Abstract
Erythrocyte development has previously been shown to depend upon the expression of the lineage-restricted trans-acting factor GATA-1. Despite predicted roles for this factor during early development, GATA-1-deficient cells in chimeric mice and embryonic stem cell cultures mature to a late proerythroblast stage and express at least certain genes that normally are thought to be regulated by GATA-1 (including erythroid Krüppel-like factor [EKLF] and the erythropoietin [Epo] receptor). Opportunities to test roles for GATA-1 in erythroid gene activation in these systems therefore are limited. In the present study, in an alternate approach to test the function of GATA-1, GATA-1 has been expressed together with the Epo receptor in myeloid FDCW2 cells and the resulting effects on cytokine-dependent proliferation and erythroid gene expression have been assessed. GATA-1 expression at low levels delayed FDCW2ER cell cycle progression at the G1 phase specifically during Epo-induced mitogenesis. Upon expression of GATA-1 at increased levels, proliferation in response to Epo, interleukin-3 (IL-3), and stem cell factor was attenuated and endogenous GATA-1, EKLF and βmaj-globin gene expression was activated. Friend of GATA-1 (FOG) transcript levels also were enhanced, and ets-1 and c-mpl but not Epo receptor gene expression was induced. Finally, in FDCW2 cells expressing increased levels of GATA-1 and a carboxyl-terminally truncated Epo receptor, Epo (with respect to IL-3 as a control) was shown to markedly promote globin transcript expression. Thus, novel evidence for select hierarchical roles for GATA-1 and Epo in erythroid lineage specification is provided.
In gene disruption experiments, erythrocyte development has been shown to depend critically upon the expression of GATA-1 (28) and the Epo receptor (51). GATA-1 is a member of a family of zinc finger transcription factors that includes GATA-1, GATA-2, and GATA-3 as essential hematopoietic factors (25, 40, 42) and GATA-4, GATA-5, and GATA-6 as regulators of heart, lung, and gut cell development (8, 20, 21). GATA-1 is expressed in cells of erythroid, megakaryocytic, and mast lineages (17) and, in studies of isolated erythroid gene promoters, has been demonstrated to activate transcription from W/GATA/R elements within late (glycophorin IIB, pyruvate kinase, and ferrochelatase) (24, 46) and early (Epo receptor, EKLF, and GATA-1 per se) erythrocyte genes (4, 34, 53). Therefore, GATA-1 may act in a dominant and possibly autoregulatory fashion to promote erythroid cell development. However, studies of GATA-1 activation of endogenous erythroid gene expression are complicated in several ways. First, while GATA-1-deficient progenitor cells in chimeric mice and embryonic stem cell-derived lines fail to develop as erythrocytes, these cells nonetheless advance to a proerythrocyte stage and somewhat unexpectedly express certain early erythroid genes including EKLF and the Epo receptor (28). This may depend in part upon compensatory events, and GATA-2 expression in these cells is increased markedly (50). In addition, GATA-2 can activate endogenous GATA-1 gene expression (47) and is expressed prior to GATA-1 during embryonic blood cell formation (12), and a functional GATA-1 cofactor, FOG, also recently has been cloned (45). Thus, factors that regulate GATA-1 gene expression are not well defined, nor are specific hierarchical roles for GATA-1 in erythroid cell development.
To address these issues, effects of exogenous GATA-1 expression previously have been investigated in myeloid FDC-P2 and HD50 cell lines. Like the FDC-P1 subline studied in this work, FDC-P2 cells are an IL-3-dependent line derived from murine marrow (5) but are less lineage restricted and express endogenous GATA-1 transcripts (1). In FDC-P2 cells, expression of endogenous Epo receptor, GATA-1, and βmaj-globin genes is stimulated upon the expression of an avian retrovirus-derived Gag-Myb-Ets fusion protein (1). Based on the ability of GATA-1 to activate transcription from isolated Epo receptor promoter constructs (54), a hierarchical mechanism involving Gag-Myb-Ets activation of GATA-1 gene expression was proposed to explain these effects. Evidence that GATA-1 may act to drive erythrocyte development also has been provided by studies in promyeloblast lines prepared from chickens infected with the E26 retrovirus (16). In these cells, spontaneous erythroid differentiation is observed, and erythroblastic cell development is promoted upon exposure to serum from anemic birds (32). Beyond this, E26-transformed promyeloblasts that express low levels of GATA-1 have been isolated, and in one such line (HD50), the forced expression of GATA-1 promotes endogenous GATA-1 gene expression (16). Upon the temperature-sensitive inactivation of v-ets and upon culture in anemic serum, exogenous GATA-1 expression in these cells also promoted erythroblast formation. These studies more directly support the notion that GATA-1 gene expression may be autoregulatory and indicate that GATA-1 can act in a concentration-dependent, v-ets-dependent, and anemic serum-dependent fashion to drive erythroid gene events. As in FDC-P2 cells, however, these conclusions are complicated by lineage-modulating and transforming effects of Gag-Myb-Ets (32).
In the present study of GATA-1 action, an in vitro model has been sought that provides a null background for erythroid gene expression and lacks any requirement for Gag-Myb-Ets in potentiating erythroid cell commitment. A subline of murine myeloid FDC-P1 cells, FDCP1-WEHI2 (FDCW2) cells, has proven to comprise such a model, and as a factor-dependent line, it has also provided the opportunity to test the possible effects of Epo on growth and erythroid cell differentiation.
MATERIALS AND METHODS
Abbreviations used in this paper.
FOG, Friend of GATA-1; EKLF, erythroid Krüppel-like factor; NF-E2, nuclear factor-erythroid 2; Epo, erythropoietin; IL-3, interleukin-3; SCF, stem cell factor; FDC-P, factor-dependent continuous cell lines, Patterson Laboratories; BFU-e, burst forming unit-erythroid; CFU-e, colony forming unit-erythroid; wt, wild type; SDS, sodium dodecyl sulfate; MTS, 3-(4,5-dimethylthiazol-2-yl)-5-carboxymethoxyphenyl)-2-(4-sulfophenyl-2H-tetrazolium); GAPDH, glyceraldehyde-3-phosphate dehydrogenase; FBS, fetal bovine serum.
Expression vectors.
GATA-1 was expressed with either pMK1059 (13), pXM (11), or pEFNeo as the vector. pMK1059GATA-1 was constructed by cloning a murine wt GATA-1 cDNA (43) stepwise to pSL1180 (Pharmacia Biotech, Piscataway, N.J.) (KpnI and NotI sites) and then to pMK1059 as a 1.6-kb XbaI fragment. pXM vectors encoding GATA-1 (43), the murine wt Epo receptor, and the truncated Epo receptor from ER372 have been described previously (31). pEFNeoGATA-1 was constructed by cloning a wt GATA-1 cDNA from pSL1180 into pEFNeo as a 1.6-kb XbaI-SpeI fragment. In transfections with pXM vectors, pCINeo (Promega, Madison, Wis.) or a pREP4 construct disrupted within the EBNA-1 cDNA (pREP4ΔEB) was cotransfected to confer resistance to G418 (1 mg/ml) and hygromycin B (250 μg/ml), respectively. For EKLF expression, a wt murine cDNA was cloned as a 1,220-bp XbaI fragment from pBOSEKLF (19) into pCINeo. To increase GATA-1 expression in FDCW2ER-pXG1 cells, a pCINeoGATA-1 vector also was constructed by cloning the above GATA-1 cDNA to SalI as a XhoI fragment.
Electrotransfection and culture of FDCW2 cells and derived cell lines.
The IL-3-dependent murine myeloid cell line used in this study, FDCW2, is a subclone of FDC-P1 cells (5) that was isolated based on stable retention of factor-dependent growth. FDCW2 cells routinely were maintained at 37°C under 5% CO2 in Opti-MEM I medium (Life Technologies, Gaithersburg, Md.) supplemented with 8% FBS and 5% conditioned medium from WEHI-3B cells (WEHI-3 CM) as a source of IL-3 (9). Derived FDCW2 cell lines ectopically expressing the Epo receptor were maintained in medium supplemented with either WEHI-3 CM (5%) or Epo (25 U/ml). Cells expressing the Epo receptor were prepared by stable coelectrotransfection with pXMER (55 μg) and pCINeo (5 μg) and by stepwise selection in G418 (1 mg/ml) and Epo (50 U/ml). Alternatively, cells were cotransfected with pXMER or pXMER372 vectors and pREP4ΔEB and were selected in hygromycin B (250 μg/ml) and Epo (50 U/ml). Expression of GATA-1 in hygromycin B-resistant cells was accomplished by transfection with pMK1059GATA-1 (55 μg) and selection in G418 (0.8 mg/ml). Alternatively, FDCW2 cells coexpressing GATA-1 and the Epo receptor were prepared by cotransfection with pXM expression vectors (55 μg of pXMGATA-1 and 5 μg of pXMER) and direct selection in Epo (50 U/ml). In addition, in derived FDCW2ER-pXG1 cells, the expression of either GATA-1 (or EKLF as a control) was reinforced via transfection with pCINeoGATA-1 (or pCINeoEKLF) vector and selection in G418 (1 mg/ml). FDCW2ER372 cells expressing increased levels of GATA-1 expression were prepared by electrotransfection with pEFNeoGATA-1 (7 μg) and pXMGATA-1 (53 μg) followed by selection in G418 (1 mg/ml).
