Short abstract
Opsins, G-protein-coupled receptors including rhodopsin, are found in animals, and more than a thousand have been identified so far. Most opsins act as pigments that activate G proteins in a light-dependent manner in both visual and non-visual systems.
Abstract
The photosensitive molecule rhodopsin and its relatives consist of a protein moiety - an opsin - and a non-protein moiety - the chromophore retinal. Opsins, which are G-protein-coupled receptors (GPCRs), are found in animals, and more than a thousand have been identified so far. Detailed molecular phylogenetic analyses show that the opsin family is divided into seven subfamilies, which correspond well to functional classifications within the family: the vertebrate visual (transducin-coupled) and non-visual opsin subfamily, the encephalopsin/tmt-opsin subfamily, the Gq-coupled opsin/melanopsin subfamily, the Go-coupled opsin subfamily, the neuropsin subfamily, the peropsin subfamily and the retinal photoisomerase subfamily. The subfamilies diversified before the deuterostomes (including vertebrates) split from the protostomes (most invertebrates), suggesting that a common animal ancestor had multiple opsin genes. Opsins have a seven-transmembrane structure similar to that of other GPCRs, but are distinguished by a lysine residue that is a retinal-binding site in the seventh helix. Accumulated evidence suggests that most opsins act as pigments that activate G proteins in a light-dependent manner in both visual and non-visual systems, whereas a few serve as retinal photoisomerases, generating the chromophore used by other opsins, and some opsins have unknown functions.
Opsins are membrane proteins with molecular masses of 30-50 kDa that are related to the protein moiety of the photoreceptive molecule rhodopsin; they typically act as light sensors in animals [1-4]. Photoreceptive proteins similar to the animal opsins in three-dimensional structure but not in amino-acid sequence have been found in archaea, bacteria, fungi, and a green alga, Chlamydomonas reinhardtii [5,6]. These non-animal opsins function as light-driven ion pumps or light sensors but there is no evidence that they are structurally related to animal opsins, so they are not considered further here.
Gene organization and evolutionary history
Since the first sequence of an opsin, bovine rhodopsin, was determined by conventional protein sequencing in 1982 [7,8] and cDNA sequencing in 1983 [9], more than 1,000 opsins have been identified. The molecular phylogenetic tree shows three large clusters, and detailed analyses have revealed that the opsin family is divided into seven subfamilies; there is less than about 25% amino-acid similarity between subfamilies but more than about 40% among members of a single family (Figure 1). The division into subfamilies corresponds well to functional classification of opsins, which is based partly on the type of G protein coupled to each of these G-protein-coupled receptors (GPCRs). The seven subfamilies are as follows: the vertebrate visual (transducin-coupled) and non-visual opsin subfamily; the encephalopsin/tmt-opsin subfamily; the Gq-coupled opsin/melanopsin subfamily; the Go-coupled opsin subfamily; the peropsin subfamily; the retinal photoisomerase subfamily; and the neuropsin subfamily. Members of the Gq-coupled opsin/melanopsin, Go-coupled opsin, encephalopsin/tmt-opsin and retinal photoisomerase subfamilies are found in both deuterostomes (such as cephalochordates and vertebrates) and protostomes (such as molluscs and insects; Figure 1), suggesting that diversification of the subfamilies occurred much earlier in animal evolution than the deuterostome-protostome split [10].
Figure 1.
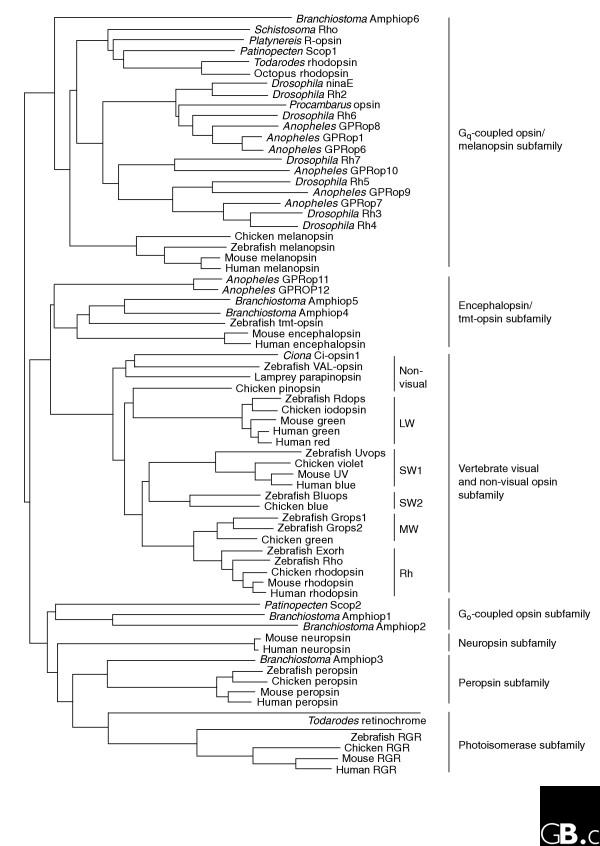
A molecular phylogenetic tree of the opsin family. The tree was inferred by the neighbor-joining method [81]. It shows that members of opsin family are divided into seven subfamilies, whose names are given on the right of the tree. Common names of species shown: Anopheles, mosquito; Branchiostoma, amphioxus; Ciona, ascidian; Drosophila, fruit fly; Patinopecten, scallop; Platynereis, polychaete annelid worm; Procambarus, crayfish; Schistosoma, blood fluke; Todarodes, squid. Abbreviations: LW, long-wavelength-sensitive opsin; SW, short-wavelength-sensitive opsin; MW, middle-wavelength-sensitive opsin; Rh, rhodopsin; RGR, retinal G-protein-coupled receptor. Other abbreviations are protein names; where only a color is given for a protein name, it refers to a cone opsin that detects that color.
