Abstract
Postural orthostatic tachycardia syndrome (POTS) is a common accompaniment of a variety of chronic, inflammatory diseases, including long COVID, as are small, insoluble, ‘fibrinaloid’ microclots. We here develop the argument, with accompanying evidence, that fibrinaloid microclots, through their ability to block the flow of blood through microcapillaries and thus cause tissue hypoxia, are not simply correlated with but in fact, by preceding it, may be a chief intermediary cause of POTS, in which tachycardia is simply the body’s exaggerated ‘physiological’ response to hypoxia. Similar reasoning accounts for the symptoms bundled under the term ‘fatigue’. Amyloids are known to be membrane disruptors, and when their targets are nerve membranes, this can explain neurotoxicity and hence the autonomic nervous system dysfunction that contributes to POTS. Taken together as a system view, we indicate that fibrinaloid microclots can serve to link POTS and fatigue in long COVID in a manner that is at once both mechanistic and explanatory. This has clear implications for the treatment of such diseases.
Keywords: fibrinaloid microclots, postural orthostatic tachycardia syndrome (POTS), Long COVID, TeamClots
1. Introduction
Orthostasis, Orthostatic Intolerance, and POTS
Human beings have evolved to maintain a largely erect posture [1] and can adopt it from recumbent poses. Orthostasis describes the (normal) physiological response used to counteract the potential fall in blood pressure when a person who has been lying down assumes the upright position. This tendency occurs because, in an adult, gravity causes a shift of some 300 to 800 mL of blood from the upper to the lower body. This orthostasis depends strongly on the autonomic nervous system.
However, if the system does not respond properly, there can be a significant decrease in the central blood pressure; common symptoms of such hypoperfusion are dizziness, lightheadedness, and syncope (fainting). The resulting intolerance of the upright posture is known as orthostatic intolerance (OI). When accompanied by a sustained postural drop in blood pressure (of more than 20 mmHg systolic or 10 mmHg diastolic [2]), the patient is diagnosed with orthostatic hypotension, which is a form of orthostatic intolerance (OI). Another variant of OI occurs when there is less of a fall in blood pressure, but the autonomic response leads instead to a rapid increase in heart rate (tachycardia). This is known as postural orthostatic tachycardia syndrome (POTS) (e.g., [3,4,5]). POTS is a manifestation of autonomic dysregulation and is clinically characterized as excessive tachycardia upon standing in the presence of symptomatic orthostatic intolerance. We recognize that POTS may be classified into subtypes such as neuropathic POTS and hyperadrenergic POTS; however, most of the papers we cite do not in fact make this distinction, and, for the present purposes, we avoid doing so as well, since our chief aim here was simply to suggest that there is, in general, significant evidence for the role of fibrinaloid microclots in POTS.
Although well known in other contexts for at least three decades [6,7] (see Table 1), with at least 500,000 cases in the USA alone [8,9,10], mostly in women (5:1) [5,9,11,12,13,14], POTS has emerged as a frequent symptom of both acute [15] and long COVID (e.g., [16,17,18,19,20,21] as part of the wider cardiovascular dysautonomia spectrum; see Table 1).
Table 1.
Some diseases and syndromes with which POTS is associated.
| Disease, State, or Syndrome | Comments | Selected Reference(s) |
|---|---|---|
| Autoimmune disorders and Autoimmunity | Some strong associations | [16,22,23,24,25,26] |
| Cognitive function | Large amount of literature; improved by plasma exchange [27] | [27,28,29,30] |
| Fatigue | [31,32,33,34,35,36,37,38] | |
| HPV or other antiviral vaccination | An example of induction by a viral protein | [39,40,41,42,43,44,45] but cf. [46] |
| Inflammation | [47] | |
| Irritable bowel disease | [48] | |
| Long COVID | A very common occurrence and a focus of our interest | [16,17,18,19,20,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68] |
| Migraine | [69] | |
| Multiple sclerosis | Now recognized as possibly caused by Epstein–Barr virus [70] (albeit much earlier evidence for an infectious origin existed [71,72], cf. [73,74]). | [75] |
| Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) | Is also usually a postviral disease and bears a number of similarities to long COVID [68,76,77,78,79] | [31,32,52,80,81,82,83,84] |
| Platelet delta granule storage pool deficiency | Causal direction unclear | [85] |
| Pregnancy | Many cardiovascular stresses accompany pregnancy, especially during hypertensive disorders [86,87] | [88,89] |
| Reviews | [22] |
The management of POTS has been the subject of prior reviews and guidelines and is beyond the aims of the present study [90,91]. Our focus in this study was mainly on microclots as a plausible, mechanistic basis for POTS, especially in relation to long COVID.
2. The Normal Control of Heart Rate
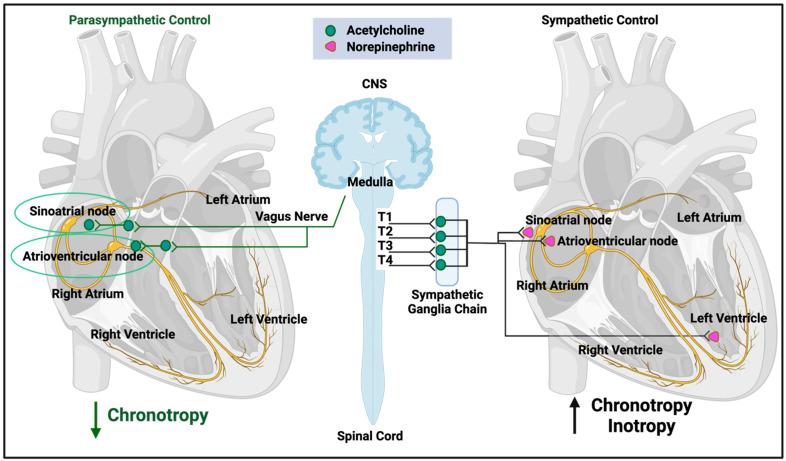
Because of the general interest in POTS in long COVID and other affected communities, we include a very brief and high-level overview. The heart rate is controlled by many genetic and lifestyle factors (e.g., [92,93]), and the required kinds of understanding are both conceptual (e.g., the need to cater for the time-varying demands of tissue oxygenation) and mechanistic (e.g., the involvement of the endocrine and autonomic nervous systems). Our overview here is very far from being comprehensive, and our focus is necessarily on short-term control, where the autonomic nervous system is predominant (Figure 1, after [92]).
Figure 1.
Autonomic nervous system regulation of heart function (after [92]). Created with BioRender.com. Access date: 26 November 2023.
As summarized in Figure 1 (redrawn from [92]), both the sympathetic and parasympathetic branches of the autonomic nervous system are involved. The former is more involved in stress responses (often called ‘fight-or-flight’) and can release noradrenaline (norepinephrine) to increase heart rate, whilst the latter (often called ‘rest-and-digest’) underpins basal activity via the vagus nerve that can release acetylcholine to decrease heart rate relative to its base rate. Multiple control steps involve baroceptors that sense pressure and other receptors that respond to pH, hypoxia, and hypercapnia. In particular, under most conditions, the heart necessarily and appropriately responds to acute hypoxia by increasing heart rate (e.g., [94,95,96,97,98]).
3. Diagnosis of POTS
Most chronic, inflammatory diseases—as their name suggests—possess multiple common symptoms [99], while those such as long COVID characterized by subsets of multiple symptoms can easily be subclustered (e.g., [49,100,101,102]). The earlier definition of POTS comes from a very small study of 16 patients in 1993, of whom, interestingly, 7 were thought to have had previous viral infections [6,103]. Nowadays, for instance, the Canadian Cardiology Society has published a position paper describing a wider heterogenous range of clinical syndromes and a spectrum of orthostatic intolerance; they propose that discrete subtypes are identified over time, each with different underlying pathophysiological phenotypes that allow for specific targeted treatment [90]. However, for present purposes, in the case of POTS, both the high-level definition and the diagnosis are relatively straightforward, as they follow virtually from the name: heart rate is monitored for tachycardia (an increase in heart rate exceeding 30 beats per minute (bpm) within the initial 10 min of standing or head-up tilt (HUT)- or a ‘final’ value exceeding 120 bpm) as the individual changes their posture from horizontal to (more) vertical [5].
Differences can occur because the transition is commonly affected either by active standing or a passive ‘tilt table’ test [104,105,106,107]. The latter, which is somewhat more controlled and considered more reliable [108], commonly involves a ‘head-up tilt’ in which an individual is strapped to a horizonal table and commonly tilted to an angle of 60–80° [106,109], and heart rate and other measurements are performed. Transcranial doppler ultrasound may be used to detect blood flow [110]. It is recognized that such ‘provocative’ tests are of most value when individuals record similar symptoms to those that they normally experience [111]. For all events, the conceptual recognition of POTS is to be seen as reasonably straightforward [112,113]. It is important to recognize that the diagnostic criteria for heart rate changes are arbitrary and based on small case series, and that patients can have disabling OI and other symptoms of autonomic dysfunction without meeting the traditional cutoffs; this is no different in long COVID patients presenting with symptoms of POTS.
4. Occurrence and Comorbidities of POTS
Although we did not cover POTS (nor even autonomic dysfunction) in our earlier review of chronic, inflammatory diseases [99], the occurrence of POTS, which is highly heterogeneous [114], broadly mirrors the kinds of disease that we did mention there. Table 1 lists some of them, implying elements of a common origin. Of particular interest is the evidence for endothelial microvascular dysfunction [50], which can occur via the microclot-mediated blockage of red cell flow to tissues.
5. Dysautonomia
Autonomic dysfunction (dysautonomia) describes any malfunction in the autonomic nervous system, especially the vagus nerve [115,116], which is a key element in (but not synonymous with [117]) POTS, and the occurrence of dysautonomia broadly mirrors the diseases in which POTS is known to occur (Table 2).
Table 2.
Some diseases and syndromes in which dysautonomia is known to occur.
| Disease, State, or Syndrome | Comments | Selected Reference(s) |
|---|---|---|
| Familial (monogenic) | Lesion in the IKBKAP gene | [118] |
| Long COVID | [57,60,62,63,67,76,119,120,121,122,123,124] | |
| Multiple sclerosis | [125,126] | |
| Myalgic encephalomyelitis/chronic fatigue syndrome | [76,82,119,127,128,129,130,131,132,133] | |
| Parkinson’s disease | [134] |
6. Fatigue and POTS
Like POTS, fatigue is a common accompaniment of many acute and chronic inflammatory diseases. It is usually based on scoring questionnaires and thus lacks a crisp definition [135,136,137,138,139,140,141,142]. However, fatigue is generally used to cover a debilitating set of symptoms in which attempts to carry out what would normally be considered a very mild exertion are followed immediately by an inability to perform or to continue such exertions and a period in which extreme rest is required. In contrast to physiological ‘tiredness’, rest and sleep are not physically or mentally rejuvenating in fatigue. As noted in Table 1 [31,32,33,34,35,36,37], fatigue is a common accompaniment of POTS and—as we shall argue—likely has a main common cause.
