Abstract
Molecular subtypes of Small Cell Lung Cancer (SCLC) have been described based on differential expression of transcription factors (TFs) ASCL1, NEUROD1, POU2F3 and immune-related genes. We previously reported an additional subtype based on expression of the neurogenic TF ATOH1 within our SCLC Circulating tumour cell-Derived eXplant (CDX) model biobank. Here we show that ATOH1 protein was detected in 7/81 preclinical models and 16/102 clinical samples of SCLC. In CDX models, ATOH1 directly regulated neurogenesis and differentiation programs consistent with roles in normal tissues. In ex vivo cultures of ATOH1-positive CDX, ATOH1 was required for cell survival. In vivo, ATOH1 depletion slowed tumour growth and suppressed liver metastasis. Our data validate ATOH1 as a bona fide oncogenic driver of SCLC with tumour cell survival and pro-metastatic functions. Further investigation to explore ATOH1 driven vulnerabilities for targeted treatment with predictive biomarkers is warranted.
INTRODUCTION
SCLC is an aggressive neuroendocrine (NE) tumour constituting ~15% of lung cancers. SCLC is the sixth most common cause of cancer-related deaths, accounting for ~250,000 diagnoses worldwide each year1–4. Most patients with SCLC present with extensive stage (ES) disease characterised by widespread metastases and rapidly acquired resistance to initially effective standard-of-care (SoC) platinum-based chemotherapy5. SoC was unchanged for >30 years6 until the recent addition of immunotherapy that extends overall survival of a minority of patients, including rare patients with durable responses7–10.
SCLC molecular subtypes were recently defined based on expression of master neurogenic transcription factors (TFs) ASCL1 (SCLC-A) and NEUROD1 (SCLC-N) and a rarer subtype defined by the non-neuroendocrine (Non-NE) Tuft Cell TF POU2F3 (SCLC-P)11,12. SCLC expressing an immune signature without these TFs was defined as ‘inflamed’ (SCLC-I)13. Preclinical studies suggest subtype-dependent therapeutic vulnerabilities14 heralding potential for stratified therapy in clinical trials, potentially guided by ctDNA methylation subtyping15 where serial liquid biopsy could assess evolving subtype plasticity16.
Patients with SCLC have prevalent circulating tumour cells (CTCs)17, prompting our establishment of CTC-Derived patient eXplant (CDX) models in immunodeficient mice to explore SCLC biology and test novel therapeutics12. ASCL1 and/or NEUROD1 subtype CDX consist primarily of NE cells with a minority Non-NE subpopulation12,18 consistent with the NE to NonNE phenotype switch brought about by Notch signalling generating intra-tumoral heterogeneity16,19. POU2F3 expressing CDX13 tumours are exclusively Non-NE12. YAP1, initially considered a subtype determinant of SCLC11, is expressed in Non-NE cells within ASCL1 or NEUROD1 CDX18.
We recently described a subset of SCLC CDX lacking expression of ASCL1 or POU2F3, that instead expressed the neurogenic, basic helix-loop-helix TF ATOH1, which could be co-expressed with NEUROD112. ATOH1 was expressed in 4 CDX models from 3/31 SCLC patients (9.6%). Two of these CDX were generated from the same patient pre- and post-treatment and maintained ATOH1 expression.
ATOH1 is homologue of Drosophila melanogaster Atonal, first identified in sensory organs of developing embryos20. In mouse models, Atoh1 (or Math1) is critical for development and differentiation of sensory cell types, including granule cells in the brain, sensory inner ear hair cells, Merkel cells in the skin, and secretory cells in the intestine21–27. Atoh1, like Ascl1, engages Notch signalling through lateral inhibition to avoid aberrant cellular differentiation in brain and intestine24,28,29. ATOH1 impact in cancer is context-dependent, described as a tumour suppressor in colorectal cancer and an oncogene in medulloblastoma30,31. Functional role(s) of ATOH1 in SCLC are unknown.
Here we explore transcriptional programmes and cellular functions(s) regulated by ATOH1 in SCLC. Although rare in our CDX biobank compared to SCLC-A, we identified ATOH1 in a subset of patients’ tumours and in additional Patient-Derived eXplants (PDX) models32. We show that in SCLC cell lines and/or CDX models, ATOH1 regulates neurogenesis, maintains cell survival in vitro and promotes tumour growth and liver metastasis in vivo. Our study adds to the emerging landscape of SCLC heterogeneity, highlighting potential for subtype-stratified approaches for improved treatment outcomes.
RESULTS
ATOH1, MYCL and chemosensitivity
We suggested ATOH1 as a SCLC subtype determinant after noting its expression in 4/38 CDX models that were distinct upon unsupervised clustering of whole transcriptomes12 (Figure 1A–i). Four ATOH1 CDX were derived from three donors: one sampled prior to chemotherapy (CDX25), one post-chemotherapy (CDX30P) and one where paired CDX were generated pre- and post-chemotherapy (CDX17, CDX17P), with maintained ATOH1 expression12 (Table S1). Whilst ATOH1 can be co-expressed with NEUROD1 (Figure 1A–i), we confirmed and extended Principal Component Analysis (PCA) of transcriptomic data from 39 CDX (including SCLC-A CDX31P18) that separated ATOH1 models from NEUROD1-only models and from models expressing ASCL1 or POU2F3 (Figure 1A–ii). As ATOH1 is expressed in Merkel cells and most Merkel cell carcinomas (MCCs)33, we checked whether ATOH1 CDX were in fact derived from CTCs from mis-diagnosed MCC primary tumours. MCC is characterised by the presence of oncogenic Merkel cell polyoma virus (MCPyV) in 80% of cases34. We detected MCPyV sequences in MCC patient samples from a publicly available dataset (PRJNA775071) but not in any ATOH1 SCLC CDX (Figure S1A). Because a minority of MCC expresses neither ATOH1 nor MCPyV, we performed differential gene expression analysis (DGEA) of ATOH1 CDX compared to the entire CDX biobank and applied a Merkel cell-specific gene signature35 (Table S2), which was not significantly enriched in ATOH1 CDX (Figure S1B), further supporting that ATOH1 CDX do not have a Merkel cell origin.
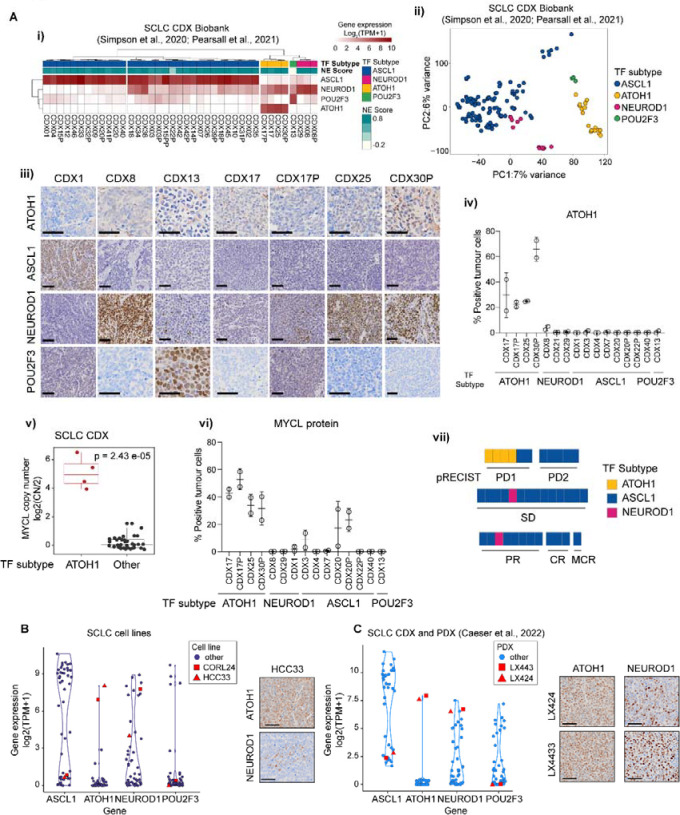
Figure 1. ATOH1 is expressed in a transcriptionally distinct subset of SCLC CDX, PDX and established cell lines.
(A-i) Heatmap illustrating expression levels of ASCL1, NEUROD1, ATOH1 and POU2F3 in the SCLC CDX biobank, annotated by SCLC subtype and NE score12,18. Gene expression is shown as log2(TPM+1). (A-ii) Unbiased principal component analysis (PCA) of SCLC CDX in the biobank annotated by SCLC molecular subtypes. Key: blue, ASCL1; pink, NEUROD1; yellow, ATOH1; green, POU2F3. (A-iii) Representative IHC images for ATOH1, ASCL1, NEUROD1 and POU2F3 in a panel of CDX models belonging to different SCLC molecular subtypes. Scale bars: 50 μm. (A-iv) Quantification of ATOH1 expression in N=2 CDX tumours in a panel of CDX models. (A-v) Boxplot of MYCL copy number (CN), reported as CN ratio (Log2(CN/2)), in CDX grouped by molecular subtype (ATOH1 or other). Statistics reported as per Wilcoxon rank sum exact test. (A-vi) Quantification of MYCL expression by IHC in N=2 CDX tumours in a panel of CDX models belonging to different SCLC molecular subtypes (annotated below). (A-vii) Chemosensitivity scores of the SCLC CDX biobank according to pRECIST criteria, coloured by SCLC molecular subtypes. Key: yellow, ATOH1; blue, ASCL1; pink, NEUROD1. Data are reported after 1 cycle of cisplatin/etoposide treatment and as average of N>3 mice for N=29 CDX (see methods). Statistical analysis was performed with a Fisher’s exact test between ATOH1 CDX and the remaining CDX; p = 0.0049. (B-C) Violin plot representing expression of indicated NE and Non-NE TFs in SCLC established cell lines (B) and the SCLC CDX and PDX biobank32 (C); ATOH1-expressing HCC33, CORL24 (B) and LX424, LX443 (C) are highlighted in red. Gene expression is reported as Log2(TPM+1). Inserts are representative images of ATOH1 and NEUROD1 IHC staining for HCC33 (B) and LX424, LX443 (C).
