Abstract
The BamHI restriction enzyme mediates integration of nonhomologous DNA into the Saccharomyces cerevisiae genome (R. H. Schiestl and T. D. Petes, Proc. Natl. Acad. Sci. USA 88:7585–7589, 1991). The present study investigates the mechanism of such events: in particular, the mediating activity of various restriction enzymes and the processing of resultant fragment ends. Our results show that in addition to BamHI, BglII and KpnI increase DNA integration efficiencies severalfold, while Asp718, HindIII, EcoRI, SalI, SmaI, HpaI, MscI, and SnaBI do not. Secondly, the three active enzymes stimulated integrations only of fragments containing 5′ or 3′ overhangs but not of blunt-ended fragments. Thirdly, integrations mediated by one enzyme and utilizing a substrate created by another required at least 2 bp of homology. Furthermore, an Asp718 fragment possessing a 5′ overhang integrated into a KpnI (isoschizomer) site possessing a 3′ overhang, most likely by filling of the 5′ overhang followed by 5′ exonuclease digestion to produce a 3′ end. We classified and analyzed the restriction enzyme-mediated integration events in the context of their genomic positions. The majority of events integrated into single sites. In the remaining 6 of 19 cases each end of the plasmid inserted into a different sequence, producing rearrangements such as duplications, deletions, and translocations.
DNA double-strand breaks occur either as a result of assaults by external agents or spontaneously during DNA metabolism, repair, or replication. Double-strand breaks may cause genome rearrangements, such as deletions, duplications, and translocations, which have been implicated in carcinogenesis. For any cell, double-strand break repair is essential, since these cytotoxic DNA lesions may cause potentially lethal losses of chromosomes. In the yeast Saccharomyces cerevisiae, DNA repair enzymes encoded by genes belonging to the RAD52 epistasis group repair double-strand breaks by homologous recombination. This process requires homologous DNA sequences, usually present on sister chromatids and on homologous chromosomes in diploids. In mammalian cells, however, the majority of double-strand breaks are repaired by nonhomologous end joining (NHEJ) (32). This event in S. cerevisiae occurs either in rad52 mutants in the presence of homology (18) or in the wild type in the absence of homology (26, 36). Joining reactions of restriction enzyme-produced DNA ends have frequently been used to study NHEJ both in vivo and in vitro. NHEJ of substrates with defined terminal configurations produced by different enzyme digestions were studied in vitro in the presence of Xenopus laevis extracts (2, 30, 43) and in vivo in mammalian cells (32) and fission yeast cells (12). In S. cerevisiae, illegitimate repair of a double-strand break in a plasmid was studied by Mezard and Nicolas (25) and the repair of double-strand breaks produced by an inducible HO endonuclease in the absence of homology was studied by Moore and Haber (26) (for the conclusions of those studies see Discussion).
Schiestl and Petes (36) studied illegitimate integration events by transforming a BamHI URA3 fragment into yeast cells lacking homology to the transforming DNA (in a ura3 deletion mutant), so that integration into the genome was by illegitimate recombination. With BamHI in the transformation mixture, the URA3 fragment integrated into genomic BamHI sites and the frequency of integration increased sixfold (36). These experiments suggest that the BamHI restriction enzyme can cut chromosomal DNA in vivo and thus mediate integration of the transforming DNA into that site. Subsequently, restriction enzyme-mediated integration (REMI) has been used in a variety of organisms for insertional mutagenesis. For example, Kuspa and Loomis (20) first adapted this technology to Dictyostelium discoideum, where previously cloning of developmental genes by complementation of mutant phenotypes was not feasible. Application of REMI has led to construction of REMI-restriction fragment length polymorphism maps (19, 23) and the cloning of most developmental genes in Dictyostelium. REMI has also been adapted successfully to the ascomycete Cochliobolus heterostrophus (24) and the maize pathogenic fungus Ustilago maydis (3) to tag genes by insertional mutagenesis.
Here we find that enzymes vary in their ability to mediate integration into the yeast genome. Furthermore, we present model mechanisms based on the products created from various blunt 5′ protruding single strand (PSS) and 3′ PSS joining combinations.
MATERIALS AND METHODS
Strains and media.
REMI experiments utilized S. cerevisiae RSY12 (MATa leu2-3,112 his3-11,15 ura3::HIS3), which contains a replacement of the entire open reading frame (ORF) of URA3 with the HIS3 gene (36).
A top1 deletion derivative of RSY12 (top1Δ) was used to prepare yeast lysate for in vitro determination of restriction enzyme activity. As previously described (47), this strain has LEU2 in place of 849 bp of the TOP1 coding sequence.
Growth conditions and media preparation were standard (38).
Plasmids.
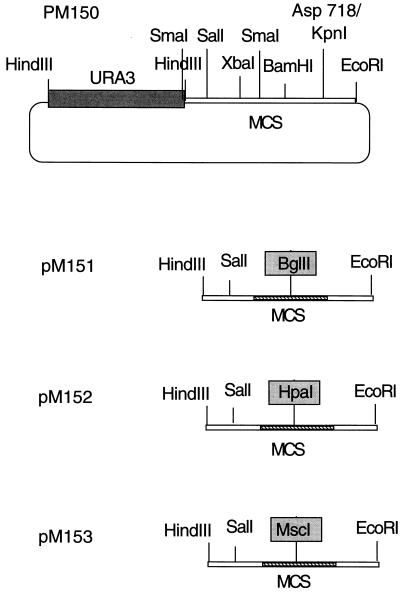
DNA manipulations were done by standard procedures (33). Plasmid pM150 was constructed by cloning the 1.1-kb HindIII fragment containing the URA3 gene into the HindIII site of pUC18. All multicloning sites except PstI and SmaI remained unique in this plasmid. Plasmid pM151 was constructed by digesting pM150 with Asp718 and XbaI, filling in the PSS ends, and inserting a BglII linker. This eliminates the sites between Asp718 and XbaI, including BamHI. Due to similar linker insertions, pM152 and pM153 contain unique MscI and HpaI sites, respectively, instead of the BglII site of pM151 (Fig. 1). YEplac195 contains the URA3 marker for selection and the 2μm origin of replication (11). Escherichia coli DH5α was used for the maintenance and amplification of plasmid DNA.
FIG. 1.
Plasmids used in the study. A 1.5-kb HindIII URA3 fragment was cloned into the HindIII site of the multicloning sites (MCS) of PUC19. The sites used to digest the plasmids (pM151 to -153) are shown. The sequences between XbaI and KpnI were replaced by BglII, HpaI, and MscI linkers in pM151, pM152, and pM153, respectively.
Molecular techniques.
Standard methods were followed (33) except as noted below. The Wizard miniprep DNA purification system (Promega) was used for small-scale preparation of plasmid DNA.
For yeast transformation the lithium acetate–single-stranded DNA–polyethylene glycol transformation method (10, 35) was used. Twenty micrograms each of CsCl-purified plasmids pM150 to -153 was treated with 100 U of restriction enzymes. After complete digestion, the DNA was precipitated with ethanol and resuspended in 200 μl of 0.01 M Tris-HCl (pH 7.5), 0.05 M EDTA, 1% sodium dodecyl sulfate, and 100 μg of proteinase K/ml. After a 30-min incubation at 37°C, the sample was extracted with phenol-chloroform-isoamyl alcohol, precipitated with ethanol, washed with 70% ethanol, and vacuum dried. The pellet was dissolved in sterile water, and the yeast cells were then transformed with this solution. For each transformation 5 to 6 μg of plasmid DNA with 200 μg of single-stranded carrier DNA was used per 200 μl of solution in each reaction tube. Transformants were selected on SC-Ura plates. For REMI experiments, 200 U of restriction enzyme and 1/10 volume of the restriction enzyme buffer were added to the transformation mixtures. Transformants were selected on SD plates lacking uracil (SD-Ura).
