Abstract
Transfer RNAs (tRNAs) are fundamental for both cellular and viral gene expression during viral infection. Moreover, mounting evidence supports a noncanonical role for tRNA cleavage products in the control of gene expression during diverse conditions of stress and infection. We previously reported that infection with the model murine gammaherpesvirus, MHV68, leads to altered tRNA transcription, suggesting that tRNA regulation may play an important role in mediating viral replication or the host response. To better understand how viral infection alters tRNA expression, we combined Ordered Two Template Relay (OTTR) with tRNA-specific bioinformatic software called tRAX to profile full-length tRNAs and fragmented tRNA-derived RNAs (tDRs) during infection with MHV68. We find that OTTR-tRAX is a powerful sequencing strategy for combined tRNA/tDR profiling and reveals that MHV68 infection triggers pre-tRNA and mature tRNA cleavage, resulting in the accumulation of specific tDRs. Fragments of virally-encoded tRNAs (virtRNAs), as well as virtRNA base modification signatures are also detectable during infection. We present evidence that tRNA splicing factors are involved in the biogenesis of MHV68-induced cleavage products from pre-tRNAs and, in the case of CLP1 kinase, impact infectious virus production. Our data offers new insights into the importance of tRNA processing during gammaherpesvirus infection.
Introduction
Advances in small RNA sequencing technology have revealed an abundance of cleavage products made from transfer RNAs (tRNAs) during the cellular response to stress, viral infection, and other disease states1–6. Recent evidence supports the idea that stress or infection incites cellular endonucleases to cleave tRNAs during the cellular response, producing small tRNA-derived RNAs (tDRs), also known as tRNA fragments or tRNA halves. While most of these cleavage products are likely degraded by RNA turnover machinery, certain tDR species are stable in stressed or infected cells, suggesting that they are protected from exonucleolytic degradation. Additionally, stable tDRs have been shown to have protein binding partners that would impart functionality, such as Argonaute or Ybx17, 8. There are diverse classes of tDRs that can be made from tRNAs—fragments of differing lengths can be generated from the 5’ end, 3’ end, or internal region of the tRNA, and sequence diversity is imparted by the parental tRNA family origin. This diversity of sequence and structure likely explains the diversity of function and protein interactors observed for tDRs7–9. Thus, comprehensively identifying the tDRs produced in response to stress and infection is fundamental to understanding the role tDRs play in diverse cellular responses.
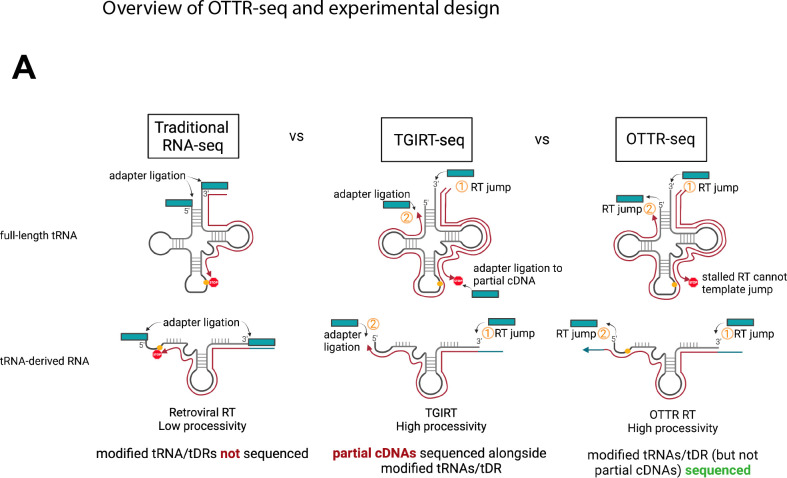
The ability to accurately profile tRNA and tDR expression by deep sequencing is difficult due to both technical and bioinformatic challenges. Standard small RNA library preparations use retroviral reverse transcriptases (RTs) that lack sufficient processivity to reverse transcribe highly modified and structured tRNAs and tDRs from end to end (Fig 1).10 Coverage of full-length tRNAs is dramatically improved with the use of more processive RTs, such as TGIRT11 used in DM-tRNA-seq12. TGIRT uses a template-jumping mechanism to initiate reverse transcription, adding the first sequencing adaptor during cDNA production. This eliminates the need to ligate adaptors prior to reverse transcription, which is convenient for low RNA input and workflow. However, this workflow allows partial cDNA products resulting from disengagement of the RT at modified bases into the sequencing pool; thus, the incomplete cDNA products are indistinguishable from biologically-relevant tDRs. Because of these technical issues, it is current standard practice to analyze full-length tRNAs and tDRs using separate library preparation techniques, such as DM-tRNA-seq for full-length tRNAs and ARM-seq for tDRs. To overcome these challenges and facilitate a single approach to monitor both tRNAs and tDRs by sequencing, we applied Ordered Two Template Relay (OTTR)13 to prepare tRNA sequencing libraries. OTTR harnesses the template jumping activity and high processivity of an engineered eukaryotic retrotransposon RT to sequentially add both adapters to RNA (rather than only one adaptor like with TGIRT)13, 14. This technology is well-suited for simultaneous tRNA and tDR analysis, as it both minimizes and excludes partial cDNA synthesis products from the sequenced pool. We have combined this technology with best-practice and user-friendly tRNA and tDR mapping software, called tRAX15, 16, into a pipeline we refer to as “OTTR-tRAX”.
Figure 1. Benefits of OTTR-seq for tRNA sequencing.
Due to the high processivity and template jumping activity of OTTR RT, OTTR-seq ensures that highly modified tRNAs and genuine tDRs (and not partial cDNA products) are sequenced.
There is mounting evidence that tDRs derived from tRNAs can alter cellular gene expression and affect the replication of diverse viruses2, 7, 9, 17, 18. Here, we applied OTTR-tRAX to murine gammaherpesvirus 68 (MHV68)-infected cells and successfully recapitulated pre-tRNA upregulation in response to MHV68 infection, as we reported prior6. In addition, we found that tDR production is a molecular signature of herpesvirus infection. We hypothesize that increased levels of nascent tRNA transcripts are mechanistically tied to tDR production, as a host shutoff mutant MHV68 with dampened expression of pre-tRNAs (MHV68.R443I) during infection6 shows dramatically decreased tDR production. Our methods also allowed for analysis of the 8 viral tRNA-miRNA encoded RNAs (TMERs) found in the MHV68 genome, leading to the observation that viral tRNAs (here referred to as virtRNAs), like host tRNAs, undergo base modification and can be cleaved into stable tDRs. Using OTTR-tRAX, we were able to map the ends of infection-induced host tDRs and predict the endonuclease machinery responsible for tRNA cleavage. As proof-of-principle, we show that a tRNA splicing endonuclease, TSEN2, as well as the tRNA splicing regulatory kinase, CLP1, both modulate the generation of pre-tRNA-derived tDRs. Further, we show that CLP1 is required for efficient MHV68 replication. Altogether, we introduce a novel sequencing approach for combined tRNA/tDR profiling and reveal the breadth of tRNA cleavage that can occur in response to infection by a DNA virus.
Materials and Methods
Cell culturing conditions.
NIH 3T3 murine fibroblasts, NIH 3T12 murine fibroblasts, and MC57G fibrosarcoma cells (ATCC) were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Cultures were screened regularly for mycoplasma and cultured in the absence of antibiotics.
Virus infections.
Green fluorescent protein (GFP) expressing MHV68-R443I and the MHV68-MR mutant revertant were amplified in NIH 3T12s 19, 20. The MHV68-MR was used as the source of wild-type MHV68 for all experiments in this work. MHV68-infected NIH 3T12 cells and media were subjected to two freeze-thaws, then cellular material was pelleted and discarded. Viruses present in the supernatant were pelleted at high-speed (12K × g) for 2 h, then treated with DNase (Fisher #EN0523) for 1 h at 37 °C. Media was added and virus was pelleted at high-speed (12K × g) for 2 h. Virus pellet was dissolved in DMEM +10% FBS media, aliquoted, and stored at −80 °C. MHV68-GFP titer was measured on NIH 3T3 cells for the 50% tissue culture infective dose (TCID50). For high MOI/single-step infection, MHV68 virus was added to ½ culture volume of media, placed on NIH 3T3 or MC57G cells as indicated for 1 h at multiplicity of infection (MOI) of 5 to allow viral entry, then replaced with fresh DMEM +10% FBS medium.
siRNA treatment.
