Abstract
This study aimed to investigate the effects of xylanase on growth performance and intestinal health of nursery pigs fed diets with reduced metabolizable energy (ME). One hundred ninety-two pigs at 8.7 kg ± 0.7 body weight (BW) after 7 d of weaning were allotted in a randomized complete block design with initial BW and sex as blocks. Eight dietary treatments consisted of 5 ME levels (3,400, 3,375, 3,350, 3,325, and 3,300 kcal ME/kg) below the NRC (2012) requirement and 4 levels of xylanase (0, 1,200, 2,400, and 3,600 XU/kg) to a diet with 3,300 kcal ME/kg. All pigs received their respective treatments for 35 d in 2 phases, pre-starter (14 d) and starter (21 d). On day 35, eight pigs in 3,400 kcal/kg (CON), 3,300 kcal/kg (LE), and 3,300 kcal/kg + 3,600 XU xylanase/kg (LEX) were euthanized to collect jejunal tissues and digesta for the evaluation of mucosa-associated microbiota, intestinal immune response, oxidative stress status, intestinal morphology, crypt cell proliferation, and digesta viscosity as well as ileal digesta to measure apparent ileal digestibility. Data were analyzed using the MIXED procedure on SAS 9.4. The LE increased (P < 0.05) jejunal digesta viscosity, tended to have decreased (P = 0.053) relative abundance of Prevotella, and tended to increase (P = 0.055) Lactobacillus. The LE also increased (P < 0.05) the concentration of protein carbonyl whereas malondialdehyde, villus height (VH), villus height to crypt depth ratio (VH:CD), apparent ileal digestibility (AID) of nutrients, and finally average daily feed intake were decreased (P < 0.05). The LE did not affect average daily gain (ADG). The LEX decreased (P < 0.05) digesta viscosity, increased (P < 0.05) the relative abundance of Prevotella, decreased (P < 0.05) Helicobacter, decreased (P < 0.05) the concentration of protein carbonyl, tended to increase (P = 0.065) VH, and decreased (P < 0.05) VH:CD and crypt cell proliferation. Moreover, LEX increased (P < 0.05) the AID of dry matter and gross energy and tended to increase (P = 0.099; P = 0.076) AID of crude protein, and ether extract. The LEX did not affect ADG but did tend to decrease (P = 0.070) fecal score during the starter phase. Overall, reducing ME negatively affected intestinal health parameters and nutrient digestibility without affecting growth. Supplementation of xylanase mitigated some of the negative effects observed by ME reduction on intestinal health and digestibility of nutrients without affecting growth.
Keywords: intestinal health, metabolizable energy, mucosa-associated microbiota, nursery pig, xylanase
Reduction of metabolizable energy through the removal of dietary fat can have detrimental effects on immune status, intestinal health, and mucosa-associated microbiota of nursery pigs. The supplementation of an exogenous xylanase in energy-reduced feeds up to 100 kcal ME/kg can mediate some of the negative effects of reduced dietary fat inclusion in nursery pigs.
Introduction
Highly digestible and nutrient-dense feedstuffs are commonly used in nursery pig feeds to combat the detrimental effects imposed by weaning stress in addition to compensating for the subsequent reduction of feed intake. Energy density in feeds is a critical component affecting feed intake, as an increase in energy level can decrease feed intake and a decrease in energy level can hamper growth and protein deposition (Southern et al., 1989). The requirement of metabolizable energy (ME) for pigs suggested by the NRC increased when the 11th revision was published (NRC, 1998, 2012). However, according to Tokach et al. (1995), varying ME levels in feeds did not affect the growth performance of newly weaned pigs. One practical method of increasing the energy in feeds is to include supplemental fats in the formulation. However, in the case of newly weaned pigs, their ability to digest typical supplemental fats is limited reaching only 65% to 80% whereas milk fat is highly digestible at 95% (Cera et al., 1988; Lauridsen, 2020). In addition, the growing demand for fats and oils by external industries such as biodiesel production (Toldrá-Reig et al., 2020), has increased the price and decreased the availability of typical supplemental fats that can be used in feeds (Mielke, 2018). Dietary fats go beyond just meeting the energy specification of the feed but become a source of fatty acids with specific functional roles in the body (Kim et al., 2007; Liu, 2015). Dietary supplementation of functional fatty acids including omega-3 series fatty acids, lysophospholipids, and conjugated linolenic acid has been shown to modulate the intestinal immune status, intestinal morphology, epithelial barrier function, and intestinal microbiota (Bassaganya-Riera et al., 2001; Jang et al., 2020; Lauridsen, 2020). In addition, increasing levels of dietary fat in feeds slow down the passage rate through the digestive tract, allowing relatively more time for increased digestion and absorption of other nutrients (Valaja and Siljander-Rasi, 2001). Poultry fat, the fat source used in this study, is composed of approximately 20% linoleic acid, a polyunsaturated fatty acid (PUFA) (NRC, 2012). Long-chain PUFA is a precursor of prostanoids, which can stimulate the recovery of intestinal barrier function (Ferrer and Moreno, 2010). In pigs, PUFA supplementation has been shown to improve the repair of previously damaged small intestines (Lopez-Pedrosa et al., 1998; López-Pedrosa et al., 1999; Jacobi et al., 2012).
Price volatility and availability of feedstuffs used in pig feeds drive the need to efficiently use readily available and economically favorable coproducts in formulation. Typically, increased inclusion of coproducts results in an increase in nonstarch polysaccharides (NSP) that are not digestible by endogenous enzymes of pigs and thus the supplementation of feed enzymes targeting specific types of NSP has been implemented (Kim and Baker, 2003; Adeola and Cowieson, 2011).
Common cereal grains and related coproducts contain variable amounts of NSP that can alter digesta viscosity in the small intestine and subsequently lead to morphological changes on the mucosal surface (Bedford and Classen, 1992; Lindberg, 2014), increased proliferation of potentially pathogenic microorganisms (Shakouri et al., 2009; Moita et al., 2022), and an increase or decrease in the retention time and passage rate of digesta depending on the type of NSP (Jørgensen et al., 1996; Wilfart et al., 2007; Schop et al., 2019; Chassé et al., 2023; Dorado-Montenegro et al., 2023). Xylanase targets the glycosidic bonds present in xylan, a structurally diverse family of NSP that share a β-1,4-linked xylopyranose backbone as a common feature (Scheller and Ulvskov, 2010). Arabinoxylan (AX) is one of the most common NSP present in cereal grains such as corn, wheat, barley, and their associated coproducts and is composed of a linear β-1,4 xylan backbone with α-1,3 and/or α-1,2-L-arabinofuranose branch points with varying degrees of substitution (Wang et al., 2020; Baker et al., 2021). Once ingested, AX can form viscous gels, increasing the viscosity of digesta and blocking the accessibility of endogenous digestive enzymes to feed particles entrapped in the viscous structure (McDonald et al., 2001; Passos et al., 2015; Duarte et al., 2019). Traditionally, xylanase has been used to increase the utilization of nutrients in the feed by lessening digesta viscosity as the xylanase breaks down the structural architecture of AX (Bedford and Schulze, 1998; Bedford, 2018). Past studies have indeed reported increases in nutrient digestibility with xylanase supplementation (Newman, 2014; Kiarie et al., 2016; Passos et al., 2023), in addition to increases in average daily gain (ADG) and feed conversion (Myers and Patience, 2014; Yang et al., 2016). Further research has elucidated functional roles of xylanase in pig feeds such as the ability of xylanase to beneficially modulate intestinal health by reducing the digesta viscosity (Passos et al., 2015; Tiwari et al., 2018), releasing prebiotic xylooligosaccharides and arabinoxylooligosaccharides (Masey-O’Neill et al., 2014; Dale et al., 2020), positively impacting the relative abundance and diversity of intestinal microbiota (Zhang et al., 2018; Chen et al., 2020; Moita et al., 2022), reducing inflammatory and oxidative damage products in the jejunum (Duarte et al., 2019; Petry et al., 2020), and thus improving intestinal integrity (Tiwari et al., 2018; Chen et al., 2020).
Based on previous findings, it is hypothesized that reducing ME by 100 kcal ME/kg feed through the reduction of supplemental fat during the nursery phase may negatively affect growth performance and intestinal health. It is also hypothesized that xylanase supplementation will mediate some of the negative effects on growth performance and intestinal health seen by the reduction of supplemental fat and thus reduced ME. To test this hypothesis, the objective of the study was to investigate the effects of xylanase supplementation on growth performance and intestinal health of nursery pigs fed diets with reduced ME.
Materials and Methods
The procedure of this study was reviewed and approved by North Carolina State University Animal Care and Use Committee (Raleigh, NC). The experiment was conducted at the Central Crops Research Station (Clayton, NC).
Experimental design, animals, and diets
One hundred and ninety-two newly weaned pigs (96 barrows and 96 gilts) at 7.3 ± 0.6 kg body weight (BW) were weaned at day 21 of age and fed a common early weaner diet for 7 d. After 7 d feeding, pigs reached 8.7 ± 0.7 kg BW and were allotted to eight dietary treatments in a randomized complete block design with initial BW and sex as blocks. Three pigs were housed per pen. The power test was completed to have 8 replicates per treatment, requiring 64 pens to handle eight dietary treatments. Dietary treatments consisted of diets with five levels of ME meeting or below NRC (2012) at 3,400 kcal ME/kg (CON), 3,375 kcal ME/kg, 3,350 kcal ME/kg, 3,325 kcal ME/kg, and 3,300 kcal ME/kg (LE) as well as diets with 4 levels of xylanase to LE (3,300 kcal ME/kg) at 0 XU/kg, 1,200 XU/kg, 2,400 XU/kg, and 3,600 XU/kg feed (LEX). All dietary treatments retained the same lysine to energy ratio. All pigs received their respective treatments for 35 d in two phases: pre-starter phase (14 d) and starter phase (21 d). Pigs had ad libitum access to water and feed during the entire 35 d period. Xylanase was supplied by CJ BIO (Seoul, Korea). All dietary treatments were offered in the form of mash feed. Titanium dioxide (0.4%) was mixed into the diet as an indigestible external marker and fed during the last 5 d of the starter phase.
Compositions of the experimental diets from all phases are shown in Table 1. All experimental diets were produced at Feed Mill Educational Unit (Raleigh, NC) at North Carolina State University. All experimental diets were sampled and sent to the North Carolina Department of Agriculture and Consumer Services for proximate analysis of nutrient composition.
Table 1.
