Abstract
Research has identified clinical, genomic, and neurophysiological markers associated with suicide attempts (SA) among individuals with psychiatric illness. However, there is limited research among those with an alcohol use disorder (AUD), despite their disproportionately higher rates of SA. We examined lifetime SA in 4,068 individuals with DSM-IV alcohol dependence from the Collaborative Study on the Genetics of Alcoholism (23% lifetime suicide attempt; 53% female; 17% Admixed African American ancestries; mean age: 38). We 1) conducted a genome-wide association study (GWAS) of SA and performed downstream analyses to determine whether we could identify specific biological pathways of risk, and 2) explored risk in aggregate across other clinical conditions, polygenic scores (PGS) for comorbid psychiatric problems, and neurocognitive functioning between those with AD who have and have not reported a lifetime suicide attempt. The GWAS and downstream analyses did not produce any significant associations. Participants with an AUD who had attempted suicide had greater rates of trauma exposure, major depressive disorder, post-traumatic stress disorder, and other substance use disorders compared to those who had not attempted suicide. Polygenic scores for suicide attempt, depression, and PTSD were associated with reporting a suicide attempt (ORs = 1.22–1.44). Participants who reported a SA also had decreased right hemispheric frontal-parietal theta and decreased interhemispheric temporal-parietal alpha electroencephalogram resting-state coherences relative to those who did not, but differences were small. Overall, individuals with alcohol dependence who report SA appear to experience a variety of severe comorbidities and elevated polygenic risk for SA. Our results demonstrate the need to further investigate suicide attempts in the presence of substance use disorders.
Introduction
Approximately 2–5% of U.S. adults report having attempted suicide in their lifetimes [1–3], with the prevalence increasing in more recent birth cohorts [4]. Additionally, deaths by suicide are one of the leading causes in the recent decline in U.S. life expectancy, alongside other “deaths of despair” such as drug and alcohol related deaths [5, 6]. While the rate of suicide attempts in the general population is alarming, the rate of lifetime suicide attempts is greater than triple (17.5%) among those with an alcohol use disorder (AUD) [7]. Among those seeking treatment for AUD, 40% report at least one suicide attempt at some point in their lives [8–11]. A history of past suicide attempts is among the most prominent predictors of subsequent suicide death and contributes significant health care and disability costs per attempt [12]. Research focused on correlates of suicide attempts can potentially help identify and treat those with non-fatal suicide attempts, with the goal of reducing suicide deaths and saving lives [13]. Individuals with AUD have emerged from these data as a particularly high-risk group.
Genome-wide association studies (GWAS) have identified numerous genomic markers associated with AUD and similar phenotypes [14–17]. For AUD, a recent GWAS from the Million Veteran Program (MVP) [15] identified three loci previously associated with alcohol dependence [16] —ADH1B, ADH1C, and ADH4, and seven novel loci -- GCKR, SIX3, SLC39A8, DRD2, an intergenic variant on chr10q25.1 (rs7906104), and FTO. A meta-analysis of AUD between MVP, PGC and the Collaborative Study on the Genetics of Alcoholism (COGA), which included 48,545 AUD cases and 187,065 controls, identified 10 genome-wide significant loci. In terms of AUD-adjacent phenotypes, Sanchez-Roige et al. (2019) meta-analyzed GWAS of the alcohol use disorders identification test (AUDIT) in 141,932 individuals from the UK Biobank and 23andme [14]; replicating previously identified signals in the genes ADH1B, ADH1C, KLB, and GCKR and finding novel associations localized to genes including JCAD and SLC39A13. Zhou et al [17] identified 110 independent risk variants in a GWAS of “problematic alcohol use,” meta-analyzing results from MVP, UK Biobank, FinnGen, PGC, and others.
Recent GWASs have also identified genomic markers associated with suicide attempts (SA) broadly [18, 19] and among individuals with psychiatric illness [20, 21]. The Suicide Working Group of the Psychiatric Genomics Consortium, or PGC (formerly the International Suicide Genetics Consortium) recently identified 12 loci in a large scale meta-analysis of 43,871 individual who had a lifetime suicide attempt and 915,025 controls. Importantly, in prior analyses, the top loci SNP on chromosome 7 remained significant after conditioning on GWAS results for depression [20]. Other GWASs have identified genomewide significant variants for SA within those with other psychiatric disorders: rs45593736 was associated with suicide attempt in major depressive disorder, chr4_23273116_D was associated with SA in bipolar disorder, and rs138689899 was associated in the meta-analysis of suicide attempt in mood disorders (bipolar disorder + major depressive disorder). Levey et al [21] found one genome-wide significant signal near LDHB (rs1677091) in individuals of European ancestries and three associations among individuals of African ancestries, including: rs683813 (ARNTL2), rs72740082 (FAH), and rs11876255. Variants within LDHB and FAH replicated in an independent sample. However, despite the higher rates of suicide attempts, no prior GWAS has examined suicide attempt in the presence of AUD.
In a similar manner to the genetics of suicide and AUD, two separate literatures have explored neurocognitive differences between (a) individuals who have attempted suicide to those who have not [22–24] and (b) individuals with AUD [25–27] compared to those unaffected with AUD. Among those with AUD, deficits in many domains of brain functioning have been observed, including neuropsychological performance, and neurophysiological indices [25–27]. Executive functioning is the primary focus of such studies, with a large literature demonstrating that individuals with AUD display poorer executive functioning and atypical neurophysiological profiles (e.g., EEG connectivity) than individuals without AUD [28–31]. These areas of brain functioning have also been examined among individuals who have exhibited suicidal ideation and related mental health problems (depression) [22–24], though research focused on SA is limited.
While no previous studies have examined EEG connectivity and SA, Leuchter et al. [32] examined EEG connectivity in depressed patients and found evidence of higher alpha and theta coherences in frontal, temporal, and parietal regions, and higher beta coherence in frontal and temporal regions. Further, a recent study found other neurophysiological differences associated with binge drinking and suicidal behaviors in Mexican American and American Indian adolescents [33]. To our knowledge, no prior study has examined neural connectivity among those with AUD who have attempted suicide.
