Abstract
Psoriasis is an immune-mediated inflammatory disease associated with an increased risk of cardiovascular disease (CVD). The risk of CVD increases with the severity of psoriasis, and exposure to systemic inflammation may partly explain the increased risk of CVD in these patients. This raises the question of whether anti-psoriatic treatment, in addition to treating the skin lesions, also lowers the risk of developing CVD. Different types of studies have examined the impact of systemic anti-psoriatic treatments on the risk of CVD in patients with psoriasis and epidemiological observational studies with, e.g., myocardial infarction and stroke as outcomes, and clinical studies investigating circulating inflammatory biomarkers in the blood indicate that anti-psoriatic therapy has a protective effect; however, no randomized controlled trial (RCT) has examined the impact of systemic anti-psoriatic treatment on future hard cardiovascular endpoints. This narrative review provides an overview of the clinical cardiovascular imaging studies examining the effect of systemic anti-psoriatic treatment on the risk of subclinical CVD in patients with psoriasis. We found a total of 24 clinical imaging studies, where 16 of these were observational cohort studies and eight were RCTs. The observational studies suggest an improvement in the risk of subclinical CVD based on different cardiovascular imaging biomarkers; however, the RCTs showed inconsistent results and mainly included vascular inflammation as the outcome. Future RCTs including other imaging biomarkers as surrogates for subclinical CVD, with longer follow-up and with hard cardiovascular endpoints are warranted to address whether systemic anti-psoriatic treatments reduce the risk of CVD.
Keywords: Psoriasis, Cardiovascular disease, Cardiovascular risk, Cardiovascular imaging, Treatment, Biologics
Key Summary Points
| The systemic inflammation seen in patients with psoriasis may partly explain the increased risk of cardiovascular disease in these patients. |
| Epidemiological studies suggest that systemic anti-psoriatic treatment may reduce the risk of cardiovascular disease in patients with psoriasis. |
| The observational cardiovascular imaging studies included in this review indicate that systemic anti-psoriatic treatment, mainly with biologics, has a protective effect on subclinical cardiovascular disease (CVD); however, these studies vary greatly in design, sample size and included different cardiovascular imaging biomarkers as outcomes. |
| The results from randomized controlled trials (RCTs) are inconsistent and mainly include biologics as intervention and vascular inflammation detected by fluorodeoxyglucose positron emission tomography/computed tomography as the outcome. |
| RCTs with longer follow-up time including different cardiovascular imaging outcomes and hard cardiovascular endpoints are warranted to elucidate whether different anti-psoriatic treatments reduce the risk of CVD in patients with psoriasis. |
Introduction
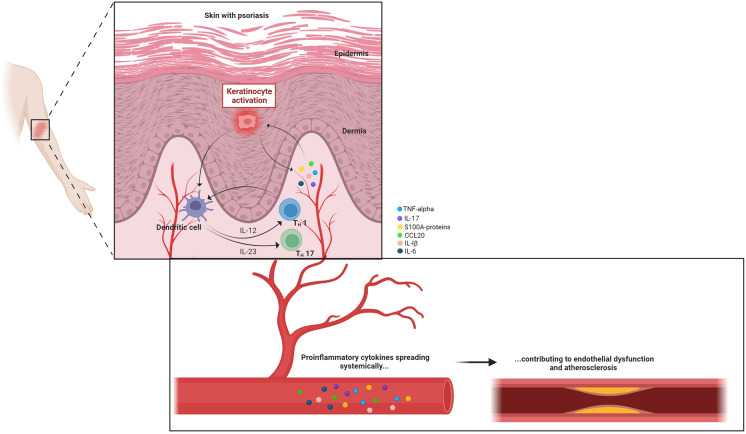
Psoriasis is an immune-mediated inflammatory disease characterized by scaly and erythematous well-demarcated skin lesions [1]. A dysregulated immune system, involving both the innate and adaptive system, is the cornerstone of the pathogenesis [2]. Interestingly, increased levels of immune cells and pro-inflammatory cytokines are not found only in the skin but also in the blood [3–6]. This chronic systemic inflammation is suggested to be part of the explanation for the increased risk of several comorbidities in patients with psoriasis, such as psoriatic arthritis, metabolic syndrome, diabetes, mental disorders, inflammatory bowel disease, liver disease and, in particular, cardiovascular disease (CVD) [7]. The chronic systemic inflammation in patients with psoriasis is thought to enhance endothelial dysfunction, leading to atherosclerosis and cardiovascular events [8] (Fig. 1). Moreover, patients with psoriasis have a high prevalence of unfavourable lifestyle factors such as obesity [9], low physical activity [10], smoking [11], hypertension [9] and dyslipidaemia [9], which also contributes to the development of CVD. Interestingly, a recent Mendelian randomization study also found causality in the other direction, where coronary artery disease appears as a causal risk factor for developing psoriasis [12].
Fig. 1.
Visual illustration of the concept that the chronic systemic inflammation in patients with psoriasis enhances endothelial dysfunction leading to atherosclerosis and cardiovascular events. TNF tumor necrosis factor, IL interleukin, CCL20 chemokine ligand 20, TH T helper
During the last 20 years, biologics have been introduced as highly effective treatment options for patients with moderate to severe psoriasis [1]. Biologics target specific cytokines or receptors along the inflammatory pathways of the disease. In the wake of that, biologics and other systemic anti-psoriatic treatments have been suggested to reduce skin symptoms and the systemic inflammatory burden and consequently reduce the risk of atherosclerosis. In 2017, a landmark study from the Canakinumab Antiinflammatory Thrombosis Outcome Study (CANTOS) Trial Group found that anti-inflammatory therapy with canakinumab reduced the risk of recurrent cardiovascular events compared with placebo in patients with previous myocardial infarction [13]. The emerging question for dermatologists is whether we can reduce the risk of CVD in our patients if we treat the psoriatic skin lesions early and more aggressively [14]. Since CVD develops slowly over several years, gold-standard randomized controlled trials (RCTs) examining the impact of systemic anti-psoriatic treatment on cardiovascular disease in patients with psoriasis is difficult to conduct. With the desire to answer this question, four types of generic studies have been conducted. First, RCTs examining the efficacy of a new drug, and herein also reporting safety data. These studies focus on acute adverse events, such as major cardiovascular events (MACE) caused by the drug during a short trial period (12–52 weeks). Second, observational register-based studies examining the long-term risk of developing CVD according to different anti-psoriatic treatments. The advantage of register-based studies is the long follow-up time; however, these studies are subject to different kinds of bias and confounding. Although these studies are good for generating hypotheses, they are not suitable for determining causal relationships. Third, clinical studies examining the effect of anti-psoriatic treatment on subclinical atherosclerosis using pro-inflammatory cytokines and other mediators of inflammation as surrogate biomarkers for future CVD. The clinical validity of these biomarkers is still uncertain and may not be a good measure of future CVD. Fourth, clinical studies examining the effect of anti-psoriatic treatment on subclinical atherosclerosis using imaging methods (imaging biomarkers) as a surrogate for future risk of CVD. Imaging biomarkers detect existing (but subclinical) disease and may therefore be closer to the clinical outcome (CVD) compared with biomarkers measured in the blood [15] and might be a better surrogate for future CVD.
