Abstract
The yeast protein Rbl2p suppresses the deleterious effects of excess β-tubulin as efficiently as does α-tubulin. Both in vivo and in vitro, Rbl2p forms a complex with β-tubulin that does not contain α-tubulin, thus defining a second pool of β-tubulin in the cell. Formation of the complex depends upon the conformation of β-tubulin. Newly synthesized β-tubulin can bind to Rbl2p before it binds to α-tubulin. Rbl2p can also bind β-tubulin from the α/β-tubulin heterodimer, apparently by competing with α-tubulin. The Rbl2p–β-tubulin complex has a half-life of ∼2.5 h and is less stable than the α/β-tubulin heterodimer. The results of our experiments explain both how excess Rbl2p can rescue cells overexpressing β-tubulin and how it can be deleterious in a wild-type background. They also suggest that the Rbl2p–β-tubulin complex is part of a cellular mechanism for regulating the levels and dimerization of tubulin chains.
Much of the work on microtubules has focused on the assembly reaction from α/β-tubulin heterodimer to polymer. This reaction is well characterized in vitro, and genetic and pharmacological studies demonstrate its importance and possible in vivo mechanisms for its regulation. Less well understood are the steps leading to the formation of the heterodimer in the cell. There is now considerable evidence that these steps are themselves subject to cellular controls crucial for microtubule function.
The proper folding of the tubulin chains in vivo (22, 23) and in vitro (6, 12, 26) apparently requires the action of chaperone complexes (variously abbreviated as TriC/CCT/TCP/c-cpn). Unlike other proteins that are TriC substrates, however, α- and β-tubulin require other proteins in vitro to exchange into exogenous heterodimers, as assayed by native gel electrophoresis (2, 7, 8). The extent to which this in vitro reaction is applicable to the in vivo situation is unknown, beginning as it does with fully denatured protein rather than newly synthesized protein (5). Comparison of elements of the in vitro reaction with cellular activities reveals both similarities and differences. For example, yeast strains with altered forms of TCP-1 genes do exhibit cytoskeleton defects (3, 15, 22–24). On the other hand, a protein that is required for the in vitro reaction is the homolog of a yeast protein, Cin1p, that is not essential in vivo but which may be involved in microtubule functions (10, 20, 21).
A recent study of the in vitro folding reaction identified cofactor A, which promotes the recovery of β-tubulin, as a monomer from the chaperonin (7). However, in this assay, the form of β-tubulin released by cofactor A does not exchange into exogenous dimer. A genetic analysis of cellular responses to β-tubulin levels identified Rbl2p as a yeast structural homolog of cofactor A; Rbl2p is a nonessential protein that suppresses the lethality associated with overexpression of β-tubulin (1). The murine cofactor A was shown to partially replace Rbl2p in this in vivo assay (1). Although results of the in vitro assay first suggested that cofactor A was a co-chaperonin, the yeast experiments demonstrated that Rbl2p interacts with β-tubulin directly, rather than with TCP-1. Results of a revised version of the in vitro assay agree with the observation that Rbl2p/cofactor A interacts with β-tubulin rather than with TriC and that Rbl2p is not essential for β-tubulin folding (21). More recently, Melki and colleagues (14) have shown that cofactor A, like Rbl2p, binds noncovalently to β-tubulin. This cofactor A–β-tubulin complex elutes from a gel filtration column in a position consistent with it being a 1:1 heterodimer.
To analyze the function of Rbl2p in the cell, we have isolated and characterized a stable complex of Rbl2p and β-tubulin, formed both in vivo and in vitro, that lacks α-tubulin. The data suggest that Rbl2p binds to a folded form of β-tubulin and predict possible roles for Rbl2p in the regulation of tubulin assembly.
MATERIALS AND METHODS
Plasmids, strains, and media.
pQE-60/RBL2 was used to produce recombinant His6-Rbl2p in Escherichia coli. This plasmid was constructed by PCR to add NcoI and BglII sites to the RBL2 gene just before the start codon and just after the penultimate codon, respectively. The PCR primers were 5′TAGGACACCATGGCACCCACACAATTG3′ and 5′AATCTGAGATCTTTTAGAATCGAGTAATTC3′. The PCR product was cloned into the NcoI and BglII sites of Qiagen vector pQE-60.
pGRH allows inducible expression of His6-Rbl2p in Saccharomyces cerevisiae. pQE-60/RBL2 was digested with HindIII, blunted, and then digested with MfeI. This fragment was cloned into pA5 (URA3 CEN GAL1-10 promoter [1]) that had been digested with NotI, blunted, and then digested with MfeI. pMM11 is a 2-μm plasmid encoding His6-Tub2p under control of the GAL1-10 promoter. Starting with the vector pBW47 containing the 3′ half of TUB2 (25), we used PCR to generate a fragment containing an NcoI site 5′ of codon 291 and a BglII site just after the penultimate codon. The 5′ primer was 5′CCGGACACCATGGCAGCAAATGTTTGAT3′. The 3′ primer was 5′CAATCTTAGATCTTTCAAAATTCTCAGTGAT3′. This fragment was cloned into the NcoI and BglII sites of pQE-60, placing six histidine codons at the carboxy terminus followed by a stop codon. A SalI-HindIII fragment was cut from this construct and cloned into pBW54 (25) from which the SalI-SacI fragment had been removed.