Given the higher rates of SA observed among those with AUD, we explored whether there are clinical, genomic, and neurophysiological markers of SA within this population. Among participants diagnosed with DSM-IV Alcohol Dependence (AD) drawn from the Collaborative Study on the Genetics of Alcoholism (COGA), we first conducted a genome-wide association study (GWAS) of SA and performed downstream analyses to determine whether we could identify specific biological pathways of risk. Next, to explore risk in aggregate, we examined whether clinical risk factors, polygenic scores (PGS) for comorbid psychiatric problems, and neurocognitive functioning differed between those with AD who have and have not reported a lifetime suicide attempt.
Methods
Sample and Measures
The Collaborative Study on the Genetics of Alcoholism (COGA) is a large, multi-site study of 17,854 participants from 2,255 families affected with AUD, designed to identify and understand genetic factors involved in the predisposition to alcoholism and related disorders, as previously described [34–36]. Participants 18 or older completed the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) which is a poly-diagnostic interview [34], and participants ages 12–17 completed an adolescent SSAGA. All participants were queried about whether they had “ever tried to kill” themselves (suicide attempt), regardless of a history of suicidal ideation (i.e., thoughts about killing yourself). Importantly, suicide attempt items were not exclusively nested within the diagnostic section for major depressive disorder (MDD), although individuals who reported suicide attempts in that section were coded accordingly as having reported the behavior.
Suicide attempt (SA) data derived from the SSAGA was available on 4,068 COGA participants with an alcohol dependence diagnosis (lifetime) and GWAS data (including 3,270 individuals of European ancestries and 798 individuals of African ancestries). For the current analyses, we included individuals reporting any suicide attempt, including those reporting drug-related suicide attempt (14% of all attempts). We created diagnoses of alcohol dependence, other substance dependence, other psychiatric disorders, suicidal thoughts and behaviors, and trauma exposure based upon DSM-IV criteria using the child and adult versions of the SSAGA. We assessed nicotine dependence using the Fagerström Test for Nicotine Dependence (FTND) scores [35]. Additionally, we included measures of sociodemographic characteristics, extended family histories of AD, and other alcohol-related problems (see supplementary information for a full description).
GWAS data
Genotyping, imputation and quality control have been described previously [36]. Briefly, genetic data were used to assign ancestry and families were classified as primarily European (EUR) or Admixed African American (AFR) ancestries according to the ancestry of the greatest proportion of family members [36]. Genotyping of 798 AFR individuals and 3270 EUR individuals included in the analytic sample was performed using the Illumina 2.5M array (Illumina, San Diego, CA, USA), the Illumina OmniExpress [37], the Illumina 1M array, or the Affymetrix Smokescreen array [38]. SNPs with a genotyping rate <98%, Hardy-Weinberg equilibrium violations (p<10−6), or with minor allele frequency (MAF) less than 3% were excluded from analyses. Mendelian inconsistencies were removed, after which data were imputed to 1000 genomes (Phase 3) using SHAPEIT [39] and IMPUTE2 [40]. Following imputation, dosage probabilities ≥ 0.90 were converted to hard calls. Mendelian errors in the imputed SNPs were reviewed and resolved as described previously [41, 42]. SNPs with an imputation information score < 0.30 or MAF < 0.03 were excluded from subsequent analysis.
Polygenic scores (PGS)
We estimated polygenic scores (PGS), which are aggregate measures of the number of risk alleles individuals carry weighted by effect sizes from GWAS summary statistics, for a variety of psychiatric and substance use phenotypes. We included PGS derived from recent GWAS of (1) alcohol use disorders (AUD) [43], (2) depression (DEP, 23andMe excluded) [44], (3) post-traumatic stress disorder (PTSD) [45], (4) bipolar disorders (BIP) [46, 47], (5) schizophrenia (SCZ) [47, 48] (6) smoking initiation (SMOK, as a proxy for externalizing risk) [49, 50] and (7) suicide attempt (SUI) [19]. For AUD and BIP, we meta-analyzed published GWAS results with corresponding results from FinnGen (release 9, see supplemental information for results) [51]. We focus on these PGS specifically because: 1) these disorders are phenotypically correlated with suicide attempt, and 2) they contain GWAS results for both European and African ancestries. For GWAS that originally included COGA in the discovery sample, we obtained summary statistics with COGA removed.
To date, GWAS have been overwhelmingly limited to individuals of European ancestries [52]. Because of variation in allele frequencies and linkage disequilibrium (LD) patterns, PGS often lose predictive accuracy when there is mismatch between the ancestries of the discovery GWAS and target sample [53, 54]. COGA includes participants of both African and European ancestries, thus we used PRS-CSx [55], a method that integrates GWAS summary statistics from well-powered GWAS (typically of European ancestries) with those from other populations to improve the predictive power of PGS in the participants of African ancestries in COGA. PRS-CSx employs a Bayesian approach to correct GWAS summary statistics for the non-independence of SNPs in LD. We converted PGS into Z-scores for ease of interpretation
Electroencephalogram (EEG) data
EEG recording and processing have been detailed previously [56]. Briefly, resting (eyes-closed) EEG was recorded for 4.25 min; a continuous interval of 256 seconds was analyzed. Each subject wore a fitted electrode cap using the 61-channel montage as specified according to the extended 10–20 International system. The nose served as reference and the ground electrode was placed on the forehead. Electrode impedances were always maintained below 5 kΩ. EEG was recorded with subjects seated comfortably in a dimly lit sound-attenuated temperature-regulated booth. They were instructed to keep their eyes closed and remain relaxed, but not to fall asleep. Electrical activity was amplified 10,000 times by Neuroscan and Masscomp amplifiers, with a bandpass between 0.02 Hz to 100 Hz and recorded using the Neuroscan system (Compumedics Limited; El Paso, TX). EEG procedures were identical at all COGA collection sites. Bipolar electrode pairs were derived to reduce volume conduction effects, and 27 representative coherence pairs were selected based on previous EEG coherence work in COGA [56]. Magnitude squared coherence was calculated from power spectral values derived from Fourier Conventional Fourier transform methods [57]. Coherence measures were generated between bipolar pairs at the following frequency bands: theta (3–7 Hz), alpha (7–12 Hz), beta (12–28 Hz).