Composition of experimental diets with varying metabolizable energy (ME)
| ME1, kcal/kg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | Early weaner | Pre-starter | Starter | ||||||||
| Basal | 3,400 | 3,375 | 3,350 | 3,325 | 3,3002 | 3,400 | 3,375 | 3,350 | 3,325 | 3,3002 | |
| Feedstuff, % | |||||||||||
| Corn, yellow dent | 23.6 | 27.5 | 28.0 | 28.5 | 29.0 | 29.5 | 32.7 | 33.2 | 33.7 | 34.2 | 34.7 |
| Wheat | 15.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 25.0 | 25.0 | 25.0 | 25.0 | 25.0 |
| Whey permeate | 24.0 | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Soybean meal | 17.0 | 24.0 | 24.0 | 24.0 | 24.0 | 24.0 | 27.0 | 27.0 | 27.0 | 27.0 | 27.0 |
| Poultry meal | 10.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | — | — | — | — | — |
| Blood plasma | 6.0 | — | — | — | — | — | — | — | — | — | — |
| Fish meal | — | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | — | — | — | — | — |
| Poultry fat | 2.0 | 3.0 | 2.5 | 2.0 | 1.5 | 1.0 | 2.5 | 2.0 | 1.5 | 1.0 | 0.50 |
| L-Lys HCl | 0.50 | 0.56 | 0.56 | 0.56 | 0.56 | 0.56 | 0.47 | 0.47 | 0.47 | 0.47 | 0.47 |
| L-Met | 0.22 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| L-Thr | 0.15 | 0.18 | 0.18 | 0.18 | 0.18 | 0.18 | 0.14 | 0.14 | 0.14 | 0.14 | 0.14 |
| L-Val | 0.00 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Dicalcium phosphate | 0.10 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.65 | 0.65 | 0.65 | 0.65 | 0.65 |
| Limestone, ground | 0.80 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Vitamin premix3 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 |
| Mineral premix4 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Salt | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| ZnO | 0.25 | — | — | — | — | — | — | — | — | — | — |
| BMD30 | 0.03 | — | — | — | — | — | — | — | — | — | — |
| Calculated composition | |||||||||||
| Dry matter, % | 91.07 | 90.52 | 90.46 | 90.40 | 90.34 | 90.28 | 89.85 | 89.79 | 89.73 | 89.67 | 89.61 |
| ME, kcal/kg | 3,409 | 3,401 | 3,376 | 3,351 | 3,326 | 3,301 | 3,357 | 3,332 | 3,307 | 3,282 | 3,258 |
| Crude protein, % | 24.74 | 21.71 | 21.76 | 21.80 | 21.84 | 21.88 | 20.11 | 20.15 | 20.20 | 20.24 | 20.28 |
| Crude fiber, % | — | — | — | — | — | — | 1.72 | 1.73 | 1.74 | 1.75 | 1.76 |
| SID5 Lys, % | 1.50 | 1.35 | 1.35 | 1.35 | 1.36 | 1.36 | 1.23 | 1.23 | 1.23 | 1.23 | 1.23 |
| SID Met + Cys, % | 0.82 | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 | 0.68 | 0.69 | 0.69 | 0.69 | 0.69 |
| SID Thr, % | 0.89 | 0.79 | 0.80 | 0.80 | 0.80 | 0.80 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 |
| SID Trp, % | 0.26 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| SID Val, % | 1.01 | 0.86 | 0.86 | 0.86 | 0.87 | 0.87 | 0.78 | 0.79 | 0.79 | 0.79 | 0.79 |
| SID Ile, % | 0.81 | 0.75 | 0.75 | 0.75 | 0.75 | 0.75 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 |
| Ca, % | 0.86 | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 |
| STTD6 P, % | 0.46 | 0.40 | 0.40 | 0.40 | 0.41 | 0.41 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 |
| Total P, % | 0.68 | 0.63 | 0.63 | 0.64 | 0.64 | 0.64 | 0.56 | 0.56 | 0.56 | 0.56 | 0.56 |
| Analyzed composition | |||||||||||
| Dry matter, % | 91.24 | 89.98 | 89.95 | 89.84 | 89.80 | 89.73 | 88.23 | 88.73 | 88.62 | 88.44 | 88.40 |
| Gross energy, kcal | 4,175 | 4,120 | 4,131 | 4,108 | 4,086 | 4,019 | 4,069 | 4,040 | 4,022 | 4,009 | 3,921 |
| Crude protein, % | 23.37 | 21.60 | 20.12 | 20.18 | 20.41 | 19.80 | 18.74 | 18.77 | 18.93 | 18.82 | 19.03 |
| Crude fiber, % | — | — | — | — | — | — | 2.47 | 2.33 | 2.61 | 1.10 | 1.06 |
| NDF, % | 6.77 | 6.75 | 6.66 | 6.09 | 6.80 | 5.68 | 6.70 | 7.02 | 6.56 | 6.37 | 6.34 |
| ADF, % | 3.25 | 3.04 | 3.09 | 2.84 | 3.17 | 3.35 | 2.49 | 2.83 | 2.67 | 2.36 | 2.31 |
| Ca, % | 0.95 | 0.87 | 0.76 | 0.79 | 0.80 | 0.80 | 0.79 | 0.70 | 0.71 | 0.72 | 0.67 |
| P, % | 0.66 | 0.58 | 0.55 | 0.59 | 0.58 | 0.55 | 0.50 | 0.50 | 0.50 | 0.51 | 0.50 |
1Metabolizable energy level at 3,400 kcal/kg (CON), 3,375 kcal/kg, 3,350 kcal/kg, 3,325 kcal/kg, and 3,300 (LE) kcal/kg of feed.
2Xylanase supplemented at 0, 1,200, 2,400, and 3,600 XU/kg (LEX) to a diet with 3,300 kcal/kg (LE).
3The vitamin premix provided per kilogram of complete diet: 6,614 IU of vitamin A as vitamin A acetate, 992 IU of vitamin D3, 19.8 IU of vitamin E, 2.64 mg of vitamin K as menadione sodium bisulfate, 0.03 mg of vitamin B12, 4.63 mg of riboflavin, 18.52 mg of D-pantothenic acid as calcium panthonate, 24.96 mg of niacin, and 0.07 mg of biotin.
4The trace mineral premix provided per kilogram of complete diet: 33 mg of Mn as manganous oxide, 110 mg of Fe as ferrous sulfate, 110 mg of Zn as zinc sulfate, 16.5 mg of Cu as copper sulfate, 0.30 mg of I as ethylenediamine dihydroiodide, and 0.30 mg of Se as sodium selenite.
5Standardized ileal digestible.
6Standardized total tract digestible phosphorus.
Experimental procedures and sample collection
The BW and feed intake were recorded every 7 d to calculate average BW, ADG, average daily feed intake (ADFI), and gain:feed (G:F). Fecal scores were recorded every 3 d based on a 1 to 5 scale: (1) very hard and dry feces, (2) firm stool, (3) normal stool, (4) loose stool, and (5) watery stool with no shape as described in Weaver and Kim (2014).
At the end of day 35 of feeding, a total of 24 pigs in three dietary treatments were selected for further research to investigate the intestinal health. Specifically, one pig representing a median BW of each pen in 3,400 kcal ME/kg (CON), 3,300 kcal ME/kg (LE), and 3,300 kcal ME/kg + 3,600 XU xylanase/kg (LEX) were selected and euthanized by a captive bolt gun followed by exsanguination and removal of the gastrointestinal tract for sample collection. Mid-jejunum segments (3 m after the pyloric duodenal junction) were taken digesta was collected in 50-mL Falcon tubes to measure digesta viscosity. Mucosal samples from the mid-jejunum were scraped by a glass slide and collected in Eppendorf tubes (2 mL), then put into liquid nitrogen immediately and stored at −80 °C for subsequent mucosa-associated microbiota measurements. Mid-jejunum tissues were rinsed with 0.9% saline solution and collected in a 50-mL Falcon tube with 10% buffered formaldehyde to evaluate histology. Ileal digesta was collected in a 100 mL container and put on the ice, then stored at −20 °C for measurement of apparent ileal digestibility (AID) of nutrients.
Digesta Viscosity
Following the procedure by Passos et al. (2015) and Duarte et al. (2019), samples of jejunum digesta from 50 mL tubes were divided into 2 falcon tubes (15 mL) and centrifuged at 1,000 × g at 4 °C for 10 min to obtain the liquid phase. The liquid phase was then removed and transferred to an Eppendorf tube (2 mL) to centrifuge at 10,000 × g at 4 °C for 10 min. The supernatant obtained was transferred to another Eppendorf tube (1.5 mL) for further measurement. About 0.5 mL of digesta supernatant was placed in the viscometer (Brookfield Digital Viscometer, Model DV-II Version 2.0, Brookfield Engineering Laboratories Inc., Stoughton, MA), set at 25 °C. The viscosity measurement was the average between 45.0 s−1 and 22.5 s−1 shear rates, and the viscosity values were recorded as apparent viscosity in centipoise (cP).
Relative abundance and diversity of jejunal mucosa-associated microbiota
Mid-jejunum mucosa samples were utilized for mucosa-associated microbiome sequencing using 16S rRNA gene sequence analysis. The DNA was extracted from the mucosa samples using the DNA Stool Mini Kit (#51604, Qiagen; Germantown, MD) as previously described by Duarte et al. (2020). Extracted DNA samples were then sent to the Genomics Department of Mako Medical Laboratories (Raleigh, NC) for 16S rRNA gene sequencing. In short, extracted DNA samples were prepared for the template on an Ion Chef and then sequenced on the Ion S5 system (Thermo Fisher Scientific Inc., Waltham, MA, USA). The variable regions analyzed were V2, V3, V4, V6, V7, V8, and V9 of the 16S rRNA gene and amplified via Ion 16S Metagenomics Kit (Thermo Fisher Scientific Inc.). Hypervariable regions were processed using Torrent Suite software (version 5.2.2) (Thermo Fisher Scientific Inc.) to produce.bam files for further analysis. The taxonomy was assigned against the GreenGenes (anybody) and MicroSeq (experts) databases, specific primers for microbiota. Alpha diversity rare fraction plot generation, and the Operational Taxonomic Unit (OTU) table generation were performed by the Ion Reporter Software Suite (version 5.2.2) of bioinformatics analysis tools (Thermo Fisher Scientific Inc.) with 98% similarity. The alpha diversity was calculated using Chao1 index (Chao, 1984), Shannon index (Shannon and Weaver, 1949), and Simpson index (Simpson, 1949). The Ion Reporter’s Metagenomics 16S workflow powered by Qiime (version w1.1) was used to analyze the samples. The depth of sequencing coverage was >1,000× sample preparation. To initiate the statistical analysis of the microbiota, OTU data were transformed to relative abundance as previously described by Kim et al. (2019). The OTU with a relative abundance <0.5% within each level were combined as “Others”.
Inflammatory cytokines, immunoglobulins, and oxidative damage products
Jejunal mucosa samples were weighed (1 g) and suspended in 1 mL of phosphate-buffered saline (PBS) on ice, then homogenized using a tissue homogenizer (Tissuemiser; Thermo Fisher Scientific Inc.). Following Holanda and Kim (2021), the processed samples were then transferred into a new 2 mL microcentrifuge tube and centrifuged at 14,000 × g for 15 min. The supernatant was pipetted into five aliquots and stored at −80 °C.
The concentration of total protein, interleukin-8 (IL-8), tumor necrosis factor-alpha (TNF-α), immunoglobulin G (IgG), immunoglobulin A (IgA), protein carbonyl, and malondialdehyde (MDA) were measured by using commercial kits based on the instruction manual. The OD value was read by the ELISA plate reader (Synergy HT, BioTek Instruments, Winooski, VT) and software (Gen5 Data Analysis Software, BioTek Instruments). The corresponding concentrations were calculated according to the absorbance of the standard curves and instruction manual.
