Abstract
Purpose
Overweight and obesity are common in Australia and among the leading risk factors for ill health. Maintained weight loss of >5–10% can prevent and reduce the risk of obesity-related comorbidities. Prescription weight loss medications plus lifestyle interventions can result in additional weight loss compared with lifestyle interventions alone, but these medications are under-prescribed in Australia. Our aim was to develop a greater understanding of the treatment preferences of people with overweight or obesity and the healthcare practitioners (HCPs) who treat them.
Participants and Methods
An online survey of Australian adults with overweight or obesity and treating HCPs was conducted in 2020. A discrete choice experiment (DCE) approach was used to determine what is most important to people when evaluating oral and injectable prescription weight loss medications. Participants were asked to choose between three hypothetical treatment alternatives: “Oral pill”; “Subcutaneous injection pen (replaceable needle)”; “Disposable subcutaneous injection pen (hidden needle)”; and an opt-out option (“None of these”).
Results
The online survey and DCE were completed by 193 patients and 104 HCPs. For both patients and HCPs, all treatment alternatives (oral, replaceable injection and disposable injection) were preferred over the opt-out. Gastrointestinal side effects, followed by success rate, percentage body weight lost, and cost were the most important attributes to patients. For HCPs, percentage body weight loss was the most important treatment attribute, followed by success rate, gastrointestinal side effects and cost. While most patients reported relatively low needle fear, physicians reported relatively high perceived patient needle fear.
Conclusion
Clinician-patient discussions about treatments for weight loss should cover the option of prescription weight loss medications, including injectable medications, which patients may be less apprehensive about than physicians believe. Treatments with a high success rate and low or manageable risk of gastrointestinal side effects may be preferred over alternatives.
Keywords: discrete choice experiment, overweight, obesity, treatment preferences, treatment, shared decision-making
Plain Language Summary
Overweight and obesity are the leading cause of health problems in Australia. Medications can be effective for people with this condition when combined with diet and exercise changes, but weight-loss medications in Australia are under-used.
To understand why, we surveyed 193 people living with overweight and obesity (“patients”), and 104 healthcare professionals (“HCPs”/doctors) who prescribe weight-loss medications. The online survey asked patients and HCPs to choose between medications with different benefits and risks, to uncover what features are most important to them (eg, would they prefer a medication that helps lose more weight, even if it means more side effects?).
We found that both patients and HCPs would prefer to take/prescribe weight-loss medication than go medication-free. Patients preferred to take medications with low risk of gastrointestinal side effects (nausea, diarrhea/vomiting), that would help them lose the most amount of weight, and were not too expensive.
HCPs preferred to prescribe medications that would help their patients lose the most amount of weight, that had the highest chance of success, had low risk of gastrointestinal side effects, and low-cost. HCPs also thought patients were more afraid of injectable medications than they actually were (most patients said they did not mind injections).
If HCPs have a better understanding of what their patients want from weight-loss medication, they can have more meaningful conversations and offer medications which align with patients’ personal values and long-term health goals. With this approach, patients are more likely to stick with treatment, which means better long-term results.
Introduction
Overweight and obesity is a chronic, relapsing, and progressive health condition and one of the leading risk factors for chronic conditions and mortality.1 Caucasian adults with a body mass index (BMI) of 25 to 29.9 kg/m2 are typically considered overweight and those with a BMI of 30kg/m2 or more are categorized as having obesity.2–4 About two-thirds of Australian adults have overweight or obesity, and its prevalence has increased significantly in Australia over the past two decades.5 Australia is ranked fifth out of twenty-three OECD member countries in terms of the proportion of people aged 15 years and older who are obese.6
Obesity is implicated in many health conditions, such as cardiovascular disease (including coronary heart disease, hypertensive heart disease and ischemic stroke), type 2 diabetes, musculoskeletal disorders (including osteoarthritis and back pain) and some cancers.1,4,7 Body weight loss of 5% or more can prevent and reduce the risk of obesity-related comorbidities, but long-term benefits rely on maintenance of weight loss.8 This in turn has resulted in demand for effective long-term weight management strategies, including prescription weight loss medication as an adjunct to lifestyle interventions such as diet and exercise8,9 to manage this chronic disease.
The use of currently available prescription weight loss medications in Australia in conjunction with lifestyle interventions can result in an additional 3% to 9% weight loss over 12 months compared with lifestyle interventions alone.8 Weight loss achieved with prescription weight loss medication has been shown to be associated with significant improvements in glycemic measures and improvements in blood pressure and lipid profiles.8 Despite this, prescription weight loss medications are currently under prescribed by healthcare professionals (HCPs), more so among male compared to female patients. In addition, both HCPs and patients consider the trade-offs between efficacy and side effects.10,11 Lack of HCP willingness to discuss weight management with patients is a further barrier to prescribing8 Emergent prescription weight loss medication in conjunction with lifestyle interventions has demonstrated an additional 12.4% weight loss over 68 weeks compared with lifestyle interventions alone.12
Given the different risk-benefit profiles of existing and emergent prescription weight loss medications, it is important to understand the treatment attributes that patients and prescribers consider most important. This can assist patient and HCP dialogue and enable clinicians to tailor treatment management plans including the use of prescription medications, based on expectations around clinical outcomes (eg, % weight loss), and address barriers such as side effects and cost. Improving HCP-patient discussions around weight loss is important, especially considering the results from the Australian cohort of the ACTION-IO study. This online, cross-sectional survey of 1000 adults with obesity and 200 HCPs involved with direct patient care found that it takes people with overweight or obesity an average of 8.9 years to have an initial discussion with a healthcare professional about their weight.13
The discrete choice experiment (DCE) approach is a quantitative method used to understand preferences, which is increasingly being used to investigate health-related preferences in both patients and healthcare professionals.14–16 In a DCE, participants are required to select their preferred treatment option from a set of competing alternatives, trading off the different risks and benefits of each option in reaching their decision. This allows the relative importance of specific treatment features of both existing and new treatment alternatives to be measured. The DCE methodology has previously been used to evaluate treatment preferences among people with diabetes,17 schizophrenia,18 multiple myeloma19 and treatment-resistant depression.20
The aim of this research was to develop a greater understanding of the treatment preferences of patients with overweight or obesity and HCPs who treat them. Two DCEs were conducted to evaluate how patients and HCPs trade off the potential risks and benefits of oral and injectable prescription weight loss medications. This allowed the treatment preferences of patients and HCPs to be compared. No hypotheses were prespecified for this research.
Methods
Preference research methods were used to measure how patients and HCPs trade off product attributes of oral and injectable prescription weight loss medications. A DCE approach was implemented to find out how these treatments are perceived, and what is most important to people when evaluating treatment options.21 Formal ethics approval was not required for this study. Under section 5.1.22 of the National Statement on Ethical Conduct in Human Research (2007; updated 2018),22 this study met the definition of “negligible risk research” and qualified to be exempt from ethical review. This study complied with the Declaration of Helsinki and all participants were required to read an information sheet and provide electronic consent to take part in the research.
