Abstract
Initiation of the T-helper lymphocyte activation program is regulated through the T-cell receptor (TCR) and costimulatory receptors. Analysis of TCR and either anti-CD28- or interleukin 1 (IL-1)-mediated activation of the IL-2 promoter shows that costimulatory signals augment promoter activity through NF-κB sites. This study comparatively evaluates the mechanisms whereby signals initiated from the TCR and these two costimulatory receptors converge to synergistically increase NF-κB transcriptional activity. IL-1 alone stimulates an acute but transient NF-κB nuclear localization and a suboptimal NF-κB transcriptional response. In contrast, anti-CD3–anti-CD28 or anti-CD3–IL-1 synergistically stimulate prolonged NF-κB nuclear localization and NF-κB-mediated transcription. Both TCR- and costimulatory receptor-initiated synergistic NF-κB responses result from prolonging high rates of cytosolic IκB degradation during the second phase of the biphasic NF-κB nuclear localization. However, in contrast to previous reports, prolonged nuclear localization of NF-κB complexes is not necessarily associated with long-term depletion of IκBβ. In response to either costimulus, c-Rel selectively translocated to the nucleus as a result of induced c-Rel expression and the continued production of c-Rel–IκBα complexes, which turn over rapidly due to the high rate of IκBα degradation in the cytosol during the second phase of the response. In contrast, IκBβ is nearly completely degraded during the acute response to either IL-1 or anti-CD3–IL-1 while anti-CD3–anti-CD28 stimulates only a partial reduction (35 to 40%) in cytosolic IκBβ. Cyclosporine (CsA), which inhibits stimulus-induced NF-κB transcriptional activity, selectively inhibits the stimulus-induced c-Rel nuclear localization and the rapid formation and degradation of c-Rel–IκBα complexes in the cytosol. CsA also inhibits both the prolonged, high rate of IκBα degradation and the lower level of IκBβ turnover during the second phase of the activation response. Together, these results suggest a mechanism by which signals from the T-cell antigen receptor and either CD28 or IL-1 synergistically regulate IL-2 gene transcription by modulating NF-κB nuclear translocation.
NF-κB is a transcription factor that regulates a large number of cellular genes in response to signals from cytokine receptors, inflammatory mediators, UV light, or mitogens (2). NF-κB is comprised of dimeric complexes of RelA, c-Rel, RelB, p50, and p52. In resting cells, NF-κB is sequestered in the cytosol as a complex associated with inhibitor family molecules such as IκBα and IκBβ or the precursors of translocating subunits p50 and p52, p105 and p100, respectively. p105 and p100 are not as stimulus responsive as IκBα and IκBβ in Jurkat cells (24) but may control levels of NF-κB needed for housekeeping functions. In response to NF-κB-activating signals, both IκBα and IκBβ are inducibly phosphorylated at two amino-terminal serine residues, ubiquitinated, and degraded by the proteosome machinery (5, 7, 31, 36). Dissociation from IκB exposes the NF-κB nuclear localization signal, resulting in NF-κB nuclear translocation. The IκBα gene promoter is regulated by NF-κB (20), resulting in the stimulus-induced synthesis of IκBα protein to levels that are often higher than those in unstimulated cells. NF-κB component polypeptides c-Rel and RelB also are inducibly synthesized in response to NF-κB-activating stimuli. The initial report of IκBβ characterized this molecule as being degraded in murine 70Z/3 cells by stimuli that resulted in a persistent activation of NF-κB, such as lipopolysaccharide or interleukin 1 (IL-1), but not being targeted by transient activators such as tumor necrosis factor alpha (30). Subsequent reports with different cell types and stimulation conditions show that the kinetics of IκBβ degradation vary depending on stimulus and cell type (12, 13, 15, 36). It is currently unknown whether the same kinase is responsible for phosphorylation of the homologous serine residues in IκBα and IκBβ. The lack of a correlation between IκBα and IκBβ degradation caused by different stimuli suggests that these inhibitory molecules can be controlled by separate signaling pathways.
Several reports have indicated that NF-κB activation in T cells stimulated with tetradecanoyl phorbol acetate (TPA)-ionomycin or TPA–anti-CD28 can be regulated in a biphasic manner, resulting in a rapid but transient nuclear localization of p50–RelA–NF-κB complexes followed by nuclear translocation of c-Rel-containing complexes (13, 19, 33). The acute NF-κB response has been attributed to the rapid phosphorylation and subsequent degradation of IκBα and IκBβ. However, the mechanism responsible for the persistent nuclear localization of c-Rel during the second phase of the response has been controversial. Suyang et al. (29) proposed that following the acute degradation of IκBβ, newly synthesized IκBβ molecules were unphosphorylated, formed a stable complex with NF-κB, and translocated to the nucleus. Thus, persistent activation of NF-κB was proposed to be due to the stimulus-induced degradation of IκBβ and the subsequent nuclear localization of IκBβ–NF-κB complexes. However, nonphosphorylated IκBβ also has been reported not to associate with c-Rel–NF-κB complexes (6). Stimulation conditions that cause the accumulation of nonphosphorylated IκBβ in the cytosol could result in IκB-independent nuclear translocation of newly synthesized c-Rel complexes. The mechanisms responsible for the sustained nuclear localization of c-Rel in Jurkat cells stimulated with TPA–anti-CD28 also are controversial. One report attributes c-Rel nuclear localization to the prolonged degradation of cytosolic IκBα (19), while another report associates it with prolonged degradation of IκBβ (13). Although pharmacologic agonists can be useful in analyzing specific components of signaling pathways, they elicit superphysiological responses that may not reflect biologically relevant responses elicited from cell surface receptors.
Studies presented here characterize the mechanisms responsible for the NF-κB nuclear translocation that regulates IL-2 promoter activity in response to cell surface receptor-initiated signals from the T-cell receptor (TCR) and either CD28 or IL-1 costimulatory receptors. Results from these analyses demonstrate the existence of two independent receptor-initiated costimulatory pathways that control c-Rel nuclear localization and differentially regulate IκBα and IκBβ degradation. In this model system, IκBα provides a critical role in T-cell activation by mediating the prolonged phase of NF-κB nuclear translocation initiated synergistically by TCR and costimulatory receptor signals.
MATERIALS AND METHODS
Cells and reagents.
Ju.1 cells are stable transfectants of the human T-cell leukemia line Jurkat with the murine type 1 IL-1 receptor (IL-1R) (23). Antisera against the IκBα and IκBβ proteins were made by immunizing rabbits with recombinant IκBα or IκBβ. Antisera to c-Rel, RelB, and RelA were purchased from Santa Cruz (Santa Cruz, Calif.). Unless otherwise noted, all additional reagents were purchased from Sigma Chemical Co. (St. Louis, Mo.). Recombinant human IL-1 (a gift from John Sims, Immunex Corporation, Seattle, Wash.) had a specific activity of 5.7 × 105 U/μg and was used in assays at 0.3 ng/ml (approximately 170 U/ml). Biotinylated monoclonal antibodies against the human CD3ɛ subunit, OKT3 (32), were complexed with avidin prior to being added to cell cultures. Unless otherwise noted, 10 μg of avidin per ml and 5 μg of biotinylated OKT3 per ml were used; these concentrations were maximally effective in costimulating IL-2 production and luciferase activity driven by the wild-type IL-2 promoter from the cells. Luciferase activity was measured with the Luciferase Assay System (Promega Corporation, Madison, Wis.). CD28 monoclonal antibody 9.3 in ascites form was generously provided by K. Cabrian (Bristol-Myers Squibb, Seattle, Wash.) and was used in assays at a dilution of 1:100,000.
Construction of reporter genes.
