Abstract
Craniometaphyseal dysplasia (CMD), a rare craniotubular disorder, occurs in an autosomal dominant (AD) or autosomal recessive (AR) form. CMD is characterized by hyperostosis of craniofacial bones and flaring metaphyses of long bones. Many patients with CMD suffer from neurological symptoms. To date, the pathogenesis of CMD is not fully understood. Treatment is limited to decompression surgery. Here, we report a knock in (KI) mouse model for AR CMD carrying a R239Q mutation in CX43. Cx43KI/KI mice replicate many features of AR CMD in craniofacial and long bones. In contrast to Cx43+/+ littermates, Cx43KI/KI mice exhibit periosteal bone deposition and increased osteoclast (OC) numbers in the endosteum of long bones, leading to an expanded bone marrow cavity and increased cortical bone thickness. Although formation of Cx43+/+ and Cx43KI/KI resting OCs are comparable, on bone chips the actively resorbing Cx43KI/KI OCs resorb less bone. Cortical bones of Cx43KI/KI mice have an increase in degenerating osteocytes and empty lacunae. Osteocyte dendrite formation is decreased with reduced expression levels of Fgf23, Sost, Tnf-α, IL-1β, Esr1, Esr2, and a lower Rankl/Opg ratio. Female Cx43KI/KI mice display a more severe phenotype. Sexual dimorphism in bone becomes more evident as mice age. Our data show that the CX43R239Q mutation results in mislocalization of CX43 protein and impairment of gap junction and hemichannel activity. Different from CX43 ablation mouse models, the CX43R239Q mutation leads to the AR CMD-like phenotype in Cx43KI/KI mice not only by loss-of-function but also via a not yet revealed dominant function.
Keywords: Genetic animal models, Bone μCT, Bone histomorphometry, Osteocytes, Osteoclasts
Introduction
Craniometaphyseal dysplasia (CMD) is a rare genetic bone disorder characterized by progressive hyperostosis of craniofacial bones and flared metaphyses of long bones.1 Diagnosis of CMD is based on clinical findings, radiographic examination, and genetic mutations. Craniofacial features of patients with CMD include hypertelorism, widened nasal bridge, paranasal bossing, widely spaced eyes with increased zygomatic width, and prominent mandibles.2 Progressive thickening of craniofacial bones can lead to compression of cranial nerves resulting in neurological symptoms, including visual or hearing impairment, and facial palsy.3–5 Associated Chiari I malformation can result in severe headaches.6 CMD is often diagnosed early during infancy due to difficulties in breathing and feeding. Radiographic images show hyperostotic calvarial and facial bones, sclerotic skull base, and thickened cortical bones of all proximal phalanges.3 CMD occurs as an autosomal dominant (AD, OMIM 605145) trait with mutations in the progressive ankylosis (ANKH) gene and in an autosomal recessive form (AR, OMIM 121014) carrying a R239Q mutation in Connexin 43 (CX43), encoded by the gap junction protein alpha 1 (GJA1) gene.7–9 Laboratory findings include normal or transiently decreased blood calcium and phosphate, elevated serum alkaline phosphatase (ALP), normal parathyroid hormone (PTH).10,11 There are no specific biomarkers for CMD. To date, CMD is managed by decompression surgeries to relieve neurological symptoms. Effective pharmaceutical therapy for CMD is still missing largely because that the pathogenesis of CMD remains incompletely understood.
To study the pathogenesis of CMD, we have generated an AD CMD mouse model (AnkKI/KI mice) expressing an ANKF377del mutation.12 AnkKI/KI mice mimic characteristic features, including hyperostosis of craniofacial bones and flaring metaphyses of long bones. Young Ank+/KI mice are phenotypically similar to Ank+/+ mice but 1-year-old Ank+/KI mice display an AD CMD-like phenotype. Our studies have shown that mutant ANK severely impairs OC formation and resorption via cytoskeleton disruption and by reducing the migration of OC progenitors.13 The pathogenesis of CMD is complex as CMD mutant ANK acts partially via loss-of-function due to rapid protein degradation but at the same time has gain-of-function properties via altered protein-protein interaction.14 It is still under investigation whether ANKHF377del and CX43R239Q mutations contribute to CMD via different mechanisms or whether there are any common targets involved. Compared to ANKH, the structure, function, effect on bone, regulation or downstream targets of CX43 has been more extensively studied. While AnkKI/KI mice are a powerful tool, we propose that the generation of an AR CMD mouse model (Cx43KI/KI) by introducing the CX43R239Q mutation greatly facilitates CMD research because more molecular tools for studying CX43 are available.
Connexins are encoded by 21 genes in humans and 20 genes in mice and are widely expressed in nearly all tissues.15 Each connexin has four transmembrane domains, two extracellular loops, one intracellular loop, and intracellular amino and carboxyl termini.16 Six connexins form hemichannels that may serve as pores or may join head to head with hemichannels in a neighboring cell to form gap junctions that allow exchange of small molecules (<1 kDa) and ions between adjacent cells.17 18 CX43 is the predominant gap junction detected in osteoblasts, osteoclasts, osteocytes, and chondrocytes, although CX45, CX46, CX26, and CX37 are also expressed in bone cells.19–25 Mutations in connexin genes are associated with a large variety of disorders affecting many organs.26 Specific to bone, mutations in CX43 are responsible for AR CMD and oculodentodigital dysplasia (ODDD), characterized by syndactyly, microphthalmia, craniofacial and dental abnormalities.9,27,28 CX43-related diseases suggest that other connexins cannot sufficiently compensate for mutated CX43, which has predominant effects on functions of bone cells.
The roles of CX43 in skeletal development and bone homeostasis have been demonstrated in multiple mouse models with CX43 deficiencies. Global Cx43-null mice exhibit delayed endochondral and intramembranous ossification in addition to neural crest cell migration defects.29 CX43 has been conditionally deleted in early osteochondro progenitors (Dermo1-Cre;Cx43−/fl mice),30,31 osteoblasts (ColI-Cre;Cx43−/fl mice),32–34 mature osteoblasts and osteocytes (Ocn-Cre;Cx43−/fl mice; Dmp1-Cre;Cx43fl/fl mice).35–38 Moreover, mice carrying different missense point mutations in Gja1 have been generated to study ODDD.39–41 These Cx43 mutant mice replicate human ODDD phenotypes including craniofacial bone anomalies, syndactyly, and enamel hypoplasia. ODDD mutations in CX43 result in reduced formation and function of CX43.39–43 Conditional CX43 knockout mouse studies have shown that CX43 affects mineral density, geometrical, and biomechanical properties of the skeleton as well as bone cell response to fracture and mechanotransduction by acting on osteoblasts, osteoclasts, and osteocytes, however, some results remain controversial.30–38
Our group identified a novel missense mutation (c.716G>A, p.Arg239Gln), located in the C-terminus of GJA1, in six patients with AR CMD by whole-exome sequencing.9 We introduced the CX43R239Q mutation in mice by CRISPR/Cas9 technology. Cx43KI/KI mice display many features of AR CMD and do not completely phenocopy AnkKI/KI mice, a mouse model for AD CMD. Cx43KI/KI mice also exhibit the unique bone remodeling features that have not been reported in other global or conditional Cx43 knockout mice. Interestingly, sex dimorphism in bone is observed and becomes more evident in aged female Cx43KI/KI mice. This model, together with the AD CMD model, can be used to elucidate how skeletal development, remodeling, and metabolism are altered in CMD.
Results
Generation and characterization of CX43R239Q KI mice, a model for AR CMD
We introduced a CX43R239Q mutation identified in AR CMD into the genome of C57Bl/6J mice using CRISPR/Cas9 mediated gene editing. Cx43KI/KI mice were viable and fertile. The ratio of Cx43+/+, Cx43+/KI and Cx43KI/KI litters followed Mendelian distribution (Cx43+/+: Cx43+/KI: Cx43KI/KI = 64: 96: 58, n=218). Approximately 40% of Cx43+/KI breeder pairs lost some pups during nursing, likely due to inappropriate feeding capability indicated by lack of milk spots in some mice (Fig. S1a). Cx43+/+ and Cx43KI/KI mice were indistinguishable at birth and had comparable weight gain between 3–12 weeks of age (Fig. S1b). Cx43KI/KI mice showed significantly increased femur length compared to wild type littermates (Fig. S1c).
