Abstract
Arthritis is the most prevalent joint disease and is characterized by articular cartilage degradation, synovial inflammation, and changes in periarticular and subchondral bone. Recent studies have reported that Yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ) have significant effects on the proliferation, migration, and survival of chondrocytes and fibroblast-like synovial cells (FLSs). YAP/TAZ signaling pathway, as well as the related Hippo–YAP signaling pathway, are responsible for the condition of cells and articular cartilage in joints. They are tightly regulated to maintain metabolism in chondrocytes and FLSs because abnormal expression may result in cartilage damage. However, the roles and mechanisms of the Hippo–YAP pathway in arthritis remain largely unknown. This review summarizes the roles and key functions of YAP/TAZ and the Hippo–YAP signaling pathway in FLSs and chondrocytes for the induction of proliferation, migration, survival, and differentiation in rheumatoid arthritis (RA) and osteoarthritis (OA) research. We also discuss the therapeutic strategies involving YAP/TAZ and the related Hippo-YAP signaling pathway involved in OA.
Keywords: YAP/TAZ, osteoarthritis, rheumatoid arthritis, chondrocytes, therapy
Introduction
Arthritis is the most common musculoskeletal disease in the world. Approximately 9% of the world’s adult population is affected by osteoarthritis (OA), which mainly causes joint disability and predominantly affects the joints of the knee, hip and hand.1,2 Radiographic evidence indicates that OA usually occurs in elderly individuals older than 65 years, while most of them are aged older than 75 years.3,4 Although OA is often defined as a joint disease characterized by low-grade inflammation and cartilage destruction, its pathogenesis is complex and needs to be fully investigated.
OA is a complex joint disorder in which a variety of pathogenic factors contribute to the pathological process. The common factors that induce the initiation and aggravation of OA include chronic mechanical stress, trauma and obesity, which lead to cartilage degeneration, synovial and joint inflammation, subchondral bone sclerosis, and osteophyte formation.5–7 The extracellular matrix (ECM) and chondrocytes are the main components of articular cartilage, and their functional status is important for joints. Since articular cartilage has a limited capacity for intrinsic repair due to a lack of blood vessels and nerves, chondrocytes exiting only in the ECM are essential for joint health.8–10 The morphology and function of chondrocytes are altered after receiving external or internal physical or chemical stimulation, leading to increased secretion of enzymes that promote the degradation of the cartilage matrix, such as matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs).11–13 These enzymes mainly accelerated the degradation of OA and resulted in catabolic-degrading effects that overwhelm the anabolic-protective function of OA chondrocytes. A large number of proinflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor (TNF-α) and IL-6, stimulate chondrocytes, and synovial cells secrete inflammatory factors to aggravate articular cartilage and synovial inflammatory responses and promote the progression of OA.14,15 Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) activated by these inflammatory cytokines not only induce catabolic gene transcription but also stimulate inflammatory mediators such as TNF-α, IL-1β, and IL-6 to accelerate OA via feedback.16
Recent studies have shown that synovial inflammation is also an important factor in the pathogenesis of rheumatoid arthritis (RA).15,17,18 The synovial membrane is composed of fibroblast-like synovial cells (FLSs) that line the space between the joint cavity and the joint capsule. The synovial membrane not only protects the articular cavity and the articular cartilage inside it but also secretes synovial fluid, which lubricates and provides essential nutrients to the articular cartilage. FLSs play an important role in synovitis by producing inflammatory cytokines in OA and RA, and these cytokines mainly include TNF-α, IL-1β, and IL-6, which are present at higher levels in the synovium in RA patients than in the synovium in normal individuals.14,18 Moreover, all these factors contribute to the development of RA. The degradation of enzymes such as MMP-1, MMP-3, and MMP-13 produced and mediated by FLS was correlated with the duration of RA and the severity of cartilage erosion.19,20 In synovial tissue extracted from RA patients, the number of FLSs was significantly increased, leading to the transformation of synovial hyperplasia into invasive tissue. These activities are controlled and regulated by various signaling mechanisms and proteins, such as Hippo-YAP/TAZ and Wnt/β-catenin. Therefore, it is particularly important to clarify these proteins and their regulatory mechanisms in RA.
YAP and TAZ are primary targets of the Hippo signaling pathway, which is an evolutionarily conserved signaling pathway that plays important roles in the development of tissues and organs. The downstream regulators of the Hippo signaling pathway, YAP and TAZ, are required for regeneration in different organs, mainly by promoting the differentiation and development of mature cell types, driving the growth of tissue and regulating metabolic processes.21–24 Generally, the Hippo pathway negatively regulates the activity of YAP and TAZ, and dysregulation of the Hippo pathway may have severe effects on the metabolic progress in cells and organs. Inappropriately low YAP/TAZ activity can lead to tissue maturation disorders, defective tissue repair and cell differentiation deficiency, while abnormally high YAP/TAZ activity may result in tissue overgrowth and tumor formation.25 Recently, a large number of studies have investigated the role of YAP/TAZ in cellular metabolism, but research on YAP/TAZ in chondrocytes or FLS related to the development of RA and OA is rare. In this review, we aimed to review recent research on YAP/TAZ activity in RA and OA to outline the current findings on the regulation of YAP/TAZ in arthritis.
A Brief History of the Hippo Signaling Pathway
The initial knowledge about the Hippo signaling pathway was derived from genetic screening of genes regulating organ size in Drosophila. First, two independent researchers discovered a gene that causes tissue overgrowth and dysfunction.26 This gene was named Warts/Wts and is homologous to mammalian tumor suppressor genes. Subsequently, the wart-binding protein Salvador/Sav, which is derived from the Drosophila homolog of the mammalian hWW45 gene, was identified. Its mutation leads to elevated expression of inhibitory apoptotic genes and cyclin E.27 The homologous genes of this protein contain the WW domain to bind with Wts, indicating its conservative effect on the regulation of organ volume growth.28 The protein kinase Hippo/Hpo, which was identified as a Drosophila homolog of the mammalian MST1/2 kinase, is responsible for activating this pathway.29–32 Due to the “big-headed” phenotype of Drosophila Hippo mutants, this pathway was named the Hippo signaling pathway.32 Through its important role as a scaffold protein, Sav interacts with Wts and Hippo via its two WW domains.33 Another gene, Mob, was also found to interact with Wts to induce kinase activation and suppress tumor growth.32,33 Subsequently, in vitro experiments revealed that Yorkie/Yki is a transcriptional activator of this signaling pathway, and they also revealed its regulatory effect on gene expression and the connection between Wts and transcriptional regulation.34 In addition, the definition and biological activity of YAP and the binding protein TAZ were described in detail in another earlier study.35
The effect of the Hippo–YAP signaling pathway is mainly focused on regulating embryonic development, cell apoptosis, differentiation and organ growth.36 Hippo-YAP was first discovered due to its critical role in regulating organ size and is highly conserved among mammals.36 Because of its influence on tissue growth and organ size, the Hippo–YAP signaling pathway has been extensively studied in oncology and other diseases. Previous studies have shown that low YAP/TAZ mRNA levels are related to the severity of cancer, especially in myeloma, lymphoma and leukemia, which at least in part indicates the role of focal deletion of YAP1 in a fraction of these tumors.37–39 In support of this notion, other results have confirmed the suppressive effect of YAP through upregulation of YAP1 to disrupt cell proliferation and induce cell death in human multiple myeloma and cells.37,39 Furthermore, YAP/TAZ are thought to be hallmarks of YAP/TAZ activity in cancer because of their phenotypic plasticity, drug resistance, cell proliferation and ability to metastasize.40,41 With respect to cardiovascular disease, increased activity of the Hippo–YAP signaling pathway kinase induced by hypoxia in myocardial infarction could promote cardiomyocyte apoptosis and increase phosphorylated YAP levels.42 Previous studies have shown that YAP can interact with human heart protein extracts and platelet hemoglobin in cultured cells.43 The relevance of this interaction has not been demonstrated in vivo. However, inhibiting YAP activity could suppress the target gene of the YAP-catenin-β signaling pathway in arrhythmogenic cardiomyopathy.44,45
YAP/TAZ Signaling and Regulation via the Hippo Pathway