Pooled siRNA purchased from Dharmacon was nucleofected into 5×10 NIH 3T3 cells at 200nM. The Neon™ Transfection System (Thermo Fisher), with buffer E2 and 100uL tips, was used to perform the nucleofection (1400 volts/20 ms/2 pulses). Cells were infected 24 h post-nucleofection and harvested 24 h post-infection (hpi). Total RNA was analyzed via RT-qPCR.
RT-qPCR and SL-qPCR.
Total RNA was isolated from cells using TRIzol (Invitrogen) and was treated with Turbo DNase (Ambion). Turbo-treated RNA was then reverse transcribed with AMV RT (Promega) primed with random 9-mer (IDT) for total RNA measurements by RT-qPCR or stem-loop (SL) primers for 5’ tDR measurements by SL-qPCR. Binding of SL primers requires an exact 3’ end for successful amplification, thus we designed primers using the most frequent 3’ end of upregulated 5’ tDRs in our dataset. Our SL-qPCR assays will detect the following using tDRnamer21 nomenclature using mm10 as a sequence source: tDR-1:37-Tyr-GTA-1-M2, tDR-1:17-Asn-GTT-1, and tDR-1:24-Gln-CTG-1-M3. Gene expression was measured by amplifying cDNA using iTaq SYBR Green Supermix (Biorad) on a QuantStudio 3 (Applied Biosystems), and all primers are listed in Table 1. RT-qPCR was analyzed using the delta delta CT (ΔΔCT) method. Average fold changes were calculated relative to 18S or U6 internal controls.
Table 1.
Oligonucleotide sequences.
| RNA oligos for SL-qPCR validation | |
| Mm pre-tRNA-Tyr | 5’ rUrArUrCrUrCrCrCrUrUrCrGrArUrArGrCrUrCrArGrUrUrGrGrUrArGrArGrCrGrGrArGrGrArCrUrGrUrArGrUrArUrArGrGrUrGrUrUrGrArUrArUrCrCrUrUrArGrGrUrCrGrCrUrGrGrUrUrCrGrArArU |
| Mm 5’ pre-tDR-Tyr | 5’ rUrArUrCrUrCrCrCrUrUrCrGrArUrArGrCrUrCrArGrUrUrGrGrUrArGrArGrCrGrGrArGrGrArCrUrGrUrArG |
| Northern probes | |
| Mm-tRNA-Tyr-GTA-1-3_probe | 5’-CTACAGTCCTCCGCTCTACCA |
| 5S_probe | 5’-AGCCTACAGCACCCGGTATT |
| Primers used for reverse transcription | |
| 5’tDR-Arg-TCT-3-1_RT | 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTAGAAGTC |
| 5’tDR-Gln-CTG_RT | 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTGCTAACC |
| 5’tDR-Leu-CAG-3_RT | 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGTCCTTAGA |
| 5’tDR-Tyr-GTA-1-3_RT | 5’-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTACAGTC |
| Primers used for qPCR | |
| Mm-tRNA-Tyr-GTA-1-3_qPCRf | 5’-CCTTCGATAGCTCAGTTGGTAGAGC |
| Mm-tRNA-Tyr-GTA-1-3_qPCRr | 5’-GGATTCGAACCAGCGACCTAAGGATATC |
| 5’tDR-Arg_TCT-3-1_qPCRf | 5’- GGCTCTGTGGCGCAATGG |
| 5’tDR-Gln-CTG_qPCRf | 5’- GGTTCCATGGTGTAATGGT |
| 5’tDR-Leu-CAG-3_qPCRf | 5’-GTCAGGATGGCCGAGTGG |
| 5’tDR-Tyr-GTA-1-3_qPCRf | 5’-CCTTCGATAGCTCAGTTGGTAGAGC |
| SL-univ_qPCRr | 5’-GTGTCGTGGAGTCGGC |
| Mm_Clp1_qPCRf | 5’-ATGAGCGAGGAATCCAATGATG |
| Mm_Clp1_qPCRr | 5’-CTCCAACTGAACCGATTGAGAG |
| Mm_Tsen2_qPCRf | 5’-CCAAACTTCACAATCGCCAAC |
| Mm_Tsen2_qPCRr | 5’-AGTGGCTCTGTGTAGTCTGTAA |
| MHV68_Orf50_qPCRf | 5’-GGCCGCAGACATTTAATGAC |
| MHV68_Orf50_qPCRr | 5’-GCCTCAACTTCTCTGGATATGCC |
| MHV68_gB_qPCRf | 5’-GGCCCAAATTCAATTTGCCT |
| MHV68_gB_qPCRr | 5’-CCCTGGACAACTCCTCAAGC |
| 18S_qPCRf | 5’-GTGGAGCGATTTGTCTGGTT |
| 18S_qPCRr | 5’-CGCTGAGCCAGTCAGTGTAG |
Northern analysis.
Total RNA was loaded onto 8 to 12% PAGE–7 M urea gels and transferred to Hybond-N+ (GE) membranes using a Trans-Blot Turbo Transfer system (Bio-Rad) in 1X TBE. Blots were crosslinked using the auto-crosslink setting on a UV Crosslinker FB-UV-XL-1000 (1,200 μJ × 100) (Fisher Scientific) and prehybridized in ULTRAhyb buffer (Thermo Fisher) at 42°C for 1hr before adding radiolabeled probe. Probes were generated by end labeling oligonucleotides specific for Tyr or 5S (Table 2) as a loading control using T4 PNK and [γ−32P] ATP. Blots were probed overnight at 55°C and washed 3 times for 10 minutes in 0.5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) and 0.1% SDS. Blots were imaged using a Typhoon imager and processed on Photoshop (Adobe). Densitometry was performed using BioRad ImageLab software.
Ordered two template relay sequencing (OTTR-seq).
RNA isolated from mock-infected, MHV68-MR-infected, or MHV68-R443I-infected MC57G cells (MOI=5, 24 h) was used for generating OTTR-seq libraries. OTTR libraries were generated as previously described13. Briefly, total PNK-treated RNA was 3’ tailed using mutant BoMoC RT in buffer containing only ddATP for 90 minutes at 30 °C, with the addition of ddGTP for another 30 minutes at 30 °C. This was then heat-inactivated at 65 °C for 5 minutes, and unincorporated ddATP/ddGTP were hydrolyzed by incubation in 5 mM MgCl2 and 0.5 units of shrimp alkaline phosphatase (rSAP) at 37 °C for 15 minutes. 5 mM EGTA was added and incubated at 65 °C for 5 minutes to stop this reaction. Reverse transcription was then performed at 37 °C for 30 minutes, followed by heat inactivation at 70 °C for 5 minutes. The remaining RNA and RNA/DNA hybrids were then degraded using 1 unit of RNase A at 50 °C for 10 minutes. cDNA was then cleaned up using a MinElute Reaction CleanUp Kit (Qiagen). To reduce adaptor dimers, cDNA was run on a 9% UREA page gel, and the size range of interest was cut out and eluted into gel extraction buffer (300mM NaCl, 10mM Tris; pH 8.0, 1mM EDTA, 0.25% SDS) and concentrated using EtOH precipitation. Size-selected cDNA was then PCR amplified for 12 cycles using Q5 High-fidelity polymerase (NEB #M0491S). Amplified libraries were then run on a 6% TBE gel, and the size range of interest was extracted to reduce adaptor dimers further. Gel slices were eluted into gel extraction buffer (300mM NaCl, 10mM Tris; pH 8.0, 1mM EDTA) followed by concentration using EtOH precipitation. Final libraries were pooled and sequenced using 150 SE and 200 cycle kit on an Illumina NovaSeq.
tRNA Analysis of eXpression (tRAX).
