Abstract
Objectives:
To describe epinephrine dosing distribution using time-stamped data and assess the impact of dosing strategy on survival after ECPR in children.
Methods:
This was a retrospective study at five pediatric hospitals of children <18 years with an in-hospital ECPR event. Mean number of epinephrine doses was calculated for each 10-minute CPR interval and compared between survivors and non-survivors. Patients were also divided by dosing strategy into a frequent epinephrine group (dosing interval of ≤5 min/dose throughout the first 30 minutes of the event), and a limited epinephrine group (dosing interval of ≤5 min/dose for the first 10 minutes then >5 min/dose for the time between 10 and 30 minutes).
Results:
A total of 191 patients were included. Epinephrine was not evenly distributed throughout ECPR, with 66% of doses being given during the first half of the event. Mean number of epinephrine doses was similar between survivors and non-survivors the first 10 minutes (2.7 doses). After 10 minutes, survivors received fewer doses than non-survivors during each subsequent 10-minute interval. Adjusted survival was not different between strategy groups [OR of survival for frequent epinephrine strategy: 0.78 (95% CI 0.36–1.69), p = 0.53].
Conclusions:
Survivors received fewer doses than non-survivors after the first 10 minutes of CPR and although there was no statistical difference in survival based on dosing strategy, the findings of this study question the conventional approach to EPCR analysis that assumes dosing is evenly distributed.
Keywords: Extracorporeal cardiopulmonary resuscitation, ECPR, Pediatrics, Cardiac arrest, Epinephrine
Introduction
Cardiopulmonary resuscitation (CPR) guidelines recommend dosing of epinephrine every 3–5 minutes during cardiac arrest, though literature to support this practice is lacking and several recent publications have challenged these guidelines.1–4 While prior attempts to understand best practices surrounding epinephrine dosing during CPR have been reported, a common challenge encountered while investigating epinephrine’s role in resuscitation is the lack of time-stamped dosing data. This limitation necessitates the use of calculated average epinephrine interval as the variable of interest. This method assumes that doses are evenly distributed throughout the event and may introduce biases that lead to incorrect conclusions regarding the efficacy or harm of epinephrine.
Understanding the true frequency of epinephrine dosing and how it is distributed through resuscitation is vital in cases of extracorporeal cardiopulmonary resuscitation (ECPR), which involves lengthy periods of CPR as the patient is cannulated onto extracorporeal membrane oxygenation (ECMO). Due to the length of ECPR events patients can be exposed to high amounts of cumulative epinephrine that may increase the risk for cerebrovascular injury.5–6 In a survey of pediatric providers with experience utilizing ECPR for children with underlying cardiac disease, 36% reported giving epinephrine doses less frequently than is recommended.7
To date there are few published reports investigating actual practice using epinephrine dosing time-stamps or the impact of epinephrine dosing strategy on pediatric ECPR outcomes. We sought to describe epinephrine dosing distribution and its impact on survival after ECPR in children. We hypothesized that epinephrine doses would not be uniformly distributed throughout ECPR events and that increased spacing between doses are resuscitation progressed would be associated with improved hospital survival.
Materials and methods
Study design and patients
This study was approved or deemed exempt with waiver of consent by the respective institutional review boards at each participating center. This was a retrospective review of in-hospital ECPR events from January 2012 to December 2019. Patients less than 18 years of age were included if they suffered a cardiac arrest ending with successful cannulation onto ECMO. Patients with return of spontaneous circulation (ROSC) >20 minutes prior to cannulation were excluded. Events where CPR started before arrival at the hospital, events without adequate documentation of epinephrine dosing, events where zero or only one dose of epinephrine was documented, and cardiac arrests in the neonatal intensive care unit were also excluded. Only the first ECPR event for a hospital stay was included.
Individual sites provided de-identified data using Research Electronic Data Capture housed at the University of Nebraska Medical Center.8 Data elements collected followed Utstein-style definitions for standardized cardiac arrest reporting and included demographics, pre-existing conditions, illness category, interventions in place at the time of arrest, first documented rhythm, and resuscitation therapies including vasoactive medication administration.9 Only one surgical non-cardiac patient met inclusion criteria, and thus surgical non-cardiac and medical non-cardiac illness categories were combined. Documentation included dosage and time stamp for each dose of epinephrine.
