Abstract
This study was conducted to investigate the effects of sodium butyrate (SB) supplementation on growth performance, intestinal barrier functions, and intestinal bacterial communities in sucking lambs. Forty lambs of 7 d old, with an average body weight (BW) of 4.46 ± 0.45 kg, were allocated into the control (CON) or SB group, with each group having five replicate pens (n = 5). Lambs were orally administered SB at 1.8 mL/kg BW in the SB group or the same volume of saline in the CON group. Treatments were administered from 7 to 35 d of age, when one lamb from each replicate was slaughtered to obtain intestinal tissues and contents. The results showed that supplementation with SB tended to increase the BW (P = 0.079) and the starter intake (P = 0.089) of lambs at 35 d of age. The average daily gain of lambs in the SB group was significantly greater than that in the CON group (P < 0.05). The villus height of jejunum in the SB group was markedly higher (P < 0.05) than that in the CON group. In ileum, lambs in the SB group had lower (P < 0.05) crypt depth and greater (P < 0.05) villus-to-crypt ratio than those in the CON group. Compared with the CON group, the mRNA and protein expressions of Claudin-1 and Occludin were increased (P < 0.05) in the SB group. Supplementation with SB decreased the relative abundances of pathogenic bacteria, including Clostridia_UCG-014 (P = 0.094) and Romboutsia (P < 0.05), which were negatively associated with the intestinal barrier function genes (P < 0.05). The relative abundance of Succiniclasticum (P < 0.05) was higher in the SB group, and it was positively correlated with the ratio of villi height to crypt depth in the jejunum (P < 0.05). Compared with the CON group, the function “Metabolism of Cofactors and Vitamins” was increased in the SB group lambs (P < 0.05). In conclusion, SB orally administration during suckling period could improve the small intestine development and growth performance of lambs by inhibiting the harmful bacteria (Clostridia_UCG-014, Romboutsia) colonization, and enhancing intestinal barrier functions.
Keywords: intestinal barrier, intestinal microbiota, sodium butyrate, suckling lambs
Sodium butyrate orally administered during suckling period could improve the small intestine development and growth performance of suckling lambs by inhibiting the harmful bacteria colonization, and enhancing intestinal barrier functions.
Introduction
Since the rumen plays a crucial role in the growth performance and health of ruminants (Diao et al., 2019), and the small intestine is primarily responsible for digesting and absorbing nutrients (Blum, 2006), timely development of the rumen and small intestine is desired in ruminants. In the preweaning stage, the rumen is not fully developed (Górka et al., 2018), small intestine therefore is the primary site for liquid feeds digestion and nutrient absorption in pre-ruminant calves and lambs (Piao et al., 2021). Although the small intestine is particularly important in pre-ruminants, the development of the small intestine relative to the rumen has received little attention from researchers (Steele et al., 2016).
Short-chain fatty acids (SCFAs), the main metabolites produced by bacterial fermentation in the gastrointestinal tract, have important metabolic functions and are crucial to intestinal health (Liu et al., 2022). As one of the most abundant SCFAs in the gut, butyrate has received particular attention due to its beneficial effects on homeostasis in the gut (Guilloteau et al., 2010). Numerous studies demonstrated that butyrate supplementation could increase growth performance (Liu et al., 2019), promote rumen epithelial development and function (Liu et al., 2019; McCurdy et al., 2019; Fukumori et al., 2022), improve intestinal development and health of animals (Liu et al., 2022; Wu et al., 2022; Zhong et al., 2023a). Moreover, gut microbiota are the main producers of SCFAs, Liu et al. (2022) reported that supplementing butyrate in milk replacers could stimulate SCFAs-producing bacteria colonization. So far, there have been few studies on the effects of exogenous butyrate on intestinal morphology, intestinal barrier function, and microbiota of suckling lambs. Therefore, sodium butyrate (SB) (salt form of butyric acid) would be used in this study, we hypothesized that SB administration in suckling lambs could promote growth performance, improve intestinal development, and alter the diversity of intestinal bacterial community.
Materials and Methods
All experimental protocols involving animals were approved by the Animal Use and Care Committee, Zhejiang A & F University (No. ZAFUAC2016002, Hangzhou, China), and the experimental procedures used in this study were in accordance with the university’s guidelines for animal research.
Animals, diets, and experimental design
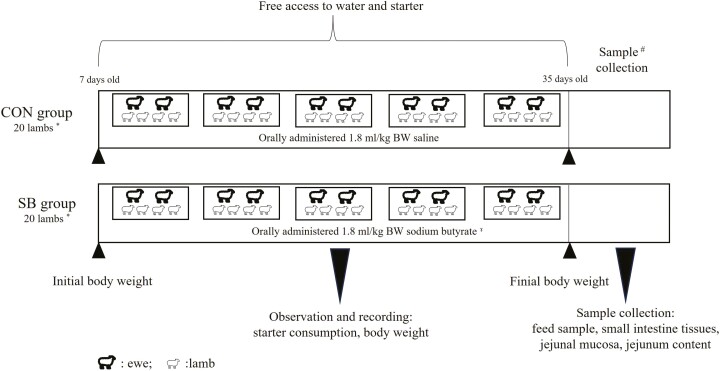
Forty healthy male Hu lambs (from twins or triplets), with a mean body weight (BW) of 4.46 ± 0.45 kg at the age of 7 d, were allotted to control (CON, no SB supplementation) or SB-supplemented groups (SB), with each group having 20 lambs. Four lambs of each group, born on the same day and with similar BW, were placed and housed in one pen (1.0 × 2.0 m), which was considered an experimental unit. Thus, there were five replicates in each treatment (n = 5). In the SB group, SB (98 g/100 g purity. King Technology Feed Co., Ltd, Hangzhou, China) was orally administered at 1.8 mL/kg BW, while in the CON group, saline was administered at the same volume. The SB powder was dissolved in saline at a concentration of 0.2 g/mL. Treatments were administered from 7 to 35 d of age at 0830 hours and the amount was adjusted weekly according to the BW. The lambs were separated from their mothers for 1 h after being orally administered. To reduce stress, the ewes were removed while the lambs remained in their pens. In addition to the suckling, the lambs also had ad libitum access to pellets of a starter (Table 1). To stimulate consumption of the starter, lambs were separated from their mothers (0700 to 1200 and 1500 to 2100 hours) from 21 to 35 d of age. The amount of starters offered and refused, and the BW were recorded every week. The schematic diagram for the animals and experimental design is shown in Figure 1.
Table 1.
Chemical composition of the starter (g/kg, dry matter basis)1
| Items2 | Content |
|---|---|
| Dry matter | 886 |
| Crude protein | 198 |
| Neutral detergent fiber | 396 |
| Acid detergent fiber | 144 |
| Ash | 74.1 |
| Calcium | 9.7 |
| Phosphorus | 6.1 |
1The starter was purchased from AB Agri China (Anhui, China) and consisted of corn, soybean meal, wheat bran, soybean hull, stone powder, NaCl, CuSO4, MnSO4, ZnSO4, VA, VD3, and VE.
2The chemical compositions were all measured values.
Figure 1.
Animal and experimental design. * Four lambs, born on the same day and with similar BW, were placed and housed in one pen, which was considered an experimental replicate. Thus, there were five replicates in each treatment (n = 5). ɤ The SB powder was dissolved in saline at a concentration of 0.2 g/mL, the administrated amount was adjusted weekly according to the BW. # One lamb of each replicate in the two groups was randomly selected for slaughter. CON, control, SB, sodium butyrate
Sample collection and analysis
Feed samples were dried in a forced-air oven at 65 °C for 48 h and stored in sealed plastic containers at 4 °C until analysis. All feed samples were ground to allow passage through a 1-mm sieve (HK-08 A ground mill; Xu Lang Machinery, China) before analysis for DM, CP, acid detergent fiber, neutral detergent fiber ash, calcium, and phosphorus. The methods were described by Mao et al. (2019).
At 35 d of age, one lamb of each replicate was slaughtered 1 h after SB infusion but before morning feeding. The process of slaughter was in accordance with the Animal protection law of the People’s Republic of China (2009). After the lumen contents were removed, the rumen, reticulum, omasum, abomasum, small intestine, and large intestine weights were recorded. The digestive tracts of small intestine tissue samples, approximately 1.5 × 1.5 cm2 each, were collected and fixed in 4% paraformaldehyde until morphological measurement. Jejunal mucosa and jejunum content samples were collected and stored at −80 °C until analysis.
