10.3390/ijms19010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verschoor C.P., Puchta A., Bowdish D.M.E. The Macrophage. Methods Mol. Biol. 2012;844:139–156. doi: 10.1007/978-1-61779-527-5_10. [DOI] [PubMed] [Google Scholar]
- 3.Taylor P.R., Gordon S. Monocyte Heterogeneity and Innate Immunity. Immunity. 2003;19:2–4. doi: 10.1016/S1074-7613(03)00178-X. [DOI] [PubMed] [Google Scholar]
- 4.Mueller M.M., Fusenig N.E. Friends or Foes—Bipolar Effects of the Tumour Stroma in Cancer. Nat. Rev. Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- 5.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-Related Inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Dwyer A.R., Greenland E.L., Pixley F.J. Promotion of Tumor Invasion by Tumor-Associated Macrophages: The Role of CSF-1-Activated Phosphatidylinositol 3 Kinase and Src Family Kinase Motility Signaling. Cancers. 2017;9:68. doi: 10.3390/cancers9060068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bain C.C., Scott C.L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W.W., Mowat A.M. Resident and Pro-Inflammatory Macrophages in the Colon Represent Alternative Context-Dependent Fates of the Same Ly6Chi Monocyte Precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulz C., Gomez Perdiguero E., Chorro L., Szabo-Rogers H., Cagnard N., Kierdorf K., Prinz M., Wu B., Jacobsen S.E.W., Pollard J.W., et al. A Lineage of Myeloid Cells Independent of Myb and Hematopoietic Stem Cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- 9.Sharma S.K., Chintala N.K., Vadrevu S.K., Patel J., Karbowniczek M., Markiewski M.M. Pulmonary Alveolar Macrophages Contribute to the Premetastatic Niche by Suppressing Antitumor T Cell Responses in the Lungs. J. Immunol. 2015;194:5529–5538. doi: 10.4049/jimmunol.1403215. [DOI] [PubMed] [Google Scholar]
- 10.Wang N., Liang H., Zen K. Molecular Mechanisms That Influence the Macrophage M1-M2 Polarization Balance. Front. Immunol. 2014;5:614. doi: 10.3389/fimmu.2014.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagemann T., Biswas S.K., Lawrence T., Sica A., Lewis C.E. Regulation of Macrophage Function in Tumors: The Multifaceted Role of NF-ΚB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray P.J. Macrophage Polarization. Annu. Rev. Physiol. 2017;79:541–566. doi: 10.1146/annurev-physiol-022516-034339. [DOI] [PubMed] [Google Scholar]
- 13.Martinez F.O., Gordon S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The Chemokine System in Diverse Forms of Macrophage Activation and Polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Biswas S.K., Lewis C.E. NF-ΚB as a Central Regulator of Macrophage Function in Tumors. J. Leukoc. Biol. 2010;88:877–884. doi: 10.1189/jlb.0310153. [DOI] [PubMed] [Google Scholar]
- 16.Mulder R., Banete A., Basta S. Spleen-Derived Macrophages Are Readily Polarized into Classically Activated (M1) or Alternatively Activated (M2) States. Immunobiology. 2014;219:737–745. doi: 10.1016/j.imbio.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Yao Y., Xu X.-H., Jin L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019;10:792. doi: 10.3389/fimmu.2019.00792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon S., Martinez F.O. Alternative Activation of Macrophages: Mechanism and Functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang L.-X., Zhang S.-X., Wu H.-J., Rong X.-L., Guo J. M2b Macrophage Polarization and Its Roles in Diseases. J. Leukoc. Biol. 2019;106:345–358. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zizzo G., Hilliard B.A., Monestier M., Cohen P.L. Efficient Clearance of Early Apoptotic Cells by Human Macrophages Requires M2c Polarization and MerTK Induction. J. Immunol. 2012;189:3508–3520. doi: 10.4049/jimmunol.1200662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graff J.W., Dickson A.M., Clay G., McCaffrey A.P., Wilson M.E. Identifying Functional MicroRNAs in Macrophages with Polarized Phenotypes. J. Biol. Chem. 2012;287:21816–21825. doi: 10.1074/jbc.M111.327031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaikkonen M.U., Lam M.T.Y., Glass C.K. Non-Coding RNAs as Regulators of Gene Expression and Epigenetics. Cardiovasc. Res. 2011;90:430–440. doi: 10.1093/cvr/cvr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X., Liu Q., Wang X., Yao X., Zhang B., Wu J., Sun C. Exosomal NcRNAs Facilitate Interactive “dialogue” between Tumor Cells and Tumor-Associated Macrophages. Cancer Lett. 2023;552:215975. doi: 10.1016/j.canlet.2022.215975. [DOI] [PubMed] [Google Scholar]
- 24.Ludwig N., Rubenich D.S., Zaręba Ł., Siewiera J., Pieper J., Braganhol E., Reichert T.E., Szczepański M.J. Potential Roles of Tumor Cell- and Stroma Cell-Derived Small Extracellular Vesicles in Promoting a Pro-Angiogenic Tumor Microenvironment. Cancers. 2020;12:3599. doi: 10.3390/cancers12123599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bowdridge S., Gause W.C. Regulation of Alternative Macrophage Activation by Chromatin Remodeling. Nat. Immunol. 2010;11:879–881. doi: 10.1038/ni1010-879. [DOI] [PubMed] [Google Scholar]
- 26.Lin W.-W., Karin M. A Cytokine-Mediated Link between Innate Immunity, Inflammation, and Cancer. J. Clin. Investig. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balkwill F. Cancer and the Chemokine Network. Nat. Rev. Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 28.Langowski J.L., Zhang X., Wu L., Mattson J.D., Chen T., Smith K., Basham B., McClanahan T., Kastelein R.A., Oft M. IL-23 Promotes Tumour Incidence and Growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 29.Vogel D.Y.S., Vereyken E.J.F., Glim J.E., Heijnen P.D.A.M., Moeton M., van der Valk P., Amor S., Teunissen C.E., van Horssen J., Dijkstra C.D. Macrophages in Inflammatory Multiple Sclerosis Lesions Have an Intermediate Activation Status. J. Neuroinflamm. 2013;10:35. doi: 10.1186/1742-2094-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettersen J.S., Fuentes-Duculan J., Suárez-Fariñas M., Pierson K.C., Pitts-Kiefer A., Fan L., Belkin D.A., Wang C.Q.F., Bhuvanendran S., Johnson-Huang L.M., et al. Tumor-Associated Macrophages in the Cutaneous SCC Microenvironment Are Heterogeneously Activated. J. Investig. Dermatol. 2011;131:1322–1330. doi: 10.1038/jid.2011.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kerneur C., Cano C.E., Olive D. Major Pathways Involved in Macrophage Polarization in Cancer. Front. Immunol. 2022;13:1026954. doi: 10.3389/fimmu.2022.1026954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang D., Yang L., Cai J., Li H., Xing Z., Hou Y. Phosphoinositide 3-Kinase/Akt and Its Related Signaling Pathways in the Regulation of Tumor-Associated Macrophages Polarization. Mol. Cell. Biochem. 2022;477:2469–2480. doi: 10.1007/s11010-022-04461-w. [DOI] [PubMed] [Google Scholar]
- 33.Capece D., Verzella D., Flati I., Arboretto P., Cornice J., Franzoso G. NF-ΚB: Blending Metabolism, Immunity, and Inflammation. Trends Immunol. 2022;43:757–775. doi: 10.1016/j.it.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Moretti M., Bennett J., Tornatore L., Thotakura A.K., Franzoso G. Cancer: NF-ΚB Regulates Energy Metabolism. Int. J. Biochem. Cell Biol. 2012;44:2238–2243. doi: 10.1016/j.biocel.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 35.Tak P.P., Firestein G.S. NF-KappaB: A Key Role in Inflammatory Diseases. J. Clin. Investig. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baeuerle P.A., Henkel T. Function and Activation of NF-Kappa B in the Immune System. Annu. Rev. Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 37.Mittal A., Papa S., Franzoso G., Sen R. NF-kappaB-Dependent Regulation of the Timing Of. J. Immunol. 2006;174:2183–2189. doi: 10.4049/jimmunol.176.4.2183. [DOI] [PubMed] [Google Scholar]