Outcomes
The primary outcome was survival to hospital discharge. Other outcomes included acute kidney injury (AKI) and favorable neurologic outcomes. AKI was defined by the Kidney Disease Improving Global Outcomes creatinine classification: Stage 1, increase in creatinine of ≥50% or absolute increase in creatinine of 0.3 mg/dl; Stage 2, increase in creatinine of ≥100%; Stage 3, increase in creatinine of ≥200% and/or renal replacement therapy.10
Neurologic function was assessed at admission for all patients and again at discharge for survivors using the Pediatric Cerebral Performance Category (PCPC).11 The PCPC rates patients’ overall function with the following scale: 1 = normal, 2 = mild disability, 3 = moderate disability, 4 = severe disability, 5 = coma/vegetative. Favorable neurologic outcome was defined as a PCPC score of 1, 2, or no worsening from baseline.12
To assess whether excessive vasoconstriction was limiting the ability of ECMO to provide support, ECMO flow at 1 hour after cannulation was recorded in addition to the use of vasodilators (continuous infusions of nicardipine and nitroprusside). Continuous inotrope and vasopressor use was assessed using the Vasoactive-Inotropic Score, which equals dopamine dose (μg/kg/min) + dobuta mine dose (μg/kg/min) + 100 × epinephrine dose (μg/kg/min) + 10 × milrinone dose (μg/kg/min) + 10,000 × vasopressin dose (units/kg/min) + 100 × norepinephrine dose (μg/kg/min).13
Analysis
Demographic and clinical characteristics of the patients and events were summarized using median (interquartile range) or frequency (percent). Chi-square, Fisher’s exact, or Kruskal-Wallis tests were used as appropriate to analyze patient demographics, event characteristics and clinical outcomes associated with survival. To visualize the distribution of epinephrine doses throughout ECPR, times of individual doses were reported relative to the duration of each event, e.g., a dose given 30 minutes into a 60-minute event would be coded as given during the 50th percentile of event duration. To compare whether patients with different average dosing intervals had different dosing distributions, the cohort was divided into <3, 3–5, and >5 min/-dose average epinephrine dosing intervals to create unique histogram plots. Previous published calculations1 for average epinephrine interval were modified to create the following formula:
The interval between the last dose of epinephrine and cannulation onto ECMO was included given ECPR resuscitations can be prolonged and may involve longer periods between last epinephrine dose and ECMO cannulation. Ignoring this last interval would introduce a bias towards shorter average dosing intervals.
To evaluate dosing pattern differences between survivors and non-survivors, the mean number of epinephrine doses during each 10-minute period was calculated and the difference tested with the Mann-Whitney U test. Only epinephrine doses from patients that received CPR during the entire 10-minute interval were used to calculate the mean during that interval. This prevents CPR duration from confounding the relationship between dosing frequency and survival.
To analyze survival based on epinephrine dosing strategies that deviated from guidelines, we selected a subgroup of events lasting ≥30 minutes to analyze longer resuscitations and mitigate the impact of CPR duration on outcome. The number of doses during the first 10 minutes of the event and for the time period between 10 and 30 minutes were counted. Patients who received zero or one epinephrine dose during the first 10 minutes were excluded, as we were interested in a strategy of epinephrine dosing per guidelines early in the event followed by spacing of doses later in resuscitation, similar to dosing strategy modifications that may occur after ECPR team activation.7 Epinephrine dosing strategy was categorized as either “frequent epinephrine” which was defined as 4 or greater doses of epinephrine given between 10 and 30 minutes (≤5 min/dose), or “limited epinephrine”, defined as less than 4 doses given between 10 and 30 minutes (>5 min/dose). Multivariable logistic regression analysis was performed and included confounders that were different between groups and previously demonstrated to impact outcomes after ECPR: illness category, first pulseless rhythm, and time of day (day vs. night).
Lastly, an analysis was performed to evaluate whether current epinephrine dosing guidelines were associated with survival to hospital discharge using average epinephrine dosing intervals for the entire duration of the ECPR event prior to ECMO cannulation as described above. Similar to previous epinephrine dosing interval studies we categorized events based on resuscitation guidelines for epinephrine dosing: < 3 min/dose, 3–5 min/dose, > 5 min/dose.
All analysis was performed using Statistical Analysis Software (version 9.4, SAS Incorporated, Cary, North Carolina). A p-value < 0.05 was considered statistically significant.
Results
A total of 204 patients from 5 centers met inclusion criteria; thirteen were excluded for only receiving 0 or 1 doses of epinephrine, leaving 191 patients for analysis. Overall survival to hospital discharge was 43%. Median age (IQR) for all patients was 5 months (0.7–44) and median weight 6 kg (3.5–9.5). Patient and resuscitation event characteristics grouped by survival are presented in Table 1. Survivors were younger, more likely to be surgical cardiac patients, had better baseline neurologic function, and experienced shorter total duration of CPR prior to initiation of ECMO. Outcomes of interest are presented in Table 2.
Table 1 –
Patient and event characteristics of hospital survivors and non-survivors.
