Abstract
Suppression of the basal extracellular signal-regulated kinase (ERK) activity in PC12 cells markedly altered their phenotype. Wild-type cells grew in a dissociated pattern adherent to the substrate. The stable expression of an ERK inhibitory mutant resulted in the formation of calcium-dependent aggregates which were less adherent to the substrate. Concomitantly, the cells reorganized their actin cytoskeleton and increased their expression of several adherens junction proteins, particularly cadherin. Metabolic labeling demonstrated an increased synthesis of cadherin and β-catenin in these cells. Nontransfected PC12 cells and a ras-transformed MDCK cell line also formed aggregates and increased their expression of adherens junction proteins following treatment with the selective MEK inhibitor PD98059. A peptide containing the HAV cadherin recognition sequence attenuated the aggregation. These studies suggest that in PC12 and epithelial cells, ERKs are pivotally positioned to enhance substrate interactions when active or to release homotypic interactions when suppressed.
To achieve the complexity of multicellularity, organisms must strike a highly regulated balance between cell-cell interactions, such as the adherens junction, and interactions with the substrate, such as adhesion plaques. Although first described as morphological entities, many of the individual molecules within various cellular contacts have been identified (18, 22), and some of the components involved in adhesive interactions, such as β-catenin, also function in signaling systems that control early development and differentiation. The necessity to generate diverse types of junctions on individual cells must require highly intertwined signaling cascades that can readily modulate responses between various pathways. In situ cells engage in both homotypic contacts with other cells and interactions with the substrate; however, for most adherent cells in culture, focal adhesions and interactions with the substrate predominate. The formation of adherens junctions and the expression of those proteins which reside in these junctions, the cadherins and catenins, are associated with the transition of cell populations from a dispersed pattern to an aggregated compact one.
The mitogen-activated protein (MAP) kinases integrate multiple intracellular signals following activation by a variety of external signals. The extracellular signal-regulated kinases (ERKs) are the most highly studied members of the MAP kinase family, which in mammalian cells also includes the JNK/SAPK and p38/RK subfamilies (10). The ERK subfamily is defined by dual phosphorylation on the TEY motif in domain VIII (1). These kinases lie downstream in a highly conserved signal transduction cascade that begins with ligand binding to a receptor tyrosine kinase or a G protein-coupled receptor (23). The ERKs exert control over cellular proliferation (41, 43) and morphological transformation (41) by phosphorylating both nuclear and cytoplasmic substrates, including the transcription factors elk-1/p62TCF (19, 26, 49), c-jun (3, 37), c-myc (3), NF-IL6 (33), and TAL1 (12), as well as RNA polymerase II (15), and the cytoplasmic substrates pp90rsk (13, 44), cytosolic phospholipase A2, stathmin (25), epidermal growth factor (EGF) receptor (34, 46), Raf-1 (4, 24), and MAP kinase kinase (27). The cascade of regulatory molecules involved in the attachment of cells to the substrate can lead to the activation of ERKs (11, 30, 40). Signaling via integrin receptors, which includes the activation of ERKs as one of many downstream elements (28), plays an important role in the formation of substrate interactions. On the other hand, little is known regarding the relationship of ERK signal transduction to cell-cell adhesion.
We prepared stable PC12 cell lines in which both the basal activity of the ERKs and their activity after nerve growth factor (NGF) stimulation were inhibited. These cells had a markedly altered phenotype: they developed cell-cell contacts, became less adherent to the substrate, reorganized their actin filaments, and increased expression of proteins found in adherens junctions. We conclude that the maintenance of substrate interactions in preference to cell-cell contacts requires a basal level of ERK activity.
MATERIALS AND METHODS
Materials.
p42 MAP kinase (ERK2) cDNAs were from M. Cobb (Dallas, Tex.). The mammalian expression vector pRc/CMV was from Invitrogen (San Diego, Calif.). The lipofectin transfection kit was from GIBCO (Grand Island, N.Y.). Flag antibodies were obtained from Eastman Kodak (New Haven, Conn.) and Santa Cruz Biotech (Santa Cruz, Calif.). Affinity-purified polyclonal anti-MAP kinases were from Santa Cruz Biotech, and anti-phosphorylated MAP kinase was a gift from M. Greenberg (Boston, Mass.). Monoclonal antibodies against α-, β-, and γ-catenins, β1 and β3 integrins, and paxillin were from Transduction Laboratories (Lexington, Ky.). Monoclonal anti-integrin 3A3 was a gift from D. Turner (Syracuse, N.Y.). Monoclonal and polyclonal antibodies against the carboxyl terminus of chick N-cadherins as well as monoclonal anti-E-cadherin (DECMA-1) were from Sigma (St. Louis, Mo.). Rhodamine phalloidin was from Molecular Probes (Eugene, Oreg.). [γ-32P]ATP and [35S]methionine were from Amersham (Arlington Heights, Ill.), and unless otherwise indicated, all chemicals were from Sigma.
Subcloning and transfection.
The cloning and mutagenesis of ERK2 were described previously (8, 39). The product was then subcloned into pRc/CMV (Invitrogen), and the flag sequence DYKDDDDK was fused to the amino-terminal end as an epitope tag. The primary structure of p42 flag-ERK2 and the mutation sites were validated by DNA sequencing. Transfections of PC12 cells were performed in accordance with the GIBCO instructions for the lipofectin transfection assay. The stable PC12 cells were selected and maintained in medium containing neomycin.
Cell cultures and immunohistochemistry.
PC12 cells were plated onto Nunc culture dishes with a Nunclon δ-treated surface (Fisher Scientific, Pittsburgh, Pa.), and they were maintained in Dulbecco’s modified Eagle medium (DMEM) with 10% horse serum and 5% fetal bovine serum. For some experiments, the dishes were coated with rat tail type I collagen (Becton Dickinson, Bedford, Mass.) to confirm the growth characteristics. For those cells which were stimulated with 100 ng of NGF per ml to study PC12 cell differentiation, the culture dishes were coated with polylysine. Neurite outgrowth was scored when the length of the processes exceeded the cell diameter. Ras-transformed MDCKf3 cells were grown in DMEM with 10% fetal bovine serum (5). Cells used for fluorescence studies were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Following staining, the cells were mounted and photographed with a Zeiss Axioskope equipped with epifluorescence.
Aggregation/reaggregation assays and suspension cultures.