The visual and non-visual opsin subfamily contains vertebrate visual and non-visual opsins. The visual opsins can be further subdivided into cone opsins and rhodopsin, which have distinct molecular properties arising from differences in the residues at positions 122 and 189 of the amino-acid sequence [11,12]. The cone opsins can be further divided into four subgroups, which correspond well with their absorption spectra: long-wavelength opsins (LW or red), short-wavelength opsins (SW1 or UV/violet and SW2 or blue), and middle-wavelength opsins (MW or green; see Figure 1) [1,3,13]. Note that other nomenclatures are also used to specify these four groups. Most vertebrates, including the lamprey [14], have four kinds of cone-opsin genes, whereas mammals lack the SW2 and MW genes. Interestingly, humans have regained the green-sensitive opsin by duplication of the LW gene, so the green cone opsins of humans and lower vertebrates belong to different opsin subgroups (LW and MW) [15,16]. In the human genome, the red and green opsin genes are localized in tandem.
Lower vertebrates, including lampreys, have several non-visual opsin genes that are members of the same subfamily as the vertebrate visual opsins. The first non-visual opsin to be discovered was pinopsin [17], which is involved in photoreception in the pineal organs of birds [17,18] and lizards [19]. Parapinopsin was first found in the pineal complex of the catfish [20], and it has also been found in zebrafish and Xenopus and more recently in the lamprey pineal [21]. 'Vertebrate ancient' opsin (VA-opsin) was first found in the salmon retina [22]; the lamprey also has an ortholog of VA-opsin, called P-opsin [23]. The ascidian chordate Ciona has an opsin (Ci-opsin1) that is closely related to the vertebrate non-visual opsins [24].
Within the other six subfamilies of opsins, members of the encephalopsin/tmt-opsin subfamily were first found in mouse and human [25], and homologs were recently identified in the teleosts [26] and interestingly in invertebrates, the mosquito Anopheles [27] and the marine ragworm Platynereis [28]. Phylogenetic analysis shows that encephalopsin/tmt-opsin subfamily probably clusters most closely with the vertebrate visual and non-visual opsin subfamily (see Figure 1). Melanopsin is an important vertebrate non-visual opsin, but because it is more similar in amino-acid sequence to invertebrate Gq-coupled visual opsins, it is not classified as a member of the vertebrate visual and non-visual opsin subfamily (see Figure 1); melanopsins have been found in many vertebrates, from fish to humans [29,30]. Members of the Go-coupled opsin subfamily have been found in molluscs and in the chordate amphioxus [10,31] but not in human, mouse, zebrafish or Drosophila. Neuropsins, recently identified in mouse and human [32], are phylogenetically distinguishable as a subfamily but little is known about them. Peropsins are known from a range of vertebrates, from fish to human [33], and an ortholog was recently found in amphioxus [31]. Finally, members of the retinal-photoisomerase subfamily, which includes retinal G-protein-coupled receptor (RGR) and retinochrome, are found in vertebrates and molluscs [34,35]; an RGR homolog has also been found in an ascidian [36].
The gene organization of different vertebrate opsins provides further information about relationships among the subfamilies [32,37,38]. The numbers of introns in the human opsin genes are shown in Table 1 as an example. Three of the four or five introns in the vertebrate visual and non-visual opsin genes are shared at conserved positions with encephalopsin/tmt-opsin genes, consistent with the close relationship between these subfamilies found by phylogenetic analysis. The peropsin, retinal photoisomerase (RGR) and neuropsin subfamily genes have six introns, which are at positions different from those of vertebrate visual and non-visual opsin genes. Two and three of the peropsin introns are conserved in the RGR of the retinal photoisomerase subfamily and the neuropsin gene, respectively, again confirming a close evolutionary relationship between these subfamilies. The melanopsin gene has nine introns at positions different from those of other opsin genes.
Table 1.
Chromosomal locations and numbers of introns of the nine human opsin genes
| Opsin | Chromosomal location | Number of introns |
| Rhodopsin | 3q22.1 | 4 |
| Blue opsin | 7q32.1 | 4 |
| Red opsin | Xq28 | 5 |
| Green opsin | Xq28 | 5 |
| Encephalopsin | 1q43 | 3 |
| Melanopsin | 10q23.2 | 9 |
| Peropsin | 4q25 | 6 |
| RGR | 10q23.1 | 6 |
| Neuropsin | 6p12.3 | 6 |
Recent genome studies have also provided us with information on the loss of opsin genes during animal evolution. No opsin gene has been found in Caenorhabditis elegans [39,40]. Drosophila has seven opsin genes, all of which belong to the Gq-coupled opsin/melanopsin subfamily [41]. In comparison, humans have nine opsin genes (Table 1), which are spread over six of the seven subfamilies (Figure 1). A PCR study [31] revealed that amphioxus has at least six opsin genes from four subfamilies (Figure 1); deuterostomes therefore appear to have opsins from more subfamilies than do protostomes.
Characteristic structural features
Opsins share several amino-acid motifs, including seven transmembrane helices, with other G-protein-coupled receptors (GPCRs) of the rhodopsin superfamily. The first primary sequence of a member of the rhodopsin superfamily, the β-adrenergic receptor, was determined in 1986 [42], and since then, the opsin family has been considered one of the typical members of the superfamily. As shown in Figure 2a, several amino-acid residues are highly conserved among the opsin family members; about half of these are conserved in all GPCRs of the rhodopsin superfamily [43]. All opsins bind a chromophore: the vertebrate visual and non-visual opsins, the invertebrate Gq-coupled opsins, and the Go-coupled opsins all bind 11-cis-retinal, whereas the photoisomerases and the peropsins bind all-trans-retinal (Figure 2b). The chromophores of the other opsins are uncertain.
Figure 2.