The homogenized mucosal supernatant was diluted (1:60) in PBS to get the appropriate range (20 to 2000 μg/mL), and then the total protein concentration was measured by using Pierce BCA Protein Assay Kit (#23225, Thermo Fisher Scientific Inc.) as described by Holanda et al. (2020). The absorbance was measured at 562 nm and the concentration of total protein were further used to normalize the concentration of other measurements in mucosa. The concentration of IL-8 was measured by using Porcine IL-8/CXCL8 DuoSet ELISA kit (#DY535, R&D Systems) as described by Jang and Kim (2019). All samples were diluted in reagent diluent to 1:5 to analyze. Absorbance was read at 450 nm and corrected at 570 nm. The TNF-α concentration was measured by using the following Porcine TNF-α Immunoassay Kit (#PTA00, R&D Systems, Minneapolis, MN, USA) as described by Cheng et al. (2021). Absorbance was read at 450 nm and corrected at 570 nm. The concentration of TNF-α was expressed as pg/mL protein. The concentration of IgA and IgG was measured by using the ELISA kits (E101-102 and E101-104, Bethyl Laboratories, Inc., Montgomery, TX) as described by Holanda et al., (2020). The mucosal supernatants were diluted with PBS to 1:1200 and 1:2400, respectively, to get the appropriate working range for measurement. Absorbance was read at 450 nm and the concentration was expressed as μg/mg of protein. The concentration was expressed as pg/mL protein. Protein carbonyl was measured by using OxiSelect Protein Carbonyl ELISA Kit (#STA-310, Cell Biolabs, Inc., San Diego, CA, USA) as described by Moita et al. (2021). All supernatants were diluted in PBS to get 10 µg/mL before measurement. The standard was prepared that range was from 0.375 to 7.5 nmol/mg protein. All processes are conducted following the manufacturer’s protocol. The absorbance was measured at 450 nm and the concentration was described as nmol/mg protein. The concentration of MDA in mucosa was measured by using OxiSelect TBARS MDA Quantitation Assay Kit (#STA-330, Cell Biolabs, Inc.) as described by Moita et al. (2021). The working range of the standard is from 0.98 to 125 µM/L. The absorbance was read under 532 nm wavelength. The concentration was calculated according to the standard and expressed as µmol/mg protein.
Intestinal morphology and Ki-67 in crypt cells
Two sections of mid jejunum per pig were fixed in 10% formalin and then transferred to a 70% ethanol solution for 2 d. The processed samples were sent to North Carolina State University Histology Laboratory (College of Veterinary Medicine, Raleigh, NC) for dehydration, embedment, staining, and immunohistochemistry of Ki-67 proteins. Villus height (VH), villus width, and crypt depth (CD) were measured using a microscope Olympus CX31 (Lumenera Corporation, Ottawa, Canada) with software Infinity 2-2 digital CCD. In each slide, 10 intact villi and their associated crypts were measured as described by Cheng et al. (2021). The villus length was measured from the top of the villus to the junction of villus and crypt; the villus width was measured in the middle of the villus; the crypt depth was measured from the junction of villus and crypt to the bottom of the crypt. The villus height to crypt depth (VH:CD) ratio was calculated using the villus height divided by the CD. Images of 10 intact crypts from each slide were cropped, and the ImageJS software was used for calculating the percentage of Ki-67 positive cells to total cells in the crypt and to count the number of Ki-67 positive cells per crypt. All analyses of the intestinal morphology were executed by the same person. The averages of the 10 measurements per pig were calculated and reported as one number per pig.
Apparent ileal digestibility
Titanium dioxide was added at an inclusion rate of 0.4% to starter diets to serve as an indigestible marker to determine the AID of nutrients. Ileal digesta were freeze-dried for 48 h (24D 48, Virtis, Gardiner, NY). The concentration of titanium dioxide in the feed and digesta were measured based on the approach of Myers et al. (2004). The feed and digesta samples were used to measure the content of dry matter (DM, method 934.01), crude fiber (method 978.10), and ether extract (EE, method 2003.06) were measured based on AOAC (2006). Gross energy (GE) was measured using a bomb calorimeter (Model 6200, Parr Instrument Company, Moline, IL). The nitrogen content was measured using TruSpec N Nitrogen Determinator (LECO CN-2000, LECO Corp., St. Joseph, MI) and the CP concentration was calculated (6.25 × N). The AID of DM, GE, EE, and CP were calculated by using the following function:
Which TiO2feed and TiO2digesta were the measured concentration of titanium dioxide in the feed and in the digesta; Nutrientdigesta and Nutrientfeed were the measured concentration of nutrient in the digesta and in the feed as previously described by Moita et al. (2021).
Statistical analysis
Data were analyzed with MIXED procedure by using SAS 9.4 (SAS Inc., Cary, NC, USA). Main effect was dietary treatment, considered a fixed effect. Initial BW and sex were blocks which were considered random effects. The experimental unit was the pen for growth performance and fecal score data. The linear and quadratic effects of reducing ME levels and increasing xylanase levels were tested using polynomial contrasts. A preplanned contrast was executed using the CONTRAST statement to analyze the effects ME (ME 3,400 vs. others) and xylanase supplementation (0 vs. others). The individual pig representing the median BW of each pen from the selected treatments (CON, LE, and LEX) was the experimental unit for all additional measurements. A preplanned contrast was executed using the CONTRAST statement to analyze the effects of ME (CON vs. LE) and xylanase supplementation (LE vs. LEX). Results were considered statistically significant when P value was less than 0.05 and considered a tendency when P value was between 0.05 and 0.10.
Results
Digesta viscosity
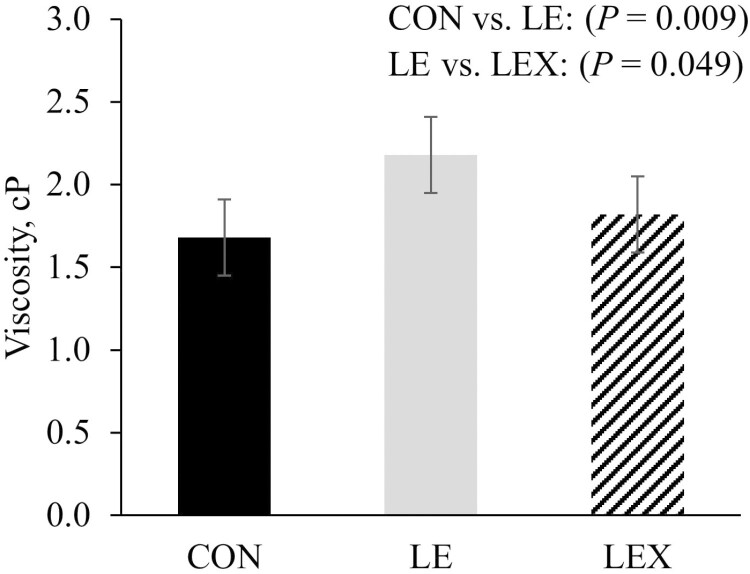
The viscosity of jejunal digesta was increased (P < 0.05) by LE, whereas was decreased (P < 0.05) by LEX (Figure 1).
Figure 1.
Changes in the viscosity of jejunal digesta in nursery pigs fed diets with reduced ME and xylanase supplementation. CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
Diversity and relative abundance of jejunal mucosa-associated microbiota
At the family level, LE had no effect on Chao1 α-diversity, but tended to increase (P = 0.088) by LEX (Table 2). The LE tended to increase Simpson index at family (P = 0.058) and genus (P = 0.087) levels. The Shannon index was not affected by the treatments. Relative abundance of Bacteroidetes tended to be reduced (P = 0.071) by LE compared to CON, whereas it was increased (P < 0.05) by LEX (Table 3). Relative abundance of Proteobacteria was not affected by LE, whereas it was reduced (P < 0.05) by LEX. Relative abundance of Prevotellaceae tended to be reduced (P = 0.062) by LE compared to CON, whereas it was increased (P < 0.05) by LEX (Table 4). Relative abundance of Helicobacteraceae was not affected by LE, whereas it tended to be reduced (P = 0.072) by LEX. Relative abundance of Prevotella tended to be reduced (P = 0.053) by LE, whereas it was increased (P < 0.05) by LEX. Relative abundance of Helicobacter was unaffected by LE; however, tended to be reduced (P = 0.076) by LEX (Table 5). At the species level, LE had no effect on the relative abundance of Prevotella copri, whereas it was increased (P < 0.05) by LEX (Table 6). Interestingly, LE tended to increase the relative abundance of Lactobacillus kitasatonis (P = 0.073) and Lactobacillus delbrueckii (P = 0.054), whereas xylanase supplementation decreased (P < 0.05) the relative abundance of L. delbrueckii.
Table 2.
α-Diversity of jejunal mucosa-associated microbiota at the family and genus level estimated with Chao1 richness, Shannon diversity, and Simpson diversity in nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| Family | ||||||
| Chao1 | 6.54 | 7.02 | 10.70 | 1.81 | 0.823 | 0.088 |
| Shannon | 0.80 | 1.18 | 1.34 | 0.28 | 0.106 | 0.471 |
| Simpson | 0.37 | 0.58 | 0.58 | 0.13 | 0.058 | 1.000 |
| Genus | ||||||
| Chao1 | 6.00 | 5.57 | 7.86 | 1.61 | 0.794 | 0.142 |
| Shannon | 0.76 | 1.02 | 1.03 | 0.20 | 0.196 | 0.965 |
| Simpson | 0.35 | 0.54 | 0.48 | 0.11 | 0.087 | 0.540 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
Table 3.
Relative abundance of jejunal mucosa-associated microbiota at the phylum level in nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| Bacteroidetes | 42.09 | 21.54 | 62.54 | 8.92 | 0.071 | 0.001 |
| Proteobacteria | 40.04 | 51.52 | 7.26 | 16.41 | 0.301 | 0.001 |
| Firmicutes | 16.32 | 25.52 | 24.14 | 7.81 | 0.258 | 0.839 |
| Cyanobacteria | 1.41 | 0.46 | 0.00 | 0.62 | 0.179 | 0.431 |
| Deferribacteres | 0.93 | 0.15 | 3.43 | 1.47 | 0.608 | 0.022 |
| Lentisphaerae | 0.00 | 0.00 | 0.07 | 0.03 | 1.000 | 0.112 |
| Tenericutes | 0.00 | 0.00 | 0.96 | 0.36 | 0.927 | 0.019 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
Table 4.