To assess the calcium dependence of cell aggregation, extracellular calcium was depleted by adding 5 mM EDTA to the culture medium. Reaggregation was assessed after the cells were returned to normal calcium-containing medium for 1, 3, 7, 10, and 21 days. To study the aggregation properties of nontransfected PC12 cells, spinner cultures with magnetic flasks (Bellco Biotech, Vineland, N.J.) were prepared. The MEK inhibitor PD98059 (>95% pure as determined by a high-performance liquid chromatograph [New England Biolabs, Beverly, Mass.]) was dissolved in dimethylsulfoxide and stored at 25 mM as stock. Control cultures were treated simply with 0.1% dimethyl sulfoxide. PD98059 at a concentration of 25 μM was included in the medium for 2 days before cell lysis. Similar experiments with PD98059 were performed on MDCKf3 cells.
To test whether the aggregation of PC12 cells in suspension is mediated by cadherin, the synthetic decapeptide LRAHAVDVNG-amide (Peninsula Lab, Belmont, Calif.) was used in control or PD98059-treated PC12 cells. Before transferring the PC12 cells to spinner flasks, the peptide was incubated in culture medium for 30 min. Afterward, the PC12 cells were returned to the incubator for 2 days in the presence or absence of the peptide. The cells were then photographed with a Leitz inverted light microscope.
Protein kinase assay.
Stable PC12 cells were lysed in immunoprecipitation buffer (10 mM Tris [pH 7.4], 50 mM NaCl, 1 mM EDTA, 1 mM EGTA, 0.2% Nonidet P-40, 0.1% deoxycholate) containing protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, 10 μg of aprotinin per ml, 10 μg of pepstatin A per ml, 10 μg of leupeptin per ml, 1 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 20 mM sodium fluoride). The cell lysates were centrifuged to remove cell debris, and the supernatants were immunoprecipitated with affinity-purified antibodies. To assay for total ERK activity, the cell lysates were immunoprecipitated with polyclonal anti-MAP kinase C16 and polyclonal anti-flag D8 was used to immunoprecipitate flag-ERK kinase only. The immunoprecipitates were captured by protein A-agarose and incubated with myelin basic protein in the presence of 150 μM ATP and 1 to 2 μCi of [γ-32P]ATP. After 30 min of incubation, the samples were then resolved by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis (SDS–15% PAGE) and exposed to Kodak film.
Metabolic labeling.
PC12 cells were preincubated in labeling medium (DMEM-fetal bovine serum minus methionine) for 20 min. The medium was then removed, and 800 μCi of [35S]methionine was added to the cells in a total volume of 4 ml of labeling medium in 10-cm dishes. After the cells were labeled for several time points, they were rinsed and then solubilized in a mixture containing 50 mM NaCl, 10 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid); pH 6.8], 3 mM MgCl2, 0.5% Triton X-100, 300 mM sucrose, 1.2 mM phenylmethylsulfonyl fluoride, and 10 μg of leupeptin per ml for 20 min at 4°C on a rocking platform. The cells were scraped off from the dishes and centrifuged in a microcentrifuge for 10 min to collect both the supernatant (Triton-soluble fraction) and the pellet (Triton-insoluble fraction).
Immunoprecipitation.
Before incubation with primary antibody, cell lysates (soluble and insoluble fractions) were precleared with protein G plus protein A-agarose beads followed by centrifugation to remove the beads. The supernatants were then immunoprecipitated with the respective cadherin or catenin antibody for 1 h at 4°C. Thirty microliters of protein G plus protein A-agarose beads was added to the samples for an additional 2 h at 4°C. The samples were washed sequentially with immunoprecipitation buffer (15 mM Tris [pH 7.5], 5 mM EDTA, 2.5 mM EGTA, 1% Triton X-100, 1% Na deoxycholate, 0.1% SDS, 120 mM NaCl, 25 mM KCl), high-salt buffer (15 mM Tris [pH 7.5], 5 mM EDTA, 2.5 mM EGTA, 1% Triton X-100, 1% Na deoxycholate, 0.1% SDS, and 1 M NaCl), and a low-salt buffer (15 mM Tris [pH 7.5], 5 mM EDTA). Immunoprecipitates were separated by SDS-PAGE and transferred to polyvinylidene difluoride for immunoblotting.
The stable PC12 cells were lysed as described above. Thirty micrograms of total protein was loaded and resolved by SDS–10% PAGE. Proteins transferred to a polyvinylidene difluoride membrane were blotted and then developed by using the Amersham enhanced chemiluminescence system.
RESULTS
Phenotype induced by the expression of an ERK inhibitory mutant (p42YF185) in PC12 cells.
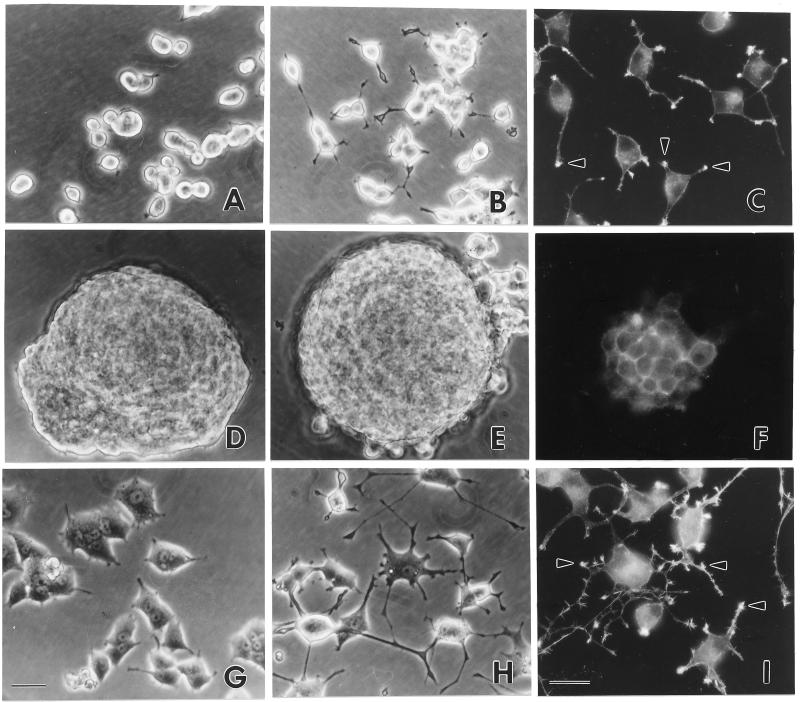
A mutant ERK2, p42YF185, in which tyrosine 185 was replaced with phenylalanine in kinase subdomain VIII, was fused to a flag sequence and subcloned into pRc/CMV. The substitution of the tyrosine within the TEY motif is believed to function as a dominant negative by binding to the upstream kinase, MEK, and preventing it from phosphorylating all of the ERK isoforms (35). The flag sequence can be detected specifically with monoclonal antibody M5 or polyclonal antibody D8. Wild-type ERK2, designated p42wt, was subcloned in the same way. Studies utilizing these stable transfectants were done with independent isolates of p42YF185 and p42wt. Immunoblots demonstrated the stable expression of flagged constructs in the PC12 cells (Fig. 1). Although the mock-transfected PC12 cells remained isolated and rounded in culture (Fig. 2A), PC12 cells expressing the inhibitory mutant p42YF185 formed large aggregates and did not adhere well to the culture dishes (Fig. 2D). On culture dishes coated with collagen, these cells grew as clusters and remained aggregated when they were triturated for passage. In contrast, PC12 cells that overexpressed p42wt became flatter and were more adherent to the culture dishes (Fig. 2G).
