Abstract
Vascular endothelial growth factor (VEGF) is a hypoxia-inducible angiogenic growth factor that promotes compensatory angiogenesis in circumstances of oxygen shortage. The requirement for translational regulation of VEGF is imposed by the cumbersome structure of the 5′ untranslated region (5′UTR), which is incompatible with efficient translation by ribosomal scanning, and by the physiologic requirement for maximal VEGF production under conditions of hypoxia, where overall protein synthesis is compromised. Using bicistronic reporter gene constructs, we show that the 1,014-bp 5′UTR of VEGF contains a functional internal ribosome entry site (IRES). Efficient cap-independent translation is maintained under hypoxia, thereby securing efficient production of VEGF even under unfavorable stress conditions. To identify sequences within the 5′UTR required for maximal IRES activity, deletion mutants were analyzed. Elimination of the majority (851 nucleotides) of internal 5′UTR sequences not only maintained full IRES activity but also generated a significantly more potent IRES. Activity of the 163-bp long “improved” IRES element was abrogated, however, following substitution of a few bases near the 5′ terminus as well as substitutions close to the translation start codon. Both the full-length 5′UTR and its truncated version function as translational enhancers in the context of a monocistronic mRNA.
Vascular endothelial growth factor (VEGF) plays a key role in the initiation of blood vessel formation. VEGF is produced by and secreted from the tissue toward which new blood vessels eventually extend. A large body of evidence supports the notion that the amount of VEGF produced determines the magnitude of the angiogenic response through interaction with cognate receptors expressed on nearby endothelial cells. VEGF expression is tightly regulated in vivo, and a deviation from the normal levels of VEGF might be detrimental to the vasculature. For example, a reduction of VEGF gene dosage by one-half (in mice heterozygous for an inactivating mutation in VEGF) leads to severe vascular defects and early embryonic death (6, 9). Conversely, overexpression of VEGF may lead to pathologies like retinopathy, underlined by excessive proliferation of blood vessels (1, 28).
VEGF expression can be modulated in vitro by a variety of agents, including a number of cytokines, growth factors, and steroid hormones. However, from a physiological point of view, regulation of VEGF by hypoxia is the most significant. Insufficient vascular supply and the resultant reduction in tissue oxygen tension often lead to compensatory neovascularization acting to satisfy the metabolic needs of the tissue. Hypoxia-induced VEGF was found to be the key mediator of this feedback response (30, 33). VEGF is regulated by hypoxia at both the transcriptional and posttranscriptional levels. Transcriptional regulation of VEGF is mediated by the transcription factor hypoxia-inducible factor 1, which accumulates under conditions of hypoxia and activates VEGF transcription through binding to specific promoter sequences (10). Hypoxia also leads to stabilization of VEGF mRNA (13, 32, 36). The intrinsically short half-life of VEGF mRNA (approximately 30 min) is significantly extended under stress, presumably through hypoxia-augmented binding of an unidentified protein(s) to its 3′ untranslated region (3′UTR) (22). Both mechanisms act to increase the steady-state levels of VEGF mRNA. However, it is not known whether the production of VEGF is also regulated at the level of mRNA translation or, in particular, whether the mode or extent of VEGF translation is affected by hypoxia.
Initiation of eukaryotic protein synthesis involves about 10 initiation factors (eIFs). The members of the eIF4 group of initiation factors collectively catalyze the recognition of the mRNA cap, the unwinding of mRNA secondary structure, and the binding of mRNA to the 43S preinitiation complex (27). The selection of a particular mRNA from the pool of translatable RNAs is determined by the relative efficiency by which eIF4E binds to the cap structure, and the efficiency of translation initiation by ribosome scanning is governed largely by the composition and structure of the 5′UTR of the mRNA (19, 35).
The 5′UTR of VEGF has several features which are incompatible with efficient ribosomal scanning. First, it is considerably longer (1,014 nucleotides in the mouse) than most eukaryotic 5′UTRs. Second, it has a high G+C content (comprising 64% of 5′UTR sequences and 80% of the 100 nucleotides preceding the translation initiation codon) and can potentially form complex, stable secondary structures. Third, the 5′UTR contains a short open reading frame bounded by in-frame initiation and termination codons. The inherent difficulty for efficient ribosome scanning, in conjunction with findings that certain cellular genes may also use internal ribosome entry sites (IRESs), has prompted us to examine the possibility that VEGF mRNA is translated in a cap-independent mode.
IRES elements were first discovered in picornavirus mRNAs, which are naturally uncapped but nonetheless efficiently translated (15, 29). Subsequently, it was found that some cellular RNAs which are normally capped can also be translated by an internal ribosome binding mechanism. The list of cellular genes shown to contain sequences mediating internal initiation within their 5′UTR includes the immunoglobulin heavy-chain binding protein (BiP) (24), antennapedia (26), fibroblast growth factor (39), platelet-derived growth factor B (PDGF-B) (4), insulin-like growth factor II (38), and the translation initiation factor eIF4G (12). We present evidence here that the 5′UTR of VEGF mRNA directs internal translation initiation in vivo.
The option of internal initiation is an advantage for competition with other mRNAs when a certain component of the eIF4 complex becomes rate limiting and provides a given mRNA with the ability to be translated at times when cap-dependent translation is compromised. Such circumstances may develop under conditions of hypoxia, when overall protein synthesis is significantly inhibited (20). Paradoxically, however, VEGF ought to be maximally produced during hypoxia to fulfill its function as a mediator of hypoxia-driven angiogenesis and to rescue the tissue from ischemic injury. These considerations have led us to examine the performance of the 5′UTR under conditions of hypoxic stress.
MATERIALS AND METHODS
Construction of bicistronic and monocistronic expression plasmids.