Relative abundance of jejunal mucosa-associated microbiota at the family level in nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| Prevotellaceae | 42.03 | 20.22 | 61.56 | 9.51 | 0.062 | 0.001 |
| Helicobacteraceae | 29.83 | 31.89 | 5.21 | 14.70 | 0.900 | 0.072 |
| Lactobacillaceae | 7.01 | 19.11 | 9.40 | 3.97 | 0.086 | 0.104 |
| Veillonellaceae | 5.57 | 4.20 | 7.47 | 2.90 | 0.463 | 0.053 |
| Enterobacteriaceae | 4.31 | 5.48 | 0.27 | 4.49 | 0.796 | 0.190 |
| Lachnospiraceae | 2.58 | 0.12 | 2.55 | 1.05 | 0.136 | 0.089 |
| Succinivibrionaceae | 2.07 | 0.00 | 0.72 | 0.75 | 0.099 | 0.480 |
| Campylobacteraceae | 1.24 | 12.07 | 1.08 | 5.20 | 0.231 | 0.159 |
| Clostridiaceae | 0.94 | 1.37 | 1.02 | 0.85 | 0.652 | 0.666 |
| Deferribacteraceae | 0.93 | 0.15 | 3.43 | 1.47 | 0.609 | 0.022 |
| Brachyspiraceae | 0.84 | 0.92 | 0.33 | 0.54 | 0.926 | 0.410 |
| Streptococcaceae | 0.37 | 0.59 | 1.28 | 0.48 | 0.775 | 0.299 |
| Bacteroidaceae | 0.00 | 1.41 | 0.15 | 0.90 | 0.360 | 0.338 |
| Moraxellaceae | 0.00 | 1.84 | 0.43 | 1.16 | 0.257 | 0.351 |
| Others | 4.27 | 1.79 | 5.21 | 2.1 | 0.399 | 0.182 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
Table 5.
Relative abundance of jejunal mucosa-associated microbiota at the Genus level in nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| Prevotella | 49.32 | 23.49 | 68.66 | 10.26 | 0.053 | 0.001 |
| Helicobacter | 33.67 | 34.38 | 6.75 | 14.89 | 0.967 | 0.076 |
| Lactobacillus | 9.19 | 26.98 | 10.66 | 5.09 | 0.055 | 0.041 |
| Succinivibrio | 2.32 | 0.00 | 0.86 | 1.13 | 0.102 | 0.447 |
| Campylobacter | 1.60 | 4.94 | 1.46 | 2.69 | 0.341 | 0.248 |
| Brachyspira | 1.05 | 0.38 | 0.50 | 0.72 | 0.324 | 0.830 |
| Clostridium | 0.82 | 1.96 | 1.25 | 1.29 | 0.395 | 0.534 |
| Megasphaera | 0.74 | 2.60 | 1.43 | 1.29 | 0.269 | 0.408 |
| Roseburia | 0.66 | 0.06 | 1.58 | 0.84 | 0.567 | 0.107 |
| Mucispirillum | 0.57 | 0.11 | 2.26 | 1.36 | 0.729 | 0.074 |
| Streptococcus | 0.53 | 0.74 | 1.44 | 0.73 | 0.478 | 0.235 |
| Acinetobacter | 0.00 | 2.78 | 0.56 | 2.22 | 0.270 | 0.336 |
| Pseudomonas | 0.00 | 1.47 | 0.21 | 1.26 | 0.358 | 0.359 |
| Others | 1.96 | 1.19 | 2.17 | 1.30 | 0.622 | 0.462 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
Table 6.
Relative abundance of jejunal mucosa-associated microbiota at the Species level in nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| Prevotella copri | 52.46 | 28.26 | 65.61 | 11.21 | 0.164 | 0.028 |
| Helicobacter mastomyrinus | 19.69 | 23.70 | 4.28 | 8.12 | 0.770 | 0.138 |
| Prevotella stercorea | 5.66 | 5.37 | 12.42 | 3.35 | 0.960 | 0.187 |
| Helicobacter rappini | 13.06 | 7.72 | 1.37 | 6.21 | 0.096 | 0.267 |
| Lactobacillus kitasatonis | 2.87 | 11.30 | 1.75 | 2.87 | 0.073 | 0.029 |
| Campylobacterhyo intestinalis | 0.41 | 0.03 | 0.77 | 0.34 | 0.440 | 0.107 |
| Mucispirillum schaedleri | 0.00 | 0.17 | 3.32 | 1.68 | 0.940 | 0.136 |
| Roseburia faecis | 1.03 | 0.13 | 2.49 | 1.05 | 0.613 | 0.162 |
| Lactobacillus delbrueckii | 0.00 | 3.78 | 0.06 | 1.31 | 0.054 | 0.040 |
| Acinetobacter lwoffii | 0.00 | 2.90 | 0.00 | 1.75 | 0.220 | 0.205 |
| Brachyspira hampsonii | 1.30 | 0.96 | 0.59 | 1.09 | 0.744 | 0.697 |
| Lactobacillus mucosae | 0.18 | 1.57 | 0.22 | 0.79 | 0.139 | 0.112 |
| Others | 1.96 | 1.19 | 2.17 | 1.30 | 0.622 | 0.462 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
Intestinal inflammatory status, humoral immune status, and oxidative stress status
The concentrations of pro-inflammatory cytokines (IL-8 and TNF-a) and immunoglobulins (IgA and IgG) in the jejunal mucosa were not affected by LE or LEX (Table 7). Protein carbonyl concentration in the jejunal mucosa was increased (P < 0.05) by LE, whereas it was reduced (P < 0.05) by LEX. Malondialdehyde (MDA) concentration in the jejunal mucosa was reduced (P < 0.05) by LE, whereas it was not influenced by LEX.
Table 7.
Pro-inflammatory cytokines, oxidative damage products, and immunoglobulins in the jejunal mucosa (mg protein) of pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| Unit/mg protein | ||||||
| IL-82, ng | 0.80 | 0.62 | 0.65 | 0.08 | 0.137 | 0.792 |
| TNF-α3, pg | 1.17 | 1.20 | 0.95 | 0.33 | 0.942 | 0.535 |
| PC4, nmol | 0.18 | 0.40 | 0.17 | 0.04 | 0.033 | 0.049 |
| MDA5, nmol | 0.70 | 0.39 | 0.40 | 0.10 | 0.036 | 0.928 |
| IgG6, µg | 2.41 | 2.74 | 2.74 | 0.52 | 0.537 | 0.993 |
| IgA7, µg | 6.50 | 7.72 | 7.08 | 0.35 | 0.572 | 0.766 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
2Interlukin-8.
3Tumor necrosis factor α.
4Protein carbonyl.
5Malondialdehyde.
6Immunoglobulin A.
7Immunoglobulin G.
Intestinal morphology and crypt cell proliferation
VH was reduced (P < 0.05) by LE, whereas it tended to be increased (P = 0.065) by LEX (Table 8). Crypt depth (CD) was not influenced by LE, whereas it was reduced (P < 0.05) by LEX. VH:CD was reduced (P < 0.05) by LE, whereas it increased (P < 0.05) by LEX. Percentage of proliferating crypt cells was not influenced by LE and LEX. However, the number of proliferating cells in a crypt was reduced (P < 0.05) by LEX.
Table 8.
Morphology of jejunum in nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| Villus height, µm | 467 | 433 | 462 | 14.73 | 0.032 | 0.065 |
| Crypt depth, µm | 264 | 263 | 238 | 8.47 | 0.916 | 0.039 |
| VH:CD2 | 1.80 | 1.64 | 1.99 | 0.12 | 0.041 | 0.022 |
| Ki-67+3, % | 26.1 | 27.5 | 24.3 | 0.02 | 0.548 | 0.160 |
| Ki-67+4, count | 78.3 | 82.8 | 69.9 | 3.1 | 0.324 | 0.009 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
2Villus height to crypt depth ratio.
3Ratio of Ki-67 positive cells to total cells in the crypt.
4Number of Ki-67 positive cells in the crypt.
Apparent ileal digestibility of nutrients
Apparent ileal digestibility of DM, GE, CP, and EE was reduced (P < 0.05) by LE, whereas digestibility of DM and GE was increased (P < 0.05) by LEX and digestibility of CP (P = 0.099), EE (P = 0.076), and crude fiber (P = 0.060) tended to be increased by LEX (Table 9).
Table 9.
AID of nutrients in nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment1 | CON | LE | LEX | P value | ||
|---|---|---|---|---|---|---|
| ME/xylanase | 3,400/0 | 3,300/0 | 3,300/3,600 | SEM | CON vs. LE | LE vs. LEX |
| AID2 of nutrients, % | ||||||
| Dry matter | 59.52 | 47.28 | 58.66 | 3.80 | 0.008 | 0.019 |
| Gross energy | 59.14 | 42.98 | 56.00 | 3.48 | 0.001 | 0.001 |
| Crude protein | 63.40 | 44.63 | 57.15 | 5.13 | 0.012 | 0.099 |
| Crude fiber | 67.73 | 62.44 | 69.10 | 3.31 | 0.102 | 0.060 |
| Ether extract | 67.73 | 55.22 | 64.39 | 4.84 | 0.013 | 0.076 |
1CON: metabolizable energy at 3,400 kcal ME/kg feed; LE: metabolizable energy at 3,300 kcal ME/kg feed; LEX: metabolizable energy at 3,300 kcal ME/kg feed and xylanase supplementation (3,600 XU/kg feed).
2Apparent ileal digestibility.
Growth performance and fecal score
BW, ADG, and G:F were not affected during any of the experimental periods by reducing energy up to 100 kcal ME/kg of feed or the supplementation of xylanase to LE (Table 10). Pigs fed the CON treatment had increased (P < 0.05) ADFI compared to the 3,375 ME kcal/kg, 3,350 ME kcal/kg, and LE (3,300 ME kcal/kg) treatments during the pre-starter phase and tended to have higher (P = 0.064) ADFI for the overall period compared to 3,375 ME kcal/kg and 3,350 ME kcal/kg treatments. Reducing energy up to 100 kcal ME/kg of feed had no effect on fecal score throughout the trial, however, increasing xylanase supplementation to LE tended to decrease (P = 0.070) the fecal score during starter phase (Table 11).
Table 10.