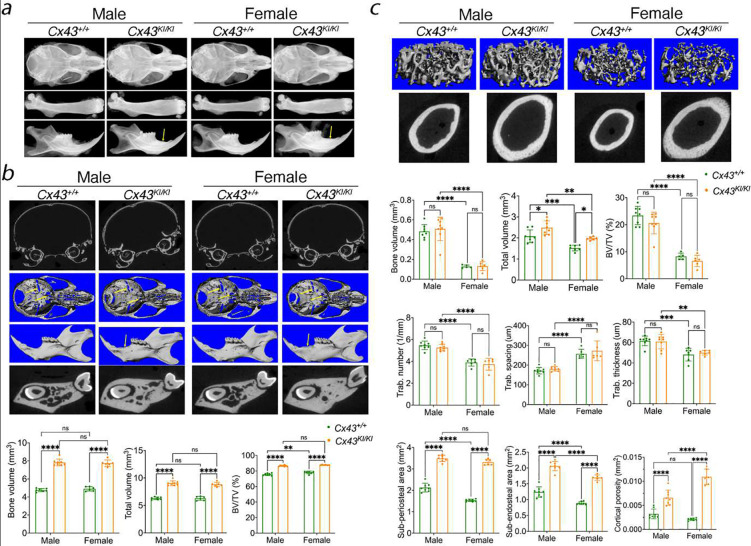
To examine whether Cx43KI/KI mice replicate CMD-like skeletal phenotypes, we first performed radiographic imaging and μCT analysis in Cx43+/+ and Cx43KI/KI male and female mice at age of 3 months. Cx43KI/KI mice had increased radiopacity of craniofacial bones, club-shaped femurs with thickened diaphyseal cortical bone, and increased alveolar bone mass with normal tooth eruption and positioning of cervical loops (Fig. 1a). μCT analysis showed that Cx43KI/KI mice exhibited thickening of skull bones, narrowed neural foramina at the cranial base, increased bone volume (BV), total volume (TV), and BV/TV in jawbones (Fig. 1b). Cx43KI/KI mice displayed significantly increased periosteal and endosteal perimeter, increased cortical diaphyseal porosity, as well as increased cortical thickness (Cx43+/+: Cx43KI/KI male mice = 0.18±0.01: 0.23±0.01, p<0.01; Cx43+/+: Cx43KI/KI female mice = 0.15±0.01: 0.28±0.02, p<0.01) (Fig. 1c). Cx43+/+ and Cx43KI/KI mice did not significantly differ in metaphyseal measurements like trabecular bone mass, trabecular number, trabecular spacing, and trabecular thickness but Cx43KI/KI mice showed increased total volume indicating widened metaphyses (Fig. 1c). There were no significant differences in vertebrae in Cx43+/+ and Cx43KI/KI mice (Fig. S2). While both sexes exhibited skeletal abnormalities, female Cx43KI/KI mice presented with a more prominent phenotype. Taken together, these data confirmed that Cx43KI/KI mice replicated many features of AR CMD patients, including skull and jawbone thickening, narrowed neural foramina of the cranial base, and widened metaphyses with hypersclerotic diaphyseal cortical bone in femurs.
Figure 1:
Skeletal analysis of male and female Cx43+/+ and Cx43KI/KI mice at age of 3 months. a) Representative radiographic images of skulls, femurs, and mandibles; Representative μCT images of b) Cross-sections of calvariae, 3D images of cranial base, mandibles, and cross-sections of mandibles through the furcation of 1st molar; yellow arrows indicate thickened mandibular alveolar bone and cranial foramina, which are narrowed in Cx43KI/KI mice. c) Trabecular bone in metaphyses and cortical bone in the mid-diaphyses. Histograms shows quantitative measurements of bone parameters by μCT analysis. Statistics were performed by two-way ANOVA followed by Tukey’s post-hoc test (* p<0.05, ** p<0.01, *** and **** p<0.001). Data presented = mean ± SD.
Effects of CX43R239Q mutation on bone turnover in Cx43KI/KI mice
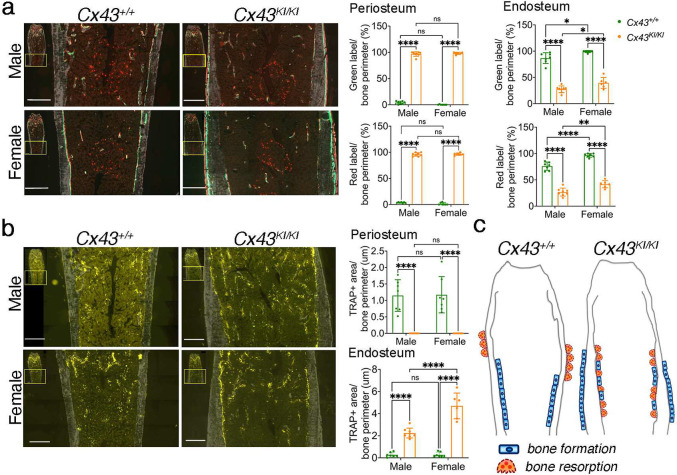
To dissect out the effects of CMD-mutant CX43 on bone turnover, we first performed static and dynamic histomorphometry in femurs of 3-month-old Cx43+/+ and Cx43KI/KI male and female mice. Consistent with findings from μCT, the CX43R239Q mutation affects turnover in cortical bone more than in metaphyseal trabeculation. There were no significant differences in mineral apposition rate (MAR), bone formation rate (BFR), osteoblast surface (AP/BS) including bone forming (AP_L/BS) and bone lining surfaces (AP_NL/BS), osteoclast surface (TRAP/BS) including bone resorbing surface (TRAP_L/BS) and bone remodeling surface (TRAP_NL/BS) in femoral metaphyses of Cx43+/+ and Cx43KI/KI mice (Table 1). In general, female mice exhibited increased osteoclast surface (TRAP/BS) and bone remodeling unit surface (AP_TRAP_R/BS) than male mice (Table 1). On the other hand, diaphyseal cortical bones of Cx43+/+ and Cx43KI/KI mice showed very different patterns. Active bone formation, suggested by the presence of calcein (green) and alizarin complexone (red) labeling, was observed on the periosteal surface of Cx43KI/KI mice but mostly on the endosteal surface of Cx43+/+ mice (Fig. 2a). We next analyzed TRAP-positive cells, indicative of bone resorption activity, in cortical bones in the mid-diaphyseal region of femurs. While Cx43+/+ mice showed some TRAP staining on the periosteal surface, mostly below the widest portion of metaphyses, Cx43KI/KI exhibited significantly increased TRAP-positive cells on the endosteal surface and very little on periosteum (Fig. 2b). In comparison to male Cx43KI/KI mice, the interlabel distance in cortical bone was increased in female Cx43KI/KI mice (male Cx43KI/KI mice: female Cx43KI/KI mice = 54.44 ± 10.93: 104.7 ± 13.43 μm, p < 0.001 by Student’s t-test). Our data, summarized in Fig. 2c, showed that Cx43KI/KI mice have increased periosteal bone formation and endosteal bone resorption whereas Cx43+/+ mice mostly exhibited endosteal bone formation and periosteal resorption. This abnormal pattern of bone formation and resorption results in cortical bone thickening in the diaphysis and an enlarged bone marrow cavity with club-shaped femurs in Cx43KI/KI mice.
Table 1:
Dynamic and static histomorphometry of trabecular parameters in metaphyses of femurs of 13-week-old Cx43+/+ and Cx43KI/KI male and female mice
| Parameters | Male | Female | ||
|---|---|---|---|---|
| Cx43+/+ (n=8) | Cx43KI/KI (n=8) | Cx43+/+ (n=8) | Cx43KI/KI (n=8) | |
| MAR (μm/day) | 1.31±0.05 | 1.48±0.11 | 1.37±0.23 | 1.26±0.27 |
| BFR (μm3/μm2/day) | 0.49±0.03 | 0.56±0.07 | 0.47±0.08 | 0.42±0.12 |
| AP/BS (%) | 77.94±2.91 | 76.65±7.35a | 80.9±7.12 | 85.86±2.29a |
| AP_L/BS (%) | 53.83±1.12 | 50.64±7.30 | 51.42±4.16 | 53.16±5.18 |
| AP_NL/BS (%) | 24.73±2.04 | 24.96±3.25b | 29.58±3.34 | 32.84±4.57b |
| TRAP/BS (%) | 18.27±5.11a | 13.41±2.17c | 25.73±2.92a | 29.74±4.85c |
| TRAP_L/BS (%) | 11.05±2.62b | 8.72±1.74c | 16.96±2.52b | 18.08±2.09c |
| TRAP_NL/BS (%) | 5.48±1.47b | 4.83±0.71c | 10.34±1.83b | 12.30±3.58c |
| AP_TRAP_ R/BS (%) | 8.31±2.22a | 6.13±1.77c | 11.58±1.97a | 13.13±1.55c |
MAR: mineral apposition rate; BFR: bone formation rate; AP: alkaline phosphatase staining; BS: bone surface; AP/BS: AP+ surface per bone surface, L: labeling surface; AP_L/BS: AP+ over labeling surface per bone surface; NL: non-labeling surface; AP_NL /BS: AP+ over non-labeling surface per bone surface; TRAP: tartrate-resistant acid phosphatase; TRAP/BS: fraction of bone surface with TRAP label; TRAP_L/BS: proportion of mineralizing surface that is covered with the TRAP label; TRAP_NL/BS: proportion of non-mineralizing surface that is covered with the TRAP label; AP_TRAP_R/BS: proportion of bone surface where the AP, TRAP and mineralization signals are co-localized. R: alizarin complexone labeling. Data presented: mean ± SD. Significant differences between groups are noted by
p<0.05,
p<0.01,
p<0.0001 by two-way ANOVA with Tukey’s multiple comparison test comparing Cx43+/+ to Cx43KI/KI mice, male to female Cx43+/+ mice, and male to female Cx43KI/KI mice.
Figure 2:
Static and dynamic histomorphometry of femoral cortical bones in male and female Cx43+/+ and Cx43KI/KI mice at age of 3 months. a) Representative images showing calcein (green) and alizarin complexone (red) double staining. Histograms show quantitative measurements of labeling on periosteum and endosteum; b) Representative images showing TRAP staining. Histograms showed quantitative measurements of TRAP positive stained area on periosteum and endosteum; c) Schematic summarizing the different patterns of bone formation and resorption activities in Cx43+/+ and Cx43KI/KI mice. Statistics were performed by two-way ANOVA followed by Tukey’s post-hoc test. Scale bar = 500 μm. Data presented = mean ± SD (* p<0.05, ** p<0.01, *** and **** p<0.001).
