Abstract
Background:
Trauma care in Nunavik, Quebec, is highly challenging. Geographic distances and delays in transport can translate into precarious patient transfers to tertiary trauma care centres. The objective of this study was to identify predictors of clinical deterioration during transport and eventual intensive care unit (ICU) admission for trauma patients transferred from Nunavik to a tertiary trauma care centre.
Methods:
This is a retrospective cohort study using the Montreal General Hospital (MGH) trauma registry. All adult trauma patients transferred from Nunavik and admitted to the MGH from 2010 to 2019 were included. Main outcomes of interest were hemodynamic and neurologic deterioration during transport and ICU admission.
Results:
In total, 704 patients were transferred from Nunavik and admitted to the MGH during the study period. The median age was 33 (interquartile range [IQR] 23–47) years and the median Injury Severity Score was 10 (IQR 5–17). On multiple regression analysis, transport time from site of injury to the MGH (odds ratio [OR] 1.04, 95% confidence interval [CI] 1.01–1.06), thoracic injuries (OR 1.75, 95% CI 1.03–2.99), and head and neck injuries (OR 3.76, 95% CI 2.10–6.76) predicted clinical deterioration during transfer. Injury Severity Score (OR 1.04, 95% CI 1.01–1.08), abnormal local Glasgow Coma Scale score (OR 2.57, 95% CI 1.34–4.95), clinical deterioration during transfer (OR 4.22, 95% CI 1.99–8.93), traumatic brain injury (OR 2.44, 95% CI 1.05–5.68), and transfusion requirement at the MGH (OR 4.63, 95% CI 2.35–9.09) were independent predictors of ICU admission.
Conclusion:
Our study identified several predictors of clinical deterioration during transfer and eventual ICU admission for trauma patients transferred from Nunavik. These factors could be used to refine triage criteria in Nunavik for more timely evacuation and higher level care during transport.
Abstract
Contexte:
Au Nunavik, au Québec, la traumatologie est confrontée à un énorme défi. Les distances et les temps de transport peuvent donner lieu à des transferts de cas précaires vers les centres de traumatologie tertiaires. L’objectif de cette étude était de dégager les prédicteurs d’une détérioration de l’état clinique durant le transport et d’une éventuelle admission à l’unité des soins intensifs (USI) pour les cas de traumatologie transférés du Nunavik vers un centre de traumatologie tertiaire.
Méthodes:
Il s’agit d’une étude de cohorte rétrospective menée à partir du registre de traumatologie de l’Hôpital général de Montréal (HGM). Tous les cas de traumatologie touchant des adultes transférés du Nunavik et admis à l’HGM de 2010 à 2019 ont été inclus. Les principaux paramètres d’intérêt concernaient la détérioration des variables hémodynamiques et neurologiques durant le transport et l’admission à l’USI.
Résultats:
En tout, 704 cas ont été transférés du Nunavik et admis à l’HGM durant la période de l’étude. L’âge moyen était de 33 (écart interquartile [ÉI] 23–47) ans et l’indice de gravité des blessures (IGB) médian était de 10 (ÉI 5–17). À l’analyse de régression multiple, le temps de transport entre le lieu où la blessure est survenue et l’arrivée à l’HGM (rapport des cotes [RC] 1,04, intervalle de confiance [IC] de 95 % 1,01–1,06), les blessures thoraciques (RC 1,75, 95 % IC 1,03–2,99), et les blessures à la tête et au cou (RC 3,76, IC de 95 % 2,10–6,76) ont été des prédicteurs de la détérioration de l’état clinique durant le transfert. L’IGB (RC 1,04, IC de 95 % 1,01–1,08), le score de Glasgow selon l’échelle locale (RC 2,57, IC de 95 % 1,34–4,95), la détérioration de l’état clinique durant le transfert (RC 4,22, IC de 95 % 1,99–8,93), un traumatisme crânien (RC 2,44, IC de 95 % 1,05–5,68), et le recours à des transfusions une fois à l’HGM (RC 4,63, IC de 95 % 2,35–9,09) ont été des prédicteurs indépendants d’admission à l’USI.