Cytokine-induced mitogenesis and cell cycle analyses.
Cytokine-induced mitogenesis was assayed based on stimulated rates of reduction of the tetrazolium compound MTS to formazan (Promega) (31) or stimulated incorporation of [methyl-3H]thymidine (53). Briefly, cells (3 × 105 cells/ml, 50 μl/assay) were exposed to cytokines (50 μl) for 48 h, MTS and phenazone methosulfate were added, and absorbance at 490 nm was measured after 2 h of incubation (model 550 microplate reader; Bio-Rad, Hercules, Calif.). Alternatively, incubations were carried out with [methyl-3H]thymidine (1 μCi per well for 2 h) and scintillation counting of harvested cells was performed with a 1205 Betaplate counter (KBL Pharmacia). Possible effects of GATA-1 expression on cell cycle progression were assayed as follows. Cultures were initiated at 2.5 × 105 cells/ml, grown to 7 × 105 to 8 × 105 cells/ml (exponential growth phase), washed in Opti-MEM I medium, and cultured for 7.5 h at 3 × 105 to 5 × 105 cells/ml in medium containing 1.5% FBS and 10 μM 2-mercaptoethanol. The cells then were stimulated with Epo (50 U/ml) or IL-3 (WEHI-3 CM at 8%), collected at 4-h intervals (2 × 106 to 3 × 106 cells total), fixed in 35% ethanol, stained with propidium iodide (6), and analyzed for cell cycle distribution by flow cytometry (Coulter XL-MCL, Miami, Fla.) with Multicycle software (Theorix Flow System, San Diego, Calif.).
RNA isolation and Northern blot analyses.
Total RNA was isolated from FDCW2 and derived cell lines by the method of Chomczynski and Sacchi (3) with 1 ml of TRIzol reagent per 107 cells (Life Technologies). Polyadenylated RNA was isolated on Oligotex spin columns (Qiagen, Chatsworth, Calif.). In Northern blotting, RNA was electrophoresed in 1.2% agarose gels containing formaldehyde (6% in gels and 3% in electrophoresis buffer) and blotted to Nytran membranes (Schleicher & Schuell, Keene, N.H.). The membranes were fixed by UV irradiation (312 nm for 3 min) and heating (1 h at 68°C under vacuum). For use in hybridizations, 32P-labeled probes were prepared by random priming (Prime-a-Gene system; Promega) with DNA polymerase I (Klenow fragment), 50 μCi of [α-32P]dATP (3,000 Ci/mmol), and 25 ng of the following cDNA fragments: the 1.5-kb XhoI fragment of pXMwtER (murine Epo receptor) (10); the 1.8-kb KpnI-NotI fragment of pXMGATA-1 (murine GATA-1) (43); the 1.2-kb XbaI fragment of pBOSEKLF (murine EKLF) (19); the 1.6-kb SphI-ScaI fragment of pCDM8-ckit (murine c-kit) (30); the murine FOG cDNA; the 1.32-kb HindIII-SstII fragment of pSK+Ets-1 (human Ets-1) (41); the 1.3-kb BglII fragment of pNTKFli (murine Fli 1) (49); the 1.2-kb EcoRI fragment of pBSMac 1 (murine Mac 1 α chain) (29); the 2.9-kb XhoI-EcoRI fragment of pBSmpl; and the 0.8-kb KpnI-XhoI fragment of pSP-GAPDH (murine GAPDH). For detection of βmaj-globin transcripts, a 1,100-bp murine βmaj-globin 5′ BglII-3′ XbaI fragment was prepared by PCR with a genomic clone (44) and the primers 5′-CTGACAGATGCTCTCTTGGG-3′ and 3′-ACAACCCCAGAAACAGACA-5′. 32P-labeled probes were purified on Sephadex G-50 microcolumns (Pharmacia Biotech), and hybridizations were performed with 2 × 106 cpm of QuickHyb solution (Stratagene, La Jolla, Calif.) per ml for 2 h at 68°C. The membranes were washed in 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS at 55°C and exposed to X-Omat film (Kodak, Rochester, N.Y.). For reprobing, the membranes were stripped in 50% formamide–0.1× SSC at 65°C for 1 h.
Western blotting.
In Western blotting of GATA-1, samples were prepared by direct lysis of FDCW2 and derived cell lines in 2.5% SDS–0.1 M dithiothreitol–7.5% glycerol–8.75 mM Tris Cl (pH 6.8) (100 μl per 106 washed cells). The samples were incubated at 100°C for 5 min, and soluble proteins were electrophoresed in an SDS–7.5% polyacrylamide gel and blotted to nitrocellulose (Micron Separations Inc., Westborough, Mass.). The rat N6 monoclonal antibody to GATA-1 (Santa Cruz Biotechnology Inc., Santa Cruz, Calif.) was used at a 1:300 dilution and was detected by using a horseradish peroxidase-linked secondary antibody and enhanced chemiluminescence (Amersham Life Science, Arlington Heights, Ill.).
RESULTS
FDCW2 cells as a heterologous model for studies of GATA-1 and Epo receptor action.
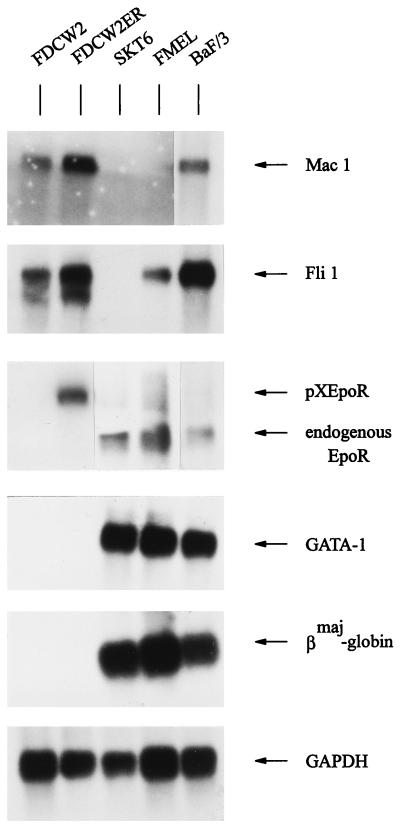
One goal of this investigation was to test whether it would be possible activate a program of erythroid gene expression in a definitively nonerythroid model through the ectopic expression of GATA-1 and the Epo receptor. In related studies, murine FDC-P2 (1) BaF/3 (15), and 32D (36) cell lines have been used to investigate effects of exogenously expressed GATA-1 and/or Epo on growth and βmaj-globin gene expression. However, preliminary analyses showed that endogenous GATA-1 and/or Epo receptor transcripts were expressed in each of these cell lines (50a). In contrast, FDCW2 cells proved to be essentially negative for GATA-1 and erythroid gene expression but supported mitogenic activities of exogenously expressed Epo receptor forms. Northern blot analyses served to establish the myeloid nature of this cell line, and Mac 1 and Fli 1 (Friend virus integration site) transcripts were observed at high levels (Fig. 1). In contrast, no expression of transcripts for GATA-1, Epo receptor, or βmaj-globin was detectable in FDCW2 cells in analyses of polyadenylated RNA. For comparison, RNA from erythroleukemic SKT6 and FMEL cells and from BaF/3 cells was coanalyzed. GATA-1, Epo receptor, and βmaj-globin transcripts were detected in each of these lines, and in BaF/3 cells the detection of low levels of Epo receptor transcripts served to confirm the high sensitivity of these analyses. Expectedly, Fli 1 transcripts also were expressed in FMEL cells, while both Mac 1 and Fli 1 transcripts also were expressed in BaF/3 cells. Also analyzed was polyadenylated RNA from FDCW2 cells transfected stably with a murine wt Epo receptor expression vector (FDCW2ER cells). As in parental FDCW2 cells, no endogenous GATA-1, Epo receptor or βmaj-globin transcripts were detected in these derived, Epo-selected cells. Also, expression of Mac 1 and Fli 1 transcripts was essentially unaffected.
FIG. 1.
Factor-dependent myeloid FDCW2 and FDCW2ER cells as a null background for studies of GATA-1 function. In FDCW2 and derived FDCW2ER cells, expression of Mac 1, Fli 1, Epo receptor, GATA-1, and βmaj-globin transcripts was assayed by Northern blotting of polyadenylated RNA. As positive controls for erythroid transcripts, polyadenylated RNAs from SKT6, FMEL, and BaF/3 cells were coanalyzed. Hybridizations were performed sequentially with a single blot, and equivalence in loading was assessed by hybridization to a GAPDH probe.