Intestinal morphology analysis
The fixed intestinal segments were embedded in paraffin, cut into 4 µm thick sections, and stained with hematoxylin-eosin. Each stratum was measured under a 40 × objective lens (OPTEC Instrument Co., Chongqing, China), and six microscopic fields per sample were selected to measure villus height and crypt depth using an image analyzer (Image Pro Plus 6.0, Media Cybernetic, Inc., MD).
Quantitative real-time PCR for tight junction proteins
Quantitative real-time PCR was used to quantitate the relative expression of claudin-1 and occludin at the mRNA level. Total RNA was isolated from jejunal mucosa using an RNApure Total RNA kit (Aidlab, Beijing, China) in accordance with the manufacturer’s instructions. RNA concentrations were measured using a NanoDrop 2,000 spectrophotometer (AllSheng, Hangzhou, China). A ReverTra Ace qPCR RT Kit (Toyobo Co., Ltd, Osaka, Japan) was used to denature RNA (65 °C, thermal denaturation for 5 min) and perform reverse transcription (37 °C, reverse transcription reaction for 15 min; 98 °C, enzyme inactivation for 5 min). The mRNA abundance was determined with a CFX96TM Real-Time System (Bio-Rad, CA, USA) using SYBR Green Real-time PCR Master Mix (Toyobo Co., Ltd). The PCR cycle procedure (Toyobo Co., Ltd) was as follows: 95 °C for 1 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The relative expressions of two genes were calculated according to the 2−ΔΔCt method with β-actin as the housekeeping gene. The primers for the genes (Supplementary Table S1) were synthesized by Sangon Biotech Co., Ltd (Shanghai, China).
Western blotting analysis for tight junction proteins
Total protein was extracted from jejunal mucosa using RIPA lysis buffer (Strong), protease inhibitor cocktail (100×), and phosphatase inhibitor cocktail (100×) (CWBiotech, Beijing, China). A total protein assay kit (employing the standard BCA method) was used to determine the protein concentration. Immunoblotting was carried out using rabbit anti-claudin-1 (1:1,000 dilution) and rabbit anti-occludin (1:1,000 dilution) antibodies (both from Huabio). After washing with tris-buffered saline tween several times, membranes were incubated with the secondary antibody of goat anti-rabbit (1:5,000 dilution, AB clonal) for 1 h at room temperature. To normalize the densitometry results, membranes were incubated with a rabbit monoclonal β-actin antibody (1:5,000 dilution, AB clonal). Electrophoretic solution, transfer solution, and TBST were prepared according to laboratory formulas. PVDF membranes (Millipore) were incubated with an ECL substrate luminescence solution and imaged in a gel imaging system (Tanon, Shanghai, China). The western blotting instruments were from Bio-Rad. Finally, ImageJ software was used to analyze the gray values on the protein strip.
DNA extraction and high-throughput sequencing
The cetyltrimethylammonium bromide method as described by Gagen et al. (2010) was used to extract the total DNA of the jejunum content samples. The hypervariable V4 region of the 16S rRNA gene was amplified using universal primers 515F (5ʹ-CCTAYGGGRBGCASCAG-3ʹ) and 806R (5ʹ-GGACTACHVGGGTATCTAAT-3ʹ). Each primer had six base barcodes to identify samples. The amplicon libraries were generated by pooling in an equal ratio and sequenced on an Illumina NovaSeq6000 at Novogene Bioinformatics Technology Co., Ltd. (Tianjin, China) to generate 2 × 250 bp paired-end reads.
The paired-end sequencing reads were assembled by using FLASH (V1.2.11) and assigned to each sample according to the unique barcodes. Denoise-paired method with DADA2 was performed to filter out the quality filtered, denoised, merged, and chimeric sequences. Operational taxonomic units (OTUs) were clustered at 97% similarity using the Greengenes 16S reference database (gg_13). Alpha diversity measurements included Chao1 (Chao, 1984), Pielou’s evenness (Pielou, 1966), Shannon (Shannon, 1948), and Simpson (Simpson, 1949) calculated by QIIME 2. Shared and unique species among groups were used to generate a Venn diagram. Principal coordinate analysis (PCoA) based on weighted UniFrac distances (Lozupone et al., 2007) was performed to compare the dissimilarity of microbiota shaped by SB supplementation. The linear discriminant analysis effect size (LEfSe) method (Segata et al., 2011) was used to identify bacterial taxa with significant differences between two groups. Taxa with linear discriminant analysis scores greater than three was identified as an important contributor to each group. The functional capabilities of the jejunal microbiota were predicted by using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt, Langille et al., 2013). Besides, co-occurrence was generated based on the Pearson correlation coefficients between the major ruminal microbiota (top 20 genera) and phenotypes (intestinal morphology variables and the relative mRNA expression of tight junction proteins). Visualization of the co-occurrence network was performed by the Genes Cloud tools, a free online platform for data analysis (https://www.genescloud.cn).
The jejunal bacterial 16S rRNA gene sequencing data have been deposited in NCBI Sequence Read Archive database (accession number: PRJNA993379).
Statistical analysis
The data were statistically analyzed as a completely randomized design. Each pen with four lambs was considered as one experimental unit for the analysis of starter intake and BW, while one slaughtered lamb from each pen was regarded as one experimental unit for other analyses. The PROC MIXED procedure of SAS (version 9.0, SAS Inst. Inc. Cary, NC, USA) was used with time as repeated measures for BW, starter intake, and average daily gain (ADG). The model included time, treatment, and time × treatment interaction as fixed effects, and lamb within treatment as a random effect. Data on gastrointestinal tract organs, intestinal morphology, jejunal tight junction proteins, and alpha diversity indices of jejunal bacteria, which were collected only once, were analyzed with one-way ANOVA procedure of SAS. The bacterial relative abundance (phylum and genus level) was compared using Kruskal–Wallis test. Statistical significance was declared at P ≤ 0.05 and trend at 0.05 < P ≤ 0.10.
Results
Growth performance
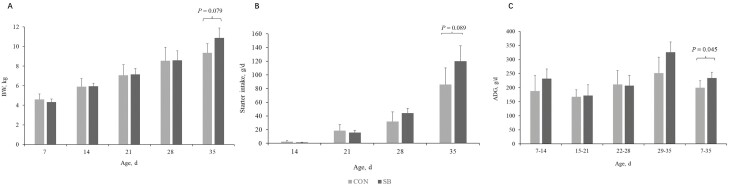
At 35 d of age, lambs in the SB group tended to have higher BW (Figure 2A, P = 0.079) and greater starter intake (Figure 2B, P = 0.089) than lambs in the CON group. Supplementation with SB significantly increased the ADG of lambs during the whole period (Figure 2C, P < 0.05).
Figure 2.
Effect of sodium butyrate supplementation on growth performance of suckling lambs. (A) Body weight (BW), (B) Starter intake, (C) Average daily gain (ADG). CON, control; SB, sodium butyrate.
Gastrointestinal tract development
As shown in Table 2, the rumen index of lambs in the SB group tended to be higher than it in the CON group (P = 0.079). Supplementation with SB increased the small intestine index of lambs (P < 0.05).
Table 2.
Effect of sodium butyrate supplementation on gastrointestinal tract organ index1 of suckling lambs
| Items, % | Treatments2 | SEM | P-value | |
|---|---|---|---|---|
| CON | SB | |||
| Rumen | 0.66 | 1.02 | 0.16 | 0.079 |
| Reticulum | 0.12 | 0.13 | 0.02 | 0.531 |
| Omasum | 0.09 | 0.09 | 0.02 | 0.892 |
| Abomasum | 0.58 | 0.59 | 0.09 | 0.921 |
| Small intestine | 3.53b | 5.84a | 0.83 | 0.038 |
| Large intestine | 2.81 | 3.71 | 0.18 | 0.466 |
1Gastrointestinal tract organ index = gastrointestinal tract organ weight/body weight.