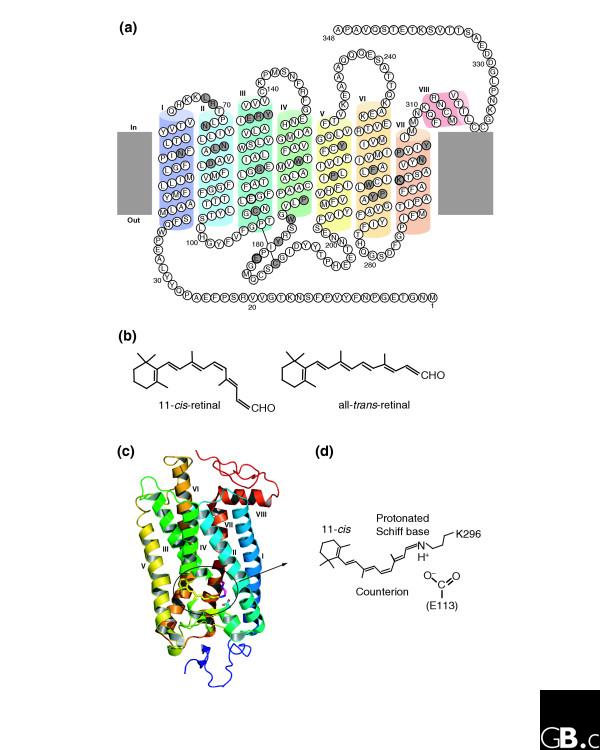
Structures of opsins and of the chromophore retinal. (a) A model of the secondary structure of bovine rhodopsin. Amino-acid residues that are highly conserved in the whole opsin family are shown with a gray background. The retinal-binding site (K296) and the counterion position (E113) are marked with bold circles, as is E181, the counterion in opsins other than the vertebrate visual and non-visual ones. C110 and C187 form a disulfide bond. (b) The chemical structures of the 11-cis and all-trans forms of retinal. (c) The crystal structure of bovine rhodopsin (Protein DataBank ID: 1U19 [PDB:1U19]). The chromophore 11-cis-retinal, K296 and E113 are shown in stick representation in the ringed area. (d) The structure of the Schiff base linkage formed by retinal within the bovine opsin, together with the counterion that stabilizes it.
The crystal structure of bovine rhodopsin has been solved [44-46] (Figure 2c). K296 (in the single-letter amino-acid code) in helix VII binds retinal via a Schiff-base linkage, in which the nitrogen atom of the K296 amino group forms a double bond with the carbon atom at one end of the retinal (Figure 2d). The key residue K296 is important for light absorption and its presence or absence can be used to judge whether or not a newly found rhodopsin-type GPCR is really an opsin. The counterion is another important residue: it is a negatively charged amino acid that helps to stabilize the protonated Schiff base (see below). In the vertebrate visual and non-visual opsin subfamily, the highly conserved residue E113 serves as the counterion [47-49], whereas in other opsins position 113 is occupied by other amino acids (tyrosine, phenylalanine, methionine, or histidine) and the highly conserved E181 serves as the counterion. This difference suggests that counterion replacement has occurred during the molecular evolution of vertebrate visual and non-visual opsins [50,51].
Localization and function
Functions of the vertebrate visual and non-visual opsins
Two photoreceptor cells are involved in vision in most vertebrates - rod and cone cells - and they are distinguishable by their shapes. The rod and cone cells contain different opsins: rods have rhodopsin, which underlies twilight vision, and cones have cone opsins, which underlie daylight (color) vision [1]. When excited by light in rod and cone cells, rhodopsin and cone pigments drive an enzyme cascade involving G proteins and their effectors: the excited pigments activate the G-protein transducin, which stimulates cGMP phosphodiesterase, resulting in a decrease in intracellular cGMP concentration. This decrease leads to closure of a cGMP-gated cation channel, leading to the hyperpolarization of the visual photoreceptor cell. In general, rods and cones contain distinct sets of phototransduction molecules (transducin, phosphodiesterases and channels) [52]. It should be noted that the visual opsins are also expressed in non-visual photoreceptor cells, including the pineal photoreceptor cells that are found in most non-mammalian vertebrates.
The lower-vertebrate non-visual opsin genes are expressed in photoreceptor cells other than rods and cones. For example, pinopsin is involved in photoreception in the pineal organs of birds [17,18] and lizards [19]. It is suggested to activate both transducin and the G-protein G11 and therefore to drive two different phototransduction cascades [53,54]. The parapinopsin recently found in the pineal organ of the lamprey [21] is a UV-sensitive and bistable opsin with stable dark and light-activated states. VA-opsin is found in the salmon retina [22] but in amacrine and horizontal cells (two kinds of neural cell in the retina), not in rod and cone visual cells [55]. A splice valiant of VA-opsin called VAL-opsin is localized to deep parts of the brain and the horizontal cells of the zebrafish [56].
Functions of other subfamilies
The visual opsins of arthropods and molluscs belong to the Gq-coupled opsin group, which is different from the vertebrate visual opsin group. They are localized to the microvilli of the rhabdomeric photoreceptor cells, which are typical visual cells of arthropods and molluscs and are morphologically different from vertebrate rods and cones. These opsins are coupled to the signal-transduction cascades involving the G protein Gq and phosopholipase C [2,57-60] and leading to depolarization of the cells in response to light. The different subgroups of insect opsins have distinct absorption spectra; this underlies insect color vision. Vertebrate melanopsins are very similar to the Gq-coupled invertebrate opsins [29,30]; mouse melanopsin has been reported from knockout studies to be involved in the response of the pupil to light [61] and in the entrainment of circadian rhythm by light [62]. As suggested by their close relationship to the Gq-coupled opsins, melanopsin can be coupled to a Gq/phosopholipase-C cascade, similar to that used by the invertebrate opsins [63-65].
Mouse encephalopsin (also called panopsin) is strongly expressed in the brain and testes and weakly in other tissues [25], and the teleost homologs are localized to multiple tissues (they are therefore named teleost multiple tissue (tmt) opsins) [26]. The functions of the encephalopsins and tmt-opsins are unknown, but their close but distinct position in the phylogenetic tree relative to the vertebrate visual and non-visual opsins may mean that they are more llikely to have distinct functions.
