Abstract
Depression and cognitive impairment are prevalent conditions among people with HIV (PWH), likely attributable to shared causes and common risk factors. Identifying subtypes of PWH with similar patterns of neurocognitive impairment (NCI) and depressive symptoms may inform development of patient-centered interventions that target-specific profiles. This study aimed to (1) classify PWH based on patterns of domain-specific NCI and depression; and (2) determine the relationship between latent class membership and pertinent clinical characteristics. PWH (N = 580, 86.2% male, 57.1% non-Hispanic White, 69.2% unemployed) completed a comprehensive neuropsychological test battery assessing global and domain-specific cognition. Domain-specific NCI was classified as deficit score >0.5. Participants completed the Beck Depression Inventory-II (BDI-II), and domain-specific BDI-II scores reflecting cognitive, affective, and somatic symptoms were computed. Latent profile analysis (LPA) was used to determine latent subgroups of NCI and depression. The optimal LPA solution consisted of five classes: minimal NCI and minimal depression (Class 1), amnestic and minimal depression (Class 2), severe multi-domain NCI and moderate depression (somatic and affective; Class 3), mild NCI and mild depression (Class 4), and moderate multi-domain NCI and severe depression (Class 5). Despite similar levels of functional impairment, Class 5 had a significant psychiatric profile, whereas Class 3 had a complex medical profile (i.e., higher frailty index, higher medications, greater proportion of AIDS diagnosis). In contrast, Class 1 had the lowest medication use and frailty index, with similar HIV disease characteristics to Classes 3 and 5. Our results suggest there are multiple pathways to cognitive and functional impairment among PWH with co-occurring depression and cognitive impairment, and these groups may respond differently to interventions. Of note, our sample was majority non-Hispanic White and male, which is nonrepresentative of the US population of PWH. Future interventions should consider a more integrated, person-centered approach that addresses cognitive and emotional health to optimize health outcomes in PWH.
Keywords: cognition, depression, AIDS, mental health, neuropsychology, latent class analysis
Introduction
Following the introduction of antiretroviral therapy (ART), HIV is now considered a chronic medical condition when treated with ART, and people with HIV (PWH) are living longer.1,2 With prolonged life span, PWH are at an increased risk for age-related comorbidities [e.g., neurocognitive impairment (NCI), frailty] compared to the general population, despite virologic suppression, which have been associated with poor HIV disease outcomes, including medication adherence.3–5 It is hypothesized that HIV is associated with accelerated aging phenotypes; therefore, these complex comorbidities may be accruing early in life and PWH will live with greater multi-morbidity throughout the life span.2,6 While many different combinations of comorbidities are possible, evidence suggests that certain comorbidities are more likely to co-occur within an individual due to shared causes or common risk factors.7,8
Depression and NCI are a prevalent pair of comorbidities among PWH, likely attributable to several common factors, including (1) common neurobiological pathways (e.g., dopaminergic changes, neuroinflammation); (2) common psychosocial determinants (e.g., poverty, stress, discrimination, childhood trauma); and (3) common behavioral symptoms (e.g., substance use, ART nonadherence, sleep disturbance, poor nutrition).9,10 Depression remains a common and serious comorbidity in PWH.10,11 The prevalence of depression among PWH is twofold to fourfold higher compared to the general population.10–13 Studies estimating the national prevalence of depression in PWH indicate that approximately one-quarter of PWH have symptoms of current depression10,12,14–18 and 42% have a diagnosis of lifetime depression,9 with higher rates among men who have sex with men living with HIV19 and women with HIV.18
Several factors may contribute to the elevated burden of depression among PWH, including socioenvironmental (e.g., limited access to care, social isolation), neurobiological (e.g., impaired neurogenesis, dopamine dysregulation), positive and negative psychological factors (e.g., resilience, stigma, grief, stress), and psychosocial factors (e.g., financial instability, underemployment, substance use).10,12,13,20,21 Behavioral risk factors such as limited physical activity, poor nutrition, and substance use are also highly prevalent and confer an increased risk of poor health outcomes and ART nonadherence.12,22–25 With regard to HIV clinical outcomes, PWH who report high levels of depressive symptoms are less likely to engage in clinical care, initiate and adhere to ART treatment, and achieve viral suppression.14,26–30
In a large, multi-site clinical cohort study, greater chronicity of depression increased the likelihood of negative health outcomes (i.e., missed HIV primary care appointments, detectable viral load, higher mortality rates) at multiple points on the HIV care continuum.28 An observational study examined four depression trajectory groups over 6 years and found that patients in the “low-chronic” depression group had higher odds of low CD4 count over time compared to the “high-chronic” group.27 These studies highlight that the longitudinal pattern of depression, rather than depression at any single time point, may be more highly associated with poor HIV clinical outcomes.
NCI is another major complication of HIV and is associated with depressive symptoms, decreased daily functioning, and poorer quality of life.3,31–33 A recent literature review shows that PWH with depressive symptoms or major depressive disorder (MDD) have a 1.5-fold to 3-fold increased odds of cognitive impairment compared to nondepressed PWH.34,35 Moreover, PWH with MDD report more subjective cognitive complaints and exhibit worse neuropsychological performance compared to PWH without MDD.3,36 Across recent cross-sectional studies examining specific cognitive phenotypes in PWH with depression, processing speed, executive function, learning and memory, and motor function are the most common domains associated with depressive symptoms.9,36
Higher rates and severity of depression have been linked to severity of cognitive impairment, including HIV-associated dementia and major neurocognitive disorder, even among virally suppressed PWH on ART.9 A recent longitudinal study of 448 PWH showed that a high cumulative burden of depression related to declining global neurocognition compared to low depression burden, driven by declines in executive functioning, delayed recall, and verbal fluency.37 Considering an individual may experience recurrent depressive episodes in the long-term course of HIV infection, one study examined whether the chronicity, recurrence, and treatment stability of depression impacted neurocognitive functioning.38 Among their sample of middle-aged (<45 years old) and virally suppressed PWH, lack of full depression remission, recurrence of depressive episodes, instability on treatment in chronic MDD, and severe symptoms of current MDD increased the likelihood of poor neurocognitive performance. HIV-related stigma has been linked to both depression and poor neurocognitive functioning and may be a potential intervention target to promote healthy brain aging in PWH.39 These studies suggest that the severity and chronicity of depression, as well as internalized stigma, may be key moderators in the relationship between depression and neurocognition in PWH because they relate to lifetime neuropsychiatric burden.
Considering the prevalence of depression and NCI among aging PWH, a detailed latent profile analysis (LPA) of subgroups of PWH with similar patterns of NCI and symptoms of depression is warranted. Further understanding risk and protective factors (e.g., demographic and clinical factors) of the combined presentation of depression and NCI in PWH may inform the development of appropriate interventions and clinical practice guidelines for the management of these two comorbid conditions in HIV. Therefore, the aims of this study are to (1) perform an LPA to determine latent class membership based on NCI status and symptoms of depression and (2) determine the relationship between latent class membership and relevant demographic and clinical factors.
Methods
Participants
Participants were 580 PWH enrolled in NIH-funded research studies at the UCSD HIV Neurobehavioral Research Program. Participants provided written, informed consent to undergo study procedures, which were approved by the UCSD Institutional Review Board. Participants were included in this analysis if they were on ART with an HIV RNA viral load below 200 copies/mL and had complete neuropsychological testing and Beck Depression Inventory-II (BDI-II) data. Consistent with Frascati research criteria for HIV-associated neurocognitive disorders (HAND),40 participants were excluded from analysis if they presented with severe comorbidity factors that confounded the interpretation of neuropsychological test results.4,41 These exclusionary factors included diagnosis of a psychotic or mood disorder with psychotic features, severe learning disability, major neurological condition (e.g., epilepsy), current substance use disorder (SUD) diagnosis, or positive urine toxicology screen for nonprescribed substance use (except marijuana), or Breathalyzer test for alcohol on the day of testing.
Neuropsychological assessment
All participants completed a comprehensive and validated neuropsychological test battery (Table 1) covering seven neurocognitive domains impacted by HIV: verbal fluency, executive function, processing speed, learning, delayed recall, working memory, and motor skills.4,42,43 Raw test scores were converted to T-scores that adjusted for known demographic influences (i.e., age, sex, education, and race/ethnicity) on neurocognitive performance.44–46 T-scores were converted to deficit scores that give differential weight to impaired over normal performance and ranged as follows: 0 (T-scores ≥40; no impairment), 1 (T-score: 39–35; mild impairment), 2 (T-score: 34–30; mild-to-moderate impairment), 3 (T-score: 29–25; moderate impairment), 4 (T-score: 24–20; moderate-to-severe impairment), and 5 (T < 19; severe impairment).47
Table 1.