For Southern blot analysis, cells from individual Ura+ yeast colonies were grown in SD-Ura liquid medium overnight. Genomic DNA was isolated from the transformed clones by standard methods (38), digested with either BglII or KpnI, and hybridized to the internal fragment (from +16 to +875 coding sequence) of the URA3 gene. For cloning of REMI target sites, approximately 5 μg of DNA was digested in a 50-μl digestion reaction mixture with the ClaI restriction enzyme, for which there are no sites on the integrated plasmids. This digestion liberates the plasmid and junction sequences. The DNA was precipitated with ethanol and allowed to self-ligate in a 100-μl ligation mixture. The ligated DNA was precipitated, washed with 70% ethanol, and suspended in 20 μl of sterile water and was used for transformation into E. coli. Plasmids from these E. coli transformants were prescreened for the size of inserts as follows. Two to three single transformed E. coli clones were streaked onto LBAmp plates and grown overnight. Cells of single colonies were picked from the plates with a toothpick and suspended in 40 μl of suspension solution (50 mM Tris-HCl [pH 8.0], 10 mM EDTA) in an Eppendorf tube. After 10 μl of 5× lysis solution (0.25 M Tris-HCl [pH 8.0], 100 mM EDTA, 0.2% sodium dodecyl sulfate, 20% sucrose, 0.4% bromophenol blue) was added, the contents were mixed gently and incubated at room temperature for 5 min. The lysed cells were pelleted, and the supernatants were loaded into the wells of an agarose gel. Plasmids with flanking chromosomal sequences can be identified by a difference in migration of the supercoiled DNA. The sequences flanking the integrated fragment were determined by using a Promega sequencing kit as recommended by the supplier. The primers 2963 (URA3 primer; CGGAGATTACCGAATCAA) and 1233 (24-mer reverse-sequencing primer) from New England Biolabs were used for DNA sequencing. Asp718 was purchased from Boehringer Mannheim. All other enzymes were purchased from New England Biolabs.
Determination of the genomic distribution of REMI and illegitimate integration target sites.
About 50 bp of genomic DNA sequence of each junction flanking the integration target sites was determined as described above. These flanking sequences were compared with sequences in the Saccharomyces Genome Databases. All genomic target sites were analyzed in terms of position and special features (such as positions relative to known or putative ORFs, tRNA, and centromere and telomere sequences) and in terms of positions relative to each other to determine preferential integration sites.
RESULTS
Previous studies (34, 37) demonstrated that transformations of S. cerevisiae, in the presence of BamHI, with URA3 DNA fragments digested with BamHI integrate into genomic BamHI sites. In the absence of the mediating BamHI enzyme, the DNA fragments integrate into the genome by illegitimate recombination. The present study investigates the mechanism of such REMI events: in particular, the mediating activity of various restriction enzymes and the processing of resultant fragment ends.
BamHI, BglII, and KpnI mediate REMI events.
To study REMI catalyzed by different enzymes, we constructed plasmid pM150, which carried the URA3 gene succeeded by a polyclonal site, as well as plasmids pM151, pM152, and pM153, which contained the unique sites BglII, HpaI, and MscI (Fig. 1). After linearization, purified plasmid DNA was transformed into yeast strain RSY12, which lacks URA3, and integration efficiencies were scored. In a parallel tube, cells from the same culture were transformed with a plasmid containing the 2μm origin. To normalize for transformation efficiency, REMI events were calculated per 104 transformants with the 2μm plasmid (47). Typical transformation of 1 μg of linear plasmid yielded 4 to 7 URA3+ colonies. Under conditions that yield 104 transformants with the 2μm plasmid we found two illegitimate integration events with plasmids pM150 and pM151 carrying 5′ PSS ends and one such event with blunt-ended pM152 or pM153 (Table 1).
TABLE 1.
Activities of different enzyme combinations for REMI
| Plasmid | Digesting enzyme | Added enzymea | Relative frequency of integrationb ± SE | Fold increase in integrations ± SE | Significancec |
|---|---|---|---|---|---|
| pM150 | 0 (0) | ||||
| BglII | 0 (1) | ||||
| BamHI | 1.1 ± 0.8 (38) | ||||
| pM151 | 0 (0) | ||||
| BamHI | 0 (0) | ||||
| BglII | 0.5 ± 0.1 (31) | ||||
| pM150 | BamHI | 2.1 ± 0.1 (118) | 1.0 ± 0.6 | ||
| BamHI | BamHI | 11.5 ± 0.7 (692) | 5.5 ± 1.2 | ** | |
| BamHI | BglII | 20.7 ± 1.7 (1,323) | 9.9 ± 0.2 | ** | |
| BamHI | BamHI-E77K | 1.7 ± 0.4 (93) | 1.0 ± 0.1 | N.S. | |
| pM151 | BglII | 2.1 ± 0.2 (230) | 1.0 | ||
| BglII | BglII | 9.2 ± 1.8 (1,884) | 4.4 ± 1.2 | ** | |
| BglII | BamHI | 7.6 ± 0.7 (786) | 3.6 ± 0.8 | ** | |
| BglII | KpnI | 2.2 ± 0.8 (168) | 1.1 ± 0.1 | N.S. | |
| pM150 | SalI | 1.3 ± 0.1 (128) | 1.0 | ||
| SalI | SalI | 1.4 ± 0.1 (125) | 1.1 ± 0.1 | N.S. | |
| SalI | BglII | 10.8 ± 2.1 (663) | 8.3 ± 1.2 | ** | |
| pM151 | SalI | 1.4 ± 0.2 (108) | 1.0 | ||
| SalI | SalI | 1.3 ± 0.2 (99) | 0.9 ± 0.1 | N.S. | |
| SalI | BamHI | 9.1 ± 1.3 (1,436) | 6.5 ± 1.1 | ** | |
| pM150 | EcoRI | 1.1 ± 0.1 (56) | 1.0 | ||
| EcoRI | EcoRI | 1.1 ± 0.1 (57) | 1.0 ± 0.1 | N.S. | |
| EcoRI | BglII | 3.2 ± 0.3 (166) | 2.9 ± 0.3 | ** | |
| pM151 | EcoRI | 0.6 ± 0.1 (23) | 1.0 | ||
| EcoRI | EcoRI | 0.6 ± 0.1 (24) | 1.0 ± 0.1 | N.S. | |
| EcoRI | BamHI | 1.8 ± 0.4 (167) | 3.0 ± 0.5 | ** | |
| pM150 | Asp718 | 2.5 ± 0.4 (37) | 1.0 | ||
| Asp718 | Asp718 | 3.5 ± 0.5 (76) | 1.4 ± 0.3 | N.S. | |
| Asp718 | KpnI | 5.8 ± 0.8 (108) | 2.3 ± 0.3 | ** | |
| Asp718 | BglII | 6.9 ± 1.0 (83) | 2.8 ± 0.4 | ** | |
| pM150 | KpnI | 1.8 ± 0.4 (63) | 1.0 | ||
| KpnI | KpnI | 8.5 ± 0.9 (381) | 5.0 ± 0.6 | ** | |
| KpnI | BglII | 4.3 ± 0.8 (144) | 2.4 ± 0.8 | ** | |
| KpnI | SnaBI | 1.9 ± 0.3 (62) | 1.0 ± 0.1 | N.S. | |
| pM151 | HindIII | 0.6 ± 0.1 (27) | 1.0 | ||
| HindIII | HindIII | 0.6 ± 0.1 (31) | 1.0 ± 0.1 | N.S. | |
| HindIII | BamHI | 1.9 ± 0.4 (153) | 3.2 ± 0.4 | ** | |
| pM152 | SmaI | 0.9 ± 0.1 (68) | 1.0 | ||
| SmaI | SmaI | 1.2 ± 0.1 (84) | 1.3 ± 0.1 | N.S. | |
| SmaI | BglII | 1.4 ± 0.3 (60) | 1.5 ± 0.2 | N.S. | |
| SmaI | KpnI | 0.9 ± 0.1 (24) | 1.0 ± 0.1 | N.S. | |
| SmaI | BamHI | 1.0 ± 0.1 (26) | 1.1 ± 0.1 | N.S. | |
| SmaI | SnaBI | 1.4 ± 0.1 (47) | 1.0 ± 0.1 | N.S. | |
| pM152 | HpaI | 1.1 ± 0.1 (84) | 1.0 | ||
| HpaI | HpaI | 1.0 ± 0.1 (74) | 0.9 ± 0.1 | N.S. | |
| HpaI | BglII | 1.2 ± 0.1 (88) | 1.1 ± 0.2 | N.S. | |
| HpaI | BamHI | 0.9 ± 0.2 (62) | 0.8 ± 0.1 | N.S. | |
| HpaI | KpnI | 1.0 ± 0.1 (80) | 0.9 ± 0.1 | N.S. | |
| HpaI | SnaBI | 1.5 ± 0.2 (41) | 1.3 ± 0.2 | N.S. | |
| pM153 | MscI | 1.1 ± 0.1 (83) | 1.0 | ||
| MscI | MscI | 0.8 ± 0.1 (61) | 0.8 ± 0.1 | N.S. | |
| MscI | BglII | 0.9 ± 0.1 (64) | 0.8 ± 0.1 | N.S. | |
| MscI | BamHI | 0.8 ± 0.1 (61) | 0.7 ± 0.1 | N.S. | |
| MscI | KpnI | 0.7 ± 0.1 (48) | 0.7 ± 0.1 | N.S. | |
| MscI | SnaBI | 1.3 ± 0.1 (44) | 1.3 ± 0.2 | N.S. | |
| pM150 | BamHI | SmaI | 1.9 ± 0.3 (74) | 1.1 ± 0.2 | N.S. |
| BamHI | HpaI | 1.6 ± 0.3 (67) | 1.1 ± 0.3 | N.S. | |
| BamHI | MscI | 1.8 ± 0.2 (68) | 0.9 ± 0.1 | N.S. | |
| BamHI | SnaBI | 2.0 ± 0.7 (65) | 1.0 ± 0.1 | N.S. | |
| pM151 | BglII | SmaI | 1.4 ± 0.2 (70) | 1.1 ± 0.5 | N.S. |
| BglII | HpaI | 1.6 ± 0.2 (76) | 1.0 ± 0.1 | N.S. | |
| BglII | MscI | 1.5 ± 0.1 (71) | 0.9 ± 0.1 | N.S. | |
| BglII | SnaBI | 1.4 ± 0.4 (59) | 1.0 ± 0.1 | N.S. |
There were no sites in the plasmids for enzymes added to the transformation mix.