2CON, control; SB, sodium butyrate.
a, bMean values in rows without a common letter are significantly different (P < 0.05)
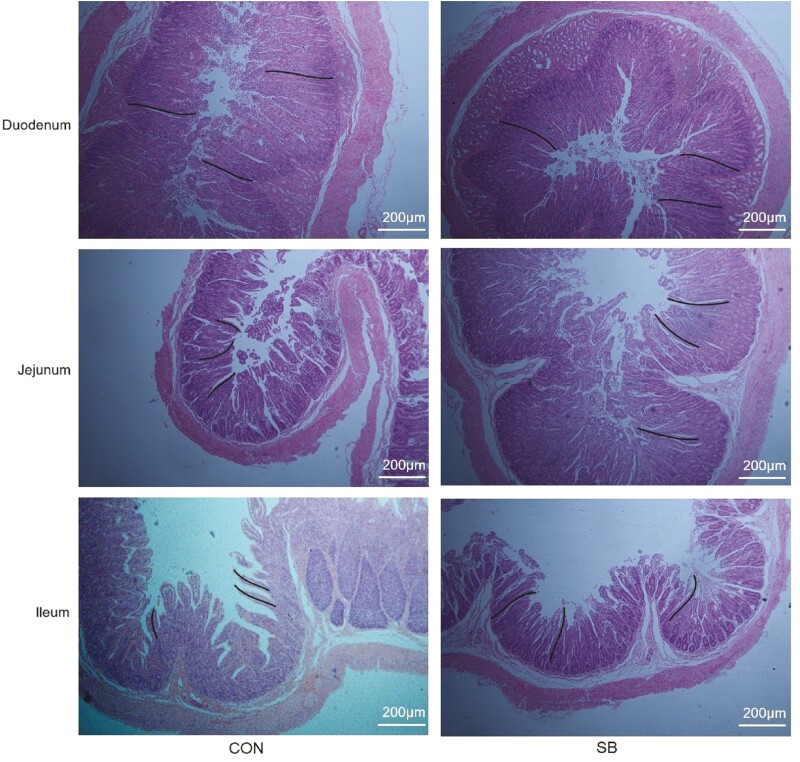
The intestinal morphology of the duodenum, jejunum, and ileum are shown in Figure 3. No significant difference in duodenum morphology was found between the CON and SB groups (Table 3, P > 0.05). In jejunum, lambs supplemented with SB had higher (P < 0.05) villus height, while there was no difference (P > 0.05) in the crypt depth or villus-to-crypt ratio. Supplementation with SB had no effect (P > 0.05) on the height of ileum villus. However, lambs in the SB group had lower (P < 0.05) ileum crypt depth and greater (P < 0.05) villus-to-crypt ratio than those in the CON group.
Figure 3.
The intestinal morphology of the duodenum, jejunum, and ileum. The black line means the villus height. CON, control; SB, sodium butyrate.
Table 3.
Effect of sodium butyrate supplementation on intestinal morphology of suckling lambs
| Items | Treatments1 | SEM | P-value | |
|---|---|---|---|---|
| CON | SB | |||
| Duodenum | ||||
| Villus height, µm | 375 | 361 | 50.8 | 0.799 |
| Crypt depth, µm | 320 | 393 | 38.7 | 0.116 |
| V/C2 | 1.12 | 1.11 | 0.22 | 0.968 |
| Jejunum | ||||
| Villus height, µm | 402b | 556a | 36.7 | 0.017 |
| Crypt depth, µm | 357 | 339 | 25.4 | 0.769 |
| V/C | 1.29 | 1.73 | 0.19 | 0.294 |
| Ileum | ||||
| Villus height, µm | 461 | 486 | 39.3 | 0.563 |
| Crypt depth, µm | 223a | 176b | 12.4 | 0.044 |
| V/C | 2.10b | 2.72a | 0.15 | 0.004 |
1CON, control; SB, sodium butyrate.
2V/C, villus height/crypt depth.
a, bMean values in rows without a common letter are significantly different (P < 0.05).
Tight junction protein expression in the jejunum
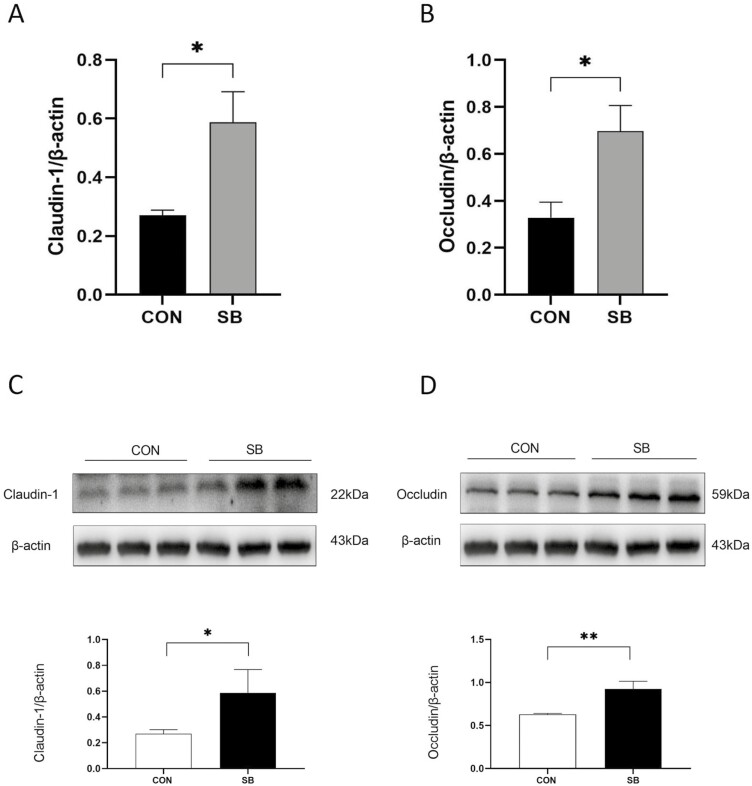
Overall, supplementation with SB significantly increased the mRNA expressions of claudin-1 (Figure 4A, P < 0.05) and occludin (Figure 4B, P < 0.01) in the jejunum of lambs. At the protein level, lambs in the SB group had higher claudin-1 (Figure 4C, P < 0.05) and occludin (Figure 4D, P < 0.05) expressions in the jejunum as compared to the CON group.
Figure 4.
Effect of sodium butyrate supplementation on the relative mRNA and protein expressions of tight junction protein in the jejunum. (A) Claudin-1 mRNA expression; (B) Occludin mRNA expression; (C) Relative protein level of Claudin-1; (D) Relative protein level of Claudin-1. * P < 0.05; ** P < 0.01.
Bacterial community composition of the jejunal microbiota
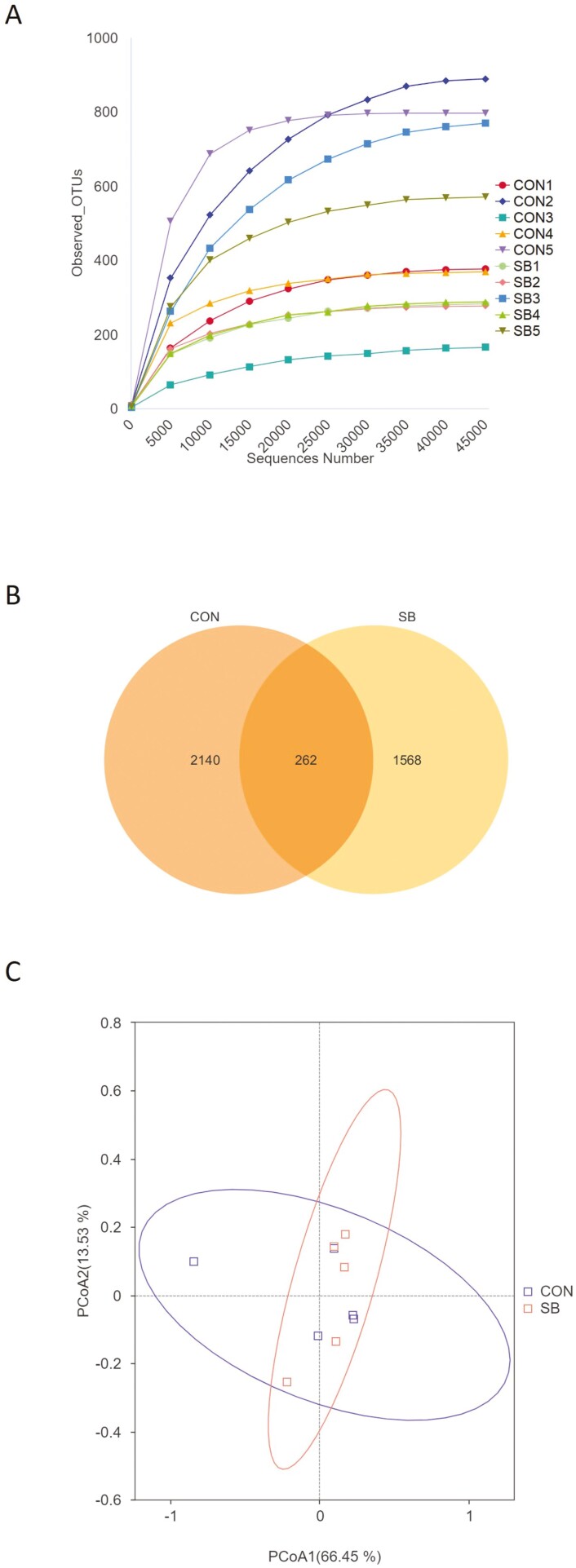
A total of 830,763 raw reads were generated from 10 jejunum content samples of sheep, and 581,817 effective reads were obtained after quality control, with an average of 58,182 ± 13,962 reads per sample. As shown in Figure 5A, the rarefaction curves tended toward saturation, which means the number of microbial sequences represented the microbial communities. Based on 97% similarity, 3,970 OTUs were annotated, among which there were 262 shared bacterial OTUs (Figure 5B).