Some invertebrates have photoreceptor cells - distinct from the rhabdomeric photoreceptors - that are called ciliary photoreceptors because their photoreceptive portions originate from cilia. Interestingly, the scallop (a bivalve mollusc) has both kinds, and in the ciliary photoreceptor cells a novel opsin has been found that is different from the Gq-coupled one [10]. It colocalizes with a large amount of Go-type G protein and is thought to activate Go in vivo; it is therefore named Go-coupled rhodopsin (or Go-coupled opsin). Electrophysiological evidence suggests that scallop Go-coupled rhodopsin elevates the intracellular cGMP concentration through light-dependent activation of Go, which leads to hyperpolarization of the cell [66].
Neuropsins are localized to the eye, brain, testes and spinal cord, but their functions are unknown. Peropsin was first found in the RPE of the mammalian eye [33]. It binds all-trans-retinal as a chromophore, and light isomerizes it to the 11-cis form [31] (Figure 2b). This photochemical property indicates that peropsin may serve as a retinal photoisomerase, like retinochromes and RGRs [34,35]. Retinochrome and RGR, the members of the retinal-photoisomerase subfamily, bind all-trans retinal (Figure 2b) as a chromophore [67,68] and are not coupled to G proteins, unlike the visual opsins, which bind the 11-cis form of retinal. Retinochrome and RGR have been identified in the mollusc and vertebrate retinas, specifically in the inner segments of the visual cells [69,70] and in the retinal pigment epithelium (RPE) [34], respectively. Irradiation of these two pigments causes the isomerization of all-trans retinal to the 11-cis form [67,68], suggesting that these opsins enzymatically generate the chromophore and supply it to the visual opsins [70,71].
Mechanism
The function of most opsins except for the photoisomerases can be divided into two parts: light absorption and G-protein activation. Most opsins function through absorption of visible light, but the chromophore retinal itself has an absorption maximum in the UV region, not in the visible region. This potential problem is solved by the opsins as follows. As previously described, retinal binds to K296 in helix VII through the protonated Schiff base (Figure 2d); the protonation, which results in the delocalization of π electrons within the retinal molecule, shifts its absorption spectrum towards visible light. In the protein, the proton on the Schiff base is unstable and a counterion, a negatively charged amino-acid residue, therefore needs to be present in order to stabilize it.
Absorption of light (a photon) by retinal results in its photoisomerization from the 11-cis to the all-trans form (Figure 2b). This is followed by a conformational change of the protein moiety, eventually resulting in activation of the G protein. Photochemical studies have identified some spectroscopically distinguished intermediates that form during bleaching of the vertebrate rhodopsin - 'batho', 'lumi', 'meta I', and 'meta II' - which appear on the picosecond, nanosecond, microsecond and millisecond timescales after light absorption, respectively [1]. Many biochemical and biophysical studies have focused on the question of what conformational changes take place in the protein moiety during the formation of the active state of opsins, especially the meta II intermediate of bovine rhodopsin. The most notable hypothesis is that light triggers the relative outward movement of helices III and VI [72,73] to form meta II, most likely following flipping-over of the retinal ring [74]. This movement of the helices could expose G-protein-binding sites, such as the cytoplasmic loop between helices V and VI [75,76]. This loop varies in sequence among the different subfamilies and underlies their selective coupling to different subtypes of G protein [77,78]. It is believed that a similar helix motion occurs in most members of the rhodopsin superfamily.
Frontiers
There are many unanswered questions concerning the functions of the different opsins. Recently, genetic approaches using knockout and/or transgenic animals have been used to understand the function and/or the expression mechanism of some opsins. Recent progress with RGR knockout mice concluded that RGR serves as the photoisomerase of retinal in vivo [71], and the function of melanopsin in the circadian clock has been studied with mutant mice lacking photoreception through rods and cones [62]. One interesting approach for investigating why there are so many kinds of opsins is functional replacement of one opsin with another and observing the altered phenotype. The first example of this approach was the experimental replacement of rhodopsin with cone opsin in transgenic Xenopus, by selective stimulation of the cone opsin in a single rod cell that contained both cone opsins and rhodopsins [79]. The 'knock-in' technique could be most useful for this kind of opsin-replacement experiment (H. Imai and Y. Shichida, unpublished observations).
GPCRs are important targets for drug discovery, and the opsin family is currently a good subject for such studies because it is the only family for which the structure of a member has been solved at high resolution. Structural studies of opsins could provide valuable information for understanding how GPCRs in general activate G proteins. Okada et al. [45] have investigated the photochemistry of the rhodopsin crystal, which raises the possibility of solving the structure of an active form of rhodopsin (meta II) at high resolution (2.5 Å). The crystal structure of the meta I photointermediate of rhodopsin has recently been solved to around 5.5 Å resolution [80]. The crystallization of a complex of active rhodopsin (meta II) with a G protein could be one of the breakthroughs that help to elucidate the G-protein-activating mechanism.
Acknowledgments
Acknowledgements
The author thanks Yoshinori Shichida of Kyoto University for valuable comments on this manuscript, Mitsumasa Koyanagi of Osaka University for useful discussions and help in preparation of Figure 1, and Hisao Tsukamoto for help in preparing the manuscript. This work was supported by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture and the Grant for the Biodiversity Research of the 21st Century COE (A14).