Number of integration events per microgram of DNA per 104 2μm transformants. This number is standardized for the transformation efficiency and represents the average of five or more experiments ± the standard error. The actual number of colonies counted is shown in parentheses.
N.S., not significant; **, t test indicates a statistically significant difference (P < 0.05) from control values.
The success of transformation events depended on the linearization of the plasmid substrate. No integrations were found if circular plasmids were transformed in the absence of enzyme or in the presence of those enzymes for which there were no recognition sites in the plasmid substrate (such as BglII for plasmid pM150 and BamHI for plasmid pM151). Transformation of circular plasmids in the presence of a restriction enzyme having a site in the plasmid, such as BamHI for pM150 and BglII for pM151, however, produced a low integration frequency when covalently closed circular DNA was transformed. This may be attributed to endonucleolytic cleavage in chromosomal sites and in the plasmid during transformation (Table 1).
Integration frequencies of pM150 linearized with BamHI (pM150-BamHI) increased fivefold upon the addition of BamHI during transformation, which was similar to previous results (36). In principle, it is possible that the BamHI enzyme creates a double-strand break to mediate integration events or that it binds to BamHI recognition sites to bring the recombination partners together. Furthermore, since restriction enzymes show nonspecific weak DNA binding (46), restriction enzymes could possibly bind to the transforming DNA and enhance uptake of such DNA indirectly, leading to an increase in the number of integration events. To address this possibility, we used purified BamHI-E77K protein, which binds to the BamHI site but cleaves DNA at a rate 1,000-fold lower than that of wild-type enzyme (46). The BamHI-E77K protein did not increase the efficiency of integration (Table 1), indicating that the cutting activity of the restriction enzyme is necessary to mediate REMI events.
Digestion of plasmids with enzymes BamHI, BglII, or KpnI and addition of the same enzyme during transformation caused significant increases (5.5, 4.4, and 5.0-fold, respectively) in the efficiencies of linear DNA integrations (Table 1). Enzymes producing other 5′ PSSs, namely, SalI, EcoRI, Asp718, and HindIII, did not cause any increase in REMI efficiency (Table 1). Furthermore, none of the enzymes producing blunt ends (SmaI, HpaI, or MscI) showed any increase in REMI efficiency (Table 1).
Restriction enzymes could mediate integrations into their respective genomic sites, as BamHI does (36). Alternatively, occasional double-strand breaks in DNA could change the conformation of chromosomal DNA, creating a larger region that attracts integration events. Since BamHI sites are conserved after integration of BamHI fragments into BamHI sites (36), Southern blots of BglII- or KpnI-mediated REMI events were used to determine whether the integrated fragments were flanked by BglII or KpnI sites, respectively. Colonies resulting from BglII or KpnI transformations were digested with the same enzyme and analyzed by Southern blotting. Indeed, 8 of 10 colonies obtained after BglII transformation contained integrations flanked by BglII sites while 11 of 13 colonies obtained after KpnI transformations contained integrations flanked by KpnI sites. These results suggest that BamHI and BglII enzymes, which produce 5′ PSS ends, as well as KpnI, which produces 3′ PSS ends, mediate “conservative” REMI events.
BamHI, BglII, and KpnI mediate integrations of fragments with different PSS ends.
We determined whether restriction enzymes that catalyzed integration events with substrates created by digestion with the same enzymes would also mediate events with substrates created by different enzymes. BamHI and BglII create the same 5′ PSS (5′GATC) after digestion of DNA. Addition of BglII to BamHI-digested pM150 increased the REMI efficiency 10-fold, and addition of BamHI to BglII-digested pM151 increased REMI efficiency fourfold (Table 1). PSS ends produced by SalI (5′TCGA), EcoRI (5′AATT), HindIII (5′AGCT), and Asp718 (5′GTAC) are different from the BamHI and BglII 5′ PSS ends. Addition of BglII to a SalI fragment increased the integration efficiency eightfold (pM150), and addition of BamHI increased efficiency 6.5-fold (pM151). Addition of BglII or BamHI to an EcoRI fragment increased the integration efficiency threefold (Table 1). Addition of BglII to an Asp718 fragment or addition of BamHI to a HindIII fragment increased the efficiency about threefold (Table 1). For short, we call a combination of an enzyme to digest the plasmid that creats a 5′ PSS end and an enzyme catalyzing REMI that creates a 3′ PSS end a “5′-3′ combination.” In conclusion, all 5′-5′ combinations of the enzymes used catalyzed REMI events.
Next we determined whether restriction enzymes producing 5′ PSS ends would also increase the efficiency of integration of a DNA fragment having 3′ PSS ends and vice versa. Asp718 and KpnI recognize the same DNA sequence, but Asp718 produces a 5′ PSS end and KpnI produces a 3′ PSS end. Addition of KpnI to an Asp718 pM150 fragment increased the efficiency more than twofold (Table 1). Addition of BglII to a KpnI pM150 fragment during transformation also resulted in more than a twofold increase in integration efficiency (Table 1). However, addition of KpnI to a BglII pM151 fragment did not significantly increase the transformation efficiency. Restriction enzymes that mediate REMI events increased efficiencies 3- to 10-fold in 5′-5′ or 3′-3′ combinations. In contrast, these 5′-3′ combinations increased the efficiencies to a lesser degree or not at all. Thus, it was not clear whether the same mechanism of increase operates for these 5′-3′ combinations (see below).
Next, we examined whether restriction enzymes producing blunt ends would work in different combinations with other enzymes. The addition of BamHI, BglII, or KpnI did not increase the integration efficiency of blunt-ended fragments from SmaI, MscI, or HpaI digests (Table 1). The lack of activity for REMI of restriction enzymes producing blunt ends might be due to the fact that blunt-ended fragments are not suitable substrates for REMI. Therefore, we investigated whether restriction enzymes producing blunt ends would mediate REMI events with PSS-ended DNA fragments. Addition of SmaI, HpaI, MscI, or SnaBI to BamHI or BglII fragments did not increase the efficiency of integration either. SnaBI also did not increase the integration efficiency of a KpnI fragment. Thus, blunt-ended fragments do not work for REMI with any restriction enzyme tested and enzymes producing blunt ends do not work for REMI with plasmids containing various ends.
Analyses of junction sequences.