Neuropsychological Battery
| Verbal fluency | |
| Controlled oral word fluency test | |
| Category fluency (animals) | |
| Action (verb) fluency | |
| Executive function | |
| Trail making test, part B | |
| Stroop color and word test | |
| Wisconsin card sorting test (64 item) | |
| Category test (halstead) | |
| Working memory | |
| WAIS-III letter-number sequencing | |
| Paced auditory serial addition test (channel 1) | |
| WMS-III spatial span | |
| Processing speed | |
| Trail making test, part A | |
| WAIS-III digit symbol | |
| WAIS-III symbol search | |
| Learning and memory | |
| Hopkins verbal learning test-revised | |
| Brief visuospatial memory test-revised | |
| Motor skills | |
| Grooved pegboard test | |
WAIS-III, Weschler Adult Intelligence Scale, Third Edition; WMS-III, Weschler Memory Scale, Third Edition.
Deficit scores were averaged within domains and across the entire battery to derive domain-specific scores (DDS) and a global deficit score (GDS). Consistent with prior studies, global NCI was classified using a validated GDS cut point of ≥0.5 and domain-specific NCI was classified as DDS >0.5.42,47 Similar to prior work,43 the seven, dichotomous domain-specific NCI classifications (impaired vs. unimpaired) were included as indicator variables in the LPA model. Pre-morbid verbal intelligence was estimated using standardized scores from the Reading subtest of the Wide Range Achievement Test, version 4 (WRAT4).48
Neuropsychiatric assessment
To measure the frequency and severity of current depressive symptoms, participants completed the BDI-II.49 The BDI-II consists of 21 items, each rated on a 4-point scale increasing in severity from 0 to 3 (possible total score range: 0–63). Based on a previous factor analysis of the BDI-II in 1583 PWH,50 we computed domain-specific BDI-II scores reflecting cognitive (possible range: 0–27), affective (possible range: 0–12), and somatic (possible range: 0–24) symptoms of depression. These three domain-specific BDI-II scores were included as indicator variables in the LPA.
In addition, participants were evaluated for lifetime and current (past 30 days) MDD and SUD using the DSM-IV-based Composite International Diagnostic Interview.51 For individuals meeting lifetime criteria for MDD, we determined the age at first depressive episode and whether this first episode occurred before HIV disease.
Neuromedical evaluation
Participants underwent a comprehensive neuromedical assessment and blood draw. Detailed history of HIV-specific disease and treatment factors, as well as indicators of non-HIV health status was ascertained. Self-reported HIV disease was corroborated by enzyme-linked immunosorbent assay with Western blot confirmation. Routine clinical chemistry panels, complete blood counts, rapid plasma reagin, hepatitis C virus antibody, and CD4+ T cell count (flow cytometry) were also performed. HIV viral load in plasma was measured using reverse transcriptase-polymerase chain reaction (Amplicor, Roche Diagnostics, Indianapolis, IN). Of the 580 study participants, 515 completed the AIDS Clinical Trials Group 4-day adherence self-report questionnaire to assess for nonadherence to ART medications over the last 4 days.52 Report of any missed dose over the last 4 days was classified as nonadherence.
Established methods for constructing a frailty index53–55 were followed to generate a cumulative metric for non-HIV medical disease burden. Based on available health deficit data, a frailty index variable was composed of 32 non-HIV health variables encompassing a range of physiologic systems, including routine clinical laboratory measures (e.g., glucose, lipids) and medical comorbidities (e.g., hepatitis C co-infection, diabetes).
Each health variable was dichotomized as normal or deficient (normal = “0” and deficit = “1”) based on criteria from previous studies56–58 and frailty index scores were constructed by dividing the total sum of health deficits by the total number of available variables, with a possible range of 0 (no deficits) to 1 (all 32 deficits). For a full list of health deficit variables and cutoff values, see Table 2. In addition to medical disease burden, participants underwent a detailed interview of current medication use to determine total number of current medications.
Table 2.
Frailty Index Criteria
| Variable | Deficit criteria |
|---|---|
| Clinical measurements | |
| (1) Abnormal BMI | >25 or <18 kg/m2 |
| (2) Low white blood cell count | <4000 cells/μL |
| (3) Abnormal MCHC | Male: <27.8 or >33.8; Female: <26.9 or >33.3 |
| (4) Abnormal BUN | <8 or >23 mg/dL |
| (5) Abnormal creatinine | <0.6 or >1.2 mg/dL |
| (6) Abnormal calcium | <9.2 or >10.8 mg/dL |
| (7) Abnormal chloride | <96 or >106 mEq/L |
| (8) Abnormal total protein (serum) | <6 or >7.8 mg |
| (9) Low albumin (serum) | <3.5 mg |
| (10) Elevated fibrinogen | >3.25 |
| (11) Low eGFR | <60 |
| (12) Low hemoglobin | Male: <12 μmol/L; Female: <10 μmol/L |
| (13) Elevated AST | >31 U/L |
| (14) Elevated ALT | >31 U/L |
| (15) Abnormal ALP | <38 U/L or >126 U/L |
| (16) Abnormal potassium | <3.5 or >5.3 mEq/L |
| (17) Elevated total bilirubin | >1.1 mg/dL |
| (18) Elevated triglycerides | ≥150 mg/dL |
| (19) Elevated total cholesterol | >200 mg/dL |
| (20) Low HDL cholesterol | Male: <40 mg/dL; Female: <50 mg/dL |
| (21) Elevated glucose | >200 mg/dL |
| (22) Abnormal phosphorus | <2.5 or >5.1 mg/dL |
| (23) Low platelets | <150 billion/L |
| Comorbidities | |
| (24) HCV | Positive |
| (25) Diabetes mellitus | Positive |
| (26) COPD | Positive |
| (27) Malignancy | Positive |
| (28) Myocardial infarction | Positive |
| (29) Renal disease | Positive |
| (30) Hypertension | Positive or >130 mmHg systolic or >85 mmHg diastolic |
| (31) Hyperlipidemia | Positive |
| (32) Cerebrovascular accident | Positive |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HCV, hepatitis C virus; HDL, high-density lipoprotein; MCHC, mean corpuscular hemoglobin concentration.
Daily functioning
The 33-item self-report Patient's Assessment of Own Functioning Inventory (PAOFI) was used to measure perceived neurocognitive symptoms in everyday life.59 Items endorsed as fairly often or greater are considered clinically significant neurocognitive symptoms and the PAOFI total score is the cumulative sum of these clinically significant neurocognitive symptoms. The PAOFI also includes a single item assessing employment status (employed vs. unemployed). Additional information regarding reason for unemployment (e.g., disability, retired) was not captured.
Dependence in instrumental activities of daily living (IADL) was assessed using a revised version of the Lawton and Brody (1969) self-report measure of everyday functioning,60,61 in which participants rated current abilities compared to previous abilities across 13 everyday functioning domains. The dichotomous classification for IADL dependence was defined as ≥2 declines at least partially attributable to cognitive problems.
Statistical analysis
LPA was used to empirically derive homogenous subgroups of PWH with similar patterns of NCI across the seven neurocognitive domains coupled with symptoms of depression across the three BDI-II domains. To determine the optimal number of latent classes, we iteratively compared indices of model fit for solutions ranging from 1 to 5 classes. For each solution, the best log-likelihood was replicated to avoid convergence at a local maximum.
The best-fitting solution was determined based on a combination of (1) statistical fit indices, specifically Akaike information criterion (AIC), Bayesian information Criterion (BIC), entropy, and the bootstrapped likelihood ratio test (BLRT); (2) adequate class size, with recommendations of at least 25 individuals per class and each class representing at least 5% of the total sample;62 and (3) theoretical interpretability of classes. After the optimal class-solution was identified, classes were substantively interpreted based on their pattern of domain-specific NCI and depression. To determine the relationship between latent class membership and relevant clinical factors, a series of omnibus analysis of variance (ANOVAs) and chi-square tests with follow-up pairwise comparisons examined group differences on demographic, medical, neuropsychiatric, and daily functioning variables. LPA was conducted in Mplus Version 8.6.63 Descriptive and group comparison analyses were conducted in JMP Pro version 16.0.0 (JMP®, Version <16.0.0>; SAS Institute, Inc., Cary, NC).
Results
Participant characteristics
The full sample of 580 PWH on suppressive ART was 86% male with a mean baseline age of 52.5 years (age range, 18–87) and mean education of 13.9 years. The overall sample was 57.1% non-Hispanic White, 25.3% Hispanic, 14.5% non-Hispanic Black, and 3.1% other. The average estimated duration of HIV disease was 18 years and most participants exhibited evidence of ART-induced immune reconstitution, indicated by higher current CD4 counts (median = 596 cells/mm3) compared to nadir CD4 counts (median = 175 cells/mm3).