All yeast strains used in this study are derivatives of FSY182, -183, or -185 (25). JAY47 is a diploid containing a third copy of the TUB2 gene integrated at the TUB2 locus and under control of the GAL promoter (1). JAY614 is FSY185 plus pGRH. JAY570 is JAY47 plus pGRH. FSY820 is a derivative of FSY182 containing a deletion of the chromosomal RBL2 locus (1). This strain was transformed with a CEN plasmid (marked with URA3) bearing tub2-590 under control of the GAL promoter (25) and with a CEN plasmid (HIS3) encoding His6-Rbl2p under control of the RBL2 promoter. The latter plasmid was created by cutting pGRH with MfeI and PvuII and subcloning the fragment into pJA33, a plasmid containing the entire RBL2 gene (1) from which the MfeI-EcoRV fragment had been removed. FSY821 is similar to FSY820 except that it constitutively expresses tub2-590 and contains TUB2 under control of the GAL promoter. We crossed FSY127 (tub2-590 [11]) with FSY 611 (rbl2::URA3-hisG). We identified sporulated segregants bearing the marker for the Δrbl2 allele (by the URA3 gene) and the tub2-590 allele by Western blotting. These segregants were plated on 5-fluoro-orotic acid, and cells that had looped out the URA3 sequence were recovered. Finally, these cells were transformed with plasmids pBW54, containing GAL-TUB2 (25), and pJA33, a CEN plasmid encoding His6-Rbl2p under control of the RBL2 promoter. LTY333 contains the pMM11 plasmid in FSY182 (Δtub1 Δtub3 PRB539) (16). LTY292 is FSY182 plus pGRH (16).
Purification of His6-tagged proteins.
The Ni-nitrilotriacetic acid (NTA) slurry and column materials were from Qiagen. We used protocols that are slight modifications of both those described earlier (13) and those recommended by the manufacturer. Immunoblot signals were quantified from multiple burns within the linear range by using the IS-1000 Digital Imaging System (Alpha Innotech Corporation).
In vivo association experiments.
We grew LTY292 overnight in selective raffinose medium and then induced expression with galactose for 10 to 16 h. We harvested protein by glass bead smash (19), using approximately 4 × 109 cells per experiment. We used a volume of PME buffer plus protease inhibitors (19) equal to the volume of the cell pellet. After centrifugation (13,000 × g, 30 min), 850 μl of extract was mixed with 130 μl of Ni-NTA slurry that had been preincubated with buffer I (20 mM imidazole, 300 mM NaCl, 50 mM sodium phosphate buffer [pH 8.0]). After a 1-h incubation at 4°C, we washed the Ni-NTA beads three times with 10 ml of buffer I plus 10% glycerol. Bound proteins were eluted by incubation with an equal volume of buffer I containing 400 mM imidazole or with 2× gel sample buffer (4% sodium dodecyl sulfate [SDS], 0.2 M dithiothreitol, 20% glycerol).
In vitro association experiments.
We harvested protein from FSY185 (wild-type) cells. After breaking the cells in PME buffer with a French press, we immediately added 300 to 500 μl of bacterial lysate containing recombinant His6-Rbl2p and then proceeded as described above. The lysate was prepared from E. coli cells containing pQE-60/RBL2 which had been induced with isopropyl-β-d-thiogalactopyranoside for 6 h. Approximately 2.5 ml of packed cells were opened by sonication in 20 ml of buffer I, followed by centrifugation at 31,000 × g for 20 min. To assay denatured proteins, we brought 1 ml of extract to a final concentration of 6 M guanidine hydrochloride for 5 min at 0°C. We diluted the sample (or untreated control) 100-fold into PME buffer plus protease inhibitors plus 500 μl of recombinant His6-Rbl2p (∼0.1 mg/ml), incubated the mixture for 1 h at 4°C, and then isolated the complex as described for the in vivo association experiments.
Dissociation experiments.
We prepared His6-Rbl2p–β-tubulin complex from JAY570 protein extracts, and His6–α/β-tubulin heterodimer from LTY333 protein extracts. Cells were opened in PME buffer by French press as described above, and the extracts were centrifuged at 13,000 × g and then mixed with Ni-NTA slurries also as described above. Unbound proteins were washed away by three washes (15 ml per 130 μl of resin), and we resuspended the samples in PME buffer plus protease inhibitors (25-fold dilution). At various times, we centrifuged aliquots of the samples, removed the supernatant, and eluted bound proteins with buffer I containing 400 mM imidazole or with 2× gel sample buffer.