- 38.Park M.H., Hong J.T. Roles of NF-ΚB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells. 2016;5:15. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu T., Zhang L., Joo D., Sun S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Begalli F., Bennett J., Capece D., Verzella D., D’Andrea D., Tornatore L., Franzoso G. Unlocking the NF-ΚB Conundrum: Embracing Complexity to Achieve Specificity. Biomedicines. 2017;5:50. doi: 10.3390/biomedicines5030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verzella D., Cornice J., Arboretto P., Vecchiotti D., Di Vito Nolfi M., Capece D., Zazzeroni F., Franzoso G. The NF-ΚB Pharmacopeia: Novel Strategies to Subdue an Intractable Target. Biomedicines. 2022;10:2233. doi: 10.3390/biomedicines10092233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bennett J., Capece D., Begalli F., Verzella D., D’Andrea D., Tornatore L., Franzoso G. NF-ΚB in the Crosshairs: Rethinking an Old Riddle. Int. J. Biochem. Cell Biol. 2018;95:108–112. doi: 10.1016/j.biocel.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hinz M., Scheidereit C. The IκB Kinase Complex in NF-ΚB Regulation and Beyond. EMBO Rep. 2014;15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oeckinghaus A., Ghosh S. The NF-KappaB Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collins P.E., Mitxitorena I., Carmody R.J. The Ubiquitination of NF-ΚB Subunits in the Control of Transcription. Cells. 2016;5:23. doi: 10.3390/cells5020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayden M.S., Ghosh S. NF-ΚB, the First Quarter-Century: Remarkable Progress and Outstanding Questions. Genes Dev. 2012;26:203–234. doi: 10.1101/gad.183434.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H., Sun S.-C. NF-ΚB in Inflammation and Renal Diseases. Cell Biosci. 2015;5:63. doi: 10.1186/s13578-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perkins N.D., Gilmore T.D. Good Cop, Bad Cop: The Different Faces of NF-KappaB. Cell Death Differ. 2006;13:759–772. doi: 10.1038/sj.cdd.4401838. [DOI] [PubMed] [Google Scholar]
- 49.Sun S.-C. Non-Canonical NF-ΚB Signaling Pathway. Cell Res. 2011;21:71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kriete A., Mayo K.L. Atypical Pathways of NF-KappaB Activation and Aging. Exp. Gerontol. 2009;44:250–255. doi: 10.1016/j.exger.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 51.O’Neill L.A.J. How Toll-like Receptors Signal: What We Know and What We Don’t Know. Curr. Opin. Immunol. 2006;18:3–9. doi: 10.1016/j.coi.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 52.Kawai T., Akira S. Toll-like Receptor Downstream Signaling. Arthritis Res. Ther. 2005;7:12–19. doi: 10.1186/ar1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozato K., Tsujimura H., Tamura T. Toll-like Receptor Signaling and Regulation of Cytokine Gene Expression in the Immune System. Biotechniques. 2002;33:S66–S75. doi: 10.2144/Oct0208. [DOI] [PubMed] [Google Scholar]
- 54.Weigert A., Tzieply N., von Knethen A., Johann A.M., Schmidt H., Geisslinger G., Brüne B. Tumor Cell Apoptosis Polarizes Macrophages Role of Sphingosine-1-Phosphate. Mol. Biol. Cell. 2007;18:3810–3819. doi: 10.1091/mbc.e06-12-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauh M.J., Ho V., Pereira C., Sham A., Sly L.M., Lam V., Huxham L., Minchinton A.I., Mui A., Krystal G. SHIP Represses the Generation of Alternatively Activated Macrophages. Immunity. 2005;23:361–374. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Biswas S.K., Gangi L., Paul S., Schioppa T., Saccani A., Sironi M., Bottazzi B., Doni A., Vincenzo B., Pasqualini F., et al. A Distinct and Unique Transcriptional Program Expressed by Tumor-Associated Macrophages (Defective NF-KappaB and Enhanced IRF-3/STAT1 Activation) Blood. 2006;107:2112–2122. doi: 10.1182/blood-2005-01-0428. [DOI] [PubMed] [Google Scholar]
- 57.He S., Wang S., Liu S., Li Z., Liu X., Wu J. Baicalein Potentiated M1 Macrophage Polarization in Cancer Through Targeting PI3Kγ/ NF-ΚB Signaling. Front. Pharmacol. 2021;12:743837. doi: 10.3389/fphar.2021.743837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shan S., Fang B., Zhang Y., Wang C., Zhou J., Niu C., Gao Y., Zhao D., He J., Wang J., et al. Mechanical Stretch Promotes Tumoricidal M1 Polarization via the FAK/NF-ΚB Signaling Pathway. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019;33:13254–13266. doi: 10.1096/fj.201900799RR. [DOI] [PubMed] [Google Scholar]
- 59.Gao J., Wang D., Liu D., Liu M., Ge Y., Jiang M., Liu Y., Zheng D. Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand Induces the Expression of Proinflammatory Cytokines in Macrophages and Re-Educates Tumor-Associated Macrophages to an Antitumor Phenotype. Mol. Biol. Cell. 2015;26:3178–3189. doi: 10.1091/mbc.e15-04-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee C.-H., Wu C.-L., Shiau A.-L. Toll-like Receptor 4 Signaling Promotes Tumor Growth. J. Immunother. 2010;33:73–82. doi: 10.1097/CJI.0b013e3181b7a0a4. [DOI] [PubMed] [Google Scholar]
- 61.He R., He Y., Du R., Liu C., Chen Z., Zeng A., Song L. Revisiting of TAMs in Tumor Immune Microenvironment: Insight from NF-ΚB Signaling Pathway. Biomed. Pharmacother. 2023;165:115090. doi: 10.1016/j.biopha.2023.115090. [DOI] [PubMed] [Google Scholar]
- 62.Yang Y., Qin J., Lan L., Li N., Wang C., He P., Liu F., Ni H., Wang Y. M-CSF Cooperating with NFκB Induces Macrophage Transformation from M1 to M2 by Upregulating c-Jun. Cancer Biol. Ther. 2014;15:99–107. doi: 10.4161/cbt.26718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mancino A., Lawrence T. Nuclear Factor-KappaB and Tumor-Associated Macrophages. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010;16:784–789. doi: 10.1158/1078-0432.CCR-09-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arkan M.C., Greten F.R. IKK- and NF-ΚB-Mediated Functions in Carcinogenesis. Curr. Top. Microbiol. Immunol. 2011;349:159–169. doi: 10.1007/82_2010_97. [DOI] [PubMed] [Google Scholar]
- 65.Saccani A., Schioppa T., Porta C., Biswas S.K., Nebuloni M., Vago L., Bottazzi B., Colombo M.P., Mantovani A., Sica A. P50 Nuclear Factor-ΚB Overexpression in Tumor-Associated Macrophages Inhibits M1 Inflammatory Responses and Antitumor Resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 66.Kühnemuth B., Michl P. The Role of CUX1 in Antagonizing NF-ΚB Signaling in TAMs. Oncoimmunology. 2014;3:e28270. doi: 10.4161/onci.28270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chang C.P., Su Y.C., Hu C.W., Lei H.Y. TLR2-Dependent Selective Autophagy Regulates NF-ΚB Lysosomal Degradation in Hepatoma-Derived M2 Macrophage Differentiation. Cell Death Differ. 2013;20:515–523. doi: 10.1038/cdd.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andersson P., Yang Y., Hosaka K., Zhang Y., Fischer C., Braun H., Liu S., Yu G., Liu S., Beyaert R., et al. Molecular Mechanisms of IL-33-Mediated Stromal Interactions in Cancer Metastasis. JCI Insight. 2018;3:e122375. doi: 10.1172/jci.insight.122375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pollard J.W. Tumour-Educated Macrophages Promote Tumour Progression and Metastasis. Nat. Rev. Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 70.Span P.N., Bussink J. Biology of Hypoxia. Semin. Nucl. Med. 2015;45:101–109. doi: 10.1053/j.semnuclmed.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 71.Li Y., Zhao L., Li X.-F. Hypoxia and the Tumor Microenvironment. Technol. Cancer Res. Treat. 2021;20:15330338211036304. doi: 10.1177/15330338211036304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Capece D., Fischietti M., Verzella D., Gaggiano A., Cicciarelli G., Tessitore A., Zazzeroni F., Alesse E. The Inflammatory Microenvironment in Hepatocellular Carcinoma: A Pivotal Role for Tumor-Associated Macrophages. Biomed. Res. Int. 2013;2013:187204. doi: 10.1155/2013/187204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A.S., Nizet V., Johnson R.S., Haddad G.G., Karin M. NF-KappaB Links Innate Immunity to the Hypoxic Response through Transcriptional Regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noman M.Z., Hasmim M., Messai Y., Terry S., Kieda C., Janji B., Chouaib S. Hypoxia: A Key Player in Antitumor Immune Response. