Abstract
Cyclin D2 is a member of the family of D-type cyclins that is implicated in cell cycle regulation, differentiation, and oncogenic transformation. To better understand the role of this cyclin in the control of cell proliferation, cyclin D2 expression was monitored under various growth conditions in primary human and established murine fibroblasts. In different states of cellular growth arrest initiated by contact inhibition, serum starvation, or cellular senescence, marked increases (5- to 20-fold) were seen in the expression levels of cyclin D2 mRNA and protein. Indirect immunofluorescence studies showed that cyclin D2 protein localized to the nucleus in G0, suggesting a nuclear function for cyclin D2 in quiescent cells. Cyclin D2 was also found to be associated with the cyclin-dependent kinases CDK2 and CDK4 but not CDK6 during growth arrest. Cyclin D2-CDK2 complexes increased in amounts but were inactive as histone H1 kinases in quiescent cells. Transient transfection and needle microinjection of cyclin D2 expression constructs demonstrated that overexpression of cyclin D2 protein efficiently inhibited cell cycle progression and DNA synthesis. These data suggest that in addition to a role in promoting cell cycle progression through phosphorylation of retinoblastoma family proteins in some cell systems, cyclin D2 may contribute to the induction and/or maintenance of a nonproliferative state, possibly through sequestration of the CDK2 catalytic subunit.
In eukaryotes, cell proliferation is regulated by the cooperative activity of a set of cell cycle control genes (reviewed in references 56 and 57). These genes include those that encode cyclin-dependent kinases (CDKs) and the proteins that regulate their behavior, cyclins and CDK inhibitors. Analysis of cyclin and CDK inhibitor expression has indicated that phase-specific oscillations in the abundance of some of these proteins are responsible, in part, for the orderly and linear progression of cells through key cell cycle checkpoints. In mammalian cells, an important cell cycle checkpoint called the restriction point is located at a position just prior to the onset of DNA synthesis (51). The expression of a subset of cell cycle control genes occurs during the G1 phase of the cycle, placing them in a temporal position to influence this key cell cycle decision (56). Among such candidate G1 control genes are those encoding cyclins D and E and the CDK inhibitors belonging to the p21WAF/CIP/SDI and p16INK4 families.
The D-type cyclins consist of three family members, cyclins D1, D2, and D3. The cyclin D1 gene was identified as a delayed-early gene that was inducible by colony-stimulating factor (37), by its ability to complement G1 cyclin-deficient yeast strains (33, 74), and as the PRAD-1/bcl-1 proto-oncogene that underwent gene rearrangements, gene amplification, and deregulated expression in a variety of tumor types (reviewed in reference 43). Microinjection experiments with anti-cyclin D1 antibodies have suggested that cyclin D1 may be required for progression of cells through G1 (4, 35, 52). Cyclins D2 and D3 were cloned as a consequence of their homology to cyclin D1 (25, 30, 37, 44, 74, 75), and cyclin D2 was also independently identified as encoded by a gene mutated by proviral insertion (20). Aberrant expression of cyclin D2 has been linked to human male germ cell tumorigenesis (24).
The G1 regulatory function of D-type cyclins is thought to be mediated by their interactions with CDK2, -4, and -6 (38, 39, 41, 76) and the retinoblastoma susceptibility gene product, Rb (9, 12, 27). Phosphorylation of Rb by cyclin D in complexes with CDK4 or CDK6 in mid- to late G1 is believed to trigger the onset of S phase by inducing the release of E2F transcription factors from growth-inhibitory Rb complexes (67). The free E2F proteins are then thought to transcriptionally activate genes involved in the activation and maintenance of DNA synthesis. However, given the experimental results obtained from E2F knockout mice, the precise molecular function of Rb-E2F complexes is currently less clear (68). Also, cyclin D1 has recently been shown to regulate gene expression independent of kinase activity (46, 78).
We and others have previously reported that in addition to positively regulating traverse through specific points of the cell cycle, overexpression of cyclin D1 in primary human fibroblasts inhibits proliferation in chronic growth assays and blocks cells from entering into S phase in acute growth assays (2, 49). Mice lacking cyclin D1 appear to develop normally except for a subset of cells within the retina and breast epithelium (13, 58) which may be related to the high levels of Rb seen in the retina (26) and the CDK-independent activation of the estrogen receptor by cyclin D1 (78), respectively. As for cyclin D1, targeted inactivation of the cyclin D2 gene in mice affects few cell types, resulting in hypoplastic ovaries and testes (59). Furthermore, the D-type cyclins have been shown to be induced during exit from the cell cycle seen upon differentiation of a wide variety of cell types (15), and cyclin D1 has been reported by many groups to be upregulated during cellular senescence (reviewed in reference 42). A brain-specific form of cyclin D2 (MN20) has been linked to differentiation of particular neural cell populations (54, 55), and coexpression of cyclin D2 and Ha-Ras under low-serum conditions can induce a senescence-like phenotype (28). These results suggest that D-type cyclins may have different roles depending on their levels of expression and cell type, which may be independent of CDK activity. Here we provide additional evidence that one function of D-type cyclins may be to act as inhibitors of cell proliferation. Our results demonstrate that cyclin D2 expression is unique among the D-type cyclins, being upregulated manyfold under three distinct conditions of growth arrest in phenotypically normal human and murine fibroblasts. Increased levels of cyclin D2 preferentially associate with CDK2 and CDK4 but not CDK6, and these complexes are inactive as histone H1 kinases. Furthermore, ectopic overexpression of cyclin D2 efficiently blocks cell cycle progression, suggesting an alternate role for cyclin D2 in promoting exit from the cell cycle and maintaining cells in a nonproliferative state.
MATERIALS AND METHODS
Cells and cell culture.
Established NIH 3T3 cells (CRL 1658) and the Hs68 (CRL 1635) and WI-38 (CCL 75) primary human diploid fibroblast (HDF) strains were obtained from the American Type Culture Collection. Forearm epidermal (A2) fibroblasts were a kind gift from S. Goldstein. HDFs were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco-BRL) containing low glucose, while NIH 3T3 fibroblasts were maintained in DMEM containing high glucose. The media for both human and murine cells were supplemented with penicillin-streptomycin (Gibco) and 10% fetal calf serum.
For growth studies, we defined senescence as a state where less than 10% (typically 3 to 5%) of cells enter DNA synthesis in response to mitogens in a 36-h period (2). Senescence occurs at different passage numbers for the primary strains used; under our culture conditions, cells reach this point as follows: Hs68 cells by 80 to 85 mean population doublings (MPDs), WI-38 cells by 45 to 50 MPDs, and A2 cells by 75 to 80 MPDs. All cell strains and lines were analyzed during exponential growth unless otherwise stated. Cells were grown in 95% air–5% CO2 and maintained at a temperature of 37°C. For study of cyclin expression during contact inhibition of growth, ∼5 × 104 Hs68 (MPD 32) or NIH 3T3 cells were seeded on 10-cm-diameter plates in complete medium containing 10% fetal bovine serum (FBS) and harvested for total RNA and protein at the intervals indicated. For release from contact inhibition, a single 10-cm-diameter plate of 8-day density-arrested Hs68 fibroblasts (∼5 × 105 cells at 32 MPDs) was split at a ratio of 1:12 (∼4 × 104 cells) into 12 10-cm-diameter plates. Cells were harvested at 2 h following replating and every 3 h thereafter for 30 h. Isolated RNA from each plate, representing ∼0.4 μg of total RNA, was used for cDNA synthesis by reverse transcription. For serum deprivation/stimulation experiments, subconfluent Hs68 HDFs (MPD 34) or NIH 3T3 fibroblasts were incubated in 0.1 to 0.2% FBS for 40 to 48 h prior to harvesting or stimulation with 10% FBS.
RNA preparation and RT-PCR.