YAP and TAZ are transcriptional coactivator proteins that are thought to be largely redundant and similarly regulated. They regulate gene expression through binding to the TEAD (TEA/ATTS domain) family of transcription factors (TEAD1-4) in the cell nucleus.46 Due to the great structural similarities between them, they have many synergistic effects on the regulation of many biological functions. The major structural difference is that the YAP protein has a WW domain (65 kDa), while TAZ is a smaller protein with a molecular weight of 43 kDa lacking the SH3-BM proline-rich region.46,47 To date, two subtypes of the YAP protein have been identified and named YAP-1 and YAP-2. The difference between them is that the molecular domain of YAP-2 has an additional WW (38 amino acids) compared to that of YAP-1.46,48 Since YAP/TAZ lack DNA-binding domains, they regulate transcription by binding target transcription factors. TEAD is one of the most important transcription factors that bind to YAP/TAZ to promote gene expression.49,50
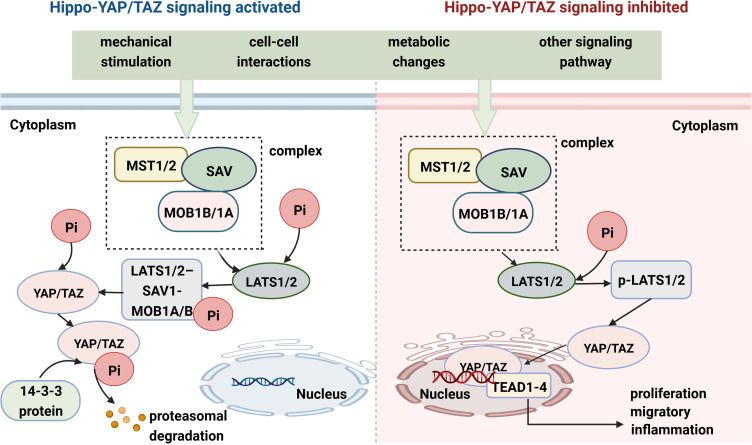
The Hippo pathway was first discovered and elucidated in Drosophila; this pathway plays an important role in encoding genes and controlling cell proliferation and organ size.31,51–53 It is regulated by many factors, mainly mechanical stress, pressure changes, extracellular stimulation and the exchange of intracellular signals. The upstream part of the Hippo signaling pathway is composed mainly of Ste20 family kinases [macrophage stimulating 1 (MST1) and MST2] and large tumor suppressor 1 and 2 kinases (LATS1 and LATS2).54–56 In general, the Hippo pathway has a negative effect on the activity of YAP/TAZ. Once the Hippo signaling pathway is activated by multiple factors, MST1/2 bind to Salvador 1 (SAV1) and MOB kinase activators 1A and 1B (MOB1A and MOB1B) to form a protein complex aimed at phosphorylating the MOB1A/B subunits of LATS1/2.57 The activated LATS1/2-SAV1-MOB1A/B protein complex binds to the YAP/TAZ protein and further promotes YAP/TAZ protein phosphorylation.58 Moreover, LAST1/2 phosphorylates the YAP/TAZ protein at multiple sites to promote its transportation from the nucleus to the cytoplasm, where it combines with the 14-3-3 proteins to form a degradation complex. The phosphorylated YAP/TAZ complex and its complex are sequestered, ubiquitinated and degraded in the cytoplasm (Figure 1).59,60 When the Hippo signaling pathway is inhibited by various stimuli and factors, the LATS1/LATS2 kinases are inactivated. Loss of function of LATS1/LATS2, SAV1 or MST1/MST2 restrains the formation of degradation complexes and inhibits YAP/TAZ protein phosphorylation, promoting YAP/TAZ translocation to the nucleus.61–64 YAP/TAZ do not directly contain a DNA-binding domain; rather, they bind to transcriptional coactivators (TEAD1-4) to regulate gene expression in the nucleus, which plays an important role in promoting cell growth, proliferation, migration, and survival (Figure 1).64–66
Figure 1.
YAP/TAZ activity is tightly regulated via Hippo signaling. YAP/TAZ subcellular localization and transcriptional co-activator activity in specific contexts are mainly regulated by phosphorylation events. When core Hippo signaling are activated, YAP/TAZ are phosphorylated and promoted to be degradation in cytoplasmic, whereas an inactive Hippo kinase cascade could result in the transportation of YAP/TAZ from cytoplasm to the nucleus and the nuclear accumulation of YAP/TAZ. YAP/TAZ act as prominent links in the Hippo-YAP/TAZ signaling pathway and integrators of several other signal pathways.
Role of YAP/TAZ in RA
YAP/TAZ activity and the Hippo pathway are known to participate in the development of cell differentiation and organ development. Many studies have attempted to elucidate the roles of these genes in pathologic development and disease progression.67 YAP and TAZ were also found to be highly upregulated in RA patients.68 Robin et al67 demonstrated the role of YAP/TAZ in synovial inflammation and the severity of rheumatoid arthritis disease. Abnormal regulation of the Hippo signaling pathway in the synovium and FLSs could aggravate the degree of inflammation and joint damage in RA.69 Other scholars have proven that YAP/TAZ has transcriptional activity in FLSs and affects the severity of RA through enhancing the aggressive phenotype of synovial cells and inflammatory responses.67,70 Overexpression of YAP in the nucleus leads to the upregulation of MMP-1 and MMP-13 induced by stimulation with TNF-α and IL-1β in FLS, and increased expression of YAP could also promote the invasion of synovial fibroblasts and aggravate the severity of synovitis in vivo and in vitro.67 Chen et al71 reported that Ezrin regulates YAP expression through interactions between the phosphatidylinositol 3 kinase/protein kinase (PI3K/Akt) pathway and the Hippo pathway. Increased YAP levels contributed to angiogenesis in synovial tissue and the severity of RA. It can promote the proliferation, migration and angiogenesis of vascular endothelial cells in RA via the upregulation of the YAP protein in the nucleus. Another study confirmed that the overexpression of nuclear YAP or protein tyrosine phosphatase 14 (PTPN14) contributes to the progression of pathological development in RA. FLSs displayed overexpression of PTPN14 and YAP, and PTPN14 combined with YAP increased the nuclear location of YAP and promoted TGF-β expression. Inhibition of YAP reduces the FLS pathogenic phenotype and pathogenic behavior and ameliorates arthritis severity in RA.72 The YAP level is also closely related to the inflammatory response to multiple factors in RA. YAP was upregulated in hyperplastic human RA synovial fibroblasts and mouse synovium. Increased expression of YAP results in an erosive phenotype, invading cartilage and bone in RA, and IL-6 activates YAP through Jak and induces the YAP–Snail interaction in synovial fibroblasts to promote invasion and the inflammatory response in RA.70 YAP/TAZ and the Hippo signaling pathway are also associated with tissue-size homeostasis.68 Recent research has shown that YAP, especially YAP that rejects entry into the nucleus, functions as a transport factor to enhance the antiapoptotic effect of synovial fibroblasts.73 The inhibition of YAP/TAZ transcriptional activity could reduce the invasive phenotype of FLSs by reducing the resistance to apoptosis, proliferation, invasion and inflammatory response of synovial cells.67,71 Moreover, another study also indicated that knockdown of YAP or TAZ inhibited the migration and invasion of FLSs and ameliorated arthritis severity. The suppression of the YAP/TAZ signaling pathway promoted autophagy, as indicated by the accumulation of microtubule-associated protein 1 light chain 3 (LC3B-II) and unc-51-like autophagy activating kinase 1 (ULK1).74 YAP/TAZ and autophagy play important roles in the migration and invasion of synoviocytes in RA.74 These studies focused mainly on the effect of YAP and synovial cells in RA and elucidated the relationship of YAP with the inflammatory response, invasiveness and angiogenesis of synovial cells to clarify the related pathological mechanism through which YAP aggravates RA, as summarized in Table 1. YAP/TAZ expression is strongly ascended in FLSs, and its activity is associated with the severity of RA. Thus, targeting YAP/TAZ is an ideal therapeutic strategy for RA (Figure 2).
Table 1.
YAP Involves the Regulation of Pathophysiological Process in RA
| Signaling | Cell Types | Main Results | Reference |
|---|---|---|---|
| TGF-β signaling | Fibroblast-like synoviocytes (FLSs) |
FLSs displayed overexpression of PTPN14 and YAP, PTPN14 combined with YAP increased the nuclear location YAP, promoted the TGF-β-dependent SMAD3 nuclear localization. Inhibition of YAP reduced FLSs pathogenic phenotype and ameliorated arthritis severity. | Angel Bottini, et al72 |
| YAP-Akt signaling | Human umbilical vein endothelial cells | YAP expression and nuclear translocation were increased in umbilical vein endothelial cells. Increased YAP expression contributed the angiogenesis of the synovial tissue patients of RA. The YAP interacted with the PI3K/Akt pathway regulated the proliferation and invasion of cells, pathogenic progress of RA. | Qiyue Chen, et al71 |
| YAP/TAZ signaling | Fibroblast-like synoviocytes (FLSs) | YAP/TAZ transcriptional activity was increased in RA FLSs. Increased YAP/TAZ transcriptional activity induced a common phenotype in RA FLSs with an elevated in apoptosis, proliferation, invasion, and inflammatory response. | Robin Caire, et al67 |
| IL-6–YAP–Snail signaling | Synovial fibroblasts | YAP was upregulated in hyperplastic human RA synovial fibroblasts and mouse synovium. Increased expression of YAP showed an erosive phenotype, invading cartilage and bone in RA, IL-6 activates YAP through Jak and induces YAP–Snail interaction in SF to promote the invasiveness activity and inflammatory response in RA. | Rebecca A Symons, et al70 |
| YAP/TAZ signaling | Fibroblast Synovial Cells (FLSs) | YAP and TAZ were upregulated in RA-FLSs, knockdown of YAP or TAZ inhibited the migration and invasion of FLSs, ameliorated arthritis severity. The suppression of YAP/TAZ signaling pathway promoted the activity of autophagy implied by the accumulation of LC3B-II and ULK1. YAP/TAZ and autophagy play important roles in the migration and invasion of RA-FLSs. | Wei Zhou, et al74 |
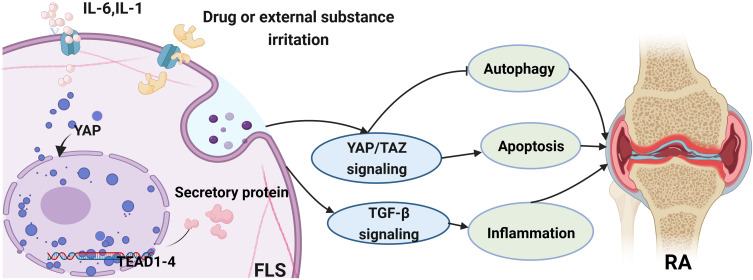
Figure 2.