SCLC subtyping was based predominantly on transcriptomes11,13,36. To examine ATOH1 protein expression we optimised an IHC assay using a commercially available antibody (from here on referred to as Ptech), that revealed nuclear ATOH1 staining only in ATOH1 subtype CDX (Figure 1A–iii, quantified in 1A–iv). Like ASCL1 and POU2F3 and in contrast to NEUROD1, ATOH1 transcript and protein expression followed a bimodal pattern; ATOH1 was either highly expressed or undetectable (Figure 1A–i, 1A–iii, 1A–iv). Whilst ATOH1 CDX expressed neither ASCL1 nor POU2F3 (Figure 1A–i), ATOH1 was expressed alone (CDX17P) or in combination with NEUROD1 at the transcript (Figure 1A–i) and protein level (Figure 1A–iii, CDX25, CDX30P: high NEUROD1 expression, 78% positive tumour cells; CDX17: moderate NEUROD1 expression, 30% positive tumour cells).
MYCL amplification is often observed in SCLC and MCC37,38. ATOH1 expression in CDX strongly correlates with MYCL focal amplification (Figure 1A–v, p=2.43*10−5), resulting in higher levels of MYCL transcript (Figure S1C) and MYCL protein (Figure 1A–vi, S1D) compared to other subtypes.
CDX reflect chemosensitivity profiles of their patient donors12,39. We investigated responses of ATOH1 CDX models to SoC (cisplatin/etoposide) in vivo adopting a modified version of preclinical RECIST (pRECIST) (see methods); tumour growth data are transformed to progressive disease (PD1, PD2), stable disease (SD) and partial (PR), complete (CR) and maintained responses (MCR)40,41. Compared to other molecular subtype CDX (31 SCLC-A, 25 patients, 2 SCLC-N, 2 patients) which displayed variable chemotherapy responses, all 4 ATOH1 CDX (3 patients) were the most chemoresistant, scoring as PD1 (Figure 1A–vii, Fisher’s exact test, p = 0.0049; Table S1). This finding was mirrored in clinical data from the 3 ATOH1 CDX patient donors who all had chemorefractory disease (Table S1). Whilst a larger number of ATOH1 models are required, our early findings imply a putative association of ATOH1 with chemotherapy resistance.
ATOH1 was expressed (transcript and protein) in 2/51 SCLC cell lines42 (Figure 1B) and 2/42 SCLC PDX32 (Figure 1C). The PDX and cell lines also exhibited bimodal ATOH1 expression accompanied by either low (HCC33) or high expression of NEUROD1 (CORL24, LX424, LX443) (Figure 1B–C, inserts). MYCL amplification was observed in ATOH1-expressing SCLC cell lines43 (HCC33 CN ratio ~5, CORL24 CN ratio ~2) and PDX (LX424/44332) and all ATOH1 preclinical models express amongst the highest reported levels of MYCL (Figure S1E-F). The ATOH1 expressing PDX were obtained from one chemorefractory donor (Table S1). Overall, whilst requiring larger sample sizes, these findings indicate that ATOH1 expression in SCLC CDX, PDX and cell lines, with or without NEUROD1, correlates with high MYCL expression and chemoresistance.
ATOH1 in SCLC clinical specimens
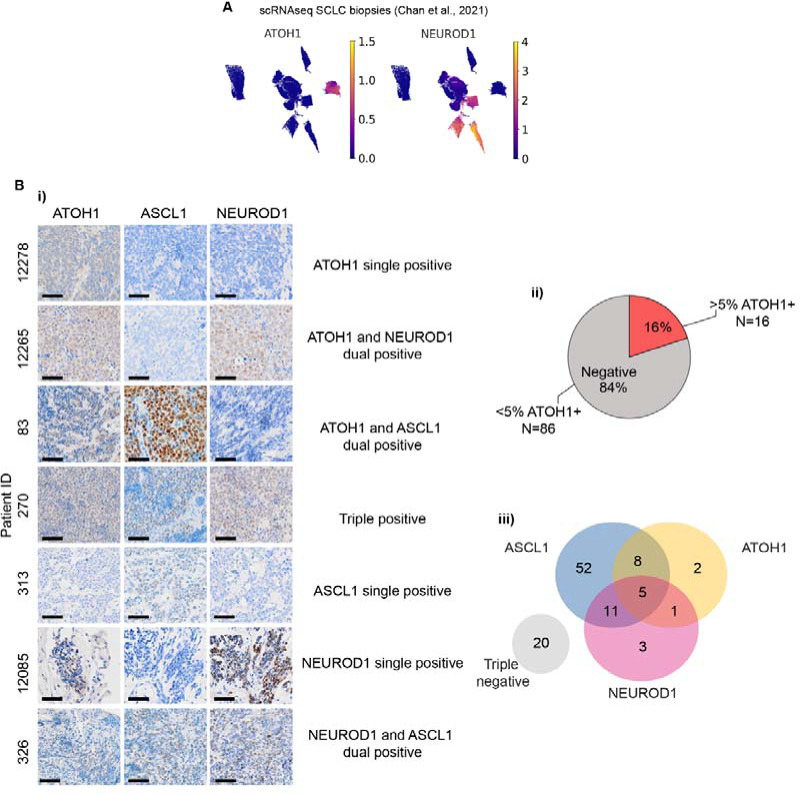
ATOH1 was detected in 1/81 SCLC tumours36 and in 3/100 small cell NE pulmonary and extrapulmonary carcinoma (SCNC) biopsies44. We detected ATOH1 in 1/19 SCLC tumours profiled by single cell RNA-Seq (scRNA-Seq)45, previously classified as NEUROD1 subtype with expression of NEUROD2 and NEUROD4 (Figure 2A). We quantified ATOH1 protein in 65 specimens from 11 LS and 54 ES SCLC patients from the CHEMORES protocol and 37 specimens from LS patients enrolled in the CONVERT trial (methods, Table S4). ATOH1 was detected in 16/102 (16%) cases (Figure 2Ai–ii). One patient sample co-expressed ATOH1 and NEUROD1 (1/16, 6%) (Figure 2A–iii, Table S5) but in contrast to CDX and PDX, 8/16 (50%) ATOH1+ samples also had detectable ASCL1 expression and all three neurogenic TFs were detectable in 3/16 (19%) cases (Figure 2A–iii). Due to scant biopsies, we could not investigate cellular co-expression of TFs. ATOH1 expression did not correlate with altered OS or PFS compared to other SCLC subtypes (data not shown) in this cohort. Nevertheless, the relatively high prevalence of ATOH1 expression in clinical samples either alone or combined with ASCL1 and/or NEUROD1 encouraged further study of ATOH1-driven biology.
Figure 2. ATOH1 protein is expressed in SCLC clinical samples.
(A) UMAP plots of single cell RNA-Seq (scRNA-Seq) from SCLC biopsies from the publicly available MSK SCLC Atlas45 reporting expression of ATOH1 (left panel) and NEUROD1 (right panel). Gene expression reported in units of log2(X + 1) where X = normalized counts. (B-i) Representative IHC images for ATOH1, ASCL1 and NEUROD1 in SCLC tissue biopsies presenting with single, dual or triple positivity (annotated). (B-ii) Pie chart illustrating the prevalence of ATOH1-positive (>5% positive tumour cells) clinical specimens (N=16/102). (B-iii) Venn diagram illustrating overlap of ASCL1, ATOH1 and NEUROD1 expression in N=102 clinical specimens as detected by IHC. Positivity determined as >1.5% positive tumour cells for ASCL1 and NEUROD1; positivity for ATOH1 determined as in B-ii.
ATOH1 regulates a neurogenesis program by binding to E-boxes at promoter and enhancer regions in SCLC CDX
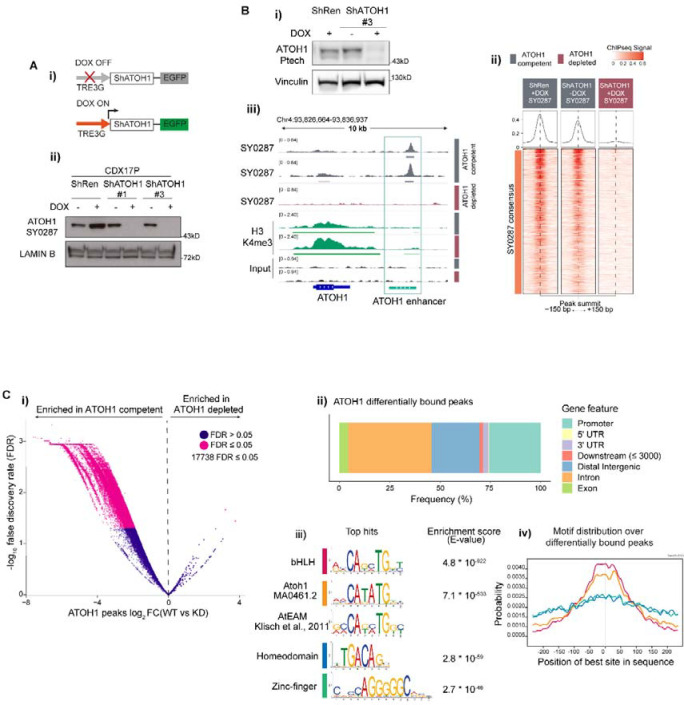
To interrogate the biological role of ATOH1 in CDX, we developed stable CDX17P lines carrying doxycycline-inducible (DOX) ATOH1 knock down (KD) ShRNA constructs (ShATOH1#1, -#3) or a control ShRNA targeting Renilla luciferase46 (ShRen) which also expressed GFP following DOX induction (Figure 3A–i). GFP expression enabled flow cytometric sorting of transduced cells. Maximal ATOH1 KD was observed after 7 days with both the Ptech antibody (Figure S2A) shown previously for IHC, as well as a previously in-house generated antibody (SY0287) (S2B-E, 3A–ii).
Figure 3. High confidence ATOH1 binding sites are located at promoter and enhancer regions and are enriched for E-box motifs.