Sequencing adaptors were trimmed from raw reads using cutadapt, v1.18, and read counts were generated for host and viral small RNA types using tRAX16. Briefly, trimmed reads were mapped to a combined mouse (GRCm38/mm10) and gammaherpesvirus (MHV68) reference containing mature tRNAs obtained from GtRNAdb22 and their corresponding genomic sequences using Bowtie2 in very-sensitive mode with the following parameters to allow for a maximum of 100 alignments per read: --very-sensitive --ignore-quals --np 5 -k 100. Mapped reads were filtered to retain only the “best mapping” alignments. Raw read counts of tRNAs and other small RNA types were computed using tRNA annotations from GtRNAdb, and annotations from GENCODE M23 and miRBase v2223. Raw read counts were then normalized using DESeq2. Data has been deposited with NCBI GEO, identification number: GSE255627.
Statistical analysis.
All experiments were performed in at least biological triplicate. All RT-qPCR statistical analysis was performed using raw DCt values. The statistical test used is indicated in the figure legends.
Reagents:
| Description | Catalog Number | |
|---|---|---|
| Cell culture | DMEM, high glucose | Gibco 11965092 |
| DPBS, no Ca/Mg | Gibco 14190250 | |
| Fetal Bovine Serum | Gibco 26140079 | |
| Trypsin-EDTA (0.25%) | Gibco 25200056 | |
| RT-qPCR | SuperScript III RT | Thermo Fisher 18080044 |
| AMV RT | Promega M5108 | |
| MgCl2 | Bio-Rad 1708872 | |
| Turbo DNAse | Invitrogen AM2239 | |
| 10mM dNTPs | Thermo Scientific FERR0193 | |
| RNAsin | Promega N2515 | |
| iTaq Universal SYBR Green Supermix | Bio-Rad 1725125 | |
| Instruments | Neon transfection system | Thermo Fisher MPK5000 |
| QuantStudio 3 | Applied Biosytems A28131 | |
| Proflex thermocycler | Applied Biosytems 4483636 |
Biological Resources:
| Description | Catalog Number | |
|---|---|---|
| Cell Lines | NIH 3T3 Murine Fibroblasts | ATCC CRL-1658 |
| NIH 3T12 Murine Fibroblasts | ATCC CCL-164 | |
| MC57G Murine Fibrosarcoma | ATCC CRL-2295 | |
| Viruses | GFP-expressing MHV68-R443I | PMID: 21811408 |
| GFP-expressing MHV68-MR (mutant revertant) | PMID: 21811408 | |
| siRNAs | siNon-targeting | Horizon D-001810-10-05 |
| siClp1 | Horizon L-051374-02-0005 | |
| siTsen2 | Horizon L-050573-01-0005 |
Results
OTTR-seq is a robust tool for the quantification of tRNAs and tDRs
We previously observed expression by northern blot of 5’ tDR-Tyr and -Arg, two tDR species generated from abundant pre-tRNAs during MHV68 infection6. To profile all tDRs expressed during viral infection, we pursued a sequencing strategy that avoided two major pitfalls common in available tDR sequencing strategies: 1) the use of RTs that perform poorly on modified tDRs and 2) library assembly strategies that do not exclude incomplete cDNA products (Fig 1). We then applied OTTR-seq, which avoids these two pitfalls and accurately sequences modified tRNAs and tDRs using a single library preparation.
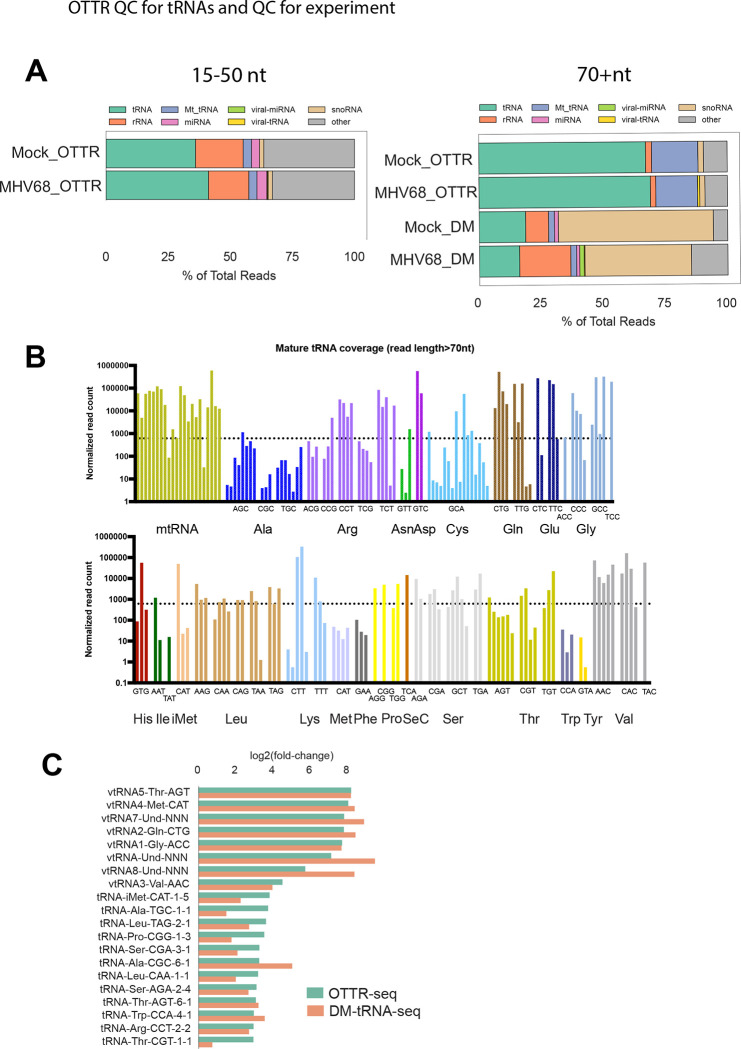
We performed OTTR-seq on small RNA extracted from the murine fibroblast cell line MC57G +/− infection with MHV68, conditions for which we have previously analyzed by DM-tRNA-seq6. To enhance our sequencing coverage of full-length tRNAs and pre-tRNAs over smaller fragments, the final cDNA library was size selected and sequenced in pools corresponding to 15–50 nt or 50–200 nt inserts. We analyzed our sequencing data using the tRNA Analysis of eXpression (tRAX) software package for the analysis of tRNAs and tDRs16. More than 60% of the reads were from tRNAs (green) in the larger size class, indicating adequate coverage of full-length tRNAs by OTTR (Fig 2A, right top 2 bars). In comparison, our previous DM-tRNA-seq library contained a much lower percentage of reads mapping to tRNAs (20%) and was instead enriched for snoRNAs (tan) at ~50% of mapped reads6 (Fig 2A, right bottom 2 bars). The composition of the 15–50 nt size class library using OTTR was derived from a variety of sources, including tDRs (green), which comprised the largest percentage at 30–40%, but also included sequences mapping to rRNAs (orange), miRNAs (pink), and snoRNAs (tan). Notably, viral-derived TMERs comprised less than 1% of the libraries from infected cells.
Figure 2. OTTR-seq robustly captures host tRNAs.
(A) Distribution of reads mapping to various small RNA classes using OTTR-seq (OTTR) or DM-tRNA-seq (DM) prepared from mock and MHV68 infected MC57G mouse fibroblasts at an MOI=5 for 24 hours. (B) Normalized read counts for host tRNA isodecoders detected in mock-infected libraries using OTTR-seq. The dotted line marks the median normalized read count for all tRNA isodecoders as reference. (C) Log2(fold-change) values between MHV68 infected (MR) versus Mock infection from OTTR-seq (green) or DM-tRNA-seq (orange) are consistent for viral-tRNAs (“vtRNA” prefix) and host pre-tRNAs.