With respect to NCI, 41.6% of participants met criteria for global NCI and rates of domain-specific NCI ranged from 38.6% (learning) to 25.0% (processing speed). With respect to depression, 56.6% met criteria for lifetime MDD, 8.1% met criteria for current MDD, and the median BDI-II score in the total cohort was 8 (interquartile range = 3–16). Of those with a lifetime MDD diagnosis, 19.1% had their first depressive episode before their estimated acquisition of HIV. Approximately 69.2% of participants identified as unemployed.
Optimal latent class solution
Table 3 presents the AIC, BIC, entropy, BLRT, and class sizes for 1- to 5-class LPA models. All models exhibited strong class separation based on entropy (i.e., ≥0.80). The AIC and BIC metrics progressively decreased with higher class solutions and the BLRT value was significant for each iterative comparison, suggesting the 5-class solution improved model fit based on log-likelihood compared to the 4-class solution.
Table 3.
Latent Profile Analysis Fit Statistics
| Number of classes | Log-likelihood | AIC | BIC | Entropy | Number (%) per class | BLRT |
|---|---|---|---|---|---|---|
| 1 | −7169 | 14364 | 14421 | 1.00 | 580 (100) | |
| 2 | −6741 | 13530 | 13634 | 0.91 | 456 (79) 124 (21) |
<0.001 |
| 3 | −6568 | 13206 | 13359 | 0.80 | 281 (48) 186 (32) 113 (19) |
<0.001 |
| 4 | −6425 | 12942 | 13143 | 0.85 | 247 (43) 174 (30) 134 (23) 25 (4) |
<0.001 |
| 5 | −6350 | 12814 | 13063 | 0.84 | 204 (35) 152 (26) 35 (6) 144 (25) 45 (8) |
<0.001 |
AIC, Akaike information criterion; BIC, Bayesian information criterion; BLRT, bootstrapped likelihood ratio test.
The distribution of class size in the 5-class solution was also favorable compared to the 4-class solution, given that the smallest subgroup of the 4-class solution comprised less than 5% of the total sample (n = 25) and was smaller than the smallest subgroup of the 5-class solution (n = 35). Further examination investigated a 6-class solution, which did not improve class separation and the smallest subgroup of the 6-class solution included less than 4% of the overall total sample. Thus, the 5-class solution was selected as the best fitting model.
Neurocognitive impairment and depression profiles by latent class
Table 4 presents latent class differences on indicator variables from the LPA model, specifically rates of impairment across the seven neurocognitive domains and BDI-II domain scores. The five latent classes exhibited differential patterns of domain-specific NCI and BDI-II scores (Fig. 1): Minimal NCI and minimal depression (Class 1), amnestic and minimal depression (Class 2), severe multi-domain NCI and moderate depression (somatic and affective; Class 3), mild NCI and mild depression (Class 4), and moderate multi-domain NCI and severe depression (Class 5).
Table 4.
5-Class Solution Differences on Latent Profile Indicators
| 1 Minimal NCI/minimal depression (n = 204) | 2 Amnestic/minimal depression (n = 152) | 3 Severe multi-domain NCI/moderate depression (n = 35) | 4 Mild NCI/mild depression (n = 144) | 5 Moderate multi-domain NCI/severe depression (n = 45) | Pairwise differencesa | |
|---|---|---|---|---|---|---|
| Neurocognitive impairment, N (%) | ||||||
| Globalb | 13 (6.4) | 123 (80.9) | 35 (100.0) | 45 (31.2) | 25 (55.6) | 3 > 2 > 5 > 4 > 1 |
| Verbal Fluency | 21 (10.3) | 87 (57.2) | 26 (74.3) | 30 (20.8) | 20 (44.4) | 3 > 2, 5 > 4 > 1 |
| Executive Function | 34 (16.7) | 77 (50.7) | 29 (82.9) | 30 (20.8) | 18 (40.0) | 3 > 2, 5 > 4, 1 |
| Processing Speed | 3 (1.5) | 71 (46.7) | 27 (77.1) | 28 (19.4) | 16 (35.6) | 3 > 2, 5 > 4 > 1 |
| Learning | 11 (5.4) | 114 (75.0) | 33 (94.3) | 48 (33.3) | 18 (40.0) | 3 > 2 > 5, 4 > 1 |
| Recall | 16 (7.8) | 98 (64.5) | 33 (94.3) | 40 (27.8) | 15 (33.3) | 3 > 2 > 5, 4 > 1 |
| Working Memory | 21 (10.3) | 66 (43.4) | 27 (77.1) | 28 (19.4) | 15 (33.3) | 3 > 2, 5 > 4 > 1 |
| Motor | 17 (8.3) | 74 (48.7) | 24 (68.6) | 24 (16.7) | 17 (37.8) | 3 > 2, 5 > 4 > 1 |
| Beck depression inventory-II, mean (SD) | ||||||
| Totalc | 4.4 (3.7) | 4.6 (3.9) | 19.8 (4.7) | 16.4 (4.9) | 34.4 (7.5) | 5 > 3 > 4 > 2, 1 |
| Cognitive (max. 27) | 1.1 (1.8) | 0.9 (1.5) | 5.3 (3.0) | 5.2 (3.7) | 13.0 (4.9) | 5 > 3, 4 > 2, 1 |
| Somatic (max. 24) | 2.8 (2.4) | 3.2 (2.8) | 9.8 (3.5) | 8.0 (2.8) | 13.9 (3.1) | 5 > 3 > 4 > 2, 1 |
| Affective (max. 12) | 0.6 (0.8) | 0.6 (0.8) | 4.7 (1.3) | 3.2 (1.0) | 7.4 (1.7) | 5 > 3 > 4 > 2, 1 |
Pairwise differences are significant at p < 0.05. Significance tested with ANOVA for BDI-II variables and chi-square analysis for NCI classifications.
Global NCI and total BDI-II score were not included as indicator variables.
BDI-II ranges: 0–12 (minimal), 14–19 (mild), 20–28 (moderate), 29–63 (severe).
ANOVA, analysis of variance; BDI-II, Beck Depression Inventory-II; NCI, neurocognitive impairment.
FIG. 1.
Depressive symptoms and NCI domains by latent class. Latent classes derived from the LPA were defined based on patterns of BDI-II domain scores (see violin plots, A–C) and rates of domain-specific NCI (D). Class 1 (blue) was characterized by minimal BDI-II scores and negligible rates of NCI. Class 2 (red) was characterized by minimal BDI-II scores and the second highest rates of NCI, with a particular vulnerability in learning and recall. Class 3 (green) was characterized by overall mild-to-moderate BDI-II scores with relative elevations in somatic and affective symptoms, and the highest rates of NCI across all domains. Class 4 (purple) was characterized by overall mild-to-moderate BDI-II scores without a domain-specific pattern and the second lowest rates of NCI. Class (5) was characterized by severe elevations across all BDI-II domains and exhibited intermediate levels of NCI across all domains. For BDI-II violin plots, dashed lines represent median and dotted lines represent 25th and 75th percentile. BDI-II, Beck Depression Inventory-II; LPA, latent profile analysis; NCI, neurocognitive impairment.
Clinical correlates of latent classes
A series of omnibus ANOVAs and chi-square tests with follow-up pairwise comparisons was conducted to determine the association between latent class membership and demographic, medical, neuropsychiatric, and daily functioning factors (Table 5).
Table 5.