Therefore, this narrative review aims to summarize what we know from clinical studies examining the effect of anti-psoriatic treatment on subclinical atherosclerosis using imaging biomarkers as a surrogate for future CVD.
Methods
Our search included clinical studies published in English examining the potential role of systemic anti-psoriatic treatment on the risk of subclinical atherosclerosis. These articles were extracted by three independent reviewers (Hannah Kaiser, Charlotte Näslund-Koch and Amanda Kvist-Hansen) from the PubMed database. The search was performed until 1 July 2023, and the following combination of search terms were used: ‘psoriasis’ or ‘psoriatic’ and ‘cardiovascular imaging’, ‘computed tomography’, ‘positron emission tomography’, ‘cardiovascular events’, ‘myocardial infarction’, ‘stroke’ and ‘biologics’, ‘IL-17 inhibitor’, ‘TNF-alpha inhibitor’, ‘TNF inhibitor’, ‘IL-12/23 inhibitor’, ‘IL-23 inhibitor’, ‘JAK-inhibitor’, ‘Infliximab’, ‘bimekizumab’, ‘etanercept’, ‘adalimumab’, ‘ustekinumab’, ‘secukinumab’, ‘ixekizumab’, ‘brodalumab’, ‘guselkumab’, ‘tildrakizumab’, ‘risankizumab’, ‘ciclosporin’, ‘methotrexate’, ‘acitretin’, ‘apremilast’ or ‘fumaric acid’. In addition, systematic reviews and meta-analyses on the subject were screened for relevant articles. We only included studies or the part of studies examining the effect of systemic anti-psoriatic treatment on imaging biomarkers in patients with psoriasis compared with a control/placebo group or studies comparing baseline and post-treatment values. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Imaging Modalities
Cardiovascular imaging measurements are widely used as biomarkers of subclinical atherosclerosis and include simple bedside ultrasound imaging and more advanced methods such as the computed tomography (CT; Fig. 2). Carotid intima-media thickness (CIMT) of the carotid, brachial and/or femoral arteries is examined by traditional 2D ultrasound, and increased CIMT is associated with increased risk of CVD including, e.g., coronary artery disease and stroke [16, 17]. In addition, echocardiography is a bedside examination of the myocardial function, and, e.g., left ventricular dysfunction measured by the global longitudinal strain (GLS) value may predict adverse cardiovascular events [18, 19]. Furthermore, other non-invasive measurements include pulse wave velocity (PWV) and flow-mediated dilatation (FMD) as measurements of arterial stiffness and endothelial dysfunction, respectively, and these values have been shown to be related to cardiovascular events and all-cause mortality [20, 21].
Fig. 2.
Imaging modalities used in the included clinical studies. CCTA coronary computed tomography angiography, E early diastolic mitral inflow velocity, e′ early diastolic mitral annular velocity, FDG-PET/CT fluorodeoxyglucose positron emission tomography/computed tomography, GLS global longitudinal strain, MRI magnetic resonance imaging
Other more time-consuming cardiovascular imaging methods include magnetic resonance imaging (MRI), coronary computed tomography angiography (CCTA) and fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT). Both MRI and CCTA can examine the total plaque burden within the arteries (mainly the aorta or coronary or carotid arteries), and coronary artery disease detected by CCTA is strongly linked with increased risk of future adverse cardiac events [22]. The coronary artery calcium score (CACS) is detected from a non-invasive low-radiation non-contrast CT. This marker describes the amount of calcification in coronary artery plaques and is also associated with increased risk of future CVD [23, 24]. In the recent two decades, vascular inflammation, primarily in the carotid arteries or the aorta detected by the FDG-PET/CT has become a frequent measurement of subclinical atherosclerosis. In brief, the mechanism is based on uptake of the intravenously injected FDG by living cells such as the macrophages, and within these cells FDG gets trapped. During inflammation within the vessel wall, especially macrophages proliferate. FDG accumulates in proportion to the cellular metabolic activity in these cells and therefore allows for FDG-PET/CT to visualize inflammation in vascular tissue, and vascular inflammation has shown to be predictive of future CVD [25, 26].
Results of Clinical Imaging Studies
We identified a total of 710 articles and excluded studies that did not examine a cardiovascular imaging endpoint, had no follow-up after the systemic anti-psoriatic treatment or lacked a control/placebo group. We included a total of 24 studies; out of these, 16 studies were observational cohort studies [18, 27–41] and 8 were RCTs [42–49] (Table 1 and Table 2).
Table 1.