Figure 5.
Number of operational taxonomic units (OTUs) in two groups. (A) Rarefaction curves of OTUs; (B) Venn diagram of shared OTUs; (C) Principal coordinate analysis (PCoA) based on Weighted UniFrac distance. CON, control; SB, sodium butyrate.
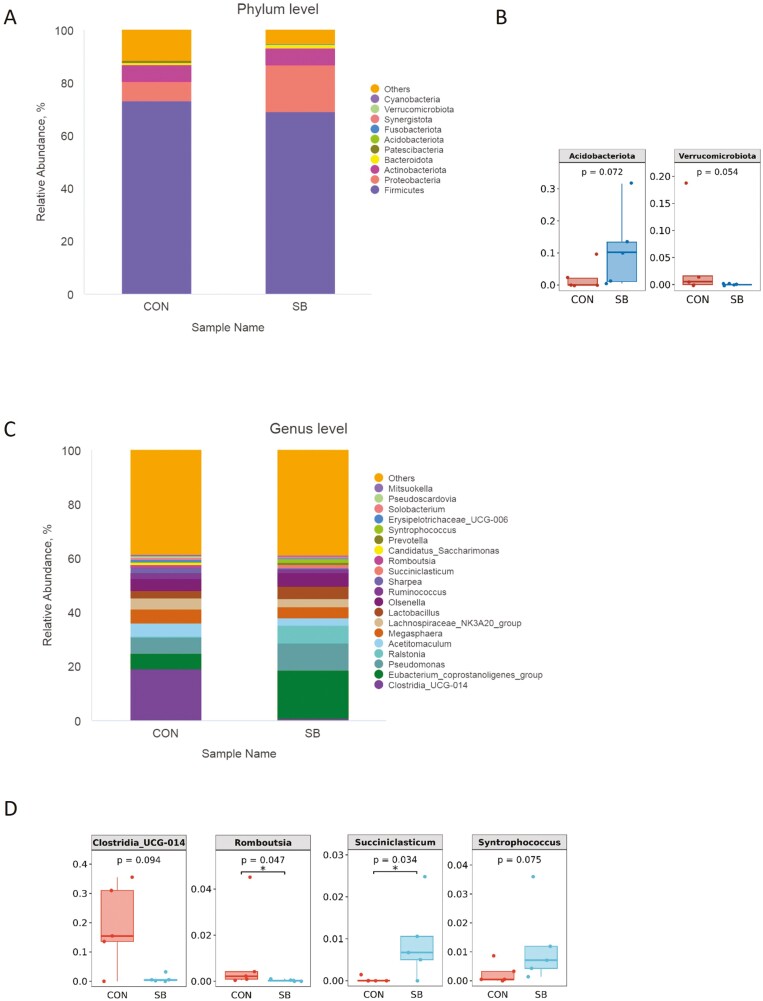
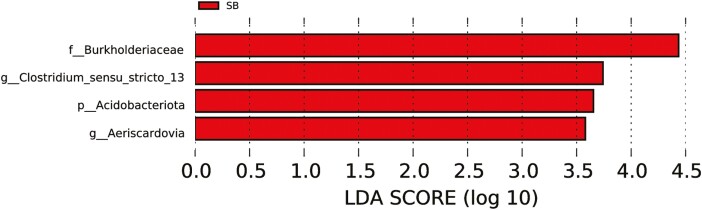
As shown in Table 4, there were no significant differences in any of the alpha diversity indices between the two groups (P > 0.05). Principal component analysis revealed that the PCo1 and PCo2 explained 66.45% and 13.53% of the variation among samples, respectively (Figure 5C). At the phylum level, Firmicutes, Proteobacteria, and Actinobacteriota were the dominant phyla in all groups (Figure 6A). The relative abundance of Acidobacteriota tended to be higher (P = 0.072) while Verrucomicrobiota tended to be lower (P = 0.054) in the SB group than those in the CON group (Figure 6B). The 20 most dominant genera in the jejunum of sheep are shown in Figure 6C. As shown in Figure 6D, the relative abundance of Succiniclasticum (P < 0.05) was higher and Syntrophococcus (P = 0.076) tended to be higher in the SB group than those in the CON group. However, supplementation with SB decreased the abundance of Romboutsia (P < 0.05) and tended to decrease the Clostridia_UCG-014 (P = 0.094). To identify the key phylotypes of jejunal microbiota between the two groups, LEfSe analysis was performed (Figure 7). The linear discriminant analysis score results showed four discriminative features in the SB group, and burkhoderiaceae was the main microbiota. However, there were no dominant microorganisms in the CON group.
Table 4.
Alpha diversity measures of jejunal bacterial communities
| Items | Treatments1 | SEM | P-value | |
|---|---|---|---|---|
| CON | SB | |||
| Chao1 | 521 | 439 | 121 | 0.643 |
| Pielou_e | 0.54 | 0.50 | 0.05 | 0.682 |
| Shannon | 4.83 | 4.20 | 0.71 | 0.549 |
| Simpson | 0.79 | 0.80 | 0.19 | 0.936 |
1CON, control; SB, sodium butyrate.
Figure 6.
Jejunal bacterial community composition of suckling lamb. Abundances of the jejunal microbiota at the (A) phylum and (C) genus levels of lamb. The top 10 phyla and the top 20 genera are listed. Comparisons of relative abundances at the phylum (B) and genus (D) levels were analyzed by the Kruskal–Wallis rank-sum test. CON, control; SB, sodium butyrate.
Figure 7.
Linear discriminant analysis effect size analysis identified the most differentially abundant jejunal microbiota between CON and SB groups. Taxa with LDA score greater than three are shown in the histogram. CON, control; SB, sodium butyrate.
Predicted jejunal microbial functions
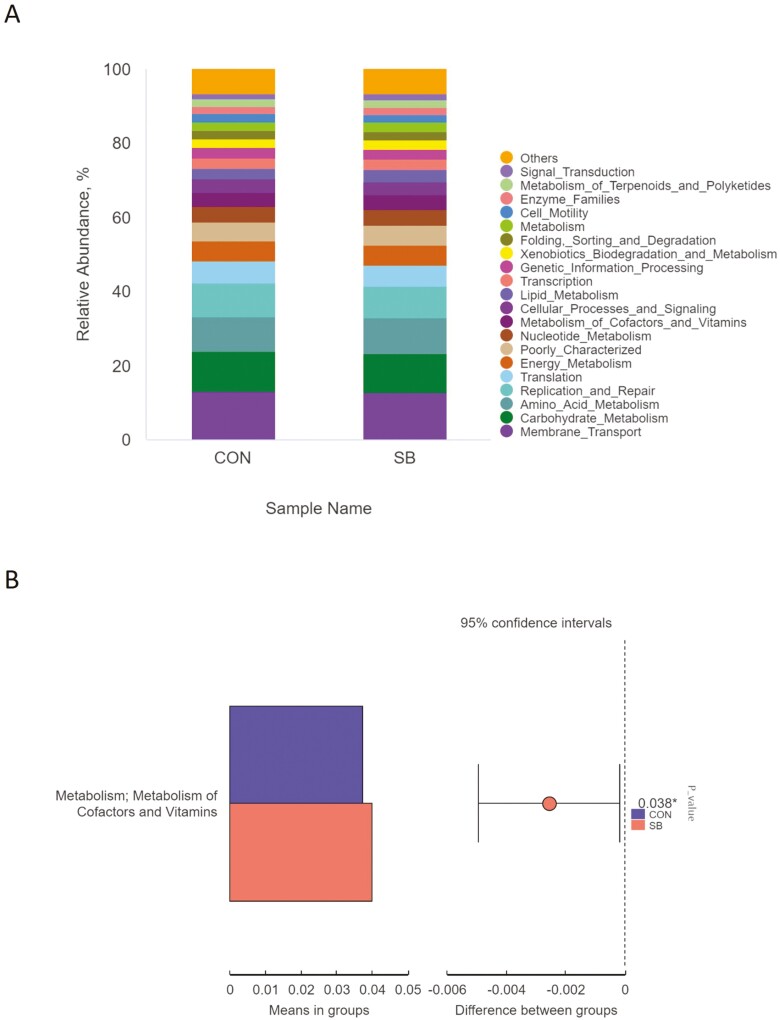
To investigate the functional differences in the jejunal microbiota of Hu sheep affected by SB, we performed a functional analysis of microbiota using PICRUSt. “Membrane_Transport” (12.9%), “Carbohydrate_Metabolism” (10.7%), “Amino_Acid_Metabolism” (9.47%), and “Replication_and_Repair” (8.78%) were identified as the top four predicted functions for the jejunal microbiota in both groups (Figure 8A). In the SB group, the function “Metabolism of Cofactors and Vitamins” increased (P < 0.05; Figure 8B).