(A-i) Schematic of DOX-inducible knock-down (KD) system: without DOX, eGFP and shRNAs targeting ATOH1 (ShATOH1) or Renilla Luciferase (ShRen) are not expressed; upon induction with DOX, both eGFP and ShATOH1 or ShRen are expressed. (A-ii) (F) Nuclear fractionation validating ATOH1 KD with the in-house ATOH1 antibody SY0287 in CDX17P ShRen, ShATOH1#1 and ShATOH1#3 upon treatment with DOX for 7 days. (B-i) Western blot showing ATOH1 expression (detected with the Ptech antibody) in the samples processed for ChIP-Seq. (B-ii) Heatmap of ChIP-Seq signal for consensus peak sets SY0287 in ATOH1 competent (grey) and depleted (red) CDX17P, generated with the generateEnrichedHeatmap function within profileplyr v1.8.1100. (B-iii) ATOH1 binding peaks at ATOH1 locus highlighting ATOH1 binding peaks at ATOH1 downstream enhancer (light green), which are lost upon ATOH1 depletion. In dark green, ChIP-Seq tracks for H3K4me3 at the ATOH1 locus. The peaks were visualized with the Integrated Genomics Viewer genome browser. (C-i) Volcano plot of ATOH1 differentially bound regions (by false discovery rate, FDR < 0.05) in ATOH1 competent vs ATOH1 depleted CDX17P. Significant peaks highlighted in pink (17,738). (C-ii) Relative frequency of ATOH1 differentially bound peaks in regulatory genetic regions. (C-iii) Motif enrichment analysis of ATOH1 differentially bound peaks with MEME ChIP101. Mouse Atoh1 E-box-associated motif (AtEAM49) reported for comparison with Atoh1 DNA binding motif and bHLH motif. (C-iv) Centrimo50 analysis of the location of enriched motifs in ATOH1 differentially bound peaks.
Transcriptional programs of ATOH1 are unexplored in SCLC. To reveal ATOH1-specific TF-DNA binding we conducted chromatin immunoprecipitation with massively parallel sequencing (ChIP-Seq) on ATOH1-competent CDX17P (ShRen, 7 days DOX and untreated ShATOH1#3) and ATOH1-depleted ShATOH1#3 CDX17P (7 days DOX). Upon ATOH1 KD (Figure 3B–i), samples clustered based on ATOH1 expression (Figure S3A). Whilst ATOH1 ChIP-Seq signal was almost completely lost upon ATOH1 KD using SY0287 (Figure 3B–ii), some ChIP-Seq signal (~50%) was retained with Ptech (Figure S3B) possibly due to non-specific antibody binding consistent with immunoblots (Figure S2A, 3B–i). Metagene analysis showed that ATOH1 peaks were located on the Transcription Start Site (TSS), near H3K4me3 peaks that identify active promoter regions47 and at intergenic regions mostly downstream of the gene body (Figure S3C) indicating that ATOH1 could regulate transcription at both promoter and distal regulatory elements. In support we found that ATOH1 binds to its own enhancer located downstream and highly conserved across species22 (Figure 3B–iii, S3D).
To identify high confidence ATOH1 binding peaks, we performed differential binding analysis between ATOH1 replete and depleted conditions, considering peaks detected by both antibodies and thus avoiding potential false positives. We found 17,738 ATOH1-specific binding events corresponding to 70% total peaks detected (25,464) (Figure 3C–i, Table S6). Amongst ATOH1-specific binding events, peaks are located at promoter regions (25%) and putative enhancer regions, such as distal intergenic (24%) and intronic regions (41%) (Figure 3C–ii) in accordance with recent results from MCC lines48. The most highly enriched motifs in ATOH1-specific peaks were basic helix-loop-helix binding motifs, including the reported ATOH1 DNA binding motif (MA0461.2) and the Atoh1 E-box-associated motif (AtEAM) identified in murine studies22,49 (Figure 3C–iii). Compared to the second and third most enriched motifs (homeodomains and zinc-fingers), E-box and ATOH1-specific motifs were found at the summit of ATOH1 peaks (Figure 3C–iv) suggesting they are uniquely present where there is highest ATOH1 signal50.
ATOH1 target genes in SCLC CDX
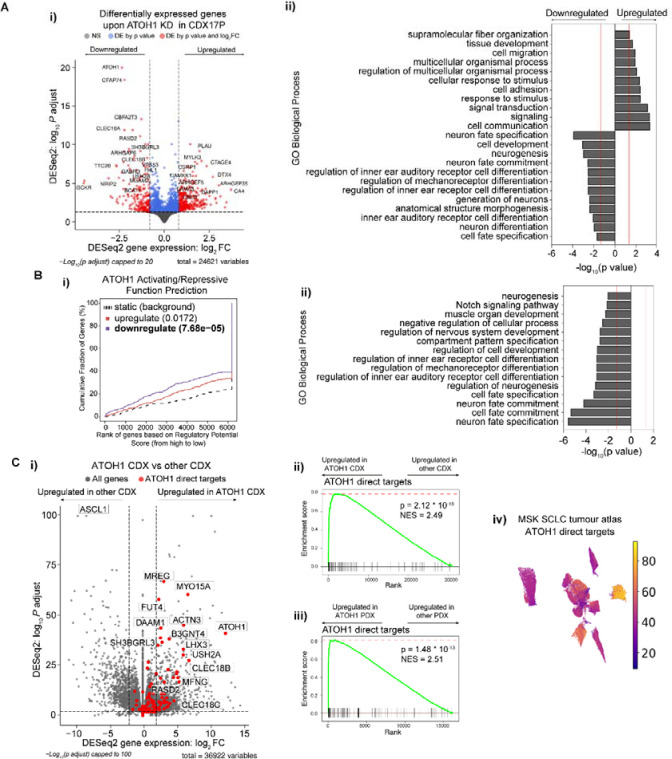
We then sought to identify the biological processes in SCLC regulated by ATOH1 and its putative target genes. Consistent with its role as a neurogenic TF, ATOH1-bound genes were enriched in pathways related to neurogenesis (Figure S3E–F, Table S7). However, this analysis only considered DNA binding events irrespective of gene expression changes. To define genes directly regulated by ATOH1, we performed global transcriptomics (RNA-Seq) of CDX17P cells cultured ex vivo in presence or absence of DOX-induced ATOH1 KD (ShATOH#1, -#3). Genes directly regulated by ATOH1 should be downregulated after ATOH1 loss. As expected, ATOH1 was the most differentially expressed (DE) gene of ~500 genes (Figure 4A–i, Table S8). Genes upregulated after ATOH1 KD included those involved in cell adhesion and migration, whereas downregulated genes play roles in neurogenesis (Figure 4A–ii, Table S9) and in inner ear hair cell differentiation, corroborated by decreased expression of independent inner ear hair cell signatures upon ATOH1 KD51,52 (Figure S4A–B, Table S10–S11). Overall, our findings agree with known ATOH1 transcriptional programs in murine developmental models whereby Atoh1 is required for inner ear hair cell and cerebellar granule cell development and differentiation21, although relevance of these processes to SCLC initiation and progression is unclear.
Figure 4. Identification of ATOH1 targetome and gene signature.
(A-i) Volcano plot illustrating differentially expressed (DE) genes upon ATOH1 depletion (DOX treatment for 6 days) in CDX17P. Key: grey, not significant; blue, significant by p value; red, significant by p value <0.01 and log2(fold change) >0.8 or <−0.8. Dotted lines represent the thresholds for determining significant gene expression changes (p value <0.01 and log2(fold change) >0.8 or <−0.8). The most significant DE genes are labelled. (A-ii) Bar plot illustrating the top 20 biological processes up- and downregulated upon ATOH1 KD in CDX17P. Analysis was performed with gProfiler2102. (B-i) Prediction of ATOH1 transcriptional function after integration of ChIP-Seq and RNA-Seq with BETA55. ATOH1 KD results in downregulation of genes with ATOH1 binding sites identified in ChIP-Seq (p = 7.68 * 10−5) and is predicted to have a function in promoting transcription. (B-ii) Bar plot illustrating biological processes (performed with gProfiler2) associated with ATOH1 target genes identified in B-i. (C-i) Volcano plot illustrating genes enriched in ATOH1 CDX (N=4) compared to the whole CDX biobank (N=35). ATOH1 gene signature (i.e. ATOH1 target genes) highlighted in red. Dotted lines represent the thresholds for determining significant gene expression changes (p value <0.01 and log2(fold change) >2 or <−2). (C-ii) Gene set enrichment analysis (GSEA) for ATOH1 direct targets in ATOH1 CDX (N=4) vs the rest of the biobank (N=35). NES: normalised enrichment score. (C-iii) GSEA for ATOH1 direct targets in ATOH1 PDX (N=2) vs the rest of the MSK PDX biobank (N=40). GSEA analysis was performed with Fgsea103. (C-iv) UMAP of cumulative expression of ATOH1 direct targets in scRNA-Seq of SCLC tumour biopsies45. Expression of ATOH1 target genes is highest in the only ATOH1-expressing tumour (identified in Figure 2A).
ASCL1 and NEUROD1 are highly expressed in NE subtypes of SCLC11,53 and drive a NE transcriptional program. Given that ATOH1 also regulates neurogenesis, we asked whether NE status was affected by ATOH1 depletion. Whilst a 25-gene NE signature54 and SYP expression were unchanged upon ATOH1 KD (Figure S4C,E, Table S10), a 25-gene Non-NE signature was upregulated54 (Figure S4D, Table S10) suggesting that ATOH1 may contribute to NE to Non-NE plasticity, albeit without increased expression of YAP1 nor MYC (Figure S4E).
Fewer significant transcriptional changes were seen upon ATOH1 KD relative to the abundance of ATOH1 binding sites (by ChIP-Seq), suggesting that ATOH1 activity might be restricted to a subset of ATOH1-bound genes in SCLC CDX. Thus, to infer direct ATOH1 transcriptional targets in SCLC, we performed an integrated analysis of ChIP-Seq and RNA-Seq with the Binding and Expression Target Analysis (BETA)55. We found that ATOH1 mainly acts as a transcriptional activator (Figure 4B–i, blue line) and identified 150 genes downregulated upon ATOH1 depletion, directly downstream of ATOH1 (Table S12). Among these genes were components of Notch signalling (including HES6, DLL1, DLL3, DLL4) consistent with the interplay between ATOH1 and Notch signalling during brain and intestinal development24,56 and genes important for inner ear hair cell development such as USH2A, LHX3 and RASD252. Concordant with transcriptomics analysis (Figure 4A–ii), ATOH1 direct targets are also involved in neurogenesis and inner ear hair cell differentiation (Figure 4B–ii, Table S13).