References
- Shichida Y, Imai H. Visual pigment: G-protein-coupled receptor for light signals. Cell Mol Life Sci. 1998;54:1299–1315. doi: 10.1007/s000180050256. A review of the properties and functions of the various visual opsins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner W. Invertebrate visual pigments. In: Stavenga DG, DeGrip WJ, Pugh Jr EN, editor. Handbook of Biological Physics. Vol. 3. Amsterdam: Elsevier Science; 2000. pp. 297–388. A detailed review of invertebrate visual and non-visual opsins. [Google Scholar]
- Takahashi Y, Ebrey TG. Photobiology of retinal proteins. In: Coohill T, Valenzeno D, editor. Photobiology for the 21th Century. Overland Park, Kansas: Valdenmar; 2001. pp. 101–133. A detailed review of vertebrate visual opsins with amino-acid sequence comparisons. [Google Scholar]
- Sakmar TP, Menon ST, Marin EP, Awad ES. Rhodopsin: insights from recent structural studies. Annu Rev Biophys Biomol Struct. 2002;31:443–484. doi: 10.1146/annurev.biophys.31.082901.134348. A recent structure-based review of vertebrate rhodopsins. [DOI] [PubMed] [Google Scholar]
- Spudich JL, Yang CS, Jung KH, Spudich EN. Retinylidene proteins: structures and functions from archaea to humans. Annu Rev Cell Dev Biol. 2000;16:365–392. doi: 10.1146/annurev.cellbio.16.1.365. A review of various non-animal opsins, comparing them with animal opsins. [DOI] [PubMed] [Google Scholar]
- Sineshchekov OA, Jung KH, Spudich JL. Two rhodopsins mediate phototaxis to low- and high-intensity light in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2002;99:8689–8694. doi: 10.1073/pnas.122243399. Identification of two opsins not related to animal opsins in a green alga. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA. Rhodopsin and bacteriorhodopsin: structure-function relationships. FEBS Lett. 1982;148:179–191. doi: 10.1016/0014-5793(82)80805-3. This article and [8,9] are the first reports of the amino-acid sequence of bovine rhodopsin. [DOI] [PubMed] [Google Scholar]
- Hargrave PA, McDowell JH, Curtis DR, Wang JK, Juszczak E, Fong SL, Rao JK, Argos P. The structure of bovine rhodopsin. Biophys Struct Mech. 1983;9:235–244. doi: 10.1007/BF00535659. See [7]. [DOI] [PubMed] [Google Scholar]
- Nathans J, Hogness DS. Isolation, sequence analysis, and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell. 1983;34:807–814. doi: 10.1016/0092-8674(83)90537-8. See [7]. [DOI] [PubMed] [Google Scholar]
- Kojima D, Terakita A, Ishikawa T, Tsukahara Y, Maeda A, Shichida Y. A novel Go-mediated phototransduction cascade in scallop visual cells. J Biol Chem. 1997;272:22979–22982. doi: 10.1074/jbc.272.37.22979. The discovery of a novel rhodopsin that activates Go-type G proteins. [DOI] [PubMed] [Google Scholar]
- Imai H, Kojima D, Oura T, Tachibanaki S, Terakita A, Shichida Y. Single amino acid residue as a functional determinant of rod and cone visual pigments. Proc Natl Acad Sci USA. 1997;94:2322–2326. doi: 10.1073/pnas.94.6.2322. This paper and [12] report the discovery of the amino-acid residues responsible for molecular properties of rod and cone visual pigments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwayama S, Imai H, Hirano T, Terakita A, Shichida Y. Conserved proline residue at position 189 in cone visual pigments as a determinant of molecular properties different from rhodopsins. Biochemistry. 2002;41:15245–15252. doi: 10.1021/bi026444k. See [11]. [DOI] [PubMed] [Google Scholar]
- Yokoyama S. Molecular evolution of vertebrate visual pigments. Prog Retin Eye Res. 2000;19:385–419. doi: 10.1016/S1350-9462(00)00002-1. A review of the molecular evolution of vertebrate rod and cone opsins. [DOI] [PubMed] [Google Scholar]
- Collin SP, Knight MA, Davies WL, Potter IC, Hunt DM, Trezise AE. Ancient colour vision: multiple opsin genes in the ancestral vertebrates. Curr Biol. 2003;13:R864–R865. doi: 10.1016/j.cub.2003.10.044. An ancestral vertebrate, the lamprey, contains four kinds of cone opsins. [DOI] [PubMed] [Google Scholar]
- Nathans J, Thomas D, Hogness DS. Molecular genetics of human color vision: the genes encoding blue, green, and red pigments. Science. 1986;232:193–202. doi: 10.1126/science.2937147. A comprehensive study of the genes and amino-acid sequences of three human cone opsins. [DOI] [PubMed] [Google Scholar]
- Okano T, Kojima D, Fukada Y, Shichida Y, Yoshizawa T. Primary structures of chicken cone visual pigments: vertebrate rhodopsins have evolved out of cone visual pigments. Proc Natl Acad Sci USA. 1992;89:5932–5936. doi: 10.1073/pnas.89.13.5932. The first report of the amino-acid sequences of four cone pigments from non-mammalian vertebrate, the chicken. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okano T, Yoshizawa T, Fukada Y. Pinopsin is a chicken pineal photoreceptive molecule. Nature. 1994;372:94–97. doi: 10.1038/372094a0. This paper and [18] report first discovery of a non-visual and extraocular opsin, pinopsin, which serves as a photoreceptive molecule in the chicken pineal. [DOI] [PubMed] [Google Scholar]