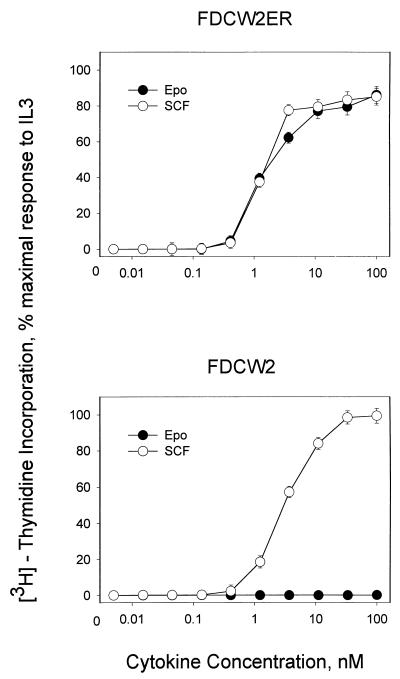
Mitogenic signaling via ectopically expressed Epo receptors in FDCW2ER cells next was assessed (Fig. 2, top). Epo supported mitogenesis at rates comparable to those activated by endogenous IL-3 and SCF receptors. In contrast, parental FDCW2 cells showed no proliferative responsiveness to Epo (Fig. 2, bottom), nor was any outgrowth of Epo-responsive (or factor-independent) sublines supported by an extended culture of FDCW2 cells in Epo at concentrations as high as 1 μM (34a). Thus, mitogenic activity of the murine wt Epo receptor is supported efficiently in this myeloid model, yet no expression of erythrocyte transcripts is detectable in either FDCW2 or derived FDCW2ER cells.
FIG. 2.
SCF-, IL-3-, and Epo-dependent mitogenesis in FDCW2 and FDCW2ER cells. Proliferative responses of factor-dependent FDCW2 and FDCW2ER cell lines to SCF, IL-3, and Epo were assayed based on rates of cytokine-stimulated incorporation of [methyl-3H]thymidine. To account for any minor differences in plating, values were normalized based on maximal responsiveness to IL-3.
GATA-1 expression in FDCW2ER cells prolongs the cell cycle G1 phase specifically during Epo-induced mitogenesis.
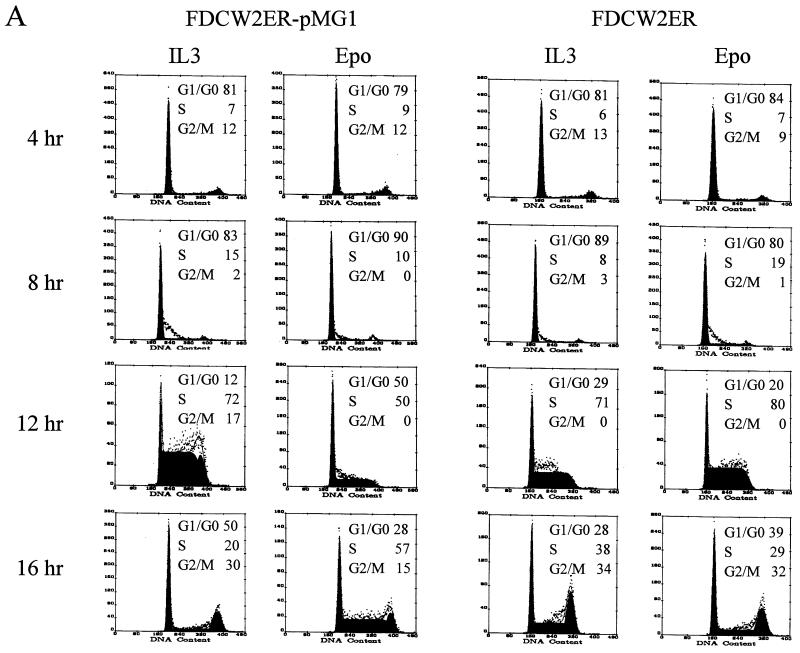
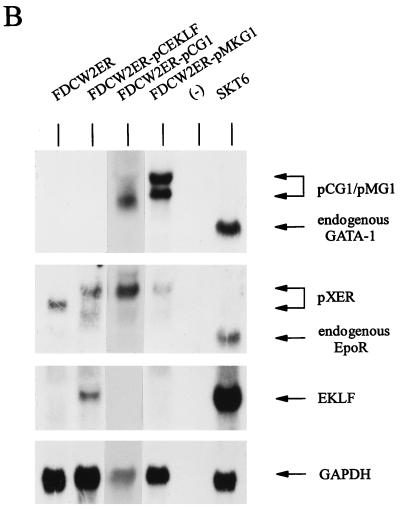
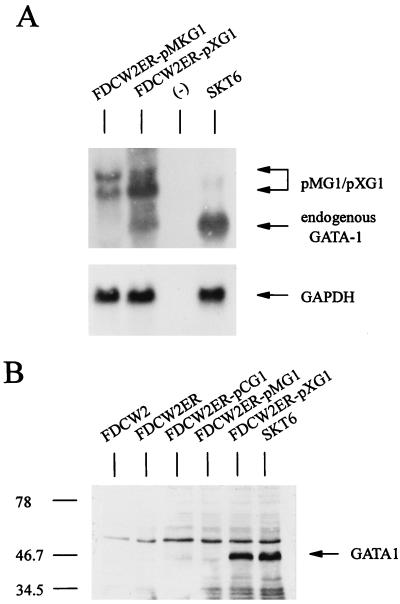
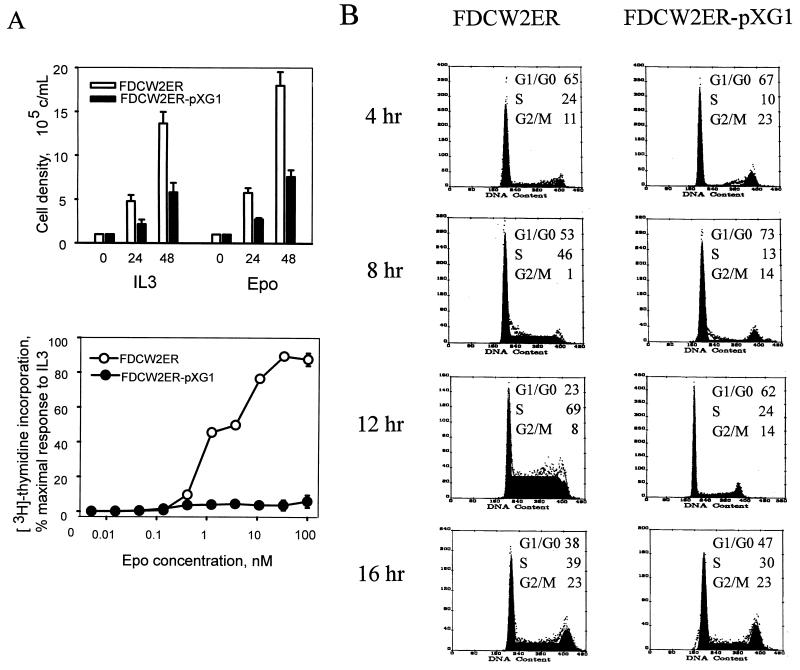
Possible effects of the ectopic expression of GATA-1 on the proliferation of FDCW2ER cells next were investigated. Based on recently suggested roles for GATA-1 in modulating mitogenesis in NIH 3T3 fibroblasts (6), effects on cell cycle distributions first were studied. In these experiments, GATA-1 was expressed stably in FDCW2ER cells by using a dicistronic vector, pMK1059. Derived FDCW2ER-pMG1 cells and control cell lines were cultured in the absence of cytokines to induce synchrony, cells were exposed to either IL-3 or Epo, and cell cycle distributions were determined at 4-h intervals. In FDCW2ER-pMG1 cells, IL-3 promoted a rapid transition from G0/G1 to S at 8 to 12 h (83% G0/G1 and 15% S at 8 h, and 12% G0/G1 and 72% S at 12 h), and the cells efficiently progressed to G2/M (30%) at 16 h. In contrast, upon exposure to Epo, a significant prolongation of the G1 phase was observed. Specifically, 50% of the cells remained in G0/G1 at 12 h and only 15% of these cells advanced to G2/M at 16 h (Fig. 3, left). In control FDCW2ER cells, no such effect was observed (Fig. 3, right). The results shown are representative of three independent analyses (data not shown). To further confirm this novel effect and its specificity, analyses also were performed with two additional FDCW2ER-derived lines: FDCW2ER-pCG1 cells in which GATA-1 was expressed with a pCINeo-derived vector, and a control line, FDCW2ER-pCEKLF, in which EKLF was ectopically expressed. As observed in FDCW2ER-pMG1 cells, FDCW2ER-pCG1 cells (but not FDCW2ER-pCEKLF cells) displayed a significant prolongation of the G1 phase selectively during Epo-stimulated mitogenesis (Table 1). In each of these FDCW2ER-derived cell lines, the ectopic expression of Epo receptor, GATA-1, and EKLF transcripts was confirmed (Fig. 3B). However, no expression of any endogenous erythroid gene transcripts was detected. Therefore, these Epo-specific effects of GATA-1 on G1-phase prolongation occurred in the apparent absence of endogenous erythroid gene activation.