This integrated analysis was performed in only CDX17P, so we next asked whether ATOH1 direct targets were conserved across all ATOH1 expressing CDX. We performed DGEA between ATOH1 CDX (CDX17, 17P, 25, 30P) and the whole CDX Biobank (35 CDX) (Figure 4C–i, Table S14), followed by gene set enrichment analysis (GSEA) for ATOH1 direct targets to demonstrate ATOH1 direct target genes were conserved (Figure 4C–ii, NES = 2.48, p = 1.13 * 10−16). We also detected high expression of ATOH1 target genes in the 2 ATOH1 SCLC PDX (Figure 4C–iii, NES = 2.44, p = 5 * 10−10) and an ATOH1 expressing tumour from the MSK SCLC tumour atlas dataset45 (Figure 4C–iv). These direct targets comprise the first SCLC-based ATOH1 gene signature consistently observed in CDX, PDX and tumour biopsies, indicative of a conserved transcriptional role for ATOH1 in SCLC.
Impact of ATOH1 on SCLC CDX cell survival ex vivo
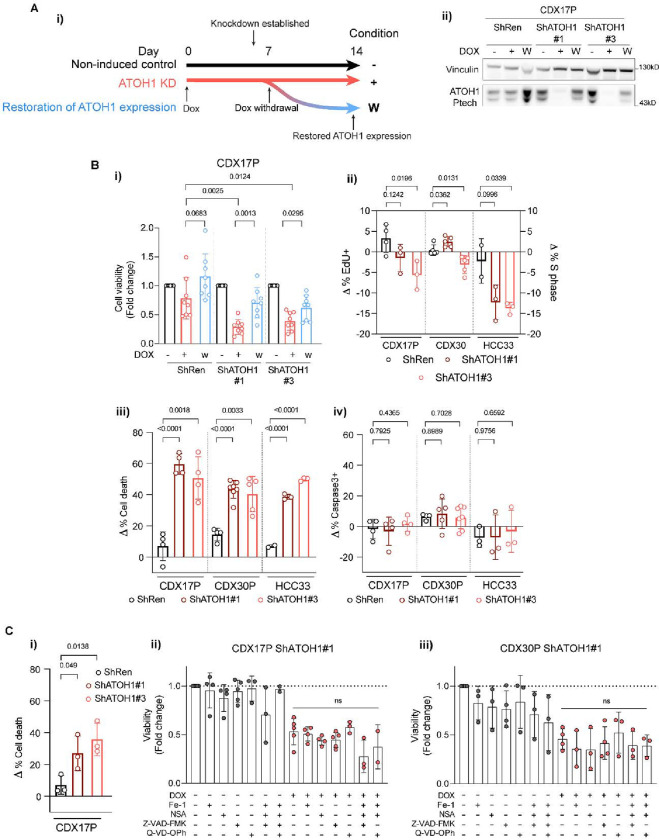
We examined the biological effects of ATOH1 depletion via DOX-inducible ATOH1 KD in CDX17P cells. Maximal ATOH1 KD was achieved after 7 days of DOX (Figure S2A) and was maintained for 14 days (the longest duration of ex vivo studies). Withdrawal of DOX restored ATOH1 expression (7 days +DOX, then 7 days −DOX) (Figure 5A–i, ii). ATOH1 depletion caused >50% decrease in cell viability (ShATOH1#1, p=0.0025; ShATOH1#3, p=0.0124), compared to un-induced and ShRen controls, which was attenuated by restoring ATOH1 expression (Figure 5B–i). To interrogate the mechanism of decreased cell viability, we established DOX-inducible ATOH1 KD in CDX30P and HCC33 SCLC cells (Figure S5A–B) and assessed cell death and cell cycle progression following ATOH1 depletion. Compared to ShRen DOX-induced controls and un-induced cells, there were no reproducible changes in cell cycle progression in CDX17P or CDX30P upon ATOH1 depletion for 14 days (Figure 5B–ii, Figure S5C). A modest ~12% decrease in cell proliferation was evident in HCC33 cells although this did not constitute a complete proliferation arrest with ~15% cells still cycling (Figure S5D). These slightly different effects on proliferation in CDX versus HCC33 may result from differences between cell lines and CDX ex vivo cultures. Instead, ATOH1 depletion increased cell death in CDX17P (55%), CDX30P (42%) and HCC33 (44%) after 14 days of ATOH1 depletion (Figure 5B–iii) via a caspase-3-independent process (Figure 5B–iv). After 7 days of DOX treatment, ATOH1 KD already induced detectable cell death (Figure 5C–i) and a decrease in ATP production, used as a proxy for viable cell number (Figure 5C–ii, iii, in red). Because other types of non-apoptotic, programmed cell death such as ferroptosis and pyroptosis have been observed in SCLC57,58, we induced ATOH1 KD in CDX17P and CDX30P ShATOH1#1 with DOX, with or without cell death pathway inhibitors for 7 days. Inhibition of apoptosis, pyroptosis, necroptosis or ferroptosis (with single or combined inhibitors) did not prevent ATOH1 KD-induced loss of cell viability (Figure 5C–ii, iii). Taken together, these findings identify ATOH1 as necessary for cell survival in CDX17P, CDX30P and HCC33 cells as its depletion induces cell death, either via an undefined programmed cell death pathway or most likely via necrosis.
Figure 5. ATOH1 is necessary for SCLC cell survival in vitro.
(A-i) Schematic of induction of ATOH1 KD. ATOH1 KD was established after 7 days induction with 1 μg/ml doxycycline (DOX). Cells were cultured for a total of 14 days with DOX (red line, +) or without DOX as controls; after the initial 7 days induction with DOX, a part of cells was plated without DOX to restore ATOH1 expression (blue line, W). Untreated parental cells served as additional control (black line, −). (A-ii) Western blot validation of ATOH1 depletion and restoration in the conditions specified in A-i. ShRen treated with DOX for 14 days and untreated ShRen, ShATOH1#1, ShATOH1#3 and were used as control. (B-i) Relative cell viability measured with CellTiter-Glo® (Promega) upon ATOH1 KD (red) and restoration (blue) compared to un-induced controls (black). N=8 independent experiments. (B-ii) Flow cytometry quantification of cell cycle progression by EdU (CDX17P, HCC33) and PI incorporation (CDX30P). Data was normalised to DOX-untreated parental controls by subtracting the proportion of cells in S phase in untreated cells to that of DOX-treated cells (Δ % S phase = % S phaseDOX-treated − % S phaseuntreated); ShATOH1 conditions were then compared to ShRen controls. CDX17P, N=4 ShRen, N=3 ShATOH1#1 and #3; CDX30P, N=5; HCC33, N=2 ShRen, N=3 ShATOH1#1 and #3 independent experiments. (B-iii) Flow cytometry quantification of cell death after 14 days induction with DOX of ATOH1 KD, normalised as in B-ii. Total cell death is reported as sum of apoptotic and necrotic cells. CDX17P: N=4; CDX30P: N=4 ShRen, N=7 ShATOH1#1, N=5 ShATOH1#3; HCC33: N=2 ShRen, N=3 ShATOH1#1 and #3 independent experiments. (B-iv) Same as B-iii, reporting total Caspase-3 positive cells. All statistics in panel B are reported as two-tailed unpaired t tests across indicated conditions. C-i) Flow cytometry quantification of cell death (as defined in B-iii) after 7 days DOX-induction of ATOH1 KD in CDX17P. N=3 independent experiments. P values are reported in panel B and C-i as per two-tailed unpaired t test. (C-ii, C-iii) ShATOH1#1 CDX17P (C-ii) and CDX30P (C-iii) cells were treated with (red) or without (black) DOX and with or without ferrostatin-1 (1μM), necrosulfonamide (NSA, 100 nM) or Z-VAD-FMK/Q-VD-OPh (20μM) and indicated combinations for 7 days. Cell viability was measured with CellTiter-Glo®, normalized to vehicle treated, DOX-untreated cells and reported as fold change. Statistics in C-ii and C-iii are reported as per one-way ANOVA test with Dunnett’s test correction for multiple comparisons between DOX-treated conditions with and without programmed cell death inhibitors. Data are shown as mean ± SD.