The IL-2 promoter-luciferase reporter gene was created by inserting the 585 bp of the murine IL-2 promoter immediately upstream of a minimal promoter and the luciferase gene in pT8luc (Promega). NF-κB-luc was made by synthesizing three copies of the consensus NF-κB site from the human immunodeficiency virus long terminal repeat and ligating them into pT8luc. Point mutations were made in the NF-κB site (14) and the CD28 response element (CD28RE) (10) of the 585-bp IL-2 promoter with the Site-Directed Mutagenesis kit (Promega) according to the manufacturer’s instructions. Primers used in the construction of the point mutations are shown as follows (underlined regions represent changes from the transcription factor binding site in the murine IL-2 promoter): NF-κB 5′ mutant, GACCAAGACTCATTTCACC; NF-κB 3′ mutant, GGTGAAATGAGTCTTGGTC; CD28RE 5′ mutant, GTATGGGGGTTTAAAGAAGCCTCAGAGAGTCATCAGAAG; and CD28RE 3′ mutant, CTTCTGATGACTCTCTGAGGCTTCTTTAAACCCCCATAC. All mutations were confirmed by sequencing. These mutations were shown by gel shift analysis to abrogate nuclear protein binding to the appropriate oligonucleotide.
Firefly luciferase reporter genes and the control plasmid, pRL-TK Renilla luciferase (Promega), were cotransfected as reported previously (22). Following electroporation, cells were divided into equal portions, cultured for 24 h at 37°C, and then stimulated in triplicate for an additional 18 h. Cells were harvested and assayed with the Dual Luciferase Assay System (Promega) and a Model LB 9501/16 Lumat luminometer (Berthold Systems, Aliquippa, Pa.). Renilla luciferase values were used as internal controls to which expression of the luciferase reporter gene was normalized.
Electrophoretic mobility shift analysis (EMSA).
Nuclear extracts were made according to the method described by Dignam et al. (8) from cells treated for various times with the indicated stimuli. EMSAs were performed with 6 to 10 μg of nuclear extract, 0.25 to 0.5 μg of poly(dI-dC), and 15,000 cpm of labeled oligonucleotide corresponding to the NF-κB site of the murine IL-2 promoter. The coding strand sequence is 5′-CCCGACCAAGAGGGATTTCACCTAAATCCAATT-3′. Samples were allowed to react for 20 min at room temperature before being loaded on 4.5% nondenaturing polyacrylamide gels. Gels were dried and exposed for autoradiography.
Immunoprecipitations and Western blots.
Immunoprecipitations were carried out sequentially from nuclei or cytosolic preps of Ju.1 cells as described elsewhere (22) under conditions of antibody excess. After sodium dodecyl sulfate-polyacrylamide gel electrophoresis, proteins were transferred to an Immobilon membrane (Millipore Corp., Bedford, Mass.) and blotted sequentially with antisera against specific proteins. Western blots were developed with the ECL system (Amersham, Arlington Heights, Ill.), and proteins were identified within the linear range of the assay. Western blots were analyzed with an Ambis 4000 densitometer (Ambis Inc., San Diego, Calif.). Data presented are representative of at least three independent experiments.
RESULTS
Costimulatory activation of the IL-2 promoter by IL-1 and anti-CD28 requires NF-κB and CD28RE sites.
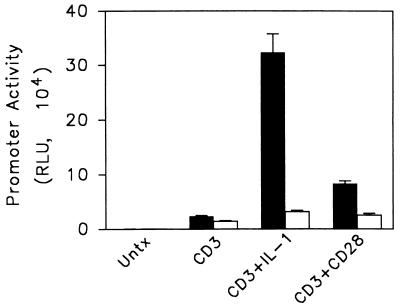
Ju.1 T cells, which stably express the type 1 IL-1R, provide a model system to characterize the activation requirements of T lymphocytes. These cells produce IL-2 (22) and activate an IL-2 promoter-luciferase reporter gene when stimulated through the TCR and costimulated either through CD28 or IL-1 receptors (Fig. 1). The importance of the NF-κB site and the CD28RE, an NF-κB-related sequence (34) that binds NF-κB proteins (11), is shown by the fact that incorporating point mutations into these two sites in the murine IL-2 promoter largely abrogates the ability of the costimulatory signals to upregulate reporter activity (Fig. 1). Reporter genes containing these mutations singly are not as severely affected (data not shown), most likely due to the known redundancy of transcription factor binding sites in the IL-2 promoter (37). Activation of the transcription factor AP-1 also has been reported to be initiated by both costimulatory pathways. However, point mutations in either or both of the two AP-1 sites do not have a detrimental effect on anti-CD3–IL-1- or anti-CD3–anti-CD28-initiated IL-2 reporter gene activity (data not shown).
FIG. 1.
Mutation of both the NF-κB and CD28RE sequences in the IL-2 promoter renders cells refractory to costimulatory activity of IL-1 and anti-CD28. Ju.1 cells were transfected with luciferase reporter genes controlled by the full-length IL-2 promoter (solid bars) or the same promoter containing nucleotide substitutions in the NF-κB and CD28RE sites (open bars). After resting overnight, cells were stimulated as indicated and harvested for luciferase assays 18 h after stimulation. Error bars indicate standard errors of the means of a representative experiment. RLU, relative light units; Untx, unstimulated.
Although IL-1 alone is an activator of NF-κB in many cell types, in Ju.1 cells it stimulates submaximal NF-κB-regulated transcriptional activity in the absence of TCR signals (22). Additionally, although cross-linking either TCR or CD28 alone results in little or no response from an NF-κB reporter gene, signals resulting from anti-CD3 plus either IL-1 or anti-CD28 synergistically activate NF-κB. We have previously demonstrated a correlation between this synergistic NF-κB transcriptional activity and prolonged NF-κB nuclear localization stimulated by anti-CD3–IL-1 in contrast to what was stimulated by IL-1 alone (22). Within minutes of stimulation, cells stimulated with IL-1, anti-CD3–IL-1, or, to a lesser extent, anti-CD3–anti-CD28 initiate rapid NF-κB nuclear localization as a result of degradation of IκB proteins that retain NF-κB complexes in the cytosol (22). In contrast, neither anti-CD3 nor anti-CD28 alone has a detectable effect on NF-κB nuclear localization (data not shown).
CsA selectively affects activation responses initiating NF-κB-mediated transcription.
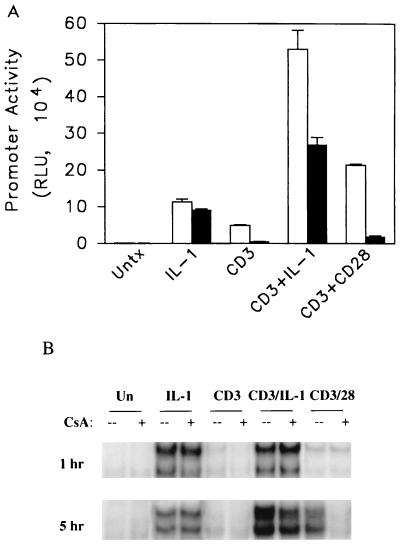
We tested the effects of cyclosporine (CsA), an inhibitor of the calcium-dependent phosphatase calcineurin, on the NF-κB reporter gene responses because of previous reports documenting its inhibitory effects on NF-κB in T cells (4, 21, 26, 28, 33). Although IL-1 does not influence calcium mobilization (1) and CD28-mediated signaling events are insensitive to inhibitors of calcineurin (16, 17), early signaling events initiated by ligation of the TCR include elevation of intracellular calcium. Ju.1 cells stimulated with either IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28 induce significant NF-κB-driven luciferase reporter gene activity. These transcriptional responses are insensitive, partially sensitive, or largely sensitive, respectively, to inhibition by CsA (Fig. 2A). The effect of CsA on NF-κB reporter gene activity is paralleled closely by the abilities of proteins in nuclear extracts from CsA-treated cells to bind a DNA probe derived from the NF-κB site in the murine IL-2 promoter in an EMSA (Fig. 2B). Although CsA has no significant effect on the acute response, it abrogates the band shift caused by nuclear extracts from cells stimulated for 5 h with anti-CD3–anti-CD28, partially reduces the intensity of shifted bands in cells stimulated with anti-CD3–IL-1, and has no effect on cells stimulated with IL-1.