Figure 8.
The relative abundance (A) and significant difference (B) in predicted metabolism pathways (KEGG) between the CON and SB groups from the jejunum using PIURUSt. CON, control; SB, sodium butyrate.
Relationships between bacterial taxa and phenotypic variables
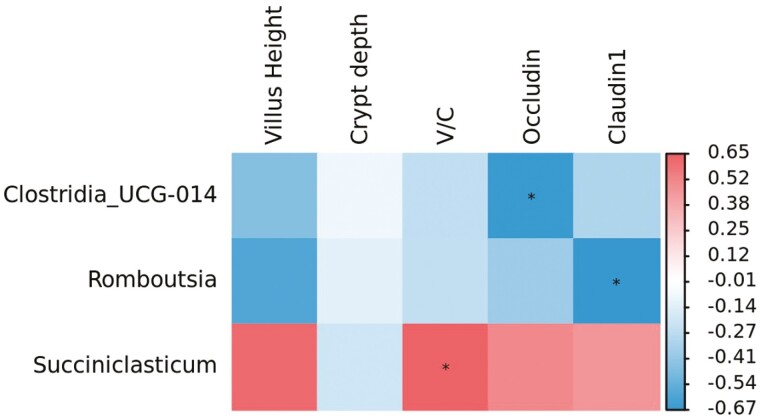
Correlation analysis showed that the ratio of villus height to crypt depth in the jejunum was positively (Figure 9, P < 0.05) correlated with the relative abundance of Succiniclasticum. The relative abundances of Clostridia_UCG-014 and Romboutsia were negatively (P < 0.05) associated with the Claudin-1 and occludin genes expression, respectively.
Figure 9.
Pearson’s correlation coefficients for individual major jejunal bacterial genera and phenotypic variables. Value represents the correlation coefficient, with positive value representing a positive correlation and negative value denoting a negative correlation. * P < 0.05.
Discussion
Many studies reported that SB has benefits on dry matter intake and feed efficiency in preweaning calves (Górka et al., 2011; Sun et al., 2019). Liu et al. (2019) indicated that the infusion of SB for 39 d at 0.36 g/kg BW can improve the performance of preweaning lambs. The natural ewe’s milk-feeding strategy were used in this study, so that only the starter intake was determined. The result showed that supplementation with SB increased the ADG of suckling lambs during the whole period, which might be due to the fact that butyrate could provide more energy for animal growth (Liu et al., 2019).
For suckling lambs, the small intestine plays a vital role in the digestion and absorption of dietary ingredients, and intestinal integrity is necessary for maintaining digestive function. In this study, SB supplementation enhanced the small intestine index of lambs, which may be due to the stimulation effect of oral infusion on the esophageal groove, so that SB made a promotion effect on small intestine maturation (Amin et al., 2022). Butyrate is considered as the primary chemical stimulator involved in the development of gastrointestinal tract epithelium (Mentschel et al., 2001), it provides greater energy to intestinal cells (through the decomposition of butyric acid), and accelerates the regeneration of intestinal epithelial cells (Hou et al., 2023). Furthermore, butyrate is also a known potent histone deacetylase inhibitor (Liu et al., 2018) that induces cellular differentiation (Donohoe et al., 2012). Our histomorphometry results showed that lambs orally administrated SB had a positive effect on the development of small intestine, as evidenced by the longer jejunum villi and smaller ileum crypt depth. The villus height, crypt depth, and villus height/crypt depth values of the small intestine are important indicators of intestinal development and function. The higher the villi height indicates a stronger capacity for digestion and absorption (Hou et al., 2023). While, crypts are involved in the generation and transportation of intestinal epithelial cells, and the smaller the crypt depth would increase the rate of mature cells (Clevers, 2013). Koch et al. (2019) observed that oral butyrate administration with milk replacer stimulated small intestinal mucosal growth of calves at weaning. Similar histological changes (increased villus size) were also found in the duodenum and jejunum epithelium of calves that had received butyrate treatment (Sun et al., 2019). Moreover, the accelerated development and maturation of small intestinal epithelium is conducive to the absorption and digestion of feed components, and provides physiological nutrients for lambs (Liu et al., 2019).
The intestinal barrier is an important defense line to prevent harmful substances from invading the body, and maintaining its integrity is very important for body health (Liu et al., 2020; Li et al., 2021). The tight junctions between intestinal epithelial cells play an important role in protecting intestinal barrier structure and development. When the intestinal epithelial barrier is destroyed, the intestinal permeability will increase, and tight junctions can limit and regulate intestinal permeability (Lee, 2015). To further investigate the effects of SB on intestinal barrier function, the mRNA and protein expressions of intestinal mucosal barrier function (claudin-1 and occludin) in the jejunum were detected. Butyrate is known to promote tight junction proteins redistribution and increase the expression level of tight junction (Peng et al., 2009). A study by Feng et al. (2018) showed that butyrate enhances the intestinal defense barrier by decreasing small intestinal permeability and increasing the expression of tight junction proteins. Tight junction proteins play an important role in controlling intestinal permeability and maintaining intestinal health barrier (Yadav et al., 2022). Claudins family and Occludin are the basis of intestinal barrier structure and composition (Feldman et al., 2005; Martínez et al., 2012). The paracellular permeability between intestinal epithelial cells is decreased by the expression of claudin-1 (Amasheh et al., 2011) and occludin (Suzuku, 2013). Claudins family constitutes a family of more than 20 proteins with four transmembrane domains. Among which Claudin-1, a major structural component of the tight junction complex (Morita et al., 1999), is the most commonly reported defective tight junction protein (Piche et al., 2009; Bertiaux-Vandaele et al., 2011). Pope et al. (2014) found that an upregulated expression of Claudin-1 could regulate intestinal epithelial homeostasis by inhibiting the goblet cell differentiation. Wu et al. (2022) reported that the incidence of diarrhea had a negative relationship with the expression of claudin-1 in the jejunum. Furthermore, the expression of Occludin affects the intestinal tight junction barrier function, and the decrease or loss of Occludin expression results in the increase of permeability between intestinal epithelial cells (Al-Sadi et al., 2011; Hu et al., 2013). Our findings revealed that SB supplementation upregulated both the mRNA and protein expressions of Claudin-1 and Occludin in the jejunum of suckling lambs, indicating that SB has a protective effect on the intestinal barrier of lambs. However, the effect of butyrate on the intestinal barrier function may be concentration-dependent, it would disrupt intestinal barrier function by inducing apoptosis at high concentrations (Huang et al., 2014). Górka et al. (2018) believed that a lower level of supplementation before weaning and a higher one after weaning seems to be a good starting point for further research.