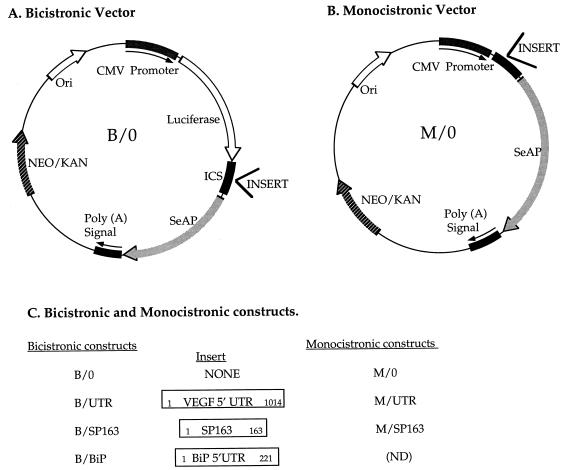
The basic bicistronic vector (designated B/0) and basic monocistronic vector (designated M/0) used in this study for insertion of 5′UTR elements were provided by QBI Enterprises (Nes-Ziona, Israel). Their structures are shown in Fig. 1A and B.
FIG. 1.
Vectors and constructs used in this study. (A) A bicistronic expression vector in which expression of a bicistronic mRNA is driven by a CMV promoter. A firefly luciferase (LUC) is translated from the first cistron, and a SeAP is translated from the second cistron. Putative IRES elements are inserted into the intercistronic space (ICS). (B) A monocistronic expression vector in which SeAP expression is driven by a CMV promoter. Sequence elements tested for a translation-modulating activity are inserted upstream of the SeAP coding region. (C) Designations of bicistronic and monocistronic constructs used in this study (see Materials and Methods for details). The nucleotide numbers inside the rectangles represent the insert length. ND, not done.
5′UTR elements were obtained by reverse transcription-PCR (RT-PCR) amplification with the specific primers indicated below. The primers were designed in a way that each amplified fragment was bounded by XhoI (5′) and NcoI (3′) sites and was inserted between the XhoI (5′) and BsmBI (3′) sites of the intercistronic spacer of the bicistronic vector B/0 or into the same sites of the monocistronic vector M/0. In either case, the hybrid NcoI-BsmBI site recreated the initiator ATG codon of the secreted alkaline phosphatase (SeAP) cistron.
For cloning VEGF and BiP 5′UTRs, RNAs from hypoxic NIH 3T3 cells or 293 cells, respectively, were reverse transcribed by using 10 μg of total RNA. PCR amplification was carried out with a Taq DNA polymerase possessing a proofreading activity (Pwo DNA polymerase; Boehringer) and the oligonucleotide primers VEGF 5′ primer (5′AGCGCAGAGGCTTGGGGC) and VEGF 3′ primer (5′GGTTTCGGAGGCCGTCCG) (corresponding to nucleotides 1218 to 1235 and 2231 to 2214, respectively, of the mouse VEGF gene; GenBank accession no. U41383).
The oligonucleotide primers used to amplify the full-length VEGF 5′UTR were also used to amplify the new splice variant of VEGF 5′UTR designated SP163 (see below), which was also inserted into the B/0 and M/0 vectors in the same way as described above. To generate SP163 mutants with nucleotide substitutions close to the termini, PCR primers similar to those described above, but containing the substitutions depicted in Fig. 5, were used. These primers are the BiP 5′ primer (5′AGGTCGACGCCGGCCAAGACA) and the BiP 3′ primer (5′CTTGCCAGCCAGTTGGGCAGC) (corresponding to nucleotides 372 to 392 and 592 to 572, respectively, of the human BiP gene; GenBank accession no. M19645).
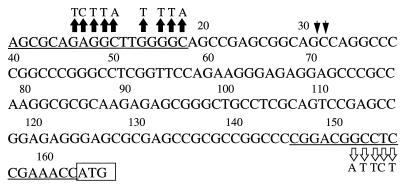
FIG. 5.
Sequence of the SP163 element. The oligonucleotide primers that were used for amplification of SP163 are underlined. These oligonucleotides correspond to the respective boundaries of the 1,014-nucleotide mouse VEGF 5′UTR. The initiation ATG codon is boxed. Arrowheads point to two possible locations of a presumptive splice junction. Nucleotide 1 of SP163 corresponds to nucleotide 1218 of the mouse VEGF gene (GenBank accession no. U41383), while nucleotide 163 of the SP163 fragment corresponds to nucleotide 2231 of the mouse gene. Substitutions depicted by solid arrows represent mutations introduced into the B/SP163/M5′ mutant, and substitutions depicted by open arrows represent mutations introduced into the B/SP163/M3′ mutant (see Fig. 6).
All the plasmid constructs were sequenced to verify their structure and the preservation of open reading frames within PCR-amplified fragments. A summary of bicistronic and monocistronic constructs used in this study is shown in Fig. 1C.
Cell culture and DNA transfections.
C6 cells, a clonal glial cell line derived from a rat glial tumor (3), were grown in Dulbecco modified Eagle medium (DMEM) containing 5% fetal calf serum and antibiotics. The human cell line 293 (adenovirus-transformed fetal kidney cells), mouse NIH 3T3 cells, and rat primary astrocytes were maintained in DMEM containing 10% fetal calf serum and antibiotics. Primary rat astrocytes were prepared as described previously (11).
The cells were exposed to hypoxia by being placed in 1% oxygen in a CO2/O2 incubator (Forma Scientific), in which oxygen levels are monitored and adjusted automatically, except for the experiment in Fig. 3C, for which the cells were incubated for 20 h in a GasPak Plus anaerobic culture chamber (BBL Microbiology Systems) utilizing hydrogen and a palladium catalyst to remove all traces of oxygen.
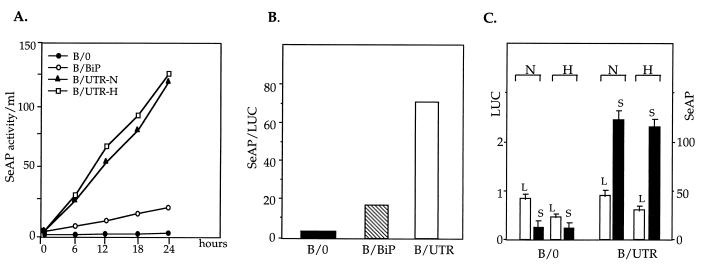
FIG. 3.