Effects of energy contents on growth performance in nursery pigs
| Treatment | ME1, kcal/kg | Xylanase2, XU/kg | P value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | 3,400 | 3,375 | 3,350 | 3,325 | 3,300 | 1,200 | 2,400 | 3,600 | SEM | ME linear3 | ME quad3 | ME 3,400 vs. others3 | Xylanase linear4 | Xylanase quad4 | LE vs. xylanase4 |
| BW, kg | |||||||||||||||
| Initial | 7.3 | 7.3 | 7.3 | 7.3 | 7.3 | 7.3 | 7.3 | 7.3 | 0.6 | 0.972 | 0.980 | 0.991 | 0.982 | 0.990 | 0.980 |
| Day 7 | 8.8 | 8.6 | 8.9 | 8.6 | 8.7 | 8.6 | 8.4 | 8.8 | 0.7 | 0.853 | 0.900 | 0.658 | 0.906 | 0.173 | 0.577 |
| Day 14 | 10.9 | 10.5 | 11.1 | 10.6 | 10.5 | 10.5 | 10.7 | 10.7 | 1.1 | 0.471 | 0.708 | 0.440 | 0.620 | 0.949 | 0.820 |
| Day 21 | 14.0 | 12.6 | 13.7 | 13.3 | 13.8 | 12.9 | 13.3 | 13.6 | 1.2 | 0.833 | 0.207 | 0.225 | 0.953 | 0.233 | 0.358 |
| Day 28 | 18.7 | 17.2 | 18.4 | 17.9 | 17.7 | 17.1 | 17.6 | 18.0 | 1.5 | 0.431 | 0.664 | 0.149 | 0.566 | 0.397 | 0.859 |
| Day 35 | 23.0 | 21.4 | 22.5 | 21.8 | 22.3 | 21.7 | 21.9 | 22.2 | 2.0 | 0.639 | 0.407 | 0.232 | 0.980 | 0.520 | 0.686 |
| ADG, g | |||||||||||||||
| Pre-starter | 260 | 227 | 275 | 237 | 232 | 227 | 244 | 241 | 35 | 0.368 | 0.633 | 0.326 | 0.533 | 0.950 | 0.775 |
| Starter | 574 | 522 | 540 | 532 | 558 | 533 | 531 | 550 | 45 | 0.779 | 0.175 | 0.204 | 0.823 | 0.387 | 0.494 |
| Overall | 449 | 404 | 434 | 414 | 427 | 410 | 417 | 426 | 40 | 0.609 | 0.368 | 0.192 | 0.975 | 0.496 | 0.670 |
| ADFI, g | |||||||||||||||
| Pre-starter | 600a | 535b | 590a | 547b | 552b | 547 | 560 | 560 | 37 | 0.116 | 0.500 | 0.025 | 0.642 | 0.895 | 0.858 |
| Starter | 860 | 744 | 837 | 807 | 812 | 762 | 805 | 823 | 78 | 0.759 | 0.368 | 0.127 | 0.615 | 0.329 | 0.707 |
| Overall | 838a | 746b | 808ab | 774ab | 783ab | 755 | 778 | 788 | 67 | 0.365 | 0.327 | 0.064 | 0.765 | 0.500 | 0.774 |
| G/F | |||||||||||||||
| Pre-starter | 0.45 | 0.42 | 0.46 | 0.42 | 0.42 | 0.41 | 0.43 | 0.43 | 0.04 | 0.462 | 0.623 | 0.571 | 0.631 | 0.772 | 0.990 |
| Starter | 0.67 | 0.70 | 0.65 | 0.66 | 0.69 | 0.70 | 0.66 | 0.67 | 0.02 | 0.977 | 0.359 | 0.772 | 0.265 | 0.984 | 0.612 |
| Overall | 0.54 | 0.54 | 0.54 | 0.53 | 0.55 | 0.54 | 0.53 | 0.54 | 0.01 | 0.691 | 0.599 | 0.812 | 0.651 | 0.582 | 0.582 |
1Metabolizable energy level at 3,400 kcal/kg of feed (CON), 3,375 kcal/kg of feed, 3,350 kcal/kg of feed, 3,325 kcal/kg of feed, and 3,300 (LE) kcal/kg of feed.
2Xylanase supplemented at 0 (LE), 1,200, 2,400, and 3,600 XU/kg feed (LEX) to LE treatment (3,300 kcal/kg feed).
3Dose–response effects of ME level (3,400 kcal/kg of feed [CON], 3,375 kcal/kg of feed, 3,350 kcal/kg of feed. 3,325 kcal/kg of feed, and 3,300 kcal/kg of feed [LE]).
4Dose response effects of xylanase supplementation (xylanase at 0 XU/kg feed [LE], 1,200 XU/kg feed, 2,400 XU/kg feed, and 3,600 XU/kg feed [LEX]).
Table 11.
Fecal score of nursery pigs fed diets with reduced ME and xylanase supplementation
| Treatment | ME1, kcal/kg | Xylanase2, XU/kg | P value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Item | 3,400 | 3,375 | 3,350 | 3,325 | 3,300 | 1,200 | 2,400 | 3,600 | SEM | ME Linear3 | ME Quad.3 | Xylanase Linear4 | Xylanase Quad.4 |
| Pre-starter | 3.3 | 3.1 | 3.2 | 3.3 | 3.1 | 3.2 | 3.2 | 3.2 | 0.1 | 0.799 | 0.830 | 0.529 | 0.840 |
| Starter | 3.3 | 3.2 | 3.3 | 3.3 | 3.4 | 3.0 | 3.3 | 3.1 | 0.1 | 0.250 | 0.515 | 0.070 | 0.288 |
| Overall | 3.3 | 3.2 | 3.3 | 3.3 | 3.3 | 3.1 | 3.3 | 3.1 | 0.1 | 0.503 | 0.572 | 0.350 | 0.526 |
1Metabolizable energy level at 3,400 kcal/kg of feed (CON), 3,375 kcal/kg of feed, 3,350 kcal/kg of feed, 3,325 kcal/kg of feed, and 3,300 (LE) kcal/kg of feed.
2Xylanase supplemented at 0 (LE), 1,200, 2,400, and 3,600 XU/kg feed (LEX) to LE treatment (3,300 kcal/kg feed).
3Dose–response effects of ME level (3,400 kcal/kg of feed (CON), 3,375 kcal/kg of feed, 3,350 kcal/kg of feed. 3,325 kcal/kg of feed, and 3,300 kcal/kg of feed (LE)).
4Dose–response effects of xylanase supplementation (xylanase at 0 XU/kg feed [LE], 1,200 XU/kg feed, 2,400 XU/kg feed, and 3,600 XU/kg feed [LEX]).
Discussion
Pigs fed LE had increased viscosity of jejunal digesta whereas, LEX decreased digesta viscosity compared to LE. Alterations to the viscosity of the digesta changes the intestinal environment and can modulate the exacerbation or onset of infectious intestinal diseases (Bertschinger et al., 1979; McDonald et al., 1999). Increased digesta viscosity in nursery pigs has been shown reduce starch digestibility and worsen postweaning diarrhea (Hopwood et al., 2004) and has been shown to increase the rate of enterocyte apoptosis leading to villus atrophy (McDonald et al., 2001,Duarte et al., 2019).
Xylanase supplementation seemed to increase Chao1 alpha diversity of mucosa-associated microbiota in the jejunum and this could be related to enhanced maintenance and stabilization of the ecosystem allowing it to retain its resilience against potential pathogenic invasion and proliferation (Konopka, 2009). Moreover, a more diverse microbiota has been regarded as a sign of a mature intestine environment in pigs (Chen et al., 2017). The changes in the alpha diversity observed in the current study resulted from distinct alterations in the composition of the microbiota in the jejunal mucosa.
Firmicutes are the predominant phylum in the lumen of the small intestine in nursery pigs whereas Bacteroidetes are the predominant phylum in the feces of postweaning pigs (Pajarillo et al., 2014; Adhikari et al., 2019). Age-dependent alterations in microbial communities in pigs have been shown by an increase in the relative abundance of Bacteroidetes in the jejunal mucosa and feces as nursery pigs age whereas Firmicutes reduced (Bian et al., 2016; Adhikari et al., 2019). Importantly, the population of microbiota in feces largely differs compared with those in the jejunal mucosa (Adhikari et al., 2019; Duarte and Kim, 2022). The relative abundance of both Proteobacteria and Bacteroidetes have been shown to be higher in mucosa compared to the lumen (Chen et al., 2017; Mu et al., 2017; Adhikari et al., 2019) which would explain the high abundance seen in the present study as samples were obtained from jejunal mucosa. Characterizing the mucosa-associated microbiota may be more relevant when evaluating their roles in the intestinal immune responses of the host (Mulder et al., 2011; Daniel et al., 2021). According to Arpaia et al. (2013) and Belkaid and Hand (2014), the microbiota associated with the mucosa has been shown to directly interact with intestinal cells. Moreover, the mucosa-associated microbiota was reported to have a greater ability to modulate the host’s immune system compared with luminal microbiota (Mu et al., 2017). Furthermore, Liu et al. (2019) reported that characterizing the fecal microbiota may be inefficient for comprehending the interactions between the intestinal microbiota and the host’s immune system.
The LEX lowered the relative abundance of Proteobacteria in the jejunal mucosa. This could have important implications as Proteobacteria contain several Gram-negative pathogenic bacteria such as Escherichia, Salmonella, Helicobacter, Campylobacter, and Vibrio that are used as potential indicators of intestinal dysbiosis (Shin et al., 2015). Gram-negative bacteria are characterized by possessing an outer cell wall with lipopolysaccharides (LPS) that provide integrity to the bacterial cell and act as a mechanism of interaction of the bacteria to outer surfaces (Li et al., 2017). Most bacterial LPS are thermostable and able to elicit a strong pro-inflammatory stimulus to the immune system of mammals (Sun and Kim, 2017). Indeed, xylanase reduced the relative abundance of Helicobacter in the jejunal mucosa which may indicate a healthier intestinal microbiota as previous reported by Cheng et al. (2021) and Xu et al. (2022). The most abundant genus in the jejunal mucosa in this study was Prevotella in the jejunal mucosa of nursery pigs fed an ME sufficient diet (CON) and an ME deficient diet with xylanase (LEX). Fecal Prevotella spp. has been shown to have positive associations with feed intake, feed efficiency, and weight gain (Mach et al; 2015; Ramayo-Caldas et al., 2016; Yang et al., 2018). However, in the present study, the enrichment of Prevotella in jejunal mucosa was not related to growth improvement. Species belonging to Prevotella have abilities to breakdown complex plant cell walls by utilizing endogenously produced NSPases such as xylanase, mannanase, and β-glucanases (Flint et al., 2008).
Interestingly, the relative abundance of Lactobacillus was high in the jejunal mucosa of nursery pigs fed an ME deficient diet (LE) compared to CON and LEX treatments. Several studies have indicated that Lactobacillus species improve growth and decrease the incidence of diarrhea (Dowarah et al., 2017; Yi et al., 2018; Wang et al., 2019). In the present study, the increase in Lactobacillus observed in LE was not related to reduced intestinal inflammation, reduced oxidative stress, enhanced intestinal integrity, and improved growth indicating the need for more research into how different microbial communities work together to produce a specific phenotype rather than increases or decreases in one particular genus. Additionally, the relative abundance of Mucispirillium was increased in the jejunal mucosa of nursery pigs fed an ME deficient diet with xylanase (LEX), mostly due to the increase in Mucispirillum schaedler, though no effect was observed at the species level. Mucispirillum schaedler has been shown to have a very limited collection of glycoside hydrolases, with only 3 family 57 α-amylases that are thought to be primarily used for processing stored glycogen (Loy et al., 2017). Therefore, M. schaedler is not a primary degrader of host-derived glycans in mucin but rather uses monosaccharides, oligopeptides, amino acids, and SCFAs as the substrates for its energy metabolism and is likely a consumer of products formed by other fermentative bacteria (El Kaoutari et al., 2013).
Feeding nursery pigs with an ME deficient diet of xylanase (LEX) did not affect the concentration of pro-inflammatory cytokines (IL-8 and TNF-α) or immunoglobulins (IgG and IgA) whereas, pigs fed an ME deficient diet (LE) had higher concentrations of protein carbonyl and lower amounts of MDA compared to pigs fed an ME sufficient diet. Xylanase supplementation to an ME deficient diet decreased concentrations of protein carbonyl. Weaning stress has been shown to induce oxidative stress in weaned pigs (Yin et al., 2014) and oxidative damage products can be detrimental to the intestinal epithelium, causing cell destruction and resulting in a reduction of villi height (Sido et al., 2017). The results in the present study support this as xylanase supplementation reduced the concentration of protein carbonyl in the mucosa compared with increased VH and VH:CD ratio. Reduction in oxidative damage products could be due to increased bioavailability of phenolic compounds with antioxidative properties. These phenolic compounds can be generated during the hydrolysis of arabinoxylan structure within the fiber fraction of corn (Duarte et al., 2019; Petry et al, 2020). Arabinoxylan in corn is highly substituted with phenolic compounds with the most abundant being ferulic acid (Boz, 2015). Ferulic acid has been shown to possess strong antioxidant abilities through the scavenging of free radicals, inhibition of enzymes that cause free radical production and stimulating antioxidase production (Ogiwara et al., 2002). In typical diets devoid of xylanase supplementation, the bioavailability of ferulic acid is limited due to esterification within arabinoxylan (Anson et al., 2009), however, supplementation of xylanase may fragment the arabinoxylan structure possibly allowing the access of esterified ferulic acid to ferulic acid esterase produced by microbiota (Mathew and Abraham, 2004).