Impact of ATOH1 on tumour growth in vivo
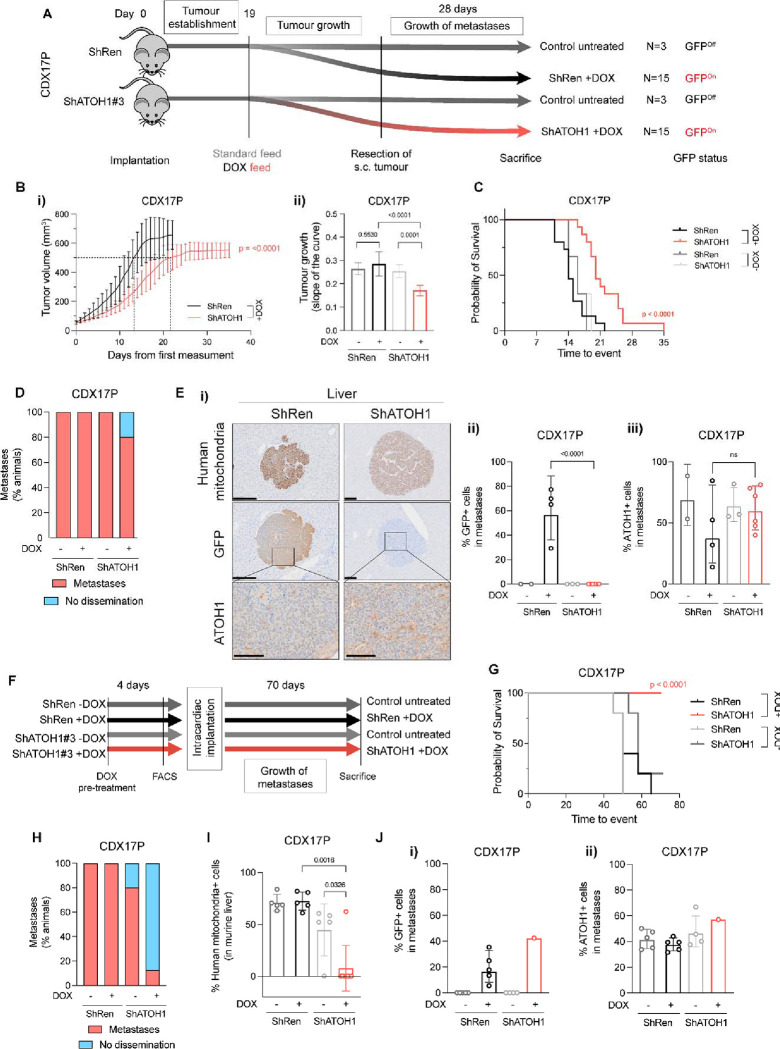
We next asked whether the role of ATOH1 in maintaining cell survival ex vivo translated to an impact on tumour growth in vivo. CDX17P control ShRen or ShATOH1(#3) cells were implanted subcutaneously (s.c.) in immunocompromised mice, and KD was induced with DOX-supplemented feed after 19 days (Figure 6A), when mice had palpable tumours. Once tumours reached 500–800 mm3 they were surgically resected and mice kept on study for 28 days to allow time for metastatic dissemination (based on previous experiments, see methods, Figure 6A).
Figure 6. ATOH1 depletion decreases tumour growth kinetics and metastasis in vivo.
(A) In vivo study design to investigate subcutaneous (s.c.) tumour growth and metastasis after s.c. tumour resection. CDX17P ShRen and ShATOH1#3 (ShATOH1) were injected s.c. in NSG mice and left for 19 days to allow for tumour establishment. After 19 days, mice were fed either standard diet (control arms, N=3) or DOX-supplemented feed (experimental arms, N=15) and s.c. tumour growth was assessed. S.c. tumours were surgically resected when at 500–800 mm3 to allow for metastatic dissemination and mice were kept on study for 28 days or until s.c. tumour reached maximum size, whichever came first. (B-i) S.c. tumour growth curves, from day of first tumour measurement to s.c. tumour resection (see methods), of mice implanted with ShRen and ShATOH1 and fed DOX-supplemented diet. Key: black, ShRen fed DOX-diet; red, ShATOH1#3 fed DOX-diet. N=15 mice per cohort; data reported as mean ± SD. Dotted lines indicate when tumours from each cohort reached 500 mm3: ShRen, 14 ± 3 days; ShATOH1, 21 ± 5 days. (B-ii) Quantification of the slope of tumour growth curves in B. Key: same as in B; shades of grey for control cohort fed standard diet for the duration of the study. P values were calculated with ANCOVA test and slope of the curve was reported as mean ± SD for each cohort. (C) Kaplan-Meier curve of time to surgical resection of s.c. tumour or maximum 800 mm3 for inoperable tumours. Control arms, fed a standard diet, reported in scales of grey. P values were calculated with Log-rank Mantel-Cox test. (D) Quantification of metastatic dissemination to the liver in N=3 mice fed standard diet, N=5 ShRen- and N=15 ShATOH1-tumour bearing mice fed DOX-diet that underwent surgical resection of s.c. tumour and survived on study for at least 22 days after resection. Data is shown as percentage of animals displaying metastatic dissemination (disseminated tumour cells and micro/macro-metastases, in red) or no metastatic dissemination in the liver (blue). Metastases were identified based on human mitochondria staining. (E-i) Representative images of human mitochondria, GFP and ATOH1 IHC staining in liver from ShRen DOX-fed and ShATOH1#3 DOX-fed cohort. Scale bars: 200 μm for human mitochondria and GFP; 100 μm for ATOH1. (E-ii, E-iii) Quantification of GFP (E-ii) and ATOH1 (E-iii) IHC staining in metastases from N=2 DOX-untreated ShRen, N=3 DOX-untreated ShATOH1#3, N=4 ShRen DOX-fed, N=6 ShATOH1#3 DOX-fed mice. Data are shown as geometric mean ± geometric SD. P values are reported as per two-tailed unpaired Mann Whitney U test. (F) In vivo study design to investigate development of metastasis following intracardiac implantation. Prior to cell implantation, ATOH1 depletion was induced by DOX treatment for 4 days in vitro, followed by sorting GFP-positive, viable cells by flow cytometry. Untreated control cells were sorted exclusively for viable cells. Animals in the DOX treatment cohorts were fed a DOX-supplemented diet 24 hours prior to implantation and they were kept on that diet until endpoint. Animals in the uninduced control groups were given a standard diet. Animals from all 4 cohorts (ShRen +/− DOX and ShATOH1 +/− DOX) were removed at the onset of symptoms (i.e., distended abdomen, detailed in methods) or after 70 days. (G) Kaplan-Meier curve of time to sacrifice. Control cohorts, fed a standard diet, reported in scales of grey. P values were calculated with Log-rank Mantel-Cox test. (H) Quantification of metastatic dissemination to the liver for each cohort. Data is shown as per Figure 6D. (I) Quantification of metastatic cells in the liver for each cohort. Metastatic cells were identified based on human mitochondria staining. Data shown as mean ± SD. P values were calculated with a two-tailed unpaired Mann Whitney U test. (J) Quantification of GFP (J-i) and ATOH1 (J-ii) IHC staining in metastases from N=5 DOX-untreated ShRen, N=5 DOX-untreated ShATOH1, N=5 ShRen DOX-fed, N=1 ShATOH1#3 DOX-fed mice. Data are shown as geometric mean ± geometric SD. No statistical test could be performed as ShATOH1 contained only one value.
A significantly delayed s.c. tumour growth was observed in mice bearing DOX-induced ATOH1 KD tumours compared to DOX-induced ShRen controls or un-induced tumours (Figure 6B–i, ii). This tumour growth delay extended time to reach the experimental endpoint tumour volume or s.c. tumour surgical resection (22 days for ShRen, 35 days for ShATOH1, p<0.0001, Figure 6C). To interpret the observed growth delay, we examined persistence of ATOH1 KD throughout the experiment by performing IHC for ATOH1 and GFP in resected s.c. tumours (mean tumour volume and time from implant: 603±54 mm3, 44±5 days ShRen +DOX; 552±48 mm3, 70±13 days ShATOH1 +DOX) (Figure 6B–i). At tumour resection, mice bearing DOX-induced ATOH1 KD tumours showed a 75% reduction in ATOH1 protein expression and both DOX-induced controls and KD tumours had high expression of GFP (Figure S6A–i, ii). However, GFP expression was ~10% lower in DOX-induced ATOH1 KD tumours (Figure S6A–ii, p=0.008) and expression of GFP and ATOH1 was heterogeneous in DOX-induced ATOH1 KD tumours, with most tumour presenting with some GFP-, ATOH1+ regions (Figure S6A–iii).
Overall, these data indicate that reduced ATOH1 expression promotes tumour growth delay in vivo, where impact may have been attenuated by outgrowth of ATOH1 positive cells which are potentially un-transduced wild-type cells or cells that escaped inducible KD, as reported in other settings59,60. These data are consistent with a selective pressure to re-instate ATOH1 expression in ATOH1 KD tumours supporting a pro-tumorigenic role for ATOH1.
A Role for ATOH1 in liver-metastatic dissemination in vivo
We previously reported metastasis to multiple organs, including brain and liver, occurs after resection of s.c. CDX17P tumours12. To investigate whether ATOH1 supports metastatic growth, s.c. tumours were resected and mice left on study for 28 days (Figure 6A) before metastasis (defined as >50 tumour cells) were quantified using a human mitochondria antibody and IHC. Dissemination, predominantly to the liver, was observed in all cohorts regardless of DOX feed, including single tumour cells, micro-or macro-metastasis (Figure 6D). Although frequency of liver metastases between control and DOX-induced ATOH1 KD mice was approximately equivalent, all liver metastases from DOX-induced ShATOH1 mice were negative for GFP and expressed similar levels of ATOH1 compared to un-induced tumours (Figure 6E–i, ii), again implying a selective pressure to retain/re-express ATOH159,60 and indirectly suggesting a role for ATOH1 in promoting liver metastasis.
In a more direct approach to investigate the role of ATOH1 in metastasis, we performed intracardiac injection of tumour cells (Figure 6F), reasoning liver metastasis would occur faster, allowing less time for outgrowth of cells with high or re-expressed ATOH1 (Figure 6E). CDX17P control ShRen or ShATOH1 cells were cultured with or without DOX for 4 days to induce ATOH1 KD in vitro and GFP-positive viable cells were sorted by flow cytometry before intra-cardiac injection. One group of mice per construct (ShRen and ShATOH1) received DOX-supplemented feed (N=5 ShRen and N=8 ShATOH1), while control animals were maintained on standard diet (N=5 ShRen and N=5 ShATOH1). Animals were removed from study 70 days after intracardiac injection (see methods, Figure 6F).
Almost all animals (14/15) in control cohorts (standard feed or implanted with DOX-induced ShRen cells) were removed before study endpoint due to extensive metastatic liver disease (Figure S6B). In contrast, 8/8 (100%) animals implanted with DOX-induced ShATOH1 cells reached study endpoint (time from implantation: 53.6 ± 7.9 ShRen+DOX; 70 ± 0 ShATOH1+DOX; Figure 6G). There was a significant reduction in metastatic burden in animals with ATOH1 KD compared to control cohorts (Figure 6H–I) and only one animal in the DOX-induced ShATOH1 group developed liver metastasis (Figure S6B). Despite showing positive GFP expression (>40% GFP+ cells), the only liver metastasis derived from ATOH1 KD cells also exhibited ATOH1 positivity in >60% of metastatic cells, indicating that ATOH1 KD was not completely retained in these cells (Figure 6Ji–ii). These data provide more direct evidence that ATOH1 KD reduced metastasis to the liver and promoted longer survival.