Sample
People with overweight and obesity (PwO) were recruited via an online general population panel (Dynata). Potential participants were eligible to take part in the study if they were aged 18 years and older; reported having weight loss discussions with their HCP in the past 12 months; and reported a BMI aligned with physician guidelines for prescribing prescription weight loss medication10 (BMI ≥27 with more than one weight-related comorbidity, or BMI ≥30 with or without comorbidities). People were excluded if they were pregnant, breastfeeding, or had an eating disorder.
HCPs were recruited via specialist medical recruiters, TKW Health. HCPs were eligible to take part if their primary medical specialty was general practice, endocrinology/diabetology, cardiology, gastroenterology, bariatric surgery or bariatric physician. In addition, they needed to report spending more than 70% of their professional time in direct patient care; having a case load of more than 100 patients per month with more than 20% of those patients having a BMI of ≥27; and managing people with overweight or obesity for more than three years.
For clarity, the two participant pools are henceforth referred to as “patients” (people with overweight and obesity) and “HCPs” (health care practitioners who treat PwO).
Discrete Choice Experiments
DCEs involve a survey (questionnaire) that presents participants with different scenarios (in this case, hypothetical treatment options) and asks them to choose a treatment alternative within each scenario that maximizes their satisfaction (utility), based on their own value framework.
Survey Instrument Development
The DCE attributes were derived from a review of clinical trial data, published preference studies in obesity and diabetes, existing market research, and expert opinion. Pilot studies were used to evaluate the content and design of the patient and HCP surveys and DCEs prior to their use in the main study. Pilot testing was carried out among 38 patients and 12 HCPs (nine general practitioners (GPs), one gastroenterologist, and two endocrinologists/diabetologists).
Discrete Choice Experiment
An online survey of Australian adults with overweight or obesity and physicians who treat overweight or obesity was conducted between November and December 2020. The patient and HCP surveys both included a multiple-scenario DCE.
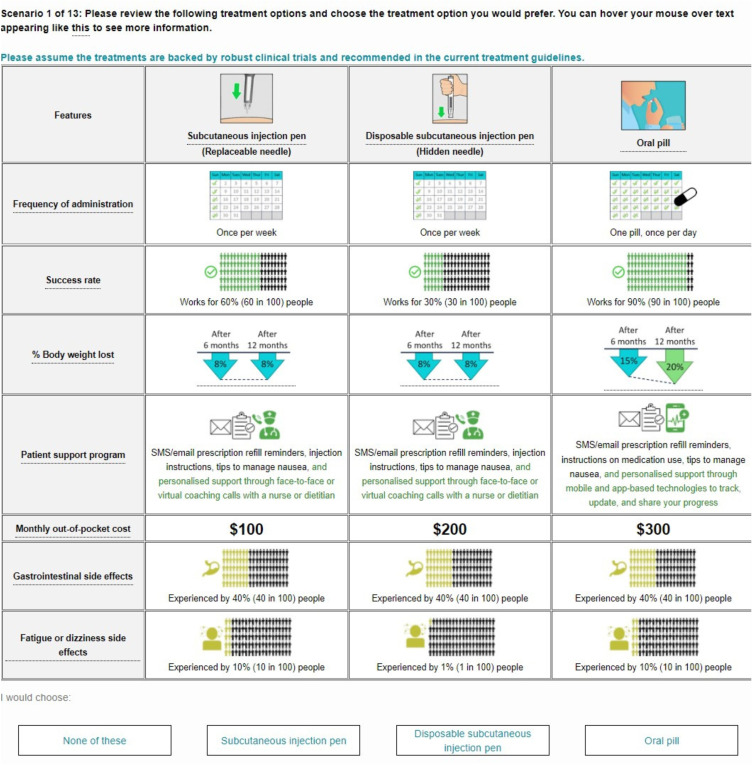
In the DCEs, participants were asked to choose between three hypothetical treatment alternatives, labelled by their mode of administration: “Oral pill”; “Subcutaneous injection pen (replaceable needle)”; “Disposable subcutaneous injection pen (hidden needle)”; and an opt-out option (“None of these”). In each scenario, patients were asked to choose their preferred treatment option, while HCPs were asked to choose the treatment they would prefer to prescribe for their patients with overweight or obesity. An example scenario is shown in Figure 1.
Figure 1.
Example of DCE choice task (wording differed slightly for patients and HCPs).
The hypothetical treatment alternatives were further described by various attributes covering the cost and administration of the medication, % body weight lost, success rate, side effects and contraindications, and available patient support program (Table 1 [patients]; Table 2 [HCPs]). Success rate and efficacy were qualitatively similar but categorically distinct attributes. Success rate was described as the proportion of patients who respond to the medication and was based on clinical outcomes defining success as achieving at least 5% body weight lost after 12 months.23,24 Efficacy was described as the amount of weight loss one could expect to lose after 12 months on the medication (percentage of body weight lost).
Table 1.
DCE Attributes and Levels for Patients
| Attributes | Description | Option A and B (Subcutaneous Injections) | Option C (Oral Pill Medication) | |
|---|---|---|---|---|
| Frequency of administration | How often the medication is needed | Two pills, twice per day | ||
| Once per day | One pill, once per day | |||
| Once per week | ||||
| Success rate | Chance the medication will work (% of people who “respond” to the medication) | Works for 30% (30 in 100) people | Works for 30% (30 in 100) people | |
| Works for 60% (60 in 100) people | Works for 60% (60 in 100) people | |||
| Works for 90% (90 in 100) people | Works for 90% (90 in 100) people | |||
| % Body weight lost | The % body weight people lose after 6 months and after 12 months on this medication. | After 6 months you will have lost 6% and after 12 months you will have lost 6% | After 6 months you will have lost 6% and after 12 months you will have lost 6% | |
| After 6 months you will have lost 7% and after 12 months you will have lost 7% | After 6 months you will have lost 7% and after 12 months you will have lost 7% | |||
| After 6 months you will have lost 8% and after 12 months you will have lost 8% | After 6 months you will have lost 8% and after 12 months you will have lost 8% | |||
| After 6 months you will have lost 12% and after 12 months you will have lost 15% | After 6 months you will have lost 12% and after 12 months you will have lost 15% | |||
| After 6 months you will have lost 15% and after 12 months you will have lost 20% | After 6 months you will have lost 15% and after 12 months you will have lost 20% | |||
| After 6 months you will have lost 20% and after 12 months you will have lost 25% | After 6 months you will have lost 20% and after 12 months you will have lost 25% | |||
| Patient support program | The type of patient support program that comes with the medication at no cost to the patient | SMS/email prescription refill reminders, injection instructions, and tips to manage nausea | SMS/email prescription refill reminders, instructions on medication use, and tips to manage nausea | |
| SMS/email prescription refill reminders, injection instructions, tips to manage nausea, and personalised support through face-to-face or virtual coaching calls with a nurse or dietitian. | SMS/email prescription refill reminders, instructions on medication use, tips to manage nausea, and personalised support through face-to-face or virtual coaching calls with a nurse or dietitian. | |||
| SMS/email prescription refill reminders, injection instructions, tips to manage nausea, personalised support through mobile and app-based technologies to track, update, and share your progress. |
SMS/email prescription refill reminders, instructions on medication use, tips to manage nausea, personalised support through mobile and app-based technologies to track, update, and share your progress. | |||
| Monthly out-of-pocket cost | How much this medication costs each month ($AUD) | $100 | $100 | |
| $200 | $200 | |||
| $300 | $300 | |||
| $400 | $400 | |||
| $500 | $500 | |||
| $600 | $600 | |||
| Side effects | Gastro-intestinal | Chance of experiencing gastrointestinal side effects (may include diarrhoea, vomiting and/or nausea) which usually resolve in a few weeks | 20% (20 in 100) experience gastrointestinal side effects | 20% (20 in 100) experience gastrointestinal side effects |
| 40% (40 in 100) experience gastrointestinal side effects | 40% (40 in 100) experience gastrointestinal side effects | |||
| 80% (80 in 100) people experience gastrointestinal side effects | 80% (80 in 100) people experience gastrointestinal side effects | |||
| Fatigue and dizziness | Chance of experiencing fatigue and dizziness which may or may not resolve | 1% (1 in 100) experience fatigue and dizziness | 1% (1 in 100) experience fatigue and dizziness | |
| 5% (5 in 100) experience fatigue and dizziness | 5% (5 in 100) experience fatigue and dizziness | |||
| 10% (10 in 100) experience fatigue and dizziness | 10% (10 in 100) experience fatigue and dizziness | |||
Table 2.