Clinical and Demographic Variables by Latent Class
| 1 Minimal NCI/minimal depression (n = 204) | 2 Amnestic/minimal depression (n = 152) | 3 Severe multi-domain NCI/moderate depression (n = 35) | 4 Mild NCI/mild depression (n = 144) | 5 Moderate multi-domain NCI/severe depression (n = 45) | p | Pairwise comparisonsa | |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age | 51.1 (12.0) | 53.9 (10.9) | 56.5 (10.8) | 53.0 (11.8) | 50.0 (11.7) | 0.022 | 3 > 1, 5; 2 > 1 |
| Sex (% male) | 170 (83.3%) | 129 (84.9%) | 31 (88.6%) | 132 (91.7%) | 38 (84.4%) | 0.20 | |
| Race/ethnicity | 0.26 | ||||||
| Asian | 2 (1.0%) | 1 (0.7%) | 1 (2.9%) | 0 (0.0%) | 1 (2.2%) | ||
| Black | 38 (18.6%) | 17 (11.2%) | 2 (5.7%) | 21 (14.6%) | 6 (13.3%) | ||
| Hispanic | 54 (26.5%) | 40 (26.3%) | 7 (20.0%) | 31 (21.5%) | 15 (33.3%) | ||
| Other | 5 (2.5%) | 5 (3.3%) | 0 (0.0%) | 2 (1.4%) | 1 (2.2%) | ||
| White | 105 (51.5%) | 89 (58.6%) | 25 (71.4%) | 90 (62.5%) | 22 (48.9%) | ||
| Education (years) | 13.5 (2.6) | 13.9 (2.8) | 14.3 (2.5) | 14.4 (2.6) | 13.5 (2.8) | 0.032 | 4 > 1, 5 |
| WRAT4b | 103.0 (12.3) | 97.7 (12.2) | 96.5 (13.0) | 104.2 (13.8) | 98.3 (12.9) | <0.001 | 1, 4 > 2, 3, 5 |
| Unemployedc | 118 (59.3%) | 107 (71.8%) | 31 (91.2%) | 97 (67.8%) | 40 (93.0%) | <0.001 | 3, 5 > 1, 2, 4; 2 > 1 |
| Medical burden | |||||||
| AIDSd | 121 (59.3%) | 94 (61.8%) | 30 (85.7%) | 89 (61.8%) | 28 (62.2%) | 0.049 | 3 > 1, 2, 4, 5 |
| Years of infection | 17.0 (9.6) | 18.9 (9.4) | 21.4 (9.2) | 18.4 (9.7) | 16.7 (9.9) | 0.06 | |
| Nadir CD4e | 199 [50, 350] | 162 [19, 292] | 99 [32, 199] | 180 [45, 300] | 159 [27, 340] | 0.06 | |
| Current CD4f | 615 [437, 798] | 560 [414, 753] | 584 [350, 889] | 640.5 [446, 864] | 617 [452, 874] | 0.61 | |
| ART non-adherentg | 12 (6.3%) | 9 (7.0%) | 3 (9.7%) | 11 (8.9%) | 11 (26.2%) | 0.011 | 5 > 1, 2, 4 |
| Frailty indexg | 0.23 (0.09) | 0.26 (0.09) | 0.28 (0.09) | 0.25 (0.08) | 0.23 (0.09) | 0.002 | 3 > 1, 4, 5; 1 < 2, 4 |
| Total medications | 5.7 (5.7) | 8.0 (7.0) | 11.0 (6.7) | 8.6 (8.4) | 9.0 (7.6) | <0.001 | 1 < 2, 3, 4, 5; 2 < 3 |
| Neuropsychiatric | |||||||
| LT MDDh | 88 (44.0%) | 80 (54.1%) | 22 (68.8%) | 96 (67.6%) | 35 (77.8%) | <0.001 | 4, 5 > 1, 2; 3 > 1 |
| Current MDDi | 5 (2.6%) | 4 (3.0%) | 7 (22.6%) | 12 (9.0%) | 15 (34.9%) | <0.001 | 3, 5 > 4 > 1, 2 |
| Age of first MDDj | 31.2 (14.1) | 34.2 (13.1) | 37.6 (16.9) | 33.0 (16.0) | 33.3 (15.0) | 0.41 | |
| MDD before HIVj | 19 (21.6%) | 12 (15.2%) | 3 (13.6%) | 20 (20.8%) | 7 (20.0%) | 0.77 | |
| LT Alcoholh | 99 (49.5%) | 74 (50.0%) | 15 (46.9%) | 77 (54.2%) | 29 (64.4%) | 0.38 | |
| LT SUDh | 128 (64.0%) | 103 (69.6%) | 20 (62.5%) | 103 (72.5%) | 37 (82.2%) | 0.10 | |
| Daily functioning | |||||||
| Total PAOFIc | 2.0 (3.3) | 3.9 (5.6) | 10.4 (7.5) | 6.3 (6.4) | 16.4 (9.4) | <0.001 | 5 > 3 > 4 > 2 > 1 |
| IADL dependentk | 15 (7.6%) | 32 (21.4%) | 19 (55.9%) | 64 (45.4%) | 26 (60.5%) | <0.001 | 3, 4, 5 > 2 > 1 |
Data presented as N (%), mean (SD), or median [IQR].
Pairwise differences are significant at p < 0.05. Significance tested with ANOVA for continuous outcomes and chi-square analysis for categorical outcomes.
N = 529; cN = 568; dN = 572; eN = 576; fN = 539; gN = 515; hN = 567; iN = 532; jN = 320; kN = 565.
ART, antiretroviral therapy; IADL, instrumental activities of daily living; IQR, interquartile range; LT, lifetime; MDD, major depressive disorder; PAOFI, Patient's Assessment of Own Functioning Inventory; SUD, substance use disorder; WRAT4, Wide Range Achievement Test, version 4 subtest.
Demographics
Significant group differences were observed for age, with Class 3 (mean age = 56.5 years) and Class 5 (mean age = 50.0 years) representing the oldest and youngest groups, respectively. Significant differences were also noted for years of education and estimated pre-morbid verbal intelligence (WRAT4). Most notably, Class 1 and Class 4, the two classes with the lowest rates of NCI, exhibited higher WRAT4 scores relative to other classes. Latent classes did not significantly differ by sex or race/ethnicity.
Medical burden
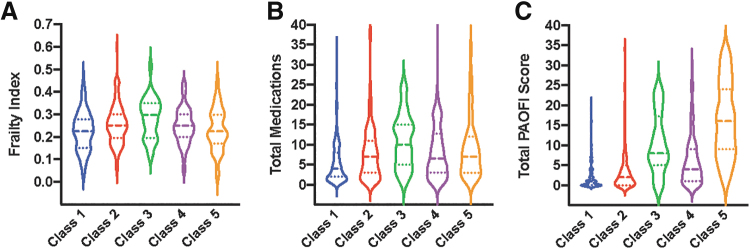
Although differences in estimated years of HIV disease and CD4 counts (both current and nadir) did not reach statistical significance, Class 3 had a markedly higher proportion of AIDS diagnoses (86%) compared to the other classes (59% to 62%). Although all participants were on prescribed ART, Class 5 exhibited a significantly elevated rate of self-reported ART nonadherence (26%), whereas rates of non-adherence were less than 10% in the other classes. Significant differences were also observed for cumulative non-HIV comorbidity and medication burden (Fig. 2A, B), with Class 3 having the highest frailty index scores (28% health deficit rate) and number of medications (mean = 11.0) and Class 1 having the lowest frailty index scores (23% health deficit rate) and number of medications (mean = 5.7).
FIG. 2.
Medical Burden and Daily Neurocognitive Symptoms by Latent Class. Violin plots depicting latent class differences in frailty index scores (A), medication burden (B), and self-reported neurocognitive symptoms in everyday life (total PAOFI score; C). Dashed lines represent median and dotted lines represent 25th and 75th percentile. PAOFI, Patient's Assessment of Own Functioning Inventory.
Neuropsychiatric
The proportion of participants meeting criteria for current MDD mirrored the pattern of BDI-II scores observed across latent classes. Specifically, rates of current MDD were highest in Class 5 (35%) and Class 3 (23%), followed by Class 4 (9%), and then Class 1 (3%) and Class 2 (3%). Class 5 also exhibited the highest rates of lifetime MDD (78%), whereas Classes 3 (69%) and Class 4 (68%) had comparable rates of lifetime MDD. Age of MDD onset and the proportion of individuals meeting MDD criteria before HIV disease did not significantly differ across latent classes. Similarly, rates of lifetime alcohol and SUDs did not differ across latent classes.
Daily functioning
A stair-step pattern was observed for self-reported neurocognitive symptoms (total PAOFI scores; Fig. 2C) such that Class 5 reported the highest number of neurocognitive symptoms, followed by Class 3, then Class 4, then Class 2, and then Class 1. Rates of unemployment were significantly higher in Class 5 (93%) and 3 (91%) relative to other classes. Similarly, rates of IADL dependence were highest in Class 5 (61%), 3 (56%), and 4 (45%), followed by Class 2 (21%) and then Class 1 (8%).
Discussion
Our study successfully identified five distinct classes of PWH on suppressive ART, varying on the basis of NCI and depression symptomatology. These classes ranged from those exhibiting minimal NCI and depression to those experiencing severe multi-domain NCI coupled with significant depressive symptoms. Clinical and demographic correlates of these classes suggested that those with moderate-to-severe NCI and depressive symptoms tended to be older, report nonadherence to ART, carry a higher burden of comorbidity, and display more significant everyday functional impairment.