The intestinal microbiota is a complex ecosystem, which is closely related to the growth and health of the intestine. When the microbiota is out of balance, it will cause animal diseases, including metabolic disorders, diarrhea, enteritis, and so on (Debnath et al., 2021). Butyrate and its derivatives have been widely used as feed additives to protect the intestinal health of animals. However, there was limited study on the microbial mechanisms of butyrate’s protective effect on the intestinal. In this study, there was no significant difference in the number of unique OTUs, α-diversity, or β-diversity index between the two groups, indicating that the supplementation with SB did not affect the composition and structure of the jejunal microflora of lambs, and did not destroy the balance of the intestinal ecosystem. Firmicutes, Proteobacteria, and Actinobacteria were the dominant bacteria in the jejunum of lambs in this study. Selim et al. (2021) found that some of Actinobacteria can produce antibiotic-like substances that have anti-inflammatory effects. In addition, the relative abundance of Actinobacteria tended to be higher in the SB group than that in the CON group. Butyric acid, the active ingredient in SB, plays a role in balancing the microbial flora in the intestine. Due to its lipid solubility and water solubility, butyric acid can pass through the cell membrane of bacteria by molecular diffusion and dissociate into H+ and butyric acid ions. Thus, the harmful bacteria with weak acid resistance in the gastrointestinal tract would die of the lower intracellular pH value. At the genus level, the relative abundance of Succiniclasticum and Syntrophococcus in the SB group were higher than those in the CON group. Succiniclasticum plays an important role in the succinic acid production and can convert succinic acid to propionic acid, which is a major ruminal short-chain fatty acid (Van Gylswyk, 1995). Chaucheyras-Durand et al. (2016) also revealed that Succiniclasticum have high fiber decomposition activity and can degrade cellulose that cannot be degraded by Ruminococcus. Syntrophococcus spp. is found to act as a one-carbon compound provider bacteria (Doré and Bryant, 1990) and convert macromolecular substances into acetic acid (Xiang et al., 2019). The higher relative abundance of Syntrophococcus was consistent with the results that SB supplementation significantly increased the concentration of acetic acid. In the current study, LEfSe results showed that the bacteria taxa with significant differences in the SB group included f_Burkholderiaceae, g_Aeriscardovia, p_Acidobacteriota, and Clostridium_sensu_stricto_13. Aeriscardovia belongs to the bifidobacteriaceae family, which produces many structurally complex enzyme preparations and antibiotic metabolites, and is involved in the degradation pathway of amino acids and fatty acids (Lugli et al., 2017). Furthermore, Clostridium_sensu_stricto_13 is a butyric acid-producing bacterium that promotes the production of butyric acid kinase (Takada et al., 2016; Louis and Flint, 2017). These results indicated that SB can promote the production of beneficial bacteria in the jejunum of lambs, so as to have a positive effect on the health of intestinal flora.
The microbial potential function analysis revealed that “Membrane Transport”, “Carbohydrate Metabolism”, “Amino Acid Metabolism,” and “Replication and Repair” are the top predicted functions. These findings were consistent with Yang et al. (2018), suggesting that the major microbial functions remain stable. Ma et al. (2022) believed that PICRUSt still has drawbacks in predicting the potential functions, therefore, functional analysis of the intestinal microbiome using metagenomic and/or metabolomic analyses would be used to investigate the effects of SB on intestinal function in the further study.
Clostridia_UCG-014, one of the proinflammatory bacteria (Wang et al., 2021a; Zhong et al., 2023b), was negatively correlated with milk lactose but positively associated with milk somatic cell counts of dairy cows (Wang et al., 2021b). Moreover, Wang et al. (2021b) reported that Romboutsia was positively associated with serum levels of four lipids and it has been correlated with obesity and its related disorders. Similarly, we found that the relative abundances of Clostridia_UCG-014 and Romboutsia were negatively associated with the Claudin-1 and occludin gene expression, respectively. These results indicated that the protects intestines from pathogenic bacteria was enhanced by the supplementation with SB.
Conclusion
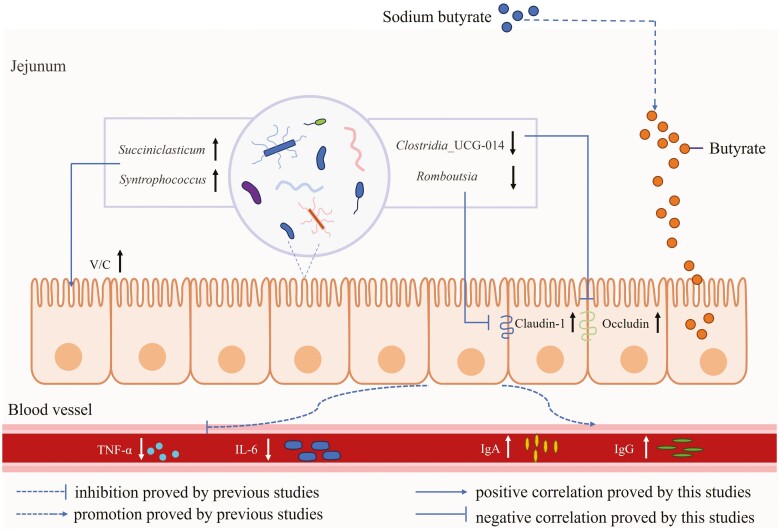
SB oral delivery could increase the small intestine index and improve the small intestinal morphology parameters. In addition, supplementation with SB upregulated the mRNA and protein expressions of intestinal mucosal barrier function (Claudin-1 and Occludin) in the jejunum. Moreover, administering sodium butyrate increased the abundance of Succiniclasticum, which was positively associated with ratio of villus height to crypt depth in the jejunum, but decreased the relative abundances of Clostridia_UCG-014 and Romboutsia, which were negatively corrected with the gene expressions of tight junction proteins. Therefore, sodium butyrate supplementation inhibited harmful bacteria colonization, enhanced intestinal barrier functions, and consequently improved intestinal development and growth performance of suckling lambs (Figure 10).
Figure 10.
Graphical summary of the effect of sodium butyrate administration on the intestinal development and health of suckling lambs. Sodium butyrate oral delivery inhibited harmful bacteria colonization, enhanced intestinal barrier functions, and consequently improved intestinal development and growth performance of suckling lambs.
Supplementary Material
Acknowledgments
The research was supported by the fund for the National Natural Science Foundation of China (31902179 and 32172742), General Scientific Research Project of Zhejiang Education Department (Y202352658) and “San Nong Jiu Fang” Technology Cooperation Project of Zhejiang Province (2022SNJF054).
Glossary
Abbreviations
- ADG
average daily gain
- BW
body weight
- CON
control
- LEfSe
linear discriminant analysis effect size
- OTUs
operational taxonomic units
- PCoA
principal coordinate analysis
- PICRUSt
phylogenetic investigation of communities by reconstruction of unobserved States
- SB
sodium butyrate
- SCFAs
short-chain fatty acids
- V/C
villus height/crypt depth
Contributor Information
Mengzhen Sun, College of Animal Science and Technology, College of Veterinary Medicine, Zhejiang A & F University; Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, Lin’an 311300, China.
Wenwen Ji, College of Animal Science and Technology, College of Veterinary Medicine, Zhejiang A & F University; Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, Lin’an 311300, China.
Hongwei Ye, Hangzhou Lin ‘an District Agroforestry Technology Extension Center, Lin’an 311300, China.
Yitao Cai, College of Animal Science and Technology, College of Veterinary Medicine, Zhejiang A & F University; Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, Lin’an 311300, China.
Yan Yun, College of Animal Science and Technology, College of Veterinary Medicine, Zhejiang A & F University; Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, Lin’an 311300, China.
Xiaoshi Wei, College of Animal Science and Technology, College of Veterinary Medicine, Zhejiang A & F University; Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, Lin’an 311300, China.
Chong Wang, College of Animal Science and Technology, College of Veterinary Medicine, Zhejiang A & F University; Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, Lin’an 311300, China.
Huiling Mao, College of Animal Science and Technology, College of Veterinary Medicine, Zhejiang A & F University; Key Laboratory of Applied Technology on Green-Eco-Healthy Animal Husbandry of Zhejiang Province, Lin’an 311300, China.
Conflict of interest statement
The authors declare no conflicts of interest in this work, and editors and editorial board members of the journal are neither included in the list of authors nor contributors.
Authors’ Contributions
M.S.: methodology, investigation, and writing-original draft preparation. W.J.: investigation, data collection, writing-original draft preparation. H.Y.: conceptualization, data collection. Y.C.: investigation, data collection, sample analysis. Y.Y.: investigation, sample analysis. X.W.: editing, statistical analysis. C.W.: editing, methodology, funding acquisition. H.M.: conceptualization, writing review, formal statistical analysis, funding acquisition.