FIG. 3.
GATA-1 expression in FDCW2ER-pMG1 cells prolongs the G1 phase of the cell cycle during Epo-stimulated mitogenesis. (A) Epo- versus IL-3-stimulated cell cycle progression in FDCW2ER-pMG1 and control FDCW2ER cells. FDCW2ER-pMG1 and FDCW2ER cells were washed free of cytokines and were cultured for 7.5 h in Opti-MEM I medium containing 1.5% FBS to promote synchrony. The cells then were exposed to either Epo or IL-3, and cell cycle distributions were analyzed at 4-h intervals by propidium iodide staining and flow cytometry. (B) GATA-1 transcript expression in FDCW2ER-pMG1 and FDCW2ER-pCG1 cells. To confirm exogenous GATA-1 expression, total RNA from FDCW2ER-pCG1 and FDCW2ER-pMG1 cells (and from FDCW2, FDCW2ER, and erythroid SKT6 cells as controls) was isolated and levels of GATA-1 and Epo receptor transcripts were assayed by Northern blotting. An additional control cell line, FDCW2ER-pCEKLF, also was analyzed for Epo receptor, EKLF, and GATA-1 transcript expression. Hybridizations were performed sequentially by using a single blot, and equivalence in loading was confirmed by hybridization to a GAPDH probe.
TABLE 1.
Epo-specific prolongation of the G1 phase in cells ectopically expressing GATA-1
| Time of cytokine exposure (h) | % of cells in G0/G1 following cytokine exposurea
|
|||||||
|---|---|---|---|---|---|---|---|---|
| FDCW2ER
|
FDCW2ER-pCEKLF
|
FDCW2ER-pMG1
|
FDCW2ER-pCG1
|
|||||
| IL-3 | Epo | IL-3 | Epo | IL-3 | Epo | IL-3 | Epo | |
| 4 | 76.0 | 75.1 | 84.0 | 78.8 | 80.6 | 79.8 | 81.6 | 72.6 |
| 8 | 73.3 | 73.1 | 68.1 | 75.1 | 82.6 | 88.5 | 85.0 | 80.0 |
| 12 | 28.6 | 27.8 | 17.4 | 26.5 | 11.8 | 41.0 | 27.4 | 53.2 |
| 16 | 34.2 | 29.8 | 51.0 | 39.9 | 50.3 | 25.2 | 34.0 | 38.5 |
Percentages represent the average of at least three independent experiments. Boldface is used to emphasize the effect of GATA-1 Epo prolongation of the G1 phase.
GATA-1-mediated inhibition of proliferation, and activation of endogenous GATA-1, EKLF, ets-1, and βmaj-globin gene expression in FDCW2ER cells.
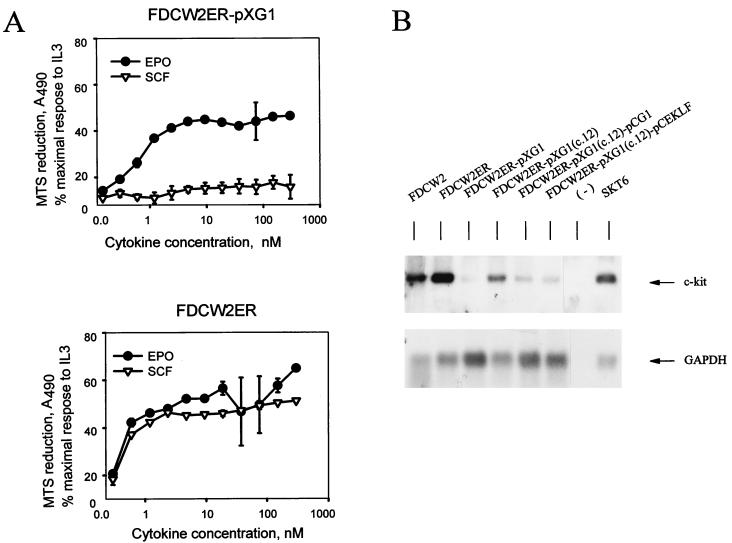
The effects of GATA-1 on erythroid gene expression recently have been suggested to depend upon its high-level expression (16). To further test whether GATA-1 might promote erythroid gene events in FDCW2ER cells, levels of exogenous expression of GATA-1 were increased by using a pXM-derived vector. Ectopic expression of GATA-1 transcripts in derived FDCW2ER-pXG1 cells was shown to be elevated compared to that in FDCW2ER-pMG1 cells (Fig. 4A). Interestingly, endogenous GATA-1 gene-derived transcripts also were detected in FDCW2ER-pXG1 cells. In these cells, levels of GATA-1 protein expression also were assayed by Western blotting and were observed to approximate the levels of GATA-1 expression in erythroleukemic SKT6 cells (Fig. 4B). By comparison, in FDCW2ER-pMG1 and FDCW2ER-pCG1 cells, GATA-1 was expressed only at the lower limits of Western blot sensitivity. The effects of GATA-1 expression on proliferation in FDCW2ER-pXG1 cells first were analyzed quantitatively. In FDCW2ER-pXG1 cells, GATA-1 expression inhibited growth in response to Epo and to IL-3, and this was observed in direct assays of cell proliferation and in [methyl-3H]thymidine incorporation assays (Fig. 5A). Also, a GATA-1- and Epo-dependent prolongation of the G1 phase of the cell cycle was observed (Fig. 5B). Finally, a selectively strong inhibition of proliferative responsiveness to SCF was effected (Fig. 6A). Interestingly, Northern blot analyses of FDCW2ER-pXG1 and control FDCW2ER cells showed that this effect was associated with an inhibition of c-kit transcript expression (Fig. 6B). In addition, in FDCW2ER-pXG1 cells in which the expression of exogenous GATA-1 was reinforced (FDCW2ER-pXG1 c.12pCG1 cells [see below]), this GATA-1-dependent suppression of c-kit transcript expression was enforced further (Fig. 6B).
FIG. 4.
Exogenous expression of GATA-1 in FDCW2ER-pXG1 cells activates endogenous GATA-1 gene expression. (A) GATA-1 transcript expression in FDCW2ER-pMG1 and FDCW2ER-pXG1 cells. Expression of GATA-1 in FDCW2ER-pMG1, FDCW2ER-pXG1, and control FDCW2 and FDCW2ER cell lines was assayed initially by Northern blotting of total RNA. As a positive control, RNA from erythroleukemic SKT6 cells was coanalyzed. Equivalence in loading was confirmed by hybridization to a GAPDH probe. (B) Levels of GATA-1 protein expression in FDCW2ER-pCG1, FDCW2ER-pMG1, and FDCW2ER-pXG1 cells. In each of these lines, GATA-1 protein expression levels were assayed by Western blotting of total cell lysates. As negative controls, lysates from FDCW2 and FDCW2ER cells were used while erythroleukemic SKT6 cells served as a positive control. Molecular weight markers (in thousands) are shown in the left margin.
FIG. 5.
Attenuation of Epo- and IL-3-dependent proliferation and cell cycle progression in FDCW2ER-pXG1 cells. (A) Effects of GATA-1 expression on Epo- and IL-3-dependent proliferation in FDCW2ER-pXG1 cells. Cytokine-induced growth of FDCW2ER-pXG1 and control FDCW2ER cells was assayed by scoring cell numbers (mean and standard deviation) every 24 h over a 72-h period (top). Also assayed were rates of Epo- and IL-3-induced incorporation of [methyl-3H]thymidine (mean and standard deviation) (bottom). The results shown for growth and [methyl-3H]thymidine incorporation are representative of three independent experiments. (B) GATA-1 expression results in an Epo-specific prolongation of the G1 phase in FDCW2ER-pXG1 cells. FDCW2ER-pXG1 and control FDCWER cells were washed free of cytokines and incubated for 7.5 h in Opti-MEM I medium–1.5% FBS to promote synchrony. Following stimulation with Epo, the cells were stained with propidium iodide and the cell cycle distributions were determined.
FIG. 6.