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015;309:C569–C579. doi: 10.1152/ajpcell.00207.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Delprat V., Tellier C., Demazy C., Raes M., Feron O., Michiels C. Cycling Hypoxia Promotes a Pro-Inflammatory Phenotype in Macrophages via JNK/P65 Signaling Pathway. Sci. Rep. 2020;10:882. doi: 10.1038/s41598-020-57677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Medvedeva G.F., Kuzmina D.O., Nuzhina J., Shtil A.A., Dukhinova M.S. How Macrophages Become Transcriptionally Dysregulated: A Hidden Impact of Antitumor Therapy. Int. J. Mol. Sci. 2021;22:2662. doi: 10.3390/ijms22052662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dijkgraaf E.M., Heusinkveld M., Tummers B., Vogelpoel L.T.C., Goedemans R., Jha V., Nortier J.W.R., Welters M.J.P., Kroep J.R., van der Burg S.H. Chemotherapy Alters Monocyte Differentiation to Favor Generation of Cancer-Supporting M2 Macrophages in the Tumor Microenvironment. Cancer Res. 2013;73:2480–2492. doi: 10.1158/0008-5472.CAN-12-3542. [DOI] [PubMed] [Google Scholar]
- 78.Beach C., MacLean D., Majorova D., Arnold J.N., Olcina M.M. The Effects of Radiation Therapy on the Macrophage Response in Cancer. Front. Oncol. 2022;12:1020606. doi: 10.3389/fonc.2022.1020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lödermann B., Wunderlich R., Frey S., Schorn C., Stangl S., Rödel F., Keilholz L., Fietkau R., Gaipl U.S., Frey B. Low Dose Ionising Radiation Leads to a NF-ΚB Dependent Decreased Secretion of Active IL-1β by Activated Macrophages with a Discontinuous Dose-Dependency. Int. J. Radiat. Biol. 2012;88:727–734. doi: 10.3109/09553002.2012.689464. [DOI] [PubMed] [Google Scholar]
- 80.Genard G., Wera A.C., Huart C., Le Calve B., Penninckx S., Fattaccioli A., Tabarrant T., Demazy C., Ninane N., Heuskin A.C., et al. Proton Irradiation Orchestrates Macrophage Reprogramming through NFκB Signaling. Cell Death Dis. 2018;9:728. doi: 10.1038/s41419-018-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crittenden M.R., Cottam B., Savage T., Nguyen C., Newell P., Gough M.J. Expression of NF-ΚB P50 in Tumor Stroma Limits the Control of Tumors by Radiation Therapy. PLoS ONE. 2012;7:e39295. doi: 10.1371/journal.pone.0039295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 83.Bruix J., Reig M., Sherman M. Evidence-Based Diagnosis, Staging, and Treatment of Patients With Hepatocellular Carcinoma. Gastroenterology. 2016;150:835–853. doi: 10.1053/j.gastro.2015.12.041. [DOI] [PubMed] [Google Scholar]
- 84.Fan Q.-M., Jing Y.-Y., Yu G.-F., Kou X.-R., Ye F., Gao L., Li R., Zhao Q.-D., Yang Y., Lu Z.-H., et al. Tumor-Associated Macrophages Promote Cancer Stem Cell-like Properties via Transforming Growth Factor-Beta1-Induced Epithelial-Mesenchymal Transition in Hepatocellular Carcinoma. Cancer Lett. 2014;352:160–168. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 85.Budhu A., Forgues M., Ye Q.-H., Jia H.-L., He P., Zanetti K.A., Kammula U.S., Chen Y., Qin L.-X., Tang Z.-Y., et al. Prediction of Venous Metastases, Recurrence, and Prognosis in Hepatocellular Carcinoma Based on a Unique Immune Response Signature of the Liver Microenvironment. Cancer Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 86.Maeda S., Kamata H., Luo J.-L., Leffert H., Karin M. IKKbeta Couples Hepatocyte Death to Cytokine-Driven Compensatory Proliferation That Promotes Chemical Hepatocarcinogenesis. Cell. 2005;121:977–990. doi: 10.1016/j.cell.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 87.Pikarsky E., Porat R.M., Stein I., Abramovitch R., Amit S., Kasem S., Gutkovich-Pyest E., Urieli-Shoval S., Galun E., Ben-Neriah Y. NF-KappaB Functions as a Tumour Promoter in Inflammation-Associated Cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 88.Luedde T., Beraza N., Kotsikoris V., van Loo G., Nenci A., De Vos R., Roskams T., Trautwein C., Pasparakis M. Deletion of NEMO/IKKgamma in Liver Parenchymal Cells Causes Steatohepatitis and Hepatocellular Carcinoma. Cancer Cell. 2007;11:119–132. doi: 10.1016/j.ccr.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 89.Sakurai T., He G., Matsuzawa A., Yu G.-Y., Maeda S., Hardiman G., Karin M. Hepatocyte Necrosis Induced by Oxidative Stress and IL-1 α Release Mediate Carcinogen-Induced Compensatory Proliferation and Liver Tumorigenesis. Cancer Cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei R., Zhu W.W., Yu G.Y., Wang X., Gao C., Zhou X., Lin Z.F., Shao W.Q., Wang S.H., Lu M., et al. S100 Calcium-Binding Protein A9 from Tumor-Associated Macrophage Enhances Cancer Stem Cell-like Properties of Hepatocellular Carcinoma. Int. J. Cancer. 2021;148:1233–1244. doi: 10.1002/ijc.33371. [DOI] [PubMed] [Google Scholar]
- 91.Fantuzzi L., Spadaro F., Purificato C., Cecchetti S., Podo F., Belardelli F., Gessani S., Ramoni C. Phosphatidylcholine-Specific Phospholipase C Activation Is Required for CCR5-Dependent, NF-KB-Driven CCL2 Secretion Elicited in Response to HIV-1 Gp120 in Human Primary Macrophages. Blood. 2008;111:3355–3363. doi: 10.1182/blood-2007-08-104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen S., Zheng P., Wang W., Yi M., Chen P., Cai J., Li J., Peng Q., Ban Y., Zhou Y., et al. Abberent Expression of NOR1 Protein in Tumor Associated Macrophages Contributes to the Development of DEN-Induced Hepatocellular Carcinoma. J. Cell. Physiol. 2018;233:5002–5013. doi: 10.1002/jcp.26349. [DOI] [PubMed] [Google Scholar]
- 93.Papa S., Zazzeroni F., Bubici C., Jayawardena S., Alvarez K., Matsuda S., Nguyen D.U., Pham C.G., Nelsbach A.H., Melis T., et al. Gadd45 β Mediates the NF-Kappa B Suppression of JNK Signalling by Targeting MKK7/JNKK2. Nat. Cell Biol. 2004;6:146–153. doi: 10.1038/ncb1093. [DOI] [PubMed] [Google Scholar]
- 94.De Smaele E., Zazzeroni F., Papa S., Nguyen D.U., Jin R., Jones J., Cong R., Franzoso G. Induction of Gadd45beta by NF-KappaB Downregulates pro-Apoptotic JNK Signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- 95.Papa S., Zazzeroni F., Fu Y.X., Bubici C., Alvarez K., Dean K., Christiansen P.A., Anders R.A., Franzoso G. Gadd45β Promotes Hepatocyte Survival during Liver Regeneration in Mice by Modulating JNK Signaling. J. Clin. Investig. 2008;118:1911–1923. doi: 10.1172/JCI33913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Papa S., Monti S.M., Vitale R.M., Bubici C., Jayawardena S., Alvarez K., De Smaele E., Dathan N., Pedone C., Ruvo M., et al. Insights into the Structural Basis of the GADD45β-Mediated Inactivation of the JNK Kinase, MKK7/JNKK2. J. Biol. Chem. 2007;282:19029–19041. doi: 10.1074/jbc.M703112200. [DOI] [PubMed] [Google Scholar]
- 97.Tornatore L., Marasco D., Dathan N., Vitale R.M., Benedetti E., Papa S., Franzoso G., Ruvo M., Monti S.M. Gadd45β Forms a Homodimeric Complex That Binds Tightly to MKK7. J. Mol. Biol. 2008;378:97–111. doi: 10.1016/j.jmb.2008.01.074. [DOI] [PubMed] [Google Scholar]
- 98.Verzella D., Bennett J., Fischietti M., Thotakura A.K., Recordati C., Pasqualini F., Capece D., Vecchiotti D., D’Andrea D., Di Francesco B., et al. GADD45β Loss Ablates Innate Immunosuppression in Cancer. Cancer Res. 2018;78:1275–1292. doi: 10.1158/0008-5472.CAN-17-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Capece D., D’Andrea D., Verzella D., Tornatore L., Begalli F., Bennett J., Zazzeroni F., Franzoso G. Turning an Old GADDget into a Troublemaker. Cell Death Differ. 2018;25:642–644. doi: 10.1038/s41418-018-0087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hu Y.C., Yi Z.J., Zhou Y., Li P.Z., Liu Z.J., Duan S.G., Gong J.P. Overexpression of RIP 140 Suppresses the Malignant Potential of Hepatocellular Carcinoma by Inhibiting NF-KB-Mediated Alternative Polarization of Macrophages. Oncol. Rep. 2017;37:2971–2979. doi: 10.3892/or.2017.5551. [DOI] [PubMed] [Google Scholar]