DISCUSSION
Emerging understanding of SCLC subtypes and phenotypic plasticity are considered key to support rational development of biomarker-directed personalised treatments14. Building upon knowledge of inter- and intra-tumoural heterogeneity32,44, we have characterised the ATOH1 subtype, defining its prevalence and demonstrating pro-tumour functions of growth and metastasis.
ASCL1, NEUROD1 and ATOH1 are all proneural TFs negatively regulated by Notch signalling24,28,61. Whilst expression of ATOH1 is not reported during normal lung development, its expression has been reported in NE lung cancer62, extrapulmonary high-grade neuroendocrine cancers44, Merkel cell carcinoma (MCC)33, medulloblastoma63,64 and rarely in NSCLC65 and colorectal cancer (CRC)30,66,67. Whilst mechanistically understudied, in medulloblastoma and MCC ATOH1 is tumour-promoting31,68–70, whereas it is a tumour suppressor in CRC30,66. These opposing context-dependent functions have been attributed to imbalance between differentiation and proliferation driven by abnormal ATOH1 expression levels71.
Co-expression of subtype TFs is commonly observed, contributing to SCLC heterogeneity12,32,72,73. ATOH1 was found to be frequently expressed in SCLC clinical samples, either alone or with ASCL1 and/or NEUROD1 (Figures 1, 2) extending existing sparse data62. In CDX30 where ATOH1 was co-expressed with NEUROD1, ATOH1 depletion impacted cell survival ex vivo (Figure 5), suggesting that NEUROD1 could not compensate for ATOH1 loss. Furthermore, NEUROD1 was not identified amongst ATOH1 direct targets and there was minimal overlap with ASCL1 and NEUROD1 target genes (Figure 4, Table S15). Like NEUROD1 and ASCL1 in their respective subtypes74–79, ATOH1 supports cell viability in ATOH1 subtype tumour cells (Figure 5).
In SCLC, ATOH1 exerts its function by binding E-box motifs at promoter and enhancer regions of target genes as in the developing mouse brain49 and in MCC80, including binding to its own downstream enhancer22 (Figure 3). In CDX, ATOH1 directly regulates expression of genes involved in neuronal fate development and mechanoreceptor differentiation (Figure 4) consistent with murine developmental studies21,81,82. This is also consistent with the role of ATOH1 in MCC33. The ability of ATOH1 to regulate neuronal fate determination and Notch ligands (DLL1, DLL3, DLL4) in mice24 mirrors the activity of ASCL1 in SCLC53,74; in CDX17P, ATOH1 depletion increased expression of Non-NE and cell adhesion genes invoking a similar role for ATOH1 in NE fate determination in SCLC (Figure S4). However, as the NE gene expression signature was retained upon ATOH1 depletion (Figure S4), additional factors, for example, MYC overexpression16, are likely required to promote full NE to Non-NE transition in ATOH1-driven SCLC. The need for additional signals to fully induce a NE to Non-NE transition is similarly posited in studies of ASCL1 and NEUROD1 depletion in SCLC, where morphological changes or a NE to Non-NE transition were not observed77,78,83,84.
Both ATOH1 and ASCL1 correlate with MYCL overexpression (Figure 1). In SCLC, overexpression/genetic amplification of MYCL was often correlated with the SCLC-A subtype and MYCL is a direct transcriptional target of ASCL135, 52, 86. A more complex relationship was recently revealed by a clinical study whereby MYCL protein was present in only ~30% of ASCL1+ samples73. Further adding to this heterogeneity, we show that all ATOH1-expressing CDX present focal amplification and overexpression of MYCL (Figure 1, S1). A correlation between ATOH1 and MYCL expression was also observed in MCC37,38. However, we did not identify MYCL as a direct ATOH1 target (Table S12) and MYCL expression was unchanged upon ATOH1 depletion (Table S8, Figure 4). Combined, these data indicate that other factors contribute to MYCL expression in ATOH1-positive SCLC.
The profound impact of metastasis on SCLC patient outcomes drives a pressing need to understand and target underlying mechanisms. Acquisition of neuronal gene expression programmes is associated with invasive and metastatic SCLC in cell lines and GEMMs59,85,86. In CDX17P, ATOH1 is pro-metastatic (Figure 6) drawing parallels with the ATOH1 pro-invasive phenotype in MCC87 and its pro-metastatic role in medulloblastoma88. ATOH1 downregulation was linked with loss of cell adhesion (Figure 4A–ii, Table S8), which was also observed in MCC33,89.
SCLC was once considered to derive from pulmonary neuroendocrine cell (PNEC) precursors90. However, elegant studies in SCLC GEMMs describe different potential cells of origin59,91–93 with differences only evident at the molecular level16,45,53. In this regard, similarities between MCC and ATOH1-driven SCLC are intriguing. MCC is a NE skin carcinoma, expressing epithelial and NE markers with morphological, ultrastructural and immunohistochemical features shared with Merkel cells91–93 yet there is no direct histo-genetic link between Merkel cells and MCC with ongoing debate on cell(s) of origin of MCC94,95. Tumour heterogeneity in MCC is attributed to variant disease aetiologies mediated by either UV exposure or Merkel cell polyomavirus (MCPyV) integration95. Virus-positive MCC has low mutation burden, whilst virus-negative MCC, like SCLC, have characteristic RB1 and TP53 mutations in a highly mutated landscape96,97. The recent identification of ‘mesenchymal-like’ MCC with an ‘inflamed’ phenotype exhibiting better response to immunotherapy draws parallels with the SCLC-I subtype13 and contrasts ‘immune-cold’ immunotherapy resistant MCC with higher expression of neuroepithelial markers including ATOH198. Altogether, that the ATOH1 subtype of SCLC CDX shares features with NE SCLC and with MCC, another NE cancer, is perhaps not surprising and might indicate convergent tumour evolution94,99.
In summary, here we validate the ATOH1 SCLC subtype (SCLC-AT) where ATOH1 suppresses cell death and promotes tumour growth and metastasis. Further studies are now needed to deepen our understanding of ATOH1-driven SCLC biology and to address whether there are therapeutic vulnerabilities of this subtype.
Supplementary Material
Acknowledgements
This work was supported through Core Funding to Cancer Research UK (CRUK) Manchester Institute (grant number A27412), Manchester CRUK Centre Award (grant number A25254), the CRUK Lung Cancer Centre of Excellence (grant number A20465), Cancer Research UK Manchester Centre award (CTRQQR-2021\100010), The Christie Charitable Fund, National Cancer Institute R35 CA263816 and U24 CA213274. Patient recruitment was supported by the National Institute for Healthcare Research (NIHR) Manchester Biomedical Research Centre, the NIHR Manchester Clinical research Facility at The Christie Hospital and the CRUK Lung Cancer Centre of Excellence. Sample collection was undertaken through the molecular mechanisms underlying chemotherapy resistance, therapeutic escape, efficacy, and toxicity improving knowledge of treatment resistance in patients with lung cancer or CHEMORES protocol, the TARGET (tumour characterization to guide experimental targeted therapy) study and the CONVERT protocol (concurrent once-daily versus twice-daily radiotherapy: a 2-arm randomised controlled trial of concurrent chemo-radiotherapy comparing twice-daily and once-daily radiotherapy schedules in patients with limited stage small cell lung cancer (SCLC) and good performance status). Dr. Frese, Dr. Simpson and Prof. Dive supervised and devised the study. Dr. Catozzi, Dr. Peiris-Pagès, Dr. Simpson, and Prof. Dive co-wrote the manuscript. Dr. Catozzi, Dr. Peiris-Pagès, Ms. Davies-Williams, Mr. Revill, and Mr. Morgan performed immunohistochemistry analysis, data analysis and interpretation. Dr. Catozzi carried out all experiments on CDX and cell lines, ChIP-Seq, RNA-Seq and western blotting, including data analysis and interpretation. Dr. Catozzi, Dr. Humphrey, Mr. Chen carried out bioinformatics analyses. Dr. Peiris-Pagès designed the in vivo metastases studies and analysed the metastatic dissemination of ATOH1 KD cells in vivo. Ms. Galvin, Mr. Roebuck and Dr. Lallo were responsible for all in vivo work described. Dr. Frese had oversight of all patients with circulating tumour cell–derived explant models and model generation and helped edit the manuscript. Dr. Pearce and Dr. Kerr had oversight of all bioinformatics analyses. Ms. Priest, Dr. Foy, Mr. Carter, and Prof. Blackhall oversaw the acquisition of ethical permission and patient consent and the collection of blood samples from patients in the CHEMORES study. Dr Rudin provided PDX and assisted with manuscript revision. Prof. Blackhall assisted with manuscript revision and is the chief investigator of the CHEMORES study. All authors read and approved the final manuscript.