Statistical analyses
We compared those with AD who reported a suicide attempt and those with AD who did not report a suicide attempt across a range of sociodemographic, clinical, and other measures. Multiple-group, multi-level regression models were conducted in Mplus [58] and adjusted for sex, age (at time of psychiatric assessment), ancestry, family history of AD, and family relatedness. We ran all models simultaneously (i.e., correlation among all variables accounted for) accounting for multiple testing.
We conducted GWAS, on 7,784,968 SNPs in the EUR sample and 16,100,604 SNPs in the AFR sample using a mixed model incorporating a genetic relationship matrix to control for relatedness [59] in the GWAF package in R [60]. We included sex, age, the first three ancestral PCs (PC1-PC3), genotype array, and birth cohort (prior to 1930, 1930–1949, 1950–1969, and 1970 and after) as covariates. GWAS were stratified by ancestries, using identical phenotypic definitions, covariates, SNP QC standards, MAF thresholds and imputation protocols. Subsequently, we meta-analyzed across the AFR and EUR results using inverse-variance fixed-effects weighting and genomic control in METAL [61]. We used established thresholds for genome wide significance (p< 5 × 10−8). We also conducted a post-hoc GWAS analysis covarying for depression, given the high prevalence.
Next we performed a series of post-GWAS analyses using a protocol outlined in previous analyses [62]. We limited results to the EUR only given the small sample size of the AFR analyses and the lack of AFR predicted transcriptomic expression results in some of the post-GWAS pipelines. To identify functional enrichment, we used MAGMA software (version 1.08), and its recent intersessions (FUMA version 1.3.6) [63], a method for gene-level and gene-set enrichment analysis using GWAS summary statistics. In all the MAGMA-based analyses, SNPs were annotated to the 20,260 coding genes from Ensembl v92, with a 1 kb window for both sides (i.e., start and end). Since GWAS contained EUR and AFR samples, we used the 1000G European and African panels [64] respectively to account for linkage disequilibrium (LD) between SNPs. Finally, we corrected all tests for multiple-testing using a Bonferroni correction.
Next, we used the summary-data-based Mendelian randomization (SMR) method to test for a joint association between GWAS summary statistics SNPs and eQTL, using the default settings in the SMR software [65] and the 1000G European ancestries reference panel. We again applied a Bonferroni correction for multiple-testing on the SMR P-value (PSMR). Moreover, a post-filtering step was applied by conducting heterogeneity in dependent instruments (HEIDI) test. The HEIDI test distinguishes the causality and pleiotropy models from the linkage model by considering the pattern of associations using all the SNPs that are significantly associated with gene expression in the cis-eQTL region. The null hypothesis is that a single variant is associated with both trait and gene expression, while the alternative hypothesis is that trait and gene expression are associated with two distinct variants. We defined significant hits based on SMR-HEIDΙ as those for which PSMR met the Bonferroni significance threshold and had PHEIDI>0.05.
Lastly, we used the JEPEGMIX2-P software [66] with default settings to conduct TWAS using only the 13 brain-specific GReX models coming from GTeX v8 [67]. This method was preferable since it relied on a covariance matrix based on 33K samples compared to other TWAS methods which use less than 3k samples. We applied the within-tissue Bonferroni correction to detect significant TWAS genes.
For polygenic scores, we first compared those with AD who had reported a suicide attempt to those with AD who had not reported a suicide attempt across all PGSs, independently, using logistic regression in R (version 4.2.1). Second, to ensure that results within those with AD were not biased by conditioning on AD [68], we also compared: 1) those with AD who had a reported suicide attempt, 2) those with AD who had not reported a suicide attempt, and 3) those without AD who had a reported suicide attempt to those who neither reported a suicide attempt nor meet criteria for AD (see Supplemental Table 1 for sample description) using a multinomial logistic regression model in the nnet package in R [69]. In both analyses, we included sex, age, the first three ancestral PCs (PC1-PC3), genotype array, and birth cohort as covariates. To adjust for familial clustering, we used cluster robust standard errors [70, 71]. We stratified analyses by ancestry and then meta-analyzed results (by PGS) within each of the analyses above. All analyses were corrected for multiple testing.
Lastly, we compared those with AD who reported a suicide attempt and those with AD who did not report a suicide attempt across neurophysiological measures (resting state EEG coherence) using multiple-group, multi-level regression models were in Mplus. We included covariates for sex, age (at time of assessment), ancestry, family history of AD, and family relatedness.
Results
GWAS and Post-GWAS analyses
Within the analytic sample we performed a GWAS of SA in those with available genetic data (no SA = 2,495 EUR and 643 AFR; SA = 775 EUR and 155 AFR). There was no individual SNP associated with suicide attempts that reached genome-wide significance (see supplemental information for full results). For the post-GWAS analyses, there were no significant gene-based or gene-set enrichment from the MAGAMA results (see supplemental tables S1-S2). Additionally, none of the results from the SMR analyses reached significance after correcting for multiple testing (see supplemental tables S3-S5).
Clinical Risk Factors Associated with Suicide Attempt in Participants with AD
The main analytic sample was limited to the 4,068 participants with a DSM-IV diagnoses of alcohol dependence (AD). We compared 3,138 COGA participants who met criteria for DSM-IV AD and did not attempt suicide in their lifetime with 930 participants with AD who attempted suicide. Overall, those with AD who attempted suicide were more likely to be female (53% vs. 32%). Rates of suicide attempt and the age distribution of participants were similar across ancestry groups (see Table S6 for ancestry stratified results). Table 1 presents the full set of comparisons across groups. The majority (58.4%) of the analytic sample endorsed suicidal ideation at some point in their lifetime; of those who attempted suicide, 97.6% endorsed prior suicidal ideation compared to 46.8% of those who did not attempt suicide. Participants with AD who had attempted suicide were more likely to have been exposed to traumatic events in their life, and to meet lifetime criteria for major depressive disorder, post-traumatic stress disorder, and other drug use disorders compared to those who had not attempted suicide. In addition, participants with AD that reported attempting suicide had higher family history densities of AD [72], started drinking at an earlier age, and had more severe indicators of alcohol-related problems.