FIG. 2.
The effect of CsA on NF-κB reporter gene activity and EMSA activity. (A) Ju.1 cells that had been transfected with an NF-κB luciferase reporter gene were stimulated with the indicated reagents in the absence (open bars) or presence (closed bars) of CsA. Error bars indicate standard errors of the means of a representative experiment. RLU, relative light units; Untx, unstimulated. (B) EMSA analysis of a radiolabeled oligonucleotide from the IL-2 promoter NF-κB sequence and nuclear extracts of unstimulated (Un) Ju.1 cells or cells stimulated for 1 or 5 h.
CsA-inhibited transcriptional responses correlate with regulation of c-Rel nuclear translocation.
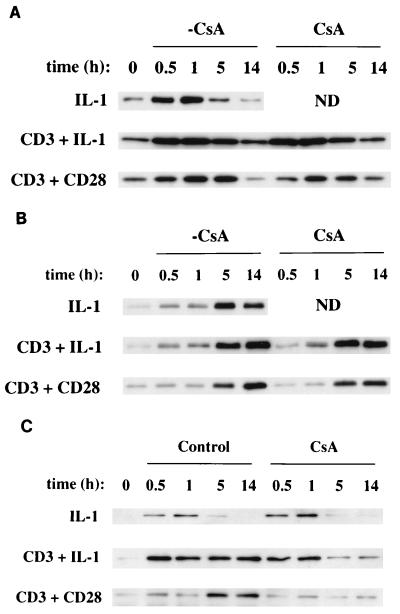
To identify which NF-κB polypeptides were affected by CsA, Ju.1 cells were stimulated either in the presence or in the absence of CsA with IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28. Levels of nuclear NF-κB polypeptides RelA, RelB, and c-Rel were evaluated by immunoprecipitation from nuclear lysates and Western blotting (Fig. 3). Each stimulus caused an acute nuclear translocation of RelA that was maximal at 30 min and diminished in intensity by 14 h, and this response was unaffected by CsA (Fig. 3A). Each stimulus also caused translocation of RelB to the nucleus at 5 and 14 h, also in a CsA-insensitive manner (Fig. 3B). In contrast, the amounts of c-Rel translocated to the nucleus in response to the various stimuli differed significantly (Fig. 3C). IL-1 alone initiated an acute but transient increase in nuclear c-Rel, and this response was unaffected by CsA. Not only did anti-CD3–IL-1 initiate an acute nuclear translocation of c-Rel, but c-Rel also remained localized in the nuclei at the 5- and 14-h time points. The addition of CsA to these cultures partially reduced the amount of nuclear c-Rel at the 5- and 14-h time points. In cells stimulated with anti-CD3–anti-CD28, c-Rel nuclear translocation was apparent at the 5- and 14-h time points and this late translocation was abrogated by CsA. Thus, the inhibitory effects of CsA on NF-κB-mediated transcriptional activity initiated by anti-CD3–IL-1 or anti-CD3–anti-CD28 correlate with the selective CsA-mediated inhibition of c-Rel nuclear localization that is initiated by anti-CD3–IL-1 or anti-CD3–anti-CD28. These results provide additional support for the conclusion that the second phase of NF-κB nuclear translocation is primarily responsible for mediating the TCR-costimulatory receptor-initiated NF-κB-dependent gene transcription in this model system. In addition, since both IL-1 and anti-CD3–IL-1 initiate c-Rel gene transcription (data not shown), differences in NF-κB-mediated transcriptional responses initiated by these stimuli are not due to an inability of IL-1 to initiate c-Rel gene expression.
FIG. 3.
Effects of CsA on the nuclear localization of c-Rel stimulated by IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28. Ju.1 cells were stimulated in the absence or presence of CsA, and nuclear lysates were prepared and immunoprecipitated with anti-RelA (A), anti-RelB (B), and anti-c-Rel (C). The amounts of RelA (A), RelB (B), and c-Rel (C) polypeptides in each sample were analyzed by Western blotting. ND, not determined.
Characterization of IκBα turnover during acute and prolonged NF-κB nuclear localization.
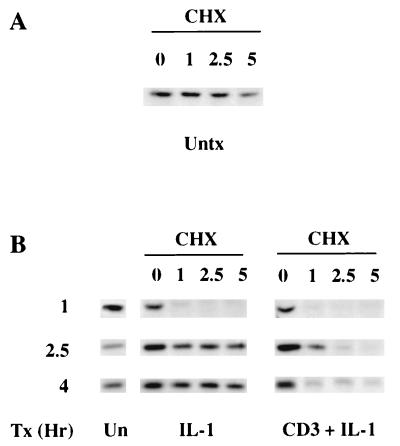
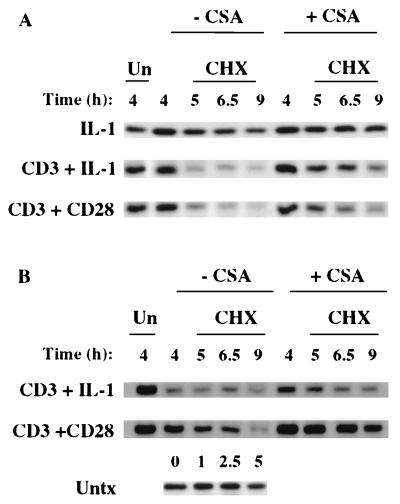
Preliminary experiments demonstrated that there are no detectable differences in the kinetics of IκBα degradation and resynthesis stimulated during the acute (0 to 2 h) response to IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28 (data not shown). However, that amount of IκBα degradation is less in cells stimulated with anti-CD3–anti-CD28 than in cells stimulated with IL-1 or anti-CD3–IL-1. To identify the NF-κB cytosolic reservoir utilized for the late NF-κB responses, we characterized the kinetics of IκB turnover stimulated by IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28 at various times after the acute NF-κB nuclear localization (Fig. 4). After the T cells were stimulated for either 0, 1, 2.5, or 4 h, the protein synthesis inhibitor cycloheximide (CHX) was added and the cells were subsequently cultured in the presence of CHX for an additional 1 to 5 h. Cytosolic lysates were analyzed by Western blotting to identify changes in IκBα turnover rates. In unstimulated cells, the half-life of IκBα is greater than 5 h (Fig. 4A). In contrast, in cells stimulated for 1 h with either IL-1 or anti-CD3–IL-1, IκBα has a half-life of less than 60 min due to ongoing stimulus-induced degradation of IκBα (Fig. 4B). Cells stimulated for 2.5 h with IL-1 alone exhibit IκBα turnover rates similar to those of untreated cells while cells treated with anti-CD3–IL-1 continue to exhibit accelerated IκBα turnover. After 4 h of stimulation, IκBα degradation in cells treated with anti-CD3–IL-1 continues to occur at the accelerated rate. Cells treated for 2.5 and 4 h with anti-CD3–anti-CD28 also exhibit an accelerated turnover of IκBα (data not shown; see Fig. 6). Together, these results demonstrate that the IL-1-induced increase in IκBα degradation is both acutely initiated and transient, returning to basal rates of degradation between 1 and 2.5 h poststimulus. In contrast, the IκBα degradation initiated by either anti-CD3–IL-1 or anti-CD3–anti-CD28 is prolonged for at least 4 h after cell stimulation. This protracted increased rate of IκBα turnover results in continued NF-κB nuclear translocation and correlates with the prolonged NF-κB nuclear localization and augmented NF-κB transcriptional activity in cells stimulated with either anti-CD3–IL-1 or anti-CD3–anti-CD28.