References
- 1.Gazdar A. F., Bunn P. A. & Minna J. D. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer 17, 725–737, doi: 10.1038/nrc.2017.87 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Forman D. Cancer incidence in five continents: volume X. (International Agency for Research on Cancer, 2014). [Google Scholar]
- 3.American Cancer Society Cancer Facts & Figures 2022. (2022). [Google Scholar]
- 4.Sabari J. K., Lok B. H., Laird J. H., Poirier J. T. & Rudin C. M. Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol 14, 549–561, doi: 10.1038/nrclinonc.2017.71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunn P. A. Jr. et al. Small Cell Lung Cancer: Can Recent Advances in Biology and Molecular Biology Be Translated into Improved Outcomes? J Thorac Oncol 11, 453–474, doi: 10.1016/j.jtho.2016.01.012 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farago A. F. & Keane F. K. Current standards for clinical management of small cell lung cancer. Transl Lung Cancer Res 7, 69–79, doi: 10.21037/tlcr.2018.01.16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horn L. et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. New England Journal of Medicine, doi: 10.1056/nejmoa1809064 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Goldman J. W. et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 22, 51–65, doi: 10.1016/S1470-2045(20)30539-8 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Rudin C. M. et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol 38, 2369–2379, doi: 10.1200/JCO.20.00793 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leal T. et al. Randomized phase II clinical trial of cisplatin/carboplatin and etoposide (CE) alone or in combination with nivolumab as frontline therapy for extensive-stage small cell lung cancer (ES-SCLC): ECOG-ACRIN EA5161. Journal of Clinical Oncology 38, 9000–9000, doi: 10.1200/JCO.2020.38.15_suppl.9000 (2020). [DOI] [Google Scholar]
- 11.Rudin C. M. et al. Molecular subtypes of small cell lung cancer: a synthesis of human and mouse model data. Nature Reviews Cancer, 1, doi: 10.1038/s41568-019-0133-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simpson K. L. et al. A biobank of small cell lung cancer CDX models elucidates inter- and intratumoral phenotypic heterogeneity. Nature Cancer 1, 437–451, doi: 10.1038/s43018-020-0046-2 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Gay C. M. et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 39, 346–360 e347, doi: 10.1016/j.ccell.2020.12.014 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poirier J. T. et al. New Approaches to SCLC Therapy: From the Laboratory to the Clinic. J Thorac Oncol 15, 520–540, doi: 10.1016/j.jtho.2020.01.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chemi F. et al. cfDNA methylome profiling for detection and subtyping of small cell lung cancers. Nat Cancer, doi: 10.1038/s43018-022-00415-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ireland A. S. et al. MYC Drives Temporal Evolution of Small Cell Lung Cancer Subtypes by Reprogramming Neuroendocrine Fate. Cancer Cell 38, 60–78 e12, doi: 10.1016/j.ccell.2020.05.001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou J. M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 30, 525–532, doi: 10.1200/JCO.2010.33.3716 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Pearsall S. M. et al. The Rare YAP1 Subtype of SCLC Revisited in a Biobank of 39 Circulating Tumor Cell Patient Derived Explant Models: A Brief Report. J Thorac Oncol 15, 1836–1843, doi: 10.1016/j.jtho.2020.07.008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart C. A. et al. Single-cell analyses reveal increased intratumoral heterogeneity after the onset of therapy resistance in small-cell lung cancer. Nature Cancer 1, 423–436, doi: 10.1038/s43018-019-0020-z (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akazawa C., Ishibashi M., Shimizu C., Nakanishi S. & Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem 270, 8730–8738, doi: 10.1074/jbc.270.15.8730 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Bermingham N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841, doi: 10.1126/science.284.5421.1837 (1999). [DOI] [PubMed] [Google Scholar]
- 22.Helms A. W., Abney A. L., Ben-Arie N., Zoghbi H. Y. & Johnson J. E. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development (Cambridge, England) 127, 1185–1196–1196 (2000). [DOI] [PubMed] [Google Scholar]
- 23.Yang Q., Bermingham N. A., Finegold M. J. & Zoghbi H. Y. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294, 2155–2158, doi: 10.1126/science.1065718 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Gazit R., Krizhanovsky V. & Ben-Arie N. Math1 controls cerebellar granule cell differentiation by regulating multiple components of the Notch signaling pathway. Development 131, 903–913–913, doi: 10.1242/dev.00982 (2004). [DOI] [PubMed] [Google Scholar]
- 25.Mulvaney J. & Dabdoub A. Atoh1, an essential transcription factor in neurogenesis and intestinal and inner ear development: function, regulation, and context dependency. Journal of the Association for Research in Otolaryngology 13, 281–293 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo Y. H. et al. Transcriptional Regulation by ATOH1 and its Target SPDEF in the Intestine. Cell Mol Gastroenterol Hepatol 3, 51–71, doi: 10.1016/j.jcmgh.2016.10.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomic G. et al. Phospho-regulation of ATOH1 Is Required for Plasticity of Secretory Progenitors and Tissue Regeneration. Cell stem cell 23, 436–443.e437–443.e437, doi: 10.1016/j.stem.2018.07.002 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Arie N. et al. Math1 is essential for genesis of cerebellar granule neurons. Nature 390, 169 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Ware M., Hamdi-Roze H. & Dupe V. Notch signaling and proneural genes work together to control the neural building blocks for the initial scaffold in the hypothalamus. Front Neuroanat 8, 140, doi: 10.3389/fnana.2014.00140 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bossuyt W. et al. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol 7, e39, doi: 10.1371/journal.pbio.1000039 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayrault O. et al. Atoh1 Inhibits Neuronal Differentiation and Collaborates with Gli1 to Generate Medulloblastoma-Initiating Cells. Cancer Research 70, 5618–5627-5627, doi: 10.1158/0008-5472.can-09-3740 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caeser R. et al. Genomic and transcriptomic analysis of a library of small cell lung cancer patient-derived xenografts. Nat Commun 13, 2144, doi: 10.1038/s41467-022-29794-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan K. et al. MCPyV Large T Antigen-Induced Atonal Homolog 1 Is a Lineage-Dependency Oncogene in Merkel Cell Carcinoma. J Invest Dermatol 140, 56–65 e53, doi: 10.1016/j.jid.2019.06.135 (2020). [DOI] [PubMed] [Google Scholar]
- 34.Feng H., Shuda M., Chang Y. & Moore P. S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319, 1096–1100, doi: 10.1126/science.1152586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menendez L. et al. Generation of inner ear hair cells by direct lineage conversion of primary somatic cells. Elife 9, doi: 10.7554/eLife.55249 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George J. et al. Comprehensive genomic profiles of small cell lung cancer. Nature 524, 47–53, doi: 10.1038/nature14664 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paulson K. G. et al. Array-CGH reveals recurrent genomic changes in Merkel cell carcinoma including amplification of L-Myc. J Invest Dermatol 129, 1547–1555, doi: 10.1038/jid.2008.365 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Starrett G. J. et al. Clinical and molecular characterization of virus-positive and virus-negative Merkel cell carcinoma. Genome Med 12, 30, doi: 10.1186/s13073-020-00727-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hodgkinson C. L. et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. Nature medicine 20, 897 (2014). [DOI] [PubMed] [Google Scholar]
- 40.Houghton P. J. et al. The pediatric preclinical testing program: description of models and early testing results. Pediatr Blood Cancer 49, 928–940, doi: 10.1002/pbc.21078 (2007). [DOI] [PubMed] [Google Scholar]
- 41.Geier B., Kurmashev D., Kurmasheva R. T. & Houghton P. J. Preclinical Childhood Sarcoma Models: Drug Efficacy Biomarker Identification and Validation. Front Oncol 5, 193, doi: 10.3389/fonc.2015.00193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghandi M. et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 569, 503–508, doi: 10.1038/s41586-019-1186-3 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barretina J. et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 483, 603–607, doi: 10.1038/nature11003 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lissa D. et al. Heterogeneity of neuroendocrine transcriptional states in metastatic small cell lung cancers and patient-derived models. Nat Commun 13, 2023, doi: 10.1038/s41467-022-29517-9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan J. M. et al. Signatures of plasticity, metastasis, and immunosuppression in an atlas of human small cell lung cancer. Cancer Cell 39, 1479–1496 e1418, doi: 10.1016/j.ccell.2021.09.008 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fellmann C. et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep 5, 1704–1713, doi: 10.1016/j.celrep.2013.11.020 (2013). [DOI] [PubMed] [Google Scholar]
- 47.Liang G. et al. Distinct localization of histone H3 acetylation and H3-K4 methylation to the transcription start sites in the human genome. Proc Natl Acad Sci U S A 101, 7357–7362, doi: 10.1073/pnas.0401866101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Park D. E. et al. Merkel cell polyomavirus activates LSD1-mediated blockade of non-canonical BAF to regulate transformation and tumorigenesis. Nat Cell Biol 22, 603–615, doi: 10.1038/s41556-020-0503-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klisch T. J. et al. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proceedings of the National Academy of Sciences 108, 3288–3293-3293, doi: 10.1073/pnas.1100230108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey T. L. & Machanick P. Inferring direct DNA binding from ChIP-seq. Nucleic Acids Res 40, e128, doi: 10.1093/nar/gks433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai T. et al. Characterization of the transcriptome of nascent hair cells and identification of direct targets of the Atoh1 transcription factor. J Neurosci 35, 5870–5883, doi: 10.1523/JNEUROSCI.5083-14.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burns J. C., Kelly M. C., Hoa M., Morell R. J. & Kelley M. W. Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat Commun 6, 8557, doi: 10.1038/ncomms9557 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borromeo M. D. et al. ASCL1 and NEUROD1 Reveal Heterogeneity in Pulmonary Neuroendocrine Tumors and Regulate Distinct Genetic Programs. Cell Rep 16, 1259–1272, doi: 10.1016/j.celrep.2016.06.081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cai L. et al. Cell-autonomous immune gene expression is repressed in pulmonary neuroendocrine cells and small cell lung cancer. Commun Biol 4, 314, doi: 10.1038/s42003-021-01842-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S. et al. Target analysis by integration of transcriptome and ChIP-seq data with BETA. Nat Protoc 8, 2502–2515, doi: 10.1038/nprot.2013.150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sancho R., Cremona C. A. & Behrens A. Stem cell and progenitor fate in the mammalian intestine: Notch and lateral inhibition in homeostasis and disease. EMBO Rep 16, 571–581, doi: 10.15252/embr.201540188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bebber C. M. et al. Ferroptosis response segregates small cell lung cancer (SCLC) neuroendocrine subtypes. Nat Commun 12, 2048, doi: 10.1038/s41467-021-22336-4 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Q. et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Sci Adv 7, eabg1850, doi: 10.1126/sciadv.abg1850 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Denny S. K. et al. Nfib Promotes Metastasis through a Widespread Increase in Chromatin Accessibility. Cell 166, 328–342, doi: 10.1016/j.cell.2016.05.052 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim M. et al. Comparative oncogenomics identifies NEDD9 as a melanoma metastasis gene. Cell 125, 1269–1281, doi: 10.1016/j.cell.2006.06.008 (2006). [DOI] [PubMed] [Google Scholar]