Conclusion:
Notre étude a permis de dégager plusieurs prédicteurs de la détérioration de l’état clinique lors du transfert et de l’éventuelle admission à l’USI des cas de traumatologie transférés du Nunavik. Ces facteurs pourraient être utilisés pour affiner les critères de triage au Nunavik, et ainsi accélérer l’évacuation et prodiguer un niveau de soins plus avancés durant le transport.
Trauma care in Nunavik involves unique challenges related to the vast and isolated geographic location, in addition to unpredictable meteorologic conditions that create substantial difficulty in transferring injured patients in a safe and timely manner. Nunavik comprises the northern third of the province of Quebec and lies in both the arctic and subarctic climate zones. Approximately 11 000 Inuit people reside in the Nunavik region, making up most of the population of northern Quebec.1
Compared with the rest of the Canadian population, Indigenous Peoples of Canada have a lower life expectancy and experience a higher incidence of chronic diseases and higher rates of substance abuse, addiction, and suicide, which collectively contribute to the trauma epidemiology in this patient population.2 Known health disparities in the Indigenous population of Canada result from major historical, economic, and governance inequities that have oppressed, and continue to oppress, this population. Examples of such inequities that have a detrimental impact on Indigenous population health include a history of assimilationist education and child welfare policies in Canada, as well as internal colonial politics that systemically marginalize Indigenous people from mainstream health care.2–5 Several studies have demonstrated that Indigenous Peoples of Canada experience more severe trauma and higher injury mortality rates than the non-Indigenous population. 6,7 A recent retrospective study from our research group demonstrated that incidence rates of major traumatic injuries in Kuujjuaq and Puvirnituq were approximately 4-fold higher than those for the Quebec general population, with an even greater disparity in trauma mortality rate.8
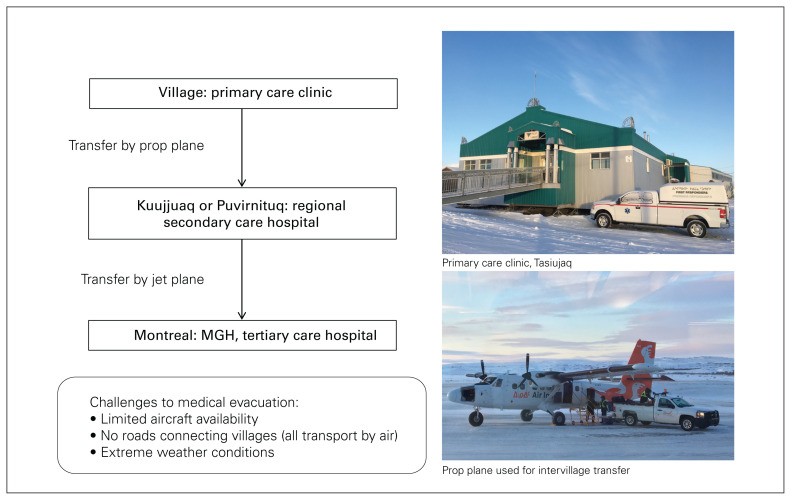
In Nunavik, 2 regional hospitals, 1 in Kuujjuaq and another in Puvirnituq, provide secondary and limited tertiary care to the resident populations of those villages and surrounding smaller communities (Figure 1). The Montreal General Hospital (MGH) is the level 1 trauma centre designated to receive all trauma transfers from the region. Currently, the request for transfer of a patient from a local hospital in Nunavik to the MGH is made by the local physician who performed the primary assessment, and the decision to accept the transfer is made by a trained trauma team leader (TTL) physician on call at the MGH. The level of expertise of the transport team is variable, and it is determined by the local physician and the TTL, taking into account the patient’s clinical status and potential need for intervention during transport. Given the inherent risks associated with the transport of critically ill patients across large geographic areas, we postulated that this process is a key target for quality improvement initiatives. Therefore, the objective of this study was to identify predictors of clinical deterioration during transport and eventual intensive care unit (ICU) admission for trauma patients transferred from Nunavik to a tertiary trauma care centre to guide future transport and triage criteria.
Fig. 1.
Trauma transport trajectory from Nunavik to Montréal. MGH = Montreal General Hospital.