RESULTS
Characterization of a His6-Rbl2p–β-tubulin complex formed in vivo.
Previous work demonstrated that Rbl2p can form a complex with β-tubulin but not α-tubulin in vivo, demonstrating the existence of a second pool of β-tubulin in the cell in addition to that of the α/β-tubulin heterodimer (1). We originally isolated this complex by immunoprecipitation with anti-β-tubulin or anti-Rbl2p antibodies. However, this isolation method has drawbacks. First, the monoclonal anti-α-tubulin antibody has only a modest level of affinity, so the immunoprecipitates are somewhat unstable. Second, on SDS-polyacrylamide gels, the sizes of the tubulin polypeptides are similar to those of the immunoglobulin G heavy chains, which are abundant in the precipitate and interfere with analyses.
To avoid these problems in analyses of the Rbl2p–β-tubulin complex, we constructed a version of the RBL2 gene encoding a form of the protein with six histidines at its carboxy terminus. By several criteria, this modified form of Rbl2p has the same activities as does the unmodified form.
First, overexpressed His6-Rbl2p suppresses the lethality associated with overexpression of β-tubulin with an efficiency of 49%, under conditions where only 0.01% of cells containing the YCpGAL control plasmid survive. This value is only slightly less than the 70% efficiency achieved with unmodified Rbl2p (1).
Second, like Rbl2p, His6-Rbl2p overexpression confers resistance to the microtubule-depolymerizing drug benomyl.
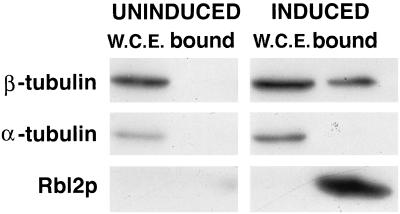
Third, protein binding to His6-Rbl2p is similar to that of unmodified Rbl2p. We mixed extracts of cells that inducibly overexpress His6-Rbl2p with Ni-NTA beads (see Materials and Methods). β-Tubulin binding to the beads strictly depended upon His6-Rbl2p expression (Fig. 1). This complex, like the one previously characterized by immunoprecipitation, contains no detectable α-tubulin.
FIG. 1.
The His6-Rbl2p–β-tubulin complex formed in vivo is devoid of α-tubulin. LTY292 cells carrying a plasmid-borne gene specifying His6-Rbl2p under control of the GAL promoter were grown for 2 h in medium containing raffinose (uninduced) or galactose (induced). The His6-Rbl2p was separated from extracts by using Ni-NTA beads. The whole-cell extracts (W.C.E.) and bound proteins were analyzed by immunoblotting for β-tubulin, α-tubulin, and Rbl2p as shown. The bottom film was exposed six times longer than the top two. The presence of β-tubulin in the bound proteins required expression of His6-Rbl2p, but no α-tubulin was detected in the bound proteins under either condition.
The level of Rbl2p–β-tubulin complex increases when both of its components are co-overexpressed (data not shown), although we detected no increase in Rbl2p levels when β-tubulin alone was overexpressed. Analysis of extracts from the co-overexpressing strains by gel filtration identified a peak containing β-tubulin and Rbl2p which eluted at an apparent molecular mass of ∼60 kDa (3a), consistent with the finding by Melki and colleagues that cofactor A and β-tubulin form a 1:1 heterodimer (14). As shown in Fig. 2 below, we also detected this complex in extracts of wild-type cells, but the level of the complex was extremely low. To estimate the relative sizes of the two pools, we used immunoprecipitation to measure the proportion of the cellular β-tubulin not associated with α-tubulin. The anti-α-tubulin antibody was covalently attached to beads and was incubated with wild-type extract. Under conditions where that antibody leaves ∼1% of the total α-tubulin in the supernatant, we found that <2% of the β-tubulin also remained. We can therefore place a limit on the proportion of β-tubulin associated with Rbl2p as being no greater than 2% of the total β-tubulin.
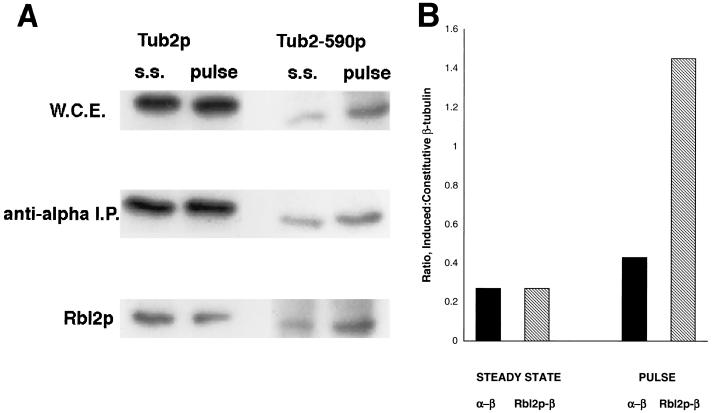
FIG. 2.