Total cellular RNA from HDFs and NIH 3T3 cells was extracted from cells and processed for reverse transcription and primer-dropping PCR essentially as described elsewhere (71). Primer sequences used for detection of both human and murine cyclin D2 transcripts were, from 5′ to 3′, TACTTCAAGTGCGTGCAGAAGGAC for the sense primer and TCCCACACTTCCAGTTGCGATCAT for the antisense primer, resulting in a predicted PCR product of 497 bp. The specificity of the primers was confirmed by amplification of the appropriate size product from a human cyclin D2 cDNA and by sequencing of the PCR product (data not shown). Primer sequences and amplification conditions for cyclins B1 and E and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) have been described previously (71). Primer-dropping reverse transcription-PCRs (RT-PCRs) were performed in 50-μl reaction volumes, using PCR tubes with screw-cap lids (Sarstedt) as described previously (71). Optimal PCR cycle numbers required for exponential amplification for each primer set were determined by preliminary range-finding experiments. Total amplification in each multiplex reaction was kept below saturation levels to permit the products to remain within the exponential range of the amplification curve and thereby provide semiquantitative data. All reactions shown in each panel of PCR results were performed under the same reaction conditions. Typically, visualization of GAPDH required 20 to 23 PCR cycles and cyclin D2 mRNA required 24 to 32 cycles, depending on cell type. Gels were illuminated with UV light, photographed with Polaroid film, and analyzed by digital image analysis using a Hewlett-Packard ScanJet IIc scanner and the NIH Image program. The intensities of the ethidium bromide fluorescence signals were determined from the area under the curve for each peak. All PCRs were repeated at least three times to verify results.
Western blotting.
Cells washed with phosphate-buffered saline (PBS) were harvested in 0.5 ml of lysis buffer (50 mM Tris-HCl [pH 7.4]–50 μM pepstatin A–0.2 mM leupeptin–10 mM EDTA–1% Triton X-100 containing freshly added 1 mM phenylmethylsulfonyl fluoride), sonicated, and stored at −20°C until required. Protein concentrations were determined by the Bradford assay and/or Coomassie staining after polyacrylamide gel electrophoresis, and 80 μg of boiled protein in 2× sample buffer was loaded per lane for electrophoresis through sodium dodecyl sulfate–12.5% polyacrylamide gels. Equal loading of protein samples was confirmed visually by Coomassie brilliant blue staining. Proteins were transferred to Polyscreen polyvinylidene difluoride membranes (NEN Research Products) for 2 h at 20 V. The membranes were blocked overnight in PBS containing 10% low-fat milk and 0.1% Tween 20, incubated with rat monoclonal anti-cyclin D2 antibody (Oncogene Science) (1:1,000 dilution in PBS containing 0.1% Tween 20 and 5% milk) for 2 h at room temperature, followed by incubation with a sheep anti-rat immunoglobulin (Ig) biotinylated conjugate (1:1,000) (Amersham) and then strepavidin-horseradish peroxidase conjugate (1:1,000) (Amersham) for 1 h each. The blots were washed for 30 min with PBS-Tween 20 following each incubation. The membrane-bound cyclin D2 protein was detected by enhanced chemiluminescence as instructed by the manufacturer (Amersham).
Indirect immunofluorescence.
NIH 3T3 and Hs68 fibroblasts were plated on glass coverslips at 20 to 40% confluence in complete medium and incubated for 2 to 3 days for analysis of exponentially proliferating cells. For study of cell cycle expression patterns, plated cells were incubated for 1 day and then starved for 36 to 48 h in 0.1% FBS prior to stimulation with 10% FBS for the time indicated. For detection of cyclin D2 protein and nonspecific rabbit IgG, cells on coverslips were fixed by sequential immersion for 10 min each in 3.7% formaldehyde (in PBS) and 0.5% Triton X-100 (in PBS) at room temperature. For detecting nuclear bromodeoxyuridine (BrdU) staining, additional incubations in 3 N HCl for 10 min and sodium borate (0.1 M, pH 8.0) for 1 min were done. Coverslips were washed by immersion in PBS for 10 min followed by incubation with primary antibodies in a humidified chamber at 37°C. Cyclin D2 was detected with a rat monoclonal antibody (1:100; AB-1; Oncogene Science), BrdU was detected with a mouse monoclonal antibody (1:300; Sigma), and rabbit nonspecific antibodies were detected with a sheep anti-rabbit IgG-Texas red conjugate (1:100; Amersham). Secondary and tertiary antibodies for cyclin D2 detection were biotinylated sheep anti-rat Ig (1:100; Amersham) followed by strepavidin-fluorescein conjugate (1:100; Amersham). BrdU was detected with goat anti-mouse IgG-Texas red conjugate (1:100; Amersham). For assaying DNA synthesis, BrdU was added to a final concentration of 5 μg/ml 2 h after serum stimulation, and cells were fixed and stained either 12 or 20 h later. Following antibody incubation, coverslips were mounted on glass slides and photographed in a Zeiss Axiophot microscope, using a Neofluar 40× lens and Kodak Tri-X pan 400 film.
Immunoprecipitation-Western blot and immunoprecipitation-kinase assays.
Lysates from exponentially growing, contact-inhibited, or serum-deprived fibroblasts were prepared by scraping and lysis in radioimmunoprecipitation assay buffer under nondenaturing conditions as described previously (2). Equal amounts of lysates quantitated by Coomassie staining after polyacrylamide gel electrophoresis were incubated for 4 h in antibody excess with rabbit anti-CDK2, anti-CDK4, anti-CDK6, anti-glutathione S-transferase (GST) (all from Santa Cruz Biotechnology Inc.), or anti-cyclin D1 (a gift from Y. Xiong) or rat anti-cyclin D2 (Calbiochem) at 4°C with gentle rocking. Solutions were pelleted for 2 min at 14,000 × g, and supernatants were transferred to fresh tubes containing 20 μl of protein G-agarose beads for incubation at 4°C with rocking for 30 min. Pellets were collected by brief centrifugation, and remaining supernatant was aspirated. Pellets were washed four times with ice-cold radioimmunoprecipitation assay buffer, and the final pellet was boiled in 2× Laemmli sample buffer for subsequent Western analysis or in 1× kinase buffer for kinase assays. Immunoprecipitates were electrophoresed and detected by Western blotting as described above. Histone H1 assays of immunoprecipitates were done with 1 μg of histone (Sigma) each as described previously (64).
Microinjection experiments.
Microinjection experiments were performed essentially as described previously (2). In brief, log-phase or synchronized HDFs plated on glass coverslips were needle microinjected in the nucleus with either cytomegalovirus (CMV)-driven expression constructs encoding cyclin D1, D2, E or B1, or the parental RcCMV plasmid and/or rabbit nonspecific antibodies. The CMV-cyclin D2 construct was obtained as a generous gift from E. Kerkhoff; for other expression vectors, see reference 2. Three independent trials for each plasmid construct were performed where a minimum of 100 cells per coverslip were microinjected and cells were scored for BrdU incorporation 24 h later. Microinjection of nonspecific antibodies was used to control for microinjection trauma in individual experiments. Tests with nonspecific antibodies showed that greater than 90% of cells survive microinjection and approximately 95% of these were able to incorporate BrdU over a 24-h period.
Transient transfection and FACS analysis.
Hs68 HDFs (MPD 30) were plated at a density of 106 cells per 150-mm-diameter plate. Cells were trypsinized and spun at 800 rpm for 5 min. Cell pellets were resuspended in 400 μl of DMEM without FBS and transferred to 4-mm gap cuvettes (BTX Inc., San Diego, Calif.). Thirty micrograms of plasmid CMV-cyclin D2, CMV-antisense cyclin D2, CMV-p16, or RcCMV vector control was added together with 10 μg of CMV-CD20 surface marker and 10 μg of salmon sperm DNA to make a total DNA content of 50 μg per cuvette. Electroporations were done with a Bio-Rad gene pulser at 250 V and 960 μF, and each was transferred to a 10-cm-diameter plate; 48 h after electroporation, cells were prepared for fluorescence-activated cell sorting (FACS) analysis.
For FACS analysis, electroporated cells were trypsinized and spun at 800 rpm for 5 min. The cell pellet was resuspended in 100 μl of complete medium (DMEM) and 20 μl of fluorescein isothiocyanate-CD20 antibody (Becton Dickinson) and incubated at 4°C with gentle rocking for 30 min. The cells were then spun down as described above, washed two times with 1× PBS, and fixed in 1 ml of 0.01% formaldehyde (in PBS) overnight at 4°C. After fixation, the cells were spun and rewashed twice with 1× PBS, 1 ml of ice-cold digitonin (10 μg/ml in 95% ethanol) was added, and cells were incubated for 5 min on ice. The cells were centrifuged as described above and washed twice with 1× PBS, and the resulting pellet was resuspended in 500 μl of 1× PBS containing 250 μg of RNase A and 6 μg of propidium iodide. After a 30-min incubation in the dark at room temperature, the cells were analyzed in a Becton Dickinson FACScan, and cell cycle analyses were performed for cells gated for positive CD20 surface marker. In experiments where transient transfections were not performed, the cells were prepared essentially as described above, omitting the addition of CD20 antibody.