Methods
Data collection
This is a retrospective cohort study using the MGH trauma registry. The MGH trauma registry is a prospectively maintained institutional database of all patients who are transferred and admitted to the MGH following traumatic injury. The database contains detailed information on patient, injury, transport, and treatment characteristics as well as patient outcomes. It is updated and maintained according to a detailed data dictionary by an experienced medical archivist team. The MGH trauma registry was queried from 2010 to 2019 to identify all adult (age ≥ 18 yr) trauma patients transferred from Nunavik and admitted to the MGH.
Ethics approval was obtained from the McGill University Institutional Review Board. The study was initiated with input from Indigenous leadership into the research questions and design, and the final product was reviewed with community leaders before presentation. In addition, all study findings were presented to the physicians in Nunavik’s largest regional hospital (Centre de santé Tulattavik de l’Ungava), and their feedback was incorporated.
Variables and outcomes
The following variables of interest were extracted from the trauma registry: patient characteristics (age, sex, village of origin), injury characteristics (Injury Severity Score [ISS], mechanism of injury, type of injury), transport time (transport time from site of injury [upon paramedic arrival] to local institution, transport time from local institution to MGH emergency department [ED]), treatment characteristics (blood product transfusion [type of product and quantity], chest tube insertion, type of surgical intervention, ICU length of stay [LOS], duration of mechanical ventilation, total LOS), in-hospital mortality, and disposition at discharge.
The primary outcomes of interest were clinical (either hemodynamic or neurologic) deterioration during transport and ICU admission. Hemodynamic deterioration was defined as a change from normal hemodynamics at the site of injury to abnormal hemodynamics upon assessment on arrival at the MGH ED. Abnormal hemodynamics was defined as a systolic blood pressure of less than 90 mm Hg or heart rate greater than 120 beats/min. Neurologic deterioration was defined as a change from normal Glasgow Coma Scale (GCS) score at the site of injury to abnormal GCS score upon assessment on arrival at the MGH ED. Abnormal GCS score was defined as any GCS score less than 15. Secondary outcomes were the requirement for transfusion or chest tube insertion or both upon arrival at the MGH, as a correlate for the need for these interventions during patient transport.
Statistical analysis
Baseline patient and injury characteristics, transport times, physiologic status, interventions, and patient outcomes were summarized using descriptive statistics. Descriptive data were reported as means with standard deviations (SD), medians with interquartile ranges (IQR), or frequencies with proportions. Continuous outcomes were compared using t tests or Wilcoxon rank-sum tests as appropriate. Distribution of categorical outcomes was examined using χ2 tests. A multiple logistic regression analysis was performed to identify predictors of clinical deterioration during transport and of ICU admission, controlling for clinically relevant factors selected on the basis of clinical knowledge to control for confounding. Complete case analysis was used to handle missing data, after confirming that there were no significant differences in important covariates when comparing those with missing versus complete data sets. Data analysis was performed using SPSS version 27.0 (IBM Corp.).
Results
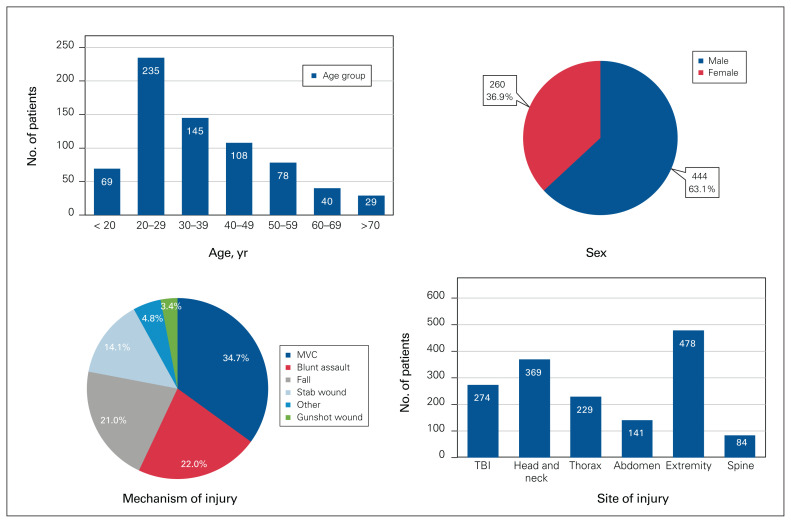
In total, 704 patients were transferred from Nunavik and admitted to the MGH during the study period. The median age was 33 (IQR 23–47) years, and the most common age range was 20–29 years (n = 235, 33.4%). There were more male than female patients (n = 444, 63.1%), and the median ISS was 10 (IQR 5–17). The most common mechanisms of injury were motor vehicle collision (n = 244, 34.7%), followed by blunt assault (n = 155, 22.0%) and fall (n = 148, 21.0%). The most common sites of injury were extremity (n = 211, 30.0%) and head and neck (n = 162, 23.0%), followed by traumatic brain injury (TBI) (n = 120, 17.0%). Patient and injury characteristics are further described in Figure 2.