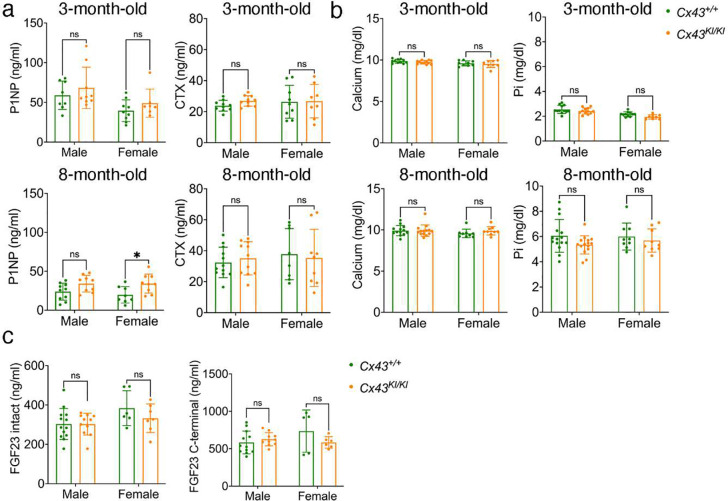
We next measured serum levels of P1NP, a marker for bone formation, and CTX, a marker for bone resorption, in male and female Cx43+/+ and Cx43KI/KI mice at ages of 3 and 8 months. Serum levels of P1NP were similar in 3-month-old Cx43+/+ and Cx43KI/KI mice but were significantly increased in 8-month-old female Cx43KI/KI mice when compared to female Cx43+/+ mice of the same age (Fig. 3a). Serum levels of CTX remained comparable between Cx43+/+ and Cx43KI/KI mice at ages of 3 and 8 months (Fig. 3a). 8-month-old mice showed remarkable reduction of P1NP levels and elevation of CTX levels compared to 3-month-old mice, suggesting somewhat decreased bone formation and increased bone resorption with aging. Our data showed that circulating P1NP and CTX levels do not reflect the tissue-specific differences in bone formation and resorption in Cx43+/+ and Cx43KI/KI mice. Serum calcium (Ca) and phosphate (Pi) levels were reported to be within normal range or slightly decreased in patients with AR CMD.9,44 Serum Ca and Pi levels in Cx43+/+ and Cx43KI/KI mice were comparable, both at ages of 3 and 8 months (Fig. 3b). We then measured fibroblast growth factor 23 (FGF23) levels, a phosphaturic factor secreted by bone to promote Pi wasting in kidney. We previously reported that the active form of FGF23 (intact FGF23) in AnkKI/KI mice, a model for AD CMD, was comparable but found an increased inactive form of FGF23 (C-terminal FGF23).10 Our data showed that there were no significant differences in intact FGF23 and C-terminal FGF23 levels between Cx43+/+ and Cx43KI/KI mice (Fig. 3c).
Figure 3:
Biochemical analysis of male and female Cx43+/+ and Cx43KI/KI mice at ages of 3 and 8 months. a) Serum levels of P1NP, a marker for bone formation, and CTX, a marker for bone resorption; b) Serum levels of Ca and Pi; and c) Serum levels of intact (active) and C-terminal form (inactive) of FGF23 at 8-month-old. Statistics were performed by two-way ANOVA followed by Tukey’s post-hoc test (* p<0.05).
Progressive worsening of bone phenotype as Cx43KI/KI mice age
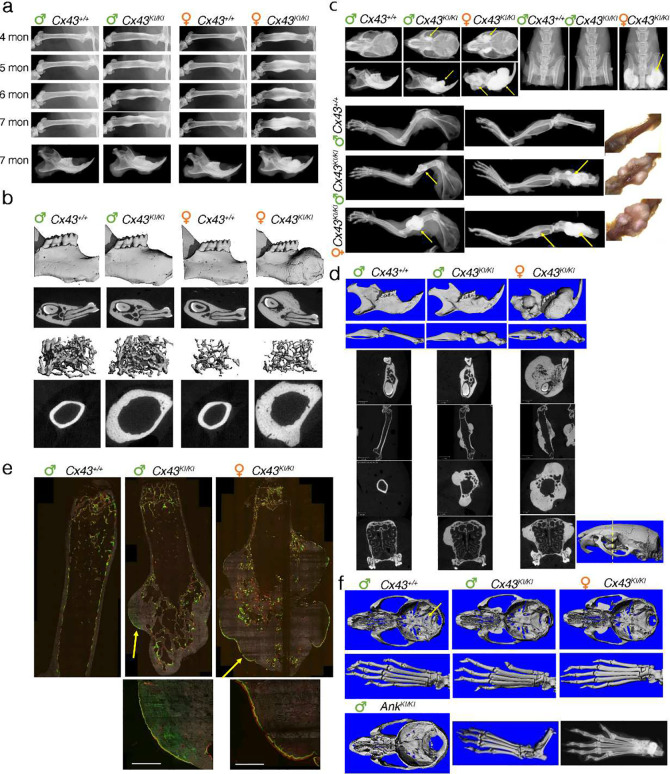
We next analyzed skeletal phenotypes in aging mice since CMD progresses throughout life. Male and female Cx43KI/KI mice progressively developed bone outgrowths in multiple sites of craniofacial and long bones, including but not limited to femurs and mandibles, shown by radiographs taken at ages of 4, 5, 6, and 7 months (Fig. 4a, data not shown). Consistent with radiographs, μCT analysis of 7-month-old bones showed grossly increased bone masses in mandibles and femurs (Fig. 4b). Sexual dimorphism became more evident with aging. At one-year-old, female Cx43KI/KI mice had significantly more prominent bone masses at multiple sites compared to male Cx43KI/KI mice (Fig. 4c–4d). Interestingly, increased interlabel distance in 3-month-old female Cx43KI/KI mice continued to be observed in 1-year-old mice, suggesting more new bone deposition in female than male Cx43KI/KI mice (Fig. 4e). When compared to 3-month-old AD CMD AnkKI/KI mice, 1-year-old Cx43KI/KI mice show less severe narrowing of cranial foramina and had no joint stiffness phenotype (Fig. 4f). In summary, our data showed progressive worsening of bone thickening and sexual dimorphism in skeletal abnormalities in Cx43KI/KI mice.
Figure 4:
Skeletal analysis of male and female Cx43+/+ and Cx43KI/KI aged mice. a) Representative radiographs of femurs and mandibles from 4- to 7-month-old mice; b) Representative μCT images of 3D mandibles, cross-sections of mandibles through the furcation of 1st molar, femoral trabecular and cortical bones from 7-month-old mice; c) Radiographs of skulls, mandibles, forearms, legs from 1-year-old Cx43+/+ and Cx43KI/KI mice; photos showing femoral bones; d) Representative μCT 3D images of mandibles and femurs, 2D images of cross-sections of mandibles, femurs (longitudinal and cross sections), and skull (cross section through the yellow dotted line) from 1-year-old Cx43+/+ and Cx43KI/KI mice; e) Dynamic histomorphometry of 1-year-old Cx43+/+ and Cx43KI/KI mice; f) Cranial neural foramina and joint phenotype from 1-year-old Cx43+/+, Cx43KI/KI and 3-month-old AnkKI/KI mice.
Effect of CX43R239Q on osteoblast and osteoclast cultures
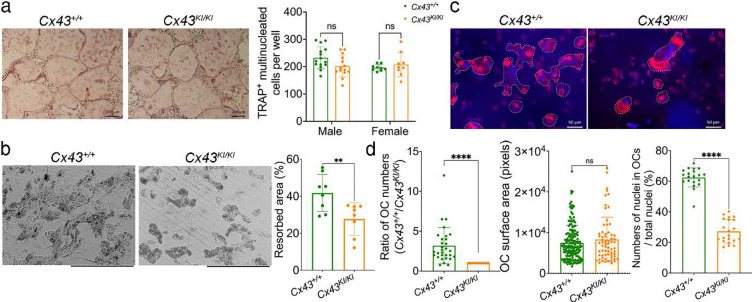
To examine mutational effects of CX43R239Q on osteoblasts, we isolated calvarial osteoblasts (mCOBs) and bone marrow stromal cells (BMSCs) from Cx43+/+ and Cx43KI/KI mice and cultured cells in osteogenic differentiation medium for 21 days. We examined cell proliferation and apoptosis 2 days after plating mCOBs by EdU and Tunel assays, respectively. Cx43KI/KI mCOBs displayed significantly increased proliferation but comparable apoptosis in comparison to Cx43+/+ mCOBs (Fig. 5a). Mineral nodule formation of mCOBs cultured in osteogenic medium for 14 and 21 days were comparable between Cx43+/+ and Cx43KI/KI mice (Fig. 5b). Expression levels of osteoblast marker genes examined by qPCR including ColI, Alp, Opn, Osx, Runx2, Ocn were comparable between Cx43+/+ and Cx43KI/KI mCOBs (Fig. S3a). However, Opg at day 0 was increased and Rankl at day 21 was decreased in Cx43KI/KI mCOBs, although the Rankl/Opg ratio was not significantly different for any time points (Fig. 5c). Similarly, ALP staining and mineral nodule formation were not significantly different in Cx43+/+ and Cx43KI/KI BMSCs from male and female mice (Fig. 5d). These data are consistent with histomorphometry data that show no significant difference in OB activity in the metaphyseal region of femurs in Cx43+/+ and Cx43KI/KI mice. Data obtained from mCOB or BMSC cultures, however, do not reflect the increased bone formation in the periosteum of Cx43KI/KI femurs shown by dynamic histomorphometry described above. To examine the Cx43R239Q mutational effects on OCs, we first cultured bone marrow-derived macrophages (BMMs) on culture plates and induced the formation of resting osteoclasts (rOCs) by supplementation with M-CSF and RANKL. TRAP+ cells with more than 3 nuclei were counted as OCs. There was no significant difference in the formation of rOCs between Cx43+/+ and Cx43KI/KI cultures (Fig. 6a). Expression levels of OC marker genes that included Nfatc1, Rank, Cathepsin K, αv integrin, β3 integrin, and Mmp9 were comparable between Cx43+/+ and Cx43KI/KI rOCs (Fig. S3b). We next plated BMMs on bone chips to examine the formation and function of actively resorbing osteoclasts (aOCs). Interestingly, the bone resorption pit assay showed a significant reduction of the resorptive activity of Cx43KI/KI aOCs when compared to Cx43+/+ aOCs (Fig. 6b). We next examined the aOCs by rhodamine phalloidin staining and found that reduced numbers of aOCs formed on bone chips (Fig. 6c & 6d). The size of Cx43+/+ and Cx43KI/KI aOCs were not significantly different but there was decreased fusion activity in Cx43KI/KI aOCs, suggested by reduced numbers of nuclei in OCs normalized to total nuclei numbers (Fig. 6d).
