Abstract
Mild Behavioral Impairment (MBI) leverages later-life emergent and persistent neuropsychiatric symptoms (NPS) to identify a high-risk group for incident dementia. Phosphorylated tau (p-tau) is a hallmark biological manifestation of Alzheimer disease (AD). We investigated associations between MBI and tau accumulation in early-stage AD cortical regions. In 442 Alzheimer’s Disease Neuroimaging Initiative participants with normal cognition or mild cognitive impairment, MBI status was determined alongside corresponding p-tau and Aβ. Two meta-regions of interest were generated to represent Braak I and III neuropathological stages. Multivariable linear regression modelled the association between MBI as independent variable and tau tracer uptake as dependent variable. Among Aβ positive individuals, MBI was associated with tau uptake in Braak I (β =0.45(0.15), p<.01) and Braak III (β =0.24(0.07), p<.01) regions. In Aβ negative individuals, MBI was not associated with tau in the Braak I region (p=.11) with a negative association in Braak III (p=.01). These findings suggest MBI may be a sequela of neurodegeneration, and can be implemented as a cost-effective framework to help improve screening efficiency for AD.
Keywords: Mild Behavioral Impairment, Neuropsychiatric Symptoms, Preclinical, Prodromal, Alzheimer disease, Tau
1.0. Introduction:
Alzheimer Disease (AD) is prevalent in 5.8 million Americans over the age of 65, characterized by progressive cognitive decline and functional impairment (Association, 2020). Current medications are mostly symptomatic treatments, and the search for disease modifying therapies has been challenging. From 2003 until recently, no clinical trial had met all primary endpoints, possibly due to intervention too late in the disease course (Gauthier et al., 2016; Mortby, M.E et al., 2018). Notwithstanding the recent success of the disease modifying therapy lecanemab and subsequent approval in the United States (Hoy, 2023), many other DMTs have failed, sometimes due to challenges recruiting participants with early-stage disease. The hallmarks of AD are Amyloid-β (Aβ) plaques and phospho-tau (p-tau) tangles; the bulk of research has focused on Aβ. However, tau is clinically meaningful, correlating better with cognitive symptoms, severity of dementia, and function, thus becoming an emerging focus of therapeutics (Congdon et al., 2023; Jack Jr et al., 2019; Malpas et al., 2020). Detecting prodromal and preclinical disease often requires neuropsychological testing, followed by ligand-based PET imaging or CSF biomarker analyses for AD biomarker confirmation, although plasma biomarkers are gaining traction (Balogun et al., 2023). Nonetheless, barriers remain to widespread screening for plasma biomarkers, including access for those in remote geographies, high cost of assays on top of blood draws, and the risk of false positives. In clinical trials, biomarkers are costly and inefficient if used to screen for AD+ cases. Neuropsychiatric symptoms (NPS) may offer an inexpensive and efficient opportunity for trial enrichment for biomarker positivity. If applied before biomarker assays, adding NPS assessments may improve accuracy of risk estimates generated from cognitive symptoms alone (especially in preclinical AD), identifying a sub-group with substantially higher risk, therefore increasing screening yield and detection of disease (Soto et al., 2023).
Historically overshadowed by cognitive symptoms in AD, NPS have emerged as a key component of the disease. NPS are almost ubiquitous in dementia (Lanctôt et al., 2017), but are also common in mild cognitive impairment (MCI) where symptoms are associated with faster progression to dementia (Martin and Velayudhan, 2020). NPS emerge in advance of cognitive symptoms in 59% of all-cause dementia, including 30% of those who develop AD (Wise et al., 2019). In cognitively normal (NC) older adults, the presence of NPS has been associated with cognitive decline and dementia (Burhanullah et al., 2019; Geda et al., 2014; Liew, 2020). However, the assessment of NPS in older adults can be challenging; in many cases psychiatric diagnoses are provided, and neurodegenerative disease is not initially considered on the differential diagnosis (Cieslak et al., 2018; Matsuoka et al., 2019; Mortby et al., 2017).
Mild Behavioral Impairment (MBI) is a validated dementia risk syndrome (Ismail et al., 2016) distinct from chronic and/or recurrent psychiatric illness (Matsuoka et al., 2019; Taragano et al., 2018). While conventionally measured NPS are associated with incident dementia, MBI leverages neurodegenerative disease associations with later-life emergent and persistent NPS to identify a group at much higher risk (Bateman et al., 2020; Creese et al., 2023; Creese et al., 2019; Ebrahim et al., 2023; Gill et al., 2020; Gill, Sascha et al., 2021; Ismail et al., 2023a; Ismail et al., 2021; Kan et al., 2022; Matsuoka et al., 2019; McGirr, A. et al., 2022; Rouse et al., 2023; Vellone et al., 2022; Yoon et al., 2022). Several recent papers have demonstrated this point when MBI was compared to psychiatric disorders or NPS not meeting MBI criteria, with the MBI group having faster cognitive decline and progressing more rapidly to dementia (Ebrahim et al., 2023; Ghahremani, Maryam et al., 2023; Ismail et al., 2023a; Ismail et al., 2023b; Matsuoka et al., 2019; Taragano et al., 2018; Vellone et al., 2022), and even lower reversion rates from MCI to NC (McGirr, Alexander et al., 2022). For some, MBI is a proxy marker of underlying neurodegenerative disease pathology, associated with AD risk genes, amyloid, tau, and neurodegeneration (Andrews et al., 2018; Creese et al., 2021; Ghahremani, Maryam et al., 2023; Gill, S. et al., 2021; Ismail et al., 2023b; Johansson et al., 2021; Lussier et al., 2020; Matsuoka et al., 2023; Matsuoka et al., 2021; Matuskova et al., 2021; Miao et al., 2021a; Naude et al., 2020). Thus, a greater proportion of the MBI group has underlying AD compared to the conventional psychiatric/NPS group; this represents prodromal AD for MBI in MCI, and preclinical AD for MBI in NC. These data tell us that risk estimates based on the emergence and persistence of NPS are useful, and that MBI should be reported in conjunction with cognitive status, contributing an estimate of behavioral risk as a complement to the determination of risk based on cognition.
Much of the MBI biomarker literature has focused on fluid biomarkers and structural (Matsuoka et al., 2023) and functional (Ghahremani, M. et al., 2023) imaging. Relatively little data have explored ligand-based PET imaging. PET studies are important to determine locations and patterns of amyloid and tau binding, to explore how presentation of MBI aligns with known patterns in AD. The Canadian TRIAD study first explored this concept in 96 NC participants, finding a correlation between MBI Checklist (MBI-C) (Ismail et al., 2017) score and global and striatal Aβ-PET tracer uptake was shown; however, the same was not demonstrated for tau-PET tracer (Lussier et al., 2020). Subsequently, a Swedish Biofinder2 study explored this same relationship in a sample of 50 Aβ + NC participants, finding an association between MBI-C score and tau-PET in the entorhinal cortex and hippocampus (Johansson et al., 2021). These conflicting results necessitate further exploration of the association of tau-PET with MBI in the presence of Aβ. Here, in a sample of NC/MCI individuals including both Aβ+ and Aβ− participants, we aimed to determine whether persons with NPS had tau-PET tracer uptake in regions of the brain affected early in the course of AD. We hypothesized that participants with a NPS profile consistent with MBI, would have a greater standardized uptake value ratio (SUVR) of AV1451 (flortaucipir), compared with persons with NPS profiles not consistent with MBI.
2.0. Methods and Materials:
2.1. Alzheimer’s Disease Neuroimaging Initiative (ADNI)
Data are from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a partnership involving multiple centers across North America with the goal of tracking participants through periods of cognitive decline and dementia. Launched in 2003, ADNI continues to evaluate biomarker, neuroimaging, and neuropsychological status in participants. Informed consent was obtained from all participants prior to enrolling in the study. Data in this study were collected prior to August 2022.