RESULTS
Contact-inhibited cells express high levels of cyclin D2.
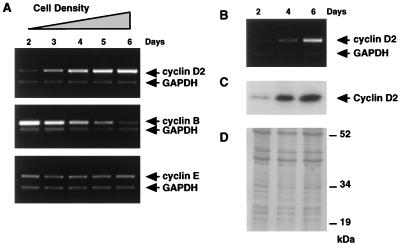
Cyclin D2 mRNA expression as a function of cell density was examined by semiquantitative RT-PCR (71). After plating of Hs68 fibroblasts at low density, cells were harvested up to 6 days later under conditions where cells began to reach confluence by 3 days. As shown in Fig. 1A, the expression levels of cyclin D2 mRNA progressively increased, attaining 20- to 30-fold higher levels over 6 days as estimated by scanning densitometry. The expression of cyclin B, which is required for progression through mitosis, was downregulated, while cyclin E, another G1 cyclin, remained relatively unchanged in the same samples. To confirm that cells plated for 2 days were at low density and were exponentially proliferating compared to cells at day 6, FACS analysis was performed. As expected, a significantly higher S-phase fraction was present in day 2 cells (32%) than in day 6 cells (4%). Human lung WI-38, IMR-90, and human skin (epidermal) A2 HDFs also exhibited a similar upregulation of cyclin D2 mRNA in response to contact inhibition (data not shown).
FIG. 1.
Increased expression of cyclin D2 during contact inhibition. (A) Primary human diploid fibroblasts at low passage numbers (Hs68, MPD 32) were split at a ratio of 1:8 and harvested daily over a 6-day period starting on the second day. Each sample was tested for cyclin B1, E, and D2 mRNA expression by using primer-dropping RT-PCR, with GAPDH as an internal control (71). Initially subconfluent NIH 3T3 murine fibroblasts were harvested at the days shown and analyzed for cyclin D2 expression by RT-PCR (B) and by Western immunoblotting using a monoclonal antibody against cyclin D2 protein (C). Amplification of cyclin D2 mRNA from NIH 3T3 cells consistently required five to six fewer PCR cycles than for similar detection of the transcript from HDFs. Assuming 80% efficiency for the PCR, this represents a 25- to 50-fold difference in abundance. (D) For Western blots, approximately 80 μg of total protein was loaded per lane, and equivalent protein loading of lanes was confirmed by Coomassie blue staining of gels.
Since the mouse homolog of cyclin D2 (CYL2) was cloned from NIH 3T3 cells (37), suggesting that its levels might be higher in this established cell line than in HDFs, and because NIH 3T3 fibroblasts are well known to contact inhibit, we also tested for changes in cyclin D2 expression in these cells. As shown in Fig. 1B, cyclin D2 mRNA levels increased to a similar degree in response to contact inhibition as seen for HDFs. Immunoblotting experiments with whole-cell lysates from NIH 3T3 cells confirmed that cyclin D2 protein levels also increased approximately 20-fold as estimated by scanning densitometry (Fig. 1C). These results demonstrate that cyclin D2 expression is highly sensitive to the degree of cell contact in both primary human and established murine fibroblasts.
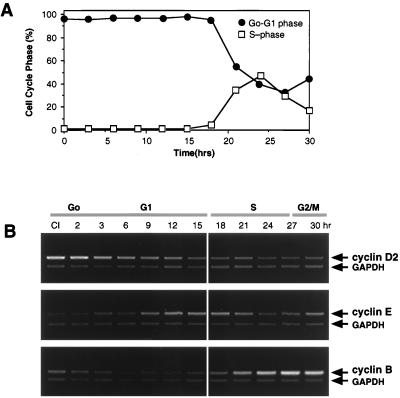
We next examined whether the cell density-related increase in cyclin D2 expression was a reversible event. Hs68 cells were released from contact inhibition by replating confluent HDFs (day 8 cells) at low density (1:12) and allowing them to synchronously reenter the cell cycle. Total RNA for analysis of mRNA expression patterns and whole cells for flow cytometry experiments were harvested in parallel at 1- to 3-h intervals following replating. Results from flow cytometry are plotted in Fig. 2A, which shows the high degree of synchrony of cells progressing through the cell cycle when released from contact inhibition. Figure 2B presents the results of RT-PCR amplifications for cyclins D2, E, and B1. Release from contact inhibition resulted in the continuous downregulation of cyclin D2 expression during the first 15 h. A comparison of cyclin D2 expression kinetics with FACS data indicated that reduction of cyclin D2 mRNA occurred continually through G1, reaching low steady-state levels during S-phase and thereafter. As controls, cyclins E and B1 were analyzed from the same pool of cDNA samples and were expressed at levels similar to those previously reported (48, 71, 72). These results show that cyclin D2 expression is downregulated in response to release from contact inhibition.
FIG. 2.
Kinetics of cyclin D2 mRNA expression upon release from contact inhibition. (A) Flow cytometry analysis of Hs68 fibroblasts released from contact inhibition. To perform the time course study, one 10-cm-diameter plate of day 8, contact-inhibited HDF cells (Hs68, MPD 36) were trypsinized and split equally into 12 plates containing fresh medium and 10% FBS. Cells were harvested at the time points shown. (B) In a parallel experiment, total RNA was harvested at the times indicated and analyzed for cyclin D2, E, and B1 mRNA expression patterns from the same pool of cDNA by RT-PCR.
Serum deprivation induces cyclin D2 expression.
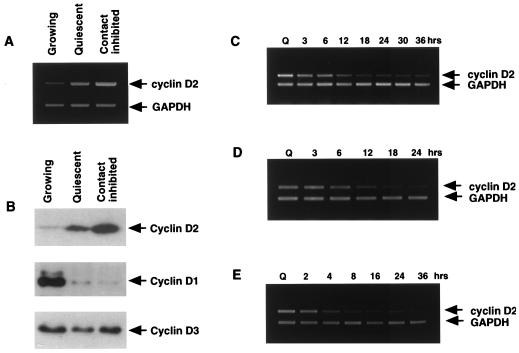
We next examined cyclin D2 expression by RT-PCR and Western immunoblotting in NIH 3T3 cells made quiescent by serum deprivation. As shown in Fig. 3, relative to exponentially proliferating cells, serum-starved (quiescent) cells express elevated levels of both cyclin D2 mRNA (Fig. 3A) and protein (Fig. 3B), although the increase was not as great as that seen in contact-inhibited cells. Upregulation of cyclin D2 in quiescent and contact-inhibited cells appeared unique among the D-type cyclins, with cyclin D1 showing decreased levels and cyclin D3 changing little in serum-deprived or contact-inhibited NIH 3T3 cells (Fig. 3B).
FIG. 3.
Cyclin D2 expression is upregulated in serum-deprived cells. NIH 3T3 fibroblasts were analyzed for cyclin D2 expression by RT-PCR (A) and by Western immunoblotting of cells that were exponentially proliferating, serum deprived for 40 to 48 h in 0.2% FBS (Quiescent), or contact inhibited (B). Subconfluent quiescent HDFs (MPD ∼26) (C), NIH 3T3 fibroblasts (D), and murine macrophages (E) were stimulated by the addition of serum to induce entry into the cell cycle. Total RNA was harvested at the time points shown and assayed for cyclin D2 mRNA expression.
We next examined whether accumulation of cyclin D2 in response to serum deprivation was reversible. When subconfluent or low-density cells were serum deprived for 48 h and restimulated with 10% serum to induce cells to exit G0 and reenter the cell cycle, cyclin D2 mRNA expression was downregulated in primary Hs68 fibroblasts (Fig. 3C), established NIH 3T3 cells (Fig. 3D), and established murine macrophages (strain J774 [Fig. 3E]). Similar patterns of repression of cyclin D2 expression by serum addition were seen in three other primary HDF strains (WI38, A2, and HF [data not shown]). Consistent with these data, other groups have reported that both cyclin D2 protein and mRNA levels are lower in S phase than during G1, although in these studies levels of cyclin D2 were not examined during quiescence (1, 30, 36).
Cyclin D2 levels increase during cellular senescence.