Figure 5:
Osteoblast cultures of Cx43+/+ and Cx43KI/KI mice. a) Cell proliferation and apoptosis in mCOB cultures analyzed by EdU and Tunel assays, respectively; b) Alkaline phosphatase (ALP) and mineral nodule formation by von Kossa staining; c) Rankl and Opg mRNA expression levels in mCOB cultures; d) ALP and mineral nodule formation in bone marrow stromal cultures (BMSCs). Histograms showing no significant differences in male mice. Statistical analysis was performed by two-way ANOVA followed by Tukey’s post-hoc test.
Figure 6:
Cx43R239Q mutational effects on osteoclasts (OCs). a) Formation of resting OCs (rOCs) was comparable between Cx43+/+ and Cx43KI/KI BMM cultures; scale bar = 200 μm. b) Resorption on bone chips by actively resorbing Cx43KI/KI OCs (aOCs) was reduced; scale bar = 300 μm. c) Cx43+/+ and Cx43KI/KI aOCs stained by rhodamine phalloidin; scale bar = 50 μm. d) Quantitative measurements of aOCs numbers that formed, surface area of aOCs, and aOC fusion, which is measured by nuclei numbers in aOCs divided by total nuclei numbers on bone chips. Statistical analysis was performed by Student’s t-test (* p<0.05, ** p< 0.01, **** p<0.001).
Effect of CX43R239Q on osteocytes
Osteocytes are the most abundant cell type in bone and regulate the quality of bone matrix by directing osteoblasts and osteoclasts via dendrites and secreted molecules. To examine the effects of CX43R239Q on osteocytes, we examined osteocytes in diaphyseal cortical bone. We categorized osteocytes into three types: type I, viable osteocytes; type II, degenerating osteocytes; and type III, empty lacunae (Fig. S4). While the number of type II and III osteocytes were significantly increased, type I osteocytes were less in Cx43KI/KI mice (Fig. 7a). The dendrites of Cx43KI/KI osteocytes were reduced in number and length shown by rhodamine phalloidin staining (Fig. 7b). We next visualized osteocytes by scanning electron microscopy. The lacunar area of Cx43KI/KI osteocytes was significantly increased due to increased perilacunar space but the cell body area was comparable between Cx43+/+ and Cx43KI/KI osteocytes (Fig. 7c). Expression levels of Fgf23 and Phex in the cortex of femurs were significantly decreased in Cx43KI/KI bones while Dmp1 was comparable to Cx43+/+ bones (Fig. 7d). Osteocytes can express pro-inflammatory cytokines such as Tnf-α, and IL-1β, which can lead to increased osteoclastogenesis and inhibit osteoblast formation.45–47 Tnf-α and IL-1β were also significantly reduced in Cx43KI/KI bones (Fig. 7d). Female Cx43KI/KI mice displayed a stronger bone phenotype than male Cx43KI/KI mice. Therefore, we examined expression levels of Esr1 and Esr2 encoding estrogen receptor α and β. Esr2 was significantly decreased in both male and female Cx43KI/KI bones while Esr1 was only negatively affected in female Cx43KI/KI bones (Fig. 7e). Osteocytes can express Sost to inhibit bone formation and Rankl to stimulate osteoclast activities.48,49 Sost mRNA and protein levels were significantly decreased and the Rankl/Opg ratio was reduced in Cx43KI/KI bones, suggesting that the increased bone mass in Cx43KI/KI mice may be due to osteocyte-promoted bone formation and osteocyte-mediated inhibition of bone resorption (Fig. 7f & 7g).
Figure 7:
Osteocyte phenotype in Cx43KI/KI mice. a) Diaphyseal cortical bone showing increased numbers of degenerating osteocytes and empty lacunae in Cx43KI/KI mice; b) Dendrite formation of osteocytes stained by rhodamine phalloidin; scale bar = 50 μm; c) Osteocytes imaged by scanning electron microscope (SEM) and quantitative measurements of areas of osteocyte bodies, lacunar area, and perilacunar space; (scale bar = 5 μm; statistical analysis was performed by Student’s t-test (ns: no significant difference, * p<0.05, **** p<0.001)). d) Expression levels of Fgf23, Phex, Dmp1, Tnfα, and IL-1β by qPCR; e) mRNA levels of Esr1 and Esr2; f) Sost mRNA and protein levels; g) Rankl and Opg mRNA levels. The ratio of Rankl/Opg is decreased in Cx43KI/KI mice. Statistical analysis was performed by two-way ANOVA followed by Tukey’s post-hoc test (* p<0.05, ** p<0.01, *** and **** p<0.001).
Expression and function of Cx43R239Q mutant protein
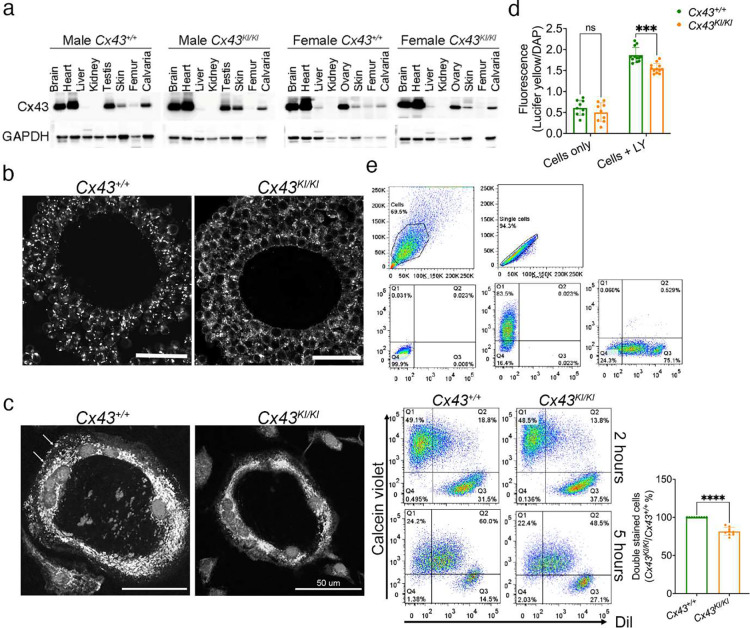
We found CX43 and CX43R239Q protein most abundantly expressed in brain, heart, testis/ovary and to a lesser amount in skin, bone, and liver but were undetectable in kidney (Fig. 8a). Expression levels of Cx43 mRNA during osteoblastogenesis and osteoclastogenesis were comparable between Cx43+/+ and Cx43KI/KI mCOB/BMSC and BMM cultures, respectively (data not shown). We next examined the localization of wt and mutant CX43 protein in ovaries and multinucleated OCs. CX43 immunostaining of ovaries showed large punctae associated with the plasma membrane in Cx43+/+ mice compared to a weaker staining and less concentrated distribution in Cx43KI/KI mice (Fig. 8b). Similarly, CX43 was detected on the plasma membrane in Cx43+/+ OCs while expression of mutant CX43 was mostly inside the cells in Cx43KI/KI OCs (Fig. 8c). CX43 serves as a hemichannel and gap junction protein when located on the plasma membrane. To examine whether the hemichannel function is compromised, skin fibroblasts isolated from Cx43+/+ and Cx43KI/KI mice were loaded with Lucifer Yellow dye and the amount of dye transfer was measured by a microplate reader. Dye transfer was reduced in Cx43KI/KI cells compared to Cx43+/+ cells (Fig. 8d). Furthermore, the gap junction activity measured by a parachute assay with DilC18 dye (recipient cells) and calcein violet AM dye (donor cells) also showed reduced activity in Cx43KI/KI cells (Fig. 8e). In summary, the CX43R239Q mutation does not affect expression levels but results in the mislocalization of mutant CX43 protein leading to the compromised hemichannel and gap junction function.
Figure 8:
Expression, localization, and function of mutant Cx43KI/KI protein. a) Tissue expression of wild type and mutant CX43 by immunoblotting; b) Localization of wild type and mutant Cx43 in ovaries; scale bar = 50 μm; c) Localization of wild type and mutant CX43 in OCs; scale bar = 50 μm; d) Hemichannel activity; statistical analysis performed by two-way ANOVA followed by Tukey’s post-hoc test (ns: no significant difference, *** p < 0.001). e) Gap junction activity by flow cytometry. Forward and side scatter has been applied to select the single-cell population and to remove debris (top panel). The gating strategies are defined by parameters obtained from cells with no staining and single staining (Calcein violet or Dil) (second panel from the top). Double stained cells (Q2) in Cx43+/+ and Cx43KI/KI groups were quantified in the histogram. Statistical analysis was performed by Student’s t-test (ns: no significant difference, **** p < 0.001).