Despite similar levels of functional impairment (i.e., IADLs and employment), Class 5 had a stronger psychiatric profile (i.e., higher BDI scores, current MDD), whereas Class 3 has a stronger medical profile (i.e., higher frailty index, higher medications, greater proportion of AIDS diagnosis). In contrast, Class 1 had the lowest medication use and frailty index, with similar HIV disease characteristics to Classes 3 and 5. These findings underscore there are multiple pathways to cognitive and functional impairment among PWH with co-occurring depression and cognitive impairment, and these groups may respond to different interventions.
LPA identified NCI phenotypes that were broadly characterized by severity of NCI rather than by cognitive domain, except for the amnestic and minimal depression group (Class 2), which had higher levels of learning and recall deficits relative to other domains. Although this group with high rates of NCI (81%) and minimal depression was identified (Class 2), a group with the reverse pattern was not (i.e., moderate or severe depression and minimal NCI). This finding suggests that PWH with depression are more likely to exhibit impairment on objective neuropsychological testing than PWH with NCI are to experience depression.
There was also no marked impairment in domains of executive functioning, processing speed, and psychomotor functioning for the classes with at least moderate NCI and depression (Classes 5 and 3), which are commonly observed in depressed PWH.9 These findings support some prior work using data-driven approaches to identify cognitive profiles. Although Molsberry and colleagues64 did not include depressive symptoms in their LPA, they identified similar cognitive profiles: One amnestic, in addition to other cognitive profiles characterized by global cognitive performance (e.g., average, below average). Paul and colleagues65 used hierarchical clustering to identify cognitive profiles in PWH and found four clusters with domain-specific effects (i.e., action fluency, verbal learning and memory, executive functioning) and one cluster with global average performance. Similarly, those in the cognitively impaired group exhibited greater depressive symptoms.65
Although HIV has been predominately regarded as a “subcortical” phenotype in the pre-ART era, heterogeneous cognitive profiles in the post-ART era (including learning and memory deficits) have implicated both cortical and subcortical involvement.66,67 Poor learning due to encoding deficits, as opposed to frank memory impairment like in Alzheimer's disease,68 and unique biological disease features (i.e., biotypes)69 are possible explanations for amnestic and heterogeneous cognitive profiles. In addition, PWH with HAND may be at greater risk for amnestic mild cognitive impairment (aMCI), a precursor to Alzheimer's disease, due to synergistic effects of HIV and aging.70 Differentiating HAND from aMCI remains an ongoing area of research as the majority of PWH in the United States reach older adulthood.71,72
LPA also identified depression phenotypes varying from minimal to severe levels using a self-report depression questionnaire. These self-reported depressive symptoms were corroborated by rates of current and lifetime history of MDD, which were most prevalent in Class 5 (35% and 78%, respectively). Depression is characterized by cognitive, affective, and somatic domains; thus, depressive symptoms were analyzed by domain in this study. Cognitive symptoms characteristic of depression such as concentration difficulties may manifest behaviorally as forgetting to take medication and have greater effects on nonadherence than vegetative symptoms such as fatigue.73
This study only found differentiation of depressive symptom domain for Class 3, in which somatic and affective, and less so cognitive symptoms of depression, characterized the severe multi-domain NCI and moderate depression group (Class 3). Across groups, Class 3 exhibited the highest elevations in somatic symptoms of depression, highest frailty score (i.e., non-HIV medical burden), and greatest number of total medications. Due to the overlap between HIV, depression, and elevated prevalence of medical comorbidities among PWH,74,75 self-reported somatic symptoms on the depression questionnaire likely overlapped with somatic symptoms associated with medical disease burden.76
Consistent with prior research, this study supports that PWH with moderate-to-severe NCI and depression are at elevated risk for poorer disease outcomes.9,10,77 The severe multi-domain NCI and moderate depression group (Class 3) had the highest rate of AIDS (86%), and the moderate multi-domain NCI and severe depression group (Class 5) had the highest rate of ART nonadherence (26%). Psychosocial barriers frequently experienced by PWH,78–80 including mental health disorders (e.g., depression), stressful life events, and stigma, are among the strongest predictors of ART adherence.77,81,82 Indeed, the cumulative effect of psychosocial burden has an incrementally negative impact on ART adherence.83,84 Among these psychosocial barriers, depression is strongly and consistently associated with ART nonadherence30,85 and progression to AIDS.77,86
Moreover, PWH with greater NCI and depression demonstrated poorer daily functioning outcomes. Substantial evidence supports that NCI among PWH, an important complication of HIV and/or depression, is associated with unemployment and functional difficulties, particularly in more complex tasks such as medication management and driving.87 PWH in Classes 3 and 5 with at least moderate depression had the highest self-reported cognitive symptoms, functional declines, and unemployment rates.
Although the amnestic and minimal depression group (Class 2) exhibited a high rate of global NCI (81%), only second to the most cognitively impaired class (Class 3), PWH in Class 2 had modest self-reported cognitive symptoms and functional declines. These findings suggest that the combined effect of depression and NCI results in functional difficulties, rather than NCI alone. It is noteworthy, however, that PWH with NCI may have limited insight regarding their current level of functioning and underreport functional difficulties, while PWH with depression may overreport functional difficulties.88,89 This self-appraisal of one's own functioning may explain why PWH with the most severe depression (Class 5) did not also exhibit the highest rate of NCI on objective neuropsychological testing.
Several possible risk and protective factors were associated with depression and NCI in this study. Interestingly, the youngest and oldest age groups were reflected by Classes 5 (mean age = 50) and 3 (mean age = 57), respectively, which had the greatest levels of NCI and depression. Although the age range represented in this sample was relatively limited, PWH in these classes may experience accelerated and/or accentuated aging with earlier and more severe onset of NCI compared to the general population.90,91
Alternatively, cognitive reserve or individual differences in cognitive processes or neural networks, which explain discrepant resilience to brain pathology,92 suggest that PWH with higher cognitive reserve may withstand greater neurological insult and maintain neurocognitive function relative to PWH with lower cognitive reserve.88 In line with prior research,93–95 proxies for cognitive reserve (i.e., higher pre-morbid IQ and employment) were highest among Classes 1 and 4, which had minimal to mild levels of NCI and depression. Further, substance use history in this sample was common across groups: 47–64% for lifetime alcohol use disorder and 63–82% for lifetime SUDs in this sample. Rates of lifetime alcohol and SUDs, however, did not differentiate cognitive profiles, as found in prior work.96 Nonetheless, future research should account for current substance use as there is significant overlap with depression in PWH.97
This study was not without limitations. The sample was majority White, which is nonrepresentative of the US population of PWH.2 Black/African American and Hispanic/Latino populations remain disproportionately impacted by HIV, but our limited sample size did not allow for analyses to be conducted by individual racial/ethnic group. The sample was also predominately male, which did not allow for examination of gender-specific effects. As disparity in depression exists by race/ethnicity and gender among PWH, different subgroups may be at elevated risk for NCI and depression than others (e.g., Black men).98–101
Moreover, examination of functional difficulties was limited to self-report as opposed to performance-based or informant report, which may be biased by depression.89 Future studies may acquire functional status through alternate methods to bolster the validity of self-report. Finally, longitudinal study is warranted as PWH with high depression burden may exhibit steeper neurocognitive decline compared to those with low depression burden.37 Moreover, the cognitive domains driving neurocognitive decline may vary among individuals with varying levels of depression.37,102
Our results suggest that ART alone may not be sufficient to address the health needs of all PWH, particularly in the context of co-occurring NCI and depression. Future interventions should consider a more integrated, person-centered approach that addresses both cognitive and emotional health, in conjunction with ART adherence, to optimize overall health outcomes in PWH. Moreover, our findings add to the literature, underscoring the need for routine neuropsychiatric assessment in HIV care settings to identify and address mental health symptoms that may impede optimal daily functioning and medication adherence. Tailoring interventions according to the cognitive and mood profiles of individuals could potentially enhance their effectiveness and acceptability.103
Our study paves the way for further research investigating the pathophysiological mechanisms linking NCI, depression, ART nonadherence, and everyday functioning in PWH. Longitudinal studies would be particularly informative to clarify the directionality and temporal patterns of these relationships. For example, it remains unclear whether cognitive impairment and depressive symptoms precede and contribute to functional difficulties and medication nonadherence, or conversely, whether these factors contribute to worsening cognitive and mood symptoms over time.
Further, future studies should investigate the role of potential moderating factors such as resilience, social support, and lifestyle factors (e.g., physical activity, sleep quality, nutrition) in the association between NCI, depressive symptoms, and health outcomes in PWH. Intervention studies are also needed to evaluate the effectiveness of cognitive remediation strategies and psychological therapies, individually or in combination, in improving cognitive function, mood, and health outcomes in PWH. Understanding the extent to which these interventions can mitigate the impact of NCI and depressive symptoms could be critical to enhancing the quality of life and health outcomes of PWH.