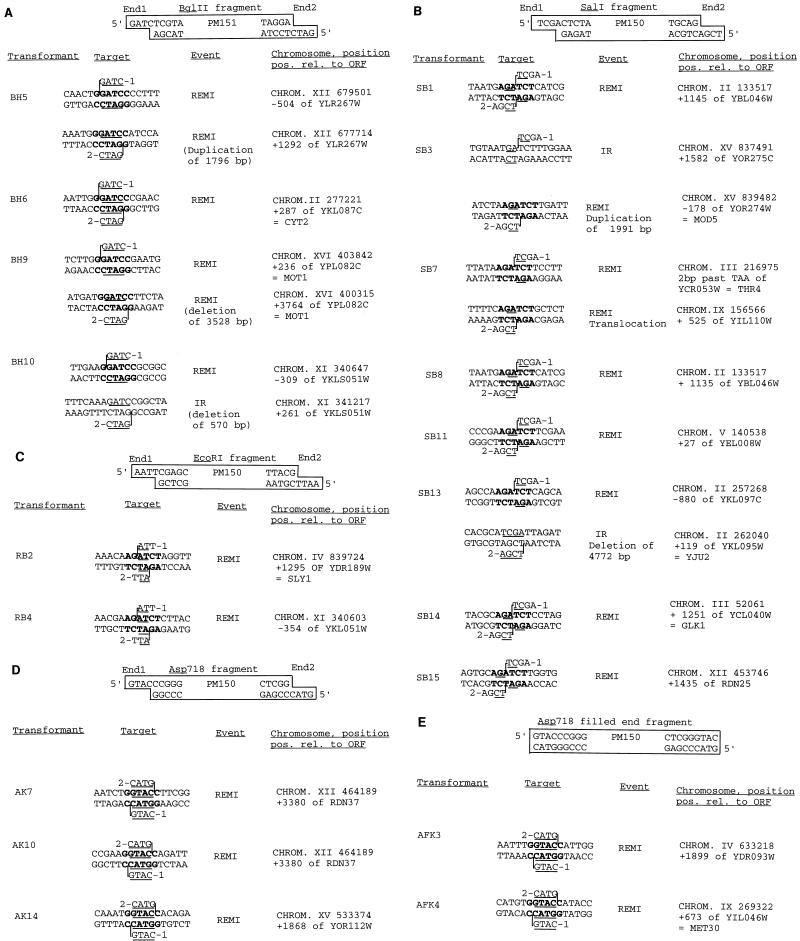
DNA sequencing of junctions between integrated fragments and genomic DNA led to further insight into the mechanism of REMI end joining. The REMI junction sequences with integrated plasmids were rescued from the genomic DNA and amplified in E. coli, and the junction sequences were determined with appropriate primers. We compared the chromosomal sequences beyond the junction sequences with sequences in the Saccharomyces GenBank database. The junction sequences and sites of integration of events mediated by restriction enzymes are shown in Fig. 2.
FIG. 2.
Flanking sequences and target sequences of linear fragments of plasmid PM150 or PM151, whose integration was mediated by restriction enzymes. Targets for 19 events are shown. The integrated DNA fragment (pM150 or pM151) is shown at the top of each panel with the two PSS ends (End1 and End2). These fragments and flanking sequences were cloned as described in Materials and Methods. The ends of the rescued plasmids and junction sequences were determined with oligonucleotide primers. At least 100 nucleotides from each junction were analyzed and compared with the sequences in the Saccharomyces Genome Database. All of the query sequences gave matches. From the sequences of these target sites we inferred the positions of the insertions. The DNA sequences of the target sites are shown as double-stranded DNA. The sequences shown above and below the target sequences are the ends of the transforming DNA (-1 indicates end 1 and 2- indicates end 2 of the DNA fragment). Sixteen base pairs of the target sites are shown to visualize the potential mechanism of integration. The vertical lines drawn between bases in the target sequences and the ends of the transforming DNA represent predicted sites at which the targets were ligated to the integrating fragments. The underlined regions in the target and fragment ends represent homologies between the plasmid ends and the target sites; we show only those homologies involving 2 or more bp immediately adjacent to the point of insertion of the transforming fragment. The recombination substrates, the target sequences into which the plasmids integrated, the types of events (whether restriction enzyme mediated or illegitimate recombination [IR]), and the genomic positions and positions relative (pos. rel.) to ORFs are shown. The names of genes or hypothetical ORFs are also shown. When the ends of the integrating plasmid integrated into different sequences, both target sites are shown. Only events where at least one junction was mediated by restriction enzymes are shown. The possible mode of integration is shown in Fig. 3. CHROM., chromosome. (A) BglII-BamHI (BH) events. Plasmid pM151 was digested with BglII to create 5′GATC PSS ends. BamHI was added to the transformation mixture. (B) SalI-BglII (SB) events. Plasmid pM150 was digested with SalI to create 5′TCGA PSS ends. BglII was added to the transformation mixture. (C) EcoRI-BglII (RB) events. Plasmid pM150 was digested with EcoRI to create 5′AATT PSS ends. BglII was added to the transformation mixture. (D) Asp718-KpnI (AK) events. Plasmid pM151 was digested with Asp718 to create 5′GTAC ends. KpnI was added to the transformation mixture. (E) Asp718 (filled)-KpnI (AFK) events. Plasmid pM151 was digested with Asp718, and the ends were filled before transformation. KpnI was added to the transformation mixture.
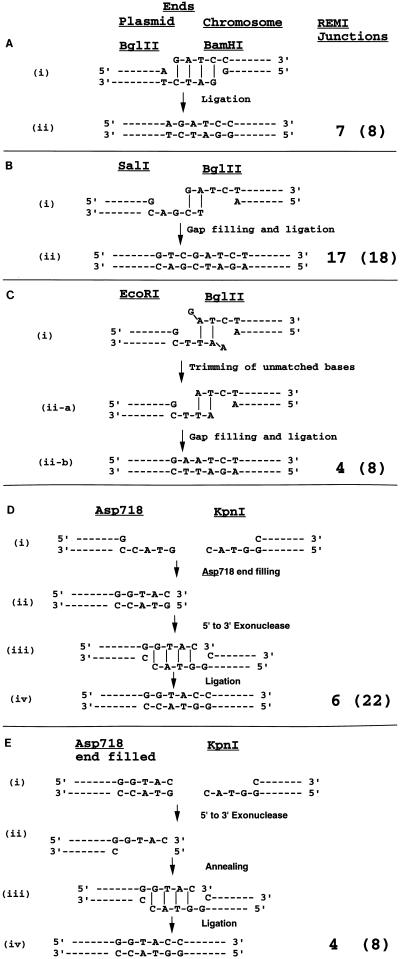
BamHI increased the integration efficiency of a BglII fragment. Eight junctions were analyzed, and matches to both junctions were found for all four integrants. In one isolate (BH6), the BglII fragment integrated into a single BamHI site. In two more isolates, BH5 and BH9 (Fig. 2A), the two ends of the plasmid integrated into different BamHI sites on the same chromosome, resulting in a duplication of 1.8 kb and a deletion of 3.5 kb, respectively. Deletions may occur simply by replacement of the deleted sequence with the integration plasmid. Duplications, on the other hand, may occur by invasion of different regions of a replication bubble as suggested previously (31, 34) or, alternatively, by a mechanism similar to that proposed for the repair of gapped plasmids (41) (i.e., the two ends could invade different positions within a single DNA molecule, priming DNA synthesis and generating the duplication). In the last isolate, BH10, one end of the plasmid integrated into a BamHI site whereas the other end integrated by microhomology-mediated illegitimate recombination (36) into a GATC sequence on the same chromosome, resulting in a deletion of 570 bp. Since BamHI and BglII have complementing PSS ends in all cases of REMI, simple ligation into the BamHI sites can explain these events (Fig. 3A).
FIG. 3.