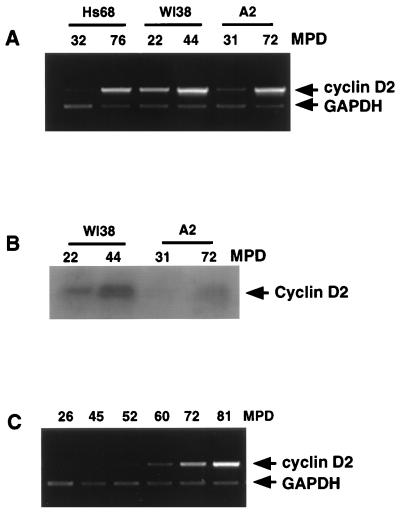
As cyclin D2 levels were consistently upregulated during contact inhibition and quiescence, we tested whether a similar upregulation occurs during cellular senescence (22) where primary cells lose their proliferative capacity and become irreversibly growth arrested with a 2N DNA content, presumably within the G1 phase of the cell cycle (17). As shown in Fig. 4A, all strains of primary HDFs examined showed 5- to 20-fold increases in cyclin D2 mRNA levels as cells became senescent, similar to the changes previously reported for cyclin D1 (2, 10, 34) and the CDK inhibitors p21WAF/CIP/SDI (2, 47) and p16INK4 (21, 72). As in contact-inhibited cells, the increase in mRNA resulted in increased levels of the cyclin D2 protein in senescent cells as shown for two primary HDF strains in Fig. 4B, although signals are relatively weak due to low levels of this cyclin in primary cells (4, 63). To determine the kinetics of cyclin D2 expression in relationship to in vitro age, Hs68 fibroblasts were assayed at several passage numbers indicated in Fig. 4C. This experiment revealed that readily detectable upregulation of cyclin D2 mRNA was first observed in “middle-aged” cells (MPD 52 to 60) and continued to progressively increase with greater passage numbers. Coincidental with the increase in cyclin D2 expression, a reduction in the rate of cellular proliferation (assayed as described in Materials and Methods), increases in the proportion of cells possessing a large flat morphology, and increased expression of the p16INK4 and p33ING1 genes (data not shown), both of which accompany the process of replicative senescence (16, 72), were observed. In summary, these experiments show that states of cellular growth arrest induced by three distinct physiological conditions, growth factor deprivation, contact inhibition, and cellular senescence, are accompanied by increased expression of cyclin D2 mRNA and protein in all phenotypically normal primary and immortal cell types examined.
FIG. 4.
Overexpression of cyclin D2 during cellular senescence. (A) Cyclin D2 primers were used to monitor the relative mRNA expression levels from three independent HDF strains at the MPDs shown, representing young (low-MPD) and old, near-senescent (high-MPD) cells. In each case, RNA was harvested from subconfluent cells grown under exponential growth conditions. (B) Western blot of cyclin D2 expression in WI38 and A2 fibroblasts at different population doublings. (C) Cyclin D2 mRNA expression levels assayed in Hs68 HDFs at increasing passage numbers.
Cyclin D2 protein is localized to the nucleus during growth arrest.
As growth-arrested cells exhibited enhanced levels of cyclin D2 mRNA and protein, we next determined the distribution of cyclin D2 protein within fibroblasts made quiescent by serum starvation and contact inhibition, using indirect immunofluorescence. As shown in Fig. 5A and B, cyclin D2 protein localized to the nucleus in both serum-starved and contact-inhibited NIH 3T3 fibroblasts. Cyclin D2 protein in subconfluent, near-senescent HDFs (MPD 84) also showed a nuclear staining pattern, but the overall immunofluorescent signal was very weak (data not shown), likely due to the relatively low levels of expression in primary fibroblasts (4, 63). Thus, nuclear cyclin D2 protein was closely associated with all three states of growth arrest.
FIG. 5.
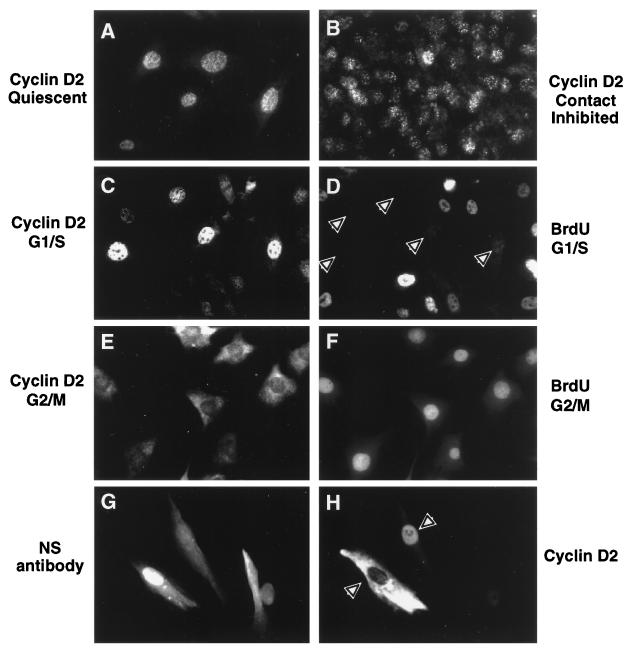
Localization of cyclin D2 protein in growth-arrested and proliferating fibroblasts by indirect immunofluorescence. Fibroblasts plated on glass coverslips were fixed and stained for cyclin D2 protein, BrdU, or nonspecific rabbit IgG. (A) Localization of cyclin D2 in serum-deprived NIH 3T3 fibroblasts. (B) Cyclin D2 in contact-inhibited (day 6) NIH 3T3 fibroblasts. (C) Cyclin D2 in NIH 3T3 cells at the G1/S boundary. (D) Cells shown in panel C stained for BrdU. Quiescent NIH 3T3 cells were stimulated for 14 h with serum prior to fixing and staining for cyclin D2 protein and BrdU. Arrowheads in panel D indicate cells that stained positively for nuclear cyclin D2 protein but had not progressed into S phase, as determined by lack of BrdU staining. (E) NIH 3T3 cells in G2/M phase stained for cyclin D2 protein and photographed with long exposure times. (F) Cells from panel E stained for BrdU. Quiescent NIH 3T3 cells were stimulated for 20 to 22 h with serum and BrdU and were fixed and stained for cyclin D2 protein and BrdU. (G) Hs68 HDFs microinjected with cyclin D2 expression constructs and nonspecific antibodies. The cells shown are stained for nonspecific rabbit IgGs. (H) HDFs from panel G stained for ectopic overexpression of cyclin D2 protein. Arrowheads in panel H show microinjected cells staining brightly for cyclin D2 protein that localized to either the nucleus or the cytoplasm.
Cyclin D2 was not, however, confined to the nucleus during all phases of the cell cycle. As shown in Fig. 5C, NIH 3T3 cells in late G1/early S phase expressed highly variable levels of cyclin D2 protein, and cells that stained brightly for nuclear cyclin D2 had not progressed through S phase, as determined by a lack of BrdU staining (Fig. 5D), similar to staining patterns reported for a variety of human cell types (36). Following DNA replication as evidenced by BrdU incorporation and staining (Fig. 5F), fibroblasts showed an overall lower intensity of cyclin D2 staining that was predominantly cytoplasmic (Fig. 5E). However, because it was formally possible that the cytoplasmic staining following entry into S phase was nonspecific, we microinjected Hs68 fibroblasts that express low endogenous levels of cyclin D2 with a CMV promoter-driven human cyclin D2 expression construct together with nonspecific rabbit antibodies to identify microinjected cells. Cells injected with expression constructs (Fig. 5G) showed distinct nuclear and cytoplasmic cyclin D2 staining patterns (Fig. 5H), indicating that these cells possess the capacity to actively partition the protein. We could not determine the position in the cell cycle of these microinjected cells by using BrdU costaining because overexpression of exogenous cyclin D2 protein interfered with DNA synthesis (see Fig. 7). Nevertheless, although total cyclin D2 expression does not change appreciably from S to M phases of the cell cycle (Fig. 3), these data support the idea that removal of the protein from the nucleus might be required to allow the G1-S phase transition and progression through S phase as suggested previously for D-type cyclins (4, 35, 36).
FIG. 7.