Fig. 2.
Patient and injury characteristics. MVC = motor vehicle collision; TBI = traumatic brain injury.
The median transport time from the site of injury to the MGH ED was 9.3 (IQR 5.9–16.5) hours among 448 patients whose transport time was recorded. After we selected for patients with ISS greater than 15, the median transport time from the site of injury to the MGH ED was 8.8 (IQR 6.1–13.8) hours, with a wide range (1.8–44.6 h). Of the 569 patients whose hemodynamics were recorded upon initial assessment in Nunavik, 128 (22.5%) had abnormal hemodynamics. For 567 patients whose hemodynamics were recorded both in Nunavik and in the MGH ED, 93 (16.4%) patients experienced deterioration of their hemodynamics during transport. In 42.3% (168/397) of patients, the local GCS score (i.e., the GSC score recorded in Nunavik) was abnormal. For 355 patients whose GCS score was recorded in both Nunavik and the MGH ED, 101 (28.5%) patients experienced deterioration of their GCS score, by a mean score of 6.0 (SD 3.7). Transferred patients who experienced clinical deterioration during transfer (as defined by deterioration in either hemodynamics or GCS), when compared with those who did not, had a higher ISS score (median 16 v. 11, p < 0.001) and were more likely to have experienced a TBI (62.5% v. 50.7%, p < 0.001), a head and neck injury (71.0% v. 49.3%, p < 0.001), or a thoracic injury (48.9% v. 34.3%, p = 0.004). They were more likely to require blood product transfusion upon arrival at the MGH ED (33.5% v. 10.1%, p < 0.001), require ICU admission (51.1% v. 23.7%, p < 0.001), and have longer total hospital LOS (median 8 v. 5 d, p < 0.001), and they were more likely to be discharged to a rehabilitation centre or other institution (30.8% v. 15.3%, p < 0.001) rather than home. On multiple regression analysis, transport time from the site of injury to the MGH (odds ratio [OR] 1.04, 95% confidence interval [CI] 1.01–1.06) and thoracic (OR 1.75, 95% CI 1.03–2.99) and head and neck (OR 3.76, 95% CI 2.10–6.76) injuries independently predicted clinical deterioration during transport, after controlling for relevant clinical variables.
Upon arrival at the MGH, 133 (18.9%) patients required chest tube insertion and 105 (14.9%) received blood product transfusions. Of all transferred patients, 174 (24.7%) were admitted to the ICU, and median ICU LOS was 2.2 (SD 5.9) days. Mechanical ventilation was required in 151 patients (21.4%), and the average duration of mechanical ventilation was 118.7 (IQR 41.5–229.1) hours. The majority of the patients (n = 419, 59.5%) required operative intervention, of which orthopedic surgery (n = 220, 52.5%) was the most commonly performed, followed by head and neck surgery (n = 103, 24.6%) and thoracoabdominal surgery (n = 60, 14.3%). The average total LOS was 9.4 (SD 14.6) days. On discharge, 575 (81.7%) patients were discharged home, 28 (4.0%) were transferred to another hospital, and 78 (11.1%) were transferred to a rehabilitation centre. Ten (1.4%) patients died in hospital.