Observational cohort studies
| Author | Year | Imaging method | PsO or PsA | N (treatment group/controls) | Controls | Treatment | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Jókai et al. [27] | 2013 | Ultrasound | PsO | 16 | None (change from baseline) | Anti-TNF (etanercept, infliximab or adalimumab) | Carotid and brachial IMT | Anti-TNF was associated with reduced IMT after 6 months of treatment, but not in those with pre-existing atherosclerotic plaques. |
| Hjuler et al. [34] | 2016 | Non-contrast CACS CT and CCTA | PsO | 56 (28/28) | Patients with PsO not receiving systemic treatment | Biologics (adalimumab, etanercept, infliximab or ustekinumab) | CACS, number of segments with luminal narrowing and vessel wall volume index | Biologics were associated with reduced coronary artery disease progression after 13 months of treatment. |
| Pina et al. [41] | 2016 | Ultrasound | PsO | 29 | None (change from baseline) | Adalimumab | FMD and PWV | Adalimumab was associated with improvement of endothelial function (FMD) and arterial stiffness (PWV) after 6 months of treatment. |
| Ahlehoff et al. [18] | 2016 | Echocardiography | PsO | 18 | None (change from baseline) | Biologics (adalimumab N = 14, ustekinumab N = 4) | Left ventricular GLS and E/e′ ratio | Biologics were associated with improvements in myocardial dysfunction (GLS and E/e′ ratio) after 3 months of treatment. |
| Heredi et al. [28] | 2016 | Echocardiography | PsO | 44 | None (change from baseline) | Anti-TNF (infliximab, adalimumab or etanercept) | TAPSE and right ventricular free wall peak systolic velocity (cm/s) among others | Anti-TNF resulted in improvement of right ventricular systolic function after a mean of 30 months of treatment. |
| Dey et al. [29] | 2017 | FDG-PET/CT | PsO | 115 (N = 17 at follow-up) | None (change from baseline) | Biologics and other systemic treatment (anti-TNF N = 17) | Vascular inflammation (TBR) in aorta | Improvement in PASI score was associated with improvement in vascular inflammation after 1 year of treatment, mainly driven by those with higher response in PASI score. The strongest association was seen in patients treated with anti-TNF. |
| Eder et al. [30] | 2018 |
Stage 1: ultrasound Stage 2: FDG-PET/CT |
PsO (stage 1) and PsA (stage 2) | Stage 1: 319 (111/208); Stage 2: 34 (21/ 13) | Patients with PsO/PsA not receiving systemic treatment | Anti-TNF (infliximab, etanercept, adalimumab, golimumab or certolizumab) for at least 50% of the follow-up period |
Stage 1: TPA in carotid arteries Stage 2: vascular inflammation (TBR) in aorta |
Anti-TNF treatment was associated with reduced progression of carotid plaques in men (not in women) after 2–3 years of treatment (Stage 1) and improvement in vascular inflammation in patients with psoriatic disease after 52 weeks of treatment (Stage 2). |
| Sorokin et al. [31] | 2018 | CCTA | PsO | 20 | None (change from baseline) | Biologic therapy (anti-TNF N = 9, anti-IL-17 N = 7, anti-IL-12/23 N = 5) | Total burden, non-calcified plaque burden, dense-calcified burden | Biologics were associated with a decrease in total plaque burden and non-calcified burden after 3–5 months of treatment. |
| Kim et al. [32] | 2018 | FDG-PET/CT | PsO | 25 (N = 10 at follow-up) | None (change from baseline) | Ustekinumab | Vascular inflammation (TBR) in aorta | Ustekinumab was associated with decreased vascular inflammation after 5 months of treatment. |
| Elnabawi et al. [35] | 2019 | CCTA | PsO | 134 (82/52) | PsO patients only receiving topical treatment or phototherapy | Biologics (anti–TNF, anti–IL-12/23 or anti–IL-17) | Perivascular FAI | Biologics were associated with a reduction in coronary inflammation assessed as FAI after 52 weeks of treatment. |
| Elnabawi et al. [33] | 2019 | CCTA | PsO | 121 (89/32) | Patients with PsO only receiving topical treatment or phototherapy | Biologics (adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab) | Non-calcified coronary plaque burden, total plaque burden in the main coronary arteries | Treatment with biologics was associated with reduced non-calcified coronary artery plaques and improvement in plaque morphology after 52 weeks of treatment. |
| Choi et al. [36] | 2020 | CCTA | PsO | 209 (124/85) | Patients with PsO not receiving biologic therapy | Biologics, anti-TNF (adalimumab, etanercept), anti-IL-12/23 (ustekinumab) and anti-IL-17 inhibitors (ixekizumab, secukinumab) | LRNC | Biologics were associated with favourable modification of LRNC after 52 weeks of treatment. |
| Makavos et al. [37] | 2020 | Echocardiography | Both (PsO N = 78, PsA N = 72) | 150 (50/50/50) | Patients with Pso/PsA receiving methotrexate or cyclosporine | Secukinumab (N = 50), methotrexate (N = 50) and cyclosporine (N = 50) | PWV, coronary flow reserve, left ventricular myocardial deformation | Secukinumab was associated with improvement of arterial elasticity, myocardial deformation indices and coronary artery function after 16 and 52 weeks of treatment. Treatment with methotrexate and cyclosporine had no effect on coronary artery function and myocardial deformation after 52 weeks of treatment. |
| Marovt et al. [38] | 2020 | Ultrasonography and PWA | PsO | 15 | None (change from baseline) | Biologics [(anti-IL-12/23 and IL-17) secukinumab, ustekinumab and ixekizumab] | PWV, augmentation index and IMT | Biologics targeting the IL-23/IL-17 axis had no effect on vascular structure (IMT) and PWV after 6 months of treatment. |
| Piros et al. [39] | 2021 | Ultrasound | PsO | 31 | None (change from baseline) | Anti-IL-17 [secukinumab, (N = 20), ixekizumab (N = 11)] | The carotid, brachial and femoral IMT | Anti-IL-17 treatment was associated with reduced carotid, brachial and femoral atherosclerosis (IMT) after 6 months of treatment. |
| Gelfand et al. [40] | 2022 | FDG-PET/CT | PsO | 70 | None (change from baseline) | Apremilast | Vascular inflammation (TBR) in entire aorta | Apremilast had no effect on aortic vascular inflammation after 16 and 52 weeks of treatment. |
CACS coronary artery calcium score, CT computed tomography, CCTA coronary computed tomography angiography, E early diastolic mitral inflow velocity, e′ early diastolic mitral annular velocity, FAI fat attenuation index, FDG-PET/CT fluorodeoxyglucose-positron emission tomography/computed tomography, FMD flow-mediated dilation, GLS global longitudinal strain, IL interleukin, IMT intima-media thickness, TBR target-to-background ratio, TAPSE tricuspid annular plane systolic excursion, anti-TNF anti-tumour necrosis factor, TPA total plaque area, PASI Psoriasis Area and Severity Index, PsO psoriasis, PsA psoriatic arthritis, PWA pulse wave analysis, PWV pulse wave velocity
Table 2.
