Abstract
Proteoglycans are core proteins associated with carbohydrate/sugar moieties that are highly variable in disaccharide composition, which dictates their function. These carbohydrates are named glycosaminoglycans, and they can be attached to proteoglycans or found free in tissues or on cell surfaces. Glycosaminoglycans such as hyaluronan, chondroitin sulfate, dermatan sulfate, keratan sulfate and heparin/heparan sulfate have multiple functions including involvement in inflammation, immunity and connective tissue structure and integrity. Heparan sulfate is a highly sulfated polysaccharide that is abundant in the periodontium including alveolar bone. Recent evidence supports the contention that heparan sulfate is an important player in modulating interactions between damage associated molecular patterns and inflammatory receptors expressed by various cell types. The structure of heparan sulfate is reported to dictate its function, thus, utilization of a homogenous and structurally defined heparan sulfate polysaccharide for modulation of cell function offers therapeutic potential. Recently, a chemoenzymatic approach was developed to allow production of many structurally defined heparan sulfate carbohydrates. These oligosaccharides have been studied in various pathological inflammatory conditions to better understand their function and their potential application in promoting tissue homeostasis. We have observed that specific size and sulfation patterns can modulate inflammation and promote tissue maintenance including an anabolic effect in alveolar bone. Thus, new evidence provides a strong impetus to explore heparan sulfate as a potential novel therapeutic agent to treat periodontitis, support alveolar bone maintenance and promote bone formation.
Keywords: glycosaminoglycans, heparan sulfate, inflammation, extracellular matrix, periodontitis, periodontal health, bone regeneration
1. INTRODUCTION
This article aims to review key extracellular matrix components in the periodontium that have been found to be actively involved in immunity, tissue homeostasis and cell signaling besides their well-known role as scaffolds to promote cell growth and migration. More recently, these extracellular matrix components have been investigated as therapeutic candidates to promote connective tissue homeostasis and regeneration. The organic environment in the periodontal tissues is primarily composed of collagen type I followed by non-collagenous proteins. A large component of the non-collagenous milieu are proteins rich in carbohydrate/sugar moieties called proteoglycans. These proteoglycans carry sugars/carbohydrates named glycosaminoglycans that can be of different lengths, disaccharide combinations and levels of sulfation, ultimately generating a series of diverse molecular signatures that act as codes to unlock specific functions. The application of glycosaminoglycan-based therapeutics represents a promising candidate as a next-generation biomaterial in bone and periodontal tissue homeostasis. The discovery of (a) specific glycosaminoglycan code(s) that is/are key in inflammation control, bone homeostasis and regeneration may lead to a novel line of cutting-edge regenerative therapeutics.
2. PROTEOGLYCANS AND GLYCOSAMINOGLYCANS IN THE PERIODONTIUM MICROENVIRONMENT
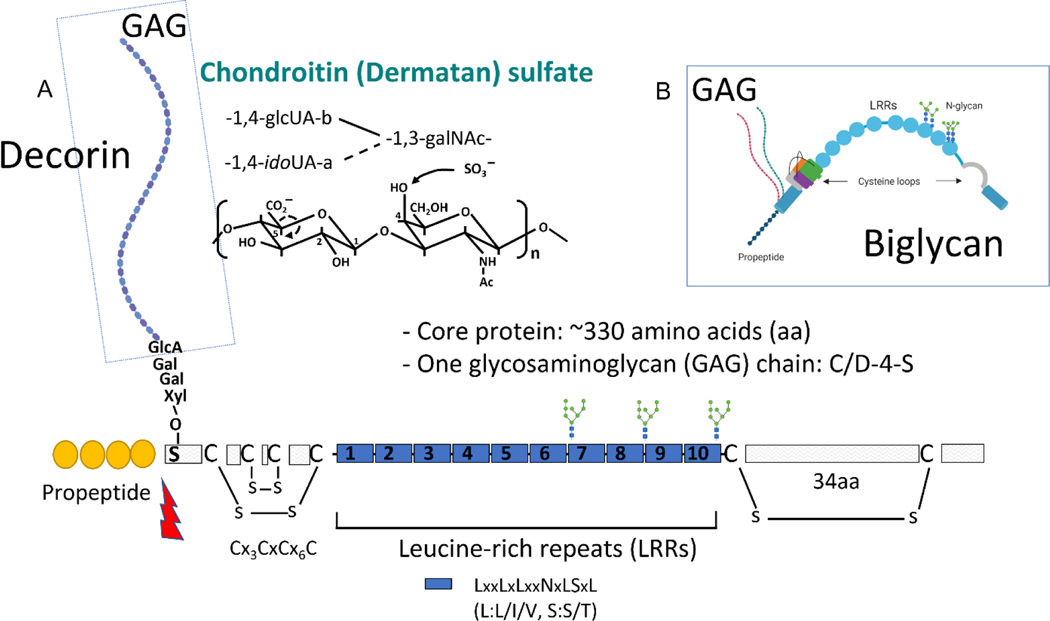
The most abundant non-collagenous extracellular matrix proteins are proteoglycans. This class of glycosylated proteins is composed of an amino acid sequence that is post-translationally modified 1. Post-translational modification happens to the core of a protein following its assembly and can be, for example, glycanation, glycosylation, enzymatic cross-linking or cleavage of core protein, all of which can ultimately dictate the protein function 2. One of these post-translational modifications, the addition of a carbohydrate covalently bound to a core protein, leads to generation of proteoglycans (a polypeptide core with short N-linked oligosaccharides and large O-linked disaccharides). These disaccharides are, in summary, carbohydrates with a repeating and specific sequence called glycosaminoglycans. Glycosaminoglycans are classified according to their composition into 6 types: heparin, heparan sulfate, chondroitin sulfate, dermatan sulfate, keratan sulfate, hyaluronic acid or hyaluronan 3,4 (Figure 1). The diversity of the glycosaminoglycan chains confers a variety of functions upon proteoglycans including collagen alignment, mineralization, cell signaling, mechanical property modulation, immune regulation, etc. 5 (supplementary table 1). The disaccharide units of glycosaminoglycans consist of an N-acetyl–hexosamine [e.g., galactosamine or glucosamine, a hexose or hexuronic acid (e.g., galactose or glucuronic acid or iduronic acid, where either or both may be sulfated). The combination of the sulfate group and the carboxylate groups gives them a very high-density negative charge. Dermatan sulfate is distinguished from chondroitin sulfate by the presence of iduronic acid. The first steps of the synthesis of glycosaminoglycan chains of chondroitin sulfate/dermatan sulfate and heparan sulfate share a common pathway involving xylosyl transferases 1 and 2, β4-galactosyltransferase, β3-galactosyltransferase and β3-glucuronosyltransferases. Chondroitin sulfate/dermatan sulfate linkage region biosynthesis and chain extension require two additional enzymes, β4-galactose N-acetyl transferase, and chondroitin synthases 1, 2 and 3-like, which has both β3 and β4 glucuronosyltransferases activities required to form the repeating disaccharide unit. Lastly, several enzymes are involved in the modification of the chondroitin sulfate/dermatan sulfate glycosaminoglycan repeating unit (5 enzymes) that adds sulfate to glucuronic acid/iduronic acid (2S) or N-acetyl galactosamine (4S and/or 6S) 6. Thus, their synthesis represents a highly complex event, and by default, difficult to replicate. Proteoglycans and their glycosaminoglycans are vastly and strategically distributed in mineralized and non-mineralized connective tissues 7–10. Many of the proteoglycans within the periodontium (cementum, periodontal ligament, and alveolar bone), are key members of each specific cell environment and play a role in physiological turnover, cell differentiation, mineralization control, mechanical strength, and inflammation 11–14. The small leucine-rich proteoglycan family are abundant proteoglycans within the periodontium 15–18. Small leucine-rich proteoglycans have been classified into five sub-families, a topic extensively reviewed in Iozzo et al., 2015 5. These proteoglycans present a cluster of leucine repeats in their core that is known to promote protein-protein interactions 19. They have been extensively studied in the context of connective tissue development and more recently in regeneration due to their ability to bind growth factors of the transforming growth factor-β family, such as bone morphogenetic proteins 20. An example of small leucine-rich proteoglycans commonly found in connective tissues, decorin and biglycan, are depicted in Figure 2.
Figure 1:
Proteoglycans and their glycosaminoglycans. (A) One or more glycosaminoglycans are attached to a protein core and can provide spacing (black arrows), mechanical support, influence water retention (blue droplet), tissue shape, mineralization, and signaling in connective tissues. Some proteoglycans have hundreds of glycosaminoglycans creating a centipede-like structure (i.e. aggrecan). (B) Glycosaminoglycans are linear polymers of repeating disaccharides that contain one hexosamine N-acetyl glucosamine or N-acetyl galactosamine and a uronic acid/galactose. Different combinations lead to 6 types of glycosaminoglycans. Partly created with Biorender.
GAG: glycosaminoglycan, N: N-terminal, C: C-terminal, HA: hyaluronic acid, HS: heparan sulfate, Hep: heparin, KS: keratan sulfate, CS: chondroitin sulfate, DS: dermatan sulfate, GlcNAc: N-acetyl glucosamine, GalNAc: N-acetyl galactosamine, GlcUA: glucuronic acid, IdoUa: iduronic acid
Figure 2:
Small leucine-rich proteoglycans. (A) Decorin is a type of small leucine-rich proteoglycan highly abundant in connective tissues of the periodontium. It has one glycosaminoglycan of chondroitin sulfate or dermatan sulfate origin attached to the N-terminal. The leucine-rich repeats favor protein-protein interactions. Its N-terminal propeptide can be cleaved by bone morphogenic protein 1 (lightning shape). (B) Decorin, like biglycan, has a tertiary structure characterized by a C-shape and often the glycosaminoglycan(s) is larger than the core protein itself.
GlcUA: glucuronic acid, idoUA: iduronic acid, GalNAc: N-acetyl galactosamine, Gal: galactose, Xyl: xylose, C/D: chondroitin/dermatan
Although proteoglycans have been widely studied, the specific involvement of their glycosaminoglycan chains, either associated or not associated with the core (i.e. released free in tissue by enzymes) in signaling processes has only recently gained traction. Glycosaminoglycans are emerging as critical components in many physiological processes, yet there is still a lack of understanding of their structure-function relationship.
A major challenge in studying glycosaminoglycan-protein interactions is the difficulty in acquiring an adequate and structurally homogeneous pool of glycosaminoglycans for research due to the extensive number and complexity of glycosides that are extracted from tissues 21. Generating glycosaminoglycans in vitro through overexpression of glycosaminoglycan-synthesizing enzymes is also a challenge due to the involvement of multiple enzymes in the synthesis of glycosaminoglycans. Therefore, there is a need to develop new methods and strategies to produce glycosaminoglycans in larger quantities, with higher purity levels and intentional and predictable homogeny 22. This will enable more detailed studies of the structural-functional relationship of glycosaminoglycans and their interactions with proteins 23. The ability to generate synthetic glycosaminoglycans of heparan sulfate and chondroitin sulfate nature has given a new understanding of the role of these glycosaminoglycans in important events such as inflammation and tissue homeostasis 24–27. This is a critical step in development of new agents and provides a novel avenue of therapeutics in the field of inflammatory diseases and bone ailments, which has the potential to make a paradigm shift in periodontal biology.
2.1. Proteoglycans and glycosaminoglycans in periodontal ligament
The most abundant extracellular proteoglycans in periodontal ligament are reported to be members of the small leucine-rich family (which are considered small proteoglycans), such as lumican (with keratan sulfate glycosaminoglycans, thought to provide the exact alignment of collagen and aid in tissue repair), asporin (which does not have a glycosaminoglycan attached, is rich in aspartic acid and is thought to inhibit mineralization), biglycan (known for its contribution to the regulation of transforming growth factor-β/bone morphogenic protein signaling) and decorin (primarily known for its “decorating” ability of collagen and the regulation of its organization and mineralization)28–31 (Figure 3). Decorin and biglycan are “sister” proteoglycans of the small leucine-rich class with very similar amino acid sequences, although decorin has one glycosaminoglycan and biglycan can have two. They both bind to collagen (decorin having the strongest affinity) and their glycosaminoglycans are primarily of chondroitin sulfate/dermatan sulfate nature. These small leucine-rich proteoglycans and their respective glycosaminoglycans have been implicated in attracting water, modulating mineralization, participating in collagen matrix organization and regulating cell differentiation and signaling 32–34.
Figure 3:
Classification of main proteoglycans and glycosaminoglycans found in periodontium based on their location and associated functions.
PDL: periodontal ligament; ECM: extracellular matrix; GAG: glycosaminoglycan; PGs: proteoglycan; CS: chondroitin sulfate; DS: dermatan sulfate; KS: keratan sulfate; HS: heparan sulfate; LRR: leucine-rich repeat. Teeth and cells schematics created with Biorender.
Of the cell surface proteoglycans in periodontal ligament, syndecans and glypicans are examples commonly found around teeth and both present with heparan sulfate-type glycosaminoglycans 35,36. Syndecans have been implicated in wound healing and modulation of inflammation, 37 and glypicans control cell growth and angiogenesis through various signaling pathways leading to Smads, β-catenin and AP-1 activation downstream of receptors such as bone morphogenetic, frizzled, low density lipoprotein receptor-related protein and fibroblast growth factor receptors 38–40. Classification of the proteoglycans based on their location, homology and associated glycosaminoglycans, is displayed in Figure 3. Proteoglycans and glycosaminoglycans that have been implicated in bone morphogenetic protein function, bind to bone morphogenetic proteins both via the negative charge of proteoglycans conferred by glycosaminoglycans. They also bind the leucine-rich repeat to the N-terminal region containing basic amino acids and known to attract other proteins such as bone morphogenetic proteins 41–43. Their critical role in transforming growth factor-β and bone morphogenetic protein signaling has not been as widely explored in periodontal ligament homeostasis as in bone 44–46. Particularly for heparan sulfate, it has been demonstrated that the degree of sulfation of heparan sulfate can impact the strength of the electrostatic interactions between heparan sulfate proteoglycans and bone morphogenetic proteins 47. However, the binding of bone morphogenetic proteins to heparan sulfate proteoglycans does not entirely depend on sulfated glycosaminoglycans 48. The significance of regulating bone morphogenetic protein biding through sulfation is of interest in development of heparan sulfate-based regenerative materials.