Newly synthesized β-tubulin can bind to Rbl2p in vivo. Distribution of β-tubulin between the Rbl2p and α-tubulin pools in FSY 820 cells is shown. (A) Immunoblots of whole-cell extracts (W.C.E.), anti-α-tubulin immunoprecipitates (I.P.), and proteins bound to His6-Rbl2p either at steady state (s.s.) or after a brief (galactose for 10 min followed by glucose for 10 min) induction of Tub2-590p (pulse) are shown. The blots were probed with antibodies specific for either wild-type Tub2p (206) or the faster-migrating Tub2-590p (339). Sample volumes and exposure times were adjusted to give detectable signals from all fractions. (B) Analysis of the data shown in panel A. The ratios represent the proportions of the induced β-tubulin (Tub2-590p) relative to the constitutive β-tubulin (Tub2p) present as the α/β-tubulin heterodimer or associated with Rbl2p from uninduced cells (steady state) and after a brief induction of Tub2-590p expression (pulse).
Ordering the formation of Rbl2p–β-tubulin complex and α/β-tubulin heterodimer in vivo.
To order the formation of these two β-tubulin complexes with respect to one another, we constructed yeast strains that would allow us to monitor the compartmentalization of newly synthesized β-tubulin relative to that of the steady-state pool. In FSY820 cells, the only source of Rbl2p is a low-copy-number plasmid that constitutively expresses His6-Rbl2p under the control of the RBL2 promoter. On the chromosome the constitutively expressed β-tubulin gene is wild-type TUB2. An inducibly expressed β-tubulin gene, tub2-590, is on a second plasmid under control of the GAL promoter. The product of that gene, Tub2-590p, is a fully functional β-tubulin protein. Because it lacks the carboxy-terminal 12 amino acids, we could distinguish between the β-tubulin proteins by using two antibodies (206 and 339) that bind specifically to the wild-type and truncated forms, respectively (11). In addition, Tub2-590p migrates faster on SDS-polyacrylamide gels than the wild-type Tub2p.
To examine the partitioning of newly synthesized β-tubulin, a culture of FSY820 cells grown in raffinose was exposed to galactose for 10 min and then to glucose for an additional 10 min. We fractionated extracts of these cells with anti-α-tubulin antibodies to isolate the α/β-tubulin heterodimers and with Ni-NTA beads to bind the His6-Rbl2p–β-tubulin complexes. As a steady-state control, an identical culture of raffinose-grown FSY820 cells was shifted to glucose for 20 min. The distributions of the two β-tubulin proteins in whole-cell extracts and in the two fractions were assayed by SDS-polyacrylamide gel electrophoresis followed by immunoblotting.
Results from a representative experiment are shown in Fig. 2A. The different fractions are represented by very different exposures because they are so different in abundance. Some Tub2-590p, the induced β-tubulin protein, is detectable in the steady-state cell extracts because the GAL promoter is weakly expressed in raffinose medium.
To monitor the newly synthesized β-tubulin, we recovered the fractions associated with α-tubulin and with Rbl2p and normalized the recoveries using the constitutively expressed Tub2p. Figure 2B presents an analysis of the results shown in Fig. 2A. The short exposure to galactose increased the level of Tub2-590p approximately fourfold, while the levels of wild-type β-tubulin were unaffected. The ratio of Tub2-590p to Tub2p associated with Rbl2p increased about 5.4-fold after the induction. In contrast, the ratio for the two β-tubulin proteins present as α/β-tubulin heterodimer increased only about 1.6-fold.
This difference in partitioning of newly synthesized β-tubulin is not the consequence of a subtle difference in the properties of the two proteins. We repeated this experiment using FSY821 cells, in which the constitutive and inducible β-tubulin genes are switched. In this experiment, the levels of the inducible Tub2p increased sixfold after the same induction protocol. After the induction, the relative proportion of this newly synthesized β-tubulin protein to the constitutive Tub2-590p was 10-fold higher in the Rbl2p pool but only about 1.5-fold higher in the α/β-tubulin heterodimer (data not shown).
These data demonstrate that newly synthesized β-tubulin can bind to Rbl2p before it incorporates into α/β-tubulin. If the opposite were true, i.e., if β-tubulin could bind to Rbl2p only after it had been in heterodimer, we would not detect any enrichment for the induced protein in the Rbl2p pool, since the heterodimer pool is at least 50-fold larger than the Rbl2p pool. It is important to note, however, that this result does not demonstrate that this order is obligatory (see Discussion).