2.2. Sample
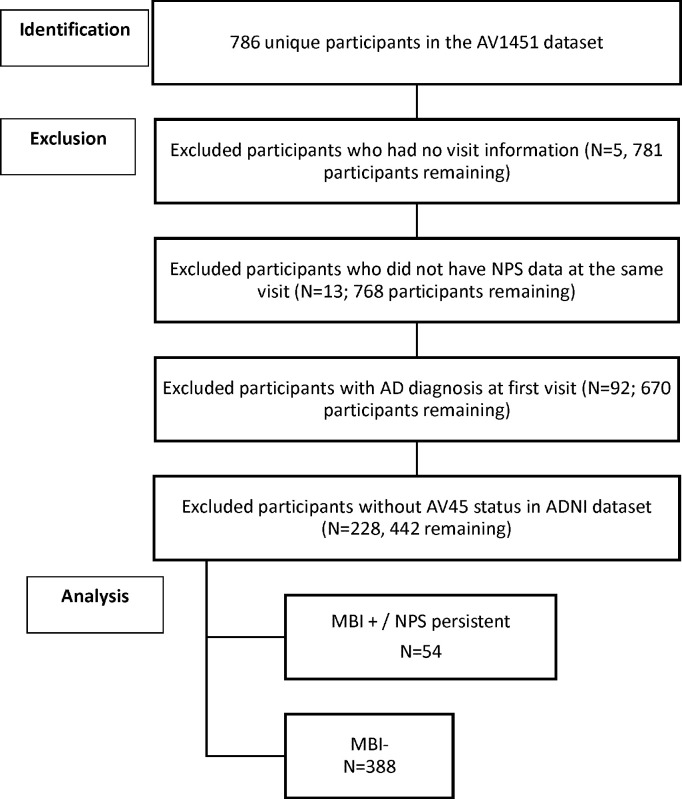
Participants enrolled in ADNI with AV1451 tau-PET and AV45 amyloid-PET imaging data available were included (n=442). Participants were classified as either NC or MCI at the time of tau-PET imaging. Participants free of memory complaints, scoring between 24–30 on the MMSE, with a Clinical Dementia Rating (CDR) of 0, and a Memory Box Score of 0 were NC. MCI was diagnosed if there were subjective memory concerns, MMSE between 24–30, CDR of 0.5, and Memory Box Score ≥0.5. Participants were excluded from this study if at the imaging visit NPS score was not available for either the visit associated with tau-PET or the subsequent visit, Aβ-PET status was not determined, or diagnosis was dementia. Demographic information including age, sex, and years of education were obtained at the time of the AV1451 imaging visit. Detailed participant selection is displayed in Figure 1.
Figure 1:
Depicts derivation of the sample population from ADNI datasets.
2.3. NPS Case Ascertainment and MBI Status
The Neuropsychiatric Inventory (NPI) (Cummings, 2020) was used to generate a NPS score that mapped NPI items onto MBI domains (Mortby, Moyra E et al., 2018; Sheikh et al., 2018). The NPI, clinician-rated after informant interview, consists of 12 items relating to frequency and severity of NPS over the previous month. The 5 MBI domains were derived from 10 NPI domains as follows: MBI motivation/drive from NPI apathy/indifference; MBI emotional regulation from the sum of NPI depression, anxiety, and elation/euphoria; MBI impulse control from the sum of NPI irritability, agitation/aggression, and aberrant motor behavior; MBI social cognition from NPI disinhibition; and MBI thoughts/perception from NPI delusions/hallucinations. Neurovegetative NPI items for sleep and appetite were not included as neither map well onto MBI criteria. If score=0 on all selected NPI items, the visit was coded NPS−. If score was >0 on any of the select NPI domains, the visit was coded NPS+. MBI status was determined from NPS status at the tau-PET scan visit and the visit 6 months later (Guan et al., 2023). A two-level variable for MBI was utilized (MBI+, MBI−), informed by recent ADNI data demonstrating that MBI was associated with significantly higher p-tau levels relative to no NPS, but NPS not meeting MBI criteria did not differ from the no NPS group (Ghahremani, Maryam et al., 2023). Thus, NPS+ status at both visits (symptom persistence) was classified as MBI+, and NPS− status at either or both visits was classified as MBI−. If NPI data were available for only one visit, the participant was conservatively classified as MBI−.
2.4. AV1451 and Regions Included
Partial Volume Corrected (PVC) AV1451 PET data were processed to render standardized uptake values (SUVs), which were reported for left and right hemispheric components of structures. Each regional SUV was normalized by dividing the raw SUV by the inferior cerebellar gray matter SUV to generate a ratio, the SUVR. Left and right hemispheric components of structures were summed and meaned. Brain regions affected early in the AD course were chosen based on previous research on tau binding and spread pattern (Cho et al., 2016; Jack et al., 2018; Ossenkoppele et al., 2018). Two meta-regions of interest (ROIs) representing early Braak neuropathological stages (Braak and Braak, 1991) were generated for use in the analysis. The Braak neuropathological staging system is based on the distribution of neurofibrillary tangles (NFTs), composed of tau. According to the staging system, NFTs are first seen in the entorhinal cortex, then hippocampus, and then other regions (Braak and Braak, 1991). For our analysis, the first ROI represented Braak stage I, composed of both left and right entorhinal cortex. The second ROI was generated to represent Braak III, composed of bihemispheric components of the parahippocampus, fusiform, posterior cingulate gyrus, lingual gyrus, and amygdala. A ROI was not generated for the hippocampus, representing Braak stage II, due to substantial off-target binding of AV1451 (Lee et al., 2018). Aβ+ status was determined based on AV45 SUVR ≤1.11 (Landau et al., 2012). Further information on the image processing pipeline for this study is available at adni.loni.usc.edu.
2.5. Statistical Analysis
Sample characteristics were described using mean, standard deviation, quartile, and frequency distributions. To assess the relationship between MBI status and AV1451 tau-PET SUVR for both ROIs, multivariable linear regression models were fitted with MBI status as the independent variable and tau-PET SUVR as dependent variable, adjusting for age, sex, and education years, with testing of interaction terms for MBI*cognitive status (NC/MCI) and MBI*Aβ status (+/−). The assumptions of linear regression were confirmed by examination of the residuals. All results were considered statistically significant with a two-sided p-value of <0.05. Analyses were conducted in SAS (v9.4 SAS Institute Inc., 2013).
2.6. Data availability
Data used in this study are available from adni.loni.usc.edu for download.
3.0. Results
Participant mean age was 75 years (SD=7.6), 64% had normal cognition, and about half were females (50.9%), with median educational attainment of 16 years (Table 1). Of the 442 participants, 338 were MBI− and 54 MBI+, 252 Aβ− and 188 Aβ+, 283 NC and 157 MCI (Table 2).
Table 1.
Sample Characteristics for overall sample and by MBI status
| Overall N=442 | MBI- N=388 | MBI + N=54 | |
|---|---|---|---|
|
|
|||
| Age, mean (SD) | 74.79 (7.63) | 74.69 (7.47) | 75.48 (8.77) |
| Female sex, n (%) | 225 (50.90) | 202 (52.06) | 23 (42.59) |
| Diagnosis, n (%) | |||
| Cognitively Normal | 283 (64.32) | 271 (70.21) | 12 (22.22) |
| MCI | 157 (35.68) | 115 (29.79) | 42 (77.78) |
| Education years, median (Q1-Q3) | 16 (15–18) | 16.50 (15–18) | 16 (14–18.75) |
| Braak I SUVR, mean (SD) | 1.88 (0.60) | 1.84 (0.57) | 2.15 (0.69) |
| Braak III SUVR, mean (SD) | 1.41 (0.28) | 1.40 (0.27) | 1.53 (0.32) |
| Braak I SUVR, median (Q1-Q3) | 1.70 (1.52–2.03) | 1.69 (1.51–1.98) | 1.97 (1.57–2.58) |
| Braak III SUVR, median (Q1-Q3) | 1.33 (1.26–1.47) | 1.33 (1.26–1.46) | 1.45 (1.31–1.70) |
| AV45 SUVR, mean (SD) | 1.16 (0.22) | 1.15 (0.21) | 1.20 (0.24) |
| AV45 SUVR binary, n (%) | 189 (42.76) | 157 (40.46) | 32 (59.26) |
Abbreviations: SD=standard deviation; MBI=mild behavioral impairment; MCI=mild cognitive impairment; SUVR, standardized uptake value ratio.
Q1=the first quartile; Q3= the third quartile; there were 2 participants missing cognitive diagnosis.
Table 2.