Production of LUC and SeAP from a bicistronic mRNA. (A) Production of SeAP from the downstream cistron. Pools of stably transfected C6 clones were grown to 70% confluence, medium was replaced with a fresh medium (t = 0) and aliquots were withdrawn at the indicated time points and analyzed for SeAP activity as described in Materials and Methods. Activity is expressed as cumulative SeAP activity (in arbitrary units of the colorogenic reaction product) per ml of medium and is normalized to total cellular protein and corrected for differences in VEGF mRNA levels (Fig. 4; also see the text). For testing SeAP production during hypoxia, cells were shifted to 1% oxygen at t = 0 and further analyzed as above. N, normoxia; H, hypoxia. (B) Ratio of SeAP to LUC production in the different transfectants in panel A. The SeAP/LUC ratio was calculated from the respective activities determined at the end point of the experiment (t = 24 h). (C) Effect of hypoxia on LUC and SeAP production in transiently transfected 293 cells. At 14 h posttransfection, the medium was changed, half of the culture plates were kept under normoxic conditions, and the other half were placed in a hypoxic chamber (see Materials and Methods). SeAP activity in the culture media and LUC activity in cell extracts were determined 20 h later. Results shown are the average of four independent transfections with each plasmid. The plasmid designations are as shown in Fig. 1. L, LUC activity in arbitrary units; S, SeAP activity in arbitrary units per milliliter of medium; N, normoxia; H, hypoxia.
Transfections were performed with Lipofectin (Gibco BRL) as follows. Cells were plated at a density of 1 × 105 to 2 × 105 cells per 60-mm plate and grown for 24 h. Medium was replaced with serum-free DMEM, and 200 μl of DNA-Lipofectin suspension (containing 2 μg of supercoiled plasmid and 15 μg of Lipofectin, preincubated at room temperature for 40 min) was added in a dropwise manner. The medium was replaced 14 h later with a medium supplemented with serum and antibiotics, and the cells were incubated for an additional 48 h before being split into a selection medium containing G418 (2 mg/ml). Selection continued for 10 to 14 days, and more than 50 individual G418-resistant clones were pooled for each plasmid.
For transient transfections, cells were seeded at a density of 2 × 105 cells per 1-cm well and transfected 24 h later with 1 μg of DNA per well by the calcium phosphate procedure (17). Cells and media were harvested 36 h posttransfection. Cell extracts were analyzed for luciferase (LUC) activity or used for mRNA preparation (in the latter case, 10 μg of DNA was used to transfect 2 × 106 to 2.5 × 106 cells per 100-mm plate), while the medium was used for analysis of SeAP activity.
RNA analysis.
Total RNA was prepared by the guanidinium thiocyanate/phenol-chloroform extraction procedure (7).
(i) Analysis of mRNA in polysomal fractions.
Protein synthesis was instantaneously arrested by treating the culture with cycloheximide (90 μg/ml) for 10 min. Cells were collected, washed with phosphate-buffered saline, and kept at −70°C until analyzed. Cell lysis, size fractionation of polysomes by sedimentation through sucrose gradients, and preparation of ribosome-associated RNAs were performed as described previously (25).
(ii) Northern blotting.
RNA was denatured in glyoxal and electrophoresed through a 1.0% agarose gel. RNAs were transferred onto a nylon-based membrane (GeneScreen Plus; NEN) by the capillary blot procedure and were hybridized with the indicated cDNAs labeled with 32P by randomly primed DNA synthesis. For standardization, rRNAs were visualized by staining with methylene blue before hybridization. For relative quantification, autoradiograms were scanned with a phosphorimager (FUJIX BAS 1000 bioimaging analyzer). The hybridization probes used were Luciferase (a 1.7-kb cDNA fragment including all of the coding region of firefly luciferase) and SeAP (a 1.5-kb cDNA fragment including the coding region of the human SeAP).
(iii) RT-PCR.
Semiquantitative RT-PCR was performed with a single-tube, one-step RT-PCR system (Access RT-PCR system; Promega) and 20 cycles of amplification. The oligonucleotide primers used for reverse transcription and amplification of the various splicing variants of VEGF mRNA were 5′GAGAGAATGAGCTTCCTACAG3′ and 5′TCACCGCCTTGGCTTGTCACA3′ (derived from the common fourth and eighth exons, respectively). PCR products were resolved by agarose gel electrophoresis and detected by blot hybridization with a VEGF-specific, 32P-labeled cDNA probe (a 0.6-kb mouse cDNA fragment encoding VEGF165 [33rsqb;).
LUC and SeAP analysis.
LUC enzymatic activity in cell extracts was determined by using a commercial LUC assay system (Promega) and the procedure recommended by the supplier. The light generated was measured with a Lumac/3M BIOCOUNTER M2010− luminometer.
SeAP activity released into the growth medium was determined as follows. The medium was heated for 5 min at 65°C and clarified by centrifugation. Aliquots were diluted in a SeAP buffer (1 M diethanolamine [pH 9.8], 0.5 mM MgCl2, 10 mM l-homoarginine) in a 96-well plate, and the enzymatic reaction (at 37°C) was initiated by the addition of 20 μl of 120 mM p-nitrophenyl phosphate. The reaction product was determined by measuring the absorbance at 405 to 630 nm with an enzyme-linked immunosorbent assay reader. The amounts of conditioned medium added and the incubation times used were adjusted so that the readings were within the linear range of the calibration curve obtained with a purified SeAP standard. The total protein was determined by the Bradford method (5).
RESULTS
To evaluate the efficiency at which VEGF mRNA is translated, cytoplasmic extracts were prepared from primary astrocytes and fractionated by centrifugation through a sucrose gradient into subpolysomal and polysomal fractions. The relative abundance of VEGF mRNA in these fractions was determined by quantitative RT-PCR. As shown in Fig. 2B, the bulk of VEGF mRNA was associated with polyribosomes, indicating that the majority of VEGF mRNA is engaged in active protein synthesis. This result suggested that VEGF mRNA is efficiently translated under normal growth conditions despite its cumbersome 5′UTR.
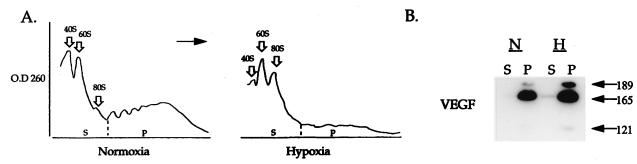
FIG. 2.