FIG. 4.
Degradation of IκBα during the T-cell activation response. (A) IκBα turnover in unstimulated (Untx) Ju.1 cells was evaluated by culturing cells with CHX (100 μg/ml). Cells were harvested after the indicated times of CHX treatment (in hours), cytosolic lysates were prepared, and IκBα immunoprecipitates were analyzed by Western blotting with anti-IκBα. (B) IκBα turnover in Ju.1 cells stimulated for 1, 2.5, or 4 h with IL-1 with or without anti-CD3 and then cultured in CHX for indicated times (in hours). IκBα was immunoprecipitated from cytosolic lysates and analyzed by Western blotting. Tx, stimulation; Un, unstimulated.
FIG. 6.
Effect of CsA on the degradation of IκBα and IκBβ in Ju.1 cells stimulated for 4 h with IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28. Ju.1 cells were stimulated for 4 h in the absence or presence of CsA, and then the cells were treated with CHX for 0 to 5 h. IκBα (A) or IκBβ (B) was immunoprecipitated from cytosolic lysates and analyzed by Western blotting. Un, unstimulated; Untx, untreated.
IκBβ degradation is differentially regulated by IL-1 and anti-CD28.
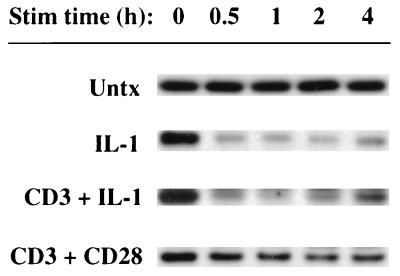
IκBβ differs from IκBα in that the IκBβ gene is not transcriptionally regulated by NF-κB, so NF-κB responses do not increase the amount of IκBβ protein (30). It has been proposed that IκBβ also differs functionally from IκBα in that persistent NF-κB nuclear translocation is controlled by IκBβ whereas transient NF-κB nuclear translocation is controlled by IκBα (30). To evaluate the role of IκBβ in the Ju.1 cell model, IκBβ was isolated from cytosolic lysates of cells stimulated with IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28 and analyzed by Western blotting (Fig. 5). While IκBβ exhibits a long half-life in untreated cells (>5 h), stimulation with IL-1 alone or in combination with anti-CD3 caused rapid and nearly complete degradation of IκBβ within 1 h of stimulation. The kinetics of the recovery of cytosolic IκBβ in cells stimulated by IL-1 and in cells stimulated by anti-CD3 plus IL-1 were comparable, returning to basal levels by 15 h (data not shown). Thus, comparable effects on cytosolic IκBβ degradation were observed in cells stimulated to produce transient (IL-1) and in cells stimulated to produce prolonged (anti-CD3–IL-1) NF-κB nuclear localization. Neither anti-CD3 nor anti-CD28 treatment alone affected IκBβ degradation (data not shown). In contrast, cells stimulated with anti-CD3–anti-CD28 responded with a much smaller loss of IκBβ during the acute response. In multiple experiments, cells stimulated with anti-CD3–anti-CD28 for 1 h contained 35 to 40% (determined by densitometry) less IκBβ than did untreated cells. These results indicate that IκBβ degradation responses are stimulus dependent and range from transient NF-κB responses (IL-1) in which degradation of IκBβ is acute and nearly complete to prolonged NF-κB responses in which IκBβ degradation is acute and either nearly complete (anti-CD3–IL-1) or only partial (anti-CD3–anti-CD28). Thus, in contrast to the model proposed by Thompson et al. (30), neither the IL-1-induced nor the anti-CD3–anti-CD28-induced effects on IκBβ degradation correlate with prolonged NF-κB nuclear localization.
FIG. 5.
Degradation of IκBβ in untreated (Untx) Ju.1 cells or during the acute phase of the activation response stimulated (Stim) by IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28. IκBβ was immunoprecipitated from cytosolic lysates and analyzed by Western blotting.
CsA inhibits the enhanced rate of IκB degradation during the second phase of the activation response.
We next investigated whether the CsA-dependent reduction in NF-κB reporter gene activity and EMSA activity correlated with stimulus-induced effects on IκBα or IκBβ turnover during the second phase of the activation response. The influence of CsA on the half-life of cytosolic IκBα was analyzed by stimulating cells in the presence or absence of CsA for 4 h with IL-1, anti-CD3–IL-1, or anti-CD3–anti-CD28 (Fig. 6). After stimulation, cells were treated for varying periods of time with CHX, and turnover of cytosolic IκBα was analyzed by Western blotting. As expected from the results shown above in Fig. 2, in cells treated for 4 h with IL-1 the half-life of IκBα was greater than 5 h, and this turnover rate was unaffected by CsA (Fig. 6A). Cells treated for 4 h with anti-CD3–IL-1 show a rapid turnover of IκBα. The addition of CsA greatly prolonged the IκBα half-life, albeit to a length that was shorter than the half-life observed in untreated (Fig. 4) or IL-1-treated cells. Similar effects of CsA were seen in cells treated for 4 h with anti-CD3–anti-CD28 (Fig. 6A). Thus, the early NF-κB activation events (nuclear translocation of p65, RelB, and c-Rel [Fig. 3]) initiated by the three stimuli were resistant to CsA effects while the prolonged accelerated turnover of IκBα at later time points in cells treated with anti-CD3 plus either anti-CD28 or IL-1 was inhibited by CsA. CsA treatment, therefore, allows separation of signals necessary for acute activation of the IκBα degradative pathway from subsequent signals responsible for prolonging the high IκBα turnover rate.
We also analyzed the effect of CsA on IκBβ turnover in cells stimulated for 4 h with anti-CD3–IL-1 or anti-CD3–anti-CD28 (Fig. 6B). Compared to that in untreated cells, IκBβ was nearly completely degraded in cells stimulated with anti-CD3–IL-1 for 4 h (Fig. 6B; Un, 4 h, versus anti-CD3–IL-1, 4 h). The addition of CsA to cells stimulated with anti-CD3–IL-1 for 4 h resulted in a small increase in cytosolic IκBβ (<20%) (Fig. 6B; CD3–IL-1, 4 h, − CsA, versus 4 h, + CsA). Such results indicate that anti-CD3–IL-1 stimulates the nearly complete degradation of IκBβ during the acute response and that CsA inhibits the low rate of degradation of IκBβ that was resynthesized during the second phase of the activation response. CsA only partially inhibits the rate of IκBβ degradation since the turnover rate in stimulated cells remains higher than that observed in untreated cells. The amount of IκBβ was partially reduced in cells treated for 4 h with anti-CD3–anti-CD28 (30 to 40%) (Fig. 6B; Un, 4 h, versus anti-CD3–anti-CD28, 4 h) as a cumulative result of the acute degradation response and 4 h of stimulation-induced degradation of IκBβ, which was produced as part of the constitutive resynthesis of IκBβ. The addition of CsA to cells stimulated with anti-CD3–anti-CD28 increased cytosolic IκBβ by partially inhibiting IκBβ degradation (Fig. 6B; anti-CD3–anti-CD28, 4 h, − CsA, versus 4 h, + CsA).