Patients who required ICU admission, when compared with those who did not, were more likely to have experienced more severe injury as measured by the ISS, to have abnormal hemodynamic and neurologic status at MGH, and to have abnormal neurologic status in Nunavik and neurologic deterioration during transport. The 2 groups also differed in their injury location, treatment characteristics and outcomes, as summarized in Table 1. On multiple logistic regression, ISS (OR 1.04, 95% CI 1.01–1.08), abnormal local GCS score (OR 2.57, 95% CI 1.34–4.95), clinical deterioration during transfer (OR 4.22, 95% CI 1.99–8.93), TBI (OR 2.44, 95% CI 1.05–5.68), and transfusion requirement at the MGH (OR 4.63, 95% CI 2.35–9.09) independently predicted ICU admission. Patients who experienced an extremity injury alone were less likely to require ICU admission (OR 0.47, 95% CI 0.24–0.91). A complete list of clinical variables included in the regression analysis and their effect measures is presented in Table 2.
Table 1.
Univariate analysis comparing trauma patients admitted to the intensive care unit with those who were not
| Variable | No. (%) of patients;* ICU status | p value | |
|---|---|---|---|
| ICU n = 172 |
Non-ICU n = 532 |
||
| ISS, median (IQR) | 21 (11–30) | 9 (4–14) | < 0.001 |
| Age, yr, mean ± SD | 15.5 ± 1.2 | 15.8 ± 0.7 | 0.75 |
| Female | 55 (32.0) | 205 (38.5) | 0.12 |
| Hemodynamic abnormality | |||
| Nunavik | 57 (34.5) | 71 (17.6) | < 0.001 |
| MGH | 47 (27.5) | 26 (5.1) | < 0.001 |
| Hemodynamic deterioration during transport | 29 (17.7) | 64 (15.9) | 0.60 |
| Neurologic abnormality | |||
| Nunavik | 97 (67.4) | 71 (28.1) | < 0.001 |
| Montreal General Hospital | 118 (76.6) | 52 (13.9) | < 0.001 |
| Neurologic deterioration during transport | 73 (56.2) | 28 (12.4) | < 0.001 |
| Injury location | |||
| TBI | 126 (73.3) | 148 (27.8) | < 0.001 |
| Head and neck | 117 (68.0) | 209 (29.3) | < 0.001 |
| Thorax | 79 (45.9) | 150 (28.2) | < 0.001 |
| Abdomen | 58 (33.7) | 83 (15.6) | < 0.001 |
| Extremity | 96 (55.8) | 382 (71.8) | < 0.001 |
| Operative management | 88 (51.2) | 331 (62.2) | 0.01 |
| Transfusion in MGH ED | 61 (35.5) | 44 (8.3) | < 0.001 |
| Thoracostomy in MGH ED | 16 (9.3) | 8 (1.5) | < 0.001 |
| Total LOS, median (IQR) | 14 (7–28) | 3 (2–6) | < 0.001 |
| Disposition | < 0.001 | ||
| Home | 93 (55.0) | 482 (92.3) | |
| Transfer to rehabiltation centre or another hospital | 76 (45.0) | 40 (7.7) | |
| Deceased | 9 (5.2) | 1 (0.2) | |
ED = emergency department; ICU = intensive care unit; IQR = interquartile range; ISS = Injury Severity Score; LOS = length of stay; MGH = Montreal General Hospital; SD = standard deviation; TBI = traumatic brain injury.
Unless indicated otherwise.
Table 2.