- 101.Sharen G., Cheng H., Hu X., Miao J., Zhao D. M1-like Tumor-Associated Macrophages Enhance Proliferation and Anti-Apoptotic Ability of Liver Cancer Cells via Activating the NF-ΚB Signaling Pathway. Mol. Med. Rep. 2022;26:331. doi: 10.3892/mmr.2022.12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wu S.-P., Liao R.-Q., Tu H.-Y., Wang W.-J., Dong Z.-Y., Huang S.-M., Guo W.-B., Gou L.-Y., Sun H.-W., Zhang Q., et al. Stromal PD-L1-Positive Regulatory T Cells and PD-1-Positive CD8-Positive T Cells Define the Response of Different Subsets of Non-Small Cell Lung Cancer to PD-1/PD-L1 Blockade Immunotherapy. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2018;13:521–532. doi: 10.1016/j.jtho.2017.11.132. [DOI] [PubMed] [Google Scholar]
- 103.Hamid O., Robert C., Daud A., Hodi F.S., Hwu W.-J., Kefford R., Wolchok J.D., Hersey P., Joseph R.W., Weber J.S., et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N. Engl. J. Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Motzer R.J., Rini B.I., McDermott D.F., Redman B.G., Kuzel T.M., Harrison M.R., Vaishampayan U.N., Drabkin H.A., George S., Logan T.F., et al. Nivolumab for Metastatic Renal Cell Carcinoma: Results of a Randomized Phase II Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:1430–1437. doi: 10.1200/JCO.2014.59.0703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Atkins M.B., Clark J.I., Quinn D.I. Immune Checkpoint Inhibitors in Advanced Renal Cell Carcinoma: Experience to Date and Future Directions. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017;28:1484–1494. doi: 10.1093/annonc/mdx151. [DOI] [PubMed] [Google Scholar]
- 106.Topalian S.L., Hodi F.S., Brahmer J.R., Gettinger S.N., Smith D.C., McDermott D.F., Powderly J.D., Carvajal R.D., Sosman J.A., Atkins M.B., et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu X., Horner J.W., Paul E., Shang X., Troncoso P., Deng P., Jiang S., Chang Q., Spring D.J., Sharma P., et al. Effective Combinatorial Immunotherapy for Castration-Resistant Prostate Cancer. Nature. 2017;543:728–732. doi: 10.1038/nature21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martini D.J., Lalani A.-K.A., Bossé D., Steinharter J.A., Harshman L.C., Hodi F.S., Ott P.A., Choueiri T.K. Response to Single Agent PD-1 Inhibitor after Progression on Previous PD-1/PD-L1 Inhibitors: A Case Series. J. Immunother. Cancer. 2017;5:66. doi: 10.1186/s40425-017-0273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu G., Feng D., Yao Y., Li P., Sun H., Yang H., Li C., Jiang R., Sun B., Chen Y. Listeria-Based Hepatocellular Carcinoma Vaccine Facilitates Anti-PD-1 Therapy by Regulating Macrophage Polarization. Oncogene. 2020;39:1429–1444. doi: 10.1038/s41388-019-1072-3. [DOI] [PubMed] [Google Scholar]
- 110.Van den Eynden G.G., Majeed A.W., Illemann M., Vermeulen P.B., Bird N.C., Høyer-Hansen G., Eefsen R.L., Reynolds A.R., Brodt P. The Multifaceted Role of the Microenvironment in Liver Metastasis: Biology and Clinical Implications. Cancer Res. 2013;73:2031–2043. doi: 10.1158/0008-5472.CAN-12-3931. [DOI] [PubMed] [Google Scholar]
- 111.Voorneveld P.W., Kodach L.L., Jacobs R.J., Liv N., Zonnevylle A.C., Hoogenboom J.P., Biemond I., Verspaget H.W., Hommes D.W., de Rooij K., et al. Loss of SMAD4 Alters BMP Signaling to Promote Colorectal Cancer Cell Metastasis via Activation of Rho and ROCK. Gastroenterology. 2014;147:196–208.e13. doi: 10.1053/j.gastro.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 112.Li M., Lai X., Zhao Y., Zhang Y., Li M., Li D., Kong J., Zhang Y., Jing P., Li H., et al. Loss of NDRG2 in Liver Microenvironment Inhibits Cancer Liver Metastasis by Regulating Tumor Associate Macrophages Polarization Article /13/1 /13/21 /13/31 /13/95 /14/5 /14/19 /38/77 /38/109 /64/60 /82/80. Cell Death Dis. 2018;9:248. doi: 10.1038/s41419-018-0284-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang L., Li Y.S., Yu L.G., Zhang X.K., Zhao L., Gong F.L., Yang X.X., Guo X.L. Galectin-3 Expression and Secretion by Tumor-Associated Macrophages in Hypoxia Promotes Breast Cancer Progression. Biochem. Pharmacol. 2020;178:114113. doi: 10.1016/j.bcp.2020.114113. [DOI] [PubMed] [Google Scholar]
- 114.Wang N., Liu W., Zheng Y., Wang S., Yang B., Li M., Song J., Zhang F., Zhang X., Wang Q., et al. CXCL1 Derived from Tumor-Associated Macrophages Promotes Breast Cancer Metastasis via Activating NF-ΚB/SOX4 Signaling. Cell Death Dis. 2018;9:880. doi: 10.1038/s41419-018-0876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moraes L.A., Kar S., Foo S.L., Gu T., Toh Y.Q., Ampomah P.B., Sachaphibulkij K., Yap G., Zharkova O., Lukman H.M., et al. Annexin-A1 Enhances Breast Cancer Growth and Migration by Promoting Alternative Macrophage Polarization in the Tumour Microenvironment. Sci. Rep. 2017;7:17925. doi: 10.1038/s41598-017-17622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.De Paolis V., Maiullari F., Chirivì M., Milan M., Cordiglieri C., Pagano F., La Manna A.R., De Falco E., Bearzi C., Rizzi R., et al. Unusual Association of NF-ΚB Components in Tumor-Associated Macrophages (TAMs) Promotes HSPG2-Mediated Immune-Escaping Mechanism in Breast Cancer. Int. J. Mol. Sci. 2022;23:7902. doi: 10.3390/ijms23147902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Guo W., Keckesova Z., Donaher J.L., Shibue T., Tischler V., Reinhardt F., Itzkovitz S., Noske A., Zürrer-Härdi U., Bell G., et al. Slug and Sox9 Cooperatively Determine the Mammary Stem Cell State. Cell. 2012;148:1015–1028. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mani S.A., Guo W., Liao M.-J., Eaton E.N., Ayyanan A., Zhou A.Y., Brooks M., Reinhard F., Zhang C.C., Shipitsin M., et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Morel A.-P., Lièvre M., Thomas C., Hinkal G., Ansieau S., Puisieux A. Generation of Breast Cancer Stem Cells through Epithelial-Mesenchymal Transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lu H., Clauser K.R., Tam W.L., Fröse J., Ye X., Eaton E.N., Reinhardt F., Donnenberg V.S., Bhargava R., Carr S.A., et al. A Breast Cancer Stem Cell Niche Supported by Juxtacrine Signalling from Monocytes and Macrophages. Nat. Cell Biol. 2014;16:1105–1117. doi: 10.1038/ncb3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sarrio D., Franklin C.K., Mackay A., Reis-Filho J.S., Isacke C.M. Epithelial and Mesenchymal Subpopulations within Normal Basal Breast Cell Lines Exhibit Distinct Stem Cell/Progenitor Properties. Stem Cells. 2012;30:292–303. doi: 10.1002/stem.791. [DOI] [PubMed] [Google Scholar]
- 122.Di Vito Nolfi M., Vecchiotti D., Flati I., Verzella D., Di Padova M., Alesse E., Capece D., Zazzeroni F. EV-Mediated Chemoresistance in the Tumor Microenvironment: Is NF-ΚB a Player? Front. Oncol. 2022;12:933922. doi: 10.3389/fonc.2022.933922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li C., Li R., Hu X., Zhou G., Jiang G. Tumor-Promoting Mechanisms of Macrophage-Derived Extracellular Vesicles-Enclosed MicroRNA-660 in Breast Cancer Progression. Breast Cancer Res. Treat. 2022;192:353–368. doi: 10.1007/s10549-021-06433-y. [DOI] [PubMed] [Google Scholar]
- 124.Wang H., Tian T., Zhang J. Tumor-Associated Macrophages (Tams) in Colorectal Cancer (Crc): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021;22:8470. doi: 10.3390/ijms22168470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Forssell J., Oberg A., Henriksson M.L., Stenling R., Jung A., Palmqvist R. High Macrophage Infiltration along the Tumor Front Correlates with Improved Survival in Colon Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007;13:1472–1479. doi: 10.1158/1078-0432.CCR-06-2073. [DOI] [PubMed] [Google Scholar]