Model for end joining during REMI. The number of REMI junctions that showed the structures depicted are shown in the “REMI junctions” column, and the total number of events analyzed for the particular combination is shown in parentheses. (A) BH events. Plasmid pM151 was digested with BglII and contains 5′GATC PSS ends. BamHI is added to the transformation mixture and should create the same GATC 5′ PSS ends (i). As the ends are compatible, they can anneal by microhomology. Ligation of nicks completes the integration process (ii). (B) SB events. Plasmid pM150 was digested with SalI and contains 5′TCGA PSS ends. The ends produced in vivo by BglII (GATC) have the terminal 2 bp compatible with the SalI ends (i). All the junction sequences are GTCGATCT sequences, indicating that integration took place through annealing of the two terminal bases, gap filling, and ligation of nicks (ii). (C) RB events. Plasmid pM150 was digested with EcoRI and contains 5′AATT PSS ends. The 5′ PSS ends produced in vivo by BglII (GATC) have the central 2 bp compatible with the EcoRI ends. Before or after annealing of the central two bases, the terminal unmatched bases are cleaved off, leaving a gap on both sides which is filled by DNA polymerase and ligated to seal the gap. (D) AK events. Plasmid pM150 was digested with Asp718 and contains 5′GTAC PSS ends. The 3′ PSS ends (GTAC) were produced in vivo by KpnI. Since the integration events recreated the KpnI sites, we proposed that filling of the Asp718 site by polymerase followed by 5′-3′ exonuclease digestion would create a 3′GTAC PSS end that was used as a substrate for KpnI-mediated integrations. (E) In vitro filling of an Asp718 site creates a blunt end that also works for REMI with KpnI, suggesting that the blunt-ended substrate is converted to a 3′GTAC PSS sequence by 5′-3′ exonuclease. Event types are defined in the legend to Fig. 2.
BglII (5′GATC PSS) increased the integration efficiency of a SalI (5′TCGA PSS)-digested plasmid. Eighteen junctions were analyzed and matched to sequences in the database. Nine BglII sites were found as target sites, and 14 junctions contained BglII sites. Five fragments (SB1, SB8, SB11, SB14, and SB15 [Fig. 2B]) integrated into single BglII (5′GATC PSS) sites. In SB7, each end of the fragment integrated into a different BglII site, producing a translocation between chromosomes III and IX. In two additional cases (SB3 and SB13), one of the two ends integrated into a BglII site and the other integrated by microhomology-mediated recombination. In SB13, 4 bp of target site homology was involved (5′TCGA) in a microhomology-mediated event, producing a deletion of 4.7 kb (Fig. 2B). While in SB3 the illegitimate integration junction shares 2 bp of homology (TC), the target (TGATCT) is similar to a BglII site (AGATCT) and the integration event occurred in a way similar to those of all the other BglII-mediated events joining the GA sequence with the 5′ end of the plasmid. On the basis of homology to the PSS ends there is no reason why 5 bp of the target should be similar to a BglII site. Therefore, this junction might be due to a digestion of the BglII enzyme with lower specificity (“star activity”). This event resulted in a duplication of about 2 kb. In each case, all plasmid and chromosomal sequences were maintained during integration. Thus, 15 of 16 cases of REMI occurred by pairing of the terminal 2 nucleotides (5′GA), filling of the 2-nucleotide gap, and religating (Fig. 3B).
BglII (5′GATC PSS) increased the integration efficiency of an EcoRI (5′AATT PSS)-digested plasmid. Eight junctions were sequenced, and four junctions contained BglII sites at their targets. In the cases of RB2 and RB4 (Fig. 2C), both ends of the plasmids integrated into a single BglII site. The junction sequences (AGATCT) again represent an end-joining reaction between the two restriction sites. These events may have arisen by pairing of the central two bases, trimming of unmatched single bases at each end, and filling of the gap and ligation (Fig. 3C).
Fourteen junctions resulting from integrations of KpnI fragments in the presence of BglII were sequenced. However, none of these events integrated into BglII sites (data not shown). Possibly the cleaving at one BglII site may open up the chromatin and facilitate the access of chromosomal DNA for illegitimate integration.
Addition of KpnI (3′ PSS) to an Asp718 (5′ PSS)-digested plasmid significantly increased the efficiency of integration (Table 1), and 6 of 22 junctions recreated the KpnI site (Fig. 2D). Surprisingly, in three cases (AK7, AK10, and AK14) both ends of the plasmid integrated into a single KpnI site (see below).
We also classified and analyzed the REMI events in the context of their genomic positions. Twice we recovered two events integrated into the same target sites. Both ends in two of these events (AK7 and AK10 [Fig. 2D]) integrated into the same KpnI site. However, the orientation of integration of AK7 was opposite to the orientation of AK10, excluding the possibility of cross-contamination and proving that the two events were truly independent. Since 7 to 14% of the genome consists of ribosomal DNA (29) organized in tandem repeats, it does not seem unusual that two KpnI-mediated events integrated into the “same” KpnI site. The integration probably occurred in different repeats in the ribosomal DNA. The second duplication of events is more unusual. Two events, SB1 and SB8, integrated into the same BglII site. These events were isolated from different experiments, indicating that they were independent events. However, since they integrated in the same orientation it remains a formal possibility that cross-contaminations during plasmid isolation might have occurred.
Effects of filling of 5′ PSSs on REMI efficiencies.
The integration of Asp718 fragments into KpnI sites (see above) suggests some sort of processing of ends during integration. The simplest explanation for these events is that the 5′ Asp718 ends were filled and a 5′-3′ exonuclease created a 3′ overhang to be annealed and ligated (Fig. 3D). To test this mechanism, we determined whether addition of KpnI to an Asp718 fragment after the filling of PSS ends would increase the integration efficiency. Plasmids were digested separately with enzymes Asp718, BglII, or SalI, and the enzymes were removed by phenol-chloroform treatment and precipitation of DNA. DNA was run on a gel to confirm complete digestion. The DNA sample was resuspended and split in half, and the 5′ PSS ends were filled with the Klenow fragment of DNA polymerase. As a control to test for the efficiency of end filling, DNA was religated before and after end filling and redigested with the same enzyme. Religation efficiency was reduced after end filling, and the religated plasmid molecules were resistant to redigestion, whereas in the control without end filling all of the religated plasmid could be redigested (not shown).
Transformation of strain RSY12 was carried out with plasmids with filled ends in the presence or absence of the different enzymes (Table 2). Addition of KpnI to an Asp718-digested plasmid significantly increased the transformation efficiency whether or not the Asp718 PSS end was filled (Table 2). One plausible explanation for this result is that a blunt end can, in fact, be converted into a 3′ PSS end to produce a substrate for KpnI-mediated integration events.
TABLE 2.
Effect of PSS end filling on REMIa
| Digesting enzyme | End filling | Added enzyme | Relative frequency of integration ± SE | Fold increase |
|---|---|---|---|---|
| Asp718 | − | KpnI | 3.10 ± 0.28 (68) | 1.9b |
| Asp718 | + | 2.36 ± 0.52 (52) | ||
| Asp718 | + | KpnI | 3.26 ± 0.56 (78) | 1.4b |
| BglII | − | 1.63 ± 0.30 (36) | ||
| BglII | − | BamHI | 6.34 ± 1.79 (140) | 3.9b |
| BglII | + | 3.10 ± 0.28 (64) | ||
| BglII | + | BamHI | 2.18 ± 0.52 (48) | 0.7 |
| SalI | − | 1.63 ± 0.25 (37) | ||
| SalI | − | BamHI | 8.88 ± 1.28 (196) | 5.5b |
| SalI | + | 3.08 ± 0.26 (44) | ||
| SalI | + | BamHI | 3.01 ± 0.27 (63) | 1 |
The plasmid pM151 was digested with restriction enzymes, and the ends were filled in with Klenow and deoxyribonucleoside triphosphates. The frequency of REMI has been determined in strain RSY12 as described in footnote b to Table 1. The data are representative of four independent experiments.
t test indicates a statistically significant difference (P < 0.05) from control values.
We also determined the integration target sites to test whether these events integrated into KpnI sites after the filling of the Asp718 site. In fact, two of four retrieved Asp718 fragments integrated into single genomic KpnI sites and recreated the KpnI site (Fig. 2E). A third event integrated by illegitimate recombination, and the fourth event integrated into mitochondrial DNA.