- Max M, McKinnon PJ, Seidenman KJ, Barrett RK, Applebury ML, Takahashi JS, Margolskee RF. Pineal opsin: a nonvisual opsin expressed in chick pineal. Science. 1995;267:1502–1506. doi: 10.1126/science.7878470. See [17]. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Hisatomi O, Yoshida M, Tokunaga F. Pinopsin expressed in the retinal photoreceptors of a diurnal gecko. FEBS Lett. 2001;496:69–74. doi: 10.1016/S0014-5793(01)02395-X. Identification of pinopsin and its localization in the gecko (a lizard). [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Snyder SH. Parapinopsin, a novel catfish opsin localized to the parapineal organ, defines a new gene family. J Neurosci. 1997;17:8083–8092. doi: 10.1523/JNEUROSCI.17-21-08083.1997. Identification of a novel opsin, parapinopsin, which defines a new opsin subfamily. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Kawano E, Kinugawa Y, Oishi T, Shichida Y, Tamotsu S, Terakita A. Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci USA. 2004;101:6687–6691. doi: 10.1073/pnas.0400819101. This paper reports that the lamprey parapinopsin is a UV pigment and its photoproduct is stable, with a bistable nature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni BG, Foster RG. A novel and ancient vertebrate opsin. FEBS Lett. 1997;406:279–283. doi: 10.1016/S0014-5793(97)00287-1. Identification of a novel opsin, VA opsin, in the salmon, demonstrating that the diversification of vertebrate opsins occurred early in vertebrate evolution. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Zhang H. Cloning and characterization of the pineal gland-specific opsin gene of marine lamprey (Petromyzon marinus). Gene. 1997;202:89–93. doi: 10.1016/S0378-1119(97)00458-7. Identification of a novel opsin, P-opsin (VA-opsin), in the lamprey. [DOI] [PubMed] [Google Scholar]
- Kusakabe T, Kusakabe R, Kawakami I, Satou Y, Satoh N, Tsuda M. Ci-opsin1, a vertebrate-type opsin gene, expressed in the larval ocellus of the ascidian Ciona intestinalis. FEBS Lett. 2001;506:69–72. doi: 10.1016/S0014-5793(01)02877-0. Identification of an opsin in the larval ocellus of an ascidian. [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Snyder SH. Encephalopsin: a novel mammalian extraretinal opsin discretely localized in the brain. J Neurosci. 1999;19:3681–3690. doi: 10.1523/JNEUROSCI.19-10-03681.1999. The discovery of a novel opsin, encephalopsin, which is expressed in the preoptic area and paraventricular nucleus of the hypothalamus among other regions of the brain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsaki P, Whitmore D, Bellingham J, Sakamoto K, David-Gray ZK, Foster RG. Teleost multiple tissue (tmt) opsin: a candidate photopigment regulating the peripheral clocks of zebrafish? Brain Res Mol Brain Res. 2003;112:135–145. doi: 10.1016/S0169-328X(03)00059-7. Identification of encephalopsin homologs (tmt-opsins) in the zebrafish. [DOI] [PubMed] [Google Scholar]
- Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. A description of the GPCRs in the genome of the mosquito Anopheles. [DOI] [PubMed] [Google Scholar]
- Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. The discovery of an encephalopsin homolog in the ciliary photoreceptor of the marine ragworm. [DOI] [PubMed] [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. This paper and [30] report the identification and localization of melanopsin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. See [29]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Terakita A, Kubokawa K, Shichida Y. Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis - and all-trans-retinals as their chromophores. FEBS Lett. 2002;531:525–528. doi: 10.1016/S0014-5793(02)03616-5. Heterologous expression of the amphioxus Go-rhodopsin and peropsin reveals their spectroscopic and biochemical properties. [DOI] [PubMed] [Google Scholar]
- Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–416. doi: 10.1016/S0014-5793(03)01212-2. Identification of a novel opsin, neuropsin, in neural tissue of mice and humans. [DOI] [PubMed] [Google Scholar]
- Sun H, Gilbert DJ, Copeland NG, Jenkins NA, Nathans J. Peropsin, a novel visual pigment-like protein located in the apical microvilli of the retinal pigment epithelium. Proc Natl Acad Sci USA. 1997;94:9893–9898. doi: 10.1073/pnas.94.18.9893. Identification of a novel opsin, peropsin, in the mammalian retinal pigment epithelium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Hara R. Rhodopsin and retinochrome in the squid retina. Nature. 1967;214:573–575. doi: 10.1038/214573a0. An important discovery of a retinochrome that is distinct from visual rhodopsin. [DOI] [PubMed] [Google Scholar]
- Jiang M, Pandey S, Fong HK. An opsin homologue in the retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1993;34:3669–3678. Identification and localization of a new opsin, an RGR, in the mammalian retina and retinal pigment epithelium. [PubMed] [Google Scholar]
- Nakashima Y, Kusakabe T, Kusakabe R, Terakita A, Shichida Y, Tsuda M. Origin of the vertebrate visual cycle: genes encoding retinal photoisomerase and two putative visual cycle proteins are expressed in whole brain of a primitive chordate. J Comp Neurol. 2003;460:180–190. doi: 10.1002/cne.10645. Identification and characterization of an RGR ortholog in the ascidian. [DOI] [PubMed] [Google Scholar]
- Bellingham J, Foster RG. Opsins and mammalian photoentrainment. Cell Tissue Res. 2002;309:57–71. doi: 10.1007/s00441-002-0573-4. A review of several opsins on the basis of their gene organization. [DOI] [PubMed] [Google Scholar]
- Bellingham J, Wells DJ, Foster RG. In silico characterization and chromosomal localization of human RRH (peropsin) - implications for opsin evolution. BMC Genomics. 2003;4:3. doi: 10.1186/1471-2164-4-3. A report of the chromosomal localization and gene organization of the human peropsin gene. [DOI] [PMC free article] [PubMed] [Google Scholar]
- C. elegans sequencing at the GSC http://genome.wustl.edu/projects/celegans/ This website and [40] are the home pages of the C. elegans genome projects.
- Caenorhabditis genome sequencing projects http://www.sanger.ac.uk/Projects/C_elegans/ See [39].