Formation of Rbl2p–β-tubulin in vitro.
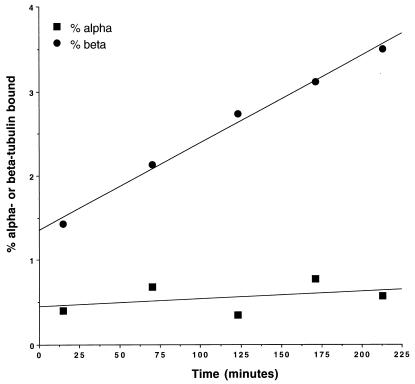
To determine if Rbl2p could bind only to newly synthesized β-tubulin or if instead it could bind to β-tubulin that had previously been in α/β-tubulin heterodimers, we used an in vitro assay. We expressed His6-Rbl2p in E. coli and incubated it with extracts of wild-type yeast cells and then assayed for bound proteins by using Ni-NTA beads. We found that Rbl2p bound β-tubulin in a time-dependent fashion (Fig. 3). Like the complex formed in vivo, this in vitro complex contained only a trace amount of α-tubulin, which amount did not increase with time. Therefore, it is likely that the α-tubulin detected represents adventitious binding.
FIG. 3.
Formation of His6-Rbl2p–β-tubulin complex in vitro. Bacterial extract containing His6-Rbl2p was incubated with wild-type yeast cell extracts at 4°C. At various times, an aliquot was removed and added to Ni-NTA beads for 15 min. The beads were washed, and the bound proteins were assayed by elution followed by immunoblotting with anti-tubulin antibodies. The data are reported as percent β-tubulin and α-tubulin bound as a function of time.
The time course demonstrates a linear rate of association between Rbl2p and β-tubulin for at least 4 h. Extrapolated back to zero time, the kinetics give evidence for a small but reproducible initial burst of complex formation. One interpretation of this biphasic time course is that it represents reaction with two distinct in vitro pools of β-tubulin. The low rate at which the majority of the complex forms may represent a rate-determining release of free β-tubulin from the heterodimer, by far the predominant population of tubulin in the extract. The initial burst could represent the diffusion-controlled reaction of a small equilibrium population of undimerized β-tubulin in the yeast extracts, which should bind to Rbl2p at the diffusion-controlled limit. The level of β-tubulin that reacts at this high rate fits well with our estimate of the level of β-tubulin not associated with α-tubulin in extracts (see above).
These results suggest that Rbl2p can interact with β-tubulin molecules that have previously been dimerized and hence completely folded. Conversely, the ability of β-tubulin to bind to Rbl2p in vitro is abolished by denaturation. We treated wild-type yeast protein extracts with 6 M guanidine hydrochloride and then diluted the protein into solutions containing His6-Rbl2p. Conventional chaperone binding and folding assays often make use of substrates that are denatured by treatment with 6 M guanidine hydrochloride. Relative to the control reaction mixtures that were diluted but not exposed to denaturing agent, the amount of β-tubulin bound to Rbl2p was only barely detectable (Fig. 4). In contrast, virtually no bound α-tubulin was detected in either the denatured or untreated samples. Immunoblots of these samples with anti-Rbl2p demonstrated that the amounts of Rbl2p bound to beads were the same in the treated and untreated samples. This result is consistent with the failure of β-tubulin denatured in this fashion to bind the murine Rbl2p homolog, cofactor A, in the in vitro system (7, 8).
FIG. 4.
Rbl2p does not bind to denatured β-tubulin. Extracts from wild-type cells (W.C.E.) were incubated either with control buffer (−) or with 6 M guanidine hydrochloride (+) for 5 min, diluted 100-fold, and then incubated with His6-Rbl2p plus Ni-NTA beads. The specifically bound proteins were eluted from the beads and assayed for the presence of both β-tubulin and α-tubulin by immunoblotting. The binding of β-tubulin to Rbl2p was essentially abolished by the preincubation with denaturing agent. No bound α-tubulin was detected under either condition.
Stability of the Rbl2p–β-tubulin complex.
The in vitro experiment described above suggests that β-tubulin can transfer from α-tubulin to Rbl2p. The in vivo experiment suggests that β-tubulin can interact with Rbl2p before it interacts with α-tubulin. A crucial issue for understanding Rbl2p function is how these two complexes of β-tubulin compare to one another. Accordingly, we measured their stabilities in vitro.
