Abstract
Introduction
Cannabis has been reported to have both anxiogenic and anxiolytic effects. Habitual cannabis use has been associated with anxiety disorders (AD). The causal pathways and mechanisms underlying the association between cannabis use (CU)/cannabis use disorder (CUD) and anxiety remain unclear. We examined the literature via a systematic review to investigate the link between cannabis and anxiety. The hypotheses studied include causality, the common factor theory, and the self-medication hypothesis.
Methods
Critical systematic review of published literature examining the relationship of CU/CUD to AD or state-anxiety, including case reports, literature reviews, observational studies, and preclinical and clinical studies. A systematic MEDline search was conducted of terms including: [anxiety], [anxiogenic], [anxiolytic], [PTSD], [OCD], [GAD], [cannabis], [marijuana], [tetrahydrocannabinol], [THC].
Results
While several case-control and cohort studies have reported no correlation between CU/CUD and AD or state anxiety (N = 5), other cross-sectional, and longitudinal studies report significant relationships (N = 20). Meta-analysis supports anxiety correlating with CU (N = 15 studies, OR = 1.24, 95% CI: 1.06–1.45, p = 0.006) or CUD (N = 13 studies, OR = 1.68, 95% CI: 1.23–2.31, p = 0.001). PATH analysis identifies the self-medication hypothesis (N = 8) as the model that best explains the association between CU/CUD and AD or state-anxiety. Despite the support of multiple large cohort studies, causal interpretations (N = 17) are less plausible, while the common factor theory (N = 5), stress-misattribution hypothesis, and reciprocal feedback theory lack substantial evidential support.
Conclusion
The association between cannabis and anxiety is best explained by anxiety predisposing individuals toward CU as a method of self-medication. A causal relationship in which CU causes AD incidence is less likely despite multiple longitudinal studies suggesting so.
Keywords: Adverse effects, Anxiety, Cannabis, Endocannabinoids, Tetrahydrocannabinol
Introduction
The use of cannabis for medicinal purposes dates back to B.C. among Chinese, Tibetan, and Indian cultures [1]. The 1973 merger of the Bureau of Narcotics and Dangerous Drugs (BNDD) and the Office of Drug Abuse Law Enforcement (ODALE) to create the Drug Enforcement Agency (DEA) reinforced the status of cannabis as a Schedule I banned substance with abuse potential and no medicinal properties. Complex governmental regulation involving the DEA, the National Institute on Drug Abuse (NIDA), and the University of Mississippi’s cannabis growth and distribution center has made it difficult to study cannabis’s potentially therapeutic properties. In August of 2016, the DEA announced that more growers would be licensed to produce and distribute marijuana for research purposes [2], along with more recent developments that work to streamline access to marijuana for research purposes. All in all, the shifting marijuana research landscape has the potential to facilitate the study of cannabis in humans, and further our knowledge of both the risks and benefits of cannabis overall.
Anxiety is a medical indication of particular interest given the millions of Americans suffering from anxiety disorders (ADs). Chief among these is post-traumatic stress disorder (PTSD), with lifetime prevalence between 3.6% and 9.7%, although the use of cannabis in social anxiety disorder (SAD), panic disorder (PD), generalized anxiety disorder (GAD), and obsessive-compulsive disorder (OCD) is also under study [3]. Correct interpretation of any association between cannabis and ADs and an understanding of the dose-dependence effect on the anxiety circuit are of increased importance to clinicians and researchers alike. However, there are a paucity of studies examining the effect of cannabis on anxiety, and the effect of anxiety on cannabis consumption.
Therefore, the purpose of this review was to critically appraise the literature with respect to the bidirectional interplay between cannabis and anxiety. Various theories seeking to explain the relationship between cannabis and anxiety have been proposed. This study is predicated on certain theoretical frameworks that are based in the literature; for example, we collect data on the possibility of a causal relationship, where cannabis use (CU) may be thought to cause anxiety. We also collect data on the common factor theory, which supports the idea that confounding factors create a false relationship between cannabis and anxiety. We also test the self-medication hypothesis, or the idea that patients with anxiety issues self-medicate with CU [4]. Our overarching hypothesis is that evidence supporting causal links would be limited; that is, that cannabis does not necessarily cause anxiety, and instead, that anxiety may predispose individuals toward using cannabis via the self-medication hypothesis (Tables 1–5).
Table 1.
Studies reporting no relationship between cannabis and anxiety
| Citation | Methodology | Results | Conclusion |
|---|---|---|---|
| Brook et al. [5] (1998) | Adolescent cohort given psychiatric and drug assessments utilizing DSM-III-R criteria over 9 years in the USA | Level of marijuana use was found to have an insignificant association with AD controlling for age and gender covariates (OR: 1.15, 95% CI: 0.99–1.34) and controlling for comorbid psychiatric disorders (OR: 1.16, 95% CI: 1.00–1.35) | There is no association between level of marijuana use and AD incidence when adjusted for covariates or comorbid psychiatric disorders |
| Gilder et al. [6] (2006) | Case-control data on 513 SWC Indian adults, with demographic information and DSM-III diagnoses | Despite high rates of cannabis dependence (43% in men and 24% in women), cannabis-induced psychiatric disorders each occurred in 1% or less of the sample | No significant association between CU and psychiatric illness in the SWC Indian population |
| Roberts et al. [7] (2007) | Sample of 4,175 youths aged 11–17 assessed for SUDs in the past year using NIMH DISC-IV and self-administered questionnaires | Marijuana abuse is not associated with increased odds of AD (OR: 0.6, 95% CI: 0.1–7.3), and marijuana dependence fails to increase odds of AD development (OR: 2.2, 95% CI: 0.9–4.8) | Neither marijuana abuse nor marijuana dependence increase incident AD |
| Low et al. [8] (2008) | Cross-sectional assessment of 642 adolescents using the Primary Care Evaluation of Mental Disorders guidelines to evaluate anxiety, mood and substance abuse disorders | No association between state-anxiety and cannabis abuse (OR: 1.4; 95% CI: 0.4–4.7) | No association between state-anxiety and cannabis abuse, perhaps due to the anxiolytic effects of cannabis or less exposure in aggregate |
| Feingold et al. [9] (2016) | 3-year multi-wave prospective study using data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) | CU was not associated with increased incidence of any AD (AOR: 1.12; 95% CI: 0.63–0.9). Baseline AD (except PD) was not associated with future initiation of CU (AOR: 1.0; 95% CI: 0.62–1.69) or onset of a CUD (AOR: 0.68; 95% CI: 0.41–1.14) | CU/CUDs are not associated with increased incidence of AD. AD (excluding PD) do not predispose toward initiation of CU |
AD, anxiety disorder; CU, cannabis use; NIMH; OR, odds ratio; SWC, Southwest CA; SUDs, substance use disorders.