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. The report of the genome sequence of Drosophila melanogaster. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–79. doi: 10.1038/321075a0. The first complete amino-acid sequence of a GPCR, suggesting the seven-transmembrane structure. [DOI] [PubMed] [Google Scholar]
- Mirzadegan T, Benko G, Filipek S, Palczewski K. Sequence analyses of G-protein-coupled receptors: similarities to rhodopsin. Biochemistry. 2003;42:2759–2767. doi: 10.1021/bi027224+. Sequence comparison of the members of the rhodopsin superfamily highlights several highly conserved amino-acid residues. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, et al. Crystal structure of rhodopsin: a G protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. The 2.8 Å crystal structure of bovine rhodopsin, the first high-resolution structure of a GPCR. [DOI] [PubMed] [Google Scholar]
- Okada T, Fujiyoshi Y, Silow M, Navarro J, Landau EM, Shichida Y. Functional role of internal water molecules in rhodopsin revealed by X-ray crystallography. Proc Natl Acad Sci USA. 2002;99:5982–5987. doi: 10.1073/pnas.082666399. The 2.5 Å three-dimensional crystal structure of bovine rhodopsin and the photochemistry of the crystal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V. The retinal conformation and its environment in rhodopsin in light of a new 2.2 Å crystal structure. J Mol Biol. 2004;342:571–583. doi: 10.1016/j.jmb.2004.07.044. The recent 2.2 Å crystal structure of bovine rhodopsin. [DOI] [PubMed] [Google Scholar]
- Zhukovsky EA, Oprian DD. Effect of carboxylic acid side chains on the absorption maximum of visual pigments. Science. 1989;246:928–930. doi: 10.1126/science.2573154. This paper and [48,49] are three independent studies that determined the retinylidene Schiff base counterion of bovine rhodopsin. [DOI] [PubMed] [Google Scholar]
- Sakmar TP, Franke RR, Khorana HG. Glutamic acid-113 serves as the retinylidene Schiff base counterion in bovine rhodopsin. Proc Natl Acad Sci USA. 1989;86:8309–8313. doi: 10.1073/pnas.86.21.8309. See [47]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans J. Determinants of visual pigment absorbance: identification of the retinylidene Schiff's base counterion in bovine rhodopsin. Biochemistry. 1990;29:9746–9752. doi: 10.1021/bi00493a034. See [47]. [DOI] [PubMed] [Google Scholar]
- Terakita A, Yamashita T, Shichida Y. Highly conserved glutamic acid in the extracellular IV-V loop in rhodopsins acts as the counterion in retinochrome, a member of the rhodopsin family. Proc Natl Acad Sci USA. 2000;97:14263–14267. doi: 10.1073/pnas.260349597. Identification of the retinylidene Schiff base counterion of retinochrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terakita A, Koyanagi M, Tsukamoto H, Yamashita T, Miyata T, Shichida Y. Counterion displacement in the molecular evolution of the rhodopsin family. Nat Struct Mol Biol. 2004;11:284–289. doi: 10.1038/nsmb731. A comprehensive determination of the retinylidene Schiff base counterions of diverged rhodopsins, showing displacement of the counterion to a different position in the protein during the molecular evolution of vertebrate opsins. [DOI] [PubMed] [Google Scholar]
- Hisatomi O, Tokunaga F. Molecular evolution of proteins involved in vertebrate phototransduction. Comp Biochem Physiol B Biochem Mol Biol. 2002;133:509–522. doi: 10.1016/S1096-4959(02)00127-6. A review comparing the functional molecules involved in rod and cone phototransduction. [DOI] [PubMed] [Google Scholar]
- Nakamura A, Kojima D, Imai H, Terakita A, Okano T, Shichida Y, Fukada Y. Chimeric nature of pinopsin between rod and cone visual pigments. Biochemistry. 1999;38:14738–14745. doi: 10.1021/bi9913496. The biochemical and photochemical properties of pinopsin. [DOI] [PubMed] [Google Scholar]
- Kasahara T, Okano T, Haga T, Fukada Y. Opsin-G11-mediated signaling pathway for photic entrainment of the chicken pineal circadian clock. J Neurosci. 2002;22:7321–7325. doi: 10.1523/JNEUROSCI.22-17-07321.2002. This paper suggests from cell biology experiments that the chicken pineal contains an opsin and G11-mediated signal-transduction cascade that enable the circadian clock to be entrained by light. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni BG, Philp AR, Foster RG, Knox BE. Novel retinal photo-receptors. Nature. 1998;394:27–28. doi: 10.1038/27794. This paper reports that VA opsin is localized to neural cells in the retina. [DOI] [PubMed] [Google Scholar]
- Kojima D, Mano H, Fukada Y. Vertebrate ancient-long opsin: a green-sensitive photoreceptive molecule present in zebrafish deep brain and retinal horizontal cells. J Neurosci. 2000;20:2845–2851. doi: 10.1523/JNEUROSCI.20-08-02845.2000. Identification of a splice valiant of VA opsin in zebrafish and its localization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Shah S, Suzuki E, Zars T, O'Day PM, Hyde DR. The Drosophila dgq gene encodes a G alpha protein that mediates phototransduction. Neuron. 1994;13:1143–1157. doi: 10.1016/0896-6273(94)90052-3. This paper concludes using mutant flies that the G protein Gq mediates the visual transduction cascade in Drosophila. [DOI] [PubMed] [Google Scholar]
- Terakita A, Hariyama T, Tsukahara Y, Katsukura Y, Tashiro H. Interaction of GTP-binding protein Gq with photoactivated rhodopsin in the photoreceptor membranes of crayfish. FEBS Lett. 1993;330:197–200. doi: 10.1016/0014-5793(93)80272-V. The first report on the interaction between an invertebrate opsin and Gq. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Terakita A, Narita K, Nagai K, Tsukahara Y, Kito Y. Squid photoreceptor phospholipase C is stimulated by membrane Gq alpha but not by soluble Gq alpha. FEBS Lett. 1995;377:333–337. doi: 10.1016/0014-5793(95)01364-4. Activation of PLCβ by Gqα using a reconstituted system. [DOI] [PubMed] [Google Scholar]
- Yarfitz S, Hurley JB. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994;269:14329–14332. A review of the phototransduction cascade in both vertebrate and invertebrate visual cells. [PubMed] [Google Scholar]