Distribution of VEGF mRNA between subpolysomal and polysomal fractions. (A) Size fractionation of ribosomes by centrifugation through a sucrose density gradient. Cytoplasmic extracts were prepared from cultures of primary astrocytes grown under normoxia (left) or hypoxia (right). Sedimentation was from left to right, and the vertical line indicates the point of division into the subpolysomal (S) and polysomal (P) pooled fractions. OD 260, optical density at 260 nm. (B) Relative abundance of VEGF mRNA in the subpolysomal and polysomal fractions. RNA was extracted from the entire pool, and aliquots representing an equal portion of each pool were analyzed by a quantitative RT-PCR/blot hybridization as described in Materials and Methods. The different splicing variants of VEGF mRNA are indicated by the respective number of amino acids in the encoded protein. The proportion of mRNA in polysomes for the most abundant mRNA species, VEGF165 (as determined by densitometric scanning) was 87 and 88% for normoxia and hypoxia, respectively.
To determine whether translation of VEGF mRNA is inhibited under conditions of severe hypoxia, a similar analysis was carried out with astrocytes grown in culture under 1% oxygen for 16 h. Astrocytes were chosen for this study since they are the first cells to respond to hypoxia of neuronal tissues in vivo by upregulating VEGF mRNA expression and are therefore the cells responsible for the feedback angiogenic response (37). As shown in Fig. 2A, the fraction of RNA associated with polyribosomes was reduced by 50% in comparison with that in cells grown under normoxia. Metabolic labeling experiments, measuring the incorporation of [35S]methionine into newly made proteins, showed that under the same hypoxic conditions, protein synthesis was reduced by 55% (data not shown). This result is consistent with previous observations that hypoxia causes about 30 to 50% inhibition in overall protein synthesis (20). Despite the general impairment of protein synthesis, the majority of VEGF RNA remained associated with polysomes (Fig. 2B). Notably, all isoforms of VEGF mRNA remained associated with the polyribosomal fraction, including the shortest, VEGF121, which can accommodate no more than three or four ribosomes when translated at a theoretically maximal rate. In contrast, the fraction of polysome-associated mRNA encoding a reference ribosomal protein L19 was reduced twofold following exposure to hypoxia (as determined by densitometry of the respective hybridizations in the subpolysomal and polysomal fractions). These results suggest that the translation machinery can handle most, if not all, VEGF mRNA, even when the amount of available mRNA increases by 1 order of magnitude due to hypoxia (33).
The fact that translation of VEGF mRNA is very efficient despite its length and high G+C content prompted us to examine whether VEGF mRNA can be translated by internal ribosome entry. Bicistronic mRNAs have been effectively used in vivo to demonstrate the existence of IRESs in both picornavirus (29) and cellular (4, 24, 39) mRNAs. A eukaryotic expression vector was constructed in which a cytomegalovirus (CMV) promoter drives the expression of a bicistronic RNA. The first cistron in the bicistronic mRNA used in this study, encoding LUC, should be translated by a conventional cap-dependent scanning mechanism. As ribosomes fail to continue scanning through the intercistronic region, the second cistron, encoding SeAP, should be translated only if preceded by an IRES. The SeAP cistron was chosen as the downstream cistron since secretion of the enzyme into the culture medium allows accurate quantification and continuous monitoring of protein synthesis. To prevent leaky translation into the second cistron, the intercistronic region included termination codons in all possible reading frames. Furthermore, the fact that the protein translated from the first cistron is an intracellular protein whereas the protein translated from the second cistron is secreted ruled out the possibility of a fused protein retaining both enzymatic activities. The 5′UTR of VEGF was subcloned by precise PCR amplification, inserted into the intercistronic spacer region, and transfected into a C6 rat glioma cell line. Pools of stably transfected clones were prepared, each composed of at least 50 individual clones. Analysis of pools ensured that possible differences in gene expression due to different integration sites were averaged. The reporter gene activity in transfectants obtained with the bicistronic vector alone (designated B/0) and with the bicistronic vector containing the VEGF 5′UTR (designated B/UTR) was determined.
The two constructs expressed a bicistronic mRNA from which LUC was translated with a comparable efficiency. Analysis of the downstream cistron, however, revealed that B/0 produced a negligible amount of SeAP activity, confirming minimal, if any, readthrough of ribosomes from the LUC to the SeAP cistron (Fig. 3). In contrast, B/UTR directed the production of high levels of SeAP in a continuous manner, indicating that the 5′UTR of VEGF contains a functional IRES element. To appreciate the relative strength of the VEGF IRES, a comparison was made with the well-characterized cellular IRES contained in the 5′UTR of BiP mRNA (24). To this end, the 5′UTR of BiP was inserted into the same site of the bicistronic vector and pools of stable transfectants were prepared and similarly analyzed for LUC and SeAP production. As shown in Fig. 3B, the VEGF IRES was fivefold more efficient than the BiP IRES in directing SeAP production.
While there is a clear rationale for the use of internal initiation in viruses, the advantage of internal initiation in cellular mRNAs is not always clear. In the case of VEGF, however, the capacity for cap-independent translation might be particularly advantageous during hypoxia, when overall protein synthesis is compromised. Therefore, we have also examined the translation of the bicistronic mRNA in cultures grown under hypoxia. Under conditions of hypoxic stress, when translation of LUC from the upstream cistron was reduced, the rate of SeAP production, presumably by internal initiation, was unaffected (Fig. 3C) and SeAP was secreted into the culture medium at the same constant rate as under normoxic conditions (Fig. 3A).