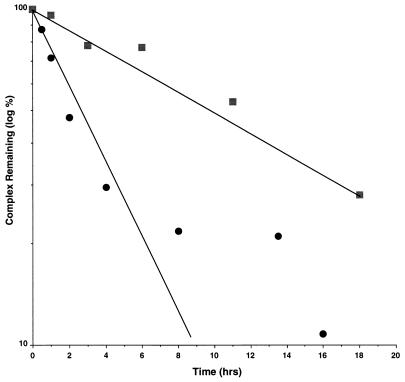
We isolated the His6-Rbl2p–β-tubulin complex from extracts of yeast cells that overproduce both proteins and then measured the dissociation of the complex by monitoring the loss of β-tubulin from the Ni-NTA beads (see Materials and Methods). Under the conditions of this experiment, the His6-Rbl2p protein does not dissociate from the beads. As shown in Fig. 5, the Rbl2p–β-tubulin complex dissociates exponentially through about two half-lives. These results are consistent with a simple dissociation reaction with a half-life of about 2.5 h, corresponding to a dissociation rate constant, koff, of 8 × 10−5 s−1.
FIG. 5.
Dissociation of His6-Rbl2p–β-tubulin and α-His6–β-tubulin in vitro. The dissociation rates of these two complexes were measured by incubating cell extracts with Ni-NTA beads, resuspending the beads in PME buffer at 4°C, and then measuring the levels of the complexes remaining at various times by immunoblotting. The data are reported as semi-log plots of β-tubulin (circles) and α-tubulin (squares) dissociation. Extracts from cells expressing either Tub2p and His6-Rbl2p or His6-Tub2p alone were used to measure the dissociation of β-tubulin or α-tubulin, respectively.
The stability of this complex should be compared to that of the α/β-tubulin heterodimer with which it can interact. The equilibrium dissociation constant for that heterodimer is reported to be ∼8 × 10−7 M (4). We cannot measure the comparable constant for the Rbl2p–β-tubulin complex, either directly or indirectly (by measuring its association rate constant), since we do not have a source of native monomeric β-tubulin. However, the bimolecular association constants for molecules of similar sizes are on the order of 107 to 108 M−1 s−1 (9). If we choose the conservative value of 106 M−1 s−1, the Rbl2p–β-tubulin complex would have a Kd of ∼10−10. That value would make the Rbl2p–β-tubulin complex significantly more stable than the α/β-tubulin heterodimer. To compare the stabilities of the complexes more directly by similar assays, we prepared α/β-tubulin heterodimer from cells expressing His6-Tub2p (see Materials and Methods). We isolated this complex on Ni-NTA beads and then monitored its dissociation by assaying for loss of the α-tubulin polypeptide. The rate of α-tubulin loss from the beads is low, consistent with a half-life for the heterodimer of about 10 h (Fig. 5). Therefore, by using essentially the same method to assay the stabilities of the two complexes, it can be concluded that the α/β-tubulin heterodimer dissociates much more slowly than does the Rbl2p–β-tubulin complex.
DISCUSSION
That β-tubulin can interact specifically with a protein other than α-tubulin suggests several possible functions for such a complex. The results presented above characterize the formation and properties of the Rbl2p–β-tubulin complex.
The results demonstrate that the formation in vitro of the Rbl2p–β-tubulin complex is dependent upon the conformation of β-tubulin. Although there may be conformational alterations of β-tubulin prior or subsequent to binding Rbl2p, the form of β-tubulin that binds Rbl2p is at least in equilibrium with the form that binds α-tubulin. That both the in vivo and in vitro complexes are essentially devoid of α-tubulin argues that Rbl2p competes with α-tubulin for binding to β-tubulin, perhaps because both ligands bind to similar sites on β-tubulin. These characteristics of the Rbl2p–β-tubulin complex are consistent with the comparable efficiencies of Rbl2p and α-tubulin in rescuing cells from β-tubulin lethality. In cell extracts, it is clear that much more β-tubulin is associated with α-tubulin than with Rbl2p, reflecting not only relative stabilities but also the likelihood that there is much less Rbl2p than α-tubulin in wild-type cells. We note that the overproduction of Rbl2p is modestly toxic (1), a phenotype that may be explained by the ability of high levels to compete successfully with α-tubulin and so to sequester β-tubulin.
Two results are consistent with a role for Rbl2p in the pathway leading to heterodimer formation. First, the in vitro data suggest that the α/β-tubulin heterodimer is more stable than the Rbl2p–β-tubulin complex. Of course this comparison is of dissociation rates that need not reflect conditions in vivo. For example, that the tubulin heterodimer can have an alternative fate to dissociation, i.e., polymerization, may affect its apparent stability in the cytoplasm. In addition, there may be effectors that modify the stability of either β-tubulin complex. Second, the pulse induction experiment shows that β-tubulin can interact with Rbl2p before it interacts with α-tubulin. This result is consistent with the interactions in vitro reported for the refolding of completely denatured β-tubulin (21), which suggest that the murine homolog of Rbl2p, cofactor A, binds β-tubulin shortly after its release from the Tcp-1 complex. However, at steady state the amount of α-tubulin available for dimerization with the newly synthesized material may be limiting. Under that circumstance, the induced β-tubulin may be forced into association with uncomplexed Rbl2p. Therefore, this experiment does not permit us to conclude that this sequence of formation of the two β-tubulin complexes is obligatory or that β-tubulin ordinarily passes through the Rbl2p complex as part of dimer formation. The experiment does establish that Rbl2p may be on the pathway of heterodimer formation for newly synthesized β-tubulin.
