Abstract
The retina is one of the most complex and extraordinary human organs affected by genetic, metabolic, and degenerative diseases, resulting in blindness for ∼1.3 million people in the United States and over 40 million people worldwide. This translates into a huge loss of productivity, especially among younger patients with inherited retinal diseases (IRDs) and diabetic retinopathy. Age-related macular degeneration accounts for 90% of all blindness cases worldwide. The prevalence of this condition is projected to reach over 5 million individuals over the next 3 decades. There are also >20 IRD phenotypes, affecting >2 million people worldwide. Nanobiotechnology uses nanotechnology for biological applications, making use of biological materials either conceptually or directly in the fabrication of new materials. Bionanotechnology, on the other hand, uses molecular biology for the purpose of creating nanostructures (ie, structures with at least 1 dimension <100 nm). Retinal applications of these technologies are developing at a rapid pace. This review includes the most current nanotechnological applications in retinal diagnostics, theranostics, drug delivery, and targeting, including the potential for nonviral vehicles such as liposomes, micelles, and dendrimers, which pose advantages over viral vectors in retinal drug delivery. Furthermore, we discuss current and future applications as surgical adjuncts and in regenerative medicine as they pertain to retinal disease. Structure and function of nanoparticles such as carbon nanotubules, quantum dots, and magnetic nanoparticles, as well as diagnostic technologies such as next-generation DNA sequencing and single-molecule bionanosensing, will also be discussed.
Keywords: biotechnology, retina, drug delivery, nanotechnology, gene therapy, ophthalmology
Background
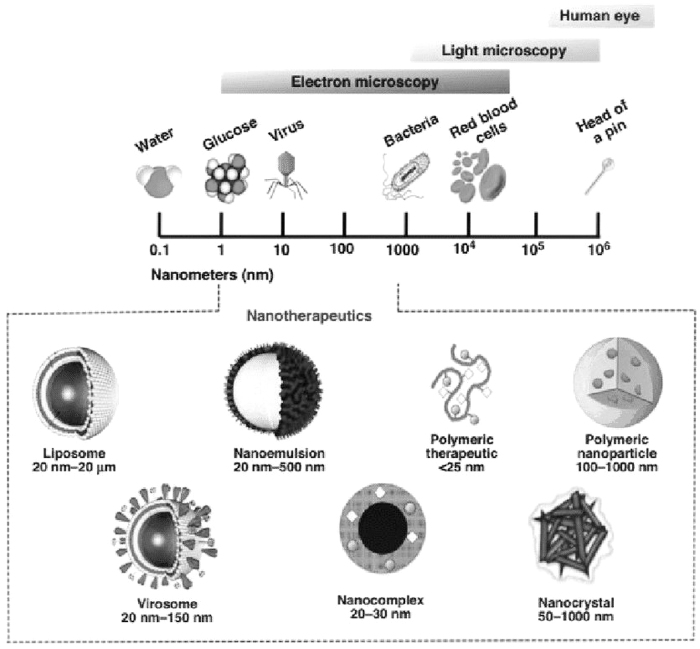
Nanotechnology is defined as technology on the scale of 1 to 100 nm in at least 1 dimension (Fig. 1).1 At this scale, the laws of gravity and inertia are replaced by charge–charge interactions and quantum mechanics; fluid flow loses its turbulence; photonic properties become size dependent; and particles become highly reactive because of their enormous surface-to-volume ratios.
FIG. 1.
Nanoscale comparison and types of nanotherapeutics used in drug delivery. Reprinted with permission from Maria Malamatari, MPhar, PhD, AFHEA.1
Nanobiotechnology can prove useful in retinal diagnostics and pharmacological and surgical therapeutics, as well as drug delivery (Tables 1 and 2). Retinal regenerative medicine also stands to benefit from nanoscience. The diminutive size of the retina, where therapeutics and surgery are in the range of micrograms and microns, respectively, combined with its relative immune privilege, and easy accessibility for both observation and treatment make it ideally suited for nanobiotechnological innovations.
Table 1.
Potential Retinal Nanodiagnostics
| Diagnostics | Nanoparticle | Model | Results |
|---|---|---|---|
| NP-enhanced optical coherence tomography | Au nanoparticles2 Fe-TiO2 nanoparticles3 |
Rabbit skin2 Chicken breast3 |
Skin layer border contrast2 210–780-μm penetration3 |
| NP-enhanced magnetic resonance imaging | Gd-PFCL emulsion linked with biotinylated antibody7 | Rabbit corneal neovascularization | Enhances magnetic resonance signal intensity in the corneal capillary bed |
| Atomic force microscopy | Rhodopsin molecule visualization8 | Murine rod outer-segment disc membranes in vitro | Rhodopsin molecule stability is a function of strength of interconnected segments |
| Photoacoustic microscopy | Oxygen metabolic studies9 Choroidal imaging10 |
Rat9 Rat and rabbit10 |
O2 saturation measurements9 Measurement of melanin, O2 saturation, and blood flow10 |
| Bionanosensors | Gold nano-interdigitated arrays12 | Ixodes ricinus im munosuppressor (anti-iris) protein | Detection of protein–antibody reaction at a concentration <1 ng/mL |
| Biologic assays | Q-dots and antibody-functionalized MNPs11 | Nanotechnology-on-a-chip | Multicolor optical coding; potential for identification of pathologic substances ex vivo and in vitro |
| Nanopore gene sequencing | cDNA-functionalized AuNPs11,13 | Nanotechnology-on-a-chip | Potential identification of inherited retinal diseases |
AuNPs, gold nanoparticles; GD-PFCL, gadolinium–perfluorocarbon liquid; MNPs, magnetic nanoparticles; NP, nanoparticle; Q-dots, quantum dots.
Table 2.