Randomized controlled trials
| Author | Year | Imaging method | PsO or PsA | N (intervention/controls) | Controls | Treatment | Outcome | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Bissonnette et al. [42] | 2013 | FDG-PET/CT | PsO | 30 (20/10) | Patients with PsO receiving topical therapies or phototherapy | Adalimumab | Vascular inflammation (TBR and MDS) in carotid arteries and ascending aorta | Reduced vascular inflammation after treatment with adalimumab for 15 weeks. No change in maximum TBR, but significant changes in mean TBR and MDS were found between patients receiving adalimumab versus controls. |
| Bissonnette et al. [43] | 2017 | FDG-PET/CT | PsO | 107 (54/53) | Patients with PsO receiving placebo | Adalimumab | Vascular inflammation (TBR) in carotid arteries and ascending aorta | No difference in vascular inflammation in patients treated with adalimumab or placebo for 16 weeks. A small increase in vascular inflammation in the carotid arteries was seen after 52 weeks of treatment with adalimumab. |
| Ikonomidis et al. [44] | 2017 | Echocardiography, pulse wave doppler, PWA | PsO | 150 (intervention N = 50, anti-TNF N = 50, cyclosporine N = 50) | Patients with PsO receiving etanercept or cyclosporine | Ustekinumab (N = 50) | Left ventricular GLS, coronary flow reserve, PWV, augmentation index, twisting–untwisting | Ustekinumab was associated with greater improvement in coronary, arterial and myocardial function compared with treatment with anti-TNF and cyclosporine after 4 months. |
| Kaur et al. [45] | 2018 | FDG-PET/CT | PsO | 16 (8/8) | Patients with PsO receiving placebo with or without treatment of plioglitazone | Methotrexate | Vascular inflammation (maximum SUV) in aorta | No difference in vascular inflammation (SUV) between those receiving methotrexate versus placebo after 12 weeks. |
| Mehta et al. [46] | 2018 | FDG-PET/CT | PsO | 97 (intervention N = 32, phototherapy N = 32, placebo N = 32) | Patients with PsO receiving placebo or phototherapy | Adalimumab | Vascular inflammation (TBR) in aorta | No change in vascular inflammation was found after treatment with adalimumab for 12 or 52 weeks compared with patients receiving phototherapy or placebo. |
| Esther von Stebut et al. [47] | 2019 | Ultrasound and MRI | PsO | 151 (102/49) | Patients with PsO receiving placebo | Secukinumab | FMD, arterial atherosclerotic plaque burden (vessel wall MRI) and PWV and augmentation index | No difference was found after 12 weeks between patients receiving secukinumab versus placebo. But secukinumab was associated with improved FMD in patients with psoriasis after 52 weeks of treatment compared with placebo. No changes were found in proatherogenic vessel wall. |
| Gelfand et al. [48] | 2020 | FDG-PET/CT | PsO | 43 (22/21) | Patients with PsO receiving placebo | Ustekinumab | Vascular inflammation (TBR) in aorta | Ustekinumab was associated with reduced vascular inflammation after 12 weeks of treatment but not at 52 weeks of treatment. |
| Gelfand et al. [49] | 2020 | FDG-PET/CT | PsO | 91 (46/45) | Patients with PsO receiving placebo | Secukinumab | Vascular inflammation (TBR) in aorta | Secukinumab had no effect on aortic vascular inflammation after 12 or 52 weeks of treatment. |
CT computed tomography, FDG-PET/CT fluorodeoxyglucose-positron emission tomography/computed tomography, FMD flow-mediated dilation, GLS global longitudinal strain, MDS most diseased segment, MRI magnetic resonance imaging, TBR target-to-background ratio, anti-TNF anti-tumour necrosis factor, PsO psoriasis, PsA psoriatic arthritis, PWA pulse wave analysis, PWV pulse wave velocity
Observational Cohort Studies
A total of 1406 patients with psoriasis and/or psoriatic arthritis were included in the 16 observational studies, with follow-up times ranging from 3 months [31] to approximately 3 years [28, 29], but 6–12 months were most common. The designs of the studies differed; five of them included a control group of patients with psoriasis not receiving systemic anti-psoriatic treatment or biologics [30, 33–36], whereas the remaining 11 studies measured changes from baseline (Table 1). Overall, the studies had small sample sizes: eight of the studies had a sample size of ≥ 50 patients [29, 30, 33–37, 40], and the largest study included 209 patients [36]. Only one study investigated the effect of a non-biologic drug, apremilast [40], whereas the rest of the studies examined the effect of biologics. Seven studies examined the effect of biologics as a group [18, 29, 31, 33–36], and five of these studies subsequently stratified according to specific target (TNF, IL-17 or IL-12/23) [29, 33–36]. In addition, four other studies focused only on the effect of anti-TNF [27, 28, 30, 41]. Two studies restricted their analyses to the effect of anti-IL-17 agents [37, 39], and one study to the effect of anti-IL-12/23 agents [32].
The observational cohort studies used different imaging modalities as surrogates for subclinical atherosclerosis including vascular inflammation, myocardial dysfunction, endothelial dysfunction, coronary-, carotid-, brachial- and femoral atherosclerosis, coronary artery plaque burden and arterial stiffness (Fig. 2). All, except two [38, 40] of the observational cohort studies showed a significant improvement or reduction in disease progression of the specific cardiovascular imaging biomarker during follow-up examinations (Table 1). Studies examining aortic vascular inflammation found that 1 year of anti-TNF treatment was associated with both improvement in vascular inflammation independent of CVD risk factors [30] and reduced psoriasis severity and improvement in vascular inflammation [29]. Treatment for 5 months with ustekinumab was associated with reduced vascular inflammation in almost all segments of aorta in 10 patients with psoriasis [32], while no significant effect on vascular inflammation was found after 16 or 52 weeks of treatment with apremilast [40]. In addition, coronary inflammation assessed by perivascular fat attenuation index (FAI) was reduced after 1 year of treatment with biologics, which remained consistent after stratification of biologic agents (anti-TNF, anti-IL-12/23 and anti-IL-17) [35]. Studies examining coronary atherosclerosis and coronary artery plaque burden found reduced coronary artery progression [34] (significant difference in those who received biologics versus control group), improvement in coronary plaque morphology and reduced total and high-risk coronary plaques [31, 33, 36] after treatment with biologics as a group including anti-TNF, anti-IL12/23- and anti-IL-17 agents. Moreover, carotid-, brachial- and femoral atherosclerosis, arterial stiffness, and myocardial and endothelial function improved during treatment with anti-TNF, anti-IL-12/23 or anti-IL-17 agents (Table 1). However, one study did not find any significant effect of either anti-IL-12/23 or anti-IL-17 agents on arterial stiffness or carotid atherosclerosis [38]. Another study only found reduced carotid and brachial intima-media thickness (IMT) in those without pre-existing atherosclerotic plaques after 6 months of treatment with anti-TNF [27].
Overall, the observational studies varied greatly in terms of design, sample size and outcome (measured imaging biomarker), but the studies generally reported reductions or improvements in imaging biomarkers associated with CVD after treatment with biologics.
Randomized Controlled Trials
In total, all the eight RCTs included 685 patients with psoriasis (both patients receiving intervention and placebo). In five out of the eight included RCTs, patients were randomized to intervention or placebo for the first 12 or 16 weeks, and following this, the placebo group crossed over and received the intervention for approximately 1 year [43, 46–49]. Two studies had included control groups that either received etanercept and cyclosporine [44] or phototherapy and/or local therapy [42]. Only one study examined the effect of a non-biologic treatment (methotrexate), and this study had a control group that received placebo, with or without plioglitazone [45].
Vascular inflammation was the main outcome in six of eight studies [42, 43, 45, 46, 48, 49]. Two of these studies found a significant reduction in vascular inflammation after treatment with adalimumab and ustekinumab for 15 weeks and 12 weeks, respectively [42, 48]. However, the effect of ustekinumab did not remain significant at follow-up after 52 weeks [48]. Moreover, four studies showed no effect on vascular inflammation after treatment with adalimumab (12, 16 or 52 weeks), methotrexate (12 weeks) and secukinumab (12 or 52 weeks) [43, 45, 46, 49].
Treatment with ustekinumab was associated with improvements in myocardial function and arterial stiffness in one study after 4 months [37], and treatment with secukinumab reduced the endothelial dysfunction measured by flow-mediated dilation (FMD) after 1 year, but no difference was found in arterial stiffness or total plaque burden in the carotid arteries or aorta [47].