Thus, the addition of CHX to cells stimulated with either anti-CD3–IL-1 or anti-CD28 permits detection of the low rate of IκBβ degradation that is normally offset by constitutive resynthesis. CsA inhibited this low rate of IκBβ degradation in cells stimulated for 4 to 9 h with either anti-CD3–IL-1 or anti-CD3–anti-CD28. Together, these results indicate that, during the second phase of NF-κB nuclear localization initiated by anti-CD3 plus either IL-1 or anti-CD28, CsA partially inhibits both the prolonged high rate of degradation of cytosolic IκBα and the relatively low rate of stimulus-induced degradation of IκBβ.
TCR and costimulatory signals converge to regulate quantitative and qualitative changes in cytosolic and nuclear NF-κB complexes.
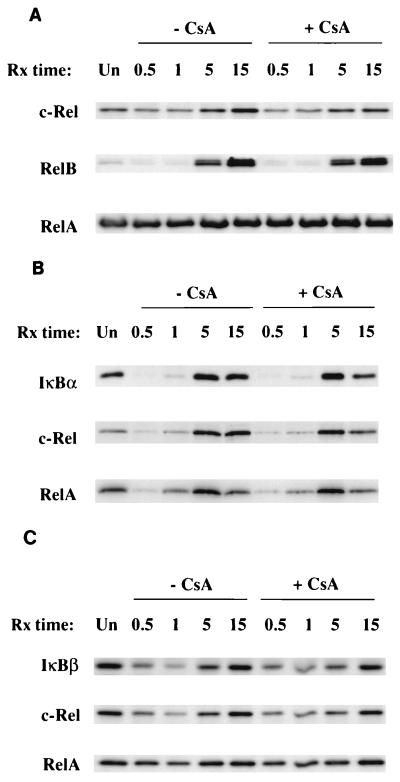
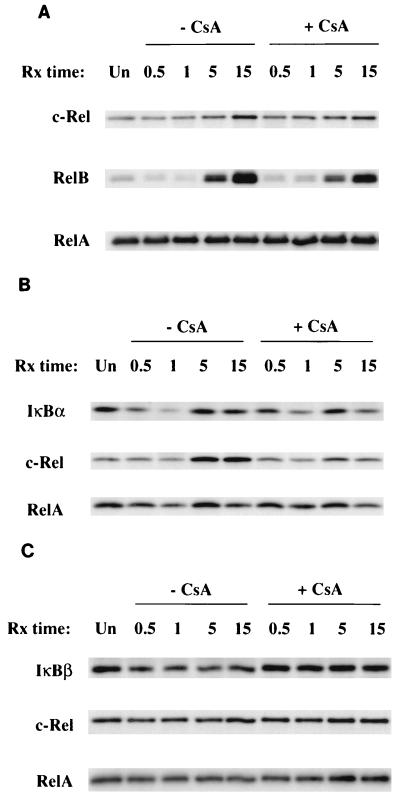
The prolonged degradation of IκBα stimulated by anti-CD3 and either IL-1 or anti-CD28 correlates with continued NF-κB nuclear localization and also could affect the composition of the NF-κB complexes that associate with the newly synthesized IκB proteins. c-Rel and RelB are inducibly regulated at a transcriptional level by NF-κB while RelA exhibits an uninducible, low rate of turnover. Thus, c-Rel and RelB may comprise a higher percentage of the NF-κB complexes associated with IκB proteins that turn over rapidly during the second phase of the stimulus-induced NF-κB response. To evaluate this possibility, c-Rel, RelB, and RelA polypeptides as well as IκBα-associated NF-κB complexes were isolated from the cytosol of cells stimulated with either anti-CD3–IL-1 (Fig. 7) or anti-CD3–anti-CD28 (Fig. 8). Densitometric analysis of Western blot data shows that both stimuli initiate increases in total c-Rel (untreated versus 15 h: 3-fold, anti-CD3–anti-CD28; 1.6 fold, anti-CD3–IL-1) and RelB (7-fold, anti-CD3–anti-CD28; 14-fold, anti-CD3–IL-1) proteins during the second phase of the activation response (5 to 15 h) while levels of p65 remain unaltered (Fig. 7A and 8A). The stimulus-induced increase in total cytosolic c-Rel is inhibited only slightly by CsA while RelB and RelA levels were unaffected (Fig. 7A and 8A). Analysis of IκBα precipitates shows that there is an increase in the amount of IκBα–c-Rel complexes in the activated cells during the second phase of the response (untreated versus 15 h: fourfold by anti-CD3–anti-CD28; twofold stimulated by anti-CD3–IL-1). This increase in cells stimulated with anti-CD3–IL-1 is partially inhibited (approximately 40%) by CsA while the increase in cells stimulated with anti-CD3–anti-CD28 is completely inhibited by CsA. Although RelB does not associate efficiently with IκBα (9), the amount of RelA associated with IκBα during the second phase of the response does not increase from the amount associated in untreated cells. CsA has no effect on the amount of RelA-IκBα complexes in the cytosol (Fig. 7B and 8B).
FIG. 7.
Effects of CsA on the anti-CD3–IL-1-induced alteration of cytosolic NF-κB component polypeptides. (A) Isolation of c-Rel, RelB, and RelA polypeptides from cytosolic lysates prepared from Ju.1 cells treated with CsA and/or anti-CD3–IL-1. Lysates were sequentially precipitated with anti-c-Rel, anti-RelB, and anti-RelA; immunoprecipitates were analyzed by Western blotting; and the blots were probed with the same antibodies as those used for immunoprecipitation. (B) Effects of CsA on the anti-CD3–IL-1-induced association of NF-κB component polypeptides c-Rel and RelA with IκBα. Ju.1 cells were stimulated for 0.5 to 15 h in the absence or presence of CsA. IκBα immunoprecipitates from the cytosolic lysates were analyzed by Western blotting, and the blot was probed sequentially with anti-IκBα, anti-c-Rel, and anti-RelA. (C) Effects of CsA on the anti-CD3–IL-1-induced association of NF-κB component polypeptides c-Rel and RelA with IκBβ. Ju.1 cells were stimulated for 0.5 to 15 h in the absence or presence of CsA. IκBβ immunoprecipitates from the cytosolic lysates were analyzed by Western blotting, and the blot was probed sequentially with anti-IκBβ, anti-c-Rel, and anti-RelA. Rx, treatment; Un, untreated.
FIG. 8.
Effects of CsA on the anti-CD3–anti-CD28-induced alteration of cytosolic NF-κB component polypeptides. (A) Isolation of c-Rel, RelB, and RelA polypeptides from cytosolic lysates prepared from Ju.1 cells treated with CsA and/or anti-CD3–anti-CD28. Lysates were sequentially precipitated with anti-c-Rel, anti-RelB, and RelA; immunoprecipitates were analyzed by Western blotting; and blots were probed with the same antibodies as those used for immunoprecipitation. (B) Effects of CsA on the anti-CD3–anti-CD28-induced association of NF-κB component polypeptides c-Rel and RelA with IκBα. Ju.1 cells were stimulated for 0.5 to 15 h in the absence or presence of CsA. IκBα immunoprecipitates from the cytosolic lysates were analyzed by Western blotting, and the blot was probed sequentially with anti-IκBα, anti-c-Rel, and anti-RelA. (C) Effects of CsA on the anti-CD3–anti-CD28-induced association of NF-κB component polypeptides c-Rel and RelA with IκBβ. Ju.1 cells were stimulated for 0.5 to 15 h in the absence or presence of CsA. IκBβ immunoprecipitates from the cytosolic lysates were analyzed by Western blotting, and the blot was probed sequentially with anti-IκBβ, anti-c-Rel, and anti-RelA. Rx, treatment; Un, untreated.