Current and Potential Retinal Nanotherapeutics and Theranostics
| Retinal application | Nanoparticle | Model | Conclusion |
|---|---|---|---|
| Precision cell ablation (ie, tumor cells) | AuNPs14 | Various cellular targets | Aggregate AuNPs enhance detection and treatment over single nanoparticles |
| Gene therapy | AuNPs15 | Various plasmid, minivector DNA, and siRNA vectors | AuNPs enhance nucleic acid delivery |
| AMD | Antiangiogenic peptides19 | Murine | Angiogenesis decreased for at least 14 weeks after a single dose |
| AMD | Cell-penetrating peptides20 | Rat | Single dose improves retinal permeability, increases antioxidant retention, and suppresses neovascularization for 56 days |
| Ocular tumors | Functionalized Q-dots16 | Human osteosarcoma cells | For single-cell microscopy, cells exhibit strong fluorescence and hypersensitivity; Q-dots are nontoxic and biologically inactive |
| Ocular tumors | MNP hyperthermia17,18 | Zebrafish embryos17 Humans18 |
Functionalized MNPs preferentially localized to the choroid and retinal pigment epithelium 17 Thermotherapy using MNPs was proven safe and effective18 |
| AMD | Topical nanoemulsions22–24 | Primate22 Human embryonic kidney cells and rat Müller cells23 Rats24 |
Drug in eyedrop form was suggested to penetrate both the cornea and blood–retinal barrier to reach the fundus22 Improved long-acting intraocular bioavailability of hydrophobic celecoxib23 Enhanced retinal accumulation of anti-VEGF apatinib24 |
| AMD | Dendrimers28 | Human donor eyes and rats |
Pathology-dependent biodistribution; suppression of choroidal neovascularization; and cytokine suppression |
| AMD | Self-assembling polymeric micelles25 | Various in vitro and in vivo models | Improved bioavailability, bioactivity, intracellular penetration, controlled delivery, and retention time |
| AMD and inherited retinal diseases | Viral | Human subjects | RGX-314, ADVM-022, and voretigene neparvovec-rzyl |
| AMD | Self-assembling polymers29 | Human subjects | Targeted delivery of siRNA |
| AMD | Nanoceria62 | Human ARPE-19 and umbilical endothelium cell lines | Anti-inflammatory, antiangiogenic, and antiapoptotic properties |
AMD, age-related macular degeneration; siRNA, small interfering RNA; VEGF, vascular endothelial growth factor.
Diagnostics
Current modalities of retinal imaging, including optical coherence tomography (OCT), ultrasonography, and angiography, can all benefit from nanotechnological innovation, with the potential to combine diagnostic modalities with therapeutic intervention (ie, theranostics).
Commercial OCT technologies have axial resolutions approaching <4 microns, with scan speeds approaching 80,000 scans/s, which allow for retinal angiographic imaging without the assistance of intravenous dyes. One of the challenges with this technology, however, is that although fine neovascular structures can be identified, the specificity can be limited by optical artifacts from adjacent vascular structures. Retinal angiography, moreover, can demonstrate chorioretinal vascular leakage, whereas OCT angiography, lacking contrast agents, is unable to demonstrate leakage directly.
One solution being investigated is the use of gold nanoparticles (AuNPs) as contrast agents for OCT, given the ability to create particles small enough to pass through even the smallest retinal or choroidal vessels (almost 3 microns in diameter) with wavelength absorption efficiencies higher than conventional angiographic dyes.2 Iron–titanium dioxide nanoparticles have also been investigated as contrast agents in swept-source OCT.3
The use of ultrasonography in the diagnosis of retinal pathology has been widespread for decades. Using frequencies of 6–20 MHz, ocular ultrasonography has a resolution that is an order of magnitude lower than OCT.4 The use of microbubbles of gas as an ultrasonic graphic contrast agent was first reported by Gramiak and Shah in the 1960s as an adjunct to echocardiography.5
One concern with this technique for ophthalmic use on a nanoscale is creating nanosized bubbles with size consistency and stability, as too large a gas bubble in a small capillary could result in sight-threatening vascular occlusion. In addition, longevity is an important factor as they would need to be sustained from the time of administration to imaging.
Perhaps long-acting gases such as perfluoropropane (C3F8) or sulfur hexafluoride (SF6), currently used for long-term postoperative retinal tamponade during retinal detachment surgery, may be able to fulfill this requirement.
MRI on its own has low sensitivity for microscopic retinal structures. To enhance corneal neovascular diagnostic sensitivity, Anderson et al. have developed a gadolinium–perfluorocarbon nanoparticle emulsion linked with a biotinylated antibody. Given that perfluorocarbon liquids are radiopaque, this combination would allow for targeted enhancement of neovascularization using MRI.6,7 Similar technology could be applied to the retinal and choroidal vasculature for enhanced imaging of neovascularization associated with age-related macular degeneration (AMD) or vascular neoplasms, such as choroidal melanomas.
Visualization of biomarkers on a molecular level requires nanoscale imaging techniques. Atomic force microscopy (AFM), with subnanometer resolution, is able to fulfill this role. AFM detects changes in the reflective angle of a laser beam focused upon a nanoscale cantilever as it traces the surface of subcellular structures, creating three-dimensional surface profiles not only of retinal cell and organelle membranes but also nanostructures such as rhodopsin molecules.8
Photoacoustic microscopy (PAM) allows for not only structural but also functional assessment of the retina and choroid on a molecular level by using high-resolution identification of endogenous chromophores such as hemoglobin and melanin. PAM detects the thermoelastic expansion of chromophores derived from rapid temperature changes generated by single-wavelength nanopulse laser absorption; an ultrasound transducer is used to detect the resulting photoacoustic waves. PAM can be integrated with OCT for enhanced chorioretinal localization of oxygen saturation measurements.9,10
Another facet of diagnostics involves identification of the hundreds of genetic variants that may contribute to inherited retinal diseases (IRDs). Treatments are actively being investigated for several of these IRDs such as Stargardt disease and retinitis pigmentosa. However, current point-of-service (POS) testing relies on saliva samples that are then sent to an off-site laboratory for analysis utilizing amplification techniques such as polymerase chain reaction and subsequent mass spectroscopy, which take days to perform. Next-generation sequencing (NGS) holds promise for more sensitive, efficient, and portable diagnostics, which can be utilized in an office setting with rapid results.
Nanoscale molecular diagnostics can revolutionize detection capabilities and sensitivities. Magnetic nanoparticles (MNPs) functionalized with antibodies, for example, can be used for localization of specific intracellular molecules, which could aid in identification of pathologic accumulation of materials [ie, lipofuscin in retinal pigment epithelium (RPE) cells].