Multiple logistic regression of variables associated with clinical deterioration during transfer and intensive care unit admission upon transfer
| Variable | OR (95% CI) | p value |
|---|---|---|
| Predictors of clinical deterioration * | ||
| ISS | 0.83 (1.00–1.06) | 0.83 |
| Age > 65 yr | 1.40 (0.35–5.65) | 0.63 |
| Female | 0.98 (0.58–1.66) | 0.94 |
| Transport time (site of injury to MGH) | 1.04 (1.01–1.06) | 0.01 |
| Injury location | ||
| TBI | 0.88 (0.47–1.66) | 0.70 |
| Head and neck | 3.76 (2.10–6.76) | < 0.01 |
| Thorax | 1.75 (1.03–2.99) | 0.04 |
| Abdomen | 0.94 (0.54–1.65) | 0.85 |
| Extremity | 0.81 (0.48–1.38) | 0.44 |
| Predictors of ICU admission * | ||
| ISS | 1.04 (1.01–1.08) | 0.03 |
| Age > 65 yr | 0.78 (0.12–4.90) | 0.79 |
| Female | 0.95 (0.49–1.85) | 0.88 |
| Abnormal local hemodynamics | 0.46 (0.21–1.05) | 0.06 |
| Abnormal local GCS score | 2.57 (1.34–4.95) | 0.01 |
| Transport time (site of injury to MGH) | 0.98 (0.95–1.01) | 0.23 |
| Clinical deterioration during transport | 4.22 (1.99–8.93) | < 0.01 |
| Injury location | ||
| TBI | 2.44 (1.05–5.68) | 0.04 |
| Head and neck | 0.90 (0.42–1.95) | 0.80 |
| Thorax | 0.82 (0.36–1.84) | 0.63 |
| Abdomen | 0.80 (0.40–1.61) | 0.54 |
| Extremity | 0.47 (0.24–0.91) | 0.03 |
| Thoracostomy in MGH ED | 1.55 (0.64–3.77) | 0.33 |
| Transfusion in MGH ED | 4.63 (2.35–9.09) | < 0.01 |
CI = confidence interval; ED = emergency department; GCS = Glasgow Coma Scale; ICU = intensive care unit; ISS = Injury Severity Score; MGH = Montreal General Hospital; OR = odds ratio; TBI = traumatic brain injury.
Regression model was adjusted for all covariates listed for each outcome separately.
Discussion
Our study identified several predictors of clinical deterioration during transport as well as eventual ICU admission for trauma patients transferred from Nunavik. These predictors were associated with critical illness (i.e., higher ISS, abnormal local GCS score), suboptimal transport conditions (i.e., longer transport time from site of injury to trauma centre ED, clinical deterioration during transport, transfusion requirement on arrival at the trauma centre), and specific injury patterns (i.e., TBI, thoracic injuries, head and neck injuries). These factors could be used to refine triage criteria in Nunavik to improve the outcomes of transferred trauma patients.
Patients are transported from Nunavik to the Montréal airport either on a medevac for urgent cases or by scheduled Challenger aircraft for nonurgent cases. They must be accompanied by at least 1 health care professional (first responder, nurse, or physician) during transport. The level of expertise of the transport team varies. The majority of transfers involve 1 first responder or a nurse or both accompanying the patient, whereas critically ill patients are accompanied by a physician.
The “golden hour” principle in trauma is that trauma patients who reach definitive care within 1 hour of injury have a greater chance of survival.9 Transport times from Nunavik to a tertiary care hospital are well outside of this golden hour. Longer transport time was associated with clinical deterioration during transfer, and we believe that this is further evidence that reducing time from injury to definitive care has the potential to improve patient outcomes. Future efforts should therefore focus on decreasing transport times to the tertiary care setting.
Educational efforts to improve outcomes in rural trauma patients across North America have been shown to be effective, as demonstrated by the Rural Trauma Team Development Course. This course uses a multidisciplinary team-based approach, with a focus on early initiation of the transfer process, and its use has resulted in significantly shortened prehospital times.10,11 Such educational interventions could be strengthened by the addition of systematic triage and transfer protocols, focusing on specific clinical factors associated with poorer outcomes in Nunavik trauma patients. In fact, rural trauma systems with predefined triage and transfer criteria have been associated with reduced trauma mortality in several rural settings.12,13 The findings of this study will further guide this process. Our trauma research group is also in the process of discussing the clinical predictors identified in the current study with local health authorities to refine and implement these triage criteria to improve the transfer of trauma patients from Nunavik.
Our study also demonstrated an important need for transfusions and thoracostomy upon arrival at the MGH ED, which is a surrogate of interventions that may have been required before or during transport had the resources been available. Currently, there is inconsistent access to blood products in Nunavik’s regional hospitals and during transport, and the expertise to perform thoracostomy varies among local health care professionals and transport teams.