FIG. 1.
Expression of p42 flag-ERK in PC12 cells. (A) Immunoblots of lysates taken from representative stable PC12 clones expressing the p42wt or p42YF185 mutant labeled with anti-MAP kinase (Erk2); (B) immunoblots of lysates taken from representative stable PC12 clones expressing the p42wt or p42YF185 mutant labeled with anti-flag (M5 monoclonal antibody). Molecular weight markers (in thousands) are indicated to the left of the immunoblots.
FIG. 2.
Morphology and growth characteristics of stable PC12 transfectants expressing p42 ERK2. (A to C) Mock-transfected PC12 cells; (D to F) PC12 cells transfected with p42YF185, the mutant ERK2 in which tyrosine 185 was mutated to phenylalanine; (G to I) PC12 cells transfected with p42wt. (A, D, and G) Unstimulated PC12 cells; (B, C, E, F, H, and I) PC12 cells stimulated with NGF for 4 days; (C, F, and I) PC12 cells labeled with rhodamine phalloidin to stain F-actin. Arrowheads indicate processes where F-actin is concentrated in mock-transfected cells and p42wt-transfected PC12 cells. A honeycomb pattern indicates that actin extends uniformly around the periphery of cells transfected with p42YF185. Bars, 24 μm.
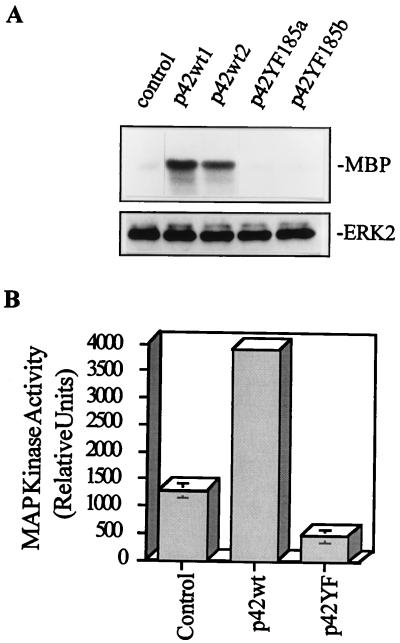
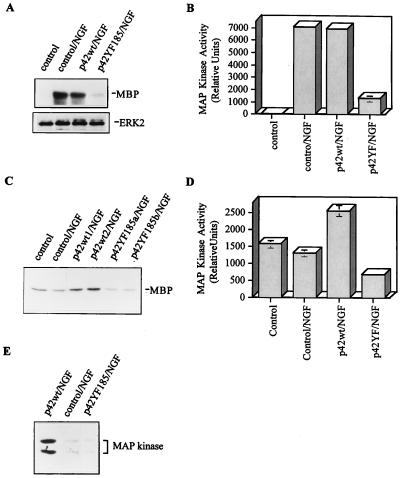
To determine whether the transfections altered ERK activity, myelin basic protein (MBP) was used as a substrate for the kinase immunoprecipitated from total cell extracts with either an anti-flag or an anti-ERK antibody (Fig. 3 and 4A to D). Compared to control mock-transfected cells, which have a low, but clearly detectable, basal ERK activity that rapidly increases after NGF treatment, the flag-p42YF185 mutant had reduced basal activity (Fig. 3A). Densitometric analysis showed approximately 50% lower basal ERK activity in p42YF185-transfected cells than in mock-transfected cells and more than 90% lower activity in p42YF185-transfected cells than in p42wt-transfected cells (Fig. 3B). p42YF185 cells also visibly failed to differentiate after NGF stimulation (Fig. 2E). In addition, the flag-p42YF185 mutant had a minimal response to NGF stimulation in comparison to control mock-transfected cells (Fig. 4A to D). NGF-stimulated ERK activity peaked at 5 min, and we found that p42YF185 cells followed a similar time course as the control and p42wt cells did but with reduced activity at each time point tested (data not shown). The inhibition of flag-ERK activity in the flag-p42YF185 cells was confirmed in a kinase immunocomplex assay using anti-flag immunoprecipitates (data not shown). Transfection of flag-p42wt resulted in increased basal ERK activity in PC12 cells (Fig. 3), but, compared to control PC12 cells, p42wt-transfected cells did not show a significantly higher ERK activity in response to NGF stimulation (Fig. 4A and B). However, p42wt cells displayed a more sustained activation after NGF treatment that lasted for several days (Fig. 4C and D). Although these cells did not spontaneously develop long processes, they did elaborate stubby protrusions (Fig. 2G) that were focally reactive for F-actin. Upon NGF stimulation, p42wt cells formed neurites in less than 1 day, whereas control PC12 cells required 3 to 4 days to attain a similar degree of differentiation (compare Fig. 2G to I with Fig. 2A to C). After NGF stimulation for 6 days, ERK activity returned to near-basal levels in control PC12 cells, remained high in p42wt cells, and dropped below basal levels in p42YF185 cells (Fig. 4C and D). Similar changes were observed when ERK activity was examined with an anti-phosphorylated ERK antibody (Fig. 4E).
FIG. 3.
Assay for basal MAP kinase (ERK) activity in PC12 transfectants. (A) Basal ERK activity is altered in PC12 cells transfected with ERK cDNAs. (Upper panel) Basal activity of total ERK in transfected PC12 cells as measured by immunoprecipitation and incubation with MBP and [γ-32P]ATP. (Lower panel) ERK immunoblot showing equal amounts of immunoprecipitated ERK. (B) Quantification of ERK activity in p42YF185 cells, mock-transfected cells, and p42wt cells. The values are means ± standard errors of the means from three experiments.
FIG. 4.