7. The Role of Fibrinaloid Microclots in POTS
Although the origins of our discoveries that blood could clot into a very anomalous form lie earlier- in observations using the electron microscope (e.g., [143,144,145,146])- it was not until 2016 [147] that we determined using fluorescence microscopy that these anomalous forms were in fact amyloid in nature [148,149,150,151,152], that they could be induced by highly substoichiometric amounts of bacterial lipopolysaccharide [147], and that the electron and optical microscopies were congruent [153]. Essentially all the clots visible using fluorescence staining were those visible in the bright field [154,155]. The microclots were found to be particularly prevalent in diabetes [156,157,158] and in particular in both acute [158] and long COVID [159,160,161,162,163,164,165,166], where they could be induced by miniscule concentrations of the spike protein [167,168]. They were also much raised over those in controls in individuals with ME/CFS [169,170]. Note that the generation of fibrinaloid microclots is essentially instantaneous (on the timescale of normal clotting) (e.g., [147,167]), whereas the time taken to develop POTS is slower. This is at least consistent with a causative role of the earlier-appearing microclots in the generation of the later-appearing POTS.
Microclots differ from clots mostly by being considerably smaller (broadly in the range of 1–200 μm, mostly at the lower end) (see Figure 2) and by virtue both of the adoption of an amyloid form [148,159,161] and their entrapment of molecules such as α2-antiplasmin [163]. These and other properties [171] make them particularly resistant to fibrinolysis, so they are removed far less quickly than would normally be the case.
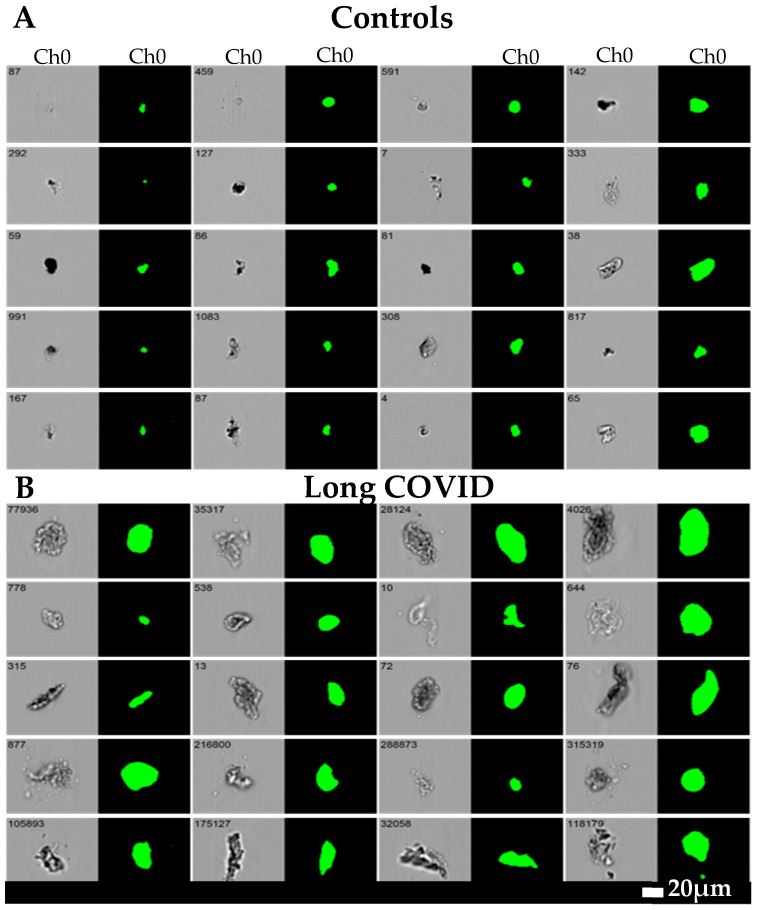
Figure 2.
Microclot size distribution as seen with imaging flow cytometry (taken from [166]). Representative micrographs of microclots in (A) controls and (B) long COVID patients using an imaging flow cytometer. The brightfield images are displayed in Channel 1 (Ch01) and fluorescence intensity due to ThT binding in Channel 7 (Ch07). All images were captured using a 20x objective. The event number is displayed in the top-left corner of each image. NB: In these pictures, the POTS status of the individuals was not assessed.
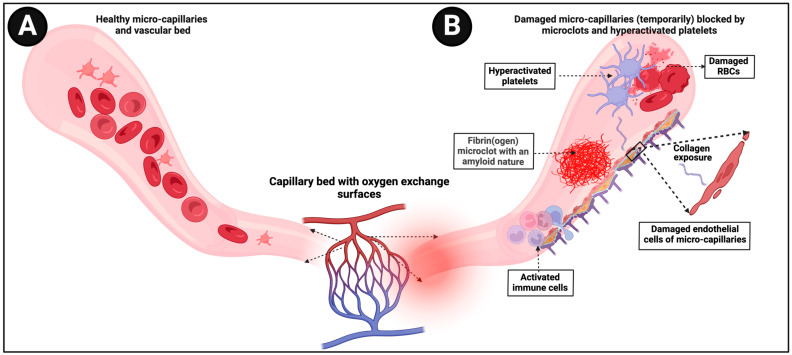
A straightforward consequence of these insoluble fibrinaloid microclots is that as blood flow pushes them along, they can block up microcapillaries, thereby inhibiting the flux of oxygen-carrying red blood cells and thus inducing tissue hypoxia. Sensing low tissue oxygen concentrations naturally (as when exercising) may induce tachycardia, and this would provide a very ready explanation of both POTS and the fatigue that is a common occurrence in both ME/CFS and long COVID (see Figure 3).
Figure 3.
(A) Representation of healthy blood flow in microcapillaries (B) versus in an individual where damaged microcapillaries are (temporarily) blocked by microclots. Created with BioRender.com (accessed on 26 November 2023).
Other mechanisms for POTS in long COVID may include:
Relative hypovolemia secondary to inadequate peripheral vasoconstriction. This results in a reduction in stroke volume and cardiac output, causing the inhibition of tissue oxygen supply and the consequent compensatory tachycardia.
Small fiber neuropathy (SFN) has been well described in long COVID (e.g., [63,65,68,172]) and is a recognized cause of dysautonomia in the condition. SFN in long COVID can be driven by autoantibodies (already known to be associated with POTS and OH) or, potentially, by ischemia of the small fibres due to microclots.
8. The Role of Microclots in Fatigue
Just as the blocking of microcapillaries by microclots gives a ready explanation for POTS, it also gives a ready explanation for fatigue as tissues that rely on aerobic respiration for their normal function are deprived of oxygen. Specifically, the microclots vary widely in diameter, so they can migrate to those parts of the capillary bed where they can block the flow of red blood cells most effectively. Consequently, the affected tissues simply cannot perform their normal functions. While details vary for every individual, the existence and capillary-blocking behavior of the microclots also provide a simple and mechanistic explanation for the co-occurrence [31,32,33,35,36,37] of POTS and fatigue.
9. Relationship between Dysautonomia and Microclots
We know that molecules such as LPS (e.g., [147,149,150]) and the spike protein of SARS-CoV-2 (e.g., [154,158,159,163,164,165,166,167,173]) can cause microclots, such that any damage such molecules may cause to nerves may be indirect [174,175,176]. This said, it is reasonable that any damage to the membranes of nerves might be mediated via fibrinaloid microclots.
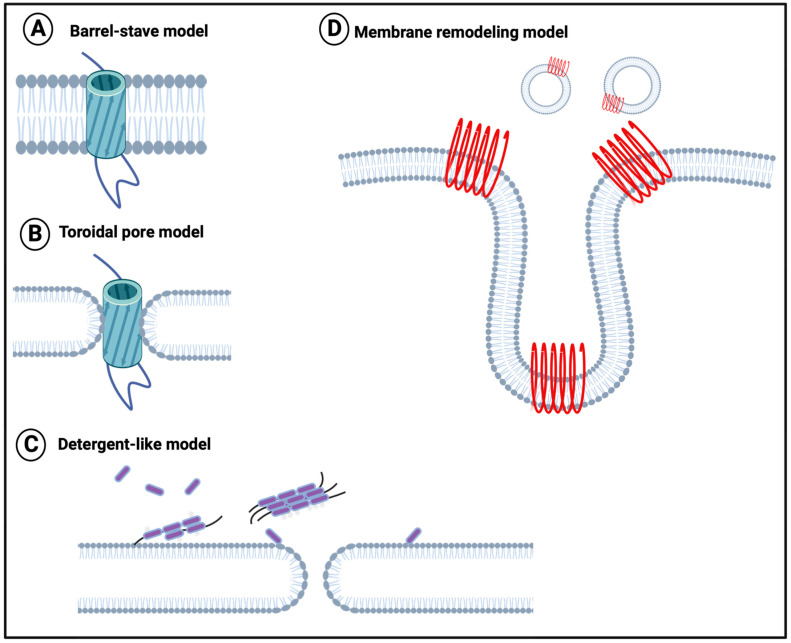
To this end, although the direct experiments have not been performed with fibrinaloid microclots (nor is it easy to conduct them in vivo), it is at least worth repeating that it is well established that amyloid forms of proteins (including those binding cations [177]) generally can effect damage to all kinds of phospholipid membranes directly (e.g., [177,178,179,180,181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201,202]). A variety of mechanisms have been proposed, such as those in Figure 4 [201].
Figure 4.
Membrane disruption models (redrawn from [201]). (A) The barrel-stave model suggests that proteins perpen-dicularly insert into the phospholipid bilayer plane, with the hydrophobic regions of protein oligomers contacting the hydrophobic interior of the membrane. (B) The toroidal pore model suggests that proteins insert perpendicular to the phospholipid bilayer, with the protein hydrophilic ends remaining in contact with the lipid head layer. (C) The deter-gent-like model, suggests that positively charged residues in the amyloidogenic protein bind to the membrane. (D) The membrane remodeling model suggests that membrane-bound peptides self-assemble into β-sheets that subsequently either form pores on the membrane surface (Pore formation model) or drag lipids out of the bilayer core (Detergent-like model). Created with BioRender.com (accessed on 26 November 2023).
When the membrane in question is a nerve membrane, neurotoxicity (e.g., [198,203,204,205,206,207,208,209] (leading to autonomic nervous system dysfunction) may result.
10. Systems Overview and Conclusions
We established that fibrinaloid microclots accompany a variety of diseases in which POTS is frequently diagnosed, with fatigue as a frequent feature, as are autoantibodies [161], implying a similar kind of cause or at least intermediate. The microclots do seem to fulfill this intermediary role, as they also provide a realistic set of mechanisms. This said, it should be admitted that detailed temporal studies have not been conducted in animals (which may not even provide a decent model), while those studies that did test, e.g., SARS-CoV-2 infection, in human volunteers directly [210] did not seek to measure microclots.