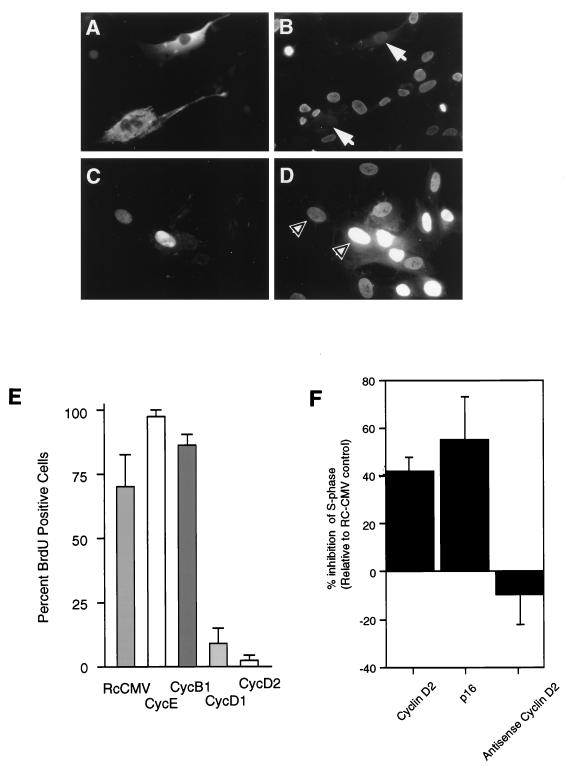
Ectopic overexpression of cyclin D2 inhibits S-phase progression. Serum-synchronized HDFs microinjected with CMV-cyclin D2 (A) and CMV-cyclin E expression constructs (C) and analyzed for BrdU incorporation (B and D, respectively). The microinjected cells were detected by staining for cyclin D2 protein (A) or nonspecific IgG (C). Arrowheads in panels B and D indicate cells injected with cyclin D2 and cyclin E expression constructs, respectively. (E) Effect on DNA synthesis (as determined by BrdU incorporation) in HDFs microinjected with the control plasmid (RcCMV) or with a CMV construct expressing cyclin B1, D1, D2, or E. Each microinjection experiment was performed at least three times with approximately 100 cells injected in total for each experiment. (F) Inhibition of entry into the S phase of the cell cycle by transient transfection of expression constructs encoding p16, cyclin D2, and anti-sense cyclin D2. Results are plotted relative to RcCMV vector controls for cells positive for CD20 surface marker staining. Bars represent the averages and standard deviations of three independent transfections.
Cyclin D2 associates with CDK2 (in a catalytically inactive complex) and with CDK4 during contact inhibition.
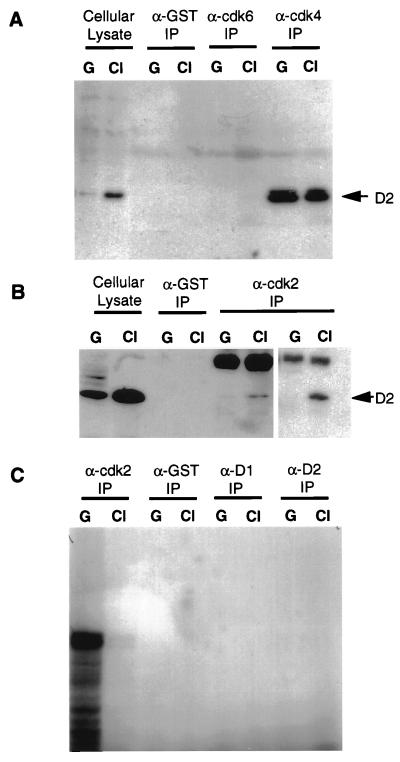
To assess the relative levels of CDKs that might interact with cyclin D2, we performed a series of immunoprecipitation-Western assays. Cyclin D2 Western blots against cell lysates from rapidly growing (lanes G) and contact-inhibited (lanes Cl) cells, or against immunoprecipitations from such lysates with control (anti-GST) or the indicated CDK antibodies, are shown in Fig. 6. Consistent with earlier blots (Fig. 1C and Fig. 3B), the levels of cyclin D2 were higher in contact-inhibited than in growing cells (Fig. 6A and B), and relatively high levels of cyclin D2 binding to CDK4 but not CDK6 were seen in both growing and contact-inhibited cell lysates (Fig. 6A). Although generally not considered to be a major binding partner of cyclin D2, CDK2 has been reported to bind cyclin D2 in some human breast epithelial cells (62). Consistent with this report, we found increased binding of CDK2 with cyclin D2 in contact-inhibited cells as shown in Fig. 6B in independent trials. No signal was seen in GST immunoprecipitates, indicating that the cyclin D2-CDK2 signal was specific. To determine if the CDK2 bound to cyclin D2 was active as a kinase, a series of immunoprecipitations were done with lysates from growing and contact-inhibited cells and antibodies directed against cyclin D2, CDK2 (as a positive control), as well as ones recognizing GST and cyclin D1 (as negative controls). Immunoprecipitates were then used in kinase assays using histone H1 as the substrate. As shown in Fig. 6C, anti-CDK2 immunoprecipitates from growing but not contact-inhibited cells contained very high levels of kinase activity, whereas anti-cyclin D2 immunoprecipitates, as well as negative control immunoprecipitates, contained no detectable activity in either growing or contact-inhibited cells.
FIG. 6.
CDK associations with cyclin D2. (A) Whole-cell lysates or immunoprecipitates (IP) from growing (lanes G) or contact-inhibited (lanes CI) NIH 3T3 cells were immunoblotted with a cyclin D2 monoclonal antibody as described in Materials and Methods. The anti-GST (α-GST) antibody served as a negative control, and the lysate served as a positive control. (B) Cyclin D2 immunoblot of cell lysates and immunoprecipitates using anti-GST and anti-CDK2 antibodies. In the case of anti-CDK2, two independent immunoprecipitations are shown. (C) Kinase assays of immunoprecipitates from growing and contact-inhibited cells, using histone H1 as the substrate. The autoradiogram was overexposed in order to detect very low amounts of activity as seen for contact-inhibited cells in the α-CDK2 lane.
Overexpression of cyclin D2 inhibits DNA synthesis in HDFs.
Because cyclin D2 expression correlated with multiple states of growth arrest, we examined whether its overexpression might prevent proliferation-competent primary HDFs from progressing through the cell cycle. HDFs were starved for 48 h and stimulated by the addition of 10% FBS to synchronously enter the cell cycle. Two to four hours later, cells were microinjected with a CMV-cyclin D2 expression construct, 1 h later BrdU was added to monitor DNA synthesis, and cells were fixed and stained for coinjected IgG and for incorporation of BrdU 24 to 30 h after stimulation. In parallel, similar microinjection experiments were conducted with a cyclin D1, cyclin E, or cyclin B1 expression construct, the empty control vector (RcCMV), and rabbit nonspecific IgG (to assess the degree of trauma from microinjection). Cells microinjected with cyclin D2 plasmids exhibited a large flat cell morphology similar to that of senescent fibroblasts (Fig. 7A) and failed to incorporate BrdU (Fig. 7B), whereas neighboring uninjected cells were BrdU positive. These observations indicate that cells microinjected with cyclin D2 expression constructs were unable to efficiently replicate their DNA. In contrast, cells microinjected with the CMV-cyclin E construct (Fig. 7C) readily incorporated BrdU (Fig. 7D).
Each of the microinjection experiments was performed in three independent trials, results of which are graphically presented in Fig. 7E. Cyclin D2 expression blocked BrdU incorporation in more than 90% of the cells. Ectopic expression of cyclin D1 in HDFs also efficiently inhibited S-phase progression, in agreement with previous reports (2, 49). Cyclins B and E appeared to have minimal effects on BrdU incorporation, although cyclin E injection resulted in a slight increase in cells entering S phase, consistent with this cyclin playing a positive role in cell cycle regulation (48, 53). Injection of the control vector itself inhibited the ability of cells to incorporate BrdU somewhat, but this effect was not seen when injecting vector at concentrations lower than 0.02 μg/μl.
To further confirm our results obtained from microinjection experiments, transient transfections of Hs68 HDFs were performed with expression vectors encoding cyclin D2, antisense-cyclin D2, and p16INK4. The combined FACS analysis results from three independent experiments (Fig. 7F) show that ectopic expression of cyclin D2 decreased the S-phase fraction by 45 to 50% compared to the control plasmid. Under the same conditions, a 55 to 70% decrease in S-phase fraction was seen for p16INK4 expressed from the same vector and a 10 to 20% increase in S-phase fraction was seen for antisense cyclin D2. Thus, in this normal diploid primary cell strain, cyclin D2 was approximately as effective as p16 in blocking cell growth. Taken together, these results indicate that ectopic overexpression of cyclin D2 in normal diploid fibroblasts blocks progression through G1 and entry into S phase.