These results do encourage further comparisons with the in vitro assay for Rbl2p/cofactor A in heterodimer formation. We show here that Rbl2p can bind to β-tubulin that has been in the α/β-tubulin heterodimer. In contrast, Gao et al. originally reported that β-tubulin bound to cofactor A fails to exchange into exogenous heterodimer in the in vitro reaction (7). That result could mean that the formation of Rbl2p–β-tubulin from tubulin heterodimer is not reversible. Alternatively, the inability to detect this exchange reaction may reflect the slow dissociation of β-tubulin from the Rbl2p complex relative to the length of the in vitro assay. It may also reflect the fact that the level of α-tubulin available to bind the released β-tubulin in that assay may be very low, limited by the rate of dissociation from the heterodimer.
What might Rbl2p do in cells? We can consider here two possible roles. First, the demonstration that Rbl2p–β-tubulin can form from newly synthesized protein, before that β-tubulin is incorporated into heterodimer, demonstrates that Rbl2p may participate as a scaffolding protein for β-tubulin in the assembly of the tubulin heterodimer. If so, it obviously does not define the sole pathway for formation of this essential protein, since RBL2 is itself not essential in wild-type cells. Alternatively, Rbl2p could serve as a buffer to sequester free β-tubulin. Even modest excesses of β-tubulin are deleterious to the cell. For example, strains deleted for the TUB3 gene, and so lacking about 15% of their normal α-tubulin complement, show distinct microtubule phenotypes (17), which are completely suppressed by an extra copy of RBL2 under control of its own promoter (1). Experimentally, the extreme toxicity of β-tubulin is best remedied by two proteins that bind to it specifically, α-tubulin and Rbl2p. The cell could find an advantage in using Rbl2p rather than excess α-tubulin in this role. Increased levels of α-tubulin would have the consequence of changing the level of heterodimer, which in turn could affect the balanced dynamics likely to be an important part of successful microtubule function.
In some genetic backgrounds, including those carrying mutations in α-tubulin genes, RBL2 function is essential (1). Detailed analysis of these situations may provide more insight both into Rbl2p function and into cellular mechanisms for regulating tubulin assembly.
ACKNOWLEDGMENTS
We thank R. Williams (Vanderbilt University) and M. Caplow (University of North Carolina) for discussions of heterodimer dissociation and the members of our laboratory for critical contributions.
J.E.A. was supported in part by an NSF predoctoral fellowship. L.R.V. was supported in part by a predoctoral fellowship from HHMI. This work was supported by a grant from the NIH to F.S.
REFERENCES
- 1.Archer J E, Vega L R, Solomon F. Rbl2p, a yeast protein that binds to β-tubulin and participates in microtubule function in vivo. Cell. 1995;82:425–434. doi: 10.1016/0092-8674(95)90431-x. [DOI] [PubMed] [Google Scholar]
- 2.Campo R, Fontalba A, Sanchez L, Zabala J. A 14kDa release factor is involved in GTP-dependent beta-tubulin folding. FEBS Lett. 1994;353:162–166. doi: 10.1016/0014-5793(94)01036-6. [DOI] [PubMed] [Google Scholar]
- 3.Chen X, Sullivan D, Huffaker T. Two yeast genes with similarity to TCP-1 are required for microtubule and actin function in vivo. Proc Natl Acad Sci USA. 1994;91:9111–9115. doi: 10.1073/pnas.91.19.9111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Compton, K., and F. Solomon. Unpublished results.