The role of YAP/TAZ in fibroblast-like synovial cells (FLS) and its related signaling pathway involved in RA. General speaking, the activation of YAP/TAZ could regulate the pathological progress of RA via inhibiting the autophagy process or promoting apoptosis activity. Furthermore, YAP/TAZ also have an influence on the inflammatory response in RA through the TGF-β signaling pathway.
Role of YAP/TAZ in OA
OA is a complex disease caused by multiple factors that lead to the destruction of articular cartilage and resultant inflammation, stiffness, swelling and dysfunction of joints. Chondrocytes are the sole cells in cartilage and are mainly responsible for maintaining the metabolic balance of the cartilage, responding to stimulation caused by joint loading, cytokines, growth factors and so on.75 YAP and TAZ are key regulators that mediate chondrocyte survival, differentiation and tissue morphogenesis during cartilage development.76 Chondrocyte differentiation, proliferation and maturation are closely influenced by YAP/TAZ, while suppressing the activities of these two proteins may damage chondrocytes, eventually impairing articular cartilage and the growth plate.77,78 In recent years, a great number of studies have described YAP/TAZ activity in the progression of OA, as summarized in Table 2. YAP/TAZ can regulate the state of chondrocytes and restrain their maturation progress by suppressing the expression of collagen type X alpha 1 chain (Col10a1) via interaction with runt-related transcription factor 2 (Runx2).73 YAP expression in chondrocytes was accompanied by cartilage, and overexpression of YAP resulted in increased expression of catabolic genes, such as MMP-3, MMP-9, MMP-13 and ADAMTS-5, in response to IL-1β; otherwise, suppression of YAP inhibited catabolic gene expression and chondrocyte apoptosis.79 Increased expression and nuclear location of YAP not only promote catabolism gene expression in chondrocytes but also inhibit the expression of anabolic genes, such as Col2a1 and SRY-box transcription factor 9 (Sox9), ultimately resulting in extracellular matrix stiffness and degeneration of articular cartilage.80 The severity of cartilage damage was also confirmed by higher Osteoarthritis Research Society International (OARSI) scores, which were used to assess the degeneration of articular cartilage.81 YAP also influences the proliferation, migration and survival of chondrocytes. Overexpression of YAP significantly suppressed chondrocyte chondrogenic cell proliferation; decreased the expression of differentiation-related genes, including Runx2, osteocalcin, and collagen I; and increased cell apoptosis, whereas these cellular processes were reversed by knockdown of YAP. In addition, YAP regulates chondrocyte autophagy activity and cartilage matrix secretion through the Wnt–YAP signaling pathway.82,83 However, some scholars have shown other interesting results through investigating the effect of YAP on chondrocytes in OA. Yang et al84 studied the effect of YAP on regulating cell proliferation and differentiation in ADTC5 cells. YAP-1 overexpression promoted chondrocyte proliferation but inhibited chondrocyte differentiation by activating the Wnt/β-catenin signaling pathway, which contradicts the findings of YAP knockout. YAP mainly attenuated ATDC5 cell chondrogenic and hypertrophic differentiation in OA. Since the YAP level is strongly related to the proliferation, differentiation and maturation of cells, several studies have evaluated the effect of YAP on stem cells during the development of OA. Overexpression of YAP could increase the differentiation of mesenchymal stem cells into chondrocytes, reduce the number of senescent cells, inhibit articular inflammation and cartilage erosion and ameliorate pathological symptoms in OA.85,86 Lorthongpanich et al87 studied the effect of fisetin on mesenchymal stem cells (MSCs) and reported that fisetin inhibited the proliferation, migration, and osteogenic differentiation of MSCs. Fisetin could reduce YAP activity, which resulted in the downregulation of osteogenic genes and upregulation of fibroblast genes. Therefore, YAP/TAZ should be maintained at an appropriate level in OA. Abnormal expression of YAP/TAZ may lead to degeneration of articular cartilage and affect the pathological process of OA through various signaling pathways in MSCs, FLSs and chondrocytes, as shown in Figure 3.
Table 2.
YAP Involves the Regulation of Pathophysiological Process in OA
| Signaling | Cell types | Main Results | Reference |
|---|---|---|---|
| YAP–FOXD1 signaling | Human mesenchymal stem cell (hMSC) | Overexpression of YAP or FOXD1 reduces the number of senescent cells, inhibits articular inflammation and cartilage erosion and ameliorates the pathological symptoms in OA. | Lina Fu et al86 |
| YAP/TAZ -NF-κB signaling | HEK293A, HEK293T, HeLa cells | YAP is both necessary and sufficient for the maintenance of cartilage homeostasis in osteoarthritis, Treatment of IL-1β or TNF-α showed increased expression of matrix degrading enzymes and ECM consistent with the result of promoting YAP degradation. Interaction of YAP/TAZ and NF-κB signaling pathway regulates the cartilage metabolism in OA. | Yujie Deng, et al88 |
| / | Chondrocytes | YAP overexpression resulted in increased expression of catabolic genes, suppression of YAP inhibited catabolic genes expression and chondrocytes apoptosis. Intra-articular injection of YAP siRNA ameliorated OA pathological progress in mice. | Yong Gong, et al79 |
| Wnt-YAP signaling | C3H10T1/2 cells, HEK293 cells, C28/I2 cells | Inhibiting the expression of YAP improved the cartilage integrity and relieved pain in OA, the mechanical stress and inflammation factors could activate the Wnt5a and ROR2, induced the nuclear localization of YAP and activity of catabolism. | Anne-Sophie Thorup, et al89 |
| Wnt-YAP signaling | Chondrocytes | The exosomes derived from synovial mesenchymal stem cells could activate the YAP to localize in the cell nucleus via the Wnt5a and Wnt5b, finally resulted in the proliferation and migration of chondrocytes with the side-effect of significantly decreasing ECM secretion through Wnt-YAP signaling pathway. | Shi-Cong Tao, et al82 |
| / | Chondrocytes | Increased YAP expression and nuclear localization resulted chondrocytes presented an OA phenotype mainly include the decreasing expression of Col2a1 and Sox9, increased extracellular matrix stiffness and degeneration of articular cartilage confirmed by the higher OARSI histological scores. Deletion of YAP or pharmacological inhibited the expression of the YAP showed an opposite result. | Xianzhu Zhang, et al80 |
| / | ATDC5 cells | Overexpression of YAP1 significantly suppressed ATDC5 chondrogenic cell proliferation and decreased the expression of differentiation-related genes including Runx2, osteocalcin, and collagen I, and elevated cell apoptosis, whereas these cellular processes were reversed by knockdown of YAP1. The mechanism of YAP regulated the progress of OA mainly through interacting with beclin-1 and promoting the apoptosis of ATDC5 cells, suggesting that YAP1 functions as a negative regulator of autophagy. | Qiang Zhang, et al83 |
| Hippo-YAP signaling | Fibroblast-like synoviocytes | Resolvin D1 inhibited OA- fibroblast-like synoviocytes proliferation and reduced MMP-13 and IL-1β secretion. Furthermore, resolvin D1 inhibits the proliferation of OA-fibroblast-like synoviocytes by arresting the cell cycle via the Hippo-YAP signaling pathway and promoted YAP phosphorylation. | Siwei Su, et al90 |
| YAP-ERK signaling | Chondrocytes | YAP and ERK activation in response to mechanical strain was time and magnitude dependent. Activated YAP and ERK could induce cell cycle progression and promote cell proliferation by up-regulating the expression of cycle-related genes. | Kaixiang Yang, et al91 |
| / | Chondrocytes | YAP1 was highly expressed in OA chondrocytes. MiR-582-3p inhibited chondrocyte apoptosis, reduced the proinflammatory cytokine production and suppressed extracellular matrix degradation via regulation of the expression of YAP1 in chondrocytes. | Jun He, et al92 |
| YAP-BMP signaling | Human mesenchymal stem cells | Overexpression of YAP inhibited chondrogenic differentiation, YAP is a negative regulator of chondrogenic differentiation in human mesenchymal stem cells. High expression of YAP suppressed chondrogenic differentiation and proliferation of stem cells by inhibiting BMP signaling pathway. | Alexandra Karystinou, et al93 |
| RhoA/YAP signaling | Chondrocytes | Mechano growth factor could inhibit chondrocytes apoptosis and inflammation, but have no effect on chondrocyte proliferation and differentiation activity. Mechano growth factor promoted chondrocytes migration which was accompanied with YAP activation and nucleus translocation via the activation of RhoA-YAP signaling pathway. | Xingzhi Jing, et al94 |