2.2. Proteoglycans and glycosaminoglycans in the mineralized tissues of the periodontium
2.2.1. Cementum
Large proteoglycans within cementum such as versican (with chondroitin sulfate-type of glycosaminoglycan), retain hydrostatic pressure and significantly influence the shape of connective tissues given their bulkiness (i.e. aggrecan in cartilage, has several chondroitin sulfate-type of glycosaminoglycan, has a bottle-brush/centipede shape, it is over 2,500kDa, thus attracting large amounts of water, Figure 1) 28,49–51, while lower molecular weight extracellular proteoglycans (such as small leucine-rich proteoglycans) can maintain and fine tune the immediate cell-matrix interactions such as cementoblasts and cementocytes. Around cementum cells, biglycan is found with greater abundance, although versican and syndecan are also highly expressed 52,53. Syndecans and glypicans, the heparan sulfate proteoglycans, play crucial role in cell signaling during tooth movement and inflammatory insult 35.
The distribution of glycosaminoglycans in cementum was thoroughly characterized in the late 80’s and early 90’s and proved them to be mostly composed of chondroitin sulfate, dermatan sulfate and heparan sulfate with some free hyaluronan 54–56. Hyaluronan is highly abundant in periodontium, is non-sulfated and is the most studied glycosaminoglycan in the context of periodontal homeostasis 57–59. Its role in cell migration, inflammation, anti-fibrosis and as a spatial regulator is well recognized 60. However, the role of hyaluronan size in the immune response needs to be further elucidated as low and high molecular weight hyaluronan have opposite effects on inflammation 61.
It has been pointed out that some small leucine-rich proteoglycans (such as biglycan and decorin), bind to collagen fibrils at very specific sites that are critical for hydroxyapatite deposition 62,63 preventing mineralization at those sites but ordering mineral deposition where their glycosaminoglycans extend to 64,65. At the periodontal ligament-cementum and dentin-cementum interfaces, biglycan, fibromodulin and their glycosaminoglycans, connect with collagen and this protective proteoglycan-glycosaminoglycan layer is thought to maintain tooth structure integrity during orthodontics as well as to control osteoclastogenic activity to reach dentin during tooth exfoliation 63,66. As teeth are subjected to loading, these glycosaminoglycans provide cushioning, protection via electrostatic interactions, steric hindrance, water retention, lubrication and prevent mineralization and resorption of these sites during tooth movement 67. In a similar manner, the cementoid secreted by progenitor cells contains higher ratios of organic to inorganic matter due to, primarily, the presence of glycosaminoglycans. During mineralization, proteoglycans abundance diminishes in mineralized tissues such as dentin and bone 68. This finding implies that proteoglycans and glycosaminoglycans are important for induction of mineralized tissue formation and, therefore, exploring this function in tissue regeneration is highly relevant to advance regenerative therapies.
2.2.2. Bone
For some tissues such as bone, information regarding absolute amounts of extracellular matrix components has not been appropriately recorded. Estimates have been generated for several connective tissues in health and disease demonstrating that there can be variations in sulfation of glycosaminoglycans and collagen content. The predominant proteoglycans in bone have been reported to be small leucine-rich and heparan sulfate proteoglycans 69–72. The predominant glycosaminoglycans in human alveolar bone have been described to be chondroitin sulfate (4-S), although hyaluronan, dermatan sulfate and heparan sulfate are all attached to proteins, cells or free within tissue 73–77. Interestingly, with age, glycosaminoglycans have been demonstrated to shorten and proteoglycans can be found completely deglycanated (so called non-proteoglycan form) or partially degraded (small leucine-rich proteoglycan fragmentation)15,72.
Glycosaminoglycan composition has also been shown to change in ectopic bone, inflammatory diseases such as diabetes, rheumatoid arthritis and lupus 33,78. The expression of proteoglycans and glycosaminoglycans on cell surfaces forming a glycocalyx dictates cell interactions with chemokines, receptors and other molecules in the endosteal and mineralized areas of bone 33,79. Thus, changes with aging and with disease can have considerable impact on cell signaling and health.
Another important aspect of proteoglycans and glycosaminoglycans in bone, is the fact that they are critical mediators of mechanical properties 11,80. Altered proteoglycans and glycosaminoglycan content can lead to brittle bone 32. The common signaling pathways found across connective tissues that are modulated by proteoglycans and glycosaminoglycans are Wnt, Smads, mitogen-activated protein kinase, etc. all resulting from modulation of bone morphogenetic protein, fibroblast growth factor, platelet-derived growth factor, insulin growth factor, vascular endothelial growth factor and transforming growth factor signaling 81,82. Many receptors have been found to interact with proteoglycans and glycosaminoglycans and the complexity of the interactions are illustrated in Figure 4.
Figure 4:
Various proteoglycans and glycosaminoglycans around progenitor cells in periodontal ligament and bone are in extracellular space or on the cell surface or peri-surface. They bind with various classes of protein (enzymes, growth factors, receptors, adhesion molecules, chemokines, pathogens). The nature of the interactions is thought to be determined by the length, sulfation pattern and type of disaccharides. The glycocalyx is composed of glycosaminoglycans surrounding cells is of key importance in ligand/receptor interactions, virus/bacteria interactions, and its shedding (via heparanase) leads to binding of glycosaminoglycans and proteoglycans with other soluble molecules, mediating the inflammatory response and microenvironment of cells. Created with Biorender.
BGN: biglycan, PGs: proteoglycans, DCN: decorin, BGN: biglycan, HS: heparan sulfate, HSPG: heparan sulfate proteoglycan, CS: chondroitin sulfate, DS: dermatan sulfate, KS: keratan sulfate, HA: hyaluronic acid, BMP2: bone morphogenetic protein 2, BMPR: bone morphogenetic protein receptor, FGF: fibroblast growth factor receptor, FGFR: fibroblast growth factor receptor, TGF-β: transforming growth factor beta, TLR: toll-like receptor, FZ: frizzled, LRP: low density lipoprotein receptor-related protein
Mice lacking proteoglycans and/or glycosaminoglycans provide clues to the function of such a variety of proteoglycans and glycosaminoglycans in bone formation and maintenance at both structural and signaling levels 5,83–87. Decorin deficient mice are primarily characterized by a phenotype of skin fragility due to abnormal collagen fibrillogenesis and assembly 88. In mice there are problems in dentin mineralization and periodontal homeostasis due to, in part, collagen fragility 89–91 as well as effects on cell adhesion, migration and differentiation. More recently, there have been several reports on the effect of decorin on innate immunity, angiogenesis and tumor progression 92–96. Biglycan ablation has a more pronounced effect in bone leading to reduced bone mass with age 83,84. Osteoblast numbers are decreased, and reduced bone morphogenetic protein signaling as well as osteoclast function is altered. Biglycan was also found to promote angiogenesis and lack of biglycan hinders vascular endothelial growth factor receptor 2 activation leading to delayed fracture healing 97.
Overall, of all the 6 types of glycosaminoglycans, hyaluronan and heparan sulfate have been the most studied in the context of inflammation and, in turn, there is growing interest in these glycosaminoglycans in bone homeostasis. Heparan sulfate is well known for interactions with growth factors and cytokines, control of bone morphogenetic protein signaling, heparanase expression (an enzyme that cleaves heparan sulfate and is abundant in response to stress such as sterile inflammation or infection) 98, fibroblast growth factor/bone morphogenetic protein interaction and noggin/gremlin (bone morphogenetic protein antagonists) function as heparan sulfate is ubiquitously expressed at cell surfaces and in extracellular matrix. Heparan sulfate has been proposed as a master regulator of stem cell fate due to its ability to modulate protein gradients and signal transduction 99. Lack of heparan sulfate production causes excessive bone morphogenetic protein signaling and can provoke ectopic chondrogenesis and osteochondroma formation 100,101.
Of the many cytokines of the transforming growth factor-β superfamily, around a third bind heparin and heparan sulfate. Growing evidence reveals that antagonists to these growth factors are also heparin binding proteins 102. Thus, it is likely that the structure of heparan sulfate plays a critical role in the fate of the molecule towards an anabolic or catabolic pathway. Since heparan sulfate, via variations in sulfation, can be constructed for a specific function (rather than using just size as dictator of function in the case of hyaluronan), this molecule is an attractive and pliable candidate for therapeutic development.
3. PROTEOGLYCANSS AND GLYCOSAMINOGLYCANS AS MEDIATORS OF INFLAMMATION
The past 15 years of research has shown that small leucine-rich proteoglycans can act as modulators of inflammation via interaction with innate immune receptors 103,104. Fascinating evidence has revealed small leucine-rich proteoglycans to promote a switch between pro-and anti-inflammatory signaling pathways by choosing which receptor to interact with. Pathogen- and danger-associated molecular patterns trigger a series of receptors that orchestrate inflammation such as toll-like receptors, and the receptor for advanced glycation end products. Small leucine-rich proteoglycans can orchestrate both pathogen-mediated- and sterile inflammation during innate and adaptive immune response 105–107 (Figure 4).
A paradigm shift in understanding the role of proteoglycans in inflammation arose when the small leucine-rich proteoglycan biglycan was found to have a pro-inflammatory function by binding to toll-like receptors-2 and −4 106. Biglycan knock out mice were subjected to lethal doses of bacterial lipopolysaccharide to induce sepsis and mutant mice presented with less systemic inflammation and had lower mortality rates. Macrophages secrete biglycan which behaves as danger-associated molecular pattern triggering the innate immune toll-like receptors-2 and 4 106 and the purinergic P2X receptors 108. Other evidence of biglycan as a pro-inflammatory molecule was found in toll-like receptors-2/4 activation in ischemic acute renal injury 103,109. Its detection as a soluble molecule has raised the idea of detection of biglycan as a biomarker of renal stress 110. The dermatan sulfate and chondroitin sulfate type of glycosaminoglycans have also been described to influence inflammation depending upon their length and by modulating nuclear factor kappa-light-chain-enhancer of activated B cells activation 105,111. Our group 112,113 found that the core of biglycan was more prone to mediate osteogenic function as compared to glycosaminoglycan-rich biglycan. This is likely because the chondroitin sulfate/dermatan sulfate type of glycosaminoglycan of such small leucine-rich proteoglycan may favor inflammatory-receptor interactions rather than bone morphogenetic protein receptor(s) activation.
Decorin, similarly structured to biglycan, with either a dermatan sulfate or chondroitin sulfate single glycosaminoglycan has various binding partners that are beyond the extracellular matrix component collagen. Some of the interactions involve glycosaminoglycan chains 114. However, decorin can also serve as a ligand to toll-like receptor-2 and 4 and, similarly to biglycan, only when associated with a glycosaminoglycan can trigger signaling through these receptors 115. Certain inflammatory conditions exhibit overexpression of decorin corroborating its proposed role as a pro-inflammatory mediator, dependent upon its structure 116,117.
Lumican, with its keratan sulfate glycosaminoglycan, has also been shown to mediate pathogen-induced inflammation. One proposed mechanism is that it promotes the binding of lipopolysaccharide-CD14 to toll-like receptor-4 leading to phagocytosis and subsequent stimulation of type I interferon production. Thus, lumican promotes further secretion of pro-inflammatory cytokines and mice lacking lumican exhibit a diminished inflammatory response 118,119.
Another small leucine-rich proteoglycan, fibromodulin, is believed to play a role in the complement system in rheumatoid arthritis and osteoarthritis, which show evidence of fibromodulin activation of complement via C1q and C3b binding. Subsequently, following activation of the complement system, toll-like receptor activation and influence on adaptive immunity is reported. Its role in wound healing is now accepted via modulation of angiogenesis and extracellular matrix organization 120,121. Of the cell bound proteoglycans, heparan sulfate proteoglycans, glypicans (heparan sulfate only glycosaminoglycans) and syndecans (heparan sulfate and chondroitin sulfate glycosaminoglycans), have heterogeneous glycosaminoglycan composition, and therefore exhibit large differences in charge and flexibility at the cell surface 122,123.
The different types of syndecans are characterized by differences in glycosaminoglycan position 124. Syndecan-1 knockout mice have a hyper-inflammatory phenotype 125. After myocardial infarction, inflammatory cell recruitment is increased in these mice and syndecan-1 deficiency accelerated the deposition of granulation tissue in the infarcted heart 126. Human umbilical vein endothelial cells, when exposed to bacteria lipopolysaccharide, show increase in syndecan-4 and decrease in both −1 and −2 mRNA levels. Without syndecan-4, these cells express angiogenic growth factors and in vivo, the knockout mice upon lipopolysaccharide injection have a reduced immune response 127,128.
Syndecan plays an important role in the migration of leukocytes via its heparan sulfate chains and this process has been described in several studies and is summarized in a recent review in Gopal 2020 129. Interestingly, this proteoglycan can undergo morphological changes, which acts as a trigger for the migration process. It has been reported to act on the actin cytoskeleton, via a feedback loop and to effect the release of matrix metalloproteinases, which are responsible for degrading the extracellular matrix allowing for leukocyte migration 104.
Shedding of syndecan-1 (with heparan sulfate and chondroitin sulfate glycosaminoglycans) occurs by the action of heparanase as well as of matrix metalloproteinases (that are increased due to heparanase) 130. The exposure of receptors due to shedding of heparan sulfate (the main component of glycocalyx) that are players in the activation of the inflammatory response is a significant consequence of the glycocalyx degradation 131,132. A systematic review evaluated if glycocalyx components change in a variety of diseases and conditions. It was found that surgery and trauma can lead to glycocalyx degradation 133. Thus, this process happens in both acute and chronic inflammation and is of high relevance to disease progression and tissue healing.
Regarding free heparan sulfate and heparin, research on inflammation has also explored them as anti-inflammatory molecules partly due to incidental findings in heparin-based research 134. Heparin is a widely-used anticoagulant, but it has also been found to have excellent anti-inflammatory properties in both animal models and human clinical trials as well as anti-apoptosis and anti-cancer properties as noted in a systematic review 135.