To assess the overall inclusion of different tRNA isodecoders using OTTR, we surveyed the normalized read counts from mock-treated samples (Fig 2B). The vast majority of isodecoders had at least one representative with expression over the median read count of all measured tRNAs. This supports the fact that OTTR robustly captures a diverse pool of tRNA species. Finally, to assess the quantitative capacity of our method versus those previously described, we compared tRNA differential expression reported by OTTR-tRAX and DM-tRNA-seq following MHV68 infection (Fig 2C). Log2(fold-change) of representative upregulated viral or host pre-tRNA species was more homogenous with OTTR-tRAX than DM-tRNA-seq, and our OTTR-tRAX experiment recapitulated enhanced tRNA expression in MHV68-infected cells reported prior6.
OTTR-tRAX reveals host shutoff-dependent changes in tRNA expression during MHV68 infection
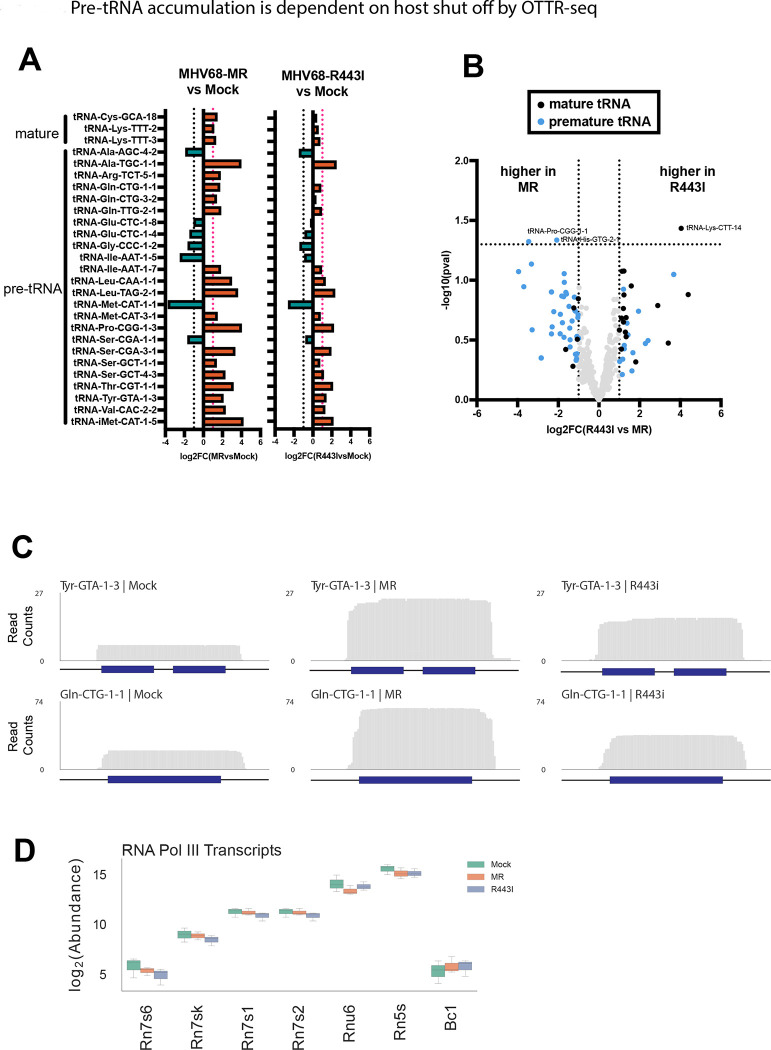
Viruses often downregulate cellular gene expression using diverse strategies in a process known as host shutoff. MHV68 performs host shutoff through the degradation of cellular mRNAs by the viral endonuclease muSOX (ORF37). Our previous work suggested host shutoff results in the accumulation of pre-tRNAs in infected cells, perhaps due to the depletion of post-transcriptional processing factors required for pre-tRNA maturation or turnover6, 24. We applied OTTR-tRAX to both wild type/mutant revertant (MR)-infected cells and those infected with the host shutoff mutant MHV68-R443I virus, which contains a single point mutation in muSOX that restricts its mRNA endonucleolytic activity20. Differentially expressed (p<0.05) pre- or mature tRNAs from the 50–200 nt library upon MHV68-MR infection compared to mock are shown in Fig 3A (MHV68-MR vs. mock infection). The majority of differentially expressed transcripts are premature tRNA sequences, further supporting our previous results using DM-tRNA-seq6. MHV68-R443I infection also resulted in differential pre-tRNA expression (Fig 3A, MHV68-R443I vs. mock infection). However, the magnitude of differential expression for pre-tRNAs from MHV68-R443I vs. mock was decreased compared to MHV68-MR vs mock, confirming that pre-tRNA accumulation is dependent on host shutoff6. Comparing tRNA expression in MHV68-R443I versus MHV68-MR contexts yielded very few differential hits reaching statistical significance, emphasizing that similar tRNA profiles exist upon MHV68-R443I and -MR infections (Fig 3B). However, pre-tRNAs tended to have a higher expression level in MHV68-MR infected cells, while mature tRNAs showed the opposite pattern (Fig 3B, blue vs. black dots). Representative read coverage plots of pre-tRNAs shown in Fig 3C illustrate the reduced pre-tRNA expression with MHV68-R443I versus MHV68-MR virus. There are no obvious changes in the 5’ or 3’ ends of pre-tRNAs upon infection, suggestive of an accumulation of nascent pre-tRNAs prior to trimming or splicing. Differential upregulation is specific to a subset of pre-tRNAs, as other non-coding RNAs made by RNA polymerase III do not increase in abundance during infection with MHV68-MR or -R443I (Fig 3D). Overall, OTTR-tRAX reveals global changes in pre-tRNA abundance that are dependent on the host shutoff functionality of MHV68 muSOX.
Figure 3. Pre-tRNA accumulation is dependent on host shut off by OTTR-seq.
(A) Log2(fold-change) values of MHV68 infected (left) or R443I (right) versus Mock infection for differentially expressed host mature and premature tRNAs (p<0.05). Horizontal dotted lines indicate a fold change of > or < 2. (B) Volcano plot of log2(fold-change or FC) values for full-length tRNAs from MHV68-MR vs. -R443I infections, plotted against -log10(pval). tRNAs with fold changes > 2 are colored blue for pre-tRNAs or black for mature tRNAs. (C) Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across the Tyr-GTA-1–3 (top) and Gln-CTG-1–1 (bottom) tRNA gene loci. Dark blue boxes correspond to the gene body of the tRNA, while the horizontal black lines correspond to the 30 bp upstream, 30 bp downstream, and/or intronic regions. (D) Box plot showing the log2(abundance) of seven RNA polymerase III transcripts colored by infection conditions.