| Variable | Survivors | Non-survivors | p |
|---|---|---|---|
| n = 83 | n = 108 | ||
| Age, median (IQR), months | 2.2 (0.4–12) | 10.5 (1.3–80.5) | <0.001 |
| Gestational agea, median (IQR), weeks | 38.3 (37–39.2) | 38 (36–39) | 0.034 |
| Weight, median (IQR), kg | 4.5 (3.3–8.5) | 8.5 (3.9–20.2) | 0.006 |
| Weight for age z-score, median (IQR) | −0.7 (−1.5–0.2) | −0.8 (−1.6–0.3) | 0.249 |
| Male gender, n (%) | 48 (58) | 48 (44) | 0.067 |
| Race, n (%) | 0.49 | ||
| Black | 9 (11) | 16 (15) | |
| White | 54 (65) | 75 (69) | |
| Other/unknown | 20 (24) | 17 (16) | |
| Illness category, n (%) | 0.001 | ||
| Medical cardiac | 27 (32) | 37 (34) | |
| Surgical cardiac | 53 (64) | 49 (45) | |
| Non-cardiac | 3 (4) | 22 (20) | |
| Genetic syndrome, n (%) | 17 (20) | 41 (38) | 0.009 |
| Admission PCPC = 1, n (%) | 68 (86) | 68 (67) | 0.004 |
| Location of arrest, n (%) | 0.436 | ||
| Cardiac intensive care unit | 50 (60) | 55 (51) | |
| Pediatric intensive care unit | 16 (19) | 25 (23) | |
| Other | 17 (21) | 28 (26) | |
| First pulseless rhythm, n (%) | <0.001 | ||
| Asystole | 2 (2) | 19 (18) | |
| Pulseless electrical activity | 65 (78) | 79 (73) | |
| Ventricular tachycardia/fibrillation | 12 (14) | 6 (6) | |
| Unknown | 4 (5) | 4 (4) | |
| Weekend event, n (%) | 20 (24) | 30 (28) | 0.566 |
| Night event, 1800–0600, n (%) | 38 (46) | 53 (49) | 0.652 |
| Interventions in place at start of CPR, n (%) | |||
| Mechanical ventilation | 48 (58) | 58 (54) | 0.569 |
| Arterial line | 51 (61) | 56 (52) | 0.186 |
| Central venous line | 53 (64) | 68 (63) | 0.899 |
| VIS score at start of CPR, median (IQR) | 3 (0–7) | 1 (0–13) | 0.786 |
| Epinephrine drip at start of CPR, n (%) | 21 (25) | 45 (42) | 0.018 |
| Duration of CPR, median (IQR), minutes | 42 (31–53) | 53 (38–66) | <0.001 |
| Time to first epinephrine dose, median (IQR), minutes | 1 (0–3) | 1 (0–3) | 0.684 |
| Total # epinephrine doses, median (IQR) | 5 (3–8) | 7 (4–11) | 0.005 |
| Total epinephrine dose, median (IQR), mg/kg | 0.05 (0.03–0.09) | 0.07 (0.04–0.16) | 0.034 |
| Average epinephrine dosing interval, median (IQR), min/dose | 7.7 (4.5–13) | 6.5 (3.7–14) | 0.329 |
| Time between last epinephrine dose and ECMO cannulation, median (IQR), minutes | 18 (6–32) | 12 (3.5–28) | 0.194 |
For patients <12 months of age. CPR, cardiopulmonary resuscitation; ECMO, extracorporeal membranous oxygenation; PCPC, Pediatric Cerebral Performance Category; VIS, vasoactive-inotropic score.
Table 2 –
Outcomes of hospital survivors and non-survivors.
| Variable | Survivors n = 83 |
Non-survivors n = 108 |
p |
|---|---|---|---|
| Length of ECMO, median (IQR), hours | 93 (59–151) | 69 (29–141) | 0.081 |
| ECMO flow 1 hour after cannulation, median (IQR), ml/kg/min | 105 (84–142) | 102 (77–147) | 0.512 |
| VIS 1 hour after cannulation, median (IQR) | 2.5 (0–5) | 3 (0–8) | 0.11 |
| Vasodilator use, n (%) | 41 (49) | 30 (28) | 0.002 |
| Any AKIa, n (%) | 36 (49) | 67 (71) | 0.004 |
| AKI stage 3a, n (%) | 10 (14) | 33 (35) | 0.002 |
| Favorable neurologic outcomeb, n (%) | 62 (77) | NA | NA |
| Brain death | NA | 25 (23) | NA |
| Hypoxic ischemic encephalopathy | 14 (17) | 24 (22) | 0.358 |
Kidney function not available in all patients, n = 169.
Survivors, n = 81.
AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; VIS, vasoactive-inotropic score.
Overall, epinephrine doses were not uniformly distributed, with 66% of doses being given during the first half of the ECPR event. This skewed distribution was most pronounced in the patient group with an average epinephrine dosing interval of >5 min/dose (Fig. 1). Fifty percent of epinephrine doses were given during the first 50% of the event for the <3 min/dose group, during the first 43% of the event for the 3–5 min/dose group, and during the first 21% of the event for the >5 min/dose group (p < 0.001).