Cognitive and MBI characteristics stratified by amyloid status
| Sample Characteristic | Beta amyloid + N=188 | Beta amyloid - N=252 |
|---|---|---|
|
|
||
| MBI +, n (%) | 32 (17) | 22 (9) |
|
|
||
| Cognitively Normal, n (%) | 9 (5) | 3 (1) |
|
|
||
| MCI, n (%) | 23 (12) | 19 (8) |
|
|
||
| MBI -, n (%) | 156 (83) | 230 (91) |
|
|
||
| Cognitively Normal, n (%) | 100 (53) | 171 (7) |
|
|
||
| MCI, n (%) | 56 (30) | 59 (23) |
Abbreviations: MBI=mild behavior impairment; MCI=mild cognitive impairment
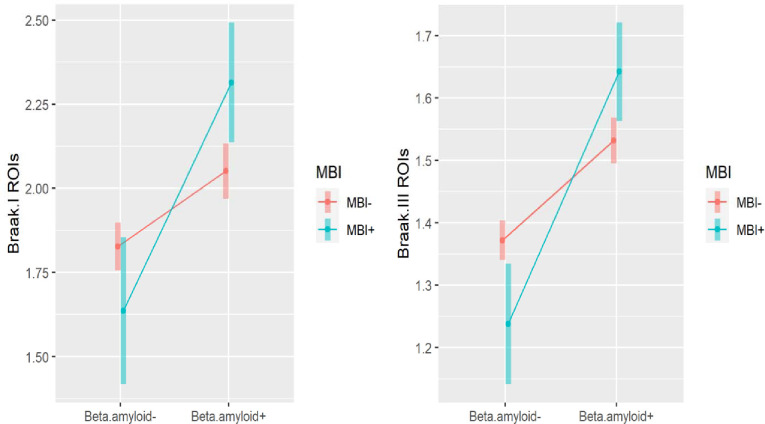
In the ROIs representing Braak I and Braak III neuropathological stages, Aβ status moderated the relationship between MBI status and AV1451 SUVR, after controlling for age, sex, education, and cognitive diagnosis (Table 3). Specifically, MBI*Aβ interaction coefficients were 0.45 (95%CI: 0.16–0.75, p< 0.01) for the Braak I ROI, and 0.24 (95%CI: 0.11–0.38, p<0.01) for the Braak III ROI. In the Aβ+ stratum, MBI was significantly associated with greater AV1451 SUVR in the Braak I ROI (, 95%CI: 0.07–0.46, p<0.01) and the Braak III ROI (, 95%CI: 0.02–0.20, p=0.01). In the Aβ− stratum, MBI status was not associated with AV1451 SUVR in the Braak I ROI (, 95%CI: −0.42 to 0.04, p=0.11) and was negatively associated with SUVR in the Braak III ROI (, 95%CI: −0.24 to −0.03, p=0.01). The adjusted marginal means of tau SUVR with 95% CI stratified by MBI status and Aβ status are shown in Figure 2.
Table 3.
The association between MBI and Braak I and III SUVR adjusted for age, sex, education, MCI, and AV45 using multiple linear regression models.
| Outcome variable Braak I tau SUVR N=440 | Outcome variable Braak III tau SUVR N=440 | |||
|---|---|---|---|---|
|
| ||||
| (95%CI) | p value | (95%CI) | p value | |
|
|
||||
| Independent Variables: | ||||
| Aβ + | ||||
| MBI + vs MBI - | 0.26 (0.07, 0.46) | <0.01 | 0.11 (0.02, 0.20) | 0.01 |
| Aβ - | ||||
| MBI + vs MBI - | −0.19 (−0.42, 0.04) | 0.11 | −0.13 (−0.24, −0.03) | 0.01 |
| MCI vs normal cognition | 0.44 (0.34, 0.55) | <0.01 | 0.22 (0.17, 0.26) | <0.01 |
| Age | 0.002 (−0.004, 0.01) | 0.45 | 0.002 (−0.0004, 0.01) | 0.10 |
| Female vs male | −0.10 (−0.20, 0.003) | 0.06 | −0.02 (−0.07, 0.02) | 0.32 |
| Education years | 0.01 (−0.01, 0.03) | 0.28 | 0.005 (−0.003, 0.01) | 0.23 |
Abbreviations: SD=standard deviation; MBI=mild behavioral impairment; MCI=mild cognitive impairment; SUVR, standardized uptake value ratio. SE= standard error. is the estimated regression coefficient.
Figure 2.
Association of MBI with TAU (Braak I and Braak III), by beta-amyloid status. Plots of the marginal means of tau with 95% CI stratified by MBI status and beta-amyloid status are based on multiple linear regression, for Braak I and Braak III, respectively.
MCI cognitive status was associated with higher AV1451 SUVR, in both the Braak I ROI (, 95%CI: 0.34–0.55, p<0.01) and the Braak III ROI (, 95%CI:0.17–0.26, p<0.01), but significant interaction effects between cognitive status and MBI were not found (p=0.24). Age, sex, and education were not associated with AV1451 SUVR in either ROI.
4.0. Discussion
In this relatively large study of 442 dementia-free participants, the majority of whom were cognitively normal, Aβ status moderated the association between MBI and tau-PET SUVR in the ROIs representing Braak I and Braak III stages of AD progression. In the Aβ+ stratum, when compared to the group with no NPS or NPS not meeting the MBI symptom persistence criterion, participants with MBI had higher tau tracer uptake in the Braak I and Braak III ROIs adjusted for age, sex, education, and cognitive diagnosis. In the Aβ− stratum, MBI was not significantly associated with tau uptake in the Braak I ROI and had a negative association with SUVR in Braak III. These findings suggest that AD and not minor age-related tauopathy (Bell et al., 2019) may be the main driver of MBI.
4.1. Braak Stages
Our representation of Braak I consisted of a weighted mean of the left and right entorhinal cortices only, normalized to the inferior cerebellar grey matter consistent with other ADNI studies (Yasuno et al., 2020). Tauopathies first become apparent within the entorhinal cortex during AD pathological progression (Cho et al., 2016), advancing to the hippocampus and then other temporal lobe structures. Entorhinal tau accumulation was consistent with our hypothesis. A post-mortem study presented compatible findings, with NPI-derived NPS such as anxiety, agitation, and depression associating with tau pathology and neurofibrillary tangles in Braak I and II regions (Ehrenberg et al., 2018). These findings suggest that both cognitive and behavioral manifestations of AD relate to p-tau neurofibrillary tangles in the entorhinal cortex, supporting the notion that NPS are core AD features. Due to technical constraints associated with off-target radiotracer binding, however, the hippocampal regions were not included in this study. The Johansson (2021) study, however, did find MBI associations with hippocampal tau SUVR. As Braak III is a stage of tau accumulation downstream from Braak stages I and II, our findings support MBI as a behavioral manifestation of AD that follows a classical progression of tau pathology (Braak et al., 2006; Davidson et al., 2018; Vogel et al., 2021).
4.2. Tau
Accumulation of Aβ pathology is considered the initial event in the AD pathological process, long before clinical symptoms (Jack Jr et al., 2019) with several MBI studies demonstrating MBI associations. Tau accrues temporally downstream from Aβ (Gordon and Tijms, 2019); abnormal tau-PET rarely occurs in the absence of abnormal Aβ-PET, with the tauopathy associating with clinical symptoms (Jack Jr et al., 2019). The tau protein stabilizes the microtubule network to ensure proper neuronal function and communication (Iqbal et al., 2010). In AD, tau becomes hyperphosphorylated, leading to production of neurofibrillary tangles, preventing normal cytoskeletal activities of microtubule associated proteins, which results in cognitive symptoms (Iqbal et al., 2010; Jack Jr et al., 2019). Post-mortem studies have determined that tau is the best predictor of global cognitive status, clinical dementia stage, functional abilities, and NPS (Malpas et al., 2020). In persons with dementia, or mixed samples including dementia, some studies have demonstrated associations between tau and NPS (Banning et al., 2020; Bloniecki et al., 2014; Koppel et al., 2013; Tommasi et al., 2021; Yasuno et al., 2020). In persons without dementia, some small to mid-size studies have found associations between tau and NPS (Ng et al., 2021). Tau has been linked with affective symptoms (Babulal et al., 2020; Gatchel et al., 2017; Yasuno et al., 2020), anxiety (Ramakers et al., 2013), and apathy (Binette et al., 2020; Johansson et al., 2022). However, there have also been a number of studies that failed to find associations between tau and overall NPS burden (Binette et al., 2020) or specific NPS domains including agitation, anxiety, apathy, depression, hyperactivity, irritability, and psychosis (Auning et al., 2015; Binette et al., 2020; Johansson et al., 2022; Ramakers et al., 2013; Yasuno et al., 2020). Interestingly, in one study of NC participants, no baseline associations between CSF p-tau181 and NPS were found, but p-tau was associated with an increase in NPS at 1 year (Babulal et al., 2016). These findings suggest that tau may be associated with incident or worsening NPS, consistent with the findings of our study.