tRNA-derived RNAs are generated in response to MHV68 infection
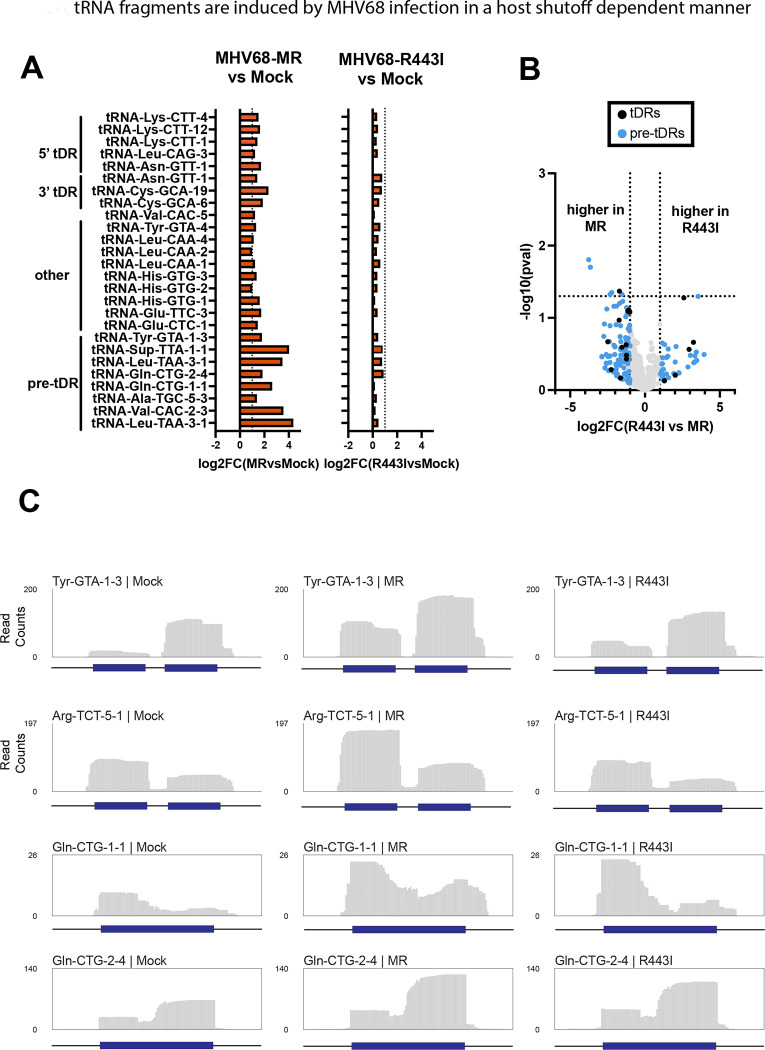
We next analyzed the expression of tDRs and other small RNAs during MHV68 infection. OTTR-tRAX analysis of the 15–50 nt size class revealed differential induction of tDRs in MHV68-infected cells (Fig 4A, MHV68-MR vs. mock infection, p<0.05, Supp Figs 1–3). Approximately one-third of these were derived from pre-tRNAs, suggesting that upregulated pre-tRNAs are a source of tDRs during infection. tDRs were more highly expressed in MHV68-MR compared to MHV68-R443I (Fig 4, Supp Fig 1–3), with few exceptions (see Glu-CTC-5 in Supp Fig 1). Overall, the dependence on muSOX activity was more striking for tDRs than observed for pre-tRNAs. For many of the pre-tDRs, there were distinct 5’ and 3’ tDRs produced from the same transcript (Fig 4C). Among the other tDRs upregulated in infected fibroblasts were 5’, 3’ and internally derived tDRs (Supp Figs 1–3). Almost all of the significantly upregulated 5’ tDRs had distinct 3’ ends, which resided in or around the D-loop (Supp Fig 1). In contrast, 3’ and internally derived tDRs exhibited more heterogenous ends, suggestive of ongoing exonucleolytic degradation (Supp Fig 2,3). In some cases, the tDRs could be mapped uniquely to a specific tRNA locus of origin (e.g., 3’ tDR-Asn-GTT-1 or int-tDR-His-GTG-1, purple reads). These results illustrate that MHV68 triggers tDR production, and that this phenomenon is dependent on host shutoff activity by MHV68 muSOX.
Figure 4. tRNA-derived RNAs (tDRs) are induced by MHV68 in a host shutoff-dependent manner.
(A) Log2(fold-change) values of MHV68 infected (left) or R443I (right) versus Mock infection for differentially expressed host cytosolic 5’tDRs, 3’tDRs, internal tDRs, and premature-derived tDRs (p<0.05). Horizontal dotted lines indicate a fold change of > or < 2. (B) Volcano plot of log2(FC) values for tDRs from MHV68-MR vs. -R443I infections, plotted against −log10(pval). tDRs up- or down-regulated more than 2-fold are colored blue for pre-tDRs or black for tDRs generated from mature tRNAs. (C) Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across tRNA gene loci. Dark blue boxes correspond to the gene body of the tRNA, while the horizontal black lines correspond to the 30 bp upstream, 30 bp downstream, and/or intronic regions.
Viral tRNA and miRNA expression profiles during MHV68 replication
MHV68 produces non-coding RNAs, called tRNA-miRNA encoded RNAs or TMERs, from 8 loci in the viral genome. Transcription of the TMERs results in a hybrid RNA molecule consisting of a viral tRNAs (virtRNAs) fused to miRNA stem-loops (Fig 5A). We use the “virtRNA” nomenclature here instead of the previously used “vtRNA” to avoid confusion with host vault RNAs. Processing of TMERs involves cleavage by the host enzyme Elac2, normally responsible for removing the 3’ trailer during host tRNA maturation, to separate the viral miRNA stem loops from the virtRNA25. Dicer then further processes the viral miRNAs for use by host silencing machinery. There is evidence that virtRNAs undergo maturation in the form of post-transcriptional -CCA addition at the 3’ ends26, which is a required step in host tRNA maturation to form the site of amino acid attachment. However, virtRNAs are not detectably charged with amino acids, at least for the virtRNAs previously tested (virtRNA3–6)26. We expected OTTR-tRAX might be a useful tool to examine changes in viral TMER and TMER-derived small RNAs during MHV68 infection. We mapped OTTR-seq reads from both small and large-size classes to the left arm of the MHV68 genome (Fig 5B,C). Our size selection at 200 nt excluded full-length TMER transcripts (~200–250nt); however, we detected robust expression of virtRNAs from TMERs 1, 2, 4, 5, and 6 (Fig 5B). For the smaller size class, we were able to detect the relative expression of viral miRNAs during infection in fibroblasts (Fig 5C). Interestingly, we detected fragments made from virtRNAs, with equal or higher abundance to viral miRNAs (see TMER4, 5, 7 as examples). TMER7 produced a highly abundant 3’ fragment, yet the virtRNA from this locus was not detected (compare Fig 5A and 5B). MHV68-R443I infection resulted in globally reduced expression of both virtRNAs and viral miRNAs compared to MHV68-MR, supporting previous microarray analysis24.
Figure 5. Viral TMERs are induced in a host-shutoff-dependent manner.
(A) Schematic of a viral TMER highlighting the 5’ virtRNA and 3’ miRNA regions. (B) Normalized read coverage across the eight viral TMERs from the 50–200nt size selected libraries for Mock (top), MR (middle), and R433I (bottom). (C) Normalized read coverage across the eight viral TMERs from the 15–50nt size selected libraries for Mock (top), MR (middle), and R433I (bottom). Color bars below indicate either a viral-tRNA feature (green) or a viral-miRNA feature (orange).
tRAX detects abundant misincorporations in transcripts, which reveal putative RNA modifications. We find 1-methyladenosine (m1a) modification-induced misincorporation at position 58A (seen as A>T in sequencing reads) in the majority of virtRNA transcripts detected (Supp Fig 4). Additionally, virtRNA5 contained near penetrant A>G substitutions, suggesting that virtRNA5 undergoes deamination at the wobble base position 34, resulting in inosine within the anticodon. These results demonstrate that, similar to host tRNAs, virtRNAs can undergo modification and cleavage into stable fragments in MHV68-infected cells.
