Abstract
Background
Despite the efficacy of immune checkpoint inhibitors (ICIs), adverse events including hepatotoxicity limit their ongoing use. We investigated the outcomes and management of patients with immune-mediated hepatitis (IMH) and clinical predictors of toxicity resolution.
Methods
Patients referred to our multidisciplinary immunotherapy-related toxicity group from August 2017 to December 2020 for IMH were evaluated. Toxicity was defined according to CTCAEv4.0. IMH resolution was defined as liver enzyme normalization after steroid initiation.
Results
Thirty-three patients were included in the study, 62% female, and 71% Caucasian. The most common ICI used was PD-1/PD-L1 (76%). Peak IMH occurred at a median of 89 [45,193] days, for which most patients received 1–2 mg/kg/day prednisone equivalent with 35% requiring MMF. Median follow-up was 123 [33,472] days with IMH resolution seen in 48% of patients at a median of 111 [41,214] days. While high-dose steroid use was not associated with IMH resolution, liver enzyme improvement one week after steroids predicted resolution in univariate analysis (p = 0.041). All 11 patients without IMH resolution died from cancer progression or complications with three patients having acute liver failure. Available liver biopsies showed bile duct injury, with varying degrees of portal and lobular inflammation.
Conclusion
IMH improvement one week after steroid initiation may predict ultimate IMH resolution.
Keywords: immunotherapy, Checkpoint inhibitor, Drug toxicity, Hepatitis
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapy for solid malignancies. Cytotoxic T-lymphocyte antigen-4 (CTLA-4) and programmed cell death protein-1 (PD-1) and its ligand (PD-L1) are two major immune checkpoint pathways that inhibit T-cell activity against tumors. CTLA-4 and PD-1 attenuate T-cell activation through non-redundant pathways through co-stimulatory signals. While antibodies that block CTLA-4 and PD-1 can activate T-cells against cancer, the same pathways mediate inflammation at off-target sites (Dougan et al. 2021a). These untended off-target effects are collectively termed immune-related adverse events (irAEs), one of which is immune-mediated hepatotoxicity (IMH) and thus complicate the continued use.
While IMH typically presents as asymptomatic elevations in alanine aminotransferase (ALT) and aspartate aminotransferase (AST), more severe cases can develop with clinical symptoms such as jaundice, pruritus, or malaise. Roughly 1–2% of patients receiving anti-PD-1/anti-PD-L1 therapy will develop grade 3 or higher IMH, and up to 10% of those receiving anti-CTLA-4/PD-1 combination therapy (Brahmer et al. 2018). The combination of ICI with other targeted chemotherapy and ICI has also been associated with high-grade or severe IMH high-grade IMH (Gandhi et al. 2018; Paz-Ares et al. 2020). ICI therapy is usually discontinued with high-grade toxicities despite limited data on whether continuing ICI alters tumor prognosis and subsequent toxicity. Multiple guidelines from anti American Society of Clinical Oncology (ASCO), the European Society for Medical Oncology (ESMO), and the American Gastroenterology Association (AGA) have provided general recommendations regarding the management of IMH (Brahmer et al. 2018; Haanen et al. 2017; Dougan et al. 2021b) with the use of high dose (1–2 mg/kg/day) of methylprednisolone reserved for high-grade toxicity (Schneider et al. 2021; Brahmer et al. 2021), but controversies still remain including the question of re-treatment after IMH and the role of liver biopsy in the management of IMH (Martin et al. 2018; Jennings et al. 2019). Only a few published studies provide an in-depth analysis of the outcomes of patients who have developed immune-mediated hepatotoxicity (IMH) from ICIs for cancer.
To identify the risk factors for the development of immune-mediated hepatitis and guide treatment choices including specific steroid doses, we investigated detailed baseline clinical features, treatment course and their clinical outcomes of patients who developed IMH from ICI therapy from our institution (Naidoo et al. 2019). We also report common pathologic features in patients who had liver biopsied collected during their IMH event.
