Short abstract
Recent microarray analyses of lymphomas suggest that the prognosis of cancer patients is related to an interplay between cancer cells and their microenvironment, including the immune response.
Abstract
The study of cancer immunology has recently been reinvigorated by the application of new research tools and technologies, as well as by refined bioinformatics methods for interpretation of complex datasets. Recent microarray analyses of lymphomas suggest that the prognosis of cancer patients is related to an interplay between cancer cells and their microenvironment, including the immune response.
That the immune system plays an important role in the regulation and outcome of cancer has been an intriguing concept for almost a century. As discussed by Dunn et al. [1,2], although many observations supported the notion that the ability of cancer to escape the tumor-controlling features of the immune system can be considered a hallmark of cancer, for many years the scientific evidence was conflicting and consensus did not emerge. More recently, however, advances in approaches that perturb specific gene functions in well-defined mouse models of cancer have convincingly demonstrated the importance of the interface between cancer and the immune system [2]. Together with a large body of evidence from human cancers, these advances have generated renewed interest in understanding the role of the host inflammatory response in cancer and in using that knowledge towards the development of new approaches to cancer immunotherapy and vaccination [3-10]. The recently reported results of two groups [11,12] give new insights into the factors that affect survival of patients with lymphomas, including the importance of the immune system.
Initially, the study of cancer immunobiology was framed within the context of 'immunosurveillance' [13], with focus on the role of the immune system in recognizing and inhibiting cancer growth. More recently, it has been recognized that the interrelationship between cancer and the immune system is highly complex and can take very different paths - for instance, from suppression of tumor growth by the immune system or enhancement of tumor progression through the selection of cells so that they lack signals recognized by the immune system. Given this complex biology, it has been suggested that the term immunosurveillance [13] be replaced with the more comprehensive term 'immunoediting', encompassing three phases: elimination, equilibrium, and escape [1] (Figure 1). In the elimination phase, which is perhaps the most similar to the original concept of immunosurveillance, the immune system attempts to eradicate the cancer. If this process is unsuccessful, the cancer and the immune system achieve a balance, referred to here as equilibrium, in which the immune system is able to contain but not eliminate the cancer. During the equilibrium phase the cancer is under constant pressure from the immune system but can also undergo genetic changes that can lead to increased immune resistance. If, following many rounds of selection and genetic change, the cancer cells become resistant to immune attack, the escape phase commences, in which the cancer cells are now free to progress, even in the presence of an intact immune system.
Figure 1.
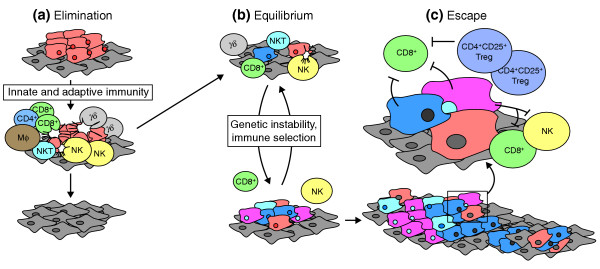
The three Es of cancer immunoediting: elimination, equilibrium, and escape. (a) After transformation of cells in a normal layer (diamond-shaped cells) into cancerous cells (with irregular shapes), attack by various different cell types of the immune system (indicated by round cells) may lead to elimination of the cancerous cells. (b) If elimination is unsuccessful, the immune system and the cancer can reach an equilibrium in which immune cells keep the cancer in check but cannot remove it completely. During the elimination phase, there is selection on the cancer cells, whose genomes are also unstable. This can lead to escape (c), in which mutated cancer cells become able to inhibit the immune system. The cancer can then grow unchecked. Figure modified from [2]. CD4+, CD8+, CD4+CD25+ Treg, γδ and NKT cells are all types of T cell; Mφ cells are macrophages and NK cells are natural killer cells.
Microarrays and cancer immunology
Out of this background have emerged new technological advances, including microarrays, which provide the opportunity comprehensively to assess gene expression in tumors, their component cells, and their microenvironment. Among the important advances that have derived from these technologies is the molecular classification of tumors on the basis of only their gene-expression patterns, resulting in the identification of 'diseases within diseases', different subtypes of known cancers that differ in both gene expression patterns and prognosis (survival time or likelihood of relapse) [14-24]. Until recently, microarray-based correlations between gene expression and clinical outcome have been attributed directly to malignant cells within tumors.
The recently published results of two studies [11,12] not only give a new opportunity to probe the biology of cancer immunology more comprehensively but also emphasize the general importance of tumor-infiltrating immune cells in disease progression. For the diseases that are the subject of these studies, namely follicular lymphoma and diffuse B-cell lymphoma, patient survival is very heterogeneous, and it is important to generate improved diagnostics for assessing predicted disease progression in individual patients. Attainment of this goal will help physicians to decide the best course of treatment and will also increase our understanding of how the underlying biology correlates with survival, thereby suggesting potential new avenues for intervention.