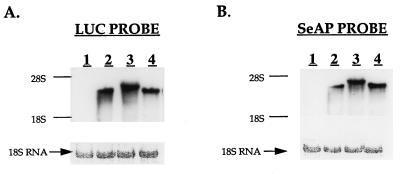
To rule out the possibility that in B/UTR-transfected cells the protein encoded by the second cistron is translated from a monocistronic SeAP mRNA, an RNA blot hybridization analysis was carried out with both a LUC-specific probe and a SeAP-specific probe (Fig. 4). A single band of RNA was detected in each case, corresponding to the expected size of the respective bicistronic transcript (the larger size of hybridized mRNA in B/UTR-transfected cells is due to the addition of 1,014 bp of 5′UTR sequence, and the slightly larger size in B/BiP is due to the addition of 221 bp of 5′UTR sequence). Hybridization with both a LUC-specific probe and a SeAP-specific probe detected a single mRNA species of a comparable size in each case, indicating that both cistrons are contained in a single transcript. While a limited electrophoretic resolution prevents the conclusion of an identical transcript, we note the failure to detect more than one transcript. Importantly, the failure to detect a band corresponding in size to that of a presumptive monocistronic SeAP mRNA ruled out the possibility that a significant amount of SeAP is translated from a monocistronic SeAP mRNA. Also, the inclusion of the 5′UTR had no significant effect on the steady-state level of the bicistronic mRNA expressed, indicating that differences in measured LUC and SeAP activities are due mostly to differences in translation efficiencies. Nevertheless, the steady-state level of the bicistronic mRNA was determined in each transfectant by densitometry of Northern blots and was used for normalizing enzymatic activity.
FIG. 4.
Northern blot analysis of mRNAs transcribed from bicistronic plasmids. RNA was extracted from untransfected C6 cells and from the same stably transfected C6 pools used in the experiment in Fig. 3. A 20-μg portion of each RNA was electrophoresed in two parallel lanes, blotted, and hybridized with either a LUC-specific probe (A) or a SeAP-specific probe (B). To ensure equal loading, rRNAs were stained with methylene blue prior to hybridization. Lanes: 1, untransfected C6 cells; 2, B/0; 3, B/UTR; 4, B/BiP.
A deletion analysis of the VEGF 5′UTR was carried out to identify sequences within the 5′UTR that are both necessary and sufficient for internal ribosome entry. This analysis was greatly facilitated by the isolation of an RT-PCR-amplified DNA fragment representing a truncated version of the 5′UTR. The 163-nucleotide sequence, designated SP163, is shown in Fig. 5. SP163 is composed of the 5′ cap-containing segment of 31 (or 32) nucleotides juxtaposed to the 132 (or 131) nucleotides preceding the initiator AUG codon. Although it is not known whether this particular truncated version of the 5′UTR represents a naturally occurring variant of the 5′UTR or an RT-PCR artifact, it was nevertheless instrumental in elucidating a structure-function relationship for the VEGF IRES.
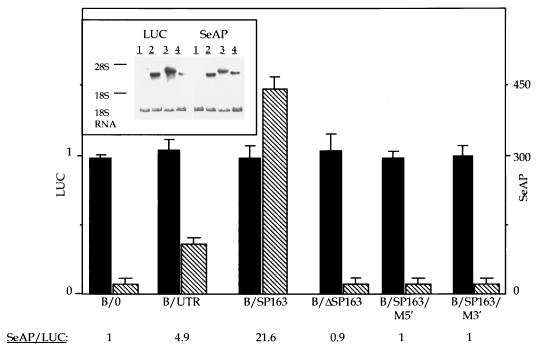
To determine whether the SP163 element can direct internal ribosome entry, it was inserted into the ICS region of the same bicistronic vector used to analyze the full-length 5′UTR. This plasmid, designated B/SP163, was transfected into the human 293 cell line alongside plasmid B/0 (serving as a negative control) and plasmid B/UTR (for comparison of relative IRES strength). The proteins encoded by the first and second cistrons were quantified following a transient transfection (Fig. 6). Consistent with the results of the stable transfections (Fig. 3), the full-length 5′UTR also directed a significant production of SeAP in cells of human origin. Remarkably, SP163 was 4.4-fold more efficient as an IRES element than was the full-length 5′UTR.
FIG. 6.
IRES activity of SP163. 293 cells were transiently transfected with each of the indicated bicistronic plasmids, and SeAP activity in culture media and LUC activity in cell extracts were determined 36 h posttransfection as described in Materials and Methods. The results are the average of four independent transfections with each plasmid and are expressed as arbitrary LUC activity units and arbitrary SeAP activity units per milliliter. Solid bars, LUC; hatched bars, SeAP. The SeAP/LUC ratios, corrected for differences in levels of the bicistronic mRNAs, are also shown. Plasmid designations are as in Fig. 1. B/ΔSP163 is a deletion mutant of SP163 missing the first 31 nucleotides. B/SP163/M5′ and B/SP163/M3′ are SP163 mutants containing the respective substitutions indicated in Fig. 5. (Inset) Northern blot analysis with a SeAP-specific probe and a LUC-specific probe performed on 20 μg of the total RNA extracted from transfected 293 cells 36 h posttransfection. Lanes: 1, mock-transfected cells; 2, B/0-transfected cells; 3, B/UTR-transfected cells; 4, B/SP163-transfected cells.
RNA blot hybridization analysis was performed to ensure that SP163-directed translation of SeAP occurred from a bicistronic mRNA. As shown in Fig. 6 (inset), a single mRNA species, corresponding in size to a bicistronic B/SP163 mRNA, cohybridized with the LUC-specific probe and the SeAP-specific probe and no mRNA corresponding in size to a monocistronic SeAP mRNA could be detected. This result indicated that SeAP was translated from a bicistronic RNA by using SP163 for internal ribosome entry.
The finding that SP163 functions as an IRES much better than does the full-length 5′UTR is of interest considering that, in terms of sequence, the former is a subset of the latter. Moreover, the relatively small size of SP163 rendered it a better substrate for mutational analysis of IRES function than the full-length 5′UTR. To identify SP163 sequences required for IRES activity, we first deleted the 5′-terminal 31 nucleotides spliced to the downstream 5′UTR segment and analyzed the resultant element (designated B/ΔSP163) for LUC and SeAP activities. As shown in Fig. 6, this construct, while permitting translation of the first cistron, was inactive in directing the translation of the second cistron, indicating that nucleotides within the first 31 nucleotides from the cap site are essential for formation of the SP163 IRES. A finer structure-function analysis of SP163 was performed with substitutions instead of a deletion. Nucleotides chosen for substitution were selected on the basis of a computer-aided folding model of SP163, which predicted that a particular pattern of base pairing brings the 5′ terminus and the initiator ATG codon into proximity, and were intended to disrupt this secondary structure. Indeed, substituting a cluster of 9 nucleotides in a region close to the 5′ terminus (solid arrows in Fig. 5) completely abolished IRES activity (Fig. 6). Likewise, translation of the downstream cistron was abolished as a result of substituting a cluster of 5 nucleotides in a region close to the initiator ATG codon (open arrows in Fig. 5). These results suggested that sequences residing close to both termini are essential for the formation of the SP163 IRES.