Table 5.
Studies supporting the self-medication hypothesis
| Citation | Methodology | Results | Conclusion |
|---|---|---|---|
| Stewart et al. [29] (1997) | Anxiety Sensitivity Index (ASI) surveys, drug use surveys, and diagnostic interviews among university students (N = 229) | Marijuana users reported lower ASI scores than nonusers, supporting a negative relationship between anxiety and CU | CU is inversely related to anxiety |
| Reilly et al. [10] (1998) | Cross-sectional “snowball” sample of N = 268 long-term CUs who had regularly used for at least 10 years | The most commonly reported negative effects were feelings of anxiety, paranoia, or depression (21%), tiredness, lack of motivation and low energy (21%), and effects of smoke on the respiratory system (18%) | Anxiety and feelings of paranoia are experienced by more than one out of every fiver CUs |
| Boys et al. [30] (1999) | Qualitative “snowball” sample (N = 50) involving interviews exploring factors in cannabis consumption decisions | Perceived functions of drug use, physical/psychological states, and social and contextual-level influences had a role in CU decisions, with the perceived function for using a particular substance being particularly influential | Perceived drug function is particularly influential in choosing to use cannabis |
| Agosti et al. [31] (2002) | Cross-Sectional National Comorbidity Survey (NCS) sample (N = 8,098) of US individuals aged 15–54 years assessed using diagnostic and risk factor interviews | 90% of respondents with cannabis dependence had a lifetime mental disorder compared to 54.7% without cannabis dependence. Alcohol dependence, antisocial personality disorder (ASP), and conduct disorder (CD) had the strongest associations with cannabis dependence, followed by anxiety and mood disorders | CU is associated with increased risk of AMD, with 55–89% of CUD patients reporting anxiety symptomology prior to CU |
| Clough et al. [32] (2005) | Cluster analysis of N = 103 CUs stratified into five groups (anxiety, dependency, mood, vegetative, and psychosis) depending on 28 mental health symptoms and criteria | “Anxiety-dependency” was positively associated with number of “cones” usually smoked per week and this remained significant when adjusted for confounders (p = 0.02). Users of more than five cones per week were more likely to display “anxiety-dependency” symptoms than those who used one cone per week (OR: 15.8, 1.8–141.2, p = 0.013) | Risk of “anxiety-dependency” symptoms in CUs increased as their level of use increased |
| Arendt et al. [33] (2007) | N = 119 cannabis-dependent subjects assessed using the Schedules of Clinical Assessment in Neuropsychiatry (SCAN), parts of the Addiction Severity Index (ASI), and questionnaires on reasons for CU and reactions to CU while intoxicated | Subjects with lifetime depression used cannabis for the same self-medication reason as other psychoses. While under the influence of cannabis, they more often experienced depression, sadness, anxiety, and paranoia, and they were less likely to report happiness or euphoria | Support for self-medication and the stress-misattribution hypothesis |
| Dorard et al. [34] (2008) | N = 32 cannabis abusers and N = 30 healthy controls completed self-reports measuring depression (BDI-13), anxiety (STAI-Y), alexithymia (TAS20; BVAQ-B), anhedonia (PAS; SAS), and sensation seeking (SSS) with subsequent MINI evaluating cannabis dependence | Of those with chronic CU, 37.5% report symptom relief associated with relaxation and anxiety sedation. Logistic regressions demonstrated that cannabis-abusing subjects were more likely to experience high levels of anxiety, physical anhedonia, and sensation seeking than the controls | Cannabis is often used for the purpose of symptom relief in chronic users seeking to alleviate anxiety |
| Temple et al. [35] (2014) | N = 316 participants aged 18–71 assessed for state-anxiety and stress using questionnaire with subsequent PATH analysis testing cannabis-anxiety hypotheses | Past and current CUs reported higher incidence of lifetime anxiety than participants who had never used cannabis, without group differences in state-anxiety, stress, or age of anxiety onset. State-anxiety and stress were not associated with frequency of CU, but reported use to self-medicate for anxiety was positively associated with all three. Preexisting and high-state anxiety was associated with frequency of CU mediating effects of self-medication | The self-medication hypothesis is the most supported hypothesis explaining the association of cannabis and ADs. The strongest predictor of CU is the use of cannabis for self-medication purposes |
AD, anxiety disorder; AMD, anxiety and mood disorder; CU, cannabis use; CUD, cannabis use disorder.
Methods
Article Inclusion and Exclusion Criteria
This systematic review utilized the 2009 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [4] was used for the purpose of article identification and information extraction. A query was performed in August 2022 to identify all relevant literature concerning anxiety and cannabis intake, with respect to ADs (i.e., PTSD, GAD, etc). Studies included in the search range in date of publication from 2008 to August 2022. The search was performed used the PubMED and MEDLINE databases (2008–2022) using the following terminology: [anxiety], [anxiogenic], [anxiolytic], [PTSD], [OCD], [GAD], [cannabis], [marijuana], [tetrahydrocannabinol], [THC].