| Hippo- YAP signaling | ATDC cells | The silencing of SETD7 inhibited the Hippo signaling pathway in ATDC5, decreased YAP phosphorylation and increased the levels of YAP and hypoxia inducible factor-1α (HIF-1α) in the nucleus. YAP combined with HIF-1α to form a complex that promoted the expression of genes involved in chondrogenic differentiation and the glycolytic pathway. | Maoquan Li, et al95 |
| / | Chondrocytes | Cyclic mechanical stress promoted HIF-1α and YAP expression in a magnitude dependent manner. Activation of YAP promoted HIF-1α stabilization and expression in chondrocytes. | Xingzhi Jing, et al96 |
| Hippo- YAP signaling | ATDC5 cells | Fluoride exposure could lead to the decrease of YAP in cytoplasm of chondrocytes accompanied by the increase of YAP location in the nucleus, finally resulted in the decrease of type II collagen and increase repression of MMP-13, and the degeneration and injury of articular cartilage. | Fang-fang Yu, et al97 |
| Src/Hippo-YAP Signaling | Mesenchymal stem cells | Dasatinib promoted chondrogenic differentiation and inhibited osteogenic differentiation of MSCs. Dasatinib inhibited the expression of YAP and TAZ and the phosphorylation of Src. Inhibition of the Hippo pathway dramatically suppressed the serine phosphorylation of YAP and chondrogenic differentiation of MSCs. Dasatinib promoted chondrogenic differentiation of MSCs via the Src/Hippo-YAP signaling pathway. | Ping Nie, et al98 |
| HIF-1α/YAP signaling | Chondrocytes | Hypoxia leads to OA phenotype in chondrocytes through HIF-1α-YAP pathway accompanied by the increased nuclear location of YAP, while inhibited the expression of HIF-1α decreased the activity and expression of YAP and Sox9 under hypoxia. | Hao Li, et al99 |
| Hippo- YAP signaling | Chondrocytes | The response of chondrocytes to substrate stiffness is associated with changes in YAP localization. Down-regulation of YAP expression helps maintain chondrocyte phenotype and inhibit chondrocyte proliferation. | Weiliang Zhong, et al85 |
| YAP-Wnt/β-catenin signaling | ATDC5 cells | Overexpression of YAP1 could promote chondrocyte proliferation but inhibit chondrocyte differentiation, which is contrary to the result of YAP1 knockout. YAP1 attenuated chondrogenesis and hypertrophic differentiation of ATDC5 cells via activating the Wnt/β-catenin signaling pathway. | Beining Yang, et al84 |
| / | Chondrocytes | YAP1 differentially regulates chondrocyte differentiation in skeletal development and bone repair. YAP1 regulated Sox6 expression to promote chondrocyte proliferation, inhibited Col10a1 expression through interaction with Runx2 during chondrocyte maturation. | Yujie Deng, et al73 |
| Autotaxin–YAP Signaling | Bone marrow mesenchymal stem cells | Exosomes derived from mesenchymal stem cells regulate the expression of YAP in chondrocytes, and the increased expression and nuclear localization of YAP in chondrocytes played an important role in promoting cell proliferation and cartilage matrix synthesis, inhibiting inflammatory response and anti-apoptosis through the YAP/TAZ signaling pathway. | Yingnan Wang, et al16 |
| / | Mesenchymal stem cells | Declined YAP activity inhibited the proliferation, migration and osteogenic of mesenchymal stem cells, accompanied by the down regulation of osteogenic and upregulation of fibroblast genes. | Chanchao Lorthongpanich, et al87 |
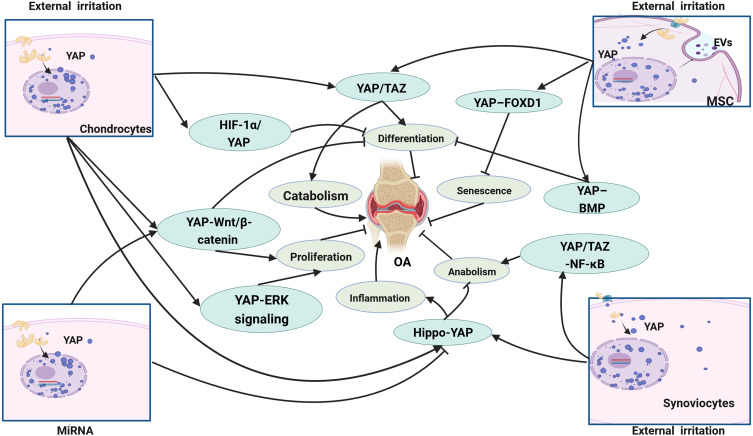
Figure 3.
The main signaling pathway YAP/TAZ involved in OA. The research of YAP/TAZ in OA is mainly carried out in chondrocytes, mesenchymal stem cells (MSC) and fibroblast-like synovial cells (FLSs), which mainly regulate the proliferation, differentiation, anabolism and catabolism of cells and affect OA through a variety of signaling pathways.
YAP Signaling Pathway Involvement in the Pathological Process of OA
The YAP/TAZ signaling pathway is important not only for cell survival, proliferation, and migration but also for tissue morphogenesis and cartilage development.76,100 YAP signaling is regulated by the upstream signaling pathway Hippo-YAP signaling and other factors, such as mechanical cues, cytokines, other mechanical stimuli and/or metabolic factors.101,102 Research has indicated that YAP can inhibit chondrocyte maturation by suppressing Col10a1 levels through interaction with Runx2.73 Overexpression of YAP in the nucleus accompanied by a decrease in YAP in the cytoplasm induced by fluoride exposure could result in a decrease in Col2a1 and increase the expression of MMP-13 and in the degeneration and injury of articular cartilage.97 While the inhibition of YAP expression could ameliorate osteoarthritic cartilage degradation, YAP is both necessary and sufficient for the maintenance of cartilage homeostasis in osteoarthritis, and treatment with IL-1β or TNF-α increased the expression of matrix-degrading enzymes and the ECM, consistent with the promotion of YAP degradation.88 Therefore, YAP should be regulated at a proper level to maintain cartilage homeostasis.
Silencing of su enhancer of zeste and trithorax domain containing 7 (SETD7) inhibited the Hippo signaling pathway in ATDC5 cells, decreased YAP phosphorylation and increased the levels of YAP and hypoxia inducible factor-1α (HIF-1α) in the nucleus. YAP binds to HIF-1α to form a complex that promotes the expression of genes involved in chondrogenic differentiation and the glycolytic pathway.95 Jing et al96 also confirmed that the levels of HIF-1α and YAP regulate chondrocyte survival and that the activation of YAP promotes HIF-1α stabilization and expression in chondrocytes by stimulating mechanical stress. Hypoxia leads to an OA phenotype in chondrocytes through the HIF-1α-YAP pathway, which is accompanied by increased nuclear localization of YAP, while inhibiting the expression of HIF-1α decreases the activity and expression of YAP and Sox9 under hypoxia.99
The Wnt signaling pathway could also mediate YAP activity in Hippo signaling independent of the signaling pathway. Synovial mesenchymal stem cell-derived exosomes can activate YAP to localize to the cell nucleus via Wnt5a and Wnt5b, ultimately resulting in the proliferation and migration of chondrocytes via the side effects of significantly decreasing ECM secretion through the regulation of the Wnt–YAP signaling pathway.82 Inhibiting the expression of YAP improved cartilage integrity and relieved pain in OA patients, mechanical stress and inflammatory factors could trigger chondrogenic differentiation and suppress the expression of the cartilage-degrading enzymes ADAMTS-4 and ADAMTS-5 by activating Wnt5a through inducing the nuclear localization of YAP and catabolism.89 Another study also indicated that YAP-1 could affect chondrocyte differentiation by activating the Wnt/β-catenin signaling pathway.84
YAP also interacts with other signaling pathways and regulates cell proliferation, migration and differentiation. YAP and extracellular signal-regulated kinase (ERK) activation in response to mechanical strain was time and magnitude dependent. Activated YAP and ERK can induce cell cycle progression and promote cell proliferation by upregulating the expression of cell cycle-related genes.91 High expression of YAP suppressed chondrogenic differentiation and proliferation of stem cells by inhibiting the bone morphogenetic protein signaling pathway.93 Nie et al98 reported that dasatinib promoted chondrogenic differentiation and inhibited osteogenic differentiation of mesenchymal stem cells (MSCs). Dasatinib inhibited the expression of YAP and TAZ. Dasatinib promoted chondrogenic differentiation of MSCs via the Src/Hippo-YAP signaling pathway. Jing et al94 suggested that mechano growth factor could inhibit chondrocyte apoptosis and inflammation but had no effect on chondrocyte proliferation or differentiation activity. Mechano growth factor promoted chondrocyte migration, which was accompanied by YAP activation and nuclear translocation via activation of the RhoA–YAP signaling pathway.94
Role of miRNAs in the Hippo–YAP Signaling Pathway in OA and RA
MicroRNAs (miRNAs) are small noncoding RNAs that play significant roles in regulating genes and modulating essential biological processes in animals and plants.103 Since it was first discovered in 1993 in Caenorhabditis elegans, many miRNAs were found to affect gene expression levels.104 In recent decades, more than 2000 miRNAs have been shown to regulate the expression of human genes, accounting for two-thirds of the human genome.105 Currently, the levels of miRNAs, which are closely related to posttranscriptional modifications, have been extensively studied in arthritis, especially in OA and RA. These results indicate that miRNAs have both protective and destructive effects on articular cartilage metabolism.