Validation of 5’ tDR production using stem-loop qPCR
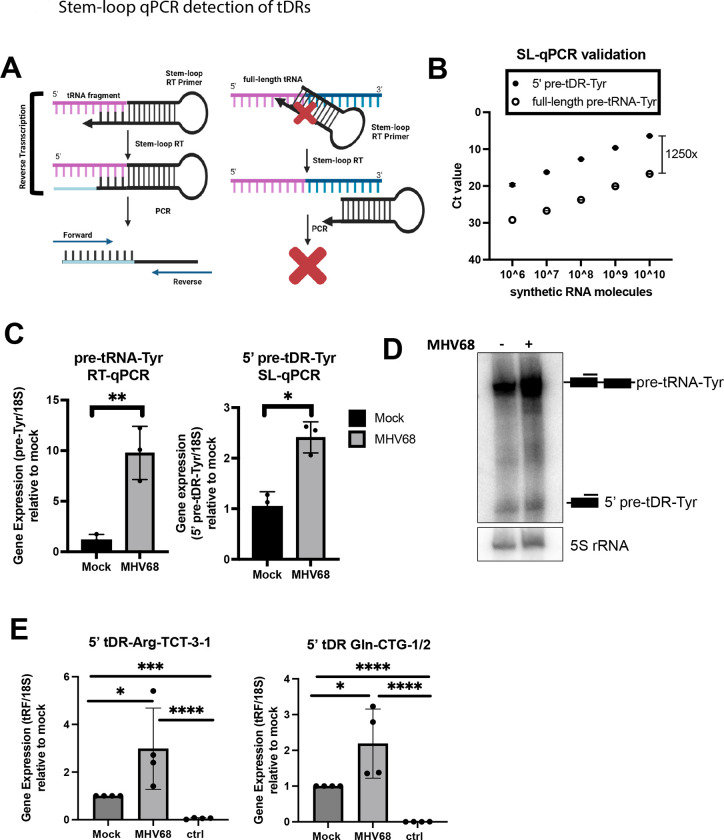
To validate host tDR production in response to MHV68 infection, we applied stem-loop reverse transcription quantitative polymerase chain reaction (SL-qPCR), which allows the selective amplification of 5’ cleavage products versus their full-length counterparts27 (Fig 6A). Briefly, stem-loop reverse transcription (SL-RT) primers are designed to be specific for the 3’ end of the cleavage product. This SL-RT primer should preferentially bind to the cleavage product, not the precursor, as the precursor has a different 3’ end and additionally cannot be internally primed due to the steric hindrance with the SL-RT primer. We designed SL-RT primers for the upregulated 5’ pre-tDR-Tyr identified by OTTR-tRAX (Fig 4A). We assessed the specificity of SL-qPCR for detecting 5’ pre-tDR-Tyr rather than full-length pre-tRNA-Tyr by using synthetic RNAs ordered from IDT (Fig 6B). SL-qPCR was performed using a dilution series of equimolecular full-length and fragment synthetic RNAs as templates and confirmed >1000-fold specificity for fragment detection. We then applied the SL-qPCR assay for 5’ pre-tDR-Tyr to cellular RNA from cells +/− MHV68 infection (Fig 6C). We measured full-length pre-tRNA-Tyr using standard RT-qPCR and a reverse qPCR primer specific for the intron (absent in 5’ pre-tDR-Tyr) alongside as a control6. There was a 2.5-fold increase in 5’ pre-tDR-Tyr levels upon infection, which was equivalent to the increase observed by northern blot done in parallel (Fig 6C,D). Importantly, the increase did not reflect the ~40-fold increase in full-length pre-tRNA-Tyr observed by both standard RT-qPCR and northern blot (Fig 6C,D). We designed SL-qPCR assays for 5’ pre-tDR-Arg-TCT-3–1 and 5’ tDR-Gln-CTG-1/2 and confirmed their upregulation during MHV68 infection (Fig 6E). Thus, SL-qPCR successfully validated 5’ tDR expression revealed by OTTR-tRAX and is a useful molecular assay to quantify 5’ tDRs.
Figure 6. Stem-loop qPCR detection of tDRs.
(A) Schematic illustrating the specificity of the stem-loop (SL) RT primer for tDR vs. full-length parental tRNA. (B) Synthetic full-length pre-tRNA-Tyr or 5’ pre-tDR-Tyr were used in known quantities for SL-qPCR using Tyr forward and SL reverse primers. Raw Ct values are shown. (C) Standard- and SL-qPCR was performed using total RNA from mock or MHV68 infected NIH 3T3s to detect the full-length pre-tRNA-Tyr or 5’ pre-tDR-Tyr, respectively. Data depicts means +/− SD from three independent experiments relative to the mock sample, with p-values calculated using raw DCt values and paired t-test. (D) Northern blot using 5’ exon Tyr probes was performed using RNA samples as described in (C). (E) SL-qPCR was performed from four independent experiments to detect 5’ tDR-Arg-TCT and - Gln-CTG as in (C). The sample labeled “ctrl” was reverse transcribed with a SL RT primer for an unrelated tDR species. tDRnamer21 nomenclature for amplified tDRs is reported in Materials and Methods. ns = p>0.05; * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001
5’ pre-tDR-Tyr expression is dependent on tRNA splicing factors
5’ pre-tDR-Tyr upregulation was previously reported in fibroblasts and tissue extracted from kinase-dead Clp1K/K mice.28 CLP1 kinase associates with the tRNA splicing complex (TSEN2,15,34,54) and negatively regulates tRNA splicing29–31. Additionally, we noticed that the 3’ end of 5’ pre-tDR-Tyr corresponds to the identical end of the 5’ tRNA-Tyr exon, suggesting it might be the result of aberrant or dysregulated splicing. To test whether the 5’ pre-tDRs we observe during MHV68 infection are sensitive to decreased expression of tRNA splicing factors, we used siRNAs to knockdown expression of both Clp1 and Tsen2 (Fig 7). We validated knockdown of Clp1 and Tsen2 mRNA expression by RT-qPCR, as we were unable to obtain commercial antibodies that specifically detected our protein of interest (Fig 7A). Both knockdowns reduced expression of their target mRNA in mock-treated cells. However, this did not reach statistical significance for Clp1 in MHV68-infected cells, which notably exhibit low Clp1 and Tsen2 mRNA expression due to host shut-off, even with non-targeting siRNA treatment. Clp1 knockdown was associated with enhanced 5’ pre-tDR-Tyr expression in both Mock- and MHV68-infected cells, confirming that CLP1 negatively regulates the production of 5’ pre-tDR-Tyr in both contexts (Fig 7B). In contrast, Tsen2 knockdown decreased 5’ pre-tDR-Tyr levels, suggesting that TSEN2 is required for accumulation of 5’ pre-tDR-Tyr in mock and MHV68-infected cells. Together, these data suggest that tRNA splicing may be dysregulated during MHV68 infection, leading to the production of pre-tRNA fragments. Finally, we measured infectious titer following a single round of MHV68 replication in cells treated with non-targeting, Clp1, or Tsen2- targeting siRNAs (Fig 7C). Clp1 knockdown resulted in an 8-fold decrease in MHV68 titer compared to the non-targeting control, while Tsen2 knockdown had no effect. These data suggest that the regulation of tRNA splicing by CLP1 contributes to MHV68 replication.
Figure 7. The tRNA splicing factors TSEN2 and CLP1 modulate 5’ pre-tDR-Tyr expression during MHV68 infection.
siRNA-mediated knockdown of Tsen2 (siTsen2) and Clp1 (siClp1) or treatment with non-targeting siRNAs (siNT) was followed by mock or MHV68 infection at an MOI=5 for 24 h. (A) siRNA knockdown was confirmed using RT-qPCR using Clp1, Tsen2, and 18S-specific primers. (B) SL-qPCR was used to measure 5’ pre-tDR-Tyr upon siRNA treatment. Data from RT-qPCR and SL-qPCR experiments is depicted as mean +/− SD from four independent experiments relative to the siNT/mock sample, with p-values calculated using raw DCt values and paired t-test. (C) Viral titer of supernatants was measured by TCID50 from three independent experiments, with p-value calculated by one-way ANOVA. ns = p>0.05; * = p ≤ 0.05; ** = p ≤ 0.01; *** = p ≤ 0.001
Discussion
By combining OTTR-seq for library production with the bioinformatic software tRAX, we have conducted an in-depth analysis of tRNA and tDR expression during MHV68 infection. OTTR-tRAX offers a significant improvement over currently used tRNA sequencing strategies, as it: 1) utilizes a novel bioengineered RT that effectively copies modified tRNAs/tRFs and 2) minimizes sequencing artifacts. Applying OTTR-tRAX facilitated the discovery that a variety of tDRs are upregulated in response to MHV68, with the majority derived from pre-tRNAs that accumulate during infection. While there have been reports of tDRs produced in response to RNA virus infection2, 4, here we show that a DNA virus can also induce host tRNA cleavage. Based on MHV68-MR and -R443I sequencing, we note a correlation between elevated tDR levels and the parental transcript of origin for some transcripts. As viruses belonging to each of the three herpesvirus subfamilies (alpha, HSV-1; beta, HCMV; gamma, MHV68) have now been reported to induce tRNA transcription6, 32, 33, it will be interesting to examine if tRNA cleavage is also a universal response to herpesvirus infection.