Table 1.
Sociodemographic Indicators, Trauma Exposure, Psychiatric and Substance Use Disorders in COGA Participants with Alcohol Dependence (N = 4,068)
| No Suicide Attempt (N = 3,138) | Suicide Attempt (N = 930) | |
|---|---|---|
| Socio-demographics | ||
| Female (%) | 31.8% | 53.1%* |
| Black or African American (%) | 20.5% | 16.7% |
| Hispanic (%) | 6.2% | 8.5% |
| Mean age at last interview (SD) | 39.9 (11.9) | 38.2 (10.5) |
| Suicide related behavior | ||
| Suicidal Ideation (%) | 46.8% | 97.6%* |
| Alcohol Use Disorder Indicators | ||
| Maximum # of AD criteria endorsed | 5.1 (1.7) | 5.6 (1.6)* |
| Maximum # drinks consumed/24hrs | 28.8 (18.6) | 34.2 (22.1)* |
| Mean age of AD onset (SD) | 24.1 (8.4) | 22.5 (7.3)* |
| Mean age of first whole drink (SD) | 15.0 (2.3) | 13.7 (2.4)* |
| Ratio of first-degree relatives with AD | 0.4 | 0.5* |
| Trauma Exposure | ||
| Sexual Assaultive Trauma (%) | 23.0% | 45.1* |
| Assaultive Trauma (%) | 34.5% | 53.8* |
| Non-Assaultive Trauma (%) | 59.3% | 71.2* |
| DSM-IV Psychiatric Comorbidities | ||
| Major Depression (%) | 9.6% | 56.0%* |
| Panic disorder (%) | 2.5% | 5.3% |
| Obsessive Compulsive Disorder (%) | 0.7% | 5.3% |
| Social phobia (%) | 5.3% | 10.6% |
| Agoraphobia (%) | 3.5% | 10.8% |
| Post-Traumatic Stress Disorder (%) | 6.3% | 19.1%* |
| Anorexia Nervosa (%) | 0.0% | 2.1% |
| Bulimia (%) | 3.4% | 15.4% |
| Mania (%) | 0.5% | 2.1% |
| Attention-Deficit Hyperactive Disorder (%) | 7.1 % | 4.3% |
| Conduct Disorder (%) | 31.3% | 31.9% |
| Antisocial Personality Disorder (%) | 22.6% | 30.9% |
| Nicotine Dependence (%) | 53.8% | 73.7%* |
| Cannabis Dependence (%) | 34.7% | 28.7% |
| Cocaine Dependence (%) | 29.2% | 35.1%* |
| Stimulant Dependence (%) | 13.4% | 18.1%* |
| Sedative Dependence (%) | 5.3% | 13.7%* |
| Opioid Dependence (%) | 10.4% | 12.6% |
p < .05
Polygenic Scores
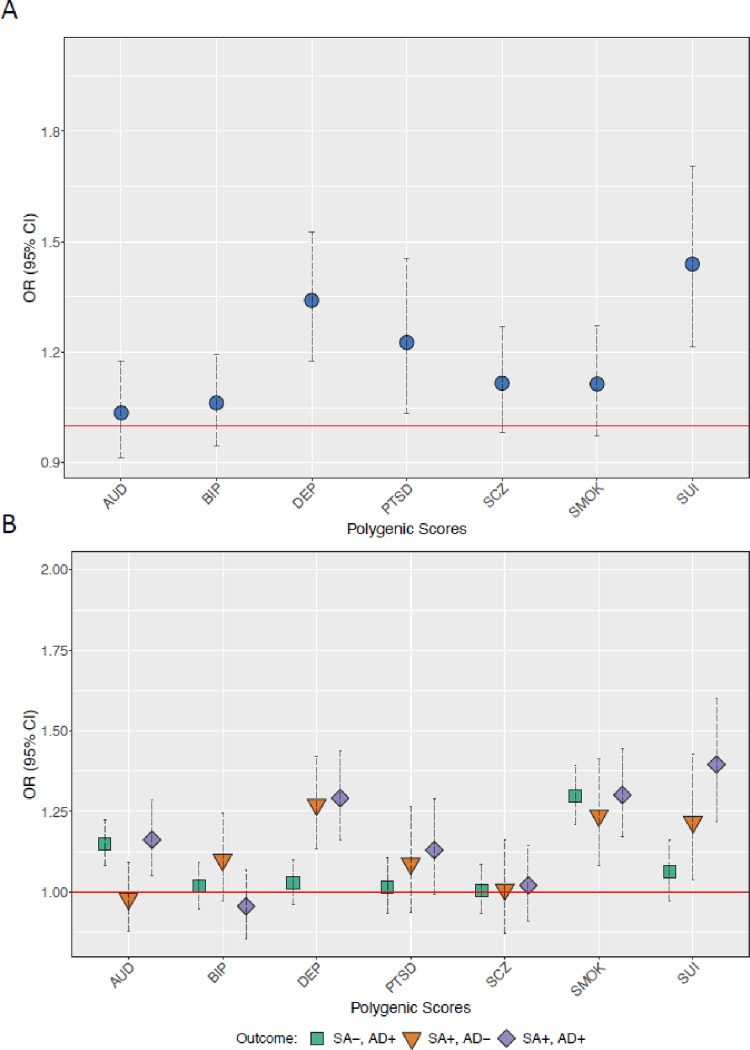
Figure 1, Panel A presents the meta-analyzed results for associations between each of the corresponding PGSs and lifetime suicide attempt within those meeting criteria for AD. PGSs for DEP (ORMETA = 1.34, 95% CI = 1.18, 1.53), PTSD (ORMETA = 1.23, 95% CI = 1.03, 1.45), and SUI (ORMETA = 1.44, 95% CI = 1.22, 1.70) were associated with increased odds of reporting suicide attempt. However, the AUD, BIP, SCZ, and SMOK PGSs were not associated with suicide attempt (ancestry-specific results in Table S8).
Figure 1.