- 126.Kang J.-C., Chen J.-S., Lee C.-H., Chang J.-J., Shieh Y.-S. Intratumoral Macrophage Counts Correlate with Tumor Progression in Colorectal Cancer. J. Surg. Oncol. 2010;102:242–248. doi: 10.1002/jso.21617. [DOI] [PubMed] [Google Scholar]
- 127.Edin S., Wikberg M.L., Dahlin A.M., Rutegård J., Öberg Å., Oldenborg P.-A., Palmqvist R. The Distribution of Macrophages with a M1 or M2 Phenotype in Relation to Prognosis and the Molecular Characteristics of Colorectal Cancer. PLoS ONE. 2012;7:e47045. doi: 10.1371/journal.pone.0047045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Porta C., Ippolito A., Consonni F.M., Carraro L., Celesti G., Correale C., Grizzi F., Pasqualini F., Tartari S., Rinaldi M., et al. Protumor Steering of Cancer Inflammation by P50 Nf-Kb Enhances Colorectal Cancer Progression. Cancer Immunol. Res. 2018;6:578–593. doi: 10.1158/2326-6066.CIR-17-0036. [DOI] [PubMed] [Google Scholar]
- 129.Rawluszko A.A., Bujnicka K.E., Horbacka K., Krokowicz P., Jagodziński P.P. Expression and DNA Methylation Levels of Prolyl Hydroxylases PHD1, PHD2, PHD3 and Asparaginyl Hydroxylase FIH in Colorectal Cancer. BMC Cancer. 2013;13:526. doi: 10.1186/1471-2407-13-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wang L., Niu Z., Wang X., Li Z., Liu Y., Luo F., Yan X. PHD2 Exerts Anti-Cancer and Anti-Inflammatory Effects in Colon Cancer Xenografts Mice via Attenuating NF-ΚB Activity. Life Sci. 2020;242:117167. doi: 10.1016/j.lfs.2019.117167. [DOI] [PubMed] [Google Scholar]
- 131.Capece D., D’Andrea D., Begalli F., Goracci L., Tornatore L., Alexander J.L., Di Veroli A., Leow S.-C., Vaiyapuri T.S., Ellis J.K., et al. Enhanced Triacylglycerol Catabolism by Carboxylesterase 1 Promotes Aggressive Colorectal Carcinoma. J. Clin. Investig. 2021;131(11):e137845. doi: 10.1172/JCI137845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Savio L.E.B., de Andrade Mello P., da Silva C.G., Coutinho-Silva R. The P2X7 Receptor in Inflammatory Diseases: Angel or Demon? Front. Pharmacol. 2018;9:52. doi: 10.3389/fphar.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shang S., Ji X., Zhang L., Chen J., Li C., Shi R., Xiang W., Kang X., Zhang D., Yang F., et al. Macrophage ABHD5 Suppresses NFκB-Dependent Matrix Metalloproteinase Expression and Cancer Metastasis. Cancer Res. 2019;79:5513–5526. doi: 10.1158/0008-5472.CAN-19-1059. [DOI] [PubMed] [Google Scholar]
- 134.Penny H.L., Sieow J.L., Adriani G., Yeap W.H., See Chi Ee P., San Luis B., Lee B., Lee T., Mak S.Y., Ho Y.S., et al. Warburg Metabolism in Tumor-Conditioned Macrophages Promotes Metastasis in Human Pancreatic Ductal Adenocarcinoma. Oncoimmunology. 2016;5:e1191731. doi: 10.1080/2162402X.2016.1191731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chappell W.H., Steelman L.S., Long J.M., Kempf R.C., Abrams S.L., Franklin R.A., Bäsecke J., Stivala F., Donia M., Fagone P., et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/MTOR Inhibitors: Rationale and Importance to Inhibiting These Pathways in Human Health. Oncotarget. 2011;2:135–164. doi: 10.18632/oncotarget.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morantz R.A., Wood G.W., Foster M., Clark M., Gollahon K. Macrophages in Experimental and Human Brain Tumors. Part 2: Studies of the Macrophage Content of Human Brain Tumors. J. Neurosurg. 1979;50:305–311. doi: 10.3171/jns.1979.50.3.0305. [DOI] [PubMed] [Google Scholar]
- 137.Chen G.G., Chu Y.S., Chak E.C.W., Leung B.C.S., Poon W.S. Induction of Apoptosis in Glioma Cells by Molecules Released from Activated Macrophages. J. Neurooncol. 2002;57:179–186. doi: 10.1023/A:1015763916020. [DOI] [PubMed] [Google Scholar]
- 138.Achyut B.R., Angara K., Jain M., Borin T.F., Rashid M.H., Iskander A.S.M., Ara R., Kolhe R., Howard S., Venugopal N., et al. Canonical NFκB Signaling in Myeloid Cells Is Required for the Glioblastoma Growth. Sci. Rep. 2017;7:13754. doi: 10.1038/s41598-017-14079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liang F., Liang J., Wang W.-Q., Sun J.-P., Udho E., Zhang Z.-Y. PRL3 Promotes Cell Invasion and Proliferation by Down-Regulation of Csk Leading to Src Activation. J. Biol. Chem. 2007;282:5413–5419. doi: 10.1074/jbc.M608940200. [DOI] [PubMed] [Google Scholar]
- 140.Zhang T., Liu L., Lai W., Zeng Y., Xu H., Lan Q., Su P., Chu Z. Interaction with Tumor-associated Macrophages Promotes PRL-3-induced Invasion of Colorectal Cancer Cells via MAPK Pathway-induced EMT and NF-κB Signaling-induced Angiogenesis. Oncol. Rep. 2019;41:2790–2802. doi: 10.3892/or.2019.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhang R., Wang Q., Chen J., Zhang N., Liu C., Wang T., Yang F., Siebert H.C., Zheng X. Ketogenic Diet Elicits Antitumor Properties through Inducing Oxidative Stress, Inhibiting MMP-9 Expression, and Rebalancing M1/ M2 Tumor-Associated Macrophage Phenotype in a Mouse Model of Colon Cancer. J. Agric. Food Chem. 2020;68:11182–11196. doi: 10.1021/acs.jafc.0c04041. [DOI] [PubMed] [Google Scholar]
- 142.Lehuédé C., Dupuy F., Rabinovitch R., Jones R.G., Siegel P.M. Metabolic Plasticity as a Determinant of Tumor Growth and Metastasis. Cancer Res. 2016;76:5201–5208. doi: 10.1158/0008-5472.CAN-16-0266. [DOI] [PubMed] [Google Scholar]
- 143.Fehri E., Ennaifer E., Bel Haj Rhouma R., Guizani-Tabbane L., Guizani I., Boubaker S. The Role of Toll-like Receptor 9 in Gynecologic Cancer. Curr. Res. Transl. Med. 2016;64:155–159. doi: 10.1016/j.retram.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 144.Taylor C.T., Colgan S.P. Regulation of Immunity and Inflammation by Hypoxia in Immunological Niches. Nat. Rev. Immunol. 2017;17:774–785. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Taylor C.T., Doherty G., Fallon P.G., Cummins E.P. Hypoxia-Dependent Regulation of Inflammatory Pathways in Immune Cells. J. Clin. Investig. 2016;126:3716–3724. doi: 10.1172/JCI84433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chittezhath M., Dhillon M.K., Lim J.Y., Laoui D., Shalova I.N., Teo Y.L., Chen J., Kamaraj R., Raman L., Lum J., et al. Molecular Profiling Reveals a Tumor-Promoting Phenotype of Monocytes and Macrophages in Human Cancer Progression. Immunity. 2014;41:815–829. doi: 10.1016/j.immuni.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 147.Chen X.J., Deng Y.R., Wang Z.C., Wei W.F., Zhou C.F., Zhang Y.M., Yan R.M., Liang L.J., Zhong M., Liang L., et al. Correction: Hypoxia-Induced ZEB1 Promotes Cervical Cancer Progression via CCL8-Dependent Tumour-Associated Macrophage Recruitment. Cell Death Dis. 2022;13:247. doi: 10.1038/s41419-022-04706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vitale C., Bottino C., Castriconi R. Monocyte and Macrophage in Neuroblastoma: Blocking Their Pro-Tumoral Functions and Strengthening Their Crosstalk with Natural Killer Cells. Cells. 2023;12:885. doi: 10.3390/cells12060885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leblond M.M., Zdimerova H., Desponds E., Verdeil G. Tumor-Associated Macrophages in Bladder Cancer: Biological Role, Impact on Therapeutic Response and Perspectives for Immunotherapy. Cancers. 2021;13:4712. doi: 10.3390/cancers13184712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun J., Park C., Guenthner N., Gurley S., Zhang L., Lubben B., Adebayo O., Bash H., Chen Y., Maksimos M., et al. Tumor-Associated Macrophages in Multiple Myeloma: Advances in Biology and Therapy. J. Immunother. Cancer. 2022;10:e003975. doi: 10.1136/jitc-2021-003975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen Y.L. Prognostic Significance of Tumor-Associated Macrophages in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis. Medicine. 