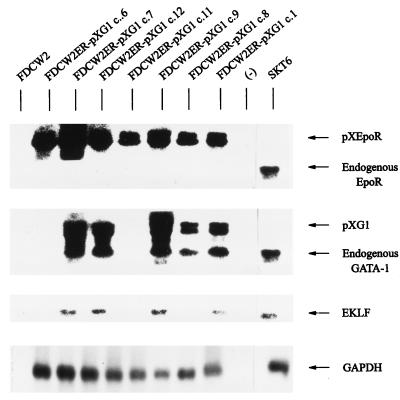
Inhibition of SCF-induced mitogenesis and c-kit transcript expression in FDCW2ER-pXG1 cells. (A) GATA-1-dependent inhibition of SCF-induced mitogenesis. The cytokine-induced mitogenesis of FDCW2ER-pXG1 (top) and control FDCW2ER (bottom) cell lines was assayed based on rates of Epo- and SCF-stimulated reduction of MTS. To account for any minor differences in plating, values were normalized based on maximal responsiveness to IL-3. (B) GATA-1-mediated inhibition of c-kit transcript expression in FDCW2ER-pXG1 cells. c-kit transcript levels in FDCW2ER-pXG1 cells versus control FDCW2 and FDCW2ER cells were assayed by Northern blotting of polyadenylated RNA. Also analyzed were FDCW2ER-pXG1 cells transfected stably with pCINeo vectors encoding either GATA-1 to reinforce its expression (FDCW2ER-pXG1 c.12pCG1 cells) or EKLF as a control (FDCW2ER-pXG1 c.12pCEKLF cells).
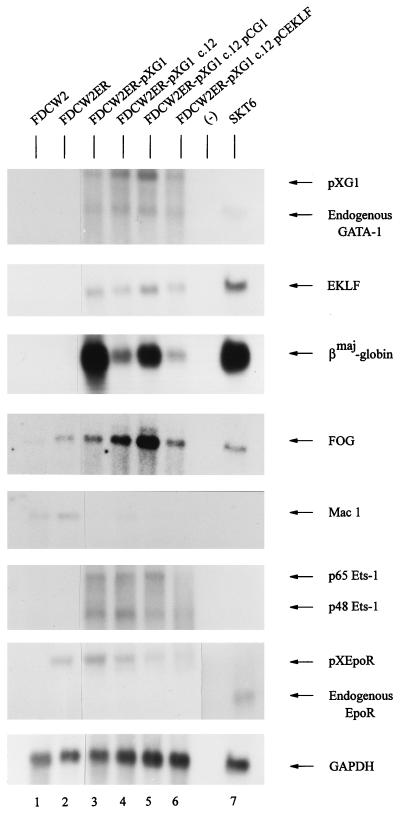
Whether GATA-1-dependent inhibition of growth was associated with an activated program of erythroid gene expression next was investigated. Polyadenylated RNA from FDCW2ER-pXG1 cells (and from control FDCW2 and FDCW2ER cells) was isolated, and possible erythroid gene expression was assayed by Northern blotting (Fig. 7, lanes 1 to 3). Apparent de novo expression of endogenous GATA-1, EKLF, and βmaj-globin genes was activated in FDCW2ER-pXG1 cells at appreciable levels, while no expression of these transcripts was detectable in control FDCW2 or FDCW2ER cells. In addition, levels of FOG transcript expression increased upon ectopic expression of GATA-1, while Mac 1 transcript expression was repressed. By comparison, levels of Fli 1, GATA-2, NF-E2, and Lmo-2 expression were not modulated (data not shown). Interestingly, the expression of transcripts encoding Ets-1 factors p48 and p65 also was activated in FDCW2ER-pXG1 cells, while no expression of the endogenous Epo receptor gene was detected. Importantly, this apparent absence of endogenous Epo receptor expression largely negates the possibility that erythroid gene transcript expression in FDCW2ER-pXG1 cells resulted simply from an Epo-promoted outgrowth of an erythroid subpopulation of FDCW2ER cells. In addition, this observation at least suggests that Epo receptor gene activation may depend upon trans-acting factors other than GATA-1.
FIG. 7.
Activation of erythroid gene expression in FDCW2ER-pXG1 cells. In polyclonal FDCW2ER-pXG1 cells (lane 3), the derived clonal subline c.12 (lane 4), c.12 cells transfected with a pCG1 expression vector (to reinforce GATA-1 expression) (lane 5), and this c.12 subline transfected with a pCEKLF expression vector (lane 6), levels of expression of the following transcripts were assayed by Northern blotting of polyadenylated RNA: GATA-1, EKLF, βmaj-globin, FOG, Mac 1, ets-1, Epo receptor, and GAPDH. Polyadenylated RNA from parental FDCW2 and FDCW2ER cells served as negative controls (lanes 1 and 2), while RNA from SKT6 cells served as a positive control (lane 7). In hybridizations, a single blot was probed sequentially and equivalence in loading was confirmed by hybridization to a GAPDH probe.
To further define effects of exogenous GATA-1 expression on endogenous erythroid gene expression, clonal sublines of FDCW2ER-pXG1 cells were isolated by limiting dilution and were assayed for levels of expression of ectopically and endogenously expressed Epo receptor, GATA-1, and EKLF transcripts (Fig. 8; Table 2). As predicted, all sublines examined expressed ectopically derived Epo receptor transcripts while no endogenous Epo receptor gene transcripts were detected in any clones. By comparison, expression of endogenous GATA-1 transcripts was observed only in FDCW2ER-pXG1 clones in which ectopic expression of GATA-1 was observed (clones 1, 2, 4, 7, 8, 9, 10, and 12). Furthermore, EKLF transcripts were detected only in clones which expressed high levels of endogenous GATA-1 transcripts (clones 1, 2, 4, 7, 9, and 12) and βmaj-globin gene transcription was activated in each of these clones (Table 2). These observed differences in the relative levels of GATA-1 and EKLF transcripts expressed among clones (together with the apparent absence of endogenous Epo receptor transcripts in all clones examined) provide further evidence that erythroid gene activation in FDCW2ER-pXG1 cells is a de novo consequence of the expression of exogenous GATA-1.
FIG. 8.
Levels of GATA-1 and EKLF transcript expression among FDCW2ER-pXG1 sublines. Clonal sublines of FDCW2ER-pXG1 cells were isolated by dilution (clones c.1, c.8, c.9, c.11, c.12, c.7, and c.6 representative of 12 clones analyzed [Table 2]) and levels of GATA-1 and EKLF transcript expression were assayed by Northern blotting of total RNA. FDCW2 and FDCW2ER cells were analyzed as negative controls, while erythroleukemic SKT6 cells were used as a positive control for erythroid gene expression. Hybridizations were performed sequentially with a single blot, and equivalence in loading was confirmed by hybridization to a GAPDH probe.
TABLE 2.
Levels of expression of GATA-1, EKLF, and βmaj-globin gene transcripts among clonal sublines of FDCW2ER-pXG1 cells
| FDCW2ER-pXG1 clone no. | Expression of:
|
|||
|---|---|---|---|---|
| Exogenous GATA-1 | Endogenous GATA-1 | Endogenous EKLF | Endogenous βmaj-globin | |
| 1 | ++ | ++ | ++ | ++ |
| 2 | ++ | ++ | +++ | ++ |
| 3 | − | − | − | − |
| 4 | ++ | ++ | ++ | ++ |
| 5 | − | − | − | − |
| 6 | − | − | − | − |
| 7 | +++ | +++ | +++ | ++ |
| 8 | + | + | − | − |
| 9 | ++ | +++ | +++ | ++ |
| 10 | + | + | − | − |
| 11 | − | − | − | − |
| 12 | +++ | +++ | +++ | +++ |
Endogenous FOG and βmaj-globin gene expression is enhanced upon the reinforced expression of GATA-1 in FDCW2ER-pXG1 cells.
The above studies suggest that ectopic expression of GATA-1 and the Epo receptor in myeloid FDCW2 cells is sufficient to activate the expression of at least a subset of erythroid genes. However, as is the case for many factor-dependent erythroid cell lines (TF-1, 32D, BaF/3, and UT7) (14, 15, 18, 22), overt conversion to morphologically identifiable erythroblasts was not demonstrable among FDCW2ER-pXG1 cell lines. To test whether erythroid differentiation might be promoted further in these cells, a clonal line of FDCW2ER-pXG1 cells (clone 12) was transfected stably with pCINeo vectors expressing either GATA-1 or EKLF (yielding FDCW2ER-pXG1 c.12pCG1 and FDCW2ER-pXG1 c.12pCEKLF cells) (Fig. 7, lanes 4 to 6). This reinforced expression of GATA-1 or EKLF did not promote overt differentiation. However, Northern blot analyses of polyadenylated RNA from FDCW2ER-pXG1 c.12pCG1 cells revealed that the enforced expression of GATA-1 did result in increased levels of endogenous GATA-1, FOG, and βmaj-globin transcript expression. To a lesser extent, levels of EKLF transcripts also were increased in these cells. In contrast, no such effects were observed in cells in which EKLF expression was enforced (i.e., FDCW2ER-pXG1 c.12pCEKLF cells). These analyses provide further evidence in FDCW2ER-pXG1 cells that GATA-1 acts as a dominant yet selective activator of endogenous erythroid gene expression.
Apparent roles for Epo in enhancing GATA-1-dependent activation of βmaj-globin gene expression.