Polygenic Scores for AUD, DEP and SUI across those who have and have not reported a suicide attempt
Panel A presents odds ratios (OR) for AUD, DEP, and SUI PGSs from logistic regression models in persons with AD who had and had not attempted suicide. Panel B presents OR from multinomial logistic models (no AD, no suicide attempt as reference group). All models include cohort, sex, PC1-PC3, array, and site as covariates. SEs adjusted for familial clustering using cluster-robust standard errors. AFR = African Ancestries, EUR = European Ancestries, AUD = alcohol use disorder polygenic score, DEP = depression polygenic score, = SUI suicide attempt polygenic score, SA− = no lifetime suicide attempt, AD− = does not meet criteria for alcohol dependence, SA+ = lifetime suicide attempt, AD+ = meets criteria for alcohol dependence.
Figure 1 (Panel B) shows conditional PGS results from the multinomial logistic models comparing those with AD who had attempted suicide (AD+, SA+), those with AD who had not attempted suicide (AD+, SA−), and those without AD who had attempted suicide (AD−, SA+) to those without an AD diagnosis and who had not attempted suicide (full results in Table S9. The AUD (ORMETA = 1.16, 95% CI = 1.05, 1.28), DEP (ORMETA = 1.29, 95% CI = 1.16, 1.44), SMOK (ORMETA = 1.30, 95% CI = 1.17, 1.44), and SUI (ORMETA = 1.40, 95% CI = 1.22, 1.60) PGSs were all associated with increased odds of being in the AD+, SA + group. Interestingly, the only differences in results between the AD+, SA + group and the AD+, SA− group was in the DEP and SUI PGSs, while the only differences in results between the AD+, SA + group and the AD−, SA+ group was in the AUD PGS.
Neurophysiological Findings
We observed nominal differences in resting state EEG coherence patterns of alcohol dependent individuals who had attempted suicide compared to those who had not attempted suicide. However, only two findings withstood multiple test correction: decreased right hemispheric frontal-parietal theta (3–7Hz @ F8-F4-P8-P4) and decreased interhemispheric temporal-parietal alpha (7–12 Hz @ T8-P8-T7-P7) EEG resting-state coherences (p < 0.001, Supplemental Fig. 3). Exploratory analyses within a subset of individuals who had available neurocognitive measures is available in the supplementary information.
Discussion
Researchers have begun to identify clinical, genomic, and neurophysiological correlates of suicide attempts among individuals with and without psychiatric illnesses (i.e., schizophrenia, bipolar disorder, depression) [13, 14, 15, 16]. However, this has yet to be examined among those with AUD, despite the higher rates of suicide attempts observed among those with AUD. The current study identified distinct clinical, genomic, and neurophysiological associations with lifetime suicide attempt among individuals who meet criteria for alcohol dependence.
In terms of genomic findings, none of the GWAS results or downstream analyses produced any robust associations. Given the large sample sizes necessary for discovery [73, 74] of genetic associations, these results are not surprising. Future studies with larger samples and even greater ancestral diversity are needed to identify genes and biological pathways implicated in SA.
When we examined risk factors in aggregate, participants with AD in COGA had elevated levels of suicidal ideation, other substance use disorders, and trauma exposure compared to the general population [2, 71]. However, among those who met criteria for AD and reported suicide attempts had even greater levels of all types of traumas (sexual, assaultive, and non-assaultive), other substance use disorders, suicidal ideation, and comorbid psychiatric conditions (PTSD and major depressive disorder) relative to those who had not attempted suicide. These results confirm that those with AD who report a lifetime suicide attempt are clinically high-risk group. Future work should utilize prospective information to determine whether these individuals have similar trajectories of psychiatric problems across time.
The polygenic scores for suicide attempt, depression, and PTSD were associated with a lifetime suicide attempt in persons with AD in the meta-analyzed results. Exploration of the ancestry-specific result demonstrate these were primarily driven by the associations in the EUR participants. The lack of associations of PGSs in those of African ancestries likely stems from the relatively small sample sizes of the discovery GWASs [73]. Importantly, in the multinomial logistic regression models, those with AD did not differ in mean levels of AUD PGS regardless of whether they had reported a lifetime suicide attempt. Similar to the logistic regression models limited to persons with AD, those who had attempted suicide had higher suicide and depression PGSs.
We also observed significant decreases in right hemispheric frontal-parietal theta (3–7 Hz @ F8-F4-P8-P4) and interhemispheric temporal-parietal alpha (7–12 Hz @ T8-P8-T7-P7) EEG resting-state coherences in the resting state among AD individuals who had attempted suicide. While there are no prior studies of suicide attempt that examined EEG coherence, differences in alpha and theta coherences in frontal, temporal, and parietal regions, and higher beta coherence in frontal and temporal regions were found previously in depressed patients [76]. Together, these data suggest that while both decreased theta and alpha resting state connectivity are likely among AD individuals with depression and suicide attempts, but more data are needed to make definitive any conclusions.
We note several important limitations. We note that suicide rates have changed significantly over the past several decades, partially spanning the interval of data collection. Additional research is needed in the area of suicide attempts among individuals with substance use disorders and other psychiatric comorbidities. While this study focused on AD, there is also a high rate of suicide attempts among individuals with other substance use disorders, particularly cocaine and opioid use disorders. Additionally, we do not have data on those who died by suicide, which may differ from those who have attempted but not taken their own lives. Lastly, larger and more diverse samples are needed so that the benefits of this research can benefit all segments of the population [77].
Research is beginning to identify risk factors for suicidal behaviors. In the current analysis, we demonstrated that polygenic scores for suicide attempt, depression, and PTSD, and lower neurophysiological functioning were associated with suicide attempts among individuals with AD. Future work with larger and more diverse samples can examine additional risk factors, such as social and environmental conditions. Identifying robust predictive markers within an already high risk group may allow for earlier intervention and prevention from unnecessary loss of human life.
Acknowledgements
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism under award numbers U01AA008401. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This research also used summary data from the Psychiatric Genomics Consortium (PGC), the Million Veterans Program (MVP), and the International Suicide Genetics Consortium (ISGC, now part of the PGC). We would like to thank the many studies that made these consortia possible, the researchers involved, and the participants in those studies, without whom this effort would not be possible.