- 61.Nakada Y., Hunsaker T. L., Henke R. M. & Johnson J. E. Distinct domains within Mash1 and Math1 are required for function in neuronal differentiation versus neuronal cell-type specification. Development 131, 1319–1330-1330, doi: 10.1242/dev.01008 (2004). [DOI] [PubMed] [Google Scholar]
- 62.Westerman B. A. et al. Basic helix-loop-helix transcription factor profiling of lung tumors shows aberrant expression of the proneural gene atonal homolog 1 (ATOH1, HATH1, MATH1) in neuroendocrine tumors. Int J Biol Markers 22, 114–123 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Ayrault O. et al. Atoh1 inhibits neuronal differentiation and collaborates with Gli1 to generate medulloblastoma-initiating cells. Cancer Res 70, 5618–5627, doi: 10.1158/0008-5472.CAN-09-3740 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flora A., Klisch T. J., Schuster G. & Zoghbi H. Y. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science 326, 1424–1427, doi: 10.1126/science.1181453 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu H. T. et al. Atonal homolog 1 expression in lung cancer correlates with inhibitors of the Wnt pathway as well as the differentiation and primary tumor stage. APMIS 121, 111–119, doi: 10.1111/j.1600-0463.2012.02946.x (2013). [DOI] [PubMed] [Google Scholar]
- 66.Peignon G. et al. Complex interplay between beta-catenin signalling and Notch effectors in intestinal tumorigenesis. Gut 60, 166–176, doi: 10.1136/gut.2009.204719 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mou W. et al. Relationship between ATOH1 and tumor microenvironment in colon adenocarcinoma patients with different microsatellite instability status. Cancer Cell Int 22, 229, doi: 10.1186/s12935-022-02651-6 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Flora A., Klisch T. J., Schuster G. & Zoghbi H. Y. Deletion of Atoh1 disrupts Sonic Hedgehog signaling in the developing cerebellum and prevents medulloblastoma. Science 326, 1424–1427 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gambichler T. et al. Prognostic relevance of high atonal homolog-1 expression in Merkel cell carcinoma. Journal of Cancer Research and Clinical Oncology 143, 43–49-49, doi: 10.1007/s00432-016-2257-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verhaegen M. E. et al. Merkel Cell Polyomavirus Small T Antigen Initiates Merkel Cell Carcinoma-like Tumor Development in Mice. Cancer Res 77, 3151–3157, doi: 10.1158/0008-5472.CAN-17-0035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fu Y., Yuan S. S., Zhang L. J., Ji Z. L. & Quan X. J. Atonal bHLH transcription factor 1 is an important factor for maintaining the balance of cell proliferation and differentiation in tumorigenesis. Oncol Lett 20, 2595–2605, doi: 10.3892/ol.2020.11833 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baine M. K. et al. SCLC Subtypes Defined by ASCL1, NEUROD1, POU2F3, and YAP1: A Comprehensive Immunohistochemical and Histopathologic Characterization. J Thorac Oncol 15, 1823–1835, doi: 10.1016/j.jtho.2020.09.009 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qu S. et al. Molecular Subtypes of Primary SCLC Tumors and Their Associations With Neuroendocrine and Therapeutic Markers. J Thorac Oncol 17, 141–153, doi: 10.1016/j.jtho.2021.08.763 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pozo K. et al. ASCL1, NKX2–1, and PROX1 co-regulate subtype-specific genes in small-cell lung cancer. iScience 24, 102953, doi: 10.1016/j.isci.2021.102953 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osborne J. K. et al. NeuroD1 regulates survival and migration of neuroendocrine lung carcinomas via signaling molecules TrkB and NCAM. Proceedings of the National Academy of Sciences, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Augustyn A. et al. ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proceedings of the National Academy of Sciences 111, 14788–14793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lenhart R. et al. Sensitivity of Small Cell Lung Cancer to BET Inhibition Is Mediated by Regulation of ASCL1 Gene Expression. Molecular Cancer Therapeutics 14, 2167–2174-2174, doi: 10.1158/1535-7163.mct-15-0037 (2015). [DOI] [PubMed] [Google Scholar]
- 78.Jiang T. et al. Achaete-Scute Complex Homologue 1 Regulates Tumor-Initiating Capacity in Human Small Cell Lung Cancer. Cancer Research 69, 845–854-854, doi: 10.1158/0008-5472.can-08-2762 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rapa I. et al. Human ASH-1 Promotes Neuroendocrine Differentiation in Androgen Deprivation Conditions and Interferes With Androgen Responsiveness in Prostate Cancer Cells. The Prostate 73, 1241–1249-1249, doi: 10.1002/pros.22679 (2013). [DOI] [PubMed] [Google Scholar]
- 80.(!!! INVALID CITATION !!! 47, 48).
- 81.Mulvaney J. & Dabdoub A. Atoh1, an essential transcription factor in neurogenesis and intestinal and inner ear development: function, regulation, and context dependency. J Assoc Res Otolaryngol 13, 281–293, doi: 10.1007/s10162-012-0317-4 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong C., Fu Y., Pan W., Yu J. & Wang J. Atoh1 and other related key regulators in the development of auditory sensory epithelium in the mammalian inner ear: function and interplay. Dev Biol 446, 133–141, doi: 10.1016/j.ydbio.2018.12.025 (2019). [DOI] [PubMed] [Google Scholar]
- 83.Augert A. et al. Targeting NOTCH activation in small cell lung cancer through LSD1 inhibition. Sci. Signal. 12, eaau2922, doi: 10.1126/scisignal.aau2922 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen H. et al. BET Inhibitors Target the SCLC-N subtype of Small Cell Lung Cancer by Blocking NEUROD1 Transactivation. Mol Cancer Res, doi: 10.1158/1541-7786.MCR-22-0594 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang D. et al. Axon-like protrusions promote small cell lung cancer migration and metastasis. Elife 8, doi: 10.7554/eLife.50616 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Semenova E. A. et al. Transcription Factor NFIB Is a Driver of Small Cell Lung Cancer Progression in Mice and Marks Metastatic Disease in Patients. Cell Rep 16, 631–643, doi: 10.1016/j.celrep.2016.06.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gambichler T. et al. Prognostic relevance of high atonal homolog-1 expression in Merkel cell carcinoma. J Cancer Res Clin Oncol 143, 43–49, doi: 10.1007/s00432-016-2257-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grausam K. B. et al. ATOH1 Promotes Leptomeningeal Dissemination and Metastasis of Sonic Hedgehog Subgroup Medulloblastomas. Cancer Res 77, 3766–3777, doi: 10.1158/0008-5472.CAN-16-1836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin T. A., Ye L., Sanders A. J., Lane J. & Jiang W. G. in Madame Curie Bioscience Database [Internet] (Landes Bioscience, 2013). [Google Scholar]
- 90.Sutherland K. D. et al. Cell of origin of small cell lung cancer: inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell 19, 754–764, doi: 10.1016/j.ccr.2011.04.019 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Yang D. et al. Intertumoral Heterogeneity in SCLC Is Influenced by the Cell Type of Origin. Cancer Discov 8, 1316–1331, doi: 10.1158/2159-8290.CD-17-0987 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ouadah Y. et al. Rare Pulmonary Neuroendocrine Cells Are Stem Cells Regulated by Rb, p53, and Notch. Cell 179, 403–416 e423, doi: 10.1016/j.cell.2019.09.010 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Semenova Ekaterina A. et al. Transcription Factor NFIB Is a Driver of Small Cell Lung Cancer Progression in Mice and Marks Metastatic Disease in Patients. Cell Reports 16, 631–643-643, doi: 10.1016/j.celrep.2016.06.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sunshine J. C., Jahchan N. S., Sage J. & Choi J. Are there multiple cells of origin of Merkel cell carcinoma? Oncogene 37, 1409–1416, doi: 10.1038/s41388-017-0073-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Becker J. C. et al. Merkel cell carcinoma. Nat Rev Dis Primers 3, 17077, doi: 10.1038/nrdp.2017.77 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goh G. et al. Mutational landscape of MCPyV-positive and MCPyV-negative Merkel cell carcinomas with implications for immunotherapy. Oncotarget 7, 3403–3415, doi: 10.18632/oncotarget.6494 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knepper T. C. et al. The Genomic Landscape of Merkel Cell Carcinoma and Clinicogenomic Biomarkers of Response to Immune Checkpoint Inhibitor Therapy. Clin Cancer Res 25, 5961–5971, doi: 10.1158/1078-0432.CCR-18-4159 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Das B. K. et al. Single-cell dissection of Merkel cell carcinoma heterogeneity unveils transcriptomic plasticity and therapeutic vulnerabilities. Cell Rep Med 4, 101101, doi: 10.1016/j.xcrm.2023.101101 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawasaki K., Rekhtman N., Quintanal-Villalonga A. & Rudin C. M. Neuroendocrine neoplasms of the lung and gastrointestinal system: convergent biology and a path to better therapies. Nat Rev Clin Oncol 20, 16–32, doi: 10.1038/s41571-022-00696-0 (2023). [DOI] [PubMed] [Google Scholar]
- 100.Barrows D. & Carroll T. profileplyr: Visualization and annotation of read signal over genomic ranges with profileplyr. Bioconductor (R package version 1.2. 0, 2019) (2022). [Google Scholar]
- 101.Bailey T. L., Johnson J., Grant C. E. & Noble W. S. The MEME Suite. Nucleic Acids Res 43, W39–49, doi: 10.1093/nar/gkv416 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kolberg L., Raudvere U., Kuzmin I., Vilo J. & Peterson H. gprofiler2 -- an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Res 9, doi: 10.12688/f1000research.24956.2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Korotkevich G. et al. Fast gene set enrichment analysis. bioRxiv, 060012, doi: 10.1101/060012 (2021). [DOI] [Google Scholar]
- 104.Ramirez F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res 44, W160–165, doi: 10.1093/nar/gkw257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luo W., Friedman M. S., Shedden K., Hankenson K. D. & Woolf P. J. GAGE: generally applicable gene set enrichment for pathway analysis. BMC Bioinformatics 10, 161, doi: 10.1186/1471-2105-10-161 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W. et al. Small cell lung cancer tumors and preclinical models display heterogeneity of neuroendocrine phenotypes. Transl Lung Cancer Res 7, 32–49, doi: 10.21037/tlcr.2018.02.02 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.