We also tested whether a filled 5′ end can act as a substrate for REMI events catalyzed by enzymes producing 5′ PSS ends. This may occur after a degradation by a 3′-5′ exonuclease or after partial melting of the base pairs at the ends of the fragments (so-called “breathing”). Addition of BamHI to a BglII-digested plasmid increased the efficiency of integration, as seen before. When the BglII PSS end was filled, however, no increase was found (Table 2). The same observation was made when the plasmid was digested with SalI and BamHI was added during transformation. In this case we observed a large increase with sticky ends but no increase after filling of the ends (Table 2). These experiments suggest that a blunt end is processed into a 3′ PSS end to create a substrate for KpnI-mediated events but that a blunt end is not processed into a 5′ PSS end. Partial melting of the filled ends was another potential explanation for the KpnI-mediated events. Since all three PSS ends have the same base composition, the same degree of partial melting should have occurred in all three cases, giving rise to an increase of integration efficiency with 5′ PSS-producing enzymes. Since this was not the case, this experiment may also exclude the possibility that partial melting of the ends was responsible for KpnI-mediated integration of a blunt-ended Asp718 fragment.
An alternative explanation was also ruled out: namely, that the plasmid was not completely cut with Asp718 and that after addition, the KpnI enzyme digested the uncut plasmid molecules and mediated integration of these molecules into KpnI sites. This is highly unlikely for the following reasons. (i) We tested for completeness of digestion by running an aliquot of all reactions on a gel, and the Asp718 digest appeared completely digested. (ii) After digestion, E. coli transformation was reduced 1,000-fold; the few transformants obtained were probably due to ligation in E. coli rather than to uncut plasmid, since filling of the PSS ends reduced the transformation efficiency another 10- to 30-fold.
DISCUSSION
As shown in this study, different restriction enzymes mediate integration into the yeast genome with varying efficiencies. Combinations of restriction fragments and different enzymes present during transformation demonstrated REMI events of compatible and noncompatible ends and revealed some characteristics of the nonhomologous end-joining process and repair during integration into the yeast genome.
Efficiency of REMI events with different enzymes.
BamHI, BglII, and KpnI increased integration efficiencies; however, SalI, EcoRI, and HindIII, as well as all tested enzymes producing blunt ends, did not. For EcoRI, no increase in the integration frequency has previously been found for plasmids YIplac211 (34) and pM20 (36a). However, transformation of an EcoRI pM20 fragment in the presence of EcoRI resulted in conservation of EcoRI sites in 3 of 10 integration events, whereas in the absence of the EcoRI enzyme none of 20 integration events resulted in such conservation of EcoRI sites (36a). This result suggests that EcoRI might possess a low activity to mediate integrations which is not sufficient to raise the integration frequency. There are many possible explanations as to why the ability of these enzymes to catalyze integration events is reduced or lacking, including the following: (i) the enzymes may not enter the cell; (ii) even if they do, they may not be active inside the nucleus or (iii) they may be degraded upon entry.
Restriction enzymes have been used to study the repair of double-strand breaks in many organisms but mostly in mammalian cells. It has been shown that they produce chromosomal double-strand breaks, cell death, chromosomal aberrations, translocations, and gene mutations such as those in CHO cells (28). For these endpoints, enzymes producing blunt ends are more active than enzymes producing PSS ends (4, 27). In contrast, our results show that some enzymes that produce PSS ends, but no enzymes that produce blunt ends, catalyze REMI events. It is possible that blunt ends cannot be efficiently ligated and the affected cells may die in the process. We did not find any decreased viability of cells after treatment with any enzyme, including enzymes producing blunt ends. This, however, does not rule out the possibility that only a small percentage of cells may take up the enzymes and that a fraction of these cells may be killed. For instance, overexpression of EcoRI is toxic to yeast cells (1). Thus, it is possible that the majority of cells in which EcoRI is active die, which possibly explains the inability of EcoRI to increase the efficiency of integration, even though integration events may be catalyzed by EcoRI to some extent (see above).
In Dictyostelium, BamHI, EcoRI, Sau3A, ClaI, and BglII catalyzed integration of plasmids containing pyr5-6, a homolog of the yeast URA3 gene (19, 20). REMI is used for insertional mutagenesis, tagging, and cloning of genes and for restriction fragment length polymorphism mapping (19). In C. heterostrophus, HindIII was used to tag the TOX1 locus with hygB (24), and in U. maydis, BamHI was used to inactivate pathogenicity genes by REMI (3). Thus, EcoRI and HindIII catalyzed an increase in integration events in Dictyostelium and Cochilobolus, respectively, but not in yeast. This indicates that the inability to raise the frequency of integrations in our experiments may not be an intrinsic property of some restriction enzymes but rather may depend on cellular environment, ability to enter cells, different transformation conditions, and so on.
Genomic position of integration events.
We classified and analyzed the REMI events in the context of their genomic positions. Overall, in 13 of 19 REMI events, integrations were into single sites. In the remaining six cases each end of the plasmid inserted into a different sequence, producing rearrangements such as duplications, deletions, and translocations. All of the integration events causing rearrangements occurred with BamHI and BglII (6 of 14 events) and none (0 of 5) with KpnI.
The above events suggest that the ends act independently during integration. For homologous recombination, Hastings et al. (14) showed that the ends of a DNA fragment can act independently during integration. Even though genome rearrangements are induced during integration, none of the enzymes showed any measurable killing effect, even in rad52 mutant cells (24a, 37). Therefore, it is unclear whether the double-strand break caused by the enzymes during integration is open or whether the ends are held together because of annealing of complementary PSS ends and/or mediation by end binding proteins. Even if there are no open double-strand breaks, the chromosomal restriction enzyme cuts may be sufficient to attract foreign DNA.
Of 78 target sites sequenced for this study, there were four events (5%) which had mitochondrial DNA at both junctions. Of previously characterized illegitimate integration events, 10% had mitochondrial DNA at the junctions (34). Such events occur by ligation of plasmid YIplac211 to mitochondrial DNA fragments that are often changed in sequence or rearranged and that presumably act as origins of replication in the yeast cells (34). The relatively lower efficiency might indicate that these events are not restriction enzyme catalyzed and thus are reduced in frequency when integrations into the genome are enhanced by the enzymes.
Insertional mutagenesis has an advantage over conventional mutagenesis in that the mutation site is marked by the inserting vector and can be cloned by plasmid excision along with adjacent sequences. To analyze the genome by insertional mutagenesis, it is important that the insertions happen randomly. Current strategies for insertional mutagenesis in yeast include transposon mutagenesis coupled with transformation of mutagenized fragments into yeast (for an example, see reference 5) and Ty mutagenesis mediated by integrations of modified Ty elements (9). The Ty insertion method is used for functional analysis of the yeast genome (for an example, see reference 39). However, target sites for Ty integrations are nonrandomly distributed and lie preferentially outside of ORFs and close to tRNA genes or long terminal repeat sequences (7, 16). In fact, only 1 of 30 integrations into chromosome III was into an ORF. Ty1 integration may be linked to RNA polymerase III transcription (7).
In 18 of 19 events, at least one of the two junctions integrated into an ORF of a known gene or a putative ORF. Since the prevalence of ORFs (including putative ORFs) in yeast is 70% (13), there may be a bias towards integration into ORFs. However, when the two junctions are considered independently and only REMI junctions are counted, 28 of 35 REMI junctions (80%) are in ORFs, a frequency not statistically different from the 70% prevalence of ORFs in yeast. Thus, REMI events readily insert into ORFs, and with further development (4-bp cutters, etc.), REMI could become a useful method for insertional mutagenesis.
PSS ends are necessary for REMI in S. cerevisiae and are protected from DNase digestion.
The end-joining reactions of different digestion products have been used as model systems in several organisms. In Xenopus oocyte extract, most combinations of PSS and blunt ends are joined, including 5′ PSS ends with blunt ends or 3′ PSS ends after the filling of ends (30, 43), suggesting the existence of an alignment protein. Such an activity seems lacking in yeast, since only some enzymes producing PSS ends but no enzymes producing blunt ends worked for REMI.