Very recently, Wüst and colleagues showed a variety of defects in the skeletal muscle of long COVID patients, including both amyloid deposition and mitochondrial dysfunction [211]. Coupled with the evidence for lactate overproduction in both COVID-19 [212,213,214,215,216,217] and ME/CFS [133,218,219,220,221,222], both of which are associated with POTS (Table 1), this provides further evidence for a role of inadequate O2 uptake in these processes.
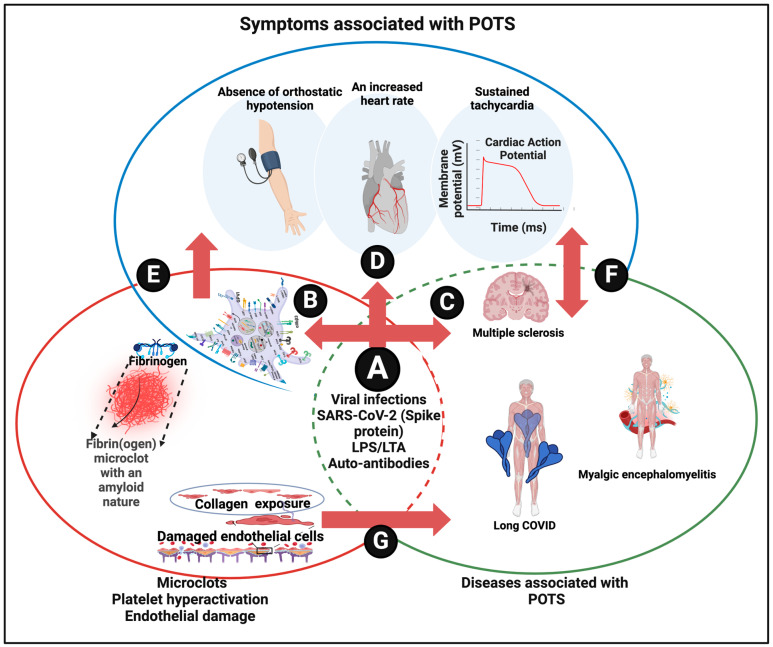
The system biology diagram linking these high-level elements is given in Figure 5.
Figure 5.
A system approach to defining dysautonomia. (A) Various causes of disease and symptoms resulting in vascular damage, microclots, and platelet hyperactivation (B) known to be involved in a variety of diseases (C) and in POTS (D). Similarly, vascular damage pathologies cause POTS (E) and other diseases (F), while POTS is found in various diseases (G). Created with BioRender.com (accessed on 26 November 2023).
We conclude that the presence of fibrinaloid microclots can indeed significantly account for the symptoms of POTS associated with long COVID (and likely other syndromes), just as they can for other symptoms [159], post-exertional symptom exacerbation [160], and the generation of autoantibodies [161].
Author Contributions
Conceptualization, D.B.K. and E.P.; writing—original draft preparation, D.B.K.; writing—review and editing, All Authors; visualization, E.P.; funding acquisition, E.P. & D.B.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
E.P. is a named inventor on a patent application covering the use of fluorescence methods for microclot detection in long COVID.
Funding Statement
EP: Funding was provided by the NRF of South Africa (grant number 142142), SA MRC (self-initiated research (SIR) grant), and the Balvi Foundation (grant B31). DBK thanks the Balvi Foundation (grant 18) and the Novo Nordisk Foundation for funding (grant NNF10CC1016517). The content and findings reported and illustrated are the sole deduction, view, and responsibility of the researchers and do not reflect the official position and sentiments of the funders.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Niemitz C. The evolution of the upright posture and gait—A review and a new synthesis. Naturwissenschaften. 2010;97:241–263. doi: 10.1007/s00114-009-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman R., Wieling W., Axelrod F.B., Benditt D.G., Benarroch E., Biaggioni I., Cheshire W.P., Chelimsky T., Cortelli P., Gibbons C.H., et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Fedorowski A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2019;285:352–366. doi: 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- 4.Vernino S., Bourne K.M., Stiles L.E., Grubb B.P., Fedorowski A., Stewart J.M., Arnold A.C., Pace L.A., Axelsson J., Boris J.R., et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 national institutes of health expert consensus meeting—Part 1. Auton. Neurosci. 2021;235:102828. doi: 10.1016/j.autneu.2021.102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grubb A.F., Grubb B.P. Postural orthostatic tachycardia syndrome: New concepts in pathophysiology and management. Trends Cardiovasc. Med. 2023;33:65–69. doi: 10.1016/j.tcm.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Schondorf R., Low P.A. Idiopathic postural orthostatic tachycardia syndrome: An attenuated form of acute pandysautonomia? Neurology. 1993;43:132–137. doi: 10.1212/WNL.43.1_Part_1.132. [DOI] [PubMed] [Google Scholar]
- 7.Safavi-Naeini P., Razavi M. Postural orthostatic tachycardia syndrome. Tex. Heart Inst. J. 2020;47:57–59. doi: 10.14503/THIJ-19-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryarly M., Phillips L.T., Fu Q., Vernino S., Levine B.D. Postural orthostatic tachycardia syndrome: JACC focus seminar. J. Am. Coll. Cardiol. 2019;73:1207–1228. doi: 10.1016/j.jacc.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 9.Shaw B.H., Stiles L.E., Bourne K., Green E.A., Shibao C.A., Okamoto L.E., Garland E.M., Gamboa A., Diedrich A., Raj V., et al. The face of postural tachycardia syndrome—Insights from a large cross-sectional online community-based survey. J. Intern. Med. 2019;286:438–448. doi: 10.1111/joim.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lecheler L., Hoffmann F., Tank J., Jordan J. Run vagus run: Cardiovagal baroreflex function and the postural tachycardia syndrome. Hypertension. 2021;77:1245–1247. doi: 10.1161/HYPERTENSIONAHA.121.16578. [DOI] [PubMed] [Google Scholar]
- 11.Agarwal A.K., Garg R., Ritch A., Sarkar P. Postural orthostatic tachycardia syndrome. Postgrad. Med. J. 2007;83:478–480. doi: 10.1136/pgmj.2006.055046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Low P.A., Sandroni P., Joyner M., Shen W.K. Postural tachycardia syndrome (POTS) J. Cardiovasc. Electrophysiol. 2009;20:352–358. doi: 10.1111/j.1540-8167.2008.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamrefors V., Spahic J.M., Nilsson D., Senneby M., Sutton R., Melander O., Fedorowski A. Syndromes of orthostatic intolerance and syncope in young adults. Open Heart. 2017;4:e000585. doi: 10.1136/openhrt-2016-000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold A.C., Ng J., Raj S.R. Postural tachycardia syndrome—Diagnosis, physiology, and prognosis. Auton. Neurosci. 2018;215:3–11. doi: 10.1016/j.autneu.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassani M., Fathi Jouzdani A., Motarjem S., Ranjbar A., Khansari N. How COVID-19 can cause autonomic dysfunctions and postural orthostatic syndrome? A review of mechanisms and evidence. Neurol. Clin. Neurosci. 2021;9:434–442. doi: 10.1111/ncn3.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Rhermoul F.Z., Fedorowski A., Eardley P., Taraborrelli P., Panagopoulos D., Sutton R., Lim P.B., Dani M. Autoimmunity in long COVID and POTS. Oxf. Open Immunol. 2023;4:iqad002. doi: 10.1093/oxfimm/iqad002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blitshteyn S., Whiteson J., Abramoff B.A., Azola A., Bartels M.N., Bhavaraju-Sanka R., Chung T., Fleming T.K., Henning E., Miglis M.G., et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) PM&R. 2022;14:1270–1291. doi: 10.1002/pmrj.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallick D., Goyal L., Chourasia P., Zapata M.R., Yashi K., Surani S. COVID-19 induced postural orthostatic tachycardia syndrome (POTS): A review. Cureus. 2023;15:e36955. doi: 10.7759/cureus.36955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narasimhan B., Calambur A., Moras E., Wu L., Aronow W. Postural orthostatic tachycardia syndrome in COVID-19: A contemporary review of mechanisms, clinical course and management. Vasc. Health Risk Manag. 2023;19:303–316. doi: 10.2147/VHRM.S380270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollack B., von Saltza E., McCorkell L., Santos L., Hultman A., Cohen A.K., Soares L. Female reproductive health impacts of long COVID and associated illnesses including ME/CFS, POTS, and connective tissue disorders: A literature review. Front. Rehabil. Sci. 2023;4:1122673. doi: 10.3389/fresc.2023.1122673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ståhlberg M., Mahdi A., Johansson M., Fedorowski A., Olshansky B. Cardiovascular dysautonomia in postacute sequelae of SARS-CoV-2 infection. J. Cardiovasc. Electrophysiol. 2023. early view . [DOI] [PubMed]
- 22.Gunning W.T., 3rd, Kvale H., Kramer P.M., Karabin B.L., Grubb B.P. Postural orthostatic tachycardia syndrome is associated with elevated g-protein coupled receptor autoantibodies. J. Am. Heart Assoc. 2019;8:e013602. doi: 10.1161/JAHA.119.013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS) Lupus. 2015;24:1364–1369. doi: 10.1177/0961203315587566. [DOI] [PubMed] [Google Scholar]
- 24.Li H., Yu X., Liles C., Khan M., Vanderlinde-Wood M., Galloway A., Zillner C., Benbrook A., Reim S., Collier D., et al. Autoimmune basis for postural tachycardia syndrome. J. Am. Heart Assoc. 2014;3:e000755. doi: 10.1161/JAHA.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahan S., Tomljenovic L., Shoenfeld Y. Postural orthostatic tachycardia syndrome (POTS)—A novel member of the autoimmune family. Lupus. 2016;25:339–342. doi: 10.1177/0961203316629558. [DOI] [PubMed] [Google Scholar]
- 26.Vernino S., Stiles L.E. Autoimmunity in postural orthostatic tachycardia syndrome: Current understanding. Auton. Neurosci. 2018;215:78–82. doi: 10.1016/j.autneu.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Seeley M.C., Hooper M., Tan J., Wells R., Gallagher C., Lau D.H. Plasma exchange improves cognitive function in long-COVID-related postural orthostatic tachycardia syndrome and autoimmune neurological dysfunction. Am. J. Med. 2023;133:e157–e159. doi: 10.1016/j.amjmed.2023.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Shanks L., Jason L.A., Evans M., Brown A. Cognitive impairments associated with CFS and POTS. Front. Physiol. 2013;4:113. doi: 10.3389/fphys.2013.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold A.C., Haman K., Garland E.M., Raj V., Dupont W.D., Biaggioni I., Robertson D., Raj S.R. Cognitive dysfunction in postural tachycardia syndrome. Clin. Sci. 2015;128:39–45. doi: 10.1042/CS20140251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raj V., Opie M., Arnold A.C. Cognitive and psychological issues in postural tachycardia syndrome. Auton. Neurosci. 2018;215:46–55. doi: 10.1016/j.autneu.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoad A., Spickett G., Elliott J., Newton J. Postural orthostatic tachycardia syndrome is an under-recognized condition in chronic fatigue syndrome. QJM. 2008;101:961–965. doi: 10.1093/qjmed/hcn123. [DOI] [PubMed] [Google Scholar]
- 32.Lewis I., Pairman J., Spickett G., Newton J.L. Clinical characteristics of a novel subgroup of chronic fatigue syndrome patients with postural orthostatic tachycardia syndrome. J. Intern. Med. 2013;273:501–510. doi: 10.1111/joim.12022. [DOI] [PubMed] [Google Scholar]