Overall, the findings from the RCTs do not indicate an effect of anti-psoriatic treatment on the risk of CVD. Most of the studies investigated changes in vascular inflammation over a short period of time (12–16 weeks) compared with placebo, and since all patients crossed over to the intervention group, the results at week 52 were not placebo controlled.
Discussion
In this narrative review, we summarize the results of clinical studies using cardiovascular imaging biomarkers of subclinical CVD to investigate effects of systemic anti-psoriatic treatment on the risk of CVD in patients with psoriasis. We included both observational clinical studies and RCTs, and the majority of the observational studies reported a protective effect of systemic anti-psoriatic treatments on subclinical CVD. In contrast, results from RCTs were inconsistent and do not confirm that systemic anti-psoriatic treatments reduce the risk of subclinical CVD.
Other types of studies have also been conducted to investigate the effect of systemic anti-psoriatic treatment on the risk of CVD. A large meta-analysis based on safety data from RCTs reported that treatment with biologics were not associated with increased risk of MACE during the short clinical trial periods, typically 12–52 weeks [50]. Safety data was also examined in a prospective cohort study investigating the risk of MACE up to 2 years after initiation of different biologics and methotrexate and did likewise not find any difference in MACE between the treatment groups [51]. These studies were not designed to examine whether systemic anti-psoriatic treatment reduces the incidence of CVD in patients with psoriasis but rather merely to investigate potential adverse events due to biologics. Registry data have suggested a protective effect of treatment with anti-TNF agents [52] and methotrexate [53] on the incidence of CVD such as myocardial infarction and stroke in patients with psoriasis. The advantages of register-based studies are long follow-up time, large sample sizes and the ability to investigate actual CV events rather than surrogates. Studies using circulating inflammatory biomarkers in blood as surrogates for subclinical CVD in patients with psoriasis have also been conducted, these biomarkers include, e.g., GlycA [54], S100A8/9 [55], growth differentiation factor-15 (GDF-15) [56], high-sensitivity (hs)-CRP [57] and the neutrophil-to-lymphocyte ratio (NLR) [5]. Some of these biomarkers are also known predictors of CVD in the general population [58–60]. Interestingly, in patients with psoriasis, decreased levels of both NLR and hs-CRP have been reported under treatment with biologics [61]. Several studies included in this review also examined blood biomarkers of systemic inflammation including, e.g., hs-CRP [27, 29, 31, 33–36, 40, 42, 43, 46–49], IL-6 [40, 43, 44, 46, 48, 49], TNF-α [40, 43, 44, 46, 48, 49] and GlycA [36, 40, 46, 48, 49], among others. The most frequently measured inflammatory biomarker was hs-CRP, and levels of hs-CRP were reduced after different systemic anti-psoriatic treatments in six out of eight and in three out of six observational studies and RCTs that included hs-CRP in their analyses, respectively. However, whether the reported decrease in circulating inflammatory biomarkers observed under systemic anti-psoriatic treatment results in a reduced risk of CVD in these patients is still unknown and needs further investigation. In summary, studies on safety data, register data, and circulating inflammatory biomarkers support the findings from the observational studies included in this review.
In contrast to results from the observational studies, the included imaging RCTs showed inconsistent results. Treatment with ustekinumab reduced vascular inflammation after 12 weeks, but this effect was not sustained after 52 weeks [48]. Additionally, adalimumab and secukinumab did not show an effect on vascular inflammation [46, 49], and contradictory adalimumab showed a small increase in vascular inflammation after 52 weeks [43]. There may be various reasons for the conflicting results between the observational imaging studies and the RCTs. In the RCTs, the most frequent investigated imaging biomarker was vascular inflammation obtained by the FDG-PET/CT. Although this imaging biomarker has been associated with future CVD [26], some limitations must be considered. The spatial resolution of FDG-PET is low, which reduces the ability to detect small early-stage plaques of atherosclerosis. Furthermore, the FDG uptake is relatively small in inflammatory plaques, leading to a reduced contrast between the plaques and the surrounding background. To compensate for this, vascular inflammation values are often corrected by the target-to-background ratio (TBR) [62]. However, TBR values depend on the time between FDG injection and imaging acquisition, which have varied between study protocols [62]. Moreover, different cardiovascular imaging biomarkers may describe different stages of the atherosclerotic disease progression with not necessarily a strict overlap, which may cause discrepancy between the results found in the observational studies and the RCT [63, 64]. Indeed, vascular inflammation and, e.g., calcifications in the arterial wall most likely represent different phases of atherosclerosis [65].
Important limitations in the included imaging RCTs are small sample sizes and short follow-up periods. A follow-up period of 12–52 weeks may be too short of a period to examine the real effects of biologics on the actual risk of CVD. The observational studies investigated different imaging biomarkers of subclinical CVD obtained by a variety of methods, and no studies examined the cardiovascular effort tolerance. The studies are therefore not directly comparable. However, the studies generally reported reductions and improvements in subclinical CVD after treatment with biologics, pointing towards a protective effect of biologics on the CVD risk in patients with psoriasis. Furthermore, several of the observational studies had a relatively long follow-up time of approximately 1 year, and two studies excelled by including up to 3 years of follow-up time [28, 30]. Another important aspect of the CVD risk in patients with psoriasis is the higher prevalence of traditional CV risk factors including hyperlipidemia, and in these patients cholesterol-lowering drugs including statins have been reported beneficial by reducing cholesterol levels and CV events [66]. Also statin treatment has been linked with reduced aortic vascular inflammation measured by the FDG-PET/CT in patients with psoriasis [67]. Indeed, statin use is an important confounder which also displays pleiotropic anti-inflammatory actions [68], and importantly, it is a strength that 12 out of the 16 observational studies adjusted for statins, equally distributed statin-users between the groups or excluded patients with dyslipidemia [18, 27, 29–37, 39]. In the RCTs, patients who received statins appeared with equal frequency between the groups [44, 46, 48, 49] were excluded from the study [45] or the use of statins was continued during the study if a constant stable dose was received by the patients [42, 43, 47]. In addition, several of the studies included different anti-psoriatic treatment, and each treatment may have different effect on the risk of subclinical CVD.
There are some ongoing RCTs investigating the impact of systemic anti-psoriatic treatment on risk of CVD using different imaging and circulating inflammatory biomarkers. As a result, more data is soon available to further examine this inconclusive field. However, RCTs with clinical hard CV endpoints are still lacking.