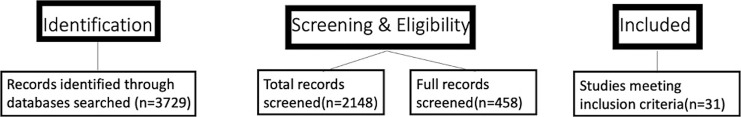
Inclusion criteria for the search comprised of all studies non-review papers on the topic of anxiety and cannabis, with a focus on modeling the relationship of cannabis and anxiety based on an afore mentioned structural model (i.e., common factor theory, self-medication hypothesis, etc). Data were primarily reported as an odds ratio, or adjusted odds ratio. A meta-analysis was not performed, however. For inclusion, studies had to be written in the English language. Abstracts were all independently reviewed by 1 investigator for determining relevance for inclusion. If an article was screened into the study, a full text was obtained for future information extraction. Two main exclusion criteria were not involving experimental data with anxiety models or wrong type of evidence (i.e., meta-analyses, review, etc). Figure 1 demonstrated a PRISMA diagram showing how inclusion criteria were applied.
Fig. 1.
PRISMA diagram. The figure demonstrates the identification, screening and inclusion of studies for review.
Statistical Modeling and Path Analysis
Various analytical techniques have been used to test the statistical validity of conceptual models; more specifically, relationships between independent and dependent variables particularly when they are not causal [36]. The framework cited in this paper consists of path analysis, which identifies both direct and indirect effects based on test hypotheses [4, 35]. The distinct purpose of this analysis is to compare different models, thereby determining which model best fits the data from a statistical standpoint. Path analysis is unique in that it can be used in conjunction with traditional statistical tests (i.e., ANOVA, regression models, χ2 tests) to examine dependent variables in the context of several contributory variables [36]. Overall, the goal is to identify which model best fits the data, and as such, it’s an approach that allows us to find the “best fit” among multiple possibilities [36].
Results
Initial research into cannabis focused on characterizing toxic and therapeutic potentials [37]. Cross-sectional studies have reported preliminary associations between CU and anxiety [10, 29, 30]. Anxiety and depression related symptoms correlated with CU among university students, with CUs reporting significantly lower levels of anxiety [29, 38]. Another similarly designed study suggested no association with state-anxiety [38]. A 10-year longitudinal study (N = 1,395) reported an association between CU/CUD and AD, both with and without adjustment for confounders [14]. Other prospective studies have reported no significant correlation between AD and CU[5–9] (Table 1) or report preliminary associations that become nonsignificant after adjustment [12, 23, 27, 28]. In resolution, a meta-analysis has reported a positive association between anxiety and CU (OR = 1.24, 95% CI: 1.06–1.45, p = 0.006; N = 15 studies) and a stronger positive association between anxiety and CUD (OR = 1.68, 95% CI: 1.23–2.31, p = 0.001; N = 13 studies) [17]. This association remained significant when stratified for confounder adjusted and confounder non-adjusted studies.
Three primary hypotheses explaining the association between CU/CUD and AD are examined in the literature: (1) causality (2) the common factor theory in which anxiety and CU have overlapping predisposing factors and (3) the self-medication hypothesis suggesting that relief from anxiety symptoms predisposes to CU. Other hypotheses include the reciprocal feedback theory as a unification of the common factor theory and causality, and the stress-misattribution hypothesis [35]. There is a lack of evidential support for the two final hypotheses (i.e., reciprocal feedback, stress-misattribution hypothesis) outside of emerging literature on the endocannabinoid system modulating the hypothalamic-pituitary-adrenal (HPA) axis [39, 40].
Causality
Assuming that the relationship between CU/CUD and AD is in fact causal, the question of directionality remains. Several studies support the idea that frequency of CU drives state-anxiety or AD incidence (Table 2). Among these studies are birth cohort studies with large sample sizes [13, 15, 16, 18]. This directional interpretation is consistent with several studies reporting the acute anxiogenic effects of cannabis [41–44]. Few studies support the reverse conclusion (Table 3), that anxiety predisposes CU. However, several observations call into question the plausibility of a causal relationship, including inconsistency of associations in the literature, relatively small, reported strengths of association, and lack of temporal information on the onset of anxious symptoms relative to CU, as noted above. Another factor weakening causality is the lack of specificity of effect, as some studies measure AD incidence [7, 9, 12, 14–16, 23, 26, 45] while others measure state-anxiety or anxiety at follow-up [8, 10, 18, 28, 32]. Iversen [46] and Boydell et al. [47] also point out that if CU were to cause AD to manifest, then we would expect said disorders to become more prevalent in the population. No such increase in AD prevalence has been reported.
Table 2.