Research on miRNAs has shown that YAP is a downstream target of miRNAs, while miRNAs can inhibit chondrocyte apoptosis, reduce proinflammatory cytokine production and suppress articular cartilage degradation.106,107 An increased level of miR-142-3p could diminish the osteogenic potential of MSCs through targeting the YAP protein; furthermore, this effect was independent of the activation of the Hippo signaling pathway.107 Another study showed that miR-23a-5p derived from exosomes could regulate the expression of Runx2, which interacts with YAP1 to suppress the activity of osteoblasts in human osteoclasts, suggesting the major role of osteoclast-derived miR-23a-5p in osteogenic differentiation, bone repair and cartilage development.108 Tao et al82 reported the therapeutic effect of miRNAs in OA through the Wnt–YAP signaling pathway. They observed that Wnt5a and Wnt5b could activate YAP and enhance the proliferation and migration of chondrocytes through the side effects of decreasing cartilage matrix secretion. A higher level of miR-140-5p alleviated joint cartilage damage by blocking this side effect.82 Another study also confirmed the therapeutic effect of miR-582-3p in OA. Research has indicated that miR-582-3p can decrease chondrocyte apoptosis, reduce proinflammatory factor secretion and suppress cartilage degradation by regulating the expression of the downstream target gene YAP1.92
In reference to RA, miRNA expression is intimately associated with the pathogenesis of RA. Experimental research has shown that the expression of microRNA genes is upregulated in male rats, which ultimately regulates the Hippo-YAP and PI3K-Akt signaling pathways to participate in biological processes such as cell adhesion, cell cycling, apoptosis, and DNA binding in RA.106
Potential Therapies for OA-Targeting YAP
The articular chondrocyte is the only cell type in articular cartilage and is responsible for maintaining the cartilaginous extracellular environment.11,109 Chondrocytes survive, proliferate, differentiate and migrate at an appropriate level, while abnormal activation or inhibition of chondrocytes may damage cartilage by synthesizing matrix-degrading enzymes.110 YAP can maintain a younger state of human mesenchymal stem cells (hMSCs) and ameliorate osteoarthritis in mice. Overexpression of YAP or forkhead Box D1 (FOXD1) rejuvenated aged hMSCs and attenuated posttraumatic osteoarthritis in mice. Moreover, intra-articular administration of lentiviral vectors encoding YAP or FOXD1 reduces the number of senescent cells, inhibits articular inflammation and cartilage erosion, and ameliorates pathological symptoms.86 Kania et al111 reported that cartilage repair and damage are strongly influenced by YAP levels in chondrocytes. The expression of genes related to cartilage matrix metabolism was negatively influenced by the upstream regulator of YAP, which is associated with chondrogenic specification. YAP is also a therapeutic target for many compounds and drugs involved in the treatment of OA. Resolvin D1 (RvD1) inhibits OA-FLS proliferation via the Hippo–YAP signaling pathway, promotes YAP phosphorylation and protects chondrocytes by inhibiting the secretion of MMP-13 and IL-1β; these findings provide an experimental basis for RvD1-mediated treatment of OA.90 Bone marrow mesenchymal stem cell (BMSC)-derived small extracellular vesicles (BMSC-sEVs) can significantly increase the expression of proliferating cell nuclear antigens and cartilage-forming factors and downregulate the expression of cartilage inflammation-related factors in OA. The therapeutic effect of BMSC-EVs mainly depends on autotaxin, which regulates the expression of YAP and phosphorylated YAP, ultimately altering the activity of the Hippo–YAP signaling pathway.16 These studies indicate that YAP is an important target gene for treating OA. Moreover, the Hippo–YAP signaling pathway is a significant regulatory mechanism for chondrocytes to decrease inflammatory cytokines, reduce chondrocyte apoptosis and alleviate articular cartilage damage in OA.
Pharmaceutical Interventions Targeting YAP in OA and RA
The complex effect of YAP and the mechanism of the Hippo signaling pathway indicate that regulating its expression and activity is a complicated process. Recently, several compounds and drugs have been used to explore and study the biological effect of YAP in OA. To date, pharmaceutical interventions targeting YAP in OA have focused mainly on YAP levels in chondrocytes and synoviocytes.
For example, Ma et al112 reported that OA plays a protective role in joints mainly through promoting the secretion of collagen II and inhibiting the secretion of inflammatory cytokines such as IL-1β, TNF-α, MMP-3 and MMP-13. Mechanistically, this therapeutic effect depended on activating the Hippo/YAP pathway, which ultimately resulted in disruption of cartilage degradation.112 Resolvin D1 can modulate the YAP signaling pathway by promoting YAP phosphorylation in synovial cells, inhibiting the secretion of inflammatory factors and promoting the proliferation of aggressive synovial cells to delay the progression of cartilage degradation.90 Aspirin also has therapeutic effects by mitigating the progression of cartilage degeneration by stabilizing YAP expression to reduce oxidative stress and inflammatory factor levels in vitro.113 Chen et al114 reported that sarsasapogenin mitigated the progression of OA by decreasing the sensitivity of chondrocytes to ferroptosis through increasing the expression of YAP1, indicating that sarsasapogenin is a potential therapeutic approach for OA. Furthermore, other scholars have shown that the YAP and Hippo signaling pathways are mediated by multiple compounds that may inhibit chondrocyte apoptosis, reduce proinflammatory factor production and suppress extracellular matrix degradation.92,115–117 Recently, small extracellular vesicles were shown to participate in chondrocyte proliferation, upregulate cartilage-forming factors and decrease cartilage inflammation-related cytokines through the YAP signaling pathway to slow the course of OA.116
The biological characteristics of the YAP protein indicate that the regulatory mechanism of YAP in RA is complex. Chen et al71 reported that Ezrin plays an important role in the proliferation, migration and angiogenesis of vascular endothelial cells in RA through regulation of the Hippo–YAP pathway. Suppression of Ezrin and its downstream YAP pathway could regulate synovial angiogenesis and aggravate the severity of RA.71 Treatment with leonurine had anti-inflammatory effects and inhibited joint swelling and bone damage in an experimental model.118 This study further confirmed that increased expression and phosphorylation of YAP and its nuclear translocation are the therapeutic mechanisms of leonurine in RA.118 Researchers have also shown that rapamycin regulates genes in the Hippo–YAP pathway, which are predicted to converge with the mTOR pathway, in RA synovial tissue.119 These activities ultimately alter rheumatoid arthritis synovial fibroblast metabolism and inhibit glycolysis and the expression of rate-limiting glycolytic enzymes in human synovial fibroblasts.119 Bottini et al72,120 reported that drugs aimed at decreasing the expression of YAP and nuclear YAP in FLS could inhibit the migration, invasion and inflammatory activity of FLS and ameliorate arthritis severity.
Taken together, these findings indicate that pharmaceutical interventions involving YAP in OA and RA have different effects on different types of cells. Generally, the current treatment mainly relies on reducing inflammatory factors in chondrocytes and synoviocytes to suppress inflammatory activity and alleviate cartilage degradation. In reference to RA, YAP is also an important mediator of synoviocyte-to-synovial cells and synovial tissue, which mainly influence the inflammatory activity of RA. It is necessary to study the use of the drug intervention YAP in both OA and RA to treat these diseases.
Conclusion
In recent years, many studies have investigated and illustrated the pathological progress of OA to identify therapeutic targets for the treatment of this disease. YAP/TAZ are transcriptional coactivator proteins that have attracted increased amounts of attention for their involvement in the growth and development of cells and the pathogenesis of OA. Increasing evidence suggests that YAP/TAZ and the signaling pathways involved play important roles in the maintenance of joint homeostasis, particularly in cartilage. YAP/TAZ and related signaling pathways, such as the Hippo–YAP signaling pathway, should be tightly regulated at the proper level. Abnormal activation of YAP/TAZ could lead to the secretion of inflammatory cytokines and degeneration of the articular cartilage matrix in chondrocytes and FLSs, while overexpression of YAP/TAZ in MSCs may induce stem cells to differentiate into chondrocytes and chondroblasts. Since YAP/TAZ have different roles and biological functions in chondrocytes, FLSs and MSCs, the expression level of YAP/TAZ should be strictly controlled and discriminatively evaluated. New strategies to target and modulate YAP/TAZ levels may lead to the preservation of cartilage or the healing of damaged joints. Further research on YAP/TAZ in more detail is absolutely necessary, not only to facilitate the development of specific therapeutic interventions but also for the discovery and use of biomarkers that could help further shape personalized medicine approaches.