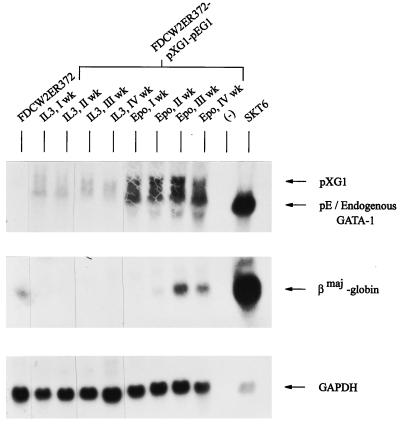
In the above studies, use of the Epo receptor as a selectable marker for cells transfected with pXMGATA-1 prevented opportunities to objectively test possible effects of Epo on erythroid differentiation events. To provide for such analyses, parental FDCW2 cells were transfected first with a pXM-Epo receptor expression vector (pXMER372) and a hygromycin B resistance vector. Derived FDCW2ER372 cells were maintained in IL-3 and then transfected with pXMGATA-1 and pEFNeoGATA-1 expression vectors. Derived FDCW2ER372-pXG1-pEG1 cells then were subdivided and were maintained in either IL-3 (WEHI-3 CM) or Epo. At defined intervals, RNA from parallel cultures was isolated and levels of GATA-1 and βmaj-globin transcripts were assayed. Exposure of FDCW2ER372-pXG1-pEG1 cells to Epo led to the expression of βmaj-globin transcripts at markedly higher levels (Fig. 9). Importantly, no such effect was observed in control FDCW2ER372 cells maintained in Epo at high concentrations for up to 1 year (34a). These findings provide novel evidence that Epo-specific signals can markedly enhance the GATA-1-dependent activation of βmaj-globin transcript expression.
FIG. 9.
Epo-enhanced activation of βmaj-globin gene expression in FDCW2ER372-pXG1-pEG1 cells. FDCW2ER372-pXG1-pEG1 cells expressing the minimal Epo receptor form ER372 and increased levels of GATA-1 were prepared via stable electrotransfection and maintained in IL-3. Cultures then were subdivided and maintained in parallel in either IL-3 (as WEHI-3 CM) or Epo (25 U/ml). At the indicated intervals of culture (weeks 1, 2, 3, and 4), total RNA was isolated and levels of GATA-1, EKLF and βmaj-globin transcripts were analyzed by Northern blotting. As negative and positive controls, RNA from parental FDCW2ER372 and erythroleukemic SKT6 cells were used, respectively. Hybridizations were performed sequentially with a single blot, and equivalence in loading was confirmed by hybridization to a GAPDH probe.
GATA-1 expression in FDCW2ER-pXG1 cells activates endogenous c-mpl gene expression.
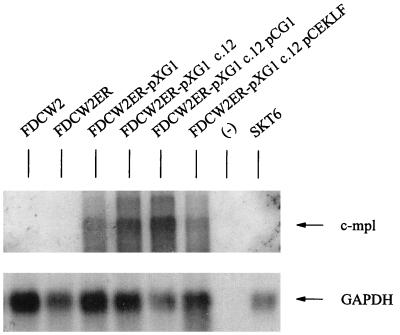
Recently, roles for GATA-1 also have been described during megakaryopoiesis (52). Whether GATA-1 expression in FDCW2ER-pXG1 cells might activate c-mpl expression therefore was assessed. Unlike the endogenous Epo receptor gene, which remained transcriptionally silent (see above), c-mpl gene expression was induced in FDCW2ER cells expressing GATA-1 from pXM vectors (Fig. 10). Thus, in this model, distinct sets of trans-acting factors regulate Epo versus Tpo receptor gene activation.
FIG. 10.
GATA-1 expression in FDCW2ER-pXG1 cells induces c-mpl gene expression. Polyadenylated RNA was isolated from parental FDCW2 and FDCW2ER cells and from polyclonal FDCW2ER-pXG1 cells, a derived clonal subline, c.12, c.12 cells transfected with a pCG1 expression vector (to reinforce GATA-1 expression), and this c.12 subline transfected with a pCEKLF expression vector. The blots were hybridized to 32P-labeled cDNA probes for murine c-mpl and GAPDH.
DISCUSSION
Evidence for GATA-1 autoregulation and hierarchical function during erythroid cell development.
Two aspects of the present study of GATA-1-dependent activation of erythroid gene expression in myeloid FDCW2ER cells merit discussion: (i) mechanisms that underlie the ability of GATA-1 to promote de novo expression of endogenous GATA-1, EKLF, βmaj-globin, and ets-1 genes and (ii) the extent to which this activation of erythroid gene expression might involve Epo-signaled events. With regard to this observed autoregulation of GATA-1 gene expression, this represents a novel finding in nontransformed cells that extends previous studies of the isolated GATA-1 promoter (34, 44). In murine and human GATA-1 promoters (23), paired GATA elements occur 760 to 665 bp upstream of transcriptional start sites that are important for transcription in erythroid FMEL cells (23). In chicken, GATA elements at 425, 150 and 125 bp upstream likewise contribute to GATA-1 gene transcription, together with CACC and Ets binding elements (7). Therefore, autoactivation has been proposed, and this was investigated previously in several cell lines. In FDC-P2 cells, GATA-1 gene transcription is stimulated upon the expression of a Gag-Myb-Ets fusion protein (1). Endogenous Epo receptor and βmaj-globin gene transcription also was enhanced. GATA-1 was suggested to mediate these effects, yet this was not tested directly and v-myb repression of c-myb expression also was implicated as a mechanism (1). In myeloid 416B cells, endogenous GATA-1 gene expression is promoted upon the expression of exogenous GATA-1 truncation mutants (including a minimal C-terminal zinc finger construct) (47). These C-terminal constructs might act through positive cofactors or via competition with negative regulators. A complicating result in this system, however, is the apparent inability of wt GATA-1 to promote any such increases in endogenous GATA-1 gene transcription (48). Consistent with a recently described dominant role for GATA-1 in megakaryopoiesis (37), acetylcholine esterase expression and increases in ploidy were induced by various forms of exogenous GATA-1. βmaj-globin transcripts (and GATA-1) also were expressed in parental 416B cells, yet no increases in globin transcripts were observed upon exogenous GATA-1 expression (48). Finally, promyeloblasts from E26 virus-infected chickens have proven useful in studies of GATA-1 action (16), and exogenous GATA-1 has been shown to promote endogenous GATA-1 gene expression and eosinophilic and thrombocytic events in HD50 cell lines. Also, upon inactivation of v-ets within an E26 Gag-Myb-Ets protein and exposure to anemic serum, βmaj-globin gene expression and erythroblastic differentiation was induced. Thus, evidence for GATA-1 autoactivation (and a dominant role in erythroid lineage specification) is suggested, yet the results are complicated by Gag-Myb-Ets-dependent potentiation of erythroid gene expression and by inhibitory effects of v-ets on erythropoiesis in this avian model. In the present investigation, the use of FDCW2 cells for studies of GATA-1 action was advantageous in two ways. As a definitive myeloid progenitor cell line, the FDCW2 cell line provides an essentially null background for studies of GATA-1 function, and Epo receptor expression could be reconstituted in the absence of any detectable lineage conversion effects. Thus, opportunities were provided to investigate possible GATA-1 autoactivation and hierarchical effects on erythroid lineage specification in a nontransformed, Epo-responsive model. With regard to GATA-1 gene expression, these studies provide the first demonstration of autoregulation in a system that is not complicated by Gag-Myb-Ets transformation or by the prior expression of endogenous GATA-1 at detectable levels. With regard to possible hierarchical roles for GATA-1 in erythroid gene activation, GATA-1 first was proposed to activate the expression of EKLF, a factor that is essential for the efficient transcription of the endogenous βmaj-globin gene (27). This is based on the occurrence of an activatable GATA element in the EKLF gene promoter (4) and upon the closely timed expression of GATA-1 and EKLF during embryogenesis (39). In clonal lines of FDCW2ER-pXG1 cells, GATA-1 expression at elevated levels was associated with increased endogenous EKLF gene transcription. While this observation does not provide direct evidence for trans activation by GATA-1, it is consistent with such a mechanism and does establish a hierarchy. In FDCW2ER-pXG1 sublines in which EKLF expression was induced, endogenous βmaj-globin gene transcription also was activated. Since disruption of EKLF gene expression inhibits βmaj-globin expression (27) and since EKLF binding at the murine βmaj-globin promoter is required for efficient transcription (2), the question whether EKLF per se might activate βmaj-globin gene transcription is raised. In FDCW2ER-pCEKLF cells, however, expression of EKLF in the absence of GATA-1 failed to detectably stimulate this event.