Methods
The study was approved by the Institutional Review Board for Human Research and complied with the Health Insurance Portability and Accountability Act regulations at Johns Hopkins Hospital. All referrals to the Immunotherapy-Related toxicity (IR-Tox) team between August 2017 and December 2020 were evaluated. IMH was defined as an acute elevation of liver enzymes of any CTCAE grade in the absence of viral hepatitis or other causes of liver injury. Patients who received at least one dose of ICI (CTLA-4, PD-1 or PD-L1 antibody) for a solid or hematological malignancy and were subsequently referred to the IR-Tox group for IMH were included. Patients with primary liver cancer were excluded from the study due to their advanced chronic liver disease. Baseline characteristics (gender, race, age), cancer type, immunotherapy specific data (treatment agent, dates and sessions, serial liver enzymes) autoimmune laboratory results [antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA), IgG, anti-mitochondrial antibody (AMA), anti-liver kidney microsomal antibody (anti-LKM)] were collected as shown in Table 1. Complications from steroid use during inpatient admissions were also evaluated. Documented complications from steroid use included hyperglycemia defined as blood glucose > 120 mg/dl requiring insulin, steroid-induced altered mental status, clinical evidence of GI bleeding, venous thromboembolism, infection, and leukocytosis (WBC > 11 × 103/mm3). For patients who had liver biopsies, their biopsy slides were obtained and reviewed by a board-certified liver pathologist, blinded to the patient’s clinical information.
Table 1.
The demographics, clinical characteristics and outcomes of patients referred for IMH evaluation
| Total | |
|---|---|
| Age (years) | 60 (10) |
| Gender (female) | 13 (62%) |
| Race | |
| Asian | 1 (5%) |
| Black or African American | 4 (19%) |
| Hispanic | 1 (5%) |
| White or Caucasian | 15 (71%) |
| History of autoimmune disease | 1 (5%) |
| BMI at referral | 28 (24–31) |
| Tumor type | |
| Bladder | 2 (10%) |
| Breast | 4 (19%) |
| Esophageal | 2 (10%) |
| GBM | 2 (10%) |
| Gastric | 2 (10%) |
| Liposarcoma | 1 (5%) |
| Melanoma | 3 (14%) |
| NSCLC | 3 (14%) |
| Ovarian | 1 (5%) |
| Pancreatic | 1 (5%) |
| Metastatic | 7 (33%) |
| Type of immunotherapy | |
| CTLA-4 | 2 (10%) |
| CTLA-4 PD-1/PD-L1 | 3 (14%) |
| PD-1/PD-L1 | 16 (76%) |
| ANA titer (> 1:80) | 6 (35%) |
| ASMA titer (> 1:40) | 2 (13%) |
| Doses of ICI before IMH | 3 (2–7) |
| Time from first ICI to IMH referral (days) | 84 (44–200) |
| Toxicity at referral | |
| Grade 1–2 | 6 (29%) |
| Grade 3–4 | 15 (71%) |
| Pattern of injury | |
| Hepatocellular | 7 (33%) |
| Mixed | 6 (29%) |
| Cholestatic | 8 (38%) |
| Toxicity at peak | |
| Grade 1–2 | 5 (24%) |
| Grade 3–4 | 16 (76%) |
| Pattern of injury | |
| Hepatocellular | 6 (29%) |
| Mixed | 8 (38%) |
| Cholestatic | 7 (33%) |
| Toxicity 1 week after treatment | |
| Grade 1–2 | 7 (44%) |
| Grade 3–4 | 9 (56%) |
| Treatment with steroid | 17 (81%) |
| Initial dosing of steroids > 1 mg/kg/day prednisone equivalent | 9 (53%) |
| Duration of steroid (days) | 58 (14–111) |
| Time from referral to death (days) | 51 (11–412) |
| Time from referral to resolution (days) | 111 (41–214) |
| Death at follow-up | 14 (67%) |
n (%), mean (STD), median (IQR)
Primary outcome of the study was complete resolution of IMH defined as the normalization of liver enzymes. Secondary outcomes were liver failure, and death. The pattern of injury was based on the R factor to differentiate a cholestatic vs hepatocellular pattern of injury (Chalasani et al. 2014). High dose steroids were defined as initial dosing of corticosteroids at > 1 mg/kg/day of oral prednisone equivalent. We graded each IMH event according to the Common Terminology Criteria for Adverse Events (Dougan et al. 2021b), with scores between 0 and 4, based on liver enzyme elevation.
Statistical analysis
Continuous and categorical variables were described as means with standard deviations (SD) or medians with interquartile ranges (IQR) [Q1–Q3], respectively. Comparison of continuous and categorical variables was conducted by ANOVA and Kruskal–Wallis test, respectively. Statistical analyses were performed using Stata v17 (StataCorp LP, College Station, TX).