2020;99:E21999. doi: 10.1097/MD.0000000000021999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fujiwara T., Healey J., Ogura K., Yoshida A., Kondo H., Hata T., Kure M., Tazawa H., Nakata E., Kunisada T., et al. Role of Tumor-Associated Macrophages in Sarcomas. Cancers. 2021;13:1086. doi: 10.3390/cancers13051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang S., Liu Q., Liao Q. Tumor-Associated Macrophages in Pancreatic Ductal Adenocarcinoma: Origin, Polarization, Function, and Reprogramming. Front. Cell Dev. Biol. 2021;8:607209. doi: 10.3389/fcell.2020.607209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lankadasari M.B., Mukhopadhyay P., Mohammed S., Harikumar K.B. TAMing Pancreatic Cancer: Combat with a Double Edged Sword. Mol. Cancer. 2019;18:48. doi: 10.1186/s12943-019-0966-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.XU F., WEI Y., TANG Z., LIU B., DONG J. Tumor-Associated Macrophages in Lung Cancer: Friend or Foe? Mol. Med. Rep. 2020;22:4107–4115. doi: 10.3892/mmr.2020.11518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shen H., Liu J., Chen S., Ma X., Ying Y., Li J., Wang W., Wang X., Xie L. Prognostic Value of Tumor-Associated Macrophages in Clear Cell Renal Cell Carcinoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021;11:657318. doi: 10.3389/fonc.2021.657318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rihawi K., Ricci A.D., Rizzo A., Brocchi S., Marasco G., Pastore L.V., Llimpe F.L.R., Golfieri R., Renzulli M. Tumor-Associated Macrophages and Inflammatory Microenvironment in Gastric Cancer: Novel Translational Implications. Int. J. Mol. Sci. 2021;22:3805. doi: 10.3390/ijms22083805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gambardella V., Castillo J., Tarazona N., Gimeno-Valiente F., Martínez-Ciarpaglini C., Cabeza-Segura M., Roselló S., Roda D., Huerta M., Cervantes A., et al. The Role of Tumor-Associated Macrophages in Gastric Cancer Development and Their Potential as a Therapeutic Target. Cancer Treat. Rev. 2020;86:102015. doi: 10.1016/j.ctrv.2020.102015. [DOI] [PubMed] [Google Scholar]
- 159.Fujimura T., Kambayashi Y., Fujisawa Y., Hidaka T., Aiba S. Tumor-Associated Macrophages: Therapeutic Targets for Skin Cancer. Front. Oncol. 2018;8:3. doi: 10.3389/fonc.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chiang E., Stafford H., Buell J., Ramesh U., Amit M., Nagarajan P., Migden M., Yaniv D. Review of the Tumor Microenvironment in Basal and Squamous Cell Carcinoma. Cancers. 2023;15:2453. doi: 10.3390/cancers15092453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhou Q., Fang T., Wei S., Chai S., Yang H., Tao M., Cao Y. Macrophages in Melanoma: A Double-edged Sword and Targeted Therapy Strategies (Review) Exp. Ther. Med. 2022;24:640. doi: 10.3892/etm.2022.11577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xue Y., Song X., Fan S., Deng R. The Role of Tumor-Associated Macrophages in Oral Squamous Cell Carcinoma. Front. Physiol. 2022;13:959747. doi: 10.3389/fphys.2022.959747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Larionova I., Tuguzbaeva G., Ponomaryova A., Stakheyeva M., Cherdyntseva N., Pavlov V., Choinzonov E., Kzhyshkowska J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020;10:566511. doi: 10.3389/fonc.2020.566511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kumari N., Choi S.H. Tumor-Associated Macrophages in Cancer: Recent Advancements in Cancer Nanoimmunotherapies. J. Exp. Clin. Cancer Res. 2022;41:68. doi: 10.1186/s13046-022-02272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qiu B., Matthay K.K. Advancing Therapy for Neuroblastoma. Nat. Rev. Clin. Oncol. 2022;19:515–533. doi: 10.1038/s41571-022-00643-z. [DOI] [PubMed] [Google Scholar]
- 166.Zhang Q., Mao Z., Sun J. NF-ΚB Inhibitor, BAY11-7082, Suppresses M2 Tumor-Associated Macrophage Induced EMT Potential via MiR-30a/NF-ΚB/Snail Signaling in Bladder Cancer Cells. Gene. 2019;710:91–97. doi: 10.1016/j.gene.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 167.Garcia J.A., Cowey C.L., Godley P.A. Renal Cell Carcinoma. Curr. Opin. Oncol. 2009;21:266–271. doi: 10.1097/CCO.0b013e32832a05c8. [DOI] [PubMed] [Google Scholar]
- 168.Mukherjee S., Baidoo J., Fried A., Atwi D., Dolai S., Boockvar J., Symons M., Ruggieri R., Raja K., Banerjee P. Curcumin Changes the Polarity of Tumor-Associated Microglia and Eliminates Glioblastoma. Int. J. cancer. 2016;139:2838–2849. doi: 10.1002/ijc.30398. [DOI] [PubMed] [Google Scholar]
- 169.Kai K., Komohara Y., Esumi S., Fujiwara Y., Yamamoto T., Uekawa K., Ohta K., Takezaki T., Kuroda J., Shinojima N., et al. Macrophage/Microglia-Derived IL-1β Induces Glioblastoma Growth via the STAT3/NF-ΚB Pathway. Hum. Cell. 2022;35:226–237. doi: 10.1007/s13577-021-00619-8. [DOI] [PubMed] [Google Scholar]
- 170.Wu H., Xu J.B., He Y.L., Peng J.J., Zhang X.H., Chen C.Q., Li W., Cai S.R. Tumor-Associated Macrophages Promote Angiogenesis and Lymphangiogenesis of Gastric Cancer. J. Surg. Oncol. 2012;106:462–468. doi: 10.1002/jso.23110. [DOI] [PubMed] [Google Scholar]
- 171.Tjiu J.W., Chen J.S., Shun C.T., Lin S.J., Liao Y.H., Chu C.Y., Tsai T.F., Chiu H.C., Dai Y.S., Inoue H., et al. Tumor-Associated Macrophage-Induced Invasion and Angiogenesis of Human Basal Cell Carcinoma Cells by Cyclooxygenase-2 Induction. J. Investig. Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 172.Gutiérrez-Seijo A., García-Martínez E., Barrio-Alonso C., Pareja-Malagón M., Acosta-Ocampo A., Fernández-Santos M.E., Puig-Kröger A., Parra-Blanco V., Mercader E., Márquez-Rodas I., et al. Ccl20/Tnf/Vegfa Cytokine Secretory Phenotype of Tumor-Associated Macrophages Is a Negative Prognostic Factor in Cutaneous Melanoma. Cancers. 2021;13:3943. doi: 10.3390/cancers13163943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paccez J.D., Vogelsang M., Parker M.I., Zerbini L.F. The Receptor Tyrosine Kinase Axl in Cancer: Biological Functions and Therapeutic Implications. Int. J. Cancer. 2014;134:1024–1033. doi: 10.1002/ijc.28246. [DOI] [PubMed] [Google Scholar]
- 174.Korshunov V.A. Axl-Dependent Signalling: A Clinical Update. Clin. Sci. 2012;122:361–368. doi: 10.1042/CS20110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chiu K.C., Lee C.H., Liu S.Y., Chou Y.T., Huang R.Y., Huang S.M., Shieh Y.S. Polarization of Tumor-Associated Macrophages and Gas6/Axl Signaling in Oral Squamous Cell Carcinoma. Oral Oncol. 2015;51:683–689. doi: 10.1016/j.oraloncology.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 176.Lee C.H., Liu S.Y., Chou K.C., Yeh C.T., Shiah S.G., Huang R.Y., Cheng J.C., Yen C.Y., Shieh Y.S. Tumor-Associated Macrophages Promote Oral Cancer Progression through Activation of the Axl Signaling Pathway. Ann. Surg. Oncol. 2014;21:1031–1037. doi: 10.1245/s10434-013-3400-0. [DOI] [PubMed] [Google Scholar]