- 33.Nijs J., Ickmans K. Postural orthostatic tachycardia syndrome as a clinically important subgroup of chronic fatigue syndrome: Further evidence for central nervous system dysfunctioning. J. Intern. Med. 2013;273:498–500. doi: 10.1111/joim.12034. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds G.K., Lewis D.P., Richardson A.M., Lidbury B.A. Comorbidity of postural orthostatic tachycardia syndrome and chronic fatigue syndrome in an Australian cohort. J. Intern. Med. 2014;275:409–417. doi: 10.1111/joim.12161. [DOI] [PubMed] [Google Scholar]
- 35.Wise S., Ross A., Brown A., Evans M., Jason L. An assessment of fatigue in patients with postural orthostatic tachycardia syndrome. J. Health Psychol. 2017;22:733–742. doi: 10.1177/1359105315613624. [DOI] [PubMed] [Google Scholar]
- 36.Boris J.R., Bernadzikowski T. Therapy for fatigue and cognitive dysfunction in postural orthostatic tachycardia syndrome. Cardiol. Young. 2018;28:1415–1420. doi: 10.1017/S1047951118001415. [DOI] [PubMed] [Google Scholar]
- 37.Strassheim V., Welford J., Ballantine R., Newton J.L. Managing fatigue in postural tachycardia syndrome (PoTS): The newcastle approach. Auton. Neurosci. 2018;215:56–61. doi: 10.1016/j.autneu.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Astudillo L., Laure A., Fabry V., Pugnet G., Maury P., Labrunee M., Sailler L., Pavy-Le Traon A. Postural tachycardia syndrome (PoTS): An up-to-date. Rev. Med. Interne. 2018;39:627–634. doi: 10.1016/j.revmed.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Blitshteyn S., Fedorowski A. The risks of POTS after COVID-19 vaccination and SARS-CoV-2 infection: More studies are needed. Nat. Rev. Cardiol. 2022;1:1119–1120. doi: 10.1038/s44161-022-00180-z. [DOI] [PubMed] [Google Scholar]
- 40.Blitshteyn S. Human papillomavirus (HPV) vaccine safety concerning POTS, CRPS and related conditions. Clin. Auton. Res. 2020;30:181–182. doi: 10.1007/s10286-019-00653-5. [DOI] [PubMed] [Google Scholar]
- 41.Butts B.N., Fischer P.R., Mack K.J. Human papillomavirus vaccine and postural orthostatic tachycardia syndrome: A review of current literature. J. Child. Neurol. 2017;32:956–965. doi: 10.1177/0883073817718731. [DOI] [PubMed] [Google Scholar]
- 42.Jefferson T., Jørgensen L. Human papillomavirus vaccines, complex regional pain syndrome, postural orthostatic tachycardia syndrome, and autonomic dysfunction—A review of the regulatory evidence from the European medicines agency. Indian J. Med. Ethics. 2017;2:30–37. doi: 10.20529/IJME.2017.006. [DOI] [PubMed] [Google Scholar]
- 43.Tomljenovic L., Colafrancesco S., Perricone C., Shoenfeld Y. Postural orthostatic tachycardia with chronic fatigue after HPV vaccination as part of the “autoimmune/auto-inflammatory syndrome induced by adjuvants”: Case report and literature review. J. Investig. Med. High Impact Case Rep. 2014;2:2324709614527812. doi: 10.1177/2324709614527812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tv P., Tran T.T., Hao H.T., Hau N.T.H., Jain N., Reinis A. Postural orthostatic tachycardia syndrome-like symptoms following COVID-19 vaccination: An overview of clinical literature. Hum. Antibodies. 2023;31:9–17. doi: 10.3233/HAB-220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arana J., Mba-Jonas A., Jankosky C., Lewis P., Moro P.L., Shimabukuro T.T., Cano M. Reports of postural orthostatic tachycardia syndrome after human papillomavirus vaccination in the vaccine adverse event reporting system. J. Adolesc. Health. 2017;61:577–582. doi: 10.1016/j.jadohealth.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 46.Barboi A., Gibbons C.H., Axelrod F., Benarroch E.E., Biaggioni I., Chapleau M.W., Chelimsky G., Chelimsky T., Cheshire W.P., Claydon V.E., et al. Human papillomavirus (HPV) vaccine and autonomic disorders: A position statement from the American autonomic society. Clin. Auton. Res. 2020;30:13–18. doi: 10.1007/s10286-019-00608-w. [DOI] [PubMed] [Google Scholar]
- 47.Gunning W.T., 3rd, Stepkowski S.M., Kramer P.M., Karabin B.L., Grubb B.P. Inflammatory biomarkers in postural orthostatic tachycardia syndrome with elevated G-protein-coupled receptor autoantibodies. J. Clin. Med. 2021;10:623. doi: 10.3390/jcm10040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mathias C.J., Low D.A., Iodice V., Owens A.P., Kirbis M., Grahame R. Postural tachycardia syndrome—Current experience and concepts. Nat. Rev. Neurol. 2011;8:22–34. doi: 10.1038/nrneurol.2011.187. [DOI] [PubMed] [Google Scholar]
- 49.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re’em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahdi A., Lodin K., Reistam U., Fedorowski A., Nygren-Bonnier M., Runold M., Bruchfeld J., Desta L., Pernow J., Nickander J., et al. Microvasular dysfunction and reduced cardiac stress reactivity in postural orthostatic tachycardia associated with postacute COVID-19. Circ. Arrhythmia Electrophysiol. 2023;16:413–414. doi: 10.1161/CIRCEP.123.011881. [DOI] [PubMed] [Google Scholar]
- 51.Johansson M., Stahlberg M., Runold M., Nygren-Bonnier M., Nilsson J., Olshansky B., Bruchfeld J., Fedorowski A. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: The swedish experience. JACC Case Rep. 2021;3:573–580. doi: 10.1016/j.jaccas.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Campen C.L.M.C., Rowe P.C., Visser F.C. Orthostatic symptoms and reductions in cerebral blood flow in long-haul COVID-19 patients: Similarities with myalgic encephalomyelitis/chronic fatigue syndrome. Medicina. 2021;58:28. doi: 10.3390/medicina58010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu L.D., Duricka D.L. Stellate ganglion block reduces symptoms of long COVID: A case series. J. Neuroimmunol. 2022;362:577784. doi: 10.1016/j.jneuroim.2021.577784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rigo S., Urechie V., Diedrich A., Okamoto L.E., Biaggioni I., Shibao C.A. Impaired parasympathetic function in long-COVID postural orthostatic tachycardia syndrome—A case-control study. Bioelectron. Med. 2023;9:19. doi: 10.1186/s42234-023-00121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seeley M.C., Gallagher C., Ong E., Langdon A., Chieng J., Bailey D., Page A., Lim H.S., Lau D.H. High incidence of autonomic dysfunction and postural orthostatic tachycardia syndrome in patients with long COVID: Implications for management and health care planning. Am. J. Med. 2023. in press . [DOI] [PMC free article] [PubMed]
- 56.Amekran Y., Damoun N., El Hangouche A.J. Postural orthostatic tachycardia syndrome and post-acute COVID-19. Glob. Cardiol. Sci. Pract. 2022;2022:e202213. doi: 10.21542/gcsp.2022.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chadda K.R., Blakey E.E., Huang C.L., Jeevaratnam K. Long COVID-19 and postural orthostatic tachycardia syndrome—Is dysautonomia to be blamed? Front. Cardiovasc. Med. 2022;9:860198. doi: 10.3389/fcvm.2022.860198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ormiston C.K., Swiatkiewicz I., Taub P.R. Postural orthostatic tachycardia syndrome as a sequela of COVID-19. Heart Rhythm. 2022;19:1880–1889. doi: 10.1016/j.hrthm.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diekman S., Chung T. Post-acute sequelae of SARS-CoV-2 syndrome presenting as postural orthostatic tachycardia syndrome. Clin. Exp. Emerg. Med. 2023;10:18–25. doi: 10.15441/ceem.22.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gómez-Moyano E., Rodríguez-Capitán J., Gaitán Román D., Reyes Bueno J.A., Villalobos Sánchez A., Espildora Hernández F., González Angulo G.E., Molina Mora M.J., Thurnhofer-Hemsi K., Molina-Ramos A.I., et al. Postural orthostatic tachycardia syndrome and other related dysautonomic disorders after SARS-CoV-2 infection and after COVID-19 messenger RNA vaccination. Front. Neurol. 2023;14:1221518. doi: 10.3389/fneur.2023.1221518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jamal S.M., Landers D.B., Hollenberg S.M., Turi Z.G., Glotzer T.V., Tancredi J., Parrillo J.E. Prospective evaluation of autonomic dysfunction in post-acute sequela of COVID-19. J. Am. Coll. Cardiol. 2022;79:2325–2330. doi: 10.1016/j.jacc.2022.03.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Minhas R., Bharadwaj A.S. COVID-19-induced postural orthostatic tachycardia syndrome and dysautonomia. Cureus. 2023;15:e40235. doi: 10.7759/cureus.40235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novak P., Giannetti M.P., Weller E., Hamilton M.J., Mukerji S.S., Alabsi H.S., Systrom D., Marciano S.P., Felsenstein D., Mullally W.J., et al. Network autonomic analysis of post-acute sequelae of COVID-19 and postural tachycardia syndrome. Neurol. Sci. 2022;43:6627–6638. doi: 10.1007/s10072-022-06423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherif Z.A., Gomez C.R., Connors T.J., Henrich T.J., Reeves W.B., Force R.M.P.T. Pathogenic mechanisms of post-acute sequelae of SARS-CoV-2 infection (PASC) eLife. 2023;12:e86002. doi: 10.7554/eLife.86002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Novak P., Mukerji S.S., Alabsi H.S., Systrom D., Marciano S.P., Felsenstein D., Mullally W.J., Pilgrim D.M. Multisystem involvement in post-acute sequelae of coronavirus disease 19. Ann. Neurol. 2022;91:367–379. doi: 10.1002/ana.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takao M., Ohira M. Neurological post-acute sequelae of SARS-CoV-2 infection. Psychiatry Clin. Neurosci. 2023;77:72–83. doi: 10.1111/pcn.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fedorowski A., Sutton R. Autonomic dysfunction and postural orthostatic tachycardia syndrome in post-acute COVID-19 syndrome. Nat. Rev. Cardiol. 2023;20:281–282. doi: 10.1038/s41569-023-00842-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis H.E., McCorkell L., Vogel J.M., Topol E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023;21:133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mueller B.R., Robinson-Papp J. Postural orthostatic tachycardia syndrome and migraine: A narrative review. Headache. 2022;62:792–800. doi: 10.1111/head.14365. [DOI] [PubMed] [Google Scholar]
- 70.Wekerle H. Epstein-barr virus sparks brain autoimmunity in multiple sclerosis. Nature. 2022;603:230–232. doi: 10.1038/d41586-022-00382-2. [DOI] [PubMed] [Google Scholar]
- 71.Kurtzke J.F., Heltberg A. Multiple sclerosis in the faroe islands: An epitome. J. Clin. Epidemiol. 2001;54:1–22. doi: 10.1016/S0895-4356(00)00268-7. [DOI] [PubMed] [Google Scholar]