Studies suggesting cannabis use causes anxiety disorders
| Citation | Methodology | Results | Conclusion |
|---|---|---|---|
| Reilly et al. [10] (1998) | Cross-sectional “snowball” sample of 268 long-term CUs with 10+ year history of regular use | Frequent CUs have higher levels of anxiety than nonusers. No between-group differences in AD diagnoses. 21% of the subjects had high levels of anxiety | Cannabis increases state-anxiety levels when used chronically or frequently |
| McGee et al. [11] (2000) | Longitudinal birth cohort study of parental attachment, childhood behavioral problems, self-reported CU, and mental health disorders through diagnostic interviews | Associations between cannabis and mental disorder were significant at 15, 18, and 21 years of age. Mental disorder at age 15 led to a small but significantly elevated risk of CU at age 18. CU at age 18 larger significant elevation of the risk of mental disorder at age 21 | Support of a causal association between CU and mental disorders, with mental disorder at age 15 predisposing to later CU whereas CU at age 18 reverses the predisposition above |
| Degenhardt et al. [12] (2001) | Cross-sectional survey data of 18 yo Australians assessing CU and AD with respect to confounders | CU and CUD increased odds of AD diagnosis. An association exists between CU in the past 12 months and AD diagnosis. Cannabis dependence (OR: 4.3; 95% CI: 2.88–6.4) and CU (OR: 1.78; 95% CI: 1.31–2.41) showed increased odds of AD reporting. Associations remained significant after controlling for demographics, neuroticism and other drug use | CU increases odds of AD diagnosis, even when adjusted for confounding factors |
| Hayatbakhsh et al. [13] (2007) | Birth cohort of 3,239 Australian adults assessing AD externalizing symptoms at 14 years and CU and AD at 21 years | Those who started using cannabis before age 15 and used it frequently at 21 years were more likely to report symptoms of AD in early adulthood (AOR: 3.4; 95% CI: 1.9–6.1) | Early-onset CU increases odds of reporting AD symptoms. Frequency of CU associates with likelihood of AD in young adults |
| Wittchen et al. [14] (2007) | Prospective (N = 1,395) 10-year study assessing substance use and mental disorders in subjects aged 14–17 at baseline | Cross-sectional and prospective analysis found that AD associated with CU/CUD. Associations of panic-anxiety with CU were significant after controlling for externalizing disorders | AD, and specifically panic-ADs, are associated with CU and CUD, even when controlling for outside factors |
| Cheung et al. [15] (2010) | Cross-sectional 5-year study of Ontario adults utilizing 12-item General Health Questionnaire assessing CU frequency | Compared to abstainers, the risk of anxiety/mood disorders was significantly greater for infrequent CUs (OR: 1.43) and heavy CUs (OR: 2.04) but not for moderate CUs | CU may increase the risk of developing anxiety and mood disorders |
| Degenhardt et al. [16] (2013) | Longitudinal, 9-wave, 15-year Australian cohort study measuring frequency of CU, dependence, and incidence of depression and AD | Daily CU associated with AD at 29 (AOR: 2.5; 95% CI: 1.2–5.2), as did cannabis dependence (AOR: 2.2, 95% CI: 1.1–4.4). Those who continued daily CU at 29 remained at increased odds of AD incidence (AOR: 3.2; 95% CI: 1.1–9.2) | Daily adolescent CU is consistently associated with anxiety. It is possible that early cannabis exposure causes enduring mental health risks in the general cannabis-using adolescent population |
| Kedzoir et al. [17] (2014) | Meta-analysis (N = 31 studies) using DSM/ICD criteria for CU and AD classification | CU at baseline was significantly associated with anxiety at follow-up in N = 5 studies adjusted for confounders (OR: 0.28; 95% CI: 1.06–1.54; p: 0.01) | There is an association between cannabis and state-anxiety at follow-up |
| Stapinski et al. [18] (2016) | Longitudinal cohort study (N = 2,508) assessing depression, AD and CU frequency | After adjustment, only generalized anxiety (not panic nor depression) symptoms showed an independent association with subsequent CU frequency (OR: 1.23; 95% CI: 1.08–1.41). Generalized anxiety symptoms were also associated with a 25% increased risk of transitioning from nonuser to user of cannabis during the study (OR: 1.25; 95% CI: 1.09–1.44) | Generalized AD symptoms are independently associated with risk of CU during adolescence, both from a frequency and new-user incident perspective |
AD, anxiety disorder; CU, cannabis use; CUD, cannabis use disorder; OR, odds ratio.
Table 3.
Studies suggesting anxiety disorders predispose to cannabis use
| Citation | Methodology | Results | Conclusion |
|---|---|---|---|
| McGee et al. [11] (2000) | Longitudinal birth cohort study of parental attachment, childhood behavioral problems, self-reported CU, and mental health disorders through diagnostic interviews | Associations between cannabis and mental disorder were significant at 15, 18 and 21 years of age. Mental disorder at age 15 led to a small but significantly elevated risk of CU at age 18. CU at age 18 larger significant elevation of the risk of mental disorder at age 21 | Support of a causal association between CU and mental disorders, with mental disorder at age 15 predisposing to later CU whereas CU at age 18 reverses the predisposition above |
| Swadi et al. [19] (2003) | Retrospective data analysis of youths aged 16 and 18 with psychiatric disorders utilizing DSM-IV criteria | Youths with psychiatric disorders (particularly AD) have increased prevalence of substance abuse disorders (SAD). The vast majority of these SADs are due to cannabis abuse | High rates of SAD due to cannabis abuse among youth with severe psychiatric illness |
| Buckner et al. [20] (2008) | Sample (N = 1,709) via the Oregon Adolescent Depression Project assess subjects at 4 different time points | Social AD (SAD) is associated with 6.5 greater odds of cannabis dependence (but not abuse) after confounder adjustment. Other ADs were not associated with subsequent cannabis or alcohol use disorder after adjustment | SAD appears to serve as a unique risk factor for the subsequent onset of cannabis dependence |
| Cougle et al. [21] (2011) | Cross-sectional study examining representative sample (N = 5,672) of US adults | After confounder adjustment, PTSD diagnoses were associated with increased odds of lifetime history of CU as well as past year daily CU | PTSD diagnosis increases the odds of CU and of daily CU use in past year |
| Martins et al. [22] (2011) | US adult data (N = 43,093) from National Epidemiological Survey on Alcohol and Related Conditions analyzed for lifetime psychiatric diagnoses and substance use | CU had increased prevalence of mood and ADs (p < 0.001), including lifetime CU, dependence and abuse-to-dependence transition. Individuals with AD diagnosis have 3.2 the odds of abuse and conditional cannabis dependence compared to individuals without AD | AD diagnosis increases the odds of cannabis abuse and conditional cannabis dependence three-fold |