Conclusion
In conclusion, the results of this narrative review suggest that systemic anti-psoriatic treatment (biologics) reduces the risk of subclinical atherosclerosis measured by cardiovascular imaging biomarkers in observational studies. When using vascular inflammation as outcome in RCTs, this could not be confirmed, which could be due to short follow-up times. Importantly, whether and how changes in cardiovascular imaging biomarkers correlate with the actual risk of CVD is still unknown. Also, the individual drug may have various impacts on the risk of CVD, and this should be taken into account in future studies. CVD develops slowly, usually over years or decades, and therefore a longer follow-time along with hard CV endpoints such as myocardial infarction and stroke should ideally be included in future RCTs to be able to answer whether systemic anti-psoriatic treatment impacts the risk of CVD.
Author Contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published. Hannah Kaiser, Charlotte Näslund-Koch, Amanda Kvist-Hansen and Lone Skov contributed to the study of the design. Hannah Kaiser, Charlotte Näslund-Koch and Amanda Kvist-Hansen drafted the first manuscript, and all authors contributed with revisions. All authors have provided important intellectual input and approved the final version of the manuscript.
Funding
No funding or sponsorship was received for this study or the publication of this article.
Data Availability
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.
Declarations
Conflict of Interest
Hannah Kaiser and Amanda Kvist-Hansen have nothing to declare. Lone Skov has received research funding from Novartis, Bristol-Myers Squibb, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the LEO Foundation and the Kgl Hofbundtmager Aage Bang Foundation, and honoraria as consultant and/or speaker for AbbVie, Eli Lilly, Novartis, Pfizer, and LEO Pharma, Janssen Cilag, UCB, Almirall, Bristol-Myers Squibb and Sanofi. She has served as an investigator for AbbVie, Pfizer, Amgen, Sanofi, Janssen Cilag, Boehringer Ingelheim, Eli Lilly, Novartis, Galderma and LEO Pharma. Charlotte Näslund Koch has served as an investigator for Galderma, Abbvie, LEO Pharma, Novartis and CSL Behring.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker JNWN. Psoriasis. Lancet. 2021;397:1301–1315. doi: 10.1016/S0140-6736(20)32549-6. [DOI] [PubMed] [Google Scholar]
- 2.Boehncke W-H, Schön MP. Psoriasis. Lancet (London, England) 2015;386:983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 3.Coimbra S, Oliveira H, Reis F, Belo L, Rocha S, Quintanilha A, et al. C-reactive protein and leucocyte activation in psoriasis vulgaris according to severity and therapy. J Eur Acad Dermatol Venereol. 2010;24:789–796. doi: 10.1111/j.1468-3083.2009.03527.x. [DOI] [PubMed] [Google Scholar]
- 4.Chhabra S, Narang T, Joshi N, Goel S, Sawatkar G, Saikia B, et al. Circulating T-helper 17 cells and associated cytokines in psoriasis. Clin Exp Dermatol. 2016;41:806–810. doi: 10.1111/ced.12845. [DOI] [PubMed] [Google Scholar]
- 5.Kvist-hansen A, Kaiser H, Wang X, Krakauer M, Gørtz PM, McCauley BD, et al. Neutrophil pathways of inflammation characterize the blood transcriptomic signature of patients with psoriasis and cardiovascular disease. Int J Mol Sci. 2021;22:10818. doi: 10.3390/ijms221910818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, et al. Inflammasome signaling and impaired vascular health in psoriasis. Arterioscler Thromb Vasc Biol. 2019;39:787–798. doi: 10.1161/ATVBAHA.118.312246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and comorbid diseases: epidemiology. J. Am. Acad. Dermatol. Mosby Inc.; 2017. p. 377–90. [DOI] [PMC free article] [PubMed]
- 8.Boehncke W-H, Boehncke S, Tobin A-M, Kirby B. The, “psoriatic march”: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20:303–307. doi: 10.1111/j.1600-0625.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 9.Miller IM, Skaaby T, Ellervik C, Jemec GBE. Quantifying cardiovascular disease risk factors in patients with psoriasis: a meta-analysis. Br J Dermatol. 2013;169:1180–1187. doi: 10.1111/bjd.12490. [DOI] [PubMed] [Google Scholar]
- 10.Torres T, Alexandre JM, Mendonça D, Vasconcelos C, Silva BM, Selores M. Levels of physical activity in patients with severe psoriasis: a cross-sectional questionnaire study. Am J Clin Dermatol. 2014;15:129–135. doi: 10.1007/s40257-014-0061-0. [DOI] [PubMed] [Google Scholar]
- 11.Näslund-Koch C, Vedel-Krogh S, Bojesen SE, Skov L. Smoking is an independent but not a causal risk factor for moderate to severe psoriasis: a Mendelian randomization study of 105,912 individuals. Front Immunol. 2023;14:737. doi: 10.3389/fimmu.2023.1119144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick MT, Li Q, Wasikowski R, Mehta N, Gudjonsson JE, Elder JT, et al. Shared genetic risk factors and causal association between psoriasis and coronary artery disease. Nat Commun. 2022;13:6565. doi: 10.1038/s41467-022-34323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 14.Girolomoni G, Griffiths CEM, Krueger J, Nestle FO, Nicolas JF, Prinz JC, et al. Early intervention in psoriasis and immune-mediated inflammatory diseases: a hypothesis paper. J Dermatol Treat. 2015;26:103–112. doi: 10.3109/09546634.2014.880396. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation. 2011;123:551–565. doi: 10.1161/CIRCULATIONAHA.109.912568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004–2006-2011) Cerebrovasc Dis Cerebrovasc Dis. 2012;34:290–296. doi: 10.1159/000343145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Øygarden H. Carotid intima-media thickness and prediction of cardiovascular disease. J Am Heart Assoc. 2017;6:1. doi: 10.1161/JAHA.116.005313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahlehoff O, Hansen PR, Gislason GH, Frydland M, Bryld LE, Elming H, et al. Myocardial function and effects of biologic therapy in patients with severe psoriasis: a prospective echocardiographic study. J Eur Acad Dermatol Venereol. 2016;30:819–823. doi: 10.1111/jdv.13152. [DOI] [PubMed] [Google Scholar]
- 19.Russo C, Jin Z, Elkind MSV, Rundek T, Homma S, Sacco RL, et al. Prevalence and prognostic value of subclinical left ventricular systolic dysfunction by global longitudinal strain in a community-based cohort. Eur J Heart Fail. 2014;16:1301–1309. doi: 10.1002/ejhf.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 21.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 22.Pundziute G, Schuijf JD, Jukema JW, Boersma E, de Roos A, van der Wall EE, et al. Prognostic value of multislice computed tomography coronary angiography in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 2007;49:62–70. doi: 10.1016/j.jacc.2006.07.070. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser H, Abdulla J, Henningsen KMA, Skov L, Hansen PR. Coronary artery disease assessed by computed tomography in patients with psoriasis: a systematic review and meta-analysis. Dermatology. 2019;235:478–487. doi: 10.1159/000502138. [DOI] [PubMed] [Google Scholar]