Heparins may protect endothelial cells from damage through their effects on histone methylation (caused by severe inflammation) and through regulation of mitogen-activated protein kinase and nuclear factor kappa-light-chain-enhancer of activated B cells signaling pathways 136–138. Heparin binds with inflammatory cytokines, inhibits neutrophil chemotaxis by modulation of the formation of neutrophil extracellular traps, has a direct effect on leukocyte migration and neutralizes the complement factor C5a 139–141. Commercially available low molecular weight heparins have been reported to limit exaggerated formation of neutrophil extracellular traps, which can result in the chronicity of periodontitis 142,143.
A number of studies have also demonstrated the effectiveness of heparin in treating, for example, inflammation associated with bronchial asthma 144. Similarly, ulcerative colitis patients treated with heparin experienced a significant reduction in their symptoms. Finally, heparin was also found to be effective in the treatment of burns, helping to reduce pain and inflammation145.
The most recent contribution of heparin in controlling inflammation was with SARS-CoV-2 146. While it was initially given to sick individuals with the purpose of preventing blood clots, its role as an anti-inflammatory is now more broadly accepted. This function likely played a key role in reducing the level of the ‘cytokine storm’ reported in several patients given the characteristic binding of the virus to heparan sulfate, reducing viral penetration of cells, decreasing glycocalyx perturbation by the virus and inhibiting heparanase 140.
A few studies of heparin have demonstrated its effect is unpredictable, likely because it is extracted from tissues and marketed for anti-coagulant purposes with little regulation 147. Due to the international source of heparin (potential contaminants) and the heterogeneity of the molecule itself, variability of its interactions is extensive and likely leads to the reported unpredictable effects. Heparin’s anti-inflammatory effect is dependent on glucosamine 6–0 sulfation, thus, other sulfation patterns did not promote the resolution of inflammation 148. Thus, we believe that finding and producing a specific heparan sulfate/heparin structure is of key importance in its potential implementation as an effective anti-inflammatory and commercially available agent.
Compared to unfractionated (tissue extracted, unmodified) heparin, the modified 2-O,3-O-desulfated heparin can block L- and P-selectins 148. A synthetic heparin mimetic developed to mimic the anti-inflammatory effects of heparin, but without any anticoagulant effects. was found to reduce airway inflammation by inhibiting human neutrophil elastase. This molecule has been shown to be a highly effective therapeutic agent for a variety of inflammatory conditions 149.
Small leucine rich proteoglycans such as biglycan, fibromodulin, lumican are being explored as therapeutics 150–152. Recombinant protein application as therapeutics in orthopedics and dentistry has led to breakthroughs in regeneration strategies (e.g. bone morphogenetic proteins, platelet derived growth factor), although concerns with cost, lack of important post-translational modifications, and evidence of induction of serious side effects are on the rise 153,154. Carbohydrates could be alternatives to these proteins, and of all glycosaminoglycans, heparan sulfate is the most studied as a modulator of inflammation in the context of infection and most recently in sterile inflammatory processes. Similarly, hyaluronan has been investigated in immunity, although hyaluronan degradation in situ (due to bacterial lyase) can lead to changes in its function 155. Due to newly developed technology, the ability to control size, degradation (sulfation can prevent cleavage) and sulfation pattern of heparan sulfate is now tangible, and this glycosaminoglycan offers the best opportunity as a therapeutic to predictably and cost-effectively control inflammation and other functions.
4. HEPARAN SULFATE, HEPARIN AND HEPARAN SULFATE PROTEOGLYCANS IN PHYSIOLOGY
As described in previous sections, heparan sulfate and heparin are known for their ability to bind proteins. While some proteins bind heparan sulfate with high affinity, others bind because of its promiscuity, thus, greater understanding of structure-function in heparan sulfate is needed.
Heparin is synthetized in mast cells and basophils in the lungs, liver and intestines 156,157. When these cells degranulate and degrade, heparin is secreted 156. Heparin is typically purified from porcine and bovine sources and is used in clinic as an anticoagulant because it binds to antithrombin and accelerates the inactivation of the procoagulation enzymes thrombin and factor Xa. It has also been demonstrated to modulate inflammation, oxidation and vasodilation while exercising its anti-coagulant function 158. Compared to heparan sulfate, heparin has a a much lower degree of sulfation leading to a decrease in the range of heparin binding sites, and it is also more susceptible to enzyme cleavage 47,159.
In a recent study, it was found that heparan sulfate has three clusters of interactions with nearly 75% of all input proteins grouped with complement factors and chemokines. Besides the complement and chemokine cluster, a second cluster, as assessed by a systems approach, revealed interaction with growth factors and growth factor receptors, including members of the hepatocyte growth factor, platelet-derived growth factor, fibroblast growth factor, family of receptor tyrosine kinases and bone morphogenetic proteins. The third cluster was composed of major extracellular matrix components, which can act as scaffolds for other proteins involved in cell support and organization 160.
Indeed, this cluster study supports the role of heparan sulfate and heparan sulfate proteoglycans in bone physiological functions such as weight bearing, endochondral biology 161, fibroblast growth factor regulation of osteogenesis 162, in skeletal development and growth 100 and their recently described role in osteoclastogenesis 163. A knock in murine model of osteoprotegerin, a known binder of receptor activator of nuclear factor kappa-B ligand, which triggers osteoclast differentiation, demonstrated that without osteoprotegerin, heparan sulfate on the cell surface of osteoblasts (as it does not engage with osteoprotegerin) rather promotes receptor activator of nuclear factor kappa-B activation. Thus, heparan sulfate plays a key role in bone homeostasis 164.
Besides its role in bone metabolism, another relevant physiological function in the context of the periodontium is the role of heparan sulfate in wound healing 165. These roles in wound healing include: re-epithelialization, modulation of coagulation, anti-inflammatory, regulation of inflammatory cell infiltration, angiogenesis, and cell proliferation and migration 159. Syndecan null mice show defective reepithelization of epidermis with thinner underlying connective tissue 165. Briefly, its role in angiogenesis has been extensively reported via binding and regulation of vascular endothelial growth factor, fibroblast growth factor, transforming growth factor and platelet derived growth factor 166. In contrast, overexpression of syndecan-1 can switch heparan sulfate mediated fibroblast growth factor-7 signaling leading to its inhibition which affected granulation tissue formation suggesting that the dose of heparan sulfate may also influence its function 165. Heparan sulfate can also modulate wound healing by its interaction with toll-like receptor-4, leading to release of proinflammatory cytokines and influencing maturation of dendritic cells. 145,167. Indeed, heparan sulfate/heparin, are found to be increased in wound fluid after injury 168.
5. HEPARAN SULFATE AND HEPARAN SULFATE PROTEOGLYCANS IN DISEASE
5.1. The glycocalyx
A large cohort of the associated diseases refer to glycocalyx degradation studies and some have found the shedding of heparan sulfate to be of diagnostic value as a biomarker for diseases 169. Several pathological conditions have detectable circulating levels of heparan sulfate and heparan sulfate proteoglycans such as cardiovascular disease, cancer, trauma, sepsis, kidney disease, diabetes, etc.169.
The degradation of glycocalyx occurs via matrix metalloproteinases and heparanase secreted by endothelial cells faced with an inflammatory trigger. Upon heparanase encounter, cleavage of the proteoglycans and heparan sulfate of syndecans and glypicans occurs. Matrix metalloproteinases 2, 7 and 9 have been reported to cleave these heparan sulfate proteoglycans mostly at sites of low sulfation or no sulfation, releasing small heparan sulfate fragments of 4–7 kDa 170,171.
Evidence suggests that a damaged glycocalyx, found in aging, may initiate endothelial dysfunction leading to cardiovascular disease 172. Accordingly, several studies have shown the association between circulating heparan sulfate proteoglycans levels, mainly syndecans-1 and −4 with myocardial infarction 169. Several reviews on the heparan sulfate and glycocalyx axis are available for more in-depth knowledge 173.
5.2. Cancer
Elevated levels of syndecans have been shown to increase significantly in the plasma of patients with certain cancers such as multiple myeloma and further, have been correlated with a poor survival and response to treatment 174,175. Glypicans have also been associated with liver carcinoma. The role of heparan sulfate proteoglycans in cancer has been found to be of diagnostic value, but has also been reported as tumor-suppressive, depending on the heparan sulfate proteoglycans and the cancer cell type 176.
5.3. Bone pathology
The development of osteochondromas in children and adults due to heparan sulfate deficiency has been extensively studied and it has been found that these tumors, rather than originate from bone marrow, are derived from progenitor cells in the perichondrium. With the loss of heparan sulfate or interference with heparan sulfate function, these cells are subjected to excessive bone morphogenetic protein signaling converting them to chondrocytes 100. Heparan sulfate regulates bone morphogenetic function by storing it at the surface of cells or in the extracellular matrix, controlling its function. Similarly, lack of heparan sulfate at the cell surface limits noggin function 177.
5.4. Viral infections
In the context of infections, mutations in the heparan sulfate proteoglycans can lead to increase susceptibility to infectious diseases 178. Several viral pathogens use heparan sulfate proteoglycans as receptors to facilitate entry in cells 179,180. In viral infections, most pathogens do not synthetize heparan sulfate or heparin but have evolved heparan sulfate-binding or degrading capacity to exploit the glycocalyx and facilitate key host-pathogen interactions. Binding to heparan sulfate was reported for several coronaviruses 180–183. Initially, heparan sulfate was found to help SARS-CoV-2 virus in cell attachment and virus entry 184,185. Later, heparan sulfate-binding to SARS-CoV-2 was found to be altered by modifying the heparan sulfate composition of the host cells 186. Extensive reviews in heparan sulfate and viral infections are available 179,187.
5.5. Periodontitis
As a chronic inflammatory disease, various factors influence the etiology of periodontitis. From the perspective of the host response, multiple players are involved and the majority of research has focused on cells, their receptors and secreted inflammatory molecules 188–191. In the early 1980’s, several glycosaminoglycans free of proteoglycans were detected in gingival crevicular fluid of inflamed gingiva 192, thus, being one of the first reports on the potential role of extracellular matrix molecules in the disease. It was initially thought that the changes in composition of the glycosaminoglycans from a healthy to a diseased periodontium was a reflection of inflammation and consequently mechanical loading of the inflamed tissue that had changed due to loss of attachment. At the time, extracellular matrix was thought to primarily serve to spatially distribute and promote support for cells 193. However, today, the role of glycosaminoglycans in promoting, sustaining, and controlling inflammation is clear although much more needs to be unveiled.
Loss of heparan sulfate proteoglycans on the surface of cells from patients with an inflamed periodontium was confirmed in subepithelial and subendothelial basement membranes and in infiltrating leukocytes 194. When fibroblasts collected from healthy verses diseased gingiva were cultured, the fibroblasts from inflamed tissue secreted less heparan sulfate and more dermatan sulfate, a phenotype that persisted after several cell culture passages, demonstrating longevity of the phenotype 195.
In different cells of the periodontium, periodontal ligament, gingival fibroblasts and osteoblasts, syndecan-2 exhibited a significant difference in expression. The presence of specific cell surface proteoglycans on periodontal cells implies distinctive functions 36. Indeed, in the presence of periodontal inflammation, and cell-surface proteoglycan expression by lymphocytes is different to that in health 14. P. gingivalis lipopolysaccharide influences the expression and processing of decorin and biglycan in extracellular matrix, altering alveolar bone cell activity and osteoblast phenotype development 196.
Overall, the role of the microenvironment in the pathogenesis of periodontitis s poorly characterized, despite the numerous reports from experimental studies about the critical involvement of these factors in modulating various aspects of the inflammatory response, such as the formation of inflammatory mediator gradients, extracellular matrix remodeling in the resolution of inflammation and report of proteoglycan vs. non-proteoglycan forms (enzymatically de-glycanated) of these proteins having different interactions (i.e. with growth factor receptors vs. inflammatory receptors) 72,106,112. Syndecans and their heparan sulfate glycosaminoglycans are likely to regulate periodontal health through the various mechanisms involved in its biosynthesis and modification of their heparan sulfate glycosaminoglycans by enzymes such as exostosins, sulfotransferases, and heparanase. A recent study clearly indicated that some syndecans display different expression profiles in healthy and diseased periodontal tissues. Expression profiles of heparan sulfate proteoglycans and modifying enzymes also correlate with the presence of inflammatory fluid in healthy and diseased periodontal tissue 197.
In a model based on data from immunofluorescence staining of human gingival samples, and reconstruction of a subset of heparan sulfate glycosaminoglycan-related proteins from the STRING protein-interaction online database, investigators reported the role of heparan sulfate glycosaminoglycans on inflammation and alveolar bone loss. Further, it was found that increased expression of heparan sulfate glycosaminoglycan could stabilize the gingival inflammatory infiltrate, alveolar bone resorption and osteoprotegerin. This study suggests that the use of exogenously added heparan sulfate glycosaminoglycans to control inflammation and periodontitis rather than just associating heparan sulfate with disease 197.
Indeed, other inflammatory conditions such as acetaminophen-induced liver toxicity, can be significantly attenuated with the addition of exogenous HS glycosaminoglycan as we describe below. The role of soluble heparan sulfate glycosaminoglycans in gingivitis and periodontitis, is likely to depend on the fine structure of the carbohydrate.
6. Biosynthesis of heparan sulfate
The biosynthesis of heparan sulfate is a posttranslational process that starts from a core protein and involves a cascade of enzymes. The heparan sulfate biosynthetic pathway encompasses the linkage region tetrasaccharide, heparan sulfate polysaccharide backbone synthesis and modification steps (Figure 5). The synthesis of linkage tetrasaccharide is initiated from the core protein of heparan sulfate proteoglycans. First, xylosyltransferase transfers a xylose unit to a serine residue of the core protein. Two galactose units are then added to the xylose sequentially by galactosyltransferase-I and galactosyltransferase-II to form a trisaccharide unit of galactose-galactose-xylose-serine. A glucuronosyltransferase adds a glucuronic acid unit to the trisaccharide to lead to the linkage region tetrasaccharide, glucuronic acid-galactose-galactose-xylose-serine. This tetrasaccharide serves as a primer for the biosynthesis of heparan sulfate polysaccharide and chondroitin sulfate polysaccharides, depending on the subsequent enzymatic modification steps. Here, our focus is on the biosynthesis of heparan sulfate.