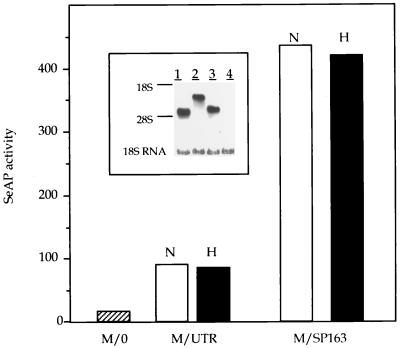
Previous studies with picornaviruses, as well as a functional analysis of the cellular fibroblast growth factor IRES, have suggested that these regulatory elements may also act as translational enhancers in the context of a monocistronic mRNA (16, 39). We examined the possibility that the 5′UTR of VEGF (both the full-length form and the truncated version) acts as a translational enhancer by testing its ability to augment the translation of a monocistronic mRNA. To this end, the full-length 5′UTR or SP163 was inserted between a CMV promoter and the coding region of SeAP in the context of a monocistronic vector (Fig. 1). These constructs, designated M/UTR and M/SP163, respectively, were transfected into C6 cells and analyzed for SeAP production in pools of stably transfected clones. Analysis of RNA extracted from transfected cells showed similar levels of SeAP-containing mRNA, which was represented in each case by a single band of the expected size (Fig. 7, inset). In contrast, the amount of SeAP activity released into the culture medium varied greatly according to the nature of the insert. Thus, the full-length 5′UTR augmented the translation of SeAP fivefold and SP163 enhanced SeAP translation much more. In the experiment in Fig. 7, enhancement by SP163 was 25-fold, and in other experiments (data not shown), enhancement was up to 40-fold.
FIG. 7.
Translation enhancing activity of VEGF 5′UTR sequences. C6 cells were stably transfected with the monocistronic constructs indicated. SeAP activity was analyzed in pools of transfected clones as described in Materials and Methods, and values were standardized to total protein. For analysis of SeAP activity under hypoxic conditions, cells were shifted to 1% oxygen 24 h before sampling. N, normoxia; H, hypoxia. (Inset) Northern blot analysis of SeAP-containing mRNAs in stably transfected pools. Lanes: 1, M/0-transfected cells; 2, M/UTR-transfected cells; 3, M/SP163-transfected cells; 4, nontransfected cells.
Considerations discussed above led us to examine whether these elements also function as translational enhancers under conditions of hypoxic stress. As shown in Fig. 7, enhancement of SeAP translation was unaffected by growth under conditions of severe hypoxia.
To demonstrate that SP163 is a strong translational enhancer in additional cell types, the same constructs were also transfected into human 293 cells and CHO cells. SP163 acted as a translation enhancer in these cells as well, augmenting SeAP production to a level comparable to that shown for C6 cells (data not shown).
DISCUSSION
Despite the key role that VEGF plays in the formation and maintenance of vascular networks and although it is tightly regulated in vivo, surprisingly little is known about its translational regulation. Here we show that, in contrast to the prediction based on the complex structure of its 5′UTR, VEGF is very efficiently translated. This is shown by the fact that the majority of VEGF mRNA expressed by cultured cells is associated with polyribosomes (Fig. 2). Importantly, translation of VEGF is not impaired under conditions of severe hypoxia, i.e., when overall translation was reduced. These findings are consistent with the physiological requirement that translation of VEGF mRNA not be adversely affected by insults that impede protein synthesis in general and be refractory to hypoxia in particular. These observations and rationales led us to explore a possible mechanism(s) acting to secure efficient translation of VEGF, particularly under conditions of hypoxic stress.
To determine whether the 5′UTR of VEGF can direct internal ribosome entry, we used a bicistronic mRNA assay. Of the various operational criteria that have been applied to examine internal initiation, including the use of cap analogs and analysis of poliovirus-infected cells, the bicistronic mRNA assay is considered the most valid test (14). It is only valid, however, if complemented by a clear demonstration that the second (downstream) cistron is indeed translated from a bicistronic mRNA. The experiments in Fig. 3 and 4 provided compelling evidence that the 5′UTR of VEGF contains a functional IRES. The possibility that the inserted 5′UTR acted to facilitate reinitiation has not been ruled out. However, the finding that translation of the downstream cistron is abolished as a result of mutations in noncontiguous regions of the 5′UTR makes this possibility less likely. Another feature of at least some known IRES elements is the activity as a translational enhancer in the context of a monocistronic RNA. The experiments in Fig. 7 demonstrate that the 5′UTR of VEGF possesses this activity.
In the case of VEGF, internal ribosome entry might improve the competition with other mRNAs which, otherwise, would have rendered the translation of this mRNA (distinguished by an exceptionally long, highly structured 5′UTR) an inefficient process (19, 35). The notion that VEGF mRNA may not compete well with other cellular mRNAs for recruitment of cap-binding proteins is supported by the finding that overexpression of eIF4E (which, as the least abundant of the initiation factors, is a rate-limiting factor in translational regulation of “weak” mRNAs) upregulates the translation of VEGF mRNA (18). Among its many functions, VEGF plays an important role in maintaining vascular homeostasis and controlling vascular permeability (31). Notably, its unscheduled downregulation at critical developmental timings may cause pathologic regression of existing vessels (2). Thus, the option of internal ribosome entry may prevent the deleterious consequences of VEGF deficiency in circumstances where cap-dependent translation is even transiently compromised.