DCE Attributes and Levels for Healthcare Professionals
| Attributes | Description | Option A and B (Subcutaneous Injections) | Option C (Oral Pill Medication) | |
|---|---|---|---|---|
| Frequency of administration | How often the medication is needed | Two pills, twice per day | ||
| Once per day | One pill, once per day | |||
| Once per week | ||||
| Dose escalation/titration | Patients start this medication on a smaller dose and increase the dose over time. The time refers to how long it takes to reach the recommended dose. | 1 months | 1 month | |
| 3 months | 3 months | |||
| 5 months | 5 months | |||
| Success rate | Chance the medication will work (% of people who “respond” to the medication) | Works for 30% (30 in 100) people | Works for 30% (30 in 100) people | |
| Works for 60% (60 in 100) people | Works for 60% (60 in 100) people | |||
| Works for 90% (90 in 100) people | Works for 90% (90 in 100) people | |||
| % Body weight lost | The % body weight people lose after 6 months and after 12 months on this medication. | After 6 months your patient will have lost 6% and after 12 months your patient will have lost 6% | After 6 months your patient will have lost 6% and after 12 months your patient will have lost 6% | |
| After 6 months your patient will have lost 7% and after 12 months your patient will have lost 7% | After 6 months your patient will have lost 7% and after 12 months your patient will have lost 7% | |||
| After 6 months your patient will have lost 8% and after 12 months your patient will have lost 8% | After 6 months your patient will have lost 8% and after 12 months your patient will have lost 8% | |||
| After 6 months your patient will have lost 12% and after 12 months your patient will have lost 15% | After 6 months your patient will have lost 12% and after 12 months your patient will have lost 15% | |||
| After 6 months your patient will have lost 15% and after 12 months your patient will have lost 20% | After 6 months your patient will have lost 15% and after 12 months your patient will have lost 20% | |||
| After 6 months your patient will have lost 20% and after 12 months your patient will have lost 25% | After 6 months your patient will have lost 20% and after 12 months your patient will have lost 25% | |||
| Patient support program | The type of patient support program that comes with the medication at no cost to the patient | SMS/email prescription refill reminders, injection instructions, and tips to manage nausea | SMS/email prescription refill reminders, instructions on medication use, and tips to manage nausea | |
| SMS/email prescription refill reminders, injection instructions, tips to manage nausea, and personalised support through face-to-face or virtual coaching calls with a nurse or dietitian. | SMS/email prescription refill reminders, instructions on medication use, tips to manage nausea, and personalised support through face-to-face or virtual coaching calls with a nurse or dietitian. | |||
| SMS/email prescription refill reminders, injection instructions, tips to manage nausea, personalised support through mobile and app-based technologies to track, update, and share progress. | SMS/email prescription refill reminders, instructions on medication use, tips to manage nausea, personalised support through mobile and app-based technologies to track, update, and share progress. | |||
| Monthly out-of-pocket cost | How much this medication costs each month ($AUD) | $100 | $100 | |
| $200 | $200 | |||
| $300 | $300 | |||
| $400 | $400 | |||
| $500 | $500 | |||
| $600 | $600 | |||
| Side effects | Gastro-intestinal | Chance your patient will experience gastrointestinal side effects (may include diarrhoea, vomiting and/or nausea) which usually resolve in a few weeks | 20% (20 in 100) experience gastrointestinal side effects | 20% (20 in 100) experience gastrointestinal side effects |
| 40% (40 in 100) experience gastrointestinal side effects | 40% (40 in 100) experience gastrointestinal side effects | |||
| 80% (80 in 100) people experience gastrointestinal side effects | 80% (80 in 100) people experience gastrointestinal side effects | |||
| Fatigue and dizziness | Chance your patient will experiencing fatigue and dizziness which may or may not resolve | 1% (1 in 100) experience fatigue and dizziness | 1% (1 in 100) experience fatigue and dizziness | |
| 5% (5 in 100) experience fatigue and dizziness | 5% (5 in 100) experience fatigue and dizziness | |||
| 10% (10 in 100) experience fatigue and dizziness | 10% (10 in 100) experience fatigue and dizziness | |||
| Contra-indications | Psychological | Other medications or conditions the weight loss medication should not be used with | None | Bipolar or eating disorders, or antidepressant medications called Monoamine Oxidase Inhibitors (MOAIs) |
| Physical | None | History of seizures, or Uncontrolled hypertension | ||
| None | None | |||
Participants were asked to assume that the medications were backed by robust clinical trials and recommended in the current treatment guidelines. They were also informed that all treatments should be taken in conjunction with a “reduced calorie diet and regular exercise” and that the medications work by making patients feel “full”, reducing hunger and cravings. Both the patient and HCP DCEs consisted of 84 unique scenarios each split into seven “blocks”, so that each participant was randomly allocated to one block containing 12 scenarios according to an experimental design. This meant that each participant was asked to assess a total of 12 choice scenarios.
Additional Background Questions
Patients were asked background questions relating to their demographics, comorbidities/health conditions, perception of weight, weight loss discussions with HCPs, weight loss goals, weight loss strategies tried and prescription weight loss medication use. HCPs were asked background questions relating to their demographics, number of patients with overweight or obesity, weight loss discussions they have had with their patients, tried and preferred weight loss strategies for patients, and experience with prescription weight loss medication prescribing.
Analysis
Participants who finished the survey too quickly (less than 8 minutes), gave non-sensical answers or who reported a poor understanding of the DCE (a rating of less than 6 on a scale from 0 [Did not understand at all] to 10 [Understood perfectly]) were excluded from the analysis. Participants with incomplete DCE data were also excluded from the final sample.
Descriptive statistics were used to summarize participants’ demographic characteristics and background information. The combinations of levels presented in the DCE scenarios were configured according to a Bayesian D-efficient experimental design method25 and generated using Ngene (ChoiceMetrics Pty Ltd, Sydney, Australia), a software tool used to generate stated choice experimental design. The DCE data were modelled using NLOGIT version 6 (Econometric Software Inc., Plainview, NY, USA).