Acknowledgments
I would like to acknowledge R.S. for his significant contribution to this research. The methodology of this work is built upon his dissertation, SuperAging in Adults with HIV Disease: Biopsychosocial Predictors of Neurocognitive Aging Trajectories. R.S.'s dedication to advancing knowledge in this field has been instrumental in shaping the direction of our research.
The San Diego HIV Neurobehavioral Research Center (HNRC) group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Health care System, and includes Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Jennifer Iudicello, Ph.D.; Donald Franklin, Jr.; Cheryl Kelley; NeuroMedical Core: Ronald J. Ellis, M.D., Ph.D. (Director/NeuroMedical Unit Head), Scott Letendre, M.D. (Co-I./Laboratory Unit Head), Christine Fennema-Notestine, Ph.D., (Co-I./Neuroimaging Unit Head); Debra Rosario, M.P.H., Neurobehavioral & Psychiatry Core: David J. Moore, Ph.D. (Co-Director/Neurobehavioral Unit Head), Murray B. Stein, M.D. (Co-Director/Psychiatry Unit Head), Erin E. Morgan, Ph.D. (Co-I./Psychiatric Coordinator), Andrew H. Miller, Matthew Dawson, NeuroVirology & Biology Core: Sara Gianella Weibel, M.D. (Co-Director/NeuroVirology Unit Head), Sarah A. LaMere, D.V.M., Ph.D. (Associate Unit Head, NeuroVirology Unit), Cristian Achim, M.D., Ph.D. (Co-Director/Neurobiology Unit Head), Ana Sanchez, Ph.D. (Co-I./Neurobiology Unit), Adam Fields, Ph.D.(Associate Unit Head, Neurobiology Unit); Microbiome Core: Rob Knight, Ph.D. (Co-Director), Pieter Dorrestein, Ph.D. (Co-Director); Developmental Core: Scott Letendre, M.D. (Director), Ajay Bharti, M.D. (Co-I.), J. Allen McCutchan, M.D., Christine Fennema-Notestine, Ph.D.; Administrative Core: Robert K. Heaton, Ph.D. (Director/Coordinating Unit Head), Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (Unit Head), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Ian Abramson, Ph.D. (Unit Head), Clint Cushman; Statistics Unit: Florin Vaida, Ph.D. (Unit Head), Anya Umlauf, M.S., Bin Tang, Ph.D.
Authors' Contributions
M.K.: Conceptualization, writing—original draft preparation, and writing—review and editing; L.H.: Conceptualization, writing—original draft preparation and writing—review and editing; R.S.: Conceptualization, methodology, data curation, formal analysis, and writing—original draft preparation; D.D.: Writing—original draft preparation, and review and editing; J.I.: Investigation, writing—review and editing, and supervision; R.J.E.: Investigation, writing—review and editing, and supervision; D.J.M.: Conceptualization, methodology, resources, review and editing, supervision, project administration, and funding acquisition.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by NIMH-funded research programs, including the HIV Neurobehavioral Research Center (HNRC), supported by award P30MH062512; the California NeuroAIDS Tissue Network (CNTN), supported by award U24MH100928; and the Multi-Dimensional Successful Aging Among HIV-Infected Adults, supported by award R01MH099987. Support for this study also includes an NIDA-funded research program that includes the Translational Methamphetamine AIDS Research Center (TMARC), supported by award P50DA026306; an NIH-funded research program that includes the CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER), supported by award HHSN271201000036C; and the NIMHD-funded study Mechanisms of Disparities in Adverse Neurocognitive Outcomes among Hispanics Aging with HIV, supported by award R01MD013502. L.H. was supported by the National Science Foundation (NSF) Graduate Research Fellowship Program (GRFP): 2038238. M.K. was supported by the National Institute on Aging (NIA): F31 AG074838.
References
- 1. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet 2008;372(9635):293–299; doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. HIV Among People Aged 50 and Over. Available from: https://www.cdc.gov/hiv/group/age/olderamericans/index.html [Last accessed: February 1, 2023].
- 3. Heaton RK, Franklin DR Jr., Deutsch R, et al. Neurocognitive change in the era of HIV combination antiretroviral therapy: The longitudinal CHARTER study. Clin Infect Dis 2015;60(3):473–480; doi: 10.1093/cid/ciu862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heaton R, Clifford D, Franklin D, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010;75(23):2087–2096; doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Olivieri-Mui B, Wilson I, Shi S, et al. Geriatric conditions associated with nonadherence to antiretroviral therapy among older people with HIV: The importance of frailty. AIDS Patient Care STDS 2022;36(6):226–235; doi: 10.1089/apc.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collini P, Mawson RL. A new era of HIV care for age-associated multimorbidity. Curr Opin Infect Dis 2023;36(1):9–14; doi: 10.1097/QCO.0000000000000890. [DOI] [PubMed] [Google Scholar]
- 7. Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J 2014;44(4):1055–1068; doi: 10.1183/09031936.00059814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De Francesco D, Sabin CA, Reiss P. Multimorbidity patterns in people with HIV. Curr Opin HIV AIDS 2020;15(2):110–117; doi: 10.1097/COH.0000000000000595. [DOI] [PubMed] [Google Scholar]
- 9. Rubin LH, Maki PM. HIV, depression, and cognitive impairment in the era of effective antiretroviral therapy. Curr HIV/AIDS Rep 2019;16(1):82–95; doi: 10.1007/s11904-019-00421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nanni MG, Caruso R, Mitchell AJ, et al. Depression in HIV infected patients: A review. Curr Psychiatry Rep 2015;17(1):1–11. [DOI] [PubMed] [Google Scholar]
- 11. Rezaei S, Ahmadi S, Rahmati J, et al. Global prevalence of depression in HIV/AIDS: A systematic review and meta-analysis. BMJ Support Palliat Care 2019;9(4):404–412; doi: 10.1136/bmjspcare-2019-001952. [DOI] [PubMed] [Google Scholar]
- 12. Cook JA, Burke-Miller JK, Steigman PJ, et al. Prevalence, comorbidity, and correlates of psychiatric and substance use disorders and associations with HIV risk behaviors in a multisite cohort of women living with HIV. AIDS Behav 2018;22(10):3141–3154; doi: 10.1007/s10461-018-2051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bernard C, Dabis F, de Rekeneire N. Prevalence and factors associated with depression in people living with HIV in sub-Saharan Africa: A systematic review and meta-analysis. PLoS One 2017;12(8):e0181960; doi: 10.1371/journal.pone.0181960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gokhale RH, Weiser J, Sullivan PS, et al. Depression prevalence, antidepressant treatment status, and association with sustained HIV viral suppression among adults living with HIV in care in the United States, 2009–2014. AIDS Behav 2019;23(12):3452–3459; doi: 10.1007/s10461-019-02613-6. [DOI] [PubMed] [Google Scholar]
- 15. Do AN, Rosenberg ES, Sullivan PS, et al. Excess burden of depression among HIV-infected persons receiving medical care in the United States: Data from the medical monitoring project and the behavioral risk factor surveillance system. PLoS One 2014;9(3):e92842; doi: 10.1371/journal.pone.0092842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orlando M, Burnam MA, Beckman R, et al. Re-estimating the prevalence of psychiatric disorders in a nationally representative sample of persons receiving care for HIV: Results from the HIV Cost and Services Utilization Study. Int J Methods Psychiatr Res 2002;11(2):75–82; doi: 10.1002/mpr.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry 2001;58(8):721–728; doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 18. Yousuf A, Musa R, Isa MLM, et al. Anxiety and depression among women living with HIV: Prevalence and correlations. Clin Pract Epidemiol Ment Health 2020;16:59–66; doi: 10.2174/1745017902016010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiao L, Qi H, Wang YY, et al. The prevalence of depression in men who have sex with men (MSM) living with HIV: A meta-analysis of comparative and epidemiological studies. Gen Hosp Psychiatry 2020;66:112–119; doi: 10.1016/j.genhosppsych.2020.04.001. [DOI] [PubMed] [Google Scholar]