Funding Statement
This work was supported by the Natural Science Foundation of Changsha City (No. kq2208380), the Natural Science Foundation of Wuxi City (No. k20231060), the youth talent project of Wuxi health commission (No. Q202150), Duo-Innovative and Excellent Doctors Project of Wuxi 9th People’s Hospital (No. YB20210) and the Top Talent Support Program for young and middle-aged people of Wuxi Health Committee (No, HB2023125).
Abbreviations
OA, osteoarthritis; YAP, Yes-associated protein; TAZ, transcriptional coactivator with PDZ-binding motif; FLS, fibroblast-like synovial cells; RA, rheumatoid arthritis; ECM, extracellular matrix; MMPs, matrix metalloproteinases; ADAMTSs, a disintegrin and metalloproteinase with thrombospondin motifs; IL, interleukin; TNF-α, tumor necrosis factor alpha; OARSI, Osteoarthritis Research Society International; TEAD: TEA/ATTS domain; MST, macrophage stimulating; LATS, large tumor suppressor; SAV1:Salvador 1; MOB1A:MOB kinase activator 1A; MOB1B: MOB kinase activator 1B; Wnt: Wingless / Integrated; Col10a1, collagen type X alpha 1 chain; Col2a1, type II collagen alpha 1 chain; Sox9, SRY-box transcription factor 9; HIF-1α, hypoxia inducible factor-1α; MSCs, mesenchymal stem cells; hMSCs, human mesenchymal stem cells; RvD1:Resolvin D1; miRNAs, microRNAs; LC3, microtubule associated protein 1 light chain 3; ULK1, unc-51 like autophagy activating kinase 1; TGF-β, transforming growth factor beta; PI3K/Akt, phosphatidylinositol 3 kinase/ protein kinase; Runx2, runt-related transcription factor 2; ERK, extracellular signal-regulated kinase; SETD7, Su(var)3-9 enhancer of zeste and trithorax domain containing 7; PTPN14, protein tyrosine phosphatase 14; OARSI, osteoarthritis research society international.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bijlsma JWJ, Berenbaum F, Lafeber FPJG. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–2126. doi: 10.1016/S0140-6736(11)60243-2 [DOI] [PubMed] [Google Scholar]
- 2.Glyn-Jones S, Palmer AJR, Agricola R, et al. Osteoarthritis. Lancet. 2015;386(9991):376–387. doi: 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- 3.Litwic A, Edwards MH, Dennison EM, Cooper C. Epidemiology and burden of osteoarthritis. Br Med Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandl LA. Osteoarthritis year in review 2018: clinical. Osteoarthritis Cartilage. 2019;27(3):359–364. doi: 10.1016/j.joca.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 5.Goldring MB, Goldring SR. Osteoarthritis. J Cell Physiol. 2007;213(3):626–634. doi: 10.1002/jcp.21258 [DOI] [PubMed] [Google Scholar]
- 6.Bian Q, Wang YJ, Liu SF, Li YP. Osteoarthritis: genetic factors, animal models, mechanisms, and therapies. Front Biosci. 2012;4(1):74–100. doi: 10.2741/e361 [DOI] [PubMed] [Google Scholar]
- 7.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8(11):665–673. doi: 10.1038/nrrheum.2012.130 [DOI] [PubMed] [Google Scholar]
- 8.Poole AR, Kobayashi M, Yasuda T, et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii78–81. doi: 10.1136/ard.61.suppl_2.ii78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang K, Wu LD. Aggrecanase and aggrecan degradation in osteoarthritis: a review. J Int Med Res. 2008;36(6):1149–1160. doi: 10.1177/147323000803600601 [DOI] [PubMed] [Google Scholar]
- 10.Choi MC, Jo J, Park J, Kang HK, Park Y. NF-kappaB signaling pathways in osteoarthritic cartilage destruction. Cells. 2019;9(1):8. doi: 10.3390/cells9010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldring MB, Marcu KB. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther. 2009;11(3):224. doi: 10.1186/ar2592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malemud CJ. Inhibition of MMPs and ADAM/ADAMTS. Biochem Pharmacol. 2019;165:33–40. doi: 10.1016/j.bcp.2019.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y, Luo L, Gui T, et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci Transl Med. 2021;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20(12):1484–1499. doi: 10.1016/j.joca.2012.08.027 [DOI] [PubMed] [Google Scholar]
- 15.Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19(1):18. doi: 10.1186/s13075-017-1229-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhao M, Li W, et al. BMSC-derived small extracellular vesicles induce cartilage reconstruction of temporomandibular joint osteoarthritis via autotaxin-YAP signaling axis. Front Cell Dev Biol. 2021;9:656153. doi: 10.3389/fcell.2021.656153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6(11):625–635. doi: 10.1038/nrrheum.2010.159 [DOI] [PubMed] [Google Scholar]
- 18.Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51(2):249–257. doi: 10.1016/j.bone.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Redlich K, Smolen JS. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat Rev Drug Discov. 2012;11(3):234–250. doi: 10.1038/nrd3669 [DOI] [PubMed] [Google Scholar]
- 20.Smolen JS, Aletaha D, Barton A, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. doi: 10.1038/nrdp.2018.1 [DOI] [PubMed] [Google Scholar]
- 21.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov. 2014;13(1):63–79. doi: 10.1038/nrd4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juan WC, Hong W. Targeting the hippo signaling pathway for tissue regeneration and cancer therapy. Genes. 2016;3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moya IM, Halder G. The Hippo pathway in cellular reprogramming and regeneration of different organs. Curr Opin Cell Biol. 2016;43:62–68. doi: 10.1016/j.ceb.2016.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Patel SH, Camargo FD, Yimlamai D. Hippo signaling in the liver regulates organ size, cell fate, and carcinogenesis. Gastroenterology. 2017;152(3):533–545. doi: 10.1053/j.gastro.2016.10.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warren JSA, Xiao Y, Lamar JM. YAP/TAZ activation as a target for treating metastatic cancer. Cancers. 2018;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–1063. doi: 10.1242/dev.121.4.1053 [DOI] [PubMed] [Google Scholar]
- 27.Tapon N, Harvey KF, Bell DW, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110(4):467–478. doi: 10.1016/S0092-8674(02)00824-3 [DOI] [PubMed] [Google Scholar]
- 28.Kango-Singh M, Nolo R, Tao C, et al. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129(24):5719–5730. doi: 10.1242/dev.00168 [DOI] [PubMed] [Google Scholar]
- 29.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114(4):457–467. doi: 10.1016/S0092-8674(03)00557-9 [DOI] [PubMed] [Google Scholar]
- 30.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17(20):2514–2519. doi: 10.1101/gad.1134003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5(10):914–920. doi: 10.1038/ncb1050 [DOI] [PubMed] [Google Scholar]
- 32.Samji P, Rajendran MK, Warrier VP, Ganesh A, Devarajan K. Regulation of Hippo signaling pathway in cancer: a MicroRNA perspective. Cell Signal. 2021;78:109858. doi: 10.1016/j.cellsig.2020.109858 [DOI] [PubMed] [Google Scholar]
- 33.Lai ZC, Wei X, Shimizu T, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120(5):675–685. doi: 10.1016/j.cell.2004.12.036 [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122(3):421–434. doi: 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- 35.Sudol M. Yes-associated protein (YAP65) is a proline-rich phosphoprotein that binds to the SH3 domain of the Yes proto-oncogene product. Oncogene. 1994;9(8):2145–2152. [PubMed] [Google Scholar]
- 36.Kong H, Han JJ, Gorbachev D, Zhang XA. Role of the Hippo pathway in autoimmune diseases. Exp Gerontol. 2024;185:112336. doi: 10.1016/j.exger.2023.112336 [DOI] [PubMed] [Google Scholar]
- 37.Cottini F, Hideshima T, Xu C, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20(6):599–606. doi: 10.1038/nm.3562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson JD, Huang K, Pacal M, et al. Binary pan-cancer classes with distinct vulnerabilities defined by pro- or anti-cancer YAP/TEAD activity. Cancer Cell. 2021;39(8):1115–1134 e12. doi: 10.1016/j.ccell.2021.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zheng B, Sun W, Yi K, et al. Integrated transcriptomic analysis reveals a distinctive role of YAP1 in extramedullary invasion and therapeutic sensitivity of multiple myeloma. Front Oncol. 2021;11:787814. doi: 10.3389/fonc.2021.787814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hanahan D. Hallmarks of Cancer: new Dimensions. Cancer Discov. 2022;12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059 [DOI] [PubMed] [Google Scholar]