Assay for MAP kinase (ERK) activity in PC12 transfectants stimulated with NGF. (A) Activity of total ERK in transfected PC12 cells treated with NGF for 45 min. It is important to note the inhibition of ERK activity by p42YF185. (B) Quantification of ERK activity in p42YF185 cells, mock-transfected cells, and p42wt cells. The values are means ± standard errors of the means from three experiments. (C) ERK activity is sustained in PC12 cells expressing p42wt but not in those expressing p42YF185. After 6 days in NGF, cell lysates were immunoprecipitated with anti-MAP kinase and incubated with MBP and [γ-32P]ATP. (D) Quantification of ERK activity in p42YF185 cells, mock-transfected cells, and p42wt cells. The values are means ± standard errors of the means from two experiments. (E) After 6 days in NGF, cell lysates were immunoblotted with affinity-purified polyclonal antibody against phosphorylated ERK. Similar to the results in panel C, there is sustained activation of ERK in PC12 cells expressing p42wt but not p42YF185.
Induction of reorganized actin filaments and adherens junction proteins in p42YF185 cells.
To understand the basis for the observed phenotypic change in the p42YF185 stable transfectants, actin filaments were labeled with rhodamine phalloidin. In control mock-transfected PC12 cells and p42wt cells, actin filaments were not uniformly distributed around the periphery; instead, they were most concentrated within lamellipodia, and particularly within their distal microspikes (Fig. 2C and I). However, actin filaments in p42YF185 cells were arranged uniformly along the cell-cell boundaries, reminiscent of adherens junctions seen among epithelial cell contacts (Fig. 2F). Consistent with the presence of adherens junctions, cadherin staining was evident at the periphery of PC12 cells expressing p42YF185 (Fig. 5A) while in control cells there was a weak, diffuse cadherin staining in the cytoplasm (Fig. 5C). Markers of focal adhesions such as α1β1 integrin were only weakly detectable in PC12 cells (Fig. 5D) and did not localize to the cell-cell contact region in p42YF185 cells (Fig. 5B).
FIG. 5.
Anti-N-cadherin (A and C) and anti-α1-integrin (B and D) immunofluorescence staining of p42YF185 (A and B) and mock-transfected (C and D) cells. Bar, 30 μM.
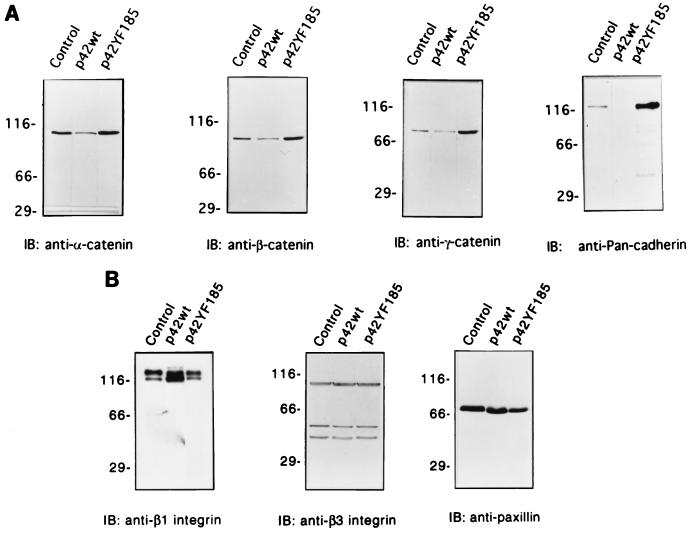
As suggested by the immunofluorescence studies, cadherin levels detected by a monoclonal antibody against N-cadherin were markedly increased in p42YF185 compared to those of control mock-transfected cells and p42wt cells (Fig. 6A). The levels of the cytoplasmic cadherin-binding proteins α-, β-, and γ-catenin (Fig. 6A) were also increased. On the other hand, proteins involved in focal adhesion and integrin function, such as β1 and β3 integrin, showed no major alterations in expression (Fig. 6B), although the levels of β1 integrin and paxillin did appear to decrease slightly in the p42YF185 cells (Fig. 6B). p42wt cells that overexpress ERK2 had focal collections of actin filaments at the plasma membrane as did the mock-transfected control cells (Fig. 2C and I), and the expression of cadherin and the catenins was suppressed even relative to that of the control cells (Fig. 6A).
FIG. 6.
Immunoblots (IB) of cell lysates from representative stable PC12 clones expressing p42wt and the inhibitory p42YF185 mutant. (A) Adherens junction protein expression is markedly enhanced. Immunoblots show that monoclonal anti-α-catenin labels a 102-kDa protein in p42YF185 cells more strongly than in p42wt cells; monoclonal anti-β-catenin specifically labels a 92-kDa protein in p42YF185 cells and minimally detects the same-molecular-weight protein in p42wt cells; monoclonal anti-γ-catenin specifically labels an 83-kDa protein in p42YF185 cells but only weakly labels the protein in p42wt cells; monoclonal antibody against N-cadherin specifically labels a 120-kDa protein in p42YF185 cells but not in p42wt cells. In all cases, the control cells show an intermediate degree of labeling. (B) Focal adhesion protein expression is only minimally affected by altered ERK expression. Immunoblots show that monoclonal anti-β1 integrin strongly labels a doublet protein at ∼130 kDa; monoclonal anti-β3 integrin strongly labels a 90-kDa protein with two relatively weaker bands in all clones; monoclonal anti-paxillin antibody specifically stains a 70-kDa protein in all clones.
The aggregation of p42YF185 cells is inhibited by low calcium.
Because adherens junctions are calcium sensitive (47), we sought to test whether the maintenance of p42YF185 cell aggregates depended on Ca2+ levels. Trituration in DMEM containing 1.8 mM Ca2+ readily dissociated control PC12 cells, but this same treatment failed to dissociate the p42YF185 cells (Fig. 7A). However, addition of 5 mM EDTA to p42YF185 cell aggregates resulted in the rapid dissociation of the cells (Fig. 7B).
FIG. 7.