- Lucas RJ, Hattar S, Takao M, Berson DM, Foster RG, Yau KW. Diminished pupillary light reflex at high irradiances in melanopsin-knockout mice. Science. 2003;299:245–247. doi: 10.1126/science.1077293. This paper reports that melanopsin is a photoreceptor molecule in the pupillary light response. [DOI] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. An excellent study showing the in vivo function of melanopsin in light-entrainment of the circadian rhythm using mutant mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. This paper and [64,65] report that melanopsin can be coupled to the Gq-signaling pathway. [DOI] [PubMed] [Google Scholar]
- Qiu X, Kumbalasiri T, Carlson SM, Wong KY, Krishna V, Provencio I, Berson DM. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. See [63]. [DOI] [PubMed] [Google Scholar]
- Isoldi MC, Rollag MD, de Lauro Castrucci AM, Provencio I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc Natl Acad Sci USA. 2005;102:1217–1221. doi: 10.1073/pnas.0409252102. See [63]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MP, Nasi E. Light transduction in invertebrate hyperpolarizing photoreceptors: possible involvement of a Go-regulated guanylate cyclase. J Neurosci. 2000;20:5254–5263. doi: 10.1523/JNEUROSCI.20-14-05254.2000. An electrophysiological study suggesting that an opsin drives a Go-mediated phototransduction cascade in the hyperpolarizing response of ciliary photoreceptor cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Hara R. Regeneration of squid retinochrome. Nature. 1968;219:450–454. doi: 10.1038/219450a0. This paper shows that the chromophore of retinochrome is an all-trans-retinal, unlike rhodopsin. [DOI] [PubMed] [Google Scholar]
- Hao W, Fong HK. The endogenous chromophore of retinal G protein-coupled receptor opsin from the pigment epithelium. J Biol Chem. 1999;274:6085–6090. doi: 10.1074/jbc.274.10.6085. This study shows that the chromophore of RGR is an all-trans-retinal. [DOI] [PubMed] [Google Scholar]
- Ozaki K, Terakita A, Hara R, Hara T. Rhodopsin and retinochrome in the retina of a marine gastropod, Conomulex luhuanus. Vision Res. 1986;26:691–705. doi: 10.1016/0042-6989(86)90083-0. The biochemical identification of a retinochrome in a marine gastropod, the conch. [DOI] [PubMed] [Google Scholar]
- Terakita A, Hara R, Hara T. Retinal-binding protein as a shuttle for retinal in the rhodopsin-retinochrome system of the squid visual cells. Vision Res. 1989;29:639–652. doi: 10.1016/0042-6989(89)90026-6. The function of retinochrome as a supplier of a photoisomerized 11-cis-retinal to light-absorbed rhodopsin via a retinal shuttle protein. [DOI] [PubMed] [Google Scholar]
- Chen P, Hao W, Rife L, Wang XP, Shen D, Chen J, Ogden T, Van Boemel GB, Wu L, Yang M, et al. A photic visual cycle of rhodopsin regeneration is dependent on Rgr. Nat Genet. 2001;28:256–260. doi: 10.1038/90089. A study using RGR-knockout mice that concludes function of RGR as an in vivo retinal photoisomerase. [DOI] [PubMed] [Google Scholar]
- Farrens DL, Altenbach C, Yang K, Hubbell WL, Khorana HG. Requirement of rigid-body motion of transmembrane helices for light activation of rhodopsin. Science. 1996;274:768–770. doi: 10.1126/science.274.5288.768. This paper and [73] are two independent studies showing the movement of helices III and VI in the formation of an active state of bovine rhodopsin. [DOI] [PubMed] [Google Scholar]
- Sheikh SP, Zvyaga TA, Lichtarge O, Sakmar TP, Bourne HR. Rhodopsin activation blocked by metal-ion-binding sites linking transmembrane helices C and F. Nature. 1996;383:347–350. doi: 10.1038/383347a0. See [72]. [DOI] [PubMed] [Google Scholar]
- Borhan B, Souto ML, Imai H, Shichida Y, Nakanishi K. Movement of retinal along the visual transduction path. Science. 2000;288:2209–2212. doi: 10.1126/science.288.5474.2209. A description of the flip-over of the ring of retinal in the formation of the active state of bovine rhodopsin. [DOI] [PubMed] [Google Scholar]
- Konig B, Arendt A, McDowell JH, Kahlert M, Hargrave PA, Hofmann KP. Three cytoplasmic loops of rhodopsin interact with transducin. Proc Natl Acad Sci USA. 1989;86:6878–6882. doi: 10.1073/pnas.86.18.6878. A pioneering study to determine the interaction site of rhodopsin with transducin, using peptide-inhibition experiments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke RR, Konig B, Sakmar TP, Khorana HG, Hofmann KP. Rhodopsin mutants that bind but fail to activate transducin. Science. 1990;250:123–125. doi: 10.1126/science.2218504. Careful mutational analyses showing the function of the third cytoplasmic loop of bovine rhodopsin in G-protein activation. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Terakita A, Shichida Y. Distinct roles of the second and third cytoplasmic loops of bovine rhodopsin in G protein activation. J Biol Chem. 2000;275:34272–34279. doi: 10.1074/jbc.M002954200. A study demonstrating that the third cytoplasmic loop of several GPCRs is involved in the selective activation of different subtypes of G protein. [DOI] [PubMed] [Google Scholar]
- Terakita A, Yamashita T, Nimbari N, Kojima D, Shichida Y. Functional interaction between bovine rhodopsin and G protein transducin. J Biol Chem. 2002;277:40–46. doi: 10.1074/jbc.M104960200. Mutational analyses of both rhodopsin and G proteins uncovered an interaction between the third intracellular loop of rhodopsin and the carboxyl terminus of the G-protein alpha subunit. [DOI] [PubMed] [Google Scholar]
- Kefalov V, Fu Y, Marsh-Armstrong N, Yau KW. Role of visual pigment properties in rod and cone phototransduction. Nature. 2003;425:526–531. doi: 10.1038/nature01992. A pioneering study to elucidate functional difference between rhodopsin and cone visual pigments in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht JJ, Mielke T, Vogel R, Villa C, Schertler GF. Electron crystallography reveals the structure of metarhodopsin I. EMBO J. 2004;23:3609–3620. doi: 10.1038/sj.emboj.7600374. A two-dimensional crystal structure of the meta I form of rhodopsin at 5.5 Å, which is remarkable as the first crystal structure of the rhodopsin photoproduct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. The neighbor-joining method for reconstruction of phylogenetic trees. [DOI] [PubMed] [Google Scholar]