Preference data were analyzed using the mixed multinomial logit (MMNL) model, which allows for preference heterogeneity (ie, variation in preferences based on distributions) between participants26 (see Supplementary Methods for details). The adjusted McFadden Pseudo R-squared was used to assess model fit. Model results (parameter coefficients, standard errors, and associated p values) are presented for the MMNL models used to analyze patient DCE and HCP DCE data. Details of the utility functions specified within each of the MMNL models are shown in the Supplementary Methods.
Using the parameter coefficients estimated from the model, the relative importance of each treatment attribute can be calculated by finding the maximum difference in utility between the attribute’s levels, expressing it as a percentage of the sum of all maximum differences.27 In addition, the demographic characteristics that influence treatment preferences can be determined. The uptake for the three treatment alternatives and the opt-out across participants can be predicted by transforming utility into probabilities (market share) for each treatment alternative.
Results
The below results report on 193 patients and 104 physicians who completed the online survey and DCE and were included in the final sample. A total of 226 patients and 106 HCPs completed the online survey, however 33 patients and two HCPs were removed in the data cleaning process due to survey duration (less than 8 minutes; patients n=14, physicians n=0), or poor DCE understanding (less than 6/10; patients n=19, physicians n=2).
Population Demographics and Participant Characteristics
The characteristics of respondents, both patients (n=193) and HCPs (n=104), are reported in Tables 3 and 4 respectively. The average BMI of patients was 35.1 kg/m2 (SD 5.91) and most patients had had weight loss discussions with their GP (95.85) compared to other HCP specialties (See Supplementary Table 1). Almost all patients (96.37%) reported having at least one comorbidity, with hypertension being the most frequently reported comorbid condition (Table 3).
Table 3.
Demographics and Clinical Characteristics of Patients
| Characteristic – Total Sample (n=193) | n (%) | |
|---|---|---|
| Gender | Male | 83 (43.01) |
| Female | 110 (56.99) | |
| Non-binary/gender fluid | 0 (0) | |
| Age | 18–30 | 13 (6.74) |
| 31–40 | 28 (14.51) | |
| 41–50 | 22 (11.40) | |
| 51–60 | 28 (14.51) | |
| 61–70 | 58 (30.05) | |
| 71–80 | 42 (21.76) | |
| 81 or older | 2 (1.04) | |
| Annual total household gross income (before tax) | Nil income | 1 (0.52) |
| $1-$25,999 (ie, $1-$499 a week) | 17 (8.81) | |
| $26,000-$51,599 (ie, $500-$999 a week) | 48 (24.87) | |
| $52,000-$88,399 (ie, $1000-$1699 a week) | 55 (28.50) | |
| $88,400-$129,999 (ie, $1700-$2499 a week) | 40 (20.73) | |
| $130,000-$181,999 (ie, $2500-$3499 a week) | 18 (9.33) | |
| $182,000 or more (ie, $3500 or more a week) | 7 (3.63) | |
| Prefer not to answer | 7 (3.63) | |
| Type of location | Metro/city | 112 (58.03) |
| Regional | 52 (26.94) | |
| Rural | 29 (15.03) | |
| Self-reported diagnosed comorbidities (n=193 reported at least 1 comorbidity) | High blood pressure | 92 (47.67) |
| High cholesterol | 79 (40.93) | |
| Depression/anxiety | 78 (40.41) | |
| Osteoarthritis | 48 (24.87) | |
| Type 2 diabetes | 46 (23.83) | |
| Obstructive sleep apnoea | 38 (19.69) | |
| Intestinal problems | 28 (14.51) | |
| Other health conditions | 26 (13.47) | |
| Cardiovascular disease | 18 (9.33) | |
| Cancer | 14 (7.25) | |
| Pre-diabetes | 13 (6.74) | |
| Polycystic ovary syndrome | 13 (6.74) | |
| Infertility | 9 (4.66) | |
| Kidney disease | 3 (1.55) | |
| Type 1 diabetes | 5 (2.59) | |
| None of these | 7 (3.63) | |
| BMI | Overweight (27–30) | 33 (17.10) |
| Obese (30–35) | 76 (39.38) | |
| Extremely obese (35+) | 84 (43.52) | |
Table 4.
Healthcare Professional Demographic Characteristics
| Characteristic – Total Sample (n=104) | n (%) | |
|---|---|---|
| Gender | Male | 68 (65.38) |
| Female | 32 (30.77) | |
| Prefer not to answer | 4 (3.85) | |
| Age | 18–30 | 0 (0) |
| 31–40 | 5 (4.81) | |
| 41–50 | 29 (27.88) | |
| 51–60 | 42 (40.38) | |
| 61–70 | 23 (22.12) | |
| 71–80 | 2 (1.92) | |
| 81 or older | 0 (0) | |
| Prefer not to answer | 3 (2.88) | |
| Primary medical specialty | General practice (GP) | 77 (74.04) |
| Specialists | 27 (25.96) | |
| Endocrinology/diabetology | 9 (8.65) | |
| Cardiology | 8 (7.69) | |
| Gastroenterology | 7 (6.73) | |
| Bariatric surgery | 3 (2.88) | |
| Professional setting | Private | 89 (85.58) |
| Public | 15 (14.42) | |
| Area | Metro/city | 90 (86.54) |
| Regional | 11 (10.58) | |
| Rural | 3 (2.88) | |
| Time spent managing and treating patients with overweight or obesity | 3–6 years | 4 (3.85) |
| 7–10 years | 8 (7.69) | |
| 10+ years | 90 (86.54) | |
| Do not know | 2 (1.92) | |
| Mean (SD) | % | |
| Total patient load per month | 504.04 (350.63) | 100 |
| Patients with overweight or obesity per month | 312.09 (240.72) | 61.92 |
Treatment and Prescription Experience
The three weight management strategies most often tried by patients were general improvements in eating habits (71.50%); being more active/increased physical activity (58.55%); and using a specific diet program, such as Get Healthy Line, Weight Watchers, CSIRO diet (53.37%) (see Supplementary Table 2 for full results on previously tried weight management strategies). Only 14 patients (7.3%) were taking prescription weight loss medications at the time of the survey, and 41 patients (21.24%) had previously taken prescription weight loss medication.
Most of the HCPs surveyed were general practitioners (n=77; 74.04%). The specialists included endocrinologists (n=9; 8.65%), cardiologists (n=8; 7.69%), gastroenterologists (n=7; 6.73%) and bariatric surgeons (n=3; 2.88%), and most of the HCPs (n=90; 86.54%) reported spending more than 10 years treating patients with overweight or obesity (Table 4).