- 41.Piccolo S, Panciera T, Contessotto P, Cordenonsi M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nat Cancer. 2023;4(1):9–26. doi: 10.1038/s43018-022-00473-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng A, Chen Q, Zhang L. The Hippo-YAP pathway in various cardiovascular diseases: focusing on the inflammatory response. Front Immunol. 2022;13:971416. doi: 10.3389/fimmu.2022.971416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Austin KM, Trembley MA, Chandler SF, et al. Molecular mechanisms of arrhythmogenic cardiomyopathy. Nat Rev Cardiol. 2019;16(9):519–537. doi: 10.1038/s41569-019-0200-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heallen T, Zhang M, Wang J, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–461. doi: 10.1126/science.1199010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen SN, Gurha P, Lombardi R, Ruggiero A, Willerson JT, Marian AJ. The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circ Res. 2014;114(3):454–468. doi: 10.1161/CIRCRESAHA.114.302810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Webb C, Upadhyay A, Giuntini F, et al. Structural features and ligand binding properties of tandem WW domains from YAP and TAZ, nuclear effectors of the Hippo pathway. Biochemistry. 2011;50(16):3300–3309. doi: 10.1021/bi2001888 [DOI] [PubMed] [Google Scholar]
- 47.Lin KC, Park HW, Guan KL. Regulation of the hippo pathway transcription factor TEAD. Trends Biochem Sci. 2017;42(11):862–872. doi: 10.1016/j.tibs.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boopathy GTK, Hong W. Role of hippo pathway-YAP/TAZ signaling in Angiogenesis. Front Cell Dev Biol. 2019;7:49. doi: 10.3389/fcell.2019.00049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22(14):1962–1971. doi: 10.1101/gad.1664408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Z, Zhao B, Wang P, et al. Structural insights into the YAP and TEAD complex. Genes Dev. 2010;24(3):235–240. doi: 10.1101/gad.1865810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5(10):921–927. doi: 10.1038/ncb1051 [DOI] [PubMed] [Google Scholar]
- 52.Praskova M, Xia F, Avruch J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Curr Biol. 2008;18(5):311–321. doi: 10.1016/j.cub.2008.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Misra JR, Irvine KD. The hippo signaling network and its biological functions. Annu Rev Genet. 2018;52(1):65–87. doi: 10.1146/annurev-genet-120417-031621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishioka N, Inoue K, Adachi K, et al. The hippo signaling pathway components lats and yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16(3):398–410. doi: 10.1016/j.devcel.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 55.Mauviel A, Nallet-Staub F, Varelas X. Integrating developmental signals: a Hippo in the (path)way. Oncogene. 2012;31(14):1743–1756. doi: 10.1038/onc.2011.363 [DOI] [PubMed] [Google Scholar]
- 56.Jiang L, Li J, Zhang C, Shang Y, Lin J. YAPmediated crosstalk between the Wnt and hippo signaling pathways (Review). Mol Med Rep. 2020;22(5):4101–4106. doi: 10.3892/mmr.2020.11529 [DOI] [PubMed] [Google Scholar]
- 57.Zhao B, Wei X, Li W, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piersma B, Bank RA, Boersema M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front Med. 2015;2:59. doi: 10.3389/fmed.2015.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei QY, Zhang H, Zhao B, et al. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28(7):2426–2436. doi: 10.1128/MCB.01874-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF β-TRCP. Genes Dev. 2010;24(1):72–85. doi: 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Camargo FD, Gokhale S, Johnnidis JB, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17(23):2054–2060. doi: 10.1016/j.cub.2007.10.039 [DOI] [PubMed] [Google Scholar]
- 62.Dong J, Feldmann G, Huang J, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130(6):1120–1133. doi: 10.1016/j.cell.2007.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107(18):8248–8253. doi: 10.1073/pnas.0912203107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishio M, Hamada K, Kawahara K, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest. 2012;122(12):4505–4518. doi: 10.1172/JCI63735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou D, Conrad C, Xia F, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16(5):425–438. doi: 10.1016/j.ccr.2009.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang N, Bai H, David KK, et al. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19(1):27–38. doi: 10.1016/j.devcel.2010.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caire R, Audoux E, Courbon G, et al. YAP/TAZ: key players for rheumatoid arthritis severity by driving fibroblast like synoviocytes phenotype and fibro-inflammatory response. Front Immunol. 2021;12:791907. doi: 10.3389/fimmu.2021.791907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang T, Wang Z, Qi W, Jiang G, Wang G. Possible future avenues for rheumatoid arthritis therapeutics: hippo pathway. J Inflamm Res. 2023;16:1283–1296. doi: 10.2147/JIR.S403925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Du Y, Cui R, Tian N, Chen M, Zhang XL, Dai SM. Regulation of type I interferon signature by VGLL3 in the fibroblast-like synoviocytes of rheumatoid arthritis patients via targeting the Hippo pathway. Arthritis Res Ther. 2022;24(1):188. doi: 10.1186/s13075-022-02880-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Symons RA, Colella F, Collins FL, et al. Targeting the IL-6-Yap-Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis. Ann Rheum Dis. 2022;81(2):214–224. doi: 10.1136/annrheumdis-2021-220875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen Q, Fan K, Chen X, et al. Ezrin regulates synovial angiogenesis in rheumatoid arthritis through YAP and Akt signalling. J Cell Mol Med. 2021;25(19):9378–9389. doi: 10.1111/jcmm.16877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bottini A, Wu DJ, Ai R, et al. PTPN14 phosphatase and YAP promote TGFbeta signalling in rheumatoid synoviocytes. Ann Rheum Dis. 2019;78(5):600–609. doi: 10.1136/annrheumdis-2018-213799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng Y, Wu A, Li P, et al. Yap1 regulates multiple steps of chondrocyte differentiation during skeletal development and bone repair. Cell Rep. 2016;14(9):2224–2237. doi: 10.1016/j.celrep.2016.02.021 [DOI] [PubMed] [Google Scholar]
- 74.Zhou W, Shen Q, Wang H, et al. Knockdown of YAP/TAZ inhibits the migration and invasion of fibroblast synovial cells in rheumatoid arthritis by regulating autophagy. J Immunol Res. 2020;2020:9510594. doi: 10.1155/2020/9510594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sacitharan PK. Ageing and Osteoarthritis. Subcell Biochem. 2019;91:123–159. [DOI] [PubMed] [Google Scholar]
- 76.Vanyai HK, Prin F, Guillermin O, et al. Control of skeletal morphogenesis by the Hippo-YAP/TAZ pathway. Development. 2020;1:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, Yang S, Qin L, Yang S. TAZ is required for chondrogenesis and skeletal development. Cell Discov. 2021;7(1):26. doi: 10.1038/s41421-021-00254-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sun K, Guo J, Guo Z, et al. The roles of the Hippo-YAP signalling pathway in cartilage and osteoarthritis. Ageing Res Rev. 2023;90:102015. doi: 10.1016/j.arr.2023.102015 [DOI] [PubMed] [Google Scholar]
- 79.Gong Y, Li SJ, Liu R, et al. Inhibition of YAP with siRNA prevents cartilage degradation and ameliorates osteoarthritis development. J Mol Med. 2019;97(1):103–114. doi: 10.1007/s00109-018-1705-y [DOI] [PubMed] [Google Scholar]
- 80.Zhang X, Cai D, Zhou F, et al. Targeting downstream subcellular YAP activity as a function of matrix stiffness with Verteporfin-encapsulated chitosan microsphere attenuates osteoarthritis. Biomaterials. 2020;232:119724. doi: 10.1016/j.biomaterials.2019.119724 [DOI] [PubMed] [Google Scholar]
- 81.Glasson SS, Chambers MG, Van Den Berg WB WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18(Suppl 3):S17–23. doi: 10.1016/j.joca.2010.05.025 [DOI] [PubMed] [Google Scholar]
- 82.Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7(1):180–195. doi: 10.7150/thno.17133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang Q, Fang X, Zhao W, Liang Q. The transcriptional coactivator YAP1 is overexpressed in osteoarthritis and promotes its progression by interacting with Beclin-1. Gene. 2019;689:210–219. doi: 10.1016/j.gene.2018.11.068 [DOI] [PubMed] [Google Scholar]