When attached to segments of complementary DNA, AuNPs can identify genetic abnormalities in cell samples obtained from relatively noninvasive extraocular sources as well as multicolor coding in biologic assays using quantum dots (Q-dots)11—semiconducting molecules with diameters from 2 to 10 nm and the ability to fluoresce in pure long-lasting colors when excited with white or UV light, which in turn transfers electrons from low-energy valence bands to high-energy conductance bands. These electrons then drop back into their respective valence bands, releasing photonic energy at specific wavelengths while doing so.
Portability could be achieved with lab-on-a-chip-type technologies incorporating bionanosensors utilizing various nanoscale technologies. For instance, functionalized carbon nanotubules (CNTs) with diameters as small as 1 nm, as well as gold nano-interdigitated arrays with digit widths as narrow as 10 nm, can be incorporated into transistor-like circuitry for single-molecule detection by functionalizing the surface with antibodies to bind to target DNA or other biologic molecules, thereby changing surface electrical conductivity.12
Similarly, nanopore DNA sequencing takes advantage of changes in conductivity when DNA nucleotides are sequentially passed through nanopores that are ∼2 nm in diameter and embedded in a protein sheet. Combined with nanofluidics, nanopore sequencing can be used to perform rapid, real-time DNA sequencing.13 The goal of these NGS technologies would be to eliminate the need for time-consuming transport and amplification of biologic samples by increasing diagnostic sensitivity at the molecular level and decentralizing the analysis to POS locations.
Theranostics
Functionalization of AuNPs can increase specificity not only for diagnostics but also theranostics. AuNPs can easily be heated using radiofrequency waves, destroying cells into which the AuNPs are introduced with a precision critical for small treatment spaces with minimal tolerance for collateral cell damage.
AuNPs can also be used for delivery of DNA and RNA for gene therapies, given their ability to bind with synthetic and biological compounds.14,15 Using this nonviral vector for gene delivery could alleviate some of the challenges associated with current adeno-associated viral (AAV) vectors—notably inflammation and payload size limitation—maximizing therapeutic effects by allowing for increased potency.
The fluorescence characteristics of Q-dots can be customized by varying particle size and, when functionalized with therapeutic agents, they can target ocular tumors for both diagnostic imaging and treatment.16
Functionalized MNPs may also be useful for identifying ocular tumors using techniques such as OCT or CT scanning; targeted drug delivery to the choroid and RPE; and precision MNP hyperthermia using alternating magnetic fields to induce high-frequency oscillations of MNPs, causing frictional heating and subsequent tumor destruction.17,18
CNTs or fullerenes (ie, buckyballs, naturally occurring carbon-60 spheres) functionalized with both therapeutic agents [ie, AuNPs and nanoceria (a “nanoenzyme” derived from cerium oxide, which has proven to be both antiangiogenic and anti-inflammatory)] and biomarkers (ie, fluorescent nanosensors) could create powerful theranostic tools for simultaneous diagnosis and treatment of retinal pathologies.
Therapeutics: Drugs, Delivery Systems, and Targeting
Nanobiotechnology has already made great strides with regard to drug delivery in the eye. Given the minute dimensions involved, the ideal therapeutic will have small volume, high potency, and accurate localization—these are the advantages to be gained by nanoscale formulations.
One of the challenges with retinal drug delivery involves the ability of the drug to migrate from the preretinal to retinal or even subretinal spaces. Analogous to the blood–brain barrier, the blood–retina barrier (BRB) prevents blood from entering extravascular spaces. Unfortunately for drug delivery, these barriers are efficient in keeping out therapeutic substances deemed foreign. However, with the help of nanotechnology, these barriers can be successfully circumvented.
Intravitreal drug delivery through injection is currently a mainstay of retinal treatments for conditions such as diabetic retinopathy and exudative AMD. Vascular endothelial growth factor (VEGF) inhibitors are antibody sized (∼10 nm); these medications readily penetrate the retina to enter the subretinal space, where choroidal neovascularization originates in patients with exudative AMD.
Intravitreally delivered agents other than anti-VEGFs also show promise, such as antiangiogenic peptides; to prevent degradation before delivery, self-assembled nanoparticles are subsequently coated to form poly(lactic-co-glycolic acid) (PLGA) nanoparticles with slow-release properties.19
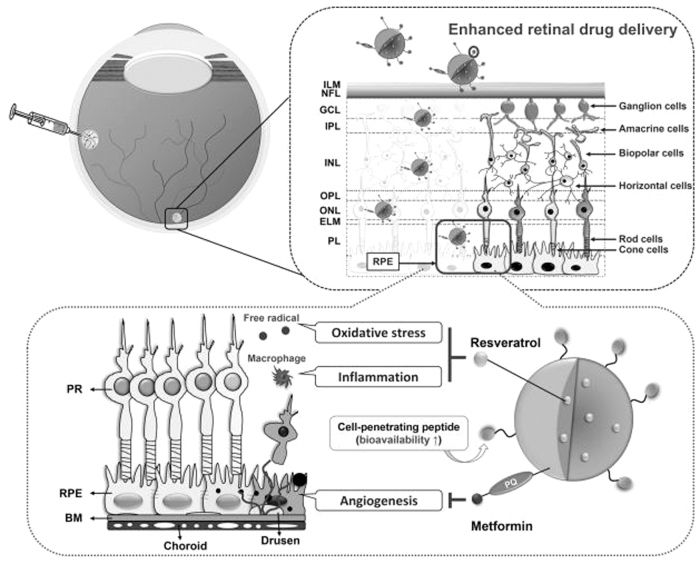
Another synthesized nanoparticle utilizes the biodegradable biopolymer, poly(E-caprolactone) (PCL), embedded with resveratrol, which demonstrates antioxidant and anti-inflammatory properties. The surface is coated with a combination of metformin, which is known to inhibit choroidal neovascularization, and cell-penetrating peptides, which increase retinal permeability by 15-fold after intravitreal injection (Fig. 2).20
FIG. 2.
Schematic representation of the pharmacological treatment of the neovascular AMD eye using PCL NPs as nanocarriers; a cell-penetrating peptide as a retinal penetration enhancer; and resveratrol and metformin as model drugs for synchronic attenuation of oxidative stress, inflammation, and angiogenesis in the diseased retina. Reprinted with permission from Nguyen et al.20 AMD, age-related macular degeneration; BM, Bruch's membrane; ELM, external limiting membrane; GCL, ganglion cell layer; ILM, inner limiting membrane; INL, inner nuclear layer; IPL, inner plexiform layer; NFL, nerve fiber layer; NPs, nanoparticles; ONL, outer nuclear layer; OPL, outer plexiform layer; PCL, poly(E-caprolactone); PL, photosensitive layer; PR, photoreceptors; RPE, retinal pigment epithelium.