Figure 5:
Biosynthesis of heparan sulfate. The biosynthesis of heparan sulfate is started with addition of a xylose followed by adding two galactose units and one glucuronic acid to yield the linkage region tetrasaccharide. EXTL3 enzyme adds one additional N-acetyl glucosamine to direct the synthesis towards heparan sulfate. Alternatively, chondroitin sulfate N acetyl galactose I/II adds N-acetyl galactosamine to proceed the synthesis of chondroitin sulfate. Detailed explanations for each enzymatic reaction step are presented in the text.
CSGalNAcT: chondroitin sulfate galactose N-acetyl transferase, CSPG: chondroitin sulfate proteoglycan, HSPG: heparan sulfate proteoglycan, NS: non-sulfated, Ser: serine, C5-epi: C5 epimerase, EXT: exostosin GlcAT: acetyl glucosamine transferase, ST: sulfotransferase, Gal: galactosamine, IdoA: iduronic acid, Glc: glucuronic acid, Xyl: xylose, GlcNAc: N-acetyl glucosamine, GalNAc: N-acetyl galactosamine
A complex of exostosin-1 and exostosin-2, from the exostosin family of proteins, catalyze the polymerization of heparan sulfate to yield the heparan sulfate proteoglycan backbone 198,199 (Fig 1). The backbone is then subjected to a series of enzymatic modifications. N-deacetylase/N-sulfotransferase transforms an N-acetyl glucosamine to an N-sulfo glucosamine. The C5-epimerase converts a glucuronic acid to an iduronic acid. 2-O-sulfotransferase transfers a sulfo group to the 2-OH position of an iduronic acid unit to form iduronic acid 2S. The 6-O-sulfation and 3-O-sulfation of glucosamine unit is carried out by 6-O-sulfotransferase and 3-O-sulfotransferase, respectively. Heparan sulfate sulfotransferases are present in multiple isoforms except for 2-sulfotransferase. The isoforms are expressed at different levels and display different substrate specificities 200. For example, N-deacetylase/N-sulfotransferase is present in four different isoforms, 6- O- sulfotransferase is present in three and 3-O-sulfotransferase is present in seven isoforms 201. The biosynthesis of heparan sulfate polysaccharides is not driven by a template. Consequently, the heparan sulfate isolated from a tissue is a complex mixture, consisting of different lengths in sugar chains and sulfation levels. Although the mechanism for the cellular control for the biosynthesis of heparan sulfate is not fully understood, it is generally accepted that the expression levels of individual heparan sulfate biosynthetic enzymes play essential roles for regulating the structures of heparan sulfate. Other factors contribute to the biosynthesis of heparan sulfate, i.e., the expression of core proteins and the level of the sulfo donor that is used by sulfotransferases or sugar nucleotides used by exostosin 1/2 for the polysaccharide backbone synthesis.
7. CHEMOENZYMATIC SYNTHESIS OF HEPARAN SULFATE OLIGOSACCHARIDES
The functions of heparan sulfate are primarily dictated by sulfation patterns and the size of the sulfated saccharide domains 202. A centerpiece in heparan sulfate-related research is to investigate the contribution of sulfation patterns in heparan sulfate to the biological functions. For this purpose, access to those structurally homogeneous or chemically pure heparan sulfate molecules is essential. Chemoenzymatic method offers a flexible and cost-effective approach for the synthesis of heparan sulfate oligosaccharides 203. The method utilizes recombinant heparan sulfate biosynthetic enzymes. Unlike cellular synthesis, chemoenzymatic synthesis emphasizes controlling the completion at each enzymatic modification step to achieve the synthesis of structurally pure oligosaccharides after multi-step enzymatic reactions.
An example for the synthesis of a heparan sulfate hexasaccharide is provided in Figure 6. The synthesis is started from a commercially available p-nitrophenyl glucuronide. The monosaccharide is elongated to a hexasaccharide using the p-nitrophenyl glucoronide (heparosan synthase 2 from Pasteurella multicide), a glycosyltransferase that can elongate the monosaccharide to a hexasaccharide backbone. In contrast to exostosin 1/2, the mammalian heparan sulfate polymerase, recombinant p-nitrophenyl glucuronid enzyme is easy to obtain, facilitating the scale up synthesis. The glucoronide uses two uridine diphosphate N-acetylglucosamine or uridine diphosphate glucuronic acid molecules for the synthesis of heparosan, a capsular polysaccharide from Pasteurella multicide that has the same structure as the heparan sulfate backbone.
Figure 6:
Chemoenzymatic synthesis of heparan sulfate hexasaccharide (i.e. 6-mer). The synthesis is initiated from a monosaccharide p-nitrophenyl glucuronide. An unnatural sugar nucleotide, diphosphate N-trifluoroacetyl glucosamine, is used in the synthesis. The structures of diphosphate N-acetyl glucosamine and diphosphate N-trifluoracetyl glusoamine are shown in the box. The difference between two donors are highlighted in a yellow box. 3’-phosphoadenosine 5’-phosphosulfate is a sulfo donor used by sulfotransferases.
GlcA-Pnp: p-nitrophenyl glucuronide, UDP-GlcNFTA: diphosphate N-trifluoroacetyl glucosamine, Udp-GlcNAc; diphosphate N-acetyl glucosamine, UDP-GlcNFTA; diphosphate N-trifluoroacetyl glucosamine, PAP; 3’-phosphoadenosine 5’-phosphate, PAPS; 3’-phosphoadenosine 5’-phosphosulfate, PmHS2; heparosan synthase 2 from Pasteurella multicide, GlcAT: glucuronyl transferase, EXTL3; exostosin-like 3
The chemoenzymatic synthetic method uses a piece of technology to position a N-sulfated glucosamine unit. An unnatural sugar nucleotide donor, diphosphate N-trifluoroacetyl glucosamine is introduced to replace uridine diphosphate N-acetylglucosamine (Figure 6). The purpose of incorporating a N-trifluoroacetyl glucosamine unit is that it that can be readily converted into a glucosamine for the subsequent N-sulfation reaction by N-sulfotransferase 204. This technique allows for precise controlling of the position of a N-sulfated glucosamine to progress the subsequent modifications. It is known that N-sulfation is essential to regulate the sites of action of C5-epimerase, 2-O sulfotransferase and 3-O sulfotransferase 205. The chemoenzymatic method is now emerging as a highly effective approach to synthesize a wide range of structurally homogeneous oligosaccharides 206,207. Access to these oligosaccharides has opened new opportunities for dissecting the biological functions of heparan sulfate and developing heparan sulfate-based therapeutics.
8. POTENTIAL THERAPEUTIC ROLES OF HEPARAN SULFATE
8.1. Anticoagulation
Heparin is a widely used anticoagulant drug in hospitals. There are several forms of heparin drugs on the marketplace, including unfractionated heparin, low-molecular weight heparin and fondaparinux. Unfractionated heparin is isolated from porcine intestine and is a highly complex mixture of polysaccharides with different sizes (MWavg ∼16,000 Da). The low molecular weight heparin is a partially depolymerized unfractionated heparin with the average molecular weight (MWavg 4,500 to 6,500 Da). Fondaparinux is a synthetic pentasaccharide which is a pure chemical with a molecular weight of 1508 Da. With heparin, its interaction with antithrombin requires a specific pentasaccharide that contains a rare 3-O-sulfated glucosamine installed by 3-O-sulfotransferase. When the 3-O-sulfation is lacking, the binding affinity to antithrombin is reduced substantially, resulting in the loss of anticoagulant activity. Fondaparinux is a synthetic pentasaccharide that encompasses the pharmacophore of the drug heparin to display the anticoagulant activity. Knowing the structural requirements for the anticoagulant activity will also allow us to engineer the heparan sulfate oligosaccharides without the anticoagulant activity for different therapeutic purposes.
There are concerns over the relevance and safety of animal-sourced heparin 208. Both the unfractionated form and its derivatives of low-molecular weight heparins are from animal sourced through a long and poorly regulated supply chain. Batches of contaminated heparin entered the world market, leading to 256 deaths in the US 209. A reliable and cost-effective method to make synthetic heparin that can replace animal-sourced heparin is being actively pursued. Although fondaparinux is a synthetic, it does not cover all the pharmacological properties of heparin drugs because the size of carbohydrate chain is too short 206. Synthesis of a dodecasacchride (12-mer) with similar pharmacological properties to low molecular weight heparin has been reported 207. The chemoenzymatic synthetic approach plays a crucial role in the effort for developing a synthetic heparin to substitute animal-sourced heparin.
8.2. Acetaminophen-induced Acute Liver Injury
Heparan sulfate also displays anti-inflammatory properties by inhibiting the activity of pro-inflammatory proteins, which offers therapeutic application beyond anticoagulation. This has been demonstrated in a recent example of treating liver damage caused by acetaminophen overdose. Acetaminophen is an active ingredient in Tylenol®, an over-the-counter pain medication. Although it is generally safe, acetaminophen overdose is the leading cause of acute liver failure in the US and Europe 210. Acetaminophen-induced acute liver failure involves the axis of high mobility group box and the receptor for advanced glycation end products 211. A synthetic heparan sulfate octadecasaccharide (18-mer) has been shown to protect against acute liver failure caused by acetaminophen overdose 212. The 18-mer does not display anticoagulant activity. The 18-mer decreased high mobility group box 1-mediated neutrophil infiltration in an air pouch model. Treatment with both 18-mer and high mobility group box 1 neutralizing antibody offered very similar protection after acetaminophen overdose, suggesting that 18-mer and the antibody engaged high mobility group box 1 target. Interestingly, the anticoagulant 18-mer did not display the hepatoprotection in the acetaminophen overdose animal model. The chemical structure of anticoagulant 18-mer is different from the 18-mer. Further studies demonstrated that the anticoagulant activity from the anticoagulant 18-mer impaired the liver regeneration process by disrupting the generation for fibrin. These findings demonstrate the need for structurally homogeneous heparan sulfate oligosaccharides to probe the functions of heparan sulfate in disease models.
8.3. Periodontitis
Periodontitis is a major chronic disease that causes alveolar bone and tooth loss and affects one out of every two American adults 213,214. Currently, the therapy for periodontitis consists of biofilm disturbance and reduction with mechanical debridement of plaque and calculus around teeth, with or without concomitant systemic antibiotics 215. Local antibiotics delivered to periodontal pockets have also been used, however, there is evidence that they are not always effective and do not prevent the re-emergence of periodontitis 216,217. There is a line of evidence supporting the idea that periodontitis patients and healthy people have a similar biofilm with variations in bacterial ratio’s causing deregulation/dysbiosis 218. Thus, aiming for bacterial eradication via debridement and antibiotic treatment is not always the best approach as a microbial balance is necessary combined with inflammatory control 218. Some studies support to primarily target the host inflammatory response to the intra-oral biofilm, alongside the reduction of biofilm accumulation (debridement, not eradication of bacteria with antibiotics). Indeed, about 30% of individuals do not respond well to any type of antibacterial periodontal therapy, including surgical therapy, and continue presenting with bone loss despite a minimal amount of pathogenic flora 219. Part of this population is thought to have a hyperinflammatory response 220.
The European Federation of Periodontology has recognized that part of the population affected does not respond to treatment, due to the therapeutic focus remaining primarily upon biofilm management (antibiotics, antiseptics) rather than modulation of inflammation, which is the primary reason for periodontal tissue damage 221. The currently available antibiotics and other therapeutics such as systemic sub-antimicrobial dose doxycycline and anti-septic mouthwashes provide limited benefit 222. Alendronates, non-steroidal anti-inflammatory drugs, statins, probiotics are all examples of off-label non-antimicrobial adjuncts with no significant evidence for improving the outcomes of periodontal therapy, thus, there is currently no effective alternative to antibiotics 222,223. An heparan sulfate of 18-mer size that specifically decreases inflammation and prevents acetaminophen-induced acute liver failure 25 has recently been investigated by our group and has shown promising results in protecting against murine liver disease and death due to inflammation. In evaluating similar-size synthetic heparan sulfate and unfractionated heparin, both heparin and the sulfated 18-mer were effective in preventing osteoclast differentiation in vitro, thus presenting potential therapeutic value in preventing bone loss. In an inflammatory bone challenge experiment in mice, heparin and the 18-mer, when locally injected into the gingiva offered protection of alveolar bone upon ligature insult (Figure 7), providing strong impetus to further investigate how this molecule affects periodontal tissue homeostasis, and the best way to explore it as a periodontal therapeutic agent. Whilst heparin also showed protective effects, its characteristic heterogeneity, since it is tissue-extracted heparin, remains a concern. The variability in bone effects has been demonstrated in several studies that use heparins and low molecular weight heparins for long term treatment (i.e. for patients at risk of embolism such as post myocardial infarction patients, deep vein thrombosis, and pregnant patients). While some of these patients present with osteopenia, others do not display any specific bone phenotype 224–226. Thus, as a therapeutic molecule in periodontitis, tissue-extracted heparin may present variable effects.
Figure 7:

(A, B) Placement of silk ligature strip in mice to induce bone loss as a model of periodontitis. (C, D) Injection of vehicle, heparin or S18-mer in a 2μl volume on the palatal tissue between mouse molars M1 and M2 was done daily during the course of the experiment (5 days) (n=7 male B6 mice/group). (E-H) At 5 days, mice were sacrificed, and maxillae harvested for microCT and quantification of bone loss performed by volumetric analyses. 2D and 3D views show representative images with clear differences in bone loss (represented by colored asterisks) in ligated mice as demonstrated in %BV/TV (I) Statistical difference between no-ligature and all groups (*) and ligature and treated groups heparin and S18-mer (**) (p<0.05). (J-L) Raw cells, a pre-osteoclast cell line, were culture for 4 days in the presence of osteoclast differentiation media. Cells were treated with the oligosaccharides daily for 5 days and stained for tatrate-resistant acid phosphatase. (M) quantification of osteoclasts/mm3 area showed that compared to control (last gray bar), S18-mer showed the largest reduction in osteoclast numbers/differentiation at 2 different concentrations (1 and 10 μg) which was statistically significant (p<0.05). Heparin significantly diminished osteoclast numbers at only one concentration (n=3 for all groups). Another synthetic heparan sulfate of 8-mer was used as a proof-of-concept that the size of the synthetic saccharide is important, showing no apparent effect on osteoclasts. Kruskal-Wallis with Dunn post hoc tests were performed to determine the differences among groups. *p<0.05 compared to negative control group (no-ligature); **p<0.05 compared to positive control (ligature).