Supplementation of xylanase has been shown to increase nutrient digestibility and thus growth performance of pigs (Patience, 2012). In this study, pigs fed an ME deficient diet with xylanase had increased AID of DM and GE and tended to have increased AID of CP, EE, and crude fiber, whereas this did not translate to improved growth. This result is in agreement with a meta-analysis published by Torres-Pitarch et al. (2019) that found regardless of diet composition, DM, GE, and CP digestibility increased with xylanase supplementation but resulting enhancements in growth performance were not as frequently observed. This is not uncommon when evaluating the efficacy of xylanase to improve growth as improvements in digestibility and subsequent responses in growth performance are inconsistent in corn-based diets and time-dependent (Kerr et al., 2013; Petry et al., 2020). Inconsistency in growth responses to xylanase among studies most likely can be attributed to inadequate determination of fiber within the feed offered, differences in age, breed, feeding duration, and feedstuffs used in conjunction with corn thus resulting in alterations to fermentation kinetics, digesta viscosity, and the microbiota composition. Additionally, the inclusion rate of xylanase in the diet, the presence or absence of supporting enzymes or xylanase inhibitors, and health status of the animal may play a role in the observation of a growth response when utilizing xylanase in formulation.
In the present study, reducing ME (LE) had no effect on overall BW, ADG, ADFI, or G:F. Although increasing energy density of diets offered to pigs through the inclusion of dietary fat typically improves ADG, G:F, and reduces ADFI (Pettigrew and Moser, 1991), this may not be completely appropriate when discussing pigs weighing less than 20 kg (Black et al., 1986) as they possess immature and limited digestive capacity which ultimately restricts their ability to adjust their feed intake relative to adult animals.
In conclusion, reducing ME up to 100 kcal/kg below the requirement by reducing supplemental fat had no effect on overall growth performance, however, it led to increased digesta viscosity, decreased diversity of mucosa-associated microbiota, increased relative abundance of potentially pathogenic bacteria in the jejunal mucosa, and elevated the concentration of oxidative damage products in the jejunal mucosa. These negative effects were seen by reducing ME manifested in negative effects on VH and VH:CD in jejunum and decreased nutrient digestibility. Supplementation of xylanase to an ME deficient diet helped to mediate some of the negative effects induced by energy restriction, resulting in increased diversity of mucosa-associated microbiota and the relative abundance of fiber-degrading bacteria, decreased oxidative damage products, reduced digesta viscosity, increased VH, and improved nutrient digestibility which are indicative of improvements in intestinal health that may be beneficial in subsequent phases of production.
Acknowledgments
This research was funded by North Carolina Agricultural Foundation (Raleigh, NC, USA), USDA-NIFA (Hatch #02893 and National Needs Fellowship #2019-38420-28970, Washington DC, USA), and CJ Cheiljedang (Seoul, Korea). The authors acknowledge technical supports from the members of the Kim lab at North Carolina State University.
Glossary
Abbreviations
- ADFI
average daily feed intake
- ADG
average daily gain
- AID
apparent ileal digestibility
- AX
arabinoxylan
- BW
body weight
- CD
crypt depth
- CP
crude protein
- cP
centipoise
- DM
dry matter
- GE
gross energy
- G:F
gain to feed ratio
- EE
ether extract
- IgA
immunoglobulin A
- IgG
immunoglobulin G
- IL-8
interleukin-8
- IL-6
interleukin-6
- LPS
lipopolysaccharide
- MDA
malondialdehyde
- ME
metabolizable energy
- mPA s
millipascal-seconds
- NSP
nonstarch polysaccharide
- PBS
phosphate-buffered saline
- PC
protein carbonyl
- PUFA
polyunsaturated fatty acid
- SCFA
short chain fatty acid
- SID
standardized ileal digestible
- TNF-α
tumor necrosis factor alpha
- VH
villus height
Contributor Information
Jonathan T Baker, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Marcos Elias Duarte, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Sung Woo Kim, Department of Animal Science, North Carolina State University, Raleigh, NC 27695, USA.
Conflict of interest statement. The authors declare no conflict of interest.
Author contributions
Sung Woo Kim (Conceptualization and design), Sung Woo Kim, Jonathan T. Baker, and Marcos Elias Duarte (Methodology), Jonathan T. Baker (Formal analysis), Sung Woo Kim, Jonathan T. Baker, and Marcos Elias Duarte (Investigation), Sung Woo Kim, Jonathan T. Baker, and Marcos Elias Duarte (Data interpretation), Sung Woo Kim, Jonathan T. Baker, and Marcos Elias Duarte (Writing—original draft preparation), Sung Woo Kim, Jonathan T. Baker, and Marcos Elias Duarte (Writing—review and editing), Sung Woo Kim (Supervision), and Sung Woo Kim (Funding acquisition). All authors have agreed and to the published version of the manuscript
Literature Cited
- Adeola, O., and Cowieson A. J... 2011. Board-invited review: opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 89:3189–3218. doi: 10.2527/jas.2010-3715 [DOI] [PubMed] [Google Scholar]
- Adhikari, B., Kim S. W., and Kwon Y... 2019. Characterization of microbiota associated with digesta and mucosa in different regions of gastrointestinal tract of nursery pigs. Int. J. Mol. Sci. 20:1630. doi: 10.3390/ijms20071630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson, N. M., van den Berg R., Havenaar R., Bast A., Haenen G. R. M. M.. 2009. Bioavailability of ferulic acid is determined by its bioaccessibility. J. Cereal Sci. 49:296–300. doi: 10.1016/j.jcs.2008.12.001 [DOI] [Google Scholar]
- AOAC. 2006. Official methods of analysis, 18th ed. In: Latimer G. W., editor. Gaithersburg (MD): AOAC International [Google Scholar]
- Arpaia, N., Campbell C., Fan X., Dikiy S., van der Veeken J., DeRoos P., Liu H., Cross J. R., Pfeffer K., Coffer P. J.,. et al. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. doi: 10.1038/nature12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, J. T., Duarte M. E., Holanda D. M., and Kim S. W... 2021. Friend or foe? Impacts of dietary xylans, xylooligosaccharides, and xylanases on intestinal health and growth performance of monogastric animals. Animals (Basel). 11:609. doi: 10.3390/ani11030609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassaganya-Riera, J., Hontecillas-Magarzo R., Bregendahl K., Wannemuehler M. J., and Zimmerman D. R... 2001. Effects of dietary conjugated linoleic acid in nursery pigs of dirty and clean environments on growth, empty body composition, and immune competence. J. Anim. Sci. 79:714–721. doi: 10.2527/2001.793714x [DOI] [PubMed] [Google Scholar]
- Bedford, M. R. 2018. The evolution and application of enzymes in the animal feed industry: the role of data interpretation. Br. Poult. Sci. 59:486–493. doi: 10.1080/00071668.2018.1484074 [DOI] [PubMed] [Google Scholar]
- Bedford, M. R., and Classen H. L... 1992. Reduction of intestinal viscosity through manipulation of dietary rye and pentosanase concentration is effected through changes in the carbohydrate composition of the intestinal aqueous phase and results in improved growth rate and food conversion efficiency of Broiler Chicks. J. Nutr. 122:560–569. doi: 10.1093/jn/122.3.560 [DOI] [PubMed] [Google Scholar]
- Bedford, M. R., and Schulze H... 1998. Exogenous enzymes for pigs and poultry. Nutr. Res. Rev. 11:91–114. doi: 10.1079/NRR19980007 [DOI] [PubMed] [Google Scholar]
- Belkaid, Y., and Hand T. W... 2014. Role of the microbiota in immunity and inflammation. Cell 157:121–141. doi: 10.1016/j.cell.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertschinger, H. U., Eggenberger E., Jucker H., and Pfirter H. P... 1979. Evaluation of low nutrient, high fibre diets for the prevention of porcine Escherichia coli enterotoxaemia. Vet. Microbiol. 3:281–290. doi: 10.1016/0378-1135(79)90004-x [DOI] [Google Scholar]
- Bian, G., Ma S., Zhu Z., Su Y., Zoetendal E. G., Mackie R., Liu J., Mu C., Huang R., Smidt H.,. et al. 2016. Age, introduction of solid feed and weaning are more important determinants of gut bacterial succession in piglets than breed and nursing mother as revealed by a reciprocal cross-fostering model. Environ. Microbiol. 18:1566–1577. doi: 10.1111/1462-2920.13272 [DOI] [PubMed] [Google Scholar]
- Black, J. L., Campbell R. G., Williams I. H., James K. J., and Davies G. T... 1986. Simulation of energy and amino acid utilisation in the pig. Agric. Res. 3:121–145 [Google Scholar]
- Boz, H. 2015. Ferulic acid in cereals—a review. Czech J. Food Sci. 33:1–7. doi: 10.17221/401/2014-cjfs [DOI] [Google Scholar]
- Cera, K. R., Mahan D. C., and Reinhart G. A... 1988. Weekly digestibilities of diets supplemented with corn oil, lard or tallow by weanling swine. J. Anim. Sci. 66:1430–1437. doi: 10.2527/jas1988.6661430x [DOI] [PubMed] [Google Scholar]
- Chao, A. 1984. Non-parametric estimation of the number of classes in a population. Scand. J. Stat. 11:265–270 [Google Scholar]
- Chassé, E., Guay F., and Létourneau-Montminy M. -P... 2023. High or low fibre diet, meal size and frequency: effect on ileal and total tract digestibility and mean retention time in growing pigs. Anim. Feed Sci. Technol. 306:115827. doi: 10.2139/ssrn.4539272 [DOI] [Google Scholar]
- Chen, L., Xu Y., Chen X., Fang C., Zhao L., and Chen F... 2017. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 8:1688. doi: 10.3389/fmicb.2017.01688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, H., Zhang S., and Kim S. W... 2020. Effects of supplemental xylanase on health of the small intestine in nursery pigs fed diets with corn distillers’ dried grains with solubles. J. Anim. Sci. 98:1–6. doi: 10.1093/jas/skaa185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Y. -C., Duarte M. E., and Kim S. W... 2021. Nutritional and functional values of lysed Corynebacterium glutamicum cell mass for intestinal health and growth of nursery pigs. J. Anim. Sci. 99:skab331. doi: 10.1093/jas/skab331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale, T., Hannay I., Bedford M. R., Tucker G. A., Brameld J. M., and Parr T... 2020. The effects of exogenous xylanase supplementation on the in vivo generation of xylooligosaccharides and monosaccharides in broilers fed a wheat-based diet. Br. Poult. Sci. 61:471–481. doi: 10.1080/00071668.2020.1751805 [DOI] [PubMed] [Google Scholar]