Few studies have explored the relationship between tau and MBI specifically. BioFINDER2 assessed tau-PET [18F]RO948 retention and CSF P-tau181 levels in 50 NC Aβ positive participants, i.e., with preclinical AD or Stage 2 disease as per the NIA-AA framework (Knopman et al., 2018). The MBI-C was used to measure MBI symptoms. Regression models determined that MBI-C score was associated with higher tau-PET SUVR in a Braak I-II composite ROI (but not Braak III-IV or V-VI). MBI was also associated with significantly higher CSF p-tau. In this NC sample, MBI-C score but not memory deficits predicted PET and CSF-tau, emphasizing the importance of MBI in advance of objective cognitive changes (Johansson et al., 2021). Our findings are consistent, demonstrating an association between MBI and tau, in Aβ+ participants with preclinical (NC) and prodromal (MCI) AD. TRIAD assessed the relationship between tau-PET [18F]MK6240 and MBI, also measured with the MBI-C, in 96 NC participants and 91 with MCI. In the NC group, while there were significant positive correlations between MBI-C score and global and striatal Aβ-PET [18F]AZD4694 SUVR, no significant associations were found between MBI and Braak Stage I or II tau-PET binding (Lussier et al., 2020). From a preliminary analysis in the MCI group, however, MBI was associated with tau signal in early AD regions particularly the precuneus and posterior cingulate cortex bilaterally (Lussier et al., 2019). We speculate that there were insufficient Aβ+ positive participants in the TRIAD NC sample to drive a tau signal, but sufficient numbers in the MCI sample to find a MBI relationship with tau.
In contrast, a recent ADNI study showed an association between MBI symptoms and Aβ-PET AV45, but no association between MBI and tau-PET AV1451 (Sun et al., 2021). Of note, a single-visit approach was taken in this study, mapping NPS onto MBI criteria, but without consideration of symptom persistence. Symptom persistence is a core MBI criterion, associated with greater progression from MCI to dementia, and lower reversion from MCI to NC (McGirr, A. et al., 2022). In our study, a complementary approach was taken, requiring NPS persistence over two study visits for MBI+ status. Consistent with the signals in BioFINDER2 and TRIAD, individuals with MBI had significantly greater burden of tau pathology in regions affected early in the course of AD, compared to the group without MBI. This finding supports the need to incorporate natural history into assessment of NPS in older adults, even those without cognitive symptoms. Rather than a cross-sectional measure of NPS at one single time point, the foundation of the MBI framework lies in assessing the natural history of NPS. Symptom emergence in later in life is a fundamental feature to help differentiate MBI from chronic and recurring psychiatric conditions. Symptom persistence differentiates MBI from transient and reactive NPS, which are less likely to represent behavioral sequelae of neurodegenerative disease than MBI. Thus MBI improves specificity and signal-to-noise ratio for disease detection. A very recent study of MBI and plasma p-tau181 has further extended this clinical approach. In a sample of 571 dementia-free participants in ADNI, MBI was significantly associated with higher p-tau181 levels compared to groups with NPS not meeting MBI criteria, and with no NPS. Longitudinally, MBI was associated with increasing p-tau, declining memory and executive function, and a 3.92-fold greater incidence rate of dementia (Ghahremani, Maryam et al., 2023). Similarly, in a study of MCI participants from ADNI and MEMENTO, MBI was associated with higher CSF p-tau levels at baseline, and increasing CSF p-tau levels over 4 years compared to the group with NPS not meeting MBI criteria (Ismail et al., 2023b). This finding was consistent in the ADNI test cohort (observational cohort) and in the MEMENTO validation cohort (memory clinic patients). In sum, this small group of novel studies are consistent in finding that MBI is associated with greater Aβ-related tau burden, supporting the biological underpinnings of MBI, and its role in capturing early-stage AD.
In our study, it was also important to resolve the role of Aβ status on the relationship between MBI and tau, given the conflicting findings in the Lussier and Johansson studies (Johansson et al., 2021; Lussier et al., 2020). MBI describes an elevated AD risk state and Aβ indicates the beginning of significant disease burden, usually preceding tau pathology (Gordon and Tijms, 2019). Thus, our findings that those with both Aβ and MBI positivity showed the greatest tau burden was unsurprising. Exploration of the Aβ negative stratum, however, was particularly informative. In both the Braak I and III ROIs, MBI positivity was negatively associated with tau-PET SUVR, although this effect was only statistically significant in Braak III regions. While these results might appear counterintuitive at first glance, they support the link between MBI and core early AD pathology, i.e., Aβ, without which there is little tau (Jack Jr et al., 2019). AV1451 binds paired helical filament tau, which is a combination of 3R and 4R tau. In contrast, for Aβ negative participants with MBI, other pathology may be involved, including vascular pathology, alpha synuclein, TDP-43, and non-AD tauopathies, e.g., straight or twisted non-helical tau, or just 3R or 4R tau, rather than the combination (Gibson et al., 2022; Mattsson et al., 2019; Miao et al., 2021b; Tsai et al., 2019). Future studies in Aβ negative participants exploring associations between MBI and other pathologies will help clarify this issue as well as explore MBI specifically in non-AD populations, once technology progresses enough to reasonably allow incorporation of fluid biomarkers and imaging tracers that bind these other pathological proteins.
Nonetheless, our study findings suggest that this MBI approach to NPS case assessment can help identify older adults, with at most MCI, at the earliest phase of cortical tauopathy due to AD. The results also lend credence to longitudinal studies finding that MBI is associated with faster cognitive decline (Creese et al., 2019), and a greater risk of developing AD (Gill et al., 2020). In a recent clinicopathological study of NACC participants, MBI in cognitively normal older adults was associated with a higher hazard of both clinically-diagnosed, and neuropathologically-confirmed AD over the next 5 years (Ruthirakuhan et al., 2022). More recently, in a functional MRI study, MBI was associated with connectivity changes in the Default Mode and Salience Networks typically seen in early-stage AD (Ghahremani, M. et al., 2023). These findings suggest a neurobiological basis of behavioral and psychological symptoms in this dementia-free sample of older adults, specifically suggesting an association to MAP systems and cytoskeletal components of neurons in varying regions of the brain (Dehmelt and Halpain, 2004). Another study suggested that cytoskeletal components such as neurofilament light were important in the presenting symptoms of MBI, and especially the emergence of those MBI symptoms, adding further credence to this approach (Naude et al., 2020).
Assessment of MBI is an effective approach that can be used in conjunction with cognitive status to identify individuals at greater risk of cognitive decline. With tau therapeutic agents on the horizon (Congdon et al., 2023) these findings may be of particular interest. The emergence and persistence of MBI symptoms might serve as a proxy marker of entorhinal tau pathology, which could be considered in clinical screening and for clinical trials (Mortby, M.E et al., 2018). For example, in dementia-free older adults, assessment of MBI could be scaled up and implemented remotely (Creese et al., 2020; Kassam et al., 2023), to flag a group for further clinical and biomarker workup. This initial remote pre-screen with an informant-rated measure of MBI could increase efficiency for preclinical or prodromal case detection and reduce costs in clinical trials due to a reduction in screen failures. Further, MBI may serve as a treatment target (Soto et al., 2023), to either reduce symptom burden, or as part of a multidimensional collection of symptoms identified in the meaningful benefits framework, to evaluate effects of disease-modifying therapies (Assunção et al., 2022).