In FDCW2ER-pXG1 cells, GATA-1 expression also promoted increases in FOG transcript levels and induced ets-1 gene expression. FOG also was expressed in parental myeloid FDCW2 and FDCW2ER cells at basal levels in the apparent absence of GATA-1. Thus, FOG might contribute to GATA-1 gene autoregulation (and possibly to the GATA-1-dependent increase in FOG gene expression). For ets-1, the observed GATA-1-dependent activation of transcript expression is of interest in two contexts. First, this effect might be interpreted to contradict the results of studies with chicken HD50 myeloblasts in which the inactivation of Ets expression was suggested to be required for erythroblastic differentiation (16). In HD50 cells, however, this refers to v-Ets. Second, c-Ets1 activates the transcription of GATA-1 (35) and transferrin receptor (38) gene promoters. Consistent with these reports, GATA-1-dependent expression of ets-1 in FDCW2ER-pXG1 cells suggests that c-Ets1 does not interfere with erythroid cell programming but may positively affect this event.
Possible roles for Epo during GATA-1-directed erythroid cell differentiation.
The present studies with FDCW2ER-G1 cells also raise the possibility that Epo-mediated events contribute to GATA-1-induced erythroid gene expression. One novel finding is that the expression of GATA-1 in FDCW2ER-pMG1 and FDCW2CR-pCG1 cells prolongs the cell cycle G1 phase specifically during Epo-stimulated mitogenesis. In IL-3-dependent BaF/3 cells ectopically expressing the wt Epo receptor, a similar cell cycle delay has been observed and was suggested to be important for Epo-induced increases in βmaj-globin transcript expression (15). Interestingly, while originally described as a pro-B cell line (26), BaF/3 cells since have been shown to express at least certain erythroid genes including GATA-1. In erythroleukemic cell lines (K562, HEL, and TF1), aphidicolin-induced interruption of G1-to-S progression likewise activates globin gene expression (22). Molecular mechanisms that might link this G1 phase effect to the onset of erythroid cell differentiation events are unresolved. Nonetheless, in FDCW2ER-pMG1 and FDCW2ER-pCG1 cells, selective effects of GATA-1 on Epo-dependent cell cycle progression suggest that such specific mechanisms normally are exerted. In FDCW2ER-pXG1-pEG1 cells, exposure to Epo (with respect to IL-3 as a control) also was shown to enhance the GATA-1-dependent activation of βmaj-globin gene expression. Epo receptor signaling is essential for development beyond the CFU-e stage (51). However, whether Epo exerts effects on erythrocyte differentiation is also unresolved. In the context of globin expression, the effects of Epo on βmaj-globin gene transcript accumulation detected in this study argue that at least certain differences exist within Epo and IL-3 signaling pathways and that Epo-specific signals can promote select late erythroid events.
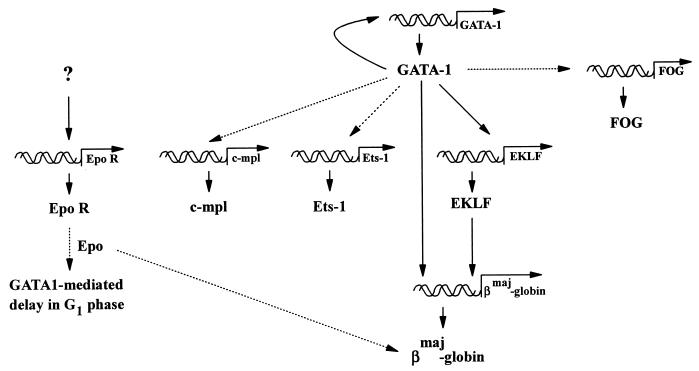
The present studies of GATA-1 action in FDCW2ER-pXG1 cells provide novel evidence for GATA-1 autoregulation and for hierarchical roles in activating endogenous EKLF, ets-1, and βmaj-globin gene expression. Also defined are an ability of GATA-1 to prolong the cell cycle G1 phase during Epo-stimulated mitogenesis, an apparent role for Epo in promoting GATA1-dependent βmaj-globin gene expression, and a possible requirement for additional trans-acting factors during Epo receptor gene transcription (Fig. 11). In future studies, this FDCW2ER-pXG1 cell model should prove useful in further defining factors that regulate GATA-1, FOG, and EKLF gene expression, and unique opportunities are provided to directly test whether (and mechanistically how) Epo might modulate GATA-1 effects on progenitor cell growth, survival, and/or differentiation.
FIG. 11.
De novo gene activation events induced by GATA-1 in FDCW2ER-pXG1 cells. Solid lines indicate pathways suggested to depend directly upon GATA-1 and EKLF trans-activation events, while broken lines depict proposed indirect events.
ACKNOWLEDGMENTS
We thank Stuart Orkin, Philip Leder, Don Blair, and Robert Pytela for the generous provision of murine FOG, c-mpl, ets-1, Fli 1, and Mac 1 cDNAs, respectively. We also thank Amy Wrentmore and Elaine Kunze for technical assistance and Amgen (and Steve Elliot and Robert Pacifici) for the provision of recombinant human Epo.
This work was supported by grants NIH R01 DK 40242 and NIH RCDA HL 03042 to D.M.W., NIH F32 HL09749 to P.G., and a Sigma Xi grant-in-aid to D.S.
REFERENCES
- 1.Aurigemma R E, Blair D G, Ruscetti S K. Transactivation of erythroid transcription factor GATA-1 by myb-ets-containing retrovirus. J Virol. 1992;66:3056–3061. doi: 10.1128/jvi.66.5.3056-3061.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieker J J, Southwood C M. The erythroid Krüppel-like factor transactivation domain is a critical component for cell-specific inducibility of a beta-globin promoter. Mol Cell Biol. 1995;15:852–860. doi: 10.1128/mcb.15.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Crossley M, Tsang A P, Bieker J J, Orkin S H. Regulation of the erythroid Krüppel-like factor (EKLF) gene promoter by the erythroid transcription factor GATA-1. J Biol Chem. 1994;269:15440–15444. [PubMed] [Google Scholar]
- 5.Dexter T M, Garlend J, Scott D, Scolnick E, Metcalf D. Growth of factor-dependent hemopoietic precursor cell lines. J Exp Med. 1980;152:1036–1047. doi: 10.1084/jem.152.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubart A, Romeo P H, Vainchenker W, Dumenil D. Constitutive expression of GATA-1 interferes with the cell-cycle regulation. Blood. 1996;87:3711–3721. [PubMed] [Google Scholar]
- 7.Hannon R, Evans T, Felsenfeld G, Gould H. Structure and promoter activity of the gene for the erythroid transcription factor GATA-1. Proc Natl Acad Sci USA. 1991;88:3004–3008. doi: 10.1073/pnas.88.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heikinheimo M, Scandrett J M, Wilson D B. Localization of transcription factor GATA-4 to regions of the mouse embryo involved in cardiac development. Dev Biol. 1994;164:361–373. doi: 10.1006/dbio.1994.1206. [DOI] [PubMed] [Google Scholar]
- 9.Ihle J N, Keller J, Greenberger J S, Henderson L, Yetter R A, Morse H C. Phenotypic characteristics of cell lines requiring interleukin 3 for growth. J Immunol. 1982;29:1377–1383. [PubMed] [Google Scholar]
- 10.Joneja B, Wojchowski D M. Mitogenic signaling and inhibition of apoptosis via the erythropoietin receptor Box-1 domain. J Biol Chem. 1997;272:11176–11184. doi: 10.1074/jbc.272.17.11176. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman R J, Murtha P. Translational control mediated by eucaryotic initiation factor-2 is restricted to specific mRNAs in transfected cells. Mol Cell Biol. 1987;7:1568–1571. doi: 10.1128/mcb.7.4.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley C, Yee K, Harland R, Zon L I. Ventral expression of GATA-1 and GATA-2 in the Xenopus embryo defines induction of hematopoietic mesoderm. Dev Biol. 1994;165:193–205. doi: 10.1006/dbio.1994.1246. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Yamauchi Y, Tanaka A, Shimamura S. Improved dicistronic mRNA expression vectors for efficient selection of transfectants highly expressing foreign genes. BioTechniques. 1996;21:398–402. doi: 10.2144/96213bm12. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu N, Yamamoto M, Fujita H, Miwa A, Hatake K, Endo T, Okano H, Katsube T, Fukumaki Y, Sassa S. Establishment and characterization of an erythropoietin-dependent subline, UT7/Epo, derived from human leukemia cell line, UT-7. Blood. 1993;82:456–464. [PubMed] [Google Scholar]
- 15.Krosl J, Damen J E, Krystal G, Humphries R K. Erythropoietin and interleukin-3 induce distinct events in erythropoietin receptor-expressing BA/F3 cells. Blood. 1995;85:50–56. [PubMed] [Google Scholar]
- 16.Kulessa H, Frampton J, Graf T. GATA-1 reprograms avian myelomonocytic cell lines into eosinophils, thromboblasts, and erythroblasts. Genes Dev. 1995;9:1250–1262. doi: 10.1101/gad.9.10.1250. [DOI] [PubMed] [Google Scholar]
- 17.Martin D I, Zon L I, Mutter G, Orkin S H. Expression of an erythroid transcription factor in megakaryocytic and mast cell lineages. Nature. 1990;344:444–447. doi: 10.1038/344444a0. [DOI] [PubMed] [Google Scholar]