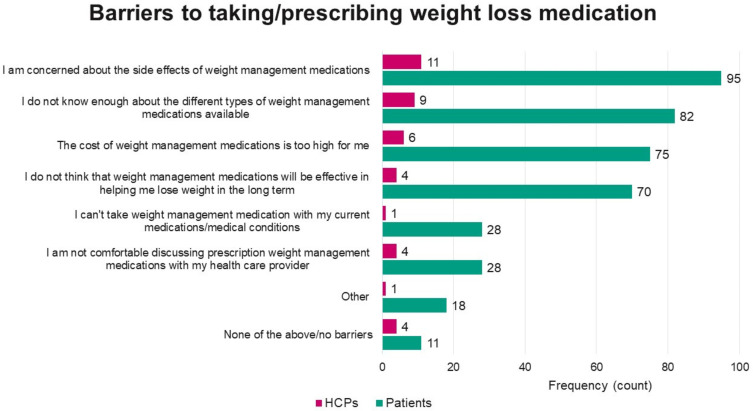
HCPs reported that based on an average monthly patient load of 504.04 (SD=350.63), 61.92% of their patients had overweight or obesity (M=312.09, SD=240.72), and reported prescribing weight loss medication for 6.89% of these patients (M=21.51, SD=19.46) in the past year. Weight loss medications had been prescribed by 85 (81.73%) HCPs in the past year, with a median recommended duration of treatment of 180 days. However, HCPs reported their patients tended to cease using the medication after only 90 days (median). Of the 19 HCPs who had not prescribed prescription weight loss medication in the past year, the most reported barrier to prescribing was concern about side effects (Figure 2).
Figure 2.
Reported barriers to taking/prescribing weight loss medications.
Note: Based on N = 147 patients who reported not currently using anti-obesity medications and N = 19 HCPs who had not recommended anti-obesity medications in the past 12 months.
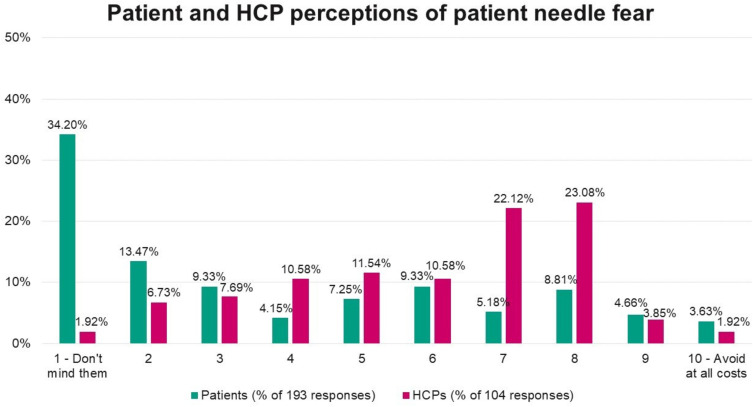
Needle Fear and Perceived Fear of Injectable Treatments
Most patients reported relatively low needle fear (47.67% rated their fear 1 to 2 out of 10), while physicians reported relatively high perceived patient needle fear (45.18% rated patients’ fear as 7 to 8 out of 10 (Figure 3). Most HCPs (n=62) reported that their patients would be less apprehensive about using an injectable medication with a hidden needle compared to an injectable medication with a replaceable needle (compared to 37.50% who reported their patients would be just as apprehensive). Only a very small proportion of patients (3.63%) reported wanting to avoid needles completely.
Figure 3.
Perceptions of patient needle fear, as reported by patients and healthcare professionals.
DCE Findings
Patient DCE – MMNL Model Results
The results from the best fitting MMNL model are shown in Table 5. There was a strong preference for any of the treatment alternatives (oral, replaceable injection and disposable injection) compared to the opt-out option. The relative size of the alternative specific constants suggest that compared to the opt out, oral medication and the disposable injection were most preferred, followed by the replaceable injection, holding all else equal (Table 5). Patients also preferred treatments with a higher success rate (proportion of patients who achieve ≥5% weight loss; parameter estimate 0.043, SE 0.003), and those that provided the greatest amount of weight loss (20% at 6 months and 25% at 12 months [1.156, SE 0.113]).
Table 5.
Parameter Estimates for the Patient MMNL Model
| Var Code | Random Parameter | Estimate | Sig. | S.E | T-ratio | |
| SUCC_C | Success rate | (Continuous variable) | 0.04277 | *** | 0.003 | 14.65 |
| WL7B | % body weight loss | 7%, 7% | −0.61867 | *** | 0.101 | −6.10 |
| WL8B | 8%, 8% | −0.57117 | *** | 0.098 | −5.83 | |
| WL12B | 12%, 15% | 0.49845 | *** | 0.089 | 5.59 | |
| WL15B | 15%, 20% | 0.56308 | *** | 0.089 | 6.33 | |
| WL20B | 20%, 25% | 1.15636 | *** | 0.113 | 10.26 | |
| (Reference category: 6%, 6%) | ||||||
| GAS_C | Gastrointestinal side effects | (Continuous variable) | −0.05311 | *** | 0.004 | −13.48 |
| DIZZ_C | Fatigue/dizziness side effects | (Continuous variable) | −0.05165 | *** | 0.013 | −4.04 |
| Oral_cb | Monthly out-of-pocket cost (cont.) | Oral cost | −0.00599 | *** | 0.001 | −9.94 |
| RInj_cb | Replaceable injection cost | −0.00348 | *** | 0.001 | −6.35 | |
| DInj_cb | Disposable injection cost | −0.00416 | *** | 0.001 | −8.13 | |
| Var Code | Non-random parameter | Estimate | Sig. | S.E | T-ratio | |
| ORAL | Alternative specific constants | Oral | 2.48089 | *** | 0.240 | 10.32 |
| Rinj | Replaceable injection | 1.95582 | *** | 0.289 | 6.77 | |
| Dinj | Disposable injection | 2.41192 | *** | 0.287 | 8.41 | |
| (Reference category: None) | ||||||
| PSP_AP | Personalised support | Mobile and app-based technologies to track, update and share progress | 0.14216 | ** | 0.062 | 2.31 |
| PSP_NUR | Face-to-face or virtual coaching calls with nurse or dietician | −0.02284 | ns | 0.068 | −0.34 | |
| (Reference category: No personalised support) | ||||||
| NEEDR | Needle fear (continuous) | Needle fear effect on replaceable injection | −0.24227 | *** | 0.043 | −5.64 |
| NEEDD | Needle fear effect on disposable injection | −0.18936 | *** | 0.038 | −4.97 | |
| AGE_Y | Ageᵻ | 18–40 years | −0.17707 | ns | 0.222 | −0.80 |
| AGE_M | 41–60 years | −0.34593 | * | 0.199 | −1.74 | |
| (Reference category: 61+ years) | ||||||
| MALE | Genderᵻ | Male | 0.44036 | *** | 0.132 | 3.34 |
| (Reference category: Female) | ||||||
Notes; *Significant at 10% level, **Significant at 5% level, ***Significant at 1% level; ns = non-significant (p>0.1). Log likelihood function: −2164.53105; Restricted log likelihood: −3210.65774; McFadden Pseudo R-squared: 0.3258294; Number of respondents: 193; Number of observations = 2316. ᵻAge and gender were included in the utility function for the opt-out and therefore positive coefficients represent preference for “No treatment” while negative coefficients represent preference for any treatment.
Patients preferred treatments with a lower chance of gastrointestinal side effects (−0.053, SE 0.004) and a lower chance of dizziness and fatigue (−0.052, SE 0.013). Preference for any of the three alternatives decreased with increased cost (Table 5).
Gender and age were shown to influence a patient’s willingness to try prescription weight loss treatment; women and those aged 41–60 years were the most likely to choose one of the treatment alternatives compared to no treatment. Patients also preferred treatments that were associated with mobile and app-based patient support (0.142, SE 0.062). Patients with a higher self-reported fear of needles resulted in a lower preference for the replaceable (−0.242, SE 0.043) and disposable (−0.189, SE 0.038) injection options.