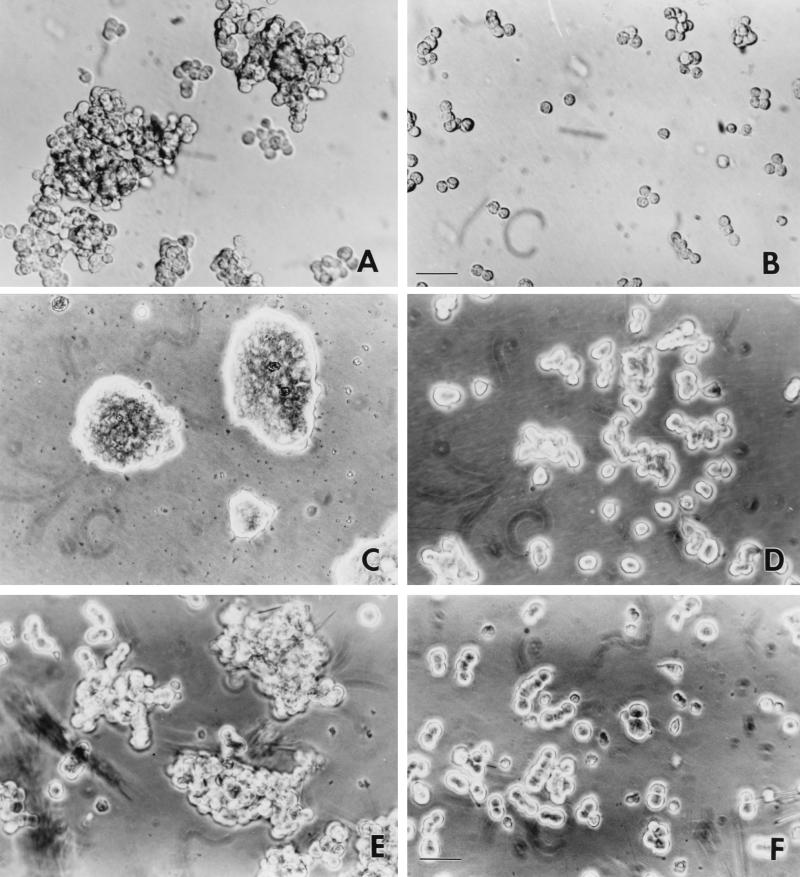
Aggregation-reaggregation properties of PC12 cells with suppressed ERK activity. (A and B) p42YF185 cell aggregation is calcium dependent. Phase images of p42YF185 cells triturated in DMEM containing 1.8 mM Ca2+ (A) or 5 mM EDTA (B) for 20 min are shown. Bar, 35 μM. (C and D) Reaggregation of p42YF185 cells. p42YF185 cells (C) and mock-transfected PC12 cells (D) were dissociated in culture medium containing both trypsin and EDTA. They were then returned to the incubator and cultured in standard calcium-containing medium for 2 weeks. Reaggregation was apparent after 2 to 3 days, and by 10 days, the cells formed large aggregates. (E and F) PC12 cell aggregation in suspension is mediated through the cadherin pathway. Aggregates of PC12 cells were grown in suspension in the presence of PD98059 (E). PC12 cell aggregation was substantially reduced when similarly grown cells were incubated with 1 mg of the cadherin cell adhesion recognition peptide LRAHAVDVNG per ml (F). Bar, 30 μM.
Reaggregation assays of wild-type PC12 cells were complicated by the fact that, unlike CHO cells, the PC12 cells spontaneously formed aggregates when dissociated from culture dishes and grown in suspension as spinner cultures. However, in contrast to p42YF185 cells, aggregates of wild-type PC12 cells in suspension were dissociable with trituration and were not accompanied by increased cadherin levels (data not shown). When p42YF185 cells were dissociated in EDTA, replating them in calcium-containing buffer induced the reaggregation of the p42YF185 cells (Fig. 7C). Reaggregation was apparent after 2 to 3 days, and by 10 days, the cells formed large aggregates. Mock-transfected PC12 cells did not aggregate (Fig. 7D).
MEK inhibitor PD98059 enhanced cadherin expression of native PC12 cells in suspension culture and in ras-transformed MDCKf3 cells.
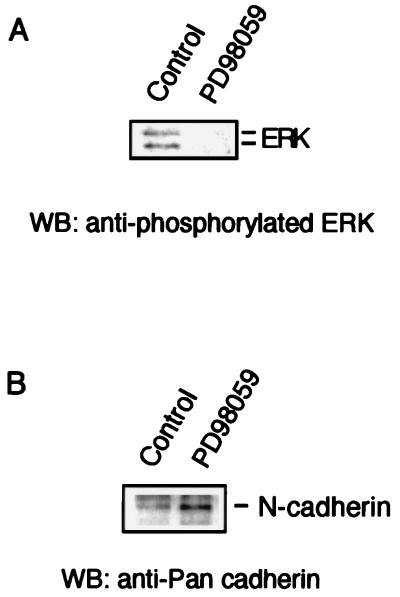
To demonstrate that the formation of adherens junctions and increased cadherin levels were not an artifact of transfection, native PC12 cells grown in suspension were treated with PD98059, a selective synthetic MEK inhibitor (16). At 25 μM, a concentration capable of inhibiting the ERKs in vitro and in vivo (2), PD98059 inhibited the phosphorylation of the ERKs in NGF-stimulated PC12 cells (Fig. 8A). Incubation of PC12 cells grown in suspension with 25 μM PD98059 enhanced cadherin (Fig. 8B) and β-catenin levels but not β1 integrin expression (data not shown). This effect was most apparent when the cells were grown in suspension; treatment with PD98059 was less effective on cells attached to culture dishes. These data supported the finding in the transfected cells that suppression of basal ERK activity releases the expression of adherens junction proteins.
FIG. 8.
Effects of the MEK inhibitor PD98059 on nontransfected PC12 cells. (A) PD98059 inhibits NGF-induced ERK activity. Blots with anti-phosphorylated ERK demonstrate immunoreactivity in control cells but not in PD98059-treated cells. (B) Immunoblot of suspended PC12 cell culture stained with monoclonal antibody against N-cadherin. Compared to control cells, PD98059-treated cells have increased N-cadherin immunoreactivity. WB, Western blot.
The synthetic decapeptide which contains the tripeptide HAV, a sequence common to all cadherins, blocks cadherin-mediated interactions (7). A 1-mg/ml concentration of the peptide LRAHAVDVNG was incubated with PD98059-treated cells in spinner culture for 2 days. The peptide significantly reduced the numbers of aggregates (Fig. 7E and F), while incubation with an unrelated protein did not (data not shown). These results demonstrated that the aggregation properties of ERK-suppressed PC12 cells are mediated through cadherin.
To demonstrate that this mechanism was not limited to PC12 cells, we treated MDCKf3 cells with PD98059. MDCKf3 cells are a ras-transformed MDCK cell line that displays a fibroblastic phenotype, does not aggregate, and has diminished E-cadherin-mediated cell-cell adhesion (5). Incubation of MDCKf3 cells with 25 μM PD98059 reversed the phenotype of these cells from a fibroblastic to an epithelial morphology and led to the reexpression of E-cadherin at the cell-cell junction (data not shown). These data strongly support the conclusion that the inhibition of basal MAP kinase activity releases the expression of proteins involved in adherens junction formation.
p42YF185 cells have increased levels of cadherin and β-catenin synthesis and increased levels of β-catenin associated with cadherin-containing complexes.