The list of cellular mRNAs with a demonstrable IRES is expanding and is likely to continue to do so in the future. However, the rationale for maintaining both modes of translation is not fully understood in all cases. Recent studies have shown that in certain systems a transition from a cap-dependent to a cap-independent mode of translation is a regulated process. For example, utilization of the anntenapedia and ultrabithorax IRESs is spatially and temporally regulated during Drosophila development (40) and the PDGF-B IRES is specifically used upon megakaryocytic differentiation (4). A physiological situation in which the predominant mode of translation can switch from cap dependent to cap independent is heat shock, where the synthesis of non-heat shock proteins (non-HSPs) decreases but the synthesis of HSPs remains the same or increases (23). While there is no definite proof that HSP70 has a functional IRES, BiP, which can be translated by internal initiation (24), has partial sequence homology to HSP70 and its level becomes elevated during certain stress conditions (e.g., hypoxia and hypoglycemia).
Hypoxia is a major cellular stress, leading to upregulation of a cohort of genes. The VEGF gene is a prominent example of a hypoxia-induced gene whose function is to allow the organism to respond better to this harmful situation. We have previously used a multicell spheroid system to simulate a continuum of microenvironments with increasing hypoxia and/or hypoglycemia and have used the system to define conditions of stress conducive for upregulation of VEGF expression (34). Interestingly, under conditions of extreme stress, VEGF mRNA expression was inhibited rather than enhanced, suggesting that excessively stressed cells are unable to elicit this compensatory response. Therefore, in a realistic setting of progressive ischemia, maximal levels of the mRNA template are available for translation during a restricted time window in which cap-dependent translation might be suboptimal. This situation underscores the need for efficient translation of VEGF, so that a sufficient amount of the protein is produced (and new blood vessels are recruited) before an ischemic injury is inflicted. Data presented here show that one solution to the problem is the use of an IRES. Importantly, we showed that internal ribosome entry is not affected by hypoxia. By way of extrapolation, it is possible that a similar mode of translation is used by other hypoxia-regulated genes.
The analysis of deleted versions of the 5′UTR may shed more light on the poorly understood structure-function relationship of IRES elements in general. The picornavirus IRESs are about 450 nucleotides long, and phylogenetic comparisons within each group suggest that most of the 450 nucleotides are required (8, 14). Among cellular IRESs, it has been shown that the complete 5′UTR of the exceedingly long (>1,500-nucleotide) antennapedia element is required for maximum IRES activity and that fragments of the 5′UTR are considerably less efficient (26). In contrast, the SP163 sequence, which is a subset of the full-length VEGF 5′UTR, gave a much better IRES activity than the full-length 5′UTR did (Fig. 6). The relatively short size of SP163 makes it a most suitable substrate for further mutational analysis aimed at elucidating the structure-function relationship.
In terms of the sequence, there is no obvious resemblance among the known cellular IRESs, nor do any of the cellular IRESs show a significant homology to picornavirus IRESs. For example, none of the cellular IRESs has a pyrimidine-rich tract located 25 nucleotides upstream of the initiation site, as in the picornavirus IRESs (8). VEGF has considerable sequence homology to another cellular gene with an IRES element, namely, the PDGF gene. However, a search for sequence homology between the respective 5′UTRs has shown that despite a similarity in length and overall high G+C content, there is no significant homology in the primary sequences. The lack of significant sequence homology among cellular IRESs, on the one hand, and the realization that RNA interactions with trans-acting proteins are likely to involve the specific recognition of sequence and/or structural elements, on the other hand, have encouraged the search for common structural motifs folded within cellular IRESs. These studies resulted in the identification of a putative common RNA structural motif that is conserved in several IRES elements and is characterized by the presence of a Y-type unusual folding region close to the initiator AUG (21). Interestingly, almost the entire SP163 element can be folded into a stable (−256.3 kJ at 37°C) Y-shaped structure in which half of the nucleotides form G · C pairs and the initiator AUG codon is preceded by a short nonpaired sequence (not shown). The functional significance of this folding pattern, however, is not clear and awaits the scrutiny of a detailed mutational analysis. The preliminary analysis shown in Fig. 6 supports the notion that sequences residing close to both termini are required for IRES activity of this element.
Finally, the strong translation enhancer activity of SP163 can be used, in principle, for biotechnological applications designed to maximize the production of genes of interest.
ACKNOWLEDGMENTS
We thank Oded Meyuhas for advice.
E.K. is the incumbent of the Woll Sisters and Brothers Chair in Cardiovascular Diseases at the Hebrew University.
REFERENCES
- 1.Aiello L P, Avery R L, Arrigg P G, Keyt B A, Jampel H D, Shah S T, Pasquale L R, Thieme H, Iwamoto M A, Park J E, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 2.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 3.Benda P, Lightbody J, Sato G, Levine L, Sweet W. Differentiated rat glial cell strain in tissue culture. Science. 1968;161:370–374. doi: 10.1126/science.161.3839.370. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein J, Sella O, Le S-U, Elroy-Stein O. PDGF2/c-sis mRNA leader contains a differentiation-linked internal ribosomal entry site (D-IRES) J Biol Chem. 1997;272:9356–9362. doi: 10.1074/jbc.272.14.9356. [DOI] [PubMed] [Google Scholar]
- 5.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 7.Chirgwin J M, Przybyla A E, McDonald R J, Rutter W J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 8.Ehrenfeld E. Initiation of translation by picornavirus RNAs. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 549–573. [Google Scholar]
- 9.Ferrara N, Carver Moore K, Chen H, Dowd M, Lu L, O’Shea K S, Powell Braxton L, Hillan K J, Moore M W. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 10.Forsythe J A, Jiang B H, Iyer N V, Agani F, Leung S W, Koos R D, Semenza G L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frangakis M V, Kimelberg H K. Dissociation of neonatal rat brain by dispase for preparation of primary astrocyte cultures. Neurochem Res. 1984;9:1689–1698. doi: 10.1007/BF00968079. [DOI] [PubMed] [Google Scholar]