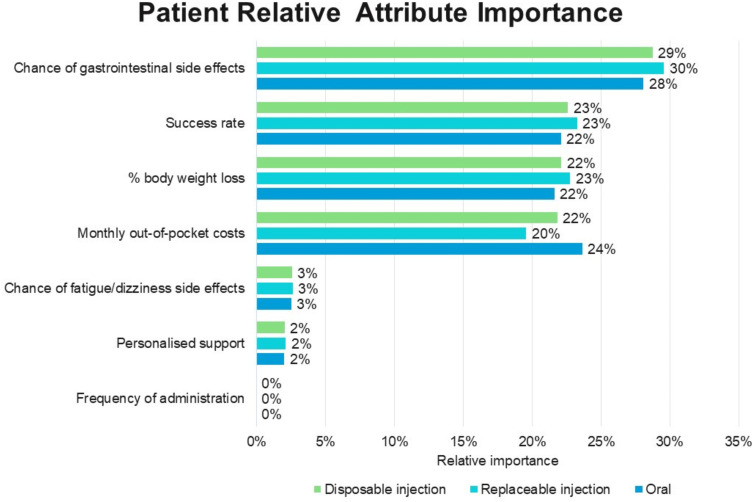
Relative attribute importance: Gastrointestinal side effects, followed by success rate, percentage body weight lost, and cost were the most important attributes to patients when deciding between weight loss medication options (Figure 4). Gastrointestinal side effects accounted for around 30% of the influence over patients’ treatment choices in the DCE (Figure 4). Patients were more sensitive to changes in cost for the oral compared to the injectable treatments such that higher priced injectables were preferred over higher priced orals.
Figure 4.
Relative importance of treatment attributes influencing patient choices in the DCE.
HCP DCE – MMNL Model Results
The results of the best fitting MMNL model demonstrating prescribing preferences for HCPs treating overweight or obesity are shown in Table 6. All treatment alternatives were preferred over the opt-out option. Oral medication demonstrated the largest share of preference for treatment mode.
Table 6.
Parameter Estimates for the HCP MMNL Model
| Var Code | Random Parameter | Estimate | Sig. | SE | T-ratio | |
| Succ_c | Success rate | (Continuous variable) | 0.041 | *** | 0.004 | 11.62 |
| WL7, WL8 | % body weight loss (after 6 months, after 12 months) | 7%, 7% OR 8%, 8% | −0.793 | *** | 0.140 | −5.66 |
| WL12 | 12%, 15% | 0.413 | *** | 0.113 | 3.64 | |
| WL15 | 15%, 20% | 0.532 | *** | 0.116 | 4.59 | |
| WL20 | 20%, 25% | 1.324 | *** | 0.138 | 9.59 | |
| (Reference category: 6%, 6%) | ||||||
| Gas | Gastrointestinal side effects | (Continuous variable) | −0.036 | *** | 0.003 | −11.25 |
| Phys | Contraindications | Psychological | −0.181 | ns | 0.142 | −1.27 |
| Psy | Psychological and physical | −0.246 | * | 0.130 | −1.89 | |
| (Reference category: None) | ||||||
| Csto | Monthly out-of-pocket cost (continuous) | Oral cost | −0.0043 | *** | 0.001 | −6.52 |
| Cstr | Replaceable injection cost | −0.0032 | *** | 0.001 | −4.75 | |
| Cstd | Disposable injection cost | −0.0033 | *** | 0.001 | −6.47 | |
| Var code | Non-random parameter | Estimate | Sig. | S.E | T-ratio | |
| Oral | Alternative specific constants | Oral | 2.572 | *** | 0.353 | 7.29 |
| Rinj | Replaceable injection | 1.602 | *** | 0.296 | 5.41 | |
| Dinj | Disposable injection | 2.019 | *** | 0.312 | 6.47 | |
| (Reference category: None) | ||||||
| Spec | Specialtyᵻ | Specialist | −0.579 | ** | 0.261 | −2.22 |
| (Reference category: GP) | ||||||
| Yng | Ageᵻ | 18–40 years | 0.566 | *** | 0.168 | 3.38 |
| (Reference category: 41+ years) | ||||||
| Male | Genderᵻ | Male | −0.123 | ns | 0.155 | −0.79 |
| (Reference category: Female) | ||||||
| OPL | Obese patient loadᵻ | ≥ 40% | −0.295 | * | 0.177 | −1.66 |
| (Reference category: <40%) | ||||||
| Error component | Estimate | Sig. | S.E | T-ratio | ||
| Oral | 1.17863 | *** | 0.18565 | 6.35 | ||
| Replaceable Injection | 0.7324 | ** | 0.2859 | 2.56 | ||
| Disposable Injection | 0.24105 | 0.27501 | 0.88 | |||
Notes: *Significant at 10% level, **Significant at 5% level, ***Significant at 1% level; ns = non-significant (p>0.1). Log likelihood function: −1179.54841; Restricted log likelihood: −1730.09536; McFadden Pseudo R-squared: 0.3182177; Number of respondents: 104; Number of observations: 1248. ᵻSpecialty, Age, Gender, and obese patient load were included in the utility function for the opt-out and therefore positive coefficients represent preference for “No treatment” while negative coefficients represent preference for any treatment.
HCPs preferred treatments with a higher success rate (parameter estimate 0.041, SE 0.004), lower gastrointestinal side effects (−0.036, SE 0.003) and without psychological and physical contraindications (−0.246, SE 0.130). Treatments associated with the highest percentage weight loss were also preferred, and the relative size of the coefficients suggest preference increases with increases in percentage of body weight lost (20% at 6 months 25% at 12 months = 1.324, SE 0.138). Similar to patients, the relative size of the coefficients suggests there was a steeper decrease in preferences for the oral treatments compared to injectables as price increased (Table 6).
Age, practitioner type, and the number of patients with overweight or obesity managed by HCPs influenced willingness to prescribe any treatment compared to the opt out, such that specialists (vs GPs), those aged 41 years and older (vs 18–40 years of age) and having a case load with at least 40% overweight patients (vs less than 40%) were more likely to prefer any treatment alternative compared to the opt out (Table 6).
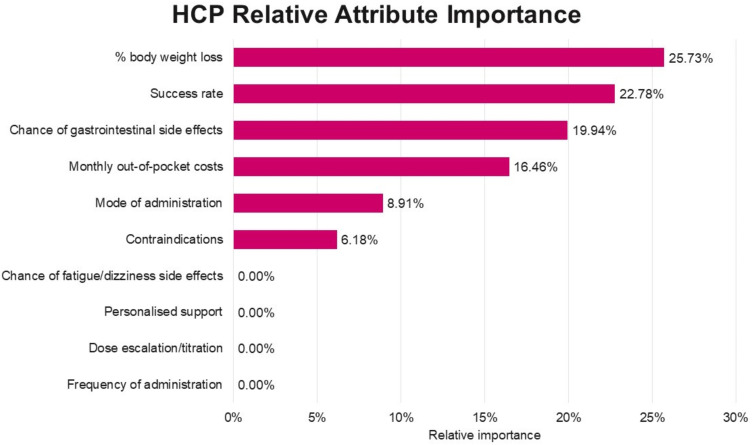
Relative attribute importance: Percentage body weight lost was the most important treatment attribute to HCPs, followed by success rate, gastrointestinal side effects and cost. Mode of administration and contraindications also contributed significantly to HCP decision making, although to a lesser extent (Figure 5).