Discussion
AD CMD is more common than the AR form. Prior to the identification of the CX43R239Q mutation for AR CMD,9 there were a small number of presumed AR CMD cases reported without genetic validation.50–53 There has been a tendency, although inconsistent, to consider more severe CMD cases as AR CMD.52,54 Characteristics common to AD and AR CMD include hyperostosis of craniofacial bones, flaring of metaphyses, widened nasal bridge, prominence of the frontal bones, hypertelorism, and mandibular prognathism. Patients with AR CMD have relatively more hypersclerotic diaphyseal cortical bones but less severe cranial neural deficits.51 Most reported patients with AR CMD have normal to mild vision or hearing impairment while most AD CMD patients have cranial nerve compression with some degree of facial paralysis, blindness, deafness, or severe headache. We have successfully generated a Cx43KI/KI mouse model for AR CMD. Cx43KI/KI mice exhibit skeletal anomalies including hyperostotic craniofacial bones and widened metaphyses with hypersclerotic cortical bones. We previously reported a mouse model for AD CMD (AnkKI/KI mice) carrying an ANKF377del mutation. AnkKI/KI mice show a CMD-like phenotype at birth and Ank+/KI mice display a milder phenotype approximately at 1-year-old.12 When comparing Cx43KI/KI mice to AnkKI/KI mice, we noticed that: 1) Cx43KI/KI mice were viable whereas AnkKI/KI mice die around 5–6 months; 2) Cx43KI/KI mice are fertile whereas AnkKI/KI mice are infertile; 3) Cx43KI/KI mice do not have joint stiffness as seen in AnkKI/KI mice. Joint stiffness is absent in Ank+/KI mice and patients with AD CMD; 4) Cx43KI/KI mice develop more hypersclerotic diaphyseal cortical bone than AnkKI/KI mice; 5) Cx43KI/KI mice have milder narrowing of cranial foramina than AnkKI/KI mice; 6) Only Cx43KI/KI mice progressively develop irregular bone bossing at multiple sites. Localized new bone on the shaft of fibulae has been reported in a patient with AR CMD.52 Excessive craniofacial bone bossing was reported in a patient with atypical CMD.55
AD and AR CMD are caused, in part of, by loss-of-function of ANKH and CX43, respectively, utilizing different mechanisms. The F377del mutation leads to rapid protein degradation of ANK resulting in significantly reduced ANKF377del protein.14 The R239Q mutation in CX43, on the other hand, does not affect protein expression levels. Loss-of-function of CX43R239Q is caused by the mislocalization of mutant CX43. Our future study will focus on whether the CX43 mutation affects the trafficking of CX43 and CX43 binding protein partners. The R239Q mutation is located at the CX43 intracellular C-terminal domain, which may interact with various protein partners, including molecules involved in adherens junctions such as the cadherins, α- and β-catenin; kinases and phosphatases that regulate the assembly or function of CX43, and with cytoskeletal proteins such as actin, actin-binding protein, and microtubules. The R239Q mutation is within the tubulin binding motif (residues 228–260).56–58 Interaction between CX43 and tubulin/microtubules can affect the intracellular trafficking of CX43, regulate gap junction function, and control the TGF-β pathway via competing with Smad2 for tubulin/microtubule binding.57
Mice with global CX43 deletion (Cx43KO/KO mice) die within one hour of delivery due to heart anomalies and muscular contractions.29 These Cx43KO/KO mice have delayed intramembranous and endochondral ossification and impaired mineralization suggesting that CX43 is important for OB differentiation.29 Because of the perinatal lethality of a global Cx43 deletion, conditional Cx43 ablation models using the Cre/LoxP system in early osteochondro progenitors (DM1Cre;Cx43−/fl), osteoblasts (ColICre; Cx43−/fl), mature osteoblasts/osteocytes (OcnCre;Cx43−/fl), and osteocytes (Dmp1Cre;Cx43fl/fl) have been developed.30–38 These CX43 mouse models and our Cx43KI/KI mice display a stronger phenotype in cortical bones than in cancellous bones, which may be explained by relatively low level of Gja1 expression in trabecular bones and growth plates.30 Osteocyte apoptosis caused by lack of CX43 in mature osteoblasts/osteocytes is more pronounced in cortical bones.37 Our Cx43KI/KI moue model differs from CX43 ablation models in several features. While DM1Cre;Cx43−/fl and ColICre; Cx43−/fl mice are osteopenic and OcnCre;Cx43−/fl mice have no bone mass abnormalities,30,32,35 our Cx43KI/KI mice display increased bone mass. Furthermore, conditional CX43 deletion mice have a thin cortex30,33,38 whereas our Cx43KI/KI mice exhibit cortical bone thickening. While deletion of CX43 leads to an increased Rankl/Opg ratio,30,36,37 our Cx43KI/KI mice show a decreased Rankl/Opg ratio. CTX, a serum marker for bone resorption, is increased in DM1Cre;Cx43−/fl and OcnCre;Cx43−/fl mice30,36 but is not altered in Cx43KI/KI mice. mCOBs and BMSCs from Cx43+/+ and Cx43KI/KI mice have comparable ALP and mineral nodule formation. This is in contrast to the reduced ALP and mineralization in osteoblast cultures with CX43 global deletion (Cx43−/−) and conditional CX43 ablation (ColCre;Cx42−/fl).29,32 In addition, the excessive bone bossing is only presented in the Cx43KI/KI mice. Based on the different phenotypes of Cx43KI/KI and Cx43KO/KO mice we propose that AR CMD is not merely caused by loss-of-function.
The club-shaped femurs in Cx43KI/KI mice are caused by an unusual pattern of periosteal bone formation and endosteal bone resorption. In Cx43KI/KI mice we suspect that osteocytes dysregulate periosteal and endosteal bone cell activities. Osteocytes control the bone quality/strength by secretion of paracrine factors, mechanosensing, and maintaining mineral homeostasis via their lacunar-canalicular network. Dendrite defects have been associated with several skeletal diseases like osteoporosis, osteoarthritis, osteogenesis imperfecta.59–61 Osteocytes control bone remodeling by sending signals to osteoblasts and osteoclasts. For instance, osteocytes can produce sclerostin (Sost), which inhibits bone formation and receptor activator of nuclear factor-κB ligand (Rankl), which promotes bone resorption.49,62 Osteocytes also express Fgf23, which is linked to hypophosphatemia and impaired bone mineralization in autosomal-dominant hypophsophatemic rickets/osteomalacia (ADHR), X-linked hypophosphatemic rickets/osteomalcia (XLH), and tumor-induced osteomalacia (TIO).63–65 The proinflammatory cytokines tumor necrosis factor alpha (Tnf-α) and interleukin 1β (IL-1β) expressed by osteocytes can upregulate Fgf23.66 Estrogen signaling may contribute to sexual dimorphism in bone.67 Our data show that ER-α is only decreased in female Cx43KI/KI mice while ER-β is reduced in both male and female Cx43KI/KI mice. ER-α and ER-β are expressed in bone but regulate bone mass differently in male and female mice.68–70 Bone remodeling in male mice is regulated only by ER-α, whereas bone turnover in female mice is controlled by ER- α and ER-β.71,72 Interestingly, the serum estradiol level is significantly increased in female but not male ER-α−/− mice.72 In female ER-β−/− mice, the bone mineral content is increased due to increased cortical bone area.73 The reduced expression of ER-α and ER-β may partially explain the increased severity of bone mass in female Cx43KI/KI mice.
In conclusion, our study presents a valid mouse model for investigating the molecular mechanisms of AR CMD. The R239Q mutation in CX43 affects osteoblasts, osteoclasts, and osteocytes, collectively contributing to the AR CMD-like skeletal phenotype. Intracellular mislocalization and differential gene expression provides insights into some of the bone abnormalities in Cx43KI/KI mice. The unique skeletal features of Cx43KI/KI mice suggest a yet unknown dominant function of CX43R239Q that supplements the loss-of-function effects of CX43R239Q in AR CMD.
Materials and Methods
Generation of the CX43R239Q knockin (KI) mouse model and study approval
To generate CX43R239Q KI mice (Cx43KI/KI), we used CRISPOR (http://crispor.tefor.net) to identify the Cx43 target site, 5’-GGGCGTTAAGGATCGCGTGAAGG with the protospacer adjacent motif (PAM) for CRISPR/Cas9 mediated gene editing. Cx43 sgRNA and Cas9 protein were mixed at room temperature for 15 min to form the RNP complex prior to adding Cx43 R239Q ssDNA donor (5’-AT*T*G*AAGTAAGCATATTTTGGAGATCCGCAGTCTTTGGATGGGCTCAGTGGGCCGGTGGTGGCGTGGTAAGGATCGCTTCTTCCTTTCACCTGATCTTTAACGCCCTTGAAGAAGACATAGAAGAGCTCAATGATATTCAGAGCGAGAGAC*A*C*C-3’) for pronuclear microinjection into C57BL/6J one-cell embryos. The concentration of sgRNA, Cas9 and ssDNA donor was 50 ng, 200 ng and 50 ng per μL, respectively (all reagents from Integrated DNA Technologies, Coralville, IA).
Injected embryos were transferred into pseudo-pregnant females and potential founders were screened by PCR using primers (CxF1: 5’-GCTTCCTCTCACGTCCCACGGAG-3’ and CxR239QR: 5’- GGATCGCTTCTTCCTTTCACCT-3’) to amplify a fragment of 145 bp specific to the KI allele. Genotype of PCR-positive founders were further confirmed by PCR using primers (CxF1 and CxR1: 5’-GCTTGTTGTAATTGCGGCAGGAGG-3’) followed by sequencing of the PCR product (Fig. S5a and S5b). The R239Q mutation (CGC → CAG, pink bolded) and two silent mutations (blue bolded) were introduced to eliminate re-cleavage of the KI allele (Fig. S5a). A positive founder was bred with wildtype C57BL/6J mice (Stock number: 000664, The Jackson Laboratory). Mice were housed in an AAALAC-accredited facility under veterinary supervision. All experiments involving animals were in accordance with animal protocol AP-200644–0025 approved by the Animal Care Committee at the University of Connecticut Health (UConn Health).