- 177.Vecchiotti D., Verzella D., Di Vito Nolfi M., D’andrea D., Flati I., Di Francesco B., Cornice J., Alesse E., Capece D., Zazzeroni F. Elevated NF-ΚB/SHh/GLI1 Signature Denotes a Worse Prognosis and Represent a Novel Potential Therapeutic Target in Advanced Prostate Cancer. Cells. 2022;11:2118. doi: 10.3390/cells11132118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chen P.-C., Cheng H.-C., Wang J., Wang S.-W., Tai H.-C., Lin C.-W., Tang C.-H. Prostate Cancer-Derived CCN3 Induces M2 Macrophage Infiltration and Contributes to Angiogenesis in Prostate Cancer Microenvironment. Oncotarget. 2014;5:1595. doi: 10.18632/oncotarget.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Beider K., Bitner H., Leiba M., Gutwein O., Koren-Michowitz M., Ostrovsky O., Abraham M., Wald H., Galun E., Peled A., et al. Multiple Myeloma Cells Recruit Tumor-Supportive Macrophages through the CXCR4/CXCL12 Axis and Promote Their Polarization toward the M2 Phenotype. Oncotarget. 2014;5:11283–11296. doi: 10.18632/oncotarget.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khalife J., Ghose J., Martella M., Viola D., Rocci A., Troadec E., Terrazas C., Satoskar A.R., Gunes E.G., Dona A., et al. MiR-16 Regulates Crosstalk in NF-ΚB Tolerogenic Inflammatory Signaling between Myeloma Cells and Bone Marrow Macrophages. JCI Insight. 2019;4:e129348. doi: 10.1172/jci.insight.129348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Song Y., Li X., Zeng Z., Li Q., Gong Z., Liao Q., Li X., Chen P., Xiang B., Zhang W., et al. Epstein-Barr Virus Encoded MiR-BART11 Promotes Inflammation-Induced Carcinogenesis by Targeting FOXP1. Oncotarget. 2016;7:36783. doi: 10.18632/oncotarget.9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Iliopoulos D., Hirsch H.A., Struhl K. An Epigenetic Switch Involving NF-KappaB, Lin28, Let-7 MicroRNA, and IL6 Links Inflammation to Cell Transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li Q., Hao Z., Hong Y., He W., Zhao W. Reprogramming Tumor Associated Macrophage Phenotype by a Polysaccharide from Ilex Asprella for Sarcoma Immunotherapy. Int. J. Mol. Sci. 2018;19:3816. doi: 10.3390/ijms19123816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kühnemuth B., Mühlberg L., Schipper M., Griesmann H., Neesse A., Milosevic N., Wissniowski T., Buchholz M., Gress T.M., Michl P. CUX1 Modulates Polarization of Tumor-Associated Macrophages by Antagonizing NF-ΚB Signaling. Oncogene. 2015;34:177–187. doi: 10.1038/onc.2013.530. [DOI] [PubMed] [Google Scholar]
- 185.Kemp S.B., Carpenter E.S., Steele N.G., Donahue K.L., Nwosu Z.C., Pacheco A., Velez-Delgado A., Menjivar R.E., Lima F., The S., et al. Apolipoprotein E Promotes Immune Suppression in Pancreatic Cancer through NF-ΚB-Mediated Production of CXCL1. Cancer Res. 2021;81:4305–4318. doi: 10.1158/0008-5472.CAN-20-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhu C., Shen H., Zhu L., Zhao F., Shu Y. Plasminogen Activator Inhibitor 1 Promotes Immunosuppression in Human Non-Small Cell Lung Cancers by Enhancing TGF-Β1 Expression in Macrophage. Cell. Physiol. Biochem. 2018;44:2201–2211. doi: 10.1159/000486025. [DOI] [PubMed] [Google Scholar]
- 187.Feng X., Yu W., Cao L., Meng F., Cong M. A Novel Chrysin Thiazole Derivative Polarizes Macrophages to an M1 Phenotype via Targeting TLR4. Int. Immunopharmacol. 2020;88:106986. doi: 10.1016/j.intimp.2020.106986. [DOI] [PubMed] [Google Scholar]
- 188.Yin L., Fan Z., Liu P., Chen L., Guan Z., Liu Y., Luo Y. Anemoside A3 Activates TLR4-Dependent M1-Phenotype Macrophage Polarization to Represses Breast Tumor Growth and Angiogenesis. Toxicol. Appl. Pharmacol. 2021;432:115755. doi: 10.1016/j.taap.2021.115755. [DOI] [PubMed] [Google Scholar]
- 189.Cheng H., Sun L., Shen D., Ren A., Ma F., Tai G., Fan L., Zhou Y. β-1,6 Glucan Converts Tumor-Associated Macrophages into an M1-like Phenotype. Carbohydr. Polym. 2020;247:116715. doi: 10.1016/j.carbpol.2020.116715. [DOI] [PubMed] [Google Scholar]
- 190.Qian S., Han X., Sha X., Tian F., Huang H., Jiang P., Huang G., Ma B., Zhang H., Zhu Y., et al. Aqueous Extract of Cimicifuga Dahurica Reprogramming Macrophage Polarization by Activating TLR4-NF-ΚB Signaling Pathway. J. Inflamm. Res. 2022;15:1027–1046. doi: 10.2147/JIR.S345497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Liu C., He D., Zhang S., Chen H., Zhao J., Li X., Zeng X. Homogeneous Polyporus Polysaccharide Inhibit Bladder Cancer by Resetting Tumor-Associated Macrophages Toward M1 Through NF-ΚB/NLRP3 Signaling. Front. Immunol. 2022;13:839460. doi: 10.3389/fimmu.2022.839460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hoover A.A., Hufnagel D.H., Harris W., Bullock K., Glass E.B., Liu E., Barham W., Crispens M.A., Khabele D., Giorgio T.D., et al. Increased Canonical NF-KappaB Signaling Specifically in Macrophages Is Sufficient to Limit Tumor Progression in Syngeneic Murine Models of Ovarian Cancer. BMC Cancer. 2020;20:970. doi: 10.1186/s12885-020-07450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ai Y., Liu S., Luo H., Wu S., Wei H., Tang Z., Li X., Zou C. LncRNA DCST1-AS1 Facilitates Oral Squamous Cell Carcinoma by Promoting M2 Macrophage Polarization through Activating NF- κ B Signaling. J. Immunol. Res. 2021;2021:5524231. doi: 10.1155/2021/5524231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang Y., Wu L., Li Z., Zhang W., Luo F., Chu Y., Chen G. Glycocalyx-Mimicking Nanoparticles Improve Anti-PD-L1 Cancer Immunotherapy through Reversion of Tumor-Associated Macrophages. Biomacromolecules. 2018;19:2098–2108. doi: 10.1021/acs.biomac.8b00305. [DOI] [PubMed] [Google Scholar]
- 195.Liu Y., Liang S., Jiang D., Gao T., Fang Y., Fu S., Guan L., Zhang Z., Mu W., Chu Q., et al. Manipulation of TAMs Functions to Facilitate the Immune Therapy Effects of Immune Checkpoint Antibodies. J. Control. Release. 2021;336:621–634. doi: 10.1016/j.jconrel.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 196.Parayath N.N., Parikh A., Amiji M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018;18:3571–3579. doi: 10.1021/acs.nanolett.8b00689. [DOI] [PubMed] [Google Scholar]
- 197.Li K., Lu L., Xue C., Liu J., He Y., Zhou J., Xia Z., Dai L., Luo Z., Mao Y., et al. Polarization of Tumor-Associated Macrophage Phenotype: Via Porous Hollow Iron Nanoparticles for Tumor Immunotherapy in Vivo. Nanoscale. 2020;12:130–144. doi: 10.1039/C9NR06505A. [DOI] [PubMed] [Google Scholar]
- 198.Liu L., Wang Y., Guo X., Zhao J., Zhou S. A Biomimetic Polymer Magnetic Nanocarrier Polarizing Tumor-Associated Macrophages for Potentiating Immunotherapy. Small. 2020;16:e2003543. doi: 10.1002/smll.202003543. [DOI] [PubMed] [Google Scholar]
- 199.Li L., Li L., Zhen M., Zhen M., Wang H., Wang H., Sun Z., Sun Z., Jia W., Jia W., et al. Functional Gadofullerene Nanoparticles Trigger Robust Cancer Immunotherapy Based on Rebuilding an Immunosuppressive Tumor Microenvironment. Nano Lett. 2020;20:4487–4496. doi: 10.1021/acs.nanolett.0c01287. [DOI] [PubMed] [Google Scholar]
- 200.Xu J., Zheng B., Zhang S., Liao X., Tong Q., Wei G., Yu S., Chen G., Wu A., Gao S., et al. Copper Sulfide Nanoparticle-Redirected Macrophages for Adoptive Transfer Therapy of Melanoma. Adv. Funct. Mater. 2021;31:2008022. doi: 10.1002/adfm.202008022. [DOI] [Google Scholar]
- 201.Zhao Y., Liu X., Huo M., Wang Y., Li Y., Xu N., Zhu H. Cetuximab Enhances the Anti-Tumor Function of Macrophages in an IL-6 Dependent Manner. Life Sci. 2021;267:118953. doi: 10.1016/j.lfs.2020.118953. [DOI] [PubMed] [Google Scholar]
- 202.Cao X., Li B., Chen J., Dang J., Chen S., Gunes E.G., Xu B., Tian L., Muend S., Raoof M., et al. Effect of Cabazitaxel on Macrophages Improves CD47-Targeted Immunotherapy for Triple-Negative Breast Cancer. J. Immunother. Cancer. 2021;9:e002022. doi: 10.1136/jitc-2020-002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jin J., Lin J., Xu A., Lou J., Qian C., Li X., Wang Y., Yu W., Tao H. CCL2: An Important Mediator Between Tumor Cells and Host Cells in Tumor Microenvironment. Front. Oncol. 2021;11:722916. doi: 10.3389/fonc.2021.722916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jin L., Guo Y., Mao W., Wang J., Jin L., Liu X., Shou Q., Fu H. Total Glucosides of Paeony Inhibit Breast Cancer Growth by Inhibiting TAMs Infiltration through NF-ΚB/CCL2 Signaling. Phytomedicine. 2022;104:154307. doi: 10.1016/j.phymed.2022.154307. [DOI] [PubMed] [Google Scholar]