The Collaborative Study on the Genetics of Alcoholism (COGA), Principal Investigators B. Porjesz, V. Hesselbrock, T. Foroud; Scientific Director, A. Agrawal; Translational Director, D. Dick, includes ten different centers: University of Connecticut (V. Hesselbrock); Indiana University (H.J. Edenberg, T. Foroud, Y. Liu, M.H. Plawecki); University of Iowa Carver College of Medicine (S. Kuperman, J. Kramer); SUNY Downstate Health Sciences University (B. Porjesz, J. Meyers, C. Kamarajan, A. Pandey); Washington University in St. Louis (L. Bierut, J. Rice, K. Bucholz, A. Agrawal); University of California at San Diego (M. Schuckit); Rutgers University (J. Tischfield, D. Dick, R. Hart, J. Salvatore); The Children’s Hospital of Philadelphia, University of Pennsylvania (L. Almasy); Icahn School of Medicine at Mount Sinai (A. Goate, P. Slesinger); and Howard University (D. Scott). Other COGA collaborators include: L. Bauer (University of Connecticut); J. Nurnberger Jr., L. Wetherill, X., Xuei, D. Lai, S. O’Connor, (Indiana University); G. Chan (University of Iowa; University of Connecticut); D.B. Chorlian, J. Zhang, P. Barr, S. Kinreich, G. Pandey (SUNY Downstate); N. Mullins (Icahn School of Medicine at Mount Sinai); A. Anokhin, S. Hartz, E. Johnson, V. McCutcheon, S. Saccone (Washington University); J. Moore, F. Aliev, Z. Pang, S. Kuo (Rutgers University); A. Merikangas (The Children’s Hospital of Philadelphia and University of Pennsylvania); H. Chin and A. Parsian are the NIAAA Staff Collaborators. We continue to be inspired by our memories of Henri Begleiter and Theodore Reich, founding PI and Co-PI of COGA, and also owe a debt of gratitude to other past organizers of COGA, including Ting-Kai Li, P. Michael Conneally, Raymond Crowe, and Wendy Reich, for their critical contributions. This national collaborative study is supported by NIH Grant U10AA008401 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the National Institute on Drug Abuse (NIDA).
Footnotes
Declarations
Disclosures
The authors do not have any conflicts of interest to report.
Contributor Information
Peter Barr, SUNY Downstate Health Sciences University.
Zoe Neale, SUNY Downstate Health Sciences University.
Chris Chatzinakos, SUNY Downstate Health Sciences University.
Jessica Schulman, Icahn School of Medicine.
Niamh Mullins, Icahn School of Medicine.
Chella Kamarajan, SUNY Downstate Health Sciences University.
Sivan Kinreich, SUNY Downstate Medical Center.
Ashwini Pandey, State University of New York Downstate Health Sciences University.
Howard Edenberg, Indiana University School of Medicine.
Victor Hesselbrock, University of Connecticut.
Emma Johnson, Washington University School of Medicine.
Dongbing Lai, Indiana University.
Martin Plawecki, Indiana University School of Medicine.
Leah Wetherill, Indiana University School of Medicine.
Arpana Agrawal, Washington University in St. Louis.
Bernice Porjesz, Downstate Medical Center.
Jacquelyn Meyers, State University of New York (SUNY) Downstate Health Sciences University.
References
- 1.Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617–626. [DOI] [PubMed] [Google Scholar]
- 2.Baca-Garcia E, Perez-Rodriguez MM, Keyes KM, Oquendo MA, Hasin DS, Grant BF, et al. Suicidal ideation and suicide attempts in the United States: 1991–1992 and 2001–2002. Mol Psychiatry. 2010;15:250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scheer V, Blanco C, Olfson M, Lemogne C, Airagnes G, Peyre H, et al. A comprehensive model of predictors of suicide attempt in individuals with panic disorder: Results from a national 3-year prospective study. Gen Hosp Psychiatry. 2020;67:127–135. [DOI] [PubMed] [Google Scholar]
- 4.Olfson M, Blanco C, Wall M, Liu SM, Saha TD, Pickering RP, et al. National Trends in Suicide Attempts Among Adults in the United States. JAMA Psychiatry. 2017;74:1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Case A, Deaton A. Rising morbidity and mortality in midlife among white non-Hispanic Americans in the 21st century. Proc Natl Acad Sci U S A. 2015;112:15078–15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tilstra AM, Simon DH, Masters RK. Trends in ‘Deaths of Despair’ among Working-Aged White and Black Americans, 1990–2017. Am J Epidemiol. 2021;190:1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potash JB, Scott Kane MH, Chiu Y, Simpson SG, Dean MacKinnon MF, McInnis MG, et al. Attempted Suicide and Alcoholism in Bipolar Disorder: Clinical and Familial Relationships. Am J Psychiatry. 2000;157:12. [DOI] [PubMed] [Google Scholar]
- 8.Sher L. Alcoholism and suicidal behavior: A clinical overview. Acta Psychiatr Scand. 2006;113:13–22. [DOI] [PubMed] [Google Scholar]
- 9.Koller G, Preuß UW, Bottlender M, Wenzel K, Soyka M. Impulsivity and aggression as predictors of suicide attempts in alcoholics. Eur Arch Psychiatry Clin Neurosci. 2002;252:155–160. [DOI] [PubMed] [Google Scholar]
- 10.Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet. 2013;382:1575–1586. [DOI] [PubMed] [Google Scholar]
- 11.Modesto-Lowe V, Brooks D, Ghani M. Alcohol dependence and suicidal behavior: From research to clinical challenges. Harv Rev Psychiatry. 2006;14:241–248. [DOI] [PubMed] [Google Scholar]
- 12.Shepard DS, Gurewich D, Lwin AK, Reed GA, Silverman MM. Suicide and Suicidal Attempts in the United States: Costs and Policy Implications. Suicide Life Threat Behav. 2016;46:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuodelis-Flores C, Ries RK. Addiction and suicide: A review. American Journal on Addictions. 2015;24:98–104. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez-Roige S, Palmer AA, Fontanillas P, Elson SL, Adams MJ, Howard DM, et al. Genome-Wide Association Study Meta-Analysis of the Alcohol Use Disorders Identification Test (AUDIT) in Two Population-Based Cohorts. American Journal of Psychiatry. 2019;176:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kranzler HR, Zhou H, Kember RL, Vickers Smith R, Justice AC, Damrauer S, et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat Commun. 2019;10:1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walters RK, Polimanti R, Johnson EOECEO, McClintick JN, Adams MJ, Adkins AE, et al. Trans-ancestral GWAS of alcohol dependence reveals common genetic underpinnings with psychiatric disorders. Nat Neurosci. 