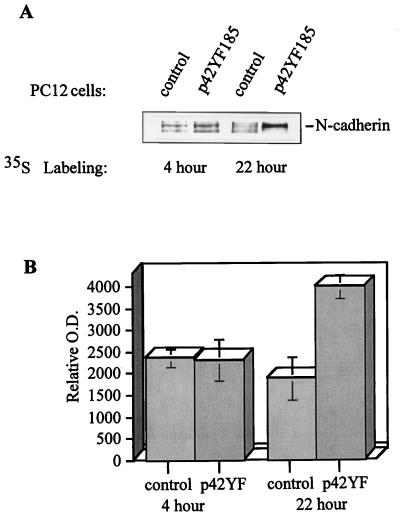
The increased levels of adherens junction proteins when ERK was suppressed may be due to increased synthesis or decreased degradation. p42YF185 cells versus control mock-transfected PC12 cells were metabolically labeled with [35S]methionine, lysed, and immunoprecipitated with an N-cadherin antibody or β-catenin antibody (Fig. 9A). Among the immunoprecipitated proteins, those bands representing N-cadherin and β-catenin were identified by immunoblotting the radiolabeled gel. In comparison to epithelial cells, the relatively low synthesis rates of the cadherins and β-catenin in PC12 cells required longer periods of labeling. After 4 h of labeling, there was no significant difference in the levels of newly synthesized cadherin between p42YF185 cells and control cells; however, by 22 h, there was approximately three times more cadherin in the p42YF185 cells (Fig. 9B). The level of newly synthesized β-catenin was also higher in p42YF185 cells than in control cells (data not shown). Thus, increased synthesis contributes to the increased levels of adherens junction proteins in p42YF185 cells. The rather long half-lives of these proteins in PC12 cells compared to MDCK cells made it difficult to assess the contribution of turnover in pulse-chase experiments. Semiquantitative support for the observation of increased synthesis in the p42YF185 cells was derived from reverse transcription-PCR analysis where, after 25 cycles, the levels of both N-cadherin and β-catenin PCR products were higher in the p42YF185 cells than in the control cells (data not shown).
FIG. 9.
Metabolic labeling demonstrates that N-cadherin synthesis is increased in p42YF185 cells compared to that in mock-transfected cells. (A) N-cadherin was immunoprecipitated from cell lysates, separated by SDS-PAGE, and visualized by autoradiography. (B) The relative amounts of newly synthesized N-cadherin in control versus p42YF185 cells were quantified. O.D., optical density.
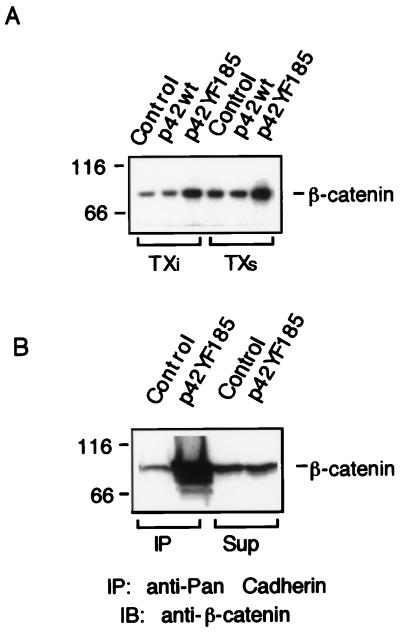
β-Catenin is present in cells in at least two pools: one associated with cadherin in the adherens junctions and a soluble pool which lies in the Wnt pathway. We wanted to determine whether the increased β-catenin in the p42YF185 cells was present in the pool associated with adherens junctions. First, β-catenin levels were determined in Triton X-100-soluble and -insoluble pools. Relative to the control cells, p42YF185 cells showed an increase in the level of β-catenin recovered by immunoprecipitation in both the insoluble pool and the soluble pool (Fig. 10A). N-cadherin antibody was then used for coimmunoprecipitations from p42YF185 cells; higher levels of β-catenin were recovered in the cadherin complex than from immunoprecipitations from control cells (Fig. 10B). Therefore, at least a portion of the increased β-catenin was associated with cadherins and Triton-insoluble actin cytoskeletons in p42YF185 cells.
FIG. 10.
β-Catenin in p42YF185 cells is associated with adherens junctions. (A) The level of β-catenin in both Triton X-100-soluble (TXs) and -insoluble pools (TXi) was higher in p42YF185 cells than in mock-transfected cells. Samples were immunoprecipitated with mouse monoclonal antibody to β-catenin and immunoblotted with β-catenin antibody. (B) The level of β-catenin pool associated with cadherin is higher in p42YF185 cells than in mock-transfected control cells. Samples were immunoprecipitated (IP) with a polyclonal antibody against N-cadherin and immunoblotted (IB) with anti-β-catenin. The cell lysates were prepared as described in Materials and Methods. The amounts of β-catenin which remained in the supernatant (sup) of control and p42YF185 cells after the cadherin complex was pelleted were approximately equal.
DISCUSSION
We have provided evidence that markers for homotypic interactions found in adherens junctions, such as cadherins and catenins, were upregulated when ERKs were inhibited in unstimulated PC12 cells. Concomitantly, these cells formed calcium-dependent cell aggregates and reorganized their actin filaments. Conversely, the overexpression of ERK2 resulted in the reduced expression of both cadherins and catenins. We suppressed basal ERK activity by using several independent experimental conditions. Confirmation of our observations that the phenotypic alterations arose from changes in ERK activity came from (i) the replication of the findings for independent clones from different transfections; (ii) the induction of a distinct and, in some senses, opposite phenotype after wild-type ERK transfection; (iii) the absence of changes after control vector transfections; and (iv) the induction of a similar expression pattern of adherens junction proteins when the ERKs were inhibited by the MEK inhibitor PD98059. Furthermore, repeated passages of the p42YF185 mutants resulted in a gradual disassembly of the aggregates as the cells lost the transfected plasmid and the expression of the mutant kinase decreased as monitored on immunoblots. The conclusion that upregulation of adherens junction proteins can occur by the inhibition of basal ERK activity also applies to an epithelially derived cell line, the MDCKf3 cells.
Many signaling systems converge upon members of the MAP kinase family, enzymes whose effects are highly dependent upon the degree and duration of their activity as well as their localization. The data here show the requirement for a basal level of ERK activity to maintain PC12 cells in a dissociated state adherent to the substrate. Unstimulated PC12 cells have a low basal ERK activity, and much of this constitutively active pool of ERKs is associated with the microtubules (31). How might the suppression of the ERKs result in the induction of homotypic interactions? It is possible that effectors in the ERK pathway also suppress homotypic adhesive interactions that are released in a default manner when ERK activity falls below a certain level. Alternatively, the regulation of homotypic and substrate adhesive interactions may operate with distinct sets of intermediates that would allow the cell to engage effectors in these pathways in parallel.