4.3. Limitations
Despite the novelty of the study and the substantial sample size, several limitations are important to consider. The AV1451 radiotracer limited assessment of Braak Stage II due to offsite choroid plexus binding (Lee et al., 2018). Future studies examining MBI and tauopathy will need to establish whether there exist any relationships within this region. Braak III was represented as a meta-ROI consisting of several regions, which might result signal loss in some regions within the composite. However, due to multicollinearity, we did not assess individual regions separately. The gold standard for MBI case ascertainment is the MBI-C, which is not utilized in ADNI. Although we approximated MBI status, transforming NPI items using a validated algorithm, this approach may not be as sensitive or specific in capturing MBI (Hu et al., 2023; Mallo et al., 2018; Mallo et al., 2019). For example, the persistent NPS group may have had emergence earlier in ADNI, before tau PET was implemented, or may have longstanding subsyndromal psychiatric symptoms, not screened out at study entry. Utilizing the MBI-C in future studies may resolve these issues and provide more clarity. Additionally, inβ A PET studies of neurodegenerative disease, lack of stratification by apolipoprotein 4 (APOE4) status has been cited as a potential contributor to inconclusive findings (Oliveira, 2023). This logic likely applies to tau-PET studies as well. Unfortunately, APOE4 data were not available for all participants; using this reduced sample would have resulted in a significant loss of statistical power for the interaction analyses. Nonetheless, addressing APOE4 effects is an important issue for studies of cognition, behavior, and function, and future studies should incorporate APOE as a covariate in the analysis. Furthermore, we were unable to assess longitudinal associations between MBI and tau tangle burden, as the implementation of tau-PET in ADNI is a relatively recent advance with insufficient sample size for analysis. By assessing these trajectories in the future, we can elucidate the differing rates of tau accumulation between those who meet MBI criteria and those who do not.
4.4. Conclusions
In this sample of dementia-free participants, the majority of whom were cognitively normal, MBI was associated with greater tau neuropathological burden in early cortical regions affected in AD, in Aβ positive participants. The findings support the biological understanding of NPS as part of the AD process, detectable at preclinical and prodromal stages. This study adds further support to the relevance of the MBI construct for dementia detection and prognostication. Future studies can explore MBI as an approach to clinical trial enrichment, to improve screening efficiency for Aβ and/or tau positivity, and to decrease study cost and duration for interventions targeting these proteins.
5.0. Acknowledgements
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (http://adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12–2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions of several others.
Z.I. is funded by the Canadian Institutes of Health BCA2633. This study was also supported by the UK National Institute for Health and Care Research Exeter Biomedical Research Centre. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Footnotes
Disclosures
Z.I. has served as a consultant/advisor for Eisai, Eli Lilly, Otsuka/Lundbeck, Novo Nordisk and Roche. E.E.S. has served as a consultant/advisor for Eli Lilly. All other authors report no conflicts of interest.
References
- Andrews S.J., Ismail Z., Anstey K.J., Mortby M., 2018. Association of Alzheimer’s genetic loci with mild behavioral impairment. Am. J. Med. Genet. B Neuropsychiatr. Genet. 177(8), 727–735. [DOI] [PubMed] [Google Scholar]
- Association, A.s., 2020. 2020. Alzheimer’s disease facts and figures. Alzheimers Dement. 16, 391–460. 10.1002/ajmg.b.32684 [DOI] [PubMed] [Google Scholar]
- Assunção S.S., Sperling R.A., Ritchie C., Kerwin D.R., Aisen P.S., Lansdall C., Atri A., Cummings J., 2022. Meaningful benefits: a framework to assess disease-modifying therapies in preclinical and early Alzheimer’s disease. Alzheimers Res. Ther. 14(1), 1–16. 10.1186/s13195-022-00984-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auning E., Selnes P., Grambaite R., Šaltytė Benth J., Haram A., Løvli Stav A., Bjørnerud A., Hessen E., Hol P., Muftuler løndalen A., 2015. Neurobiological correlates of depressive symptoms in people with subjective and mild cognitive impairment. Acta Psychiatr. Scand. 131(2), 139–147. 10.1111/acps.12352 [DOI] [PubMed] [Google Scholar]
- Babulal G.M., Ghoshal N., Head D., Vernon E.K., Holtzman D.M., Benzinger T.L., Fagan A.M., Morris J.C., Roe C.M., 2016. Mood Changes in Cognitively Normal Older Adults are Linked to Alzheimer Disease Biomarker Levels. The American Journal of Geriatric Psychiatry 24(11), 1095–1104. 10.1016/j.jagp.2016.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babulal G.M., Roe C.M., Stout S.H., Rajasekar G., Wisch J.K., Benzinger T.L., Morris J.C., Ances B.M., 2020. Depression is Associated with Tau and Not Amyloid Positron Emission Tomography in Cognitively Normal Adults. J. Alzheimers Dis. 74(4), 1045–1055. 10.3233/jad-191078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balogun W.G., Zetterberg H., Blennow K., Karikari T.K., 2023. Plasma biomarkers for neurodegenerative disorders: ready for prime time? Current Opinion in Psychiatry, 10.1097. 10.1097/yco.0000000000000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banning L.C., Ramakers I.H., Köhler S., Bron E.E., Verhey F.R., de Deyn P.P., Claassen J.A., Koek H.L., Middelkoop H.A., van der Flier W.M., 2020. The association between biomarkers and neuropsychiatric symptoms across the Alzheimer’s disease spectrum. The American Journal of Geriatric Psychiatry. 10.1016/j.jagp.2020.01.012 [DOI] [PubMed] [Google Scholar]
- Bateman D.R., Gill S., Hu S., Foster E.D., Ruthirakuhan M.T., Sellek A.F., Mortby M.E., Matušková V., Ng K.P., Tarawneh R.M., 2020. Agitation and impulsivity in mid and late life as possible risk markers for incident dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 6(1), e12016. 10.1002/Ftrc2.12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell W.R., An Y., Kageyama Y., English C., Rudow G.L., Pletnikova O., Thambisetty M., O’Brien R., Moghekar A.R., Albert M.S., 2019. Neuropathologic, genetic, and longitudinal cognitive profiles in primary age-related tauopathy (PART) and Alzheimer’s disease. Alzheimer’s & Dementia 15(1), 8–16. 10.1016/j.jalz.2018.07.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binette A.P., Vachon-Presseau É., Morris J., Bateman R., Benzinger T., Collins D.L., Poirier J., Breitner J.C., Villeneuve S., Network D.I.A., 2020. Amyloid and tau pathology associations with personality traits, neuropsychiatric symptoms and cognitive lifestyle in the preclinical phases of sporadic and autosomal dominant Alzheimer’s disease. Biol. Psychiatry. 10.1016/j.biopsych.2020.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloniecki V., Aarsland D., Cummings J., Blennow K., Freund-Levi Y., 2014. Agitation in dementia: relation to core cerebrospinal fluid biomarker levels. Dement. Geriatr. Cogn. Dis. Extra 4(2), 335–343. 10.1159/F000363500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Del Tredici K., 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112(4), 389–404. 10.1007/s00401-006-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E., 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82(4), 239–259. 10.1007/bf00308809 [DOI] [PubMed] [Google Scholar]
- Burhanullah M.H., Tschanz J.T., Peters M.E., Leoutsakos J.-M., Matyi J., Lyketsos C.G., Nowrangi M.A., Rosenberg P.B., 2019. Neuropsychiatric Symptoms as Risk Factors for Cognitive Decline in Clinically Normal Older Adults: The Cache County Study. The American Journal of Geriatric Psychiatry. 10.1016/j.jagp.2019.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H., Choi J.Y., Hwang M.S., Kim Y.J., Lee H.M., Lee H.S., Lee J.H., Ryu Y.H., Lee M.S., Lyoo C.H., 2016. In vivo cortical spreading pattern of tau and amyloid in the Alzheimer disease spectrum. Ann. Neurol. 80(2), 247–258. 10.1002/ana.24711 [DOI] [PubMed] [Google Scholar]