Fig. 1 –
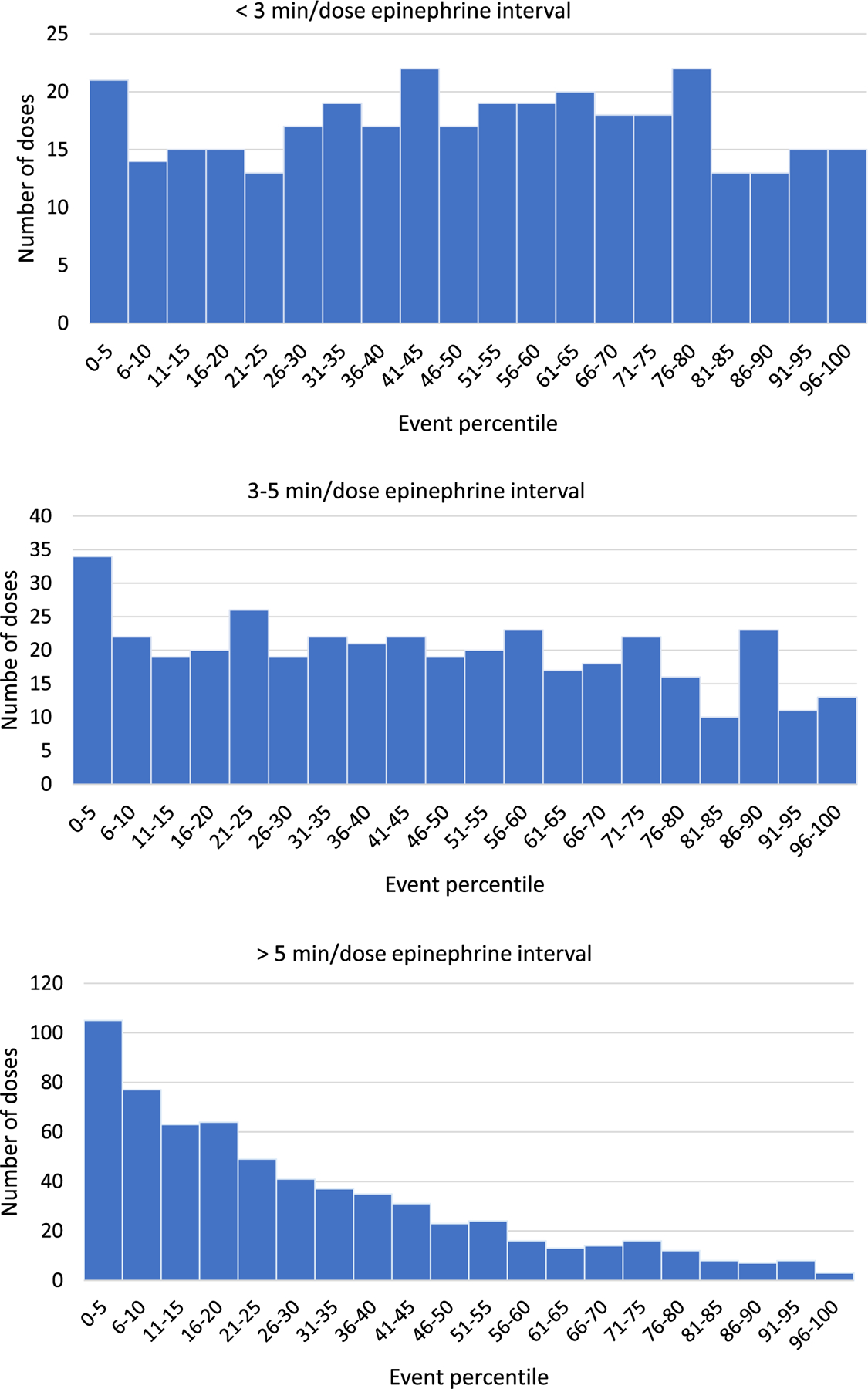
Total number of epinephrine doses given per 5%tile grouped by average epinephrine dosing interval.
Mean number of doses per 10-minute time increment is shown in Fig. 2 along with the number of patients still requiring CPR during that time period. Number of doses was similar between survivors and non-survivors during the first 10 minutes of the event at 2.7 doses. After the first 10 minutes survivors consistently received fewer epinephrine doses than non-survivors throughout all 10 min periods. The difference in mean number of doses was statistically significantly at time periods 11–20, 21–30, 41–50, and 51–60 minutes.