DISCUSSION
In this study, we present evidence that cyclin D2 may function as a negative regulator of growth during the G0 and early G1 phases of the fibroblast cell cycle. First, cyclin D2 mRNA and protein expression were upregulated in primary human diploid and murine fibroblasts during growth arrest induced by contact inhibition, serum starvation, and cellular senescence. Conversely, cyclin D2 is downregulated in cells synchronously exiting G0 as a result of release from contact inhibition or serum stimulation. Second, cyclin D2 localizes to the nucleus in quiescent cells, where it is believed to be active and is cleared from the nucleus during the G1-S phase transition, consistent with previous reports suggesting that subcellular relocalization may be a prerequisite to permit cells to progress through S phase (4, 35, 36, 49). Third, an increased association of cyclin D2 with CDK2 is seen during contact inhibition, but the cyclin D2-CDK2 complex has no histone H1 kinase activity. Fourth, overexpression of cyclin D2 in human primary fibroblasts by needle microinjection or transient transfection of expression constructs strongly inhibits entry into S phase and DNA synthesis. Thus, in addition to the previously proposed role in mediating transit through the restriction point via phosphorylation of Rb family members, cyclin D2 may also function as a negative regulator of growth by inducing exit from the cell cycle and by helping to maintain cells in a nonproliferative state.
It has been suggested that D-type cyclins may be multifunctional growth factor sensors, responsible for inactivating Rb in late G1, thus providing G1 checkpoint function, and functioning as S-phase regulators preventing unwarranted DNA replication and repair synthesis by means of titrating essential replication factors such as proliferating cell nuclear antigen (49). Presumably D-type cyclins may have differing roles depending on their relative abundance and the particular cell type. Indeed, the most extensively studied D-type cyclin, cyclin D1, has been reported to accelerate transit through G1 but inhibit S-phase traverse when moderately upregulated in some cell types (52, 53), while ectopic overexpression of cyclin D1 in normal HDFs, mammary cell lines, Dami megakaryocytic cells, and rat embryo fibroblasts inhibits DNA synthesis and cell growth (2, 18, 19, 28, 49, 70). In this and other studies, overexpression of other classes of cyclins generally has growth-promoting effects, underscoring fundamental differences in their roles within the cell. Subsets of D-type cyclins are also upregulated in irreversibly growth-arrested cells such as during replicative senescence (2, 10, 34, 72), terminal differentiation (6, 23, 29, 65, 70, 77), and apoptosis (14), lending further weight to the idea that they provide growth-suppressive functions.
Additional links between D-type cyclins and growth suppression are provided by correlations to tumor suppressor activity. Expression of the p53 tumor suppressor gene causes cell cycle arrest in G1 (32), in part due to the transcriptional upregulation of p21WAF/CIP/SDI by p53 (11). p21WAF/CIP/SDI, in turn, inhibits CDK activity and prevents the phosphorylation and inactivation of Rb that normally occurs in late G1 (67). In murine cells, in addition to p21WAF/CIP/SDI, cyclin D1 expression is also upregulated by the inducible expression of p53, using temperature-sensitive mutants or a steroid hormone-inducible system (7, 8, 60), and by ectopic expression of Rb (45). Changes in the expression patterns of a number of cell cycle-regulatory genes during replicative senescence, some of which are thought to contribute to the senescent phenotype, have been described (42, 69). For example, p21WAF/CIP/SDI, p16INK4, and p33ING1 are cell cycle inhibitors that are upregulated during replicative senescence and may have roles in maintaining or inducing cell cycle arrest associated with a senescence or cellular aging program (2, 16, 21, 47, 72). Interestingly, we and others have found that the activity of p53 increases in senescent fibroblasts (3, 66), perhaps providing a molecular mechanism for the upregulation of D-type cyclins during cellular aging. Since senescent cells possess constitutively activated hypophosphorylated Rb (61), the high levels of D-type cyclins seen during cellular senescence may be the result of the combined activities of both p53 and Rb tumor suppressors in a growth-inhibitory feedback loop.
In spite of their many similarities, D-type cyclins exhibit a number of distinctive properties as well. For example, the three cyclin D genes are more highly conserved between species than they are to each other (25), are differentially expressed in various cell lineages (5, 35, 37, 41, 50), exhibit distinctive induction kinetics (1, 4, 35, 36), and behave differently with respect to their interaction with, and phosphorylation of, Rb (9, 12, 27). These observations suggest that their functions are not fully redundant. Indeed, although we have observed upregulation of cyclin D2 during quiescence in several independent primary fibroblast strains and in murine cell lines under conditions of serum starvation, cyclins D1 and D3 were not similarly upregulated. However, all three of the D-type cyclins were found to be upregulated during cellular senescence (references 2 and 10 and data not shown), indicating that growth arrest mediated by quiescence and senescence have overlapping but distinct gene expression patterns. Cyclin D2 expression has also been shown to increase during in vitro differentiation of P19 embryonal carcinoma cells, with a concomitant increase in hypophosphorylated Rb (31). Similarly, differentiation of myoblasts into myotubes resulted in increased expression of cyclins D2 and D3 (29). It is also important to note that MN20, whose expression is restricted to postmitotic neurons in the brain, shows significant homology to cyclin D2 and is believed to function in the neuronal differentiation pathway (54). In addition, of the three D-type cyclins, expression of cyclin D2 appears to be least frequently detected in assays of a large array of cells grown in culture (5, 41, 63). For example, we find that normal HDFs express much lower levels of cyclin D2 mRNA than NIH 3T3 fibroblasts or primary T cells (data not shown). However, as shown in Fig. 1 and 3, the patterns of cyclin D2 expression are essentially identical between HDFs and NIH 3T3 cells in response to different stimuli, suggesting similar functions in these different cell types, although in the highly transforming growth factor β (TGF-β)-sensitive established Mv1Lu line, both TGF-β and contact inhibition were reported to inhibit cyclin D2 expression (73). Finally, in contrast to cultured cells, our preliminary results indicate that high levels of cyclin D2 are expressed in a variety of normal human tissues, including brain, breast, and lymphoid tissues (data not shown). The fact that these tissues are composed primarily of nonproliferating contact-inhibited cells is consistent with the upregulation of cyclin D2 seen in growth arrested cells grown in vitro.
The mechanism for the upregulation of cyclin D2 during growth arrest is presently unclear. It is possible that the increase in cyclin D2 mRNA is transcriptionally and/or posttranscriptionally regulated. Our preliminary results of assays using the transcriptional inhibitor actinomycin D suggest that the D-type cyclin transcripts are relatively stable over extended (6- to 12-h) time courses in young versus senescent and in growing versus contact-inhibited cells. Thus, a transcriptional mechanism for the upregulation of cyclins D1 and D2 during growth arrest appears a likely possibility.
The results presented here clearly illustrate that cyclin D2 expression is upregulated in quiescent cells. Our data further suggest that cyclin D2 sequesters CDK2 in a catalytically inactive complex, perhaps preventing it from being activated by other cyclins such as cyclin E, which is expressed at levels which are similar in quiescent and growing cells. This could serve a function in maintaining cells in a quiescent state, consistent with the observed effects of overexpressing cyclin D2. However, the major binding partner of cyclin D2 in quiescent cells appears to be CDK4, although we have been unable to detect kinase activity in this complex that is beyond background levels in assays using a number of substrates, including Rb. Nevertheless, given the close functional relationship between the D-type cyclins and the Rb family of pocket proteins (72), it is possible that an Rb-related protein can act as a target for cyclin D2 kinase activity. One such candidate may be the Rb-related protein p130, which is upregulated in G0 and exists in complexes containing E2F-4 and E2F-5 transcription factors (8, 25, 70). Indeed, both p130 and E2F-4 are known to be differentially phosphorylated during early and late phases of the cycle (43, 70), and p130 exists in distinct forms during the early portion of the cell cycle. These early G1 forms accumulate in cells made quiescent by serum starvation, contact inhibition, and treatment of cells with TGF-β (40), suggesting that a kinase that recognizes p130 is activated during the transition from G1 to G0. Hence, given the correlation between the expression pattern of cyclin D2 and the G0 forms of p130, it is reasonable to speculate that a cyclin D2 kinase activity may target p130 or a p130 complex containing E2F proteins. Whether quiescent cells possess such a cyclin D2 kinase activity specific for the p130 complex is currently being tested.