The serum response element (SRE), which mediates immediate-early gene expression, is one target of the ERK signal transduction pathway (19, 21, 26); however, the expression of immediate-early genes is not a prominent feature of unstimulated PC12 cells. Therefore, basal ERK activity may not be sufficient to activate these genes, but cytoplasmic substrates of the ERKs may be affected by basal ERK activity. Among these substrates is pp90rsk (13, 44), which partially suppresses GSK-3β (17, 45). GSK-3β is also inhibited by the Wg-Wnt signal (14), which stabilizes β-catenin and in PC12 cells increases the expression of adherens junction proteins (9). Because GSK-3β is positioned at a point where it could modulate signals between the MAP kinase and the Wg-Wnt pathways, changes in its activity may contribute to the observed phenotypic effects.
Cell culture is an artifactual condition which allows substrate interactions to predominate, and in PC12 cells, one pathway that may maintain basal ERK activity could be that initiated by the integrins. In 3T3 or REF52 fibroblasts, MAP kinase is activated and translocates to the nucleus when the cells adhere to integrin ligands such as fibronectin or laminin (11). This pathway involves the creation of multiple SH2 binding sites on FAK, and, in particular, the binding of GRB2 leads to the formation of signaling complexes which promote the activation of the Ras signaling pathway (40). Although proteins involved in focal adhesions such as β1 and β3 integrin and paxillin showed minimal changes in expression levels (Fig. 6B) in the p42YF185 cells, the ability of these molecules to trigger ERK activation was probably blunted. Therefore, the ability to sustain substrate interactions may require more than the presence of focal adhesion components in cells, namely, the activation of the MAP kinase pathway and possibly an undetected alteration in the functional state of focal adhesion components. Also notable was the detection of a similar basal ERK activity even when the cells were grown in suspension, suggesting that integrin-mediated pathways arising from substrate interactions are not exclusively responsible for maintaining basal ERK activity.
Two completely independent ways of suppressing ERK activity both resulted in the increased expression of adherens junctions proteins, and in the p42YF185 cells, there was increased synthesis of cadherins and β-catenin. The more prolonged half-lives of these molecules in PC12 cells did not allow us to determine whether stabilization of the adherens junction proteins also occurred. Regulation of the cadherins involves a multifactorial array of mechanisms (38), and the cadherin levels themselves serve as one regulatory control over catenin expression. For example, L cells contain significant levels of a 102-kDa catenin mRNA, but only after transfection with E-, N-, or P-cadherin did significant quantities of protein appear (32). The interaction of β-catenin and the Tcf-Lef family of transcription factors (6, 20, 29) may also lead to altered expression patterns of gene products involved in the formation of adherens junctions. Alternatively, the ERKs may directly regulate catenins via the multiple potential ERK phosphorylation sites in catenin family proteins.
PC12 cells may be able to regulate the levels of adherens junction proteins via stimulation of the Wnt signaling pathway. Wnt-1 expression in PC12 cells leads to an increased expression of γ-catenin and E-cadherin and increased calcium-dependent cell-cell adhesion (9). Following NGF stimulation, these cells do not undergo tyrosine phosphorylation of the p44 (ERK1) and p42 (ERK2) MAP kinases (48) or neurite extension (42). They also fail to induce the neuronal marker SCG10 (42), a protein related to stathmin, which is a downstream cytoplasmic substrate of MAP kinase (36). To implement a repertoire of cellular behaviors that leads to homotypic interactions, it may be necessary to suppress the ERKs. Suppression of the ERKs in PC12 cells leads to a phenotypic conversion in which homotypic interactions predominate. Basal ERK activity may be one of the key factors which establishes a set point to balance homotypic and substrate interactions.
ACKNOWLEDGMENTS
This work was supported by NIH grants.
We thank M. Cobb for providing p42 (ERK2) MAP kinase cDNAs, M. Greenberg for polyclonal anti-phosphorylated ERK, D. Turner for monoclonal anti-integrin, M. Medina for the reverse transcription-PCR, and J. Collard for MDCKf3 cells.
REFERENCES
- 1.Ahn N G, Seger R, Krebs E G. The mitogen-activated protein kinase activator. Curr Opin Cell Biol. 1992;4:992–999. doi: 10.1016/0955-0674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 2.Allessi D R, Cuenda A, Cohen P, Dudley D T, Satiel A R. PD098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez E, Northwood I C, Gonzalez F A, Latour D A, Seth A, Abate C, Curran T, Davis R J. Pro-leu-ser/thr-pro is a consensus primary sequence for substrate protein phosphorylation. J Biol Chem. 1991;266:15277–15285. [PubMed] [Google Scholar]
- 4.Anderson N G, Li P, Marsden L A, Williams N, Roberts T M, Sturgill T W. Raf-1 is a potential substrate for mitogen-activated protein kinase in vivo. Biochem J. 1991;277:573–576. doi: 10.1042/bj2770573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behrens J, Mareel M M, Van Roy F M, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol. 1989;108:2435–2447. doi: 10.1083/jcb.108.6.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Behrens J, von Kries J P, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 7.Blaschuk O W, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- 8.Boulton T G, Nye S H, Robbins D J, Ip N Y, Radziejewaska E, Morgenbesser S D, DePinho R A, Panayotatos N, Cobb M H, Yancopoulos G D. Erks: a family of protein serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991;65:663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- 9.Bradley R S, Cowin P, Brown A M C. Expression of Wnt-1 in PC12 cells results in modulation of plakoglobin and E-cadherin and increased cellular adhesion. J Cell Biol. 1993;123:1857–1865. doi: 10.1083/jcb.123.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cano E, Mahadevan L C. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;269:26602–26605. [PubMed] [Google Scholar]
- 12.Cheng J T, Cobb M H, Baer R. Phosphorylation of the TAL 1 oncoprotein by the extracellular signal-regulated protein kinase ERK 1. Mol Cell Biol. 1993;13:801–808. doi: 10.1128/mcb.13.2.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung J, Pelech S L, Blenis J. Mitogen-activated Swiss 3T3 RSK kinases I and II are related to pp44mpk from sea star oocytes and participate in the regulation of pp90rsk activity. Proc Natl Acad Sci USA. 1991;88:4981–4985. doi: 10.1073/pnas.88.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook D, Fry M J, Hughes K, Sumathipala R, Woodgett J R, Dale T C. Wingless inactivates glycogen synthase kinase-3 via an intracellular signalling pathway which involves a protein kinase C. EMBO J. 1996;15:4526–4536. [PMC free article] [PubMed] [Google Scholar]