Results
Patient demographics and clinical course of IMH
A total of 331 referrals to the Johns Hopkins IR-Tox team were evaluated, of which 33 cases were referred for suspected IMH. Twelve patients were excluded as they were either duplicates, had not received immunotherapy, did not have laboratory evidence of demonstrable IMH, or had primary liver cancer and 21 patients were included in the final analysis. The median age was 60 [57–65] years, 62% of patients were female, and the majority were Caucasian (71%), with a median BMI of 28 [24–31] kg/m2 (Table 1). Anti-PD-1 and Anti-PD-L1 antibodies were the most common types of immunotherapy used in this cohort. Most common tumor types were breast (19%), non-small cell lung cancer (14%), and melanoma (14%); Majority of patients (95%) had metastatic disease at the time of ICI initiation. Autoantibodies were evaluated in 18 out of 21 patients in which 33% (618) had elevated ANA (> 1:80) titers, 11% (2) had elevated ASMA (> 1:40) titers, 6% (1/18) had elevated IgG levels. No patient had elevated anti-LKM antibodies. Time from ICI initiation to peak, grade of toxicity at peak, and resolution of hepatitis did not differ by type of ICI.
Most patients had grade 3 or 4 toxicity at the time of referral. 81% of patients were treated with corticosteroids and 53% of patients requiring doses > 1 mg/kg of prednisone equivalent. Median duration of treatment with corticosteroids was 58 [14–111] days. Among those treated with corticosteroids, 35% of patients also received mycophenolate mofetil. Three patients had an acute liver failure from toxicity, and 14 patients had died at the time of follow-up due to progression or complications of cancer. Among those who died during the follow-up period, the time from referral to death was 51 [11–127] days. 48% (10/21) of patients had complete resolution of IMH with the meantime from referral to resolution of 111 [41–214] days. 52% (11/21) of patients did not achieve complete resolution of IMH.
Resolution of IMH
We then compared the patients who had complete resolution of IMH to those without resolution. No significant difference in demographics, clinical history at baseline, immunotherapy type, presence of auto-immune serologies or liver injury pattern at referral between the two groups was evident (Table 2). We looked at the peak level of liver enzymes between the two groups and there was no statistical difference in the toxicity grade between the two groups, indicating that the grade of toxicity at referral and at the peak of injury were similar between the two groups. However, improvement of hepatoxicity defined as > 1 decrease in IMH grading after one week of steroid therapy, was associated with complete resolution of IMH (p = 0.041).
Table 2.
The demographics, clinical characteristics and outcomes of IMH stratified by resolution
| No resolution of IMH (n = 11) | Resolution of IMH (n = 10) | p-value | |
|---|---|---|---|
| Age (years) | 59 (10) | 62 (11) | 0.64 |
| Gender (female) | 6 (55%) | 7 (70%) | 0.66 |
| Race | 0.67 | ||
| Asian | 0 (0%) | 1 (10%) | |
| Black or African American | 2 (18%) | 2 (20%) | |
| Hispanic | 0 (0%) | 1 (10%) | |
| White or Caucasian | 9 (82%) | 6 (60%) | |
| History of autoimmune disease | 0 (0%) | 1 (10%) | 0.48 |
| BMI at referral | 26 (24–30) | 28 (26–31) | 0.36 |
| Tumor type | 0.016 | ||
| Bladder | 2 (18%) | 0 (0%) | |
| Breast | 4 (36%) | 0 (0%) | |
| Esophageal | 0 (0%) | 2 (20%) | |
| GBM | 1 (9%) | 1 (10%) | |
| Gastric | 0 (0%) | 2 (20%) | |
| Liposarcoma | 0 (0%) | 1 (10%) | |
| Melanoma | 2 (18%) | 1 (10%) | |
| NSCLC | 0 (0%) | 3 (30%) | |
| Ovarian | 1 (9%) | 0 (0%) | |
| Pancreatic | 1 (9%) | 0 (0%) | |
| Metastatic | 4 (36%) | 3 (30%) | 1.00 |
| Type of immunotherapy | 0.59 | ||
| CTLA-4 | 2 (18%) | 0 (0%) | |