- 18.Migliaccio G, Migliaccio A R, Kreider B L, Rovera G, Adamson J W. Selection of lineage-restricted lines immortalized at different stages of hematopoietic differentiation from the murine cell line 32D. J Cell Biol. 1989;109:833–841. doi: 10.1083/jcb.109.2.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller I J, Bieker J J. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrisey E E, Ip H S, Lu M M, Parmacek M S. GATA-6: a zinc finger transcription factor that is expressed in multiple cell lineages derived from lateral mesoderm. Dev Biol. 1996;177:309–322. doi: 10.1006/dbio.1996.0165. [DOI] [PubMed] [Google Scholar]
- 21.Morrisey E E, Ip H S, Tang Z, Lu M M, Parmacek M S. GATA-5: a transcriptional activator expressed in a novel temporally and spatially-restricted pattern during embryonic development. Dev Biol. 1997;183:21–36. doi: 10.1006/dbio.1996.8485. [DOI] [PubMed] [Google Scholar]
- 22.Murate T, Saga S, Hotta T, Asano H, Ito T, Kato K, Tsushita K, Kinoshita T, Ichikawa A, Yoshida S, Saito H. The close relationship between DNA replication and the selection of differentiation lineages of human erythroleukemia cell lines K562, HEL, and TF1 into either erythroid or megakaryocytic lineages. Exp Cell Res. 1993;208:35–43. doi: 10.1006/excr.1993.1219. [DOI] [PubMed] [Google Scholar]
- 23.Nicolis S, Bertini C, Ronchi A, Crotta S, Lanfranco L, Moroni E, Giglioni B, Ottolenghi An erythroid specific enhancer upstream to the gene encoding the cell-type specific transcription factor GATA-1. Nucleic Acids Res. 1991;19:5285–5291. doi: 10.1093/nar/19.19.5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orkin S H. Globin gene regulation and switching: circa 1990. Cell. 1990;63:665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- 25.Orkin S H. GATA-binding transcription factors in hematopoietic cells. Blood. 1992;80:575–581. [PubMed] [Google Scholar]
- 26.Palacios R, Steinmetz M. IL3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. J Biol Chem. 1985;270:15942–15945. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 27.Perkins A C, Sharpe A H, Orkin S H. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 28.Pevny L, Simon M C, Robertson E, Klein W H, Tsai S F, D’Agati V, Orkin S H, Costantini F. Erythroid differentiation in chimeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 29.Pytela R. Amino acid sequence of the murine Mac-1 alpha chain reveals homology with the integrin family and an additional domain related to von Willebrand factor. EMBO J. 1988;7:1371–1378. doi: 10.1002/j.1460-2075.1988.tb02953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu F H, Ray P, Brown K, Barker P E, Jhanwar S, Ruddle F H, Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988;7:1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quelle D E, Wojchowski D M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1991;88:4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radke K, Beug H, Kornfeld S, Graf T. Transformation of both erythroid and myeloid cells by E26, and avian leukemia virus that contains the myb gene. Cell. 1982;31:643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- 33.Reese T T, Gregory R C, Sharlow E R, Pacifici R E, Crouse J A, Todokoro K, Wojchowski D M. Epo-induced hemoglobinization of SKT6 cells is mediated by minimal cytoplasmic domains of the Epo or prolactin receptors without modulation of GATA-1 or EKLF. Growth Factors. 1997;14:161–176. doi: 10.3109/08977199709021518. [DOI] [PubMed] [Google Scholar]
- 34.Schwartzbauer G, Schlesinger K, Evans T. Interaction of the erythroid transcription factor cGATA-1 with a critical auto-regulatory element. Nucleic Acids Res. 1992;20:4429–4436. doi: 10.1093/nar/20.17.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34a.Seshasayee, D. Unpublished data.
- 35.Seth A, Robinson L, Thompson D M, Watson D K, Papas T S. Transactivation of GATA-1 promoter with ETS1, ETS2 and ERGB/Hu-FLI-1 proteins: stabilization of the ETS1 protein binding on GATA-1 promoter sequences by monoclonal antibody. Oncogene. 1993;8:1783–1790. [PubMed] [Google Scholar]
- 36.Shimada Y, Migliaccio G, Ralph H, Migliaccio A R. Erythropoietin-specific cell cycle progression in erythroid subclones of the interleukin-3-dependent cell line 32D. Blood. 1993;81:935–941. [PubMed] [Google Scholar]
- 37.Shivdasani R A, Fujiwara Y, McDevitt M A, Orkin S H. A lineage-selective knockout establishes the critical role of transcription factor GATA-1 in megakaryocyte and platelet development. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieweke M H, Tekotte H, Frampton J, Graf T. MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentiation. Cell. 1996;85:49–60. doi: 10.1016/s0092-8674(00)81081-8. [DOI] [PubMed] [Google Scholar]
- 39.Southwood C M, Downs K M, Bieker J J. Erythroid Krüppel-like factor exhibits an early and sequentially localized pattern of expression during mammalian erythroid ontogeny. Dev Dyn. 1996;206:248–259. doi: 10.1002/(SICI)1097-0177(199607)206:3<248::AID-AJA3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.Ting C-N, Olson M C, Barton K P, Leiden J M. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature. 1996;384:474–478. doi: 10.1038/384474a0. [DOI] [PubMed] [Google Scholar]
- 41.Topol L Z, Tatosyan A G, Ascione R, Thompson D M, Blair D G, Kola I, Seth A. C-ets-1 proto oncogene expression alters the growth properties of immortalized rat fibroblasts. Cancer Lett. 1992;67:71–78. doi: 10.1016/0304-3835(92)90010-s. [DOI] [PubMed] [Google Scholar]
- 42.Tsai F-Y, Keller G, Kuo F C, Weiss M, Chen J, Rosenblatt M, Alt F W, Orkin S H. An early hematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371:221–226. doi: 10.1038/371221a0. [DOI] [PubMed] [Google Scholar]
- 43.Tsai S F, Martin D I, Zon L I, D’Andrea A D, Wong G G, Orkin S H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989;339:446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]
- 44.Tsai S F, Strauss E, Orkin S H. Functional analysis and in vivo footprinting implicate erythroid transcription factor GATA-1 as a positive regulator of its own promoter. Development. 1991;5:919–931. doi: 10.1101/gad.5.6.919. [DOI] [PubMed] [Google Scholar]
- 45.Tsang A P, Visvader J E, Turner C A, Fujiwara Y, Yu C, Weiss M J, Crossley M, Orkin S H. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. doi: 10.1016/s0092-8674(00)80318-9. [DOI] [PubMed] [Google Scholar]
- 46.Tugores A, Magness S T, Brenner D A. A single promoter directs both housekeeping and erythroid preferential expression of the human ferrochelatase gene. J Biol Chem. 1994;269:30789–30797. [PubMed] [Google Scholar]
- 47.Visvader J E, Crossley M, Hill J, Orkin S H, Adams J M. The C-terminal zinc finger of GATA-1 or GATA-2 is sufficient to induce megakaryocytic differentiation of an early myeloid cell line. Mol Cell Biol. 1995;15:634–641. doi: 10.1128/mcb.15.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Visvader J E, Elefanty A G, Strasser A, Adams J M. GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J. 1992;11:4557–4564. doi: 10.1002/j.1460-2075.1992.tb05557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watson D K, Robinson L, Hodge D R, Kola I, Papas T S, Seth A. FLI1 and EWS-FLI1 function as ternary complex factors and ELK1 and SAP1 a function as ternary and quaternary complex factors on the Egr1 promoter serum response element. Oncogene. 1997;14:213–221. doi: 10.1038/sj.onc.1200839. [DOI] [PubMed] [Google Scholar]
- 50.Weiss M J, Keller G, Orkin S H. Novel insights into erythroid development revealed through in vitro differentiation of GATA-1 embryonic stem cells. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 50a.Wojchowski, D. M. Unpublished data.
- 51.Wu H, Liu X, Jaenisch R, Lodish H F. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- 52.Yamaguchi Y, Zon L I, Ackerman S J, Yamamoto M, Suda T. Forced GATA-1 expression in the murine myeloid cell line M1: induction of c-Mpl expression and megakaryocytic/erythroid differentiation. Blood. 1998;91:450–457. [PubMed] [Google Scholar]
- 53.Zhuang H, Patel S V, He T C, Sonsteby S K, Niu Z, Wojchowski D M. Inhibition of erythropoietin-induced mitogenesis by a kinase-deficient form of Jak2. J Biol Chem. 1994;269:21411–21414. [PubMed] [Google Scholar]
- 54.Zon L I, Youssoufian H, Mather C, Lodish H F, Orkin S H. Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc Natl Acad Sci USA. 1991;88:10638–10641. doi: 10.1073/pnas.88.23.10638. [DOI] [PMC free article] [PubMed] [Google Scholar]