- 20. Wen J, Yeh TP, Xie H, et al. Resilience, self-esteem, self-efficacy, social support, depression and ART adherence among people living with HIV in Sichuan, China. AIDS Care 2021;33(11):1414–1421; doi: 10.1080/09540121.2020.1828800. [DOI] [PubMed] [Google Scholar]
- 21. McLaurin KA, Harris M, Madormo V, et al. HIV-associated apathy/depression and neurocognitive impairments reflect persistent dopamine deficits. Cells 2021;10(8):2158; doi: 10.3390/cells10082158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hernandez-Salazar P, Ortiz-Rodriguez MA, Garcia Fuentes NB, et al. Physical activity, anxiety, depression, and adherence to antiretroviral therapy in people with HIV. AIDS Res Hum Retroviruses 2023;39(6):310–316; doi: 10.1089/AID.2022.0064. [DOI] [PubMed] [Google Scholar]
- 23. Camargo CC, Cavassan NRV, Tasca KI, et al. Depression and coping are associated with failure of adherence to antiretroviral therapy among people living with HIV/AIDS. AIDS Res Hum Retroviruses 2019;35(11–12):1181–1188; doi: 10.1089/aid.2019.0050. [DOI] [PubMed] [Google Scholar]
- 24. Spence AB, Michel K, Wang C, et al. Viral suppression is associated with HIV treatment self-efficacy in a cohort of women in Washington, DC. AIDS Patient Care STDS 2021;35(3):75–83; doi: 10.1089/apc.2020.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thet D, Siritientong T, Sangarlangkarn A, et al. Deterioration of nutritional status and its negative association with depression among older HIV-infected Asian Population: A four-year longitudinal study. AIDS Res Hum Retroviruses 2022;38(7):592–600; doi: 10.1089/AID.2021.0087. [DOI] [PubMed] [Google Scholar]
- 26. Yousuf A, Mohd Arifin SR, Musa R, et al. Depression and HIV disease progression: A mini-review. Clin Pract Epidemiol Ment Health 2019;15:153–159; doi: 10.2174/1745017901915010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Owora AH. Major depression disorder trajectories and HIV disease progression: Results from a 6-year outpatient clinic cohort. Medicine (Baltimore) 2018;97(12):e0252; doi: 10.1097/MD.0000000000010252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pence BW, Mills JC, Bengtson AM, et al. Association of increased chronicity of depression with HIV appointment attendance, treatment failure, and mortality among HIV-infected adults in the United States. JAMA Psychiatry 2018;75(4):379–385; doi: 10.1001/jamapsychiatry.2017.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lesko CR, Hutton HE, Fojo AT, et al. Depression and HIV viral nonsuppression among people engaged in HIV care in an urban clinic, 2014–2019. AIDS 2021;35(12):2017–2024; doi: 10.1097/QAD.0000000000003005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gonzalez JS, Batchelder AW, Psaros C, et al. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. J Acquir Immune Defic Syndr 2011;58(2):181–187; doi: 10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alford K, Vera JH. Cognitive impairment in people living with HIV in the ART era: A review. Br Med Bull 2018;127(1):55–68; doi: 10.1093/bmb/ldy019. [DOI] [PubMed] [Google Scholar]
- 32. Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. J Neurovirol 2011;17(1):3–16; doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keng LD, Winston A, Sabin CA. The global burden of cognitive impairment in people with HIV. AIDS 2023;37(1):61–70; doi: 10.1097/QAD.0000000000003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinheiro CA, Souza LD, Motta JV, et al. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz J Med Biol Res 2016;49(10):e5344; doi: 10.1590/1414-431X20165344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cysique LA, Deutsch R, Atkinson JH, et al. Incident major depression does not affect neuropsychological functioning in HIV-infected men. J Int Neuropsychol Soc 2007;13(1):1–11; doi: 10.1017/S1355617707070026. [DOI] [PubMed] [Google Scholar]
- 36. Fellows RP, Byrd DA, Morgello S, et al. Major depressive disorder, cognitive symptoms, and neuropsychological performance among ethnically diverse HIV+ men and women. J Int Neuropsychol Soc 2013;19(2):216–225; doi: 10.1017/S1355617712001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paolillo EW, Pasipanodya EC, Moore RC, et al. Cumulative burden of depression and neurocognitive decline among persons with HIV: A longitudinal study. J Acquir Immune Defic Syndr 2020;84(3):304–312; doi: 10.1097/QAI.0000000000002346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cysique LA, Dermody N, Carr A, et al. The role of depression chronicity and recurrence on neurocognitive dysfunctions in HIV-infected adults. J Neurovirol 2016;22(1):56–65; doi: 10.1007/s13365-015-0368-5. [DOI] [PubMed] [Google Scholar]
- 39. Thompson EC, Muhammad JN, Adimora AA, et al. Internalized HIV-related stigma and neurocognitive functioning among women living with HIV. AIDS Patient Care STDs 2022;36(9):336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology 2007;69(18):1789–1799; doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saloner R, Heaton RK, Campbell LM, et al. Effects of comorbidity burden and age on brain integrity in HIV. AIDS 2019;33(7):1175–1185; doi: 10.1097/QAD.0000000000002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Carey CL, Woods SP, Gonzalez R, et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol 2004;26(3):307–319; doi: 10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- 43. Pasipanodya EC, Montoya JL, Campbell LM, et al. Metabolic risk factors as differential predictors of profiles of neurocognitive impairment among older HIV+ and HIV− adults: An observational study. Arch Clin Neuropsychol 2021;36(2):151–164; doi: 10.1093/arclin/acz040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heaton R, Miller S, Taylor M, et al. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults. Psychological Assessment Resources: Lutz, FL; 2004. [Google Scholar]
- 45. Heaton R, Taylor M, Manly J. Demographic effects and use of demographically corrected norms with the WAIS-III and WMS-III. Clinical Interpretation of the WAIS-III and WMS-III. Elsevier; 2003; pp. 181–210. [Google Scholar]
- 46. Norman MA, Moore DJ, Taylor M, et al. Demographically corrected norms for African Americans and Caucasians on the Hopkins Verbal Learning Test-Revised, Brief Visuospatial Memory Test-Revised, Stroop Color and Word Test, and Wisconsin Card Sorting Test 64-Card Version. J Clin Exp Neuropsychol 2011;33(7):793–804; doi: 10.1080/13803395.2011.559157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blackstone K, Moore DJ, Franklin DR, et al. Defining neurocognitive impairment in HIV: Deficit scores versus clinical ratings. Clin Neuropsychol 2012;26(6):894–908; doi: 10.1080/13854046.2012.694479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wilkinson G. WRAT-3: Wide Range Achievement Test Administration Manual. Journal of Experimental Neuropsychology1993. [Google Scholar]
- 49. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation: San Antonio, TX; 1996; Vol. 1, p. 82. [Google Scholar]
- 50. Hobkirk AL, Starosta AJ, De Leo JA, et al. Psychometric validation of the BDI-II among HIV-positive CHARTER study participants. Psychol Assess 2015;27(2):457–466; doi: 10.1037/pas0000040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. World Health Organization. Composite international diagnostic interview (CIDI), version 2.1. In: Composite International Diagnostic Interview, version 2.1, 1997.