Whilst these are proof-of-concept studies and more research needs to be undertaken to fully characterize the effect of the sulfate 18-mer in management of periodontitis, the data is very encouraging. To our knowledge, we are the first group to report that a specific heparan sulfate glycosaminoglycan code is beneficial in preventing bone loss in a murine model of periodontitis. The sulfated 18-mer could be a cost-effective therapeutic to promote alveolar bone homeostasis. Further mechanistic characterization of its effect at the level of inflammatory and bone-cell function is underway (likely involving high mobility group box 1 as described above, among other factors). In addition, dose-dependent studies including susceptible subjects such as with a hyper-inflammatory phenotype are being investigated.
Other studies have attempted to investigate heparan sulfate/heparin in periodontitis management. A low molecular weight heparin derived from depolymerized tissue-extracted heparin (Clexane®) was subcutaneously injected for 60 days as a treatment for ligature-induced periodontitis in rats, although researchers did not see significant differences in bone levels 227. Others have looked at a biomimetic of heparan sulfate, a regenerating agent named ReGeneraTing which is dextran-based with added sulfate groups. Coyak (2018) applied the biomimetic intra-muscularly for 8-weeks post-disease induction in a bacteria-induced mouse model of periodontitis and reported decreased levels of inflammation and fewer osteoclast numbers in mice treated with ReGeneraTing 228.
Practice guidelines for the management of various stages of periodontitis have been published recently 223,229. Besides biofilm control, plaque removal, oral hygiene instructions, and management of other risk factors (such as metabolic diseases, smoking), there is a recommendation to provide adjunctive therapy which may include host-modulation and antimicrobial agents in the treatment of periodontitis. These may be included concomitantly or after initial therapy. Lastly, therapy may include periodontal surgery (where regeneration may be attempted) and supportive periodontal maintenance at regular intervals. We envision that in the future heparan sulfate could to be used as an adjunctive therapy and host-modulating agent. Another potential application for heparan sulfate is in the treatment of residual pockets through regenerative therapy. In regeneration, it is recommended that membranes or enamel matrix derivative with or without biomaterials be applied to infrabony pockets. This is based on a rigorous evidence-based assessment that identified most promising biomaterials 223. Regenerative therapy leads to improved clinical attachment levels, including in molars with class II furcation involvement. Ultimately, heparan sulfate as a periodontal therapeutic, will need to go through careful, powerful, and with low risk of bias, randomized clinical trials in order to be recommended as a periodontal supportive and regenerative therapy in the future.
8.4. Tissue Engineering
Besides the management of inflammation, another area that has gained traction for heparan sulfate is in the tissue engineering of various connective tissues. Heparan sulfate-based biomaterials have generated interest because molecules of the transforming growth factor-β superfamily bind to heparin and heparan sulfate 102. Thus, heparan sulfate can facilitate controlled release of growth factors such as bone morphogenetic protein 2, fibroblast growth factor, platelet-derived growth factor among others 46. Heparin has been found to bind to the N-terminal of bone morphogenetic protein although further characterization of this feature is needed, thus changes in heparan sulfate structure may alter the binding pattern 230.
Heparan sulfate proteoglycans can promote the formation of a complex between bone morphogenetic protein 2 receptors I and II sustaining the activity. Heparan sulfate proteoglycans can also bind noggin, a bone morphogenetic protein antagonist, helping to block the binding epitopes of bone morphogenetic protein receptors 231,232. Heparan sulfate as a soluble molecule, can enhance bone morphogenetic protein signaling in cell culture and sustain Smad 1/5/8 phosphorylation 43,233. Interestingly, when heparan sulfate is coupled with bone morphogenetic protein 2 in vitro, noggin is not able to antagonize it, which is a promising way to deliver bone morphogenetic protein, potentially sustaining signaling and preventing canceling of its function. Heparan sulfate or heparin bound to growth factors can also protect them from enzymatic degradation 46.
There is growing interest in biomaterials development harboring glycosaminoglycans for the purpose of improving cell migration, decreasing inflammation, improving extracellular matrix organization and biomaterials that are capable of controlled and persistent release of bioactive molecules. A few studies have explored bone healing with adjunctive heparan sulfate and report improvements in the regenerative process due to improved stability, cell reception and flexibility in delivery of biomolecules in fractures, including non-union or delayed union 234,235.
The mechanism by which heparan sulfate and heparin interact with growth factors is primarily through ionic interactions and carboxylate groups 44. Various polymers, nanoparticles and composite materials have been modified with heparin or low molecular weight heparin via chemical cross-linking, encapsulation or just pooled together 236,237. Some of the reported biomaterial backbones utilized with heparan sulfate are collagen, other glycosaminoglycans, chitosan, etc. 237–239
Besides the delivery of growth factors, the addition of a heparan sulfate molecule provides the scaffold with the ability to concomitantly be (potentially) anti-inflammatory and anti-scarring 240,241. It is tempting to assume that the release of heparan sulfate could also potentially reduce viral and bacterial binding to cell receptors in the event of infection, thus, further controlling an excessive inflammatory response.
However, part of the literature has emphasized that heparin, when used long-term, can lead to osteopenia and even osteoporosis, potentially promoting poor bone regeneration 225,242. Low molecular weight treatment did not show any obvious effects on fracture risk but heparin increased the osteoclast surface 224,226,243. Low molecular weight heparin has several advantages such as increased half-life and bioavailability compared to full length heparin. Thus, added to the low concern for osteoporosis with long-term use of low molecular weight heparin, such risk is perhaps understated.
Other classes of heparins have been developed for tissue engineering such as biomimetics in an attempt to deliver a more consistently generated molecule with low degree of complexity during manufacturing (i.e. size, chemical modifications, etc.). Lallam et al., in 2006 244 and 2011 245, reported a heparan sulfate biomimetic to use in the regeneration of the periodontium of hamsters. The biomimetic, carries carboxymethyl and sulfate groups on a carbohydrate backbone and has shown promise in the resolution of inflammation and promotion of healing in muscle, skin wounds and ulcers of the cornea 246,247. This compound was delivered systemically and promoted cell proliferation and changed the organization of healing tissues in periodontium. This is the beginning of a new era of glycobiology, where defined carbohydrate structures can predictably act as adjuncts in multiple clinical scenarios. Given the new tools available to generate such therapeutics, a clear safety profile and the ability to manufacture it at low cost, human translational studies should soon be underway 45.
CONCLUSIONS
The availability of synthetic heparan sulfate oligosaccharides will accelerate the understanding of the role of heparan sulfate in health and disease and contribute to a new era in biologics as therapeutics. Of interest, periodontitis and periodontal regeneration are promising targets for a myriad of defined heparan sulfate molecules with potential anti-inflammatory and anti-clastogenic effects. Efforts are underway to also characterize them in osteogenesis. In summary, this may represent a paradigm shift in periodontics and regenerative dentistry.
Supplementary Material
Acknowledgments:
We would like to acknowledge Dr. Vinicius de Paiva Goncalves for help with technical support for microcomputed tomography and its data collection as well as support for statistical validation.
Funding Statement:
This research was partly funded by the University of North Carolina at Chapel Hill; grant 550KR211924 (to PAM) from the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UM1TR004406; grants HL094463, HL144970 and GlycoMIP (to JL) a National Science Foundation Materials Innovation Platform funded through Cooperative Agreement DMR-1933525. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing Interests:
JL is founder and chief scientific officer of Glycan Therapeutics. Other authors declare no competing interest.
Footnotes
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of the University of North Carolina at Chapel Hill, IACUC#16-162.
Data Availability Statement:
Any raw data supporting reported results can be requested by contacting the corresponding author.
References:
- 1.Merry CLR, Lindahl U, Couchman J, Esko JD. Proteoglycans and Sulfated Glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, et al. , eds. Essentials of Glycobiology. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; Copyright © 2022 The Consortium of Glycobiology Editors, La Jolla, California; published by Cold Spring Harbor Laboratory Press; doi: 10.1101/glycobiology.4e.17. All rights reserved.; 2022:217–232. [DOI] [Google Scholar]
- 2.Duan G, Walther D. The roles of post-translational modifications in the context of protein interaction networks. PLoS Comput Biol. 2015;11(2):e1004049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hogwood J, Mulloy B, Lever R, Gray E, Page CP. Pharmacology of Heparin and Related Drugs: An Update. Pharmacol Rev. 2023;75(2):328–379. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani V, Maccari F, Volpi N. Chondroitin Sulfate and Glucosamine as Disease Modifying Anti- Osteoarthritis Dru gs (DMOADs). Curr Med Chem. 2016;23(11):1139–1151. [DOI] [PubMed] [Google Scholar]
- 5.Iozzo RV, Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015;42:11–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silbert JE, Sugumaran G. A starting place for the road to function. Glycoconj J. 2002;19(4–5):227–237. [DOI] [PubMed] [Google Scholar]
- 7.Boskey AL. Amorphous calcium phosphate: the contention of bone. J Dent Res. 1997;76(8):1433–1436. [DOI] [PubMed] [Google Scholar]
- 8.Rees SG, Hughes Wassell DT, Waddington RJ, Embery G. Interaction of bone proteoglycans and proteoglycan components with hydroxyapatite. Biochim Biophys Acta. 2001;1568(2):118–128. [DOI] [PubMed] [Google Scholar]
- 9.Coulson-Thomas YM, Coulson-Thomas VJ, Norton AL, et al. The identification of proteoglycans and glycosaminoglycans in archaeological human bones and teeth. PLoS One. 2015;10(6):e0131105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nomura Y. Structural change in decorin with skin aging. Connect Tissue Res. 2006;47(5):249–255. [DOI] [PubMed] [Google Scholar]
- 11.Bertassoni LE, Swain MV. The contribution of proteoglycans to the mechanical behavior of mineralized tissues. J Mech Behav Biomed Mater. 2014;38:91–104. [DOI] [PubMed] [Google Scholar]
- 12.Landis WJ. Mineral characterization in calcifying tissues: atomic, molecular and macromolecular perspectives. Connect Tissue Res. 1996;34(4):239–246. [DOI] [PubMed] [Google Scholar]
- 13.Bartold PM. Turnover in periodontal connective tissues: dynamic homeostasis of cells, collagen and ground substances. Oral Dis. 1995;1(4):238–253. [DOI] [PubMed] [Google Scholar]
- 14.Manakil JF, Sugerman PB, Li H, Seymour GJ, Bartold PM. Cell-surface proteoglycan expression by lymphocytes from peripheral blood and gingiva in health and periodontal disease. J Dent Res. 2001;80(8):1704–1710. [DOI] [PubMed] [Google Scholar]
- 15.Zappia J, Joiret M, Sanchez C, et al. From Translation to Protein Degradation as Mechanisms for Regulating Biological Functions: A Review on the SLRP Family in Skeletal Tissues. Biomolecules. 2020;10(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Foster BL, Kram V, et al. Fibromodulin and Biglycan Modulate Periodontium through TGFβ/BMP Signaling. J Dent Res. 2014;93(8):780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu R, Li W, Herber RP, Marshall SJ, Young M, Ho SP. Effects of biglycan on physico-chemical properties of ligament-mineralized tissue attachment sites. Arch Oral Biol. 2012;57(2):177–187. [DOI] [PubMed] [Google Scholar]
- 18.Matheson S, Larjava H, Häkkinen L. Distinctive localization and function for lumican, fibromodulin and decorin to regulate collagen fibril organization in periodontal tissues. J Periodontal Res. 2005;40(4):312–324. [DOI] [PubMed] [Google Scholar]
- 19.McEwan PA, Scott PG, Bishop PN, Bella J. Structural correlations in the family of small leucine-rich repeat proteins and proteoglycans. J Struct Biol. 2006;155(2):294–305. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrand A, Romarís M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302 ( Pt 2)(Pt 2):527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Szekeres GP, Pagel K, Heiner Z. Analytical challenges of glycosaminoglycans at biological interfaces. Anal Bioanal Chem. 2022;414(1):85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Pagadala V, Jester HM, et al. Chemoenzymatic synthesis of heparan sulfate and heparin oligosaccharides and NMR analysis: paving the way to a diverse library for glycobiologists. Chem Sci. 2017;8(12):7932–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao YE, Liu J, Arnold K. Heparan sulfates and heparan sulfate binding proteins in sepsis. Front Mol Biosci. 2023;10:1146685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stancanelli E, Liu W, Wander R, et al. Chemoenzymatic Synthesis of Homogeneous Heparan Sulfate and Chondroitin Sulfate Chimeras. ACS Chem Biol. 2022;17(5):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold K, Xu Y, Sparkenbaugh EM, et al. Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced acute liver failure. Science translational medicine. 2020;12(535). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnold K, Xu Y, Liao YE, Cooley BC, Pawlinski R, Liu J. Synthetic anticoagulant heparan sulfate attenuates liver ischemia reperfusion injury. Sci Rep. 2020;10(1):17187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vidaurre M, Osborn BK, Lowak KD, et al. A 3-O-sulfated heparan sulfate dodecasaccharide (12-mer) suppresses thromboinflammation and attenuates early organ injury following trauma and hemorrhagic shock. Front Immunol. 2023;14:1158457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott JE. Proteodermatan and proteokeratan sulfate (decorin, lumican/fibromodulin) proteins are horseshoe shaped. Implications for their interactions with collagen. Biochemistry. 1996;35(27):8795–8799. [DOI] [PubMed] [Google Scholar]