- Cieslak A., Smith E.E., Lysack J., Ismail Z., 2018. Case series of mild behavioral impairment: toward an understanding of the early stages of neurodegenerative diseases affecting behavior and cognition. Int. Psychogeriatr. 30(2), 273–280. 10.1017/s1041610217001855 [DOI] [PubMed] [Google Scholar]
- Congdon E.E., Ji C., Tetlow A.M., Jiang Y., Sigurdsson E.M., 2023. Tau-targeting therapies for Alzheimer disease: current status and future directions. Nature Reviews Neurology, 1–22. 10.1038/s41582-023-00883-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese B., Arathimos R., Aarsland D., Ballard C., Brooker H., Hampshire A., Corbett A., Ismail Z., 2023. Late-life onset psychotic symptoms and incident cognitive impairment in people without dementia: Modification by genetic risk for Alzheimer’s disease. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 9(2), e12386. 10.1002/Ftrc2.12386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese B., Arathimos R., Brooker H., Aarsland D., Corbett A., Lewis C., Ballard C., Ismail Z., 2021. Genetic risk for Alzheimer’s disease, cognition, and mild behavioral impairment in healthy older adults. Alzheimers Dement (Amst) 13(1), e12164. 10.1002/Fdad2.12164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese B., Brooker H., Ismail Z., Wesnes K.A., Hampshire A., Khan Z., Megalogeni M., Corbett A., Aarsland D., Ballard C., 2019. Mild Behavioral Impairment as a Marker of Cognitive Decline in Cognitively Normal Older Adults. Am. J. Geriatr. Psychiatry 27(8), 823–834. 10.1016/j.jagp.2019.01.215 [DOI] [PubMed] [Google Scholar]
- Creese B., Griffiths A., Brooker H., Corbett A., Aarsland D., Ballard C., Ismail Z., 2020. Profile of mild behavioral impairment and factor structure of the Mild Behavioral Impairment Checklist in cognitively normal older adults. Int. Psychogeriatr. 32(6), 705–717. 10.1017/s1041610219001200 [DOI] [PubMed] [Google Scholar]
- Cummings J., 2020. The Neuropsychiatric Inventory: Development and Applications. J. Geriatr. Psychiatry Neurol. 33(2), 73–84. 10.1177/0891988719882102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson Y.S., Robinson A., Prasher V.P., Mann D.M.A., 2018. The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer’s disease in individuals with Down syndrome. Acta Neuropathologica Communications 6(1), 56. 10.1186/s40478-018-0559-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmelt L., Halpain S., 2004. The MAP2/Tau family of microtubule-associated proteins. Genome Biology 6(1), 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim I.M., Ghahremani M., Camicioli R., Smith E.E., Ismail Z., 2023. Effects of race, baseline cognition, and APOE on the association of affective dysregulation with incident dementia: A longitudinal study of dementia-free older adults. J. Affect. Disord. 332, 9–18. 10.1016/j.jad.2023.03.074 [DOI] [PubMed] [Google Scholar]
- Ehrenberg A.J., Suemoto C.K., Franca Resende E.P., Petersen C., Leite R.E.P., Rodriguez R.D., Ferretti-Rebustini R.E.L., You M., Oh J., Nitrini R., Pasqualucci C.A., Jacob-Filho W., Kramer J.H., Gatchel J.R., Grinberg L.T., 2018. Neuropathologic Correlates of Psychiatric Symptoms in Alzheimer’s Disease. J Alzheimers Dis 66(1), 115–126. 10.3233/jad-180688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatchel J.R., Donovan N.J., Locascio J.J., Schultz A.P., Becker J.A., Chhatwal J., Papp K.V., Amariglio R.E., Rentz D.M., Blacker D., 2017. Depressive symptoms and tau accumulation in the inferior temporal lobe and entorhinal cortex in cognitively normal older adults: a pilot study. J. Alzheimers Dis. 59(3), 975–985. 10.3233/jad-170001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier S., Albert M., Fox N., Goedert M., Kivipelto M., Mestre-Ferrandiz J., Middleton L.T., 2016. Why has therapy development for dementia failed in the last two decades? Alzheimer’s & Dementia 12(1), 60–64. 10.1016/j.jalz.2015.12.003 [DOI] [PubMed] [Google Scholar]
- Geda Y.E., Roberts R.O., Mielke M.M., Knopman D.S., Christianson T.J., Pankratz V.S., Boeve B.F., Sochor O., Tangalos E.G., Petersen R.C., Rocca W.A., 2014. Baseline neuropsychiatric symptoms and the risk of incident mild cognitive impairment: a population-based study. Am. J. Psychiatry 171(5), 572–581. 10.1176/appi.ajp.2014.13060821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani M., Nathan S., Smith E.E., McGirr A., Goodyear B., Ismail Z., 2023. Functional connectivity and mild behavioral impairment in dementia-free elderly. Alzheimers Dement (N Y) 9(1), e12371. 10.1002/trc2.12371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghahremani M., Wang M., Chen H.-Y., Zetterberg H., Smith E., Ismail Z., Initiative, A.s.D.N., 2023. Plasma Phosphorylated Tau at Threonine 181 and Neuropsychiatric Symptoms in Preclinical and Prodromal Alzheimer Disease. Neurology 100(7), e683–e693. 10.1212/wnl.0000000000201517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson L.L., Aarsland D., Suemoto C.K., 2022. The importance of co-pathologies on neuropsychiatric symptoms in dementia. Aging 14(23), 9384–9385. 10.18632/aging.204430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Mouches P., Hu S., Rajashekar D., MacMaster F.P., Smith E.E., Forkert N.D., Ismail Z., Initiative, A.s.D.N., 2020. Using Machine Learning to Predict Dementia from Neuropsychiatric Symptom and Neuroimaging Data. J. Alzheimers Dis. 75(1), 277–288. 10.3233/jad-191169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Wang M., Mouches P., Rajashekar D., Sajobi T., MacMaster F.P., Smith E.E., Forkert N.D., Ismail Z., Alzheimer’s Disease Neuroimaging, I., 2021. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int. J. Geriatr. Psychiatry 36(9), 1398–1406. 10.1002/gps.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S., Wang M., Mouches P., Rajashekar D., Sajobi T., MacMaster F.P., Smith E.E., Forkert N.D., Ismail Z., Initiative, A.s.D.N, 2021. Neural correlates of the impulse dyscontrol domain of mild behavioral impairment. Int. J. Geriatr. Psychiatry 36(9), 1398–1406. 10.1002/gps.5540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B.A., Tijms B.M., 2019. Elevated tau PET signal depends on abnormal amyloid levels and is uncommon in unimpaired individuals. Brain 142(10), 2903–2904. 10.3233/jad-200526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan D.X., Smith E.E., Pike G.B., Ismail Z., 2023. Persistence of neuropsychiatric symptoms and dementia prognostication: A comparison of three operational case definitions of mild behavioral impairment. Alzheimers Dement (Amst) 15(4), e12483. 10.1002/dad2.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy S.M., 2023. Lecanemab: first approval. Drugs 83(4), 359–365. [DOI] [PubMed] [Google Scholar]
- Hu S., Patten S., Charlton A., Fischer K., Fick G., Smith E.E., Ismail Z., 2023. Validating the Mild Behavioral Impairment Checklist in a Cognitive Clinic: Comparisons With the Neuropsychiatric Inventory Questionnaire. J. Geriatr. Psychiatry Neurol. 36(2), 107–120. 10.1177/08919887221093353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal K., Liu F., Gong C.X., Grundke-Iqbal I., 2010. Tau in Alzheimer disease and related tauopathies. Curr Alzheimer Res 7(8), 656–664. 10.2174/156720510793611592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z., Agüera-Ortiz L., Brodaty H., Cieslak A., Cummings J., Fischer C.E., Gauthier S., Geda Y.E., Herrmann N., Kanji J., 2017. The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J. Alzheimers Dis. 56(3), 929–938. 10.3233/jad-160979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z., Ghahremani M., Amlish Munir M., Fischer C.E., Smith E.E., Creese B., 2023a. A longitudinal study of late-life psychosis and incident dementia and the potential effects of race and cognition. Nature Mental Health 1(4), 273–283. 10.1038/s44220-023-00043-x [DOI] [Google Scholar]