Intravitreal administration of gene therapies is also being investigated using AAV vectors (∼20 nm in size) containing DNA, which codes for VEGF-inhibiting medication production (ie, REGENXBIO RGX-314; Adverum Biotechnologies ADVM-022). Given the aforementioned limitations of viral vectors, most notably inflammation, nonviral gene delivery would seem to be a favorable alternative.
Transcorneal and transconjunctival nanoemulsions are much less invasive and can enhance delivery and retention of poorly soluble drugs through these layers and into the eye through endocytosis, delivering high potency because of the high nanoparticle surface area-to-volume ratio.21 A nanoparticle formulation of aminocaproic acid-modified Dio is able to penetrate both the cornea and BRB, resulting in antiangiogenic activity with low cellular toxicity in a primate AMD model.22
Similarly, topical celecoxib-loaded poly(ortho ester) nanoparticles have been formulated, possessing both anti-inflammatory and antiproliferative properties.23 Researchers have also engineered serum albumin nanoparticles containing apatinib (an anti-VEGF receptor 2 tyrosine kinase inhibitor), which when coated with hyaluronic acid, are able to penetrate the cornea and target retinal cells.24
Other mechanisms of ocular delivery of retinal drugs could include polymeric nanoparticles because of their favorable biocompatibility and biodegradability as well as self-assembling polymeric micelles of small size (as small as 5 nm), water solubility, and controlled drug release.25,26
Dendrimers are highly branched molecules (as small as 1.5 nm in diameter) that can allow for codelivery of multiple drugs as well as effect sustained delivery. Dendrimer technology is already being used for glaucoma drug delivery.27
Liposomes (the water-insoluble “opposite” of micelles) and lipid nanoparticles (LNPs) are additional options. Although most of these strategies are being used for corneal/anterior segment disease, they could be utilized to provide noninvasive drug delivery to the vitreoretinal/subretinal structures.
Systemic delivery of retinal therapeutics is also possible. The challenge, however, is delivering the drug to target tissues in the eye while avoiding systemic degradation during transit; in addition to alteration of pharmaceutical properties, a proteinaceous biocorona can form around the active molecule, potentially altering both its pharmacologic and pharmacokinetic properties.
Systemic dendrimer-based molecules have demonstrated selective, passive RPE uptake of steroid in damaged cells, which can suppress both inflammation and choroidal neovascularization.28 Next-generation gene therapies, such as small interfering RNA, can be delivered through self-assembled polymers to which both targeting and therapeutic agents can be attached; such polymers may be suitable for systemic administration, having low toxicity and immunogenicity.29
MNPs have been used to accurately deliver induced pluripotent stem cells (iPSCs) to the trabecular meshwork in vivo; similar methods could potentially be used for iPSC-derived RPE delivery.30
Surgical Adjuncts
Despite improvements in microscope optics and resolution, and even with a surgeon's steady hand, a limiting factor in retinal surgery appears to be instrument size. Investigators are attempting to design nanoscale instruments for nanosurgical repairs. Nanotube tweezers have been designed by fusing 2 carbon nanotubes with a spacing of 10 nm.31 Others have proposed direct nerve fiber axon repairs using a knife edge with a 20-nm radius of curvature, with attachment of a transplanted axon segment performed by electrofusion.32 Current instruments can be coated with silver nanoparticles that confer both anti-infective and antioxidative properties.33
YAG laser vitreolysis, a relatively new technique that can serve as an alternative to pars plana vitrectomy for visually debilitating vitreous floaters, has been facilitated by the development of newer laser delivery systems; however, the relatively high-power settings pose potential complications with vitreous opacities close to the retina or lens. Hyaluronic acid-coated AuNPs have been found to cluster on vitreous opacities in vivo.
Low-energy, nanosecond laser pulses can subsequently be applied to create vitreous nanobubbles by ultrafast heating of AuNPs that in turn ablate these vitreous opacities with significantly reduced energy levels when compared with conventional YAG vitreolysis.34
Nanoprostheses
Retinal implants continue to evolve, from the Argus II (Second Sight Medical Products) and IRIS II (Pixium Vision), with epiretinal microelectrode arrays of up to 60 electrodes and external hardware, to the compact and self-contained Alpha MS (Retina Implant AG), which utilizes a subretinal photodiode array of 1,500 electrodes to detect light and deliver a charge to the inner retina.35,36 Even as implants become more compact, the best visual resolution is still in the 20/400 range, a limitation of sensor resolution; the technology is also quite expensive.
With the smallest nanoscale transistors now only 1 nm in length and diodes as small as 1 molecule in size, we would expect resolution to increase in the future, with a corresponding decrease in the intrusiveness and cost of the implant. Another promising technology involves poly(3-hexylthiophene) (P3HT) nanoparticles injected into the eye. These nanoparticles are injected in liquid form into the subretinal space, mimicking the spatial distribution of photoreceptors and forming a light-sensitive interface.37
CNT nanoprosthetics are being integrated with neural tissue to guide synaptic development during neuronal repair.38 Such technology could theoretically help patients with neuro-ophthalmologic trauma or degeneration.
Regenerative Nanobiotechnology
Diseases such as macular degeneration and diabetic retinopathy result in premature aging, trauma, and inflammation of retinal tissues, with cell loss from oxidative stress and subsequent apoptosis. Future therapies should therefore include the ability to not only protect retinal tissues from these ravages but also to repair and regenerate structure and function. Metallic and metal oxide nanoparticles are uniquely equipped to help with inflammation and oxidative stress.