| Danielsson et al. [23] (2016) | Swedish longitudinal population-based study (N = 10,441) assessing exposure to cannabis and comorbid mood and anxiety disorder (AMD) | Adjusted for age, anxiety at baseline increased the risk of CU onset at 3-year follow-up (RR: 1.63; 95% CI: 1.28–2.08). Associations lost significance after adjustment for other drug use | Baseline depression or anxiety each increased risk of initiation of CU, although these associations lost significance correcting for confounders |
| Buonomano et al. [24] (2022) | Two-hundred adults naive to MM and referred for any of the 23 state-approved qualifying conditions were recruited at three dispensaries in Pennsylvania between September 2020 and March 2021. All participants consented to the study; completed semi-structured interviews that included demographic questionnaires, the Short Form-36 (SF-36), and Generalized Anxiety Disorder-7 (GAD-7) | Participants had a mean age of 48.5±15.6 years, predominantly identified as female (67.5%), and were most referred for chronic pain (63.5%) and/or anxiety (58.5%). Additionally, 46.0% were living with obesity as determined by BMI. Relative to a normative sample, participants reported diminished HRQoL in several domains, most notably in role limitations due to physical health (M = 46.0±42.0), role limitations due to emotional problems (M = 52.5±42.3), energy and fatigue (M = 39.8±20.2), and pain (M = 49.4±26.0) | Patients initiating MM treatment experienced low HRQoL in multiple domains. Future studies could evaluate the relationship between HRQoL and patients’ decisions to pursue MM treatment, as well as changes in HRQoL with MM use over time |
| Ashare et al. [25] (2022) | These data are from the baseline assessment of a 12-month longitudinal study. The survey assessed certifying conditions, current medications, symptoms, and symptom severity. QoL was assessed using the Functional Assessment of Chronic Illness Therapy-Palliative Care (FACIT-Pal) which includes Physical Well-Being (PWB), Social/Family Well-Being (SWB), Emotional Well-Being (EWB), Functional Well-Being (FWB), and the Palliative Care subscale | Overall, 210 patients (114 female, 92 male, 3 nonbinary, 1 refused) completed the survey. The most common certifying conditions were pain (48.6%), anxiety (36.7%), and PTSD (15.7%) and the most common symptoms were anxiety (65.2%), pain (56.7%), sleep disturbance (38.6%), and depression (31.4%). Compared to normative data, this sample reported lower QoL, specifically EWB and SWB scores (i.e., T-Scores < 45). Opioid/benzodiazepine/sedative-hypnotic use was associated with lower QoL on all subscales (except SWB and FWB) (ps < 0.05). Greater number of self-reported symptoms and medical conditions were associated with lower QoL (ps < 0.01) | Despite mixed evidence regarding cannabis’ efficacy for anxiety, 36.7% of the current sample were certified by a physician for anxiety. Lower QoL was associated with more self-reported comorbid medical conditions, higher total symptom count, and reported use of an opioid and/or benzodiazepine. Future longitudinal data will provide critical information regarding the trajectory of these symptoms and QoL |
AD, anxiety disorder; CU, cannabis use; CUD, cannabis use disorder; HRQoL, health-related quality of life; OR, odds ratio, PTSD, post-traumatic stress disorder; SAD, society anxiety disorder.
Common Factor Theory
The common factor theory points to potential confounders creating a false relationship between CU/CUD and AD, such as biology, social factors, and environmental factors like trauma or adversity [16, 45, 48]. Studies that support this hypothesis are those in which initial positive correlations became insignificant after controlling for confounding (Table 4).
Table 4.
Studies supporting the common factor theory
| Citation | Methodology | Results | Conclusion |
|---|---|---|---|
| Brook et al. [5] (1998) | Adolescent cohort given psychiatric and drug assessments utilizing DSM-III-R criteria over 9 years in the USA. | Level of marijuana use was found to have an insignificant association with AD controlling for age and gender covariates (OR: 1.15, 95% CI: 0.99–1.34) and controlling for comorbid psychiatric disorders (OR: 1.16, 95% CI: 1.00–1.35) | There is no association between level of marijuana use and AD incidence when adjusted for covariates or comorbid psychiatric disorders |
| Fergusson et al. [26] (1997) | Christchurch Health and Development Study birth cohort examining early onset CU until age 18 | AD incidence was positively associated with frequency of CU between 3 frequency groups of abstinence, 10 time use and frequent use (p < 0.005). Association lost significance after correction for demographic characteristics, other substance use, and personality traits (p = 0.9) | Preliminary association between frequency of CU and ADs was abolished after demographic, substance use, and personality adjustment |
| Laar et al. [27] (2007) | Netherlands Mental Health Survey and Incidence Study (NEMESIS) prospective study of (N = 3,881) adults without mood disorders and (N = 3,854) adults without lifetime AD at baseline | After adjustment, CU at baseline predicted a modest increase in the risk of a first major depression (AOR: 1.62; 95% CI: 1.06–2.48) and a stronger increase in the risk of a first bipolar disorder (AOR: 4.98; 95% CI: 1.80–13.81). None of the associations between CU and AD remained significant after adjustment for confounders | The preliminary association between CU and AD lost significance after adjustment |
| Gage et al. [28] (2015) | UK birth cohort study (N = 4,561) investigating associations between self-reported CU at 16 and CIS-R interview diagnosed anxiety at 18 | Neither cannabis nor cigarette use were associated with anxiety after adjustment for pre-birth and childhood confounders | Preliminary association between CU and AD was abolished after adjustment for pre-birth and childhood confounders |
| Danielsson et al. [23] (2016) | Swedish longitudinal population-based study (N = 10,441) assessing exposure to cannabis and comorbid mood and anxiety disorder (AMD) | Adjusted for age, anxiety at baseline increased the risk of CU onset at 3-year follow-up (RR: 1.63; 95% CI: 1.28–2.08). Associations lost significance after adjustment for other drug use | Baseline depression or anxiety each increased risk of initiation of CU, although these associations lost significance correcting for confounders |
AD, anxiety disorder; CU, cannabis use; DSM, diagnostic and statistical manual; CI, confidence interval; CU, cannabis use disorder; UK, United Kingdom.