- Daniel, N., Lécuyer E., and Chassaing B... 2021. Host/microbiota interactions in health and diseases-time for mucosal microbiology! Mucosal Immunol. 14:1006–1016. doi: 10.1038/s41385-021-00383-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorado-Montenegro, S., Lammers-Jannink K., Gerrits W., and de Vries S... 2023. Insoluble fibers affect digesta transit behavior in the upper gastrointestinal tract of growing pigs, regardless of particle size. J. Anim. Sci. 101:skad299. doi: 10.1093/jas/skad299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowarah, R., Verma A. K., and Agarwal N... 2017. The use of lactobacillus as an alternative of antibiotic growth promoters in pigs: a review. Anim. Nutr. 3:1–6. doi: 10.1016/j.aninu.2016.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., and Kim S. W... 2022. Significance of mucosa-associated microbiota and its impacts on intestinal health of pigs challenged with F18+ E. coli. Pathogens. 11:589. doi: 10.3390/pathogens11050589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., Zhou F. X., Dutra W. M., and Kim S. W... 2019. Dietary supplementation of xylanase and protease on growth performance, digesta viscosity, nutrient digestibility, immune and oxidative stress status, and gut health of newly weaned pigs. Anim. Nutr. 5:351–358. doi: 10.1016/j.aninu.2019.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte, M. E., Tyus J., and Kim S. W... 2020. Synbiotic effects of enzyme and probiotics on intestinal health and growth of newly weaned pigs challenged with enterotoxigenic F18+ Escherichia coli. Front. Vet. Sci. 7:573. doi: 10.3389/fvets.2020.00573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer, R., and Moreno J. J... 2010. Role of eicosanoids on intestinal epithelial homeostasis. Biochem. Pharmacol. 80:431–438. doi: 10.1016/j.bcp.2010.04.033 [DOI] [PubMed] [Google Scholar]
- Flint, H., and Bayer E... 2008. Plant cell wall breakdown by anaerobic microorganisms from the Mammalian digestive tract. Ann. N. Y. Acad. Sci. 280:280–288. doi: 10.1196/annals.1419.022 [DOI] [PubMed] [Google Scholar]
- Holanda, D. M., and Kim S. W... 2021. Investigation of the efficacy of mycotoxin-detoxifying additive on health and growth of newly-weaned pigs under deoxynivalenol challenges. Anim. Biosci. 34:405–416. doi: 10.5713/ajas.20.0567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda, D. M., Yiannikouris A., and Kim S. W... 2020. Investigation of the efficacy of a postbiotic yeast cell wall-based blend on newly-weaned pigs under a dietary challenge of multiple mycotoxins with emphasis on deoxynivalenol. Toxins. 12:504. doi: 10.3390/toxins12080504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood, D. E., Pethick D. W., Pluske J. R., and Hampson D. J... 2004. Addition of pearl barley to a rice-based diet for newly weaned piglets increases the viscosity of the intestinal contents, reduces starch digestibility and exacerbates post-weaning colibacillosis. Br. J. Nutr. 92:419–427. doi: 10.1079/bjn20041206 [DOI] [PubMed] [Google Scholar]
- Jacobi, S. K., Moeser A. J., Corl B. A., Harrell R. J., Blikslager A. T., and Odle J... 2012. Dietary long-chain PUFA enhance acute repair of ischemia-injured intestine of suckling pigs. J. Nutr. 142: 1266–1271. doi: 10.3945/jn.111.150995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K. B., and Kim S. W... 2019. Supplemental effects of dietary nucleotides on intestinal health and growth performance of newly weaned pigs. J. Anim. Sci. 97:4875–4882. doi: 10.1093/jas/skz334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang, K., Purvis J. M., and Kim S. W.. 2020. Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health and gut associated microbiome of offspring. J. Anim. Sci. 98:skaa227. doi: 10.1093/jas/skaa227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen, H., Zhao X. -Q., and Eggum B. O... 1996. The influence of dietary fibre and environmental temperature on the development of the gastrointestinal tract, digestibility, degree of fermentation in the hind-gut and energy metabolism in pigs. Br. J. Nutr. 75:365–378. doi: 10.1079/bjn19960140 [DOI] [PubMed] [Google Scholar]
- Kaoutari, A. E., Armougom F., Gordon J. I., Raoult D., and Henrissat B... 2013. The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat. Rev. Microbiol. 11:497–504. doi: 10.1038/nrmicro3050 [DOI] [PubMed] [Google Scholar]
- Kerr, B. J., Weber T. E., and Shurson G. C... 2013. Evaluation of commercially available enzymes, probiotics, or yeast on apparent total-tract nutrient digestion and growth in nursery and finishing pigs fed diets containing corn dried distillers grains with solubles. Prof. Anim. Sci. 29:508–517. doi: 10.15232/s1080-7446(15)30272-2 [DOI] [Google Scholar]
- Kiarie, E., Liu Y., Walsh M. C., Stein H. H., and Payling L... 2016. Xylanase responses on apparent ileal digestibility of nutrients, fiber and energy in growing pigs fed corn, 30% corn co-products and soybean meal based diets as influenced by microbial phytase and acclimatization period. J. Anim. Sci. 94:116–116. doi: 10.2527/msasas2016-245 [DOI] [Google Scholar]
- Kim, S. W., and Baker D. H.. 2003. Use of enzyme supplements in pig diets based on soybean meal. Pig News and Information. CABI 24:91–96. [Google Scholar]
- Kim, S. W., Mateo R. D., Yin Y.-L., and Wu G.. 2007. Functional amino acids and fatty acids for enhancing production performance of sows and piglets. Asian-Australas. J. Anim. Sci. 20:295–306. doi: 10.5713/ajas.2007.295 [DOI] [Google Scholar]
- Kim, S. W., Holanda D. M., Gao X., Park I., and Yiannikouris A... 2019. Efficacy of a yeast cell wall extract to mitigate the effect of naturally co-occurring mycotoxins contaminating feed ingredients fed to young pigs: impact on gut health microbiome, and growth. Toxins. 11:633. doi: 10.3390/toxins11110633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka, A. 2009. What is microbial community ecology? ISME J. 3:1223–1230. doi: 10.1038/ismej.2009.88 [DOI] [PubMed] [Google Scholar]
- Lauridsen, C. 2020. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 98:1–12. doi: 10.1038/ismej.2009.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Shi Z., Radauer-Preiml I., Andosch A., Casals E., Luetz-Meindl U., Cobaleda M., Lin Z., Jaberi-Douraki M., Italiani P.,. et al. 2017. Bacterial endotoxin (lipopolysaccharide) binds to the surface of gold nanoparticles, interferes with biocorona formation and induces human monocyte inflammatory activation. Nanotoxicology. 11:1157–1175. doi: 10.1080/17435390.2017.1401142 [DOI] [PubMed] [Google Scholar]
- Lindberg, J. E. 2014. Fiber effects in nutrition and gut health in Pigs. J. Anim. Sci. Biotechnol. 5:15. doi: 10.1186/2049-1891-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y. 2015. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 6:41. doi: 10.1186/s40104-015-0040-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Zeng X., Zhang G., Hou C., Li N., Yu H., Shang L., Zhang X., Trevisi P., Yang F.,. et al. 2019. Maternal milk and fecal microbes guide the spatiotemporal development of mucosa-associated microbiota and barrier function in the porcine neonatal gut. BMC Biol. 17:1–16. doi: 10.1186/s12915-019-0729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pedrosa, J. M., Torres M. I., Fernández M. I., Ríos A., and Gil A... 1998. Severe malnutrition alters lipid composition and fatty acid profile of small intestine in newborn piglets. J. Nutr. 128:224–233. doi: 10.1093/jn/128.2.224 [DOI] [PubMed] [Google Scholar]
- López-Pedrosa, J. M., Ramírez M., Torres M. I., and Gil A... 1999. Dietary phospholipids rich in long-chain polyunsaturated fatty acids improve the repair of small intestine in previously malnourished piglets. J. Nutr. 129:1149–1155. doi: 10.1093/jn/129.6.1149 [DOI] [PubMed] [Google Scholar]
- Loy, A., Pfann C., Steinberger M., Hanson B., Herp S., Brugiroux S., Gomes Neto J. C., Boekschoten M. V., Schwab C., Urich T.,. et al. 2017. Lifestyle and horizontal gene transfer-mediated evolution of Mucispirillum schaedleri, a core member of the murine gut microbiota. mSystems. 2:e00171–e00116. doi: 10.1128/mSystems.00171-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach, N., Berri M., Estellé J., Levenez F., Lemonnier G., Denis C., Leplat J. -J., Chevaleyre C., Billon Y., Doré J.,. et al. 2015. Early-life establishment of the swine gut microbiome and impact on host phenotypes. Environ. Microbiol. Rep. 7:554–569. doi: 10.1111/1758-2229.12285 [DOI] [PubMed] [Google Scholar]
- Masey-O’neill, H. V., Singh M., and Cowieson A. J... 2014. Effects of exogenous xylanase on performance, nutrient digestibility, volatile fatty acid production and digestive tract thermal profiles of broilers fed on wheat- or maize-based diet. Br. Poult. Sci. 55:351–359. doi: 10.1080/00071668.2014.898836 [DOI] [PubMed] [Google Scholar]
- Mathew, S., and Abraham T. E... 2004. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 24:59–83. doi: 10.1080/07388550490491467 [DOI] [PubMed] [Google Scholar]
- McDonald, D. E., Pethick D. W., Pluske J. R., and Hampson D. J... 1999. Adverse effects of soluble non-starch polysaccharide (guar gum) on piglet growth and experimental colibacillosis immediately after weaning. Res. Vet. Sci. 67:245–250. doi: 10.1053/rvsc.1999.0315 [DOI] [PubMed] [Google Scholar]
- McDonald, D. E., Pethick D. W., Mullan B. P., and Hampson D. J... 2001. Increasing viscosity of the intestinal contents alters small intestinal structure and intestinal growth, and stimulates proliferation of enterotoxigenic Escherichia coli in newly-weaned pigs. Br. J. Nutr. 86:487–498. doi: 10.1079/bjn2001416 [DOI] [PubMed] [Google Scholar]
- Mielke, T.. 2018. World markets for vegetable oils and animal fats. In: Kaltschmitt, M., and Neuling U., editors, Biokerosene. Springer, Berlin, Heidelberg; p. 147–188. doi: 10.1007/978-3-662-53065-8_8 [DOI] [Google Scholar]