- 205.Ortega R.A., Barham W., Sharman K., Tikhomirov O., Giorgio T.D., Yull F.E. Manipulating the NF-ΚB Pathway in Macrophages Using Mannosylated, SiRNA-Delivering Nanoparticles Can Induce Immunostimulatory and Tumor Cytotoxic Functions. Int. J. Nanomed. 2016;11:2163–2177. doi: 10.2147/IJN.S93483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Tsagozis P., Augsten M., Pisa P. All Trans-Retinoic Acid Abrogates the pro-Tumorigenic Phenotype of Prostate Cancer Tumor-Associated Macrophages. Int. Immunopharmacol. 2014;23:8–13. doi: 10.1016/j.intimp.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 207.Wanderley C.W., Colón D.F., Luiz J.P.M., Oliveira F.F., Viacava P.R., Leite C.A., Pereira J.A., Silva C.M., Silva C.R., Silva R.L., et al. Paclitaxel Reduces Tumor Growth by Reprogramming Tumor-Associated Macrophages to an M1 Profile in a TLR4-Dependent Manner. Cancer Res. 2018;78:5891–5900. doi: 10.1158/0008-5472.CAN-17-3480. [DOI] [PubMed] [Google Scholar]
- 208.Li X., Yao W., Yuan Y., Chen P., Li B., Li J., Chu R., Song H., Xie D., Jiang X., et al. Targeting of Tumour-Infiltrating Macrophages via CCL2/CCR2 Signalling as a Therapeutic Strategy against Hepatocellular Carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 209.Glass E.B., Hoover A.A., Bullock K.K., Madden M.Z., Reinfeld B.I., Harris W., Parker D., Hufnagel D.H., Crispens M.A., Khabele D., et al. Stimulating TAM-Mediated Anti-Tumor Immunity with Mannose-Decorated Nanoparticles in Ovarian Cancer. BMC Cancer. 2022;22:497. doi: 10.1186/s12885-022-09612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Liu M., Sakamaki T., Casimiro M.C., Willmarth N.E., Quong A.A., Ju X., Ojeifo J., Jiao X., Yeow W.-S., Katiyar S., et al. The Canonical NF-KappaB Pathway Governs Mammary Tumorigenesis in Transgenic Mice and Tumor Stem Cell Expansion. Cancer Res. 2010;70:10464–10473. doi: 10.1158/0008-5472.CAN-10-0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tornatore L., Sandomenico A., Raimondo D., Low C., Rocci A., Tralau-Stewart C., Capece D., D’Andrea D., Bua M., Boyle E., et al. Cancer-Selective Targeting of the NF-ΚB Survival Pathway with GADD45β/MKK7 Inhibitors. Cancer Cell. 2014;26:495–508. doi: 10.1016/j.ccr.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tornatore L., Capece D., D’Andrea D., Begalli F., Verzella D., Bennett J., Acton G., Campbell E.A., Kelly J., Tarbit M., et al. Clinical Proof of Concept for a Safe and Effective NF-ΚB-Targeting Strategy in Multiple Myeloma. Br. J. Haematol. 2019;185:588–592. doi: 10.1111/bjh.15569. [DOI] [PubMed] [Google Scholar]
- 213.Tornatore L., Capece D., D’Andrea D., Begalli F., Verzella D., Bennett J., Acton G., Campbell E.A., Kelly J., Tarbit M., et al. Preclinical Toxicology and Safety Pharmacology of the First-in-Class GADD45β/MKK7 Inhibitor and Clinical Candidate, DTP3. Toxicol. Rep. 2019;6:369–379. doi: 10.1016/j.toxrep.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rega C., Russo R., Focà A., Sandomenico A., Iaccarino E., Raimondo D., Milanetti E., Tornatore L., Franzoso G., Pedone P.V., et al. Probing the Interaction Interface of the GADD45β/MKK7 and MKK7/DTP3 Complexes by Chemical Cross-Linking Mass Spectrometry. Int. J. Biol. Macromol. 2018;114:114–123. doi: 10.1016/j.ijbiomac.2018.03.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sandomenico A., Di Rienzo L., Calvanese L., Iaccarino E., D’auria G., Falcigno L., Chambery A., Russo R., Franzoso G., Tornatore L., et al. Insights into the Interaction Mechanism of Dtp3 with Mkk7 by Using Std-Nmr and Computational Approaches. Biomedicines. 2021;9:20. doi: 10.3390/biomedicines9010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pittet M.J., Michielin O., Migliorini D. Clinical Relevance of Tumour-Associated Macrophages. Nat. Rev. Clin. Oncol. 2022;19:402–421. doi: 10.1038/s41571-022-00620-6. [DOI] [PubMed] [Google Scholar]
- 217.Mantovani A., Allavena P., Marchesi F., Garlanda C. Macrophages as Tools and Targets in Cancer Therapy. Nat. Rev. Drug Discov. 2022;21:799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ribas A., Medina T., Kirkwood J.M., Zakharia Y., Gonzalez R., Davar D., Chmielowski B., Campbell K.M., Bao R., Kelley H., et al. Overcoming PD-1 Blockade Resistance with CpG-A Toll-like Receptor 9 Agonist Vidutolimod in Patients with Metastatic Melanoma. Cancer Discov. 2021;11:2998–3007. doi: 10.1158/2159-8290.CD-21-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chow L.Q.M., Morishima C., Eaton K.D., Baik C.S., Goulart B.H., Anderson L.N., Manjarrez K.L., Dietsch G.N., Bryan J.K., Hershberg R.M., et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin. Cancer Res. 2017;23:2442–2450. doi: 10.1158/1078-0432.CCR-16-1934. [DOI] [PubMed] [Google Scholar]
- 220.Cassier P.A., Italiano A., Gomez-Roca C.A., Le Tourneau C., Toulmonde M., Cannarile M.A., Ries C., Brillouet A., Müller C., Jegg A.M., et al. CSF1R Inhibition with Emactuzumab in Locally Advanced Diffuse-Type Tenosynovial Giant Cell Tumours of the Soft Tissue: A Dose-Escalation and Dose-Expansion Phase 1 Study. Lancet Oncol. 2015;16:949–956. doi: 10.1016/S1470-2045(15)00132-1. [DOI] [PubMed] [Google Scholar]
- 221.Gomez-Roca C., Cassier P., Zamarin D., Machiels J.P., Luis Perez Gracia J., Stephen Hodi F., Taus A., Martinez Garcia M., Boni V., Eder J.P., et al. Anti-CSF-1R Emactuzumab in Combination with Anti-PD-L1 Atezolizumab in Advanced Solid Tumor Patients Naïve or Experienced for Immune Checkpoint Blockade. J. Immunother. Cancer. 2022;10:e004076. doi: 10.1136/jitc-2021-004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gomez-Roca C.A., Italiano A., Le Tourneau C., Cassier P.A., Toulmonde M., D’Angelo S.P., Campone M., Weber K.L., Loirat D., Cannarile M.A., et al. Phase i Study of Emactuzumab Single Agent or in Combination with Paclitaxel in Patients with Advanced/Metastatic Solid Tumors Reveals Depletion of Immunosuppressive M2-like Macrophages. Ann. Oncol. 2019;30:1381–1392. doi: 10.1093/annonc/mdz163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nywening T.M., Wang-Gillam A., Sanford D.E., Belt B.A., Panni R.Z., Cusworth B.M., Toriola A.T., Nieman R.K., Worley L.A., Yano M., et al. Targeting Tumour-Associated Macrophages with CCR2 Inhibition in Combination with FOLFIRINOX in Patients with Borderline Resectable and Locally Advanced Pancreatic Cancer: A Single-Centre, Open-Label, Dose-Finding, Non-Randomised, Phase 1b Trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Halama N., Zoernig I., Berthel A., Kahlert C., Klupp F., Suarez-Carmona M., Suetterlin T., Brand K., Krauss J., Lasitschka F., et al. Tumoral Immune Cell Exploitation in Colorectal Cancer Metastases Can Be Targeted Effectively by Anti-CCR5 Therapy in Cancer Patients. Cancer Cell. 2016;29:587–601. doi: 10.1016/j.ccell.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 225.Upton R., Banuelos A., Feng D., Biswas T., Kao K., McKenna K., Willingham S., Ho P.Y., Rosental B., Tal M.C., et al. Combining CD47 Blockade with Trastuzumab Eliminates HER2-Positive Breast Cancer Cells and Overcomes Trastuzumab Tolerance. Proc. Natl. Acad. Sci. USA. 2021;118:e2026849118. doi: 10.1073/pnas.2026849118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.O’Hara M.H., O’Reilly E.M., Varadhachary G., Wolff R.A., Wainberg Z.A., Ko A.H., Fisher G., Rahma O., Lyman J.P., Cabanski C.R., et al. CD40 Agonistic Monoclonal Antibody APX005M (Sotigalimab) and Chemotherapy, with or without Nivolumab, for the Treatment of Metastatic Pancreatic Adenocarcinoma: An Open-Label, Multicentre, Phase 1b Study. Lancet Oncol. 2021;22:118–131. doi: 10.1016/S1470-2045(20)30532-5. [DOI] [PubMed] [Google Scholar]
- 227.Irenaeus S.M.M., Nielsen D., Ellmark P., Yachnin J., Deronic A., Nilsson A., Norlén P., Veitonmäki N., Wennersten C.S., Ullenhag G.J. First-in-Human Study with Intratumoral Administration of a CD40 Agonistic Antibody, ADC-1013, in Advanced Solid Malignancies. Int. J. Cancer. 2019;145:1189–1199. doi: 10.1002/ijc.32141. [DOI] [PubMed] [Google Scholar]