- Ismail Z., Leon R., Creese B., Ballard C., Robert P., Smith E.E., 2023b. Optimizing detection of Alzheimer’s disease in mild cognitive impairment: a 4-year biomarker study of mild behavioral impairment in ADNI and MEMENTO. Mol. Neurodegener. 18(1), 50. 10.1186/s13024-023-00631-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z., McGirr A., Gill S., Hu S., Forkert N.D., Smith E.E., 2021. Mild Behavioral Impairment and Subjective Cognitive Decline Predict Cognitive and Functional Decline. J. Alzheimers Dis. 80(1), 459–469. 10.3233/jad-201184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail Z., Smith E.E., Geda Y., Sultzer D., Brodaty H., Smith G., Agüera-Ortiz L., Sweet R., Miller D., Lyketsos C.G., 2016. Neuropsychiatric symptoms as early manifestations of emergent dementia: Provisional diagnostic criteria for mild behavioral impairment. Alzheimer’s & Dementia 12(2), 195–202. 10.1016/Fj.jalz.2015.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R. Jr., Wiste H.J., Schwarz C.G., Lowe V.J., Senjem M.L., Vemuri P., Weigand S.D., Therneau T.M., Knopman D.S., Gunter J.L., Jones D.T., Graff-Radford J., Kantarci K., Roberts R.O., Mielke M.M., Machulda M.M., Petersen R.C., 2018. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain 141(5), 1517–1528. 10.1093/Fbrain/Fawaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack C.R. Jr, Wiste H.J., Botha H., Weigand S.D., Therneau T.M., Knopman D.S., Graff-Radford J., Jones D.T., Ferman T.J., Boeve B.F., 2019. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain 142(10), 3230–3242. 10.1093/brain/awz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Stomrud E., Insel P.S., Leuzy A., Johansson P.M., Smith R., Ismail Z., Janelidze S., Palmqvist S., van Westen D., Mattsson-Carlgren N., Hansson O., 2021. Mild behavioral impairment and its relation to tau pathology in preclinical Alzheimer’s disease. Transl. Psychiatry 11(1), 76. 10.1038/s41398-021-01206-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Stomrud E., Johansson P.M., Svenningsson A., Palmqvist S., Janelidze S., van Westen D., Mattsson-Carlgren N., Hansson O., 2022. Development of apathy, anxiety, and depression in cognitively unimpaired older adults: effects of Alzheimer’s Disease pathology and cognitive decline. Biol. Psychiatry 92(1), 34–43. 10.1016/j.biopsych.2022.01.012 [DOI] [PubMed] [Google Scholar]
- Kan C.N., Cano J., Zhao X., Ismail Z., Chen C.L., Xu X., 2022. Prevalence, Clinical Correlates, Cognitive Trajectories, and Dementia Risk Associated With Mild Behavioral Impairment in Asians. J. Clin. Psychiatry 83(3), 40123. 10.4088/jcp.21m14105 [DOI] [PubMed] [Google Scholar]
- Kassam F., Chen H., Nosheny R.L., McGirr A., Williams T., Ng N., Camacho M., Mackin R.S., Weiner M.W., Ismail Z., 2023. Cognitive profile of people with mild behavioral impairment in Brain Health Registry participants. Int. Psychogeriatr. 35(11), 643–652. 10.1017/s1041610221002878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman D.S., Haeberlein S.B., Carrillo M.C., Hendrix J.A., Kerchner G., Margolin R., Maruff P., Miller D.S., Tong G., Tome M.B., 2018. The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: perspectives from the research roundtable. Alzheimer’s & Dementia 14(4), 563–575. 10.1016/Fj.jalz.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppel J., Sunday S., Buthorn J., Goldberg T., Davies P., Greenwald B., Initiative, A.s.D.N., 2013. Elevated CSF Tau is associated with psychosis in Alzheimer’s disease. Am. J. Psychiatry 170(10), 1212–1213. 10.1176/appi.ajp.2013.13040466 [DOI] [PubMed] [Google Scholar]
- Lanctôt K.L., Amatniek J., Ancoli-Israel S., Arnold S.E., Ballard C., Cohen-Mansfield J., Ismail Z., Lyketsos C., Miller D.S., Musiek E., Osorio R.S., Rosenberg P.B., Satlin A., Steffens D., Tariot P., Bain L.J., Carrillo M.C., Hendrix J.A., Jurgens H., Boot B., 2017. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimer’s & Dementia: Translational Research & Clinical Interventions 3, 440–449. 10.1016/j.trci.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau S.M., Mintun M.A., Joshi A.D., Koeppe R.A., Petersen R.C., Aisen P.S., Weiner M.W., Jagust W.J., Initiative, A.s.D.N., 2012. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol. 72(4), 578–586. 10.1002/ana.23650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.M., Jacobs H.I., Marquié M., Becker J.A., Andrea N.V., Jin D.S., Schultz A.P., Frosch M.P., Gomez-Isla T., Sperling R.A., 2018. 18F-flortaucipir binding in choroid plexus: related to race and hippocampus signal. J. Alzheimers Dis. 62(4), 1691–1702. 10.3233/jad-170840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew T.M., 2020. Neuropsychiatric symptoms in cognitively normal older persons, and the association with Alzheimer’s and non-Alzheimer’s dementia. Alzheimers Res. Ther. 12(1), 35. 10.1186/s13195-020-00604-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lussier F., Pascoal T., Therriault J., Chamoun M., Tissot C., Savard M., Mathotaarachchi S., Ismail Z., Rosa-Neto P., Gauthier S., 2019. Mild behavioral impairment is associated with beta-amyloid and tau across the alzheimer’s disease spectrum. J. Cereb. Blood Flow Metab. 39, 158–159. [Google Scholar]
- Lussier F.Z., Pascoal T.A., Chamoun M., Therriault J., Tissot C., Savard M., Kang M.S., Mathotaarachchi S., Benedet A.L., Parsons M., Qureshi M.N.I., Thomas E.M., Shin M., Dion L.A., Massarweh G., Soucy J.P., Tsai I.H., Vitali P., Ismail Z., Rosa-Neto P., Gauthier S., 2020. Mild behavioral impairment is associated with beta-amyloid but not tau or neurodegeneration in cognitively intact elderly individuals. Alzheimers Dement. 16(1), 192–199. 10.1002/alz.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallo S.C., Ismail Z., Pereiro A.X., Facal D., Lojo-Seoane C., Campos-Magdaleno M., Juncos-Rabadán O., 2018. Assessing mild behavioral impairment with the Mild behavioral impairment-checklist in people with mild cognitive impairment. J. Alzheimers Dis. 66(1), 83–95. 10.3233/jad-180131 [DOI] [PubMed] [Google Scholar]
- Mallo S.C., Ismail Z., Pereiro A.X., Facal D., Lojo-Seoane C., Campos-Magdaleno M., Juncos-Rabadán O., 2019. Assessing mild behavioral impairment with the mild behavioral impairment checklist in people with subjective cognitive decline. Int. Psychogeriatr. 31(2), 231–239. 10.1017/s1041610218000698 [DOI] [PubMed] [Google Scholar]
- Malpas C.B., Sharmin S., Kalincik T., 2020. The histopathological staging of tau, but not amyloid, corresponds to antemortem cognitive status, dementia stage, functional abilities and neuropsychiatric symptoms. Int. J. Neurosci., 1–10. 10.1080/00207454.2020.1758087 [DOI] [PubMed] [Google Scholar]
- Martin E., Velayudhan L., 2020. Neuropsychiatric Symptoms in Mild Cognitive Impairment: A Literature Review. Dement. Geriatr. Cogn. Disord. 49(2), 146–155. 10.1159/000507078 [DOI] [PubMed] [Google Scholar]
- Matsuoka T., Imai A., Narumoto J., 2023. Neuroimaging of mild behavioral impairment: A systematic review. Psychiatry and Clinical Neurosciences Reports 2(1), e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T., Ismail Z., Narumoto J., 2019. Prevalence of mild behavioral impairment and risk of dementia in a psychiatric outpatient clinic. J. Alzheimers Dis. 70(2), 505–513. 10.3233/jad-190278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka T., Ueno D., Ismail Z., Rubinstein E., Uchida H., Mimura M., Narumoto J., 2021. Neural Correlates of Mild Behavioral Impairment: A Functional Brain Connectivity Study Using Resting-State Functional Magnetic Resonance Imaging. J. Alzheimers Dis. 83(3), 1221–1231. 10.3233/jad-210628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N., Insel P.S., Donohue M., Jögi J., Ossenkoppele R., Olsson T., Schöll M., Smith R., Hansson O., 2019. Predicting diagnosis and cognition with 18F-AV-1451 tau PET and structural MRI in Alzheimer’s disease. Alzheimer’s & Dementia 15(4), 570–580. 10.1016/j.jalz.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Matuskova V., Ismail Z., Nikolai T., Markova H., Cechova K., Nedelska Z., Laczo J., Wang M., Hort J., Vyhnalek M., 2021. Mild behavioral impairment is associated with atrophy of entorhinal cortex and hippocampus in a memory clinic cohort. Frontiers in Aging Neuroscience 13, 236. 10.3389/fnagi.2021.643271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGirr A., Nathan S., Ghahremani M., Gill S., Smith E., Ismail Z., 2022. Progression to Dementia or Reversion to Normal Cognition in Mild Cognitive Impairment as a Function of Late Onset Neuropsychiatric Symptoms. Neurology 98(21), e2132–2139. 10.1212/wnl.0000000000200256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao R., Chen H.-Y., Gill S., Naude J., Smith E.E., Ismail Z., 2021a. Plasma β-Amyloid in Mild Behavioural Impairment – Neuropsychiatric Symptoms on the Alzheimer’s Continuum. J. Geriatr. Psychiatry Neurol., 08919887211016068. 10.1177/08919887211016068 [DOI] [PubMed] [Google Scholar]