In human cells, the filling of PSS ends, as well as the loss of one to several hundred nucleotides, was found in 24 of 25 cases during end joining (6). In fact, Derbyshire et al. (6) isolated such an end-joining activity tightly associated with the human homologous-pairing activity and an intrinsic 3′-5′ exonuclease. In contrast, Roth and Wilson (32) showed in CV1 monkey cells that about 70% of the PSS ends had not lost any nucleotides. In Schizosaccharomyces pombe more than half of all parental blunt ends remained intact, whereas almost all parental 5′ and 3′ PSS ends (96 of 98) were shortened, suggesting the presence of 3′ and 5′ exonuclease activities (12). About 90% of these deletions affected terminal PSS ends, and 10% reached further into an adjacent duplex region (1 to 9 bp); therefore, S. pombe preferentially eliminates PSS termini to produce blunt ends. In our study, none of 4 BamHI-, 10 BglII-, and 5 KpnI-mediated events lost any bases of the PSS ends. In addition, none of nine previously published BamHI-mediated events lost any bases at their PSS ends (36). For illegitimate integration events, 27 of 34 (79%) 5′GATC or 5′AATT junctions maintained all 4 bp of the PSS ends whereas 6 (18%) lost 1 bp and 1 (3%) lost 3 bp (34, 36, 37). Thus, most of the 5′ PSS ends maintain all 4 bp during integration, which suggests that exonucleases may be less active in S. cerevisiae than in the other organisms mentioned above. Alternatively, since illegitimate recombination in S. cerevisiae is relatively rare (about 20-fold less efficient than homologous integration [37]), such exonucleases might progressively destroy the substrate molecules if homology is not found. Therefore, only a minority of the molecules (i.e., those that are fully protected from digestion) may be available for illegitimate integration. In yeast it has been shown that HDF1 protein (8), a homolog of mammalian Ku70, is able to bind to DNA ends and is involved in illegitimate integration (44). In fact, integration events by REMI as well as by illegitimate integration are dramatically decreased in an hdf1 mutant (24a). HDF1 protein, alone or in complex with other proteins, may protect the DNA ends and thus may be involved in the repair of broken ends by illegitimate integration.
Mechanisms of integration.
Analysis of the integration junction sequences uncovered several different mechanisms for NHEJ (Fig. 3). Integration events involving compatible ends (5′GATC) produced by BamHI and BglII (Fig. 2A) result in hybrid junctions (5′GGATCT) and can be explained by annealing and simple ligation (Fig. 3A). SalI and BglII produce different, incompatible 5′ PSS ends (TCGA and GATC, respectively) and likely require the alignment of two terminal bases, gap filling, and ligation (Fig. 3B). Goedecke et al. (12) studied end ligation of digested plasmids in S. pombe and found that the majority of events with SalI-BglII fill in both single-stranded tails before ligation. We did not find any such end-filling events in S. cerevisiae, and annealing of the terminal 2 nucleotides (the type of event that we exclusively found) accounts for only 4 of 20 events in S. pombe (12). In Xenopus egg extracts, however, the majority of junctions formed by BamHI-SalI-cut plasmids are 2-bp overlap annealing and filling in of the gaps (30), just like the events we found in the present study.
Since EcoRI (AATT) and BglII (GATC) ends share homology at the central 2 bp and all EcoRI-BglII junction sequences contain GAATCT, the tailing, unmatched bases must be removed either after base pairing of the middle sequences (Fig. 3) or before annealing and the gaps must be filled before ligation. This mechanism contrasts with that of the end ligation in Xenopus egg extract, where EcoRI-BamHI-cut plasmids (30) filled in the overhangs prior to ligation while only 1 of 14 had sequences which suggest a mode similar to our findings for S. cerevisiae.
While the recognition sequences of Asp718 and KpnI are the same (GGTACC), Asp718 leaves the sequence (5′GTAC) as 5′ PSS and KpnI leaves it as 3′ PSS. The events where the KpnI site is restored indicate a mechanism whereby the 5′ end is converted to a 3′ end by filling of the 5′ Asp718 end (made into a 3′ overhang by a 5′-3′ exonuclease) followed by annealing and ligation (Fig. 3D). In fact, filling of the Asp718 PSS end also worked as a substrate for REMI with KpnI.
Meiotic recombination hot spots coincide with the positions of double-strand breaks (22), which are processed by 5′-3′ exonuclease activity into long 3′ single strands (40). Mitotic cells expressing the HO endonuclease (45) induce a site-specific double-strand break, and the ends are processed via similar extensive degradation by a 5′-3′ DNA exonuclease for long 3′ PSS ends, which stimulate recombination. Such a 5′-3′ exonuclease activity has been partially purified for the catalysis of in vitro recombination between linear molecules with overlapping homology (15). Possibly the same 5′-3′ exonuclease activity prepares the 3′ end of a filled Asp718 site for KpnI-mediated integration. The filling of a 5′ end prevented REMI events with a 5′ PSS-producing enzyme due to a possible lack, or relatively less abundance, of 3′-5′ exonuclease activity. On the other hand, it is possible that proteins that allow 5′-3′ but not 3′-5′ exonucleases to work bind to the ends of the integrating DNA fragment. The SPO11 protein sets a precedent for this scenario, for it stays bound to ends after catalyzing meiotic double-strand breaks, allowing 5′-3′ exonucleases to process the ends (17).
A 5′-3′ exonuclease activity creates 3′ single-stranded tails, which may be a substrate for homologous recombination. However, long single-stranded ends may lower illegitimate integration efficiency. Beyert et al. (2) studied the effect of different lengths of PSS ends on DNA end joining in Xenopus egg extracts with synthetic hairpin substrates and found end joining suppressed with PSS ends longer than 10 nucleotides. This suggests that there is a limit to the length of PSS ends for illegitimate integration, for such long 3′ single stranded tails may channel integration events into homologous positions and prevent illegitimate integrations. In yeast, homologous integration is about 20-fold more efficient than illegitimate integration (37), yet the opposite ratio of 1:20 to 1:105 is the case in mammalian cells (for an example, see reference 42). It is possible that exonucleases are more processive in yeast than in other organisms, producing the difference in homologous versus nonhomologous integration. Xenopus oocytes exemplify this explanation, since late-stage oocytes show abundant 5′-3′ exonuclease activity and are proficient in homologous recombination whereas early-stage oocytes are devoid of 5′-3′ exonuclease and show only NHEJ reactions (21). Alternatively, yeast may possess more active homology-searching factors and therefore a relatively higher efficiency of homologous integration.
ACKNOWLEDGMENTS
We thank the members of the Schiestl laboratory and Stephanie Kong for helpful suggestions and discussion. We also thank Joan Brooks from New England Biolabs for the generous gift of BamHI-E77K protein.
This work was supported by grant CN-83B from the American Cancer Society to R.H.S.