Skeletal analysis
Skulls, mandibles, and femurs of Cx43+/+ and Cx43KI/KI male and female mice at ages of 3–12 months (n ≥ 5 for each group) were radiographed with a Kubtec Radiography System (KUB Technologies Inc., Stratford, CT) and analyzed by μCT (mCT20; ScanCo Medical AG, Bassersdorf, Switzerland) in the MicroCT facility at UConn Health as previously described.74 Mandibular data were collected by measuring vertical sections covering 1st & 2nd molars. Femoral trabecular data were taken at the distal growth plate over a distance of 960 μm. Cortical bone parameters were collected from 50 cross-sectional slices of 12 μm in the mid-diaphysis.
For the analysis of bone histomorphometry, we injected Cx43+/+ (n=8) and Cx43KI/KI (n=8) male and female mice intraperitoneally with calcein (10 mg/kg body weight) and alizarin complexone (AC, 30 mg/kg body weight) at an interval of 7 days. Two days after the second injection, mice were sacrificed at 13 weeks of age and bones subjected to histomorphometry as previously described.74 Femurs were fixed in 10% formalin and frozen-embedded in OCT medium (Richard-Allan Scientific, San Diego, CA). Blocks were sectioned using a cryotome (CM3050S; Leica, Wetzlar, Germany). Three sections (7 μm thickness) revealing the central vein were collected using adhesive tape (Cryofilm type IIC; Section-Lab Co, Ltd., Yokohama, Japan).74 For dynamic histomorphometry, fluorescent images showing the mineralization status by calcein (green) and AC (red) were taken using a microscope slide scanner (Mirax Midi automated image acquisition system; Carl Zeiss, Jena, Germany). Parameters of bone forming activity were measured in the Computer Science Department at UConn.74,75
After scanning for mineralization imaging, the same sections were subjected to cellular staining. To detect OBs, ALP was stained by the fluorescent substrate fast red.76 AP-positive cells located on calcein- or AC-labeled bone surfaces were considered active osteoblastic cells whereas AP-positive cells on non-labeled bone surfaces were considered inactive bone lining cells.74,75 For OCs, tartrate resistant acid phosphatase (TRAP) staining was performed using a fluorescent substrate (ELF-97; Thermo Fisher Scientific, Waltham, MA). Bone surfaces that had both, mineralization labeling and TRAP signals, were considered remodeling surfaces whereas surfaces with TRAP-positive cells but no mineralization labeling were considered resorbing surfaces. The TRAP and AP signals were captured using filters optimized for tetracycline and tetramethyl rhodamine iso-thiocyanate (TRITC), respectively.
Biochemical analysis
Fasting sera were collected from the submandibular vein using animal lancets (Medipoint, Long Island, NY) in microtainer tubes (Becton Dickinson, Franklin Lakes, NJ) from 3-month- and 8-month-old Cx43+/+ and Cx43KI/KI mice. Total serum calcium and phosphate concentrations were determined using a calcium reagent kit and a phosphorus reagent set (Eagle Diagnostics, Cedar Hill, Tx). Concentrations of mouse CTX (RatLaps CTX-1 ELISA kit; Immunodiagnostic Systems, East Boldon, United Kingdom), P1NP (Rat/mouse P1NP ELISA kit; Immunodiagnostic Systems, Mountain Lakes, NJ), FGF-23 (intact), and FGF-23 (C-terminal) (Mouse/Rat FGF-23 (intact) ELISA, Mouse/Rat FGF-23 (C-Term) ELISA; Quidel, SanDiego, CA) were determined according to manufacturer instructions.
Mouse osteoblast cultures
To study OBs, we used mouse calvarial osteoblast (mCOB) cultures and bone marrow stromal cell (BMSC) cultures. For mCOB cultures, calvariae from postnatal day 4–7 mice were digested with 0.05% trypsin (Thermo Fisher Scientific) and 0.15% collagenase (Type II; Sigma Aldrich, St. Louis, MO) at 37° C. Cells from digests 3 to 5 were collected and plated at a density of 10,000/cm2 in DMEM (Thermo Fisher Scientific) until confluent. Cells were maintained in osteoblast differentiation medium (α-MEM (Thermo Fisher Scientific) containing 10% fetal bovine serum (FBS; Cytiva Life Sciences, Marlborough, MA), 100 IU/mL penicillin, 100 μg/mL streptomycin (Thermo Fisher Scientific), 50 μg/mL ascorbic acid and 4mM β-glycerophosphate (Sigma Aldrich)). Medium was changed every 2–3 days. For BMSCs, bone marrow was flushed out from the shafts of femurs, tibiae and humeri of 7-to 9-week-old mice.77 Cells were cultured at a density of 2×106 cells/well in 12-well culture plates in α-MEM containing 10% FBS, 100 IU/mL penicillin, and 100 μg/mL streptomycin. At day 3, half of the medium was changed. On day 7, cells were switched to 100% osteoblast differentiation medium containing 50 μg/mL ascorbic acid and 8 mM β-glycerophosphate (β-GP). Medium was changed every 2–3 days.
Mouse osteoclast cultures
Bone marrow-derived macrophage (BMM) cultures were obtained from bone marrow flushed out from femora and tibiae of 7- to 9-week-old mice and cultured for 18 to 24 hours in α-MEM containing 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin. Non-adherent cells were collected and purified by Ficoll separation (Lymphoprep; STEMMCELL Technologies, Vancouver, Canada). Cells were cultured in α-MEM medium with 10% FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, and supplemented with M-CSF (30 ng/mL; Peprotech, Cranbury NJ) for 2 days to enrich OC progenitors followed by M-CSF and RANKL (30 ng/mL; Peprotech) treatment for 4–5 days and 9–10 days to obtain mature resting OCs (on culture plates) and actively resorbing OCs (on bone chips), respectively.
In vitro osteoblast and osteoclast assays
For OB cultures, we determined matrix expression by ALP staining and mineral nodule formation by von Kossa staining. ALP staining was performed using a commercially available alkaline phosphatase kit (Sigma Aldrich) according to manufacturer instructions. Mineral deposition was stained with 5% silver nitrate (Sigma Aldrich).
We analyzed OC formation by TRAP staining with a commercially available kit (Sigma Aldrich).13 TRAP-positive stained cells ≥ 3 nuclei were counted as OCs. Actin rings of OCs were examined by rhodamine-phalloidin staining (1:40 dilution in PBS, Thermo Fisher Scientific). Nuclei were stained with Hoechst 33342 dye (trihydrochloride trihydrate; Molecular Probe, Thermo Fisher Scientific). Images were taken by a Z1 observer microscope (Carl Zeiss). For bone resorption pit assays, BMMs plated on bone chips were terminated at day 10 and bone chips were imaged using a tabletop scanning electron microscope (TM-1000, Hitachi, Tokyo, Japan). Quantitative data were obtained by Image J (National institutes of Health, NIH). Experiments were performed in triplicate.
Quantitative real-time PCR (qPCR)
Total RNA from mCOBs, BMM cultures, and long bones (shafts of femurs and tibia without bone marrow) was isolated using TRIzol (Thermo Fisher Scientific) followed by Direct-zol RNA extraction (Zymo Research, Irvine, CA) according to manufacturer instructions. RNA was treated with DNase I and cDNA was synthesized by Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). qPCR was performed as previously described.13 Relative quantification of gene expression was determined by the ΔΔCt method. Data were normalized to mouse 18S gene expression. Primer sequences are listed in Supplementary Table S1.
Protein preparation, SDS-PAGE, and Western blotting analysis
Dissected tissues were immediately frozen by liquid nitrogen and crushed to powder. Two ends of femurs and tibiae were cut off followed by brief centrifugation to remove bone marrow. Protein was extracted by lysis buffer (1% Triton X-100, 50mM Tris (pH 7.8), 150 mM NaCl, 0.1% SDS, 10% glycerol, 0.5% deoxycholic acid) with protease and phosphatase inhibitor cocktails (Thermo Fisher Scientific). Protein concentrations were measured by BCA protein assay kit (Thermo Fisher Scientific). Equal amounts of protein were run in 10% SDS-PAGE gels. Samples were transferred to PVDF membranes (BioRad, Hercules, CA) using a wet transfer apparatus. CX43 antibody (3512) was purchased from Cell Signaling Technology, Danvers, MA. GAPDH (Santa Cruz Biotechnology, Dallas, TX) served as internal control. Bands were detected using enhanced chemiluminescent detection reagent (Azure Biosystems, Dublin, CA) and visualized by an Azure c600 imaging system (Azure Biosystems). Densitometric analyses of immunoblots were performed by Image J.