Figure 5.
Relative importance of treatment attributes influencing healthcare provider (HCP) choice in the DCE.
Discussion
To the authors’ knowledge, this is the first DCE examining patient and HCP preferences for prescription weight loss medications. Our results showed that both patients with overweight or obesity and HCPs treating patients with overweight or obesity preferred oral and injectable treatment options compared with no prescription weight loss medication. Both patients and HCPs preferred treatments that provided a high success rate and low chance of gastrointestinal side effects. Patients’ choices were influenced the most by gastrointestinal side effects – this may be explained by the high chance of gastrointestinal side effects in each of the three treatment alternatives presented in the experiment (up to a maximum of 80%). Had participants been presented with treatments with relatively low gastrointestinal side effects, perhaps the influence of this attribute on treatment choices would have been smaller. Cost was important to both, but this was secondary to treatment efficacy and safety.
The attributes that influenced patient decisions the most aligned with a previous DCE evaluating preferences for bariatric surgery. Patients in Rozier et al’s study28 identified cost, expected weight loss, and resolution of medical conditions as the most important characteristics of weight loss surgery. Similarly, Mansfield et al29 conducted an online DCE study with German and Spanish patients with type 2 diabetes comparing an oral and injectable medication. The greatest preferences for German patients were for treatments with a lower risk of gastrointestinal problems, and less weight gain.
Patients in Mansfield’s study had a strong preference for oral versus injection modes of administration.29 This reflected the preferences seen in the current study for both patients and HCPs, but there was only a small difference in the size of the parameter estimates for oral and disposable injection treatments for patients (oral: 2.481, disposable injection: 2.412). Hidden needle injections (disposable) were preferred over replaceable needles, aligning with research in diabetes treatments that has demonstrated that injection pens are associated with greater acceptance and patient adherence compared to syringes with visible needles.30,31
Despite patients’ preferences for oral medications, the current results revealed a difference between patients’ self-reported fear of needles and HCPs’ perception of their patients’ needle fear, suggesting that prescribers overestimate opposition to injectable medications. This may result in fewer injectable medications being offered to patients where an oral alternative is available. Patients seeking treatment for overweight or obesity may be less concerned with the mode of administration than their treating physicians suggest.
Prescribing of weight loss medications was low in the current study, where HCPs reported prescribing prescription weight loss medication for around 7% of their patients with overweight or obesity. The DCE modelling suggested that women aged 41–60 years old were most likely to prefer prescription weight loss medication compared to men and other age groups. This aligns with research in the UK, which demonstrated that despite the low prescription rate, women aged 35–64 years were the most likely groups to receive prescription weight loss medication.11 This discrepancy exists despite relatively similar rates of obesity amongst men and women, and despite increasing health risks in older compared to younger patients.11 Such findings suggest men and those in older age groups may benefit from more targeted education on available treatment options.
In addition to low prescribing rates, HCPs reported their patients tended to cease prescription weight loss medication use three months earlier than recommended. Patients with overweight and obesity may benefit from HCPs initiating weight loss discussions as part of routine care in an effort to engage patients earlier on in their weight management journey. Once engaged, HCPs and patients should work together to improve duration on prescription weight loss medication to ensure the best possible health outcomes for patients. Currently there is a long delay in patients presenting to an HCP and a potential lack of adequate time on prescription weight loss medication to maximize potential benefits. Patient support managing gastrointestinal side effects may be pivotal to this.
Research in shared decision-making suggests that providing information alone is not sufficient for patients to make informed treatment decisions. Rather, physicians must offer a conversation around the unique needs and values of individual patients, including evidenced-based recommendations.32,33
The current patient sample showed considerable heterogeneity in treatment preferences, as well as discrepancies between patient and HCP perspectives on injectable treatments. Consideration should be given to promoting weight-management discussions using a shared-decision making framework that incorporates both patient preferences and clinical guidance on safety and efficacy. This could help improve access to quality obesity care within Australia.
Limitations
This study relied on self-reported data. Patients’ eligibility relied on self-reported height and weight (to calculate BMI), and participation was restricted to individuals with internet access. Although the survey questions were designed to ensure that only patients with overweight or obesity were included, it is possible that due to sampling requirements, those included in the study were not representative of the overall patient population. Furthermore, their recruitment through online panels and willingness to participate in online surveys may also have resulted in the inclusion of patients who are more engaged in treatment decisions than is typical of this patient population as a whole.
It is possible that participants may not have adequately understood the tasks involved in the DCE; however, to reduce this possibility, the survey and DCE were piloted among patients and HCPs and scenarios revised where needed to improve clarity. In addition, participants who completed the survey too quickly, gave non-sensical answers or rated their understanding of the choice experiment below the required level were excluded from the analysis.
Although the treatment attributes evaluated in the DCE were derived from existing market research, literature, and expert opinion, it is possible that the study did not include some attributes that participants considered important.
Our study was limited to participants in Australia, so the findings may not be generalizable to other countries or regions. The cross-sectional nature of the study did not allow for the capture of any evolution in patient preferences over time (ie, before and after prescription weight loss medication use).
Conclusions
Overweight and obesity are significant health problems in Australia, and prevalence has increased rapidly in recent years. While prescription weight loss medications are underutilized in Australia, our study results show that both patients with overweight or obesity and HCPs who treat such patients prefer medications over no prescription weight loss medication. HCPs’ perceptions of their patients’ hesitancy to use injectable medications were greater than the needle fear reported by patients. This indicates that clinician-patient treatment discussions should include the option of injectable medications, especially if they have a low or manageable risk of gastrointestinal side effects and are effective, which were the main considerations for patients in this study.
Gastrointestinal side effects, followed by success rate, percentage body weight lost, and cost were the most important attributes to patients when deciding between weight loss medication options. Percentage body weight loss was the most important treatment attribute to HCPs, followed by success rate, gastrointestinal side effects and cost. Mode of administration and contraindications also contributed significantly to HCP decision making, although to a lesser extent. Highlighting the differences in attributes of most importance to patients and HCP’s may help improve the quality of weight management discussions between HCPs and patients and the development of quality clinical management plans including the use of prescription medications in Australia.13
HCP and patient education around prescription weight loss medications is crucial, as is a shared decision-making framework between HCPs and people with overweight or obesity which is based on expectations around clinical outcomes such as efficacy and side effects. This could remove existing barriers and help support patients to achieve their weight loss goals and improve overall health.
Acknowledgments
Medical writing services provided by Anne Dyson from WriteSource Medical were funded by Novo Nordisk Australia in accordance with Good Publication Practice (GPP3) guidelines (http://ismpp.org/gpp3).
Funding Statement
This study was conducted by Community and Patient Preference Research (CaPPRe) and funded by Novo Nordisk. CaPPRe was responsible for carrying out all aspects of the research. Novo Nordisk was consulted on the research design, and were not involved in the data collection, analysis, or interpretation of the data. AP, an employee of Novo Nordisk is a co-author of the current manuscript.