Literature Cited
- Al-Sadi, R., Khatib K., Guo S., Ye D., Youssef M., and Ma T... 2011. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 300:G1054–G1064. doi: 10.1152/ajpgi.00055.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh, S., Fromm M., and Günzel D... 2011. Claudins of intestine and nephron-a correlation of molecular tight junction structure and barrier function. Acta Physiol. 201:133–140. doi: 10.1111/j.1748-1716.2010.02148.x [DOI] [PubMed] [Google Scholar]
- Amin, A. B., Trabi E. B., Zhu C., and Mao S... 2022. Role of butyrate as part of milk replacer and starter diet on intestinal development in pre-weaned calves. A systematic review. Anim. Feed Sci. Technol. 292:115423. doi: 10.1016/j.anifeedsci.2022.115423 [DOI] [Google Scholar]
- Bertiaux-Vandaele, N., Youmba S. B., Belmonte L., Lecleire S., Antonietti M., Gourcerol G., Leroi A. M., Dechelotte P., Menard J. F., Ducrotte P.,. et al. 2011. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am. J. Gastroenterol. 106:2165–2173. doi: 10.1038/ajg.2011.257 [DOI] [PubMed] [Google Scholar]
- Blum, J. W. 2006. Nutritional physiology of neonatal calves. J. Anim. Physiol. Anim. Nutr. (Berl) 90:1–11. doi: 10.1111/j.1439-0396.2005.00614.x [DOI] [PubMed] [Google Scholar]
- Chao, A. 1984. Nonparametric estimation of the number of classes in a population. Scand. J. Stat. 11:265–270. doi: 10.2307/4615964 [DOI] [Google Scholar]
- Chaucheyras-Durand, F., Ameilbonne A., Bichat A., Mosoni P., Ossa F., and Forano E... 2016. Live yeasts enhance fibre degradation in the cow rumen through an increase in plant substrate colonization by fibrolytic bacteria and fungi. J. Appl. Microbiol. 120:560–570. doi: 10.1111/jam.13005 [DOI] [PubMed] [Google Scholar]
- Clevers, H. 2013. The intestinal crypt, a prototype stem cell compartment. Cell 154:274–284. doi: 10.1016/j.cell.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Debnath, N., Kumar R., Kumar A., Mehta P. K., and Yadav A. K... 2021. Gut-microbiota derived bioactive metabolites and their functions in host physiology. Biotechnol. Genet. Eng. Rev. 37:105–153. doi: 10.1080/02648725.2021.1989847 [DOI] [PubMed] [Google Scholar]
- Diao, Q., Zhang R., and Fu T... 2019. Review of strategies to promote rumen development in calves. Animals. 9:490. doi: 10.3390/ani9080490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe, D. R., Collins L. B., Wali A., Bigler R., Sun W., and Bultman S. J... 2012. The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation. Mol. Cell. 48:612–626. doi: 10.1016/j.molcel.2012.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré, J., and Bryant M. P... 1990. Metabolism of one-carbon compounds by the ruminal acetogen syntrophococcus sucromutans. Appl. Environ. Microbiol. 56:984–989. doi: 10.1128/aem.56.4.984-989.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, G. J., Mullin J. M., and Ryan M. P... 2005. Occludin: structure, function and regulation. Adv. Drug Deliv. Rev. 57:883–917. doi: 10.1016/j.addr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Feng, W., Wu Y., Chen G., Fu S., Li B., Huang B., Wang D., Wang W., and Liu J... 2018. Sodium butyrate attenuates diarrhea in weaned piglets and promotes tight junction protein expression in colon in a GPR109A-dependent manner. Cell. Physiol. Biochem. 47:1617–1629. doi: 10.1159/000490981 [DOI] [PubMed] [Google Scholar]
- Fukumori, R., Doi K., Mochizuki T., Oikawa S., Gondaira S., Iwasaki T., and Izumi K... 2022. Sodium butyrate administration modulates the rumen villus height, inflammation-related gene expression, and plasma hormones concentration in dry cows fed a high-fiber diet. Anim. Sci. J. 93:e13791. doi: 10.1111/asj.13791 [DOI] [PubMed] [Google Scholar]
- Gagen, E. J., Denman S. E., Padmanabha J., Zadbuke S., Jassim R., Morrison M., and Mcsweeney C. S... 2010. Functional gene analysis suggests different acetogen population in the bovine rumen and tammar wallaby forestomach. Appl. Environ. Microbiol. 76:7785–7795. doi: 10.1128/AEM.01679-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górka, P., Kowalski Z. M., Pietrzak P., Kotunia A., Jagusiak W., Holst J. J., Guilloteau P., and Zabielski R... 2011. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J. Dairy Sci. 94:5578–5588. doi: 10.3168/jds.2011-4166 [DOI] [PubMed] [Google Scholar]
- Górka, P., Kowalski Z. M., Zabielski R., and Guilloteau P... 2018. Invited review: use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 101:4785–4800. doi: 10.3168/jds.2017-14086 [DOI] [PubMed] [Google Scholar]
- Guilloteau, P., Savary G., Jaguelin-Peyrault Y., Romé V., Le Normand L., and Zabielski R... 2010. Dietary sodium butyrate supplementation increases digestibility and pancreatic secretion in young milk-fed calves. J. Dairy Sci. 93:5842–5850. doi: 10.3168/jds.2009-2751 [DOI] [PubMed] [Google Scholar]
- Hou, J., Lian L., Lu L., Gu T., Zeng T., Chen L., Xu W., Li G., Wu H., and Tian Y... 2023. Effects of dietary Bacillus coagulans and tributyrin on growth performance, serum antioxidants, intestinal morphology, and cecal microbiota of growing Yellow-Feathered Broilers. Animals. 13:3534. doi: 10.3390/ani13223534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, C., Song J., Li Y., Luan Z., and Zhu K... 2013. Diosmectite–zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 110:681–688. doi: 10.1017/s0007114512005508 [DOI] [PubMed] [Google Scholar]
- Huang, X., Li Z., Zhu L., Huang H., Hou L., and Lin J... 2014. Inhibition of p38 mitogen-activated protein kinase attenuates butyrate-induced intestinal barrier impairment in a Caco-2 cell monolayer model. J. Pediatr. Gastroenterol. Nutr. 59:264–269. doi: 10.1097/MPG.0000000000000369 [DOI] [PubMed] [Google Scholar]
- Koch, C., Gerbert C., Frieten D., Dusel G., and Hammon H. M... 2019. Effects of ad libitum milk replacer feeding and butyrate supplementation on the epithelial growth and development of the gastrointestinal tract in Holstein calves. J. Dairy Sci. 102:8513–8526. doi: 10.3168/jds.2019-16328 [DOI] [PubMed] [Google Scholar]
- Langille, M. G. I., Zaneveld J., Caporaso J. G., McDonald D., Knights D., Reyes J. A., Clemente J. C., Burkepile D. E., Vega Thurber R. L., Knight R.,. et al. 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 31:814–821. doi: 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. H. 2015. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest. Res. 13:11–18. doi: 10.5217/ir.2015.13.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Wen J., Jiao L., Wang C., Hong Q., Feng J., and Hu C... 2021. Dietary copper/zinc-loaded mont-morillonite improved growth performance and intestinal barrier and changed gut microbiota in weaned piglets. J. Anim. Physiol. Anim. Nutr. (Berl) 105:678–686. doi: 10.1111/jpn.13522 [DOI] [PubMed] [Google Scholar]
- Liu, H., Wang J., He T., Becker S., Zhang G., Li D., and Ma X... 2018. Butyrate: a double-edged sword for health. Adv. Nutr. 9:21–29. doi: 10.1093/advances/nmx009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L., Sun D., Mao S., Zhu W., and Liu J... 2019. Infusion of sodium butyrate promotes rumen papillae growth and enhances expression of genes related to rumen epithelial VFA uptake and metabolism in neonatal twin lambs. J. Anim. Sci. 97:909–921. doi: 10.1093/jas/sky459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, D. Y., Lou W. J., Zhang D. Y., and Sun S. Y... 2020. ROS plays a role in the neonatal rat intestinal barrier damages induced by hyperoxia. Biomed Res. Int. 2020:8819195. doi: 10.1155/2020/8819195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S., Wu J., Wu Z., Alugongo G. M., Khan M. Z., Li J., Xiao J., He Z., Ma Y., Li S.,. et al. 2022. Tributyrin administration improves intestinal development and health in pre-weaned dairy calves fed milk replacer. Anim. Nutr. 10:399–411. doi: 10.1016/j.aninu.2022.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis, P., and Flint H. J... 2017. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19:29–41. doi: 10.1111/1462-2920.13589 [DOI] [PubMed] [Google Scholar]