Self-Medication Hypothesis
Concordant with this hypothesis are multiple studies reporting reduction in anxiety or desired sedation as the primary drivers of CU [33, 34, 49] (Table 5). Crucial to the self-mediation hypothesis is data on the temporality of anxiety or AD onset relative to CU/CUD. For example, Agosti et al. [31] reported that individuals with CUD had 2.7, 2.3, and 2.98 the odds of having a GAD, PD or PTSD diagnosis compared to controls (GAD CI = 1.78–4.0, PD CI = 1.54–3.3, PTSD CI = 2.13–4.17). An addendum to the study found that 55–89% of those with CUD reported that their anxiety symptomatology began prior to their use of cannabis, suggesting that use began as self-medication. Using PATH analytic techniques, the investigators found that state anxiety predicted self-medication with cannabis, consistent with the self-medication hypothesis [35].
Discussion
There are contradictory data on the role of CU/CUD in AD or state-anxiety. While there is a clear association between CU/CUD and AD, the direction of causality remains unclear, with studies reporting contradictory relationships as well as effects that become insignificant with covariate adjustment. The most compelling evidence lies in favor of the self-medication hypothesis, with the common factor theory, stress-misattribution hypothesis and reciprocal feedback theory lacking substantial evidential support. Inferring causality from the CU: AD correlation is problematic given the inconsistency of associations, relatively small strengths of association, lack of specificity of effect and lack of expected increased AD incidence due to increased CU.
At this juncture in time, the self-medication hypothesis is the most likely explanation associating CU with ADs and symptomatology. Longitudinal and epidemiological studies of targeted populations, tracking temporal relationships while studying both AD and state-anxiety levels, may better ascertain if CU is a true risk factor for AD incidence. As research in various domains continues to examine cannabis science from both pharmacological and neuroscientific perspectives, we will better understand the molecular and functional effects of cannabis on anxiety, allowing better separation of the toxic and therapeutic effects of cannabis for more targeted preventative and treatment purposes.
As new clinical trials seek to better define the therapeutic potential of cannabis and cannabinoid derivatives for conditions such as OCD, PD, GAD, and PTSD, elucidating the effect of cannabis and its constituents on the anxiety circuit is as important as ever. THC has been of particularly interest in the past decade due to increasing CU-cases and shifting state legal climates. Yet it is another cannabinoid – cannabidiol (CBD) – that is currently being tested for FDA approval in case of treatment-resistant epilepsy. CBD has been shown to reduce THC-induced anxiety [50] and experimentally induced anxiety [51], most likely due to agonist activity at the 5HT1A receptor [52–54]. A recent systematic review found both clinical and preclinical studies supporting the use of CBD in reducing anxiety levels [54]. Other potential therapeutic potentials include antipsychotic, analgesic, neuroprotective, anticonvulsant, and antiemetic properties [55].
This paper is not without important limitations each reader must consider. First, there are limited studies of cannabis on anxiety, and only recently (i.e., in the past 5–10 years) have more researchers attempts to study cannabis given the complex medicolegal environment surrounding the drug. Therefore, there is a true need for future studies of the cannabis, both in animal models and in human models, to better define the drug’s effect on the anxiety circuit as well as other circuits should specific therapeutic benefits be agreed upon in the literature. It’s important to note that cannabis has a dose-dependent relationship on anxiety; that is, low doses are anxiolytic and high doses are anxiogenic. The concept of dose becomes of upmost important in this setting. Another important limitation associated with this first point is heterogeneity in the data. Specifically, when anxiety, different measures of anxiety were used, including subjective reports of decreased anxiety, patient-reported outcome tools, and measures of health-related quality of life, as well as markers of sleep and mood. It is important to define and perhaps standardize a clinical outcome of interest (e.g., GAD-7, EQ-5D-5L Index Value), such as a specific patient-reported outcome measure at a distinct time points, as this will limit heterogeneity and facilitate future reviews and meta-analyses. In addition, information on the effects of cannabis on anxiety are overall limited, but with continued interest from the academic community, this area of research is growing in terms of the number of studies published. Current data on the relationship cannabis and anxiety contains mixed results that cannot be easily pooled for meta-analysis. However, emerging literature are suggesting directionality of relationships. Ultimately, future studies with a higher level of evidence will be needed. An additional limitation is our review of study process, which only involves one reviewer and not a second or third reviewer for disagreements in the reviewal process. An additional limitation has to do with our PRISMA diagram, which does not specifically list numbers of studies excluded for specific reasons. Lastly, some of the included studies are on CU in an adolescent population. Previous literature has demonstrated CU in adolescents to be associated with higher risk of cannabis abuse and dependence [56], but also associated with risk of depression and ADs [48]. Therefore, the use of cannabis between adults and adolescents may not necessarily be considered similar in terms of downstream health risks, as adolescents may be at-risk for possible dependence and anxiety/mood disorders.
Acknowledgments
This research was supported by the Center for Medicinal Cannabis Research at the University of California, San Diego (UCSD).
Statement of Ethics
This systematic review was performed in a deliberate, ethically sound manner.
Conflict of Interest Statement
The authors of this paper do not have any significant conflict of interests to report.
Funding Sources
Grant money for the study was provided by the Center for Medical Cannabis Research at the University of California, San Diego (UCSD).
Author Contributions
Alexander Beletsky was involved in data analysis, study review, and manuscript drafting and review. Cherry Liu was involved in critical manuscript drafting and review. Bryson Lochte and Nebiyou Samuel were involved in manuscript drafting and review. Igor Grant was involved in study design, manuscript drafting, and manuscript review.
Funding Statement
Grant money for the study was provided by the Center for Medical Cannabis Research at the University of California, San Diego (UCSD).
Data Availability Statement
The data that support the findings of this study are openly available online on MEDline, found on the repository PUBMED.