Abbreviations
BMI, Body mass index; GP, General practitioner; HCP, Healthcare practitioner; DCE, Discrete choice experiment; MMNL, Mixed multinomial logit; SE, standard error.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the commercially sensitive nature of the data but are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
Formal ethics approval was not required for this study. Under section 5.1.22 of the National Statement on Ethical Conduct in Human Research (2007; updated 2018),19 this study met the definition of “negligible risk research” and qualified to be exempt from ethical review. All participants were provided with an information sheet which contained details on how their data would be used, confidentiality of responses, and the benefits and risks of participation. All participants provided electronic consent before completing the online survey.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
SF and BK are employed by CaPPRe. CaPPRe has consulted to Abbvie, Amgen, AstraZeneca, Celgene, CSL Behring, Edwards, GSK, Ipsen, Janssen, Novo Nordisk, Roche, Sanofi, Shire and UCB, outside of the submitted work. AP is an employee of Novo Nordisk Australia. The authors report no other conflicts of interest in this work.
References
- 1.AIHW. Impact of Overweight and Obesity as a Risk Factor for Chronic Conditions: Australian Burden of Disease Study. Canberra: AIHW; 2017. [Google Scholar]
- 2.World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization Technical Report Series. 2000;i–xii, 1–253. [PubMed]
- 3.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Obesity and overweight; 2021. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed November, 2021.
- 5.Australian Institute of Health and Welfare (AIHW). Overweight and Obesity in Australia: A Birth Cohort Analysis. Canberra: AIHW; 2017. [Google Scholar]
- 6.AIHW. Overweight and obesity: an interactive insight: Australian government; 2021. Available from: https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity-an-interactive-insight/contents/what-is-overweight-and-obesity. Accessed February 13, 2024.
- 7.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadde KM, Atkins KD. The limits and challenges of antiobesity pharmacotherapy. Expert Opin Pharm. 2020;21(11):1319–1328. doi: 10.1080/14656566.2020.1748599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khera R, Murad MH, Chandar AK, et al. Association of pharmacological treatments for obesity with weight loss and adverse events. JAMA. 2016;315(22):2424–2434. doi: 10.1001/jama.2016.7602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huq S, Todkar S, Lahiri SW. Patient perspectives on obesity management: need for greater discussion of BMI and weight-loss options beyond diet and exercise, especially in patients with diabetes. Endocr Pract. 2020;26(5):471–483. doi: 10.4158/EP-2019-0452 [DOI] [PubMed] [Google Scholar]
- 11.Patterson L, Kee F, Hughes C, O’Reilly D. The relationship between BMI and the prescription of anti-obesity medication according to social factors: a population cross sectional study. BMC Public Health. 2014;14:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002. doi: 10.1056/NEJMoa2032183 [DOI] [PubMed] [Google Scholar]
- 13.Rigas G, Williams K, Sumithran P, et al. Delays in healthcare consultations about obesity - barriers and implications. Obes Res Clin Pract. 2020;14(5):487–490. doi: 10.1016/j.orcp.2020.08.003 [DOI] [PubMed] [Google Scholar]
- 14.Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmaco Economic. 2014;32(9):883–902. [DOI] [PubMed] [Google Scholar]
- 15.Merlo G, van Driel M, Hall L. Systematic review and validity assessment of methods used in discrete choice experiments of primary healthcare professionals. Health Economic Rev. 2020;10(1):39. doi: 10.1186/s13561-020-00295-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fifer S, Rose J, Hamrosi KK, Swain D. Valuing injection frequency and other attributes of type 2 diabetes treatments in Australia: a discrete choice experiment. BMC Health Serv Res. 2018;18(1):675. doi: 10.1186/s12913-018-3484-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fifer S, Keen B, Newton R, Puig A, McGeachie M. Understanding the treatment preferences of people living with schizophrenia in Australia: a patient value mapping study. Patient Prefer Adhe. 2022;16:1687–1701. doi: 10.2147/PPA.S366522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fifer S, Galinsky J, Richard S. Myeloma patient value mapping: a discrete choice experiment on myeloma treatment preferences in the UK. Patient Prefer Adhe. 2020;14:1283–1293. doi: 10.2147/PPA.S259612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fifer S, Puig A, Sequeira V, et al. Understanding treatment preferences of Australian patients living with treatment-resistant depression. Patient Prefer Adhe. 2021;15:1621. doi: 10.2147/PPA.S311699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. [DOI] [PubMed] [Google Scholar]
- 22.National Health and Medical Research Council; National statement on ethical conduct in human research. 2007;Available from: https://www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2007-updated-2018. Accessed February 13, 2024.
- 23.Lee PC, Dixon J. Pharmacotherapy for obesity. Aust Fam Phys. 2017;46(7):472–477. [PubMed] [Google Scholar]
- 24.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and pharmacotherapy weight loss interventions to prevent obesity-related morbidity and mortality in adults: updated evidence report and systematic review for the US preventive services task force. JAMA. 2018;320(11):1172–1191. doi: 10.1001/jama.2018.7777 [DOI] [PubMed] [Google Scholar]
- 25.Rose JM, Bliemer MC. Constructing efficient stated choice experimental designs. Transp Rev. 2009;29(5):587–617. doi: 10.1080/01441640902827623 [DOI] [Google Scholar]
- 26.Hensher D, Rose J, Greene W. Applied Choice Analysis. 2nd ed. Cambridge: Cambridge University Press; 2015. [Google Scholar]
- 27.Gonzalez JM. A guide to measuring and interpreting attribute importance. Patient. 2019;12(3):287–295. doi: 10.1007/s40271-019-00360-3 [DOI] [PubMed] [Google Scholar]
- 28.Rozier MD, Ghaferi AA, Rose A, Simon NJ, Birkmeyer N, Prosser LA. Patient preferences for bariatric surgery: findings from a survey using discrete choice experiment methodology. JAMA Surg. 2019;154(1):e184375. doi: 10.1001/jamasurg.2018.4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mansfield C, Sikirica MV, Pugh A, et al. Patient preferences for attributes of type 2 diabetes mellitus medications in Germany and Spain: an online discrete-choice experiment survey. Diabetes Ther. 2017;8(6):1365–1378. doi: 10.1007/s13300-017-0326-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyllested-Winge J, Sparre T, Pedersen LK. NovoPen Echo((R)) insulin delivery device. Med Dev. 2016;9:11–18. doi: 10.2147/MDER.S59229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson BJ, Redondo MJ. What can we learn from patient-reported outcomes of insulin pen devices? J Diabet Sci Technol. 2011;5(6):1563–1571. doi: 10.1177/193229681100500633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breslin M, Mullan RJ, Montori VM. The design of a decision aid about diabetes medications for use during the consultation with patients with type 2 diabetes. Patient Educ Couns. 2008;73(3):465–472. doi: 10.1016/j.pec.2008.07.024 [DOI] [PubMed] [Google Scholar]
- 33.Mullan RJ, Montori VM, Shah ND, et al. The diabetes mellitus medication choice decision aid: a randomized trial. Archiv Internal Med. 2009;169(17):1560–1568. doi: 10.1001/archinternmed.2009.293 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to the commercially sensitive nature of the data but are available from the corresponding author on reasonable request.