Fig. 2 –
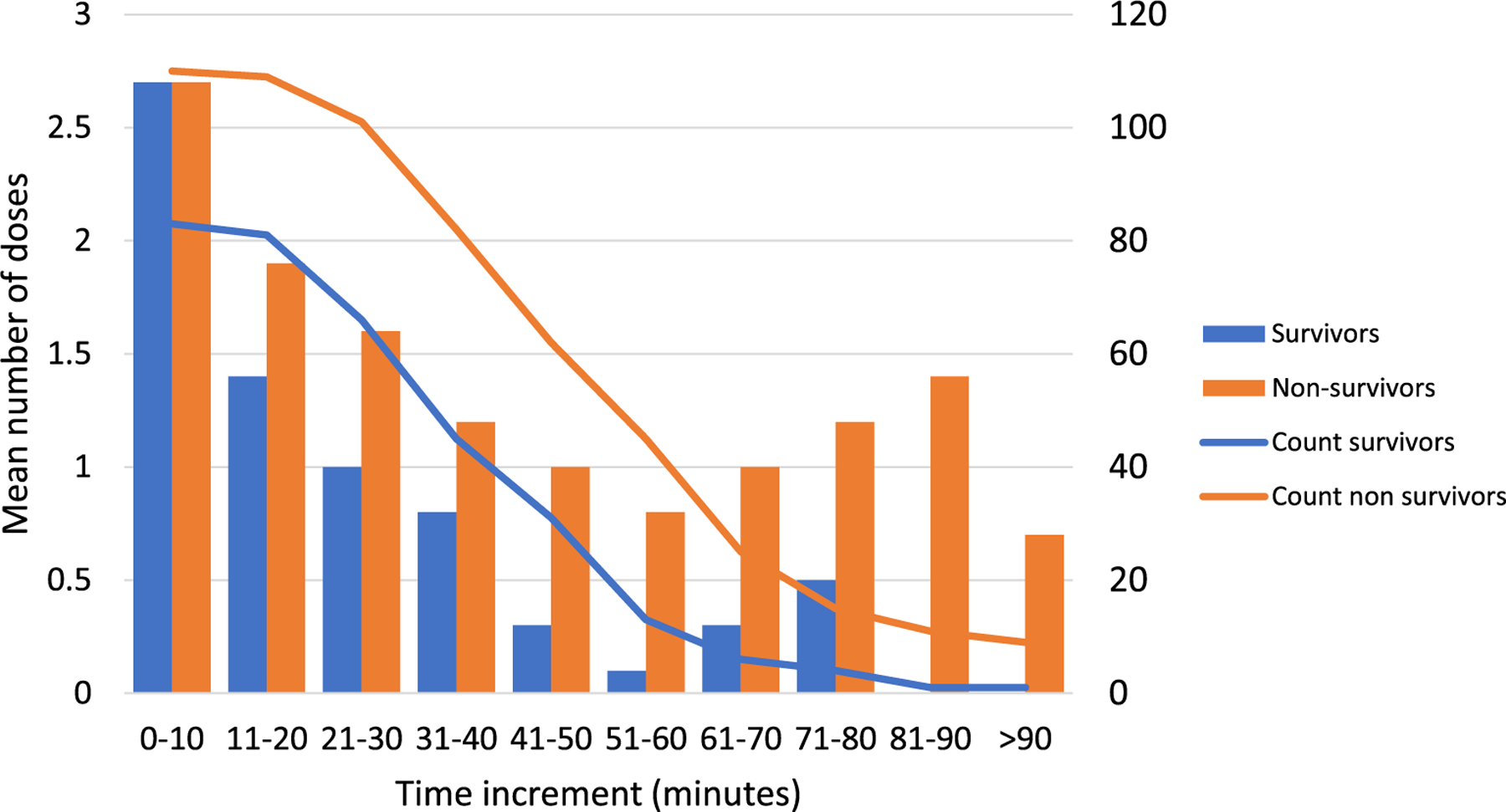
Mean number of epinephrine doses given per 10 minutes of CPR and number of patients receiving CPR during that time period. * Indicates p ≤ 0.05 for difference in mean epinephrine doses between survivors and non-survivors.
For the analysis of outcome based on frequent or limited dosing strategies, 27 patients had less than 30 minutes of CPR and 20 received zero or one dose of epinephrine during the first 10 minutes CPR so were excluded, leaving a total of 144 patients for analysis. Duration of CPR was similar between the frequent and limited epinephrine groups (49 vs. 51 minutes, p = 0.533). There were significant differences between total epinephrine doses received during the entire event [frequent 10 doses (8–14) vs. limited 4 doses (3–5), p < 0.001] and in time between last dose given and the end of CPR [frequent 7 minutes (2–22) vs. limited 25 minutes (14–39)], p < 0.001].
Patients in the frequent epinephrine group were more likely to have non-cardiac disease (frequent 22% vs. limited 7%, p = 0.012) and arrest during daytime hours (frequent 60% vs. limited 41%). The frequent epinephrine group was less likely to have an arterial line (frequent 40% vs. limited 65%, p = 0.003) or a central venous line (frequent 53% vs. limited 70%, p = 0.036). Other baseline and event characteristics are available in Table S1. There was no difference in AKI incidence or favorable neurologic outcome in survivors between the frequent and limited epinephrine groups (Table 3). The was no statistically significant difference in incidence of brain death (12% vs. 11%, p = 0.84) or hypoxic ischemic encephalopathy (29% vs. 15%, p = 0.06). There was no difference in adjusted survival between the frequent and limited epinephrine groups [OR of survival for frequent epinephrine group: 0.78 (95% CI 0.36–1.69, p = 0.53) (Table 4).