CX43 Immunofluorescence of ovary cryosections and BMMs
Ovaries from Cx43+/+and Cx43KI/KI mice were fixed in 4% paraformaldehyde (PFA, Electron Microscopy Sciences, Hatfield, PA) at 4°C for 48 hours, rinsed with 0.12M phosphate buffer, and cryo-protected with 30% sucrose at 4°C overnight. 10 μm frozen sections were cut and collected on Superfrost plus slides (Thermo Fisher Scientific). Sections were rinsed with 1x PBS, blocked with 5% normal goat serum in 1% BSA in PBS, then incubated in anti-CX43 antibody (C6219; Millipore Sigma, Burlington, MA) diluted 1:300 in blocking buffer for two hours at room temperature. After rinsing with PBS, sections were incubated in Alexa 488-goat anti-rabbit (Invitrogen) diluted 1:500 for 30 minutes at room temperature. Sections were imaged on a Zeiss Pascal confocal microscope with the same conditions for both genotypes. Multinucleated BMMs were fixed in 4% PFA for 10 minutes at room temperature. Cells were permeabilized with 0.2% Tween-20 for 3 minutes, washed with PBS, and blocked in 5% normal goat serum for 60 minutes at RT. Cells were incubated in anti-CX43 antibody (C6219; Millipore Sigma) diluted 1:500 overnight. After rinsing with PBS three times, cells were incubated with goat anti-rabbit secondary antibody (1:400) for 1 hour at RT in dark. Images were taken by a Z1 observer microscope (Carl Zeiss).
Hemichannel and gap junction activity of Cx43
To assess the hemichannel activity, we cultured Cx43+/+ and Cx43KI/KI skin fibroblasts at the density of 5000 cells per well in 96-well plates. The next day, cells were washed with PBS three times and cultured with or without 100 μM Lucifer Yellow (LY) dye in DMEM containing 3 mMM EGTA for 30 minutes. Cells were then washed and fixed in 4% PFA for 5 minutes prior to measuring dye transfer with an excitation peak at 428 nm and an emission peak at 536 nm in a microplate reader (Tecan Life Sciences, Maennedorf, Switzerland). The fluorescence reading was normalized to nuclei staining with Hoechst 33342 (excitation peak at 352 nm and emission peak at 454 nm).
To evaluate the gap junction activity, donor and recipient Cx43+/+ and Cx43KI/KI skin fibroblasts were seeded at a density of 12,000/cm2 (day 0). The following day (day 1), recipient cells were labelled with 2.4 μM DilC18 dye (Thermo Fisher Scientific) for 2 hours. On day 2, donor cells were trypsinized and stained with 5 μM Calcein Violet AM (Thermo Fisher Scientific) for 20 minutes. Donor cells were then seeded on top of the recipient cells (Ratio of donor: recipient cells=4:1) for 2 and 5 hours. At the end points, cells were harvested and analyzed by flow cytometry using a Becton-Dickinson LSRII analyzer (Becton, Dickinson and Company, Franklin Lakes, NJ).
Statistical Analysis
Statistical analysis was performed by one-way ANOVA followed by Bonferroni multiple comparison test, using Prism 5 (GraphPad Software, La Jolla, CA).
Acknowledgements:
This work was supported by NIH/NIDCR grant R01DE025664 to IPC. We thank the UConn Health core facilities for the support of our work: Flow Cytometry Core, Center for Mouse Genome Modification, MicroCT Imaging Facility, Molecular Core Services. We thank the UConn Health Fluorescent Imaging Core in the laboratory of Dr. David Rowe for tissue imaging and the UConn Computer Science Department for histomorphometric image analysis. We thank Dr. Paul Lampe for his input and discussion.
Footnotes
Conflict of Interests:
All authors have no conflict of interest.
References
- 1.Jackson W. P., lbright F., Drewry G., Hanelin J. & Rubin M. I. Metaphyseal dysplasia, epiphyseal dysplasia, diaphyseal dysplasia, and related conditions. I. Familial metaphyseal dysplasia and craniometaphyseal dysplasia; their relation to leontiasis ossea and osteopetrosis; disorders of bone remodeling. A.M.A. archives of internal medicine 94, 871–885 (1954). [DOI] [PubMed] [Google Scholar]
- 2.Reichenberger E. & Chen I. P. in GeneReviews (R) (eds Pagon R. et al. (1993).
- 3.Ramseyer L. T., Leonard J. C. & Stacy T. M. Bone scan findings in craniometaphyseal dysplasia. Clinical nuclear medicine 18, 137–139 (1993). [DOI] [PubMed] [Google Scholar]
- 4.Hayashibara T., Komura T., Sobue S. & Ooshima T. Tooth eruption in a patient with craniometaphyseal dysplasia: case report. Journal of oral pathology & medicine : official publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology 29, 460–462 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Cai C., Zhang Q., Shen C., Sun G. & Wang C. Chiari malformation caused by craniometaphyseal dysplasia: case report and review of literature. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery… [et al] = Zeitschrift fur Kinderchirurgie 18, 198–201, doi: 10.1055/s-2008-1038536 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M. et al. Chiari type I malformation caused by craniometaphyseal dysplasia. Acta Med Okayama 67, 385–389, doi: 10.18926/MO/52012 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Reichenberger E. et al. Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. American journal of human genetics 68, 1321–1326, doi: 10.1086/320612 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nurnberg P. et al. Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nature genetics 28, 37–41, doi: 10.1038/88236 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Hu Y. et al. novel autosomal recessive GJA 1 missense mutation linked to Craniometaphyseal dysplasia. PloS one 8, e73576, doi: 10.1371/journal.pone.0073576 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y., Dutra E. H., Reichenberger E. J. & Chen I. P. Dietary phosphate supplement does not rescue skeletal phenotype in a mouse model for craniometaphyseal dysplasia. J Negat Results Biomed 15, 18, doi: 10.1186/s12952-016-0061-0 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto T. et al. Bone marrow-derived osteoclast-like cells from a patient with craniometaphyseal dysplasia lack expression of osteoclast-reactive vacuolar proton pump. The Journal of clinical investigation 91, 362–367, doi: 10.1172/JCI116194 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen I. P. et al. Introduction of a Phe377del mutation in ANK creates a mouse model for craniometaphyseal dysplasia. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 24, 1206–1215, doi: 10.1359/jbmr.090218 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen I. P., Wang L., Jiang X., guila H. L. & Reichenberger E. J. A Phe377del mutation in NK leads to impaired osteoblastogenesis and osteoclastogenesis in a mouse model for craniometaphyseal dysplasia (CMD). Human molecular genetics 20, 948–961, doi: 10.1093/hmg/ddq541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanaujiya J. et al. Rapid degradation of progressive ankylosis protein (ANKH) in craniometaphyseal dysplasia. Sci Rep 8, 15710, doi: 10.1038/s41598-018-34157-5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saez J. C., Berthoud V. M., Branes M. C., Martinez, A. D. & Beyer, E. C. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83, 1359–1400, doi: 10.1152/physrev.00007.2003 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Nielsen M. S. et al. Gap junctions. Compr Physiol 2, 1981–2035, doi: 10.1002/cphy.c110051 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alexander D. B. & Goldberg G. S. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem 10, 2045–2058, doi: 10.2174/0929867033456927 (2003). [DOI] [PubMed] [Google Scholar]
- 18.Goodenough D. A., Goliger J. A. & Paul D. L. Connexins, connexons, and intercellular communication. Annu Rev Biochem 65, 475–502, doi: 10.1146/annurev.bi.65.070196.002355 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Pacheco-Costa R. et al. High bone mass in mice lacking Cx37 because of defective osteoclast differentiation. The Journal of biological chemistry 289, 8508–8520, doi: 10.1074/jbc.M113.529735 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stains J. P. & Civitelli R. Gap junctions in skeletal development and function. Biochim Biophys Acta 1719, 69–81, doi: 10.1016/j.bbamem.2005.10.012 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann B. Assembly and disassembly of gap junctions during mesenchymal cell condensation and early chondrogenesis in limb buds of mouse embryos. J Anat 138 ( Pt 2), 351–363 (1984). [PMC free article] [PubMed] [Google Scholar]
- 22.Civitelli R. et al. Connexin43 mediates direct intercellular communication in human osteoblastic cell networks. The Journal of clinical investigation 91, 1888–1896, doi: 10.1172/JCI116406 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yellowley C. E., Li Z., Zhou Z., Jacobs C. R. & Donahue H. J. Functional gap junctions between osteocytic and osteoblastic cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 15, 209–217, doi: 10.1359/jbmr.2000.15.2.209 (2000). [DOI] [PubMed] [Google Scholar]
- 24.Koval M., Harley J. E., Hick E. & Steinberg T. H. Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. The Journal of cell biology 137, 847–857, doi: 10.1083/jcb.137.4.847 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paic F. et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone 45, 682–692, doi: 10.1016/j.bone.2009.06.010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly J. J., Simek J. & Laird D. W. Mechanisms linking connexin mutations to human diseases. Cell and tissue research 360, 701–721, doi: 10.1007/s00441-014-2024-4 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Richardson R. J. et al. nonsense mutation in the first transmembrane domain of connexin 43 underlies autosomal recessive oculodentodigital syndrome. J Med Genet 43, e37, doi: 10.1136/jmg.2005.037655 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paznekas W. A. et al. Connexin 43 (GJ 1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. American journal of human genetics 72, 408–418, doi: 10.1086/346090 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lecanda F. et al. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. The Journal of cell biology 151, 931–944, doi: 10.1083/jcb.151.4.931 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins M. et al. Osteoblast connexin43 modulates skeletal architecture by regulating both arms of bone remodeling. Molecular biology of the cell 22, 1240–1251, doi: 10.1091/mbc.E10-07-0571 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grimston S. K., Watkins M. P., Brodt M. D., Silva M. J. & Civitelli R. Enhanced periosteal and endocortical responses to axial tibial compression loading in conditional connexin43 deficient mice. PloS one 7, e44222, doi: 10.1371/journal.pone.0044222 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung D. J. et al. Low peak bone mass and attenuated anabolic response to parathyroid hormone in mice with an osteoblast-specific deletion of connexin43. J Cell Sci 119, 4187–4198, doi: 10.1242/jcs.03162 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Grimston S. K., Brodt M. D., Silva M. J. & Civitelli R. Attenuated response to in vivo mechanical loading in mice with conditional osteoblast ablation of the connexin43 gene (Gja1). Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 23, 879–886, doi: 10.1359/jbmr.080222 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gonzalez-Nieto D. et al. Connexin-43 in the osteogenic BM niche regulates its cellular composition and the bidirectional traffic of hematopoietic stem cells and progenitors. Blood 119, 5144–5154, doi: 10.1182/blood-2011-07-368506 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plotkin L. I. et al. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 23, 1712–1721, doi: 10.1359/jbmr.080617 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y. et al. Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PloS one 6, e23516, doi: 10.1371/journal.pone.0023516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bivi N. et al. Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 27, 374–389, doi: 10.1002/jbmr.548 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lloyd S. A, Lewis G. S., Zhang Y., Paul E. M. & Donahue H. J. Connexin 43 deficiency attenuates loss of trabecular bone and prevents suppression of cortical bone formation during unloading. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 27, 2359–2372, doi: 10.1002/jbmr.1687 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flenniken M. et al. Gja1 missense mutation in a mouse model of oculodentodigital dysplasia. Development 132, 4375–4386, doi: 10.1242/dev.02011 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Kalcheva N. et al. Gap junction remodeling and cardiac arrhythmogenesis in a murine model of oculodentodigital dysplasia. Proceedings of the National cademy of Sciences of the United States of America 104, 20512–20516, doi: 10.1073/pnas.0705472105 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dobrowolski R. et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Human molecular genetics 17, 539–554, doi: 10.1093/hmg/ddm329 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibayama J. et al. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res 96, e83–91, doi: 10.1161/01.RES.0000168369.79972.d2 (2005). [DOI] [PubMed] [Google Scholar]
- 43.McLachlan E. et al. ODDD-linked Cx43 mutants reduce endogenous Cx43 expression and function in osteoblasts and inhibit late stage differentiation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 23, 928–938, doi: 10.1359/jbmr.080217 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Prontera P. et al. Craniometaphyseal dysplasia with severe craniofacial involvement shows homozygosity at 6q21–22.1 locus. American journal of medical genetics. Part 155, 1106–1108, doi: 10.1002/ajmg.a.33826 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Kanaji, A. et al. Co-Cr-Mo alloy particles induce tumor necrosis factor alpha production in MLO-Y4 osteocytes: a role for osteocytes in particle-induced inflammation. Bone 45, 528–533, doi: 10.1016/j.bone.2009.05.020 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pesce Viglietti A. I. et al. Brucella abortus Invasion of Osteocytes Modulates Connexin 43 and Integrin Expression and Induces Osteoclastogenesis via Receptor ctivator of NF-kappaB Ligand and Tumor Necrosis Factor lpha Secretion. Infection and immunity 84, 11–20, doi: 10.1128/II.01049-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kulkarni R. N., Bakker D., Everts V. & Klein-Nulend J. Mechanical loading prevents the stimulating effect of IL-1beta on osteocyte-modulated osteoclastogenesis. Biochemical and biophysical research communications 420, 11–16, doi: 10.1016/j.bbrc.2012.02.099 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Winkler D. G. et al. Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22, 6267–6276, doi: 10.1093/emboj/cdg599 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakashima T. et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nature medicine 17, 1231–1234, doi: 10.1038/nm.2452 (2011). [DOI] [PubMed] [Google Scholar]
- 50.Ross M. W. & ltman D. H. Familial metaphyseal dysplasia. Review of the clinical and radiologic feature of Pyle's disease. Clin Pediatr (Phila) 6, 143–149, doi: 10.1177/000992286700600309 (1967). [DOI] [PubMed] [Google Scholar]
- 51.Penchaszadeh V. B., Gutierrez E. R. & Figueroa E. Autosomal recessive craniometaphyseal dysplasia. American journal of medical genetics 5, 43–55, doi: 10.1002/ajmg.1320050107 (1980). [DOI] [PubMed] [Google Scholar]
- 52.Elcioglu N. & Hall C. M. Temporal aspects in craniometaphyseal dysplasia: autosomal recessive type. American journal of medical genetics 76, 245–251 (1998). [DOI] [PubMed] [Google Scholar]
- 53.Iughetti P., lonso L. G., Wilcox W., lonso N. & Passos-Bueno M. R. Mapping of the autosomal recessive (AR) craniometaphyseal dysplasia locus to chromosome region 6q21–22 and confirmation of genetic heterogeneity for mild AR spondylocostal dysplasia. American journal of medical genetics 95, 482–491, doi: (2000). [DOI] [PubMed] [Google Scholar]
- 54.Beighton P., Hamersma H. & Horan F. Craniometaphyseal dysplasia--variability of expression within a large family. Clinical genetics 15, 252–258 (1979). [DOI] [PubMed] [Google Scholar]
- 55.Novelli G., rdito E., Mazzoleni F., Bozzetti, A. & Sozzi D.An atypical case of craniometaphyseal dysplasia. Case report and surgical treatment. Ann Stomatol (Roma) 8, 89–94, doi: 10.11138/ads/2017.8.2.045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Giepmans B. N. et al. Gap junction protein connexin-43 interacts directly with microtubules. Curr Biol 11, 1364–1368, doi: 10.1016/s0960-9822(01)00424-9 (2001). [DOI] [PubMed] [Google Scholar]
- 57.Dai P., Nakagami T., Tanaka H., Hitomi T. & Takamatsu T. Cx43 mediates TGF-beta signaling through competitive Smads binding to microtubules. Molecular biology of the cell 18, 2264–2273, doi: 10.1091/mbc.e06-12-1064 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saidi Brikci-Nigassa A. et al. Phosphorylation controls the interaction of the connexin43 C-terminal domain with tubulin and microtubules. Biochemistry 51, 4331–4342, doi: 10.1021/bi201806j (2012). [DOI] [PubMed] [Google Scholar]
- 59.Knothe Tate M. L., damson J. R., Tami E. & Bauer T. W. The osteocyte. The international journal of biochemistry & cell biology 36, 1–8, doi: 10.1016/s1357-2725(03)00241-3 (2004). [DOI] [PubMed] [Google Scholar]
- 60.Jaiprakash, A. et al. Phenotypic characterization of osteoarthritic osteocytes from the sclerotic zones: a possible pathological role in subchondral bone sclerosis. Int J Biol Sci 8, 406–417, doi: 10.7150/ijbs.4221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zimmerman S. M., Dimori M., Heard-Lipsmeyer M. E. & Morello R. The Osteocyte Transcriptome Is Extensively Dysregulated in Mouse Models of Osteogenesis Imperfecta. JBMR Plus 3, e10171, doi: 10.1002/jbm4.10171 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Balemans W. et al. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Human molecular genetics 10, 537–543, doi: 10.1093/hmg/10.5.537 (2001). [DOI] [PubMed] [Google Scholar]
- 63.White K. E. et al. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60, 2079–2086, doi: 10.1046/j.1523-1755.2001.00064.x (2001). [DOI] [PubMed] [Google Scholar]
- 64.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nature genetics 11, 130–136, doi: 10.1038/ng1095-130 (1995). [DOI] [PubMed] [Google Scholar]
- 65.Shimada T. et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proceedings of the National cademy of Sciences of the United States of America 98, 6500–6505, doi: 10.1073/pnas.101545198 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito N. et al. Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Molecular and cellular endocrinology 399, 208–218, doi: 10.1016/j.mce.2014.10.007 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Milette S. et al. Sexual dimorphism and the role of estrogen in the immune microenvironment of liver metastases. Nat Commun 10, 5745, doi: 10.1038/s41467-019-13571-x (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komm B. S. et al. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science 241, 81–84, doi: 10.1126/science.3164526 (1988). [DOI] [PubMed] [Google Scholar]
- 69.Onoe Y., Miyaura C., Ohta H., Nozawa S. & Suda T. Expression of estrogen receptor beta in rat bone. Endocrinology 138, 4509–4512, doi: 10.1210/endo.138.10.5575 (1997). [DOI] [PubMed] [Google Scholar]
- 70.Vidal O., Kindblom L. G. & Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 14, 923–929, doi: 10.1359/jbmr.1999.14.6.923 (1999). [DOI] [PubMed] [Google Scholar]
- 71.Sims N. A. et al. functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. The Journal of clinical investigation 111, 1319–1327, doi: 10.1172/JCI17246 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sims N. A. et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 30, 18–25, doi: 10.1016/s8756-3282(01)00643-3 (2002). [DOI] [PubMed] [Google Scholar]
- 73.Windahl S. H., Vidal O., ndersson G., Gustafsson J. A. & Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(−/−) mice. The Journal of clinical investigation 104, 895–901, doi: 10.1172/JCI6730 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hong S. H. et al. Computer-Automated Static, Dynamic and Cellular Bone Histomorphometry. J Tissue Sci Eng Suppl 1, 004, doi: 10.4172/2157-7552.S1-004 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rowe D. W. et al. Screening Gene Knockout Mice for Variation in Bone Mass: Analysis by muCT and Histomorphometry. Curr Osteoporos Rep 16, 77–94, doi: 10.1007/s11914-018-0421-4 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Cox W. G. & Singer V. L. A high-resolution, fluorescence-based method for localization of endogenous alkaline phosphatase activity. J Histochem Cytochem 47, 1443–1456, doi: 10.1177/002215549904701110 (1999). [DOI] [PubMed] [Google Scholar]
- 77.Kalajzic I. et al. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 17, 15–25, doi: 10.1359/jbmr.2002.17.1.15 (2002). [DOI] [PubMed] [Google Scholar]