- 84.Yang B, Sun H, Song F, Yu M, Wu Y, Wang J. YAP1 negatively regulates chondrocyte differentiation partly by activating the beta-catenin signaling pathway. Int J Biochem Cell Biol. 2017;87:104–113. doi: 10.1016/j.biocel.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 85.Zhong W, Tian K, Zheng X, et al. Mesenchymal stem cell and chondrocyte fates in a multishear microdevice are regulated by Yes-associated protein. Stem Cells Dev. 2013;22(14):2083–2093. doi: 10.1089/scd.2012.0685 [DOI] [PubMed] [Google Scholar]
- 86.Fu L, Hu Y, Song M, et al. Up-regulation of FOXD1 by YAP alleviates senescence and osteoarthritis. PLoS Biol. 2019;17(4):e3000201. doi: 10.1371/journal.pbio.3000201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lorthongpanich C, Charoenwongpaiboon T, Supakun P, Klaewkla M, Kheolamai P, Issaragrisil S. Fisetin inhibits osteogenic differentiation of mesenchymal stem cells via the inhibition of YAP. Antioxidants. 2021;5(10):10. doi: 10.3390/antiox11010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deng Y, Lu J, Li W, et al. Reciprocal inhibition of YAP/TAZ and NF-kappaB regulates osteoarthritic cartilage degradation. Nat Commun. 2018;9(1):4564. doi: 10.1038/s41467-018-07022-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thorup AS, Strachan D, Caxaria S, et al. ROR2 blockade as a therapy for osteoarthritis. Sci Transl Med. 2020;2:12. [DOI] [PubMed] [Google Scholar]
- 90.Su S, Jiang W, Wang X, et al. Resolvin D1 inhibits the proliferation of osteoarthritis fibroblast-like synoviocytes through the Hippo-YAP signaling pathway. BMC Musculoskelet Disord. 2022;23(1):149. doi: 10.1186/s12891-022-05095-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang K, Wu Y, Cheng P, et al. YAP and ERK mediated mechanical strain-induced cell cycle progression through RhoA and cytoskeletal dynamics in rat growth plate chondrocytes. J Orthop Res. 2016;34(7):1121–1129. doi: 10.1002/jor.23138 [DOI] [PubMed] [Google Scholar]
- 92.He J, Su X, Xie W. MiR-582-3p alleviates osteoarthritis progression by targeting YAP1. Mol Immunol. 2020;128:258–267. doi: 10.1016/j.molimm.2020.10.022 [DOI] [PubMed] [Google Scholar]
- 93.Karystinou A, Roelofs AJ, Neve A, Cantatore FP, Wackerhage H, De bari C. Yes-associated protein (YAP) is a negative regulator of chondrogenesis in mesenchymal stem cells. Arthritis Res Ther. 2015;17(1):147. doi: 10.1186/s13075-015-0639-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jing X, Ye Y, Bao Y, et al. Mechano-growth factor protects against mechanical overload induced damage and promotes migration of growth plate chondrocytes through RhoA/YAP pathway. Exp Cell Res. 2018;366(2):81–91. doi: 10.1016/j.yexcr.2018.02.021 [DOI] [PubMed] [Google Scholar]
- 95.Li M, Ning J, Wang J, Yan Q, Zhao K, Jia X. SETD7 regulates chondrocyte differentiation and glycolysis via the Hippo signaling pathway and HIF1alpha. Int J Mol Med. 2021;47(4):48. doi: 10.3892/ijmm.2021.4881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jing X, Yang X, Zhang W, et al. Mechanical loading induces HIF-1alpha expression in chondrocytes via YAP. Biotechnol Lett. 2020;42(9):1645–1654. doi: 10.1007/s10529-020-02910-4 [DOI] [PubMed] [Google Scholar]
- 97.Yu FF, Zuo J, Fu X, et al. Role of the hippo signaling pathway in the extracellular matrix degradation of chondrocytes induced by fluoride exposure. Ecotoxicol Environ Saf. 2021;225:112796. doi: 10.1016/j.ecoenv.2021.112796 [DOI] [PubMed] [Google Scholar]
- 98.Nie P, Li Y, Suo H, Jiang N, Yu D, Fang B. Dasatinib promotes chondrogenic differentiation of human mesenchymal stem cells via the Src/Hippo-YAP signaling pathway. ACS Biomater Sci Eng. 2019;5(10):5255–5265. doi: 10.1021/acsbiomaterials.9b00618 [DOI] [PubMed] [Google Scholar]
- 99.Li H, Li X, Jing X, et al. Hypoxia promotes maintenance of the chondrogenic phenotype in rat growth plate chondrocytes through the HIF-1alpha/YAP signaling pathway. Int J Mol Med. 2018;42(6):3181–3192. doi: 10.3892/ijmm.2018.3921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Moya IM, Halder G. Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat Rev Mol Cell Biol. 2019;20(4):211–226. doi: 10.1038/s41580-018-0086-y [DOI] [PubMed] [Google Scholar]
- 101.Dupont S. Role of YAP/TAZ in cell-matrix adhesion-mediated signalling and mechanotransduction. Exp Cell Res. 2016;343(1):42–53. doi: 10.1016/j.yexcr.2015.10.034 [DOI] [PubMed] [Google Scholar]
- 102.Koo JH, Guan KL. Interplay between YAP/TAZ and Metabolism. Cell Metab. 2018;28(2):196–206. doi: 10.1016/j.cmet.2018.07.010 [DOI] [PubMed] [Google Scholar]
- 103.Bartel DP. Metazoan MicroRNAs. Cell. 2018;173(1):20–51. doi: 10.1016/j.cell.2018.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cheng J, Li M, Bai R. The Wnt signaling cascade in the pathogenesis of osteoarthritis and related promising treatment strategies. Front Physiol. 2022;13:954454. doi: 10.3389/fphys.2022.954454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Norouzi M, Yasamineh S, Montazeri M, et al. Recent advances on nanomaterials-based fluorimetric approaches for microRNAs detection. Mater Sci Eng C Mater Biol Appl. 2019;104:110007. doi: 10.1016/j.msec.2019.110007 [DOI] [PubMed] [Google Scholar]
- 106.Wang SR, Chen X, Ling S, Ni RZ, Guo H, Xu JW. MicroRNA expression, targeting, release dynamics and early-warning biomarkers in acute cardiotoxicity induced by triptolide in rats. Biomed Pharmacother. 2019;111:1467–1477. doi: 10.1016/j.biopha.2018.12.109 [DOI] [PubMed] [Google Scholar]
- 107.Cha S, Wang J, Lee SM, Tan Z, Zhao Q, Bai D. Clock-modified mesenchymal stromal cells therapy rescues molecular circadian oscillation and age-related bone loss via miR142-3p/Bmal1/YAP signaling axis. Cell Death Discov. 2022;8(1):111. doi: 10.1038/s41420-022-00908-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang JX, Xie P, Li YS, Wen T, Yang XC. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2020;70:109504. doi: 10.1016/j.cellsig.2019.109504 [DOI] [PubMed] [Google Scholar]
- 109.Woods A, Wang G, Beier F. Regulation of chondrocyte differentiation by the actin cytoskeleton and adhesive interactions. J Cell Physiol. 2007;213(1):1–8. doi: 10.1002/jcp.21110 [DOI] [PubMed] [Google Scholar]
- 110.Xia B, Di C, Zhang J, Hu S, Jin H, Tong P. Osteoarthritis pathogenesis: a review of molecular mechanisms. Calcif Tissue Int. 2014;95(6):495–505. doi: 10.1007/s00223-014-9917-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kania K, Colella F, Riemen AHK, et al. Regulation of Gdf5 expression in joint remodelling, repair and osteoarthritis. Sci Rep. 2020;10(1):157. doi: 10.1038/s41598-019-57011-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ma T, Ruan H, Lv L, et al. Oleanolic acid, a small-molecule natural product, inhibits ECM degeneration in osteoarthritis by regulating the Hippo/YAP and Wnt/beta-catenin pathways. Food Funct. 2023;14(22):9999–10013. doi: 10.1039/D3FO01902K [DOI] [PubMed] [Google Scholar]
- 113.Wang X, Liao H, Liu Y, et al. Aspirin reverses inflammatory suppression of chondrogenesis by stabilizing YAP. Cell Prolif. 2023;56:e13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen R, Ying C, Zou Y, et al. Sarsasapogenin inhibits YAP1-dependent chondrocyte ferroptosis to alleviate osteoarthritis. Biomed Pharmacother. 2023;168:115772. doi: 10.1016/j.biopha.2023.115772 [DOI] [PubMed] [Google Scholar]
- 115.Hao X, Zhao J, Jia L, et al. XMU-MP-1 attenuates osteoarthritis via inhibiting cartilage degradation and chondrocyte apoptosis. Front Bioeng Biotechnol. 2022;10:998077. doi: 10.3389/fbioe.2022.998077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Feng X, Li S, Wang S, et al. Piezo1 mediates the degradation of cartilage extracellular matrix in malocclusion-induced TMJOA. Oral Dis. 2023. doi: 10.1111/odi.14615 [DOI] [PubMed] [Google Scholar]
- 117.Liu R, Liu Z, Chen H, et al. Ginkgolide K delays the progression of osteoarthritis by regulating YAP to promote the formation of cartilage extracellular matrix. Phytother Res. 2023;37(11):5205–5222. doi: 10.1002/ptr.7953 [DOI] [PubMed] [Google Scholar]
- 118.Ma XN, Feng W, Li N, et al. Leonurine alleviates rheumatoid arthritis by regulating the Hippo signaling pathway. Phytomedicine. 2023;123:155243. doi: 10.1016/j.phymed.2023.155243 [DOI] [PubMed] [Google Scholar]
- 119.Barker BE, Hanlon MM, Marzaioli V, et al. The mammalian target of rapamycin contributes to synovial fibroblast pathogenicity in rheumatoid arthritis. Front Med. 2023;10:1029021. doi: 10.3389/fmed.2023.1029021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Z, Wang Y, Xu Q, et al. Methyl Canthin-6-one-2-carboxylate Restrains the Migration/Invasion Properties of Fibroblast-like Synoviocytes by Suppressing the Hippo/YAP Signaling Pathway. Pharmaceuticals. 2023;17(1):16. doi: 10.3390/ph17010016 [DOI] [PMC free article] [PubMed] [Google Scholar]