ACKNOWLEDGMENTS
We thank R. Johnston and Y. Xiong for helpful discussions, L. Robertson for FACS analysis, and C. Veillette for expert technical assistance.
This work was supported by grants to K.R. from the Canadian Breast Cancer Foundation and the National Cancer Institute of Canada. M.M. and C.H. are recipients of the Alberta Heritage Foundation for Medical Research (AHFMR) studentship awards, and K.R. is an AHFMR Senior Scholar.
REFERENCES
- 1.Ajchenbaum F, Ando K, DeCaprio J A, Griffin J D. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- 2.Atadja P, Wong H, Veillette C, Riabowol K. Overexpression of cyclin D1 blocks proliferation of normal diploid fibroblasts. Exp Cell Res. 1995;217:205–216. doi: 10.1006/excr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 3.Atadja P, Wong H, Garkavtev I, Veillette C, Riabowol K. Increased activity of p53 in senescing fibroblasts. Proc Natl Acad Sci USA. 1995;92:8348–8352. doi: 10.1073/pnas.92.18.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldin V, Lukas J, Marcote M J, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 5.Buckley M F, Sweeney K J E, Hamilton J A, Sini R L, Manning D L, Nicholson R I, deFazio A, Watts C W K, Musgrove E A, Sutherland R L. Expression and amplification of cyclin genes in human breast cancer. Oncogene. 1993;8:2127–2133. [PubMed] [Google Scholar]
- 6.Burger C, Wick M, Muller R. Lineage-specific regulation of cell cycle gene expression in differentiating myeloid cells. J Cell Sci. 1994;107:2047–2054. doi: 10.1242/jcs.107.7.2047. [DOI] [PubMed] [Google Scholar]
- 7.Chen X, Bargonetti J, Prives C. p53, through p21 (WAF1/CIP1), induces cyclin D1 synthesis. Cancer Res. 1995;55:4257–4263. [PubMed] [Google Scholar]
- 8.Del Sal G, Murphy M, Ruaro E M, Lararevic D, Levine A J, Schneider C. Cyclin D1 and p21/waf1 are both involved in p53 growth suppression. Oncogene. 1996;12:177–185. [PubMed] [Google Scholar]
- 9.Dowdy S F, Hinds P W, Louie K, Reed S I, Arnold A, Weinberg R A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- 10.Dulic V, Drullinger L F, Lees E, Reed S I, Stein G H. Altered regulation of G1 cyclins in senescent human diploid fibroblasts: accumulation of inactive E-Cdk2 and cyclin D1-Cdk2 complexes. Proc Natl Acad Sci USA. 1993;90:11034–11038. doi: 10.1073/pnas.90.23.11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Deiry W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer E, Kinzler K W, Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 12.Ewen M, Sluss H K, Sherr C J, Matsushime H, Kato J-Y, Livingston D M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993;73:487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- 13.Fantl V, Stamp G, Andrews A, Rosewell I, Dickson C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 1995;9:2364–2372. doi: 10.1101/gad.9.19.2364. [DOI] [PubMed] [Google Scholar]
- 14.Freeman R S, Estus S, Johnson E M. Analysis of cell cycle-regulated gene expression in post mitotic neurons: selective induction of cyclin D1 during programmed cell death. Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 15.Gao C Y, Zelenka P S. Cyclins, cyclin-dependent kinases and differentiation. Bioessays. 1997;19:307–315. doi: 10.1002/bies.950190408. [DOI] [PubMed] [Google Scholar]
- 16.Garkavtsev I, Riabowol K. Extension of the replicative lifespan of human diploid fibroblasts by inhibition of the p33ING1 candidate tumor suppressor. Mol Cell Biol. 1997;17:2014–2019. doi: 10.1128/mcb.17.4.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstein S. Replicative senescence: the human fibroblast comes of age. Science. 1990;249:1129–1133. doi: 10.1126/science.2204114. [DOI] [PubMed] [Google Scholar]
- 18.Han E K-H, Sgambato A, Jiang W, Zhang Y-J, Santella R M, Doki Y, Cacace A M, Schieren I, Weinstein I B. Stable overexpression of cyclin D1 in a human mammary epithelial cell line prolongs the S-phase and inhibits growth. Oncogene. 1995;10:953–961. [PubMed] [Google Scholar]
- 19.Han E K-H, Begemann M, Sgambato A, Soh J-W, Doki Y, Xing W-Q, Liu W, Weinstein I B. Increased expression of cyclin D1 in murine mammary epithelial cell line induces p27kip1, inhibits growth, and enhances apoptosis. Cell Growth Differ. 1996;7:699–710. [PubMed] [Google Scholar]
- 20.Hanna Z, Jankowski M, Tremblay P, Xiaoyan J, Milatovich A, Francke U, Jolicoeur P. The vin-1 gene, identified by proviral insertional mutagenesis, corresponds to the G1-phase cyclin D2. Oncogene. 1993;8:1661–1667. [PubMed] [Google Scholar]
- 21.Hara E, Smith R, Parry D, Tahara H, Stone S, Peters G. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol Cell Biol. 1996;16:859–867. doi: 10.1128/mcb.16.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayflick L. The limited in vitro lifetime of human diploid fibroblasts. Exp Cell Res. 1965;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 23.Horiguchi-Yamada J, Yamada H, Nakada S, Ochi K, Nemoto T. Changes of G1 cyclins, cdk2, and cyclin A during differentiation of HL60 cells induced by TPA. Mol Cell Biochem. 1994;132:31–37. doi: 10.1007/BF00925672. [DOI] [PubMed] [Google Scholar]
- 24.Houldsworth J, Reuter V, Bosl G J, Chaganti R S. Aberrant expression of cyclin D2 is an early event in human male germ cell tumorigenesis. Cell Growth Differ. 1997;8:293–299. [PubMed] [Google Scholar]
- 25.Inaba T, Matsushime H, Valentine M, Roussel M, Sherr C J, Look A T. Genomic organization, and independent expression of human cyclin D genes. Genomics. 1992;13:565–574. doi: 10.1016/0888-7543(92)90126-d. [DOI] [PubMed] [Google Scholar]
- 26.Jiang Z, Zacksenhaus E, Gallie B L, Phillips R A. The retinoblastoma gene family is differentially expressed during embryogenesis. Oncogene. 1997;14:1789–1797. doi: 10.1038/sj.onc.1201014. [DOI] [PubMed] [Google Scholar]
- 27.Kato J-Y, Matsushime H, Hiebert S W, Ewen M E, Sherr C J. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 28.Kerkhoff E, Ziff E B. Cyclin D2 and Ha-ras transformed rat embryo fibroblasts exhibit a novel deregulation of cell size control and early S phase arrest in low serum. EMBO J. 1995;14:1892–1903. doi: 10.1002/j.1460-2075.1995.tb07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kiess M, Gill R M, Hamel P A. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation of myoblasts into quiescent myotubes. Oncogene. 1995;10:159–166. [PubMed] [Google Scholar]
- 30.Kiyokawa H, Busquets X, Powell C T, Ngo L, Rifkind R A, Marks P A. Cloning of a D-type cyclin from murine erythroleukemia cells. Proc Natl Acad Sci USA. 1992;89:2444–2447. doi: 10.1073/pnas.89.6.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kranenburg O, De Groot R P, van der Eb A J, Zantema A. Differentiation of P19 EC cells leads to differential modulation of cyclin-dependent kinase activities and to changes in the cell cycle profile. Oncogene. 1995;10:87–95. [PubMed] [Google Scholar]
- 32.Levine A J. The tumor suppressor genes. Annu Rev Biochem. 1993;62:623–651. doi: 10.1146/annurev.bi.62.070193.003203. [DOI] [PubMed] [Google Scholar]
- 33.Lew D J, Dulic V, Reed S I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991;66:1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- 34.Lucibello F C, Sewing A, Brusselbach S, Burger C, Muller R. Deregulation of cyclins D1 and E and suppression of cdk2 and cdk4 in senescent human fibroblasts. J Cell Sci. 1993;105:123–133. doi: 10.1242/jcs.105.1.123. [DOI] [PubMed] [Google Scholar]
- 35.Lukas J, Pagano M, Staskova Z, Draetta G, Bartek J. Cyclin D1 protein oscillates and is essential for cell cycle progression in human tumor cell lines. Oncogene. 1994;9:707–718. [PubMed] [Google Scholar]
- 36.Lukas J, Bartkova J, Welcker M, Peterson O W, Peters G, Strauss M, Bartek J. Cyclin D2 is a moderately oscillating nucleoprotein required for G1 phase progression in specific cell types. Oncogene. 1995;10:2115–2134. [PubMed] [Google Scholar]