- 24.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tarkin JM, Joshi FR, Rudd JHF. PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol. 2014;11:443–457. doi: 10.1038/nrcardio.2014.80. [DOI] [PubMed] [Google Scholar]
- 26.Figueroa AL, Abdelbaky A, Truong QA, Corsini E, MacNabb MH, Lavender ZR, et al. Measurement of arterial activity on routine FDG PET/CT images improves prediction of risk of future CV events. JACC Cardiovasc Imaging. 2013;6:1250–1259. doi: 10.1016/j.jcmg.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Jókai H, Szakonyi J, Kontár O, Marschalkó M, Szalai K, Kárpáti S, et al. Impact of effective tumor necrosis factor-alfa inhibitor treatment on arterial intima-media thickness in psoriasis: results of a pilot study. J Am Acad Dermatol. 2013;69:523–529. doi: 10.1016/j.jaad.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Herédi E, Végh J, Pogácsás L, Gáspár K, Varga J, Kincse G, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-α inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531–1536. doi: 10.1111/jdv.13649. [DOI] [PubMed] [Google Scholar]
- 29.Dey AK, Joshi AA, Chaturvedi A, Lerman JB, Aberra TM, Rodante JA, et al. Association between skin and aortic vascular inflammation in patients with psoriasis: a case-cohort study using positron emission tomography/computed tomography. JAMA Cardiol. 2017;2:1013–1018. doi: 10.1001/jamacardio.2017.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eder L, Joshi AA, Dey AK, Cook R, Siegel EL, Gladman DD, et al. Association of tumor necrosis factor inhibitor treatment with reduced indices of subclinical atherosclerosis in patients with psoriatic disease. Arthritis Rheumatol. 2018;70:408–416. doi: 10.1002/art.40366. [DOI] [PubMed] [Google Scholar]
- 31.Sorokin AV, Kotani K, Elnabawi YA, Dey AK, Sajja AP, Yamada S, et al. Association between oxidation-modified lipoproteins and coronary plaque in psoriasis an observational cohort study. Circ Res. 2018;123:1244–1254. doi: 10.1161/CIRCRESAHA.118.313608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim BS, Lee WK, Pak K, Han J, Kim GW, Kim HS, et al. Ustekinumab treatment is associated with decreased systemic and vascular inflammation in patients with moderate-to-severe psoriasis: feasibility study using 18 F-fluorodeoxyglucose PET/CT. J Am Acad Dermatol. 2019;80:1322–1331. doi: 10.1016/j.jaad.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115:721–728. doi: 10.1093/cvr/cvz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hjuler KF, Bottcher M, Vestergaard C, Botker HE, Iversen L, Kragballe K. Association between changes in coronary artery disease progression and treatment with biologic agents for severe psoriasis. JAMA Dermatol. 2016;152:1114–1121. doi: 10.1001/jamadermatol.2016.1984. [DOI] [PubMed] [Google Scholar]
- 35.Elnabawi YA, Oikonomou EK, Dey AK, Mancio J, Rodante JA, Aksentijevich M, et al. Association of biologic therapy with coronary inflammation in patients with psoriasis as assessed by perivascular fat attenuation index. JAMA Cardiol. 2019;4:885–891. doi: 10.1001/jamacardio.2019.2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi H, Uceda DE, Dey AK, Abdelrahman KM, Aksentijevich M, Rodante JA, et al. Treatment of psoriasis with biologic therapy is associated with improvement of coronary artery plaque lipid-rich necrotic core: results from a prospective, observational study. Circ Cardiovasc Imaging. 2020 doi: 10.1161/CIRCIMAGING.120.011199. [DOI] [PubMed] [Google Scholar]
- 37.Makavos G, Ikonomidis I, Andreadou I, Varoudi M, Kapniari I, Loukeri E, et al. Effects of interleukin 17A inhibition on myocardial deformation and vascular function in psoriasis. Can J Cardiol. 2020;36:100–111. doi: 10.1016/j.cjca.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 38.Marovt M, Marko PB, Pirnat M, Ekart R. Effect of biologics targeting interleukin-23/-17 axis on subclinical atherosclerosis: results of a pilot study. Clin Exp Dermatol. 2020;45:560–564. doi: 10.1111/ced.14180. [DOI] [PubMed] [Google Scholar]
- 39.Piros ÉA, Szabó Á, Rencz F, Brodszky V, Szalai K, Galajda N, et al. Impact of interleukin-17 inhibitor therapy on arterial intima-media thickness among severe psoriatic patients. Life. 2021;11:1–13. doi: 10.3390/life11090919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gelfand JM, Shin DB, Armstrong AW, Tyring SK, Blauvelt A, Gottlieb S, et al. Association of apremilast with vascular inflammation and cardiometabolic function in patients with psoriasis: the VIP-a phase 4, open-label, nonrandomized clinical trial. JAMA Dermatol. 2022;158:1394–1403. doi: 10.1001/jamadermatol.2022.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pina T, Corrales A, Lopez-Mejias R, Armesto S, Gonzalez-Lopez MA, Gómez-Acebo I, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267–1272. doi: 10.1111/1346-8138.13398. [DOI] [PubMed] [Google Scholar]
- 42.Bissonnette R, Tardif J-C, Harel F, Pressacco J, Bolduc C, Guertin M-C. Effects of the tumor necrosis factor-α antagonist adalimumab on arterial inflammation assessed by positron emission tomography in patients with psoriasis: results of a randomized controlled trial. Circ Cardiovasc Imaging. 2013;6:83–90. doi: 10.1161/CIRCIMAGING.112.975730. [DOI] [PubMed] [Google Scholar]
- 43.Bissonnette R, Harel F, Krueger JG, Guertin MC, Chabot-Blanchet M, Gonzalez J, et al. TNF-α antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo-controlled study. J Invest Dermatol. 2017;137:1638–1645. doi: 10.1016/j.jid.2017.02.977. [DOI] [PubMed] [Google Scholar]
- 44.Ikonomidis I, Papadavid E, Makavos G, Andreadou I, Varoudi M, Gravanis K, et al. Lowering interleukin-12 activity improves myocardial and vascular function compared with tumor necrosis factor-a antagonism or cyclosporine in psoriasis. Circ Cardiovasc Imaging. 2017;10:1–13. doi: 10.1161/CIRCIMAGING.117.006283. [DOI] [PubMed] [Google Scholar]