AuNPs are not only relatively inert but also possess intrinsic anti-inflammatory and antiangiogenic properties.39 Nanoceria is another powerful example of a reactive oxygen species scavenger. In addition, nanoceria can help to both promote and regulate healthy angiogenesis vital for regenerating tissues while demonstrating anti-VEGF properties consistent with its role in angiogenic modulation without adverse effects on vision.40
Dexamethasone-conjugated dendrimers (D-Dex) demonstrate selective affinity for damaged retinal Müller glial cells, promoting regenerative stem cell-like properties in mammals. The selectivity of D-Dex, furthermore, minimizes the potential for systemic toxicity.41
CNTs, described previously, are not only useful in diagnostics but can also inherently function as free radical scavengers, potentially reducing oxidative stress thought to play a vital role in progression to wet AMD. Similarly, fullerenes are inherently antioxidative and anti-inflammatory.42
In addition to previously mentioned theranostics, MNPs are being used for targeted stem cell delivery within the eye. Yanai et al. describe a technique of magnetizing rat mesenchymal stem cells using superparamagnetic iron oxide nanoparticles and, after intravenous injection, inducing migration and localization to the inner and outer retina with a magnet placed in the orbit.43
Developing viable scaffolding is essential to retinal regeneration as both primary stem cells and iPSCs rely upon these scaffolds for proper orientation. With proper growth and differentiation/dedifferentiation factors, as well as niche formation allowing for the proper juxtaposition of cellular and extracellular matrix components, retinal regeneration has a higher probability of success. Nanoscaffolds are being developed to aide in retinal tissue regeneration.
Repopulation with normal retinal cells becomes challenging because of the multilayered nature of the retina as well as the intricate interconnectivity between different cells. Natural nanofiber scaffolds are being created from materials such as gelatin, chitosan, collagen, and hyaluronic acid. These have been shown to promote healthy RPE growth and release of regenerative factors, with superior cellular adhesion to that of synthetic scaffolds created from biodegradable polymers such as PLGA, although the latter have better strength and therefore longer half-lives.44
Electrospinning is a technique whereby nanofibers are created by extrusion of a polymer solution that is then carried to a distant electrically charged plate (analogous to a cotton candy machine, except using electrostatic rather than centrifugal forces). This technique has recently been used to create a nanofiber scaffold upon which RPE cells can be cultured with the possibility of subsequent subretinal transplantation.45
Future Directions
The future is exceedingly bright for nanobiotechnological applications in ophthalmology. With technologies such as clustered regularly interspaced short palindromic repeats (CRISPR) combined with the enzyme, CRISPR-associated protein 9 (CRISPR-Cas9), gene editing has the ability to address multiple genes simultaneously, which could prove beneficial for multifactorial diseases such as AMD, for which over a dozen single-nucleotide polymorphisms have been found to be associated with risk of disease progression.
AAV vectors are currently the most common means for retinal intracellular delivery of genetic material; however, associated risks include immunogenic responses, as mentioned previously, and off-target genomic editing contributed by the sustained presence of Cas9; pre-existing immunity to this persistent Cas9 can also negate CRISPR gene editing.46,47
These issues can potentially be avoided by using nonviral delivery methods; however, transfection efficiency is limited by the lack of effective delivery systems, and successful delivery of CRISPR-Cas9 to the outer retinal cells in vivo continues to elude researchers.48 Current methods that are under investigation and could potentially be utilized for CRISPR-Cas9 delivery to the retina in vivo include nonchemical modalities such as sonoporation, where cell membrane permeability is increased by creating pores using ultrasound irradiation to create microbubbles.
However mechanical and physical disruption of the cell membranes, however transient, risks long-term trauma to the cells with subsequent adverse effects on vision. There are also challenges in isolating target retinal cells given the microscopic dimensions as well as intricate juxtaposition of various retinal cellular components.49,50
Chemical modalities include AuNPs, LNPs, and polymeric nanoparticles, whose positive charge readily interacts with negatively charged DNA.48,51 Carefully designed inorganic nanoparticles can overcome the challenges of crossing the BRB, inefficacy from rapid degradation, and both gene and cellular toxicity from overpersistence. Such nanoparticles include polymers, silicon, and organometallic composites.51
iPSC-derived RPE cells can be derived from fibroblasts or mononuclear blood cells by incubation with various protein-coding genes such as LEFTY2.52 Müller glial cells, furthermore, have been shown to transdifferentiate to rod photoreceptors using the sonic hedgehog gene.53 Targeted delivery of messenger RNA (mRNA) to the RPE, Müller glia, and neural retina has been accomplished using LNPs, which can overcome limitations of AAV vectors.46,54
Delivery has, however, been limited to direct subretinal injection, which is highly invasive, or intravitreal injections that are nonlocalizing. Laser-enhanced delivery of genetic material is a novel technique utilizing either direct or indirect optoporation in precise areas of retinal degeneration after intravitreal injection.55,56 Similar methodology could conceivably be used to deliver gene-laden LNPs or other nonviral nanoparticles.
Exosomes are bilayered nanovesicles that range upward from 30 nm in size, originating from invagination of plasma membranes. They show promise as disease biomarkers, intercellular communication vehicles, and drug delivery vehicles.57 MicroRNA (miRNA) plays an important role in both cellular maintenance and inflammatory modulation. Exosomes have been shown to transport miRNA between RPE cells and retinal glial cells.
This finding could prove useful in modulating senescence and apoptosis of the neuroretina in conditions such as AMD, where RPE cells show an increase in exosomal production with oxidative stress, and diabetic retinopathy, where a similar increase in exosomal production occurs in retinal glial cells.58
Researchers have created helicoid, magnetic nanoscale micropropellers that are guided by external magnetic fields to traverse the vitreous cavity, with the potential to deliver therapeutics to the retina with precision (Fig. 3).59 With a diameter of 500 nm, these nanorobots are relatively large; however, given the ability to fabricate structures such as functionalized DNA nanostructures with MNPs, rather than with dyes and other nanomaterials currently being used for ultrasensitive molecular imaging, it is conceivable that such nanorobots could be engineered to obtain diameters approaching 2 nm.60
FIG. 3.