Table 3 –
Outcomes based on dosing strategy.
| Outcome variable | Frequent epinephrine n = 73 |
Limited epinephrine n = 71 |
p-value |
|---|---|---|---|
| Hospital survival, n (%) | 28 (38) | 33 (46) | 0.324 |
| Length of ECMO, median (IQR), hours | 81 (46–166) | 88 (43–159) | 0.666 |
| ECMO flow 1 hour after cannulation, median (IQR), ml/kg/min | 111 (84–152) | 103 (75–136) | 0.264 |
| VIS 1 hour after cannulation, median (IQR) | 2 (0–6) | 3 (0–5) | 0.516 |
| Vasodilator use, n (%) | 35 (48) | 20 (28) | 0.014 |
| Any AKIa, n (%) | 39 (60) | 41 (66) | 0.475 |
| AKI stage 3a, n (%) | 16 (25) | 18 (29) | 0.574 |
| Favorable neurologic outcomeb, n (%) | 20 (71) | 25 (78) | 0.55 |
| Brain death | 9 (12) | 8 (11) | 0.84 |
| Hypoxic ischemic encephalopathy | 21 (29) | 11 (15) | 0.06 |
Kidney function not available for all patients, n = 131,
Survivors, n = 61.
AKI, acute kidney injury; ECMO, extracorporeal membrane oxygenation; VIS, vasoactive-inotropic score.
Table 4 –
Association between epinephrine dosing strategy and survival.
| Variable | Adjusted OR for survival (95% CI) | P-value |
|---|---|---|
| Epinephrine strategy | ||
| Frequent epinephrine | 0.78 (0.36–1.69) | 0.528 |
| Limited epinephrine | reference | |
| Illness category | ||
| Medical Cardiac | 12.1 (1.4–102) | 0.022 |
| Surgical Cardiac | 12.2 (1.5–102) | 0.021 |
| Non-cardiac | reference | |
| First pulseless rhythm | ||
| Asystole | 0.06 (0.01–0.42) | 0.005 |
| Pulseless electrical activity | 0.45 (0.11–1.8) | 0.248 |
| Ventricular tachycardia/fibrillation | reference | |
| Day vs. night | 1.48 (0.68–3.23) | 0.324 |
Of note, twenty patients had at least 30 minutes of CPR but received no doses of epinephrine after the first 10 minutes of the event. Survival was not different for these patients compared to those that continued to receive epinephrine after 10 minutes (no epinephrine 45% vs. continued epinephrine 40%, p = 0.64). This survival difference remained non-significant when adjusted for CPR duration [OR of survival for no epinephrine group: 0.99 (95% CI 0.38–2.47), p = 0.98].
In the analysis of outcomes based on average epinephrine interval, average interval for the total cohort was <3 min/dose in 10% of patients, 3–5 min/dose in 23%, and >5 min/dose in 67%. There was a significant variation in dosing practice between hospitals, with a range of 7–38% in the 3–5 min/dose group (p < 0.001) (Table S2). Baseline characteristics, event characteristics, and outcomes by dosing interval group are available in Tables S3 and S4. There was no statistically significant difference in survival between the three dosing interval groups, although survival for patients receiving <3 min/dose epinephrine was 25% compared to 44% and 46% respectively for the 3–5 min/dose and >5 min/dose groups.
Discussion
In this multi-center study of pediatric patients undergoing ECPR for in-hospital cardiac arrest, we observed significant variation in practice between centers. Deviation from advanced life support guidelines for administration of epinephrine was common, with doses typically given at least every 5 minutes early in the event but then less frequently as the event progressed. Our analysis is the first to demonstrate a skewed distribution of epinephrine doses given across ECPR events, suggesting that the use of average dosing interval in previous resuscitation studies may result in an incomplete understanding of how epinephrine dosing impacts outcomes, at least in longer resuscitations. Although we note a significant difference in doses per 10 minute period between survivors and non-survivors, our subgroup analysis of events lasting longer than 30 minutes found no difference in survival between a frequent and limited epinephrine strategy after accounting for confounders that can impact survival.
Current accepted guidelines for both pediatric and adult advanced life support recommend epinephrine dosing every 3–5 minutes without limit during cardiac arrest.14,15 Furthermore, the employment of ECPR has been endorsed by national and international resuscitation associations, with ECPR utilization more common in children with underlying cardiac disease.14,16 In a survey querying providers of children with cardiac disease about practices related to EPCR, 64% reported giving epinephrine boluses every 3–5 minutes throughout the event.7 With time-stamped epinephrine dosing aggregated across 5 institutions, our study demonstrated that resuscitation guidelines for epinephrine dosing appear to be followed much less rigidly, as only 23% of patients in our study were in the 3–5 min/dose group as averaged across the entire CPR duration. Non-compliance with guidelines is not unique to ECPR and has been found in conventional CPR studies as well.2,17 Whether this is due to system factors or deliberate slowing of doses as CPR time lengthens is unknown. It is also conceivable that providers altered their epinephrine dosing strategy in response to patient variables such as diastolic blood pressure or end tidal CO2, factors which are associated with outcomes. It is also conceivable that providers altered their epinephrine dosing strategy in response to patient variables such as diastolic blood pressure or end tidal CO2, factors which are associated with outcomes.