IκBβ does not exhibit a comparable high rate of turnover in response to these combined stimuli and therefore should not selectively associate with c-Rel during the second phase of the response. Western blot analysis of IκBβ immunoprecipitates isolated from cytosol lysates of cells stimulated with anti-CD3 plus either IL-1 or anti-CD28 showed no preferential association of c-Rel or RelA with IκBβ (Fig. 7C and 8C). Importantly, CsA had no effect on the amount of either c-Rel or RelA associated with IκBβ in the cytosol. Thus, the signals initiated by anti-CD3 plus either IL-1 or anti-CD28 stimulate a prolonged, high rate of IκBα degradation, resulting in continued NF-κB nuclear translocation. These signals also result in a selective increase in the amount of c-Rel–IκBα cytosolic complexes that are likely a primary reservoir of c-Rel-containing NF-κB complexes that translocate to the nucleus during the second phase of stimulus-induced activation. CsA, which partially inhibits NF-κB transcriptional activity in cells stimulated with anti-CD3–IL-1, also partially inhibits c-Rel–IκBα complex formation during the second phase of the activation response. The NF-κB transcriptional activity stimulated by anti-CD3–anti-CD28 is completely inhibited by CsA, and CsA also selectively inhibits the inducible association of c-Rel with IκBα as well as the high turnover of IκBα in the cytosol.
DISCUSSION
An extensive literature documents the role of NF-κB in the transcriptional regulation of genes involved in the T-cell activation program. Several recent reports have suggested that the IL-2 promoter contains NF-κB sites that bind both RelA- and c-Rel-containing NF-κB complexes but may be more responsive to c-Rel complexes. Knockout mice lacking the c-Rel member of the NF-κB family were shown to be unresponsive to anti-CD3–anti-CD28 stimulation (18). These results suggest that c-Rel is necessary for IL-2 synthesis and that other NF-κB component polypeptides (RelA and RelB) do not effectively substitute for c-Rel in regulating this gene. Similarly, a potent pharmacologic inhibitor of IL-2 gene transcription, pentoxifylline, was shown to selectively inhibit c-Rel nuclear localization without altering RelA responses (35). Several reports also have shown that anti-CD28 and combinations of pharmacologic agonists initiate a biphasic NF-κB nuclear localization response in T lymphocytes (12, 25, 26, 30). RelA is translocated during the initial phase, and c-Rel is translocated during the subsequent second phase of the response. A biphasic response also has been associated with the synergistic NF-κB-mediated transcriptional regulation initiated through TCR and IL-1R (22). These studies led us to characterize the mechanism whereby cell surface receptor-initiated c-Rel nuclear localization and NF-κB transcriptional activity were selectively and synergistically regulated when T cells were stimulated through the TCR and either costimulatory receptor CD28 or IL-1R.
The current paradigm of NF-κB activation suggests that a variety of stimuli known to translocate NF-κB from the cytosol to the nucleus initiate phosphorylation, ubiquitination, and degradation of IκBα and IκBβ polypeptides that are complexed to NF-κB in the cytosol. The released NF-κB translocates to the nucleus where it transcriptionally regulates the production of IκBα, c-Rel, and RelB as well as many other genes involved in growth and differentiation. This acute translocation is largely complete within 1 h, which is the time needed to resynthesize IκBα to levels found in untreated cells. Recent reports (29, 30) have proposed that stimuli that initiate persistent activation of NF-κB regulate nuclear localization by lowering IκBβ levels in the cytosol. Suyang et al. (29) proposed that newly synthesized IκBβ is unphosphorylated and associates preferentially with newly synthesized NF-κB, thereby outcompeting newly synthesized IκBα for the NF-κB complexes. Association of IκBβ–NF-κB fails to mask the nuclear localization signals on the NF-κB complexes, and the unphosphorylated IκBβ–NF-κB complexes are imported into the nucleus where the NF-κB complexes bind to DNA. The very low levels of nuclear IκBβ molecules actually detected in activated cells were attributed to rapid degradation of IκBβ in the nucleus. The synergistic NF-κB transcriptional responses initiated by anti-CD3–IL-1 or anti-CD3–anti-CD28 provide a valuable experimental model to evaluate receptor-initiated mechanisms responsible for NF-κB nuclear localization and to compare these mechanisms to previous models of NF-κB regulation.
Our initial experiments demonstrated that, in the Ju.1 model system, signals initiated from the TCR and either IL-1R or CD28 converge to synergistically regulate the transcription of the IL-2 gene. In addition, the augmenting effects of the costimulatory signals were shown to require the NF-κB sites in the IL-2 promoter. Analysis of an NF-κB reporter gene demonstrated that, although IL-1 alone is capable of activating the NF-κB reporter gene, NF-κB transcriptional activity is synergistically upregulated by the signals initiated by the combination of anti-CD3 and either IL-1 or anti-CD28. Thus, the two costimulatory receptors, which have previously been shown to initiate distinct signaling pathways (3, 27), converge to affect similar transactivating components in the IL-2 promoter. The early, transient NF-κB nuclear localization initiated by IL-1 is the result of the rapid degradation of both IκBα and IκBβ, releasing NF-κB (primarily RelA-p50 complexes) and exposing the nuclear localization signals. During this acute phase of the response, IκBα is inducibly resynthesized as a consequence of NF-κB-mediated transcription, and the amount of IκBα in the cytosol returns to normal within 1 to 2 h. The high rate of IκBα degradation induced by IL-1 is short lived, returning to basal rates within 2.5 h. In contrast, the resynthesis of IκBβ is not transcriptionally regulated by NF-κB and returns to normal levels by 15 h, presumably due to a very low rate of resynthesis of IκBβ.
Although neither anti-CD28 nor anti-CD3 alone activates NF-κB, IL-1 initiates an acute but transient NF-κB nuclear translocation that results in a submaximal NF-κB-mediated transcriptional response. In contrast, both anti-CD3–IL-1 and anti-CD3–anti-CD28 initiate a biphasic NF-κB nuclear translocation that results in both acute (0 to 2 h) and prolonged (3 to >15 h) NF-κB nuclear localization. IκBα degradation during the acute phase of the IL-1 or anti-CD3–IL-1 response is indistinguishable while the response to anti-CD3–anti-CD28 is less than that initiated by IL-1. During the second phase of these responses, the degradation rates of IκBα in cells stimulated with IL-1 alone or with anti-CD3–IL-1 differed dramatically. In contrast to the transient degradation of IκBα induced by IL-1, a rapid turnover of IκBα was maintained for a prolonged period of time in cells that were stimulated through both the TCR and either IL-1R or CD28 (Fig. 4). During this second phase, IκBα–NF-κB complexes are present at normal levels in the cytosol, presumably as a consequence of degradation rates of IκBα being balanced by new synthesis. The effects on IκBβ degradation during the acute phase of the response stimulated by anti-CD3–IL-1 or anti-CD3–anti-CD28 also differed markedly. Although anti-CD3–IL-1 rapidly stimulates the acute degradation of both IκBα and IκBβ, anti-CD3–anti-CD28 preferentially stimulates the degradation of IκBα. As a result, only a very small amount of IκBβ is present in the cytosol of cells stimulated with anti-CD3–IL-1 during the initiation of the second phase of the activation response. In contrast, cytosolic IκBβ–NF-κB complexes decrease slowly during the second phase of the response in cells stimulated with anti-CD3–anti-CD28.