- 72.Kurtzke J.F. Epidemiology in multiple sclerosis: A pilgrim’s progress. Brain. 2013;136:2904–2917. doi: 10.1093/brain/awt220. [DOI] [PubMed] [Google Scholar]
- 73.Poser C.M., Hibberd P.L. Analysis of the ‘epidemic’ of multiple sclerosis in the faroe islands. II. Biostatistical aspects. Neuroepidemiology. 1988;7:181–189. doi: 10.1159/000110153. [DOI] [PubMed] [Google Scholar]
- 74.Poser C.M., Hibberd P.L., Benedikz J., Gudmundsson G. Analysis of the ‘epidemic’ of multiple sclerosis in the faroe islands. I. Clinical and epidemiological aspects. Neuroepidemiology. 1988;7:168–180. doi: 10.1159/000110152. [DOI] [PubMed] [Google Scholar]
- 75.Adamec I., Lovric M., Zaper D., Barusic A.K., Bach I., Junakovic A., Mismas A., Habek M. Postural orthostatic tachycardia syndrome associated with multiple sclerosis. Auton. Neurosci. 2013;173:65–68. doi: 10.1016/j.autneu.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 76.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021;12:698169. doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Altmann D.M., Whettlock E.M., Liu S., Arachchillage D.J., Boyton R.J. The immunology of long COVID. Nat. Rev. Immunol. 2023;23:618–634. doi: 10.1038/s41577-023-00904-7. [DOI] [PubMed] [Google Scholar]
- 78.Komaroff A.L., Lipkin W.I. ME/CFS and long COVID share similar symptoms and biological abnormalities: Road map to the literature. Front. Med. 2023;10:1187163. doi: 10.3389/fmed.2023.1187163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ryabkova V.A., Gavrilova N.Y., Fedotkina T.V., Churilov L.P., Shoenfeld Y. Myalgic encephalomyelitis/chronic fatigue syndrome and post-COVID syndrome: A common neuroimmune ground. Diagnostics. 2023;13:66. doi: 10.3390/diagnostics13010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.van Campen C.L.M.C., Visser F.C. The abnormal cardiac index and stroke volume index changes during a normal tilt table test in ME/CFS patients compared to healthy volunteers, are not related to deconditioning. J. Thromb. Circ. 2018;2018:108. [Google Scholar]
- 81.van Campen C., Rowe P.C., Visser F.C. Blood volume status in ME/CFS correlates with the presence or absence of orthostatic symptoms: Preliminary results. Front. Pediatr. 2018;6:352. doi: 10.3389/fped.2018.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bateman L., Bested A.C., Bonilla H.F., Chheda B.V., Chu L., Curtin J.M., Dempsey T.T., Dimmock M.E., Dowell T.G., Felsenstein D., et al. Myalgic encephalomyelitis/chronic fatigue syndrome: Essentials of diagnosis and management. Mayo Clin. Proc. 2021;96:2861–2878. doi: 10.1016/j.mayocp.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 83.van Campen C.L.M.C., Rowe P.C., Verheugt F.W.A., Visser F.C. Influence of end-tidal CO2 on cerebral blood flow during orthostatic stress in controls and adults with myalgic encephalomyelitis/chronic fatigue syndrome. Physiol. Rep. 2023;11:e15639. doi: 10.14814/phy2.15639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Campen C., Verheugt F.W.A., Rowe P.C., Visser F.C. Orthostatic chronotropic incompetence in patients with myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) IBRO Neurosci. Rep. 2023;15:1–10. doi: 10.1016/j.ibneur.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gunning W.T., Kramer P.M., Cichocki J.A., Karabin B.L., Khuder S.A., Grubb B.P. Platelet storage pool deficiency and elevated inflammatory biomarkers are prevalent in postural orthostatic tachycardia syndrome. Cells. 2022;11:774. doi: 10.3390/cells11050774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Staff A.C., Redman C.W., Williams D., Leeson P., Moe K., Thilaganathan B., Magnus P., Steegers E.A., Tsigas E.Z., Ness R.B., et al. Pregnancy and long-term maternal cardiovascular health: Progress through harmonization of research cohorts and biobanks. Hypertension. 2016;67:251–260. doi: 10.1161/HYPERTENSIONAHA.115.06357. [DOI] [PubMed] [Google Scholar]
- 87.Thilaganathan B., Kalafat E. Cardiovascular system in preeclampsia and beyond. Hypertension. 2019;73:522–531. doi: 10.1161/HYPERTENSIONAHA.118.11191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morgan K., Chojenta C., Tavener M., Smith A., Loxton D. Postural orthostatic tachycardia syndrome during pregnancy: A systematic review of the literature. Auton. Neurosci. 2018;215:106–118. doi: 10.1016/j.autneu.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 89.Morgan K., Smith A., Blitshteyn S. POTS and pregnancy: A review of literature and recommendations for evaluation and treatment. Int. J. Womens Health. 2022;14:1831–1847. doi: 10.2147/IJWH.S366667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Raj S.R., Guzman J.C., Harvey P., Richer L., Schondorf R., Seifer C., Thibodeau-Jarry N., Sheldon R.S. Canadian cardiovascular society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can. J. Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 91.Zadourian A., Doherty T.A., Swiatkiewicz I., Taub P.R. Postural orthostatic tachycardia syndrome: Prevalence, pathophysiology, and management. Drugs. 2018;78:983–994. doi: 10.1007/s40265-018-0931-5. [DOI] [PubMed] [Google Scholar]
- 92.Gordan R., Gwathmey J.K., Xie L.H. Autonomic and endocrine control of cardiovascular function. World J. Cardiol. 2015;7:204–214. doi: 10.4330/wjc.v7.i4.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Armstrong R., Wheen P., Brandon L., Maree A., Kenny R.A. Heart rate: Control mechanisms, pathophysiology and assessment of the neurocardiac system in health and disease. QJM. 2022;115:806–812. doi: 10.1093/qjmed/hcab016. [DOI] [PubMed] [Google Scholar]
- 94.Simon P.M., Taha B.H., Dempsey J.A., Skatrud J.B., Iber C. Role of vagal feedback from the lung in hypoxic-induced tachycardia in humans. J. Appl. Physiol. 1995;78:1522–1530. doi: 10.1152/jappl.1995.78.4.1522. [DOI] [PubMed] [Google Scholar]
- 95.Faulhaber M., Gatterer H., Haider T., Linser T., Netzer N., Burtscher M. Heart rate and blood pressure responses during hypoxic cycles of a 3-week intermittent hypoxia breathing program in patients at risk for or with mild COPD. Int. J. Chronic Obstr. Pulm. Dis. 2015;10:339–345. doi: 10.2147/COPD.S75749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Siebenmann C., Lundby C. Regulation of cardiac output in hypoxia. Scand. J. Med. Sci. Sports. 2015;25((Suppl. S4)):53–59. doi: 10.1111/sms.12619. [DOI] [PubMed] [Google Scholar]
- 97.Paleczny B., Seredynski R., Tubek S., Adamiec D., Ponikowski P., Ponikowska B. Hypoxic tachycardia is not a result of increased respiratory activity in healthy subjects. Exp. Physiol. 2019;104:476–489. doi: 10.1113/EP087233. [DOI] [PubMed] [Google Scholar]
- 98.Joyce W., Wang T. Regulation of heart rate in vertebrates during hypoxia: A comparative overview. Acta Physiol. 2022;234:e13779. doi: 10.1111/apha.13779. [DOI] [PubMed] [Google Scholar]
- 99.Kell D.B., Pretorius E. No effects without causes. The iron dysregulation and dormant microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. 2018;93:1518–1557. doi: 10.1111/brv.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Deer R.R., Rock M.A., Vasilevsky N., Carmody L., Rando H., Anzalone A.J., Basson M.D., Bennett T.D., Bergquist T., Boudreau E.A., et al. Characterizing long COVID: Deep phenotype of a complex condition. eBioMedicine. 2021;74:103722. doi: 10.1016/j.ebiom.2021.103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reese J.T., Blau H., Casiraghi E., Bergquist T., Loomba J.J., Callahan T.J., Laraway B., Antonescu C., Coleman B., Gargano M., et al. Generalisable long COVID subtypes: Findings from the NIH N3C and recover programmes. eBioMedicine. 2023;87:104413. doi: 10.1016/j.ebiom.2022.104413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yong S.J., Liu S. Proposed subtypes of post-COVID-19 syndrome (or long-COVID) and their respective potential therapies. Rev. Med. Virol. 2022;32:e2315. doi: 10.1002/rmv.2315. [DOI] [PubMed] [Google Scholar]
- 103.Low P.A., Schondorf R., Rummans T.A. Why do patients have orthostatic symptoms in POTS? Clin. Auton. Res. 2001;11:223–224. doi: 10.1007/BF02298952. [DOI] [PubMed] [Google Scholar]
- 104.Plash W.B., Diedrich A., Biaggioni I., Garland E.M., Paranjape S.Y., Black B.K., Dupont W.D., Raj S.R. Diagnosing postural tachycardia syndrome: Comparison of tilt testing compared with standing haemodynamics. Clin. Sci. 2013;124:109–114. doi: 10.1042/CS20120276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Novak P. Cerebral blood flow, heart rate, and blood pressure patterns during the tilt test in common orthostatic syndromes. Neurosci. J. 2016;2016:6127340. doi: 10.1155/2016/6127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Aponte-Becerra L., Novak P. Tilt test: A review. J. Clin. Neurophysiol. 2021;38:279–286. doi: 10.1097/WNP.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 107.Cheshire W.P., Jr., Goldstein D.S. Autonomic uprising: The tilt table test in autonomic medicine. Clin. Auton. Res. 2019;29:215–230. doi: 10.1007/s10286-019-00598-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stewart J.M., Visintainer P., Medow M.S. Standing tests lack reliability to diagnose all adolescents who have postural tachycardia syndrome. Clin. Auton. Res. 2023;33:899–901. doi: 10.1007/s10286-023-00971-9. [DOI] [PubMed] [Google Scholar]