Infection with the host shutoff mutant virus MHV68-R443I was associated with lower steady-state levels of tDRs compared to that by MHV68-MR. Of note, MHV68-R443I exhibits wild-type replication kinetics and indistinguishable levels of RNA polymerase II occupancy on the viral genome from MHV68-MR in fibroblasts.20, 34 In contrast, host and viral mRNA and protein abundance is distinct between MHV68-MR and-R443I due to posttranscriptional regulation by muSOX24. We suggest that elevated host tRNA/tDR levels in infected cells is an indirect effect of multiple downregulated host pathways, starting with the depletion of tRNA maturation or turnover machinery. It is possible that tRNA cleavage is part of a quality control mechanism triggered by the abundance of immature and/or hypomodified pre-tRNAs. Importantly, muSOX does not cleave reporter transcripts made by RNA polymerase I or II35, arguing against the possibility of cleavage of tRNAs by muSOX, though this remains to be directly tested. Altogether, our data reveal a mechanistic link between pre-tRNA accumulation and tDR production that requires further dissection.
Viruses often encode “mimics” of host machinery to facilitate infection, and TMERs encoded by MHV68 are an intriguing example of this viral strategy. TMERs are transcribed by host RNA polymerase III and undergo maturation by host tRNA and miRNA processing factors, including Elac2 and Dicer25. Early studies revealed that the virtRNAs are not charged26, and thus virtRNAs have been considered as solely promoter sequence for the expression of downstream viral miRNAs36 or other recently discovered ncRNAs37 made from TMER loci. Our data emphasize how widely the expression levels of different virtRNAs vary, with virtRNA5 being the most abundant in fibroblasts. Surprisingly, 3’ tDRs are made from TMER-derived virtRNAs as well, with abundance greater than their downstream miRNA counterparts (e.g., TMER7). Our finding that full-length virtRNA7 is not detected by RNA sequencing aligns with initial predictions based on sequence gazing that this virtRNA would not form a functional tRNA26. Additionally, we found evidence that virtRNAs undergo modification, including m1a modification at position 58 (virtRNAs1,2,4,5,6,7) and deamination at the wobble base position 34 (virtRNA5). The m1a modification at position 58 is a highly conserved modification among all branches of life, and is known to be important for proper RNA folding38, while modification of the wobble base is essential for viability due to its requirement in relaxed decoding39. We argue that the presence of base modifications and novel processing events within virtRNAs suggest additional functionality yet to be described. TMER maturation should be explored further to expand our understanding of the functional capacity of TMERs and other RNA polymerase III-transcribed viral genes.
We noticed that MHV68 infection leads to increased levels of pre-tDRs derived from pre-tRNA-Tyr and -Arg with 3’ ends that matched that of the 5’ tRNA exon. Because of this observation, we explored the possibility that these particular fragments form due to dysregulated tRNA splicing events, as reported in Clp1 kinase-dead mice28. TSEN2 is known to cleave pre-tRNAs at the 5’ exon-intron junction, matching the most abundant transcript end for 5’ pre-tDR-Tyr we detected. We found that TSEN2 activity contributes to 5’ pre-tDR-Tyr production in both mock- and MHV68-infected cells, suggesting that this tDR may be a splicing intermediate or byproduct. CLP1 is a negative regulator of tRNA splicing31, and we saw that Clp1 depletion increased the levels of detectable 5’ pre-tDR-Tyr, whether or not the cells were infected by MHV68. Because the intact 5’ leader sequence is present on 5’ pre-tDR-Tyr28, this may suggest that intron removal can occur prior to 5’ leader trimming, or that perhaps 5’ pre-tDR-Tyr is a result of dysregulated splicing or other quality control event. However, we also detected pre-tDRs from pre-tRNAs that lack introns, such as pre-tDR-Gln, -Ala, and others. We are currently investigating the mechanism leading to the cleavage of intronless pre-tRNA transcripts during infection.
Overall, we present a novel sequencing strategy and a comprehensive profile of tRNAs and tDRs induced during gammaherpesvirus infection. Our work reveals novel tRNA-centric nodes of gene expression control that may be manipulated in novel therapeutic contexts. The use of OTTR-tRAX across diverse infection and disease scenarios will help pave the way toward a comprehensive understanding of tRNA regulation and cleavage.
Supplementary Material
Supplemental Figure 1. 5’ tDRs induced by MHV68 infection. Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across tRNA gene loci. 5’ tDRs are defined by tRAX as reads that are within 10 base pairs of the start position, but do not reach the end of the full tRNA sequence. Colors of the coverage defines the specificity of read mapping. Purple “unique” reads uniquely map to the corresponding tRNA transcript sequence. Blue “multitRNA” reads map to the transcripts with the corresponding anticodon. Green “multianticodon” reads map only to transcripts of the corresponding tRNA isotype. Red “multiamino” are reads that map to more than one tRNA isotype.
Supplemental Figure 2. 3’ tDRs induced by MHV68 infection. Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across tRNA gene loci. 3’ tDRs are defined by tRAX as reads that are within 10 base pairs of the end position, but do not reach the start position of the full tRNA sequence. See Supp Fig 1 legend for coloring information.
Supplemental Figure 3. Other tDRs induced by MHV68 infection. Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across tRNA gene loci. Other tDRs are defined by tRAX as reads that cannot be defined as 5’ or 3’ tDRs. See Supp Fig 1 legend for coloring information.
Supplemental Figure 4. Viral tRNAs interact with host modification machinery. (A) Read coverage (5’->3’) across detectable virtRNAs. Grey indicates reads containing nucleotides matching the reference sequence, whereas further colors indicate the misincorporated base present. Sprinzl positions harboring a significant modification-induced misincorporation are highlighted.
Acknowledgements
ACM, MMB, ARJ, and HEU acquired the data, as supervised by KC, TML, and JMT. ACM and JMT analyzed the data and prepared the manuscript. All authors edited and approved the final manuscript. We thank Lucas Ferguson for his contributions to the development and optimization of OTTR, as well as all present and past members of the Tucker, Lowe, Glaunsinger, and Roller labs for their helpful suggestions, discussion, and careful reading of this manuscript. Data presented herein were obtained at the Genomics Division of the Iowa Institute of Human Genetics which is supported, in part, by the University of Iowa Carver College of Medicine and the Holden Comprehensive Cancer Center (National Cancer Institute of the National Institutes of Health under Award Number P30CA086862).
Funding
This work was supported by Grant IRG-21-141-46-IRG from the American Cancer Society, administered through the Holden Comprehensive Cancer Center at The University of Iowa to JMT, as well as funding from the National Human Genome Research Institute, National Institutes of Health [R01HG006753 to T.L.]. HEU and KC are supported by the Bakar Fellows Program, University of California, Berkeley.
Data Availability/Sequence Data Resources
Sequencing data was deposited in GEO: GSE255627.