REFERENCES
- 1.Barnes G, Rine J. Regulated expression of endonuclease EcoRI in Saccharomyces cerevisiae: nuclear entry and biological consequences. Proc Natl Acad Sci USA. 1985;82:1354–1358. doi: 10.1073/pnas.82.5.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beyert N, Reichenberger S, Peters M, Hartung M, Gottlich B, Goedecke W, Vielmetter W, Pfeiffer P. Nonhomologous DNA end joining of synthetic hairpin substrates in Xenopus laevis egg extracts. Nucleic Acids Res. 1994;22:1643–1650. doi: 10.1093/nar/22.9.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolker M, Bohnert H U, Braun K H, Gorl J, Kahmann R. Tagging pathogenicity genes in Ustilago maydis by restriction enzyme-mediated integration (REMI) Mol Gen Genet. 1995;248:547–552. doi: 10.1007/BF02423450. [DOI] [PubMed] [Google Scholar]
- 4.Bryant P E. Enzymatic restriction of mammalian cell DNA using Pvu II and Bam H1: evidence for the double-strand break origin of chromosomal aberrations. Int J Radiat Biol Relat Stud Phys Chem Med. 1984;46:57–65. doi: 10.1080/09553008414551061. [DOI] [PubMed] [Google Scholar]
- 5.Burns N, Grimwade B, Ross-Macdonald P B, Choi E Y, Finberg K, Roeder G S, Snyder M. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 6.Derbyshire M K, Epstein L H, Young C S, Munz P L, Fishel R. Nonhomologous recombination in human cells. Mol Cell Biol. 1994;14:156–169. doi: 10.1128/mcb.14.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devine S E, Boeke J D. Integration of the yeast retrotransposon Ty1 is targeted to regions upstream of genes transcribed by RNA polymerase III. Genes Dev. 1996;10:620–633. doi: 10.1101/gad.10.5.620. [DOI] [PubMed] [Google Scholar]
- 8.Feldmann H, Winnacker E L. A putative homologue of the human autoantigen Ku from Saccharomyces cerevisiae. J Biol Chem. 1993;268:12895–12900. [PubMed] [Google Scholar]
- 9.Garfinkel D J, Strathern J N. Ty mutagenesis in Saccharomyces cerevisiae. Methods Enzymol. 1991;194:342–361. doi: 10.1016/0076-6879(91)94026-9. [DOI] [PubMed] [Google Scholar]
- 10.Gietz D, St. Jean A, Woods R A, Schiestl R H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 12.Goedecke W, Pfeiffer P, Vielmetter W. Nonhomologous DNA end joining in Schizosaccharomyces pombe efficiently eliminates DNA double-strand-breaks from haploid sequences. Nucleic Acids Res. 1994;22:2094–2101. doi: 10.1093/nar/22.11.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goffeau A, Barrell B G, Bussey H, Davis R W, Dujon B, Feldmann H, Galibert F, Hoheisel J D, Jacq C, Johnston M, Louis E J, Mewes H W, Murakami Y, Philippsen P, Tettelin H, Oliver S G. Life with 6000 genes. Science. 1996;274:546. doi: 10.1126/science.274.5287.546. , 563–567. [DOI] [PubMed] [Google Scholar]
- 14.Hastings P J, McGill C, Shafer B, Strathern J N. Ends-in vs. ends-out recombination in yeast. Genetics. 1993;135:973–980. doi: 10.1093/genetics/135.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang K N, Symington L S. A 5′-3′ exonuclease from Saccharomyces cerevisiae is required for in vitro recombination between linear DNA molecules with overlapping homology. Mol Cell Biol. 1993;13:3125–3134. doi: 10.1128/mcb.13.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji H, Moore D P, Blomberg M A, Braiterman L T, Voytas D F, Natsoulis G, Boeke J D. Hotspots for unselected Ty1 transposition events on yeast chromosome III are near tRNA genes and LTR sequences. Cell. 1993;73:1007–1018. doi: 10.1016/0092-8674(93)90278-x. [DOI] [PubMed] [Google Scholar]
- 17.Keeney S, Giroux C N, Kleckner N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 1997;88:375–384. doi: 10.1016/s0092-8674(00)81876-0. [DOI] [PubMed] [Google Scholar]
- 18.Kramer K M, Brock J A, Bloom K, Moore J K, Haber J E. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol Cell Biol. 1994;14:1293–1301. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuspa A, Loomis W F. REMI-RFLP mapping in the Dictyostelium genome. Genetics. 1994;138:665–674. doi: 10.1093/genetics/138.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuspa A, Loomis W F. Tagging developmental genes in Dictyostelium by restriction enzyme-mediated integration of plasmid DNA. Proc Natl Acad Sci USA. 1992;89:8803–8807. doi: 10.1073/pnas.89.18.8803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehman C W, Clemens M, Worthylake D K, Trautman J K, Carroll D. Homologous and illegitimate recombination in developing Xenopus oocytes and eggs. Mol Cell Biol. 1993;13:6897–6906. doi: 10.1128/mcb.13.11.6897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichten M, Goldman A S. Meiotic recombination hotspots. Annu Rev Genet. 1995;29:423–444. doi: 10.1146/annurev.ge.29.120195.002231. [DOI] [PubMed] [Google Scholar]
- 23.Loomis W F, Welker D, Hughes J, Maghakian D, Kuspa A. Integrated maps of the chromosomes in Dictyostelium discoideum. Genetics. 1995;141:147–157. doi: 10.1093/genetics/141.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu S, Lyngholm L, Yang G, Bronson C, Yoder O C, Turgeon B G. Tagged mutations at the Tox1 locus of Cochliobolus heterostrophus by restriction enzyme-mediated integration. Proc Natl Acad Sci USA. 1994;91:12649–12653. doi: 10.1073/pnas.91.26.12649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Manivasakam, P., and R. H. Schiestl. Unpublished data.
- 25.Mezard C, Nicolas A. Homologous, homeologous, and illegitimate repair of double-strand breaks during transformation of a wild-type strain and a rad52 mutant strain of Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:1278–1292. doi: 10.1128/mcb.14.2.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore J K, Haber J E. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2164–2173. doi: 10.1128/mcb.16.5.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan W F, Phillips J W, Chung H W, Ager D D, Winegar R A. The use of restriction endonucleases to mimic cytogenetic damage induced by ionizing radiations. New York, N.Y: Wiley-Liss; 1990. [Google Scholar]
- 28.Obe G, Johannes C, Schulte-Frohlinde D. DNA double-strand breaks induced by sparsely ionizing radiation and endonucleases as critical lesions for cell death, chromosomal aberrations, mutations and oncogenic transformation. Mutagenesis. 1992;7:3–12. doi: 10.1093/mutage/7.1.3. [DOI] [PubMed] [Google Scholar]
- 29.Olson M V. The genome structure and organization in Saccharomyces cerevisiae. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1991. [Google Scholar]
- 30.Pfeiffer P, Thode S, Hancke J, Vielmetter W. Mechanisms of overlap formation in nonhomologous DNA end joining. Mol Cell Biol. 1994;14:888–895. doi: 10.1128/mcb.14.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth D, Wilson J. Illegitimate recombination in mammalian cells. In: Kucherlapati R, Smith G R, editors. Genetic recombination. Washington, D.C: American Society for Microbiology; 1988. pp. 621–653. [Google Scholar]
- 32.Roth D B, Wilson J H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986;6:4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schiestl R H, Dominska M, Petes T D. Transformation of Saccharomyces cerevisiae with nonhomologous DNA: illegitimate integration of transforming DNA into yeast chromosomes and in vivo ligation of transforming DNA to mitochondrial DNA sequences. Mol Cell Biol. 1993;13:2697–2705. doi: 10.1128/mcb.13.5.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 36.Schiestl R H, Petes T D. Integration of DNA fragments by illegitimate recombination in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1991;88:7585–7589. doi: 10.1073/pnas.88.17.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36a.Schiestl, R. H., and T. D. Petes. Unpublished data.
- 37.Schiestl R H, Zhu J, Petes T D. Effect of mutations in genes affecting homologous recombination on restriction enzyme-mediated and illegitimate recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:4493–4500. doi: 10.1128/mcb.14.7.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1986. [Google Scholar]
- 39.Smith V, Chou K N, Lashkari D, Botstein D, Brown P O. Functional analysis of the genes of yeast chromosome V by genetic footprinting. Science. 1996;274:2069–2074. doi: 10.1126/science.274.5295.2069. [DOI] [PubMed] [Google Scholar]
- 40.Sun H, Treco D, Szostak J W. Extensive 3′-overhanging, single-stranded DNA associated with the meiosis-specific double-strand breaks at the ARG4 recombination initiation site. Cell. 1991;64:1155–1161. doi: 10.1016/0092-8674(91)90270-9. [DOI] [PubMed] [Google Scholar]
- 41.Szostak J W, Orr-Weaver T L, Rothstein R J, Stahl F W. The double-strand-break repair model for recombination. Cell. 1983;33:25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- 42.te Riele H, Maandag E R, Berns A. Highly efficient gene targeting in embryonic stem cells through homologous recombination with isogenic DNA constructs. Proc Natl Acad Sci USA. 1992;89:5128–5132. doi: 10.1073/pnas.89.11.5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thode S, Schafer A, Pfeiffer P, Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990;60:921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- 44.Tsukamoto Y, Kato J, Ikeda H. Hdf1, a yeast Ku-protein homologue, is involved in illegitimate recombination, but not in homologous recombination. Nucleic Acids Res. 1996;24:2067–2072. doi: 10.1093/nar/24.11.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White C I, Haber J E. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu S Y, Schildkraut I. Cofactor requirements of BamHI mutant endonuclease E77K and its suppressor mutants. J Bacteriol. 1991;173:5030–5035. doi: 10.1128/jb.173.16.5030-5035.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Schiestl R H. Topoisomerase I involvement in illegitimate recombination in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:1805–1812. doi: 10.1128/mcb.16.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]