- 109.Petersen M.E.V., Williams T.R., Gordon C., Chamberlain-Webber R., Sutton R. The normal response to prolonged passive head up tilt testing. Heart. 2000;84:509–514. doi: 10.1136/heart.84.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Purkayastha S., Sorond F. Transcranial doppler ultrasound: Technique and application. Semin. Neurol. 2012;32:411–420. doi: 10.1055/s-0032-1331812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Thijs R.D., Brignole M., Falup-Pecurariu C., Fanciulli A., Freeman R., Guaraldi P., Jordan J., Habek M., Hilz M., Traon A.P., et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: Consensus statement of the European federation of autonomic societies (EFAS) endorsed by the American autonomic society (AAS) and the European academy of neurology (EAN) Clin. Auton. Res. 2021;31:369–384. doi: 10.1007/s10286-020-00738-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Raj S.R., Fedorowski A., Sheldon R.S. Diagnosis and management of postural orthostatic tachycardia syndrome. CMAJ. 2022;194:E378–E385. doi: 10.1503/cmaj.211373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Spahic J.M., Hamrefors V., Johansson M., Ricci F., Melander O., Sutton R., Fedorowski A. Malmö POTS symptom score: Assessing symptom burden in postural orthostatic tachycardia syndrome. J. Intern. Med. 2023;293:91–99. doi: 10.1111/joim.13566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Benarroch E.E. Postural tachycardia syndrome: A heterogeneous and multifactorial disorder. Mayo Clin. Proc. 2012;87:1214–1225. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Petelin Gadze Z., Bujan Kovac A., Adamec I., Milekic N., Sulentic V. Vagal nerve stimulation is beneficial in postural orthostatic tachycardia syndrome and epilepsy. Seizure. 2018;57:11–13. doi: 10.1016/j.seizure.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 116.Diedrich A., Urechie V., Shiffer D., Rigo S., Minonzio M., Cairo B., Smith E.C., Okamoto L.E., Barbic F., Bisoglio A., et al. Transdermal auricular vagus stimulation for the treatment of postural tachycardia syndrome. Auton. Neurosci. 2021;236:102886. doi: 10.1016/j.autneu.2021.102886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benarroch E.E. “Dysautonomia”: A plea for precision. Clin. Auton. Res. 2021;31:27–29. doi: 10.1007/s10286-020-00749-3. [DOI] [PubMed] [Google Scholar]
- 118.Palma J.A., Norcliffe-Kaufmann L., Fuente-Mora C., Percival L., Mendoza-Santiesteban C., Kaufmann H. Current treatments in familial dysautonomia. Expert. Opin. Pharmacother. 2014;15:2653–2671. doi: 10.1517/14656566.2014.970530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murga I., Aranburu L., Gargiulo P.A., Gomez Esteban J.C., Lafuente J.V. Clinical heterogeneity in ME/CFS. A way to understand long-COVID19 fatigue. Front. Psychiatry. 2021;12:735784. doi: 10.3389/fpsyt.2021.735784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carmona-Torre F., Minguez-Olaondo A., Lopez-Bravo A., Tijero B., Grozeva V., Walcker M., Azkune-Galparsoro H., Lopez de Munain A., Alcaide A.B., Quiroga J., et al. Dysautonomia in COVID-19 patients: A narrative review on clinical course, diagnostic and therapeutic strategies. Front. Neurol. 2022;13:886609. doi: 10.3389/fneur.2022.886609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bisaccia G., Ricci F., Recce V., Serio A., Iannetti G., Chahal A.A., Stahlberg M., Khanji M.Y., Fedorowski A., Gallina S. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: What do we know? J. Cardiovasc. Dev. Dis. 2021;8:156. doi: 10.3390/jcdd8110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chung T.H., Azar A. Autonomic nerve involvement in post-acute sequelae of SARS-CoV-2 syndrome (PASC) J. Clin. Med. 2022;12:73. doi: 10.3390/jcm12010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Marques K.C., Quaresma J.A.S., Falcao L.F.M. Cardiovascular autonomic dysfunction in “long COVID”: Pathophysiology, heart rate variability, and inflammatory markers. Front. Cardiovasc. Med. 2023;10:1256512. doi: 10.3389/fcvm.2023.1256512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Finsterer J. Small fiber neuropathy underlying dysautonomia in COVID-19 and in post-SARS-CoV-2 vaccination and long-COVID syndromes. Muscle Nerve. 2022;65:E31–E32. doi: 10.1002/mus.27554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Aktürk T., Turan Y., Tanik N., Karadağ M.E., Sacmaci H., Inan L.E. Vitamin D, vitamin D binding protein, vitamin D receptor levels and cardiac dysautonomia in patients with multiple sclerosis: A cross-sectional study. Arq. Neuro-Psiquiatr. 2019;77:848–854. doi: 10.1590/0004-282x20190182. [DOI] [PubMed] [Google Scholar]
- 126.Foschi M., Giannini G., Merli E., Mancinelli L., Zenesini C., Viti B., Guaraldi P., Cortelli P., Lugaresi A. Frequency and characteristics of dysautonomic symptoms in multiple sclerosis: A cross-sectional double-center study with the validated Italian version of the composite autonomic symptom score-31. Neurol. Sci. 2021;42:1395–1403. doi: 10.1007/s10072-020-04620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stewart J.M. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr. Res. 2000;48:218–226. doi: 10.1203/00006450-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 128.Naschitz J.E., Yeshurun D., Rosner I. Dysautonomia in chronic fatigue syndrome: Facts, hypotheses, implications. Med. Hypotheses. 2004;62:203–206. doi: 10.1016/S0306-9877(03)00331-1. [DOI] [PubMed] [Google Scholar]
- 129.Bested A.C., Marshall L.M. Review of myalgic encephalomyelitis/chronic fatigue syndrome: An evidence-based approach to diagnosis and management by clinicians. Rev. Environ. Health. 2015;30:223–249. doi: 10.1515/reveh-2015-0026. [DOI] [PubMed] [Google Scholar]
- 130.Nelson M.J., Bahl J.S., Buckley J.D., Thomson R.L., Davison K. Evidence of altered cardiac autonomic regulation in myalgic encephalomyelitis/chronic fatigue syndrome: A systematic review and meta-analysis. Medicine. 2019;98:e17600. doi: 10.1097/MD.0000000000017600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murga Gandasegui I., Aranburu Laka L., Gargiulo P.Á., Gómez-Esteban J.C., Lafuente Sánchez J.V. Myalgic encephalomyelitis/chronic fatigue syndrome: A neurological entity? Medicina. 2021;57:1030. doi: 10.3390/medicina57101030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nelson M.J., Buckley J.D., Thomson R.L., Bellenger C.R., Davison K. Markers of cardiac autonomic function during consecutive day peak exercise tests in people with myalgic encephalomyelitis/chronic fatigue syndrome. Front. Physiol. 2021;12:771899. doi: 10.3389/fphys.2021.771899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Renz-Polster H., Tremblay M.E., Bienzle D., Fischer J.E. The pathobiology of myalgic encephalomyelitis/chronic fatigue syndrome: The case for neuroglial failure. Front. Cell. Neurosci. 2022;16:888232. doi: 10.3389/fncel.2022.888232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pavy-Le Traon A., Amarenco G., Duerr S., Kaufmann H., Lahrmann H., Shaftman S.R., Tison F., Wenning G.K., Goetz C.G., Poewe W., et al. The movement disorders task force review of dysautonomia rating scales in Parkinson’s disease with regard to symptoms of orthostatic hypotension. Mov. Disord. 2011;26:1985–1992. doi: 10.1002/mds.23742. [DOI] [PubMed] [Google Scholar]
- 135.Krupp L.B. Fatigue in multiple sclerosis: Definition, pathophysiology and treatment. CNS Drugs. 2003;17:225–234. doi: 10.2165/00023210-200317040-00002. [DOI] [PubMed] [Google Scholar]
- 136.Shen J., Barbera J., Shapiro C.M. Distinguishing sleepiness and fatigue: Focus on definition and measurement. Sleep. Med. Rev. 2006;10:63–76. doi: 10.1016/j.smrv.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 137.Choi-Kwon S., Kim J.S. Poststroke fatigue: An emerging, critical issue in stroke medicine. Int. J. Stroke. 2011;6:328–336. doi: 10.1111/j.1747-4949.2011.00624.x. [DOI] [PubMed] [Google Scholar]
- 138.Kluger B.M., Herlofson K., Chou K.L., Lou J.S., Goetz C.G., Lang A.E., Weintraub D., Friedman J. Parkinson’s disease-related fatigue: A case definition and recommendations for clinical research. Mov. Disord. 2016;31:625–631. doi: 10.1002/mds.26511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Poulsen M.B., Skovbølling S.L., Kruuse C., Overgaard K., Rasmussen R.S. How to identify fatigue in stroke patients: An investigation of the post-stroke fatigue case definition validity. Top. Stroke Rehabil. 2020;27:369–376. doi: 10.1080/10749357.2019.1704387. [DOI] [PubMed] [Google Scholar]
- 140.Moore Y., Serafimova T., Anderson N., King H., Richards A., Brigden A., Sinai P., Higgins J., Ascough C., Clery P., et al. Recovery from chronic fatigue syndrome: A systematic review-heterogeneity of definition limits study comparison. Arch. Dis. Child. 2021;106:1087–1094. doi: 10.1136/archdischild-2020-320196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adibi I., Sanayei M., Tabibian F., Ramezani N., Pourmohammadi A., Azimzadeh K. Multiple sclerosis-related fatigue lacks a unified definition: A narrative review. J. Res. Med. Sci. 2022;27:24. doi: 10.4103/jrms.jrms_1401_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levesque A., Caru M., Duval M., Laverdiere C., Marjerrison S., Sultan S. Cancer-related fatigue: Scoping review to synthesize a definition for childhood cancer survivors. Support. Care Cancer. 2023;31:231. doi: 10.1007/s00520-023-07690-x. [DOI] [PubMed] [Google Scholar]
- 143.Pretorius E., Bronkhorst P., Briedenhann S., Smit E., Franz R.C. Comparisons of the fibrin networks during pregnancy, nonpregnancy and pregnancy during dysfibrinogenaemia using the scanning electron microscope. Blood Coagul. Fibrinolysis. 2009;20:12–16. doi: 10.1097/MBC.0b013e328322b429. [DOI] [PubMed] [Google Scholar]
- 144.Pretorius E., Oberholzer H.M., van der Spuy W.J., Meiring J.H. The changed ultrastructure of fibrin networks during use of oral contraception and hormone replacement. J. Thromb. Thrombolysis. 2010;30:502–506. doi: 10.1007/s11239-010-0502-4. [DOI] [PubMed] [Google Scholar]
- 145.Pretorius E., Steyn H., Engelbrecht M., Swanepoel A.C., Oberholzer H.M. Differences in fibrin fiber diameters in healthy individuals and thromboembolic ischemic stroke patients. Blood Coagul. Fibrinolysis. 2011;22:696–700. doi: 10.1097/MBC.0b013e32834bdb32. [DOI] [PubMed] [Google Scholar]
- 146.Pretorius E., Vermeulen N., Bester J., Lipinski B., Kell D.B. A novel method for assessing the role of iron and its functional chelation in fibrin fibril formation: The use of scanning electron microscopy. Toxicol. Mech. Methods. 2013;23:352–359. doi: 10.3109/15376516.2012.762082. [DOI] [PubMed] [Google Scholar]