| CTLA-4 PD-1/PD-L1 | 1 (9%) | 2 (20%) | |
| PD-1/PD-L1 | 8 (73%) | 8 (80%) | |
| ANA titer (> 1:80) | 2 (25%) | 4 (44%) | 0.62 |
| ASMA titer (> 1:40) | 1 (11%) | 1 (14%) | 1.00 |
| Doses of ICI before IMH | 2 (1–4) | 4 (2–9) | 0.32 |
| Time from first ICI to IMH referral (days) | 84 (44–238) | 107 (44–200) | 0.89 |
| Toxicity at referral | 1.00 | ||
| Grade 1–2 | 3 (27%) | 3 (30%) | |
| Grade 3–4 | 8 (73%) | 7 (70%) | |
| Pattern of injury | 0.30 | ||
| Hepatocellular | 3 (27%) | 4 (40%) | |
| Mixed | 2 (18%) | 4 (40%) | |
| Cholestatic | 6 (55%) | 2 (20%) | |
| Toxicity at peak | 0.15 | ||
| Grade 1–2 | 1 (9%) | 4 (40%) | |
| Grade 3–4 | 10 (91%) | 6 (60%) | |
| Pattern of injury | 1.00 | ||
| Hepatocellular | 3 (27%) | 3 (30%) | |
| Mixed | 4 (36%) | 4 (40%) | |
| Cholestatic | 4 (36%) | 3 (30%) | |
| Toxicity 1 week after treatment | 0.041 | ||
| Grade 1–2 | 1 (13%) | 6 (75%) | |
| Grade 3–4 | 7 (88%) | 2 (25%) | |
| Treatment with steroid | 9 (82%) | 8 (80%) | 1.00 |
| Initial dosing of steroids > 1 mg/kg/day prednisone equivalent | 5 (56%) | 4 (50%) | 1.00 |
| Duration of steroid (days) | 28 (12–58) | 110 (46–162) | 0.074 |
| Time from referral to death (days) | 33 (10–67) | 467 (412–472) | 0.016 |
| Death at follow-up | 11 (100%) | 3 (30%) | 0.001 |
n (%), mean (STD), median (IQR)
To understand the impact of treatment variability, we then investigated the dosing and duration of corticosteroid use. Both groups had similar proportions of high-dose steroids with 56% of patients who had no resolution of IMH and 50% of patients with complete resolution of IMH. This suggests that higher dose did not associate with hepatoxicity improvement in this study. Furthermore, it is notable that the duration of steroid treatment was longer for those who had complete resolution of IMH (110 [46–162] vs those without resolution with 28 [12–58] days) although this difference was not statistically significant (p = 0.074). Time from referral to death was longer in patients who had complete resolution of IMH toxicity [33 (10–67) vs 467 (412–472) days (p = 0.016), respectively]. For those patients who had complete resolution, the median time to resolution from referral was 111 days (41–214).
Toxicities from steroid use included hyperglycemia (82%), leukocytosis (41%), infection (18%, majority oral candidiasis/thrush), followed by altered mental status, melena, and venous thromboembolism. Rates of toxicity from steroid use were not significantly different between high and low dose steroids (Supplemental Table 2).
Liver histology
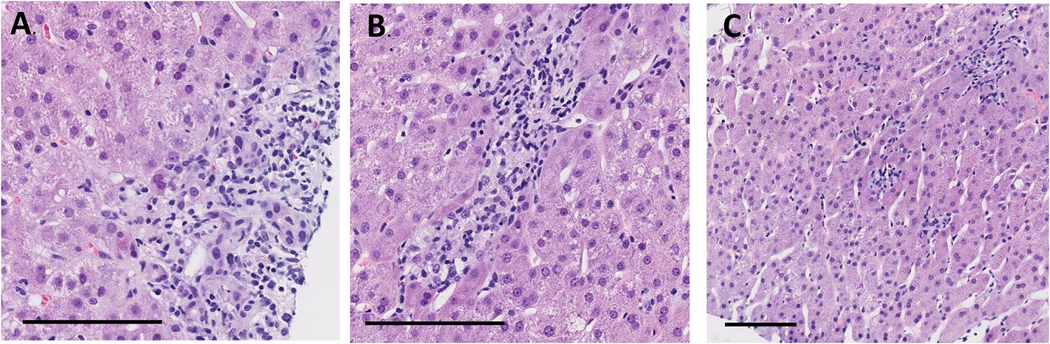
Among 21 patients, eight underwent percutaneous liver biopsies were completed to evaluate abnormal liver enzymes further to help guide further immunosuppression; six cases had slides available for review. The time from the initial referral to biopsy was 6.5 [3–40] days. All patients were on steroids at the time of biopsy with a mean duration of 9 [3–40] days. Notably, all the cases had mild to moderate bile duct injury (Fig. 1A), with 67% (4/6) demonstrating ductopenia with no visible portal tracts (Fig. 1B). Five patients also had varying degrees of lobular inflammation from mild to moderate degrees with a mix of lymphocytes and neutrophils (Fig. 1C), except one case with no lobular inflammation. Hepatitis mainly consisted of lymphocytes, with only two cases showing plasma cells. None of the cases had focal aggregates of eosinophils (> 5 cells/high power field). Some regenerative changes were found, with 50% (3/6) showing mitoses, and rare acidophil bodies, and ballooning degeneration. Furthermore, most biopsies had less than 5% steatosis with no steatohepatitis, except for one case with severe steatosis and steatohepatitis. Details of these findings are summarized in Supplemental Table 1.