References
- 1. Touw M. The religious and medicinal uses of Cannabis in China, India and Tibet. J Psychoactive Drugs. 1981;13(1):23–34. [DOI] [PubMed] [Google Scholar]
- 2. National Academies of Sciences E, Medicine, Health, Medicine D, Board on Population H, Public Health P, et al. The national academies collection: reports funded by national institutes of health. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. Washington (DC): National Academies Press (US) Copyright 2017 by the National Academy of Sciences. All rights reserved; 2017. [PubMed] [Google Scholar]
- 3. Bonn-Miller MO, Sisley S, Riggs P, Yazar-Klosinski B, Wang JB, Loflin MJE, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brook JS, Cohen P, Brook DW. Longitudinal study of co-occurring psychiatric disorders and substance use. J Am Acad Child Adolesc Psychiatry. 1998;37(3):322–30. [DOI] [PubMed] [Google Scholar]
- 6. Gilder DA, Lau P, Dixon M, Corey L, Phillips E, Ehlers CL. Co-morbidity of select anxiety, affective, and psychotic disorders with cannabis dependence in Southwest California Indians. J Addict Dis. 2006;25(4):67–79. [DOI] [PubMed] [Google Scholar]
- 7. Roberts RE, Roberts CR, Xing Y. Comorbidity of substance use disorders and other psychiatric disorders among adolescents: evidence from an epidemiologic survey. Drug Alcohol Depend. 2007;88(Suppl 1):S4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Low NC, Lee SS, Johnson JG, Williams JB, Harris ES. The association between anxiety and alcohol versus cannabis abuse disorders among adolescents in primary care settings. Fam Pract. 2008;25(5):321–7. [DOI] [PubMed] [Google Scholar]
- 9. Feingold D, Weiser M, Rehm J, Lev-Ran S. The association between cannabis use and anxiety disorders: results from a population-based representative sample. Eur Neuropsychopharmacol. 2016;26(3):493–505. [DOI] [PubMed] [Google Scholar]
- 10. Reilly D, Didcott P, Swift W, Hall W. Long-term cannabis use: characteristics of users in an Australian rural area. Addiction. 1998;93(6):837–46. [DOI] [PubMed] [Google Scholar]
- 11. McGee R, Williams S, Poulton R, Moffitt T. A longitudinal study of cannabis use and mental health from adolescence to early adulthood. Addiction. 2000;95(4):491–503. [DOI] [PubMed] [Google Scholar]
- 12. van Laar M, van Dorsselaer S, Monshouwer K, de Graaf R. Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction. 2007;102(8):1251–60. [DOI] [PubMed] [Google Scholar]
- 13. Hayatbakhsh MR, Najman JM, Jamrozik K, Mamun AA, Alati R, Bor W. Cannabis and anxiety and depression in young adults: a large prospective study. J Am Acad Child Adolesc Psychiatry. 2007;46(3):408–17. [DOI] [PubMed] [Google Scholar]
- 14. Wittchen HU, Fröhlich C, Behrendt S, Günther A, Rehm J, Zimmermann P, et al. Cannabis use and cannabis use disorders and their relationship to mental disorders: a 10-year prospective-longitudinal community study in adolescents. Drug Alcohol Depend. 2007;88(Suppl 1):S60–70. [DOI] [PubMed] [Google Scholar]
- 15. Cheung JT, Mann RE, Ialomiteanu A, Stoduto G, Chan V, Ala-Leppilampi K, et al. Anxiety and mood disorders and cannabis use. Am J Drug Alcohol Abuse. 2010;36(2):118–22. [DOI] [PubMed] [Google Scholar]
- 16. Degenhardt L, Coffey C, Romaniuk H, Swift W, Carlin JB, Hall WD, et al. The persistence of the association between adolescent cannabis use and common mental disorders into young adulthood. Addiction. 2013;108(1):124–33. [DOI] [PubMed] [Google Scholar]
- 17. Kedzior KK, Laeber LT. A positive association between anxiety disorders and cannabis use or cannabis use disorders in the general population- a meta-analysis of 31 studies. BMC Psychiatry. 2014;14(1):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stapinski LA, Montgomery AA, Araya R. Anxiety, depression and risk of cannabis use: examining the internalising pathway to use among Chilean adolescents. Drug Alcohol Depend. 2016;166:109–15. [DOI] [PubMed] [Google Scholar]
- 19. Swadi H, Bobier C. Substance use disorder comorbidity among inpatient youths with psychiatric disorder. Aust N Z J Psychiatry. 2003;37(3):294–8. [DOI] [PubMed] [Google Scholar]
- 20. Buckner JD, Schmidt NB, Lang AR, Small JW, Schlauch RC, Lewinsohn PM. Specificity of social anxiety disorder as a risk factor for alcohol and cannabis dependence. J Psychiatr Res. 2008;42(3):230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, Hawkins KA. Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav. 2011;25(3):554–8. [DOI] [PubMed] [Google Scholar]
- 22. Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159(4):379–87. [DOI] [PubMed] [Google Scholar]
- 23. Degenhardt L, Hall W, Lynskey M. The relationship between cannabis use, depression and anxiety among Australian adults: findings from the National Survey of Mental Health and Well-Being. Soc Psychiatry Psychiatr Epidemiol. 2001;36(5):219–27. [DOI] [PubMed] [Google Scholar]
- 24. Buonomano LS, Mitnick MM, McCalmont TR, Syracuse P, Dugosh KL, Festinger DS, et al. Clinical characteristics and quality of life in adults initiating medical marijuana treatment. Med Cannabis Cannabinoids. 2022;5(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ashare RL, Kelly E, Hajjar ER, Pant S, Meghani SH, Worster B. Characterizing anxiety, pain, sleep, and quality of life among patients in a state Medical Marijuana Program. Complement Ther Clin Pract. 2022;48:101612. [DOI] [PubMed] [Google Scholar]