Many researchers have cautioned against excessive epinephrine in resuscitation. Specifically, increased vasoconstriction due to repeated epinephrine doses during prolonged resuscitation has been proposed as a mechanism of cerebro-vascular injury in animal models with concerns for reduction in survival and worse neurologic outcomes.6,18 We found no difference in the rate of favorable neurologic outcomes amongst survivors between the frequent and limited epinephrine strategy groups. Further investigation into the associations between neurologic complications and epinephrine dosing in non-survivors may aid in our understanding of the impact of epinephrine dosing strategy on cerebral injury.
In addition to the potential direct risk of α-agonism to the microcirculation, more frequent epinephrine may result in excessive vasoconstriction thereby limiting ECMO flow and thus organ perfusion after cannulation.19 In our study there was no difference in ECMO flow rates between the frequent and limited epinephrine dosing strategy groups one hour after cannulation. However, use of continuous vasodilators was higher in the frequent epinephrine group. Vasodilators are often used in ECMO when elevated blood pressure restricts the circuit’s ability to flow and to prevent hypertension related complications such as bleeding and stroke. This suggests vasoconstriction was increased in the frequent epinephrine group, which could impact organ perfusion and clinical outcomes. This study did not collect hemodynamic or oxygen delivery data, limiting the ability to correlate epinephrine dosing with alterations in blood pressure, systemic vascular resistance, or tissue perfusion. Future prospective studies that include hemodynamic and oxygen delivery data would allow further assessment of the impact of epinephrine dosing on these measures during and after ECPR.
There is biologic plausibility that a limited epinephrine dosing strategy may be beneficial, or at least not harmful. Animal models of cardiac arrest show improvement in cerebral perfusion, cerebral oxygenation, and mean arterial pressure with initial doses of epinephrine, but this effect diminishes with successive boluses.20,21 A recent study of conventional CPR by Kienzle and colleagues found a higher rate of ROSC and shortened duration of CPR with an epinephrine dosing interval of less than 2 minutes.22 The median CPR time, however, was only 13 minutes and the authors suggested that continued frequent epinephrine dosing after the initial 10 minutes is unlikely to be beneficial. This is supported by our finding that outcomes were similar in patients that received no epinephrine doses after the first 10 minutes of CPR. In addition, efforts to improve care models for pre-hospital ECPR for adults suffering cardiac arrest led Lamhaut and colleagues in France to modify their vasoactive administration protocol to limit epinephrine doses, with a resulting increase in neurologically intact survival.23 At some point during a prolonged arrest at centers offering ECPR the resuscitation goal shifts from achieving ROSC to providing high quality CPR and adequate organ perfusion until ECMO cannulation. The findings from our analysis support conducting further research on when that shift should occur and whether additional doses of epinephrine provide any benefit or actually cause harm.
This study has several limitations. First, it is a retrospective analysis and dependent on the quality of documentation, which occurs during a high stress event and thus is at risk for inaccuracies. However, it is standard practice to have an individual exclusively assigned to document interventions during hospital resuscitations, so dosing times are likely reliable. This study has the advantage of being multi-center with five pediatric hospitals contributing data; in addition to the variation seen in epinephrine dosing between centers, other unmeasured organizational and practice differences between hospitals are likely to impact outcomes. CPR quality metrics were also not available at all centers and thus not accounted for in our analysis. Detailed vital sign data was also not available and thus it is unknown if more frequent epinephrine dosing was in response to poor hemodynamic conditions that could also impact outcome. Detailed vital sign data was also not available and thus it is unknown if more frequent epinephrine dosing was in response to poor hemodynamic conditions that could also impact outcome.
Conclusions
In this multi-center retrospective study of children undergoing in-hospital ECPR, epinephrine dosing frequency often did not follow recommended guidelines and doses were not evenly distributed throughout the duration of ECPR. Survivors consistently received fewer epinephrine doses than non-survivors after the first 10 minutes of resuscitation, and continued epinephrine dosing after 10 minutes did not improve survival. The prior conventional approach to ECPR analyses that assume epinephrine dosing is evenly distributed throughout prolonged resuscitations should be challenged given the findings from this study. Further research is needed to better understand the impact of epinephrine dosing on organ function during prolonged resuscitation leading to ECMO cannulation, as well as the ideal dosing distribution to maximize organ perfusion and minimize injury from excess vasoconstriction.
Supplementary Material
Acknowledgement
We thank Matthew Sandbulte, PhD of the Child Health Research Institute at Children’s Hospital & Medical Center and the University of Nebraska Medical Center for manuscript review and editing and Elizabeth Lyden, MS of the University of Nebraska Medical Center College of Public Health for statistical support.
Funding
This study was funded by the Department of Pediatrics, University of Nebraska Medical Center.
Footnotes
Financial disclosure
The authors have no disclosures to report.
CRediT authorship contribution statement
Laura A. Ortmann: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. Ron W. Reeder: Methodology, Writing – review & editing. Tia T. Raymond: Investigation, Methodology, Writing – review & editing, Data curation. Marissa A. Brunetti: Data curation, Methodology, Writing – review & editing. Adam Himebauch: Data curation, Investigation, Writing – review & editing. Rupal Bhakta: Data curation, Investigation, Writing – review & editing. Jessica Kempka: Data curation, Writing – review & editing. Shauna di Bari: Data curation, Writing – review & editing. Javier J. Lasa: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.resuscitation.2023.109855.