Early blood product transfusion is a key tenet of damage control resuscitation, leading to improved outcomes in trauma patients suffering hemorrhagic shock.14,15 A recent systematic review showed that prehospital blood transfusion reduced the odds of long-term mortality in multiple observational studies.16 Coordinated, provincial efforts to make blood products more readily and consistently available in northern Quebec and during patient transport, with an appropriate stewardship process in place to ensure that blood products are used in a manner that is clinically appropriate and minimizes waste, has the potential to improve the outcomes of transferred patients.17
Thoracic injury was a predictor of clinical deterioration during transport; these patients could potentially benefit from thoracostomy before or during transfer as needed. Prehospital thoracostomy has been shown to be safe and effective when performed by aeromedical personnel with adequate training and with set guidelines regarding appropriate indications.18,19 Training local health care professionals, including transport personnel, through a structured Advanced Trauma Life Support curriculum, has the potential to improve their comfort in performing tube thoracostomy before and during transport as needed and to improve safety for the patient.20,21 Alternatively, telemedicine could be used to provide procedural guidance to local physicians or transport personnel for potentially life-saving interventions.22
Limitations
Our study is limited by its retrospective design using a database registry, as well as missing data on local trauma mortality and transfers without admission. Data were missing for several patients on transport times and vital signs on scene, limiting our analysis. The trauma registry is also missing important variables, such as baseline comorbidities, which could represent further predictors of poor outcomes. Arbitrary cut-offs based on clinical experience and previous literature were used to define abnormal values for hemodynamics and GCS; however, given the administrative nature of the database, the context and cause of these values are unknown and may not reflect patients’ actual clinical status. Furthermore, the amount of time patients spent in smaller communities before being transferred to Kuujjuaq or Puvirnituq was not captured in our data set; therefore, overall transport times were probably systematically longer than those documented. Given that longer transport times were associated with clinical deterioration during transfer, future directions for research include obtaining more granular data regarding transfer times, with details on how much time is spent at each time point in the transfer trajectory, to identify where the delays are occurring. These data will help to determine areas in which the current transport system can be improved and guide quality improvement measures for future practice. Finally, results of logistic regression analyses of a retrospective data set should be interpreted with caution and need further validation in prospective studies.
Conclusion
Our study identified several predictors of clinical deterioration during transport as well as eventual ICU admission for trauma patients transferred from Nunavik to a level 1 trauma centre. These factors could be used to refine triage criteria in Nunavik for more timely and efficient evacuation and higher levels of care during transport for trauma patients. Improved access to blood products and formal procedural training could further improve patient outcomes.
Acknowledgement
The authors thank Johanne Prud’homme for her help with data extraction and analysis using the MGH trauma registry.
Footnotes
Presented at the 2022 American College of Surgeons Committee on Trauma Residents Trauma Papers Competition, Seattle, Wash.; the Canadian Association of General Surgeons Canadian Surgery Forum, Virtual Edition, Sept. 21–24, 2021; and the 2021 Annual Rocke Robertson Visiting Professorship Trauma Residents Competition, Montréal, Que.
Competing interests: None declared.
Contributors: J. Moon, J. Grushka, D. Deckelbaum, A. Jastaniah, and E. Wong conceived the study. T. Razek, N. Boulanger, L. Watt, K. Khwaja, P. Fata, and K. McKendy analyzed the data. J. Moon wrote the article, which T. Razek, J. Grushka, D. Deckelbaum, N. Boulanger, L. Watt, K. Khwaja, P. Fata, K. McKendy, A. Jastaniah, and E. Wong critically revised. J. Moon, T. Razek, J. Grushka, D. Deckelbaum, N. Boulanger, L. Watt, K. Khwaja, P. Fata, K. McKendy, A. Jastaniah, and E. Wong gave final approval of the version to be published.