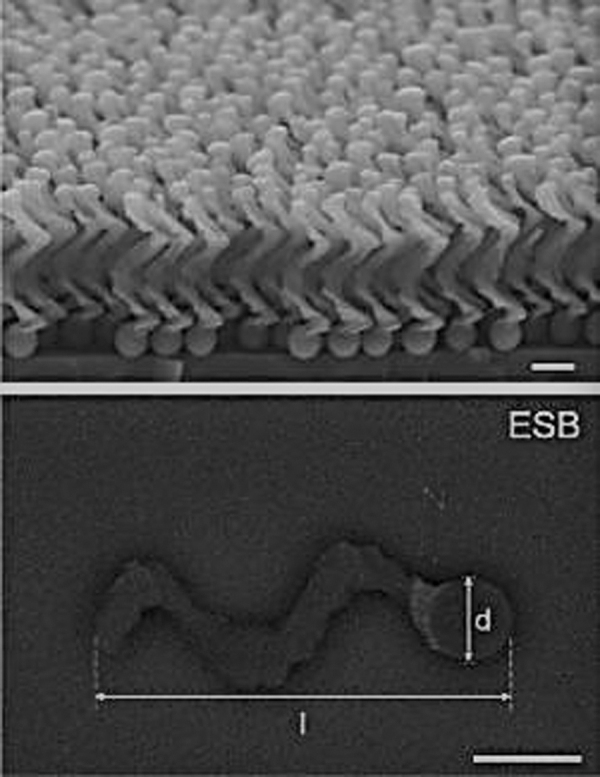
SEM (top) and ESB-SEM (bottom) images of micropropellers. Scale bar = 500 nm. Reprinted with permission from Wu et al.59 ESB-SEM, energy selective backscatter-scanning electron microscope.
Recent innovations in passive, nonviral, targeted retinal drug delivery (thereby minimizing both invasiveness and inflammation) include the development of a polyacrylamide nanoparticle (ANP) platform integrated with neuroprotective neurotrophin nerve growth factor and utilization of the targeting agent, peanut agglutinin, which has an affinity for retinal rods and cones.
This polymer-based nanoparticle has been shown to prevent retinal cell apoptosis characteristic of retinal degenerative diseases such as AMD.61 Similarly, oligochitosan-coated nanoceria demonstrates antiangiogenic, anti-inflammatory, and antiapoptotic characteristics in AMD cellular models.62
LNPs functionalized with targeting peptides conjugated with mRNA are now able to bypass retinal barriers that have, to date, limited LNP penetration into RPE and Müller glial cells, allowing the functionalized LNPs to reach photoreceptors, a critical target in designing gene therapies for IRDs.46
Reengineering the photoreceptors themselves has also been a topic of recent research. Sp et al. have succeeded in nanoparticle-mediated delivery of a full-length, human rhodopsin gene (gRHO) to murine rod photoreceptors, resulting in decreased degeneration; this could be useful in future treatment of retinitis pigmentosa.63 Kwon et al. have synthesized melanin-like nanoparticles with powerful antioxidant properties, which could be utilized as an artificial melanin substitute in murine RPE cells, in turn stemming deterioration seen in AMD.64
We have only begun to scratch the surface of what is possible with therapeutic and regenerative applications of nanotechnology in the field of retina.
Finally, the ability to engineer nanostructures at an atomic level using devices such as the scanning tunneling microscope (which utilizes the tunneling phenomenon of quantum mechanics to not only image subnanometer structures but also move individual atoms65) will open the door to an entirely new class of nanotherapeutics, where nanoscale circuits as well as pumps, such as those existing across cell membranes where they are utilized for transmembrane transport, could be fabricated and utilized in diagnostic, maintenance, and restorative functions; indeed, the best is yet to come.
Acknowledgments
The authors would like to thank Rajat N. Agrawal, MD, MS; Uday B. Kompella, PhD, FARVO, FAAPS; and Rachel R. Hartman, PRA; for their recommendations and support during the writing of this review article.
Authors' Contributions
N.J.M. contributed to conceptualization (lead); methodology (lead); resources (lead); writing—original draft (lead); and writing—review and editing (lead). S.N.M. contributed to writing—review and editing. All authors had full control of the content and made the final decision on all aspects of this publication.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
No funding was received for this article.
References
- 1. Malamatari M, Charisi A, Malamataris S, et al. Spray drying for the preparation of nanoparticle-based drug formulations as dry powders for inhalation. Processes 2020;8(7):788; doi: 10.3390/pr8070788 [DOI] [Google Scholar]
- 2. Zagaynova E, Shirmanova M, Kirillin Y, et al. Contrasting properties of gold nanoparticles for optical coherence tomography: Phantom in vivo studies and Monte Carlo simulation. Phys Med Biol 2008;53:4995–5009. [DOI] [PubMed] [Google Scholar]
- 3. Barkhade T, Indolaya A, Poddar R, et al. Iron content titanium dioxide nanoparticles as exogenous contrast agent for tissue imaging using swept-source optical coherence tomography. AIP Adv 2021;11:015023; doi: 10.1063/5.0031385 [DOI] [Google Scholar]
- 4. Silverman R. High-resolution ultrasound imaging of the eye—A review. Clin Exper Ophthalmol 2009;37(1):54–67; doi: 10.1111/j.1442-9071.2008.01892.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol 1968;3:356–366. [DOI] [PubMed] [Google Scholar]
- 6. Milsap CM, Peyman G, Mehta N. Perfluoroperhydrophenanthrene (Vitreon®) is radiopaque. Ophthalmic Surg 1993;24(7):500. [PubMed] [Google Scholar]
- 7. Anderson S, Rader R, Westlin W, et al. Magnetic resonance contrast enhancement of neovasculature with avB3-targeted nanoparticles. Magn Reson Med 2000;44:433–439. [DOI] [PubMed] [Google Scholar]
- 8. Bosshart PD, Engel A, Fotiadis D. High-resolution atomic force microscopy imaging of rhodopsin in rod outer segment disk membranes. Methods Mol Biol 2015;1271:189–203; doi: 10.1007/978-1-4939-2330-4_13 [DOI] [PubMed] [Google Scholar]
- 9. Song W, Wei Q, Liu W, et al. A combined method to quantify the retinal metabolic rate of oxygen using photoacoustic ophthalmoscopy and optical coherence tomography. Sci Rep 2014;4(6525):1–7; doi: 10.1038/srep06525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen VP, Paulus YM. Photoacoustic microscopy of the retina and choroid: Imaging by hearing the retina. Retina Phys 2020;17:42–44, 46, 47. [Google Scholar]