Fig. 1.
Key histological findings common in immune-related hepatotoxicity. Bile duct injury (a) was seen in all biopsy samples, commonly associated with ductopenia (b). Pattern of injury is mostly consistent with lobular inflammation (c). Black bar represents 100 um
Discussion
As immune-checkpoint inhibitors continue to improve the treatment response and survival of multiple cancers, immune-mediated hepatitis remains a critical complication of these treatments, with limited evidence for a clear treatment algorithm. To our knowledge, this is the largest single-center cohort study examining the clinical features of the patients who developed IMH, including their clinical course, treatment, and outcomes to identify any early risk factors and potential prognostic indicators. Our cohort study reflects a well-characterized patient population with concordant histologic assessment of IMH in response to steroids. In our study, half of the patients had complete resolution. Steroid dosing did not differ between those who had resolution vs those who did not, but the duration in those with resolution trended longer, owing likely to survival bias. Our data suggested that lab values at one-week post-treatment correlate with overall resolution. Of those who did not have resolution of IMH, all 11 died at a median time of 33 days after referral.
Our histological evaluation of the biopsy samples revealed that bile duct injury is the most consistent finding seen in all samples. This could be a subtle but important pathological finding that distinguishes IMH from other causes of liver injury. Lobular inflammation was not present in all biopsies, and if present, mostly lymphocytic infiltration rather than plasma cells that are more commonly seen in autoimmune hepatitis not related to immunotherapy. It is important to note that liver biopsies were obtained while on steroids, potentially masking an initial inflammatory response. Aside from bile duct injury, other findings are consistent with previous reports with variable inflammation and rare steatosis (Johncilla et al. 2015; Ibraheim et al. 2019; Kim et al. 2013; Patil and Zhang 2021). While there remains a question whether liver biopsy is beneficial with concerns for delay in treatment initiation (Li et al. 2021), bile duct injury in the background of lymphocytic inflammation may help in challenging cases that need a diagnosis confirmation.
In our study, most patients with IMH received monotherapy with anti-PD-1/PD-L1, in contrast to the reported combination of CTLA-4 and PD-1/PD-L1 antibody evaluated in prior studies (Cheung et al. 2019). At doses commonly advised for the treatment of severe acute autoimmune hepatitis and IMH (Dougan et al. 2021b; EASL Clinical Practice Guidelines 2015), we did not notice a difference in outcome when stratified by high and low dose steroids. A retrospective study suggests that initial steroid dosing does not affect overall resolution and normalization of ALT and did not affect median time to resolution at around 28–29 days which is in line with our findings (Li et al. 2022). Another study showed that the median time to improve by one grade was 13 days with a median time to complete resolution of 52 days (Patrinely et al. 2021). Both studies show a shorter time to resolution and improvement than our findings. Time to resolution in our cohort may be longer due to a stricter endpoint of normalization of liver enzymes.
Some limitations merit further discussion. This study is limited by a small sample size with a retrospective data collection. Additionally, our cohort may reflect the clinical course of a sicker subset of IMH which is reflected in the > 70% of patients with Grade 3 or 4 IMH at referral and majority with metastatic disease. Those with milder IMHs may have not been referred to the IR-Tox group.
Overall, our findings have suggested three important implications. First, early response to steroids one week into treatment may predict complete resolution of IMH. Second, high dosing of steroids may not offer more benefit to the patients while longer duration of treatment with longer taper regimen may be more beneficial. Last, if etiology of liver injury is unclear, a liver biopsy could offer a more definitive diagnosis with the evidence of bile duct injury that was consistently seen in our cohort. Further prospective studies will be needed to evaluate predictors of the resolution of IMH.