- 52. Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care 2000;12(3):255–266; doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 53. Bagshaw SM, McDermid RC. The role of frailty in outcomes from critical illness. Curr Opin Crit Care 2013;19(5):496–503; doi: 10.1097/MCC.0b013e328364d570. [DOI] [PubMed] [Google Scholar]
- 54. Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr 2008;8:24; doi: 10.1186/1471-2318-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011;27(1):17–26; doi: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 56. Oppenheim H, Paolillo EW, Moore RC, et al. Neurocognitive functioning predicts frailty index in HIV. Neurology 2018;91(2):e162–e170; doi: 10.1212/WNL.0000000000005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guaraldi G, Brothers TD, Zona S, et al. A frailty index predicts survival and incident multimorbidity independent of markers of HIV disease severity. AIDS 2015;29(13):1633–1641; doi: 10.1097/QAD.0000000000000753. [DOI] [PubMed] [Google Scholar]
- 58. Wallace LM, Ferrara M, Brothers TD, et al. Lower frailty is associated with successful cognitive aging among older adults with HIV. AIDS Res Hum Retroviruses 2017;33(2):157–163; doi: 10.1089/AID.2016.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Heaton RK, Chelune GJ, Lehman RA. Using neuropsychological and personality tests to assess the likelihood of patient employment. J Nerv Ment Dis 1978;166(6):408–416; doi: 10.1097/00005053-197806000-00004. [DOI] [PubMed] [Google Scholar]
- 60. Heaton R, Marcotte TD, Mindt MR, et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 2004;10(3):317–331; doi: 10.1017/S1355617704102130. [DOI] [PubMed] [Google Scholar]
- 61. Woods SP, Iudicello JE, Moran LM, et al. HIV-associated prospective memory impairment increases risk of dependence in everyday functioning. Neuropsychology 2008;22(1):110–117; doi: 10.1037/0894-4105.22.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wickrama KAS, Klopack ET, O'Neal CW. Higher-order trajectories of pain and depressive symptoms link midlife financial stress to women's well-being in later life. Aging Ment Health 2022;26(12):2358–2365; doi: 10.1080/13607863.2021.1993129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Muthén LK, Muthén BO. Mplus User's Guide: Statistical Analysis with Latent Variables: User's Guide. Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- 64. Molsberry SA, Cheng Y, Kingsley L, et al. Neuropsychological phenotypes among men with and without HIV disease in the multicenter AIDS cohort study. AIDS 2018;32(12):1679–1688; doi: 10.1097/QAD.0000000000001865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Paul RH, Cho K, Belden A, et al. Cognitive phenotypes of HIV defined using a novel data-driven approach. J Neuroimmune Pharmacol 2022;17(3–4):515–525; doi: 10.1007/s11481-021-10045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sacktor N. Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol 2018;24(2):141–145; doi: 10.1007/s13365-017-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sacktor N, Robertson K. Evolving clinical phenotypes in HIV-associated neurocognitive disorders. Curr Opin HIV AIDS 2014;9(6):517–520; doi: 10.1097/COH.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Paul R. Neurocognitive phenotyping of HIV in the era of antiretroviral therapy. Curr HIV/AIDS Rep 2019;16(3):230–235; doi: 10.1007/s11904-019-00426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Johnson TP, Nath A. Biotypes of HIV-associated neurocognitive disorders based on viral and immune pathogenesis. Curr Opin Infect Dis 2022;35(3):223–230; doi: 10.1097/QCO.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sundermann EE, Bondi MW, Campbell LM, et al. Distinguishing amnestic mild cognitive impairment from HIV-associated neurocognitive disorders. J Infect Dis 2021;224(3):435–442; doi: 10.1093/infdis/jiaa760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Milanini B, Valcour V. Differentiating HIV-associated neurocognitive disorders from Alzheimer's disease: An emerging issue in geriatric NeuroHIV. Curr HIV/AIDS Rep 2017;14(4):123–132; doi: 10.1007/s11904-017-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rubin LH, Sundermann EE, Moore DJ. The current understanding of overlap between characteristics of HIV-associated neurocognitive disorders and Alzheimer's disease. J Neurovirol 2019;25(5):661–672; doi: 10.1007/s13365-018-0702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. D. Bangsberg David RMD, MPH Liu Honghu Ph. D. MIWGJPDgroGKPDRRHPDRMISJP. A closer look at depression and its relationship to HIV antiretroviral adherence. Ann Behav Med 2011;42(3):352–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011;53(11):1120–1126. [DOI] [PubMed] [Google Scholar]
- 75. Gallant J, Hsue PY, Shreay S, et al. Comorbidities among US patients with prevalent HIV infection-a trend analysis. J Infect Dis 2017;216(12):1525–1533; doi: 10.1093/infdis/jix518. [DOI] [PubMed] [Google Scholar]
- 76. Kalichman SC, Rompa D, Cage M. Distinguishing between overlapping somatic symptoms of depression and HIV disease in people living with HIV-AIDS. J Nerv Ment Dis 2000;188(10):662–670; doi: 10.1097/00005053-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 77. Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med 2008;70(5):539–545; doi: 10.1097/PSY.0b013e3181777a5f. [DOI] [PubMed] [Google Scholar]
- 78. Katz IT, Ryu AE, Onuegbu AG, et al. Impact of HIV-related stigma on treatment adherence: Systematic review and meta-synthesis. J Int AIDS Soc 2013;16(3 Suppl 2):18640; doi: 10.7448/IAS.16.3.1864018640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Turan B, Rogers AJ, Rice WS, et al. Association between perceived discrimination in healthcare settings and HIV medication adherence: Mediating psychosocial mechanisms. AIDS Behav 2017;21:3431–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Halkitis PN, Perez-Figueroa RE, Carreiro T, et al. Psychosocial burdens negatively impact HIV antiretroviral adherence in gay, bisexual, and other men who have sex with men aged 50 and older. AIDS Care 2014;26(11):1426–1434; doi: 10.1080/09540121.2014.921276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Langebeek N, Gisolf EH, Reiss P, et al. Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: A meta-analysis. BMC Med 2014;12:142; doi: 10.1186/PREACCEPT-1453408941291432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Leserman J, Ironson G, O'Cleirigh C, et al. Stressful life events and adherence in HIV. AIDS Patient Care STDS 2008;22(5):403–411; doi: 10.1089/apc.2007.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Blashill AJ, Bedoya CA, Mayer KH, et al. Psychosocial syndemics are additively associated with worse ART adherence in HIV-infected individuals. AIDS Behav 2015;19(6):981–986; doi: 10.1007/s10461-014-0925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ham L, Montoya JL, Serrano V, et al. High psychosocial burden relates to poorer antiretroviral treatment adherence among Black/African American people with HIV. AIDS Patient Care STDS 2023;37(2):103–113; doi: 10.1089/apc.2022.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Springer SA, Dushaj A, Azar MM. The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: A systematic review. AIDS Behav 2012;16(8):2119–2143; doi: 10.1007/s10461-012-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Schuster R, Bornovalova M, Hunt E. The influence of depression on the progression of HIV: Direct and indirect effects. Behav Modif 2012;36(2):123–145; doi: 10.1177/0145445511425231. [DOI] [PubMed] [Google Scholar]
- 87. Casaletto KB, Weber E, Iudicello JE, et al. Real-World Impact of HIV-Associated Neurocognitive Impairment. In: Changes in the Brain: Impact on Daily Life. (Chiaravalloti ND, Goverover Y. eds.) New York: Springer, 2017; pp. 211–245. [Google Scholar]
- 88. Thames AD, Becker BW, Marcotte TD, et al. Depression, cognition, and self-appraisal of functional abilities in HIV: An examination of subjective appraisal versus objective performance. Clin Neuropsychol 2011;25(2):224–243; doi: 10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Blackstone K, Moore D, Heaton R, et al. Diagnosing symptomatic HIV-associated neurocognitive disorders: Self-report versus performance-based assessment of everyday functioning. J Int Neuropsychol Soc 2012;18(1):79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sheppard DP, Iudicello JE, Morgan EE, et al. Accelerated and accentuated neurocognitive aging in HIV infection. J Neurovirol 2017;23(3):492–500; doi: 10.1007/s13365-017-0523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Aung HL, Aghvinian M, Gouse H, et al. Is there any evidence of premature, accentuated and accelerated aging effects on neurocognition in people living with HIV? A systematic review. AIDS Behav 2021;25(3):917–960; doi: 10.1007/s10461-020-03053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Stern Y. Cognitive reserve. Neuropsychologia 2009;47(10):2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Foley JM, Ettenhofer ML, Kim MS, et al. Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Appl Neuropsychol 2012;19(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Morgan EE, Woods SP, Smith C, et al. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND). AIDS Behav 2012;16(8):2279–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kaur N, Fellows LK, Brouillette MJ, et al. Development and validation of a cognitive reserve index in HIV. J Int Neuropsychol Soc 2022;28(3):230–238; doi: 10.1017/S1355617721000461. [DOI] [PubMed] [Google Scholar]
- 96. Byrd DA, Fellows RP, Morgello S, et al. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr 2011;58(2):154–162; doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Earnshaw VA, Eaton LA, Collier ZK, et al. HIV stigma, depressive symptoms, and substance use. AIDS Patient Care STDS 2020;34(6):275–280; doi: 10.1089/apc.2020.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Bengtson AM, Pence BW, Crane HM, et al. Disparities in depressive symptoms and antidepressant treatment by gender and race/ethnicity among people living with HIV in the United States. PLoS One 2016;11(8):e0160738; doi: 10.1371/journal.pone.0160738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Williamson TJ, Mahmood Z, Kuhn TP, et al. Differential relationships between social adversity and depressive symptoms by HIV status and racial/ethnic identity. Health Psychol 2017;36(2):133–142; doi: 10.1037/hea0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Cross S, Onen N, Gase A, et al. Identifying risk factors for HIV-associated neurocognitive disorders using the international HIV dementia scale. J Neuroimmune Pharmacol 2013;8(5):1114–1122; doi: 10.1007/s11481-013-9505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sundermann EE, Heaton RK, Pasipanodya E, et al. Sex differences in HIV-associated cognitive impairment. AIDS 2018;32(18):2719–2726; doi: 10.1097/QAD.0000000000002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rubin LH, Sundermann EE, Dastgheyb R, et al. Sex differences in the patterns and predictors of cognitive function in HIV. Front Neurol 2020;11:551921; doi: 10.3389/fneur.2020.551921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. LeGrand SH, Davis DA, Parnell HE, et al. Integrating HIV and mental health services for Black gay, bisexual, and other men who have sex with men living with HIV: Findings from the STYLE 2.0 intervention. AIDS Patient Care STDS 2022;36(S1):S74–S85; doi: 10.1089/apc.2022.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]