- 37.Matsushime H, Roussel M, Ashmun R A, Sherr C J. Colony-stimulating factor regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991;65:701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- 38.Matsushime H, Ewen M E, Strom D K, Kato J-Y, Hanks S K, Roussel M, Sherr C J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992;71:323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- 39.Matsushime H, Quelle D E, Shurtleff S A, Shibuya M, Sherr C J, Kato J-Y. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayol X, Garriga J, Grana X. G1 cyclin/CDK-independent phosphorylation and accumulation of p130 during the transition from G1 to G0 lead to its association with E2F-4. Oncogene. 1996;13:237–246. [PubMed] [Google Scholar]
- 41.Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meyyappan M, Atadja P W, Riabowol K T. Regulation of gene expression and transcription factor binding activity during cellular aging. Biol Signals. 1996;5:130–138. doi: 10.1159/000109183. [DOI] [PubMed] [Google Scholar]
- 43.Motokura T, Arnold A. Cyclin D and oncogenesis. Curr Opin Genet Dev. 1993;3:5–10. doi: 10.1016/s0959-437x(05)80334-x. [DOI] [PubMed] [Google Scholar]
- 44.Motokura T, Keyomarsi K, Kronenberg H M, Arnold A. Cloning and characterization of human cyclin D3, a cDNA closely related in sequence to the PRAD1/cyclin D1 proto-oncogene. J Biol Chem. 1992;267:20412–20415. [PubMed] [Google Scholar]
- 45.Muller H, Lukas J, Schneider A, Warthoe P, Bartek J, Eilers M, Strauss M. Cyclin D1 expression is regulated by the retinoblastoma protein. Proc Natl Acad Sci USA. 1994;91:2945–2949. doi: 10.1073/pnas.91.8.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuman E, Ladha M H, Lin N, Upton T M, Miller S J, DiRenzo J, Pestell R G, Hinds P W, Dowdy S F, Brown M, Ewen M E. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol Cell Biol. 1997;17:5338–5347. doi: 10.1128/mcb.17.9.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noda A, Ning Y, Venable S F, Pereira-Smith O M, Smith J R. Cloning of senescent cell-derived inhibitors of DNA synthesis using an expression screen. Exp Cell Res. 1994;211:90–98. doi: 10.1006/excr.1994.1063. [DOI] [PubMed] [Google Scholar]
- 48.Ohtsubo M, Roberts J M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993;259:1908–1911. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- 49.Pagano M, Theodoras A M, Tam S W, Draetta G F. Cyclin D1-mediated inhibition of repair and replicative DNA synthesis in human fibroblasts. Genes Dev. 1994;8:1627–1639. doi: 10.1101/gad.8.14.1627. [DOI] [PubMed] [Google Scholar]
- 50.Palmero I, Holder A, Sinclair A J, Dickson C, Peters G. Cyclins D1 and D2 are differentially expressed in human B-lymphoid cell lines. Oncogene. 1993;8:1049–1054. [PubMed] [Google Scholar]
- 51.Pardee A B. G1 events and regulation of cell proliferation. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 52.Quelle D E, Ashmun R A, Shurtleff S A, Kato J-Y, Bar-Sagi D, Roussel M F, Sherr C J. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- 53.Resnitzky D, Gossen M, Bujard H, Reed S I. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ross M E, Risken M. MN20, a D2 cyclin found in brain, is implicated in neural differentiation. J Neurosci. 1994;14:6394–6391. doi: 10.1523/JNEUROSCI.14-11-06384.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross M E, Carter M L, Lee J H. MN20, a D2 cyclin, is transiently expressed in selected neural populations during embryogenesis. J Neurosci. 1996;16:210–219. doi: 10.1523/JNEUROSCI.16-01-00210.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sherr C J. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- 57.Sherr C J. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- 58.Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- 59.Sicinski P, Donaher J L, Geng Y, Parker S B, Gardner H, Park M Y, Robker R L, Richards J S, McGinnis L K, Biggers J D, Eppig J J, Bronson R T, Elledge S J, Weinberg R A. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- 60.Spikovsky D, Steiner P, Gopalkrishnan R V, Eilers M, Jansen-Durr P. The role of p53 in coordinated regulation of cyclin D1 and p21 gene expression by the adenovirus E1A and E1B oncogenes. Oncogene. 1995;10:2421–2425. [PubMed] [Google Scholar]
- 61.Stein G H, Beeson M, Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990;249:666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- 62.Sweeney K J, Sarcevic B, Sutherland R L, Musgrove E A. Cyclin D2 activates cdk2 in preference to cdk4 in human breast epithelial cells. Oncogene. 1997;14:1329–1340. doi: 10.1038/sj.onc.1200951. [DOI] [PubMed] [Google Scholar]
- 63.Tam S W, Theodoras A M, Shay J W, Draetta G F, Pagano M. Differential expression and regulation of cyclin D1 protein in normal and tumor human cells: association with Cdk4 is required for cyclin D1 function in G1 progression. Oncogene. 1994;9:2663–2674. [PubMed] [Google Scholar]
- 64.Tsai L H, Lees E, Faha B, Harlow E, Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- 65.Van Grunsven L A, Thomas A, Urdiales J L, Machenaud S, Choler P, Durand I, Rudkin B B. Nerve growth factor-induced accumulation of PC12 cells expressing cyclin D1: evidence for a G1 phase block. Oncogene. 1996;12:855–862. [PubMed] [Google Scholar]
- 66.Vaziri H, West M D, Allsopp R C, Davison T S, Wu Y S, Arrowsmith C H, Poirier G S, Benchimol S. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 1997;16:6018–6033. doi: 10.1093/emboj/16.19.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weinberg R A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 68.Weinberg R A. E2F and cell proliferation: a world turned upside down. Cell. 1996;85:457–459. doi: 10.1016/s0092-8674(00)81244-1. [DOI] [PubMed] [Google Scholar]
- 69.Wheaton K, Atadja P, Riabowol K. Regulation of transcription factor activity during cellular aging. Biochem Cell Biol. 1996;74:523–534. doi: 10.1139/o96-056. [DOI] [PubMed] [Google Scholar]
- 70.Wilhide C C, Dang C V, Dipersio J, Kenedy A A, Bray P F. Overexpression of cyclin D1 in the Dami Megakaryocytic cell line causes growth arrest. Blood. 1995;86:294–304. [PubMed] [Google Scholar]
- 71.Wong H, Anderson W D, Cheng T, Riabowol K T. Monitoring mRNA expression by polymerase chain reaction: the “primer-dropping” method. Anal Biochem. 1994;223:251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- 72.Wong H, Riabowol K T. Differential CDK-inhibitor gene expression in aging human diploid fibroblasts. Exp Gerontol. 1996;31:311–325. doi: 10.1016/0531-5565(95)00025-9. [DOI] [PubMed] [Google Scholar]
- 73.Wu F, Buckley S, Bui K C, Yee A, Wu H Y, Liu J, Warburton D. Cell cycle arrest in G0/G1 phase by contact inhibition and TGF-β1 in mink Mv1Lu lung epithelial cells. Am J Physiol. 1996;270:L879–L888. doi: 10.1152/ajplung.1996.270.5.L879. [DOI] [PubMed] [Google Scholar]
- 74.Xiong Y, Connolly T, Futcher B, Beach D. Human D-type cyclin. Cell. 1991;65:691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- 75.Xiong Y, Menninger J, Beach D, Ward D C. Molecular cloning and chromosomal mapping of CCND genes encoding human D-type cyclins. Genomics. 1992;13:575–584. doi: 10.1016/0888-7543(92)90127-e. [DOI] [PubMed] [Google Scholar]
- 76.Xiong Y, Zhang H, Beach D. D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- 77.Yan G-Z, Ziff E B. NGF regulates the PC12 cell cycle machinery through specific inhibition of the Cdk kinases and induction of cyclin D1. J Neurosci. 1995;15:6200–6212. doi: 10.1523/JNEUROSCI.15-09-06200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zwijsen R M L, Wientjens E, Klompmaker R, van der Sman J, Bernards R, Michalides R J A M. CDK-independent activation of estrogen receptor by cyclin D1. Cell. 1997;88:405–415. doi: 10.1016/s0092-8674(00)81879-6. [DOI] [PubMed] [Google Scholar]