- 12.Gan W, Rhoads R E. Internal initiation of translation directed by the 5′-untranslated region of the mRNA for eIF4G, a factor involved in the picornavirus-induced switch from cap-dependent to internal initiation. J Biol Chem. 1996;271:623–626. doi: 10.1074/jbc.271.2.623. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda E, Achen M G, Breier G, Risau W. Hypoxia-induced transcriptional activation and increased mRNA stability of vascular endothelial growth factor in C6 glioma cells. J Biol Chem. 1995;270:19761–19766. doi: 10.1074/jbc.270.34.19761. [DOI] [PubMed] [Google Scholar]
- 14.Jackson R J. A comparative view of initiation site selection mechanisms. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 71–112. [Google Scholar]
- 15.Jang S K, Krausslich H-G, Nicklin M J H, Duke G M, Palmenberg A C, Wimmer E. A segment of the 5′ nontranslated region of encephalomyocarditis virus RNA directs internal entry of ribosomes during in vitro translation. J Virol. 1988;62:2636–2643. doi: 10.1128/jvi.62.8.2636-2643.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang S K, Davies M V, Kaufman R J, Wimmer E. Initiation of protein synthesis by internal entry of ribosomes into the 5′ nontranslated region of encephalomyocarditis virus RNA in vivo. J Virol. 1989;63:1651–1660. doi: 10.1128/jvi.63.4.1651-1660.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jordan M, Schallhorn A, Wurm F M. Transfecting mammalian cells: optimization of critical parameters affecting calcium-phosphate precipitate formation. Nucleic Acids Res. 1996;24:596–601. doi: 10.1093/nar/24.4.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kevil C G, De Benedetti A, Payne D K, Coe L L, Laroux F S, Alexander J S. Translational regulation of vascular permeability factor by eukaryotic initiation factor 4E: implications for tumor angiogenesis. Int J Cancer. 1996;65:785–790. doi: 10.1002/(SICI)1097-0215(19960315)65:6<785::AID-IJC14>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 19.Kozak M. An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol. 1991;115:887–903. doi: 10.1083/jcb.115.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kraggerud S M, Sandvik J A, Pettersen E O. Regulation of protein synthesis in human cells exposed to extreme hypoxia. Anticancer Res. 1995;15:683–686. [PubMed] [Google Scholar]
- 21.Le S-Y, Maizel J V., Jr A common RNA structural motif involved in the internal initiation of translation of cellular mRNAs. Nucleic Acids Res. 1997;25:362–369. doi: 10.1093/nar/25.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy A P, Levy N S, Goldberg M A. Hypoxia-inducible protein binding to vascular endothelial growth factor mRNA and its modulation by the von Hippel-Lindau protein. J Biol Chem. 1996;271:25492–25497. doi: 10.1074/jbc.271.41.25492. [DOI] [PubMed] [Google Scholar]
- 23.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 24.Macejak D G, Sarnow P. Internal initiation of translation mediated by the 5′ leader of a cellular mRNA. Nature. 1991;353:90–94. doi: 10.1038/353090a0. [DOI] [PubMed] [Google Scholar]
- 25.Meyuhas O, Thompson E A, Jr, Perry R P. Glucocorticoids selectively inhibit translation of ribosomal protein mRNAs in P1798 lymphosarcoma cells. Mol Cell Biol. 1987;7:2691–2699. doi: 10.1128/mcb.7.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh S-K, Scott M P, Sarnow P. Homeotic gene Antennapedia mRNA contains 5′-noncoding sequences that confer translational initiation by internal ribosome binding. Genes Dev. 1992;6:1643–1653. doi: 10.1101/gad.6.9.1643. [DOI] [PubMed] [Google Scholar]
- 27.Pain V M. Initiation of protein synthesis in eukaryotic cells. Eur J Biochem. 1996;236:747–771. doi: 10.1111/j.1432-1033.1996.00747.x. [DOI] [PubMed] [Google Scholar]
- 28.Pe’er J, Shweiki D, Itin A, Hemo I, Gnessin H, Keshet E. Hypoxia-induced expression of vascular endothelial growth factor by retinal cells is a common factor in neovascularizing ocular diseases. Lab Invest. 1995;72:638–645. [PubMed] [Google Scholar]
- 29.Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- 30.Plate K H, Breier G, Weich H A, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 31.Senger D R, Van de Water L, Brown L F, Nagy J A, Yeo K T, Yeo T K, Berse B, Jackman R W, Dvorak A M, Dvorak H F. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12:303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 32.Shima D T, Deutsch U, D’Amore P A. Hypoxic induction of vascular endothelial growth factor (VEGF) in human epithelial cells is mediated by increases in mRNA stability. FEBS Lett. 1995;370:203–208. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- 33.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 34.Shweiki D, Neeman M, Itin A, Keshet E. Induction of vascular endothelial growth factor expression by hypoxia and by glucose deficiency in multicell spheroids: implications for tumor angiogenesis. Proc Natl Acad Sci USA. 1995;92:768–772. doi: 10.1073/pnas.92.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonenberg N. mRNA 5′ cap-binding protein eIF4E and control of cell growth. In: Hershey J W B, Mathews M B, Sonenberg N, editors. Translational control. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 245–269. [Google Scholar]
- 36.Stein I, Neeman M, Shweiki D, Itin A, Keshet E. Stabilization of vascular endothelial growth factor mRNA by hypoxia and hypoglycemia and coregulation with other ischemia-induced genes. Mol Cell Biol. 1995;15:5363–5368. doi: 10.1128/mcb.15.10.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stone J, Itin A, Alon T, Pe’er J, Gnessin H, Chan Ling T, Keshet E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J Neurosci. 1995;15:4738–4747. doi: 10.1523/JNEUROSCI.15-07-04738.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teerink H, Voorma H O, Thomas A A M. The human insulin-like growth factor II leader 1 contains an internal ribosomal entry site. Biochim Biophys Acta. 1995;1264:403–408. doi: 10.1016/0167-4781(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 39.Vagner S, Gensac M C, Maret A, Bayard F, Amalric F, Prats H, Prats A C. Alternative translation of human fibroblast growth factor 2 mRNA occurs by internal entry of ribosomes. Mol Cell Biol. 1995;15:35–44. doi: 10.1128/mcb.15.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye X, Fong P, Iizuka N, Choate D, Cavener D R. Ultrabithorax and Antennapedia 5′ untranslated regions promote developmentally regulated internal translation initiation. Mol Cell Biol. 1997;17:1714–1721. doi: 10.1128/mcb.17.3.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]