- 45.Kaur S, Shafiq N, Dogra S, Mittal BR, Attri SV, Bahl A, et al. 18F-fluorodeoxyglucose positron emission tomography-based evaluation of systemic and vascular inflammation and assessment of the effect of systemic treatment on inflammation in patients with moderate-to-severe psoriasis: a randomized placebo-controlled pilot study. Indian J Dermatol Venereol Leprol. 2018;84:660–666. doi: 10.4103/ijdvl.IJDVL_717_17. [DOI] [PubMed] [Google Scholar]
- 46.Mehta NN, Shin DB, Joshi AA, Dey AK, Armstrong AW, Duffin KC, et al. Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers: a randomized placebo-controlled trial. Circ Cardiovasc Imaging. 2018;11:1–11. doi: 10.1161/CIRCIMAGING.117.007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Stebut E, Reich K, Thaçi D, Koenig W, Pinter A, Körber A, et al. Impact of secukinumab on endothelial dysfunction and other cardiovascular disease parameters in psoriasis patients over 52 weeks. J Investig Dermatol. 2019;139:1054–1062. doi: 10.1016/j.jid.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 48.Gelfand JM, Shin DB, Alavi A, Torigian DA, Werner T, Papadopoulos M, et al. A phase IV, randomized, double-blind, placebo-controlled crossover study of the effects of ustekinumab on vascular inflammation in psoriasis (the VIP-U trial) J Investig Dermatol. 2020;140:85. doi: 10.1016/j.jid.2019.07.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gelfand JM, Shin DB, Duffin KC, Armstrong AW, Blauvelt A, Tyring SK, et al. A randomized placebo-controlled trial of secukinumab on aortic vascular inflammation in moderate-to-severe plaque psoriasis (VIP-S) J Invest Dermatol. 2020;140:1784–1793.e2. doi: 10.1016/j.jid.2020.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2017;176:890–901. doi: 10.1111/bjd.14964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rungapiromnan W, Mason KJ, Lunt M, McElhone K, Burden AD, Rutter MK, et al. Risk of major cardiovascular events in patients with psoriasis receiving biologic therapies: a prospective cohort study. J Eur Acad Dermatol Venereol. 2020;34:769–778. doi: 10.1111/jdv.16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu JJ, Joshi AA, Reddy SP, Batech M, Egeberg A, Ahlehoff O, et al. Anti-inflammatory therapy with tumour necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatol Venereol. 2018;32:1320–1326. doi: 10.1111/jdv.14951. [DOI] [PubMed] [Google Scholar]
- 53.Ahlehoff O, Skov L, Gislason G, Gniadecki R, Iversen L, Bryld LE, et al. Cardiovascular outcomes and systemic anti-inflammatory drugs in patients with severe psoriasis: 5-year follow-up of a Danish nationwide cohort. J Eur Acad Dermatol Venereol. 2015;29:1128–1134. doi: 10.1111/jdv.12768. [DOI] [PubMed] [Google Scholar]
- 54.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, et al. GlycA is a novel biomarker of inflammation and subclinical cardiovascular disease in psoriasis. Circ Res. 2016;119:1242–1253. doi: 10.1161/CIRCRESAHA.116.309637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grantham HJ, Hussain AB, Reynolds NJ. Serum S100A8/A9 may act as biomarker of atherosclerosis severity in psoriasis. J Invest Dermatol. 2022;142:2848–2850. doi: 10.1016/j.jid.2022.06.018. [DOI] [PubMed] [Google Scholar]
- 56.Kaiser H, Wang X, Kvist-Hansen A, Krakauer M, Gørtz PM, McCauley BD, et al. Biomarkers of subclinical atherosclerosis in patients with psoriasis. Sci Rep. 2021 doi: 10.1038/s41598-021-00999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niknezhad N, Haghighatkhah HR, Zargari O, Ghalamkarpour F, Younespour S, Niknejad N, et al. High-sensitivity C-reactive protein as a biomarker in detecting subclinical atherosclerosis in psoriasis. Dermatol Ther. 2020;33:e136228. doi: 10.1111/dth.13628. [DOI] [PubMed] [Google Scholar]
- 58.Adamstein NH, MacFadyen JG, Rose LM, Glynn RJ, Dey AK, Libby P, et al. The neutrophil–lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42:896–903. doi: 10.1093/eurheartj/ehaa1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riggs KA, Joshi PH, Khera A, Otvos JD, Greenland P, Ayers CR, et al. GlycA, hsCRP differentially associated with MI, ischemic stroke: in the Dallas Heart Study and multi-ethnic study of atherosclerosis: GlycA, hsCRP differentially associated MI, stroke. Am J Prev Cardiol. 2022;12:100373. doi: 10.1016/j.ajpc.2022.100373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kaptoge S, Di Angelantonio E, Lowe G, Pepys MB, Thompson SG, Collins R, et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Karabay EA, Çerman AA, Demir D, Altunay IK. The effects of systemic psoriasis therapies on the C-reactive protein and the neutrophil-lymphocyte ratio. Ann Dermatol. 2019;31:601. doi: 10.5021/ad.2019.31.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Alavi A, Werner TJ, Høilund-Carlsen PF, Revheim ME. Can target-to-background ratio measurement lead to detection and accurate quantification of atherosclerosis with FDG PET? Likely not. Clin Nucl Med. 2022;47:532–536. doi: 10.1097/RLU.0000000000004131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cocker MS, Mc Ardle B, Spence JD, Lum C, Hammond RR, Ongaro DC, et al. Imaging atherosclerosis with hybrid [18F]fluorodeoxyglucose positron emission tomography/computed tomography imaging: what Leonardo da Vinci could not see. J Nucl Cardiol. 2012;19:1211–1225. doi: 10.1007/s12350-012-9631-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menezes LJ, Kotze CW, Agu O, Richards T, Brookes J, Goh VJ, et al. Investigating vulnerable atheroma using combined (18)F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J Nucl Med. 2011;52:1698–1703. doi: 10.2967/jnumed.111.093724. [DOI] [PubMed] [Google Scholar]
- 65.Dunphy MPS, Freiman A, Larson SM, Strauss HW. Association of vascular 18F-FDG uptake with vascular calcification. J Nucl Med. 2005;46:1278–1284. [PubMed] [Google Scholar]
- 66.Ports WC, Fayyad R, DeMicco DA, Laskey R, Wolk R. Effectiveness of lipid-lowering statin therapy in patients with and without psoriasis. Clin Drug Investig. 2017;37:775–785. doi: 10.1007/s40261-017-0533-0. [DOI] [PubMed] [Google Scholar]
- 67.Kaiser H, Kvist-Hansen A, Krakauer M, Gørtz P, Henningsen K, Wang X, et al. Statin therapy and vascular inflammation detected by positron emission tomography computed tomography in patients with psoriasis. Acta Derm Venereol. 2021;101:adv00406. doi: 10.2340/00015555-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Antonopoulos AS, Margaritis M, Lee R, Channon K, Antoniades C. Statins as anti-inflammatory agents in atherogenesis: molecular mechanisms and lessons from the recent clinical trials. Curr Pharm Des. 2012;18:1519–1530. doi: 10.2174/138161212799504803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article, as no datasets were generated or analysed during the current study.