- 29.Thant L, Kaku M, Kakihara Y, et al. Extracellular Matrix-Oriented Proteomic Analysis of Periodontal Ligament Under Mechanical Stress. Front Physiol. 2022;13:899699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosiriluck N, Kashio H, Takada A, Mizuguchi I, Arakawa T. The profiling and analysis of gene expression in human periodontal ligament tissue and fibroblasts. Clin Exp Dent Res. 2022;8(3):658–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y, Guan Q, Han X, Bai D, Li D, Tian Y. Proteoglycans in the periodontium: A review with emphasis on specific distributions, functions, and potential applications. J Periodontal Res. 2021;56(4):617–632. [DOI] [PubMed] [Google Scholar]
- 32.Hua R, Jiang JX. Small leucine-rich proteoglycans in physiological and biomechanical function of bone. Matrix Biol Plus. 2021;11:100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salbach-Hirsch J, Rauner M, Hofbauer C, Hofbauer LC. New insights into the role of glycosaminoglycans in the endosteal bone microenvironment. Biol Chem. 2021;402(11):1415–1425. [DOI] [PubMed] [Google Scholar]
- 34.Taye N, Karoulias SZ, Hubmacher D. The “other” 15–40%: The Role of Non-Collagenous Extracellular Matrix Proteins and Minor Collagens in Tendon. J Orthop Res. 2020;38(1):23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worapamorn W, Li H, Pujic Z, Xiao Y, Young WG, Bartold PM. Expression and distribution of cell-surface proteoglycans in the normal Lewis rat molar periodontium. J Periodontal Res. 2000;35(4):214–224. [DOI] [PubMed] [Google Scholar]
- 36.Worapamorn W, Li H, Haas HR, Pujic Z, Girjes AA, Bartold PM. Cell surface proteoglycan expression by human periodontal cells. Connect Tissue Res. 2000;41(1):57–68. [DOI] [PubMed] [Google Scholar]
- 37.Worapamorn W, Xiao Y, Li H, Young WG, Bartold PM. Differential expression and distribution of syndecan-1 and −2 in periodontal wound healing of the rat. J Periodontal Res. 2002;37(4):293–299. [DOI] [PubMed] [Google Scholar]
- 38.Li N, Spetz MR, Ho M. The Role of Glypicans in Cancer Progression and Therapy. J Histochem Cytochem. 2020;68(12):841–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mun S, Kim SM, Choi MJ, Jang YJ. Transcriptome Profile of Membrane and Extracellular Matrix Components in Ligament-Fibroblastic Progenitors and Cementoblasts Differentiated from Human Periodontal Ligament Cells. Genes (Basel). 2022;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan J, Ho M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am J Physiol Cell Physiol. 2021;321(5):C846–c858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohkawara B, Iemura S, ten Dijke P, Ueno N. Action range of BMP is defined by its N-terminal basic amino acid core. Curr Biol. 2002;12(3):205–209. [DOI] [PubMed] [Google Scholar]
- 42.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237(1):295–302. [DOI] [PubMed] [Google Scholar]
- 43.Bramono DS, Murali S, Rai B, et al. Bone marrow-derived heparan sulfate potentiates the osteogenic activity of bone morphogenetic protein-2 (BMP-2). Bone. 2012;50(4):954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hettiaratchi MH, Krishnan L, Rouse T, Chou C, McDevitt TC, Guldberg RE. Heparin-mediated delivery of bone morphogenetic protein-2 improves spatial localization of bone regeneration. Sci Adv. 2020;6(1):eaay1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le BQ, Too JH, Tan TC, et al. Application of a BMP2-binding heparan sulphate to promote periodontal regeneration. Eur Cell Mater. 2021;42:139–153. [DOI] [PubMed] [Google Scholar]
- 46.Wang J, Xiao L, Wang W, et al. The Auxiliary Role of Heparin in Bone Regeneration and its Application in Bone Substitute Materials. Front Bioeng Biotechnol. 2022;10:837172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gandhi NS, Mancera RL. Prediction of heparin binding sites in bone morphogenetic proteins (BMPs). Biochim Biophys Acta. 2012;1824(12):1374–1381. [DOI] [PubMed] [Google Scholar]
- 48.Kirkpatrick CA, Knox SM, Staatz WD, Fox B, Lercher DM, Selleck SB. The function of a Drosophila glypican does not depend entirely on heparan sulfate modification. Dev Biol. 2006;300(2):570–582. [DOI] [PubMed] [Google Scholar]
- 49.Lammi MJ, Inkinen R, Parkkinen JJ, et al. Expression of reduced amounts of structurally altered aggrecan in articular cartilage chondrocytes exposed to high hydrostatic pressure. Biochem J. 1994;304 ( Pt 3)(Pt 3):723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scott JE, Haigh M. Identification of specific binding sites for keratan sulphate proteoglycans and chondroitin-dermatan sulphate proteoglycans on collagen fibrils in cornea by the use of cupromeronic blue in ‘critical-electrolyte-concentration’ techniques. Biochem J. 1988;253(2):607–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian H, Xiao Y, Bartold PM. Immunohistochemical localization and expression of fibromodulin in adult rat periodontium and inflamed human gingiva. Oral Dis. 2004;10(4):233–239. [DOI] [PubMed] [Google Scholar]
- 52.Cheng H, Caterson B, Yamauchi M. Identification and immunolocalization of chondroitin sulfate proteoglycans in tooth cementum. Connect Tissue Res. 1999;40(1):37–47. [DOI] [PubMed] [Google Scholar]
- 53.Cheng H, Caterson B, Neame PJ, Lester GE, Yamauchi M. Differential distribution of lumican and fibromodulin in tooth cementum. Connect Tissue Res. 1996;34(2):87–96. [DOI] [PubMed] [Google Scholar]
- 54.Vidal BC, Mello ML, Valdrighi L. Histochemical and anisotropical aspects of the rat cementum. Acta Anat (Basel). 1974;89(4):546–559. [DOI] [PubMed] [Google Scholar]
- 55.Bartold PM, Reinboth B, Nakae H, Narayanan AS, Page RC. Proteoglycans of bovine cementum: isolation and characterization. Matrix. 1990;10(1):10–19. [DOI] [PubMed] [Google Scholar]
- 56.Bartold PM, Miki Y, McAllister B, Narayanan AS, Page RC. Glycosaminoglycans of human cementum. J Periodontal Res. 1988;23(1):13–17. [DOI] [PubMed] [Google Scholar]
- 57.Bertl K, Bruckmann C, Isberg PE, Klinge B, Gotfredsen K, Stavropoulos A. Hyaluronan in non-surgical and surgical periodontal therapy: a systematic review. J Clin Periodontol. 2015;42(3):236–246. [DOI] [PubMed] [Google Scholar]
- 58.Asparuhova MB, Chappuis V, Stähli A, Buser D, Sculean A. Role of hyaluronan in regulating self-renewal and osteogenic differentiation of mesenchymal stromal cells and pre-osteoblasts. Clin Oral Investig. 2020;24(11):3923–3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eliezer M, Imber JC, Sculean A, Pandis N, Teich S. Hyaluronic acid as adjunctive to non-surgical and surgical periodontal therapy: a systematic review and meta-analysis. Clin Oral Investig. 2019;23(9):3423–3435. [DOI] [PubMed] [Google Scholar]
- 60.Garantziotis S, Savani RC. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biology. 2019;78–79:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Campo GM, Avenoso A, Campo S, D’Ascola A, Nastasi G, Calatroni A. Molecular size hyaluronan differently modulates toll-like receptor-4 in LPS-induced inflammation in mouse chondrocytes. Biochimie. 2010;92(2):204–215. [DOI] [PubMed] [Google Scholar]
- 62.Ababneh KT, Hall RC, Embery G. The proteoglycans of human cementum: immunohistochemical localization in healthy, periodontally involved and ageing teeth. J Periodontal Res. 1999;34(2):87–96. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T, Domon T, Takahashi S, Islam N, Suzuki R, Wakita M. The structure and function of the cemento-dentinal junction in human teeth. J Periodontal Res. 1999;34(5):261–268. [DOI] [PubMed] [Google Scholar]
- 64.Mochida Y, Parisuthiman D, Pornprasertsuk-Damrongsri S, et al. Decorin modulates collagen matrix assembly and mineralization. Matrix Biol. 2009;28(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mochida Y, Duarte WR, Tanzawa H, Paschalis EP, Yamauchi M. Decorin modulates matrix mineralization in vitro. Biochem Biophys Res Commun. 2003;305(1):6–9. [DOI] [PubMed] [Google Scholar]
- 66.Benedetto MS, Siqueira FM, Mascaro MB, Araújo VC, Bönecker MJ. Immunohistochemical expression of biglycan and decorin in the pulp tissue of human primary teeth during resorption. Braz Oral Res. 2013;27(5):438–444. [DOI] [PubMed] [Google Scholar]
- 67.Hao JX, Shen MJ, Wang CY, et al. Regulation of biomineralization by proteoglycans: From mechanisms to application. Carbohydr Polym. 2022;294:119773. [DOI] [PubMed] [Google Scholar]
- 68.Goldberg M, Takagi M. Dentine proteoglycans: composition, ultrastructure and functions. Histochem J. 1993;25(11):781–806. [PubMed] [Google Scholar]
- 69.Velleman SG. The role of the extracellular matrix in skeletal development. Poult Sci. 2000;79(7):985–989. [DOI] [PubMed] [Google Scholar]
- 70.Lamoureux F, Baud’huin M, Duplomb L, Heymann D, Rédini F. Proteoglycans: key partners in bone cell biology. Bioessays. 2007;29(8):758–771. [DOI] [PubMed] [Google Scholar]
- 71.Kram V, Shainer R, Jani P, Meester JAN, Loeys B, Young MF. Biglycan in the Skeleton. J Histochem Cytochem. 2020;68(11):747–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miguez PA. Evidence of biglycan structure-function in bone homeostasis and aging. Connect Tissue Res. 2020;61(1):19–33. [DOI] [PubMed] [Google Scholar]
- 73.Bartold PM. A biochemical and immunohistochemical study of the proteoglycans of alveolar bone. J Dent Res. 1990;69(1):7–19. [DOI] [PubMed] [Google Scholar]
- 74.Waddington RJ, Embery G, Last KS. Glycosaminoglycans of human alveolar bone. Arch Oral Biol. 1989;34(7):587–589. [DOI] [PubMed] [Google Scholar]
- 75.Mizumoto S, Yamada S. An Overview of in vivo Functions of Chondroitin Sulfate and Dermatan Sulfate Revealed by Their Deficient Mice. Front Cell Dev Biol. 2021;9:764781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papy-Garcia D, Albanese P. Heparan sulfate proteoglycans as key regulators of the mesenchymal niche of hematopoietic stem cells. Glycoconj J. 2017;34(3):377–391. [DOI] [PubMed] [Google Scholar]
- 77.McKee TJ, Perlman G, Morris M, Komarova SV. Extracellular matrix composition of connective tissues: a systematic review and meta-analysis. Sci Rep. 2019;9(1):10542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mania VM, Kallivokas AG, Malavaki C, et al. A comparative biochemical analysis of glycosaminoglycans and proteoglycans in human orthotopic and heterotopic bone. IUBMB Life. 2009;61(4):447–452. [DOI] [PubMed] [Google Scholar]
- 79.Salbach-Hirsch J, Samsonov SA, Hintze V, et al. Structural and functional insights into sclerostin-glycosaminoglycan interactions in bone. Biomaterials. 2015;67:335–345. [DOI] [PubMed] [Google Scholar]
- 80.Hua R, Ni Q, Eliason TD, et al. Biglycan and chondroitin sulfate play pivotal roles in bone toughness via retaining bound water in bone mineral matrix. Matrix Biol. 2020;94:95–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ross FP, Christiano AM. Nothing but skin and bone. J Clin Invest. 2006;116(5):1140–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ferraro F, Celso CL, Scadden D. Adult stem cels and their niches. Adv Exp Med Biol. 2010;695:155–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu T, Bianco P, Fisher LW, et al. Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet. 1998;20(1):78–82. [DOI] [PubMed] [Google Scholar]
- 84.Ameye L, Young MF. Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology. 2002;12(9):107r–116r. [DOI] [PubMed] [Google Scholar]
- 85.Karamanos NK, Piperigkou Z, Theocharis AD, et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem Rev. 2018;118(18):9152–9232. [DOI] [PubMed] [Google Scholar]
- 86.Kram V, Kilts TM, Bhattacharyya N, Li L, Young MF. Small leucine rich proteoglycans, a novel link to osteoclastogenesis. Sci Rep. 2017;7(1):12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chakravarti S. Functions of lumican and fibromodulin: lessons from knockout mice. Glycoconj J. 2002;19(4–5):287–293. [DOI] [PubMed] [Google Scholar]
- 88.Nyström A, Bruckner-Tuderman L, Kiritsi D. Dystrophic Epidermolysis Bullosa: Secondary Disease Mechanisms and Disease Modifiers. Front Genet. 2021;12:737272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldberg M, Septier D, Rapoport O, Iozzo RV, Young MF, Ameye LG. Targeted disruption of two small leucine-rich proteoglycans, biglycan and decorin, excerpts divergent effects on enamel and dentin formation. Calcif Tissue Int. 2005;77(5):297–310. [DOI] [PubMed] [Google Scholar]
- 90.Haruyama N, Sreenath TL, Suzuki S, et al. Genetic evidence for key roles of decorin and biglycan in dentin mineralization. Matrix Biol. 2009;28(3):129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Häkkinen L, Strassburger S, Kähäri VM, et al. A role for decorin in the structural organization of periodontal ligament. Lab Invest. 2000;80(12):1869–1880. [DOI] [PubMed] [Google Scholar]
- 92.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21(31):4765–4777. [DOI] [PubMed] [Google Scholar]
- 93.Schönherr E, Sunderkötter C, Schaefer L, et al. Decorin deficiency leads to impaired angiogenesis in injured mouse cornea. J Vasc Res. 2004;41(6):499–508. [DOI] [PubMed] [Google Scholar]
- 94.Neill T, Painter H, Buraschi S, et al. Decorin antagonizes the angiogenic network: concurrent inhibition of Met, hypoxia inducible factor 1α, vascular endothelial growth factor A, and induction of thrombospondin-1 and TIMP3. J Biol Chem. 2012;287(8):5492–5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bocian C, Urbanowitz AK, Owens RT, Iozzo RV, Götte M, Seidler DG. Decorin potentiates interferon-γ activity in a model of allergic inflammation. J Biol Chem. 2013;288(18):12699–12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bi Y, Stuelten CH, Kilts T, et al. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280(34):30481–30489. [DOI] [PubMed] [Google Scholar]