REFERENCES
- 1.Hoyme D, Patel S, Samson R, et al. Epinephrine dosing interval and survival outcomes during pediatric in-hospital cardiac arrest. Resuscitation 2017;117:18–23. [DOI] [PubMed] [Google Scholar]
- 2.Warren SA, Huszti E, Bradley SM, et al. Adrenaline (epinephrine) dosing period and survival after in-hospital cardiac arrest: a retrospective review of prospectively collected data. Resuscitation 2014;85:350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang C-H, Huang C-H, Chang W-T, et al. The influences of adrenaline dosing frequency and dosage on outcomes of adult in-hospital cardiac arrest: a retrospective cohort study. Resuscitation 2016;103:125–30. [DOI] [PubMed] [Google Scholar]
- 4.Baird A, Coppler PJ, Callaway CW, et al. Rate of intra-arrest epinephrine administration and early post-arrest organ failure after in-hospital cardiac arrest. Resuscitation 2020;156:15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasa J, Rogers R, Localio R, et al. Extracorporeal cardiopulmonary resuscitation (E-CPR) during pediatric in-hospital cardiopulmonary arrest is associated with improved survival to discharge. Circulation 2016;133:165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ristagno G, Tang W, Huang L, et al. Epinephrine reduces cerebral perfusion during cardiopulmonary resuscitation. Crit Care Med 2009;37:1408–15. [DOI] [PubMed] [Google Scholar]
- 7.Lasa J, Jain P, Raymond T, et al. Extracorporeal cardiopulmonary resuscitation in the pediatric cardiac population: in search of a standard of care. Pediatr Crit Car Med 2018;19:125–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummins RO, Chamberlain D, Hazinski MF, et al. Recommended guidelines for reviewing, reporting, and conducting research on in-hospital resuscitation: the in-hospital Utstein style. Circulation 1997;95:2213–39. [DOI] [PubMed] [Google Scholar]
- 10.Kellum JA, Lameire N, KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013;17:204. 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr 1992;121:68–74. [DOI] [PubMed] [Google Scholar]
- 12.Becker L, Aufderheide T, Geocadin R, et al. Primary outcomes for resuscitation science studies: a consensus statement from the American Heart Association. Circulation 2011;124:2158–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaies M, Gurney J, Yen A, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11:234–8. [DOI] [PubMed] [Google Scholar]
- 14.Topjian AA, Raymond TT, Atkins D, et al. Part 4: pediatric basic and advanced life support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020;142:S469–523. 10.1161/CIR.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 15.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care [published correction appears in Circulation. 2011 Feb 15;123(6):e236] [published correction appears in Circulation. 2013 Dec 24;128(25):e480]. Circulation 2010;122: S729–67. 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 16.Bembea M, Ng D, Rizkalla N, et al. Outcomes after extracorporeal cardiopulmonary resuscitation of pediatric in-hospital cardiac arrest: a report from the Get With the Guidelines-Resuscitation and the Extracorporeal Life Support Organization Registries. Crit Care Med 2019;47:e278–85. [DOI] [PubMed] [Google Scholar]
- 17.Meert K, Telford R, Slomine B, et al. Paediatric in-hospital cardiac arrest: factors associated with survival and neurobehavioural outcome one year later. Resuscitation 2018;124:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardig B, Gōtberg M, Rundgren M, et al. Physiologic effect of repeated adrenaline (epinephrine) doses during cardiopulmonary resuscitation in the cath lab setting: a randomized porcine study. Resuscitation 2016;101:77–83. [DOI] [PubMed] [Google Scholar]
- 19.Zorzmann V, Rilinger J, Lang C, et al. Epinephrine, inodilator, or no inotrope in venoarterial extracorporeal membrane oxygenation implantation: a single-center experience. Crit Care 2019;23:320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nosrati R, Lin S, Mohindra R, et al. Study of the effects of epinephrine on cerebral oxygenation and metabolism during cardiac arrest and resuscitation by hyperspectral near-infrared spectroscopy. Crit Care Med 2019;47:e349–57. [DOI] [PubMed] [Google Scholar]
- 21.Putzer G, Martini J, Spraider P, et al. Adrenaline improves regional cerebral blood flow, cerebral oxygenation and cerebral metabolism during CPR in a porcine cardiac arrest model using low-flow extracorporeal support. Resuscitation 2021;168:151–9. [DOI] [PubMed] [Google Scholar]
- 22.Kienzle MF, Morgan RW, Faerber JA, et al. The effect of epinephrine dosing intervals on outcomes from pediatric in-hospital cardiac arrest. Am J Respir Crit Care Med 2021;204:977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamhaut L, Hutin A, Puymirat E, et al. A pre-hospital extracorporeal cardiopulmonary resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation 2017;117:109–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