References
- 1.Blanco S, Dietmann S, Flores JV, Hussain S, Kutter C, Humphreys P, et al. Aberrant methylation of tRNAs links cellular stress to neuro-developmental disorders. EMBO J. 2014;33(18):2020–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho H, Lee W, Kim GW, Lee SH, Moon JS, Kim M, et al. Regulation of La/SSB-dependent viral gene expression by pre-tRNA 3’ trailer-derived tRNA fragments. Nucleic Acids Res. 2019;47(18):9888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun C, Fu Z, Wang S, Li J, Li Y, Zhang Y, et al. Roles of tRNA-derived fragments in human cancers. Cancer Lett. 2018;414:16–25. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Lee I, Ren J, Ajay SS, Lee YS, Bao X. Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol Ther. 2013;21(2):368–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu P, Yu J, Zhou P. Role of tRNA-derived fragments in cancer: novel diagnostic and therapeutic targets tRFs in cancer. Am J Cancer Res. 2020;10(2):393–402. [PMC free article] [PubMed] [Google Scholar]
- 6.Tucker JM, Schaller AM, Willis I, Glaunsinger BA. Alteration of the Premature tRNA Landscape by Gammaherpesvirus Infection. mBio. 2020;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodarzi H, Liu X, Nguyen HC, Zhang S, Fish L, Tavazoie SF. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell. 2015;161(4):790–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar P, Anaya J, Mudunuri SB, Dutta A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014;12:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falconi M, Giangrossi M, Zabaleta ME, Wang J, Gambini V, Tilio M, et al. A novel 3’-tRNA(Glu)-derived fragment acts as a tumor suppressor in breast cancer by targeting nucleolin. FASEB J. 2019;33(12):13228–40. [DOI] [PubMed] [Google Scholar]
- 10.Motorin Y, Muller S, Behm-Ansmant I, Branlant C. Identification of modified residues in RNAs by reverse transcription-based methods. Methods Enzymol. 2007;425:21–53. [DOI] [PubMed] [Google Scholar]
- 11.Mohr S, Ghanem E, Smith W, Sheeter D, Qin Y, King O, et al. Thermostable group II intron reverse transcriptase fusion proteins and their use in cDNA synthesis and next-generation RNA sequencing. RNA. 2013;19(7):958–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng G, Qin Y, Clark WC, Dai Q, Yi C, He C, et al. Efficient and quantitative high-throughput tRNA sequencing. Nat Methods. 2015;12(9):835–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Upton HE, Ferguson L, Temoche-Diaz MM, Liu XM, Pimentel SC, Ingolia NT, et al. Low-bias ncRNA libraries using ordered two-template relay: Serial template jumping by a modified retroelement reverse transcriptase. Proc Natl Acad Sci U S A. 2021;118(42). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bibillo A, Eickbush TH. High processivity of the reverse transcriptase from a non-long terminal repeat retrotransposon. J Biol Chem. 2002;277(38):34836–45. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Manning AC, Bagi A, Yang X, Gokulnath P, Spanos M, et al. Distinct Stress-Dependent Signatures of Cellular and Extracellular tRNA-Derived Small RNAs. Adv Sci (Weinh). 2022;9(17):e2200829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmes AD HJ, Chan PP, and Lowe TM. tRNA Analysis of eXpression (tRAX): A tool for integrating analysis of tRNAs, tRNA-derived small RNAs, and tRNA modifications. bioRxiv. 2022. [Google Scholar]
- 17.Kumar P, Kuscu C, Dutta A. Biogenesis and Function of Transfer RNA-Related Fragments (tRFs). Trends Biochem Sci. 2016;41(8):679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yeung ML, Bennasser Y, Watashi K, Le SY, Houzet L, Jeang KT. Pyrosequencing of small non-coding RNAs in HIV-1 infected cells: evidence for the processing of a viral-cellular double-stranded RNA hybrid. Nucleic Acids Res. 2009;37(19):6575–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adler H, Messerle M, Wagner M, Koszinowski UH. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J Virol. 2000;74(15):6964–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richner JM, Clyde K, Pezda AC, Cheng BY, Wang T, Kumar GR, et al. Global mRNA degradation during lytic gammaherpesvirus infection contributes to establishment of viral latency. PLoS Pathog. 2011;7(7):e1002150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmes AD, Chan PP, Chen Q, Ivanov P, Drouard L, Polacek N, et al. A standardized ontology for naming tRNA-derived RNAs based on molecular origin. Nat Methods. 2023;20(5):627–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan PP, Lowe TM. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016;44(D1):D184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42(Database issue):D68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abernathy E, Clyde K, Yeasmin R, Krug LT, Burlingame A, Coscoy L, et al. Gammaherpesviral gene expression and virion composition are broadly controlled by accelerated mRNA degradation. PLoS Pathog. 2014;10(1):e1003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bogerd HP, Karnowski HW, Cai X, Shin J, Pohlers M, Cullen BR. A mammalian herpesvirus uses noncanonical expression and processing mechanisms to generate viral MicroRNAs. Mol Cell. 2010;37(1):135–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowden RJ, Simas JP, Davis AJ, Efstathiou S. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol. 1997;78 ( Pt 7):1675–87. [DOI] [PubMed] [Google Scholar]
- 27.Kramer MF. Stem-loop RT-qPCR for miRNAs. Curr Protoc Mol Biol. 2011;Chapter 15:Unit 15 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanada T, Weitzer S, Mair B, Bernreuther C, Wainger BJ, Ichida J, et al. CLP1 links tRNA metabolism to progressive motor-neuron loss. Nature. 2013;495(7442):474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weitzer S, Martinez J. The human RNA kinase hClp1 is active on 3’ transfer RNA exons and short interfering RNAs. Nature. 2007;447(7141):222–6. [DOI] [PubMed] [Google Scholar]
- 30.Paushkin SV, Patel M, Furia BS, Peltz SW, Trotta CR. Identification of a human endonuclease complex reveals a link between tRNA splicing and pre-mRNA 3’ end formation. Cell. 2004;117(3):311–21. [DOI] [PubMed] [Google Scholar]
- 31.Hayne CK, Schmidt CA, Haque MI, Matera AG, Stanley RE. Reconstitution of the human tRNA splicing endonuclease complex: insight into the regulation of pre-tRNA cleavage. Nucleic Acids Res. 2020;48(14):7609–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ball CB, Parida M, Li M, Spector BM, Suarez GA, Meier JL, et al. Human Cytomegalovirus Infection Elicits Global Changes in Host Transcription by RNA Polymerases I, II, and III. Viruses. 2022;14(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dremel SE, Sivrich FL, Tucker JM, Glaunsinger BA, DeLuca NA. Manipulation of RNA polymerase III by Herpes Simplex Virus-1. Nat Commun. 2022;13(1):623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartenian E, Gilbertson S, Federspiel JD, Cristea IM, Glaunsinger BA. RNA decay during gammaherpesvirus infection reduces RNA polymerase II occupancy of host promoters but spares viral promoters. PLoS Pathog. 2020;16(2):e1008269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Covarrubias S, Gaglia MM, Kumar GR, Wong W, Jackson AO, Glaunsinger BA. Coordinated destruction of cellular messages in translation complexes by the gammaherpesvirus host shutoff factor and the mammalian exonuclease Xrn1. PLoS Pathog. 2011;7(10):e1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldman ER, Kara M, Coleman CB, Grau KR, Oko LM, Krueger BJ, et al. Virus-encoded microRNAs facilitate gammaherpesvirus latency and pathogenesis in vivo. mBio. 2014;5(3):e00981–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffman BA, Wang Y, Feldman ER, Tibbetts SA. Epstein-Barr virus EBER1 and murine gammaherpesvirus TMER4 share conserved in vivo function to promote B cell egress and dissemination. Proc Natl Acad Sci U S A. 2019;116(51):25392–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oerum S, Degut C, Barraud P, Tisne C. m1A Post-Transcriptional Modification in tRNAs. Biomolecules. 2017;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackman JE, Alfonzo JD. Transfer RNA modifications: nature’s combinatorial chemistry playground. Wiley Interdiscip Rev RNA. 2013;4(1):35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. 5’ tDRs induced by MHV68 infection. Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across tRNA gene loci. 5’ tDRs are defined by tRAX as reads that are within 10 base pairs of the start position, but do not reach the end of the full tRNA sequence. Colors of the coverage defines the specificity of read mapping. Purple “unique” reads uniquely map to the corresponding tRNA transcript sequence. Blue “multitRNA” reads map to the transcripts with the corresponding anticodon. Green “multianticodon” reads map only to transcripts of the corresponding tRNA isotype. Red “multiamino” are reads that map to more than one tRNA isotype.
Supplemental Figure 2. 3’ tDRs induced by MHV68 infection. Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across tRNA gene loci. 3’ tDRs are defined by tRAX as reads that are within 10 base pairs of the end position, but do not reach the start position of the full tRNA sequence. See Supp Fig 1 legend for coloring information.
Supplemental Figure 3. Other tDRs induced by MHV68 infection. Normalized read coverage (5’ -> 3’) from Mock (left), MR (middle), or R443I (right) across tRNA gene loci. Other tDRs are defined by tRAX as reads that cannot be defined as 5’ or 3’ tDRs. See Supp Fig 1 legend for coloring information.
Supplemental Figure 4. Viral tRNAs interact with host modification machinery. (A) Read coverage (5’->3’) across detectable virtRNAs. Grey indicates reads containing nucleotides matching the reference sequence, whereas further colors indicate the misincorporated base present. Sprinzl positions harboring a significant modification-induced misincorporation are highlighted.
Data Availability Statement
Sequencing data was deposited in GEO: GSE255627.