Thompson et al. (30) reported a correlation between stimuli that initiate IκBβ turnover and the ability to initiate persistent NF-κB transcriptional activity. Prolonged NF-κB nuclear localization was observed in responses that initiated degradation of both IκBα and IκBβ while transient NF-κB responses were associated with the selective degradation of IκBα (30). Results presented here are not consistent with those conclusions. IL-1 alone stimulates an acute loss of both IκBα and IκBβ in the cytosol, yet the resulting NF-κB nuclear localization response is transient and the NF-κB-mediated transcriptional activity is submaximal. Prolonged NF-κB nuclear localization with concomitant high levels of NF-κB transcriptional activity can occur when IκBβ is present in the cytosol at either very low levels (anti-CD3–IL-1) or intermediate levels (anti-CD3–anti-CD28). The low rate of stimulus-induced IκBβ turnover observed during the second phase of the activation responses initiated by anti-CD3 plus either IL-1 or anti-CD28 suggests that IκBβ may regulate a component of the late NF-κB response. However, initiation of prolonged NF-κB nuclear localization does not correlate with the extent of degradation of cytosolic IκBβ that occurs as part of the acute activation response.
In untreated Ju.1 cells, the half-life of the c-Rel protein is approximately 5 h while that of RelA is greater than 10 h (data not shown). Costimulation through either CD28 or IL-1 receptors in TCR-activated cells causes increased synthesis of c-Rel and RelB, which in turn results in an increase in the total amount of cytosolic c-Rel and RelB. In contrast, RelA is constitutively produced and is uninducible with these stimuli. Although there is little evidence for a role for RelB in the regulation of the IL-2 promoter, multiple reports have demonstrated a specific role for c-Rel in regulating this important gene. Thus, it is biologically relevant that the combination of the inducible c-Rel and IκBα synthesis and prolonged high rates of IκBα degradation result in an increase in the formation of IκBα–c-Rel complexes during the second phase of the activation response. The converging signals from these different receptors, therefore, not only prolong the period of time in which NF-κB translocates to the nucleus but also can change the composition of the NF-κB components that translocate during the later phase of the activation response.
The effects of CsA on these T-cell responses provide valuable insight into the events that are important for controlling NF-κB-mediated transcription. CsA is a potent inhibitor of NF-κB-mediated transcriptional activity initiated by TCR and either IL-1R or CD28 signals. CsA also selectively inhibits c-Rel nuclear localization during the second component of the biphasic activation response. Although it has been reported that CsA effects on c-Rel nuclear localization may be mediated by inhibition of c-Rel gene expression (14), reverse transcription-PCR analysis of c-Rel mRNA from Ju.1 cells stimulated for 4 or 8 h with anti-CD3–anti-CD28 shows that CsA reduces c-Rel message by only 40% (data not shown). Since the c-Rel promoter is incompletely characterized, we cannot rule out the possibility that c-Rel gene transcription is regulated by an NF-κB-independent transcription factor that is affected by CsA. To identify potential mechanisms by which CsA mediates its inhibitory effects on NF-κB, we focused our analyses on alterations in the rates of IκB degradation. Although we were unable to detect any effects of CsA on the acute response initiated by anti-CD3–IL-1 or anti-CD3–anti-CD28, we found that the principal effect of CsA was to inhibit the high rate of IκBα degradation during the second phase of the activation response initiated by both combinations of stimuli. We also observed that CsA inhibits a low level of IκBβ degradation that occurs during the second phase of the activation response to anti-CD3 plus either IL-1 or anti-CD28. As a consequence of the coordinate synthesis of IκBα and c-Rel, there is a selective increase in the amount of c-Rel associated with IκBα during this component of the response. Even though both stimuli increase the total amount of c-Rel in the cytosol, no comparable selective association of c-Rel with IκBβ was observed. Together, these results suggest that CsA-mediated inhibition of IκBα degradation during the second component of the activation response is an important mechanism responsible for the CsA-mediated reduction in c-Rel nuclear localization and reduced NF-κB transcriptional activity observed in cells stimulated with anti-CD3 plus either IL-1 or anti-CD28. These results also support the conclusions of Sen and collaborators (33, 35) that c-Rel-containing NF-κB complexes play a major role in NF-κB-mediated regulation of the IL-2 gene.
Suyang et al. (29) proposed that IκBβ can outcompete IκBα for newly synthesized NF-κB, resulting in nuclear translocation of the IκBβ–NF-κB complex to the nucleus. Although our experiments do not directly address this hypothesis, IκBα clearly associates effectively with NF-κB during the second phase of the activation responses evaluated in this report. In addition, IκBβ is synthesized at a low rate during the second phase of these activation responses. Preliminary experiments have comparatively evaluated the amount of c-Rel associated with either IκBα or IκBβ in lysates isolated from Ju.1 cells stimulated for 4 h with anti-CD3–anti-CD28. Western blot analysis of anti-IκBα and anti-IκBβ immunoprecipitates (isolated in antibody excess) showed comparable amounts of c-Rel associated with IκBα and IκBβ in the cytosol of these activated cells (data not shown). Compared to the prolonged high rate of turnover of IκBα during the second phase of the activation response, the observed low level of stimulus-induced IκBβ degradation suggests that IκBβ is not the primary reservoir of c-Rel-transactivating complexes during the critical second phase of these responses. Unless the NF-κB complexes originating from IκBβ are preferentially translocated to the nucleus or, once in the nucleus, preferentially interact with the DNA, it is likely that the major component of the c-Rel complexes that associate with the DNA originates from IκBα cytosolic reservoirs.
A number of reports have studied the mechanisms that regulate NF-κB nuclear localization in model systems that have been stimulated with pharmacologic agonists (13, 19, 33). Results from these analyses have demonstrated the biphasic nature of the NF-κB nuclear localization response and shown the preferential nuclear localization of c-Rel during the second phase of the activation response. However, these pharmacologic agonists elicit superphysiological responses that may not mimic biologically relevant responses. They may provide information on potential responses that can be elicited by a cell but that may not necessarily occur as a result of receptor-initiated stimulation. The results presented in this report reinforce the potential limitations of such analyses. We have demonstrated that signals initiated through the TCR and two alternative costimulatory receptors can converge to synergistically regulate a common transactivating element (NF-κB) and yet differentially affect IκB cytosolic inhibitor–NF-κB complexes. Anti-CD3–IL-1 controls NF-κB nuclear localization by initiating the acute degradation of both IκBα and IκBβ and initiating the prolonged degradation of IκBα. In contrast, anti-CD3–anti-CD28 preferentially stimulates both the acute and the prolonged degradation of IκBα. Additional efforts will be needed to clarify the biologically relevant role of the different IκB inhibitors in normal T lymphocytes as part of the larger goal of designing novel therapeutic strategies that may control NF-κB transcriptional regulation in immune responses.
ACKNOWLEDGMENT
This work was supported by a grant from the American Cancer Society.