Supplementary Material
Declarations
Conflict of interest
CF is supported by an NIH T32 GM066691. JN is supported by Merck, Astrazeneca, BMS, Amgen, Novartis, Roche/Genentech and on the consulting/advisory board of: Merck, Astrazeneca, BMS, Amgen, Novartis, Roche/Genentech, Takeda, Pfizer, Daiichi Sankyo, NGM biosciences. LCC provides consulting and has research funding support from Bristol-Myers Squibb. JRB is an advisory board member of Amgen, BMS, Genentech, Eli Lilly, GlaxoSmithKline, Merck, Sanofi, AstraZeneca, Regeneron and receives funding support from: AstraZeneca, BMS, Genentech, RAPT Therapeutics, Inc., Revolution Medicine and is a DSMB member of Sanofi, GlaxoSmithKline, Janssen. RAA is a consultant for Bristol Myers Squib. AKK is supported by NCI K08 CA237624 and DoD Award W81XWH-20-1-0605, Exelixis and AstraZeneca.
Funding
National Institutes of Health, grant nos.: NIH T32 GM06669, K08 CA237624. U.S. Department of Defense, grant no. W81XWH-20-1-0605.
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00432-022-04299-1.
References
- Brahmer JR et al. (2018) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol off J Am Soc Clin Oncol 36:1714–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer JR et al. (2021) Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 9:e002435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani NP et al. (2014) ACG Clinical Guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol 109:950–966 (quiz 967) [DOI] [PubMed] [Google Scholar]
- Cheung V et al. (2019) Immunotherapy-related hepatitis: real-world experience from a tertiary centre. Frontline Gastroenterol 10:364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martin E et al. (2018) Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol 68:1181–1190 [DOI] [PubMed] [Google Scholar]
- Dougan M, Luoma AM, Dougan SK, Wucherpfennig KW (2021a) Understanding and treating the inflammatory adverse events of cancer immunotherapy. Cell 184:1575–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Wang Y, Rubio-Tapia A, Lim JK (2021b) AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterology 160:1384–1393 [DOI] [PubMed] [Google Scholar]
- EASL Clinical Practice Guidelines (2015) Autoimmune hepatitis. J Hepatol 63:971–1004 [DOI] [PubMed] [Google Scholar]
- Gandhi L et al. (2018) Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 378:2078–2092 [DOI] [PubMed] [Google Scholar]
- Haanen JBAG et al. (2017) Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Off J Eur Soc Med Oncol 28:iv119–iv142 [DOI] [PubMed] [Google Scholar]
- Ibraheim H, Perucha E, Powell N (2019) Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatol Oxf Engl 58:vii17–vii28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JJ, Mandaliya R, Nakshabandi A, Lewis JH (2019) Hepatotoxicity induced by immune checkpoint inhibitors: a comprehensive review including current and alternative management strategies. Expert Opin Drug Metab Toxicol 15:231–244 [DOI] [PubMed] [Google Scholar]
- Johncilla M et al. (2015) Ipilimumab-associated hepatitis: clinicopathologic characterization in a series of 11 cases. Am J Surg Pathol 39:1075–1084 [DOI] [PubMed] [Google Scholar]
- Kim KW et al. (2013) Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest New Drugs 31:1071–1077 [DOI] [PubMed] [Google Scholar]
- Li M et al. (2021) Utility of liver biopsy in diagnosis and management of high-grade immune checkpoint inhibitor hepatitis in patients with cancer. JAMA Oncol 7:1711–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M et al. (2022) Effect of corticosteroid dosing on outcomes in high-grade immune checkpoint inhibitor hepatitis. Hepatol Baltim Md 75:531–540 [DOI] [PubMed] [Google Scholar]
- Naidoo J et al. (2019) A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J Natl Compr Cancer Netw JNCCN 17:712–720 [DOI] [PubMed] [Google Scholar]
- Patil PA, Zhang X (2021) Pathologic manifestations of gastrointestinal and hepatobiliary injury in immune checkpoint inhibitor therapy. Arch Pathol Lab Med 145:571–582 [DOI] [PubMed] [Google Scholar]
- Patrinely JRJ et al. (2021) A multicenter characterization of hepatitis associated with immune checkpoint inhibitors. Oncoimmunology 10:1875639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares L et al. (2020) A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: protocol-specified final analysis of KEY-NOTE-407. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer 15:1657–1669 [DOI] [PubMed] [Google Scholar]
- Schneider BJ et al. (2021) Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol Off J Am Soc Clin Oncol 39:4073–4126 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.