- Lozupone, C. A., Hamady M., Kelley S. T., and Knight R... 2007. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 73:1576–1585. doi: 10.1128/AEM.01996-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli, G. A., Milani C., Turroni F., Duranti S., Mancabelli L., Mangifesta M., Ferrario C., Modesto M., Mattarelli P., Jiří K.,. et al. 2017. Comparative genomic and phylogenomic analyses of the Bifidobacteriaceae family. BMC Genomics 18:1–15. doi: 10.1186/s12864-017-3955-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, Y., Deng X., Yang X., Wang J., Li T., Hua G., Han D., Da L., Li R., Rong W.,. et al. 2022. Characteristics of bacterial microbiota in different intestinal segments of Aohan Fine-Wool sheep. Front. Microbiol. 13:874536. doi: 10.3389/fmicb.2022.874536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, H., Wang C., and Yu Z... 2019. Dietary leucine supplementation enhances the heath of early weaned Hu lambs. Anim. Feed Sci. Technol. 247:248–254. doi: 10.1016/j.anifeedsci.2018.11.020 [DOI] [Google Scholar]
- Martínez, C., González-Castro A., Vicario M., and Santos M... 2012. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome. Gut Liver. 6:305. doi: 10.5009/gnl.2012.6.3.305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy, D. E., Wilkins K. R., Hiltz R. L., Moreland S., Klanderman K., and Laarman A. H... 2019. Effects of supplemental butyrate and weaning on rumen fermentation in Holstein calves. J. Dairy Sci. 102:8874–8882. doi: 10.3168/jds.2019-16652 [DOI] [PubMed] [Google Scholar]
- Mentschel, J., Leiser R., Mülling C., Pfarrer C., and Claus R... 2001. Butyric acid stimulates rumen mucosa development in the calf mainly by a reduction of apoptosis. Arch. Tierernahr. 55:85–102. doi: 10.1080/17450390109386185 [DOI] [PubMed] [Google Scholar]
- Morita, K., Furuse M., Fujimoto K., and Tsukita S... 1999. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. USA. 96:511–516. doi: 10.1073/pnas.96.2.511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L., Li Z. R., Green R. S., Holzman I. R., and Lin J... 2009. Butyrate enhances the intestinal barrier by facilitating tight junction assembly via activation of AMP-activated protein kinase in Caco-2 cell monolayers. J. Nutr. 139:1619–1625. doi: 10.3945/jn.109.104638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao, M. Y., Ma J. N., Diao Q. Y., and Tu Y... 2021. Effects of diets with different solid-to-liquid feed ratios with the same dry matter intake on the growth performance and gastrointestinal development of male Holstein calves. Anim. Feed Sci. Technol. 274:114846. doi: 10.1016/j.anifeedsci.2021.114846 [DOI] [Google Scholar]
- Piche, T., Barbara G., Aubert P., Bruley D. V. S., Dainese R., Nano J. L., Cremon C., Stanghellini V., De Giorgio R., Galmiche J. P.,. et al. 2009. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 58:196–201. doi: 10.1136/gut.2007.140806 [DOI] [PubMed] [Google Scholar]
- Pielou, E. C. 1966. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13:131–144. doi: 10.1016/0022-5193(66)90013-0 [DOI] [Google Scholar]
- Pope, J. L., Bhat A. A., Sharma A., Ahmad R., Krishnan M., Washington M. K., Beauchamp R. D., Singh A. B., and Dhawan P... 2014. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch signaling. Gut. 63:622–634. doi: 10.1136/gutjnl-2012-304241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segata, N., Izard J., Waldron L., Gevers D., Miropolsky L., Garrett W. S., and Huttenhower C... 2011. Metagenomic biomarker discovery and explanation. Genome Biol. 12:R60. doi: 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim, M. S. M., Abdelhamid S. A., and Mohamed S. S... 2021. Secondary metabolites and biodiversity of actinomycetes. J. Genet. Eng. Biotechnol. 19:72. doi: 10.1186/s43141-021-00156-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon, C. E. 1948. A mathematical theory of communication. Bell Syst. Tech. J. 27:623–656, 623–656. doi: 10.1002/j.1538-7305.1948.tb00917.x [DOI] [Google Scholar]
- Simpson, E. H. 1949. Measurement of diversity. Nature. 163:688. doi: 10.1136/thx.27.2.261 [DOI] [Google Scholar]
- Steele, M. A., Penner G. B., Chaucheyras-Durand F., and Guan L. L... 2016. Development and physiology of the rumen and the lower gut: targets for improving gut health. J. Dairy Sci. 99:4955–4966. doi: 10.3168/jds.2015-10351 [DOI] [PubMed] [Google Scholar]
- Sun, Y. Y., Li J., Meng Q. S., Wu D. L., and Xu M... 2019. Effects of butyric acid supplementation of acidified milk on digestive function and weaning stress of cattle calves. Livest. Sci. 225:78–84. doi: 10.1016/j.livsci.2019.04.021 [DOI] [Google Scholar]
- Suzuku, T. 2013. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 70:631–659. doi: 10.1007/s00018-012-1070-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada, T., Watanabe K., Makino H., and Kushiro A... 2016. Reclassification of Eubacterium desmolans as Butyricicoccus desmolans comb. nov., and description of Butyricicoccus faecihominis sp. nov., a butyrate-producing bacterium from human faeces. Int. J. Syst. Evol. Microbiol. 66:4125–4131. doi: 10.1099/ijsem.0.001323 [DOI] [PubMed] [Google Scholar]
- Van Gylswyk, N. O. 1995. Succiniclasticum ruminis gen. nov., sp. nov., a ruminal bacterium converting succinate to propionate as the sole energy-yielding mechanism. Int. J. Syst. Bacteriol. 45:297–300. doi: 10.1099/00207713-45-2-297 [DOI] [PubMed] [Google Scholar]
- Wang, Y., Nan X., Zhao Y., Jiang L., Wang H., Zhang F., Hua D., Liu J., Yao J., Yang L.,. et al. 2021a. Dietary supplementation of inulin ameliorates subclinical mastitis via regulation of rumen microbial community and metabolites in dairy cows. Microbiol. Spectr. 9:e0010521. doi: 10.1128/Spectrum.00105-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., Ablimit N., Zhang Y., Li J., Wang X., Liu J., Miao T., Wu L., Wang H., Wang Z.,. et al. 2021b. Novel β-mannanase/GLP-1 fusion peptide high effectively ameliorates obesity in a mouse model by modifying balance of gut microbiota. Int. J. Biol. Macromol. 191:753–763. doi: 10.1016/j.ijbiomac.2021.09.150 [DOI] [PubMed] [Google Scholar]
- Wu, D. L., Meng Q. S., Wang Y. D., Wang M. Y., Xu E. H., Xiao L., and Xu M... 2022. Dietary supplementation of free or two fat-coated sodium butyrate with varying release times on gastrointestinal development and tight junctions in preweaning Holstein calves. Anim. Feed Sci. Technol. 285:115224. doi: 10.1016/j.anifeedsci.2022.115224 [DOI] [Google Scholar]
- Xiang, Z., Tong W., Guo Z., Xu Y., Guo J., Ruan Y., and Zhao P... 2019. Rat H1 parvovirus infection leads to alterations in gut microbiota. Pathog. Dis. 77:ftz058. doi: 10.1093/femspd/ftz058 [DOI] [PubMed] [Google Scholar]
- Yadav, S., Teng P. Y., Choi J., Singh A. K., and Kim W. K... 2022. Nutrient profile and effects of carinata meal as alternative feed ingredient on broiler performance, tight junction gene expression and intestinal morphology. Poult. Sci. 101:101411. doi: 10.1016/j.psj.2021.101411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X., Yin F., Yang Y., Lepp D., Yu H., Ruan Z., Yang C., Yin Y., Hou Y., Leeson S.,. et al. 2018. Dietary butyrate glycerides modulate intestinal microbiota composition and serum metabolites in broilers. Sci. Rep. 8:4940. doi: 10.1038/s41598-018-22565-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, H., Yu W., Wang M., Lin B., Sun X., Zheng N., Wang J., and Zhao S... 2023a. Sodium butyrate promotes gastrointestinal development of preweaning bull calves via inhibiting inflammation, balancing nutrient metabolism, and optimizing microbial community functions. Anim. Nutr. 14:88–100. doi: 10.1016/j.aninu.2023.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, X., Zhao Y., Huang L., Liu J., Wang K., Gao X., Zhao X., and Wang X... 2023b. Remodeling of the gut microbiome by Lactobacillus johnsonii alleviates the development of acute myocardial infarction. Front. Microbiol. 14:1140498. doi: 10.3389/fmicb.2023.1140498 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.