In the study of Dave and colleagues [11], biopsy samples from untreated lymphoma patients were examined by gene-expression profiling on microarrays. Informatics methods were used to identify 'signatures' - expression patterns that correlated with disease outcome - which were then validated in independent samples. Among the gene-expression signatures were two, named immune-response 1 (ir1) and immune-response 2 (ir2), that together could be used to make the best predictive model of patient survival. The signatures allowed patient outcome to be segmented into quartiles, with patients in the best prognosis quartile (which had ir1 but not ir2) having median survival times of more than 13 years, and those in the worst prognosis quartile (which had ir2 but not ir1) having an average survival of less than 4 years. Among the ir1 genes (associated with favorable survival) were T-cell markers such as CD7 and CD8B1 as well as the macrophage markers ACTN1 and TNFSF13B. Importantly, the gene-expression pattern predicting good prognosis is not simply a generalized T-cell response, as other T-cell markers such as CD2 and CD4 were not correlated with survival. The presence of CD8+ (cytotoxic) T-cells is an important feature, as these probably have a direct tumor-killing role [1]. In the ir2 set (associated with poor prognosis) were both macrophage markers (distinct from those in ir1) and dendritic-cell markers. Following sorting of malignant from non-malignant cells using the CD19 marker, which malignant cells lack, it was established that the ir1 and ir2 signatures were expressed in the non-malignant cells. Therefore, in this study, patient outcome was most directly associated with the type of immune response, not the expression profile of the cancer cells themselves.
A distinctly different result was seen in the recent study by Monti and colleagues [12] of the most common lymphoma in adults, diffuse large B-cell lymphoma (DLBCL). This lymphoma has previously been the subject of a series of gene-expression profiling studies, including now-classic studies demonstrating the 'disease within disease' concept and correlating gene-expression signatures with cells of origin and patient survival [12,25-27]. The recent study [12] used whole-genome arrays, multiple clustering algorithms and knowledge of previously identified genetic aberrations to identify three distinct groups of DLBCL, including an 'oxidative phosphorylation' group (which showed elevated expression of oxidative phosphorylation, mitochondrial, and electron transport chain genes) and a group called 'BCR/proliferation' (which showed elevated expression of genes encoding cell-cycle regulators, DNA-repair genes, the B-cell receptor signaling cascade, and B-cell associated transcription factors). The third group was termed 'host response' (HR) and had expression of a suite of immune components such as T/NK-cell receptor and activation pathways, the complement cascade, macrophage and dendritic-cell markers and inflammatory mediators. Among the immune components in the HR group were markers of CD2+/CD3+ tumor-infiltrating lymphocytes. Clearly, the HR signature is very consistent with an active inflammatory response. But, patient survival was not improved in the HR group: this may reflect a different immunoediting result compared with the results observed for follicular lymphoma, perhaps for example a less effective elimination phase. Alternatively, this may reflect an overall balance in a series of complex factors that could reflect the immunoediting process. For example, tumors in the HR group had less pronounced genetic abnormalities and also occurred more frequently in younger patients. Thus, it is possible to discern the features of the host, the cancer, and the immune response that impact on the different biological features of these cancers and ultimately on patient outcome.
The importance of tumor microenvironment
The combined use of new technologies, such as monoclonal antibodies that perturb specific functions, together with improved mouse model systems that have specifically defined genetic modifications, have provided new insights into the cancer surveillance process, thereby leading to a more refined concept of immunoediting. The studies of Dave et al. [11] and Monti et al. [12] now give impetus to this reinvigorated approach. These studies highlight the importance of studying the genetics and phenotypes not only of cancer cells but also of the surrounding microenvironment. The immune system has long been known to be an important part of this microenvironment, although our biological knowledge of specific mechanisms remains incomplete.
The application of microarrays to follicular lymphoma and DLBCL [11,12] presents a remarkable new opportunity to gain a wider perspective of the biology of cancer and its microenvironment. It will be increasingly important to integrate microarray technology with immunohistochemistry, such that not only can the types of T-cells present be discerned but also their localization with respect to cancer cells [12,28,29]. Moreover, through the improved definition of the immune response to tumors, new avenues will open for learning how to harness the immune response more effectively to improve cancer outcomes. Excitingly, it is clear that this opportunity includes many other and perhaps all cancers, as tumor-infiltrating lymphocytes are associated with a diversity of tumors [2,10,30,31]. Thus, through the application of technologies such as microarrays, together with very careful annotation of tumors and patient information, it is hoped that new strategies will emerge for monitoring and controlling the development of new tumors and for more effective targeting of the tumors that have already formed.
Acknowledgments
Acknowledgements
I thank Lloyd Old, Andrew Simpson, Robert Schreiber, and Vanessa King for helpful comments.