- 97.Berendsen AD, Pinnow EL, Maeda A, et al. Biglycan modulates angiogenesis and bone formation during fracture healing. Matrix Biol. 2014;35:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vlodavsky I, Singh P, Boyango I, et al. Heparanase: From basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016;29:54–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Grünert M, Nurcombe V, Cool SM. Stem cell fate decisions: the role of heparan sulfate in the control of autocrine and paracrine signals. Curr Stem Cell Res Ther. 2008;3(1):1–8. [DOI] [PubMed] [Google Scholar]
- 100.Huegel J, Sgariglia F, Enomoto-Iwamoto M, Koyama E, Dormans JP, Pacifici M. Heparan sulfate in skeletal development, growth, and pathology: the case of hereditary multiple exostoses. Dev Dyn. 2013;242(9):1021–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pacifici M. The pathogenic roles of heparan sulfate deficiency in hereditary multiple exostoses. Matrix Biol. 2018;71–72:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rider CC, Mulloy B. Heparin, Heparan Sulphate and the TGF-β Cytokine Superfamily. Molecules. 2017;22(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zeng-Brouwers J, Beckmann J, Nastase MV, Iozzo RV, Schaefer L. De novo expression of circulating biglycan evokes an innate inflammatory tissue response via MyD88/TRIF pathways. Matrix Biol. 2014;35:132–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng-Brouwers J, Pandey S, Trebicka J, Wygrecka M, Schaefer L. Communications via the Small Leucine-rich Proteoglycans: Molecular Specificity in Inflammation and Autoimmune Diseases. J Histochem Cytochem. 2020;68(12):887–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J Biol Chem. 2014;289(51):35237–35245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schaefer L, Babelova A, Kiss E, et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J Clin Invest. 2005;115(8):2223–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schaefer L, Reinhardt DP. Special issue: Extracellular matrix: Therapeutic tools and targets in cancer treatment. Adv Drug Deliv Rev. 2016;97:1–3. [DOI] [PubMed] [Google Scholar]
- 108.Babelova A, Moreth K, Tsalastra-Greul W, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284(36):24035–24048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moreth K, Frey H, Hubo M, et al. Biglycan-triggered TLR-2- and TLR-4-signaling exacerbates the pathophysiology of ischemic acute kidney injury. Matrix Biol. 2014;35:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hsieh LT, Nastase MV, Zeng-Brouwers J, Iozzo RV, Schaefer L. Soluble biglycan as a biomarker of inflammatory renal diseases. Int J Biochem Cell Biol. 2014;54:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moreth K, Brodbeck R, Babelova A, et al. The proteoglycan biglycan regulates expression of the B cell chemoattractant CXCL13 and aggravates murine lupus nephritis. J Clin Invest. 2010;120(12):4251–4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Miguez PA, Terajima M, Nagaoka H, Mochida Y, Yamauchi M. Role of glycosaminoglycans of biglycan in BMP-2 signaling. Biochem Biophys Res Commun. 2011;405(2):262–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miguez PA, Terajima M, Nagaoka H, et al. Recombinant biglycan promotes bone morphogenetic protein-induced osteogenesis. J Dent Res. 2014;93(4):406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rühland C, Schönherr E, Robenek H, et al. The glycosaminoglycan chain of decorin plays an important role in collagen fibril formation at the early stages of fibrillogenesis. Febs j. 2007;274(16):4246–4255. [DOI] [PubMed] [Google Scholar]
- 115.Merline R, Moreth K, Beckmann J, et al. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and MicroRNA-21. Sci Signal. 2011;4(199):ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seidler DG, Mohamed NA, Bocian C, et al. The role for decorin in delayed-type hypersensitivity. J Immunol. 2011;187(11):6108–6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Köninger J, Giese NA, Bartel M, et al. The ECM proteoglycan decorin links desmoplasia and inflammation in chronic pancreatitis. J Clin Pathol. 2006;59(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karamanou K, Perrot G, Maquart FX, Brézillon S. Lumican as a multivalent effector in wound healing. Adv Drug Deliv Rev. 2018;129:344–351. [DOI] [PubMed] [Google Scholar]
- 119.Kao WW, Funderburgh JL, Xia Y, Liu CY, Conrad GW. Focus on molecules: lumican. Exp Eye Res. 2006;82(1):3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zheng Z, Jian J, Velasco O, et al. Fibromodulin Enhances Angiogenesis during Cutaneous Wound Healing. Plast Reconstr Surg Glob Open. 2014;2(12):e275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kalamajski S, Bihan D, Bonna A, Rubin K, Farndale RW. Fibromodulin Interacts with Collagen Cross-linking Sites and Activates Lysyl Oxidase. J Biol Chem. 2016;291(15):7951–7960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kokenyesi R, Bernfield M. Core protein structure and sequence determine the site and presence of heparan sulfate and chondroitin sulfate on syndecan-1. J Biol Chem. 1994;269(16):12304–12309. [PubMed] [Google Scholar]
- 123.Tarbell JM, Pahakis MY. Mechanotransduction and the glycocalyx. J Intern Med. 2006;259(4):339–350. [DOI] [PubMed] [Google Scholar]
- 124.Manon-Jensen T, Itoh Y, Couchman JR. Proteoglycans in health and disease: the multiple roles of syndecan shedding. The FEBS Journal. 2010;277(19):3876–3889. [DOI] [PubMed] [Google Scholar]
- 125.Voyvodic PL, Min D, Liu R, et al. Loss of syndecan-1 induces a pro-inflammatory phenotype in endothelial cells with a dysregulated response to atheroprotective flow. J Biol Chem. 2014;289(14):9547–9559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vanhoutte D, Schellings MW, Götte M, et al. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115(4):475–482. [DOI] [PubMed] [Google Scholar]
- 127.Strand ME, Aronsen JM, Braathen B, et al. Shedding of syndecan-4 promotes immune cell recruitment and mitigates cardiac dysfunction after lipopolysaccharide challenge in mice. J Mol Cell Cardiol. 2015;88:133–144. [DOI] [PubMed] [Google Scholar]
- 128.Vuong TT, Reine TM, Sudworth A, Jenssen TG, Kolset SO. Syndecan-4 is a major syndecan in primary human endothelial cells in vitro, modulated by inflammatory stimuli and involved in wound healing. J Histochem Cytochem. 2015;63(4):280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Gopal S. Syndecans in Inflammation at a Glance. Front Immunol. 2020;11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rangarajan S, Richter JR, Richter RP, et al. Heparanase-enhanced Shedding of Syndecan-1 and Its Role in Driving Disease Pathogenesis and Progression. J Histochem Cytochem. 2020;68(12):823–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.McDonald KK, Cooper S, Danielzak L, Leask RL. Glycocalyx Degradation Induces a Proinflammatory Phenotype and Increased Leukocyte Adhesion in Cultured Endothelial Cells under Flow. PLoS One. 2016;11(12):e0167576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hahn RG, Patel V, Dull RO. Human glycocalyx shedding: Systematic review and critical appraisal. Acta Anaesthesiol Scand. 2021;65(5):590–606. [DOI] [PubMed] [Google Scholar]
- 134.Page C. Heparin and related drugs: beyond anticoagulant activity. ISRN Pharmacol. 2013;2013:910743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-Inflammatory Effects of Heparin and Its Derivatives: A Systematic Review. Adv Pharmacol Sci. 2015;2015:507151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma J, Bai J. Protective effects of heparin on endothelial cells in sepsis. Int J Clin Exp Med. 2015;8(4):5547–5552. [PMC free article] [PubMed] [Google Scholar]
- 137.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cornet AD, Smit EG, Beishuizen A, Groeneveld AB. The role of heparin and allied compounds in the treatment of sepsis. Thromb Haemost. 2007;98(3):579–586. [PubMed] [Google Scholar]
- 139.Costanzo L, Palumbo FP, Ardita G, Antignani PL, Arosio E, Failla G. Coagulopathy, thromboembolic complications, and the use of heparin in COVID-19 pneumonia. J Vasc Surg Venous Lymphat Disord. 2020;8(5):711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thachil J. The versatile heparin in COVID-19. J Thromb Haemost. 2020;18(5):1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Saithong S, Saisorn W, Tovichayathamrong P, et al. Anti-Inflammatory Effects and Decreased Formation of Neutrophil Extracellular Traps by Enoxaparin in COVID-19 Patients. Int J Mol Sci. 2022;23(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vitkov L, Muñoz LE, Schoen J, et al. Neutrophils Orchestrate the Periodontal Pocket. Front Immunol. 2021;12:788766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Manfredi AA, Rovere-Querini P, D’Angelo A, Maugeri N. Low molecular weight heparins prevent the induction of autophagy of activated neutrophils and the formation of neutrophil extracellular traps. Pharmacol Res. 2017;123:146–156. [DOI] [PubMed] [Google Scholar]
- 144.Shute JK, Puxeddu E, Calzetta L. Therapeutic use of heparin and derivatives beyond anticoagulation in patients with bronchial asthma or COPD. Curr Opin Pharmacol. 2018;40:39–45. [DOI] [PubMed] [Google Scholar]
- 145.Olczyk P, Komosińska-Vassev K, Winsz-Szczotka K, et al. Propolis modulates vitronectin, laminin, and heparan sulfate/heparin expression during experimental burn healing. J Zhejiang Univ Sci B. 2012;13(11):932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mangiafico M, Caff A, Costanzo L. The Role of Heparin in COVID-19: An Update after Two Years of Pandemics. J Clin Med. 2022;11(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oduah EI, Linhardt RJ, Sharfstein ST. Heparin: Past, Present, and Future. Pharmaceuticals (Basel). 2016;9(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang L, Brown JR, Varki A, Esko JD. Heparin’s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins. The Journal of Clinical Investigation. 2002;110(1):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ding F, Song JH, Jung JY, et al. A biomimetic membrane device that modulates the excessive inflammatory response to sepsis. PLoS One. 2011;6(4):e18584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Amenta AR, Yilmaz A, Bogdanovich S, et al. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc Natl Acad Sci U S A. 2011;108(2):762–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jiang W, Ting K, Lee S, et al. Fibromodulin reduces scar size and increases scar tensile strength in normal and excessive-mechanical-loading porcine cutaneous wounds. J Cell Mol Med. 2018;22(4):2510–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu XJ, Kong FZ, Wang YH, et al. Lumican Accelerates Wound Healing by Enhancing α2β1 Integrin-Mediated Fibroblast Contractility. PLoS One. 2013;8(6):e67124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Aravamudhan A, Ramos DM, Nip J, et al. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr Pharm Des. 2013;19(19):3420–3428. [DOI] [PubMed] [Google Scholar]
- 154.James AW, LaChaud G, Shen J, et al. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng Part B Rev. 2016;22(4):284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zamboni F, Wong CK, Collins MN. Hyaluronic acid association with bacterial, fungal and viral infections: Can hyaluronic acid be used as an antimicrobial polymer for biomedical and pharmaceutical applications? Bioact Mater. 2023;19:458–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lindahl U, Kjellén L. Pathophysiology of heparan sulphate: many diseases, few drugs. J Intern Med. 2013;273(6):555–571. [DOI] [PubMed] [Google Scholar]
- 157.Rönnberg E, Melo FR, Pejler G. Mast cell proteoglycans. J Histochem Cytochem. 2012;60(12):950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peplow PV. Glycosaminoglycan: a candidate to stimulate the repair of chronic wounds. Thromb Haemost. 2005;94(1):4–16. [DOI] [PubMed] [Google Scholar]
- 159.Olczyk P, Mencner Ł, Komosinska-Vassev K. Diverse Roles of Heparan Sulfate and Heparin in Wound Repair. Biomed Res Int. 2015;2015:549417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gómez Toledo A, Sorrentino JT, Sandoval DR, Malmström J, Lewis NE, Esko JD. A Systems View of the Heparan Sulfate Interactome. J Histochem Cytochem. 2021;69(2):105–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Farach-Carson MC, Hecht JT, Carson DD. Heparan sulfate proteoglycans: key players in cartilage biology. Crit Rev Eukaryot Gene Expr. 2005;15(1):29–48. [DOI] [PubMed] [Google Scholar]
- 162.Jackson RA, Nurcombe V, Cool SM. Coordinated fibroblast growth factor and heparan sulfate regulation of osteogenesis. Gene. 2006;379:79–91. [DOI] [PubMed] [Google Scholar]
- 163.Li M, Yang S, Xu D. Heparan Sulfate Regulates the Structure and Function of Osteoprotegerin in Osteoclastogenesis. J Biol Chem. 2016;291(46):24160–24171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li M, Xu D. Antiresorptive activity of osteoprotegerin requires an intact heparan sulfate-binding site. Proc Natl Acad Sci U S A. 2020;117(29):17187–17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ojeh N, Hiilesvuo K, Wärri A, Salmivirta M, Henttinen T, Määttä A. Ectopic expression of syndecan-1 in basal epidermis affects keratinocyte proliferation and wound re-epithelialization. J Invest Dermatol. 2008;128(1):26–34. [DOI] [PubMed] [Google Scholar]
- 166.Fuster MM, Wang L. Endothelial heparan sulfate in angiogenesis. Prog Mol Biol Transl Sci. 2010;93:179–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wegrowski Y, Milard AL, Kotlarz G, Toulmonde E, Maquart FX, Bernard J. Cell surface proteoglycan expression during maturation of human monocytes-derived dendritic cells and macrophages. Clin Exp Immunol. 2006;144(3):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lepedda AJ, Nieddu G, Piperigkou Z, Kyriakopoulou K, Karamanos N, Formato M. Circulating Heparan Sulfate Proteoglycans as Biomarkers in Health and Disease. Semin Thromb Hemost. 2021;47(03):295–307. [DOI] [PubMed] [Google Scholar]
- 170.Meirovitz A, Goldberg R, Binder A, Rubinstein AM, Hermano E, Elkin M. Heparanase in inflammation and inflammation-associated cancer. Febs j. 2013;280(10):2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Grindel BJ, Martinez JR, Pennington CL, et al. Matrilysin/matrix metalloproteinase-7(MMP7) cleavage of perlecan/HSPG2 creates a molecular switch to alter prostate cancer cell behavior. Matrix Biol. 2014;36:64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Machin DR, Phuong TT, Donato AJ. The role of the endothelial glycocalyx in advanced age and cardiovascular disease. Curr Opin Pharmacol. 2019;45:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vlodavsky I, Barash U, Nguyen HM, Yang SM, Ilan N. Biology of the Heparanase-Heparan Sulfate Axis and Its Role in Disease Pathogenesis. Semin Thromb Hemost. 2021;47(3):240–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seidel C, Sundan A, Hjorth M, et al. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000;95(2):388–392. [PubMed] [Google Scholar]