- 147.Pretorius E., Mbotwe S., Bester J., Robinson C.J., Kell D.B. Acute induction of anomalous and amyloidogenic blood clotting by molecular amplification of highly substoichiometric levels of bacterial lipopolysaccharide. J. R. Soc. Interface. 2016;123:20160539. doi: 10.1098/rsif.2016.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kell D.B., Pretorius E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: Lessons from and for blood clotting. Prog. Biophys. Mol. Biol. 2017;123:16–41. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 149.Pretorius E., Mbotwe S., Kell D.B. Lipopolysaccharide-binding protein (LBP) reverses the amyloid state of fibrin seen in plasma of type 2 diabetics with cardiovascular comorbidities. Sci. Rep. 2017;7:9680. doi: 10.1038/s41598-017-09860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pretorius E., Page M.J., Hendricks L., Nkosi N.B., Benson S.R., Kell D.B. Both lipopolysaccharide and lipoteichoic acids potently induce anomalous fibrin amyloid formation: Assessment with novel Amytracker™ stains. J. R. Soc. Interface. 2018;15:20170941. doi: 10.1098/rsif.2017.0941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pretorius E., Page M.J., Mbotwe S., Kell D.B. Lipopolysaccharide-binding protein (LBP) can reverse the amyloid state of fibrin seen or induced in Parkinson’s disease. PLoS ONE. 2018;13:e0192121. doi: 10.1371/journal.pone.0192121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pretorius E., Bester J., Page M.J., Kell D.B. The potential of LPS-binding protein to reverse amyloid formation in plasma fibrin of individuals with Alzheimer-type dementia. Front. Aging Neurosci. 2018;10:257. doi: 10.3389/fnagi.2018.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.de Waal G.M., Engelbrecht L., Davis T., de Villiers W.J.S., Kell D.B., Pretorius E. Correlative light-electron microscopy detects lipopolysaccharide and its association with fibrin fibres in parkinson’s disease, Alzheimer’s disease and type 2 diabetes mellitus. Sci. Rep. 2018;8:16798. doi: 10.1038/s41598-018-35009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Laubscher G.J., Lourens P.J., Venter C., Kell D.B., Pretorius E. TEG®, microclot and platelet mapping for guiding early management of severe COVID-19 coagulopathy. J. Clin. Med. 2021;10:5381. doi: 10.3390/jcm10225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pretorius E., Kell D.B. Seminars in Thrombosis and Hemostasis. Thieme Medical Publishers, Inc.; New York, NY, USA: 2023. A perspective on how microscopy imaging of fibrinaloid microclots and platelet pathology may be applied in clinical investigations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pretorius E., Bester J., Vermeulen N., Alummoottil S., Soma P., Buys A.V., Kell D.B. Poorly controlled type 2 diabetes is accompanied by significant morphological and ultrastructural changes in both erythrocytes and in thrombin-generated fibrin: Implications for diagnostics. Cardiovasc. Diabetol. 2015;13:30. doi: 10.1186/s12933-015-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pretorius E., Page M.J., Engelbrecht L., Ellis G.C., Kell D.B. Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc. Diabetol. 2017;16:141. doi: 10.1186/s12933-017-0624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pretorius E., Venter C., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B. Prevalence of readily detected amyloid blood clots in ‘unclotted’ type 2 diabetes mellitus and COVID-19 plasma: A preliminary report. Cardiovasc. Diabetol. 2020;19:193. doi: 10.1186/s12933-020-01165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kell D.B., Laubscher G.J., Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: Origins and therapeutic implications. Biochem. J. 2022;479:537–559. doi: 10.1042/BCJ20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kell D.B., Pretorius E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, long COVID and ME/CFS: Evidence, mechanisms, and therapeutic implications. Biochem. J. 2022;479:1653–1708. doi: 10.1042/BCJ20220154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kell D.B., Pretorius E. Are fibrinaloid microclots a cause of autoimmunity in long COVID and other post-infection diseases? Biochem. J. 2023;480:1217–1240. doi: 10.1042/BCJ20230241. [DOI] [PubMed] [Google Scholar]
- 162.Kruger A., Vlok M., Turner S., Venter C., Laubscher G.J., Kell D.B., Pretorius E. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc. Diabetol. 2022;21:190. doi: 10.1186/s12933-022-01623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pretorius E., Vlok M., Venter C., Bezuidenhout J.A., Laubscher G.J., Steenkamp J., Kell D.B. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021;20:172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pretorius E., Venter C., Laubscher G.J., Kotze M.J., Oladejo S., Watson L.R., Rajaratnam K., Watson B.W., Kell D.B. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with long COVID/post-acute sequelae of COVID-19 (PASC) Cardiovasc. Diabetol. 2022;21:148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Turner S., Khan M.A., Putrino D., Woodcock A., Kell D.B., Pretorius E. Long COVID: Pathophysiological factors and abnormal coagulation. Trends Endocrinol. Metab. 2023;34:321–344. doi: 10.1016/j.tem.2023.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Turner S., Laubscher G.J., Khan M.A., Kell D.B., Pretorius E. Accelerating discovery: A novel flow cytometric method for detecting fibrin(ogen) amyloid microclots using long COVID as a model. Heliyon. 2023;9:e19605. doi: 10.1016/j.heliyon.2023.e19605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Grobbelaar L.M., Venter C., Vlok M., Ngoepe M., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B., Pretorius E. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: Implications for microclot formation in COVID-19. Biosci. Rep. 2021;41:BSR20210611. doi: 10.1042/BSR20210611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Grobbelaar L.M., Kruger A., Venter C., Burger E.M., Laubscher G.J., Maponga T.G., Kotze M.J., Kwaan H.C., Miller J.B., Fulkerson D., et al. Relative hypercoagulopathy of the SARS-CoV-2 beta and delta variants when compared to the less severe omicron variants is related to TEG parameters, the extent of fibrin amyloid microclots, and the severity of clinical illness. Semin. Thromb. Haemost. 2022;48:858–868. doi: 10.1055/s-0042-1756306. [DOI] [PubMed] [Google Scholar]
- 169.Nunes J.M., Kruger A., Proal A., Kell D.B., Pretorius E. The occurrence of hyperactivated platelets and fibrinaloid microclots in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Pharmaceuticals. 2022;15:931. doi: 10.3390/ph15080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nunes J.M., Kell D.B., Pretorius E. Cardiovascular and haematological pathology in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS): A role for viruses. Blood Rev. 2023;60:101075. doi: 10.1016/j.blre.2023.101075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kell D.B., Pretorius E. The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen) Integr. Biol. 2015;7:24–52. doi: 10.1039/c4ib00173g. [DOI] [PubMed] [Google Scholar]
- 172.Oaklander A.L., Mills A.J., Kelley M., Toran L.S., Smith B., Dalakas M.C., Nath A. Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol.-Neuroimmunol. Neuroinflamm. 2022;9:e1146. doi: 10.1212/NXI.0000000000001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pretorius E., Venter C., Laubscher G.J., Lourens P.J., Steenkamp J., Kell D.B. Prevalence of amyloid blood clots in COVID-19 plasma. medRxiv. 2020 doi: 10.1101/2020.07.28.20163543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Safavi F., Gustafson L., Walitt B., Lehky T., Dehbashi S., Wiebold A., Mina Y., Shin S., Pan B., Polydefkis M., et al. Neuropathic symptoms with SARS-CoV-2 vaccination. medRxiv. 2022 doi: 10.1101/2022.05.16.22274439. [DOI] [Google Scholar]
- 175.Theoharides T.C. Could SARS-CoV-2 spike protein be responsible for long-COVID syndrome? Mol. Neurobiol. 2022;59:1850–1861. doi: 10.1007/s12035-021-02696-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Theoharides T.C., Kempuraj D. Role of SARS-CoV-2 spike-protein-induced activation of microglia and mast cells in the pathogenesis of neuro-COVID. Cells. 2023;12:688. doi: 10.3390/cells12050688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roy M., Nath A.K., Pal I., Dey S.G. Second sphere interactions in amyloidogenic diseases. Chem. Rev. 2022;122:12132–12206. doi: 10.1021/acs.chemrev.1c00941. [DOI] [PubMed] [Google Scholar]
- 178.Janson J., Ashley R.H., Harrison D., McIntyre S., Butler P.C. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 179.Engel M.F.M., Khemtémourian L., Kleijer C.C., Meeldijk H.J.D., Jacobs J., Verkleij A.J., de Kruijff B., Killian J.A., Hoppener J.W.M. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc. Natl. Acad. Sci. USA. 2008;105:6033–6038. doi: 10.1073/pnas.0708354105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Friedman R., Pellarin R., Caflisch A. Amyloid aggregation on lipid bilayers and its impact on membrane permeability. J. Mol. Biol. 2009;387:407–415. doi: 10.1016/j.jmb.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 181.Butterfield S.M., Lashuel H.A. Amyloidogenic protein-membrane interactions: Mechanistic insight from model systems. Angew. Chem. Int. Ed. Engl. 2010;49:5628–5654. doi: 10.1002/anie.200906670. [DOI] [PubMed] [Google Scholar]