- 15.Dubois M F, Nguyen V T, Dahmus M E, Pages G, Pouyssegur J, Bensaude O. Enhanced phosphorylation of the C-terminal domain of RNA polymerase II upon serum stimulation of quiescent cells: possible involvement of MAP kinase. EMBO J. 1994;13:4787–4797. doi: 10.1002/j.1460-2075.1994.tb06804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eldar-Finkelman H, Seger R, Vandenheede J R, Krebs E G. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. J Biol Chem. 1995;270:987–990. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- 18.Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- 19.Gille H, Sharrocks A D, Shaw P E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 20.Huber O, Korn R, McLaughlin J, Ohsugi M, Herrmann B G, Kemler R. Nuclear localization of beta-catenin by interaction with transcription factor LEF-1. Mech Dev. 1996;59:3–10. doi: 10.1016/0925-4773(96)00597-7. [DOI] [PubMed] [Google Scholar]
- 21.Janknecht R, Ernst W H, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinase. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jockusch B M, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- 23.Johnson G L, Vaillancourt R R. Sequential protein kinase reactions controlling cell growth and differentiation. Curr Opin Cell Biol. 1994;6:230–238. doi: 10.1016/0955-0674(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 24.Lee R M, Cobb M H, Blackshear P J. Evidence that extracellular signal-regulated kinases are the insulin-activated Raf-1 kinase kinases. J Biol Chem. 1992;267:1088–1092. [PubMed] [Google Scholar]
- 25.Leighton I A, Curmi P, Campbell D G, Cohen P, Sobel A. The phosphorylation of stathmin by MAP kinase. Mol Cell Biochem. 1993;127–128:151–156. doi: 10.1007/BF01076766. [DOI] [PubMed] [Google Scholar]
- 26.Marais R, Wynne J, Treisman R. The SRF accessory protein Elk-1 contains a growth factor-regulated transcriptional activation domain. Cell. 1993;73:381–393. doi: 10.1016/0092-8674(93)90237-k. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda S, Gotoh Y, Nishida E. Phosphorylation of Xenopus mitogen-activated protein (MAP) kinase kinase by MAP kinase kinase kinase and MAP kinase. J Biol Chem. 1993;268:3277–3281. [PubMed] [Google Scholar]
- 28.Miyamoto S, Teramoto H, Coso O A, Gutkind J S, Burbelo P D, Akiyama S K, Yamada K M. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destree O, Clevers H. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 30.Morino N, Mimura T, Hamasaki K, Tobe K, Ueki K, Kikuchi K, Takehara K, Kadowski T, Yazaki Y, Nojima Y. Matrix/integrin interaction activates the mitogen-activated protein kinase, p44erk-1 and p42erk-2. J Biol Chem. 1995;270:269–273. doi: 10.1074/jbc.270.1.269. [DOI] [PubMed] [Google Scholar]
- 31.Morishima M, Kosik K S. The pool of MAP kinase associated with microtubules is small but constitutively active. Mol Biol Cell. 1996;7:893–905. doi: 10.1091/mbc.7.6.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- 33.Nakajima T, Kinoshita S, Sasagawa T, Sasaki K, Naruto M, Kishimoto T, Akira S. Phosphorylation at threonine-235 by a ras-dependent mitogen-activated protein kinase cascade is essential for transcription factor NF-IL-6. Proc Natl Acad Sci USA. 1993;90:2207–2211. doi: 10.1073/pnas.90.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Northwood I C, Gonzalez F A, Wartmann M R, Raden D L, Davis R J. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991;266:15266–15276. [PubMed] [Google Scholar]
- 35.Pages G, Lenormand P, L’Allemain G, Chambard J C, Meloche S, Pouyssegur J. Mitogen-activated protein kinase p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paolo G D, Pellier V, Catsicas M, Antonsson B, Catsicas S, Grenningloh G. The phosphoprotein stathmin is essential for nerve growth factor-stimulated differentiation. J Cell Biol. 1996;133:1383–1390. doi: 10.1083/jcb.133.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulverer B J, Kyriakis J M, Avruch J, Nikolakaki E, Woodgett J R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- 38.Roark E F, Paradies N E, Lagunowich L A, Grunwald G B. Evidence for endogenous proteases, mRNA level and insulin as multiple mechanisms of N-cadherin down-regulation during retinal development. Development. 1992;114:973–984. doi: 10.1242/dev.114.4.973. [DOI] [PubMed] [Google Scholar]
- 39.Robbins D J, Zhen E, Owaki H, Vanderbilt C A, Ebert D, Geppert T D, Cobb M H. Regulation and properties of extracellular signal-regulated protein kinase 1 and 2 in vitro. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 40.Schlaepfer D D, Hanks S K, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 41.Seger R, Seger D, Reszka A A, Munar E S, Eldar-Finkelman H, Dobrowolska G, Jensen A M, Cambell J S, Fischer E H, Krebs E G. Overexpression of mitogen-activated protein kinase kinase (MAPKK) and its mutants in NIH 3T3 cells. J Biol Chem. 1994;269:25699–25709. [PubMed] [Google Scholar]
- 42.Shackleford G M, Willert K, Wang J, Varmus H E. The Wnt-1 proto-oncogene induces changes in morphology, gene expression, and growth factor responsiveness in PC12 cells. Neuron. 1993;11:865–875. doi: 10.1016/0896-6273(93)90116-9. [DOI] [PubMed] [Google Scholar]
- 43.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the MAP kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 44.Sturgill T W, Ray L B, Erickson E, Maller J. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- 45.Sutherland C, Leighton I A, Cohen P. Inactivation of glycogen synthase kinase 3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takishima K, Griswold-Prenner I, Ingebritsen T, Rosner M R. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated “MAP” kinase. Proc Natl Acad Sci USA. 1991;88:2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volk T, Volberg T, Sabanay I, Geiger B. Cleavage of A-CAM by endogenous proteinases in cultured lens cells and in developing chick embryos. Dev Biol. 1990;139:314–326. doi: 10.1016/0012-1606(90)90301-x. [DOI] [PubMed] [Google Scholar]
- 48.Wu C F, Howard B D. K252a-potentiation of EGF-induced neurite outgrowth from PC12 cells is not mimicked or blocked by other protein kinase activators or inhibitors. Brain Res Dev Brain Res. 1995;86:217–226. doi: 10.1016/0165-3806(95)00028-c. [DOI] [PubMed] [Google Scholar]
- 49.Zinck R, Hipskind R A, Pingoud V, Nordheim A. c-fos transcriptional activation and repression correlate temporally with the phosphorylation status of TCF. EMBO J. 1993;12:2377–2387. doi: 10.1002/j.1460-2075.1993.tb05892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]