- 11. Jain K. Nanodiagnostics: Application of nanotechnology in molecular diagnostics. Expert Rev Mol Diagn 2003;3(2):153–161; doi: 10.1586/14737159.3.2.153 [DOI] [PubMed] [Google Scholar]
- 12. Tang X, Jonas A, Nysten B, et al. Direct protein detection with a nano-interdigitated array gate MOSFET. Biosens Bioelectron 2009;24(12):3531–3537. [DOI] [PubMed] [Google Scholar]
- 13. Booker R, Boysen E.. Nanotechnology for Dummies. Wiley-VCH Verlag GmbH & Co. KGaA: Germany; 2005; pp. 247. [Google Scholar]
- 14. Roca M, Haes A.. Probing cells with noble metal nanoparticle aggregates. Nanomedicine (Lond) 2008;3:555–565. [DOI] [PubMed] [Google Scholar]
- 15. Ding Y, Jiang Z, Saha K, et al. Gold nanoparticles for nucleic acid delivery. Mol Ther 2014;22(6):1075–1083; doi: 10.1038/mt.2014.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arya H, Kaul Z, Wadhwa R, et al. Quantum dots in bio-imaging: Revolution by the small. Biochem Biophys Res Commun 2005;329:1173–1177. [DOI] [PubMed] [Google Scholar]
- 17. Giannaccini, M, Pedicini, L, De Matienzo G, et al. Magnetic nanoparticles: A strategy to target the choroidal layer in the posterior segment of the eye. Sci Rep 2017;7:43092; doi: 10.1038/srep43092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and thermotherapy safety of intratumoral thermotherapy using magnetic iron oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multifore. J Neurooncol 2010;103(2):317–324; doi: 10.1007/s11060-010-0389-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shmueli R, Ohnaka M, Miki A, et al. Long-term suppression of ocular neovascularization by intraocular injection of biodegradable polymeric particles containing a serpin-derived peptide. Biomaterials 2013;34:7544–7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen D, Luo LJ, Yang CJ, et al. Highly retina-permeating and long-acting resveratrol/metformin nanotherapeutics for enhanced treatment of macular degeneration. ACS Nano 2023;17:168–183. [DOI] [PubMed] [Google Scholar]
- 21. J Zhang, Zhou T, Wang L, et al. Nanoemulsion as a vehicle to enhance the ocular absorption after topically applied cyclosporine a in the rabbit eye. Invest Ophthalmol Vis Sci 2012;53(14):488. [Google Scholar]
- 22. Xin G, Zhang M, Zhong Z, et al. Ophthalmic drops with nanoparticles derived from a natural product for treating age-related macular degeneration. ACS Appl Mater Interfaces 2020;12:57710–57720. [DOI] [PubMed] [Google Scholar]
- 23. Palamoor M, Jablonski M.. Synthesis, characterization and in vitro studies of celecoxib-loaded poly (orth ester) nanoparticles targeted for intraocular drug delivery. Colloids Surf B Biointerfaces 2013;112:474–482. [DOI] [PubMed] [Google Scholar]
- 24. Radwan S, El-Kamel A, Zaki E, et al. Hyaluronic-coated albumin nanoparticles for the non-invasive delivery of apatinib in diabetic retinopathy. Int J Nanomedicine 2021;16:4481–4494; doi: 10.2147/IJN.S316564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric naoparticles based drug delivery systems. Clloids Surf B Biointerfaces 2010;75(1):1–18. [DOI] [PubMed] [Google Scholar]
- 26. Di Tomaso C, Bourges JL, Valamanesh F, et al. Novel micelle carriers for cyclosporin A topical ocular delivery: In vivo cornea penetration, ocular distribution and efficacy studies. Eur J Pharm Biopharm 2012;81(2):257–264. [DOI] [PubMed] [Google Scholar]
- 27. Holden C, Tyagi P, Thakur A, et al. Polyamidoamine dendrimer hydrogel for enhanced delivery of anti-glaucoma drugs. Nanomedicine 2012;8(5):776–783. [DOI] [PubMed] [Google Scholar]
- 28. Kambhampati S, Bhutto I, Wu T, et al. Sysyemic dendrimer nanotherapies for targeted suppression of choroidal inflammation and neovascularization in age-related macular degeneration. J Control Release 2021;10;335:527–540; doi: 10.1016/j.jconrel.2021.05.035 [DOI] [PubMed] [Google Scholar]
- 29. Davis M. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: From concept to clinic. Mol Pharma 2009;6(3):659–668. [DOI] [PubMed] [Google Scholar]
- 30. Wang X, Cao Q, Wu S, et al. Magnetic nano-platform enhanced iPSC-derived trabecular meshwork delivery and tracking efficiency. Int J Nanomedicine 2022;22;17:1285–1307; doi: 10.2147/IJN.S346141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim P, Lieber CM. Nanotube tweezers. Science 1999;286(5447):2148–2150. [DOI] [PubMed] [Google Scholar]
- 32. Sretavan D, Chang W, Hawkes E, et al. Microscale surgery on single axons. Neurosurgery 2005;57(4):635–646. [PubMed] [Google Scholar]
- 33. Burgess R. Understanding Nanomedicine. Pan Stanford Publishing Pte. Ltd.: Singapore; 2012; pp. 1–44. [Google Scholar]
- 34. Sauvage F, Nguyen V, Li Y, et al. Laser-induced nanobubbles safely ablate vitreous opacities in vivo. Nat Nanotechnol 2022;17:552–559; doi: 10.1038/s41565-022-01086-4 [DOI] [PubMed] [Google Scholar]
- 35. Bajaj R, Dagnelie G, Handa J, et al. Retinal Implants for RP: An Update on Argus II and Others. EyeNet Magazine 2019. Available from: https://www.aao.org/eyenet/article/retinal-implants-for-rp [Last accessed: March 30, 2023].
- 36. Makawi A, Firi K, Tang P, et al. Retina prosthesis. EyeWiki 2022. Available from: https://eyewiki.aao.org/Retina_Prosthesis [Last accessed: April 22, 2023].