- 175.Lovell R, Dunn JA, Begum G, et al. Soluble syndecan-1 level at diagnosis is an independent prognostic factor in multiple myeloma and the extent of fall from diagnosis to plateau predicts for overall survival. Br J Haematol. 2005;130(4):542–548. [DOI] [PubMed] [Google Scholar]
- 176.Yang H, Wang L. Heparan sulfate proteoglycans in cancer: Pathogenesis and therapeutic potential. Adv Cancer Res. 2023;157:251–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Paine-Saunders S, Viviano BL, Economides AN, Saunders S. Heparan sulfate proteoglycans retain Noggin at the cell surface: a potential mechanism for shaping bone morphogenetic protein gradients. J Biol Chem. 2002;277(3):2089–2096. [DOI] [PubMed] [Google Scholar]
- 178.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol. 2011;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bauer S, Zhang F, Linhardt RJ. Implications of Glycosaminoglycans on Viral Zoonotic Diseases. Diseases. 2021;9(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cagno V, Tseligka ED, Jones ST, Tapparel C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses. 2019;11(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.de Haan CA, Li Z, te Lintelo E, Bosch BJ, Haijema BJ, Rottier PJ. Murine coronavirus with an extended host range uses heparan sulfate as an entry receptor. J Virol. 2005;79(22):14451–14456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hippensteel JA, LaRiviere WB, Colbert JF, Langouët-Astrié CJ, Schmidt EP. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L211–l217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Milewska A, Zarebski M, Nowak P, Stozek K, Potempa J, Pyrc K. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J Virol. 2014;88(22):13221–13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Clausen TM, Sandoval DR, Spliid CB, et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell. 2020;183(4):1043–1057.e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim SY, Jin W, Sood A, et al. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antiviral Res. 2020;181:104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhang Q, Chen CZ, Swaroop M, et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020;6(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Tiwari V, Maus E, Sigar IM, Ramsey KH, Shukla D. Role of heparan sulfate in sexually transmitted infections. Glycobiology. 2012;22(11):1402–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Van Dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. 2017;58:21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Patil CS, Kirkwood KL. p38 MAPK signaling in oral-related diseases. J Dent Res. 2007;86(9):812–825. [DOI] [PubMed] [Google Scholar]
- 190.Dong G, Song L, Tian C, et al. FOXO1 Regulates Bacteria-Induced Neutrophil Activity. Front Immunol. 2017;8:1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sanz M, Ceriello A, Buysschaert M, et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 2018;45(2):138–149. [DOI] [PubMed] [Google Scholar]
- 192.Purvis JA, Embery G, Oliver WM. Molecular size distribution of proteoglycans in human inflamed gingival tissue. Arch Oral Biol. 1984;29(7):513–519. [DOI] [PubMed] [Google Scholar]
- 193.Kirkham J, Robinson C, Smith AJ, Spence JA. The effect of periodontal disease on sulphated glycosylaminoglycan distribution in the sheep periodontium. Arch Oral Biol. 1992;37(12):1031–1037. [DOI] [PubMed] [Google Scholar]
- 194.Oksala O, Haapasalmi K, Häkkinen L, Uitto VJ, Larjava H. Expression of heparan sulphate and small dermatan/chondroitin sulphate proteoglycans in chronically inflamed human periodontium. J Dent Res. 1997;76(6):1250–1259. [DOI] [PubMed] [Google Scholar]
- 195.Bartold PM, Page RC. Proteoglycans synthesized by cultured fibroblasts derived from normal and inflamed human gingiva. In Vitro Cell Dev Biol. 1986;22(7):407–417. [DOI] [PubMed] [Google Scholar]
- 196.Roberts HC, Moseley R, Sloan AJ, Youde SJ, Waddington RJ. Lipopolysaccharide alters decorin and biglycan synthesis in rat alveolar bone osteoblasts: consequences for bone repair during periodontal disease. Eur J Oral Sci. 2008;116(3):207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Duplancic R, Roguljic M, Puhar I, et al. Syndecans and Enzymes for Heparan Sulfate Biosynthesis and Modification Differentially Correlate With Presence of Inflammatory Infiltrate in Periodontitis. Front Physiol. 2019;10:1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Li H, Chapla D, Amos RA, Ramiah A, Moreman KW, Li H. Structural basis for heparan sulfate co-polymerase action by the EXT1–2 complex. Nat Chem Biol. 2022:doi. 10.1038/s41589-41022-01220-41582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Leisico F, Omeiri J, Le Narvor C, et al. Structure of the human heparan sulfate polymerase complex EXT1-EXT2. Nat Commun. 2022;13:7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Liu J, Pedersen LC. Emerging chemical and biochemical tools for studying 3- O-sulfated heparan sulfate. Am J Physiol Cell Physiol. 2022;322:C1166–C1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu J, Pedersen LC. Anticoagulant heparan sulfate: Structural specificity and biosynthesis. Appl Microbiol Biotechnol. 2007;74:263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Gama C, Tully SE, Sotogaku N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. [DOI] [PubMed] [Google Scholar]
- 203.Liu J, Linhardt RJ. Chemoenzymatic synthesis of heparan sulfate and heparin. Nat Prod Rep. 2014;31:1676–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Liu R, Xu Y, Chen M, et al. Chemoenzymatic design of heparan sulfate oligosaccharides. J Biol Chem. 2010;285:34240–34249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu Y, Masuko S, Takieddin M, et al. Chemoenzymatic synthesis of homogeneous ultra-low molecular weight heparin. Science. 2011;334:498–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Xu Y, Cai C, Chandarajoti K, et al. Homogeneous and reversible low-molecular weight heparins with reversible anticoagulant activity. Nat Chem Biol. 2014;10:248–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Xu Y, Chandarajoti K, Zhang X, et al. Synthetic oligosaccharides can replace animal-sourced low-molecular weight heparins. Sci Transl Med. 2017;9:eaan5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Szajek A, Chess EK, Johanssen K, et al. The US regulatory and pharmacopeia responses to the global heparin contamination crisis. Nat Biotechnol. 2016;34:625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Turnbull J. Getting the farm out of pharma for heparin production. Science. 2011;334:462–463. [DOI] [PubMed] [Google Scholar]
- 210.Bernal W, Wendon J. Acute liver failure. N Eng J Med. 2013;369:2525–2534. [DOI] [PubMed] [Google Scholar]
- 211.Huebener P, Pradere JP, Hernandez C, et al. The HMGB1/RAGE axis triggers neutrophil-mediated injury amplification following necrosis. J Clin Invest. 2015;125:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Arnold KM, Xu Y, Sparkenbaugh EM, et al. Design of anti-inflammatory heparan sulfate to protect against acetaminophen-induced accute liver failure. Sci Transl Med. 2020;12:eaav8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. Journal of dental research. 2014;93(11):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Eke PI, Dye BA, Wei L, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. Journal of periodontology. 2015;86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontology 2000. 2016;71(1):82–112. [DOI] [PubMed] [Google Scholar]
- 216.Choi E, Um HS, Chang BS, Lee SY, Lee JK. Clinical and microbiological effects of adjunctive local delivery of minocycline (Periocline®) in patients receiving supportive periodontal therapy: a pilot study. J Periodontal Implant Sci. 2021;51(1):53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Killeen AC, Harn JA, Jensen J, Yu F, Custer S, Reinhardt RA. Two-Year Randomized Clinical Trial of Adjunctive Minocycline Microspheres in Periodontal Maintenance. J Dent Hyg. 2018;92(4):51–58. [PubMed] [Google Scholar]
- 218.Curtis MA, Diaz PI, Van Dyke TE. The role of the microbiota in periodontal disease. Periodontology 2000. 2020;83(1):14–25. [DOI] [PubMed] [Google Scholar]
- 219.Colombo AP, Haffajee AD, Dewhirst FE, et al. Clinical and microbiological features of refractory periodontitis subjects. J Clin Periodontol. 1998;25(2):169–180. [DOI] [PubMed] [Google Scholar]
- 220.Santos RS, Macedo RF, Souza EA, Soares RS, Feitosa DS, Sarmento CF. The use of systemic antibiotics in the treatment of refractory periodontitis: A systematic review. J Am Dent Assoc. 2016;147(7):577–585. [DOI] [PubMed] [Google Scholar]
- 221.Tonetti MS, Chapple IL. Biological approaches to the development of novel periodontal therapies--consensus of the Seventh European Workshop on Periodontology. Journal of clinical periodontology. 2011;38 Suppl 11:114–118. [DOI] [PubMed] [Google Scholar]
- 222.Walters J, Lai PC. Should Antibiotics Be Prescribed to Treat Chronic Periodontitis? Dent Clin North Am. 2015;59(4):919–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Sanz M, Herrera D, Kebschull M, et al. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. Journal of clinical periodontology. 2020;47 Suppl 22(Suppl 22):4–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Muir JM, Hirsh J, Weitz JI, Andrew M, Young E, Shaughnessy SG. A histomorphometric comparison of the effects of heparin and low-molecular-weight heparin on cancellous bone in rats. Blood. 1997;89(9):3236–3242. [PubMed] [Google Scholar]
- 225.Alban S. Adverse effects of heparin. Handb Exp Pharmacol. 2012(207):211–263. [DOI] [PubMed] [Google Scholar]
- 226.Monreal M, Lafoz E, Olive A, del Rio L, Vedia C. Comparison of subcutaneous unfractionated heparin with a low molecular weight heparin (Fragmin) in patients with venous thromboembolism and contraindications to coumarin. Thromb Haemost. 1994;71(1):7–11. [PubMed] [Google Scholar]
- 227.Oballe HJR, Lamers ML, Muniz F, Spuldaro TR, Gaio EJ, Rösing CK. Effects of Low Molecular Weight Heparin on Alveolar Bone Loss in Wistar Rats. Braz Dent J. 2019;30(1):12–21. [DOI] [PubMed] [Google Scholar]
- 228.Coyac BR, Detzen L, Doucet P, et al. Periodontal reconstruction by heparan sulfate mimetic-based matrix therapy in Porphyromonas gingivalis-infected mice. Heliyon. 2018;4(8):e00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Herrera D, Sanz M, Kebschull M, et al. Treatment of stage IV periodontitis: The EFP S3 level clinical practice guideline. J Clin Periodontol. 2022;49 Suppl 24:4–71. [DOI] [PubMed] [Google Scholar]
- 230.Migliorini E, Horn P, Haraszti T, et al. Enhanced Biological Activity of BMP-2 Bound to Surface-Grafted Heparan Sulfate. Adv Biosyst. 2017;1(4):e1600041. [DOI] [PubMed] [Google Scholar]
- 231.Kuo WJ, Digman MA, Lander AD. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell. 2010;21(22):4028–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, Saunders S. Domain-specific Modification of Heparan Sulfate by Qsulf1 Modulates the Binding of the Bone Morphogenetic Protein Antagonist Noggin*. Journal of Biological Chemistry. 2004;279(7):5604–5611. [DOI] [PubMed] [Google Scholar]
- 233.Murali S, Rai B, Dombrowski C, et al. Affinity-selected heparan sulfate for bone repair. Biomaterials. 2013;34(22):5594–5605. [DOI] [PubMed] [Google Scholar]
- 234.Gallagher ZJ, Fleetwood S, Kirley TL, et al. Heparin Mimic Material Derived from Cellulose Nanocrystals. Biomacromolecules. 2020;21(3):1103–1111. [DOI] [PubMed] [Google Scholar]
- 235.Nozawa S, Inubushi T, Irie F, et al. Osteoblastic heparan sulfate regulates osteoprotegerin function and bone mass. JCI Insight. 2018;3(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. ACS Nano. 2009;3(1):16–20. [DOI] [PubMed] [Google Scholar]
- 237.Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10(4):1588–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Laner-Plamberger S, Oeller M, Rohde E, Schallmoser K, Strunk D. Heparin and Derivatives for Advanced Cell Therapies. Int J Mol Sci. 2021;22(21). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Salbach J, Rachner TD, Rauner M, et al. Regenerative potential of glycosaminoglycans for skin and bone. J Mol Med (Berl). 2012;90(6):625–635. [DOI] [PubMed] [Google Scholar]
- 240.Li X, Ma X. The role of heparin in sepsis: much more than just an anticoagulant. Br J Haematol. 2017;179(3):389–398. [DOI] [PubMed] [Google Scholar]
- 241.Gockel LM, Nekipelov K, Ferro V, Bendas G, Schlesinger M. Tumour cell-activated platelets modulate the immunological activity of CD4(+), CD8(+), and NK cells, which is efficiently antagonized by heparin. Cancer Immunol Immunother. 2022;71(10):2523–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pettilä V, Leinonen P, Markkola A, Hiilesmaa V, Kaaja R. Postpartum bone mineral density in women treated for thromboprophylaxis with unfractionated heparin or LMW heparin. Thromb Haemost. 2002;87(2):182–186. [PubMed] [Google Scholar]
- 243.Muir JM, Andrew M, Hirsh J, et al. Histomorphometric analysis of the effects of standard heparin on trabecular bone in vivo. Blood. 1996;88(4):1314–1320. [PubMed] [Google Scholar]
- 244.Lallam-Laroye C, Escartin Q, Zlowodzki AS, et al. Periodontitis destructions are restored by synthetic glycosaminoglycan mimetic. J Biomed Mater Res A. 2006;79(3):675–683. [DOI] [PubMed] [Google Scholar]
- 245.Lallam-Laroye C, Baroukh B, Doucet P, Barritault D, Saffar JL, Colombier ML. ReGeneraTing agents matrix therapy regenerates a functional root attachment in hamsters with periodontitis. Tissue Eng Part A. 2011;17(17–18):2359–2367. [DOI] [PubMed] [Google Scholar]
- 246.Barritault D, Gilbert-Sirieix M, Rice KL, et al. RGTA(®) or ReGeneraTing Agents mimic heparan sulfate in regenerative medicine: from concept to curing patients. Glycoconj J. 2017;34(3):325–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Pereira S, Resende R, Coelho P, Sampaio F. Matrix Regenerating Agent (RGTA) in a Neurotrophic Corneal Ulcer. Cureus. 2020;12(10):e11167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Any raw data supporting reported results can be requested by contacting the corresponding author.