References
- 1.Statistics Canada. Aboriginal Peoples in Canada: First Nations People, Métis and Inuit. Ottawa: Statistics Canada; 2021. [Google Scholar]
- 2.Adelson N. The embodiment of inequity: health disparities in Aboriginal Canada. Can J Public Health 2005;96:S45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trocmé N, Knoke D, Blackstock C. Pathways to the overrepresentation of Aboriginal children in Canada’s child welfare system. Soc Serv Rev 2004;78:577–600. [Google Scholar]
- 4.Sinclair R. The Indigenous child removal system in Canada: an examination of legal decision-making and racial bias. First Peoples Child Family Rev 2016;11:8–18. [Google Scholar]
- 5.Browne AJ, Fiske J-A. First Nations women’s encounters with mainstream health care services. West J Nurs Res 2001;23:126–47. [DOI] [PubMed] [Google Scholar]
- 6.Karmali S, Laupland K, Harrop AR, et al. Epidemiology of severe trauma among status Aboriginal Canadians: a population-based study. CMAJ 2005;172:1007–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrop AR, Brant RF, Ghali WA, et al. Injury mortality rates in Native and non-Native children: a population-based study. Public Health Rep 2007;122:339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon J, Pop C, Talaat M, et al. Trauma in northern Quebec, 2005–204: epidemiologic features, transfers and patient outcomes. Can J Surg 2021;64:E527–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nathens AB, Brunet FP, Maier RV. Development of trauma systems and effect on outcomes after injury. Lancet 2004;363:1794–801. [DOI] [PubMed] [Google Scholar]
- 10.Kappel DA, Rossi DC, Polack EP, et al. Does the rural trauma team development course shorten the interval from trauma patient arrival to decision to transfer? J Trauma 2011;70:315–9. [DOI] [PubMed] [Google Scholar]
- 11.Rural Trauma Team Development Course. Chicago: American College of Surgeons; 2021. Available: https://www.facs.org/quality%20programs/trauma/education/rttdc (accessed 1 Aug. 2023). [Google Scholar]
- 12.Porter A, Karim S, Bowman S, et al. Impact of a statewide trauma system on the triage, transfer, and inpatient mortality of injured patients. J Trauma Acute Care Surg 2018;84:771–9. [DOI] [PubMed] [Google Scholar]
- 13.Tiesman H, Young T, Torner JC, et al. Effects of a rural trauma system on traumatic brain injuries. J Neurotrauma 2007;24:1189–97. [DOI] [PubMed] [Google Scholar]
- 14.Ball CG. Damage control resuscitation: history, theory and technique. Can J Surg 2014;57:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powell EK, Hinckley WR, Gottula A, et al. Shorter times to packed red blood cell transfusion are associated with decreased risk of death in traumatically injured patients. J Trauma Acute Care Surg 2016;81:458–62. [DOI] [PubMed] [Google Scholar]
- 16.Rijnhout TWH, Wever KE, Marinus RHAR, et al. Is prehospital blood transfusion effective and safe in haemorrhagic trauma patients? A systematic review and meta-analysis. Injury 2019;50:1017–27. [DOI] [PubMed] [Google Scholar]
- 17.Krook C, O’Dochartaigh D, Martin D, et al. Blood on board: the development of a prehospital blood transfusion program in a Canadian helicopter emergency medical service. CJEM 2019;21:365–73. [DOI] [PubMed] [Google Scholar]
- 18.Davis DP, Pettit K, Rom CD, et al. The safety and efficacy of prehospital needle and tube thoracostomy by aeromedical personnel. Prehosp Emerg Care 2005;9:191–7. [DOI] [PubMed] [Google Scholar]
- 19.Chesters A, Davies G, Wilson A. Four years of pre-hospital simple thoracostomy performed by a physician–paramedic helicopter emergency medical service team: a description and review of practice. Trauma 2016;18:124–8. [Google Scholar]
- 20.Berkenstadt H, Yaron Munz, Trodler G, et al. Evaluation of the Trauma-Man® simulator for training in chest drain insertion. Eur J Trauma 2006;32:523–6. [Google Scholar]
- 21.Ali J, Adam R, Stedman M, et al. Advanced trauma life support program increases emergency room application of trauma resuscitative procedures in a developing country. J Trauma 1994;36:391–4. [DOI] [PubMed] [Google Scholar]
- 22.Van Oeveren L, Donner J, Fantegrossi A, et al. Telemedicine-assisted intubation in rural emergency departments: a national emergency airway registry study. Telemed J E Health 2017;23:290–7. [DOI] [PubMed] [Google Scholar]