REFERENCES
- 1.Abraham R T, Ho S, Barna T, McKean D J. Transmembrane signalling during IL-1-dependent T cell activation: interaction of signal 1- and signal 2-type mediators with the phosphoinositide-dependent signal transduction mechanism. J Biol Chem. 1987;262:2719–2729. [PubMed] [Google Scholar]
- 2.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 3.Bankers-Fulbright J L, Kalli K J, McKean D J. IL-1 signal transduction. Life Sci. 1996;59:61–83. doi: 10.1016/0024-3205(96)00135-x. [DOI] [PubMed] [Google Scholar]
- 4.Brini A T, Harel-Bellan A, Farrar W L. Cyclosporin A inhibits induction of IL-2 receptor alpha chain expression by affecting activation of NF-κB-like factor(s) in cultured human T lymphocytes. Eur Cytokine Netw. 1990;1:131–139. [PubMed] [Google Scholar]
- 5.Chen A, Hagler J, Palombella V J, Melandri F, Scherer D, Ballard D, Maniatis T. Signal-induced site-specific phosphorylation targets IκB-α to the ubiquitin-A proteasome pathway. Genes Dev. 1995;9:1586–1597. doi: 10.1101/gad.9.13.1586. [DOI] [PubMed] [Google Scholar]
- 6.Chu Z-L, McKinsey T A, Liu L, Qi X, Ballard D W. Basal phosphorylation of the PEST domain in IκBβ regulates its functional interaction with the c-rel proto-oncogene product. Mol Cell Biol. 1996;16:5974–5984. doi: 10.1128/mcb.16.11.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dignam J D, Liebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrzanski P, Ryseck R P, Bravo R. Differential interactions of Rel-NF-kappa B complexes with I kappa B alpha determine pools of constitutive and inducible NF-kappa B activity. EMBO J. 1994;13:4608–4609. doi: 10.1002/j.1460-2075.1994.tb06782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser J D, Irving B A, Crabtree G R, Weiss A. Regulation of the interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science. 1991;251:313–316. doi: 10.1126/science.1846244. [DOI] [PubMed] [Google Scholar]
- 11.Ghosh P, Tan T-H, Rice N R, Sica A, Young H A. The interleukin 2 CD29-responsive complex contains at least three members of the NF-κB family: c-Rel, p50, and p65. Proc Natl Acad Sci USA. 1993;90:1696–1700. doi: 10.1073/pnas.90.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Y, Brasier A R. Mechanism for biphasic RelA-NF-κB1 nuclear translocation in tumor necrosis factor α-stimulated hepatocytes. J Biol Chem. 1997;272:9825–9832. doi: 10.1074/jbc.272.15.9825. [DOI] [PubMed] [Google Scholar]
- 13.Harhaj E W, Maggirwar S B, Good L, Sun S-C. CD28 mediates a potent costimulatory signal for rapid degradation of IκBβ which is associated with accelerated activation of various NF-κB/Rel heterodimers. Mol Cell Biol. 1996;16:6736–6743. doi: 10.1128/mcb.16.12.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyos B, Ballard D W, Bohnlein E, Siekevitz M, Greene W C. Kappa B-specific DNA binding proteins: role in the regulation of human interleukin-2 gene expression. Science. 1989;244:457–460. doi: 10.1126/science.2497518. [DOI] [PubMed] [Google Scholar]
- 15.Johnson D R, Douglas I, Jahnke A, Ghosh S, Pober J S. A sustained reduction in IκB-α may contribute to persistent NF-κB activation in human endothelial cells. J Biol Chem. 1996;271:16317–16322. doi: 10.1074/jbc.271.27.16317. [DOI] [PubMed] [Google Scholar]
- 16.June C H, Ledbetter J A, Gillespie M M, Lindsten T, Thompson C B. T-cell proliferation involving the CD28 pathway is associated with cyclosporin-resistant interleukin 2 gene expression. Mol Cell Biol. 1987;7:4472–4481. doi: 10.1128/mcb.7.12.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay J E, Benzie C R. /1990. T lymphocyte activation through the C28 (sic) pathway is insensitive to inhibition by the immunosuppressive drug FK-506. Immunol Lett. 1989;23:155–160. doi: 10.1016/0165-2478(89)90129-6. [DOI] [PubMed] [Google Scholar]
- 18.Köntgen F, Grumont R J, Strasser A, Metcalf D, Li R, Tarlington D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 19.Lai J H, Tan T H. CD28 signaling causes a sustained down-regulation of IκBα which can be prevented by the immunosuppressant rapamycin. J Biol Chem. 1994;269:30077–30080. [PubMed] [Google Scholar]
- 20.Le Bail O, Schmidt-Ullrich R, Israel A. Promoter analysis of the gene encoding the IκB-α/MAD3 inhibitor of NF-κB: positive regulation by members of the rel/NFκB family. EMBO J. 1993;12:5043–5049. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCaffrey P G, Kim P K, Valge-Archer V E, Sen R, Rao A. Cyclosporin A sensitivity of the NF-kappa B site of the IL2R alpha promoter in untransformed murine T cells. Nucleic Acids Res. 1994;22:2134–2142. doi: 10.1093/nar/22.11.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKean D J, Bell M, Huntoon C, Rastogi S, Van Norstrand M, Podzorski R, Nilson A, Paya C. IL-1 receptor and TCR signals synergize to activate NF-κB-mediated gene transcription. Int Immunol. 1995;7:9–20. doi: 10.1093/intimm/7.1.9. [DOI] [PubMed] [Google Scholar]
- 23.McKean D J, Huntoon C, Bell M. Ligand-induced desensitization of interleukin 1 receptor-initiated intracellular signaling events in T helper lymphocytes. J Exp Med. 1994;180:1321–1328. doi: 10.1084/jem.180.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mercurio F, DiDonato J A, Rosette C, Karin M. p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes Dev. 1993;7:705–718. doi: 10.1101/gad.7.4.705. [DOI] [PubMed] [Google Scholar]
- 25.Molitor J A, Walker W H, Doerre S, Ballard D W, Greene W C. NF-kappa B: a family of inducible and differentially expressed enhancer-binding proteins in human T cells. Proc Natl Acad Sci USA. 1990;87:10028–10032. doi: 10.1073/pnas.87.24.10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pimentel-Muinos F X, Mazana J, Fresno M. Biphasic control of nuclear factor-kappa B activation by the T cell receptor complex: role of tumor necrosis factor alpha. Eur J Immunol. 1995;25:179–186. doi: 10.1002/eji.1830250130. [DOI] [PubMed] [Google Scholar]
- 27.Rudd C E. Upstream-downstream: CD28 cosignaling pathways and T cell function. Immunity. 1996;4:527–534. doi: 10.1016/s1074-7613(00)80479-3. [DOI] [PubMed] [Google Scholar]
- 28.Su M S, Semerjian A. Activation of transcription factor NF kappa B in Jurkat cells is inhibited selectively by FK 506 in a signal-dependent manner. Transplant Proc. 1991;23:2912–2915. [PubMed] [Google Scholar]
- 29.Suyang H, Phillips R, Douglas I, Ghosh S. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol Cell Biol. 1996;16:5444–5449. doi: 10.1128/mcb.16.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson J E, Phillips R J, Erdjument-Bromage H, Tempst P, Ghosh S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 31.Traenckner E B, Pahl H L, Henkel T, Schmidt K N, Wilk S, Baeuerle P A. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 1995;14:2876–2883. doi: 10.1002/j.1460-2075.1995.tb07287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Wauwe J, Goossens J G. Monoclonal anti-human T lymphocyte antibodies: enumeration and characterization of T cell subsets. Immunology. 1981;42:157–164. [PMC free article] [PubMed] [Google Scholar]
- 33.Venkataraman L, Burakoff S J, Sen R. FK506 inhibits antigen receptor-mediated induction of c-Rel in B and T lymphoid cells. J Exp Med. 1995;181:1091–1099. doi: 10.1084/jem.181.3.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Verweij C L, Geerts M, Aarden L A. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-κB-like response element. J Biol Chem. 1991;266:14179–14182. [PubMed] [Google Scholar]
- 35.Wang W, Tam W F, Hughes C C W, Rath S, Sen R. c-Rel is a target of pentoxifylline-mediated inhibition of T-lymphocyte activation. Immunity. 1997;6:165–174. doi: 10.1016/s1074-7613(00)80423-9. [DOI] [PubMed] [Google Scholar]
- 36.Weil R, Laurent-Winter C, Israel A. Regulation of IκBβ degradation. J Biol Chem. 1997;272:9942–9949. doi: 10.1074/jbc.272.15.9942. [DOI] [PubMed] [Google Scholar]
- 37.Zhang L, Nabel G J. Positive and negative regulation of IL-2 gene expression: role of multiple regulatory sites. Cytokine. 1994;6:221–228. doi: 10.1016/1043-4666(94)90016-7. [DOI] [PubMed] [Google Scholar]