- Moita, V. H., Duarte M. E., da Silva S. N., and Kim S. W... 2021. Supplemental effects of functional oils on the modulation of mucosa-associated microbiota, intestinal health, and growth performance of Nursery Pigs. Animals. 11:1591. doi: 10.3390/ani11061591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moita, V. H., Duarte M. E., and Kim S. W... 2022. Functional roles of xylanase enhancing intestinal health and growth performance of nursery pigs by reducing the digesta viscosity and modulating the mucosa-associated microbiota in the Jejunum. J. Anim. Sci. 100:1–15. doi: 10.1093/jas/skac116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu, C., Yang Y., Su Y., Zoetendal E. G., and Zhu W... 2017. Differences in microbiota membership along the gastrointestinal tract of piglets and their differential alterations following an early-life antibiotic intervention. Front. Microbiol. 8:797. doi: 10.3389/fmicb.2017.00797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder, I. E., Schmidt B., Lewis M., Delday M., Stokes C. R., Bailey M., Aminov R. I., Gill B. P., Pluske J. R., Mayer C. -D.,. et al. 2011. Restricting microbial exposure in early life negates the immune benefits associated with gut colonization in environments of high microbial diversity. PLoS One 6:e28279. doi: 10.1371/journal.pone.0028279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, A. J., and Patience J. F... 2014. The effects of cereal type and xylanase supplementation on pig growth performance and energy digestibility. Iowa State University Animal Industry Report. Iowa State University, Ames, IA, USA. doi: 10.31274/ans_air-180814-1212 [DOI] [Google Scholar]
- Myers, W. D., Ludden P. A., Nayigihugu V., and Hess B. W... 2004. Technical note: a procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. doi: 10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- Newman, M. A. 2014. Defining the energy and nutrient content of corn grown in drought-stressed conditions and determining the relationship between energy content of corn and the response of growing pigs to xylanase supplementation. MS Thesis. Iowa State University, Ames. USA. doi: 10.31274/etd-180810-3773 [DOI] [Google Scholar]
- NRC. 1998. Nutrient Requirements of Swine. 10th ed.Washington, (DC): Natl. Acad. Press [Google Scholar]
- NRC. 2012. Nutrient Requirements of Swine. 11th ed.Washington, (DC): Natl. Acad. Press [Google Scholar]
- Ogiwara, T., K. , Satoh, Kadoma Y., Murakami Y., S. , Unten, Atsumi T., Sakagami H., and Fujisawa S... 2002. Radical scavenging activity and cytotoxicity of ferulic acid. Anticancer Res. 22:2711–2717 [PubMed] [Google Scholar]
- Pajarillo, E. A. B., Chae J. P., Balolong M. P., Kim H. B., Seo K. -S., and Kang D. -K... 2014. Pyrosequencing-based analysis of fecal microbial communities in three purebred pig lines. J. Microbiol. 52:646–651. doi: 10.1007/s12275-014-4270-2 [DOI] [PubMed] [Google Scholar]
- Passos, A. A., Park I., Ferket P., von Heimendahl E., and Kim S. W... 2015. Effect of dietary supplementation of xylanase on apparent ileal digestibility of nutrients, viscosity of digesta, and intestinal morphology of growing pigs fed corn and soybean meal based diet. Anim. Nutr. 1:19–23. doi: 10.1016/j.aninu.2015.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos, A. A., Moita V. H. C., and Kim S. W... 2023. Individual or combinational use of phytase, protease, and xylanase for the impacts on total tract digestibility of corn, soybean meal, and distillers dried grains with soluble fed to pigs. Anim. Biosci. 36:1869–1879. doi: 10.5713/ab.23.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patience, J. F., 2012. The influence of dietary energy on feed efficiency in grow-finish swine. In: Patience, J. F., editor. Feed efficiency in swine. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 101–129 [Google Scholar]
- Petry, A. L., Huntley N. F., Bedford M. R., and Patience J. F... 2020. Xylanase increased the energetic contribution of fiber and improved the oxidative status, gut barrier integrity, and growth performance of growing pigs fed insoluble corn-based fiber. J. Anim. Sci. 98:1–11. doi: 10.1093/jas/skaa233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettigrew, J. E., and Moser R. L.. 1991. Fat in swine nutrition. Swine nutrition. Miller E. R., Ullrey D. E., and Lewis A. J. ed. Butterworth-Heineman, Stoneham, MA; p. 133–145 [Google Scholar]
- Ramayo-Caldas, Y., Mach N., Lepage P., Levenez F., Denis C., Lemonnier G., Leplat J. -J., Billon Y., Berri M., Doré J.,. et al. 2016. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME J. 10:2973–2977. doi: 10.1038/ismej.2016.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller, H. V., and Ulvskov P.. 2010. Hemicelluloses. Ann. Rev. Plant Biol. 61:263–289. doi: 10.1146/annurev-arplant-042809-112315 [DOI] [PubMed] [Google Scholar]
- Schop, M., Jansman A. J., de Vries S., and Gerrits W. J... 2019. Increasing intake of dietary soluble nutrients affects digesta passage rate in the stomach of growing pigs. Br. J. Nutr. 121:529–537. doi: 10.1017/s0007114518003756 [DOI] [PubMed] [Google Scholar]
- Shakouri, M. D., Iji P. A., Mikkelsen L. L., and Cowieson A. J... 2009. Intestinal function and gut microflora of broiler chickens as influenced by cereal grains and microbial enzyme supplementation. J. Anim. Physiol. Anim. Nutr. 93:647–658. doi: 10.1111/j.1439-0396.2008.00852.x [DOI] [PubMed] [Google Scholar]
- Shannon, C. E., and Weaver, W., 1949. The mathematical theory of communication. Urbana: University of Illinois Press [Google Scholar]
- Shin, N.-R., Whon T. W., and Bae J.-W... 2015. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33:496–503. doi: 10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Sido, A., Radhakrishnan S., Kim S. W., Eriksson E., Shen F., Li Q., Bhat V., Reddivari L., and Vanamala J. K. P... 2017. A food-based approach that targets interleukin-6, a key regulator of chronic intestinal inflammation and colon carcinogenesis. J. Nutr. Biochem. 43:11–17. doi: 10.1016/j.jnutbio.2017.01.012 [DOI] [PubMed] [Google Scholar]
- Simpson, E. H. 1949. Measurement of diversity. Nature 163:688. doi: 10.1038/163688a0 [DOI] [Google Scholar]
- Southern, L. L., Watkins K. L., Ojeda A. R., and Hembry F. G... 1989. Effect of season of the year and energy density of the diet on growth, feed intake, and feed efficiency of swine. Nutr. Rep. Int. 40:1029–1039 [Google Scholar]
- Sun, Y., and Kim S. W.. 2017. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim. Nutr. 3:322–330. doi: 10.1016/j.aninu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, U. P., Chen H., Kim S. W., and Jha R... 2018. Supplemental effect of xylanase and mannanase on nutrient digestibility and gut health of nursery pigs studied using both in vivo and in vitro models. Anim. Feed Sci. Technol. 245:77–90. doi: 10.1016/j.anifeedsci.2018.07.002 [DOI] [Google Scholar]
- Tokach, M. D., Pettigrew J. E., Johnston L. J., Øverland M., Rust J. W., and Cornelius S. G... 1995. Effect of adding fat and(or) milk products to the weanling pig diet on performance in the nursery and subsequent grow-finish stages. J. Anim. Sci. 73:3358–3368. doi: 10.2527/1995.73113358x [DOI] [PubMed] [Google Scholar]
- Toldrá-Reig, F., Mora L., and Toldrá F... 2020. Trends in biodiesel production from animal fat waste. Appl. Sci. 10:3644. doi: 10.3390/app10103644 [DOI] [Google Scholar]
- Torres-Pitarch, A., Manzanilla E. G., Gardiner G. E., O’Doherty J. V., and Lawlor P. G... 2019. Systematic review and meta-analysis of the effect of feed enzymes on growth and nutrient digestibility in grow-finisher pigs: effect of enzyme type and cereal source. Anim. Feed Sci. Technol. 251:153–165. doi: 10.1016/j.anifeedsci.2018.12.007 [DOI] [Google Scholar]
- Valaja, J., and Siljander-Rasi H. S.,. 2001. Dietary fat supplementation affects apparent ileal digestibility of amino acids and digesta passage rate of rapeseed meal-based diet. Digestive Physiology of Pigs. Proceedings of the 8th Symposium. Lindberg J. E., and Ogle B. ed. Swedish University of Agricultural Sciences, Uppsala, Sweden; p. 175–177. doi: 10.1079/9780851995175.0175 [DOI] [Google Scholar]
- Wang, T., Teng K., Liu Y., Shi W., Zhang J., Dong E., Zhang X., Tao Y., and Zhong J... 2019. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front. Microbiol. 10:90. doi: 10.3389/fmicb.2019.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J., Bai J., Fan M., Li T., Li Y., Qian H., Wang L., Zhang H., Qi X., and Rao Z... 2020. Cereal-derived arabinoxylans: structural features and structure–activity correlations. Trends Food Sci. Technol. 96:157–165. doi: 10.1016/j.tifs.2019.12.016 [DOI] [Google Scholar]
- Weaver, A. C., and Kim S. W... 2014. Supplemental nucleotides high in Inosine 5ʹ-monophosphate to improve the growth and health of nursery pigs. J. Anim. Sci. 92:645–651. doi: 10.2527/jas.2013-6564 [DOI] [PubMed] [Google Scholar]
- Wilfart, A., Montagne L., Simmins H., Noblet J., and van Milgen J... 2007. Digesta transit in different segments of the gastrointestinal tract of pigs as affected by insoluble fibre supplied by wheat bran. Br. J. Nutr. 98:54–62. doi: 10.1017/S0007114507682981 [DOI] [PubMed] [Google Scholar]
- Xu, X., Duarte M. E., and Kim S. W... 2022. Postbiotic effects of Lactobacillus fermentate on intestinal health, mucosa-associated microbiota, and growth efficiency of nursery pigs challenged with F18+ Escherichia coli. J. Anim. Sci. 100:skac210. doi: 10.1093/jas/skac210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Walsh M. C., Kiarie E., Tekeste A., and Baidoo S. K... 2016. Effects of xylanase on growth performance and digestibility of fiber and energy in growing pigs fed corn, corn DDGS and soybean meal based diet supplemented with phytase. J. Anim. Sci. 94:131–131. doi: 10.2527/msasas2016-279 [DOI] [Google Scholar]
- Yang, H., Yang M., Fang S., Huang X., He M., Ke S., Gao J., Wu J., Zhou Y., Fu H.,. et al. 2018. Evaluating the profound effect of gut microbiome on host appetite in pigs. BMC Microbiol. 18:215. doi: 10.1186/s12866-018-1364-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H., Wang L., Xiong Y., Wen X., Wang Z., Yang X., Gao K., and Jiang Z... 2018. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J. Anim. Sci. 96:2342–2351. doi: 10.1093/jas/sky129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, J., Wu M. M., Xiao H., Ren W. K., Duan J. L., Yang G., Li T. J., and Yin Y. L... 2014. Development of an antioxidant system after early weaning in piglets. J. Anim. Sci. 92:612–619. doi: 10.2527/jas.2013-6986 [DOI] [PubMed] [Google Scholar]
- Zhang, Z., Tun H. M., Li R., Gonzalez B. J. M., Keenes H. C., Nyachoti C. M., Kiarie E., and Khafipour E... 2018. Impact of xylanases on gut microbiota of growing pigs fed corn- or wheat-based diets. Anim. Nutr. 4:339–350. doi: 10.1016/j.aninu.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]