- 4.Detrich H W, Williams R C. Reversible dissociation of the alpha-beta dimer of tubulin from bovine brain. Biochemistry. 1978;17:3900–3907. doi: 10.1021/bi00612a002. [DOI] [PubMed] [Google Scholar]
- 5.Frydman J, Hartl F. Principles of chaperone-assisted protein folding: differences between in vitro and in vivo mechanism. Science. 1996;272:1497–1502. doi: 10.1126/science.272.5267.1497. [DOI] [PubMed] [Google Scholar]
- 6.Frydman J, Nimmesgern E, Erdjument-Bromage H, Wall J, Tempst P, Hartl F-U. Function in protein folding of TRiC, a cytosolic ring complex containing TCP-1 and structurally related subunits. EMBO J. 1992;11:4767–4778. doi: 10.1002/j.1460-2075.1992.tb05582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao Y, Melki R, Walden P, Lewis S, Ampe C, Rommelaere H, Vandekerckhove J, Cowan N. A novel cochaperonin that modulates the ATPase activity of cytoplasmic chaperonin. Cell. 1994;125:989–996. doi: 10.1083/jcb.125.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao Y, Vainberg I, Chow R, Cowan N. Two cofactors and cytoplasmic chaperonin are required for the folding of α- and β-tubulin. Mol Cell Biol. 1993;13:2478–2485. doi: 10.1128/mcb.13.4.2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halford S E, Johnson N P. Single turnovers of the EcoRI restriction endonuclease. Biochem J. 1983;211:405–415. doi: 10.1042/bj2110405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyt M, Stearns T, Botstein D. Chromosome instability mutants of Saccharomyces cerevisiae that are defective in microtubule-mediated processes. Mol Cell Biol. 1990;10:223–234. doi: 10.1128/mcb.10.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz W, Solomon F. Diversity among β-tubulins: a carboxy-terminal domain of yeast β-tubulin is not essential in vivo. Mol Cell Biol. 1988;8:2730–2736. doi: 10.1128/mcb.8.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis V, Hynes G, Zheng D, Saibil H, Willison K. T-complex polypeptide-1 is a subunit of the heteromeric particle in the eukaryotic cytosol. Nature. 1992;358:249–252. doi: 10.1038/358249a0. [DOI] [PubMed] [Google Scholar]
- 13.Magendantz M, Henry M, Lander A, Solomon F. Inter-domain interactions of radixin in vitro. J Biol Chem. 1995;270:25324–25327. doi: 10.1074/jbc.270.43.25324. [DOI] [PubMed] [Google Scholar]
- 14.Melki R, Rommelaere H, Leguy R, Vandekerckhove J, Ampe C. Cofactor A is a molecular chaperone required for beta-tubulin folding: functional and structural characterization. Biochemistry. 1996;35:10422–10435. doi: 10.1021/bi960788r. [DOI] [PubMed] [Google Scholar]
- 15.Miklos D, Caplan S, Mertens D, Hynes G, Pitluk Z, Kashi Y, Harrison-Lavoie K, Stevenson S, Brown C, Barrell B, Horwich A, Willison K. Primary structure and function of a second essential member of the heterooligomeric TCP1 chaperonin complex of yeast, TCP1 beta. Proc Natl Acad Sci USA. 1994;91:2743–2747. doi: 10.1073/pnas.91.7.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schatz P, Solomon F, Botstein D. Isolation and characterization of conditional-lethal mutation in the TUB1 a-tubulin gene of the yeast Saccharomyces cerevisiae. Genetics. 1988;120:681–695. doi: 10.1093/genetics/120.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schatz P J, Solomon F, Botstein D. Genetically essential and nonessential α-tubulin genes specify functionally interchangeable proteins. Mol Cell Biol. 1986;6:3722–3733. doi: 10.1128/mcb.6.11.3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherman F, Fink G, Hicks J. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 19.Solomon F, Connell L, Kirkpatrick D, Praitis V, Weinstein B. Methods for studying the yeast cytoskeleton. In: Carraway K, Carraway C, editors. The cytoskeleton. Oxford, United Kingdom: Oxford University Press; 1992. pp. 197–222. [Google Scholar]
- 20.Stearns T, Hoyt M, Botstein D. Yeast mutants sensitive to antimicrotubule drugs define three genes that affect microtubule function. Genetics. 1990;124:251–262. doi: 10.1093/genetics/124.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian G, Huang Y, Rommelaere H, Vandekerckhove J, Ampe C, Cowan N. Pathway leading to correctly folded β-tubulin. Cell. 1996;86:287–296. doi: 10.1016/s0092-8674(00)80100-2. [DOI] [PubMed] [Google Scholar]
- 22.Ursic D, Culbertson M. The yeast homolog to mouse Tcp-1 affects microtubule-mediated processes. Mol Cell Biol. 1991;11:2629–2640. doi: 10.1128/mcb.11.5.2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ursic D, Sedbrook J, Himmel K, Culbertson M. The essential yeast Tcp1 protein affects actin and microtubules. Mol Biol Cell. 1994;5:1065–1080. doi: 10.1091/mbc.5.10.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vinh D, Drubin D. A yeast TCP-1-like protein is required for actin function in vivo. Proc Natl Acad Sci USA. 1994;91:9116–9120. doi: 10.1073/pnas.91.19.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstein B, Solomon F. Phenotypic consequences of tubulin overproduction in Saccharomyces cerevisiae: differences between alpha-tubulin and beta-tubulin. Mol Cell Biol. 1990;10:5295–5304. doi: 10.1128/mcb.10.10.5295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaffe M, Farr G, Miklos D, Horwich A, Sternlicht M, Sternlich H. TCP-1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]