- 37. Maya-Vetencourt JF, Manfredi G, Mete M, et al. Subretinally injected semiconducting polymer nanoparticles rescue vision in a rat model of retinal dystrophy. Nat Nanotechnol 2020;15:698–708; doi: 10.1038/s41565-020-0696-3 [DOI] [PubMed] [Google Scholar]
- 38. Pampaloni N, Scaini D, Perissinotto F, et al. Sculpting neurotransmission during synaptic development by 2D nanostructured interfaces. Nanomedicine 2018;14(7):2521–2532. [DOI] [PubMed] [Google Scholar]
- 39. Mukherjee P, Bhattacharya R, Wang P, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res 205;11(9):3530–3534. [DOI] [PubMed] [Google Scholar]
- 40. Das S, Chigurupati S, Dowding J, et al. Therapeutic potential of nanoceria in regenerative medicine. MRS Bull 2014;39:976–983. [Google Scholar]
- 41. Emmerich K, White DT, Kambhampati SV, et al. Nanoparticle-based targeting of microglia improves the neural regeneration enhancing effects of immunosuppression in the zebrafish retina. Commun Biol 2023;6(534):1–14; doi: 10.1038/s42003-023-04898-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dellinger A, Cunun P, Lee D, et al. Inhibition of inflammatory arthritis using fullerene nanomaterials. PLoS One 2015;10(4):e0126290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yanai A, Häfeli U, Metcalfe A, et al. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant 2012;21(6):1137–1148. [DOI] [PubMed] [Google Scholar]
- 44. Sahle F, Kim S, Niloy K, et al. Nanotechnology in regenerative ophthalmology. Adv Drug Deliv Rev 2019;148:290–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Egbowon BF, Fornari E, Pally JM, et al. Retinal pigment epithelial cells can be cultured on fluocinolone acetonide treated nanofibrous scaffold. Mater Design 2023;232:1121522; doi: 10.1016/j.matdes.2023.11215 [DOI] [Google Scholar]
- 46. Herrera-Barrera M, Ryals R, Gautam M, et al. Peptide-guided lipid nanoparticles deliver mRNA to the neural retina of rodents and nonhuman primates. Sci Adv 2023;9(2):eadd4623; doi: 10.1126/sciadv.add4623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li A, Tanner M, Lee C, et al. AAV-CRISPR gene editing is negated by pre-existing immunity to Cas9. Mol Ther 2020;28(6):1432–1441; doi: 10.1016/j.ymthe.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kantor A, McClements M, Peddle C, et al. CRISPR genome engineering for retinal diseases. Prog Mol Biol Transl Sci 2021;182:29–79; doi: 10.1016/bs.pmbts.2021.01.024 [DOI] [PubMed] [Google Scholar]
- 49. Rinaldi L, Folliero V, Palomba L, et al. Sonoporation by microbubbes as gene therapy approach against liver cancer. Oncotarget 2018;9(63):32182–32190; doi: 10.18632/oncotarget.25875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khokhlova T, Wang YN, Simon J, et al. Ultrasound-guided tissue fractionation by high intensity focused ultrasound in an in vivoporcine liver model. Proc Natl Acad Sci U S A 2014;111:8161–8166; doi: 10.1073/pnas.1318355111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Salman A, Kantor A, McClements M, et al. Non-viral delivery of CRISPR/Cas cargo to the retina using nanoparticles: Current possibilities, challenges, and limitations. Pharmaceutics 2022;14(9):184; doi: 10.3390/pharmaceutics14091842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bharti K, Miller S, Arnheiter H, et al. The new paradigm: Retinal pigment epithelium cells generated from embryonic or induced plutipotent stem cells. Pigment Cell Melanoma Res 2011;24(1):21–34; doi: 10.111/j.1755-148X.2010.00772.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gu D, Wang S, Zhang S, et al. Direct transdifferentiation of Müller glial cells to photoreceptors using sonic hedgehog signaling pathway agonist purmorphamine. Mol Med Rep 2017;16(6):7993–8002; doi: 10.3892/mmr.2017.7652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Patel S, Ryals R, Weler K, et al. Lipid nanoparticles for delivery of messenger RNA to the back of the eye. J Control Release 2019;303:91–100; doi: 10.1016/jconrel.2019.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Batabyal S, Gajjeraman S, Bhattacharya S, et al. Nano-enhanced optical gene delivery to retinal degenerated mice. Curr Gene Ther 2019;19(5):318–329; doi: 10.2174/1566523219666191017114044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Batabyal S, Kim S, Wright W, et al. Laser-assisted targeted gene delivery to degenerated retina improves retinal function. J Biophotonics 2021;14(1):e202000234; doi: 10.1002/jbio.202000234 [DOI] [PubMed] [Google Scholar]
- 57. Zhang Z, Mugisha A, Fransisca S, et al. Emerging role of exosomes in retinal diseases. Front Cell Dev Biol 2021;9:64360; doi: 10.3389/fcell.2021.643680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morris D, Bounds S, Liu H, et al. Exosomal miRNA transfer between retinal microglia and RPE. Int J Mol Sci 2020;21(10):3541; doi: 10.3390/ijms21103541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wu Z, Troll J, Jeong HH, et al. A swarm of slippery micropropellers penetrates the vitreous body of the eye. Sci Adv 2018;4(11):eaat4388; doi: 10.1126/sciadv.aat4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shaikh S, Younis M, Yuan L. Functionalized DNA nanostructures for bioimaging. Cordination Chem Rev 2022;469:214648; doi: 10.1016/j.ccr2022.21468 [DOI] [Google Scholar]
- 61. Colucci P, Giannaccini M, Baggiani M, et al. Neuroprotective nanoparticles targeting the retina: A polymeric platform for ocular drug delivery applications. Pharmaceutics 2023;15(4):1096; doi: 10.3390/pharmaceutics15041096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang K, Mitra RN, Zheng M, et al. Nanoceria-loaded injectable hydrogels for potential age-related macular degeneration treatment. J Biomed Mater Res A 2018. ;106(11):2795–2804; doi: 10.1002/jbm.a.36450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sp S, Mitra RN, Zheng M, et al. Gene augmentation for autosomal dominant retinitis pigmentosa using rhodopsin genomic loci nanoparticles in the P23H+/− knock-in murine model. Gene Ther 2023;30(7–8):628–640; doi: 10.1038/s41434-023-00394-1 [DOI] [PubMed] [Google Scholar]
- 64. Kwon YS, Zheng M, Zhang AY, et al. Melanin-like nanoparticles as an alternative to natural melanin in retinal pigment epithelium cells and their therapeutic effects against age-related macular degeneration. ACS Nano 2022;16(11):19412–19422. [DOI] [PubMed] [Google Scholar]
- 65. Bai C. Scanning Tunneling Microscopy and its Applications. Springer Verlag: New York; 2000. [Google Scholar]