- 182.Sciacca M.F.M., Brender J.R., Lee D.K., Ramamoorthy A. Phosphatidylethanolamine enhances amyloid fiber-dependent membrane fragmentation. Biochemistry. 2012;51:7676–7684. doi: 10.1021/bi3009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sciacca M.F.M., Kotler S.A., Brender J.R., Chen J., Lee D.K., Ramamoorthy A. Two-step mechanism of membrane disruption by abeta through membrane fragmentation and pore formation. Biophys. J. 2012;103:702–710. doi: 10.1016/j.bpj.2012.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Relini A., Marano N., Gliozzi A. Misfolding of amyloidogenic proteins and their interactions with membranes. Biomolecules. 2013;4:20–55. doi: 10.3390/biom4010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sciacca M.F.M., Milardi D., Messina G.M.L., Marletta G., Brender J.R., Ramamoorthy A., La Rosa C. Cations as switches of amyloid-mediated membrane disruption mechanisms: Calcium and IAPP. Biophys. J. 2013;104:173–184. doi: 10.1016/j.bpj.2012.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jang H., Arce F.T., Ramachandran S., Kagan B.L., Lal R., Nussinov R. Disordered amyloidogenic peptides may insert into the membrane and assemble into common cyclic structural motifs. Chem. Soc. Rev. 2014;43:6750–6764. doi: 10.1039/C3CS60459D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ow S.Y., Dunstan D.E. A brief overview of amyloids and Alzheimer’s disease. Protein Sci. 2014;23:1315–1331. doi: 10.1002/pro.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Relini A., Marano N., Gliozzi A. Probing the interplay between amyloidogenic proteins and membranes using lipid monolayers and bilayers. Adv. Colloid Interface Sci. 2014;207:81–92. doi: 10.1016/j.cis.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 189.Caillon L., Hoffmann A.R., Botz A., Khemtemourian L. Molecular structure, membrane interactions, and toxicity of the islet amyloid polypeptide in type 2 diabetes mellitus. J. Diabetes Res. 2016;2016:5639875. doi: 10.1155/2016/5639875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Bode D.C., Baker M.D., Viles J.H. Ion channel formation by amyloid-beta42 oligomers but not amyloid-beta40 in cellular membranes. J. Biol. Chem. 2017;292:1404–1413. doi: 10.1074/jbc.M116.762526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Malishev R., Shaham-Niv S., Nandi S., Kolusheva S., Gazit E., Jelinek R. Bacoside-A, an indian traditional-medicine substance, inhibits beta-amyloid cytotoxicity, fibrillation, and membrane interactions. ACS Chem. Neurosci. 2017;8:884–891. doi: 10.1021/acschemneuro.6b00438. [DOI] [PubMed] [Google Scholar]
- 192.Cheng B., Li Y., Ma L., Wang Z., Petersen R.B., Zheng L., Chen Y., Huang K. Interaction between amyloidogenic proteins and biomembranes in protein misfolding diseases: Mechanisms, contributors, and therapy. Biochim. Biophys. Acta Biomembr. 2018;1860:1876–1888. doi: 10.1016/j.bbamem.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 193.Rawat A., Langen R., Varkey J. Membranes as modulators of amyloid protein misfolding and target of toxicity. Biochim. Biophys. Acta Biomembr. 2018;1860:1863–1875. doi: 10.1016/j.bbamem.2018.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sciacca M.F.M., Chillemi R., Sciuto S., Greco V., Messineo C., Kotler S.A., Lee D.K., Brender J.R., Ramamoorthy A., La Rosa C., et al. A blend of two resveratrol derivatives abolishes hIAPP amyloid growth and membrane damage. Biochim. Biophys. Acta Biomembr. 2018;1860:1793–1802. doi: 10.1016/j.bbamem.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 195.Younan N.D., Chen K.F., Rose R.S., Crowther D.C., Viles J.H. Prion protein stabilizes amyloid-beta (abeta) oligomers and enhances abeta neurotoxicity in a Drosophila model of Alzheimer’s disease. J. Biol. Chem. 2018;293:13090–13099. doi: 10.1074/jbc.RA118.003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bode D.C., Freeley M., Nield J., Palma M., Viles J.H. Amyloid-beta oligomers have a profound detergent-like effect on lipid membrane bilayers, imaged by atomic force and electron microscopy. J. Biol. Chem. 2019;294:7566–7572. doi: 10.1074/jbc.AC118.007195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Malishev R., Kolusheva S., Jelinek R. Vesicle-based assays to study membrane interactions of amyloid peptides. Methods Mol. Biol. 2019;1873:39–51. doi: 10.1007/978-1-4939-8820-4_3. [DOI] [PubMed] [Google Scholar]
- 198.Huang Y.R., Liu R.T. The toxicity and polymorphism of beta-amyloid oligomers. Int. J. Mol. Sci. 2020;21:4477. doi: 10.3390/ijms21124477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sciacca M.F.M., La Rosa C., Milardi D. Amyloid-mediated mechanisms of membrane disruption. Biophysica. 2021;1:137–156. doi: 10.3390/biophysica1020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tempra C., Scollo F., Pannuzzo M., Lolicato F., La Rosa C. A unifying framework for amyloid-mediated membrane damage: The lipid-chaperone hypothesis. Biochim. Biophys. Acta Proteins Proteom. 2022;1870:140767. doi: 10.1016/j.bbapap.2022.140767. [DOI] [PubMed] [Google Scholar]
- 201.Ma L., Li X., Petersen R.B., Peng A., Huang K. Probing the interactions between amyloidogenic proteins and bio-membranes. Biophys. Chem. 2023;296:106984. doi: 10.1016/j.bpc.2023.106984. [DOI] [PubMed] [Google Scholar]
- 202.Viles J.H. Imaging amyloid-beta membrane interactions: Ion-channel pores and lipid-bilayer permeability in Alzheimer’s disease. Angew. Chem. Int. Ed. Engl. 2023;62:e202215785. doi: 10.1002/anie.202215785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Busciglio J., Lorenzo A., Yankner B.A. Methodological variables in the assessment of beta amyloid neurotoxicity. Neurobiol. Aging. 1992;13:609–612. doi: 10.1016/0197-4580(92)90065-6. [DOI] [PubMed] [Google Scholar]
- 204.Ono K., Condron M.M., Teplow D.B. Structure-neurotoxicity relationships of amyloid beta-protein oligomers. Proc. Natl. Acad. Sci. USA. 2009;106:14745–14750. doi: 10.1073/pnas.0905127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Broersen K., Rousseau F., Schymkowitz J. The culprit behind amyloid beta peptide related neurotoxicity in Alzheimer’s disease: Oligomer size or conformation? Alzheimers Res. Ther. 2010;2:12. doi: 10.1186/alzrt36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Malchiodi-Albedi F., Paradisi S., Matteucci A., Frank C., Diociaiuti M. Amyloid oligomer neurotoxicity, calcium dysregulation, and lipid rafts. Int. J. Alzheimers Dis. 2011;2011:906964. doi: 10.4061/2011/906964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Smith L.M., Strittmatter S.M. Binding sites for amyloid-beta oligomers and synaptic toxicity. Cold Spring Harb. Perspect. Med. 2017;7:a024075. doi: 10.1101/cshperspect.a024075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Tanokashira D., Mamada N., Yamamoto F., Taniguchi K., Tamaoka A., Lakshmana M.K., Araki W. The neurotoxicity of amyloid beta-protein oligomers is reversible in a primary neuron model. Mol. Brain. 2017;10:4. doi: 10.1186/s13041-016-0284-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kepp K.P., Robakis N.K., Hoilund-Carlsen P.F., Sensi S.L., Vissel B. The amyloid cascade hypothesis: An updated critical review. Brain. 2023;146:3969–3990. doi: 10.1093/brain/awad159. [DOI] [PubMed] [Google Scholar]
- 210.Killingley B., Mann A.J., Kalinova M., Boyers A., Goonawardane N., Zhou J., Lindsell K., Hare S.S., Brown J., Frise R., et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat. Med. 2022;28:1031–1041. doi: 10.1038/s41591-022-01780-9. [DOI] [PubMed] [Google Scholar]
- 211.Appelman B., Charlton B.T., Goulding R.P., Kerkhoff T.J., Breedveld E.A., Noort W., Offringa C., Bloemers F.W., van Weeghel M., Schomakers B.V., et al. Muscle abnormalities worsen after postexertional malaise in long COVID. Nat. Commun. 2024;15:17. doi: 10.1038/s41467-023-44432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Vassiliou A.G., Jahaj E., Ilias I., Markaki V., Malachias S., Vrettou C., Ischaki E., Mastora Z., Douka E., Keskinidou C., et al. Lactate kinetics reflect organ dysfunction and are associated with adverse outcomes in intensive care unit patients with COVID-19 pneumonia: Preliminary results from a greek single-centre study. Metabolites. 2020;10:386. doi: 10.3390/metabo10100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Iepsen U.W., Plovsing R.R., Tjelle K., Foss N.B., Meyhoff C.S., Ryrsø C.K., Berg R.M.G., Secher N.H. The role of lactate in sepsis and COVID-19: Perspective from contracting skeletal muscle metabolism. Exp. Physiol. 2021;107:665–673. doi: 10.1113/EP089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nechipurenko Y.D., Semyonov D.A., Lavrinenko I.A., Lagutkin D.A., Generalov E.A., Zaitceva A.Y., Matveeva O.V., Yegorov Y.E. The role of acidosis in the pathogenesis of severe forms of COVID-19. Biology. 2021;10:852. doi: 10.3390/biology10090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Carpenè G., Onorato D., Nocini R., Fortunato G., Rizk J.G., Henry B.M., Lippi G. Blood lactate concentration in COVID-19: A systematic literature review. Clin. Chem. Lab. Med. 2022;60:332–337. doi: 10.1515/cclm-2021-1115. [DOI] [PubMed] [Google Scholar]
- 216.de Boer E., Petrache I., Goldstein N.M., Olin J.T., Keith R.C., Modena B., Mohning M.P., Yunt Z.X., San-Millan I., Swigris J.J. Decreased fatty acid oxidation and altered lactate production during exercise in patients with post-acute COVID-19 syndrome. Am. J. Respir. Crit. Care Med. 2022;205:126–129. doi: 10.1164/rccm.202108-1903LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.van der Togt V., Rossman J.S. Hypothesis: Inflammatory acid-base disruption underpins long COVID. Front. Immunol. 2023;14:1150105. doi: 10.3389/fimmu.2023.1150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Rutherford G., Manning P., Newton J.L. Understanding muscle dysfunction in chronic fatigue syndrome. J. Aging Res. 2016;2016:2497348. doi: 10.1155/2016/2497348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fernandez-Guerra P., Gonzalez-Ebsen A.C., Boonen S.E., Courraud J., Gregersen N., Mehlsen J., Palmfeldt J., Olsen R.K.J., Brinth L.S. Bioenergetic and proteomic profiling of immune cells in myalgic encephalomyelitis/chronic fatigue syndrome patients: An exploratory study. Biomolecules. 2021;11:961. doi: 10.3390/biom11070961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Natelson B.H., Vu D., Coplan J.D., Mao X., Blate M., Kang G., Soto E., Kapusuz T., Shungu D.C. Elevations of ventricular lactate levels occur in both chronic fatigue syndrome and fibromyalgia. Fatigue. 2017;5:15–20. doi: 10.1080/21641846.2017.1280114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Murrough J.W., Mao X., Collins K.A., Kelly C., Andrade G., Nestadt P., Levine S.M., Mathew S.J., Shungu D.C. Increased ventricular lactate in chronic fatigue syndrome measured by 1H MRS imaging at 3.0 T. II: Comparison with major depressive disorder. NMR Biomed. 2010;23:643–650. doi: 10.1002/nbm.1512. [DOI] [PubMed] [Google Scholar]
- 222.Shungu D.C., Weiduschat N., Murrough J.W., Mao X., Pillemer S., Dyke J.P., Medow M.S., Natelson B.H., Stewart J.M., Mathew S.J. Increased ventricular lactate in chronic fatigue syndrome. III. Relationships to cortical glutathione and clinical symptoms implicate oxidative stress in disorder pathophysiology. NMR Biomed. 2012;25:1073–1087. doi: 10.1002/nbm.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]