- Miao R., Chen H.-Y., Robert P., Smith E.E., Ismail Z., Group M.S., 2021b. White matter hyperintensities and mild behavioral impairment: Findings from the MEMENTO cohort study. Cerebral Circulation-Cognition and Behavior 2, 100028. 10.1016/j.cccb.2021.100028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortby M.E., Black S.E., Gauthier S., Miller D.S., Porsteinsson A., Smith E.E., Ismail Z., 2018. Dementia clinical trial implications of Mild Behavioral Impairment. Int. Psychogeriatr. 30(2), 171–175. 10.1017/s1041610218000042 [DOI] [PubMed] [Google Scholar]
- Mortby M.E., Burns R., Eramudugolla R., Ismail Z., Anstey K.J., 2017. Neuropsychiatric Symptoms and Cognitive Impairment: Understanding the Importance of Co-Morbid Symptoms. J. Alzheimers Dis. 59(1), 141–153. 10.3233/jad-170050 [DOI] [PubMed] [Google Scholar]
- Mortby M.E., Ismail Z., Anstey K.J., 2018. Prevalence estimates of mild behavioral impairment in a population-based sample of pre-dementia states and cognitively healthy older adults. Int. Psychogeriatr. 30(2), 221–232. 10.1017/s1041610217001909 [DOI] [PubMed] [Google Scholar]
- Naude J., Gill S., Hu S., McGirr A., Forkert N., Monchi O., Stys P., Smith E.E., Ismail Z., 2020. Plasma Neurofilament Light: a marker of cognitive decline in Mild Behavioural Impairment. J. Alzheimers Dis. 76(3), 1017–1027. 10.3233/jad-200011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K.P., Chiew H., Rosa-Neto P., Kandiah N., Ismail Z., Gauthier S., 2021. Associations of AT (N) biomarkers with neuropsychiatric symptoms in preclinical Alzheimer’s disease and cognitively unimpaired individuals. Translational Neurodegeneration 10(1), 1–14. 10.1186/s40035-021-00236-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira F.F.d., 2023. Looking behind the curtain: patient stratification according to genetic or demographic factors may yield unexpected results in studies of neurodegenerative diseases. J. Alzheimers Dis.(Preprint), 1–4. 10.3233/jad-230561 [DOI] [PubMed] [Google Scholar]
- Ossenkoppele R., Rabinovici G.D., Smith R., Cho H., Schöll M., Strandberg O., Palmqvist S., Mattsson N., Janelidze S., Santillo A., Ohlsson T., Jögi J., Tsai R., La Joie R., Kramer J., Boxer A.L., Gorno-Tempini M.L., Miller B.L., Choi J.Y., Ryu Y.H., Lyoo C.H., Hansson O., 2018. Discriminative Accuracy of [18F]flortaucipir Positron Emission Tomography for Alzheimer Disease vs Other Neurodegenerative Disorders. Jama 320(11), 1151–1162. 10.1001/jama.2018.12917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers I., Verhey F., Scheltens P., Hampel H., Soininen H., Aalten P., Rikkert M.O., Verbeek M., Spiru L., Blennow K., 2013. Anxiety is related to Alzheimer cerebrospinal fluid markers in subjects with mild cognitive impairment. Psychol. Med. 43(5), 911–920. 10.1017/s0033291712001870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse H.J., Ismail Z., Andel R., Molinari V.A., Schinka J.A., Small B.J., 2023. Impact of Mild Behavioral Impairment on Longitudinal Changes in Cognition. The Journals of Gerontology: Series A, glad098. 10.1093/gerona/glad098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthirakuhan M., Ismail Z., Herrmann N., Gallagher D., Lanctot K.L., 2022. Mild behavioral impairment is associated with progression to Alzheimer’s disease: A clinicopathological study. Alzheimers Dement. in press. 10.1002/alz.12519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikh F., Ismail Z., Mortby M.E., Barber P., Cieslak A., Fischer K., Granger R., Hogan D.B., Mackie A., Maxwell C.J., Menon B., Mueller P., Patry D., Pearson D., Quickfall J., Sajobi T., Tse E., Wang M., Smith E.E., 2018. Prevalence of mild behavioral impairment in mild cognitive impairment and subjective cognitive decline, and its association with caregiver burden. Int. Psychogeriatr. 30(2), 233–244. 10.1017/s104161021700151x [DOI] [PubMed] [Google Scholar]
- Soto M., Rosenberg P., Ballard C., Vellas B., Miller D., Gauthier S., Carrillo M., Lyketsos C., Ismail Z., Abushakra S., 2024. Neuropsychiatric Symptoms in AD: Clinical Trials Targeting Mild Behavioral Impairment: A Report from the International CTAD Task Force. The Journal of Prevention of Alzheimer’s Disease. 11, 56–64. 10.14283/jpad.2023.125 [DOI] [PubMed] [Google Scholar]
- Sun Y., Xu W., Chen K.-L., Shen X.-N., Tan L., Yu J.-T., 2021. Mild behavioral impairment correlates of cognitive impairments in older adults without dementia: mediation by amyloid pathology. Transl. Psychiatry 11(1), 1–8. 10.1038/Fs41398-021-01675-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taragano F.E., Allegri R.F., Heisecke S.L., Martelli M.I., Feldman M.L., Sánchez V., García V.A., Tufro G., Castro D.M., Leguizamón P.P., Guelar V., Ruotolo E., Zegarra C., Dillon C., 2018. Risk of Conversion to Dementia in a Mild Behavioral Impairment Group Compared to a Psychiatric Group and to a Mild Cognitive Impairment Group. J. Alzheimers Dis. 62, 227–238. 10.3233/jad-170632 [DOI] [PubMed] [Google Scholar]
- Tommasi N.S., Gonzalez C., Briggs D., Properzi M.J., Gatchel J.R., Marshall G.A., Initiative, A.s.D.N., 2021. Affective symptoms and regional cerebral tau burden in early-stage Alzheimer’s disease. Int. J. Geriatr. Psychiatry 36(7), 1050–1058. 10.1002/gps.5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai R.M., Bejanin A., Lesman-Segev O., LaJoie R., Visani A., Bourakova V., O’Neil J.P., Janabi M., Baker S., Lee S.E., 2019. 18 F-flortaucipir (AV-1451) tau PET in frontotemporal dementia syndromes. Alzheimers Res. Ther. 11, 1–18. 10.1186/s13195-019-0470-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vellone D., Ghahremani M., Goodarzi Z., Forkert N.D., Smith E.E. and Ismail Z., 2022. Apathy and APOE in mild behavioral impairment, and risk for incident dementia. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 8(1), p.e12370. 10.1002/trc2.12370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel J.W., Young A.L., Oxtoby N.P., Smith R., Ossenkoppele R., Strandberg O.T., La Joie R., Aksman L.M., Grothe M.J., Iturria-Medina Y., 2021. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 27(5), 871–881. 10.1038/s41591-021-01309-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E.A., Rosenberg P.B., Lyketsos C.G., Leoutsakos J.-M., 2019. Time course of neuropsychiatric symptoms and cognitive diagnosis in National Alzheimer’s Coordinating Centers volunteers. Alzheimers Dement (Amst) 11, 333–339. 10.1016/j.dadm.2019.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno F., Minami H., Hattori H., Initiative, A.s.D.N., 2020. Relationship between neuropsychiatric symptoms and Alzheimer’s disease pathology: An in vivo positron emission tomography study. Int. J. Geriatr. Psychiatry. 10.1002/gps.5459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon E.J., Lee J.-Y., Kwak S., Kim Y.K., 2022. Mild behavioral impairment linked to progression to Alzheimer’s disease and cortical thinning in amnestic mild cognitive impairment. Frontiers in Aging Neuroscience 14. 10.3389/fnagi.2022.1051621 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this study are available from adni.loni.usc.edu for download.