2018;21:1656–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H, Kember RL, Deak JD, Xu H, Toikumo S, Yuan K, et al. Multi-ancestry study of the genetics of problematic alcohol use in over 1 million individuals. Nat Med. 2023;29:3184–3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mullins N, Kang JE, Campos AI, Coleman JRI, Edwards AC, Galfalvy H, et al. Dissecting the Shared Genetic Architecture of Suicide Attempt, Psychiatric Disorders, and Known Risk Factors. Biol Psychiatry. 2022;91:313–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Docherty AR, Mullins N, Ashley-Koch AE, Qin X, Coleman JRI, Shabalin A, et al. GWAS Meta-Analysis of Suicide Attempt: Identification of 12 Genome-Wide Significant Loci and Implication of Genetic Risks for Specific Health Factors. American Journal of Psychiatry. 2023;180:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullins N, Bigdeli TB, Børglum AD, Coleman JRI, Demontis D, Mehta D, et al. GWAS of suicide attempt in psychiatric disorders and association with major depression polygenic risk scores. American Journal of Psychiatry. 2019;176:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey DF, Polimanti R, Cheng Z, Zhou H, Nuñez YZ, Jain S, et al. Genetic associations with suicide attempt severity and genetic overlap with major depression. Translational Psychiatry 2019 9:1. 2019;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richard-Devantoy S, Berlim MT, Jollant F. A meta-analysis of neuropsychological markers of vulnerability to suicidal behavior in mood disorders. Psychol Med. 2014;44:1663–1673. [DOI] [PubMed] [Google Scholar]
- 23.Richard-Devantoy S, Gorwood P, Annweiler C, Olié JP, Le Gall D, Beauchet O. Suicidal behaviours in affective disorders: A deficit of cognitive inhibition? Canadian Journal of Psychiatry. 2012;57:254–262. [DOI] [PubMed] [Google Scholar]
- 24.Keilp JG, Gorlyn M, Russell M, Oquendo MA, Burke AK, Harkavy-Friedman J, et al. Neuropsychological function and suicidal behavior: Attention control, memory and executive dysfunction in suicide attempt. Psychol Med. 2013;43:539–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Berre AP. Emotional processing and social cognition in alcohol use disorder. Neuropsychology. 2019;33:808–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Berre AP, Fama R, Sullivan E V. Executive Functions, Memory, and Social Cognitive Deficits and Recovery in Chronic Alcoholism: A Critical Review to Inform Future Research. Alcohol Clin Exp Res. 2017;41:1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cabé N, Laniepce A, Ritz L, Lannuzel C, Boudehent C, Vabret F, et al. Cognitive impairments in alcohol dependence: From screening to treatment improvements. Encephale. 2016;42:74–81. [DOI] [PubMed] [Google Scholar]
- 28.Park SM, Lee JY, Kim YJ, Lee J-Y, Jung HY, Sohn BK, et al. Neural connectivity in Internet gaming disorder and alcohol use disorder: A resting-state EEG coherence study. Sci Rep. 2017;7:1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardenas VA, Price M, Fein G. EEG coherence related to fMRI resting state synchrony in long-term abstinent alcoholics. Neuroimage Clin. 2018;17:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mumtaz W, Vuong PL, Xia L, Malik AS, Rashid RBA. An EEG-based machine learning method to screen alcohol use disorder. Cogn Neurodyn. 2017;11:161–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamarajan C, Ardekani BA, Pandey AK, Chorlian DB, Kinreich S, Pandey G, et al. Random forest classification of alcohol use disorder using EEG source functional connectivity, neuropsychological functioning, and impulsivity measures. Behavioral Sciences. 2020;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leuchter AF, Cook IA, Hunter AM, Cai C, Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS One. 2012;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ehlers CL, Wills DN, Karriker-Jaffe KJ, Gilder DA, Phillips E, Bernert RA. Delta Event-Related Oscillations Are Related to a History of Extreme Binge Drinking in Adolescence and Lifetime Suicide Risk. Behavioral Sciences. 2020;10:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, et al. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55:149–158. [DOI] [PubMed] [Google Scholar]
- 35.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 36.Lai D, Kapoor M, Wetherill L, Schwandt M, Ramchandani VA, Goldman D, et al. Genome-wide admixture mapping of DSM-IV alcohol dependence, criterion count, and the self-rating of the effects of ethanol in African American populations. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2021;186:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J-C, Foroud T, Hinrichs AL, Le NXH, Bertelsen S, Budde JP, et al. A genome-wide association study of alcohol-dependence symptom counts in extended pedigrees identifies C15orf53. Mol Psychiatry. 2013;18:1218–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baurley JW, Edlund CK, Pardamean CI, Conti D V., Bergen AW. Smokescreen: a targeted genotyping array for addiction research. BMC Genomics. 2016;17:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delaneau O, Howie B, Cox AJ, Zagury J-F, Marchini J. Haplotype estimation using sequencing reads. Am J Hum Genet. 2013;93:687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wetherill L, Agrawal A, Kapoor M, Bertelsen S, Bierut LJ, Brooks A, et al. Association of substance dependence phenotypes in the COGA sample. Addiction Biology. 2015;20:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyers JL, Zhang J, Wang JC, Su J, Kuo SI, Kapoor M, et al. An endophenotype approach to the genetics of alcohol dependence: a genome wide association study of fast beta EEG in families of African ancestry. Mol Psychiatry. 2017. 2017. 10.1038/mp.2016.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou H, Sealock JM, Sanchez-Roige S, Clarke TK, Levey DF, Cheng Z, et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat Neurosci. 2020. 2020. 10.1038/s41593-020-0643-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levey DF, Stein MB, Wendt FR, Pathak GA, Zhou H, Aslan M, et al. Bi-ancestral depression GWAS in the Million Veteran Program and meta-analysis in > 1.2 million individuals highlight new therapeutic directions. Nat Neurosci. 2021. 27 May 2021. 10.1038/s41593-021-00860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019. 