References
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Atanackovic D, Altorki NK, Stockert E, Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA, Matsuo M, Selvakumar A, et al. Vaccine-induced CD4+ T cell responses to MAGE-3 protein in lung cancer patients. J Immunol. 2004;172:3289–3296. doi: 10.4049/jimmunol.172.5.3289. [DOI] [PubMed] [Google Scholar]
- Emens LA, Jaffee EM. Cancer vaccines: an old idea comes of age. Cancer Biol Ther. 2003;2(Suppl 1):S161–168. [PubMed] [Google Scholar]
- Jakobisiak M, Lasek W, Golab J. Natural mechanisms protecting against cancer. Immunol Lett. 2003;90:103–122. doi: 10.1016/j.imlet.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TJ, Scott AM, Gullick WJ, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci USA. 2003;100:639–644. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odunsi K, Jungbluth AA, Stockert E, Qian F, Gnjatic S, Tammela J, Intengan M, Beck A, Keitz B, Santiago D, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–6083. [PubMed] [Google Scholar]
- Radvanyi L. Discovery and immunologic validation of new antigens for therapeutic cancer vaccines. Int Arch Allergy Immunol. 2004;133:179–197. doi: 10.1159/000076625. [DOI] [PubMed] [Google Scholar]
- Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065X.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- Zbar AP. The immunology of colorectal cancer. Surg Oncol. 2004;13:45–53. doi: 10.1016/j.suronc.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Dave SS, Wright G, Tan B, Rosenwald A, Gascoyne RD, Chan WC, Fisher RI, Braziel RM, Rimsza LM, Grogan TM, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. New Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, Wu B, Pasqualucci L, Neuberg D, Aguiar RC, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–1861. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- Burnet F. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- Alizadeh A, Eisen M, Davis R, Ma C, Lossos I, Rosenwald A, Boldrick J, Sabet H, Tran T, Yu X, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- Davis RE, Staudt LM. Molecular diagnosis of lymphoid malignancies by gene expression profiling. Curr Opin Hematol. 2002;9:333–338. doi: 10.1097/00062752-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Gascoyne RD, Dave S, Zettl A, Bea S, Chan WC, Rosenwald A, Jaffe ES, Campo E, Delabie J, Weisenburger D, et al. Gene expression microarray analysis of de novo CD5(+) diffuse large B-cell lymphoma (LLMPP study): a distinct entity? Blood. 2003;102:178A–179A. doi: 10.1182/blood-2003-03-0786. [DOI] [Google Scholar]
- Rosenwald A, Staudt LM. Gene expression profiling of diffuse large B-cell lymphoma. Leuk Lymphoma. 2003;44:S41–S47. doi: 10.1080/10428190310001623775. [DOI] [PubMed] [Google Scholar]
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland EB, Giltnane JM, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:1937–1947. doi: 10.1056/NEJMoa012914. [DOI] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Perou CM. DNA microarrays in breast cancer: the promise of personalised medicine. Lancet. 2003;361:1576–1577. doi: 10.1016/S0140-6736(03)13322-3. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Nat Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci USA. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub TR. Genome-wide views of cancer. N Engl J Med. 2001;344:601–602. doi: 10.1056/NEJM200102223440809. [DOI] [PubMed] [Google Scholar]
- Nutt CL, Mani DR, Betensky RA, Tamayo P, Cairncross JG, Ladd C, Pohl U, Hartmann C, McLaughlin ME, Batchelor TT, et al. Gene expression-based classification of malignant gliomas correlates better with survival than histological classification. Cancer Res. 2003;63:1602–1607. [PubMed] [Google Scholar]
- Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, Dave S, Xiao L, Cao K, Zhu Q, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004;165:159–166. doi: 10.1016/s0002-9440(10)63284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt LM. Molecular diagnosis of cancer by gene expression profiling. Eur J Cancer. 2002;38:S3–S4. [Google Scholar]
- Zettl A, Bea S, Rosenwald A, Jehn P, Salaverria I, Ott G, Staudt LM, Chan WC, Jaffe ES, Weisenburger DD, et al. Different subtypes of diffuse large B-cell lymphoma defined by gene expression profiling are genetically distinct. Blood. 2003;102:178A–178A. [Google Scholar]
- Chiba T, Ohtani H, Mizoi T, Naito Y, Sato E, Nagura H, Ohuchi A, Ohuchi K, Shiiba K, Kurokawa Y, et al. Intraepithelial CD8(+) T-cell-count becomes a prognostic factor after a longer follow-up period in human colorectal carcinoma: possible association with suppression of micrometastasis. Brit J Cancer. 2004;91:1711–1717. doi: 10.1038/sj.bjc.6602201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- Riccobon A, Gunelli R, Ridolfi R, De Paola F, Flamini E, Fiori M, Saltutti C, Petrini M, Fiammenghi L, Stefanelli M, et al. Immunosuppression in renal cancer: differential expression of signal transduction molecules in tumor-infiltrating, near-tumor tissue, and peripheral blood lymphocytes. Cancer Invest. 2004;22:871–877. doi: 10.1081/CNV-200039653. [DOI] [PubMed] [Google Scholar]
- Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]