- 26. Martins SS, Gorelick DA. Conditional substance abuse and dependence by diagnosis of mood or anxiety disorder or schizophrenia in the U.S. population. Drug Alcohol Depend. 2011;119(1–2):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gage SH, Hickman M, Heron J, Munafò MR, Lewis G, Macleod J, et al. Associations of cannabis and cigarette use with depression and anxiety at age 18: findings from the Avon Longitudinal Study of Parents and Children. PLoS One. 2015;10(4):e0122896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Danielsson AK, Lundin A, Agardh E, Allebeck P, Forsell Y. Cannabis use, depression and anxiety: a 3-year prospective population-based study. J Affect Disord. 2016;193:103–8. [DOI] [PubMed] [Google Scholar]
- 29. Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. J Subst Abuse. 1997;9:223–40. [DOI] [PubMed] [Google Scholar]
- 30. Boys A, Marsden J, Fountain J, Griffiths P, Stillwell G, Strang J. What influences young people's use of drugs? A qualitative study of decision-making. Drugs Educ Prev Policy. 2009;6(3):373–87. [Google Scholar]
- 31. Agosti V, Nunes E, Levin F. Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse. 2002;28(4):643–52. [DOI] [PubMed] [Google Scholar]
- 32. Clough AR, d’Abbs P, Cairney S, Gray D, Maruff P, Parker R, et al. Adverse mental health effects of cannabis use in two indigenous communities in Arnhem Land, Northern Territory, Australia: exploratory study. Aust N Z J Psychiatry. 2005;39(7):612–20. [DOI] [PubMed] [Google Scholar]
- 33. Schofield D, Tennant C, Nash L, Degenhardt L, Cornish A, Hobbs C, et al. Reasons for cannabis use in psychosis. Aust N Z J Psychiatry. 2006;40(6–7):570–4. [DOI] [PubMed] [Google Scholar]
- 34. Dorard G, Berthoz S, Phan O, Corcos M, Bungener C. Affect dysregulation in cannabis abusers: a study in adolescents and young adults. Eur Child Adolesc Psychiatry. 2008;17(5):274–82. [DOI] [PubMed] [Google Scholar]
- 35. Temple EC, Driver M, Brown RF. Cannabis use and anxiety: is stress the missing piece of the puzzle? Front Psychiatry. 2014;5:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Streiner DL. Finding our way: an introduction to path analysis. Can J Psychiatry. 2005;50(2):115–22. [DOI] [PubMed] [Google Scholar]
- 37. Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1–20. [PubMed] [Google Scholar]
- 38. Tournier M, Sorbara F, Gindre C, Swendsen JD, Verdoux H. Cannabis use and anxiety in daily life: a naturalistic investigation in a non-clinical population. Psychiatry Res. 2003;118(1):1–8. [DOI] [PubMed] [Google Scholar]
- 39. Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. [DOI] [PubMed] [Google Scholar]
- 40. Hill MN, Tasker JG. Endocannabinoid signaling, glucocorticoid-mediated negative feedback, and regulation of the hypothalamic-pituitary-adrenal axis. Neuroscience. 2012;204:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roy-Byrne PP, Uhde TW. Exogenous factors in panic disorder: clinical and research implications. J Clin Psychiatry. 1988;49(2):56–61. [PubMed] [Google Scholar]
- 42. Langs G, Fabisch H, Fabisch K, Zapotoczky H. Can cannabis trigger recurrent panic attacks in susceptible patients? Eur Psychiatry. 1997;12(8):415–9. [DOI] [PubMed] [Google Scholar]
- 43. D’Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu YT, et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29(8):1558–72. [DOI] [PubMed] [Google Scholar]
- 44. Manzanares J, Urigüen L, Rubio G, Palomo T. Role of endocannabinoid system in mental diseases. Neurotox Res. 2004;6(3):213–24. [DOI] [PubMed] [Google Scholar]
- 45. Fergusson DM, Horwood LJ. Early onset cannabis use and psychosocial adjustment in young adults. Addiction. 1997;92(3):279–96. [PubMed] [Google Scholar]
- 46. Iversen L. Cannabis and the brain. Brain. 2003;126(Pt 6):1252–70. [DOI] [PubMed] [Google Scholar]
- 47. Boydell J, van Os J, Caspi A, Kennedy N, Giouroukou E, Fearon P, et al. Trends in cannabis use prior to first presentation with schizophrenia, in South-East London between 1965 and 1999. Psychol Med. 2006;36(10):1441–6. [DOI] [PubMed] [Google Scholar]
- 48. Patton GC, Coffey C, Carlin JB, Degenhardt L, Lynskey M, Hall W. Cannabis use and mental health in young people: cohort study. BMJ. 2002;325(7374):1195–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Arendt M, Rosenberg R, Fjordback L, Brandholdt J, Foldager L, Sher L, et al. Testing the self-medication hypothesis of depression and aggression in cannabis-dependent subjects. Psychol Med. 2007;37(7):935–45. [DOI] [PubMed] [Google Scholar]
- 50. Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9: tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28(1):172–7. [DOI] [PubMed] [Google Scholar]
- 51. Bergamaschi MM, Queiroz RHC, Chagas MHN, de Oliveira DCG, De Martinis BS, Kapczinski F, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology. 2011;36(6):1219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gomes FV, Resstel LB, Guimarães FS. The anxiolytic-like effects of cannabidiol injected into the bed nucleus of the stria terminalis are mediated by 5-HT1A receptors. Psychopharmacology. 2011;213(2–3):465–73. [DOI] [PubMed] [Google Scholar]
- 53. Zanettini C, Panlilio LV, Alicki M, Goldberg SR, Haller J, Yasar S. Effects of endocannabinoid system modulation on cognitive and emotional behavior. Front Behav Neurosci. 2011;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gomes FV, Del Bel EA, Guimarães FS. Cannabidiol attenuates catalepsy induced by distinct pharmacological mechanisms via 5-HT1A receptor activation in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:43–7. [DOI] [PubMed] [Google Scholar]
- 55. Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30(10):515–27. [DOI] [PubMed] [Google Scholar]
- 56. Rioux C, Castellanos-Ryan N, Parent S, Vitaro F, Tremblay RE, Séguin JR. Age of cannabis use onset and adult drug abuse symptoms: a prospective study of common risk factors and indirect effects. Can J Psychiatry. 2018;63(7):457–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available online on MEDline, found on the repository PUBMED.