2019. 10.1038/s41467-019-12576-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mullins N, Forstner AJ, O’Connell KS, Coombes B, Coleman JRI, Qiao Z, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021. 2021. 10.1038/s41588-021-00857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bigdeli TB, Fanous AH, Li Y, Rajeevan N, Sayward F, Genovese G, et al. Genome-Wide Association Studies of Schizophrenia and Bipolar Disorder in a Diverse Cohort of US Veterans. Schizophr Bull. 2020. 2020. 10.1093/schbul/sbaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trubetskoy V, Pardiñas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saunders GRB, Wang X, Chen F, Jang SK, Liu M, Wang C, et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature 2022 612:7941. 2022;612:720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karlsson Linnér R, Mallard TT, Barr PB, Sanchez-Roige S, Madole JW, Driver MN, et al. Multivariate analysis of 1.5 million people identifies genetic associations with traits related to self-regulation and addiction. Nat Neurosci. 2021:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurki MI, Karjalainen J, Palta P, Sipilä TP, Kristiansson K, Donner KM, et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature. 2023;613:508–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mills MC, Rahal C. A scientometric review of genome-wide association studies. Commun Biol. 2019;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin AR, Gignoux CR, Walters RK, Wojcik GL, Neale BM, Gravel S, et al. Human Demographic History Impacts Genetic Risk Prediction across Diverse Populations. Am J Hum Genet. 2017;100:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duncan L, Shen H, Gelaye B, Meijsen J, Ressler K, Feldman M, et al. Analysis of polygenic risk score usage and performance in diverse human populations. Nat Commun. 2019. 2019. 10.1038/s41467-019-11112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruan Y, Lin YF, Feng YCA, Chen CY, Lam M, Guo Z, et al. Improving polygenic prediction in ancestrally diverse populations. Nat Genet. 2022;54:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chorlian DB, Rangaswamy M, Porjesz B. EEG coherence: Topography and frequency structure. Exp Brain Res. 2009;198:59–83. [DOI] [PubMed] [Google Scholar]
- 57.Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB, et al. EEG coherency. I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalogr Clin Neurophysiol. 1997;103:499–515. [DOI] [PubMed] [Google Scholar]
- 58.Wannisa Rattana, Narunart Rattana. 16262. Journal. 2558;20:5. [Google Scholar]
- 59.Lai D, Wetherill L, Bertelsen S, Carey CE, Kamarajan C, Kapoor M, et al. Genome-wide association studies of alcohol dependence, DSM-IV criterion count and individual criteria. Genes Brain Behav. 2019;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen MH, Yang Q. GWAF: An R package for genome-wide association analyses with family data. Bioinformatics. 2009;26:580–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chatzinakos C, Pernia CD, Morrison FG, Iatrou A, McCullough KM, Schuler H, et al. Single-Nucleus Transcriptome Profiling of Dorsolateral Prefrontal Cortex: Mechanistic Roles for Neuronal Gene Expression, Including the 17q21.31 Locus, in PTSD Stress Response. Am J Psychiatry. 2023;180:739–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe K, Taskesen E, van Bochoven A, Posthuma D. Functional mapping and annotation of genetic associations with FUMA. Nat Commun. 2017;8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Auton A, Abecasis GR, Altshuler DM, Durbin RM, Bentley DR, Chakravarti A, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu Z, Zhang F, Hu H, Bakshi A, Robinson MR, Powell JE, et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat Genet. 2016;48:481–487. [DOI] [PubMed] [Google Scholar]
- 66.Chatzinakos C, Georgiadis F, Lee D, Cai N, Vladimirov VI, Docherty A, et al. TWAS pathway method greatly enhances the number of leads for uncovering the molecular underpinnings of psychiatric disorders. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2020;183:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Consortium TGte, Aguet F, Anand S, Ardlie KG, Gabriel S, Getz GA, et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science (1979). 2020;369:1318–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Akimova ET, Breen R, Brazel DM, Mills MC. Gene-environment dependencies lead to collider bias in models with polygenic scores. Sci Rep. 2021;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ripley B, Venables W. Feed-Forward Neural Networks and Multinomial Log-Linear Models. R package Version 7.3–17. 2022. 28 September 2022. [Google Scholar]
- 70.Davenport C, Soule SA, Armstrong DA. Protesting while black? the differential policing of american activism, 1960 to 1990. Am Sociol Rev. 2011;76:152–178. [Google Scholar]
- 71.Colin Cameron A, Gelbach JB, Miller DL. Robust inference with multiway clustering. Journal of Business and Economic Statistics. 2011;29:238–249. [Google Scholar]
- 72.Pandey G, Seay MJ, Meyers JL, Chorlian DB, Pandey AK, Kamarajan C, et al. Density and Dichotomous Family History Measures of Alcohol Use Disorder as Predictors of Behavioral and Neural Phenotypes: A Comparative Study Across Gender and Race/Ethnicity. Alcohol Clin Exp Res. 2020;44:697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dudbridge F. Power and Predictive Accuracy of Polygenic Risk Scores. PLoS Genet. 2013;9:e1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang M, Xu S. Statistical power in genome-wide association studies and quantitative trait locus mapping. Heredity (Edinb). 2019;123:287–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grant BF, Saha TD, June Ruan W, Goldstein RB, Patricia Chou S, Jung J, et al. Epidemiology of DSM-5 drug use disorder results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatry. 2016;73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leuchter AF, Cook IA, Hunter AM, Cai C, Horvath S. Resting-state quantitative electroencephalography reveals increased neurophysiologic connectivity in depression. PLoS One. 2012;7:e32508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet. 2019;51:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]